Abstract
In the central nervous system (CNS), neuronal functionality is highly dependent on mitochondrial integrity and activity. In the context of a damaged or diseased brain, mitochondrial dysfunction leads to reductions in ATP levels, thus impairing ATP-dependent neural firing and neurotransmitter dynamics. Restoring mitochondrial ability to generate ATP may be a basic premise to restore neuronal functionality. Recently, emerging data in rodent and human studies suggest that mitochondria and its components are surprisingly released into extracellular space and potentially transferred between cells. Transferred mitochondria may support oxidative phosphorylation in recipient cells. In this mini-review, we (a) survey recent findings in cell to cell mitochondrial transfer and the presence of cell-free extracellular mitochondria and its components, (b) review experimental details of how to detect extracellular mitochondria and mitochondrial transfer in the CNS, (c) discuss strategies and tissue sources for mitochondria isolation, and (d) explore exogenous mitochondrial transplantation as a novel approach for CNS therapies.
Keywords: Central nervous system, extracellular mitochondria, mtDNA, mitochondrial transplantation
Introduction
Mitochondria comprise the intracellular energetic core of cells. Because the brain is a high-metabolism organ, mitochondria play a vital homeostatic role in almost all aspects of cell physiology and pathophysiology. Mitochondria generate the majority of adenosine triphosphate (ATP) (Jonckheere, et al., 2012, Murphy, et al., 2019), biosynthesis of fatty acids (Kastaniotis, et al., 2017), cellular calcium buffering (David and Barrett, 2003, Pivovarova and Andrews, 2010), and also act as a platform to integrate cell signaling circuitry that modulates cell survival, immune response, and autophagy (Tait and Green, 2012). During injury or disease, mitochondria may be a key regulator for neurodegeneration as well as neurorecovery, depending on its functionality (Anne Stetler, et al., 2013, Johri and Beal, 2012, Li and Stary, 2016, Lin and Beal, 2006, McEwen, et al., 2011).
Recently, emerging data suggest that mitochondria may be released into extracellular space and potentially transferred from cell to cell in the central nervous system (CNS). In the damaged or diseased brain, extracellular mitochondria might represent a novel class of intercellular signals that can be deleterious or beneficial, depending on the context. In this regard, healthy and viable mitochondrial transplantation into the injured tissues may become a novel therapeutic approach to rescue recipient cells and restore the normal function. In this mini-review, we survey representative studies of intercellular mitochondrial transfer in the CNS, review methodologies for assessing extracellular mitochondria and mitochondrial transfer, discuss potential tissue sources for mitochondria isolation and application, and finally explore the overall hypothesis of mitochondrial transplantation as a therapeutic approach in various models of CNS injury and disease.
1. Mitochondrial transfer and extracellular mitochondria in CNS pathophysiology
Mitochondria comprise the intracellular cores for cell energetics and viability. Recent findings suggest that mitochondria might also be actively released into extracellular space and transferred between cells. Here, we survey recent findings regarding intercellular mitochondrial transfer in the CNS. Additionally, we summarize experimental techniques used to detect mitochondrial transfer from cell to cell in Table 1.
Table 1.
Summary of method or techniques to investigate mitochondrial transfer
| A. Labeling method or technique | |
| MitoTracker dyes | [9], [11], [15], [19], [20], [34], [54], [55], [66], [56], [69], [73] |
| JC1 dye for mitochondrial membrane potential | [9], [19], [20], [27], [50], [54], [56], [69], [73] |
| Furuorescent protein | [8], [15], [16], [23], [54] |
| Tag mitoDNA by BrdU | [23] |
| pDsRed2-Mito vector | [36] |
| Mito-DsRed2 vectors | [30] |
| DsRed Cox8-GFP | [51] |
| B. Detection Method or Device | |
| Confocal mictoscopy | [8], [11], [15], [16], [19], [20], [23], [34], [36], [51], [54], [56], [66], [73] |
| Fluorescence microscope | [30], [69] |
| Electron microscopy | [9], [11], [19], [20], [23], [27], [30], [56], [69], [73] |
| 3D tomographic microscopy | [30], [56] |
| Time-lapse microscopy | [55] |
| Flow cytometry | [9], [15], [19], [20], [30], [56], [69], [73] |
| Mitochondrial genotype | [56] |
| PCR (mtDNA) | [6], [19], [30], [45], [47], [55] |
| Fluorescence (detect for JC1 dye) | [9], [27], [54] |
| Genotyping for mtGFP | [51] |
DNA; deoxyribonucleic acid, BrdU; Bromodeoxyuridine, GFP; green fluorescent protein, FACS; fluorescence-activated cell sorting, PCR,; polymerase chain reaction, mtDNA; mitochondria DNA, mtGFP; mitochondria GFP.
1.1. Intercellular mitochondrial transfer in the CNS
1.1.1. neuron to astrocyte
The mitochondrial exchange between cells has been reported in retinal ganglion cell axons at the optic nerve head. In this study, AAV2-MitoEGFPmCherry was delivered into the vitreous space to visualize mitochondria at the optic nerve head. Mitochondrial degradation induced by intravitreal treatment with rotenone was assessed by fluorescence in situ hybridization (FISH) of mitochondrial DNA accompanied by TUNEL staining. Serial block-face scanning electron microscopy analysis confirmed that evulsions of retinal ganglion cell axons contained mitochondria. These axon-derived mitochondria labeled by MitoEGFPmCherry were found in retinal astrocytes labeled by GLT1-EGFP, and confocal microscopy confirmed that these axon-derived mitochondria were degraded within astrocytes. In a combination of cellular, molecular, and advanced imaging tools, authors nicely demonstrated the transcellular degradation of mitochondria as a new mechanism of mitophagy in the CNS (Davis, et al., 2014).
1.1.2. astrocyte to neuron
Recent proof-of-concept studies demonstrate that astrocytes can also release and transfer mitochondria into damaged neurons and promote neuroprotection and neurorepair via calcium-dependent CD38 signaling (Hayakawa, et al., 2016, Huang, et al., 2019, Lippert and Borlongan, 2019). First, primary cortical astrocytic mitochondria were labeled with Mitotracker CMXRos or CellLight Mitochondria-GFP. A battery of assays including confocal imaging, transmission electron microscopy, flow cytometry, and mitochondrial function assays (CellTiter-Glo, JC1) indicated the presence of functional extracellular mitochondria in astrocyte-conditioned media. When astrocyte-conditioned media were then added to primary neurons, these extracellular mitochondria appeared to enter into neurons and protect them against oxygen-glucose deprivation as well as promote dendritic markers of neuroplasticity. Similar results were obtained in vivo. In mouse models of focal cerebral ischemia, astrocyte-derived mitochondria indicated by GFAP-GFP and Tom40 also appeared to enter into neurons, and upregulate pro-survival anti-apoptotic signals. Conversely, the blockade of transfer of mitochondria from astrocytes into adjacent neurons with CD38 siRNA worsened stroke recovery outcomes in these in vivo mouse models of focal ischemia. Interestingly, mutations in GFAP disrupted the transfer of mitochondria (labeled by lentivirus mitodsred) from astrocytes to neurons was accompanied by decreasing astrocytic CD38 expression (Gao, et al., 2019), suggesting that CD38 signaling can be targeted to modify mitochondrial transfer. It has been reported that mitochondria can enter cells via endocytosis (Sun, et al., 2019, Wei, et al., 2018), integrin-src/syk dependent mechanisms (Hayakawa, et al., 2016), macro-pinocytosis (Kitani, et al., 2014), connexin 43-mediated mitochondrial transfer (Yao, et al., 2018), tunneling nanotubes or cell fusion (Spees, et al., 2006, Torralba, et al., 2016). Whether and how CD38 signaling is associated with these mitochondria uptake mechanisms may be interesting to pursue in the future study.
1.1.3. microglia to astrocyte to neuron
Accumulating studies have implicated that reactive astrocytes have phenotypes that may be impacted by the type of injury or disease. For example, reactive astrocytes in stroke may be beneficial or protective phenotype (A2), whereas treatment with LPS or neurodegenerative diseases such as Parkinson’s disease, Alzheimer’s disease, Huntington’s disease (HD), and Amyotrophic lateral sclerosis (ALS) can induce detrimental pro-inflammatory phenotype (A1) (Khakh, et al., 2017, Liddelow, et al., 2017, Trias, et al., 2018, Yun, et al., 2018, Zamanian, et al., 2012). More recently, it was found that activated microglia and A1 reactive astrocytes may release fragmented and dysfunctional mitochondria into the extracellular milieu that propagated neuronal death (Joshi, et al., 2019). In vitro cell culture system, microglia isolated from mouse models of HD and ALS secreted dysfunctional mitochondria into the culture media. Dysfunctional extracellular mitochondria in diseased microglia-conditioned media were determined by fragmentation, lower ATP levels, lower mitochondrial membrane potential, and higher mitochondrial reactive oxygen species (ROS) production using TEM, ATP measurements, TMRM accumulation, JC1, and MitoSOX. Intriguingly, transfer of these microglia-conditioned media to astrocytes induced mitochondrial fragmentation and A1 pro-inflammatory phenotype in the treated astrocytes. Then, A1 astrocytes further released dysfunctional mitochondria. When A1 astrocyte-conditioned media were treated in neurons, mitochondrial functions including ATP contents, TMRM accumulation, and oxygen consumption rates (OCR) measured by Seahouse assay were significantly decreased along with an increase of LDH release. But depleting extracellular dysfunctional mitochondria from media by 0.2 μm filtration or the blockade of Fis1-mediated mitochondrial fragmentation diminished the deleterious effects, suggesting that neuro-glial crosstalk through extracellular dysfunctional mitochondria may propagate injury in neurodegenerative diseases.
1.1.4. endothelial progenitor cell (EPC) to brain endothelial cell
Beyond neural-glial-vascular interactions within the CNS per se, emerging studies suggest that responses from outside the brain are also important for neurovascular remodeling (Carmichael, 2006, Moskowitz, et al., 2010, Thored, et al., 2007). A recent study shows that human endothelial progenitor cells (EPCs) can also produce extracellular functional mitochondria (Hayakawa, et al., 2018). To determine extracellular mitochondria, EPC-conditioned media were collected and particle fractions were analyzed by western blot, qPCR, TEM, and flow cytometry analysis. Functional extracellular mitochondria were determined by MitoTracker CMXRos and oxygen consumption assay using MITO-ID Extracellular O2 Sensor Kit. Furthermore, the transfer of EPC-derived extracellular mitochondria into brain endothelial cells improved cell viability and barrier function. Mitochondrial incorporation and functional benefits following the transfer were confirmed by confocal microscopy, and restorations of ATP and mitochondrial DNA (mtDNA) contents (Hayakawa, et al., 2018). In this study, we also examined media derived from human astrocytes, endothelial cells, and pericytes. Quantitation of flow cytometry data showed that the ability of EPCs to produce extracellular mitochondria was highest, and EPC-derived extracellular mitochondria retained high membrane potentials. Collectively, these findings suggest that cell for cell, human EPCs can be a prolific source of active extracellular mitochondria for protecting brain endothelium after ischemic stroke.
1.1.5. hematopoietic stem/progenitor cell to neuron
Another study demonstrated that infused hematopoietic stem and progenitor cells (HSPCs) appeared to transfer their mitochondria to neurons and restored mitochondrial function along with increasing frataxin expression in a mouse model of Friedreich’s ataxia (Rocca, et al., 2017). To ensure that the transfer of mitochondria occurred in vivo, HSPCs were isolated from cytosolic DsRed Cox8-GFP mice. Quantification in spinal cord tissue by confocal microscopy revealed that 50% of neurons contained Cox8-GFP. Improvements in mitochondrial function were determined by attenuation of the accumulation of oxidized proteins in HSPC-transplanted YG8R mice. Mitochondrial PCR array also revealed that genes related to the solute carrier family of inner mitochondrial membrane transporters and mitochondrial lipid metabolism were significantly upregulated. These findings may provide pieces of evidence that the crosstalk exists between the damaged brain and peripheral responses through mitochondrial transfer in CNS injury and disease.
1.2. Extracellular mitochondria and cell-free mtDNA in the CNS
Accumulating pieces of evidence suggest that mitochondria and the components may be released into extracellular space. However, good or bad effects of extracellular mitochondria may depend on the mitochondrial functional state (Miliotis, et al., 2019). We have reported that mitochondrial membrane potential measurement in cerebrospinal fluid (CSF) may provide an insight on mitochondrial functionality or integrity in the brain (Chou, et al., 2017, Hayakawa, et al., 2018). Indeed, JC1 chemical assay in human CSF samples demonstrated that extracellular mitochondrial membrane potentials were decreased after subarachnoid hemorrhage (SAH), and higher mitochondrial membrane potentials in the CSF were correlated with good clinical recovery at 3 months after SAH onset (Chou, et al., 2017). We also explored the potential cellular origin of these CSF mitochondria signals found in SAH. Because some extracellular mitochondria were contained within membranous particles observed by TEM, cell-specific markers embedded in these membranes may provide indirect information on potential cellular origins. We performed flow cytometry for all functional mitochondria labeled with MitoTracker Red CMXRos, and assessed positive events for vWF (endothelial cell origin), GLAST (astrocyte), CD45 (microglia/macrophage), and CD41/CD61 (platelet). Intriguingly, subjects with good outcomes at 3 months had a significantly higher percentage of GLAST-positive mitochondria in their CSF at day 3 post-SAH, but there was no correlation with other cellular origin markers.
Mitochondrial components may play a regulatory role in modulating immune and inflammatory responses (Galluzzi, et al., 2012). It is well known that cell-free mtDNA may contain damage-associated molecular pattern (DAMP) motifs such as CpG motifs, and thus act as “danger signals” through TLR9 receptor (Galluzzi, et al., 2012, Thurairajah, et al., 2018). mtDNA may cause sepsis-like symptoms in systemic inflammatory response syndrome in a similar manner to bacterial molecules (Wilkins, et al., 2017). Circulating levels of mtDNA molecules increase progressively after the age of 50 and are associated with chronic low-grade inflammation (Picca, et al., 2019, Pinti, et al., 2014), suggesting that an increase of mitochondrial DAMPs may be age-dependent. Extracellular mtDNA is detectable in CSF samples. Cervera-Carles and colleagues compared mtDNA levels in CSF among patients at different stages of Alzheimer’s disease (AD). They found a higher level of mtDNA in CSF from AD patients compared to ones from healthy control subjects (Cervera-Carles, et al., 2017). Furthermore, Peng and colleagues reported that mtDNA levels in CSF samples from patients with anti-N-methyl-D-aspartate receptor encephalitis were significantly higher than control subjects (Peng, et al., 2019).
Collectively, extracellular mitochondria and the components may provide a biomarker-like glimpse into severity and prognosis in pathophysiological conditions such as subarachnoid hemorrhage, neurodegeneration, and inflammation. Future studies are warranted to investigate how physiological variances such as gender and age influence extracellular mitochondria or mtDNA to optimize gender- or age-appropriate thresholds in human patients.
2. Practical strategies for mitochondrial transfer
In the CNS, crosstalk between neuronal, glial and vascular cells is important for maintaining homeostasis, a concept known as the “neurovascular unit” (del Zoppo, 2009, Hawkins and Davis, 2005, Iadecola, 2004, Lo, et al., 2004, Zacchigna, et al., 2008, Zlokovic, 2008), and disruption of homeostatic signals within the neurovascular unit may be fundamental causality of CNS disorders. If functional and viable mitochondria can indeed be released and transferred between cells within the neurovascular unit, this non-cell autonomous signaling may provide novel opportunities for CNS protection and repair. In a proof-of-concept study in heart, McCully and colleagues demonstrated that direct transplantation of autologous mitochondria isolated from the patient’s skeletal muscle into the heart was feasible without inducing immune or auto-immune responses (McCully, et al., 2016), suggesting that mitochondrial transplantation therapy may be applicable in other injuries or diseases.
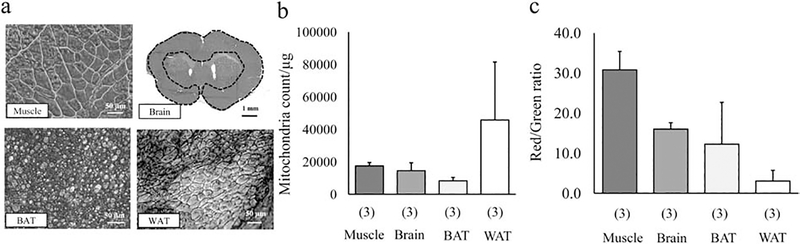
Mitochondria are present in all cells in the body except for red blood cells (Chan, 2006), and various tissues or organs can be sources for mitochondria isolation. But it has been reported that biosynthetic capacities of mitochondria could be varied among organs (Vafai and Mootha, 2012). Moreover, functional homogeneity of isolated mitochondria may be critical for achieving therapeutic efficiency (Preble, et al., 2013). Here, we isolate mitochondrial enriched fractions from various tissues including muscle, brain, brown and white adipose tissue (BAT, WAT) and compare the mitochondria number, purity, and mitochondrial membrane potential in adjusted mitochondrial protein concentration. Hematoxylin-Eosin (HE) staining shows representative tissue structures (Fig.1a). Mitochondria were isolated by a standard mitochondria Isolation Kit purchased from Thermo Fisher Scientific (#89801), and mitochondrial protein concentration was measured by Bradford assay. Isolated mitochondria were suspended in 10 mM HEPES (pH7.5) containing 25 mM sucrose, 1 mM ATP, 0.1 mM ADP, 5 mM sodium succinate, and 2 mM K2HPO4. Then, mitochondrial suspension (10 μg protein / 500 μL in muscle, brain, BAT, or 1–4 μg protein / 500 μL in WAT) was incubated with MitoTracker Deep Red (final conc. 100 nM) and JC1 (final conc. 0.8 μM) for 20 min at 37°C before FACS analysis. Intriguingly, FACS analysis demonstrated that the number of mitochondria in the protein-adjusted suspension was slightly different among tissues (Fig. 1b). Moreover, we found that muscle-derived mitochondria showed the highest JC1 value compared to other tissue-derived mitochondria (Fig. 1c). Altogether, protein content per mitochondrion and mitochondrial functionality might be varied among tissues or organs. Additionally, our data show that muscle-derived mitochondria may be a good candidate for isolation and transplantation as recently suggested (Zhang, et al., 2019), while WAT may not be suitable tissue to isolate mitochondria as shown in the lowest JC1 values after isolation. Future studies are warranted to define mitochondrial dosages with a common unit (e.g. number or protein conc.) and the therapeutic efficacy following mitochondrial transplantation.
Figure 1. Comparison of mitochondria number and JC1 value among various tissues.
a. Hematoxylin-Eosin staining in muscle, brain, brown adipose tissue (BAT) and white adipose tissue (WAT). b. Comparison of the estimated number of isolated mitochondria per protein in FACS analysis (n=3). Muscle: 17,447 ± 2,091 / μg protein, Brain: 14,493 ± 4,900 / μg protein, BAT: 8,268 ± 2,090 / μg protein, WAT: 45,766 ± 35,729 / μg protein. Samples were analyzed for 1 min in the “Lo” flow rate mode (~12 μL/min) by BD Fortessa. c. Comparison of the mitochondrial membrane potential. JC-1 ratio was calculated by the number of JC-1 red positive mitochondrial populations over JC-1 green positive mitochondrial populations. Mitochondria isolated from skeletal muscle had the highest JC1 value compared to ones derived from the cerebral cortex, BAT, or WAT (n=3). Muscle: 30.8 ± 4.6, Brain: 16.0 ± 1.6, BAT: 12.2 ± 10.5, WAT: 3.0 ± 2.7. Results were expressed as mean ± standard deviation.
Mitochondrial storage is one of the key aspect of clinical translation. It has known that isolated mitochondrial functions could be influenced by cooling rate (Araki, 1977, Fishbein and Griffin, 1976, Tsvetkov, et al., 1985, Tsvetkov, et al., 1985), thawing rate (Tsvetkov, et al., 1985, Tsvetkov, et al., 1985) and storage temperature (Araki, 1977) in cryopreservation. Freshly isolated mitochondria are the best option for mitochondrial transplantation as to mitochondrial function (Roushandeh, et al., 2019). But, is it impossible to store mitochondria? Back in 2006, mitochondrial storage has been attempted by Nukala et at. In this study, brain-derived mitochondria were suspended in 10% (v/v) dimethyl sulfoxide as a cryoprotectant, then mitochondria were cooled at a uniform rate of ~1°C/min and stored at −80°C. These stored mitochondria showed roughly 50% of normal respiratory function compared to freshly isolated mitochondria (Nukala, et al., 2006). Another study demonstrated that trehalose-frozen mitochondria preserved the ultrastructure of mitochondria along with retaining the ability to produce ATP and to import proteins (Yamaguchi, et al., 2007). If we can freeze and thaw isolated mitochondria without inducing functional disruption, mitochondrial transplantation therapy will be potentially applicable at the patient bedside. Further studies are warranted to investigate this idea and assess the feasibility of storing mitochondria until the transplantation.
3. Therapeutic use of extracellular mitochondria for CNS injury or disease
So far, more than 400 clinical trials for mitochondrial-targeted medical intervention including completed trials have been registered at ClinicalTrials.gov. However, medicines targeting mitochondrial energy production and loss of physiological ROS function remain to be developed. As a new mitochondria-targeted therapy, exogenous mitochondrial transplantation has been emerged and tested in models of various CNS injuries or diseases. In this section, we summarize “mitochondrial transplantation therapy” reported in the CNS injury or disease. Experimental details including models, dosages of mitochondria, sources, and administration route are summarized in Table 2.
Table 2.
Recent study of exogenous mitochondrial transplantation in CNS disease or injury
| Disease or injury model of animal | Source of mitochondria | Number, dose of mitochondria or number of MSCs | Recipient | Route of transplantation | Localization of mitochondria | Outcome | Reference |
|---|---|---|---|---|---|---|---|
| SCI (rats) | PC12 cell/rat skeltal muscle (allograft) | 50–150 μg | Spinal cord | DI | Macrophages, endothelial cells, pericytes, astrocytes, and oligodendrocytes | Improve OCR, no improvement in long term motor and sensory functions | [16] |
| SZ (rats) | Human lymphocyte/rat brain (heterograft/allograft) | 100 μg | Brain (prefrontal cortex) | DI | Cortical neurons | Prevented dissipation in mitochondrial membrane potential and attentional deficit | [50] |
| PD (rats) | PC12 (human osteosarcoma cybrids) | 1.05 μg | Brain neurons | DI | Pep-1-mediated cell-penetrating mitochondrial delivery | Improve locomotive activity and attenuate deterioration of dopaminergic neurons | [8] |
| PD (mice) | HepG2 | 0.5 mg/kg | Multiple tissues including brain | i.v. injection | Not discussed | Improved behavior test, ATP, increased ETC activity, decreased ROS formation, apoptosis and necrosis | [54] |
| I/R brain injury (mice) | Mouse cortical astrocytes | 1,000 | Peri-infarct cortex | DI | Cortical neurons | Localization of mitochondria in neuron | [20] |
| I/R brain injury (rats) | BHK cells | 75 μg, 750 μg | Brain | DI/intra femoral artery injection | Neuron, astrocyte and microglia in peri-infarct area of the ischemic hemisphere | Improve motor function, decrease infarct area and cell death | [23] |
| I/R brain injury (rats) | Pectoralis major muscle (autologous) | 5×106 | Brain | ICV injection | Neurons around the ischemic penumbra | Decrease infarct volume, neurological deficits, cellular oxidative stress, apoptosis, and gliosis, promote neurogenesis | [73] |
| I/R brain injury (rats) | Rat MSCs (allograft) | 5×105 MSCs | Brain | IA injection | Peri-infarct area | Improved microvasculature, mitochondria function in peri-infarct area and infarct volume and functional recovery | [36] |
CNS; central nervous system, MSCs; mesenchymal stroma cells, SCI; spinal cord injury, PC12; pheochromocytoma cell line, DI; direct injection, OCR; oxygen consumptions rate, SZ; Schizophrenia, PD; Parkinson's disease, i.v., intravenous; HepG2; hepatocellular carcinoma cell line, ATP; adenosine triphosphate, ETC; electron transfer chain, ROS; reactive oxygen species, I/R; Ischemic reperfusion, BHK; baby hamster kidney fibroblasts, ICV; intracerebroventricular, IA; intra-arterial.
3.1. Spinal Cord Injury
Mitochondrial transplantation may have a therapeutic potential in spinal cord injury (SCI). In this study, PC12 cells were labeled with a mitochondria-targeting tGFP (turbo Green Fluorescent Protein). Mitochondria (50–150 μg) were then transplanted into the site of injury within 30 min after SCI induction. Mitochondria incorporation was observed in macrophages, endothelial cells, and astrocytes, but not in neuronal colocalization in confocal microscopy. They demonstrated that mitochondrial transplantation improved acute mitochondrial bioenergetics of the injured tissue measured by oxygen consumption ratio using the metabolic analyzer, and improved mitochondria respiration in State III, V.1, and V.2 OCR. However, in the long term, it did not provide significant functional neuroprotection as assessed by behavioral recovery or tissue sparing (Gollihue, et al., 2018). Collectively, this proof-of-concept study advances the burgeoning field of mitochondrial transplantation and an increase of incorporation efficiency and cell-type targeting may be key in improving long-term functional neuroprotection in models of SCI.
3.2. Schizophrenia
Effectiveness of mitochondrial transplantation for Schizophrenia (SZ) has also been described. SZ model was prepared by maternal immune activation, which intravenously injecting poly-I:C (4 mg/kg/ml) in the pregnant female rats. The systemic inflammatory responses during embryogenesis disturb fetal brain development and consequently lead to various neuropsychiatric diseases such as SZ. On a postnatal day 21, offspring were separated from parents and housed themselves. Then, mitochondria isolated from rat brains were bilaterally injected into the intra-prefrontal cortex in SZ model rats. Before transplantation, mitochondria were labeled with JC1 for evaluate mitochondrial membrane potential. Then, images were captured by fluorescence microscopy. Intriguingly, mitochondrial transplantation prevented attention-deficit characterized cognitive impairment in SZ along with an improvement in mitochondrial membrane potential measured by JC1 ratio (Robicsek, et al., 2018). However, fundamental mechanisms of how transplanted mitochondria influence brain function in a rat model of SZ remain to be fully explored.
3.3. Parkinson’s Disease
Mitochondrial transplantation therapy in a rat model of 6-OHDA-induced Parkinson’s disease (PD) has been attempted. Therapeutic effects of mitochondrial transplantation were investigated by transferring allogeneic (pheochromocytoma cell line, PC12) or xenogeneic (human osteosarcoma cybrids) mitochondria with or without Pep-1 conjugation (Chang, et al., 2016). In order to label transplanted mitochondria, donor cells were infected with a plasmid-encoding mitochondrial matrix-localized GFP. At 3 weeks after 6-OHDA injection, rats randomly received mitochondria (1.05 μg/5 μL) by local injection. In peptide-mediated delivery of allogeneic mitochondria (PMD) group, the double-positive expression of GFP and Tom20 (mitochondrial marker) in the substantia nigra pars compacta was observed by confocal microscopy. Furthermore, the colocalized expression of GFP and calbindin (a marker of dorsal tier neuron) was further confirmed by an optical cut plain in the z projection of 3D confocal sections. Finally, dopaminergic neurons stained with tyrosine hydroxylase were positive for mitochondrial GFP, implicating that the grafted mitochondria were incorporated in dorsal tier dopaminergic neurons of substantia nigra pars compacta. These results suggest that peptide-mediated mitochondria can be internalized into neurons in vivo. Moreover, in the evaluation of behavior test, PMD and xenogeneic mitochondria (peptide-mediated delivery of xenogeneic mitochondria, xPMD) significantly improved in locomotion, travel distance, the speed of movement and number of crossed zone entries in comparison with vehicle or allogeneic mitochondrial transplantation (without Pep-1 label). Furthermore, both PMD and xPMD attenuated deterioration of dopaminergic neurons compared to the vehicle-treated group. Interestingly, allogeneic transplantation was more effective than xenogeneic transplantation in some locomotive activity tests, suggesting that mitochondrial transplantation efficacy may be influenced by the host species (i.e. allogeneic or xenogeneic). Additionally, mitochondrial modification may be a promising approach to amplify transplantation efficacy.
Another study has been reported by Shi and colleagues. A mouse model of PD was prepared by intraperitoneal injections of 10 mg/kg 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) once a day for 5 days. In this study, mitochondria were labeled by Mitotracker CMXRos or GFP, then mice exposed to MPTP were intravenously treated with fluorescent-labeled mitochondria (0.5 mg/kg body weight). After injection, confocal microscopy analysis demonstrated that mitochondria were distributed in the brain, heart, liver, kidney, and muscle. Moreover, mitochondrial transplantation significantly improved behavioral outcomes in the pole test and rotarod test along with restoring mitochondrial function including complex I activity and ATP content and ameliorating cell apoptosis and necrosis in the striatum compared to the vehicle-treated group (Shi, et al., 2017).
3.4. Ischemic Stroke
After ischemic stroke, lack of glucose and oxygen supply disturbs ATP synthesis in mitochondria, causes energy imbalance and dysregulation of cellular homeostasis, and leads to neural cell death. Therefore, targeting mitochondria can be a promising approach for neuroprotection and neuroregeneration (Baek, et al., 2014, Russo, et al., 2018, Watts, et al., 2013). Recently, it has been reported that direct transplantation of healthy mitochondria may induce neuroprotection in a rat model of focal cerebral ischemia. In this study, isolated mitochondria from hamster kidney fibroblast (BHK-21) cell lines were injected directly into ischemic striatum (IC, 75 μg) or via intra-arterial space (IA, 750 μg) after transient focal cerebral ischemia. BHK-21 cells were pre-incubated with BrdU that can also label mtDNA in order to assess the distribution of infused mitochondria. Interestingly, confocal microscopy analysis revealed that BrdU signals were detected in neuron, astrocytes, and microglia in the peri-infarct area at 4 weeks after transient focal ischemia in both IC and IA groups. Concomitantly, mitochondrial transplantation significantly improved motor function along with decreasing infarct area and TUNEL-positive cells (Huang, et al., 2016).
More recently, Zhang and colleagues allografted skeletal muscle-derived mitochondria in a rat model of focal ischemia (Zhang, et al., 2019). Mitotracker Red CMXRos-labeled mitochondria (5×106/10 μL) were infused into the lateral side of the ventricle after immediate reperfusion in transient focal ischemia. Confocal microscopy analysis showed transplanted mitochondria were indeed incorporated in neurons of the peri-infarct area. Additionally, mitochondrial allograft significantly decreased infarct volume, and attenuated neurological deficits, cellular oxidative stress, and apoptosis at 28 days after stroke. Furthermore, mitochondrial treatment attenuated reactive astrogliosis and promoted neurogenesis after stroke, implicating that mitochondrial transplantation may induce not only acute neuroprotection but also promote neurogenesis in the late phase of post-stroke.
Recent studies implicate that stem cells may have the ability to transfer mitochondria into injured cells via tunneling nanotubes, microvesicles, gap junctions, cell fusion, or direct mitochondrial uptake (Heyck, et al., 2019, Liu, et al., 2018, Paliwal, et al., 2018), and stem cell-mediated mitochondrial transfer may be a key mechanism for neuroprotection and neurorepair. Here Liu and colleagues have demonstrated the possibility of mitochondrial transfer from transplanted MSCs to damaged brain endothelial cells after transient focal cerebral ischemia in rats (Liu, et al., 2019). In this study, experimental animals were divided into 3 groups; PBS, MCSs-treated group, or MSCs with LatA- or Annexin V-treated groups. LatA or Annexin V was used to aim for inhibiting tunneling nanotubes (TNT)-mediated mitochondrial transfer. Nucleus or mitochondria of bone marrow-derived MSCs were labeled by Hoechst 33342 or pDsRed2-Mito, respectively. Confocal microscopy was used to investigate the distribution of transplanted MSCs and its mitochondrial transfer. After MSC transplantation (5×105) through a common carotid artery at 24-hour post-stroke, confocal microscopy showed that both DsRed2+/Hoechst 33342+ and DsRed2+/Hoechst 33342- cells accumulated in and around the microvessels in the peri-infarct area. Interestingly, in LatA or Annexin V treated groups, the number of DsRed2+/Hoechst 33342− cells was reduced in the peri-infarct area without affecting the number of DsRed2+/Hoechst 33342+ cells. Furthermore, OCR and extracellular acidification rate (ECAR) were assessed in isolated brain microvascular fragments by XF24 Extracellular Flux Analyzer. As a result, MSC transplantation improved maximal OCR and relative ECAR in the brain microvascular fragments in the ischemic cerebral hemisphere of stroke rats. But when TNT formation was inhibited, these effects were diminished. Collectively, MSC transplantation can be promising for stroke therapy in part through a mechanism of mitochondrial transfer to brain endothelium involved.
4. Conclusions
Emerging findings in cell, animal and human studies suggest that mitochondria can be released and potentially transferred between cells. Additionally, extracellular mitochondria are indeed detectable in human samples, and these measurements may be functionally correlated with the severity of injury or diseases, suggesting that extracellular mitochondria may represent a potentially new class of mediators and biomarkers. MitoTracker-dependent mitochondria labeling has been commonly utilized in order to assess mitochondrial transfer and the presence of extracellular mitochondria. But we are also aware that membrane potential-dependent labeling with MitoTracker dyes may cause mitochondrial toxicity and be leaked out of mitochondria when they are used at higher concentrations. Therefore, preliminary studies should be conducted in order to optimize experimental conditions particularly dye concentrations to minimize toxicity and background signals/non-specific detection. Mitochondrial labeling with fluorescent proteins can be an alternative approach with less toxicity. DNA constructs to drive expression of fluorescent proteins in cell-specific mitochondria may be useful to investigate the frequency and magnitude of mitochondrial transfer regulated by targeted cells under physiological and pathophysiological conditions. Moreover, the therapeutic use of extracellular mitochondria may be a promising approach for a wide range of CNS injury or disease, although it raises some questions regarding how extracellular mitochondria can survive in the extracellular environment and maintain their function during the process of transfer (Bertero, et al., 2018). Further studies are warranted to investigate potential therapeutic and biomarker applications for these extracellular mitochondria signals in the CNS.
Acknowledgments
The authors thank Eng H. Lo for many helpful discussions. This work was supported in part by grants from NIH NINDS (R01NS094756).
Footnotes
Competing Interests: The authors declare they have no competing financial interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anne Stetler R, Leak RK, Gao Y, and Chen J, 2013. The dynamics of the mitochondrial organelle as a potential therapeutic target. J Cereb Blood Flow Metab 33, 22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Araki T, 1977. Freezing injury in mitochondrial membrances. I. Susceptible components in the oxidation systems of frozen and thawed rabbit liver mitochondria. Cryobiology 14, 144–150. [DOI] [PubMed] [Google Scholar]
- 3.Baek SH, Noh AR, Kim KA, Akram M, Shin YJ, Kim ES, Yu SW, Majid A, and Bae ON, 2014. Modulation of mitochondrial function and autophagy mediates carnosine neuroprotection against ischemic brain damage. Stroke 45, 2438–2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertero E, Maack C, and O’Rourke B, 2018. Mitochondrial transplantation in humans: “magical” cure or cause for concern? J Clin Invest 128, 5191–5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carmichael ST, 2006. Cellular and molecular mechanisms of neural repair after stroke: making waves. Ann Neurol 59, 735–742. [DOI] [PubMed] [Google Scholar]
- 6.Cervera-Carles L, Alcolea D, Estanga A, Ecay-Torres M, Izagirre A, Clerigue M, Garcia-Sebastian M, Villanua J, Escalas C, Blesa R, Martinez-Lage P, Lleo A, Fortea J, and Clarimon J, 2017. Cerebrospinal fluid mitochondrial DNA in the Alzheimer’s disease continuum. Neurobiol Aging 53, 192.e191–192.e194. [DOI] [PubMed] [Google Scholar]
- 7.Chan DC, 2006. Mitochondria: dynamic organelles in disease, aging, and development. Cell 125, 1241–1252. [DOI] [PubMed] [Google Scholar]
- 8.Chang JC, Wu SL, Liu KH, Chen YH, Chuang CS, Cheng FC, Su HL, Wei YH, Kuo SJ, and Liu CS, 2016. Allogeneic/xenogeneic transplantation of peptide-labeled mitochondria in Parkinson’s disease: restoration of mitochondria functions and attenuation of 6-hydroxydopamine-induced neurotoxicity. Transl Res 170, 40–56.e43. [DOI] [PubMed] [Google Scholar]
- 9.Chou SH, Lan J, Esposito E, Ning M, Balaj L, Ji X, Lo EH, and Hayakawa K, 2017. Extracellular Mitochondria in Cerebrospinal Fluid and Neurological Recovery After Subarachnoid Hemorrhage. Stroke 48, 2231–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.David G, and Barrett EF, 2003. Mitochondrial Ca2+ uptake prevents desynchronization of quantal release and minimizes depletion during repetitive stimulation of mouse motor nerve terminals. J Physiol 548, 425–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis CH, Kim KY, Bushong EA, Mills EA, Boassa D, Shih T, Kinebuchi M, Phan S, Zhou Y, Bihlmeyer NA, Nguyen JV, Jin Y, Ellisman MH, and Marsh-Armstrong N, 2014. Transcellular degradation of axonal mitochondria. Proc Natl Acad Sci U S A 111, 9633–9638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.del Zoppo GJ, 2009. Inflammation and the neurovascular unit in the setting of focal cerebral ischemia. Neuroscience 158, 972–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fishbein WN, and Griffin JL, 1976. Studies on the mechanism of freezing damage to mouse liver. IV. Effects of ultrarapid freezing on structure and function of isolated mitochondria. Cryobiology 13, 542–556. [DOI] [PubMed] [Google Scholar]
- 14.Galluzzi L, Kepp O, and Kroemer G, 2012. Mitochondria: master regulators of danger signalling. Nat Rev Mol Cell Biol 13, 780–788. [DOI] [PubMed] [Google Scholar]
- 15.Gao L, Zhang Z, Lu J, and Pei G, 2019. Mitochondria Are Dynamically Transferring Between Human Neural Cells and Alexander Disease-Associated GFAP Mutations Impair the Astrocytic Transfer. Front Cell Neurosci 13, 316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gollihue JL, Patel SP, Eldahan KC, Cox DH, Donahue RR, Taylor BK, Sullivan PG, and Rabchevsky AG, 2018. Effects of Mitochondrial Transplantation on Bioenergetics, Cellular Incorporation, and Functional Recovery after Spinal Cord Injury. J Neurotrauma 35, 1800–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hawkins BT, and Davis TP, 2005. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev 57, 173–185. [DOI] [PubMed] [Google Scholar]
- 18.Hayakawa K, Bruzzese M, Chou SH, Ning M, Ji X, and Lo EH, 2018. Extracellular Mitochondria for Therapy and Diagnosis in Acute Central Nervous System Injury. JAMA Neurol 75, 119–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayakawa K, Chan SJ, Mandeville ET, Park JH, Bruzzese M, Montaner J, Arai K, Rosell A, and Lo EH, 2018. Protective Effects of Endothelial Progenitor Cell-Derived Extracellular Mitochondria in Brain Endothelium. Stem Cells 36, 1404–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayakawa K, Esposito E, Wang X, Terasaki Y, Liu Y, Xing C, Ji X, and Lo EH, 2016. Transfer of mitochondria from astrocytes to neurons after stroke. Nature 535, 551–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heyck M, Bonsack B, Zhang H, Sadanandan N, Cozene B, Kingsbury C, Lee JY, and Borlongan CV, 2019. The brain and eye: Treating cerebral and retinal ischemia through mitochondrial transfer. Exp Biol Med (Maywood), 1535370219881623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang L, Nakamura Y, Lo EH, and Hayakawa K, 2019. Astrocyte Signaling in the Neurovascular Unit After Central Nervous System Injury. Int J Mol Sci 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang PJ, Kuo CC, Lee HC, Shen CI, Cheng FC, Wu SF, Chang JC, Pan HC, Lin SZ, Liu CS, and Su HL, 2016. Transferring Xenogenic Mitochondria Provides Neural Protection Against Ischemic Stress in Ischemic Rat Brains. Cell Transplant 25, 913–927. [DOI] [PubMed] [Google Scholar]
- 24.Iadecola C, 2004. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci 5, 347–360. [DOI] [PubMed] [Google Scholar]
- 25.Johri A, and Beal MF, 2012. Mitochondrial dysfunction in neurodegenerative diseases. J Pharmacol Exp Ther 342, 619–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jonckheere AI, Smeitink JA, and Rodenburg RJ, 2012. Mitochondrial ATP synthase: architecture, function and pathology. J Inherit Metab Dis 35, 211–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joshi AU, Minhas PS, Liddelow SA, Haileselassie B, Andreasson KI, Dorn GW 2nd, and Mochly-Rosen D, 2019. Fragmented mitochondria released from microglia trigger A1 astrocytic response and propagate inflammatory neurodegeneration. Nat Neurosci 22, 1635–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kastaniotis AJ, Autio KJ, Keratar JM, Monteuuis G, Makela AM, Nair RR, Pietikainen LP, Shvetsova A, Chen Z, and Hiltunen JK, 2017. Mitochondrial fatty acid synthesis, fatty acids and mitochondrial physiology. Biochim Biophys Acta Mol Cell Biol Lipids 1862, 39–48. [DOI] [PubMed] [Google Scholar]
- 29.Khakh BS, Beaumont V, Cachope R, Munoz-Sanjuan I, Goldman SA, and Grantyn R, 2017. Unravelling and Exploiting Astrocyte Dysfunction in Huntington’s Disease. Trends Neurosci 40, 422–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kitani T, Kami D, Matoba S, and Gojo S, 2014. Internalization of isolated functional mitochondria: involvement of macropinocytosis. J Cell Mol Med 18, 1694–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li L, and Stary CM, 2016. Targeting Glial Mitochondrial Function for Protection from Cerebral Ischemia: Relevance, Mechanisms, and the Role of MicroRNAs. Oxid Med Cell Longev 2016, 6032306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Munch AE, Chung WS, Peterson TC, Wilton DK, Frouin A, Napier BA, Panicker N, Kumar M, Buckwalter MS, Rowitch DH, Dawson VL, Dawson TM, Stevens B, and Barres BA, 2017. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin MT, and Beal MF, 2006. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443, 787–795. [DOI] [PubMed] [Google Scholar]
- 34.Lippert T, and Borlongan CV, 2019. Prophylactic treatment of hyperbaric oxygen treatment mitigates inflammatory response via mitochondria transfer. CNS Neurosci Ther 25, 815–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu F, Lu J, Manaenko A, Tang J, and Hu Q, 2018. Mitochondria in Ischemic Stroke: New Insight and Implications. Aging Dis 9, 924–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu K, Guo L, Zhou Z, Pan M, and Yan C, 2019. Mesenchymal stem cells transfer mitochondria into cerebral microvasculature and promote recovery from ischemic stroke. Microvasc Res 123, 74–80. [DOI] [PubMed] [Google Scholar]
- 37.Lo EH, Broderick JP, and Moskowitz MA, 2004. tPA and proteolysis in the neurovascular unit. Stroke 35, 354–356. [DOI] [PubMed] [Google Scholar]
- 38.McCully JD, Levitsky S, Del Nido PJ, and Cowan DB, 2016. Mitochondrial transplantation for therapeutic use. Clin Transl Med 5, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McEwen ML, Sullivan PG, Rabchevsky AG, and Springer JE, 2011. Targeting mitochondrial function for the treatment of acute spinal cord injury. Neurotherapeutics 8, 168–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miliotis S, Nicolalde B, Ortega M, Yepez J, and Caicedo A, 2019. Forms of extracellular mitochondria and their impact in health. Mitochondrion. [DOI] [PubMed] [Google Scholar]
- 41.Moskowitz MA, Lo EH, and Iadecola C, 2010. The science of stroke: mechanisms in search of treatments. Neuron 67, 181–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murphy BJ, Klusch N, Langer J, Mills DJ, Yildiz O, and Kuhlbrandt W, 2019. Rotary substates of mitochondrial ATP synthase reveal the basis of flexible F1-Fo coupling. Science 364. [DOI] [PubMed] [Google Scholar]
- 43.Nukala VN, Singh IN, Davis LM, and Sullivan PG, 2006. Cryopreservation of brain mitochondria: a novel methodology for functional studies. J Neurosci Methods 152, 48–54. [DOI] [PubMed] [Google Scholar]
- 44.Paliwal S, Chaudhuri R, Agrawal A, and Mohanty S, 2018. Regenerative abilities of mesenchymal stem cells through mitochondrial transfer. J Biomed Sci 25, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peng Y, Zheng D, Zhang X, Pan S, Ji T, Zhang J, Shen HY, and Wang HH, 2019. Cell-Free Mitochondrial DNA in the CSF: A Potential Prognostic Biomarker of Anti-NMDAR Encephalitis. Front Immunol 10, 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Picca A, Guerra F, Calvani R, Bucci C, Lo Monaco MR, Bentivoglio AR, Coelho-Junior HJ, Landi F, Bernabei R, and Marzetti E, 2019. Mitochondrial Dysfunction and Aging: Insights from the Analysis of Extracellular Vesicles. Int J Mol Sci 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pinti M, Cevenini E, Nasi M, De Biasi S, Salvioli S, Monti D, Benatti S, Gibellini L, Cotichini R, Stazi MA, Trenti T, Franceschi C, and Cossarizza A, 2014. Circulating mitochondrial DNA increases with age and is a familiar trait: Implications for “inflamm-aging”. Eur J Immunol 44, 1552–1562. [DOI] [PubMed] [Google Scholar]
- 48.Pivovarova NB, and Andrews SB, 2010. Calcium-dependent mitochondrial function and dysfunction in neurons. FEBS J 277, 3622–3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Preble JM, Kondo H, Levitsky S, and McCully JD, 2013. Quality control parameters for mitochondria transplant in cardiac tissue. Mol Biol 2, 1008. [Google Scholar]
- 50.Robicsek O, Ene HM, Karry R, Ytzhaki O, Asor E, McPhie D, Cohen BM, Ben-Yehuda R, Weiner I, and Ben-Shachar D, 2018. Isolated Mitochondria Transfer Improves Neuronal Differentiation of Schizophrenia-Derived Induced Pluripotent Stem Cells and Rescues Deficits in a Rat Model of the Disorder. Schizophr Bull 44, 432–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rocca CJ, Goodman SM, Dulin JN, Haquang JH, Gertsman I, Blondelle J, Smith JLM, Heyser CJ, and Cherqui S, 2017. Transplantation of wild-type mouse hematopoietic stem and progenitor cells ameliorates deficits in a mouse model of Friedreich’s ataxia. Sci Transl Med 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roushandeh AM, Kuwahara Y, and Roudkenar MH, 2019. Mitochondrial transplantation as a potential and novel master key for treatment of various incurable diseases. Cytotechnology 71, 647–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Russo E, Nguyen H, Lippert T, Tuazon J, Borlongan CV, and Napoli E, 2018. Mitochondrial targeting as a novel therapy for stroke. Brain Circ 4, 84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shi X, Zhao M, Fu C, and Fu A, 2017. Intravenous administration of mitochondria for treating experimental Parkinson’s disease. Mitochondrion 34, 91–100. [DOI] [PubMed] [Google Scholar]
- 55.Spees JL, Olson SD, Whitney MJ, and Prockop DJ, 2006. Mitochondrial transfer between cells can rescue aerobic respiration. Proc Natl Acad Sci U S A 103, 1283–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sun C, Liu X, Wang B, Wang Z, Liu Y, Di C, Si J, Li H, Wu Q, Xu D, Li J, Li G, Wang Y, Wang F, and Zhang H, 2019. Endocytosis-mediated mitochondrial transplantation: Transferring normal human astrocytic mitochondria into glioma cells rescues aerobic respiration and enhances radiosensitivity. Theranostics 9, 3595–3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tait SW, and Green DR, 2012. Mitochondria and cell signalling. J Cell Sci 125, 807–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thored P, Wood J, Arvidsson A, Cammenga J, Kokaia Z, and Lindvall O, 2007. Long-term neuroblast migration along blood vessels in an area with transient angiogenesis and increased vascularization after stroke. Stroke 38, 3032–3039. [DOI] [PubMed] [Google Scholar]
- 59.Thurairajah K, Briggs GD, and Balogh ZJ, 2018. The source of cell-free mitochondrial DNA in trauma and potential therapeutic strategies. Eur J Trauma Emerg Surg 44, 325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Torralba D, Baixauli F, and Sanchez-Madrid F, 2016. Mitochondria Know No Boundaries: Mechanisms and Functions of Intercellular Mitochondrial Transfer. Front Cell Dev Biol 4, 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Trias E, Barbeito L, and Yamanaka K, 2018. Phenotypic heterogeneity of astrocytes in motor neuron disease. Clin Exp Neuroimmunol 9, 225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tsvetkov T, Tsonev L, Meranzov N, and Minkov I, 1985. Functional changes in mitochondrial properties as a result of their membrane cryodestruction. I. Influence of freezing and thawing on succinate-ferricyanide reductase of intact liver mitochondria. Cryobiology 22, 47–54. [DOI] [PubMed] [Google Scholar]
- 63.Tsvetkov T, Tsonev L, Meranzov N, and Minkov I, 1985. Functional changes in mitochondrial properties as a result of their membrane cryodestruction. II. Influence of freezing and thawing on ATP complex activity of intact liver mitochondria. Cryobiology 22, 111–118. [DOI] [PubMed] [Google Scholar]
- 64.Vafai SB, and Mootha VK, 2012. Mitochondrial disorders as windows into an ancient organelle. Nature 491, 374–383. [DOI] [PubMed] [Google Scholar]
- 65.Watts LT, Lloyd R, Garling RJ, and Duong T, 2013. Stroke neuroprotection: targeting mitochondria. Brain Sci 3, 540–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wei Z, Su W, Lou H, Duan S, and Chen G, 2018. Trafficking pathway between plasma membrane and mitochondria via clathrin-mediated endocytosis. J Mol Cell Biol 10, 539–548. [DOI] [PubMed] [Google Scholar]
- 67.Wilkins HM, Weidling IW, Ji Y, and Swerdlow RH, 2017. Mitochondria-Derived Damage-Associated Molecular Patterns in Neurodegeneration. Front Immunol 8, 508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yamaguchi R, Andreyev A, Murphy AN, Perkins GA, Ellisman MH, and Newmeyer DD, 2007. Mitochondria frozen with trehalose retain a number of biological functions and preserve outer membrane integrity. Cell Death Differ 14, 616–624. [DOI] [PubMed] [Google Scholar]
- 69.Yao Y, Fan XL, Jiang D, Zhang Y, Li X, Xu ZB, Fang SB, Chiu S, Tse HF, Lian Q, and Fu QL, 2018. Connexin 43-Mediated Mitochondrial Transfer of iPSC-MSCs Alleviates Asthma Inflammation. Stem Cell Reports 11, 1120–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yun SP, Kam TI, Panicker N, Kim S, Oh Y, Park JS, Kwon SH, Park YJ, Karuppagounder SS, Park H, Oh N, Kim NA, Lee S, Brahmachari S, Mao X, Lee JH, Kumar M, An D, Kang SU, Lee Y, Lee KC, Na DH, Kim D, Lee SH, Roschke VV, Liddelow SA, Mari Z, Barres BA, Dawson VL, Dawson TM, and Ko HS, 2018. Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson’s disease. Nat Med 24, 931–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zacchigna S, Lambrechts D, and Carmeliet P, 2008. Neurovascular signalling defects in neurodegeneration. Nat Rev Neurosci 9, 169–181. [DOI] [PubMed] [Google Scholar]
- 72.Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, and Barres BA, 2012. Genomic analysis of reactive astrogliosis. J Neurosci 32, 6391–6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang Z, Ma Z, Yan C, Pu K, Wu M, Bai J, Li Y, and Wang Q, 2019. Muscle-derived autologous mitochondrial transplantation: A novel strategy for treating cerebral ischemic injury. Behav Brain Res 356, 322–331. [DOI] [PubMed] [Google Scholar]
- 74.Zlokovic BV, 2008. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 57, 178–201. [DOI] [PubMed] [Google Scholar]