Abstract
Introduction:
Smoking is an established risk factor for both lung diseases and rheumatoid arthritis (RA). Chronic mucosal airway inflammation may result in immune tolerance loss, neoantigen formation, and production of RA-related autoantibodies that increase the subsequent risk of RA. In this review, we aimed to summarize the current evidence supporting the role of obstructive lung diseases and subsequent risk of RA.
Areas covered:
We identified scientific articles discussing the biologic mechanisms linking mucosal airway inflammation and RA risk. We also identified studies investigating asthma, chronic obstructive pulmonary disease, bronchiectasis, cystic fibrosis, chronic tuberculous and nontuberculous mycobacterial infections, and interstitial lung disease with subsequent risk for RA.
Expert opinion:
The current evidence supports the hypothesis that mucosal airway inflammation may increase the risk of developing RA. However, most studies investigating this relationship have been retrospective and may not have adequately addressed the role of smoking. Larger prospective studies may provide stronger evidence for obstructive lung disease and RA risk. Determining the role of obstructive lung disease in RA pathogenesis may provide opportunity for RA prevention and screening strategies, while identifying novel biologic mechanisms that could offer targets to improve treatment and outcomes.
Keywords: ACPA, asthma, autoimmunity, bronchiectasis, COPD, ILD, inflammation, obstructive lung disease, rheumatoid arthritis
1. Introduction
Rheumatoid arthritis (RA) is the most common systemic rheumatic disease, affecting nearly 1% of the population, and is characterized by a painful, chronic inflammatory polyarthritis [1]. While the etiology of RA is complex, cigarette smoking is the best-established environmental risk factor for RA [2-4]. Patients with RA are known to have excess respiratory morbidity and mortality compared to the general population [5,6]. Pulmonary involvement as a disease manifestation of RA may affect pleura, airways, parenchyma, blood vessels, and respiratory muscles [7,8]. Patients with RA are also prone to developing interstitial lung disease (ILD), which has particularly high morbidity and mortality [9,10]. Smoking increases mortality more in patients with RA than in the general population, which may be related to these respiratory complications [11]. Therefore, the lung has an established importance in outcomes among patients already diagnosed with RA. However, less is known about the role of the lung in RA pathogenesis.
RA is phenotyped as either seropositive (i.e., serum elevation of either anti-citrullinated protein antibodies [ACPA] or rheumatoid factor [RF]) or seronegative (negative blood tests for both ACPA and RF). Smoking specifically increases risk for seropositive RA [2,4]. Patients with seropositive RA are also more likely to develop respiratory complications [5,9,12]. Since many non-smokers develop seropositive RA, other causes of respiratory inflammation may be important in RA pathogenesis. The mucosal paradigm for seropositive RA pathogenesis postulates that RA may develop after loss of immune tolerance due to environmental triggers at mucosal surfaces in genetically susceptible individuals [13,14]. In particular, chronic mucosal airway inflammation could be a nidus for development of RA-related autoantibodies and subsequent risk for systemic inflammation, autoimmunity, and articular inflammation. Alternatively, the lungs may be also be a target of autoimmunity from immune tolerance breakdown that occurs at other anatomic sites.
In this review, we will detail the biologic rationale that may link obstructive lung diseases to RA risk. We focus on diseases involving the airways and parenchyma since prior research has not found an association between vocal cord dysfunction or subglottic stenosis with RA risk. We will also discuss the current literature of investigations of particular obstructive lung diseases and RA risk, focusing on asthma, chronic obstructive pulmonary disease (COPD), muco-obstructive lung diseases, and ILD. Finally, we will summarize the current literature and postulate about future steps.
2. Biologic framework for airway inflammation and increased RA risk
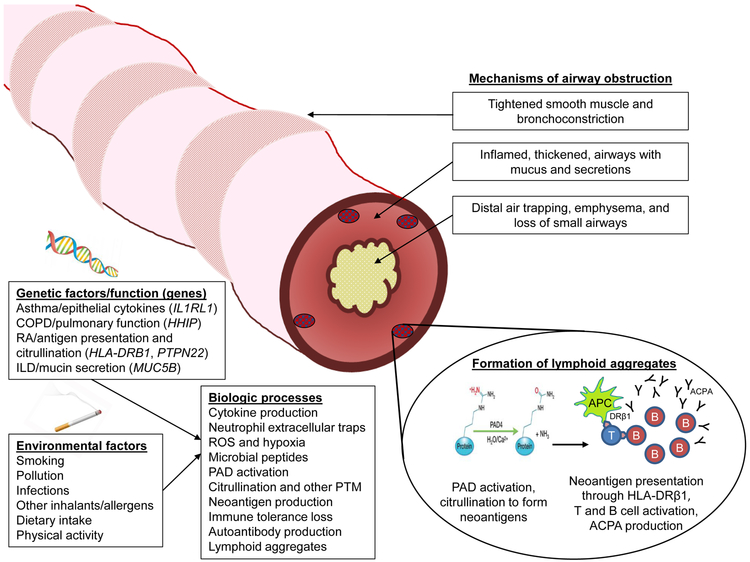
RA-related autoimmunity has been hypothesized to originate at mucosal surfaces in the lungs or other anatomic sites such as the gingiva, gut, bladder, or reproductive tract [15]. This review will specifically focus on airway mucosa as a potential site of RA genesis. Figure 1 provides a summary of the proposed biological framework linking obstructive lung disease and RA risk. Obstructive lung diseases are a group of complex and heterogeneous diseases and clinical syndromes that result from restricted airflow from a variety of mechanisms. This airflow obstruction can be due to a variety of factors, including tightened bronchiolar smooth muscles and bronchoconstriction; inflamed, thickened airways; excess mucus thickening and secretions; loss of small airways; and parenchymal destruction and loss of elastic recoil. These mechanisms of airflow obstruction are present to varying degrees in different types of obstructive lung diseases. In asthma, individuals with increased genetic risk factors and overactive allergic reactions in combination with external factors such as inhalants, chronic infections, and other pathogens may induce smooth muscle contraction. This in turn causes bronchoconstriction, promoting narrowing of the airway and tissue inflammation [16]. In emphysema, these external factors promote the recruitment of macrophages and neutrophils which promote tissue cell remodeling and destruction, ultimately leading to damaged epithelial cells and air trapping [17]. In muco-obstructive lung diseases like COPD and cystic fibrosis, activation from external factors increases mucin secretion. Elevated mucin concentration, coupled with insufficient ion transport across airway surfaces, causes thickening of airway walls with mucus and impaired airflow [18]. Though some mucus is expelled as sputum, any remaining mucus in the small airways accumulates to form obstructions and promote further inflammation as well as a nidus for infection [19]. In COPD, in addition to airway inflammation, loss of small airways may precede the development of emphysema [20]. Chronic inflammation may lead to inducible bronchus-associated lymphoid tissue (iBALT), a mucosal lymphoid collection in airways or lung parenchyma, that has been shown to occur in patients with pulmonary complications from RA or Sjögren syndrome and may be important in inducing autoimmunity [21].
Figure 1.
Schematic depicting possible biologic mechanisms linking obstructive lung disease with increased risk for rheumatoid arthritis. Mechanisms of airway obstruction include bronchoconstriction, thickened airways with secretions, loss of airways, and distal air trapping. A combination of genetic and environmental factors may contribute to many biologic processes that could increase local inflammation, recruit immune cells, produce neoantigens, produce RA-related autoantibodies, and form lymphoid aggregates in lung tissue prior to systemic involvement. Abbreviations: ACPA, anti-citrullinated protein antibodies; APC, antigen-presenting cell; COPD, chronic obstructive pulmonary disease; ILD, interstitial lung disease; PAD, peptidylarginine deiminase; PTM, post-translational modifications; RA, rheumatoid arthritis; ROS, reactive oxygen species.
Genetic susceptibility for obstructive lung diseases has mostly been studied on a disease-specific basis. In asthma, many genetic risk factors (>800) have been investigated that help explain its biologic underpinning [22]. For example, epithelial cytokines have been identified as crucial molecules in susceptibility to airway disease, perhaps due to immune-cell recruitment [22]. IL1RL1 variants have been associated with asthma risk in a diverse range of patient populations [23-26]. A meta-analysis of geographically diverse genome-wide association studies found that many asthma risk loci overlap with genetic variants of autoimmune and inflammatory diseases, suggesting shared genetics between asthma and autoimmune diseases such as RA [22].
Variants in the HLA region have been associated with lung function and COPD, including HLA-DQB1 and AGER. In addition, the IL27 locus is associated with COPD, type 1 diabetes mellitus, and inflammatory bowel disease [27,28]. A recent phenome-wide association study investigating variants previously described in association with COPD or related phenotypes found suggestive evidence of an association between the rs207488 single nucleotide polymorphism of HLA-C and increased risk of RA as well as type 1 diabetes and bronchiectasis [29]. These findings suggest that autoimmunity and COPD may be intrinsically connected. Other COPD susceptibility genes such as HHIP may increase RA risk through effects on worsened pulmonary function, resulting in chronic lung damage that may induce local inflammatory milieu and promote RA-related autoimmunity. Gene-smoking interactions have been described in both RA and COPD, which may explain bidirectional associations of both diseases increasing risk for each other [30,31].
Interstitial lung disease (ILD) has been associated with a promotor variant in the MUC5B gene [32]. MUC5B is the predominant respiratory mucin and is elevated in concentration in muco-obstructive diseases, including COPD, cystic fibrosis, primary ciliary dyskinesia, and non-cystic fibrosis bronchiectasis [18]. Hypersecretion of mucin leads to relatively dehydrated and dysfunctional mucus, causing adhesion to airway surfaces and mucus accumulation in small airways that cannot be expectorated, resulting in obstruction, infection, and inflammation [19,33]. A recent large study also found the MUC5B promoter variant is a strong genetic risk factor for RA-associated ILD [34], supporting the hypothesis that the MUC5B promoter variant has downstream effects that induce muco-obstructive lung diseases. However, the MUC5B promotor variant has not been associated with RA risk, overall or by serologic status.
RA also has its own set of unique genetic susceptibility factors. Polymorphisms in HLA-DRB1, collectively known as the shared epitope, have the strongest genetic risk factor for RA [35,36]. This excess risk is likely related to the function of HLA-DRβ1 in antigen presentation. Particular amino acid haplotypes in the peptide binding grooves of HLA-DRβ1 markedly affect RA risk [36]. It is hypothesized that individuals with polymorphisms that increase RA risk may be vulnerable to aberrant immune response from neoantigens that induces immune cell activation and subsequent ACPA production [37,38]. It is possible that these biologic processes may occur specifically in inflamed airways as a nidus for immune cell activation that eventually leads to RA onset. PTPN22, a gene that codes for a tyrosine phosphatase involved in T and B cell signaling, has also been implicated in RA risk [39]. The PTPN22 polymorphism is associated with a lower threshold for T and B cell activation, which may allow a wider range of reactive T cells to be recruited to inflamed airways [40]. The PTPN22 polymorphism also promotes hypercitrullination which may pre-dispose individuals to have aberrant responses to neoepitopes formed in inflamed airways, eventually leading to the formation of ACPA locally and then systemically even years prior to articular RA onset [41-43]. This hypothesis complements the MHC class II dependent immunity that is believed to initiate ACPA formation [44]. HLA-DRB1 and PTPN22 are two of the best described genetic risk factors for RA, though there are many other genes identified that play a role in RA risk [39]. A recent genome-wide association study meta-analysis identified many other RA risk loci, many of which are targets of currently approved RA drug therapies [39]. More research is needed to understand whether there are shared genetic factors of respiratory diseases and RA.
There are also environmental factors that are hypothesized to work in conjunction with genetics to increase susceptibility to autoimmune dysfunction. Smoking is the best-established lifestyle factor that increases both obstructive lung disease and RA risk. Smoking has been shown to increase the presence of citrullinated proteins in samples obtained in bronchoalveolar lavage (BAL) [45], which likely contributes to local production of ACPA [46]. However, ACPA elevation in BAL fluid is also present in non-smoking early RA patients, which suggests that smoking is not the only factor causing this kind of inflammation in of the pathogenesis of RA [47,48].
Other environmental factors, including non-smoking inhalants such as pollution, may also increase lung autoimmunity and affect RA risk [49,50]. Inhaled substances like silica and coal dust have been shown to activate dendritic cells and provoke an immune response through pattern-recognition cells [51]. These external substances also increase bacterial colonization in the lungs, which may modify the microbiome of the lung [51]. Changes in the microbiome may be an external factor capable of contributing to autoimmunity and associated clinical manifestations [40]. Specifically, microbiome irregularity and chronic infection are thought to serve as stimuli for autoantigen formation and subsequent ACPA production [35]. Allergens play a major role in risk of asthma and flares, and may also impact RA risk [52]. Finally, dietary intake, physical activity, and body mass index have complex contributions to many diseases including obstructive lung diseases and RA [53-56].
The diverse environmental and genetic factors described above are hypothesized to induce an inflammatory response in the airways of lungs. Various studies report a mechanism whereby long-term exposure to these stimuli induces mucosal inflammation [46,57]. Local tissue inflammation caused by exogenous factors has been shown to lead to citrullination and other post-translational modifications [14], which in turn generates neoantigens [35]. A growing body of evidence suggests that citrullination and the subsequent immune intolerance forms a positive feedback loop that prompts continued autoimmune response. A study of blood samples collected from individuals before RA onset showed that epitope spreading of ACPA occurs years before clinical RA onset but no particular ACPA has been identified that initiates this process [58]. This suggests that an immune response to one citrullinated epitope leads to further responses to other citrullinated epitopes, magnifying the inflammatory response [44].
Citrullination and neoantigen formation are established precursors to ACPA production [35,37]. Antigen presenting cells from the innate immune response present these neoantigens to T cells, stimulating additional T and B cells, inducing loss of immune tolerance, causing inflammation, and ultimately generating ACPA [37]. Assembly of these RA-related autoantibodies in the lungs is supported by several studies. Rangel-Moreno et all reported that lungs in patients with RA contain ectopic lymphoid aggregates of B cells in iBALT capable of binding citrullinated proteins [21]. Furthermore, studies have identified enriched ACPA levels in the BAL fluid of patients with RA [47] and the sputum of individuals at risk of developing RA, compared to the blood [59]. A Swedish study also found circulating CCP and RF to be associated with mucosal inflammation in non-RA patients with cystic fibrosis [57]. These combined findings implicate the lung tissue as a site of production for RA-related autoantibodies during either the preclinical or early phases of RA. In addition, chronic airway inflammation may induce autoimmunity from many other mechanisms, including cell turnover from reactive oxygen species, chronic hypoxia resulting in tissue damage and remodeling, and chronic infections with increased chance of molecular mimicry inducing autoimmunity and joint specificity.
Other autoantibodies have been implicated in RA pathogenesis, including anti-carbamylated proteins (anti-CarP). Anti-CarP are produced when cyanate and lysine residues react to form homocitrulline [35]. Neutrophils may function as the bridge from functional innate immunity to the autoimmune response that generates these posttranslational protein modifications [60]. Other novel autoantibodies such as anti-peptidylarginine deiminase (anti-PAD) and anti-malondialdehyde-acetaldehyde (anti-MAA) have been associated with RA-associated ILD and research is ongoing to understand whether these may be generated in inflamed airways [61,62]. Finally, RF is the best-known RA-associated autoantibody, but its origins are still unclear. Some research suggests that RF may also be generated at inflamed mucosa, such as airways [63]. Therefore, obstructive lung disease may particularly impact risk for seropositive RA (either ACPA or RF positivity).
The role of neutrophil extracellular traps (NETs) has recently gained increasing attention in investigations of the pathogenesis of many diseases, including RA [64]. NETs are used to combat pathogens during an immune system response in a process called NETosis, a type of cell death distinct from apoptosis or necrosis [65]. NETs are composed of histones, globular proteins, and chromatin which make up a fibrous complex [65,66]. NETs may be increased at mucosal sites including the lung in pre-clinical RA; an increased frequency of these complexes was found in first-degree relatives of RA who are at risk of developing RA [67,68]. NETs recruit other immune cells such as antigen-presenting cells and T cells, so they may be an important component in initial breakdown of immune tolerance loss on the path toward RA-related autoantibody production. Therefore, the formation and persistence of NETs in inflamed and damaged airways may be an integral part of the RA pathogenesis.
The method by which inflammation and ACPA production transition from a local process to a systemic process predominantly affecting joints is not yet understood. ACPA presence and levels are increased in the blood of individuals years before articular involvement of RA [69]. ACPAs may be produced locally by B and plasma cells at lymphoid aggregates prior to migration through the reticuloendothelial system, where ACPAs are produced in greater abundance and enter into the peripheral circulation [40]. Epitope spread to different ACPA classes also induces systemic and articular inflammation, perhaps through molecular mimicry [44,70]. There are various complement binding methods by which ACPAs have been proposed to induce articular inflammation, including cell or autoantibody targeting of antigens that are present in both sites, new joint-specific inflammatory responses as epitopes spread, and the circulation of immune complexes [71,72]. However, one would expect mucosal IgA autoantibodies to emerge prior to IgG in this schematic of autoimmune triggering at mucosal inflammation. A recent study suggested circulating levels of ACPA-IgG were elevated earlier than ACPA-IgA prior to RA onset, so more work is needed to understand the timing and role of multiple mucosal sites as it related to RA pathogenesis [73].
This biologic framework has formed the overarching hypothesis that obstructive lung disease may induce RA-related production and increase RA risk. Studies have investigated whether asthma, COPD, muco-obstructive lung diseases, and ILD may increase RA risk.
3. Asthma and RA risk
Asthma is a chronic inflammatory airway disease with a wide range of clinical phenotypes, ranging from mild exercise- or allergy-induced bronchoconstriction with infrequent flares to recurrent, intermittent severe flares, to a more chronic form that persists throughout adulthood. Asthma has traditionally been considered an atopic, Th2 (T-helper cell 2)-mediated condition, whereas autoimmune disorders like RA are thought to be Th1 (T-helper cell 1) disorders [74,75]. However, it is hypothesized that there may be a shared susceptibility for asthma and RA [76,77]. An international genome-wide association study supports this hypothesis, finding significant overlap of asthma-association signals with autoimmune diseases [22]. Additional studies further hypothesize that asthma may cause systemic inflammatory dysfunction that manifests in regions beyond the airways [78]. Several studies have assessed the relationship between asthma and RA, many of which found an association between asthma and increased RA risk. Table 1 provides a summary of all prior studies that have investigated the relationship between asthma and RA risk. Since smoking above a threshold of 10 pack-years is associated with nearly 2-fold increased risk of RA, special attention should be made on how these studies adjust for smoking [4,79,80].
Table 1.
Studies investigating the association of asthma with risk of rheumatoid arthritis.
| Reference | Study design | Population Sample (n) RA outcomes (n) |
Asthma exposure methods |
RA outcome methods |
Smoking and other adjustment variables |
Effect size (95% confidence interval) for asthma and RA risk (reference group: no asthma) |
Comments |
|---|---|---|---|---|---|---|---|
| Tirosh A, Ann Intern Med (2006) [87] | 1) Cross-sectional 2) Retrospective cohort |
Israel, soldiers 1) n=488,841 n=479 prevalent RA 2) n=486,375 n=438 incident RA |
Physician-reported | Not detailed | No smoking adjustment Analyses stratified by sex |
1) Cross-sectional RR 0.46 (0.29-0.73)* 2) Cohort RR 0.45 (0.27-0.75)* |
First and largest study to investigate asthma and RA risk; also examined many other autoimmune diseases; only study to find an inverse association of asthma and RA; study population may limit generalizability |
| De Roos AJ, Ann Epidemiol (2008) [81] | Case-control | Iowa & North Carolina, pesticide applicators and spouses (Agricultural Health Study) n=810 n=135 RA |
Self-report (asthma or reactive lung disease) | Physician-confirmed | No smoking adjustment Age, US state |
Prevalent RA: OR 1.1 (0.4-10.9) Incident RA: OR 3.7 (1.3-10.5) Incident RF+ RA: OR 3.7 (1.0-13.6) Incident RF- RA: 3.5 (0.7-16.9) |
Only study investigating by RF status; cases were mostly prevalent RA; also investigated other personal and family medical conditions |
| Hemminki K, Ann Epidemiol (2010) [74] |
Retrospective cohort | Sweden n=148,295 n=490 RA |
Billing code in hospitalization | Billing code in hospitalization | No smoking adjustment Age, sex, region, socioeconomic status |
SIR 1.81 (1.66-1.98) 1+ year follow-up: SIR 1.75 (1.59-1.92) 5+ years follow-up: SIR 1.83 (1.63-2.04) |
Large nationwide study; only those with hospital codes were identified; no direct comparator group |
| Yun HD, Mayo Clin Proc (2012) [86] | Retrospective cohort | Olmsted County, Minnesota (Rochester Epidemiology Project) n=7,176 Number of RA outcomes not reported |
Medical record review |
Billing and Berkson codes | No smoking adjustment Age, sex, race/ethnicity |
HR 1.30 (0.78-2.18) | Also investigated asthma and risk of other inflammatory conditions |
| Lai NS, Allergy Asthma Proc (2015) [85] | Retrospective cohort | Taiwan n=340,808 n=395 RA |
Billing code | Billing code after successful application for catastrophic illness certificate | No smoking adjustment Age, sex, urbanization, socioeconomic status, comorbidities |
HR 1.67 (1.32-2.10) | Large nationwide study evaluating allergic diseases and RA risk |
| Hou YC, Allergy Asthma Proc (2017) [84] | Retrospective cohort | Taiwan n=310,622 n=456 RA |
Billing code | Billing code after successful application for catastrophic illness certificate | No smoking adjustment Age, sex, urbanization, socioeconomic status, comorbidities |
HR 1.87 (1.54-2.26) | Large nationwide study evaluating atopic diseases and risk of several connective tissue diseases |
| Jeong HE, Int J Rheumatol (2018) [83] | Cross-sectional | Korea n=506 n=253 prevalent RA |
Self-report | Self-report | Smoking adjustment: never/past/current Age, sex socioeconomic status, alcohol drinking, BMI, comorbidities |
OR 3.12 (2.77-3.51) | Analyzed national survey data; rich covariates available for adjustment; cross-sectional design limits conclusions |
| Sheen YH, J Allergy Clin Immunol Pract (2018) [75] |
Case-control | Olmsted County, Minnesota (Rochester Epidemiology Project) n=439 n=221 RA |
Medical record review using a standardized definition incorporating symptoms, signs, and objective test results | Billing and Berkson codes | Smoking adjustment: never/past/current Age, sex, BMI, socioeconomic status, hypertension, coronary artery disease, dyslipidemia |
OR 1.73 (1.03-2.92) | Population-based; high accuracy of asthma and RA with rich data on covariates for adjustment; asthma phenotyping and treatment data available |
| Kronzer V, Arthritis Rheumatol (2019) [52] | Case-control | Minnesota & Florida (Mayo Clinic Biobank) n=4,084 n=1,023 RA (n=175 incident RA) |
Self-report | Self-report and at least 2 billing codes | Smoking adjustment: never/past/current Sex, age, allergies, US state, education, passive smoking |
OR 1.28 (1.04-1.67) Incident RA: OR 1.17 (0.66-2.06) |
Highest number of RA outcomes of these studies; rich data on covariates available for adjustment; RA was mostly prevalent; hospital-based study sample may not affect generalizability |
| Zaccardelli A, Arthritis Res Ther (2019) [82] |
Nested case-control | US, female nurses (Nurses’ Health Studies) n=1,133 n=284 incident RA |
Self-report | Medical record review satisfying 1987 ACR or 2010 ACR/EULAR criteria | Smoking adjustment: continuous pack years, passive smoking Matching factors, BMI |
Overall RA: OR 1.45 (0.91-2.31) Seropositive RA: OR 1.79 (1.01-3.18) Seronegative RA: OR 0.97 (0.43-2.21) Pre-RA ACPA+: OR 3.57 (1.58-8.04) Pre-RA ACPA-: OR 0.86 (0.46-1.60) |
Investigated ACPA status in blood banked 8-10 years prior to RA diagnosis; also investigated RA serostatus at time of diagnosis; rich data on covariates collected prospectively prior to RA; study only included women |
Bolded results are statistically significant.
In Tirosh A et al, Ann Intern Med (2006) [87], the effect size estimates and 95% confidence intervals for rheumatoid arthritis outcomes were originally presented comparing participants without asthma to those with asthma as the reference group. The inverse of the originally reported results are presented here to maintain a consistent comparison group to the other studies that all compared participants with asthma to those without asthma as the reference group.
ACPA, anti-citrullinated protein antibodies; ACR, American College of Rheumatology; BMI, body mass index; EULAR, European League Against Rheumatism; HR, hazard ratio; OR, odds ratio; RA, rheumatoid arthritis; RF, rheumatoid factor; RR, relative risk; SIR, standardized incidence ratio; US, United States.
Several case-control studies have found asthma to be a risk factor for RA risk. A 2018 Minnesota study found that asthma was significantly associated with increased RA risk (adjusted odds ratio [OR] 1.73; 95%confidence interval [CI], 1.03–2.92), adjusted for smoking status and other confounders [75]. Another large case-control study of 1,023 RA cases within a single-center biobank population also found asthma to be associated with increased RA risk (OR 1.28, 95%CI 1.04–1.58), adjusted for smoking status and other potential confounders, though mostly included prevalent RA [52]. Two case-control studies have also investigated asthma and RA risk by seropositivity. An investigation of the Agricultural Health Study found that asthma was associated with RF+ RA (OR 3.7, 95%CI, 1.3–10.5) [81], but was limited by small sample size and lack of adjustment for smoking. In a recent case-control study nested within the Nurses’ Health Studies assessing asthma and ACPA elevation prior to RA onset, Zaccardelli and colleagues found that females with asthma were nearly 4-fold more likely to be ACPA+ in blood banked years prior to RA onset [82]. Asthma was also associated with increased risk for seropositive RA from clinical laboratory results at time of diagnosis (OR 1.79, 95%CI 1.01–3.18) [82]. These results were adjusted for confounders that were collected prior to RA onset, including continuous smoking pack-years and passive smoking [82]. A cross-sectional study from Korea with rich covariate data from adjustment also suggested that asthma may be associated with RA (OR 3.12, 95%CI 2.77–3.51) [83]. These findings support the hypothesis that chronic airway inflammation may play an important role in the development of seropositive RA.
Several retrospective cohort studies have also found an association between asthma and RA risk. Two similarly designed Taiwanese retrospective cohort studies utilizing national databases found asthma to be associated with increased RA risk [84,85]. Adjusting for potential confounders, Lai and colleagues found that asthma was significantly associated with incident RA (hazard ratio [HR] 1.67, 95%CI 1.32–2.62), and Hou et al reported that asthma had a HR of 1.87 (95%CI 1.54–2.26) for RA compared to individuals without asthma [84,85]. A Swedish cohort study using billing codes had similar findings, finding that hospitalized asthmatic patients were significantly more likely to develop RA than the general population (standardized incidence ratio 1.81, 95%CI 1.66–1.98) [74]. These results also suggest that asthma may increase RA risk. However, a retrospective cohort study investigating asthma and risk of RA and other inflammatory conditions using the Rochester Epidemiology Project found no significant association between asthma and RA (HR 1.30, 95%CI 0.78–2.18), but had small sample size [86].
Though the majority of studies investigating asthma and RA risk have found an association, the largest study on this found an inverse association between asthma and RA risk as well as other autoimmune diseases [87]. In this study of 488,841 Israeli soldiers between 18 and 21 years of age, non-asthmatic individuals had a higher prevalence of RA than their asthmatic counterparts (relative risk [RR] 2.17, 95%CI 1.37–3.43) in the cross-sectional analysis [87]. The researchers also performed a retrospective cohort study and found that RA was also diagnosed more frequently in individuals without asthma (RR 2.21, 95%CI 1.34–3.64) compared to those with asthma [87]. These findings led to the contentious hypothesis that asthma may actually be protective against autoimmune disorders like RA, potentially through an inverse relationship between Th2 disorders and Th1 diseases [87]. However, this study did not adjust for covariates, including smoking, allowing for the possibility of confounding. Since all participants were all active soldiers and mostly men, the findings may not be generalizable to other populations. It is possible that some subtypes of asthma, or their treatments, may have different effects on RA risk. For example, childhood-onset asthma with intermittent or lack of symptoms into adulthood may impact RA risk differently than chronic refractory or adult-onset asthma. However, this is the largest study to date to investigate asthma and RA risk and is in conflict with the subsequent studies. Prospective studies with phenotypes of asthma type, severity, age of onset, and duration are needed to resolve this conflicting literature and determine whether asthma may increase or decrease RA risk.
There are several limitations to consider when interpreting these results. None of the current literature was prospective and many studies had limited ability to adjust for confounders, most importantly smoking. However, it could be argued that adjusting for smoking may not be sufficient since this may be on the causal pathway between mucosal inflammation and RA risk. To help untangle this complex relationship, analyses should be performed that stratify by smoking status (never or ever), include mediation analyses, and account for smoking intensity and duration. Many of the current studies have only had smoking status at baseline so could not analyze in this way. Prospective studies carefully accounting for time-varying measures of smoking are necessary to establish a relationship between obstructive lung diseases and RA. Additionally, only two studies investigating this relationship accounted for RA serostatus, one finding an association specifically for seropositive RA [82]. It is possible that asthma may specifically affect seropositive RA risk based on the hypothesized biologic mechanisms. Since asthma is a heterogeneous condition often associated with atopy, future studies should investigate subtypes of asthma (such as age of onset, duration, severity, and other atopic conditions). Finally, only one of these investigations considered the type and severity of asthma in its analyses [75]. Simply assessing for presence versus absence of asthma may not account for the complexity of asthma and may explain the conflicting results. Accounting for subtypes of asthma may provide more insights into the relationship between Th1- and Th2-mediated pathways and the disorders they are associated with. Though there is certainly evidence to suggest that asthma is associated with increased RA risk, future studies accounting for RA serostatus, smoking, and asthma phenotypic features may allow researchers to draw stronger conclusions about the association between asthma and RA risk.
4. COPD and RA risk
COPD is defined by the presence of chronic, irreversible airflow limitation in the presence of risk factors and absent other etiologies of [88]. The current definition of COPD overlaps with other entities such as chronic bronchitis and emphysema. Heavy smoking is a well-established risk factor for COPD, but up to 25% may have relatively low smoking history or never smoked [89]. Other inhalants such as pollution and occupational exposures may also contribute to COPD risk. Patients with COPD may also experience heterogeneous disease severity, some with relatively mild shortness of breath or cough and some that progress to respiratory failure and death [90]. A small minority of patients may have alpha-1 antitrypsin deficiency [91]. Patients may also have asthma-COPD overlap syndrome, which presents as clinical symptoms of both asthma and COPD [92]. The pathogenesis of COPD involves neutrophil and macrophage infiltration with smoking as a strong environmental factor [93]. It has been shown that there are higher levels of citrullination in COPD patient lung samples compared to patients with no airway disease [94]. Patients with COPD are also more likely to produce autoantibodies to a broad spectrum of self-antigens, which may increase susceptibility to RA [95]. Prior research has also found RA to be significantly associated with subsequent risk of developing COPD [96]. A phenome-wide association study found an association between HLA-C and autoimmune diseases and bronchiectasis, suggesting a genetic link between autoimmune disorders and obstructive lung disease [29].
These findings provide rationale for investigating an association between COPD and risk of RA. However, only a few studies have investigated this relationship, summarized in Table 2. Since COPD is typically diagnosed late in life after heavy smoking exposure, studies of COPD and RA have been limited. This is likely related to insufficient sample size, given that patients with RA are usually diagnosed at younger ages. As already discussed above in the asthma section, adjustment for smoking is of particular significance.
Table 2.
Studies investigating the association of chronic obstructive pulmonary disease with risk of rheumatoid arthritis.
| Reference | Study design | Population Sample (n) RA outcomes (n) |
COPD exposure methods |
RA outcome methods |
Smoking and other adjustment variables |
Effect size (95% confidence interval) for COPD and RA risk (reference group: no COPD) |
Comments |
|---|---|---|---|---|---|---|---|
| De Roos AJ, Ann Epidemiol (2008) [81] | Case-control | Iowa & North Carolina, pesticide applicators and spouses (Agricultural Health Study) n=810 n=135 RA |
Self-report (chronic bronchitis) | Physician-confirmed | No smoking adjustment Age, US state |
Prevalent RA: OR 0.8 (0.3-2.2) Incident RA: OR 1.3 (0.3-5.7) |
Cases were mostly prevalent RA; limited sample size and adjustment variables; also investigated other personal and family medical conditions |
| Bergström U, Rheumatology (2011) [97] | Nested case-control | Sweden (Malmö Preventive Medicine Program) n=1,450 n=290 incident RA |
Presence and severity by GOLD criteria from research pulmonary function testing | Medical record review meeting 1987 ACR criteria | Smoking adjustment: not current/current Age, sex, socioeconomic status |
Mild/stage I COPD: OR 1.34 (0.67-2.68) Moderate to very severe/stages II-IV COPD: OR 1.08 (0.59-1.99) |
Pulmonary function tests were measured prospectively prior to RA diagnosis; suggestion of increased risk for RA among women with COPD, but not men; limited sample size and adjustment variables; also investigated restrictive lung disease |
| Sheen YH, J Allergy Clin Immunol Pract (2018) [75] |
Case-control | Olmsted County, Minnesota (Rochester Epidemiology Project) n=439 n=221 RA |
Not described | Billing and Berkson codes | No smoking adjustment No covariates |
OR 0.62 (0.32-1.23) | Only unadjusted COPD results reported in this study that was focused on asthma; limited sample size |
ACR, American College of Rheumatology; COPD, chronic obstructive pulmonary disease; GOLD, Global initiative for Obstructive Lung Disease; OR, odds ratio; RA, rheumatoid arthritis.
Prior case-control studies investigating COPD and RA risk have found no statistically significant association between COPD and RA. Bergström et al found that neither mild COPD nor moderate to very severe COPD was associated with RA risk [97]. This study used pulmonary function test results to identify the presence and severity of COPD prior to RA diagnosis [97]. Since there were few participants identified with COPD, power was limited to detect an association. There was a suggestion that women with COPD may have an increased risk for RA (OR 3.26, 95%CI 0.84–12.7) [97]. However, smoking was only adjusted for smoking status (current vs. noncurrent) and RA serostatus was unavailable.
A case-control study in Minnesota of 221 RA cases and 218 controls also found no association between COPD and RA risk (unadjusted OR 0.62, 95%CI 0.32–1.23), but was limited by small sample size [75]. Similarly, self-reported chronic bronchitis was not associated with RA in the Agricultural Health Study (OR 0.8, 95%CI 0.3–2.2) [81].
Though these studies similarly failed to detect an association between COPD and RA risk, all had small sample sizes and typically investigated other primary exposures in their analyses. Additionally, the difficulty in adjusting for smoking and other variables make these results susceptible to confounding. As cigarette smoking may have a causal role in both COPD and RA, properly accounting for smoking in analyses presents methodologic challenges, as detailed above, and is particularly important in studying this relationship. COPD is also a heterogenous condition so future studies should focus on subtypes of COPD based on age of onset, duration, severity, and treatment. The chronic airway damage and heavy smoking of COPD may particularly predispose to increased risk for seropositive RA, but no published studies have adequately investigated this hypothesis. In addition, no currently published studies have investigated the asthma-COPD overlap syndrome and risk for RA since this would likely require a very large sample size with long follow-up.
Given this limited evidence but strong biologic rationale, larger, prospective studies are necessary to investigate the relationship between COPD and RA risk. Specifically, future studies with larger sample sizes and enhanced ability to adjust for smoking are necessary to accurately assess the way in which COPD may affect RA risk. Additionally, studies examining RA serostatus would provide a more nuanced understanding of the relationship between these conditions, perhaps providing insights into the potential shared etiology of COPD and RA.
5. Muco-obstructive lung diseases and RA risk
Bronchiectasis
Bronchiectasis, characterized by abnormal widening of the airways leading to mucus buildup and subsequent risk of infection, has frequently been identified as a long-term consequence of seropositive RA [12], though the reported prevalence of bronchiectasis in RA varies widely from approximately 3–62% in the literature [98,99]. The coexistence of bronchiectasis and RA is associated with increased mortality compared to bronchiectasis alone [100], and concomitant RA and bronchiectasis or other chronic pulmonary diseases is associated with increased mortality as well as RA disease activity and severity [101,102]. Patients with RA and bronchiectasis have been shown to have higher levels of autoantibodies compared to patients with RA and no bronchiectasis, perhaps due to chronic bacterial infection [103]. As bronchiectasis can be detected both prior to or well after articular RA onset [12], it is challenging to determine whether bronchiectasis may be a risk factor for RA-related autoantibody production and RA risk or whether this is a consequence of longstanding RA. Imaging evidence of pulmonary inflammation and structural airway abnormalities are common in patients with RA-specific autoantibodies in absence of classifiable RA. A study of 42 RF-positive or ACPA-positive patients without detectable inflammatory arthritis found that 14% had bronchiectasis by high-resolution chest CT imaging, with 76% having any pulmonary abnormality which included air trapping, bronchial wall thickening, and centrilobular opacities [15]. This supports the hypothesis that chronic inflammation in the lung or airways may be a site for genesis of RA-related autoimmunity.
Cystic fibrosis
Cystic fibrosis (CF) is a multisystem genetic disorder in which mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene result in a defect in the chloride transporter protein in the lung, pancreas, and exocrine glands [104]. The lungs are predominantly affected in patients with CF, with thickened mucus leading to airway obstruction, remodeling, and chronic infections, all of which cause chronic airway damage. NETs may play a particularly important role in the production of many autoantibodies, including ACPA and anti-carbamylated protein antibodies, in patients with CF [105]. A syndrome of CF arthropathy consisting of recurrent, episodic mono- or polyarthritis distinct from other classifiable rheumatic diseases has been described [106]. In a retrospective study of 186 patients with CF, 29% were considered to have CF arthropathy as defined by at least one episode of joint pain and/or swelling, primarily involving the hands and feet [107]. Some suggest that CF patients that receive inhaled antibiotics may develop arthralgias perhaps as an immune response from bactericidal products. Therefore, CF arthropathy and RA may have some overlapping features. A clear association between CF and increased risk of classifiable RA has not been demonstrated in the limited literature to date. However, there is evidence to support an association between CF and RA-related autoimmunity. Janssen et al compared sera of non-RA patients with CF to those of healthy controls as well as patients with periodontitis, bronchiectasis, and RA [57]. Elevated IgA anti-CCP and IgA RF were significantly associated with both CF and RA [57]. Furthermore, 33% of patients with CF arthropathy tested positive for anti-CCP, and 10% tested positive for RF [57]. There may also be genetic linkages between CF and RA; a family-based association study comprised of RA-associated bronchiectasis probands and their families found an increased frequency of CFTR gene mutations in subjects with RA-associated bronchiectasis compared to unaffected relatives as well as healthy controls [108].
Chronic tuberculous and nontuberculous mycobacterial infections
While tuberculosis is typically viewed as a consequence of treatment of RA, patients with tuberculosis may have higher levels of ACPA than non-infected individuals, perhaps not dependent on citrullination [109,110]. Interestingly, some of these patients may not develop clinical manifestations of RA and it is thought that some patients may turn ACPA negative after treatment [109]. A link between tuberculosis and RA risk is less established. More research is needed to understand different biologic mechanisms for producing ACPA and the subsequent progression to RA.
Nontuberculous mycobacteria (NTM) are organisms ubiquitous in the environment that can colonize mucosal surfaces and sometimes progress to subclinical infection or clinically-apparent disease in immunosuppressed individuals, but occasionally in patients who are otherwise healthy [111]. Chronic pulmonary NTM infection may be linked to increased RA risk, perhaps due to chronic mucosal inflammation in airways or other sites. Pulmonary NTM and RA both typically affect middle-aged or older women with underlying lung disease, particularly bronchiectasis [111]. A population-based study in Northern California found higher NTM rates among RA patients compared to similarly aged patients without RA [112]. Among users of anti-tumor necrosis factor inhibitors, patients with RA had higher NTM rates compared to patients with other inflammatory diseases (e.g., inflammatory bowel disease) using these drugs [112]. Another study investigated whether concentrations of lung microbiota in BAL fluid are different in early RA and healthy controls and found less microbial diversity in early RA, perhaps due to overgrowth of commensal pathogens such as NTM [113]. These studies provide some evidence that perhaps chronic airway infections might pre-dispose individuals to developing RA, though patients with RA may develop these chronic infections due to altered immunity, immunosuppressive medications, or smoking.
6. ILD and RA risk
In patients with RA-ILD, ILD typically manifests clinically after patients have already been diagnosed with RA. Increasingly, there is evidence that ILD onset may precede the articular onset of RA, particularly in patients with RA-related seropositivity. Subtypes of ILD exist on a spectrum and can involve airways and the interstitium, which may be difficult to quantify by imaging alone without lung biopsy. A large cross-sectional study of community-dwelling adults tested RA-related autoantibodies for research purposes and also obtained cardiac computed tomography scans that were read for features of subclinical ILD [114]. This showed strong relationships between elevated RF and ACPA and subclinical ILD, suggesting autoantibody production and pulmonary inflammation are linked prior to clinical RA onset [114]. A retrospective study analyzed the predictive role of ACPA positivity in patients diagnosed with idiopathic interstitial pneumonia (IIP), but who did not fulfill the diagnostic criteria for RA [115]. They found that one-third of those with IIP and who were ACPA-positive developed RA within three years of their ILD diagnosis [115]. Several case reports have reported similar instances of ILD with ACPA positivity preceding the onset of RA. Two cases of patients with organizing pneumonia, which often involves the small airways, were found to be ACPA positive and subsequently developed RA within a year of their OP diagnoses [116,117]. A single-center study in Colorado described 74 patients with respiratory symptoms who were referred to a pulmonary clinic and had elevated anti-CCP without RA or other connective tissue disease [118]. They found that 54% had isolated airways diseases, 14% had isolated ILD, 26% had a mix of airway diseases and ILD, and 7% had combined pulmonary fibrosis and ILD. Usual interstitial pneumonia was the most common ILD subtype [118]. Among 33 patients with high-titer anti-CCP, only 3 developed articular RA during median follow-up of about 1 year [118]. Another study reviewed 340 patients in a single-center health system in Massachusetts with anti-CCP positivity but without diagnosed RA [119]. This study found that lung disease was the second most common indication for checking anti-CCP after arthralgias, but these patients were unlikely to progress to classifiable RA during median follow-up of 2.7 years [119]. It is possible that patients with severe enough lung disease to be detected clinically may have markedly shortened longevity and treatment with potent immunosuppressants for lung disease could mask the articular onset of RA [120]. A large population-based study in Denmark showed that only 2.2% of patients with incident RA have ILD at articular diagnosis, but 34.0% of all RA-associated ILD cases occurred within one year of articular RA onset [121]. Therefore, it is possible subclinical ILD may be relatively common in patients, even prior to the articular clinical presentation of RA. Future work is needed to determine a timeline of pulmonary inflammation and damage leading to inflammatory arthritis in RA.
6. Conclusion
The current evidence supports a biologic role in airway inflammation and RA-related autoantibody production with subsequent increased risk for RA. Airway inflammation induces a local immune response and may alter proteins to form neoantigens. In addition, chronic airway damage may increase risk for infections which may cause more damage and molecular mimicry. All these mechanisms may result in increased immune activation, immune tolerance, and production of autoantibodies in local lymphoid aggregates. These processes may occur years prior to the detection of RA-related autoantibodies in the peripheral circulation and the articular symptom onset that results in a clinical diagnosis of RA.
The literature is richest for investigating asthma and RA risk, with a total of 10 epidemiologic investigations identified (Table 1). Eight of these publications reported an increased risk for RA (either overall or for seropositive RA). However, the largest study to date found an inverse relationship between asthma and RA risk [87]. None of these studies were prospective, except for a case-control nested within a prospective cohort [82]. Therefore, the evidence of asthma and RA risk is currently conflicted and mostly relies on retrospective evidence.
The literature is relatively sparse for other obstructive lung diseases and RA risk. Only 3 epidemiologic investigations have investigated COPD and RA risk (Table 2). All of these were underpowered to detect an association. These studies were also limited in the ability to account for smoking and none investigated COPD subtypes or RA by serologic phenotypes. The current data are therefore limited to determine whether a relationship between COPD and RA exists. The data on muco-obstructive lung diseases such as bronchiectasis, cystic fibrosis, and tuberculosis and NTM are limited to case reports, but suggest possible increased risk for RA. Finally, some studies suggest that ILD may precede the onset of RA diagnosis, particularly in patients with seropositivity. However, these are limited to cross-sectional studies and case series and it is possible that treatment of clinically-apparent ILD may alter the natural history related to emergence of articular symptoms.
7. Expert opinion
Despite many improvements in the treatment of RA, patients continue to have high rates of respiratory complications that do not seem to have improved over time, even in recent years [5,10,101]. While smoking is the major environmental risk factor for RA, the prevalence of RA has not decreased even with decreasing rates of smoking [122]. Therefore, other causes of mucosal airway inflammation may be important for RA development. Determining the biologic mechanisms linking obstructive lung diseases with RA risk may offer novel targets for RA prevention as well as reduction of respiratory outcomes among patients already diagnosed with RA. Most studies have focused on airways disease and parenchymal involvement, both seeming to be associated with increased RA risk but perhaps due to different biologic mechanisms. Less research has focused on other forms of obstruction such as vocal cord dysfunction or subglottic stenosis.
More epidemiologic investigations are needed to firmly establish that obstructive lung diseases are associated with RA risk. Prospective studies with the ability to phenotype asthma and COPD (both to accurately identify their presence and investigate subtypes based on clinical severity, age of onset, and duration) will be crucial to establish these patient populations as being at risk for developing RA. These studies should also have the ability to control for the possible confounding/mediating effect of smoking on possible associations. Longitudinal, repeated measures of smoking incorporating status, intensity, and duration are needed to establish these diseases as RA risk factors. The current conflicting evidence for the relationship between asthma and RA risk (as well as COPD and RA risk) should be a research priority. If these relationships are established, future studies should investigate whether treatments for obstructive lung diseases affect RA risk. Studies on prevention and screening could be considered in patients with asthma and COPD. Given current strong evidence implicating smoking as an RA risk factor, public health interventions should focus on smoking cessation for first-degree relatives and individuals with RA-related seropositivity [4]. Permanent smoking cessation has been shown to reduce risk for seropositive RA [4]. More research is also needed to understand the timing and risk related to muco-obstructive lung diseases and ILD for RA. Research should focus on identifying subclinical bronchiectasis and ILD in patients with RA-related seropositivity but no articular RA, since these patients are very likely to either develop clinical lung disease or RA.
Research studies should consider advanced pulmonary imaging for research purposes, including chest computed tomography, magnetic resonance imaging, positron emission tomography, and pulmonary function testing. Leveraging the increasing amount of molecular data in genetic, gene expression, and other -omic studies may also help elucidate mechanisms of disease susceptibility and causal pathways. Enhancing automated methods of detecting and quantifying airway and interstitial abnormalities [123] and disseminating these tools widely will increase efficiency and accuracy of studies. Patients with RA should be monitored closely for obstructive lung diseases and subclinical RA involvement, since these may be highly predictive of poor outcomes. Among patients with RA, research should focus on detection of airway and interstitial lung abnormalities to determine the natural history of lung involvement in RA and to move toward optimizing management to reduce respiratory morbidity and mortality beyond smoking cessation alone. Since progress is being made on many of these issues, the next five years in research focused on obstructive lung disease and RA risk are likely to offer important breakthroughs that may enhance the understanding of RA pathogenesis and move toward both RA prevention and reduction of the respiratory burden of RA.
ARTICLE HIGHLIGHTS.
Many potential biologic mechanisms may place patients with obstructive lung diseases at increased risk for rheumatoid arthritis (RA).
Chronic airway inflammation may lead to neoantigen production, immune tolerance breakdown, and RA-related autoantibody production prior to the systemic and articular involvement of RA.
Asthma is the most studied obstructive lung disease for risk of RA and there are conflicting results; most studies suggest that asthma may increase RA risk, but one study found a possible protective effect of asthma on RA risk. Many of these studies had limited ability to account for the possible effect of smoking in explaining this relationship and most were retrospective.
Fewer studies have investigated chronic obstructive pulmonary disease, bronchiectasis, cystic fibrosis, chronic tuberculous and nontuberculous mycobacterial infections, and interstitial lung disease for subsequent risk of RA, but these are hypothesized to play a role in RA pathogenesis.
Acknowledgments
Funding
JA Sparks is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (grant numbers K23 AR069688, R03 AR075886, L30 AR066953, P30 AR070253, and P30 AR072577), the Rheumatology Research Foundation K Supplement Award, and the Brigham Research Institute. The funders had no role in the decision to publish or preparation of this manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard University, its affiliated academic health care centers, or the National Institutes of Health.
Footnotes
Declaration of interest
JA Sparks has received research support from Amgen and Bristol-Myers Squibb and performed consultancy for Optum, Janssen, and Gilead unrelated to this work. M Cho has received grant funding from GSK, consulting fees from Genentech, and speaking fees from Illumina unrelated to this work. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
REFERENCES
- 1.Sparks JA. Rheumatoid Arthritis. Ann Intern Med. 2019;170(1):ITC1–ITC16. [DOI] [PubMed] [Google Scholar]
- 2.Sugiyama D, Nishimura K, Tamaki K et al. Impact of smoking as a risk factor for developing rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis. 2010;69(1):70–81. [DOI] [PubMed] [Google Scholar]
- 3.Di Giuseppe D, Discacciati A, Orsini N, Wolk A. Cigarette smoking and risk of rheumatoid arthritis: a dose-response meta-analysis. Arthritis Res Ther. 2014;16(2):R61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu X, Tedeschi SK, Barbhaiya M et al. Impact and Timing of Smoking Cessation on Reducing Risk of Rheumatoid Arthritis Among Women in the Nurses’ Health Studies. Arthritis Care Res (Hoboken). 2019;71(7):914–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sparks JA, Chang SC, Liao KP et al. Rheumatoid Arthritis and Mortality Among Women During 36 Years of Prospective Follow-Up: Results From the Nurses’ Health Study. Arthritis Care Res (Hoboken). 2016;68(6):753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.England BR, Sayles H, Michaud K et al. Cause-Specific Mortality in Male US Veterans With Rheumatoid Arthritis. Arthritis Care Res (Hoboken). 2016;68(1):36–45. [DOI] [PubMed] [Google Scholar]
- 7.Nannini C, Ryu JH, Matteson EL. Lung disease in rheumatoid arthritis. Curr Opin Rheumatol. 2008;20(3):340–346. [DOI] [PubMed] [Google Scholar]
- 8.Duarte AC, Porter JC, Leandro MJ. The lung in a cohort of rheumatoid arthritis patients-an overview of different types of involvement and treatment. Rheumatology (Oxford). 2019; [DOI] [PubMed] [Google Scholar]
- 9.Bongartz T, Nannini C, Medina-Velasquez YF et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum. 2010;62(6):1583–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raimundo K, Solomon JJ, Olson AL et al. Rheumatoid Arthritis-Interstitial Lung Disease in the United States: Prevalence, Incidence, and Healthcare Costs and Mortality. J Rheumatol. 2019;46(4):360–369. [DOI] [PubMed] [Google Scholar]
- 11.Sparks JA, Chang SC, Nguyen UDT et al. Smoking Behavior Changes in the Early Rheumatoid Arthritis Period and Risk of Mortality During Thirty-Six Years of Prospective Followup. Arthritis Care Res (Hoboken). 2018;70(1):19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shadick NA, Fanta CH, Weinblatt ME, O’Donnell W, Coblyn JS. Bronchiectasis. A late feature of severe rheumatoid arthritis. Medicine (Baltimore). 1994;73(3):161–170. [PubMed] [Google Scholar]
- 13.Holers VM, Demoruelle MK, Kuhn KA et al. Rheumatoid arthritis and the mucosal origins hypothesis: protection turns to destruction. Nat Rev Rheumatol. 2018;14(9):542–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klareskog L, Catrina AI. Autoimmunity: lungs and citrullination. Nat Rev Rheumatol. 2015;11(5):261–262. [DOI] [PubMed] [Google Scholar]
- 15.Demoruelle MK, Weisman MH, Simonian PL et al. Brief report: airways abnormalities and rheumatoid arthritis-related autoantibodies in subjects without arthritis: early injury or initiating site of autoimmunity? Arthritis Rheum. 2012;64(6):1756–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barnes PJ. Cellular and molecular mechanisms of asthma and COPD. Clin Sci (Lond). 2017;131(13):1541–1558. [DOI] [PubMed] [Google Scholar]
- 17.Sharafkhaneh A, Hanania NA, Kim V. Pathogenesis of emphysema: from the bench to the bedside. Proc Am Thorac Soc. 2008;5(4):475–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boucher RC. Muco-Obstructive Lung Diseases. N Engl J Med. 2019;380(20):1941–1953. [DOI] [PubMed] [Google Scholar]
- 19.Button B, Goodell HP, Atieh E et al. Roles of mucus adhesion and cohesion in cough clearance. Proc Natl Acad Sci U S A. 2018;115(49):12501–12506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McDonough JE, Yuan R, Suzuki M et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med. 2011;365(17):1567–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rangel-Moreno J, Hartson L, Navarro C, Gaxiola M, Selman M, Randall TD. Inducible bronchus-associated lymphoid tissue (iBALT) in patients with pulmonary complications of rheumatoid arthritis. J Clin Invest. 2006;116(12):3183–3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Demenais F, Margaritte-Jeannin P, Barnes KC et al. Multiancestry association study identifies new asthma risk loci that colocalize with immune-cell enhancer marks. Nat Genet. 2018;50(1):42–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferreira MA, McRae AF, Medland SE et al. Association between ORMDL3, IL1RL1 and a deletion on chromosome 17q21 with asthma risk in Australia. Eur J Hum Genet. 2011;19(4):458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen J, Zhang J, Hu H, Jin Y, Xue M. Polymorphisms of RAD50, IL33 and IL1RL1 are associated with atopic asthma in Chinese population. Tissue Antigens. 2015;86(6):443–447. [DOI] [PubMed] [Google Scholar]
- 25.Queiroz GA, Costa RS, Alcantara-Neves NM et al. IL33 and IL1RL1 variants are associated with asthma and atopy in a Brazilian population. Int J Immunogenet. 2017;44(2):51–61. [DOI] [PubMed] [Google Scholar]
- 26.Ober C, Yao TC. The genetics of asthma and allergic disease: a 21st century perspective. Immunol Rev. 2011;242(1):10–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wain LV, Shrine N, Artigas MS et al. Genome-wide association analyses for lung function and chronic obstructive pulmonary disease identify new loci and potential druggable targets. Nat Genet. 2017;49(3):416–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hobbs BD, Parker MM, Chen H et al. Exome Array Analysis Identifies a Common Variant in IL27 Associated with Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2016;194(1):48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ji X, Niu X, Qian J et al. A Phenome-Wide Association Study Uncovers a Role for Autoimmunity in the Development of Chronic Obstructive Pulmonary Disease. Am J Respir Cell Mol Biol. 2018;58(6):777–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim K, Jiang X, Cui J et al. Interactions between amino acid-defined major histocompatibility complex class II variants and smoking in seropositive rheumatoid arthritis. Arthritis Rheumatol. 2015;67(10):2611–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park B, Koo SM, An J et al. Genome-wide assessment of gene-by-smoking interactions in COPD. Sci Rep. 2018;8(1):9319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hunninghake GM, Hatabu H, Okajima Y et al. MUC5B promoter polymorphism and interstitial lung abnormalities. N Engl J Med. 2013;368(23):2192–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hancock LA, Hennessy CE, Solomon GM et al. Muc5b overexpression causes mucociliary dysfunction and enhances lung fibrosis in mice. Nat Commun. 2018;9(1):5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Juge PA, Lee JS, Ebstein E et al. MUC5B Promoter Variant and Rheumatoid Arthritis with Interstitial Lung Disease. N Engl J Med. 2018;379(23):2209–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mikuls TR, Payne JB, Deane KD, Thiele GM. Autoimmunity of the lung and oral mucosa in a multisystem inflammatory disease: The spark that lights the fire in rheumatoid arthritis? J Allergy Clin Immunol. 2016;137(1):28–34. [DOI] [PubMed] [Google Scholar]
- 36.Raychaudhuri S, Sandor C, Stahl EA et al. Five amino acids in three HLA proteins explain most of the association between MHC and seropositive rheumatoid arthritis. Nat Genet. 2012;44(3):291–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sparks JA, Karlson EW. The Roles of Cigarette Smoking and the Lung in the Transitions Between Phases of Preclinical Rheumatoid Arthritis. Curr Rheumatol Rep. 2016;18(3):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kokkonen H, Brink M, Hansson M et al. Associations of antibodies against citrullinated peptides with human leukocyte antigen-shared epitope and smoking prior to the development of rheumatoid arthritis. Arthritis Res Ther. 2015;17(125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okada Y, Wu D, Trynka G et al. Genetics of rheumatoid arthritis contributes to biology and drug discovery. Nature. 2014;506(7488):376–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Catrina AI, Deane KD, Scher JU. Gene, environment, microbiome and mucosal immune tolerance in rheumatoid arthritis. Rheumatology (Oxford). 2016;55(3):391–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chang HH, Liu GY, Dwivedi N et al. A molecular signature of preclinical rheumatoid arthritis triggered by dysregulated PTPN22. JCI Insight. 2016;1(17):e90045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sokolove J, Bromberg R, Deane KD et al. Autoantibody epitope spreading in the pre-clinical phase predicts progression to rheumatoid arthritis. PLoS One. 2012;7(5):e35296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arkema EV, Goldstein BL, Robinson W et al. Anti-citrullinated peptide autoantibodies, human leukocyte antigen shared epitope and risk of future rheumatoid arthritis: a nested case-control study. Arthritis Res Ther. 2013;15(5):R159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Catrina AI, Ytterberg AJ, Reynisdottir G, Malmstrom V, Klareskog L. Lungs, joints and immunity against citrullinated proteins in rheumatoid arthritis. Nat Rev Rheumatol. 2014;10(11):645–653. [DOI] [PubMed] [Google Scholar]
- 45.Klareskog L, Stolt P, Lundberg K et al. A new model for an etiology of rheumatoid arthritis: smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006;54(1):38–46. [DOI] [PubMed] [Google Scholar]
- 46.Makrygiannakis D, Hermansson M, Ulfgren AK et al. Smoking increases peptidylarginine deiminase 2 enzyme expression in human lungs and increases citrullination in BAL cells. Ann Rheum Dis. 2008;67(10):1488–1492. [DOI] [PubMed] [Google Scholar]
- 47.Reynisdottir G, Karimi R, Joshua V et al. Structural changes and antibody enrichment in the lungs are early features of anti-citrullinated protein antibody-positive rheumatoid arthritis. Arthritis Rheumatol. 2014;66(1):31–39. [DOI] [PubMed] [Google Scholar]
- 48.Joshua V, Reynisdottir G, Ytterberg J et al. A1.1 Characterisation of lung inflammation and identification of shared citrullinated targets in the lungs and joints of early rheumatoid arthritis. Annals of the Rheumatic Diseases. 2014;73(Suppl 1):A4–A5. [Google Scholar]
- 49.Hart JE, Laden F, Puett RC, Costenbader KH, Karlson EW. Exposure to traffic pollution and increased risk of rheumatoid arthritis. Environ Health Perspect. 2009;117(7):1065–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hart JE, Kallberg H, Laden F et al. Ambient air pollution exposures and risk of rheumatoid arthritis: results from the Swedish EIRA case-control study. Ann Rheum Dis. 2013;72(6):888–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arnson Y, Shoenfeld Y, Amital H. Effects of tobacco smoke on immunity, inflammation and autoimmunity. J Autoimmun. 2010;34(3):J258–265. [DOI] [PubMed] [Google Scholar]
- 52.Kronzer VL, Crowson CS, Sparks JA, Vassallo R, Davis JM 3rd. Investigating Asthma, Allergic Disease, Passive Smoke Exposure, and Risk of Rheumatoid Arthritis. Arthritis Rheumatol. 2019;71(8):1217–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sparks JA, Barbhaiya M, Tedeschi SK et al. Inflammatory dietary pattern and risk of developing rheumatoid arthritis in women. Clin Rheumatol. 2019;38(1):243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu X, Tedeschi SK, Lu B et al. Long-Term Physical Activity and Subsequent Risk for Rheumatoid Arthritis Among Women: A Prospective Cohort Study. Arthritis Rheumatol. 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hu Y, Sparks JA, Malspeis S et al. Long-term dietary quality and risk of developing rheumatoid arthritis in women. Ann Rheum Dis. 2017;76(8):1357–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lu B, Hiraki LT, Sparks JA et al. Being overweight or obese and risk of developing rheumatoid arthritis among women: a prospective cohort study. Ann Rheum Dis. 2014;73(11):1914–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Janssen KM, de Smit MJ, Brouwer E et al. Rheumatoid arthritis-associated autoantibodies in non-rheumatoid arthritis patients with mucosal inflammation: a case-control study. Arthritis Res Ther. 2015;17(174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brink M, Hansson M, Mathsson L et al. Multiplex analyses of antibodies against citrullinated peptides in individuals prior to development of rheumatoid arthritis. Arthritis Rheum. 2013;65(4):899–910. [DOI] [PubMed] [Google Scholar]
- 59.Willis VC, Demoruelle MK, Derber LA et al. Sputum autoantibodies in patients with established rheumatoid arthritis and subjects at risk of future clinically apparent disease. Arthritis Rheum. 2013;65(10):2545–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Darrah E, Andrade F. Editorial: citrullination, and carbamylation, and malondialdehyde-acetaldehyde! Oh my! Entering the forest of autoantigen modifications in rheumatoid arthritis. Arthritis Rheumatol. 2015;67(3):604–608. [DOI] [PubMed] [Google Scholar]
- 61.England BR, Duryee MJ, Roul P et al. Malondialdehyde-Acetaldehyde Adducts and Antibody Responses in Rheumatoid Arthritis-Associated Interstitial Lung Disease. Arthritis Rheumatol. 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Giles JT, Darrah E, Danoff S et al. Association of cross-reactive antibodies targeting peptidyl-arginine deiminase 3 and 4 with rheumatoid arthritis-associated interstitial lung disease. PLoS One. 2014;9(6):e98794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lucchino B, Spinelli FR, Iannuccelli C, Guzzo MP, Conti F, Di Franco M. Mucosa-Environment Interactions in the Pathogenesis of Rheumatoid Arthritis. Cells. 2019;8(7): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee KH, Kronbichler A, Park DD et al. Neutrophil extracellular traps (NETs) in autoimmune diseases: A comprehensive review. Autoimmun Rev. 2017;16(11):1160–1173. [DOI] [PubMed] [Google Scholar]
- 65.Zawrotniak M, Rapala-Kozik M. Neutrophil extracellular traps (NETs) - formation and implications. Acta Biochim Pol. 2013;60(3):277–284. [PubMed] [Google Scholar]
- 66.Yu HC, Lu MC. The roles of anti-citrullinated protein antibodies in the immunopathogenesis of rheumatoid arthritis. Ci Ji Yi Xue Za Zhi. 2019;31(1):5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.O’Neil LJ, Kaplan MJ. Neutrophils in Rheumatoid Arthritis: Breaking Immune Tolerance and Fueling Disease. Trends Mol Med. 2019;25(3):215–227. [DOI] [PubMed] [Google Scholar]
- 68.Demoruelle MK, Harrall KK, Ho L et al. Anti-Citrullinated Protein Antibodies Are Associated With Neutrophil Extracellular Traps in the Sputum in Relatives of Rheumatoid Arthritis Patients. Arthritis Rheumatol. 2017;69(6):1165–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rantapaa-Dahlqvist S, de Jong BA, Berglin E et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 2003;48(10):2741–2749. [DOI] [PubMed] [Google Scholar]
- 70.Falkenburg WJJ, van Schaardenburg D. Evolution of autoantibody responses in individuals at risk of rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2017;31(1):42–52. [DOI] [PubMed] [Google Scholar]
- 71.Kelmenson LB, Demoruelle MK, Deane KD. The Complex Role of the Lung in the Pathogenesis and Clinical Outcomes of Rheumatoid Arthritis. Curr Rheumatol Rep. 2016;18(11):69. [DOI] [PubMed] [Google Scholar]
- 72.Ytterberg AJ, Joshua V, Reynisdottir G et al. Shared immunological targets in the lungs and joints of patients with rheumatoid arthritis: identification and validation. Ann Rheum Dis. 2015;74(9):1772–1777. [DOI] [PubMed] [Google Scholar]
- 73.Kelmenson LB, Wagner BD, McNair BK et al. Timing of elevations of autoantibody isotypes in rheumatoid arthritis prior to disease diagnosis. Arthritis Rheumatol. 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hemminki K, Li X, Sundquist J, Sundquist K. Risk of asthma and autoimmune diseases and related conditions in patients hospitalized for obesity. Ann Med. 2012;44(3):289–295. [DOI] [PubMed] [Google Scholar]
- 75.Sheen YH, Rolfes MC, Wi CI et al. Association of Asthma with Rheumatoid Arthritis: A Population-Based Case-Control Study. J Allergy Clin Immunol Pract. 2018;6(1):219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rottem M, Shoenfeld Y. Asthma as a paradigm for autoimmune disease. Int Arch Allergy Immunol. 2003;132(3):210–214. [DOI] [PubMed] [Google Scholar]
- 77.Li X, Ampleford EJ, Howard TD et al. Genome-wide association studies of asthma indicate opposite immunopathogenesis direction from autoimmune diseases. J Allergy Clin Immunol. 2012;130(4):861–868 e867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Juhn YJ. Risks for infection in patients with asthma (or other atopic conditions): is asthma more than a chronic airway disease? J Allergy Clin Immunol. 2014;134(2):247–257; quiz 258–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hedstrom AK, Ronnelid J, Klareskog L, Alfredsson L. Complex Relationships of Smoking, HLA-DRB1 Genes, and Serologic Profiles in Patients With Early Rheumatoid Arthritis: Update From a Swedish Population-Based Case-Control Study. Arthritis Rheumatol. 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zaccardelli A, Friedlander HM, Ford JA, Sparks JA. Potential of Lifestyle Changes for Reducing the Risk of Developing Rheumatoid Arthritis: Is an Ounce of Prevention Worth a Pound of Cure? Clin Ther. 2019;41(7):1323–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.de Roos AJ, Cooper GS, Alavanja MC, Sandler DP. Personal and family medical history correlates of rheumatoid arthritis. Ann Epidemiol. 2008;18(6):433–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zaccardelli A, Liu X, Ford JA et al. Asthma and elevation of anti-citrullinated protein antibodies prior to the onset of rheumatoid arthritis. Arthritis Res Ther. 2019;21(1):246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jeong HE, Jung SM, Cho SI. Association between Rheumatoid Arthritis and Respiratory Allergic Diseases in Korean Adults: A Propensity Score Matched Case-Control Study. Int J Rheumatol. 2018;2018(3798124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hou YC, Hu HY, Liu IL, Chang YT, Wu CY. The risk of autoimmune connective tissue diseases in patients with atopy: A nationwide population-based cohort study. Allergy Asthma Proc. 2017;38(5):383–389. [DOI] [PubMed] [Google Scholar]
- 85.Lai NS, Tsai TY, Koo M, Lu MC. Association of rheumatoid arthritis with allergic diseases: A nationwide population-based cohort study. Allergy Asthma Proc. 2015;36(5):99–103. [DOI] [PubMed] [Google Scholar]
- 86.Yun HD, Knoebel E, Fenta Y et al. Asthma and proinflammatory conditions: a population-based retrospective matched cohort study. Mayo Clin Proc. 2012;87(10):953–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tirosh A, Mandel D, Mimouni FB, Zimlichman E, Shochat T, Kochba I. Autoimmune diseases in asthma. Ann Intern Med. 2006;144(12):877–883. [DOI] [PubMed] [Google Scholar]
- 88.Vogelmeier CF, Criner GJ, Martinez FJ et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am J Respir Crit Care Med. 2017;195(5):557–582. [DOI] [PubMed] [Google Scholar]
- 89.Wheaton AG, Liu Y, Croft JB et al. Chronic Obstructive Pulmonary Disease and Smoking Status - United States, 2017. MMWR Morb Mortal Wkly Rep. 2019;68(24):533–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Barnes PJ. Chronic obstructive pulmonary disease. N Engl J Med. 2000;343(4):269–280. [DOI] [PubMed] [Google Scholar]
- 91.Silverman EK, Sandhaus RA. Clinical practice. Alpha1-antitrypsin deficiency. N Engl J Med. 2009;360(26):2749–2757. [DOI] [PubMed] [Google Scholar]
- 92.Postma DS, Rabe KF. The Asthma-COPD Overlap Syndrome. N Engl J Med. 2015;373(13):1241–1249. [DOI] [PubMed] [Google Scholar]
- 93.Rogliani P, Ora J, Puxeddu E, Cazzola M. Airflow obstruction: is it asthma or is it COPD? Int J Chron Obstruct Pulmon Dis. 2016;11(3007–3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lugli EB, Correia RE, Fischer R et al. Expression of citrulline and homocitrulline residues in the lungs of non-smokers and smokers: implications for autoimmunity in rheumatoid arthritis. Arthritis Res Ther. 2015;17(9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Packard TA, Li QZ, Cosgrove GP, Bowler RP, Cambier JC. COPD is associated with production of autoantibodies to a broad spectrum of self-antigens, correlative with disease phenotype. Immunol Res. 2013;55(1–3):48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sparks JA, Lin TC, Camargo CA Jr. et al. Rheumatoid arthritis and risk of chronic obstructive pulmonary disease or asthma among women: A marginal structural model analysis in the Nurses’ Health Study. Semin Arthritis Rheum. 2018;47(5):639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bergstrom U, Jacobsson LT, Nilsson JA, Berglund G, Turesson C. Pulmonary dysfunction, smoking, socioeconomic status and the risk of developing rheumatoid arthritis. Rheumatology (Oxford). 2011;50(11):2005–2013. [DOI] [PubMed] [Google Scholar]
- 98.Walker WC. Pulmonary infections and rheumatoid arthritis. Q J Med. 1967;36(142):239–251. [PubMed] [Google Scholar]
- 99.Wilczynska MM, Condliffe AM, McKeon DJ. Coexistence of bronchiectasis and rheumatoid arthritis: revisited. Respir Care. 2013;58(4):694–701. [DOI] [PubMed] [Google Scholar]
- 100.De Soyza A, McDonnell MJ, Goeminne PC et al. Bronchiectasis Rheumatoid Overlap Syndrome Is an Independent Risk Factor for Mortality in Patients With Bronchiectasis: A Multicenter Cohort Study. Chest. 2017;151(6):1247–1254. [DOI] [PubMed] [Google Scholar]
- 101.England BR, Sayles H, Michaud K et al. Chronic lung disease in U.S. Veterans with rheumatoid arthritis and the impact on survival. Clin Rheumatol. 2018;37(11):2907–2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Perry E, Eggleton P, De Soyza A, Hutchinson D, Kelly C. Increased disease activity, severity and autoantibody positivity in rheumatoid arthritis patients with co-existent bronchiectasis. Int J Rheum Dis. 2017;20(12):2003–2011. [DOI] [PubMed] [Google Scholar]
- 103.Quirke AM, Perry E, Cartwright A et al. Bronchiectasis is a Model for Chronic Bacterial Infection Inducing Autoimmunity in Rheumatoid Arthritis. Arthritis Rheumatol. 2015;67(9):2335–2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Stoltz DA, Meyerholz DK, Welsh MJ. Origins of cystic fibrosis lung disease. N Engl J Med. 2015;372(4):351–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Skopelja S, Hamilton BJ, Jones JD et al. The role for neutrophil extracellular traps in cystic fibrosis autoimmunity. JCI Insight. 2016;1(17):e88912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Merkel PA. Rheumatic disease and cystic fibrosis. Arthritis Rheum. 1999;42(8):1563–1571. [DOI] [PubMed] [Google Scholar]
- 107.Roehmel JF, Kallinich T, Staab D, Schwarz C. Clinical manifestations and risk factors of arthropathy in cystic fibrosis. Respir Med. 2019;147(66–71. [DOI] [PubMed] [Google Scholar]
- 108.Puechal X, Bienvenu T, Genin E et al. Mutations of the cystic fibrosis gene in patients with bronchiectasis associated with rheumatoid arthritis. Ann Rheum Dis. 2011;70(4):653–659. [DOI] [PubMed] [Google Scholar]
- 109.Elkayam O, Segal R, Bendayan D, van Uitert R, Onnekink C, Pruijn GJ. The anti-cyclic citrullinated peptide response in tuberculosis patients is not citrulline-dependent and sensitive to treatment. Arthritis Res Ther. 2010;12(1):R12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Elkayam O, Segal R, Lidgi M, Caspi D. Positive anti-cyclic citrullinated proteins and rheumatoid factor during active lung tuberculosis. Ann Rheum Dis. 2006;65(8):1110–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Winthrop KL, Iseman M. Bedfellows: mycobacteria and rheumatoid arthritis in the era of biologic therapy. Nat Rev Rheumatol. 2013;9(9):524–531. [DOI] [PubMed] [Google Scholar]
- 112.Winthrop KL, Baxter R, Liu L et al. Mycobacterial diseases and antitumour necrosis factor therapy in USA. Ann Rheum Dis. 2013;72(1):37–42. [DOI] [PubMed] [Google Scholar]
- 113.Scher JU, Joshua V, Artacho A et al. The lung microbiota in early rheumatoid arthritis and autoimmunity. Microbiome. 2016;4(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bernstein EJ, Barr RG, Austin JHM et al. Rheumatoid arthritis-associated autoantibodies and subclinical interstitial lung disease: the Multi-Ethnic Study of Atherosclerosis. Thorax. 2016;71(12):1082–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Katsumata M, Hozumi H, Yasui H et al. Frequency and clinical relevance of anti-cyclic citrullinated peptide antibody in idiopathic interstitial pneumonias. Respir Med. 2019;154(102–108. [DOI] [PubMed] [Google Scholar]
- 116.Komiya K, Teramoto S, Kurosaki Y et al. Organizing pneumonia with a positive result for anti-CCP antibodies as the first clinical presentation of rheumatoid arthritis. Intern Med. 2010;49(15):1605–1607. [DOI] [PubMed] [Google Scholar]
- 117.Hoshino C, Satoh N, Narita M, Kikuchi A, Inoue M. Organising pneumonia as the first manifestation of rheumatoid arthritis. BMJ Case Rep. 2011;2011( [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fischer A, Solomon JJ, du Bois RM et al. Lung disease with anti-CCP antibodies but not rheumatoid arthritis or connective tissue disease. Respir Med. 2012;106(7):1040–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ford JA, Liu X, Marshall AA et al. Impact of Cyclic Citrullinated Peptide Antibody Level on Progression to Rheumatoid Arthritis in Clinically Tested CCP-Positive Patients Without RA. Arthritis Care Res (Hoboken). 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Olson AL, Swigris JJ, Sprunger DB et al. Rheumatoid arthritis-interstitial lung disease-associated mortality. Am J Respir Crit Care Med. 2011;183(3):372–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hyldgaard C, Hilberg O, Pedersen AB et al. A population-based cohort study of rheumatoid arthritis-associated interstitial lung disease: comorbidity and mortality. Ann Rheum Dis. 2017;76(10):1700–1706. [DOI] [PubMed] [Google Scholar]
- 122.Hunter TM, Boytsov NN, Zhang X, Schroeder K, Michaud K, Araujo AB. Prevalence of rheumatoid arthritis in the United States adult population in healthcare claims databases, 2004–2014. Rheumatol Int. 2017;37(9):1551–1557. [DOI] [PubMed] [Google Scholar]
- 123.Ash SY, Harmouche R, Vallejo DL et al. Densitometric and local histogram based analysis of computed tomography images in patients with idiopathic pulmonary fibrosis. Respir Res. 2017;18(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]