Abstract
Background:
Chronic cocaine use is associated with structural brain abnormalities within prefrontal regions implicated in impulsivity. Despite high levels of impulsivity among persons who use cocaine, it is not known how reductions in gray matter volume (GMV) may relate to trait and behavioral measures of impulsivity.
Methods:
The sample included 39 active cocaine users (COC+) and 40 controls with no history of cocaine use (COC−). Participants had a brain scan on a 3T MRI machine and completed out-of-scanner measures of trait impulsivity and delayed reward discounting. Whole-brain voxel-based morphometry was used to compare GMV between COC+ and COC−. Within regions that differed between groups, voxelwise correlations were conducted to examine the relationship between GMV and impulsivity.
Results:
In a whole-brain analysis, COC+ had broad reductions in GMV compared to COC− in bilateral frontal, parietal, occipital, and cerebellar regions. Lower GMV correlated with trait impulsivity in lateral prefrontal regions and with delayed reward discounting in medial prefrontal regions, while lower GMV correlated with both measures in the posterior parietal cortex. COC+ demonstrated significantly higher impulsivity than COC− on all measures, but the nature of the correlation with GMV was similar in both groups.
Conclusions:
Reflecting the multi-faceted nature of impulsivity, these results show that trait and behavioral measures of impulsivity map differentially onto altered brain morphology. While the brain-behavior patterns were similar in COC+ and COC−, suggesting that impulsivity varies on a continuous spectrum, cocaine-related abnormalities in frontal-parietal brain systems may contribute to heightened impulsivity.
Keywords: Cocaine, Impulsivity, delayed reward discounting, MRI, voxel-based morphometry, gray matter volume
1. INTRODUCTION
Cocaine use remains a significant public health problem, with multiple indicators suggesting that it is on the rise globally. After a sustained decrease, cocaine manufacture rose by 56% from 2013 to 2016, reaching its highest level in recorded history (UNODC, 2018). In 2016, approximately 18.2 million people worldwide used cocaine, a nearly 7% increase from 2015 (UNODC, 2018). The prevalence of cocaine use is highest in the United States, with an estimated 1.9 million current users (Ahrnsbrak et al., 2017). Chronic cocaine use is associated with deficits in reward-based decision making that are often associated with impulsive behaviors (Spronk et al., 2013). Many of the negative consequences of cocaine use, such as sexually transmitted infections, criminal behaviors, and violence (Butler et al., 2017; Enns et al., 2017), likely stem from impulsivity.
Impulsivity is a multidimensional construct that describes a propensity toward rapid, unplanned reactions without regard for possible undesirable consequences (de Wit, 2009; Robbins et al., 2012). Most commonly, impulsivity is measured as a personality trait using self-report questionnaires that require introspection. As a personality trait, there are three distinct substrates of impulsivity – attentional, non-planning, and motor (Stanford et al., 2009). In contrast, behavioral measures provide a direct assessment of specific processes involved in impulsivity. Among these, delayed reward discounting describes the subjective devaluing of rewards that are delayed in time (Mazur, 1987; Rachlin and Green, 1972). Delayed reward discounting can be estimated using decision-making tasks that ask participants to choose between smaller, immediate rewards and larger, delayed rewards. Together, trait and behavioral measures of impulsivity may provide complementary information related to the neural mechanisms underlying the expression of impulsive behavior.
Cocaine users consistently demonstrate greater trait impulsivity and delayed reward discounting compared to non-cocaine users (Dalley et al., 2011; MacKillop et al., 2011; J. L. Smith et al., 2014). Impulsivity is thought to be related to abnormalities in the mesocorticolimbic system, including lower gray matter volume (GMV). In healthy adults, trait impulsivity is most consistently associated with reduced GMV in medial frontal regions, specifically orbitofrontal cortex (OFC) and medial prefrontal cortex (MPFC) (Fuentes et al., 2012; Korponay et al., 2017; Matsuo et al., 2009). In contrast, delayed reward discounting appears to be associated with reduced GMV in the lateral prefrontal cortex, including dorsal (DLPFC) and ventral (VLPFC) regions, as well as abnormalities in the OFC (Bjork et al., 2009; Mohammadi et al., 2016; Owens et al., 2017; Wang et al., 2016).
Many studies have identified cocaine-related abnormalities in the mesocorticolimbic system (Mackey and Paulus, 2013), largely overlapping with the regions implicated in impulsivity. The most consistent finding is reduced GMV in lateral and medial prefrontal cortices, including DLPFC, VLPFC, and OFC, among cocaine users compared to healthy controls, although broader reductions in temporal, hippocampal, and cerebellar regions have also been reported (Alia-Klein et al., 2011; Crunelle et al., 2014; Ersche et al., 2011; Moreno-Lopez et al., 2012; Sim et al., 2007; Yip et al., 2018). The largest of these studies also demonstrated cocaine-related increases in striatal volume (Ersche et al., 2011).
Only three of the aforementioned studies examined the link between brain morphology and impulsivity in cocaine users. Two studies correlated whole-brain GMV to trait impulsivity. In the first, which included only cocaine users, attentional impulsivity correlated negatively with GMV in the OFC and precentral gyrus (Crunelle et al., 2014). Unexpectedly, there were also positive correlations between motor impulsivity and GMV in the DLPFC and between non-planning impulsivity and GMV in the posterior parietal cortex (PPC) (Crunelle et al., 2014). In the second study, which included cocaine users, gamblers, and healthy controls, trait impulsivity correlated negatively with GMV in insular, limbic, and temporo-parietal regions (Yip et al., 2018). A third study combined trait measures with behavioral measures of inhibitory control and sustained attention (Ersche et al., 2011). Restricting the analyses to regions that differed between cocaine users and controls, greater impairment in attentional control, as measured primarily by a stop-signal task, was negatively correlated with GMV in the insula and middle temporal area, and positively with GMV in the striatum (Ersche et al., 2011). These studies suggest that brain morphology may contribute to impulsivity in cocaine users, but findings are inconsistent. Moreover, prior studies have not examined the relationship between GMV and delayed reward discounting, a signature of drug addiction, in persons who use cocaine.
Building upon this foundational research, the present study investigated the relationship of GMV to impulsivity in a community-recruited sample of cocaine users. Using voxel-based morphometry, we expected to find reduced GMV in lateral and medial PFC and increased GMV in the striatum among cocaine users compared to a closely matched comparison group of non-cocaine users. To investigate the potential implications of cocaine-related abnormalities in brain structure, we examined the relationship of GMV to both trait impulsivity and delayed reward discounting. We hypothesized that prefrontal GMV would be negatively correlated with both measures, and that the strength of the correlation would be strongest in cocaine users.
2. METHODS
2.1. Participants
The study was open to adults aged 18–55 years who were active cocaine users (COC+) or non-cocaine users (COC−). The COC+ groups met the following criteria: lifetime cocaine dependence as defined by the DSM-IV-TR, regular cocaine use for ≥1 year, repeated use in the past month (defined as ≥2 days or positive drug screen), and cocaine as a principal drug of abuse (as determined by structured clinical interviews). The COC− groups had no history of cocaine abuse and met the following criteria: no lifetime cocaine use disorder (abuse or dependence), no history of regular cocaine use, no cocaine use in the past year, and a cocaine-negative drug screen. Alcohol and marijuana use were permitted in all groups. For other drugs, individuals were excluded for any history of dependence, lifetime regular use for >2 years, and regular use in the past year. Additional exclusion criteria were: English non-fluency or illiteracy; <8th grade education; severe learning disability; serious neurological disorders or history of neuroinfections; severe head trauma with loss of consciousness >30 minutes and persistent functional decline; severe mental illness; current use of antipsychotic or mood stabilizing medications; MRI contraindications; and/or impaired mental status.
2.2. Procedures
Participants were recruited via advertisements in local newspapers, websites, and community-based organizations. After a brief pre-screener conducted over the telephone, individuals completed an in-person screening. Eligible participants returned for the MRI scan and additional assessments. All participants provided written informed consent, and procedures were approved by the institutional review board at Duke University Health System. Participants were compensated up to $180 for their participation.
2.3. Screening measures
All participants had a confirmed blood alcohol level of 0.00 prior to engaging in any study activities. Participants completed clinical interviews, computerized surveys, urine drug screening, and pregnancy testing. Module E of the Structured Clinical Interview for DSM-IV-TR identified substance use disorders (First et al., 1996), and the Addiction Severity Index-Lite assessed current and lifetime substance use and associated impairments (McLellan et al., 1992). This comprehensive substance abuse assessment covered cocaine, marijuana, alcohol, opioids, amphetamines, benzodiazepines, and other illicit drugs. Participants reported on demographic characteristics, including age, gender, race, and education. HIV status was verified using an OraQuick© rapid test; all participants were negative. Healthcare records were reviewed to ensure no exclusionary medical history, including substance abuse.
2.4. Behavioral assessments
Substance abuse.
On the day of the MRI scan, participants had another breathalyzer test, which had to be 0.00 before proceeding with the visit. Timeline follow-back methodology was used to assess substance use in the past 30 days (Robinson et al., 2014). Participants also completed another urine toxicology screen for cocaine, cannabis, methamphetamine, opioids, and benzodiazepines.
Trait impulsivity.
Trait impulsivity was assessed with the widely-used the Barratt Impulsivity Scale (BSI-11) (Patton et al., 1995). This 30-item self-report measure includes three factors that capture non-planning, attentional, and motor impulsivity. Non-planning impulsivity describes a present orientation without consideration of the future (e.g., “I plan tasks carefully.” “I am self-controlled.” “I am more interested in the present than the future.”). Attentional impulsivity is characterized by an inability to focus or concentrate (e.g., “I don’t pay attention.” “I concentrate easily.” “I have racing thoughts.”). Motor impulsivity involves acting without thinking (e.g., “I do things without thinking.” “I act on the spur of the moment.” “I can only think about one thing at a time.”). Participants rate the frequency with which each item applies to them using a 4-point scale.
Delayed reward discounting.
The Monetary Choice Questionnaire (MCQ) is a commonly used delayed reward discounting task that asks participants to choose between a series of smaller, immediate rewards and larger, delayed rewards (e.g., “Would you prefer $33 today or $80 in 14 days?”) (Kirby et al., 1999). To accommodate for extreme discounting among cocaine users, we used the extended version (MCQ-36), which includes a fixed set of 36 items (Towe et al., 2015). Immediate rewards ranged from $7-$80 and delayed rewards ranged from $25-$85 with a delay of 1–186 days. The task was programed using ePrime (Psychology Software Tools, Inc., http://www.pstnet.com), and choices were presented in random order. Participant k-values were estimated using standard scoring procedures (Towe et al., 2015). Since the range of possible k-values increases exponentially from 0.00016 to 4.00, the data was normalized using a natural logarithm transformation.
2.5. MRI data acquisition
MRI data was acquired with a 3.0T GE Discovery MR750 whole-body scanner. All scans occurred on the same scanner at the Brain Imaging and Analysis Center at Duke University using an eight-channel head coil. High-resolution T1-weighted structural images were acquired with the following parameters: TR = 8.10 ms, TE = 3.18 ms, FOV = 25.6 cm, flip angle = 12°, in-plane matrix = 256*256; slice thickness = 1 mm, and number of slices = 166.
2.6. Data Analysis
Case-specific pre-processing steps were implemented to improve data quality. The images were first visually checked to ensure full coverage and identify significant motion-related artifacts that could affect processing. Structural data was analyzed with FSL-VBM (Douaud et al., 2007, http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSLVBM), an optimized VBM protocol (Good et al., 2001) carried out with FSL tools (Smith et al., 2004). First, structural images were brain-extracted and segmented for maximum gray matter (GM) inclusion before being registered to the MNI 152 standard space using non-linear registration (Andersson et al., 2007). The resulting images were averaged and flipped along the x-axis to create a left-right symmetric, study-specific GM template that contained equal representation from both groups. Second, all native GM images were non-linearly registered to this study-specific template and “modulated” to correct for local expansion/contraction due to the non-linear component of the spatial transformation. The modulated GM images were then smoothed with an isotropic Gaussian kernel with a sigma of 2 mm. Finally, a voxelwise GLM was performed between groups using permutation-based non-parametric testing (p < .05; 10,000 permutations), correcting for multiple comparisons across space using FSL’s Threshold-Free Cluster Enhancement (TFCE) (S. M. Smith and Nichols, 2009). Age (in years) and other substance use (alcohol use, marijuana use, and daily cigarette use; each yes/no) were included as covariates. Analyses were restricted to a mean GM mask created from the study sample. Any significant regional differences between groups in the whole-brain analysis were then masked, and the mean GMV within each cluster was extracted using fslmeants. Cortical regions were defined using the Human Connectome Project’s multi-modal cortical parcellation (HCP_MMP1.0) that delineates 180 regions per hemisphere (Glasser et al., 2016), while sub-cortical regions and cerebellum were identified using the Harvard-Oxford Atlas (Desikan et al., 2006). Among COC+, in regions that differed between groups, we examined the relationships between GMV to years of regular cocaine use and days of use in the past 30 days (log 10 transformed) while controlling for age utilizing a voxelwise correlation analysis.
These regional volumes were then examined across the entire sample for any relationship to trait impulsivity (BIS-11 factors) and delayed reward discounting (MCQ ln k-value) utilizing voxelwise correlations restricted to a mask of significant results from the whole-brain analyses with age and other substance use included as covariates. These voxelwise correlations were conducted using permutation testing (p < .05; 10,000 permutations) with TFCE correction. Scatter plots were used to visualize the partial correlations between GMV and impulsivity controlling for age and other substance use. To test whether COC status moderated the relationship between GMV and impulsivity scores, step-wise multiple regression models were conducted in SPSS Statistics 26. For each significant cluster, variables were entered in blocks as follows: (1) age and other substance abuse, (2) COC status and impulsivity measure, and (3) interaction product of COC status and impulsivity measure.
3. RESULTS
3.1. Sample characteristics
The sample included 39 COC+ and 40 COC− participants (Table 1). Participants were primarily male (61%) and African-American (77%), ranging in age from 28 to 55 (M= 44.43, SD= 6.87), and had a mean of 13.16 years of education (SD= 2.36). Participants were well matched on gender, race, and age, but COC+ had significantly fewer years of education than COC−.
Table 1.
Sample characteristics by cocaine status
| COC+ (N = 39) |
COC− (N = 40) |
Statistic | P-value | |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Male, N (%) | 24 (61.5%) | 24 (60.0%) | χ2(1) = .020 | .889 |
| Age in years, M (SD) | 45.44 (6.21) | 43.45 (7.40) | t(77) = 1.29 | .201 |
| Race, N (%) | χ2(2) = 1.28 | .528 | ||
| African American | 32 (82.1%) | 29 (72.5%) | ||
| White | 5 (12.8%) | 9 (22.5%) | ||
| Other or mixed | 2 (5.1%) | 2 (5.0%) | ||
| Education in years, M (SD) | 12.56 (2.46) | 13.75 (2.12) | t(77) = −2.30 | .024 |
| Impulsivity | ||||
| BIS-11 non-planning, M (SD) | 28.03 (4.73) | 21.50 (4.28) | t(77) = 6.43 | < .001 |
| BIS-11 attention, M (SD) | 15.10 (3.75) | 13.23 (3.45) | t(77) = 2.31 | .023 |
| BIS-11 motor, M (SD) | 23.74 (4.99) | 19.93 (3.52) | t(77) = 3.94 | < .001 |
| MCQ ln(k), M (SD) 1 | −2.52 (1.19) | −3.51 (1.83) | t(74) = 2.77 | .007 |
| Other substance use in past 30 days | ||||
| Any alcohol use, n (%) | 34 (87.2%) | 22 (55.0%) | χ2(1) = 9.91 | .002 |
| Days of alcohol use, M (SD) | 12.38 (9.39) | 5.09 (6.51) | t(54) = 3.18 | .002 |
| Any cannabis use, n (%) | 18 (46.2%) | 10 (25.0%) | χ2(1) = 3.86 | .049 |
| Days of cannabis use, M (SD) | 12.00 (11.25) | 24.20 (9.16) | t(26) = −2.93 | .007 |
| Daily cigarette use, N (%) | 25 (64.1%) | 14 (35.0%) | χ2(1) = 6.69 | .010 |
Three participants had MCQ scores that did not pass the standard QA checks.
Participants in the COC+ group had been using cocaine regularly for an average of 17.87 years (SD = 8.20), with the majority (92%) reporting smoking as their primary route of administration. In the 30 days prior to screening, they had used on an average of 10.69 days (SD = 7.66). On the day of the scan, 76% of participants had a urine toxicology screen positive for cocaine. The mean number of days since last use was 4.31 (SD = 5.67), with 69% reporting use within the past 3 days.
Polysubstance use was common (Table 1). In the past 30 days, 71% used alcohol, 35% used cannabis, and 49% smoked cigarettes daily, with COC+ more likely than COC− to use each substance. Among users of each substance, COC+ had used alcohol on more days, while COC− had used cannabis on more days.
As expected, COC+ had significantly higher scores than COC− on each subscale of the BIS-11, indicating higher trait impulsivity. They also demonstrated steeper delayed reward discounting on the MCQ, with higher k-value estimates.
3.2. Group differences in GM volume
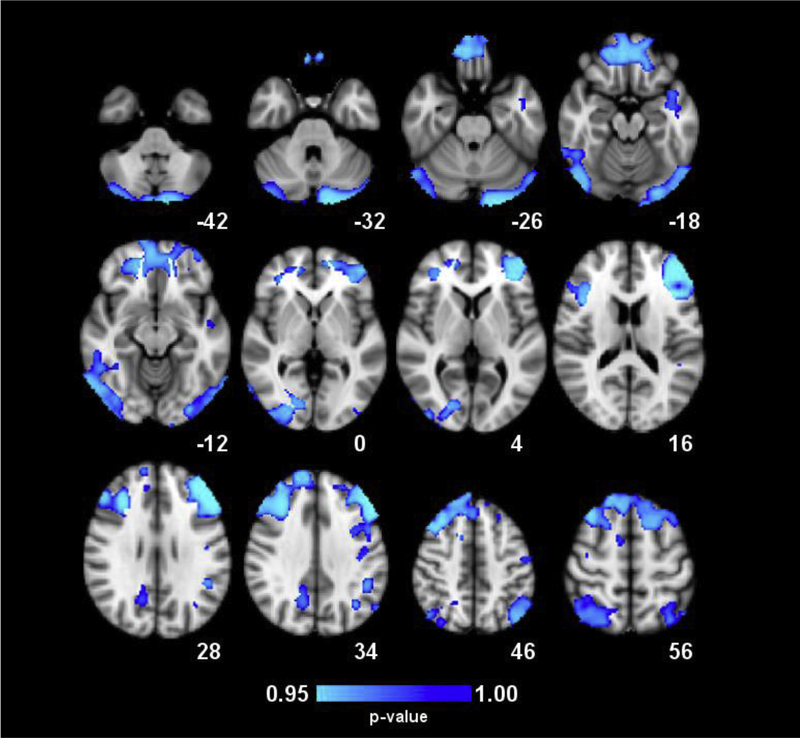
In the whole-brain analysis, voxel-wise group comparisons revealed significant group differences in seven clusters that spanned bilateral regions throughout frontal, parietal, and occipital lobes and the cerebellum (Figure 1, Table 2). In each cluster, COC+ had significantly reduced GMV compared to COC−. The largest cluster of >110,000 mm3 included bilateral DLPFC, VLPFC, OFC, MPFC, frontopolar cortex, anterior cingulate cortex, and motor cortices. Additional clusters included bilateral cerebellum, right and left PPC, right and left visual cortices, and left insula. Among COC+, years of regular cocaine use and days of cocaine use in the past 30 days was unrelated to GMV in all clusters (p > .05).
Figure 1.
Whole-brain maps of significant differences in gray matter volume (GMV) between groups. Regions in blue denote lower GMV in COC+ relative to COC− participants. Results were generated through permutation testing of voxel cluster statistics with cluster-wise p < .05 using TFCE to correct for multiple comparisons. Clusters are described in Table 2. Statistical results are overlaid on the MNI152 2mm standard-space T1-weighted structural template. The numbers below the images indicate the Z coordinate for each axial slice. Images are in radiological orientation (left = right, right = left).
Table 2:
Description of clusters that differed between COC groups in a whole-brain analysis
| Anatomical regions in cluster | HCP_MMP1.9 area name | Volum e in mm3 | X Y Z at max | COC+ M (SE) | COC− M (SE) |
|---|---|---|---|---|---|
| B. Dorsolateral prefrontal cortex | L/R_p9-46v*, L/R_46, L/R_8Ad, L/R_s6-8, L/R_SFL, L/R_9p, L_a9-46v, L_9-46d, R_8Av, R_8C, R_8BL, R_i6-8 | 110,83 2 | −44, 42, 16 | .383 (.005) | .425 (.005) |
| B. Orbitofrontal cortex | L/R_OFC, L/R_13l, L/R_11l, L_a47r | ||||
| B. Medial prefrontal cortex | L/R_8BM, L/R_10r, L/R_10v | ||||
| B. Ventrolateral prefrontal cortex | L/R_44, L/R_p47r, L/R_IFSa, L_IFJp, | ||||
| B. Frontopolar cortex | L/R_a10p, L/R_10pp | ||||
| B. Motor cortices | L/R_4, L/R_6mp, L/R_6ma, L/R_SCEF, L_PEF | ||||
| B. Anterior and mid cingulate cortices | L/R_s32, R_p32pr, R_24dv, R_24dd | ||||
| L. Primary somatosensory cortex | L_3a | ||||
| B. Cerebellum* | n/a | 21,784 | −16, −94, −32 | .384 (.011) | .441 (.011) |
| L. Ventral stream visual cortex | L_PIT | ||||
| L. Early visual cortex | L_V2, L_V3, L_V4 | ||||
| L. Lateral occipital cortex | L_LO2 | ||||
| R. Posterior parietal cortex* | R_7PC*, R_7AL, R_PGs, R_IP1, R_LIPv | 13,960 | 32, −52, 66 | .352 (.007) | .395 (.007) |
| R. Somatosensory cortices | R_2 | ||||
| R. Posterior cingulate cortex | R_POS2, R_31pd, R_7m | ||||
| L. Posterior parietal cortex* | L_PFm*, L_PGs, L_PGp, L_PF, L_7PC, L_IP2, L_LIPv, L_7AL | 12,616 | −44, −72, 46 | .360 (.006) | .403 (.006) |
| L. Temporal parietal occipital junction | L_TPOJ3 | ||||
| R. Lateral temporal cortex | R_TE1p*, R_TE2p, R_PHT | 6,112 | 62, −64, −12 | .380 (.008) | .432 (.008) |
| R. Cerebellum | n/a | ||||
| R. Primary and early visual cortices | R_V3*, R_V2, R_V4, R_V1 | 4,736 | 28, −86, −2 | .394 (.010) | .450 (.010) |
| R. Lateral occipital cortex | R_LO2 | ||||
| L. Insula* | L_PI*, L_Pol1 | 280 | −42, −2, −22 | .383 (.007) | .430 (.007) |
| L. Auditory association cortex | L_STGa |
Region at maximum value. Mean values are estimated marginal means controlling for age and other substance use.
3.3. Correlation with impulsivity measures
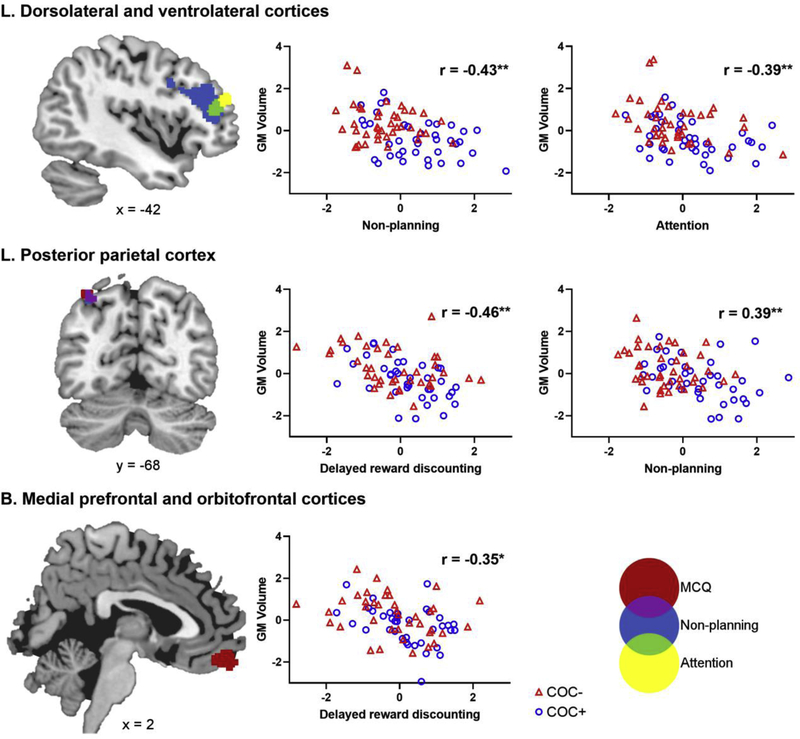
Within the clusters that differed significantly between COC+ and COC−, there were significant voxel-wise correlations of GMV with BIS attention, BIS non-planning, and MCQ ln k-value, but not BIS motor (Figure 2). In each case, reduced GMV correlated with increased impulsivity. Table 3 describes the clusters that correlated with each measure, including the overlap across measures. Reduced volume in the left DLPFC and VLPFC correlated with greater attentional and non-planning impulsivity. For non-planning, this correlation was present in a larger area of the DLPFC and VLPFC. Reduced volume in the PPC correlated with greater non-planning and delayed reward discounting, with substantial overlap for the two measures. Delayed reward discounting was also uniquely correlated with reduced volume in the MPFC and OFC.
Figure 2.
Images on the left show clusters in which gray matter volume (GMV) correlated with impulsivity measures. Voxelwise correlations were conducted using permutation testing with TFCE at p < .05 to correct for multiple comparisons. Each impulsivity measure and their overlap is coded with a different color. Clusters are described in Table 3. Statistical results are overlaid on the MNI152 2mm standard-space T1-weighted structural template. The numbers below the images indicate the appropriate coordinate for each slice. Images are in radiological orientation (left = right, right = left). Scatterplots on the right show the correlation between GMV and impulsivity within each significant cluster. The standardized residuals from the partial correlations controlling for age and other substance use are plotted. The r-values represent the partial correlation coefficients. *p<.005, **p<.001.
Table 3:
Description of clusters that correlated with impulsivity measures in regions with volumetric differences between COC+ and COC− groups
| Anatomical regions in cluster | HCP_MMP1.9 area name | Volume (mm3) | X Y Z at max |
|---|---|---|---|
| Primary clusters | |||
| BIS-11 attention | |||
| L. Dorsolateral and ventrolateral prefrontal cortices | L_a9-46v*, L_46, L_IFSa | 1,352 | −44 40 16 |
| BIS-11 non-planning | |||
| L. Dorsolateral and ventrolateral prefrontal cortices | L_a9-46v*, L_p9-46v, L_46, L_8C, L_p47r, L_IFJa, L_IFSp, L_IFSa | 7,496 | −42 38 14 |
| L. Posterior parietal cortex | L_PFm*, L_PGs, L_IP1 | 568 | −34 −66 54 |
| MCQ discounting | |||
| B. Medial prefrontal and orbitofrontal cortices | L/R_10v*, L_10r, L_OFC | 2,104 | 0 54 −18 |
| L. Posterior parietal cortex | L_PFm*, L_IP1 | 648 | −36 −68 58 |
| Overlap of clusters | |||
| BIS-11 non-planning and BIS-11 attention | |||
| L. Dorsolateral prefrontal cortex | L_a9-46v*, L_46, L_IFSa | 792 | −40 40 12 |
| BIS-11 non-planning and MCQ discounting | |||
| L. Posterior parietal cortex | L_PFm*, L_IP1 | 464 | −36 −60 52 |
| BIS-11 attention and MCQ discounting | |||
| None | |||
Region at maximum value. Only clusters with >25 voxels are presented.
Figure 2 shows the scatterplots for each significant correlation. Moderator analyses confirmed that the strength of the correlations were similar between COC+ and COC− for each impulsivity measure. The COC*impulsivity interaction term was non-significant for attentional impulsivity in the left DLPFC (β= .24, p= .58), for non-planning impulsivity in the left DLPFC (β= −.74, p= .25) and left PPC (β= −.32, p= .64), and for delayed reward discounting in the bilateral OFC (β= −.22, p= .33) and left PPC (β= −.31, p= .13).
4. DISCUSSION
Our key finding is that cocaine-related alterations in fronto-parietal GMV are associated with both trait and behavioral measures of impulsivity. Consistent with prior studies, cocaine users had reduced GMV compared to non-cocaine users broadly in the bilateral PFC, including DLPFC, VLPFC, OFC, and MPFC, and throughout occipital, parietal, and cerebellar regions. As expected, cocaine users also had higher trait impulsivity and steeper delayed reward discounting, which were associated with morphological differences in the PFC and PPC. Specifically, lower GMV correlated with trait impulsivity in the lateral PFC and with delayed reward discounting in the medial PFC, while lower GMV correlated with both measures in the PPC. The nature of these correlations was similar for cocaine users and non-users, underscoring the relevance of brain structure for understanding both normal personality differences and more extreme impulsivity in clinical populations.
Self-reported measures of trait impulsivity capture an individual’s self-perception of behavior, and thus rely heavily on regions of the cognitive control system. Specifically, both non-planning and attentional impulsivity correlated with GMV in the left DLPFC/VLPFC. The lateral PFC plays a key role in cognitive and inhibitory control processes across multiple domains (Miller and Cohen, 2001). Specifically, the DLPFC is associated with impulsive choice, attention, and planning, while the VLPFC is activated during response inhibition (Bari and Robbins, 2013; Dalley and Robbins, 2017; Morein-Zamir and Robbins, 2015; Zhang et al., 2017). The non-planning cluster extended into a larger area of the lateral PFC, suggesting that this is a more complex component of impulsivity that draws on broader set of neural substrates. Moreover, non-planning also correlated with GMV in the PPC, which is involved in decision making processes that require cognitive flexibility or abstraction, including the evaluation of reward magnitude and probability (Dorris and Glimcher, 2004; Zhou and Freedman, 2019). These regions map onto the lateralized fronto-parietal network that is critical for planning and other complex attentional tasks (Huettel et al., 2005; Laird et al., 2011; Majdandzic et al., 2007; Newman et al., 2003; Reyna and Huettel, 2014).
In contrast, delayed reward discounting correlated with GMV in the OFC and MPFC, suggesting that discrete regions of the PFC are involved in different aspects of impulsivity. Medial prefrontal activation is associated with a preference for immediate rewards (Dalley and Robbins, 2017; Hariri et al., 2006). Specifically, the OFC is thought to guide behavior based on the represented value of expected outcomes (Lucantonio et al., 2012), and has been shown to be involved in delayed reward discounting tasks (Mobini et al., 2002; Winstanley et al., 2004). In functional MRI studies, the medial OFC shows greater activation when choosing the smaller, sooner reward (McClure et al., 2007; McClure et al., 2004), while the PPC encodes the subjective value of the delayed option (Essex et al., 2012; Louie and Glimcher, 2010; Massar et al., 2015). In sum, structural abnormalities in the OFC/MPFC and PPC may disrupt reward processing and top-down control over impulses, leading to an exaggerated preference for immediate rewards.
While multiple studies have examined brain volume differences associated with cocaine addiction, there is considerable inconsistency in results (Mackey and Paulus, 2013). This could stem from differences in methodologies, such as alternate software packages and variations in smoothing kernel size. In addition, some of the earlier studies had relatively small sample sizes (Button et al., 2013). Given the current crisis of reproducibility in neuroscience, including a lack of attention to reproducing well-powered studies (Poldrack et al., 2017), we report whole-brain GMV analyses. The most robust study to date included a sample of 60 cocaine users and 60 healthy controls (Ersche, 2011). We reproduced most of their findings, including reduced GMV in prefrontal, parietal, temporal, and cerebellar cortices, but did not find increases in striatum or cerebellum. We also found more extensive reductions in GMV in the lateral PFC, including DLPFC. These differences may relate to our use of TFCE, rather than cluster-based probability thresholding, which minimizes problems associated with defining the arbitrary initial cluster-forming threshold and amount of spatial smoothing (S. M. Smith and Nichols, 2009). Despite these inconsistencies, collectively these studies indicate that cocaine addiction is associated with broad reductions in GMV.
Several biological mechanisms are believed to drive neuronal loss in cocaine users. Vasoconstriction following cocaine intake decreases blood flow in the cortex that can result in “mini-strokes” or cell death (Siniscalchi et al., 2015). In addition, increases in dopamine following cocaine use can lead to cell death via oxidative stress (Pereira et al., 2015). Chronic cocaine use is associated with neuroinflammation resulting from microglial activation, which can lead to tissue damage (Kohno 2019; Ersche 2017). While it might be expected that longer duration of cocaine use would be associated with greater neuronal loss, we did not find a correlation between years of regular use and GMV. However, the COC+ sample was relatively homogenous in regard to cocaine use, with all participants reporting a chronic history of use and meeting diagnostic criteria for dependence. Therefore, this study was not well-suited to test the effects of varying duration of cocaine exposure.
Poly-substance use is highly prevalent among persons who use cocaine (John and Wu, 2017). A strength of this study is the comprehensive assessment of lifetime cocaine and other substance use, and the exclusion of participants with any regular use of “hard” drugs such as opioids and amphetamines. However, we permitted the use of alcohol, marijuana, and nicotine to ensure generalizability of results. Importantly, use of these substances was represented in both groups, although they were more common in the COC+ group. To control for potential effects of these other substances, our analyses controlled for concurrent use of alcohol, marijuana, and cigarettes. As our sample was comprised of persons with cocaine as their principal drug of abuse, additional studies are needed to distinguish the effects of polysubstance abuse on brain morphology.
This is the first MRI study conducted among cocaine users to examine the relationship of GMV to both trait and behavioral measures of impulsivity. Additional strengths of our study include a robust sample size with demographically-matched groups and subject-specific skull stripping parameters to optimize the amount of GM included in the analyses. Nevertheless, there are several limitations to consider. First, due to the cross-sectional design, it is unknown whether reductions in GMV are due to chronic exposure to cocaine or whether individual differences in brain morphology predispose individuals to drug abuse. Longitudinal studies that begin assessments prior to the onset of cocaine use disorder are needed to determine this. Second, our study included only a single behavioral measure that taps delayed reward discounting. Future studies should examine additional processes such as inhibitory control and sustained attention. Finally, while impulsivity is a robust predictor of risk behaviors (Bickel et al., 2012; Hayaki et al., 2006; Stanford et al., 2009), additional research is needed to determine whether cocaine-related abnormalities in GMV relate to real-world outcomes, such as sexual risk behavior.
4.1. Conclusion
Impulsive behaviors in cocaine users are associated with a variety of adverse outcomes, such as use addiction severity (Moeller, Dougherty, et al., 2001), poor treatment adherence (Winhusen et al., 2013), and the development of persistent, compulsive drug-taking (Belin, Mar, Dalley, Robbins, & Everitt, 2008). Consistent with the broader literature, our study suggests that cocaine-related alterations in PFC and PPC structures may contribute to heightened impulsivity. However, trait and behavioral measures mapped differentially onto brain morphology in the PFC, such that self-reported non-planning and attentional impulsivity were linked to the left DLPFC/VLPFC, while delayed reward discounting was linked to the OFC and MPFC. Our results support the multifaceted nature of impulsivity and the importance of supplementing trait measures with behavioral task data, particularly in clinical populations characterized by elevated impulsivity. Interestingly, the brain-behavior patterns were similar between cocaine users and non-users, suggesting that impulsivity varies along a continuous spectrum, rather than there being a quantitative distinction. While cocaine-related structural abnormalities in fronto-parietal brain systems appear to contribute to impulsive behaviors, longitudinal research is critical to elucidate the mechanisms driving this link.
Highlights.
Cocaine users had broad reductions in gray matter volume in the brain.
Reduced volume in prefrontal and posterior parietal cortices correlated with impulsivity.
Trait impulsivity correlated with left lateral prefrontal and posterior parietal regions.
Behavioral impulsivity mapped onto left medial prefrontal and posterior parietal regions.
The link between brain structure and impulsivity is similar in non-cocaine users.
Acknowledgements
We thank all of the participants who were part of the original studies and the research staff who assisted with data collection.
Role of Funding Source
This study was funded by grant R01-DA045565 from the United States National Institutes of Health. The NIH had no further role in study design, data collection, analysis and interpretation of data, writing of the report, or the decision to submit the paper for publication.
Funding: This work was supported by the National Institute on Drug Abuse of the National Institutes of Health [R01-DA045565]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST STATEMENT
The authors declare that there are no conflicts of interest.
REFERENCES
- Ahrnsbrak R, Bose J, Hedden SL, Lipari RN, Park-Lee E. (2017). Key substance use and mental health indicators in the United States: results from the 2016 national survey on drug use and health. Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration; Retrieved from https://www.samhsa.gov/data/. [Google Scholar]
- Alia-Klein N, Parvaz MA, Woicik PA, Konova AB, Maloney T, Shumay E, Wang R, Telang F, Biegon A, Wang GJ, Fowler JS, Tomasi D, Volkow ND, Goldstein RZ, 2011. Gene x disease interaction on orbitofrontal gray matter in cocaine addiction. Arch. Gen. Psychiatry 68, 283–294. doi: 10.1001/archgenpsychiatry.2011.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari A, Robbins TW, 2013. Inhibition and impulsivity: behavioral and neural basis of response control. Prog. Neurobiol 108, 44–79. doi: 10.1016/j.pneurobio.2013.06.005 [DOI] [PubMed] [Google Scholar]
- Bickel WK, Jarmolowicz DP, Mueller ET, Koffarnus MN, Gatchalian KM, 2012. Excessive discounting of delayed reinforcers as a trans-disease process contributing to addiction and other disease-related vulnerabilities: emerging evidence. Pharmacol. Ther 134, 287–297. doi: 10.1016/j.pharmthera.2012.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork JM, Momenan R, Hommer DW, 2009. Delay discounting correlates with proportional lateral frontal cortex volumes. Biol. Psychiatry 65, 710–713. doi: 10.1016/j.biopsych.2008.11.023 [DOI] [PubMed] [Google Scholar]
- Butler AJ, Rehm J, Fischer B, 2017. Health outcomes associated with crack-cocaine use: Systematic review and meta-analyses. Drug Alcohol Depend. 180, 401–416. doi: 10.1016/j.drugalcdep.2017.08.036 [DOI] [PubMed] [Google Scholar]
- Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES, Munafo MR, 2013. Power failure: why small sample size undermines the reliability of neuroscience. Nature Reviews. Neuroscience. 14, 365–376. doi: 10.1038/nrn3475 [DOI] [PubMed] [Google Scholar]
- Crunelle CL, Kaag AM, van Wingen G, van den Munkhof HE, Homberg JR, Reneman L, van den Brink W, 2014. Reduced frontal brain volume in non-treatment-seeking cocaine-dependent individuals: exploring the role of impulsivity, depression, and smoking. Frontiers in Human Neuroscience. 8, 7. doi: 10.3389/fnhum.2014.00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Everitt BJ, Robbins TW, 2011. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 69, 680–694. doi: 10.1016/j.neuron.2011.01.020 [DOI] [PubMed] [Google Scholar]
- Dalley JW, Robbins TW, 2017. Fractionating impulsivity: neuropsychiatric implications. Nature Reviews. Neuroscience 18, 158–171. doi: 10.1038/nrn.2017.8 [DOI] [PubMed] [Google Scholar]
- de Wit H, 2009. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict. Biol 14, 22–31. doi: 10.1111/j.1369-1600.2008.00129.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ, 2006. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 31, 968–980. doi: 10.1016/j.neuroimage.2006.01.021 [DOI] [PubMed] [Google Scholar]
- Dorris MC, Glimcher PW, 2004. Activity in posterior parietal cortex is correlated with the relative subjective desirability of action. Neuron. 44, 365–378. doi: 10.1016/j.neuron.2004.09.009 [DOI] [PubMed] [Google Scholar]
- Enns B, Krebs E, DeBeck K, Hayashi K, Milloy MJ, Richardson L, Wood E, Nosyk B, 2017. The costs of crime associated with stimulant use in a Canadian setting. Drug Alcohol Depend. 180, 304–310. doi: 10.1016/j.drugalcdep.2017.08.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Barnes A, Jones PS, Morein-Zamir S, Robbins TW, Bullmore ET, 2011. Abnormal structure of frontostriatal brain systems is associated with aspects of impulsivity and compulsivity in cocaine dependence. Brain. 134, 2013–2024. doi: 10.1093/brain/awr138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essex BG, Clinton SA, Wonderley LR, Zald DH, 2012. The impact of the posterior parietal and dorsolateral prefrontal cortices on the optimization of long-term versus immediate value. The Journal of Neuroscience. 32, 15403–15413. doi: 10.1523/JNEUROSCI.6106-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW, 1996. Structured Clinical Interview for DSM-IV Axis I Disorders, Research Version, Patient/Non-patient Edition. Biometrics Research, New York State Psychiatric Institute, New York.
- Fuentes P, Barros-Loscertales A, Bustamante JC, Rosell P, Costumero V, Avila C, 2012. Individual differences in the behavioral inhibition system are associated with orbitofrontal cortex and precuneus gray matter volume. Cognitive, Affective & Behavioral Neuroscience. 12, 491–498. doi: 10.3758/s13415-012-0099-5 [DOI] [PubMed] [Google Scholar]
- Glasser MF, Coalson TS, Robinson EC, Hacker CD, Harwell J, Yacoub E, Ugurbil K, Andersson J, Beckmann CF, Jenkinson M, Smith SM, Van Essen DC, 2016. A multi-modal parcellation of human cerebral cortex. Natur. 536, 171–178. doi: 10.1038/nature18933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Brown SM, Williamson DE, Flory JD, de Wit H, Manuck SB, 2006. Preference for immediate over delayed rewards is associated with magnitude of ventral striatal activity. J. Neurosci 26, 13213–13217. doi: 10.1523/JNEUROSCI.3446-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayaki J, Anderson B, Stein M, 2006. Sexual risk behaviors among substance users: Relationship to impulsivity. Psychol. Addict. Behav 20, 328–332. [DOI] [PubMed] [Google Scholar]
- Huettel SA, Song AW, McCarthy G, 2005. Decisions under uncertainty: probabilistic context influences activation of prefrontal and parietal cortices. J. Neurosci 25, 3304–3311. doi: 10.1523/JNEUROSCI.5070-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- John WS, Wu LT, 2017. Trends and correlates of cocaine use and cocaine use disorder in the United States from 2011 to 2015. Drug Alcohol Depend. 180, 376–384. doi: 10.1016/j.drugalcdep.2017.08.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby KN, Petry NM, Bickel WK, 1999. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J. Exp. Psychol. Gen 128, 78–87. [DOI] [PubMed] [Google Scholar]
- Korponay C, Dentico D, Kral T, Ly M, Kruis A, Goldman R, Lutz A, Davidson RJ, 2017. Neurobiological correlates of impulsivity in healthy adults: lower prefrontal gray matter volume and spontaneous eye-blink rate but greater resting-state functional connectivity in basal ganglia-thalamo-cortical circuitry. NeuroImage. 157, 288–296. doi: 10.1016/j.neuroimage.2017.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Eickhoff SB, Turner JA, Ray KL, McKay DR, Glahn DC, Beckmann CF, Smith SM, Fox PT, 2011. Behavioral interpretations of intrinsic connectivity networks. J. Cogn. Neurosci 23, 4022–4037. doi: 10.1162/jocn_a_00077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louie K, Glimcher PW, 2010. Separating value from choice: delay discounting activity in the lateral intraparietal area. The Journal of Neuroscience. 30, 5498–5507. doi: 10.1523/JNEUROSCI.5742-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucantonio F, Stalnaker TA, Shaham Y, Niv Y, Schoenbaum G, 2012. The impact of orbitofrontal dysfunction on cocaine addiction. Nat. Neurosci 15, 358–366. doi: 10.1038/nn.3014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey S, Paulus M, 2013. Are there volumetric brain differences associated with the use of cocaine and amphetamine-type stimulants? Neurosci. Biobehav. Rev 37, 300–316. doi: 10.1016/j.neubiorev.2012.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J, Amlung MT, Few LR, Ray LA, Sweet LH, Munafò MR, 2011. Delayed reward discounting and addictive behavior: a meta-analysis. Psychopharmacology. 216, 305–321. doi: 10.1007/s00213-011-2229-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majdandzic J, Grol MJ, van Schie HT, Verhagen L, Toni I, Bekkering H, 2007. The role of immediate and final goals in action planning: an fMRI study. NeuroImage. 37, 589–598. doi: 10.1016/j.neuroimage.2007.04.071 [DOI] [PubMed] [Google Scholar]
- Massar SAA, Libedinsky C, Weiyan C, Huettel SA, Chee MWL, 2015. Separate and overlapping brain areas encode subjective value during delay and effort discounting. NeuroImage. 120, 104–113. doi: 10.1016/j.neuroimage.2015.06.080 [DOI] [PubMed] [Google Scholar]
- Matsuo K, Nicoletti M, Nemoto K, Hatch JP, Peluso MA, Nery FG, Soares JC, 2009. A voxel-based morphometry study of frontal gray matter correlates of impulsivity. Hum. Brain Mapp 30, 1188–1195. doi: 10.1002/hbm.20588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur James E. (1987). An adjusting procedure for studying delayed reinforcement In Commons ML, Mazur JE, Nevin JA & Rachlin H. (Eds.), The Effect of Delay and of Intervening Events on Reinforcement Value (pp. 55–73). Hillsdale, NJ: Lawrence Erlbaum Associates, Inc. [Google Scholar]
- McClure SM, Ericson KM, Laibson DI, Loewenstein G, Cohen JD, 2007. Time discounting for primary rewards. The Journal of Neuroscience 27, 5796–5804. doi: 10.1523/JNEUROSCI.4246-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure SM, Laibson DI, Loewenstein G, Cohen JD, 2004. Separate neural systems value immediate and delayed monetary rewards. Science. 306, 503–507. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M, 1992. The fifth edition of the Addiction Severity Index. J. Subst. Abuse Treat 9, 199–213. doi: 10.1016/0740-5472(92)90062-S [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD, 2001. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci 24, 167–202. [DOI] [PubMed] [Google Scholar]
- Mobini S, Body S, Ho MY, Bradshaw CM, Szabadi E, Deakin JF, Anderson IM, 2002. Effects of lesions of the orbitofrontal cortex on sensitivity to delayed and probabilistic reinforcement. Psychopharmacology. 160, 290–298. doi: 10.1007/s00213-001-0983-0 [DOI] [PubMed] [Google Scholar]
- Mohammadi B, Hammer A, Miedl SF, Wiswede D, Marco-Pallares J, Herrmann M, Munte TF, 2016. Intertemporal choice behavior is constrained by brain structure in healthy participants and pathological gamblers. Brain Structure & Function. 221, 3157–3170. doi: 10.1007/s00429-015-1093-9 [DOI] [PubMed] [Google Scholar]
- Morein-Zamir S, Robbins TW, 2015. Fronto-striatal circuits in response-inhibition: Relevance to addiction. Brain Res. 1628, 117–129. doi: 10.1016/j.brainres.2014.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Lopez L, Catena A, Fernandez-Serrano MJ, Delgado-Rico E, Stamatakis EA, Perez-Garcia M, Verdejo-Garcia A, 2012. Trait impulsivity and prefrontal gray matter reductions in cocaine dependent individuals. Drug Alcohol Depend. 125, 208–214. doi: 10.1016/j.drugalcdep.2012.02.012 [DOI] [PubMed] [Google Scholar]
- Newman SD, Carpenter PA, Varma S, Just MA, 2003. Frontal and parietal participation in problem solving in the Tower of London: fMRI and computational modeling of planning and high-level perception. Neuropsychologia. 41, 1668–1682. doi: 10.1016/s0028-3932(03)00091-5 [DOI] [PubMed] [Google Scholar]
- Owens MM, Gray JC, Amlung MT, Oshri A, Sweet LH, MacKillop J, 2017. Neuroanatomical foundations of delayed reward discounting decision making. NeuroImage. 161, 261–270. doi: 10.1016/j.neuroimage.2017.08.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. 1995. Factor structure of the Barratt Impulsiveness Scale. J. Clin. Psychol 51, 768–774. [DOI] [PubMed] [Google Scholar]
- Pereira RB, Andrade PB, Valentao P, 2015. A Comprehensive view of the neurotoxicity mechanisms of cocaine and ethanol. Neurotoxicity Research. 28, 253–267. doi: 10.1007/s12640-015-9536-x [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Baker CI, Durnez J, Gorgolewski KJ, Matthews PM, Munafo MR, Nichols TE, Poline JB, Vul E, Yarkoni T, 2017. Scanning the horizon: towards transparent and reproducible neuroimaging research. Nature Reviews. Neuroscience. 18, 115–126. doi: 10.1038/nrn.2016.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachlin H, Green L, 1972. Commitment, choice and self-control. J Exp Anal Behav. 17, 15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyna VF, Huettel SA. (2014). Reward, representation, and impulsivity: A theoretical framework for the neuroscience of risky decision making In Reyna VF & Zayas V. (Eds.), The Neuroscience of Risky Decision Making (pp. 11–42). Washington, DC: American Psychological Association. [Google Scholar]
- Robbins TW, Gillan CM, Smith DG, de Wit S, Ersche KD, 2012. Neurocognitive endophenotypes of impulsivity and compulsivity: towards dimensional psychiatry. Trends Cogn. Sci 16, 81–91. doi: 10.1016/j.tics.2011.11.009 [DOI] [PubMed] [Google Scholar]
- Robinson SM, Sobell LC, Sobell MB, Leo GI, 2014. Reliability of the Timeline Followback for cocaine, cannabis, and cigarette use. Psychol. Addict. Behav 28, 154–162. doi: 10.1037/a0030992 [DOI] [PubMed] [Google Scholar]
- Sim ME, Lyoo IK, Streeter CC, Covell J, Sarid-Segal O, Ciraulo DA, Kim MJ, Kaufman MJ, Yurgelun-Todd DA, Renshaw PF, 2007. Cerebellar gray matter volume correlates with duration of cocaine use in cocaine-dependent subjects. Neuropsychopharmacology. 32, 2229–2237. doi: 10.1038/sj.npp.1301346 [DOI] [PubMed] [Google Scholar]
- Siniscalchi A, Bonci A, Mercuri NB, De Siena A, De Sarro G, Malferrari G, Diana M, Gallelli L, 2015. Cocaine dependence and stroke: pathogenesis and management. Current Neurovascular Research. 12, 163–172. [DOI] [PubMed] [Google Scholar]
- Smith JL, Mattick RP, Jamadar SD, Iredale JM, 2014. Deficits in behavioural inhibition in substance abuse and addiction: a meta-analysis. Drug Alcohol Depend. 145, 1–33. doi: 10.1016/j.drugalcdep.2014.08.009 [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE, 2009. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage. 44, 83–98. doi: 10.1016/j.neuroimage.2008.03.061 [DOI] [PubMed] [Google Scholar]
- Spronk DB, van Wel JHP, Ramaekers JG, Verkes RJ, 2013. Characterizing the cognitive effects of cocaine: A comprehensive review. Neurosci. Biobehav. Rev 37, 1838–1859. doi: 10.1016/j.neubiorev.2013.07.003 [DOI] [PubMed] [Google Scholar]
- Stanford MS, Mathias CW, Dougherty DM, Lake SL, Anderson NE, Patton JH, 2009. Fifty years of the Barratt Impulsiveness Scale: An update and review. Pers Individ Dif. 47, 385–395. doi: 10.1016/j.paid.2009.04.008 [DOI] [Google Scholar]
- Towe SL, Hobkirk AL, Ye DG, Meade CS, 2015. Adaptation of the Monetary Choice Questionnaire to accommodate extreme monetary discounting in cocaine users. Psychol. Addict. Behav 29, 1048–1055. doi: 10.1037/adb0000101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNODC. (2018). World Drug Report 2018. United Nations; Retrieved from https://www.unodc.org/wdr2018/prelaunch/WDR18_Booklet_3_DRUG_MARKETS.pdf. [Google Scholar]
- Wang Q, Chen C, Cai Y, Li S, Zhao X, Zheng L, Zhang H, Liu J, Chen C, Xue G, 2016. Dissociated neural substrates underlying impulsive choice and impulsive action. NeuroImage. 134, 540–549. doi: 10.1016/j.neuroimage.2016.04.010 [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Cardinal RN, Robbins TW, 2004. Contrasting roles of basolateral amygdala and orbitofrontal cortex in impulsive choice. J. Neurosci 24, 4718–4722. doi: 10.1523/JNEUROSCI.5606-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip SW, Worhunsky PD, Xu J, Morie KP, Constable RT, Malison RT, Carroll KM, Potenza MN, 2018. Gray-matter relationships to diagnostic and transdiagnostic features of drug and behavioral addictions. Addict. Biol 23, 394–402. doi: 10.1111/adb.12492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Geng X, Lee TMC, 2017. Large-scale functional neural network correlates of response inhibition: an fMRI meta-analysis. Brain Structure & Function. 222, 3973–3990. doi: 10.1007/s00429-017-1443-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Freedman DJ, 2019. Posterior parietal cortex plays a causal role in perceptual and categorical decisions. Science. 365, 180–185. doi: 10.1126/science.aaw8347 [DOI] [PMC free article] [PubMed] [Google Scholar]