Abstract
Objective:
Suicide is a public health threat. Nevertheless, the research literature on actively suicidal participants is relatively sparse, in part because they are often excluded from medical, psychiatric, and psychological research for a host of logistical, ethical, and safety concerns. These obstacles to research participation and enrollment may contribute to our lack of understanding regarding the neurobiology of the suicidal crisis as well as to the dearth of evidence concerning both risk prediction and treatment.
Method:
In order to directly investigate neurobiological markers of acute suicide risk, the National Institute of Mental Health Intramural Research Program (NIMH-IRP) implemented the Neurobiology of Suicide protocol. In this protocol, actively suicidal individuals consent to research for both neurobiological assessment and potential rapid-acting interventions.
Results and Conclusions:
This article reviews lessons learned from implementing this protocol in the hopes of assisting future research on the neurobiology of suicide. Areas of specific discussion include the failure modes and effects analysis (FMEA), recruitment and informed consent, participant monitoring, and the safety of the physical environment.
Keywords: Suicide, Clinical Trials, Implementation
1. Introduction
Suicide is a major public health crisis. In 2017 alone, more than 44,000 people in the United States died by suicide [1]. Despite research and legislative policy prevention efforts, suicide rates have increased, with age-adjusted rates rising by 30% since 2000 [2, 3]. Over the past 20 years, several national efforts have been initiated to address the dearth of suicide research data and to develop prevention strategies. In 2010, the U.S. Federal Government and the private sector joined together to form the National Action Alliance for Suicide Prevention, an organization striving to reduce suicide rates nationwide. The Action Alliance released a two-pronged agenda for research prioritization in 2014 [4]; short-term objectives included developing rapid-acting interventions, and long-term objectives included identifying neurobiological markers to indicate treatment response for individuals at risk for suicide.
As such efforts underscore, a tremendous need exists for high-quality research into suicide risk and prevention. At the same time, several obstacles have led to the scarcity of suicide research. Chief among these are ethical concerns surrounding a suicidal patient’s participation due to perceived high vulnerability, including whether these participants lack the ability to give informed consent to research procedures, whether they can receive adequate clinical care throughout their participation in a research study, and how to determine effective levels of data and safety monitoring [5]. In addition to limiting our knowledge base to the few studies conducted, these obstacles may lead to decreased generalizability. Specifically, few clinical trials include participants with a history of suicidal thoughts and behavior, even as suicide rates are increasing [6, 7]. Therefore, these most vulnerable individuals are arguably the least researched, leaving clinicians with inadequate resources to identify, assess, and treat suicidal individuals. This stark discrepancy between clinical need and the research literature suggests that new methods and policies are needed to study suicide risk.
In this context, a critical need exists for investigators worldwide to directly research suicidal thoughts and behaviors while still maintaining the highest standards of participant safety and clinical care. This article seeks to help investigators conduct research with suicidal participants, with the ultimate goal of increasing knowledge regarding the neurobiology of suicide risk and making progress towards addressing the current public health crisis of suicide. Here, we describe the lessons learned from our implementation of the Neurobiology of Suicide protocol at the National Institute of Mental Health Intramural Research Program (NIMH-IRP). This article outlines concerns and safeguards within the following processes: 1) approval and implementation of suicide-focused research; 2) recruitment and consent of suicidal participants; and 3) study monitoring of participant safety. Wherever possible, we highlight potential safeguards that may be particularly applicable to clinical settings with limited research infrastructure. We hope that this framework can help both neuropsychiatric researchers who would like to study suicide but do not know how to comprehensively address safety issues inherent in this population as well as suicide researchers who want to add neurobiological procedures to their current efforts.
2. Overview: The Neurobiology of Suicide Protocol
The Neurobiology of Suicide protocol seeks to evaluate neurobiological, psychological, psychosocial, and environmental factors associated with active suicidal thoughts and behaviors; therefore, data are collected using a dimensional approach from participants in acute crisis as well as individuals with no history of psychiatric disorders. The protocol includes four study groups: participants who are currently experiencing an acute suicidal crisis; individuals with a history of suicidal behavior who are not in acute crisis (i.e., suicide attempt or ideation with a plan that occurred at least one year ago); depressed or anxious participants with no history of suicidal behavior; and healthy control participants with no personal or family history of psychiatric disorder. Participants considered “in crisis” are those who, within the last two weeks, either attempted suicide or had suicidal ideation with intent to attempt. Research procedures associated with the protocol include clinical interviews, self-report ratings, blood draws, urine collection, computerized cognitive tasks, pencil and paper cognitive tasks, overnight sleep studies and polysomnography, functional magnetic resonance imaging (fMRI), magnetoencephalography (MEG), and open-label administration of subanesthetic-dose ketamine. Due to the vulnerabilities associated with participants in active suicide crisis—and because they are often the ones excluded from research participation—this population will be the focus of the rest of this review.
All research takes place on the Mood Disorders Research Unit at the NIMH IRP, which is well-versed in high-risk research with participants with treatment-resistant depression [8] and monitors suicide risk according to the American Psychiatric Association Practice Guidelines for the Assessment and Treatment of Patients with Suicidal Behaviors as well as Joint Commission guidelines for the management of suicidal individuals [9–11]. However, as described below, several suicide-specific risk factors raised additional concerns for this protocol that impacted the entire hospital environment. All of these factors were taken into account when designing, implementing, and conducting the protocol.
3. Approval and Implementation
Like any scientific investigation conducted within a particular institution, the Neurobiology of Suicide protocol was reviewed by the NIH Institutional Review Board (IRB). However, due to the sensitive nature of the protocol and the vulnerability of the participants, three additional procedures were also part of the review and implementation process. First, before submission to the IRB, the protocol was reviewed by the NIH Department of Bioethics Consultation Service, which comprises clinicians, hospital chaplains, individuals with advanced training in ethics and the law, and members of the public. The Bioethics Consultation Service consults when ethical questions arise at the NIH Clinical Center; such consults can range from questions about one specific participant to entire research projects. In this case, the entire research protocol was reviewed by the Bioethics Consultation Service. Second, all individuals involved with the study, including researchers, clinicians, administrators, and administrative staff participated in a Failure Modes and Effects Analysis (FMEA) to prospectively identify risk, as described in the next section. Third, before recruitment began, multiple training sessions were conducted to orient all clinical and research staff to the nature of the research protocol and to prepare them to work directly with suicidal participants.
3.1. Prospective Identification of Risk: The Failure Modes and Effects Analysis (FMEA)
Both health care delivery and the conduct of cutting-edge clinical research are processes that inherently involve risk. Identifying and mitigating these real and potential risks is essential for safely delivering patient care in the context of a complex research protocol. Understanding and managing these risks is the shared responsibility of the clinical research team and the leadership of the healthcare organization. Fortunately, over the last 15–20 years, high reliability organizations (HROs) have provided the healthcare industry with a roadmap for managing these risks. HROs are organizations whose operations are extraordinarily complex and potentially risk-laden but who consistently operate with a high level of safety. Example industries include nuclear power, aviation, chemical manufacturing, and aerospace. HROs achieve this high degree of safety through their attention to five principles of high reliability: preoccupation with failure, sensitivity to operations, reluctance to simplify, deference to expertise, and resilience [9]. The principle of “pre-occupation with failure” is particularly germane to the design of processes supporting the conduct of clinical research. An effective high reliability tool used to assist organizations in characterizing real and potential risks associated with clinical research is the FMEA. FMEA is used to identify and prioritize areas of risk before the system is implemented, making it a proactive rather than reactive process. Hospitals have adapted FMEAs for their own use to identify potential risks across entire healthcare systems and processes [10]. While many forms of the FMEA process exist, a brief description of the steps taken for the Neurobiology of Suicide protocol are detailed in Table 1.
Table 1:
Steps for the Use of a Failure Mode and Effects Analysis (FMEA) for the Neurobiology of Suicide Protocol
| 1. Construct a process chart of the steps of the protocol |
| 2. Identify all potential risks or “failure modes” within the system |
| 3. Assign a “Hazard Score” to each failure mode to establish prioritization of mitigation strategies. The Hazard Score is a product of: - The ‘probability’ of an event occurring - The ‘severity’ of the impact of the failure mode - The presence of ‘controls’ to mitigate the risk associated with the failure mode |
| 4. Using the Hazard Score, staff identify the top priorities to address |
| 5. Create list of action items to address potential risks |
As an example of the thoroughness of this process, NIH Clinical Center and NIMH clinical leadership, research staff, physicians, the medical director of the psychiatric inpatient unit, nurse managers, nursing leadership, and representatives from social work were all involved in the FMEA discussions for this protocol, sharing their concerns and ideas about potential areas of risk or “failure modes”. Briefly, after identifying over 40 potential failure modes associated with the Neurobiology of Suicide protocol, the team then assigned each failure mode a “Hazard Score” representing the likelihood of the failure mode occurring, the severity of the subsequent consequences, and the presence of ‘controls’ to mitigate the associated risk. Failure modes associated with the highest Hazard Scores included recruiting patients, determining eligibility, ensuring informed consent, transferring participants to the NIH from local emergency departments, establishing admissions processes, providing clinical care (in particular, ensuring adequate staffing for actively suicidal patients), maintaining a safe care environment, planning for discharge, and establishing post-discharge procedures. At the conclusion of the FMEA process, the study staff had developed a comprehensive list of actions that could be taken prior to enrolling participants in order to prevent or minimize these risks. Examples included interdisciplinary staff training sessions, a review of staffing needs and competencies, and the establishment of specific participant safeguards such as safety checklists; all are detailed later in this review.
3.2. Training and Launching the Study
Training emerged as a key area of concern across all forms of the initial review process. All inpatient clinicians who would be working directly with suicidal participants were re-trained by an expert in suicide assessment and psychotherapy. In addition, many other members of the clinical research environment also required training and orientation to work with suicidal individuals. These included: on-call psychiatrists, psychologists, social workers, nurses, recreation therapists, and occupational therapists, as well as individuals in patient transport services, admissions, housecleaning, spiritual ministries, the neuroimaging center, sleep laboratories, and dietary services. Because of these training efforts, the entire hospital system learned about their role in the research endeavor, even if they were not providing direct clinical care to the participants.
These training efforts had two fundamental messages. The first concerned the importance of vigilance in safeguarding the physical environment, and the second underscored that discussing suicide with participants is not only critical for suicide prevention, it is also not associated with any iatrogenic increase in suicide risk [11]. Additionally, in our unit, studies are usually “launched” with a formal presentation to research and clinical staff before enrolling the first participant. To provide further orientation for the study, a series of smaller presentations were also given to clinical staff, specifically nurses, in order to facilitate the opportunity to ask questions, provide feedback, and subsequently make any policy changes as needed.
3.2.1. Lessons Learned: Consider an FMEA
Although some of the relevant approval processes for the Neurobiology of Suicide protocol were specific to the NIH (e.g., the Bioethics Consultation Service), the FMEA was a valuable process that can be completed by any institution using publicly available worksheets and websites. Even within a non-hospital environment, such as an outpatient practice, such thorough and organized discussions of risk can be useful in identifying all potential points of failure.
The FMEA had the added benefit of bringing together key stakeholders to discuss safety concerns in a relatively low-stress environment. The perspective of the researchers was often quite different from that of nursing staff or hospital administrators, which provided opportunities for education on all sides. Arguably, bringing these individuals together before the study began rather than after a negative incident (such as a root cause analysis related to a suicide attempt) facilitated a more open discussion with creative problem-solving and less defensiveness and blaming.
3.2.2. Lessons Learned: Training benefits all staff members regardless of their level of mental health training
Over the course of a research study, suicidal participants can encounter many different types of staff members with varying levels of mental health training. Staff members—particularly technicians and administrative staff—appreciated clear guidance and reminders of key safety considerations for working with individuals at risk for suicide. Often, these training sessions identified new areas of risk (such as orienting the check-in staff in the neuroimaging suite about a suicidal participant) that were identified proactively rather than after a negative event occurred.
4. Recruitment and Consent
The NIH Clinical Research Center is a research hospital where all participants are enrolled in research protocols. Because the NIH does not have an emergency department, suicidal participants are often recruited from local community hospitals. Consequently, participants are recruited in a purely clinical setting and asked to transfer to a research setting. It is therefore essential that participants understand the difference between an admission for clinical care and an admission for research where many additional procedures will be conducted. It is also critical for participants to understand that they will be transferred from one hospital setting to another, often via ambulance. Similarly, the referring clinician must also understand the distinction between clinical research and clinical care, such that referring a patient for research participation is not simply “finding a bed” for potential discharge.
Another area of significant concern is the validity of informed consent for suicidal participants. It is common for individuals to experience side effects from overdoses or other medical complications resulting from a suicide attempt that may cloud their judgment. Concerns may also exist regarding a suicidal participant’s capacity to provide informed consent for research and remain safe on a research unit; by virtue of their suicidal ideation or organic injury from unsuccessful past suicide attempts, suicidal participants may (or may not) have challenged decisional capacity. For these participants, special considerations need to be taken to evaluate capacity to participate in research, to provide information about the benefits and risks of suicide research, and to conduct the consent process in a safe and supportive manner [12].
Therefore, we implemented several safety checks throughout the recruitment and consenting process to facilitate understanding and avoid confusing clinical research with clinical care; these included a study summary, multiple informed consents, and comprehension “quizzes” (see Figure 1). In addition, the presence of the clinical research advocate during the informed consent process ensured that participants had many opportunities to ask questions about their research participation.
Figure 1:
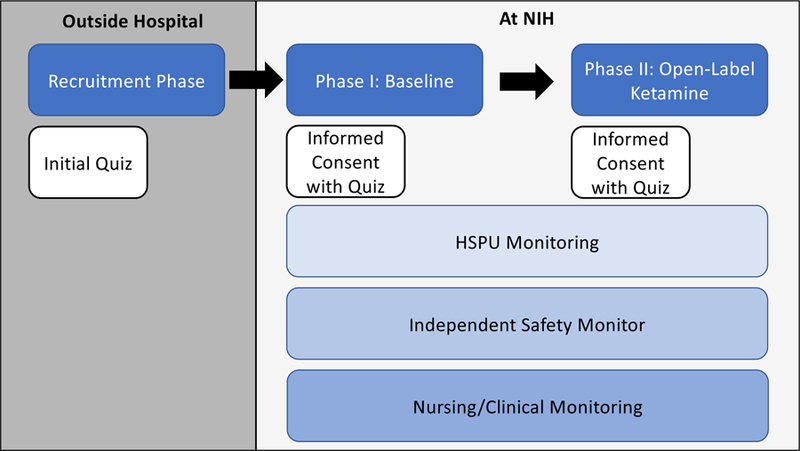
Phases of Study, Informed Consent, and Quizzes
HSPU: Human Subjects Protection Unit
4.1. Study Summary
To ensure that all participants receive the same information about the research study, an IRB-approved study summary is distributed to referring clinicians as well as potential participants who hear about the research protocol while in the community and before they agree to be transported to the NIH. The summary was formatted as a series of questions and answers about the study, with a specific focus on addressing therapeutic misconceptions. An example question and answer are depicted below:
“How is this study different than regular clinical treatment? —
This is a research study, which is different from clinical care that you would receive from your doctors in the community. In clinical care, you can get new treatments, like medications, such as antidepressants, mood stabilizers, or electroconvulsive therapy, or psychotherapy, such as individual or group psychotherapy, right away. In this research study, you will not receive new treatments for up to 11 days. If you do not want to wait for new medications, then you should not participate in this study.”
4.2. Multiple Informed Consents
In the Neurobiology of Suicide Protocol, participants are asked to consent to a wide range of procedures including neuroimaging scans, blood draws, and an open-label administration of subanesthetic-dose ketamine, which has been associated with rapid decreases in depressive symptoms and suicidal thoughts [13]. To facilitate understanding and avoid overwhelming participants, protocol participation was divided into two phases, each with its own informed consent. Participants initially consented to the research procedures and then, a few days later, to ketamine administration.
4.3. Quizzes
Three IRB-reviewed quizzes are administered before the participant is recruited and at the time of each informed consent. These quizzes are designed to ensure participants’ understanding and are meant to safeguard participants’ rights to autonomy and decision-making. They also serve to ensure that researchers are acting in participants’ best interests. The content of these quizzes are as follows:
A five-question true/false quiz is administered during recruitment to ensure that participants understand the voluntary nature of research participation (example question: “This study is voluntary and you are free to withdraw at any time.” [Answer: True]). This quiz is administered before the participant is transported to the NIH.
A 10-question true/false quiz is administered on admission day at the conclusion of the informed consent meeting to ensure that participants understand the general nature of the baseline phase of the study (Phase I) (example question: “You may not get any benefit from participating in this study.” [Answer: True]). This quiz is administered at the time of study consent and after the participant is transported to the NIH.
An additional five-question true/false quiz is administered before Phase II, when the participants have the option to receive open-label ketamine. The quiz ensures participants’ understanding of the general nature of Phase II, which involves an open-label trial of ketamine (example question: “You will receive a placebo during this study.” [Answer: False]), thereby also assessing informed consent.
Together, these quizzes ascertain participants’ comprehension. If the participant does not attain a ≥90% score on the first two quizzes and a ≥80% score on the third, the participant can raise any concerns, discuss specific issues with the research team, and/or retake any quiz again to ensure understanding of what is taking place. If the participant again fails to meet the above criteria, a formal, independent, and protocol-specific capacity assessment is conducted by the Human Subjects Protection Unit (HSPU; described below) to determine whether cognitive or other reasons are present and that would prevent the participant from making appropriate decisions.
4.4. Clinical Research Advocate
One facet of the Neurobiology of Suicide protocol that may be comparatively rare in other organizations is the existence of the Clinical Research Advocate (CRA). The CRA is a member of the NIMH-specific HSPU and serves as an independent advocate for the participant throughout the duration of the protocol; notably, the CRA is not part of the Principal Investigator’s research team [14]. The CRA, who is also a trained social worker, regularly assesses the participant’s capacity to enroll in psychiatric research studies and provides ongoing monitoring throughout the course of the study. During this informed consent meeting, the participant’s primary clinician reviews the protocol consent forms, and the participant may ask questions. If the participant indicates a desire to participate in the study at the end of this meeting, s/he is asked to sign consent forms. The CRA further monitors participation by meeting regularly with participants throughout the study to check understanding, appreciation, reasoning, and decision to participate in the study. The goal of the CRA monitoring process is to make sure the participant understands the voluntary nature of agreeing to research and their choice to stop participation at any time.
4.4.1. Lessons Learned: Recruitment and Consent is an Ongoing Process
Ethical research can only be completed with the full informed consent of the participants. In recruiting and consenting a suicidal participant, the process may need to “slow down” in order to ensure that the participant fully understands each step. Each of the safeguards in the recruitment and consenting process is meant to allow the participant to ask questions and learn more about the research study before providing their informed consent, particularly about the relationship between clinical research and clinical care.
5. Study Monitoring and Safety
Most staff working in psychiatric inpatient settings are comfortable monitoring suicide risk while patients are on the unit. Typical procedures include regular assessments and checks of the physical environment to ensure participant safety [15]. However, no objective biomarkers exist for suicide risk. Clinicians must rely on the self-report of participants for their suicidal thoughts, intent, and plan, as well as for other suicide risk factors, including previous history of attempt and psychiatric diagnosis; even then, these factors are limited in their ability to predict suicidal behavior [16, 17]. However, a neurobiological research study involves exposure to additional environments that are not part of clinical care and were not designed with psychiatric patients in mind. For example, sleep studies using sleep electroencephalography (EEG) or polysomnography are often conducted in specific hospital rooms equipped with objects such as cords that participants could use to harm themselves. Sleep technicians monitor the sleep study environment but may lack the appropriate psychiatric training to manage suicide risk. Similarly, neuroimaging scans, including MEG and magnetic resonance imaging (MRI) scans, are also conducted in distinct areas of the hospital. These environments also often include cords and other potential lethal means and are staffed by technicians who are not specifically trained to work with suicidal participants and maintain the required constant vigilance.
As a result of these concerns about suicide risk while in research, a number of safeguards were developed for study monitoring, including a suicide safety plan, daily suicide assessments, and physical environment checklists, detailed in the following sections.
5.1. Suicide Safety Plan
Shortly after admission, the participant works with a study investigator to create a suicide safety plan, developed from previous work by Brown and Stanley [18]. The safety plan details the participant’s specific warning signs of suicide; individualized coping strategies; activities identified by the participant that refocus his/her attention; people the participant feels comfortable asking for support; and NIH professionals the participant feels comfortable reaching out to in the event of a crisis. The identified warning signs and coping strategies focus on what the participant can do while on the psychiatric inpatient unit rather than after discharge. The creation of this safety plan helps establish a communication-based relationship between the participant and the clinical research team. Once completed, a copy is given to the participant, a copy is entered into the medical record, and a copy is entered into the research record (which is reviewed by nursing staff daily). Therefore, the individual suicide risk factors and coping strategies identified by the participants themselves are shared throughout the entire clinical and research team to ensure continuity of care.
5.2. Daily Suicide Assessment
A daily suicide assessment provides another level of study monitoring of daily suicide risk as distinct from clinical interviews conducted for the purpose of research. This 19-question assessment administered by the participant’s primary nurse consists of suicide-focused questions drawn from the following rating scales: Scale for Suicide Ideation (SSI) [19]; Columbia-Suicide Severity Rating Scale (C-SSRS) [20]; Suicide Status Form (SSF) [21]; Montgomery-Åsberg Depression Rating Scale (MADRS) [22]; and Hamilton Depression Rating Scale (HAM-D) [23]. Integrating both reported suicidal thoughts as well as clinical judgment, the participant’s primary nurse rates overall functioning through the Clinical Global Impression – Severity Scale (CGI-S) [24]. If this CGI-S score worsens by two points or more during research participation, nurses are instructed to inform the primary clinician and a safety monitor who is not part of the research team. The clinicians work with the safety monitor to determine whether the participant should remain in research or should be removed to immediately start new clinical treatments. Any other relevant questions regarding whether or not the participant can remain in the study are also discussed with this safety monitor.
5.3. Physical Environment
Traditional environmental safeguards—such as removing knives, hooks, cords, and non-shatterproof glass—are in place in psychiatric hospitals nationwide. To ensure participant safety during off-unit MEGs, MRIs, and sleep EEGs, checklists are completed by participants’ nurses before any procedures take place. The safety checklists ensure that potentially hazardous objects (e.g., “sharps”) are removed from the area. In addition, during all imaging and sleep procedures, the participant is observed by psychiatric nurses on a video monitor. Examples from the sleep study checklist include: “Visually inspect the sleep lab/room to ensure sharps have been removed” and “Set up the wireless video monitor so that nursing staff can see the patient from the nurses’ station”. Examples from the neuroimaging checklist include: “Ensure the patient has gone to the bathroom prior to leaving the unit” (to avoid using a bathroom that has not been evaluated for suicide risk) and “Remove any metal objects and empty all pockets before the patient’s scan” (so that the nurse can quickly enter the scanning environment in case of an emergency). Research staff who work in neuroimaging or sleep lab areas are also informed when they will be working with a suicidal participant so that they can take additional precautions in monitoring the physical environment for safety concerns.
5.3.1. Lessons Learned: Integrate Standardized Suicide Assessment with Clinical Judgement and Use Language Consistently
In training clinical staff to work with suicidal individuals, we received repeated requests for specific language regarding suicide risk assessment. Therefore, it was important to provide standard language for discussing suicidal thoughts and behaviors that could be used consistently by clinicians. In addition, because no objective markers of suicide risk exist, integrating clinicians’ general assessments of overall participant functioning with standardized assessments provides a fuller picture than clinical observation alone. At the same time, each individual has their own distinct suicide warning signs and drivers that lead to suicidal behavior; consequently, working with each participant to create a suicide safety plan tailored to their specific suicide risk factors helps to further safeguard this vulnerable population.
5.3.2. Lessons Learned: Maintain Constant Checks of the Physical Environment
Neurobiological research with suicidal individuals may involve taking these individuals into new and possibly unsafe physical environments. Repeated reminders of the potential dangers of these research environments, including checklists, can prompt research and clinical staff to re-orient for each procedure.
6. Conclusions
To lower the suicide rate, we need to expand our knowledge of risk factors and treatment methods. In this context, recruiting suicidal participants into research settings is an important goal. As the Neurobiology of Suicide protocol has shown, it is possible to implement special safety measures and procedures to protect this vulnerable population, thus increasing their enrollment into clinical trials and settings. To date, recruitment of participants at high risk for suicide has been both feasible and successful. We encourage other psychiatric research facilities to consider the importance and feasibility of suicide research.
Highlights.
Research into the neurobiology of suicide is critically needed.
Suicidal individuals are often excluded from research due to logistical challenges.
A framework for research with suicidal individuals is presented.
Lessons learned include multidisciplinary communication and safety checklists.
A failure modes and effects analysis may be useful for suicide researchers.
Acknowledgements
The authors thank the 7SE research unit and staff for their support. Ioline Henter (NIMH) provided invaluable editorial assistance.
Funding and Role of Funding Source
Funding for this work was supported by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH; and 15-M-0188, ZIAMH002927), by a NARSAD Independent Investigator Award to Dr. Zarate, and by a Brain and Behavior Mood Disorders Research Award to Dr. Zarate. These organizations had no further role in study design; in the collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement
Dr. Zarate is listed as a co-inventor on a patent for the use of ketamine in major depression and suicidal ideation; as a co-inventor on a patent for the use of (2R,6R)-hydroxynorketamine, (S)-dehydronorketamine, and other stereoisomeric dehydro and hydroxylated metabolites of (R,S)-ketamine metabolites in the treatment of depression and neuropathic pain; and as a co-inventor on a patent application for the use of (2R,6R)-hydroxynorketamine and (2S,6S)-hydroxynorketamine in the treatment of depression, anxiety, anhedonia, suicidal ideation, and post-traumatic stress disorders. He has assigned his patent rights to the U.S. government but will share a percentage of any royalties that may be received by the government. All other authors have no conflict of interest to disclose, financial or otherwise.
References
- 1.Centers for Disease Control and Prevention: National Center for Injury Prevention and Control: National Violent Death Reporting System, 2018,
- 2.Stone DM, Simon TR, Fowler KA, Kegler SR, Yuan K, Holland KM, et al. : Vital Signs: Trends in State Suicide Rates - United States, 1999–2016 and Circumstances Contributing to Suicide - 27 States, 2015. MMWR Morb Mortal Wkly Rep 2018;67:617–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Curtin SC, Warner M, Hedegaard H: Increase in suicide in the United States, 1999–2014: NCHS data brief, . Hyattsville, MD, 2016, [PubMed] [Google Scholar]
- 4.National Action Alliance for Suicide Prevention: Research Prioritization Task Force: A prioritized research agenda for suicide prevention: An action plan to save lives. Rockville, MD: National Institute of Mental Health and the Research Prioritization Task Force, 2014, [Google Scholar]
- 5.Pearson JL, Stanley B, King CA, Fisher CB: Intervention research with persons at high risk for suicidality: safety and ethical considerations. J Clin Psychiatry 2001;62 Suppl 25:17–26. [PubMed] [Google Scholar]
- 6.Zimmerman M, Clark HL, Multach MD, Walsh E, Rosenstein LK, Gazarian D: Have treatment studies of depression become even less generalizable? A review of the inclusion and exclusion criteria used in placebo-controlled antidepressant efficacy trials published during the past 20 years. Mayo Clin Proc 2015;90:1180–1186. [DOI] [PubMed] [Google Scholar]
- 7.Zimmerman M, Mattia JI, Posternak MA: Are subjects in pharmacological treatment trials of depression representative of patients in routine clinical practice? Am J Psychiatry 2002;159:469–473. [DOI] [PubMed] [Google Scholar]
- 8.Nugent AC, Iadarola ND, Miller FG, Luckenbaugh DA, Zarate CA Jr.: Safety of research into severe and treatment-resistant mood disorders: analysis of outcome data from 12 years of clinical trials at the US National Institute of Mental Health. Lancet Psychiatry 2016;3:436–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sutcliffe KM: High reliability organizations (HROs). Best Pract Res Clin Anaesthesiol 2011;25:133–144. [DOI] [PubMed] [Google Scholar]
- 10.DeRosier J, Stalhandske E, Bagian JP, Nudell T: Using health care Failure Mode and Effect Analysis: the VA National Center for Patient Safety’s prospective risk analysis system. Jt Comm J Qual Improv 2002;28:248–267, 209. [DOI] [PubMed] [Google Scholar]
- 11.Gould MS, Marrocco FA, Kleinman M, Thomas JG, Mostkoff K, Cote J, et al. : Evaluating iatrogenic risk of youth suicide screening programs: a randomized controlled trial. JAMA 2005;293:1635–1643. [DOI] [PubMed] [Google Scholar]
- 12.Nugent AC, Miller FG, Henter ID, Zarate CA Jr.: The ethics of clinical trials research in severe mood disorders. Bioethics 2017;31:443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilkinson ST, Ballard ED, Bloch MH, Mathew SJ, Murrough JW, Feder A, et al. : The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am J Psychiatry 2017:appiajp201717040472. [DOI] [PMC free article] [PubMed]
- 14.Cadman ME, Murphy JH, Brintnall-Karabelas J, Squires C, Whorton K, Pao M: A training program for improving the informed consent discussion between clinical researchers and their subjects. J Empir Res Hum Res Ethics 2014;9:71–75.24572085 [Google Scholar]
- 15.The Joint Commission: National Patient Safety Goals Effective January 2019: Behavioral Health Care Accreditation Program; in Commission TJ (ed). Washington, DC, 2019, [Google Scholar]
- 16.Joiner TE Jr., Conwell Y, Fitzpatrick KK, Witte TK, Schmidt NB, Berlim MT, et al. : Four studies on how past and current suicidality relate even when “everything but the kitchen sink” is covaried. J Abnorm Psychol 2005;114:291–303. [DOI] [PubMed] [Google Scholar]
- 17.Nock MK, Hwang I, Sampson N, Kessler RC, Angermeyer M, Beautrais A, et al. : Cross-national analysis of the associations among mental disorders and suicidal behavior: findings from the WHO World Mental Health Surveys. PLoS Med 2009;6:e1000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stanley B, Brown GK: Safety planning intervention: A brief intervention to mitigate suicide risk. Cogn Behav Pract 2012;19:256–264. [Google Scholar]
- 19.Beck AT, Kovacs M, Weissman A: Assessment of suicidal intention: the Scale for Suicide Ideation. J Consult Clin Psychol 1979;47:343–352. [DOI] [PubMed] [Google Scholar]
- 20.Posner K, Brown GK, Stanley B, Brent DA, Yershova KV, Oquendo MA, et al. : The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry 2011;168:1266–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jobes DA, Nelson KN, Peterson EM, Pentiuc D, Downing V, Francini K, et al. : Describing suicidality: an investigation of qualitative SSF responses. Suicide Life Threat Behav 2004;34:99–112. [DOI] [PubMed] [Google Scholar]
- 22.Montgomery SA, Asberg M: A new depression scale designed to be sensitive to change. Br J Psychiatry 1979;134:382–389. [DOI] [PubMed] [Google Scholar]
- 23.Hamilton M: A rating scale for depression. J Neurol Neurosurg Psychiatry 1960;23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Busner J, Targum SD: The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont) 2007;4:28–37. [PMC free article] [PubMed] [Google Scholar]


