Abstract
The reactions between disinfectants and organic matter or inorganic matter in source water generates disinfection by-products (DBPs) such as iodoacetic acid (IAA). DBPs are associated with health effects such as bladder cancer and adverse reproductive outcomes, but the effects of IAA on the ovary are not well known. This study determined whether IAA exposure affects ovarian follicle growth, steroidogenesis, and expression of apoptotic factors, cell cycle regulators, estrogen receptors, and steroidogenic factors in vitro. IAA exposure significantly decreased follicle growth, expression of cell cycle stimulators, and the proliferation marker Ki67. In contrast, IAA increased expression of the cell cycle inhibitor Cdkn1a. Moreover, IAA exposure increased expression of pro-apoptotic factors, whereas it decreased expression of anti-apoptotic factors. IAA exposure also altered expression of steroidogenic factors and estrogen receptors, disrupting steroidogenesis. These data demonstrate that IAA exposure inhibits follicle growth, decreases cell proliferation, and alters steroidogenesis in mouse ovarian follicles in vitro.
Keywords: Disinfection by-products, iodoacetic acid, ovary, folliculogenesis, steroidogenesis
1. Introduction
Disinfectants are extensively applied to source water to control pathogens and prevent waterborne diseases. However, these chemicals can react with organic or inorganic matter in source water to form compounds called water disinfection by-products (DBPs) [1]. To date, more than 700 DBPs have been found in drinking water; however, only eleven of these compounds are regulated in the United States [1, 2]. People are exposed to DBPs on a daily basis, mostly through drinking water treated with disinfectants, but inhalation and dermal absorption also can occur. Another potential source of exposure is consumption of food and beverages that were prepared with treated water [3].
The presence of DBPs in the drinking water has become a health concern because epidemiological studies have demonstrated associations between DBP exposure and increased risk of cancer development and adverse reproductive outcomes in humans [4–7]. Specifically, exposure to DBPs in drinking water has been associated with an increased risk of bladder cancer (adjusted odds ratio: 1.24, 95% confidence interval: 1.09–1.41) [8] and rectal cancer (adjusted odds ratio: 1.92, 95% confidence interval: 1.20–3.09) [5]. Further, exposure to DBPs has been associated with increased risk of stillbirth (adjusted odds ratio: 1.74, 95% confidence interval: 1.03–2.95) [7], cardiac birth defects (adjusted odds ratio: 1.56, 95% confidence interval: 1.01–2.43) [9], birth defects such as septal defects (adjusted odds ratio 1.81, 95% confidence interval: 0.98–3.35), cleft palate (adjusted odds ratio 1.56, 95% confidence interval: 1.00–2.41), and anencephalus (adjusted odds ratio 1.96, 95% confidence interval: 0.94–4.07) [10].
High levels of iodide in source waters can lead to formation of iodinated DBPs, such as iodoacetic acid (IAA) in drinking water. Studies have demonstrated that the concentration of IAA in drinking water is up to 1.7 μg/L in the United States and Canada [11] and up to 2.18 μg/L in China [12]. Although the levels of IAA in drinking water are not as high as other DBPs, IAA is known to be more cytotoxic and genotoxic than its chlorinated or brominated analogues [13, 14]. Studies have demonstrated that IAA is toxic in cell lines. IAA reduced pyruvate and ATP levels by inhibiting glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in Chinese hamster ovarian (CHO) cells [15]. Wei et al. [12] showed that prolonged exposure to IAA increased the frequency of transformed cells in the NIH3T3 mouse cell line. Ali et al. [16] showed that IAA causes DNA damage in human lymphocytes and sperm. Toxicogenomic and reporter gene analyses demonstrated that in non-transformed human FHs 74 Int cells, IAA altered gene expression in DNA repair and oxidative stress pathways [17, 18].
IAA has also been shown to be an ovarian toxicant. Our laboratory established that IAA inhibited antral follicle growth and reduced estradiol production by mouse ovarian follicles in vitro [19], however, the mechanisms by which IAA inhibits ovarian follicle growth and reduces estradiol levels were not determined. In addition, it is estimated that adult women absorb 3.42 mg/day of DBPs and about 3 μg/L/h reach the ovaries, which equates to approximately 0.09 μg/ml/day reaching the ovary [20], raising concern that the ovary is a target tissue for DBP-induced toxicity. Thus, the objective of this study was to test the hypothesis that IAA disrupts ovarian function through mechanisms that include cell growth/death and steroidogenesis pathways. To test this hypothesis, we conducted mouse ovarian follicle cultures to determine the effects of IAA on follicle growth, expression of genes related to apoptosis, the cell cycle, ovarian steroidogenesis, estrogen receptors, and proliferation markers, and on the levels of sex steroid hormones.
2. Material and Methods
2.1. Chemical
IAA was purchased from Sigma-Aldrich (St. Louis, MO). A 1 M stock solution was prepared in dimethyl sulfoxide (DMSO) and stored in sealed sterile glass vials (Supelco, Bellefonte, PA) at −20 °C. We decided to test IAA specifically because it was shown that this compound has the highest cytotoxic and genotoxic potential in CHO cells compared to other haloacetic acids [14]. To our knowledge, data are not available on the plasma or follicular IAA levels in humans. Taking into account this lack of information in the literature, the concentration range was chosen based on a study by Plewa et al. [14] reporting that the lowest toxic concentration for IAA was 0.5 μM, and a previous study in our laboratory indicating that IAA reduces follicle growth and estradiol levels in vitro at 10 and 15 μM [19]. Based on these reports, we chose the concentrations for our experiment as 2, 5, 10 and 15 μM of IAA.
2.2. Animals
Cycling female CD-1 mice (postnatal days 28–30) were purchased from Charles River Laboratories (Charles River, CA). Mice were housed at the University of Illinois at Urbana-Champaign, College of Veterinary Medicine Animal Facility. Mice were kept under 12 h light-dark cycles at 22 ± 1 °C and were provided food and water ad libitum. The Institutional Animal Use and Care Committee at the University of Illinois at Urbana-Champaign approved all procedures involving animal care, euthanasia, and tissue collection.
2.3. Antral follicle cultures
Female CD-1 mice were euthanized on postnatal days 38–40 and antral follicles were meticulously dissected from the ovaries based on size (220–400 μm) using watchmaker’s forceps as described previously [19, 21]. Ovaries from 2 to 3 mice were used for each culture and approximately 25–30 antral follicles were obtained per mouse per culture. Isolated antral follicles were individually placed in wells of a 96-well culture microplate with unsupplemented α-MEM prior to treatment. The follicles were treated with either 0.075% dimethyl sulfoxide (DMSO; vehicle control) or IAA (2, 5, 10, or 15 μM), which were prepared in supplemented α-MEM. Importantly, the percentage of DMSO used in the cultures was 0.075%. This amount is very low and does not produce difference in follicle growth, gene expression, or hormonal levels compared to media without DMSO [22, 23]. Supplemented α-MEM contained unsupplemented α-MEM, 1% ITS (10 μg/ml insulin, 5.5 μg/ml transferrin, 5.5 ng/ml sodium selenite, Sigma-Aldrich, St. Louis, MO), 100 U/ml penicillin, and 100 μg/ml streptomycin (Sigma-Aldrich, St. Louis, MO), 5 IU/ml human recombinant follicle-stimulating hormone (FSH; Dr. A.F. Parlow, National Hormone and Peptide Program, Harbor-UCLA Medical Center, Torrance, CA), and 5% fetal bovine serum (FBS; Atlanta Biological, Lawrenceville, GA). Follicles were incubated for 96 h at 37 °C, 5% CO2 in 150 μl of supplemented α-MEM medium. Follicle growth was measured every 24 h, and after 96 h of culture, media were collected and stored at −80 °C for further measurements of sex steroid hormone levels. Antral follicles were collected, snap frozen, and then stored at –80 °C for gene expression analyses as described below. Each culture was performed with 11–12 follicles per treatment group, and cultures were performed three times.
2.4. Analysis of antral follicle growth
Follicles were cultured for 96 h and their growth was evaluated every 24 h by measuring follicle diameters across the perpendicular axes with an inverted microscope equipped with a calibrated ocular micrometer. The sizes of follicles in each treatment group were averaged first and then converted to percentage of the initial follicle size at the start of the culture. These percent data were averaged and analyzed across cultures (n = 3 separate cultures).
2.5. Analysis of gene expression
For quantitative polymerase chain reaction (qPCR) analyses, follicles were collected after culture, snap-frozen in liquid nitrogen, and then stored at −80 °C until RNA extraction. Total RNA was then isolated from follicles (n = 3, 11–12 follicles per culture) using an RNeasy Micro kit (Qiagen, Inc., Valencia, California) following the manufacturer’s instructions. RNA was eluted in 14 μl of RNase-free water and the concentration was determined using a NanoDrop (λ = 260/280 nm; ND 1000; Nanodrop Technologies Inc., Wilmington, Delaware). Total RNA (100 ng) was reverse transcribed to complementary DNA (cDNA) using the iScript RT kit (Bio-Rad Laboratories, Inc., Hercules, California) according to the manufacturer’s protocol. Analysis of qPCR was conducted using the C and FX96 Real-Time PCR Detection System (Bio-Rad Laboratories) and accompanying CFX Manager Software according to the manufacturer’s protocol. The machine quantifies the amount of PCR product generated by measuring SsoFastEvaGreen dye (Bio-Rad Laboratories) that fluoresces when bound to double-stranded DNA. All qPCR reactions were conducted in duplicate with 1.67ng of cDNA, forward and reverse primers (0.75 pmol) for beta-actin (Actb), B cell leukemia/lymphoma 2 (Bcl2), Bcl2 like protein 10 (Bcl2l10), Bcl2-associated X protein (Bax), apoptosis-inducing factor 1 (Aimf1), cyclin A2 (Ccna2), cyclin B1 (Ccnb1), cyclin D2 (Ccnd2), cyclin E1 (Ccne1), cyclin-dependent kinase 4 (Cdk4), cyclin-dependent kinase inhibitor 1a (Cdkn1a), proliferation marker protein Ki-67 (Ki67), proliferating cell nuclear antigen (Pcna), steroidogenic acute regulatory protein (Star), cytochrome-P450 cholesterol side-chain cleavage (Cyp11a1), cytochrome P450 steroid 17-α-hydroxylase 1 (Cyp17a1), 3β-hydroxysteroid dehydrogenase (Hsd3b1), 17β-hydroxysteroid dehydrogenase 1 (Hsd17b1), cytochrome P450 aromatase (Cyp19a1), estrogen receptor α (Esr1), estrogen receptor β (Esr2) (Table 1), and SsoFastEvaGreen Supermix for a final reaction volume of 10 μl. The qPCR program consisted of an enzyme activation step (95 °C for 1 min), an amplification and quantification program (36 cycles of 94 °C for 10 s, and 72 °C for 10 s, a melt curve (65 °C–95 °C heating, 0.4 °C/sec with continuous fluorescence readings), and a final step at 72 °C for 2 min per the manufacturer’s protocol. Actb mRNA expression was used in qPCR analyses as a housekeeping gene. All gene expression data were normalized to the housekeeping gene. Relative fold changes were calculated as the ratio of each treatment group to the control group level from the same culture and were analyzed using a mathematical model for relative quantification of qPCR data developed by Pfaffl [24].
Table 1.
Primers used in quantitative real-time polymerase chain reactions (qPCR)
| Gene | Gene Symbol | Primer Sequence | |
|---|---|---|---|
| Forward | Reverse | ||
| Beta actin | Actb | GGGCACAGTGTGGGTGAC | CTGGCACCACACCTTCTAC |
| Steroidogenic acute regulatory protein | StAR | CAGGGAGAGGTGGCTATGCA | CCGTGTCTTTTCCAATCCTCTG |
| Cytochrome P450 cholesterol side-chain cleavage | Cyp11a1 | AGATCCCTTCCCCTGGCGACAATG | CGCATGAGAAGAGTATCGACGCATC |
| 3b-Hydroxysteroid dehydrogenase 1 | Hsd3b1 | CAGGAGAAAGAACTGCAGGAGGTC | GCACACTTGCTTGAACACAGGC |
| Cytochrome P450 steroid 17-a-hydroxylase 1 | Cyp17a1 | CCAGGACCCAAGTGTGTTCT | CCTGATACGAAGCACTTCTCG |
| 17b-Hydroxysteroid dehydrogenase 1 | Hsd17b1 | ACTGTGCCAGCAAGTTTGCG | AAGCGGTTCGTGGAGAAGTAG |
| Cytochrome P450 aromatase | Cyp19a1 | CATGGTCCCGCAAACTGTGA | GTAGTAGTTGCAGGCACTTC |
| Estrogen receptor 1 (alpha) | Esr1 | CCGTGTGCAATGACTATGCC | GTGCTTCAACATTCTCCCTCCTC |
| Estrogen receptor 2 (beta) | Esr2 | GGAATCTCTTCCCAGCAGCA | GGGACCACATTTTTGCACTT |
| Cyclin A2 | Ccna2 | GCTCTACTGCCCGGAGGCTGA | TGGCCTACATGTCCTCTGGGGAA |
| Cyclin B1 | Ccnb1 | TGCATTCTCTCAGTGCCCTCCACA | AGACAGGAGTGGCGCCTTGGT |
| Cyclin D2 | Ccnd2 | CCTTTGACGCAGGCTCCCTTCT | ACCCTGGTGCACGCATGCAAA |
| Cyclin E1 | Ccne1 | GGTGTCCTCGCTGCTTCTGCTT | CCGGCTAACCATGGCGAACGGA |
| Cyclin-dependent kinase 4 | Cdk4 | AGAAACCCTCGCTGAAGCGGCA | TGGGGGTGAACCTCGTAAGGAGA |
| Cyclin-dependent kinase inhibitor 1A (p21) | Cdkn1a | TTAGGCAGCTCCAGTGGCAACC | ACCCCCACCACCACACACCATA |
| Proliferation marker protein Ki-67 | Ki67 | GCTCACCTGGTCACCATCAA | ACTACAGGCAGCTGGATACG |
| Proliferating cell nuclear antigen | Pcna | CGGCGTGAACCTGCAGAGCA | GGTTGCGGTCGCAGCGGTAT |
| B cell lymphoma 2 | Bcl2 | ATGCCTTTGTGGAACTATATGGC | GGTATGCACCCAGAGTGATGC |
| Bcl2 like protein 10 | Bcl2l10 | CGCTACACACACTGACTGGA | CTTTAGGATCCCCTGCCCTG |
| Bcl2-associated X protein | Bax | TGAAGACAGGGGCCTTTTTG | AATTCGCCGGAGACACTCG |
| Apoptosis-inducing factor 1, mitochondrion-associated 1 | Aimf1 | AGGACGGTGAGCAACATGAA | GTTCTATCCACCCATCCCGC |
2.6. Analysis of sex steroid hormone levels
Culture media were collected at 96 h of culture from 3 separate antral follicle cultures, and subjected to enzyme-linked immunosorbent assays (ELISAs, DRG International Inc., Springfield, New Jersey) for measurement of progesterone (analytical sensitivity was 0.034 ng/ml and both intra- and inter-assay coefficients of variation were below 8%), pregnenolone (analytical sensitivity was 0.05 ng/ml and both intra- and inter-assay coefficients of variation were below 15%), androstenedione (analytical sensitivity was 0.021 ng/ml and both intra- and inter-assay coefficients of variation were below 10%), testosterone (analytical sensitivity was 0.083 ng/mL and both intra- and inter-assay coefficients of variation were below 10%), and estradiol (analytical sensitivity was 10.6 pg/mL and both intra- and inter-assay coefficients of variation were below 15%). Assays were run according to the manufacturer’s instructions. Samples were diluted if necessary and run in duplicate for measurements of progesterone (no dilution), pregnenolone (no dilution), androstenedione (no dilution), testosterone (no dilution), and estradiol (1:10 dilution) to match the dynamic range of each ELISA kit.
2.7. Statistical analysis
All data analyses were performed using SPSS statistical software (SPSS Inc., Chicago, Illinois). Data were expressed as means ± SEM (standard error of the mean) from three separate experiments. Multiple comparisons between normally distributed experimental groups were made using one-way analysis of variance (ANOVA) followed by Dunnett post hoc comparison, if equal variances were assumed, or Games–Howell post hoc comparisons if equal variances were not assumed. If data were not normally distributed, comparison between 2 groups was done using Kruskal–Wallis H test for several independent samples and Mann–Whitney U 2-independent sample tests. Statistical significance was assigned at P ≤ 0.05.
3. Results
3.1. The effects of IAA exposure on antral follicle growth in vitro
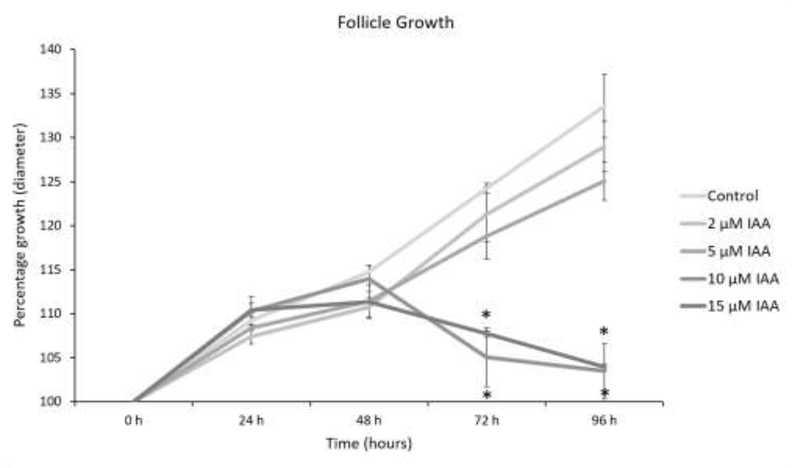
Similar to a previous publication [19], IAA exposure at 10 and 15 μM significantly decreased antral follicle growth compared to control, beginning at 72 h and continuing through 96 h of culture (Fig. 1, n = 3, P < 0.05). However, the lowest doses of IAA (2 and 5 μM) did not significantly affect follicle growth compared to control (Fig. 1).
Fig.1. Effect of IAA on antral follicles growth.
Antral follicles were cultured with IAA for 96 h. Growth of follicles was recorded every 24 h and reported as percent change compared to the follicle size at the beginning of treatment (0 h = 100%). Control = DMSO, IAA = iodoacetic acid. Data represent means ± SEM from three separate cultures. Asterisks (*) represent significant differences from control at each time point (P ≤ 0.05).
3.2. The Effects of IAA exposure on gene expression in antral follicles
3.2.1. Apoptotic factors
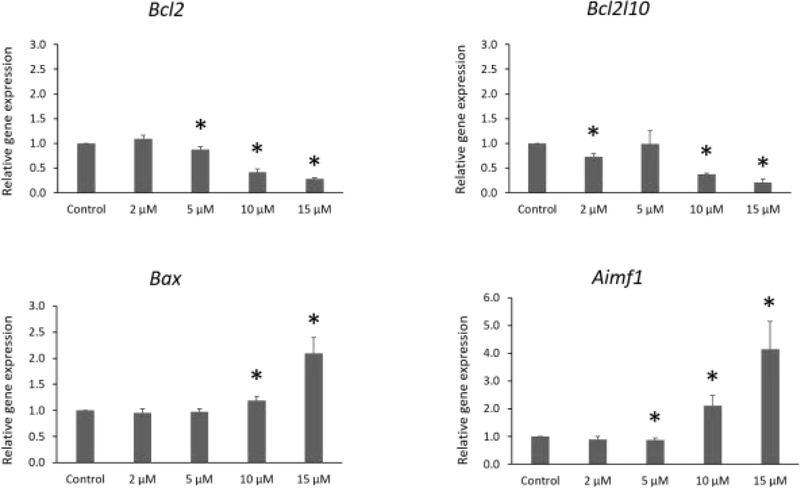
After 96 h of exposure, IAA significantly decreased expression of the anti-apoptotic factors Bcl2 (5, 10, and 15 μM) and Bcl2l10 (2, 10, and 15 μM) compared to control. In addition, IAA exposure at 5 μM slightly decreased expression of the pro-apoptotic factor Aimf1 compared to control. Interestingly, IAA exposure at 10 and 15 μM increased the expression of the pro-apoptotic factors Bax and Aimf1 compared to control at the same time point (Fig. 2, n = 3 separate cultures, 34 follicles, P ≤ 0.05).
Fig.2.
Effects of IAA exposure on gene expression of Bcl2, Bcl2l10, Bax and Aifm1, in follicles treated with 2, 5, 10 and 15 μM of IAA. RNA was extracted from snap-frozen follicles, reversed transcribed to cDNA, and subjected to quantitative real time PCR. Graphs represent means ± SEM from 3 separate cultures. Asterisks (*) indicate significant differences compared to control (P ≤ 0.05).
3.2.2. Cell Cycle Regulators
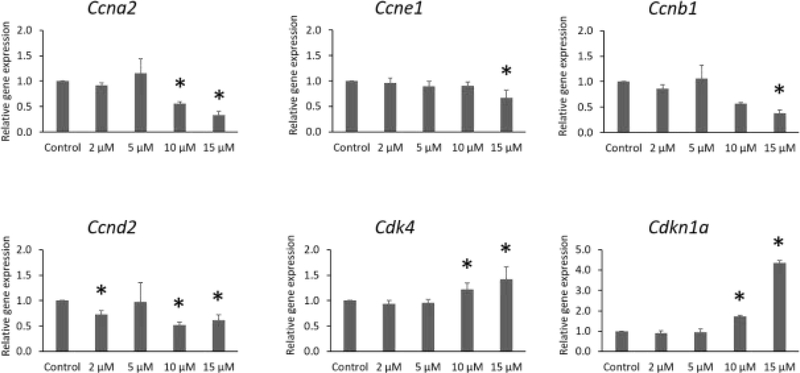
IAA exposure significantly altered expression of cell cycle stimulators (Fig. 3). Specifically, after 96 h of culture, IAA exposure decreased expression of Ccna2 (10 and 15 μM), Ccne1 (15 μM), Ccnb1 (15 μM), and Ccnd2 (2, 10, and 15 μM). Further, IAA slightly increased expression of Cdk4 (10 and 15 μM) compared to control. Interestingly, IAA exposure at 10 and 15 μM substantially increased expression of the cell cycle inhibitor Cdkn1a compared to the control group (Fig. 3, n = 3 separate cultures, 34 follicles, P ≤ 0.05).
Fig.3.
Effects of IAA exposure on gene expression of Ccna2, Ccne1, Ccnb1, Ccnd2, Cdk4, and Cdkn1a, in follicles treated with 2, 5, 10 and 15 μM of IAA. RNA was extracted from snap-frozen follicles, reversed transcribed to cDNA, and subjected to quantitative real time PCR. Graphs represent means ± SEM from 3 separate cultures. Asterisks (*) indicate significant differences compared to control (P ≤ 0.05).
3.2.3. Proliferation markers
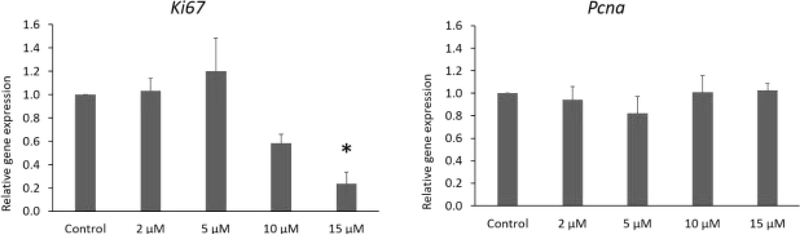
IAA significantly reduced expression of the proliferation marker Ki67 (15 μM) at 96 h of culture compared to control. However, IAA exposure did not affect Pcna at any dose compared to control. (Fig. 4, 3 separate cultures, 34 follicles, P ≤ 0.05).
Fig.4.
Effects of IAA exposure on gene expression of Ki67 and Pcna in follicles treated with 2, 5, 10 and 15 μM of IAA. RNA was extracted from snap-frozen follicles, reversed transcribed to cDNA, and subjected to quantitative real time PCR. Graphs represent means ± SEM from 3 separate cultures. Asterisks (*) indicate significant differences compared to the control (P ≤ 0.05).
3.2.4. Steroidogenesis genes
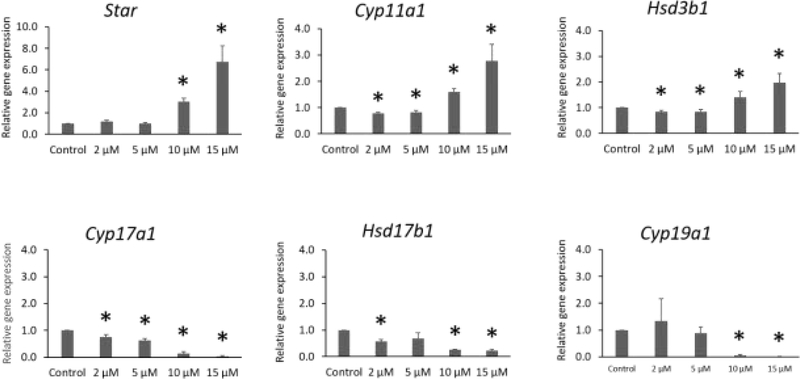
IAA exposure increased expression of Star (10 and 15 μM), Cyp11a1 (10 and 15 μM), and Hsd3b1 (10 and 15 μM) compared to control (Fig. 5). In contrast, IAA exposure decreased expression of Cyp17a1 (2, 5, 10, and 15 μM), Hsd17b1 (2, 10, and 15 μM), and Cyp19a1 (10 and 15μM) compared to control. In addition, IAA exposure slightly decreased of expression of Cyp11a1 (2 and 5 μM) and Hsd3b1 (2 and 5 μM) compared to control (Fig. 5, n = 3 separate cultures, 34 follicles, P ≤ 0.05).
Fig.5.
Effects of IAA exposure on gene expression of Star, Cyp11a1, Hsd3b1, Cyp17a1, Hsd17b, and Cyp19a1 in follicles treated with 2, 5, 10 and 15 μM of IAA. RNA was extracted from snap-frozen follicles, reversed transcribed to cDNA, and subjected to quantitative real time PCR. Graphs represent means ± SEM from 3 separate cultures. Asterisks (*) indicate significant differences compared to control (P ≤ 0.05).
3.2.5. Estrogen receptor genes
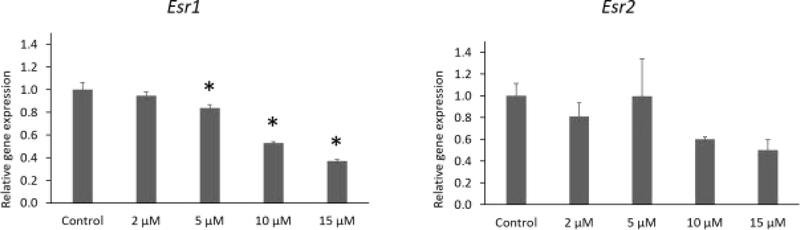
IAA exposure at 5, 10, and 15 μM significantly decreased expression of Esr1 compared to control. IAA exposure did not alter expression Esr2 compared to control (Fig. 6, n = 3 separate cultures, 34 follicles, P ≤ 0.05).
Fig.6.
Effects of IAA exposure on gene expression of Esr1 and Esr2 in follicles treated with 2, 5, 10 and 15 μM of IAA. RNA was extracted from snap-frozen follicles, reversed transcribed to cDNA, and subjected to quantitative real time PCR. Graphs represent means ± SEM from 3 separate cultures. Asterisks (*) indicate significant differences compared to the control (P ≤ 0.05).
3.3. The effects of IAA exposure on sex steroid hormone levels
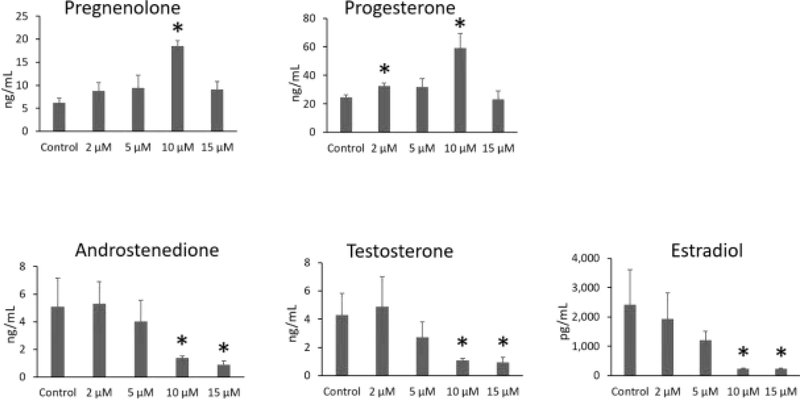
IAA exposure significantly increased the levels of pregnenolone (10 μM) and progesterone (2 μM and 10 μM) compared to control. In contrast, IAA exposure decreased the levels of androstenedione (10 μM and 15 μM), testosterone (10 μM and 15 μM), and estradiol (10 μM and 15 μM) compared to control (Fig. 7, n = 3 separate cultures, 34 follicles, P ≤ 0.05).
Fig.7.
Effects of IAA exposure on sex steroid hormone levels. Culture media were collected at 96 h of culture from 3 separate antral follicle cultures, and subjected to enzyme-linked immunosorbent assays for measurement of pregnenolone, progesterone, androstenedione, testosterone, and estradiol. Asterisks (*) indicate significant differences compared to control (P ≤ 0.05).
4. Discussion
Our results show that exposure to IAA inhibits follicle growth, dysregulates steroidogenesis, and alters the expression of estrogen receptor 1, and genes involved in apoptosis and the cell cycle in mouse ovarian follicles. Previously, a study in our laboratory demonstrated that exposure to haloacetic acids, including IAA, inhibited follicle growth and decreased the levels of estradiol in a culture system [19]. However, the previous study did not determine the mechanisms by which IAA inhibits follicle growth and decreases estradiol levels. In this study, IAA exposure affected follicle growth by altering apoptosis and cell cycle progression and IAA interfered with steroidogenesis by disrupting expression of steroidogenic enzymes and production of precursor hormones.
We demonstrated that IAA impaired follicle growth in vitro. Environmental chemicals can disrupt the expression of proteins involved in cell cycle regulation, affecting follicular cell proliferation [21, 25, 26]. We determined that IAA exposure decreased expression of cell cycle stimulators (Ccna2, Ccne1, Ccnb1, and Ccnd2) and increased expression of the cell cycle inhibitor Cdkn1a. These data are consistent with IAA inhibiting the cell cycle, which would lead to inhibition of follicle growth. Interestingly, IAA exposure slightly increased the expression of the cell cycle stimulator Cdk4 after 96 h in culture. In a similar manner, Hannon et al. [26] observed that di(2-ethylhexyl) phthalate (DEHP) increased the expression of cell cycle regulators, including Cdk4, in mouse ovarian follicles in vitro. They suggested that the early exposure to DEHP may have resulted in an initial push for cell cycle progression and proliferation to combat the toxicity of the chemical. In our study, the upregulation of the cell cycle stimulator Cdk4 might have been a compensation for the upregulation of the cell cycle inhibitor Cdkn1a, essentially a last effort to maintain normal cell proliferation. However, this compensation was not sufficient to overcome IAA-induced follicle growth inhibition. Additionally, IAA exposure decreased expression of the proliferation marker Ki67, an important factor that regulates the cell cycle progression and cell proliferation [27]. These data are also consistent with the decreased follicle growth observed in this study. In contrast, IAA exposure did not affect the expression of the proliferation marker Pcna.
We measured the effects of IAA exposure on the relative gene expression of the anti-apoptotic factors Bcl2 and Bcl2l10 and the pro-apoptotic factors Bax and Aimf1 in isolated follicles. Exposure to IAA significantly decreased expression of anti-apoptotic factors Bcl2 and Bcl2l10, whereas it increased expression of pro-apoptotic factors Bax and Aimf1. Interestingly, IAA exposure at 5 μM slightly decreased expression of Aimf1. Overall, these changes in expression will drive follicular cell death. In contrast, Zhou and Flaws [21] observed an increase in the expression of the anti-apoptotic factor Bcl-2 in mouse ovarian follicles that were exposed to an environmentally relevant phthalate mixture. Moreover, Hannon et al. [26] observed increased expression of pro- and anti-apoptotic factors following 24 h and 72 h of DEHP exposure in mouse ovarian follicles. These findings differ from our results, likely because different environmental chemicals may interfere with follicle growth via diverse mechanisms.
Ovarian antral follicles are an important source of sex steroid hormones. We measured the effects off IAA exposure on the levels of androstenedione, testosterone, estradiol, progesterone, and its precursor pregnenolone. IAA significantly increased the levels of pregnenolone and progesterone, and it decreased the levels of androstenedione, testosterone and estradiol. These data suggest that IAA exposure inhibits production of androgens (androstenedione and testosterone) from precursor hormones (pregnenolone and progesterone), resulting in a backup of progesterone and a decrease in downstream hormones. These alterations in hormones are consistent with our gene expression data. Exposure to IAA significantly increased expression of Star, Hsd3b1, and Cyp11a1 in the highest concentration groups. Interestingly, IAA exposure at 2 and 5 μM slightly decreased expression of Hsd3b1 and Cyp11a1. These factors are important for production of pregnenolone and progesterone and their upregulation would increase levels of pregnenolone and progesterone. Moreover, IAA exposure significantly decreased expression of Cyp17a1, Hsd17b1, and Cyp19a1, which are important for the production of androstenedione, testosterone and, estradiol. The downregulation of Cyp17a1, Hsd17b1, and Cyp19a1 would lead to decreases in androstenedione, testosterone, and estradiol levels.
The growth and atresia of ovarian follicles depends on the action of estrogens. Estrogens act through ESR1 and ESR2 to induce follicular cell proliferation and differentiation [28]. Mice lacking Esr1 are infertile because they are unable to ovulate [29]. Moreover, female mice lacking Esr2 have reduced litter number and size [29]. We observed that IAA not only reduced estradiol production, but it also decreased expression of Esr1 in mouse ovarian follicles, likely interfering with the follicles ability to respond to estradiol. This could explain, in part, the IAA-induced inhibition of follicle growth and steroidogenesis, as well as the increase in cell death.
In summary, IAA exposure decreased follicle growth, altered steroidogenesis, and impacted gene expression that interfered with cell proliferation and death, and ultimately impaired growth and steroidogenesis. Based on our data, we hypothesize that IAA disrupts cell proliferation and steroidogenesis to reduce the antral follicle growth. However, further studies are necessary to verify these alterations at different time points. Such studies would elucidate whether changes in gene expression precede changes in hormones or vice-versa and will be useful in determining the initial target of IAA in the ovary. Further, it has been shown that oxidative stress might play a role in IAA toxicity [30–32]; thus, it will be important for future studies to analyze oxidative stress markers in the follicles cultured with IAA. Although these data suggest that IAA could impact female fertility, further studies are in progress to investigate the effects of IAA on the female reproduction system in vivo.
Highlights.
IAA inhibits mouse antral follicle growth.
IAA interferes with ovarian steroidogenesis.
IAA reduces expression of cell cycle stimulators.
IAA alters expression of steroidogenic factors.
IAA is an ovarian toxicant.
Acknowledgements
This work was supported by grant numbers NIH R21 ES028963 and NIH T32 ES007326.
Abbreviations
- CHO cells
Chinese hamster ovary cells
- DBP
disinfection by-product
- DMSO
dimethylsulfoxide
- E2
estradiol
- IAA
iodoacetic acid
- α-MEM
α-minimal essential medium
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Transparency document
The Transparency document associated with this article can be found in the online version.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Richardson SD, et al. , Occurrence, genotoxicity, and carcinogenicity of regulated and emerging disinfection by-products in drinking water: a review and roadmap for research. Mutat Res, 2007. 636(1–3): p. 178–242 DOI: 10.1016/j.mrrev.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 2.EPA, U.S.E.P.A., Comprehensive Disinfectants and Disinfection Byproducts Rules (Stage 1 and Stage 2): Quick Reference Guide, Systems D.W.R.f.S.a.P.W., Editor. 2010, EPA: US. [Google Scholar]
- 3.NTP, N.T.P., Revised Draft: Report on Carcinogens Monograph on Haloacetic Acids Found as Water Disinfection By-Products, Services U.S.D.o.H.a.H., Editor. 2017, Office of the Report on Carcinogens, Division of the National Toxicology Program, National Institute of Environmental Health Sciences, U.S. Department of Health and Human Services USA; p. 194. [Google Scholar]
- 4.Grellier J, et al. , Assessing the human health impacts of exposure to disinfection by-products--a critical review of concepts and methods. Environ Int, 2015. 78: p. 61–81 DOI: 10.1016/j.envint.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Jones RR, et al. , Ingested nitrate, disinfection by-products, and risk of colon and rectal cancers in the Iowa Women’s Health Study cohort. Environ Int, 2019. 126: p. 242–251 DOI: 10.1016/j.envint.2019.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Righi E, et al. , Trihalomethanes, chlorite, chlorate in drinking water and risk of congenital anomalies: a population-based case-control study in Northern Italy. Environ Res, 2012. 116: p. 66–73 DOI: 10.1016/j.envres.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 7.Rivera-Nunez Z, Wright JM, and Meyer A, Exposure to disinfectant by-products and the risk of stillbirth in Massachusetts. Occup Environ Med, 2018. 75(10): p. 742–751 DOI: 10.1136/oemed-2017-104861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Villanueva CM, et al. , Disinfection byproducts and bladder cancer: a pooled analysis. Epidemiology, 2004. 15(3): p. 357–67. [DOI] [PubMed] [Google Scholar]
- 9.Wright JM, et al. , Disinfection By-Product Exposures and the Risk of Specific Cardiac Birth Defects. Environ Health Perspect, 2017. 125(2): p. 269–277 DOI: 10.1289/ehp103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hwang BF, Jaakkola JJ, and Guo HR, Water disinfection by-products and the risk of specific birth defects: a population-based cross-sectional study in Taiwan. Environ Health, 2008. 7: p. 23 DOI: 10.1186/1476-069x-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richardson SD, et al. , Occurrence and mammalian cell toxicity of iodinated disinfection byproducts in drinking water. Environ Sci Technol, 2008. 42(22): p. 8330–8 DOI: 10.1021/es801169k. [DOI] [PubMed] [Google Scholar]
- 12.Wei X, et al. , Drinking water disinfection byproduct iodoacetic acid induces tumorigenic transformation of NIH3T3 cells. Environ Sci Technol, 2013. 47(11): p. 5913–20 DOI: 10.1021/es304786b. [DOI] [PubMed] [Google Scholar]
- 13.Plewa MJ, et al. , Chemical and biological characterization of newly discovered iodoacid drinking water disinfection byproducts. Environ Sci Technol, 2004. 38(18): p. 4713–22 DOI: 10.1021/es049971v. [DOI] [PubMed] [Google Scholar]
- 14.Plewa MJ, et al. , Mammalian cell cytotoxicity and genotoxicity of the haloacetic acids, a major class of drinking water disinfection by-products. Environ Mol Mutagen, 2010. 51(8–9): p. 871–8 DOI: 10.1002/em.20585. [DOI] [PubMed] [Google Scholar]
- 15.Pals JA, et al. , Biological mechanism for the toxicity of haloacetic acid drinking water disinfection byproducts. Environ Sci Technol, 2011. 45(13): p. 5791–7 DOI: 10.1021/es2008159. [DOI] [PubMed] [Google Scholar]
- 16.Ali A, et al. , Effect of drinking water disinfection by-products in human peripheral blood lymphocytes and sperm. Mutat Res, 2014. 770: p. 136–43 DOI: 10.1016/j.mrfmmm.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 17.Attene-Ramos MS, Wagner ED, and Plewa MJ, Comparative human cell toxicogenomic analysis of monohaloacetic acid drinking water disinfection byproducts. Environ Sci Technol, 2010. 44(19): p. 7206–12 DOI: 10.1021/es1000193. [DOI] [PubMed] [Google Scholar]
- 18.Pals J, et al. , Human cell toxicogenomic analysis linking reactive oxygen species to the toxicity of monohaloacetic acid drinking water disinfection byproducts. Environ Sci Technol, 2013. 47(21): p. 12514–23 DOI: 10.1021/es403171b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeong CH, et al. , Monohaloacetic acid drinking water disinfection by-products inhibit follicle growth and steroidogenesis in mouse ovarian antral follicles in vitro. Reprod Toxicol, 2016. 62: p. 71–6 DOI: 10.1016/j.reprotox.2016.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teuschler LK, et al. , A feasibility study of cumulative risk assessment methods for drinking water disinfection by-product mixtures. J Toxicol Environ Health A, 2004. 67(8–10): p. 755–77 DOI: 10.1080/15287390490428224. [DOI] [PubMed] [Google Scholar]
- 21.Zhou C and Flaws JA, Effects of an Environmentally Relevant Phthalate Mixture on Cultured Mouse Antral Follicles. Toxicol Sci, 2017. 156(1): p. 217–229 DOI: 10.1093/toxsci/kfw245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta RK, et al. , Methoxychlor inhibits growth and induces atresia of antral follicles through an oxidative stress pathway. Toxicol Sci, 2006. 93(2): p. 382–9 DOI: 10.1093/toxsci/kfl052. [DOI] [PubMed] [Google Scholar]
- 23.Miller KP, et al. , Methoxychlor directly affects ovarian antral follicle growth and atresia through Bcl-2- and Bax-mediated pathways. Toxicol Sci, 2005. 88(1): p. 213–21 DOI: 10.1093/toxsci/kfi276. [DOI] [PubMed] [Google Scholar]
- 24.Pfaffl MW, A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res, 2001. 29(9): p. e45 DOI: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta RK, et al. , Di-(2-ethylhexyl) phthalate and mono-(2-ethylhexyl) phthalate inhibit growth and reduce estradiol levels of antral follicles in vitro. Toxicol Appl Pharmacol, 2010. 242(2): p. 224–30 DOI: 10.1016/j.taap.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hannon PR, et al. , Di(2-ethylhexyl) phthalate inhibits antral follicle growth, induces atresia, and inhibits steroid hormone production in cultured mouse antral follicles. Toxicol Appl Pharmacol, 2015. 284(1): p. 42–53 DOI: 10.1016/j.taap.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun X and Kaufman PD, Ki-67: more than a proliferation marker. Chromosoma, 2018. 127(2): p. 175–186 DOI: 10.1007/s00412-018-0659-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Britt KL and Findlay JK, Estrogen actions in the ovary revisited. J Endocrinol, 2002. 175(2): p. 269–76. [DOI] [PubMed] [Google Scholar]
- 29.Hamilton KJ, et al. , Estrogen Hormone Biology. Curr Top Dev Biol, 2017. 125: p. 109–146 DOI: 10.1016/bs.ctdb.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang J, et al. , Drinking water disinfection byproduct iodoacetic acid interacts with catalase and induces cytotoxicity in mouse primary hepatocytes. Chemosphere, 2018. 210: p. 824–830 DOI: 10.1016/j.chemosphere.2018.07.061. [DOI] [PubMed] [Google Scholar]
- 31.Cemeli E, et al. , Modulation of the cytotoxicity and genotoxicity of the drinking water disinfection byproduct lodoacetic acid by suppressors of oxidative stress. Environ Sci Technol, 2006. 40(6): p. 1878–83 DOI: 10.1021/es051602r. [DOI] [PubMed] [Google Scholar]
- 32.Wang S, et al. , Iodoacetic acid activates Nrf2-mediated antioxidant response in vitro and in vivo. Environ Sci Technol, 2014. 48(22): p. 13478–88 DOI: 10.1021/es502855x. [DOI] [PubMed] [Google Scholar]