Abstract
Recent work indicates that the bed nucleus of the stria terminalis (BNST) is critically involved in the regulation of conditioned fear responses to unpredictable threats. Here we examined whether the involvement of the BNST in contextual fear conditioning in male rats depends on the imminence of shock after placement in the conditioning chamber. Specifically, we hypothesized that the BNST supports contextual freezing after conditioning with delayed, but not imminent, footshock (relative to placement in the context). Rats were implanted with cannulae targeting the BNST and underwent a contextual fear conditioning procedure in which a single footshock unconditioned stimulus (US) was delivered either 1 minute or 9 minutes after the rat was placed in the context; the rats received a total of four identical conditioning sessions over two days, all with equivalent exposure to the context. Contexts associated with either imminent or delayed US onsets produced distinct patterns of freezing and shock-induced activity but freezing in each case was context-dependent. Reversible inactivation of the BNST reduced the expression of contextual freezing in the context paired with delayed (9 min), but not imminent (1 min), footshock onset. Implications of these data are discussed in the light of recent conceptualizations of BNST function, as well as for anxiety behaviors.
Keywords: anxiety, bed nucleus of the stria terminalis, context, fear, rat
1. INTRODUCTION
Anxiety disorders, such as generalized anxiety disorder (GAD), social anxiety disorder, and panic disorder, as well as stress and trauma disorders, such as posttraumatic stress disorder (PTSD), are among the most common and debilitating of mental illnesses (Craske et al., 2017; Essau et al., 2018; Kilpatrick et al., 2013; McMillan et al., 2014; Ravindran and Stein, 2010; Stein et al., 2017). Despite their prevalence and severity, a complete understanding of the brain mechanisms of anxiety-related behaviors has been elusive. Current models indicate that anxiety and trauma disorders involve a complex network of highly interconnected brain regions (Adhikari, 2014; Avery et al., 2016; Brooks and Stein, 2015; Calhoon and Tye, 2015; Ch’ng et al., 2018; Dunsmoor and Paz, 2015; Fenster et al., 2018; Fox and Shackman, 2019; Janak and Tye, 2015; Knight and Depue, 2019; Lebow and Chen, 2016; Maren et al., 2013; Miles and Maren, 2019; Robinson et al., 2019; Shackman and Fox, 2016); these include (but are not limited to) the medial prefrontal cortex, amygdala, hippocampus, and bed nucleus of the stria terminalis (BNST). In recent years, growing interest has centered on the BNST as a potential target of therapeutic interventions. However, the precise circumstances that engage the BNST in the learning and memory processes involved in anxiety are poorly understood.
To address these lingering questions, we have used Pavlovian fear conditioning procedures to probe the contributions of the BNST to aversive learning and memory. In this form of learning, a neutral conditioned stimulus (CS), such as an auditory tone, is paired with a salient and aversive stimulus, such as footshock [unconditioned stimulus (US)] (Maren, 2001a; Pavlov, 1927; Rescorla, 1968, 1988). The US itself elicits a number of unconditioned behaviors (URs), including bursts in activity and ultrasonic vocalizations (Fanselow, 1994). With as little as a single pairing with the US, the CS alone will elicit conditioned defensive responses (CRs; including defensive immobility or “freezing”, which often serves as the index of conditioning). During fear conditioning, animals not only learn that the CS predicts the US, but also learn to fear the context in which conditioning occurs. Interestingly, numerous studies implicate the BNST in the acquisition and expression of conditioned fear to contexts, but not discrete CSs (Goode et al., 2015; LeDoux et al., 1988; Luyten et al., 2011; Poulos et al., 2010; Resstel et al., 2008; Sullivan et al., 2004; Waddell et al., 2006; Walker and Davis, 1997; Zimmerman and Maren, 2011).
A number of interpretations have been developed to explain the selective role for the BNST in contextual fear (Davis et al., 2010, 1997b, 1997a; Fox et al., 2015; Fox and Shackman, 2019; Gafford and Ressler, 2015; Goode and Maren, 2017; Gungor and Paré, 2016; Klumpers and Kroes, 2019; Luyck et al., 2019; Miles and Maren, 2019; Robinson et al., 2019; Shackman and Fox, 2016; Walker et al., 2009, 2003, Walker and Davis, 2008, 2002; Waraczynski, 2016). A dominant view has been that the BNST mediates the sustained (anxiety-like) fear responses to long-duration threats, including contexts (Hammack et al., 2015; Lee and Davis, 1997; Waddell et al., 2006; Walker and Davis, 1997). However, more recent data suggest that the BNST mediates conditioned fear to threat CSs (whether short or long in duration) that are poor predictors of when aversive outcomes may occur (Daldrup et al., 2016; Goode et al., 2019; Goode and Maren, 2017; Lange et al., 2017). Consistent with this, we have recently reported that pharmacological inactivation of the BNST attenuates fear elicited by discrete auditory CSs that poorly signaled shock onset (Goode et al., 2019).
If temporal predictability, rather than stimulus modality or duration, is the critical factor determining BNST involvement in conditioned fear, then there should be factors in which contextual fear conditioning is independent of the BNST. Indeed, it has recently been reported that BNST lesions do not affect context fear conditioning when the footshock US occurs relatively soon (1 min) after an animal is placed in the conditioning context relative to those shocked after a long delay (10 min) (Hammack et al., 2015). However, in this study, total exposure to the conditioning context was not equated, which therefore confounded the timing of shock onset (early or late) with the duration of the context CS (short or long).
Here we sought to disentangle these factors by using a fear conditioning procedure that equated total context and shock exposure, while varying the placement-to-shock interval (1 min or 9 min). Animals experienced four 10-min contextual conditioning sessions in which they received a single shock per session that either occurred 1 min after the animal was placed in the conditioning context (imminent, 1-MIN) or 9 min after placement in the context (delayed, 9-MIN). We hypothesized that the reversible inactivation of the BNST would impair the expression of contextual freezing in animals conditioned with delayed (9-MIN), but not imminent (1-MIN), USs. We also examined the context-dependence of conditioning by assessing the degree of context discrimination supported by the two conditioning procedures. Furthermore, we characterized behavioral features of each procedure, including shock-induced activity during conditioning, and the freezing latencies and bout durations of the rats during fear retrieval. Overall, we found that pharmacological disruption of the BNST was most effective in disrupting delayed but not imminent shock.
2. MATERIALS AND METHODS
2.1. Subjects.
All subjects were adult (200-240 g) male Long-Evans (Blue Spruce) rats (n = 96, before exclusions) obtained from Envigo (Indianapolis, IN). Rats were individually housed in a climate-controlled vivarium and kept on a fixed light/dark cycle (lights on from 7:00 AM to 9:00 PM). Home cages consisted of a clear plastic cage layered with sawdust bedding (changed weekly), with access for the animals to standard rodent chow and water ad libitum. Home cages were housed on a rotating cage rack. Group assignments for all behavioral testing was randomized for cage position on the racks. Prior to the start of any surgical or behavioral procedures, experimenters handled the rats (~30 sec/day) for five consecutive days. All procedures were performed in accordance with the US National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals and were approved by the Texas A&M University Institutional Animal Care and Use Committee.
2.2. Apparatuses.
Conditioning/testing chambers (30 cm × 24 cm × 21 cm; MED Associates, Inc.) were housed in two distinct rooms of the laboratory (eight chambers per room). Each chamber rested in larger external sound-attenuating cabinets. The chambers were comprised of aluminum (sidewalls) and Plexiglas (rear walls, ceilings, and front doors). The floors of the chambers consisted of a grid of nineteen stainless steel bars (4 mm in diameter), spaced 1.5 cm apart (center to center). For delivery of footshock, the grid floors were connected to an electric shock source and a solid-state grid scrambler (MED Associates, Inc.). The chambers were also equipped with 15 W house lights (as needed for distinct contexts). Fans in each cabinet provided background noise (~70 dB) as needed. Aluminum pans collected animal waste below each chamber. Speakers were attached to the chambers (for delivery of auditory tones), but these were not used in the current study.
To measure freezing over time, our lab utilized an automated and unbiased scoring system (Maren, 1998). Specifically, each chamber rests on a load-cell platform that detects chamber displacement as the animal moves. Load-cell activity values (in a range of −10 to +10 V) were digitized at 5 Hz and recorded using Threshold Activity Software (MED Associates, Inc.). These measurements are converted offline to generate absolute values ranging from 0 to 100; low values correspond to minimal chamber displacement. Thus, freezing bouts were set as absolute values of ≤10 for 1 s or more. Percentages of freezing can then be calculated for periods of time as defined in each experiment. For shock-induced activity measurements, we reported the absolute values generated by the Threshold Activity Software, such that larger values indicate more displacement of the chamber as a result of the animal’s activity (Goode et al., 2019; Maren, 1998).
Experiments utilized distinct contextual features to generate two different contexts. Each context was assigned to a separate behavioral room in the laboratory. Chambers were cleaned with the context’s respective odor before and after each squad of rats. For Context A, the following procedures and features were used: testing chambers were wiped down with 3% acetic acid odor, and a small amount of the solution was poured into the pans beneath the chambers. Chamber lights were turned on, while the cabinet fans remained off. The cupboard doors of the cabinets were closed. A dim red light was used for illuminating the room. Rats were transported to and from the context using small white plastic boxes. For Context B, the following procedures were used: 1% ammonium hydroxide odor was used to scent the chambers. Chamber lights were turned off, while the cabinet fans remained on. Thin black plastic sheets were set on top of the grid floors. The cupboard doors of the cabinet were left open. White lights were used to illuminate the behavioral room. Rats were transported to and from Context B using sawdust-containing black plastic transport boxes.
2.3. Surgeries.
For the data corresponding to Figs. 5–8, animals were first implanted with bilateral guide aimed at the BNST [similar to prior reports: (Acca et al., 2017; Goode et al., 2019, 2015; Nagaya et al., 2015; Zimmerman and Maren, 2011)]. On the day of surgery, animals were individually transported from the vivarium to a surgical suite and prepped for surgery. Animals were deeply anesthetized using gaseous isoflurane (5% for induction; maintained during surgery at 1-2%). Once deeply anesthetized, animals were secured in a stereotactic frame (Kopf Instruments, Inc.), the hair on the top of the head was clipped, artificial tear ointment was applied, and the skin at the site of the incision was treated with povidone-iodine. A small incision was made in the skin and the skull exposed. Small holes were drilled into the skull to attach jeweler’s screws. Bregma and lambda of the skull were aligned on an even plane and small holes were drilled in the skull to allow for insertion of bilateral stainless-steel guide cannulas (26-gauge, 8 mm from the bottom of their plastic pedestals; Small Parts). The guide cannulas were lowered into the brain at the following coordinates: −0.15 mm posterior to bregma, ± 2.65 mm lateral to the midline, and −5.85 mm dorsal to dura (guide cannulas were angled at 10° with their needles directed at the midline). Dental cement was applied to cover the skull and to secure the guide cannulas to the screws. Stainless steel obturators (33-gauge, 9 mm; Small Parts) were inserted into the guide cannulas. Subsequently, rats were removed from the stereotaxic frame, topical antibiotic ointment was applied to the head, and the rats were monitored for recovery. Rats were provided rimadyl-containing bacon-flavored tablets for post-operative pain management. Animals recovered for one week in their homecages prior to the onset of behavioral training.
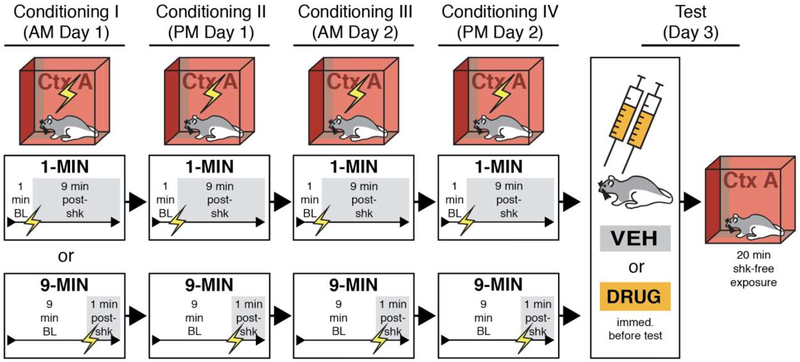
Figure 5. Behavioral design for contextual fear conditioning with imminent or delayed footshock.
BNST-cannulated animals were randomly assigned to undergo four separate sessions of contextual fear conditioning using 1-MIN (1-min placement-to-shock interval) or 9-MIN (9-min placement-to-shock interval) unsignaled shock. Each conditioning session was 10 min. After conditioning, 1-MIN and 9-MIN rats were infused with DRUG (MUS or NBQX) (collapsed into a single group in the figures) or vehicle (VEH) into the BNST prior to a 20-min shock-free retrieval session in the conditioning context.
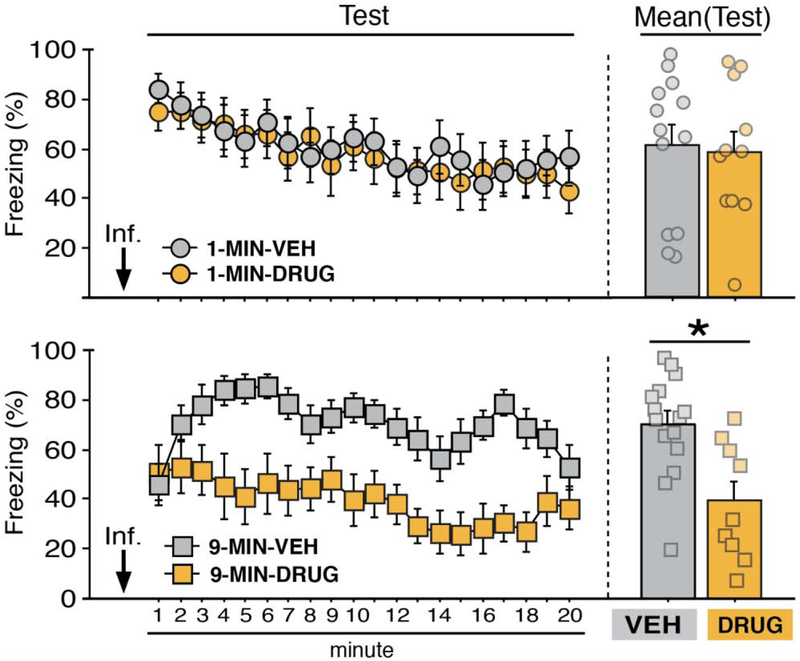
Figure 8. BNST inactivation attenuates freezing in a context conditioned with delayed but not imminent shock onset.
Mean percentage freezing (± s.e.m.) of 1-MIN (top) and 9-MIN (bottom) rats in the conditioning context following intra-BNST microinfusions of VEH or DRUG. 1-MIN-VEH (n = 13); 1-MIN-DRUG (n = 11); 9-MIN-VEH (n = 15); 9-MIN-DRUG (n = 9). *p < 0.05.
2.4. Intracranial infusions.
In the week of recovery following surgery, animals were acclimated to the process of intracranial microinfusions. This involved transporting the animals (in sawdust-containing five-gallon buckets) from the vivarium (in squads of eight) to the separate room used for the infusions in the laboratory. The stainless-steel obturators were gently removed from the guide cannulas and replaced with clean ones. Animals were then returned to their homecages. This process was repeated twice on separate days.
For the data shown in Figures 5–8, the γ-aminobutyric acid (GABA)A receptor agonist, muscimol (MUS), or the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor antagonist 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione, NBQX, was used to temporarily inactivate the BNST. MUS (Sigma-Aldrich) was dissolved in physiological saline to a concentration of 0.1 μg/μl. NBQX (Sigma Life Sciences) was dissolved in physiological saline to a concentration of 10.0 μg/μl. Physiological saline served as vehicle (“VEH” for all cases). An equal number of animals were assigned to received muscimol (“MUS”; n = 16 prior to exclusions) or NBQX (n = 16 prior to exclusions). Both MUS (Bangasser et al., 2005; Breitfeld et al., 2015; Buffalari and See, 2011; Fendt et al., 2003; Goode et al., 2019, 2015; Markham et al., 2009; Pina et al., 2015; Sajdyk et al., 2008; Xu et al., 2012) and NBQX (Adami et al., 2017; Davis and Walker, 2014; Goode et al., 2019, 2015; Zimmerman and Maren, 2011) have been used to reversibly inactivate the BNST. We initially chose to use both drugs to determine whether one was more effective (or more specific) than the other in modulating freezing in either paradigm. Ultimately, MUS- [n = 8, after exclusions; included in figures (see next section for exclusion details)] and NBQX-treated (n = 12, after exclusions; included in figures) animals were collapsed into a single group (“DRUG”) as each manipulation produced similar effects on conditioned freezing (see Results for additional details).
For microinjections, 9-mm stainless steel injectors (33 gauge, Small Parts) were connected to water-filled polyethylene tubing (PE-20; Braintree Scientific), which was in turn connected to 10-μl syringes (Hamilton, Co.). Syringes were secured to an infusion pump (KD Scientific, Inc.). The infusion pump was set to deliver a total volume of 0.275 μl (per injector) of MUS, NBQX, or VEH (infused at a rate of 0.275 μl/min). On infusion day, MUS, NBQX, or VEH was drawn into the injectors and tubing, with a small air bubble separating the drug solution or vehicle from the water in the tubing. Rats (in squads of eight) were brought to the infusion room and the obturators were removed. The drug- and vehicle-filled injectors were inserted into the guide cannulas; microinjections occurred simultaneously for the entire squad of rats (injectors were left in the cannulas for 1 min after the infusions to allow for diffusion). Once the infusions were completed, the injectors were removed and new obturators were inserted. Animals were then immediately transported to the behavioral chambers as necessary for testing.
2.5. Behavioral procedures and exclusions.
Summaries of the behavioral procedures can be found in Figs. 1 and 4. For all experiments, footshock (2 sec, 1.0 mA) served as the unconditioned stimulus (US).
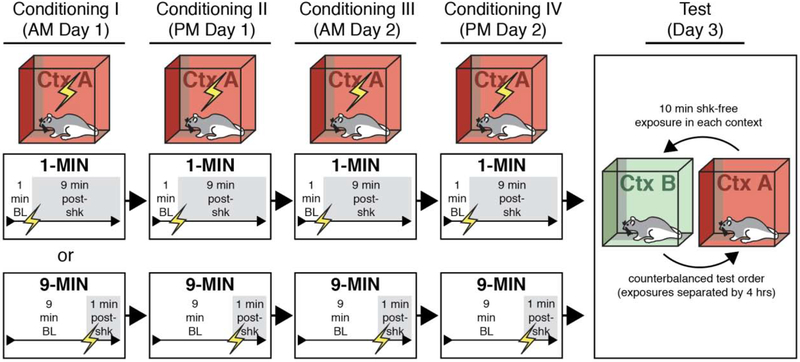
Figure 1. Behavioral design for testing context discrimination following conditioning using imminent or delayed shock onset.
Rats were randomly assigned to undergo four separate sessions of contextual fear conditioning in which shock was delivered either 1 minute (1-MIN) or 9 minutes (9-MIN) after placement in the conditioning chamber. Each conditioning session was 10 min. After conditioning, rats received counterbalanced retrieval tests in the conditioning context (Ctx A) or a novel context (Ctxt B) for 10 min in the absence of shock.
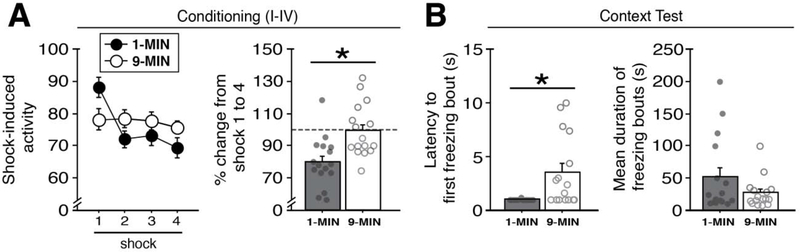
Figure 4. Shock-induced activity and conditioned freezing topography in animals trained with imminent or delayed shock.
(A) Left panel shows mean values of shock-induced activity (± s.e.m.) of 1-MIN (n = 16) and 9-MIN (n = 16) rats (freezing for these rats shown in Figure 2). Right panel shows the percentage change in magnitude of shock-induced activity (± s.e.m.) from shock 1 to shock 4. Note that the dotted line (at 100%) indicates the level at which there is no change in shock-induced activity. (B) Left panel depicts latency (in seconds; ± s.e.m.) of 1-MIN and 9-MIN rats to exhibit their first freezing bout in the conditioning context during testing (corresponding to Figure 3). Right panel shows mean duration of each bout (± s.e.m.) for the entire test. *p < 0.05.
Contextual conditioning and discrimination with imminent (1-MIN) or delayed (9-MIN) footshock.
Thirty-two rats (n = 8 per group) were randomly assigned to a 2 × 2 design with variables of placement-to-shock interval [imminent (1-MIN) or delayed (9-MIN)] and retrieval test order (context A→B or context B→A); the within-subjects tests in the conditioning context (A) and a novel context (B) were counterbalanced. On the morning of the first day of conditioning, animals (in squads of eight rats) were transported from the vivarium to context A. We alternated squads in the 1-MIN and 9-MIN groups. Drug and vehicle assignments were counterbalanced across each squad and for chamber position during conditioning and retrieval testing. For rats in the 1-MIN group, animals were placed in the chamber and allowed to acclimate to the context for 1 min before the onset of the US; animals remained in the chamber for 9 min after shock onset before being removed and returned to their homecages (session I). Later that afternoon (~4 hrs later), this process was repeated (session II). The following day, this process was repeated for a morning session (III) and an afternoon session (IV). Rats in the 9-MIN group were treated identically, except that the shock US was delivered 9 minutes after the rats were placed in the conditioning chamber. Thus, both groups of rats experienced a total of four 10-min conditioning sessions in total (2 per day for 2 days). On the day after the final conditioning session, rats (in squads comprised of equal numbers of 1- and 9-MIN animals) were placed in either the conditioning context (A) or a novel context (B) for a 10 min retrieval test in the absence of shock. The retrieval tests were separated by ~4 hours (morning vs. afternoon sessions). Both of these retrieval tests occurred on the same day (day 3). After each retrieval test, animals were returned to their homecages.
Effect of BNST inactivation on expression of contextual fear conditioned with imminent (1-MIN) or delayed (9-MIN) shock.
In a 2 × 2 design, rats (n = 64, prior to exclusions) were randomly selected to receive contextual conditioning with imminent (1-MIN) or delayed (9-MIN) foot shock; the rats received MUS or NBQX (DRUG) or vehicle (VEH) infusions into the BNST prior to retrieval testing. Of the original sixty-four rats, fifteen were found to have off-target cannulas and were excluded from the final analyses [exclusions were determined with the experimenter(s) blind to the group identity of the animal]. An additional rat was euthanized to due illness and was excluded from the study. This resulted in final group sizes of : 1-MIN-VEH (n = 13); 1-MIN-DRUG (n = 11; comprised of five MUS-treated and six NBQX-treated animals); 9-MIN-VEH (n = 15); 9-MIN-DRUG (n = 9; comprised of seven MUS-treated and two NBQX-treated animals). For contextual fear conditioning, rats in the 1-MIN and 9-MIN groups experienced identical conditioning procedures to those described in the first experiment; drug assignments were counterbalanced for each squad. After completing the final conditioning session, and on the following day, animals were infused with DRUG (MUS or NBQX) or VEH immediately before a 20-min shock-free retrieval test in the conditioning context. In this case, test squads included equal numbers of 1-MIN and 9- MIN rats (counterbalanced for drug assignments). After the test session, animals were returned to their homecages.
2.6. Histological procedures and image analyses.
For animals implanted with cannulas in the BNST, rats were overdosed on sodium pentobarbital (Fatal Plus; 100 mg/ml, 0.5 ml, i.p.) and transcardially perfused using chilled physiological saline followed by 10% formalin. Brains were extracted and placed in 10% formalin for 24 hrs at 4° C. Brains were then transferred to a 30% sucrose-formalin solution before sectioning (stored at 4° C). For sectioning, brains were flash frozen using crushed dry ice and coronal sections (40 μm) containing the BNST were collected using a cryostat set to −20° C (Leica Microsystems). Sections were wet-mounted onto gelatin-subbed glass microscope slides. Subsequently, the tissue was stained with 0.25% thionin using a standard staining procedure. Glass coverslips were glued (Permount, Sigma) to the microscope slides, and the slides were allowed to dry before imaging. Photomicrographs of the thionin-stained tissue were generated at 10× using a Leica Microscope (MZFLIII) and Leica Firecam software. Data shown in Figures 5–8 include only those animals with injector tips localized to within the borders of the BNST.
2.7. Statistics.
All data were submitted to repeated or factorial analysis of variance (ANOVA) or two tailed t-tests as described for each experiment. Only after a significant omnibus F ratio in the ANOVA (α was set at 0.05) were data submitted to post-hoc comparisons in the form of Fisher’s protected least significant difference (PLSD). All data are shown as means (± s.e.m.). No statistical methods were used to predetermine group sizes (group sizes were based on prior work). Data distributions were assumed to be normal, although these were not formally tested.
3. RESULTS
Conditioning using imminent (1-MIN) or delayed (9-MIN) shock onset is context-dependent
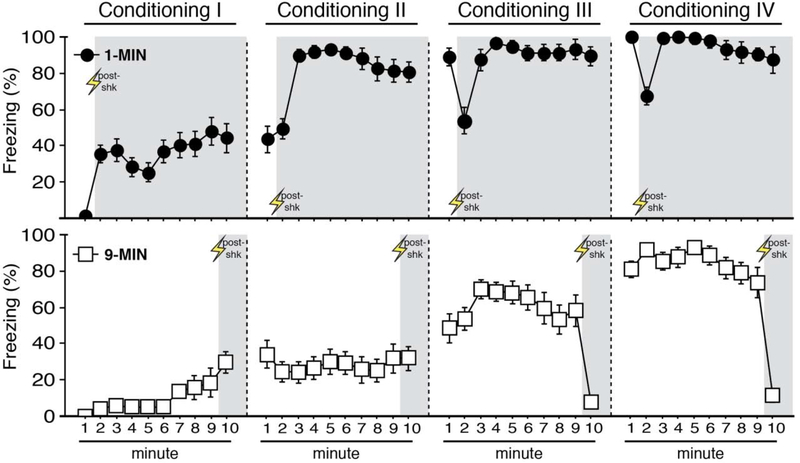
As a first step, we began by characterizing the topography and specificity of conditioned freezing produced by imminent (1 min after placement into the context) or delayed (9 min after placement into the context) shock onset. Rats were placed in the conditioning context and received a footshock with either 1 or 9 min placement-to-shock intervals (1-MIN and 9-MIN, respectively); each rat received a total of four 10-min conditioning sessions over two days. One day after the final conditioning session, animals underwent a counterbalanced test for context discrimination. A schematic of the behavioral design is shown in Figure 1. As shown in Figure 2, freezing behavior increased across each conditioning session in both the 1-MIN and 9-MIN groups. ANOVAs of freezing during conditioning sessions I-IV revealed a main effect of time for each session (session I, repeated measures: F9,252 = 10.05, p < 0.0001; session II, repeated measures: F9,252 = 6.80, p < 0.0001; session III, repeated measures: F9,252 = 14.90, p < 0.0001; session IV, repeated measures: F9,252 = 20.88, p < 0.0001). Additionally, a main effect of conditioning procedure was detected for each session (session I: F1,28 = 32.02, p < 0.0001; session II: F1,28 = 79.42, p < 0.0001; session III: F1,28 = 25.41, p < 0.0001; session IV: F1,28 = 18.41, p < 0.0005). Significant time × conditioning procedure interactions were detected across conditioning (session I, repeated measures: F9,252 = 2.67, p < 0.01; session II, repeated measures: F9,252 = 8.21, p < 0.0001; session III, repeated measures: F9,252 = 12.09, p < 0.0001; session IV, repeated measures: F9,252 = 16.94, p < 0.0001). No main effects of test order or any other interactions were detected for any of the conditioning sessions (F’s < 2.70, p’s > 0.10). Nonetheless, it is worth noting that post-shock freezing greatly differed between the two procedures by the end of training. Specifically, a factorial ANOVA comparing freezing behavior during the 1-min post-shock period of session IV in each group (minute 2 in the 1-MIN group and minute 10 in the 9-MIN groups) revealed a main effect of conditioning procedure (main effect of conditioning procedure: F1,28 = 86.33, p < 0.0001; no other main effects or interactions, F’s < 0,75, p’s > 0.40). Although we have not formally characterized the nature of these differences in the post-shock response, reduced post-shock freezing in the 9-MIN animals may be due anticipatory increases in activity that precede removal from the conditioning context 1-min after footshock.
Figure 2. Context conditioning using imminent or delayed shock.
Mean percentage freezing (± s.e.m.) of 1-MIN (n = 16, top) and 9-MIN (n = 16, bottom) rats for each minute of the four conditioning sessions (I-IV). Shaded areas indicate the post-shock period for during each session.
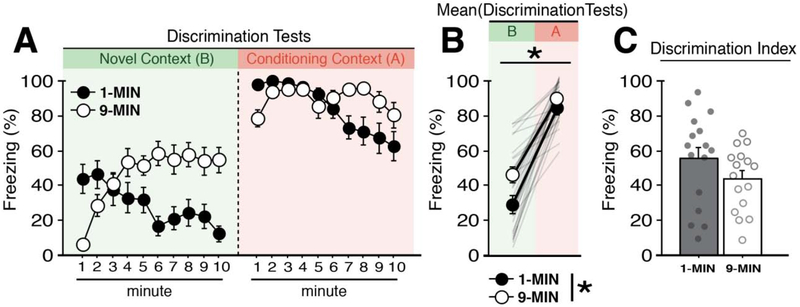
To test for retrieval and context discrimination, animals received counterbalanced retrieval tests (10 minutes) in the conditioning (A) and novel (B) context; the tests were separated by ~4 hrs. As shown in Figure 3A and 3B, animals exhibited robust freezing in the conditioned context and lower fear in the novel context. Specifically, a three-way ANOVA of freezing (with test context as a factor) revealed a main effect of context (F1,56 = 142.43, p < 0.0001), indicating robust context discrimination. Additionally, we observed a main effect of conditioning procedure (F1,56 = 7.38, p < 0.01), a main effect of time (repeated measures: F9,504 = 5.32, p < 0.0001), a time × context interaction (repeated measures: F9,504 = 3.30, p < 0.001), a time × conditioning procedure interaction (repeated measures: F9,504 = 17.85, p < 0.0001), a time × context × conditioning procedure interaction (repeated measures: F9,504 = 3.12, p < 0.005), and a time × context × test order interaction (repeated measures: F9,504 = 4.03, p < 0.0001). However, there was no overall main effect of test order (F < 0.09, p > 0.75). No other main effects or interactions were detected (F’s < 1.80, p’s > 0.06). To further assess performance during the retrieval tests, we calculated a context discrimination index (i.e., mean freezing of animals in the conditioning context subtracted from freezing percentages in the novel context) (Figure 3C). The groups did not significantly differ in the degree of their discrimination (t < 1.5, p > 0.15). In total, these data indicate that although both 1-MIN and 9-MIN exhibited similar degrees of context discrimination between the conditioning and novel contexts, indicating that the two conditioning procedures yielded context-dependent freezing. Interestingly, in both the conditioning and novel contexts, rats in the 1-MIN group showed the greatest freezing in the first half of the retention tests, whereas rats in the 9-MIN group showed their highest level of freezing in the latter half of the retrieval test. This suggests that freezing may be determined, at least in part, by an expectancy of when shock had occurred during conditioning.
Figure 3. Context discrimination after imminent or delayed shock.
(A) Mean percentage freezing (± s.e.m.) of 1-MIN (n = 16) and 9-MIN (n = 16) rats during counterbalanced retrieval tests in a novel context (Context B) or the conditioning context (Context A). (B) Mean percentage freezing (± s.e.m.) of 1-MIN and 9-MIN rats across the entire test in each context. Shaded lines denote individual performance of each animal. (C) Mean freezing percentages (± s.e.m.) in Context B were subtracted from mean responding in Context A to generate a discrimination index for 1-MIN and 9-MIN rats. *p < 0.05.
Conditioning procedure influences both activity-burst URs and freezing CRs
To determine whether the placement-to-shock interval influences the unconditioned response to footshock, we examined the magnitude of shock-evoked activity bursts (as assessed by cage displacement) during the 2-sec period of each shock. Levels of shock-induced activity are shown in Figure 4A (left). Repeated measures ANOVA revealed a significant main effect of shock number (F3,90 = 7.25, p < 0.0005), indicating that shock-induced activity changed across the course of the conditioning sessions. A shock number × conditioning procedure interaction was detected in the ANOVA (F3,90 = 5.38, p < 0.005), indicating differences in the shock-induced activity between the two conditioning procedures across the conditioning sessions (no other main effects were detected: F < 0.5, p > 0.5). These differences are further apparent when comparing the percent change in shock-induced activity from the first to the final shock (Figure 4A; right). An unpaired t-test revealed that 1-MIN rats exhibited a significant reduction in the magnitude of the shock-induced activity across the trials as compared to 9-MIN rats (t30 = −3.48, p < 0.005). These findings parallel our recent work (Goode et al., 2019), which found that conditioning-related decreases in shock-induced activity were lower in backward (unpredictable)-conditioned compared to forward (predictable)-conditioned animals. These outcomes may reflect a greater regulation of US responding in procedures in which animals can better predict the onset of footshock (i.e., in the imminent group). Nonetheless, shock-induced activity may also interact with contextual novelty (and the extent of exploration) which differs between the two conditioning procedures at the time that the first shock is delivered.
In addition to affecting the expression of shock-induced URs, the conditioning procedures produced differences in the nature of conditioned freezing to the conditioning context. To elucidate these differences, we examined the latency to the first freezing bout, as well as the average length of the freezing bouts in the conditioning context at test (Maren, 2001b). Although percentages of freezing are commonly reported as an index of learning, how animals reach certain magnitudes of freezing can differ. For example, an animal could express 50% freezing across a 10-min session by freezing for a sustained 300-sec bout, or by engaging in ten separate 30-sec bouts across the session. Thus, by examining latencies and durations of bouts over time, we may reveal important differences in BNST-dependent or -independent defensive strategies.
Latencies and bout durations of freezing of 1-MIN and 9-MIN rats in the conditioned context are shown in Figure 4B (left). We opted to perform these analyses on the retrieval responses of 1-MIN and 9-MIN rats from Figure 3 because these two groups exhibited similar overall magnitudes of freezing percentages across the 10-min session. Although both groups exhibited relatively short latencies for initiating freezing (all rats exhibited freezing within the first 15 sec of the exposure), 1-MIN rats were freezing almost immediately upon entering the context. Indeed, an unpaired t-test revealed that 1-MIN rats exhibited significantly shorter latencies to their first bout of freezing as compared to 9-MIN rats (t30 = −3.02, p < 0.01). Concerning the duration of the conditioned responses, we observed no significant difference between 1-MIN and 9-MIN rats in the average length of freezing bouts (t < 1.5, p > 0.15). Thus, these data identified distinct (as well as overlapping) features of URs and CRs in 1-MIN and 9-MIN rats, which may be factors the contribution of the BNST to the expression of contextual fear.
Reversible inactivation of the BNST disrupts fear to contexts conditioned with delayed, but not imminent, shock onset
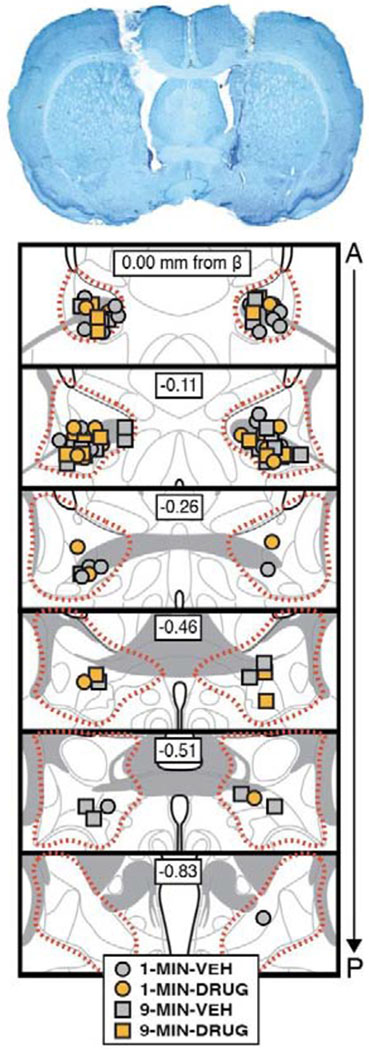
In this experiment we tested whether the placement-to-shock interval influences the role of the BNST in the expression of contextual freezing. Rats with cannula targeting the BNST were placed in the conditioning context and received a footshock either 1 min (1-MIN) or 9 min (9-MIN) after placement in the chamber (identical to the previous experiment); a schematic of the behavioral design is shown in Figure 5. Cannula placements for all rats included in the final analyses, as well as a representative image of cannula tracts in the BNST, are displayed in Figure 6.
Figure 6. Bilateral cannula placements in the BNST.
Representative photomicrograph of a thionin-stained coronal section (40 μm) depicting bilateral cannula tracts and injector tips in the BNST (top image). Bottom image shows the positions of injector tips for each animal for each group included in the final analyses of the experiment (approximate borders of the BNST are outline by the dotted red line). 1-MIN-VEH (n = 13); 1-MIN-DRUG (n = 11); 9-MIN-VEH (n = 15); 9-MIN-DRUG (n = 9).
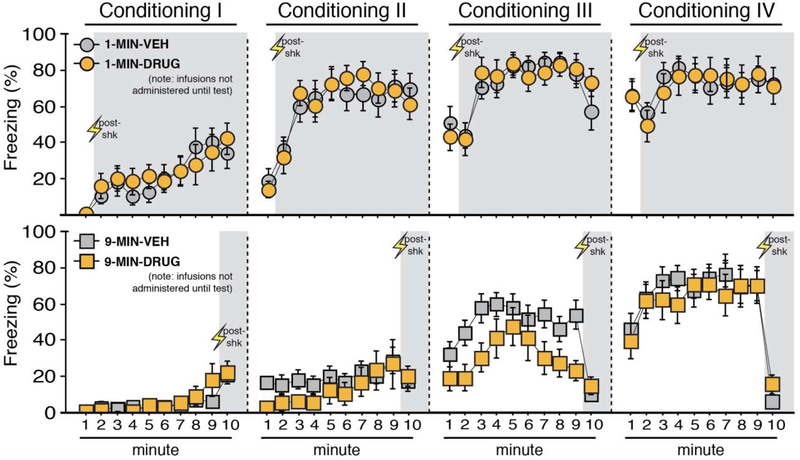
Freezing during each minute of each of the four conditioning sessions is shown in Figure 7. Freezing behavior reliably increased across the conditioning sessions. Separate ANOVAs of freezing during each conditioning session (I-IV) revealed a main effect of time for each session (session I, repeated measures: F9,396 = 17.91, p < 0.0001; session II, repeated measures: F9,396 = 22.58, p < 0.0001; session III, repeated measures: F9,396 = 24.39, p < 0.0001; session IV, repeated measures: F9,396 = 17.17, p < 0.0001). Additionally, a main effect of conditioning procedure was detected for the first three sessions (session I: F1,44 = 14.62, p < 0.0005; session II: F1,44 = 50.11, p < 0.0001; session III: F1,44 = 27.85, p < 0.0001). Moreover, a time × conditioning procedure interaction was detected for each conditioning session (session I, repeated measures: F9,396 = 3.16, p < 0.005; session II, repeated measures: F9,396 = 11.17, p < 0.0001; session III, repeated measures: F9,396 = 6.46, p < 0.0001; session IV, repeated measures: F9,396 = 13.272, p < 0.0001). These data indicate that rats in the 1-MIN shock group generally increased their freezing more rapidly across each session. A time × drug assignment interaction was found for session 3 (repeated measures: F9,396 = 2.20, p < 0.05), but this difference was not apparent by session 4; no other main effects or interactions of drug assignment were found (F’s < 2.15, p’s > 0.15). Post-shock freezing in the final session appeared to mirror patterns seen in the prior experiment. Specifically, 9-MIN animals exhibited significantly less freezing during minute 10 of session IV vs. minute 2 of session IV of 1-MIN animals (factorial ANOVA, main effect of conditioning procedure: F1,44 = 59.387, p < 0.0001; no other main effects or interactions, F’s < 2.25, p’s > 0.14). Collectively, these data reveal reliable increases in freezing during conditioning; results that are similar to the prior experiment.
Figure 7. Context conditioning using imminent or delayed shock onset.
Mean percentage freezing (± s.e.m.) of 1-MIN (top) and 9-MIN (bottom) rats at each minute of each conditioning session (I-IV). Shaded areas indicate minutes post-shock for both procedures at each session. Drug and vehicle assignments are shown for comparison but no BNST infusions occurred during this pre-test phase. 1-MIN-VEH (n = 13); 1-MIN-DRUG (n = 11); 9-MIN-VEH (n = 15); 9-MIN-DRUG (n = 9). *p < 0.05.
After conditioning, MUS (a GABAA receptor agonist) or NBQX (an AMPA receptor antagonist) was used to inactivate the BNST prior to a shock-free retrieval test in the conditioning context (Figure 8). Neither drug on its own was effective in attenuating freezing in 1-MIN animals [NBQX vs. VEH: F’s < 0.45, p’s > 0.90; mean percentage freezing attest (± s.e.m.), NBQX: 75.40 (± 7.70) vs. VEH (for the NBQX cohort): 74.69 (± 8.07); MUS vs. VEH: F’s < 0.70, p’s > 0.85; mean percentage freezing at test (± s.e.m.), MUS: 37.58 (± 9.90) vs. VEH (for the MUS cohort): 39.56 (± 12.25)]. Therefore, the MUS and NBQX conditions were collapsed (DRUG, collapsed in the figures). However, we note that the number of NBQX animals in the 9-MIN drug-treated animals (after exclusions) is limited. A significant main effect of time (repeated measures: F19,836 = 7.02, p < 0.0001) and a significant time × conditioning procedure interaction (repeated measures: F19,836 = 1.90, p < 0.05) were found in the ANOVA, indicating that 1-MIN and 9-MIN rats exhibited changes in freezing across the retrieval test. A main effect of drug treatment was also apparent (F1,44 = 5.215, p < 0.05). A significant time × conditioning procedure × drug assignment interaction was also found in the ANOVA (repeated measures: F19.836 = 1.81, p < 0.05). Fisher’s PLSD for mean freezing during the test indicated that 9-MIN-DRUG rats exhibited significantly less freezing as compared to 9-MIN-VEH rats (p < 0.01). No other main effects or interactions were detected for these analyses (F’s < 3,60, p’s > 0.05). Additionally, we examined whether off-target infusions in the excluded DRUG animals (twelve rats) had any effect on freezing responses relative to VEH-treated animals. We did not see any significant main effects or interactions for off-target drug treatment on freezing performance [repeated measures of test responding, independent of training paradigm (data not shown in figures for these animals): F’s < 2.1, p’s > 0.15). In total, these data indicate that BNST inactivation impaired the expression of contextual freezing in rats in the 9-MIN, but not 1-MIN, group.
4. DISCUSSION
We demonstrate a dissociable role for the BNST in the expression of fear to contexts that signal imminent vs. delayed shock onset. Inactivation of the BNST impaired conditioned freezing in a context associated with a placement-to-shock interval of 9 min but had no effect on freezing in a context associated with imminent shock onset (1 min). Importantly, the conditioning procedures equated total context and shock exposure, suggesting that it is the timing of shock (with respect to placement) in the conditioning context that determines involvement of the BNST in contextual freezing (at least with the number and intensity of footshocks used here). Interestingly, despite differences in latency to freeze, the overall magnitude of freezing was similar in animals conditioned with imminent and delayed footshocks. These data indicate that although freezing was similarly sustained across the context retrieval test [at least based on mean percentage freezing at retrieval (Figs. 3 and 8) and mean bout duration in the shock context (Fig. 4B)], BNST inactivation only reduced freezing conditioned with delayed footshock. Accordingly, this suggests that it is not the duration of the CR, but rather the degree to which the context or CS signals when footshock will occur that determines the necessity of the BNST in conditioned freezing.
Importantly, the current work replicates the findings of a significant prior study that showed that context fear was insensitive to permanent lesions of the BNST when trained with imminent shock onset (Hammack et al., 2015). The current study builds on and expands on these findings in several critical ways. First, the study by Hammack and colleagues (2015) utilized lesions that persisted throughout conditioning and retrieval, making it difficult to isolate whether the role of the BNST in context fear was specific to processes of conditioning, consolidation, or retrieval. Although the BNST may have roles during these other stages, our current data suggest that the BNST is necessary for the expression of conditioned freezing after delayed- but not imminent-shock conditioning. Additionally, Hammack and colleagues (2015) compared context fear expression in two groups of animals that not only differed in the timing of shock, but also in total context exposure. In the current study, all animals had equal exposure to the context, indicating that our effects on retrieval were modulated by the timing of shock onset, rather than the overall duration of the exposure to the context.
Additionally, the current results build on a prior study from our lab (Goode et al., 2019), in which we found that CSs that reliably signaled imminent shock (e.g., forward-trained CSs) were insensitive to BNST inactivation, whereas CSs that were poor predictors of shock onset (e.g., backward or randomized CSs) were sensitive to the manipulation. Similarly, and in the present study, we have found a dissociable role for the BNST in contextual fear expression when the context signaled imminent shock vs. a context that signaled more delayed shock onset. This pattern of results is consistent with predator imminence theory (Fanselow, 2018, 1994; Fanselow et al., 2019; Fanselow and Lester, 1988; Perusini and Fanselow, 2015), which proposes that defensive behaviors (and the brain structures mediating each defensive mode) scale with the spatio-temporal proximity of threat. The short latency between context placement and shock onset in the 1-MIN group engenders high threat imminence and is, as we report here, independent of the BNST. In contrast, the long latency between context placement and shock onset in the 9-MIN group engenders low threat imminence and is BNST-dependent. Interestingly, both conditioning procedures yielded robust freezing behavior, suggesting that different levels of threat imminence (imminent or delayed shock) can yield similar defensive topographies under some conditions (though the topography of freezing in the two groups was somewhat different).
Prior work has suggested that the BNST mediates distinct forms of fear expression, particularly sustained responses, that are dissociable from phasic fear responses, which may be governed by other structures such as the central amygdala [e.g., (Sullivan et al., 2004; Walker and Davis, 1997)]. In contrast, we observed robust freezing in both 1-MIN and 9-MIN animals, including similar overall durations of freezing bouts (at least in animals that did not undergo any surgery; Fig. 4B), but only 9-MIN training was sensitive to BNST inactivation. Thus, we believe a more accurate depiction of BNST function is that it mediates responses to delayed or unpredictable threats, and that these responses may in some cases be sustained (perhaps as the risk of threat persists), but that response duration itself is not always predictive of whether BNST is involved.
We also observed differences in the magnitude of shock-evoked URs of 1-MIN vs. 9-MIN animals across the conditioning sessions. Specifically, imminent shock onset appeared to coincide with reductions in shock-induced activity across the sessions, whereas delayed-shock animals largely expressed consistent levels of activity-burst URs across the sessions. We recently reported a similar outcome in the US-induced activity of animals subjected to auditory forward vs. backward fear conditioning (Goode et al., 2019), insofar as forwards-conditioned animals more rapidly exhibited reductions in their activity across conditioning. These outcomes may reflect a greater regulation of US responding in signaled or imminent threat paradigms, which may be a factor in BNST recruitment to fear. Nonetheless, we acknowledge that UR magnitudes and their changes over conditioning may also be influenced by contextual novelty that leads to exploration prior to the onset of shock (particularly during exploration of a novel context).
Recent human imaging work [e.g., (Clauss et al., 2019; Figel et al., 2019; Naaz et al., 2019)] suggests that the BNST is engaged by unpredictable threat. Assuming that a short latency between context entry (CS onset) and shock onset increases predictability of the aversive US (and decreases reliance on the BNST), our data agree with these findings. Thus, delayed or unpredictable onset of aversive events may be a critical feature that recruits the BNST to anxiety-related behaviors, as well as for fear and drug relapse in the aftermath of unpredictable stressors (Goode et al., 2018; Goode and Maren, 2019; Harris and Winder, 2018; Mantsch et al., 2016; Miles et al., 2018; Stamatakis et al., 2014; Vranjkovic et al., 2017). Nonetheless, the BNST is an intricate, sexually dimorphic and heterogeneous structure, with diverse functions (Avery et al., 2014; Crestani et al., 2013; Daniel and Rainnie, 2016; Flavin and Winder, 2013; Hammack et al., 2012, 2010, 2009; Kash, 2012; Kash et al., 2015; Radley and Johnson, 2018; Waraczynski, 2016)—more work is needed to fully characterize its complex neural responses and contributions to aversive stimuli [(Acca et al., 2017; Duvarci et al., 2009; Haufler et al., 2013; Jennings et al., 2013; Luyck et al., 2018, 2017, Marcinkiewcz et al., 2019, 2016; Martinon et al., 2019; Moaddab and Dabrowska, 2017); also, see (Bjomi et al., 2019)]. Our findings relied on local pharmacological inactivation of this complex circuitry; other drugs or cell-type specific manipulations may reveal unique roles of BNST circuits in aversive learning and memory. To conclude, we build on our prior study (Goode et al., 2019) by demonstrating a dissociable role of the BNST in the behavioral expression of contextual fear, an effect that was modulated by the timing of shock onset.
Supplementary Material
HIGHLIGHTS.
Placement-to-shock intervals of 1 minute or 9 minutes resulted in context-dependent fear conditioning
Timing of shock onset critically modulated the topography of context-dependent fear
Pharmacological inactivation of the BNST disrupted the expression of contextual freezing after conditioning with delayed (9 min), but not imminent (1 min), shock
ACKNOWLEDGEMENTS
The authors thank Carolyn Evemy and Sohmee Kim for technical assistance. Supported by grants from the National Institutes of Health (R01MH065961 and R01MH117852 to S.M. and F31MH107113 to T.D.G.), as well as a McKnight Foundation Memory and Cognitive Disorders Award and a Brain & Behavior Research Foundation NARSAD Distinguished Investigator Grant to S.M.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF COMPETING INTEREST
The authors declare no conflict of interest.
References
- Acca GM, Mathew AS, Jin J, Maren S, Nagaya N, 2017. Allopregnanolone induces state-dependent fear via the bed nucleus of the stria terminalis. Horm. Behav 89, 137–144. doi: 10.1016/j.yhbeh.2017.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adami MB, Barretto-de-Souza L, Duarte JO, Almeida J, Crestani CC, 2017. Both N-methyl-D-aspartate and non-N-methyl-D-aspartate glutamate receptors in the bed nucleus of the stria terminalis modulate the cardiovascular responses to acute restraint stress in rats. J. Psychopharmacol. (Oxford) 31, 674–681. doi: 10.1177/0269881117691468 [DOI] [PubMed] [Google Scholar]
- Adhikari A, 2014. Distributed circuits underlying anxiety. Front. Behav. Neurosci 8, 112. doi: 10.3389/fnbeh.2014.00112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery SN, Clauss JA, Blackford JU, 2016. The human BNST: functional role in anxiety and addiction. Neuropsychopharmacology 41, 126–141. doi: 10.1038/npp.2015.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery SN, Clauss JA, Winder DG, Woodward N, Heckers S, Blackford JU, 2014. BNST neurocircuitry in humans. Neuroimage 91, 311–323. doi: 10.1016/j.neuroimage.2014.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Santollo J, Shors TJ, 2005. The bed nucleus of the stria terminalis is critically involved in enhancing associative learning after stressful experience. Behav. Neurosci 119, 1459–1466. doi: 10.1037/0735-7044.119.6.1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorni M, Rovero NG, Yang ER, Holmes A, Halladay LR, 2019. Phasic signaling in the bed nucleus of the stria terminalis during fear learning predicts within- and across-session cued fear expression. BioRxiv. doi: 10.1101/768416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitfeld T, Bruning JEA, Inagaki H, Takeuchi Y, Kiyokawa Y, Fendt M, 2015. Temporary inactivation of the anterior part of the bed nucleus of the stria terminalis blocks alarm pheromone-induced defensive behavior in rats. Front. Neurosci 9, 321. doi: 10.3389/fnins.2015.00321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks SJ, Stein DJ, 2015. A systematic review of the neural bases of psychotherapy for anxiety and related disorders. Dialogues Clin Neurosci 17, 261–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffalari DM, See RE, 2011. Inactivation of the bed nucleus of the stria terminalis in an animal model of relapse: effects on conditioned cue-induced reinstatement and its enhancement by yohimbine. Psychopharmacology 213, 19–27. doi: 10.1007/s00213-010-2008-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoon GG, Tye KM, 2015. Resolving the neural circuits of anxiety. Nat. Neurosci 18, 1394–1404. doi: 10.1038/nn.4101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ch’ng S, Fu J, Brown RM, McDougall SJ, Lawrence AJ, 2018. The intersection of stress and reward: BNST modulation of aversive and appetitive states. Prog. Neuropsychopharmacol. Biol. Psychiatry 87, 108–125. doi: 10.1016/j.pnpbp.2018.01.005 [DOI] [PubMed] [Google Scholar]
- Clauss JA, Avery SN, Benningfield MM, Blackford JU, 2019. Social anxiety is associated with BNST response to unpredictability. Depress. Anxiety, doi: 10.1002/da.22891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craske MG, Stein MB, Eley TC, Milad MR, Holmes A, Rapee RM, Wittchen H-U, 2017. Anxiety disorders. Nat. Rev. Dis. Primers 3, 17024. doi: 10.1038/nrdp.2017.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestani CC, Alves FH, Gomes FV, Resstel LB, Correa FM, Herman JP, 2013. Mechanisms in the bed nucleus of the stria terminalis involved in control of autonomic and neuroendocrine functions: a review. Curr Neuropharmacol 11, 141–159. doi: 10.2174/1570159X11311020002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daldrup T, Lesting J, Meuth P, Seidenbecher T, Pape H-C, 2016. Neuronal correlates of sustained fear in the anterolateral part of the bed nucleus of stria terminalis. Neurobiol. Learn. Mem 131, 137–146. doi: 10.1016/j.nlm.2016.03.020 [DOI] [PubMed] [Google Scholar]
- Daniel SE, Rainnie DG, 2016. Stress modulation of opposing circuits in the bed nucleus of the stria terminalis. Neuropsychopharmacology 41, 103–125. doi: 10.1038/npp.2015.178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Walker DL, 2014. Role of bed nucleus of the stria terminalis and amygdala AMPA receptors in the development and expression of context conditioning and sensitization of startle by prior shock. Brain Struct. Funct 219, 1969–1982. doi: 10.1007/s00429-013-0616-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Walker DL, Lee Y, 1997b. Roles of the amygdala and bed nucleus of the stria terminalis in fear and anxiety measured with the acoustic startle reflex. Possible relevance to PTSD. Ann. N. Y. Acad. Sci 821, 305–331. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Lee Y, 1997a. Amygdala and bed nucleus of the stria terminalis: differential roles in fear and anxiety measured with the acoustic startle reflex. Philos. Trans. R. Soc. Lond. B, Biol. Sci 352, 1675–1687. doi: 10.1098/rstb.1997.0149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C, 2010. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology 35, 105–135. doi: 10.1038/npp.2009.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmoor JE, Paz R, 2015. Fear generalization and anxiety: behavioral and neural mechanisms. Biol. Psychiatry 78, 336–343. doi: 10.1016/j.biopsych.2015.04.010 [DOI] [PubMed] [Google Scholar]
- Duvarci S, Bauer EP, Pare D, 2009. The bed nucleus of the stria terminalis mediates interindividual variations in anxiety and fear. J. Neurosci 29, 10357–10361. doi: 10.1523/JNEUROSCI.2119-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essau CA, Lewinsohn PM, Lim JX, Ho M-HR, Rohde P, 2018. Incidence, recurrence and comorbidity of anxiety disorders in four major developmental stages. J. Affect. Disord 228, 248–253. doi: 10.1016/j.jad.2017.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, 1994. Neural organization of the defensive behavior system responsible for fear. Psychon. Bull. Rev 1, 429–438. doi: 10.3758/BF03210947 [DOI] [PubMed] [Google Scholar]
- Fanselow MS, 2018. The role of learning in threat imminence and defensive behaviors. Curr. Opin. Behav. Sci 24, 44–49. doi: 10.1016/j.cobeha.2018.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Hoffman AN, Zhuravka I, 2019. Timing and the transition between modes in the defensive behavior system. Behav. Processes 166, 103890. doi: 10.1016/j.beproc.2019.103890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Lester LS, 1988. A functional behavioristic approach to aversively motivated behavior: predatory imminence as a determinant of the topography of defensive behavior, in: Bolles RC, Beecher MD (Eds.), Evolution and Learning. Erlbaum, Hillsdale, NJ, pp. 185–211. [Google Scholar]
- Fendt M, Endres T, Apfelbach R, 2003. Temporary inactivation of the bed nucleus of the stria terminalis but not of the amygdala blocks freezing induced by trimethylthiazoline, a component of fox feces. J. Neurosci 23, 23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenster RJ, Lebois LAM, Ressler KJ, Suh J, 2018. Brain circuit dysfunction in posttraumatic stress disorder: from mouse to man. Nat. Rev. Neurosci 19, 535–551. doi: 10.1038/s41583-018-0039-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figel B, Brinkmann L, Buff C, Heitmann CY, Hofmann D, Bruchmann M, Becker MPI, Herrmann MJ, Straube T, 2019. Phasic amygdala and BNST activation during the anticipation of temporally unpredictable social observation in social anxiety disorder patients. Neuroimage Clin. 22, 101735. doi: 10.1016/j.nicl.2019.101735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavin SA, Winder DG, 2013. Noradrenergic control of the bed nucleus of the stria terminalis in stress and reward. Neuropharmacology 70, 324–330. doi: 10.1016/j.neuropharm.2013.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AS, Oler JA, Tromp DPM, Fudge JL, Kalin NH, 2015. Extending the amygdala in theories of threat processing. Trends Neurosci. 38, 319–329. doi: 10.1016/j.tins.2015.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AS, Shackman AJ, 2019. The central extended amygdala in fear and anxiety: Closing the gap between mechanistic and neuroimaging research. Neurosci. Lett 693, 58–67. doi: 10.1016/j.neulet.2017.11.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gafford GM, Ressler KJ, 2015. GABA and NMDA receptors in CRF neurons have opposing effects in fear acquisition and anxiety in central amygdala vs. bed nucleus of the stria terminalis. Horm. Behav 76, 136–142. doi: 10.1016/j.yhbeh.2015.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode TD, Jin J, Maren S, 2018. Neural circuits for fear relapse, in: Neurobiology of Abnormal Emotion and Motivated Behaviors. Elsevier, pp. 182–202. doi: 10.1016/B978-0-12-813693-5.00010-1 [DOI] [Google Scholar]
- Goode TD, Kim JJ, Maren S, 2015. Reversible inactivation of the bed nucleus of the stria terminalis prevents reinstatement but not renewal of extinguished fear. Eneuro 2. doi: 10.1523/ENEURO.0037-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode TD, Maren S, 2017. Role of the bed nucleus of the stria terminalis in aversive learning and memory. Learn. Mem 24, 480–491. doi: 10.1101/lm.044206.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode TD, Maren S, 2019. Common neurocircuitry mediating drug and fear relapse in preclinical models. Psychopharmacology 236, 415–437. doi: 10.1007/s00213-018-5024-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode TD, Ressler RL, Acca GM, Miles OW, Maren S, 2019. Bed nucleus of the stria terminalis regulates fear to unpredictable threat signals. Elife 8. doi: 10.7554/eLife.46525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gungor NZ, Paré D, 2016. Functional heterogeneity in the bed nucleus of the stria terminalis. J. Neurosci 36, 8038–8049. doi: 10.1523/JNEUROSCI.0856-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Cooper MA, Lezak KR, 2012. Overlapping neurobiology of learned helplessness and conditioned defeat: implications for PTSD and mood disorders. Neuropharmacology 62, 565–575. doi: 10.1016/j.neuropharm.2011.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Guo J-D, Hazra R, Dabrowska J, Myers KM, Rainnie DG, 2009. The response of neurons in the bed nucleus of the stria terminalis to serotonin: implications for anxiety. Prog. Neuropsychopharmacol. Biol. Psychiatry 33, 1309–1320. doi: 10.1016/j.pnpbp.2009.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Roman CW, Lezak KR, Kocho-Shellenberg M, Grimmig B, Falls WA, Braas K, May V, 2010. Roles for pituitary adenylate cyclase-activating peptide (PACAP) expression and signaling in the bed nucleus of the stria terminalis (BNST) in mediating the behavioral consequences of chronic stress. J. Mol. Neurosci 42, 327–340. doi: 10.1007/s12031-010-9364-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammack SE, Todd TP, Kocho-Schellenberg M, Bouton ME, 2015. Role of the bed nucleus of the stria terminalis in the acquisition of contextual fear at long or short context-shock intervals. Behav. Neurosci 129, 673–678. doi: 10.1037/bne0000088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris NA, Winder DG, 2018. Synaptic Plasticity in the Bed Nucleus of the Stria Terminalis: Underlying Mechanisms and Potential Ramifications for Reinstatement of Drug- and Alcohol-Seeking Behaviors. ACS Chem. Neurosci 9, 2173–2187. doi: 10.1021/acschemneuro.8b00169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haufler D, Nagy FZ, Pare D, 2013. Neuronal correlates of fear conditioning in the bed nucleus of the stria terminalis. Learn. Mem. 20, 633–641. doi: 10.1101/lm.031799.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janak PH, Tye KM, 2015. From circuits to behaviour in the amygdala. Nature 517, 284–292 doi: 10.1038/naturel4188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JH, Sparta DR, Stamatakis AM, Ung RL, Pleil KE, Kash TL, Stuber GD, 2013. Distinct extended amygdala circuits for divergent motivational states. Nature 496, 224–228. doi: 10.1038/nature12041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kash TL, 2012. The role of biogenic amine signaling in the bed nucleus of the stria terminals in alcohol abuse. Alcohol 46, 303–308. doi: 10.1016/j.alcohol.2011.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kash TL, Pleil KE, Marcinkiewcz CA, Lowery-Gionta EG, Crowley N, Mazzone C, Sugam J, Hardaway JA, McElligott ZA, 2015. Neuropeptide regulation of signaling and behavior in the BNST. Mol. Cells 38, 1–13. doi: 10.14348/molcells.2015.2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick DG, Resnick HS, Milanak ME, Miller MW, Keyes KM, Friedman MJ, 2013. National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. J. Trauma. Stress 26, 537–547. doi: 10.1002/jts.21848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpers F, Kroes MCW, 2019. Roles of the amygdala and basal forebrain in defense: a reply to luyck et al. and implications for defensive action. Neuropsychol Rev 29, 186–189. doi: 10.1007/s11065-019-09401-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight LK, Depue BE, 2019. New frontiers in anxiety research: the translational potential of the bed nucleus of the stria terminalis. Front. Psychiatry 10, 510. doi: 10.3389/fpsyt.2019.00510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange MD, Daldrup T, Remmers F, Szkudlarek HJ, Lesting J, Guggenhuber S, Ruehle S, Jüngling K, Seidenbecher T, Lutz B, Pape HC, 2017. Cannabinoid CB1 receptors in distinct circuits of the extended amygdala determine fear responsiveness to unpredictable threat. Mol. Psychiatry 22, 1422–1430. doi: 10.1038/mp.2016.156 [DOI] [PubMed] [Google Scholar]
- Lebow MA, Chen A, 2016. Overshadowed by the amygdala: the bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Mol. Psychiatry 21, 450–463. doi: 10.1038/mp.2016.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Iwata J, Cicchetti P, Reis DJ, 1988. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J. Neurosci 8, 2517–2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Davis M, 1997. Role of the hippocampus, the bed nucleus of the stria terminalis, and the amygdala in the excitatory effect of corticotropin-releasing hormone on the acoustic startle reflex. J. Neurosci 17, 6434–6446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyck K, Goode TD, Lee Masson H, Luyten L, 2019. Distinct Activity Patterns of the Human Bed Nucleus of the Stria Terminalis and Amygdala during Fear Learning. Neuropsychol Rev 29, 181–185. doi: 10.1007/s11065-018-9383-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyck K, Nuttin B, Luyten L, 2018. Electrolytic post-training lesions of the bed nucleus of the stria terminalis block startle potentiation in a cued fear conditioning procedure. Brain Struct. Funct. 223, 1839–1848. doi: 10.1007/s00429-017-1591-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyck K, Tambuyzer T, Deprez M, Rangarajan J, Nuttin B, Luyten L, 2017. Electrical stimulation of the bed nucleus of the stria terminalis reduces anxiety in a rat model. Transl. Psychiatry 7, e1033. doi: 10.1038/tp.2017.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyten L, van Kuyck K, Vansteenwegen D, Nuttin B, 2011. Electrolytic lesions of the bed nucleus of the stria terminalis disrupt freezing and startle potentiation in a conditioned context. Behav. Brain Res. 222, 357–362. doi: 10.1016/j.bbr.2011.03.066 [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Baker DA, Funk D, Lê AD, Shaham Y, 2016. Stress-Induced Reinstatement of Drug Seeking: 20 Years of Progress. Neuropsychopharmacology 41, 335–356. doi: 10.1038/npp.2015.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcinkiewcz CA, Bierlein-De La Rosa G, Dorrier CE, McKnight M, DiBerto JF, Pati D, Gianessi CA, Hon OJ, Tipton G, McElligott ZA, Delpire E, Kash TL, 2019. Sex-Dependent Modulation of Anxiety and Fear by 5-HT1A Receptors in the Bed Nucleus of the Stria Terminalis. ACS Chem. Neurosci 10, 3154–3166. doi: 10.1021/acschemneuro.8b00594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcinkiewcz CA, Mazzone CM, D’Agostino G, Halladay LR, Hardaway JA, DiBerto JF, Navarro M, Burnham N, Cristiano C, Dorrier CE, Tipton GJ, Ramakrishnan C, Kozicz T, Deisseroth K, Thiele TE, McElligott ZA, Holmes A, Heisler LK, Kash TL, 2016. Serotonin engages an anxiety and fear-promoting circuit in the extended amygdala. Nature 537, 97–101. doi: 10.1038/naturel9318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, 1998. Overtraining does not mitigate contextual fear conditioning deficits produced by neurotoxic lesions of the basolateral amygdala. J. Neurosci 18, 3088–3097. doi: 10.1523/JNEUROSCI.18-08-03088.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, 2001a. Neurobiology of Pavlovian fear conditioning. Annu. Rev. Neurosci 24, 897–931. doi: 10.1146/annurev.neuro.24.1.897 [DOI] [PubMed] [Google Scholar]
- Maren S, 2001b. Is there savings for pavlovian fear conditioning after neurotoxic basolateral amygdala lesions in rats? Neurobiol. Learn. Mem 76, 268–283. doi: 10.1006/nlme.2001.4042 [DOI] [PubMed] [Google Scholar]
- Maren S, Phan KL, Liberzon I, 2013. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat. Rev. Neurosci. 14, 417–428. doi: 10.1038/nrn3492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham CM, Norvelle A, Huhman KL, 2009. Role of the bed nucleus of the stria terminalis in the acquisition and expression of conditioned defeat in Syrian hamsters. Behav. Brain Res 198, 69–73. doi: 10.1016/j.bbr.2008.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinon D, Lis P, Roman AN, Tornesi P, Applebey SV, Buechner G, Olivera V, Dabrowska J, 2019. Oxytocin receptors in the dorsolateral bed nucleus of the stria terminalis (BNST) bias fear learning toward temporally predictable cued fear. Transl. Psychiatry 9, 140. doi: 10.1038/s41398-019-0474-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan KA, Sareen J, Asmundson GJG, 2014. Social anxiety disorder is associated with PTSD symptom presentation: an exploratory study within a nationally representative sample. J. Trauma. Stress 27, 602–609. doi: 10.1002/jts.21952 [DOI] [PubMed] [Google Scholar]
- Miles OW, Maren S, 2019. Role of the bed nucleus of the stria terminalis in PTSD: insights from preclinical models. Front. Behav. Neurosci 13, 68. doi: 10.3389/fnbeh.2019.00068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles OW, May V, Hammack SE, 2018. Pituitary Adenylate Cyclase-Activating Peptide (PACAP) Signaling and the Dark Side of Addiction. J. Mol. Neurosci doi: 10.1007/sl2031-018-1147-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moaddab M, Dabrowska J, 2017. Oxytocin receptor neurotransmission in the dorsolateral bed nucleus of the stria terminalis facilitates the acquisition of cued fear in the fear-potentiated startle paradigm in rats. Neuropharmacology 121, 130–139. doi: 10.1016/j.neuropharm.2017.04.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naaz F, Knight LK, Depue BE, 2019. Explicit and ambiguous threat processing: functionally dissociable roles of the amygdala and bed nucleus of the stria terminalis. J. Cogn. Neurosci 31, 543–559. doi: 10.1162/jocn_a_01369 [DOI] [PubMed] [Google Scholar]
- Nagaya N, Acca GM, Maren S, 2015. Allopregnanolone in the bed nucleus of the stria terminalis modulates contextual fear in rats. Front. Behav. Neurosci 9, 205. doi: 10.3389/fnbeh.2015.00205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov I, 1927. Conditioned reflexes: an investigation of the physiological activity of the cerebral cortex Oxford Univ. Press, Oxford, England. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perusini JN, Fanselow MS, 2015. Neurobehavioral perspectives on the distinction between fear and anxiety. Learn. Mem 22, 417–425. doi: 10.1101/lm.039180.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pina MM, Young EA, Ryabinin AE, Cunningham CL, 2015. The bed nucleus of the stria terminalis regulates ethanol-seeking behavior in mice. Neuropharmacology 99, 627–638. doi: 10.1016/j.neuropharm.2015.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulos AM, Ponnusamy R, Dong H-W, Fanselow MS, 2010. Compensation in the neural circuitry of fear conditioning awakens learning circuits in the bed nuclei of the stria terminalis. Proc. Natl. Acad. Sci. USA 107, 14881–14886. doi: 10.1073/pnas.1005754107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Johnson SB, 2018. Anteroventral bed nuclei of the stria terminalis neurocircuitry: Towards an integration of HPA axis modulation with coping behaviors - Curt Richter Award Paper 2017. Psychoneuroendocrinology 89, 239–249. doi: 10.1016/j.psyneuen.2017.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindran LN, Stein MB, 2010. The pharmacologic treatment of anxiety disorders: a review of progress. J. Clin. Psychiatry 71, 839–854. doi: 10.4088/JCP.10r06218blu [DOI] [PubMed] [Google Scholar]
- Rescorla RA, 1968. Probability of shock in the presence and absence of CS in fear conditioning. J Comp Physiol Psychol 66, 1–5. doi: 10.1037/h0025984 [DOI] [PubMed] [Google Scholar]
- Rescorla RA, 1988. Pavlovian conditioning. It’s not what you think it is. Am. Psychol 43, 151–160. doi: 10.1037/0003-066X.43.3.151 [DOI] [PubMed] [Google Scholar]
- Resstel LBM, Alves FHF, Reis DG, Crestani CC, Corrêa FMA, Guimarães FS, 2008. Anxiolytic-like effects induced by acute reversible inactivation of the bed nucleus of stria terminalis. Neuroscience 154, 869–876. doi: 10.1016/j.neuroscience.2008.04.007 [DOI] [PubMed] [Google Scholar]
- Robinson OJ, Pike AC, Cornwell B, Grillon C, 2019. The translational neural circuitry of anxiety. J. Neurol. Neurosurg. Psychiatry doi: 10.1136/jnnp-2019-321400 [DOI] [PubMed] [Google Scholar]
- Sajdyk T, Johnson P, Fitz S, Shekhar A, 2008. Chronic inhibition of GABA synthesis in the bed nucleus of the stria terminalis elicits anxiety-like behavior. J. Psychopharmacol. (Oxford) 22, 633–641. doi: 10.1177/0269881107082902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Fox AS, 2016. Contributions of the central extended amygdala to fear and anxiety. J. Neurosci 36, 8050–8063. doi: 10.1523/JNEUROSCI.0982-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis AM, Sparta DR, Jennings JH, McElligott ZA, Decot H, Stuber GD, 2014. Amygdala and bed nucleus of the stria terminalis circuitry: Implications for addiction-related behaviors. Neuropharmacology 76 Pt B, 320–328. doi: 10.1016/j.neuropharm.2013.05.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein DJ, Scott KM, de Jonge P, Kessler RC, 2017. Epidemiology of anxiety disorders: from surveys to nosology and back. Dialogues Clin Neurosci 19, 127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan GM, Apergis J, Bush DEA, Johnson LR, Hou M, Ledoux JE, 2004. Lesions in the bed nucleus of the stria terminalis disrupt corticosterone and freezing responses elicited by a contextual but not by a specific cue-conditioned fear stimulus. Neuroscience 128, 7–14. doi: 10.1016/j.neuroscience.2004.06.015 [DOI] [PubMed] [Google Scholar]
- Vranjkovic O, Pina M, Kash TL, Winder DG, 2017. The bed nucleus of the stria terminalis in drug-associated behavior and affect: A circuit-based perspective. Neuropharmacology 122, 100–106. doi: 10.1016/j.neuropharm.2017.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell J, Morris RW, Bouton ME, 2006. Effects of bed nucleus of the stria terminalis lesions on conditioned anxiety: aversive conditioning with long-duration conditional stimuli and reinstatement of extinguished fear. Behav. Neurosci 120, 324–336. doi: 10.1037/0735-7044.120.2.324 [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M, 1997. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. J. Neurosci 17, 9375–9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Davis M, 2002. The role of amygdala glutamate receptors in fear learning, fear-potentiated startle, and extinction. Pharmacol. Biochem. Behav 71, 379–392. doi: 10.1016/S0091-3057(01)00698-0 [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M, 2008. Role of the extended amygdala in short-duration versus sustained fear: a tribute to Dr. Lennart Heimer. Brain Struct. Funct. 213, 29–42. doi: 10.1007/s00429-008-0183-3 [DOI] [PubMed] [Google Scholar]
- Walker DL, Miles LA, Davis M, 2009. Selective participation of the bed nucleus of the stria terminalis and CRF in sustained anxiety-like versus phasic fear-like responses. Prog. Neuropsychopharmacol. Biol. Psychiatry 33, 1291–1308. doi: 10.1016/j.pnpbp.2009.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M, 2003. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur. J. Pharmacol 463, 199–216. doi: 10.1016/S0014-2999(03)01282-2 [DOI] [PubMed] [Google Scholar]
- Waraczynski M, 2016. Toward a systems-oriented approach to the role of the extended amygdala in adaptive responding. Neurosci. Biobehav. Rev 68, 177–194. doi: 10.1016/j.neubiorev.2016.05.015 [DOI] [PubMed] [Google Scholar]
- Xu HY, Liu YJ, Xu MY, Zhang YH, Zhang JX, Wu YJ, 2012. Inactivation of the bed nucleus of the stria terminalis suppresses the innate fear responses of rats induced by the odor of cat urine. Neuroscience 221, 21–27. doi: 10.1016/j.neuroscience.2012.06.056 [DOI] [PubMed] [Google Scholar]
- Zimmerman JM, Maren S, 2011. The bed nucleus of the stria terminalis is required for the expression of contextual but not auditory freezing in rats with basolateral amygdala lesions. Neurobiol. Learn. Mem 95, 199–205. doi: 10.1016/j.nlm.2010.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.