Abstract
Background:
To optimize treatment and prevent cardiovascular disease in subjects with type 1 diabetes, it is important to determine how cholesterol metabolism changes with type 1 diabetes.
Objective:
To compare plasma levels of campesterol and β-sitosterol, markers of cholesterol absorption, as well as lathosterol, a marker of cholesterol synthesis, in youth with and without type 1 diabetes.
Methods:
Serum samples were obtained from adolescent subjects with type 1 diabetes [n= 175, mean age 15.2 years, mean duration of diabetes 8.2 years] and without diabetes [n=74, mean age 15.4 years]. Campesterol, β-sitosterol, and lathosterol, were measured using targeted liquid chromatography tandem mass spectrometry, normalized to the control serum mean, and expressed in arbitrary units. The markers were then compared between groups and correlated with the available cardiometabolic variables.
Results:
Campesterol and β-sitosterol levels were 30% higher in subjects with type 1 diabetes and positively correlated with hemoglobin A1c levels. In contrast, lathosterol levels were 20% lower in subjects with type 1 diabetes and positively correlated with triglycerides, body mass index, and systolic blood pressure.
Conclusion:
Plasma markers suggest that cholesterol absorption is increased whereas cholesterol synthesis is decreased in type 1 diabetes. Further studies to address the impact of these changes on the relative efficacy of cholesterol absorption and synthesis inhibitors in subjects with type 1 diabetes are urgently needed.
Keywords: cholesterol metabolism, dyslipidemia, cholesterol-lowering therapy, cardiovascular disease risk, youth, type 1 diabetes
Introduction
The risk of cardiovascular disease (CVD) in subjects with type 1 diabetes (T1D) remains exceedingly high, up to 30-fold higher than in those without diabetes in some populations1, despite the numerous recent advancements in therapy2. Furthermore, lifespan is compromised by 11–13 years in subjects with T1D compared to non-diabetic subjects, with CVD as the main cause of this discrepancy3,4. Therefore, an important goal of treatment for T1D subjects is the prevention of CVD.
The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) trial showed conclusively that intensive insulin treatment lowers cardiovascular risk5, and, indeed, subjects with good glycemic control show lipid profiles comparable to controls6,7. However, most T1D subjects do not achieve their glycemic target1, and poor control is associated with high rates of dyslipidemia, including elevated total cholesterol (TC), LDL-cholesterol (LDL-C), non-HDL cholesterol, and ApoB levels8–12. Thus, lipid-lowering drugs can become very important. Indeed, up to 30% of the excess cardiovascular death associated with poor control may be secondary to high cholesterol levels13.
Cholesterol synthesis inhibitors (statin drugs) have generally been found to be much more effective in lipid-lowering than cholesterol absorption inhibitors (such as ezetimibe): thus, statins reduced LDL-C levels by 32–38% in non-diabetic and type 2 diabetic subjects, whereas ezetimibe only reduced LDL-C by 15–19%14–16. Similarly, both adults and adolescents with hypercholesterolemia showed a 35–50% reduction in LDL-C with statins, but a 19–42% reduction with ezetimibe17–21.
Large studies comparing the effectiveness of statins versus ezetimibe in T1D are lacking. T1D subjects appeared to respond to statins, as they showed the same reduction in CVD for a given reduction in LDL as type 2 diabetic subjects22. However, a direct comparison of the two drugs in a small cohort of patients showed ezetimibe to be more effective than atorvastatin in lowering LDL-C in T1D subjects (32% vs 19% reduction, respectively)23.
These studies point to potential differences in efficacy of different lipid-lowering drugs in T1D subjects. In this regard, it is important to consider the possibility that subjects with T1D may have unique changes in cholesterol metabolism that alter the relative effectiveness of different drugs. Rodent models of T1D show increased cholesterol absorption but decreased cholesterol synthesis24–29. Consistent with this, intensive insulin treatment lowers cholesterol absorption in T1D subjects30. Moreover, several excellent studies have found increased cholesterol absorption and decreased cholesterol synthesis in T1D patients30–33. However, limitations in sample size have precluded their generalizability.
Here, we measured markers of cholesterol synthesis and absorption in a large cohort of adolescent subjects with T1D and similar-aged controls34. The use of an adolescent cohort enabled us to avoid the confounding effects of lipid-lowering drugs, as this population is largely drug-naïve.
Methods
Study Population
As previously described34, the study population consisted of individuals with T1D and individuals in the Denver area with no chronic disease who participated in a study of cardiovascular risk factors at the Barbara Davis Center for Childhood Diabetes. All subjects were 12–20 years of age. Individuals with T1D (diagnosed by the presence of islet cell antibodies or provider clinical diagnosis), had diabetes duration > 5 years. For the current study, all subjects (n=252) with plasma samples and data available for fasting metabolic measurements and lipid parameters were included. Subjects who reported taking statin medications at the time of the study (n=3) were excluded from the analyses since these medications have been shown to increase cholesterol synthesis35. The study was approved by the Colorado Multiple Institution Review Board, and informed consent and assent (for subjects <18 years of age) were obtained in all subjects prior to participation in the study.
Anthropometric Measures and Laboratory Assays
Anthropometric measures were obtained during study visits, including height to the nearest 0.1 cm, and weight to the nearest 0.1 kg using a Detecto scale (Detecto, Webb City, Missouri). Subjects fasted for at least 8 hours prior to the study visit. Hemoglobin A1c (HbA1c) was measured on the DCA Vantage (Siemens, Princeton, New Jersey) at the Children’s Hospital Colorado lab. Lipid parameters, including total cholesterol, high-density lipoprotein cholesterol (HDL-C), and triglycerides (TG), were obtained in the Clinical Translational Research Core lab using a Beckman Coulter AU system (Beckman Coulter Inc, Brea, CA). LDL-C was calculated using the Friedwald formula (no subjects showed TG >400mg/dL). High-sensitivity C-reactive protein (Hs-CRP) was measured at the Children’s Hospital Colorado lab using a multiplex assay platform Siemens BNII Nephelometer (Siemens, Princeton, New Jersey).
Sample preparation and mass spectrometry analysis
Sterols were extracted from serum and derivatized to picolinyl esters as previously described36. Dried sterol derivatives were reconstituted in acetonitrile and analyzed using a targeted liquid chromatography tandem mass spectrometry method operated on an Agilent 1290 U-HPLC coupled to Agilent 6495 triple quadrupole mass spectrometer. Briefly, samples (10 µL) were injected onto a Hypersil GOLD column (150x 2.1 mm, 3µm, Thermo Electron) that was eluted at flow rate of 300 µL/min with acetonitrile/methanol/ water (40/40/20, v/v/v) with 0.1% acetic acid (mobile phase A) for 0.5 minutes followed by a linear gradient to acetonitrile/methanol/water (45/45/10, v/v/v) with 0.1% acetic acid (mobile phase B) over 19.5 minutes and held for 21 minutes. Mass spectra were acquired using electrospray ionization in the positive ion mode and using dynamic multiple reaction monitoring scanning. Collision energies and precursor-to-product ion transitions were determined using derivatized authentic reference standards. Mass spectrometer setting were: 3.5 kV, ionization voltage; 200°C, gas temperature; 14 L/min, gas flow; 40 psi, nebulizer pressure; 325°C, sheath gas temperature; 11 L/min, sheath gas flow; 0.5kV, nozzle voltage; 150, high pressure RF; 90, low pressure RF. Peak integration was performed using Agilent MassHunter software (Agilent, G3336AA); the area under the curve was normalized to a standardized pooled serum sample and values were reported as arbitrary units (A.U.).
Biostatistical analysis
Values are reported as mean ± standard deviation (range) or number (% from total). Sterol values were normalized to the control mean value. Bivariate Spearman correlations were calculated between cholesterol absorption and synthesis markers and potential covariates (HbA1c, fasting glucose, C-peptide, BMI, Hs-CRP, TG, TC, LDL-C, HDL-C, systolic and diastolic blood pressure). Significance was determined using Student’s t-test, Fisher’s exact test, or two-way ANOVA. P-value lower than 0.05 was considered significant for all analyses; n=249 total, 74 controls, 175 T1D for all measurements, except for HbA1c (n=246 total, 71 controls), C-peptide and Hs-CRP (n=248 total, 73 controls).
Results
Demographic, anthropometric, and cardiovascular risk factors by study group.
There were no statistically-significant differences by age, sex, BMI, Hs-CRP, and tobacco use between the groups. As expected, HbA1c (p<0.001) and fasting plasma glucose levels (p<0.001) were significantly higher, and C-peptide levels were significantly lower (p<0.001) in T1D subjects. TG levels were similar between controls and T1D subjects. In contrast, TC (p<0.01), LDL-C (p<0.05), HDL-C (p<0.05), systolic blood pressure (SBP, p<0.001) and diastolic blood pressure (DBP, p<0.001) were significantly higher in T1D subjects (Table 1).
Table 1.
Demographics and clinical parameters of subjects with T1D and non-diabetic controls. Values are presented as mean ± SD (range) or n (%).
| Control (n=74) | T1D (n=175) | p-value* | |
|---|---|---|---|
| Age (yrs) | 15.4 ± 2.2 (12.0 − 20.0) | 15.2 ± 2.2 (12.0 − 19.4) | 0.547 |
| Sex (%female) | 42 F/ 32 M (56.8%) | 99 F/ 76 M (56.6%) | 1.000 |
| Tobacco Use Now | 6 (8.1%) | 12 (6.9%) | 0.790 |
| HbA1c (%) | 5.3 ± 0.3 (4.6 − 5.9) | 9.0 ± 1.6 (5.8 − 14.0) | < 0.001 |
| Fasting glucose (mg/dL) | 82±7(64 − 99) | 185 ± 80 (40 − 451) | < 0.001 |
| C-peptide (ng/mL) | 1.7 ± 0.6 (0.1 − 3.3) | 0.1 ± 0.1 (0.1 − 1.3) | < 0.001 |
| BMI (kg/m2) | 21.8 ± 4.2 (13.8 − 33.5) | 22.4 ± 3.4 (15.3 − 35.3) | 0.259 |
| Hs-CRP (mg/dL) | 0.9 ±1.4 (0.0 – 6.4) | 1.3 ± 2.3 (0.1 – 22.0) | 0.084 |
| TG (mg/dL) | 83.5 ± 41.5 (34.0 − 235.0) | 86.8 ± 50.5 (28.0 − 333.0) | 0.586 |
| TC (mg/dL) | 147.7 ± 28.0 (96.0 − 235.0) | 159.4 ± 34.8 (89.0 − 347.0) | 0.006 |
| LDL-C (mg/dL) | 82.4 ± 22.9 (43.0 − 168.0) | 89.9 ± 27.8 (42.0 − 250.0) | 0.028 |
| HDL-C (mg/dL) | 48.6 ± 9.3 (28.0 − 74.0) | 52.1 ± 10.6 (30.0 − 81.0) | 0.010 |
| SBP (mmHg) | 109.0 ± 8.5 (89.0 − 129.0) | 113.1 ± 8.5 (93.0 − 137.0) | < 0.001 |
| DBP (mmHg) | 64.3 ± 6.1 (49.0 − 79.0) | 69.0 ± 6.5 (50.0 − 89.0) | < 0.001 |
| Campesterol (A.U.) | 1.00 ± 0.35 (0.41 – 1.95) | 1.31 ± 0.41 (0.47 – 2.60) | < 0.001 |
| β-Sitosterol (A.U.) | 1.00 ± 0.36 (0.39 – 2.25) | 1.29 ± 0.44 (0.50 – 2.72) | < 0.001 |
| Lathosterol (A.U.) | 1.00 ± 0.51 (0.32 – 2.52) | 0.79 ± 0.38 (0.26 – 2.77) | 0.001 |
p-value based on t-tests or Fisher’s exact test. Boldface type highlights statistical significance with p<0.05.
Cholesterol absorption and synthesis markers.
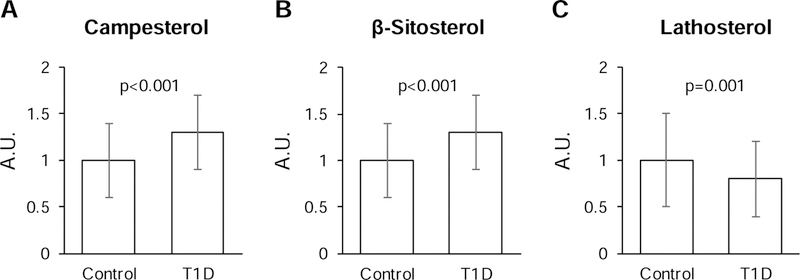
The serum levels of campesterol and β-sitosterol (cholesterol absorption markers), were 30% higher in T1D subjects compared to controls (p<0.001, Table 1 and Figure 1). In contrast, the serum levels of lathosterol (cholesterol synthesis marker) were 20% lower in T1D subjects compared to controls. These markers also differed significantly when comparing T1D to controls for male and for female subjects separately (p<0.05), but not when comparing male and female subjects within the T1D and control groups (p>0.05) (Supplemental Figure 1).
Figure 1.
Abundance of markers of cholesterol absorption (A, B) and synthesis (C) in T1D subjects and non-diabetic controls. Values are presented as mean ± SD; *p-value based on t-tests. A.U., arbitrary units.
In the complete cohort, serum levels of campesterol and β-sitosterol correlated positively with HbA1c and fasting glucose, and negatively with C-peptide. With regard to plasma lipids, campesterol and β-sitosterol were positively associated with TC, LDL-C, and HDL-C. Both markers were negatively associated with BMI, while β-sitosterol was negatively associated with SBP. When the T1D and control subjects were analyzed separately, the association with HbA1c remained significant in T1D subjects only. However, the associations with TC, LDL-C, and HDL-C remained significant in both groups. A significant negative association with Hs-CRP emerged for both campesterol and β-sitosterol in the control group only (Table 2).
Table 2.
Spearman correlation coefficients (p-value) for the markers of cholesterol absorption and clinical parameters in subjects with T1D and non-diabetic controls. Boldface type highlights statistical significance with p<0.05; asterisk indicates statistically-significant difference between controls and T1D subjects.
| Campesterol | β-Sitosterol | ||||||
|---|---|---|---|---|---|---|---|
| All (n=249) | Control (n=74) | T1D (n=175) | All (n=249) | Control (n=74) | T1D (n=175) | ||
| HbA1c (%) | 0.41 (<0.001) | 0.11 (0.379) | 0.25 (0.001)* | 0.35 (<0.001) | 0.06 (0.636) | 0.20 (0.007)* | |
| Fasting glucose (mg/dL) | 0.2 (0.002) | −0.04 (0.742) | −0.03 (0.668) | 0.18 (0.005) | −0.14 (0.233) | −0.03 (0.680) | |
| C-peptide (ng/mL) | −0.35 (<0.001) | −0.21 (0.080) | 0.06 (0.414) | −0.32 (<0.001) | −0.24 (0.040)* | 0.01 (0.889) | |
| BMI (kg/m2) | −0.18 (0.005) | −0.36 (0.002) | −0.15 (0.049) | −0.22 (<0.001) | −0.41 (<0.001) | −0.18 (0.020) | |
| Hs-CRP (mg/dL) | −0.04 (0.539) | −0.24 (0.044)* | −0.04 (0.582) | −0.05 (0.420) | −0.26 (0.026)* | −0.04 (0.628) | |
| TG (mg/dL) | −0.004 (0.951) | −0.12 (0.294) | 0.06 (0.467) | 0.01 (0.831) | −0.11 (0.329) | 0.07 (0.363) | |
| TC (mg/dL) | 0.37 (<0.001) | 0.33 (0.004) | 0.33 (<0.001) | 0.38 (<0.001) | 0.31 (0.007) | 0.36 (<0.001) | |
| LDL-C (mg/dL) | 0.33 (<0.001) | 0.37 (0.001) | 0.28 (<0.001) | 0.33 (<0.001) | 0.31 (0.007) | 0.30 (<0.001) | |
| HDL-C (mg/dL) | 0.26 (<0.001) | 0.26 (0.023) | 0.2 (0.008) | 0.28 (<0.001) | 0.28 (0.014) | 0.24 (0.002) | |
| SBP (mmHg) | −0.11 (0.085) | −0.23 (0.050) | −0.17 (0.022) | −0.14 (0.022) | −0.30 (0.009) | −0.19 (0.014) | |
| DBP (mmHg) | 0.08 (0.184) | −0.10 (0.404) | 0.01 (0.847) | 0.04 (0.549) | −0.14 (0.232) | −0.02 (0.760) | |
Serum levels of lathosterol correlated negatively with HbA1c and positively with C-peptide. Lathosterol was positively correlated with Hs-CRP, particularly in the controls; SBP and BMI in all subjects; and DBP in T1D subjects. For the lipids, lathosterol was not correlated with HDL-C, but was positively correlated with TG. On the other hand, lathosterol, like campesterol and β-sitosterol, was positively correlated with TC and LDL-C in all subjects (Table 3).
Table 3.
Spearman correlation coefficients (p-value) for the marker of cholesterol synthesis and clinical parameters in subjects with T1D and non-diabetic controls. Boldface type highlights statistical significance with p<0.05; asterisk indicates statistically-significant difference between controls and T1D subjects.
| Lathosterol | |||
|---|---|---|---|
| All (n=249) | Control (n=74) | T1D (n=175) | |
| HbA1c (%) | −0.13 (0.046) | −0.17 (0.149) | 0.10 (0.188) |
| Fasting glucose (mg/dL) | −0.04 (0.581) | 0.16 (0.162) | 0.11 (0.143) |
| C-peptide (ng/mL) | 0.22 (<0.001) | 0.34 (0.003)* | −0.03 (0.666) |
| BMI (kg/m2) | 0.36 (<0.001) | 0.58 (<0.001) | 0.31 (<0.001) |
| Hs-CRP (mg/dL) | 0.18 (0.004) | 0.61 (<0.001)* | 0.05 (0.525) |
| TG (mg/dL) | 0.39 (<0.001) | 0.56 (<0.001) | 0.35 (<0.001) |
| TC (mg/dL) | 0.28 (<0.001) | 0.46 (<0.001) | 0.29 (<0.001) |
| LDL-C (mg/dL) | 0.23 (<0.001) | 0.30 (0.008) | 0.27 (<0.001) |
| HDL-C (mg/dL) | −0.03 (0.608) | 0.08 (0.492) | −0.04 (0.630) |
| SBP (mmHg) | 0.25 (<0.001) | 0.34 (0.003) | 0.30 (<0.001) |
| DBP (mmHg) | 0.12 (0.057) | 0.21 (0.077) | 0.21 (0.006) |
Discussion
In a cohort of 175 T1D and 74 control subjects, we find that T1D is associated with higher campesterol and β-sitosterol and lower lathosterol. While campesterol, β-sitosterol, and lathosterol are all associated with TC and LDL-C levels in the complete cohort, campesterol and β-sitosterol are positively correlated with HbA1c and fasting glucose levels, and lathosterol is positively correlated with C-peptide, TG, BMI, SBP, and DBP. Moreover, Hs-CRP is negatively associated with campesterol and β-sitosterol in controls, but positively associated with lathosterol.
These findings extend previous studies to a larger, younger cohort30, and confirm a positive correlation between markers of cholesterol synthesis with TG, BMI, SBP, and DBP33,37,38. In addition, they document a positive correlation between markers of cholesterol absorption and HbA1c not observed in previous studies39,40, perhaps because of the inclusion of subjects treated with medications that could interfere with cholesterol absorption41,42.
An important limitation of the present study is the use of serum markers of cholesterol synthesis and absorption rather than direct measurements with isotope tracers. Increases in dietary plant sterol content or reduced biliary sterol secretion could increase plasma β-sitosterol and campesterol levels, independently of intestinal absorption. In addition, β-sitosterol and campesterol are associated with HDL31; therefore, their increased abundance in T1D subjects could be due, to some degree, to their increased HDL levels. Nonetheless, serum markers correlate well with direct synthesis and absorption measurements43–47, which were not practical in a cohort of this size.
These data raise the possibility that drugs that reduce cholesterol absorption may be relatively more effective in T1D subjects than non-diabetic and T2D subjects14–16. Thus, additional, large-scale studies in T1D subjects, comparing the effectiveness of cholesterol absorption inhibitors versus cholesterol synthesis inhibitors in lowering TC and LDL-C, as well as CVD risk, are urgently needed48.
Supplementary Material
Cholesterol absorption markers are increased in type 1 diabetes
Cholesterol synthesis markers are decreased in type 1 diabetes
Cholesterol absorption markers correlate with HbA1c in type 1 diabetes
Cholesterol synthesis markers correlate with BMI, TG, and BP in type 1 diabetes
Acknowledgements
This study was supported by the Juvenile Diabetes Research Foundation (grant 11-2007-694), National Institute of Diabetes and Digestive and Kidney Diseases (grant DK075360), NIH/NCRR Colorado CTSI grant UL1 RR025780, American Diabetes Association grant 9-18-CVD1-003 (IS), NIH training grant No. T32 DK007260 (JK), National Institute of Diabetes and Digestive and Kidney Diseases grants K23 DK075360 and P30 DK116074 (DMM), National Institute of Health National Heart, Lung, and Blood Institute grant R01-HL-109650 (SBB), American Heart Association Established Investigator Award (SBB), National Institute of Diabetes and Digestive and Kidney Diseases grant 5K12-DK-094721-04 (AEL), and a SPARC grant from the Broad Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest: none
References
- 1.Miller RG et al. A Contemporary Estimate of Total Mortality and Cardiovascular Disease Risk in Young Adults With Type 1 Diabetes: The Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes care 39, 2296–2303, doi: 10.2337/dc16-1162 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lind M et al. Glycemic control and excess mortality in type 1 diabetes. The New England journal of medicine 371, 1972–1982, doi: 10.1056/NEJMoa1408214 (2014). [DOI] [PubMed] [Google Scholar]
- 3.Livingstone SJ et al. Estimated life expectancy in a Scottish cohort with type 1 diabetes, 2008–2010. JAMA : the journal of the American Medical Association 313, 37–44, doi: 10.1001/jama.2014.16425 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huo L, Harding JL, Peeters A, Shaw JE & Magliano DJ Life expectancy of type 1 diabetic patients during 1997–2010: a national Australian registry-based cohort study. Diabetologia 59, 1177–1185, doi: 10.1007/s00125-015-3857-4 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Nathan DM et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. The New England journal of medicine 353, 2643–2653, doi: 10.1056/NEJMoa052187 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lipid and lipoprotein levels in patients with IDDM diabetes control and complication. Trial experience. The DCCT Research Group. Diabetes care 15, 886–894, doi: 10.2337/diacare.15.7.886 (1992). [DOI] [PubMed] [Google Scholar]
- 7.Sosenko JM, Breslow JL, Miettinen OS & Gabbay KH Hyperglycemia and plasma lipid levels: a prospective study of young insulin-dependent diabetic patients. The New England journal of medicine 302, 650–654, doi: 10.1056/NEJM198003203021202 (1980). [DOI] [PubMed] [Google Scholar]
- 8.Guy J et al. Lipid and lipoprotein profiles in youth with and without type 1 diabetes: the SEARCH for Diabetes in Youth case-control study. Diabetes care 32, 416–420, doi: 10.2337/dc08-1775 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maahs DM et al. Longitudinal lipid screening and use of lipid-lowering medications in pediatric type 1 diabetes. The Journal of pediatrics 150, 146–150, 150 e141–142, doi: 10.1016/j.jpeds.2006.10.054 (2007). [DOI] [PubMed] [Google Scholar]
- 10.Petitti DB et al. Serum lipids and glucose control: the SEARCH for Diabetes in Youth study. Arch Pediatr Adolesc Med 161, 159–165, doi: 10.1001/archpedi.161.2.159 (2007). [DOI] [PubMed] [Google Scholar]
- 11.Vaid S, Hanks L, Griffin R & Ashraf AP Body mass index and glycemic control influence lipoproteins in children with type 1 diabetes. J Clin Lipidol 10, 1240–1247, doi: 10.1016/j.jacl.2016.07.010 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwab KO et al. Spectrum and prevalence of atherogenic risk factors in 27,358 children, adolescents, and young adults with type 1 diabetes: cross-sectional data from the German diabetes documentation and quality management system (DPV). Diabetes care 29, 218–225, doi: 10.2337/diacare.29.02.06.dc05-0724 (2006). [DOI] [PubMed] [Google Scholar]
- 13.Bebu I et al. The relationship of blood glucose with cardiovascular disease is mediated over time by traditional risk factors in type 1 diabetes: the DCCT/EDIC study. Diabetologia 60, 2084–2091, doi: 10.1007/s00125-017-4374-4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guyton JR et al. Achievement of recommended lipid and lipoprotein levels with combined ezetimibe/statin therapy versus statin alone in patients with and without diabetes. Diab Vasc Dis Res 8, 160–172, doi: 10.1177/1479164111406457 (2011). [DOI] [PubMed] [Google Scholar]
- 15.Winkler K, Jacob S, Muller-Schewe T, Hoffmann MM & Konrad T Ezetimibe alone and in combination lowers the concentration of small, dense low-density lipoproteins in type 2 diabetes mellitus. Atherosclerosis 220, 189–193, doi: 10.1016/j.atherosclerosis.2011.10.043 (2012). [DOI] [PubMed] [Google Scholar]
- 16.Lakoski SG et al. Indices of cholesterol metabolism and relative responsiveness to ezetimibe and simvastatin. The Journal of clinical endocrinology and metabolism 95, 800–809, doi: 10.1210/jc.2009-1952 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.John CC, Regier MD, Lilly CL & Aly S Long-term pharmacotherapy for elevated low density lipoprotein levels in children: A retrospective analysis. J Clin Lipidol 10, 265–272, doi: 10.1016/j.jacl.2015.11.012 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clauss S, Wai KM, Kavey RE & Kuehl K Ezetimibe treatment of pediatric patients with hypercholesterolemia. The Journal of pediatrics 154, 869–872, doi: 10.1016/j.jpeds.2008.12.044 (2009). [DOI] [PubMed] [Google Scholar]
- 19.Yeste D et al. Ezetimibe as monotherapy in the treatment of hypercholesterolemia in children and adolescents. Journal of pediatric endocrinology & metabolism : JPEM 22, 487–492 (2009). [DOI] [PubMed] [Google Scholar]
- 20.Farnier M et al. Effects of ezetimibe, simvastatin and ezetimibe/simvastatin on correlations between apolipoprotein B, LDL cholesterol and non-HDL cholesterol in patients with primary hypercholesterolemia. Atherosclerosis 229, 415–422, doi: 10.1016/j.atherosclerosis.2013.05.010 (2013). [DOI] [PubMed] [Google Scholar]
- 21.Avis HJ et al. Efficacy and safety of rosuvastatin therapy for children with familial hypercholesterolemia. Journal of the American College of Cardiology 55, 1121–1126, doi: 10.1016/j.jacc.2009.10.042 (2010). [DOI] [PubMed] [Google Scholar]
- 22.Cholesterol Treatment Trialists C et al. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet 371, 117–125, doi: 10.1016/S0140-6736(08)60104-X (2008). [DOI] [PubMed] [Google Scholar]
- 23.Ciriacks K, Coly G, Krishnaswami S, Patel SB & Kidambi S Effects of simvastatin and ezetimibe in lowering low-density lipoprotein cholesterol in subjects with type 1 and type 2 diabetes mellitus. Metab Syndr Relat Disord 13, 84–90, doi: 10.1089/met.2014.0114 (2015). [DOI] [PubMed] [Google Scholar]
- 24.Uchida K et al. Altered bile acid metabolism in alloxan diabetic rats. Jpn.J Pharmacol 29, 553–562 (1979). [DOI] [PubMed] [Google Scholar]
- 25.Akiyoshi T, Uchida K, Takase H, Nomura Y & Takeuchi N Cholesterol gallstones in alloxan-diabetic mice. J Lipid.Res 27, 915–924 (1986). [PubMed] [Google Scholar]
- 26.Uchida K, Makino S & Akiyoshi T Altered bile acid metabolism in nonobese, spontaneously diabetic (NOD) mice. Diabetes 34, 79–83 (1985). [DOI] [PubMed] [Google Scholar]
- 27.Milliat F et al. Short and long-term effects of streptozotocin on dietary cholesterol absorption, plasma lipoproteins and liver lipoprotein receptors in RICO rats. Experimental and clinical endocrinology & diabetes : official journal, German Society of Endocrinology [and] German Diabetes Association 108, 436–446, doi: 10.1055/s-2000-8401 (2000). [DOI] [PubMed] [Google Scholar]
- 28.Biddinger SB et al. Hepatic insulin resistance is sufficient to produce dyslipidemia and susceptibility to atherosclerosis. Cell metabolism 7, 125–134, doi: 10.1016/j.cmet.2007.11.013 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miao J et al. Hepatic insulin receptor deficiency impairs the SREBP-2 response to feeding and statins. Journal of lipid research 55, 659–667, doi: 10.1194/jlr.M043711 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kojima H et al. Effect of glycemic control on plasma plant sterol levels and post-heparin diamine oxidase activity in type 1 diabetic patients. Atherosclerosis 145, 389–397 (1999). [DOI] [PubMed] [Google Scholar]
- 31.Miettinen TA, Gylling H, Tuominen J, Simonen P & Koivisto V Low synthesis and high absorption of cholesterol characterize type 1 diabetes. Diabetes care 27, 53–58 (2004). [DOI] [PubMed] [Google Scholar]
- 32.Gylling H et al. Markers of absorption and synthesis of cholesterol in men with type 1 diabetes. Diabetes Metab Res Rev 23, 372–377, doi: 10.1002/dmrr.697 (2007). [DOI] [PubMed] [Google Scholar]
- 33.Jarvisalo M, Raitakari O, Gylling H & Miettinen TA Cholesterol absorption and synthesis in children with type 1 diabetes. Diabetes care 29, 2300–2304, doi: 10.2337/dc05-2235 (2006). [DOI] [PubMed] [Google Scholar]
- 34.Brown TL, Maahs DM, Bishop FK, Snell-Bergeon JK & Wadwa RP Influences of gender on cardiovascular disease risk factors in adolescents with and without type 1 diabetes. Int J Pediatr Endocrinol 2016, 8, doi: 10.1186/s13633-016-0026-6 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kallien G, Lange K, Stange EF & Scheibner J The pravastatin-induced decrease of biliary cholesterol secretion is not directly related to an inhibition of cholesterol synthesis in humans. Hepatology 30, 14–20, doi: 10.1002/hep.510300119 (1999). [DOI] [PubMed] [Google Scholar]
- 36.Honda A et al. Highly sensitive analysis of sterol profiles in human serum by LC-ESI-MS/MS. Journal of lipid research 49, 2063–2073, doi: 10.1194/jlr.D800017-JLR200 (2008). [DOI] [PubMed] [Google Scholar]
- 37.Pihlajamaki J, Gylling H, Miettinen TA & Laakso M Insulin resistance is associated with increased cholesterol synthesis and decreased cholesterol absorption in normoglycemic men. J Lipid.Res 45, 507–512 (2004). [DOI] [PubMed] [Google Scholar]
- 38.Lupattelli G et al. Visceral fat positively correlates with cholesterol synthesis in dyslipidaemic patients. European journal of clinical investigation 42, 164–170, doi: 10.1111/j.1365-2362.2011.02572.x (2012). [DOI] [PubMed] [Google Scholar]
- 39.Hallikainen M, Kurl S, Laakso M, Miettinen TA & Gylling H Plant stanol esters lower LDL cholesterol level in statin-treated subjects with type 1 diabetes by interfering the absorption and synthesis of cholesterol. Atherosclerosis 217, 473–478, doi: 10.1016/j.atherosclerosis.2011.03.041 (2011). [DOI] [PubMed] [Google Scholar]
- 40.Sittiwet C et al. Cholesterol metabolism and non-cholesterol sterol distribution in lipoproteins of type 1 diabetes: the effect of improved glycemic control. Atherosclerosis 194, 465–472, doi: 10.1016/j.atherosclerosis.2006.08.044 (2007). [DOI] [PubMed] [Google Scholar]
- 41.Nakamura K, Matsui T, Adachi H & Yamagishi S Involvement of angiotensin II in intestinal cholesterol absorption. Pharmacol Res 61, 460–465, doi: 10.1016/j.phrs.2009.12.002 (2010). [DOI] [PubMed] [Google Scholar]
- 42.Inoue T et al. Inhibition of intestinal cholesterol absorption might explain cholesterol-lowering effect of telmisartan. J Clin Pharm Ther 36, 103–110, doi: 10.1111/j.1365-2710.2010.01161.x (2011). [DOI] [PubMed] [Google Scholar]
- 43.Miettinen TA, Tilvis RS & Kesaniemi YA Serum cholestanol and plant sterol levels in relation to cholesterol metabolism in middle-aged men. Metabolism: clinical and experimental 38, 136–140 (1989). [DOI] [PubMed] [Google Scholar]
- 44.Miettinen TA, Tilvis RS & Kesaniemi YA Serum plant sterols and cholesterol precursors reflect cholesterol absorption and synthesis in volunteers of a randomly selected male population. Am J Epidemiol 131, 20–31, doi: 10.1093/oxfordjournals.aje.a115479 (1990). [DOI] [PubMed] [Google Scholar]
- 45.Tilvis RS & Miettinen TA Serum plant sterols and their relation to cholesterol absorption. The American journal of clinical nutrition 43, 92–97, doi: 10.1093/ajcn/43.1.92 (1986). [DOI] [PubMed] [Google Scholar]
- 46.Simonen P, Gylling H & Miettinen TA The validity of serum squalene and non-cholesterol sterols as surrogate markers of cholesterol synthesis and absorption in type 2 diabetes. Atherosclerosis 197, 883–888, doi: 10.1016/j.atherosclerosis.2007.08.003 (2008). [DOI] [PubMed] [Google Scholar]
- 47.Mashnafi S, Plat J, Mensink RP & Baumgartner S Non-Cholesterol Sterol Concentrations as Biomarkers for Cholesterol Absorption and Synthesis in Different Metabolic Disorders: A Systematic Review. Nutrients 11, doi: 10.3390/nu11010124 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bishop FK, Wadwa RP, Ellis S, Rewers M & Maahs DM Lessons learned from a lipid lowering trial in adolescents with type 1 diabetes. Int J Pediatr Endocrinol 2012, 24, doi: 10.1186/1687-9856-2012-24 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.