Abstract
Vesicular acetylcholine transporter (VAChT) is a promising target for a PET measure of cholinergic deficits which contribute to cognitive impairments. Dopamine D2-like agonists and antagonists are frequently used in the elderly and could alter cholinergic function and VAChT level. Therefore, pretreatment with dopamine D2-like drugs may interfere with PET measures using [18F]VAT, a specific VAChT radioligand. Herein, we investigated the impact of dopaminergic D2-like antagonist/agonist on VAChT level in the brain of macaques using [18F]VAT PET. PET imaging studies were carried out on macaques at baseline or pretreatment conditions. For pretreatment, animals were injected using a VAChT inhibitor (−)-vesamicol, a D2-like antagonist (−)-eticlopride, and a D2-like agonist (−)-quinpirole, separately. (−)-Vesamicol was injected at escalating doses of 0.025, 0.05, 0.125, 0.25 and 0.35 mg/kg; (−)-eticlopride was injected at escalating doses of 0.01, 0.10 and 0.30 mg/kg; (−)-quinpirole was injected at escalating doses of 0.20, 0.30, and 0.50 mg/kg. PET data showed [18F]VAT uptake declined in a dose-dependent manner by (−)-vesamicol pretreatment, demonstrating [18F]VAT uptake is sensitive to reflect the availability of VAChT binding sites. Furthermore, (−)-eticlopride increased [18F]VAT striatal uptake in a dose-dependent manner, while (−)-quinpirole decreased its uptake, suggesting striatal VAChT levels can be regulated by D2-like drug administration. Our findings confirmed [18F]VAT offers a reliable tool to in vivo assess the availability of VAChT binding sites. More importantly, PET with [18F]VAT successfully quantified the impact of dopaminergic D2-like drugs on striatal VAChT level, suggesting [18F]VAT has great potential for investigating the interaction between dopaminergic and cholinergic systems in vivo.
Keywords: Vesicular acetylcholine transporter, [18F]VAT, PET, dopaminergic D2-like drugs, nonhuman primates
Graphical abstract
1. Introduction
Cholinergic pathways are widely distributed throughout the brain and play a critical role in modulating cognitive performances, learning and memory processes, cortical activity, cerebral blood flow, and other essential physiological functions (Schliebs and Arendt, 2006). As such, cholinergic dysfunction contributes to the pathogenesis of various neurodegenerative disorders such as Alzheimer disease (AD) and Parkinson disease (PD) (Bohnen and Albin, 2011; Hampel et al., 2018). A wealth of recent literature has also revealed the complex interactions between cholinergic dysfunction and other pathophysiological hallmarks of AD including amyloid-β pathology, neurofibrillary tangles, and dysregulation of other major neurotransmitter and neurohormonal systems (Hampel et al., 2018). Concordantly, interventions improving cholinergic function are essential and critical components of AD therapy; four of the five drugs approved by US Food and Drug Administration (FDA) for AD are cholinesterase inhibitors: donepezil, galantamine, rivastigmine, and tacrine (the latter discontinued in the US due to safety concerns) (Hampel et al., 2018). Cholinergic pathology is less well recognized in PD but may play an important role in pathophysiology with the classic hypothesis that reduced dopaminergic input to the striatum causes relative cholinergic hyperactivity (Duvoisin, 1967). However, emerging evidence suggests cholinergic degeneration, especially in the basal forebrain, appears early in PD and worsens with the appearance of dementia (Bohnen and Albin, 2011). To better understand the critical role that cholinergic dysfunction plays in the pathophysiological events of neurodegenerative disorders, methodologies for in vivo quantification of the changes in cholinergic neurons/synapses are urgently needed. Positron emission tomography (PET) imaging with specific radioligands targeting cholinergic biomarkers could provide such a unique imaging methodology.
The most specific and widely used presynaptic biomarker is ChAT, however, there is no PET radiotracer currently available for it. The vesicular acetylcholine transporter (VAChT), which localizes on synaptic vesicle membranes and is responsible for translocating acetylcholine from the cytoplasm into vesicles, correlates well with ChAT distribution (Prado et al., 2013; Weihe et al., 1996). Two radioligands targeting VAChT, [123I]IBVM and [18F]FEOBV, have been used in patients with single-photon emission computerized tomography (SPECT) or PET. Decreased [123I]IBVM binding is reported in cortex and/or hippocampus of AD and PD brains (Kuhl et al., 1996). In agreement with that, [18F]FEOBV uptake is significantly reduced in cortical areas in AD patients and the tracer uptake positively correlates with cognitive performances (Aghourian et al., 2017). Patients with PD or Lewy body dementia also showed remarkable reduction of [18F]FEOBV uptake in various brain areas including neocortical, limbic, striatal and thalamic regions (Bohnen et al., 2019; Nejad-Davarani et al., 2019). Yet both radioligands require at least 180 min to reach the equilibrium in vivo, which might pose a challenge for aged patients and patients with dementia or motor disorders. Our group have developed a potent and specific VAChT radioligand [18F]VAT with favorable kinetics for clinical use. The Ki value is 0.59 nM for VAChT, over 1000-fold affinity than the binding to σ receptors (Tu et al., 2015), as shown in Fig.1 and Table 1. Our prior studies demonstrated that [18F]VAT possesses promising radiopharmaceutical profiles in rodents and nonhuman primates (NHPs), and favorable in vivo radiation dosimetry (Jin et al., 2018; Karimi et al., 2015; Tu et al., 2015). USA Food and Drug Administration (FDA) has approved the research use of the radiopharmaceutical [18F]VAT for quantifying VAChT level in human brain, including healthy participants and patients with PD, dystonia and other neurodegenerative diseases. To better characterize [18F]VAT in vivo, we examined the in vivo specificity of [18F]VAT binding to VAChT using a well-known VAChT inhibitor (−)-vesamicol. Furthermore, target engagement by PET imaging is a powerful tool to optimize drug dosing to maximal pharmacological outcomes (Zhang and Fox, 2012). Therefore, target engagement studies were conducted using [18F]VAT PET to establish the relationship between the dose of (−)-vesamicol and target occupancy percentage.
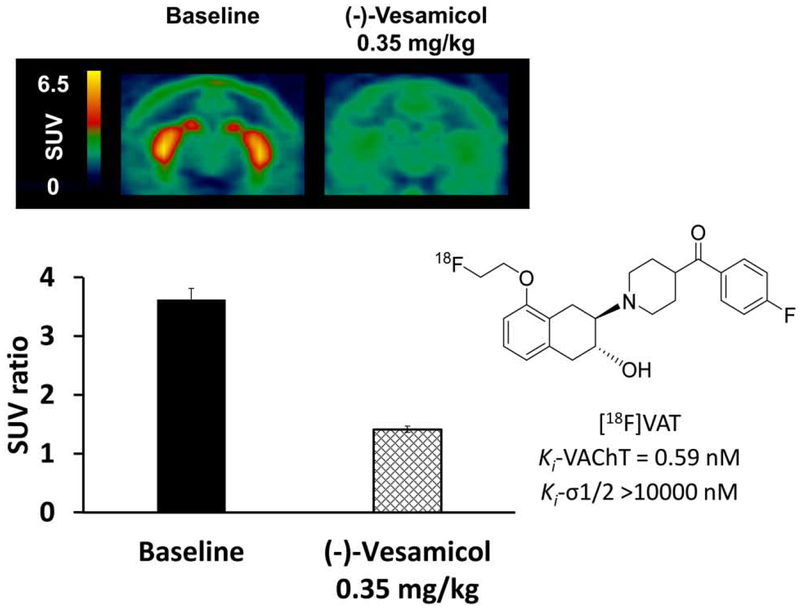
Figure 1.
Representative PET images and SUV ratio (striatum-to-cerebellar hemispheres) of [18F]VAT in the NHP brain at baseline and following (−)-vesamicol pretreatment (summed images from 0-120min). SUV, standardized uptake value. The chemical structure and binding affinities of [18F]VAT were also included.
Table 1.
The binding affinities of compounds used in this study*
| Compound names | Binding affinity (Ki, nM) | |||
|---|---|---|---|---|
| VAChT | σ1 | σ2 | D2 | |
| VAT | 0.59 | >10000 | >10000 | N/A |
| (−)-Vesamicol | 15.20 | 73.80 | 346.00 | N/A |
| (−)-Eticlopride | N/A | N/A | N/A | 0.50 |
| (−)-Quinpirole | N/A | N/A | N/A | 3.69 (Kd) |
All the values were obtained from previous publications (see citation in “2.2 study compounds” and “references” sections)
Notably, dopaminergic drugs, especially those targeting D2-like receptors, which are frequently used in the elderly (Johnell and Fischer, 2011), may change VAChT levels (Terry et al., 2007) and interfere with PET measurement of VAChT in the brain using a VAChT radiotracer. Specifically, D2-like receptor agonists or antagonists are commonly prescribed for patients with PD and AD(Antonini et al., 2018). In addition, D2-like antagonists are used for managing behavioral and neuropsychiatric symptoms in a variety of patients (Beaulieu and Gainetdinov, 2011). Therefore, it is critical to determine the impact of D2-like drugs on VAChT level in vivo. In this manuscript, in addition to the pretreatment studies in animals using (−)-vesamicol, we also evaluated the effect of D2-like drugs on striatal VAChT level by quantifying the brain uptake of [18F]VAT following pretreatment with a D2-like antagonist (−)-eticlopride or a D2-like agonist (−)-quinpirole.
2. Experimental procedures
2.1. Animals
All animal experiments were conducted by following the Guidelines for the Care and Use of Research Animals under a research protocol approved by Washington University Institutional Animal Care and Use Committee (IACUC). This work was conducted in the nonhuman primate microPET facility at the Washington University School of Medicine in St. Louis. Three adult male Macaca fascicularis (8-10 kg) were used in this study. Animals were prepared for microPET studies as previously reported (Jin et al., 2018), with minor changes in anesthesia. Each animal was initially anesthetized with ketamine (10–20 mg/kg) and injected with glycopyrrolate (0.013–0.017 mg/kg) to reduce saliva secretions. 40% N20 and 60% 02 with 10-1.5% isoflurane inhalation anesthesia was maintained throughout the microPET imaging sessions. A 20-gauge plastic catheter was inserted into a limb vein to permit hydration and injection of the radiotracer. To minimize the potential impact of ketamine on the microPET measure such as cerebral blood flow and brain glucose metabolism (Black et al., 1997), the tracer injection and microPET scan were started at least two hours after ketamine administration, and all PET scans followed the same procedure.
2.2. Study compounds
Three compounds, (−)-vesamicol, (−)-eticlopride, and (−)-quinpirole were used for pretreatment in the current study. The binding affinities of these compounds are obtained from extant literature (Hall et al., 1985; Malmberg and Mohell, 1995; Tu et al., 2015) and are listed in Table 1. Compounds (±)-vesamicol, (−)-eticlopride, and (−)-quinpirole were purchased from Sigma-Aldrich Corporation in Saint Louis, MO. (−)-Vesamicol was resolved from (±)-vesamicol and then converted to hydrochloride salt in-house. All compounds were dissolved in 100% sterile water to yield the desired concentration within 24 hours prior to the microPET studies.
2.3. PET data acquisition
PET imaging studies were carried out with a microPET Focus-220 scanner (Concorde/CTI/Siemens Microsystems, Knoxville, TN). Prior to each PET emission acquisition, a 45 min transmission scan for attenuation correction was performed following a 10 min transmission that was used to check the position of the brain within the scanner.
[18F]VAT was radiosynthesized according to our published standard protocol (Tu et al., 2015). Intravenous (iv) injection of 8-10 mCi of [18F]VAT was applied for each PET. PET scans were either baseline scans or pretreatment studies with the unlabeled VAChT inhibitor (−)-vesamicol at doses of 0.025, 0.05, 0.125, 0.25 and 0.35 mg/kg (iv, animal A), a D2-like antagonist (−)-eticlopride at doses of 0.01, 0.10 and 0.30 mg/kg, (iv, animal A) or a D2-like agonist (−)-quinpirole at doses of 0.20, 0.30, and 0.50 mg/kg (iv, animal B). Dynamic scans continued from 0 to 120 or 0 to 180 min. In additional studies, all three animals (A, B, C) received high dose pretreatment of each D2-like drug ((−)-eticlopride 0.30 mg/kg, or (−)-quinpirole 0.50 mg/kg, one drug each time) and followed by [18F]VAT PET. All compounds for pretreatment were administered 5 min prior to tracer injection, except (−)-quinpirole which was injected 30 min ahead. The dosages and administration times were chosen based on previous reports and/or in vivo kinetic properties of each compound (Horvitz et al., 2001; Jin et al., 2016; Van Hartesveldt, 1997; Yue et al., 2017). The interval between two consecutive scans on the same subject was at least 4 weeks.
2.4. PET data process and quantitative analysis
The PET/CT images were processed according to our published procedure (Jin et al., 2018). In brief, sinogram data were corrected for attenuation, randoms and scatter, and reconstructed using filtered back projection. The first baseline PET image for each animal acted as the target image. The magnetization-prepared rapid gradient echo (MP-RAGE) MR image and subsequent PET images were co-registered to the target PET image using an automated image registration program Automated Image Registration (AIR), and superimposed using Analyze 10.0 (AnalyzeDirect, Overland Park, KS). Regions of interest (ROIs), including striatum and cerebellar hemispheres, were manually drawn on MP-RAGE images for brain, and yield the volumes of interest (VOIs). The VOIs were transformed to the baseline PET space and then overlaid on all reconstructed PET images. Time-activity curves (TACs) were generated from the dynamic PET images. Activity concentration measures were calculated and standardized to the body weight and the injected dose of radioactivity, and yielded standardized uptake value (SUV). SUV ratio (SUVR) of striatum to cerebellar hemispheres was then obtained. To minimize noise signal in the presentation, data in TAC graphs have been smoothed by “LOWESS” using GraphPad Prism 6.0 (GraphPad Software, Inc., San Diego, CA), while the original data was used for tracer kinetic analysis.
We use the Logan Reference model (LoganREF) and simplified reference tissue model (SRTM) to calculate the non-displaceable binding potential (BPND) to quantify the change of brain tracer uptake in response to different pretreatments. Logan REF and SRTM were performed using an in house program, with cerebellar hemispheres as the reference region (Jin et al., 2018). The area under the time-activity curve (AUC), which is a model and input function-independent method to assess tracer accumulation in the brain, was also calculated. The target occupancy (OccM) following blockade with (−)-vesamicol was calculated as the relative change in BPND and refers to the percentage of VAChT binding sites bound by (−)-vesamicol.
3. Results
3.1. In vivo block studies using (−)-vesamicol
To explore whether PET with the VAChT-specific radioligand [18F]VAT could reflect the availability of VAChT binding sites, escalating doses of (−)-vesamicol were i.v. injected prior to tracer injection. In baseline studies, [18F]VAT accumulated highly in striatum with modest uptake in midbrain and cortex (Fig.1), which is in accordance with previous findings in NHPs and humans (Jin et al., 2018; Jin et al., 2015; Tu et al., 2015). The striatum-to-cerebellar hemispheres SUV ratio from 90-120 min post injection was 3.62 at baseline. Blocking studies using the VAChT ligand (−)-vesamicol at the max dose of 0.35 mg/kg significantly decreased striatal uptake of [18F]VAT, and the SUV ratio dropped to 1.41 (Fig.1).
Using escalating doses of (−)-vesamicol at 0, 0.025, 0.05, 0.125, 0.25, and 0.35 mg/kg, striatal binding potential significantly declined in a dose-dependent manner. The striatal BPND values were 2.35, 1.37, 1.28, 0.55, 0.30, 0.25 by LoganREF and 2.47, 1.58, 1.52, 0.41, 0.23, 0.23 by SRTM (Table 2). Furthermore, the target occupancy was determined by the change of binding potentials estimated with LoganREF or SRTM. The vesamicol occupancy levels were 42%, 46%, 77%, 87% and 89% using LoganREF, and 36%, 38%, 83%, 91% and 91% using SRTM (Table 2).
Table 2.
Striatal BPND estimates of vesamicol pretreatment in animal A
| BP-Logan (0-120 min, t* = 90 min) | OccM, %* | BP-SRTM (0-120min) | OccM, %* | ||
|---|---|---|---|---|---|
| Baseline (n = 3 scans) | 2.35 ± 0.30 | N/A | 2.47 ± 0.24 | N/A | |
| (−)-Vesamicol, mg/kg | 0.025 | 1.37 | 41.70 | 1.58 | 36.03 |
| 0.05 | 1.28 | 45.53 | 1.52 | 38.46 | |
| 0.125 | 0.55 | 76.60 | 0.41 | 83.40 | |
| 0.25 | 0.30 | 87.23 | 0.23 | 90.69 | |
| 0.35 | 0.25 | 89.36 | 0.23 | 90.69 | |
OccM = (BPND-Baseline - BPND-pretreatment) / BPND-Baseline * 100%.
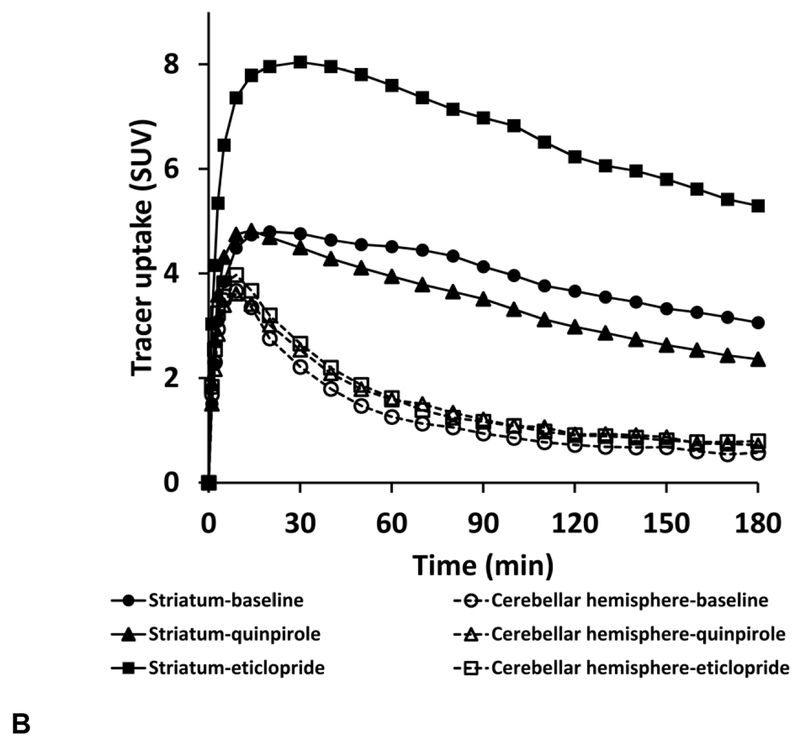
In addition, the averaged TAC curves of cerebellar hemispheres at baseline and with (−)-vesamicol blocking were plotted, and showed no significant difference (Fig.3A). This demonstrates the binding of [18F]VAT to cerebellar hemispheres is nonspecific, which further supports the use of cerebellar hemispheres as the reference region for [18F]VAT imaging analysis. In line with our finding, cerebellar hemispheres have been used as the reference region in PET studies for other VAChT radiotracers (Jin et al., 2016; Mazere et al., 2015; Petrou et al., 2014).
Figure 3.
Averaged tissue-activity curves of [18F]VAT in the cerebellar hemispheres showing negligible difference between baseline and pretreatment with (−)-vesamicol (A) or D2-like drugs (B).
3.2. Studies of the impact of D2-like drugs on [18F]VAT uptake
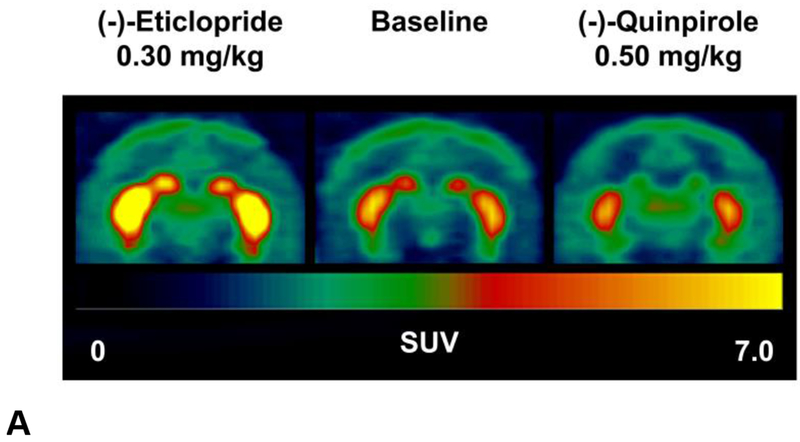
To investigate the acute effect of D2-like drugs on the uptake of [18F]VAT in the brain, a D2-like antagonist (−)-eticlopride or a D2-like agonist (−)-quinpirole was administrated 5 min or 30 min prior to [18F]VAT injection, respectively. (−)-Eticlopride pretreatment 0.30 mg/kg dramatically increased striatal uptake of [18F]VAT as revealed in the summed PET images and TACs (Fig.2). Conversely, (−)-quinpirole at the dose of 0.50 mg/kg modestly reduced [18F]VAT uptake in the striatum (Fig.2). Pretreatment with either (−)-eticlopride or quinpirole did not significantly alter cerebellar hemisphere uptake, indicating that cerebellar hemispheres can serve as the reference region possessing very low VAChT specific binding sites.
Figure 2.
Representative PET images and tissue-activity curves of [18F]VAT in the NHP brain at baseline and following (−)-eticlopride or (−)-quinpirole pretreatment (summed images from 0-180 min). SUV, standardized uptake value.
The entire 180 min PET data from 3 NHPs total were applied for quantitative analysis. Striatal BPND values for baseline, (−)-eticlopride (0.30 mg/kg) and (−)-quinpirole (0.50 mg/kg) were 3.22 ± 0.25, 6.85 ± 1.99, and 2.09 ± 0.56, estimated by LoganREF using 0-180 min data with t* = 90 min; and 3.09 ± 0.25, 6.42 ± 1.76, and 2.10 ± 0.52 by SRTM (Table 3). To verify if 120 min scan time would be sufficient to detect the change induced by the above pharmacological challenge, BPND calculation was performed with truncation to 120 min of data. The results showed that, when the scan duration for analysis was reduced to 120min, BPND values were largely comparable to 180 min data, although a slight decrease was observed: 2.81 ± 0.13, 6.26 ± 2.04, and 1.93 ± 0.65 by LoganREF, and 2.87 ± 0.22, 5.80 ± 1.69, and 2.04 ± 0.50 by SRTM (Table 3).
Table 3.
Averaged striatal BPND estimates of high dose (−)-eticlopride and (−)-quinpirole pretreatment (n = 3 NHPs)
| BP-Logan (0-180 min, t* = 90 min) | BP-Logan (0-120 min, t* = 90 min) | BP-SRTM (0-180min) | BP-SRTM (0-120min) | |
|---|---|---|---|---|
| Baseline | 3.22 ± 0.25 | 2.81 ± 0.13 | 3.09 ± 0.25 | 2.87 ± 0.22 |
| (−)-Eticlopride, 0.30 mg/kg | 6.85 ± 1.99 | 6.26 ± 2.04 | 6.42 ± 1.76 | 5.80 ± 1.69 |
| (−)-Quinpirole, 0.50 mg/kg | 2.09 ± 0.56 | 1.93 ± 0.65 | 2.10 ± 0.52 | 2.04 ± 0.50 |
In addition, to explore the potential dose-dependent response of the PET measures of VAChT, escalating doses of (−)-eticlopride ranging from 0.01 to 0.30 mg/kg and (−)-quinpirole ranging from 0.20 to 0.50 mg/kg were administrated to two individual NHPs respectively. As shown in Table 4, increasing pretreatment doses of (−)-eticlopride (0, 0.01, 0.10 and 0.30 mg/kg) yielded striatal BPND values of 2.73, 3.40, 4.12, and 4.15 by LoganREF using data from 0-120 min with t* = 90 min; and 2.85, 3.49, 4.55, and 4.45 by SRTM (0-120 min). The AUC of striatal uptake revealed the same trend, which were 95.73, 118.6, 163.4, and 164.8 sequentially (Table 4). Cerebellar hemispheres displayed comparatively stable AUC values, except a slight increase at high doses of (−)-eticlopride (0.10 and 0.30 mg/kg, Table 4).
Table 4.
BPND and AUC estimates of (−)-eticlopride pretreatment in Animal A
| Striatal BP | AUC (90-120 min) | ||||
|---|---|---|---|---|---|
| Logan (0-120 min, t* = 90 min) | SRTM (0-120min) | Striatum | Cerebellar hemispheres | ||
| Baseline | 2.73 | 2.85 | 95.73 | 19.94 | |
| (−)-Eticlopride, mg/kg | 0.01 | 3.40 | 3.49 | 118.6 | 21.77 |
| 0.10 | 4.12 | 4.55 | 163.4 | 25.90 | |
| 0.30 | 4.15 | 4.45 | 164.8 | 25.27 | |
Increasing doses of (−)-quinpirole (0, 0.20, 0.30, and 0.50 mg/kg) yielded striatal BPND values of 2.96, 2.71, 2.59 and 2.67 by LoganREF using data from 0-120 min with t* = 90 min; and 3.09, 2.75, 2.87 and 2.59 by SRTM (0-120 min) (Table 5). The AUC of striatal uptake revealed the same trend, which were 94.57, 87.57, 84.43, and 87.31 sequentially (Table 5). Cerebellar hemisphere uptake remained unchanged; the AUC values were 19.59, 19.55, 18.68, and 20.72 (Table 5).
Table 5.
Striatal BPND and AUC estimates of (−)-quinpirole pretreatment in Animal B
| Striatal BP | AUC (90-120 min) | ||||
|---|---|---|---|---|---|
| Logan (0-120 min, t* = 90 min) | SRTM (0-120min) | Striatum | Cerebellar hemisphere | ||
| Baseline | 2.96 | 3.09 | 94.57 | 19.59 | |
| (−)-Quinpirole, mg/kg | 0.20 | 2.71 | 2.75 | 87.57 | 19.55 |
| 0.30 | 2.59 | 2.87 | 84.43 | 18.68 | |
| 0.50 | 2.67 | 2.59 | 87.31 | 20.72 | |
In addition, negligible difference was observed between the averaged TAC curves of cerebellar hemispheres at baseline and with D2-like drug pretreatment (Fig.3B), which suggested that cerebellar hemispheres are also suitable as the reference region for [18F]VAT imaging analysis in the D2-like drug microPET study.
4. Discussion
In the current study, in vivo block studies using escalating doses of (−)-vesamicol clearly showed that striatal [18F]VAT uptake declined in a dose-dependent manner by (−)-vesamicol pretreatment, suggesting the tracer uptake of [18F]VAT positively correlates with the availability of VAChT binding sites. This provides the basis for the following investigation into the impact of dopaminergic D2-like antagonist/agonist on PET measures of VAChT. Our results revealed that D2-like antagonism by (−)-eticlopride increased striatal uptake of [18F]VAT in NHPs while D2-like agonism by (−)-quinpirole decreased the striatal uptake of [18F]VAT, and demonstrated that striatal VAChT levels can be regulated by D2-like drug administration and that [18F]VAT PET can detect the change of VAChT level in vivo.
The dopaminergic and cholinergic systems dynamically and reciprocally influence each other, particularly in the striatum and substantia nigra. In general, activation of nicotinic receptors (nAChRs) by ACh facilitates dopamine release in the striatum (Gallezot et al., 2014; Naylor et al., 2017). In contrast, the predominant effect of dopamine release on ACh is inhibition, which is mediated by D2-like receptors (Jin et al., 2016). With a specific focus on the impact of D2-like drugs on cholinergic system in the striatum, in vivo microdialysis measurement was widely used to evaluate ACh release in response to dopaminergic drugs, especially when specific PET/SPECT radiotracers were not available decades ago. In 1990 Bertorelli reported dopaminergic regulation of the extracellular ACh content in rat striatum measured by in vivo microdialysis (Bertorelli and Consolo, 1990). The D2-like agonist (−)-quinpirole (LY171555, 0.20 mg/kg) was i.p. injected into rats, and reduced ACh output by −30% within 20 min; whereas injection of D2-like antagonists remoxipride (10mg/kg, s.c.) or L-sulpiride (50 mg/kg, i.p.) induced ~50% maximal increases within 10 - 20 min. Another series of studies conducted by DeBoer in 1996 investigated the dose-related effects of (−)-quinpirole on the extracellular ACh concentration in rat striatum by microdialysis (DeBoer et al., 1996). (−)-Quinpirole administration produced a significant decrease in the extracellular ACh concentration at a large range of doses (30 μg/kg, 0.30 mg/kg, and 3.0 mg/kg). These data provided solid in vivo evidence for ACh level regulation by dopamine D2-like receptors. However, the mechanism of dopaminergic regulation of ACh release remains obscure. In vivo whole-cell patch-clamp recording studies suggested activation of D2-like dopamine receptors in cholinergic interneurons reduces N-type Ca2+ currents via a membrane-delimited, Gi/o class G protein pathway (Yan et al., 1997), which may underlie the ability of D2-like receptors to reduce striatal acetylcholine release.
VAChT mediates ACh storage by synaptic vesicles and is the rate limiting factor for ACh transmission in vivo (Prado et al., 2013). More importantly, recent studies using VAChT knockout mice illustrated that synaptosomes obtained from brains of homozygous knockouts were incapable of releasing ACh (de Castro et al., 2009). Brain microdialysis also revealed decreased extracellular ACh level in frontal cortex and striatum of VAChT homozygous knockout mice (Prado et al., 2006). These data firmly suggest that VAChT is essential to ACh release. Accordingly, VAChT levels can be modulated by D2-like antagonism or agonism to facilitate or hinder ACh release, respectively, though its mechanism of action is not fully understood yet. In agreement with our findings, a microPET study using [18F]NEFA, a VAChT radioligand, found elevated tracer uptake in NHP striatum after pretreatment with nonspecific dopaminergic 2 antagonists haloperidol orraclopride (Ingvar et al., 1993). Notably, the impact of (−)-quinpirole on [18F]VAT striatal uptake is smaller compared to (−)-eticlopride. Bertorelli’s study mentioned above also revealed the difference in D2 agonists and antagonists on ACh release (Bertorelli and Consolo, 1990). The different effects of D2 compounds may be partly attributed to the relatively low dose of (−)-quinpirole used in this study. Also, there might be a discrepancy between the availability of VAChT binding sites and the ACh concentration, which still needs further investigation.
It is intriguing that pharmacological challenges might cause changes in cerebral blood flow/volume and subsequently impact tracer delivery and washout in the brain. Chen recently utilized so-called “pharmacological MRI (phMRI)” with iron oxide contrast agents to map the relative cerebral blood volume (rCBV) in response to (−)-eticlopride or (−)-quinpirole pretreatment (Chen et al., 2005). Both (−)-eticlopride and (−)-quinpirole produced detectable rCBV changes in striatum, nucleus accumbens and frontal cortex, but the changes were very small (5-10% at high doses, 2.0 mg/kg for both drugs) and lasted less than 60 min post injection. In addition, a more recent study using simultaneous acquisition of dynamic PET data and pseudo-continuous arterial spin labeling (pcASL) with MRI demonstrated that cerebral blood flow induced by hypercapnia did not change the shape of TACs or the quantification of binding potentials (Sander et al., 2017). Therefore, based on these previous reports, the changes in brain uptake of [18F]VAT cannot be attributed to the effect of the hemodynamic response following D2-like drug administration. In addition, we also determined the in vitro binding of (−)-eticlopride and (−)-quinpirole to VAChT by [3H]VAT raioligand competitive binding assay. Our unpublished data revealed no binding of (−)-eticlopride or (−)-quinpirole to VAChT, which excluded the direct interaction between these two drugs and VAChT.
Interestingly, there is a discrepancy between clinical observations and preclinical findings: significant degeneration of cholinergic cells has been observed in PD patient brains whereas striatal cholinergic interneuron activity is elevated in MPTP-induced Parkinsonian model in NHPs (Bohnen and Albin, 2011; Raz et al., 1996). In PD patients, cholinergic denervation may occur as early as nigral dopaminergic pathology, rather than a delayed pathologic event (Bohnen and Albin, 2011; Braak et al., 2003). A recent study further revealed the interaction of α-synuclein and GluN2D-expressing N-methyl-D-aspartate (NMDA) receptors directly contribute to striatal cholinergic dysfunction (Tozzi et al., 2016). The chronic course of disease (years), as well as the aging, might also be confounding factors. In the NHP Parkinsonian model, dopaminergic neurons and terminals are depleted by MPTP administration acutely (weeks) (Perlmutter and Norris, 2014). Cholinergic activity would not be altered by the direct toxic effect of MPTP. D2-like receptors mediate the predominant effect of dopamine on cholinergic system, thus MPTP alters cholinergic function similarly to the action of D2-like antagonists. We found that [18F]VAT striatal uptake is elevated by D2-like antagonism is in line with previous reports in PD animals.
In conclusion, [18F]VAT offers a reliable tool to in vivo assess the availability of VAChT binding sites. More importantly, PET with [18F]VAT successfully quantified the impact of dopaminergic D2-like drugs on [18F]VAT striatal uptake, suggesting [18F]VAT has great potential for investigating the interaction between dopaminergic and cholinergic systems in vivo. Future investigations are warranted to further characterize the radioligand [18F]VAT with arterial input function in human. Given that the current D2-like drugs also target D3 receptors (Le Foil et al., 2014), future studies with more D3 specific ligands might also assist to explore the unique contribution of D3 receptor agonism and antagonism on [18F]VAT binding in vivo (Nakajima et al., 2013).
Acknowledgements
This work was supported by United States NIH Grants NS061025, NS075527, MH092797, and NS103988. The authors thank John Hood, Emily Williams, and Darryl Craig for their assistance with the nonhuman primate microPET studies. The authors also thank Washington University Cyclotron Facility for [18F]fluoride production.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
All authors declare no conflicts of interest.
References
- Aghourian M, Legault-Denis C, Soucy JP, Rosa-Neto P, Gauthier S, Kostikov A, Gravel P, Bedard MA, 2017. Quantification of brain cholinergic denervation in Alzheimer’s disease using PET imaging with [18F]-FEOBV. Mol. Psychiatry. 22, 1531–1538. 10.1038/mp.2017.183. [DOI] [PubMed] [Google Scholar]
- Antonini A, Moro E, Godeiro C, Reichmann H, 2018. Medical and surgical management of advanced Parkinson’s disease. Mov. Disord 33, 900–908. 10.1002/mds.27340. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR, 2011. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol. Rev 63, 182–217. 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- Bertorelli R, Consolo S, 1990. D1 and D2 dopaminergic regulation of acetylcholine release from striata of freely moving rats. J. Neurochem 54, 2145–2148. 10.1111/j.1471-4159.1990.tb04922.x. [DOI] [PubMed] [Google Scholar]
- Black KJ, Gado MH, Perlmutter JS, 1997. PET measurement of dopamine D2 receptor-mediated changes in striatopallidal function. J Neurosci 17, 3168–3177. 10.1523/JNEUROSCI.17-09-03168.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnen NI, Albin RL, 2011. The cholinergic system and Parkinson disease. Behav. Brain. Res 221, 564–573. 10.1016/j.bbr.2009.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnen NI, Kanel P, Zhou Z, Koeppe RA, Frey KA, Dauer WT, Albin RL, Muller M, 2019. Cholinergic system changes of falls and freezing of gait in Parkinson disease. Ann. Neurol [Epub ahead of print]. 10.1002/ana.25430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E, 2003. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging. 24, 197–211. 10.1016/S0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Chen YC, Choi JK, Andersen SL, Rosen BR, Jenkins BG, 2005. Mapping dopamine D2/D3 receptor function using pharmacological magnetic resonance imaging. Psychopharmacology (Berl) 180, 705–715. 10.1007/s00213-004-2034-0. [DOI] [PubMed] [Google Scholar]
- de Castro BM, De Jaeger X, Martins-Silva C, Lima RD, Amaral E, Menezes C, Lima P, Neves CM, Pires RG, Gould TW, Welch I, Kushmerick C, Guatimosim C, Izquierdo I, Cammarota M, Rylett RJ, Gomez MV, Caron MG, Oppenheim RW, Prado MA, Prado VF, 2009. The vesicular acetylcholine transporter is required for neuromuscular development and function. Mol. Cell. Biol 29, 5238–5250. 10.1128/MCB.00245-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBoer P, Heeringa MJ, Abercrombie ED, 1996. Spontaneous release of acetylcholine in striatum is preferentially regulated by inhibitory dopamine D2 receptors. Eur. J. Pharmacol 317, 257–262. 10.1016/S0014-2999(96)00761-3. [DOI] [PubMed] [Google Scholar]
- Duvoisin RC, 1967. Cholinergic-anticholinergic antagonism in parkinsonism. Arch. Neurol 17, 124–136. 10.1001/archneur.1967.00470260014002. [DOI] [PubMed] [Google Scholar]
- Gallezot JD, Kloczynski T, Weinzimmer D, Labaree D, Zheng MQ, Lim K, Rabiner EA, Ridler K, Pittman B, Huang Y, Carson RE, Morris ED, Cosgrove KP, 2014. Imaging nicotine- and amphetamine-induced dopamine release in rhesus monkeys with [(11)C]PHNO vs [(11)C]raclopridePET.Neuropsychopharmacology 39, 866–874. 10.1038/npp.2013.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall H, Kohler C, Gawell L, 1985. Some in vitro receptor binding properties of [3H]eticlopride, a novel substituted benzamide, selective for dopamine-D2 receptors in the rat brain. Eur. J. Pharmacol 111, 191–199. 10.1016/0014-2999(85)90756-3. [DOI] [PubMed] [Google Scholar]
- Hampel H, Mesulam MM, Cuello AC, Farlow MR, Giacobini E, Grossberg GT, Khachaturian AS, Vergallo A, Cavedo E, Snyder PJ, Khachaturian ZS, 2018. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain 141, 1917–1933. 10.1093/brain/awy132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvitz JC, Williams G, Joy R, 2001. Time-dependent actions of D2 family agonist quinpirole on spontaneous behavior in the rat: dissociation between sniffing and locomotion. Psychopharmacology (Berl) 154, 350–355. 10.1007/s002130000677. [DOI] [PubMed] [Google Scholar]
- Ingvar M, Stone-Elander S, Rogers GA, Johansson B, Eriksson L, Parsons SM, Widen L, 1993. Striatal D2/acetylcholine interactions: PET studies of the vesamicol receptor. Neuroreport 4, 1311–1314. [PubMed] [Google Scholar]
- Jin H, Yue X, Liu H, Han J, Flores H, Su Y, Parsons SM, Perlmutter JS, Tu Z, 2018. Kinetic modeling of [18F]VAT, a novel radioligand for positron emission tomography imaging vesicular acetylcholine transporter in non-human primate brain. J. Neurochem 144,791–804. 10.1111/inc.14291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Yue X, Zhang X, Li J, Yang H, Flores HP, Karimi M, Perlmutter JS, Parsons SM, Tu Z, 2015. A promising F-18 labeled PET radiotracer (−)-[18F]VAT for assessing the VAChT in vivo. J. Nucl. Med 56, 4. [Google Scholar]
- Jin H, Zhang X, Yue X, Liu H, Li J, Yang H, Flores H, Su Y, Parsons SM, Perlmutter JS, Tu Z, 2016. Kinetics modeling and occupancy studies of a novel C-11 PET tracer for VAChT in nonhuman primates. Nucl. Med. Biol 43, 131–139. 10.1016/i.nucmedbio.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnell K, Fischer H, 2011. Dopaminergic and serotonergic drug use: a nationwide register-based study of over 1,300,000 older people. PLoS One 6, e23750 http://dx.doi.orq/10.1371/iournal.pone.0023750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi M, Tu Z, Yue X, Zhang X, Jin H, Perlmutter JS, Laforest R, 2015. Radiation dosimetry of [18F]VAT in nonhuman primates. EJNMMI. Res 5, 73 10.1186/s13550-015-0149-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl DE, Minoshima S, Fessler JA, Frey KA, Foster NL, Ficaro EP, Wieland DM, Koeppe RA, 1996. In vivo mapping of cholinergic terminals in normal aging, Alzheimer’s disease, and Parkinson’s disease. Ann. Neurol 40, 399–410. 10.1002/ana.410400309. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Wilson AA, Graff A, Boileau I, Di Ciano P, 2014. Recent methods for measuring dopamine D3 receptor occupancy in vivo: importance for drug development. Front Pharmacol 5, 161 10.3389/fphar.2014.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmberg A, Mohell N, 1995. Characterization of [3H]quinpirole binding to human dopamine D2A and D3 receptors: effects of ions and guanine nucleotides. J. Pharmacol. Exp. Ther 274, 790–797. [PubMed] [Google Scholar]
- Mazere J, Mayo W, Pariscoat G, Schulz J, Allard M, Fernandez P, Lamare F, 2015. Simplified Quantification Method for In Vivo SPECT Imaging of the Vesicular Acetylcholine Transporter with 123I-Iodobenzovesamicol. J Nucl Med 56, 862–868. 10.2967/jnumed.114.147074. [DOI] [PubMed] [Google Scholar]
- Nakajima S, Gerretsen P, Takeuchi H, Caravaggio F, Chow T, Le Foil B, Mulsant B, Pollock B, Graff-Guerrero A, 2013. The potential role of dopamine D(3) receptor neurotransmission in cognition. Eur Neuropsychopharmacol 23, 799–813. 10.1016/i.euroneuro.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor JE, Hiranita T, Matazel KS, Zhang X, Paule MG, Goodwin AK, 2017. Positron emission tomography (PET) imaging of nicotine-induced dopamine release in squirrel monkeys using [F-18]Fallypride. Drug Alcohol Depen 179, 254–259. 10.1016/i.drugalcdep.2017.07.013. [DOI] [PubMed] [Google Scholar]
- Nejad-Davarani S, Koeppe RA, Albin RL, Frey KA, Muller M, Bohnen NI, 2019. Quantification of brain cholinergic denervation in dementia with Lewy bodies using PET imaging with [18F]-FEOBV. Mol. Psychiatry. 24, 322–327. 10.1038/s41380-018-0130-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter JS, Norris SA, 2014. Neuroimaging biomarkers for Parkinson disease: facts and fantasy. Ann. Neurol 76, 769–783. 10.1002/ana.24291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrou M, Frey KA, Kilbourn MR, Scott PJ, Raffel DM, Bohnen NI, Muller ML, Albin RL, Koeppe RA, 2014. In vivo imaging of human cholinergic nerve terminals with (−)-5-(18)F-fluoroethoxybenzovesamicol: biodistribution, dosimetry, and tracer kinetic analyses. J Nucl Med 55, 396–404. 10.2967/jnumed.113.124792. [DOI] [PubMed] [Google Scholar]
- Prado VF, Martins-Silva C, de Castro BM, Lima RF, Barros DM, Amaral E, Ramsey AJ, Sotnikova TD, Ramirez MR, Kim HG, Rossato JI, Koenen J, Quan H, Cota VR, Moraes MF, Gomez MV, Guatimosim C, Wetsel WC, Kushmerick C, Pereira GS, Gainetdinov RR, Izquierdo I, Caron MG, Prado MA, 2006. Mice deficient for the vesicular acetylcholine transporter are myasthenic and have deficits in object and social recognition. Neuron 51, 601–612. http://dx.doi.orq/10.1016/i.neuron.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Prado VF, Roy A, Kolisnyk B, Gros R, Prado MA, 2013. Regulation of cholinergic activity by the vesicular acetylcholine transporter. Biochem. J 450, 265–274. 10.1042/BJ20121662. [DOI] [PubMed] [Google Scholar]
- Raz A, Feingold A, Zelanskaya V, Vaadia E, Bergman H, 1996. Neuronal synchronization of tonically active neurons in the striatum of normal and parkinsonian primates. J. Neurophysiol 76, 2083–2088. http://dx.doi.orq/10.1152/in.1996.76.3.2083. [DOI] [PubMed] [Google Scholar]
- Sander CY, Mandeville JB, Wey HY, Catana C, Hooker JM, Rosen BR, 2017. Effects of flow changes on radiotracer binding: Simultaneous measurement of neuroreceptor binding and cerebral blood flow modulation. J. Cereb. Blood. Flow. Metab 39, 131–146. 10.1177/0271678X17725418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schliebs R, Arendt T, 2006. The significance of the cholinergic system in the brain during aging and in Alzheimer’s disease. J. Neural. Transm. (Vienna) 113, 1625–1644. http://dx.doi.orq/10.1007/s00702-006-0579-2. [DOI] [PubMed] [Google Scholar]
- Terry AV Jr., Gearhart DA, Warner SE, Zhang G, Bartlett MG, Middlemore ML, Beck WD Jr., Mahadik SP, Waller JL, 2007. Oral haloperidol or risperidone treatment in rats: temporal effects on nerve growth factor receptors, cholinergic neurons, and memory performance. Neuroscience 146, 1316–1332. http://dx.doi.orq/10.1016/i.neuroscience.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozzi A, de lure A, Bagetta V, Tantucci M, Durante V, Quiroga-Varela A, Costa C, Di Filippo M, Ghiglieri V, Latagliata EC, Wegrzynowicz M, Decressac M, Giampa C, Dalley JW, Xia J, Gardoni F, Mellone M, El-Agnaf OM, Ardah MT, Puglisi-Allegra S, Bjorklund A, Spillantini MG, Picconi B, Calabresi P, 2016. Alpha-Synuclein Produces Early Behavioral Alterations via Striatal Cholinergic Synaptic Dysfunction by Interacting With GluN2D N-Methyl-D-Aspartate Receptor Subunit. Biol. Psychiatry. 79, 402–414. 10.1016/i.biopsych.2015.08.013. [DOI] [PubMed] [Google Scholar]
- Tu Z, Zhang X, Jin H, Yue X, Padakanti PK, Yu L, Liu H, Flores HP, Kaneshige K, Parsons SM, Perlmutter JS, 2015. Synthesis and biological characterization of a promising F-18 PET tracer for vesicular acetylcholine transporter. Bioorg. Med. Chem 23, 4699–4709. 10.1016/i.bmc.2015.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hartesveldt C, 1997. Temporal and environmental effects on quinpirole-induced biphasic locomotion in rats. Pharmacol. Biochem. Behav 58, 955–960. 10.1016/S0091-3057(97)00332-8. [DOI] [PubMed] [Google Scholar]
- Weihe E, Tao-Cheng JH, Schafer MK, Erickson JD, Eiden LE, 1996. Visualization of the vesicular acetylcholine transporter in cholinergic nerve terminals and its targeting to a specific population of small synaptic vesicles. Proc. Natl. Acad. Sci. U. S. A 93, 3547–3552. 10.1073/pnas.93.8.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Song WJ, Surmeier J, 1997. D2 dopamine receptors reduce N-type Ca2+ currents in rat neostriatal cholinergic interneurons through a membrane-delimited, protein-kinase-C-insensitive pathway. J. Neurophysiol 77, 1003–1015. 10.1152/in.1997.77.2.1003. [DOI] [PubMed] [Google Scholar]
- Yue X, Jin H, Luo Z, Liu H, Zhang X, McSpadden ED, Tian L, Flores HP, Perlmutter JS, Parsons SM, Tu Z, 2017. Chiral resolution of serial potent and selective sigma1 ligands and biological evaluation of (−)-[18F]TZ3108 in rodent and the nonhuman primate brain. Bioorg. Med. Chem 25, 1533–1542. 10.1016/i.bmc.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Fox GB, 2012. PET imaging for receptor occupancy: meditations on calculation and simplification. J. Biomed. Res 26, 69–76. 10.1016/S1674-8301(12)60014-1. [DOI] [PMC free article] [PubMed] [Google Scholar]