Abstract
Objectives:
The purpose of this review was to provide a novel perspective utilizing an assessment of biomarkers to evaluate the impact of stress-related disorders on the progression of periodontal disease and evaluate the growing body of evidence of stress as a risk indicator for periodontal disease progression.
Methods:
Cross sectional, case control, and biomarker studies associating psychological disorders and periodontal disease were included in the literature search. Computational studies, animal studies, reviews, and studies lacking healthy controls were excluded. Electronic and manual literature searches were conducted by two independent reviewers in several databases as well as a manual search for relevant articles published up to January 2018.
Results:
Twenty-six articles fulfilled the inclusion criteria and were included in the qualitative synthesis. Relationships between stress-related disorders and serum and salivary biomarkers such as cortisol, dehydroepiandrosterone (DHEA), chromogranin A (CgA), and pro-inflammatory cytokines were identified.
Conclusions:
Use of salivary pro-inflammatory cytokines alone is not sufficient for identification of periodontal disease severity/progression with or without the presence of stress-associated diseases. Keeping in mind the limitations of this review, a positive qualitative correlation was observed in the literature among stress-related biomarkers and severity of periodontal disease. This correlation may serve as an important reporter of patient susceptibility for periodontal breakdown in the future.
Clinical Relevance:
Stress related disorders should be included in the list of globally screened diseases because it can change the biochemistry of both the local periodontal microenvironment as well as the global systemic inflammatory burden.
Keywords: Psychological stress, periodontitis, salivary cortisol, serum cortisol, interleukins, DHEA
Introduction:
The skeleton is a dynamic tissue that has the capacity to adapt to mechanical strain, withstand forces generated during bodily movement, while acting as a reservoir for calcemic demands. The dynamic balance of bone is primarily provided by the cellular building blocks: osteoblasts and osteoclasts, which carry out bone formation and bone destruction, respectively. Endocrine control of this multicellular balance is well studied. However, central regulation of bone mass is poorly understood. Interestingly the hypothalamic neuronal signaling axis and autonomic nervous system complex has been identified as a new participant in regulation of bone homeostasis and disease development.
The brain and its neuronal extensions have long been regarded as a master regulator of peripheral tissues across many organ systems, and recent studies have outlined regulatory processes extending between the hypothalamus (located in the brainstem) and the bone. This connection was established when neural tracts between the central nervous system and femoral bone marrow were identified using retrograde trans-synaptic signaling 1. From that point, study of these processes has revealed novel mechanisms whereby control of bone mass its regulatory connections that have a complexity that was previously unappreciated2. Stress-related disorders function through a two-fold process to affect bone mass and remodeling: 1) hypothalamic/adrenal cortex/cortisol signaling axis and 2) direct adrenergic nerve signaling, which collectively affects immune capacity, alters inflammatory signaling, and disrupts the bone remodeling balance.
Furthermore, data has emerged from the fields of psychoneuroimmunology demonstrating, through both animal models and clinical research, that stress-related disorders have increased expression of systemic inflammatory burden and anti-inflammatory agents may be a useful adjunctive psychiatric intervention3. Increased inflammatory burden and its co-factors remains a relevant measure in periodontal disease risk assessment, and its relevance to periodontal diseases is discussed in the present study.
Interestingly, there have been many reports linking stress-related disorders as a risk indicator for periodontal disease 4 The association of psychological stress was first ascertained in development of acute necrotizing ulcerative gingivitis, and now there is a growing consensus backed by new mechanistic insights between psychological stress and chronic periodontal diseases. The purpose of this review is to 1) provide a novel perspective utilizing an assessment of biomarkers and bone remodeling cytokines to evaluate the impacts of stress-related disorders on the progression of periodontal disease and 2) evaluate the growing body of evidence of stress as a risk indicator for periodontal disease progression.s
Methods:
Study Registration
The review protocol was registered and allocated the identification number CRD42018081541 in the PROSPERO International Prospective Register of Systematic Reviews hosted by the National Institute for Health Research, University of York, Centre for Reviews and Dissemination.
Objective of the review
The objective of this review was to address the following focused questions:
-
–
Are stress-related disorders associated with periodontal status or treatment outcomes?
-
–
Are there significant biological effects (biomarkers) of stress-related disorders on clinically measurable periodontal outcomes (probing depth (PD), clinical attachment level (CAL))?
A focused research question that would aid the literature search was developed, where the assigned PECO5 measures were the following:
Population (P): human subjects with periodontal diseases;
Exposure (E): Psychological stress evaluated via specialized questionnaires or biological biomarkers, serum cortisol, salivary cortisol, DHEA if present;
Comparison (C): Periodontally healthy subjects exhibiting no stress or anxiety;
Outcomes (O): The association between psychological disorders and the presence of periodontal disease.
Population:
The population of interest consisted of periodontally diseased patients with disease.classified based on clinical description of periodontal conditions (gingivitis, chronic periodontitis and aggressive periodontitis) or periodontal probing depths.
Exposure:
Psychological stress would be evaluated via specialized questionnaires or detecting the levels of various associated biomarkers, such as salivary cortisol, serum cortisol, Dehydroepiandrosterone (DHEA), crevicular interleukin (IL)-1, or crevicular IL-6.
Comparison:
To obtain a control group, only studies comprising a group of periodontally involved patients exhibiting no characteristics of stress/anxiety were included.
Outcomes:
The outcomes were the presence or the absence of the relationship between different forms of periodontal diseases and psychological stress.
Information sources for data extraction:
Electronic and manual literature searches were conducted independently by two authors (HA & MT) in multiple databases including MEDLINE (OVID), EMBASE (OVID) and Cochrane Central Register of Controlled Trials (Cochrane Library) for reports published up to December 2017 without any language restrictions. Moreover, the grey literature at the New York Academy of Medicine Grey Literature Report (http://greylit.org) and the register of clinical studies hosted by the US National Institutes of Health (www.clinicaltrials.gov) were searched to further identify potential candidates for inclusion. Additionally, a manual search of periodontics- and endocrinology-related journal issues was performed: Journal of Dental Research, Journal of periodontology, Journal of clinical periodontology, International Journal of Periodontics & Restorative Dentistry, and Journal of Endocrinology. Furthermore, reference lists/bibliographies of all candidate full-text articles were searched. Finally, two experts in the field (HW, WB) were consulted whether any additional reports can be included to our final search results.
Literature screening strategies:
-
–
MEDLINE: ((“periodontitis”[MeSH Terms] OR “periodontitis”[All Fields]) OR (“periodontal diseases”[MeSH Terms] OR (“periodontal”[All Fields] AND “diseases”[All Fields]) OR “periodontal diseases”[All Fields] OR (“periodontal”[All Fields] AND “disease”[All Fields]) OR “periodontal disease”[All Fields])) AND (“stress, psychological”[MeSH Terms] OR (“stress”[All Fields] AND “psychological”[All Fields]) OR “psychological stress”[All Fields] OR (“psychological”[All Fields] AND “stress”[All Fields]))
-
–
Embase: (((Anxiety[Title] OR stress[Title] OR depression[Title]))) AND ((periodontitis[MeSH Terms]) OR (periodontitis[Title] OR periodontal[Title]))
-
–
Cochrane: Periodontitis AND Salivary Cortisol OR Psychological stress OR Salivary Cortisol
Two independent reviewers (HA and MT) screened the titles, abstracts, and full texts of the articles that were identified. Data on the following issues were extracted and recorded: 1) citation, publication status, and year of publication; 2) study design; 3) characteristics of participants and group(s); 4) methodological characteristics of the trial and the approach used to evaluate periodontal health and presence of a psychological disorder; and 5) outcome measures.
Study Inclusion and Exclusion Criteria:
The titles and abstracts of identified articles were managed using a commercial citation manager (Endnote X6 Thomson, London, UK). The following inclusion criteria were applied:
-
1-
Cross sectional and case-control studies that investigated the association between psychological disorders and periodontal disease using predefined psychological scales.
-
2-
Studies that investigated the association between various biomarkers used to identify psychological disorders and periodontal disease.
At the second stage of selection, all full text articles identified during the first stage were reviewed. During this stage, the following exclusion criteria were followed:
Computational studies
Review papers
Animal studies
Studies that lack a healthy control group
During each stage, any disagreement was resolved via discussion with a third reviewer (AD).
Reporting format
The PRISMA-P checklist (supplemental Checklist 1) was followed for protocol preparation of this systematic review.6 All review methods were established entirely prior to conducting the review. The 27-item Preferred Reporting Items for Systematic Reviews and Meta- Analyses (PRISMA) statement 7 was employed in outlining and summarizing the results of search progression. The outline of the PRISMA guidelines screening process is summarized in figure 1.
Figure 1:
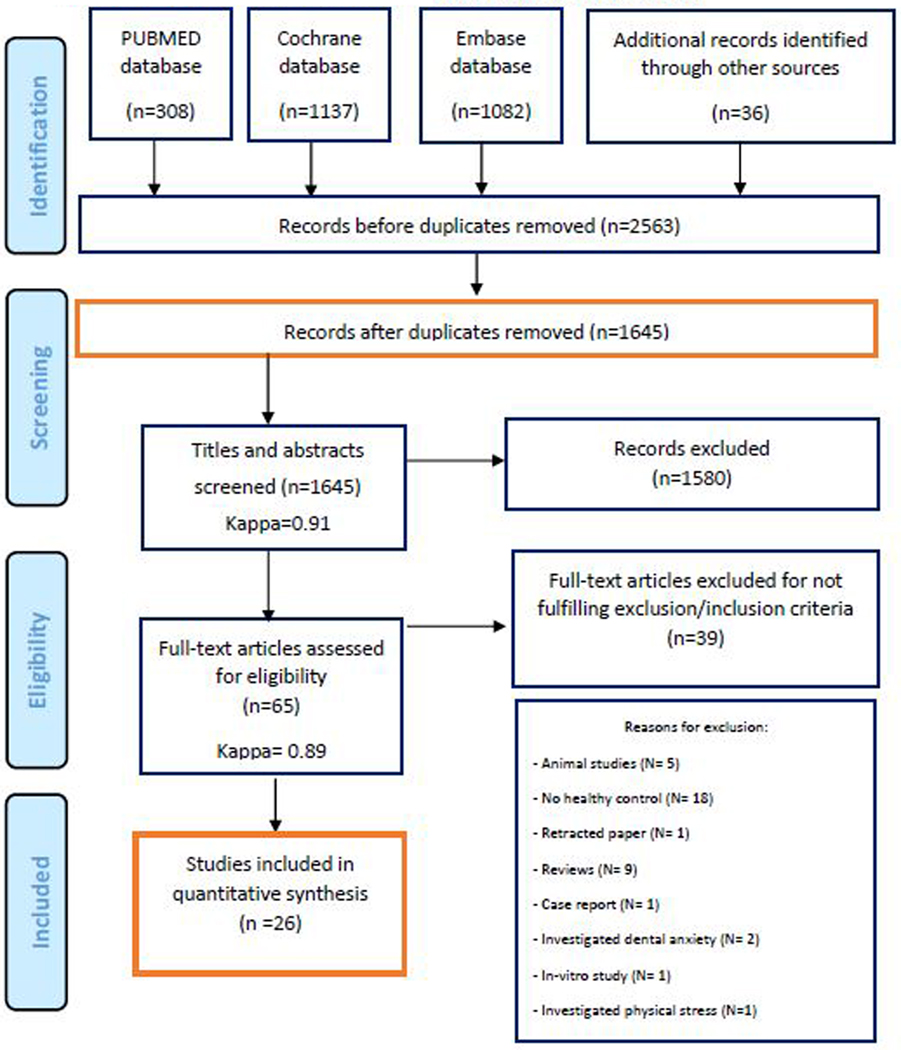
PRISMA flowchart of the screening process in the different databases.
Assessment of Methodological Quality and Risk of Bias and Data Extraction
The Newcastle-Ottawa Scale (NOS) was used to evaluate each of the included cross-sectional and case control studies. A study that achieved at least 7 out of 9 points was considered of high quality. Two authors (HA, MT) independently have evaluated the included studies and, based on obtaining mutual agreement, eliminated the discrepancies between each individual assessment. This inter-rater agreement was achieved through the Cohen kappa coefficient. The articles that fulfilled the inclusion criteria were selected for full text review and data extraction.
Results
Study protocol
The performed search and data extraction did not deviate from the proposed study protocol. The search for full-text articles, as well as the quality of selected studies were independently assessed by two reviewers using a standard method to enable us to appraise our internal validity (Supplementary Tables 1 and 2).
Study Selection and quality assessment:
Initial electronic screening yielded a total of 2527 articles (308 PubMed, 1137 EMBASE, 1082 Cochrane) and an additional 36 articles were found through manual screening. Overall, 1645 potentially relevant articles were selected to be evaluated by their titles and abstracts, of which, 65 full text articles were obtained for thorough evaluation. Only 26 of these articles fulfilled the inclusion criteria and were included in the qualitative synthesis of this review; excluding the remaining 39 articles. Figure 1 depicts the screening process based on the PRISMA guidelines and Supplementary Table 1 shows the exclusion justification following full-text evaluation. The k value for inter-reviewer agreement when screening for potentially relevant articles was 0.91 (titles and abstracts) and 0.98 (full-text articles), indicating a consistent agreement between the two reviewers. The results of the quality assessments pertaining to the included studies are outlined in Supplementary Table 2. Fifteen publications scored at least 7 out of 9 and were, thus, considered high quality. Whereas, 11 publications were considered as low quality due to scoring below 7 out of 9.
Cortisol levels and periodontal disease:
Hypercortisolemia appears to be correlated with progression of the periodontal disease. The majority of literature concurs with the assessment that cortisol levels are correlated with presence of periodontal disease clinical characteristics. Seven of the total included studies investigated the association between salivary or serum cortisol levels and the occurrence of periodontal disease (Table 1). All the included studies compared periodontally healthy to periodontally diseased subjects. Only one study8 exclusively used serum cortisol instead of salivary cortisol as a stress biomarker, reporting a positive correlation between the occurrence of chronic periodontitis and elevated levels of serum cortisol (psychological stress). The remainder of the studies (6) used salivary cortisol levels, where 4 9–12 demonstrated that elevated salivary cortisol positively correlated with periodontal disease and 2 13, 14 negated this correlation.
Table 1:
Studies that measured serum or salivary cortisol.
| Author, Year |
Sample Characteristics | Biomarkers | Stress Scale |
Outcomes | Cortisol Correlation |
||
|---|---|---|---|---|---|---|---|
| Country, Study design | Size | Mean Age (yrs) |
Group | ||||
| Haririan et al. 2017 | 21 | NS | AP | PSQ | |||
| Austria, CS | 35 | NS | CP | WS | N/C | ||
| SA PL Cortisol | WSS | No relationship established | N/C | ||||
| 44 | NS | H | SCQ | ||||
| NS | PD >4 & <6 | ||||||
| Fenol et al. 2017 | SA Cortisol | DASS | Increased stress scores correlated with PD | Positive | |||
| NS | 38.56 ± 10.878 | 4+ sites, ≥6 | |||||
| India, CS | NS | PD ≤3 | - | ||||
| Jaiswal et al. 2016 | 20 | NS | CP | PL cortisol | PSQ | Increased cortisol (PL) and psychological stress correlated with CP | Positive |
| India, CS | 20 | NS | H | ||||
| Seraphim et al. 2016 | 46 | N/S | H - Pregnant | SA cortisol | GQ | Increased perceived stress levels correlated with PD and G. | N/C |
| Brazil, CS | 24 | CP, Pregnant | |||||
| Mesa et al. 2014 | 41 | 53.7 ± 14.7 | CP | SA cortisol, SA sIgA | GQ | Cortisol (SA) correlated with PI, BOP, and tooth loss. | Positive |
| Spain, CS | 36 | 37.8 ± 19.1 | H | ||||
| Refulio et al. 2013 | 12 | NS | Mild CP | SA cortisol | ZSRDS | Cortisol (SA) correlated with CP | Positive |
| Brazil, CS | 3 | NS | Sev. CP | ||||
| Mannem et al. 2012 | 111 | 53.40 ± 9.00 | CP | SA cortisol | LSSAI | Cortisol (SA), work tension, economic problems, clinical stress syndrome correlated with CP | Positive |
| India, CS | 50.50 ± 9.00 | H | |||||
CS: Cross-sectional, CC: Case Control, CP: Chronic periodontitis, H: Healthy periodontium, G: Gingivitis, AP: Aggressive periodontitis, PL: Plasma, SA: Salivary, CRE: Crevicular, N/C: No correlation, Positive: Positive correlation. PSQ: Perceived Stress Questionnaire, SCQ: Stress Coping Questionnaire, WSS: Warning Signals for Stress, WS: Weariness scale, DASS: Depression, Anxiety, and Stress Scale, LSSAI: Lipp’s Stress Symptoms for Adults Inventory, ZSRDS: Zung Self Rating Depression, GQ: General questionnaire.
Inflammatory mediator levels, psychological stress and periodontal disease:
High levels of IL-β and IL-6 are correlated with periodontal disease. Five studies 15–19 investigated the relationship between stress-related inflammatory mediators and periodontal disease (Table 2). According to Giannopoulou et al. (2003), mediators IL-1b, IL-6 and IL-8 were positively correlated with levels of stress, in addition to reflecting chronic periodontal destruction 16. Conversely, Mousavijazi and coworkers (2013) reported the relationship with stress being limited to IL-1b, with no observed correlation with IL-6 19 Meanwhile, Kamma et al. (2004) and Aurer et al. (1999) reported an exclusive association between IL-6 and generalized aggressive periodontitis only 15, 17. However, despite the relationships observed in the remaining investigations, Mengel and coworkers (2002) have found no such correlation between the inflammatory mediators IL-1b and IL-6, and the occurrence of periodontal disease18 .
Table 2:
Studies that measured the inflammatory Interleukins.
| Author, Year | Sample Characteristics | Interleukins | Stress Scale | Outcomes | ||
|---|---|---|---|---|---|---|
| Country, Study Design | Size | Mean Age (yrs) |
Group | |||
| Giannopoulou et al.2003 | 20 | 52± 8 | AP | IL-1β, IL-6 and IL-8 (CRE) were significantly associated with stress and reflected periodontal destruction in G, CP, AP groups compared to H | ||
| 20 | 32 ± 2 | CP | CRE IL-1β, IL- | N/A | ||
| Greece, CS | 20 | 31 ± 8 | G | 4, IL-6 & IL-8 | ||
| 20 | 38 ± 11 | H | ||||
| MousaviJazi et al. 2013 | 25 | 44 | CP | |||
| Iran, CS | 25 25 |
41 24 |
AP H |
CRE IL-6 and IL-1β levels | KSQ | Stress severity, IL-1β correlated with AP & CP |
| Mengel et al. 2002 | 16 | 28 | Untreated GAP | |||
| Germany, CC | 14 | 28 | Treated GAP | IL-1β, IL-6 and cortisol | UMQ | N/C |
| 5 | 18 | Untreated LAP | levels | |||
| 5 | 52 | GCP | ||||
| Kamma et al. 2004 | 16 | 38 ± 8.8 | H / Smokers | |||
| Greece, CS | 19 | 38 ± 11.8 | H / Non-smokers | CRE IL-1β IL-4 | MPPSQ | IL-6 correlated with stress severity in smoking and AP status. |
| 39 | 32.4 ± 2.9 | AP / Smokers | IL-6 and I L-8 | |||
| 26 | 32.6 ± 2.8 | AP / Non-smokers | ||||
| Aurer et al. 1999 | 10 | 23 to 25 | H | |||
| Croatia, CS | 10 | 65 to 81 | Edentulous | SA Calcium, IL-6 | PTSDQ | IL-6 correlated with stress- related symptoms and GAP. |
| 20 | 26 to 46 | cp | ||||
| 20 | 19 to 46 | ap | ||||
CS: Cross-sectional, CC: Case Control, CP: Chronic periodontitis, H: Healthy periodontium, G: Gingivitis, AP: Aggressive periodontitis, PL: Plasma, SA: Salivary, CRE: Crevicular, N/C: No correlation, Positive: Positive correlation, KSQ: Kettle Stress Questionnaire, UMQ: University of Marburg Questionnaire, MPPSQ: Modified and Perceived Stress Scale Questionnaire, PTSDQ: PTSD-related symptoms Questionnaire.
Dehydroepiandrosterone (DHEA) and chromogranin A (CgA) levels and periodontal disease:
Dehydroepiandrosterone levels were associated with the presence of periodontitis. Two of the included studies20, 21 investigated the possible association between crevicular DHEA levels and periodontal disease, where one reported a positive correlation with aggressive periodontitis20 and the other with ascending severity of chronic periodontitis21. In another study, the relationship between (both, salivary and plasma) CgA and chronic periodontitis was investigated22. The results of this trial depicted significantly higher values of the stress biomarker CgA in periodontally compromised patients (Table 3).
Table 3:
Studies that measured other biomarkers.
| Author Year Country Study Design |
Size | Mean Age (yrs) |
Periodontal Status |
Biomarkers Tested | Stress Scale |
Outcomes |
|---|---|---|---|---|---|---|
| Cakmak et al. 2016 | 34 | 37 | GCP | DHEA (PL, SA), Cortisol (PL, SA) | BDI | Cortisol (SA) and DHEA (SA) values were significantly higher in more severely stress-scoring GAP |
| Turkey, CS | 27 | 32 | GAP | |||
| 31 | 36 | H | ||||
| Cakmak et al. 2014 | 39 | 41 | GCP | DHEA(CRE), Cortisol (CRE) | GQ | DHEA (CRE) significantly higher in more severely stress-scoring GCP compared to healthy controls |
| Turkey, CS | 41 | 36 | LCP | |||
| 40 | 35 | H | ||||
| Reshma et al. 2013 | 30 | NS | CP | CgA (PL, SA) | HRS | CgA (PL, SA) levels were higher in more severely stress-scoring CP |
| India, CC | 30 | NS | H |
CS: Cross-sectional, CC: Case Control, GCP: Generalized Chronic Periodontitis, LCP: Localized Chronic Periodontitis, H: Healthy Periodontium, GAP: Generalized Aggressive Periodontitis, PL: Plasma, SA: Salivary, CRE: Crevicular, BDI: Beck Depression Inventory, HRS: Holmes and Rahe Stress Scale, STAI: State Trait Anxiety Inventory Scale, GQ: General questionnaire
Scales of psychological assessment and periodontal disease:
From among the identified studies that have used scales of psychological assessment to investigate the relationship between quality and quantity of psychological stress and the occurrence of periodontal disease, only 11 successfully fulfilled the inclusion criteria (Table 4). While 9 of the total 11 studies reported a positive correlation between the occurrence of periodontal disease and elevated frequency of psychological stress, the remaining 2 studies found no correlation between the two factors. The scales used in evaluation psychological disorders to be related with the occurrence of periodontal disease included: Beck Anxiety Inventory (BAI)23, Beck Depression Inventory (BDI)24, Beck Hopelessness Scale25, State-Trait Anxiety Inventory (STAI)26, Life Events Scale (LES) , Life Events Scale modified by Savoia 27, Self-Report Screening Questionnaire-2028, Stress Symptom Inventory (SSI)29, the Social Readjustment Rating Scale (SRRS)30, Stress coping questionnaire 31, UCLA loneliness scale32, The Daily Strains Scale33, The Brief Symptom Inventory (SCL-90-R)34, and COPE Inventory35.
Table 4.
Studies that only used pre-prepared scale to measure the correlation between periodontitis and stress.
| Author, Year | Sample | Scale | Outcomes | Correlation between stress and PD |
||
|---|---|---|---|---|---|---|
| Country, Study Design | Size | Mean Age (yrs) |
Periodontal Status |
|||
| Kolte et al. 2016 | 30 | N/S | H, non-smokers | Stress from poor socioeconomic status correlated with increased PPD and CP in both smokers and non-smokers | ||
| Socioeconomic data | Positive | |||||
| India, CS | 30 | N/S | CP, nonsmokers | |||
| Castro et al. 2006 | 96 | CP | BAI, BDI, STAI, LES | Similar levels of anxiety observed in both CP and H groups. | ||
| 45.65±6.23 | N/C | |||||
| Brazil, CC | ||||||
| Vettore et al. 2005 | 20 | 45.9 ± 8.3 | H | Increased anxiety traits correlated with poor response to NSPT | ||
| STAI | Positive | |||||
| Brazil, CC | 20 | 46.1 ±7.8 | Severe CP | |||
| Johannsen et al. 2005 | 119 | 36.2 ± 2.8 | CP | Increased anxiety correlated with severity of CP in smokers | ||
| 22 | 36.2 ± 2.8 | AP | GQ | Positive | ||
| Sweden, CS | 26 | 35.4 ± 3.5 | H | |||
| Solis et al. 2004 | 160 | 42.91 ± 10.45 | CP | STAI, BDI, LES, BHS, GQ | No association was observed | N/C |
| 160 | ||||||
| Brazil, CS | 34.92 ± 10.21 | H | ||||
| 97 | 46.26 ± 8.59 | Moderate CP | SSI, SRRS, STAI | Increased anxiety scores correlated with increased PPD (>4mm) and AL | ||
| Vettore et al. 2003 | 46.30 ± 6.53 | Severe CP | Positive | |||
| Brazil, CS | 46.00 ± 8.19 | H | ||||
| Wimmer et al. 2002 | 89 | 42.5 ± 8.9 | CP | Poor/defensive coping strategies correlated with AL | ||
| SCQ | Positive | |||||
| Austria, CS | 63 | 31.6 ± 10.4 | H | |||
| Pistorius et al. 2002 | 242 | 41.3 ± 10.7 | H | Life event-related stress correlated with severe CP | ||
| H | GQ | Positive | ||||
| Sweden, CS | 242 | 43.2 ± 10.3 | CP | |||
| Croucher et al. 1997 | 50 | 42.4 ± 7.2 | H | SRRS | Negative life events correlated with both CP and smoking status. | Positive |
| England, CC | 50 | 42.3 ± 6.7 | CP | |||
| Silva et al. 1996 | 50 | 37.8 ± 5.24 | AP | STAI, MPSSQ | Depression and loneliness scores correlated with AP compared to CP and H | Positive |
| 50 | 45.2 ± 6.36 | CP | ||||
| England, CS | 50 | 38.8 ± 8.83 | H | |||
| Moss et al. 1996 | 71 | N/S | CP | DSS, SCL-90R, CI | Risk of CP approximately triples (OR=2.84) with a high strain score | |
| Positive | ||||||
| NY, USA, CC | 77 | N/S | H |
CS: Cross-sectional, CC: Case Control, CP: Chronic periodontitis, H: Healthy periodontium, G: Gingivitis, AP: Aggressive periodontitis, PL: Plasma, SA: Salivary, CRE: Crevicular, N/C: No correlation, Positive: Positive correlation. BDI: Beck Depression Inventory, BAI: Beck anxiety inventory, STAI: State Trait Anxiety Inventory Scale, LES: Life Events Scale, GQ: General Questionnaire, BHS: Beck Hopelessness Scale, SSI: Stress symptom inventory, SRRS: Social readjustment rating scale, SCQ: Stress coping questionnaire, MPPSQ: Modified and Perceived Stress Scale Questionnaire, DSS: Daily strains scale, SCL90R: Brief symptom inventory, CI: Cope inventory.
Discussion:
Periodontal disease is characterized by exacerbated bone loss/clinical attachment loss in the presence of an etiological stimulus of bacterial biofilm in a susceptible host. The extent and progression of the CAL loss is unique to the individual due to the presence of modifying factors that ultimately disrupt the bone remodeling apparatus. Increased understanding of stress related disorders and its biochemical tenants show both a physical and chemically connection capable of modifying the rate of periodontal tissue destruction in the presence of initiating factors. As such, through this research we propose the use of the following biomarkers in a comprehensive assessment of categorization in an attempt to achieve the reality of personalized medicine within the field of periodontology.
Salivary/crevicular/serum cortisol levels.
Of the articles reporting cortisol levels, 7 followed the inclusion criteria. There was significant heterogeneity among type of periodontal condition, stress scale used, and patient inclusion criteria between these articles, and as such statistical analysis could not be completed. However, a few trends were noted. Increasing stress scores correlated with increased levels of salivary and plasma cortisol levels in 5 of the 7 articles, with 2 of the 7 included articles observing no correlation was observed between stress scores and salivary cortisol levels, specifically. One of the two articles that did not observe a correlation (Seraphim et al., 2016) sampled a patient population that was pregnant, suggesting that these data might have a different relative cytokine and paracrine profile than other studies that met our inclusion criteria13. The second article (Haririan et al., 2017) that did not find a correlation between stress and salivary/plasma cortisol levels evaluated stress with cortisol in combination with periodontal disease severity14. The authors reported low response rate of fully completed questionnaires (at 66%) and it was unclear how they devised homologous comparisons of stress levels using data four different types of stress surveys, possibly contributing to the lack of correlation observed in that study.
Increasing evidence suggests that salivary/plasma cortisol may be correlated with stress and periodontal disease. However, due to the nature of cross-sectional study design, separation of disease severity and stress cannot be confirmed at this time. In line with this trend, Hilgert et al., 2006 previously reported salivary cortisol levels were positively correlated with deeper probing pocket depths and attachment loss36. Cakmak et al. (2016) concluded that salivary and GCF cortisol levels were higher in aggressive periodontitis and chronic periodontitis groups compared to healthy cohort20. Stress (as assessed by the Beck Depression Score) was found in the aggressive periodontitis group. Furthermore, Jaiswal et al (2016), recently investigated the correlation between serum cortisol values and self-assessed stress levels, finding a positive correlation with chronic periodontitis and thereby proposing a risk profile for periodontitis that involved stress and serum cortisol levels8. More recent work, involving accepted scale systems, showed that higher values on stress scales and anxiety ratings were correlated with salivary cortisol levels and larger probing pocket depth values. Toward this end, lower values on these accepted anxiety/depression scalers were associated with increased periodontal health9, 37. These data suggest that glucocorticoids travel systemically and locally affect the periodontal tissues. As such, biomarker assessment tools might be keen in the future to include glucocorticoid assessment as a tool to further identify personalized strategies in assessment of periodontal disease progression and treatment.
Glucocorticoid-induced osteoporosis is one of the most frequent forms of secondary osteoporosis in males and females38. Though the majority of glucocorticoid-induced osteoporosis is primarily caused by exogenous glucocorticoids through treatment of autoimmune, pulmonary, and gastrointestinal disorders, more recent reports of endogenous glucocorticoid (i.e. cortisol) levels are surfacing with important results and applications39. Furthermore, the incidence of hypercortisolism is growing with increased awareness for screening. Chiodini et al. (2007) showed that up to 10% of males attending an outpatient clinic of osteoporosis may have subclinical Cushing’s syndrome40. One endogenous glucocorticoid of particular interest to the medical community at-large is cortisol, an endocrine molecule released during psychological and physical stress. Dennison et al. (1999) reported that there was a statistically significant positive association between serum cortisol levels and bone loss rate at the lumbar spine, femoral neck, and trochanteric region over a 4-year follow-up period even after adjustment for adiposity, cigarette smoking, alcohol consumption, dietary calcium intake, physical activity, and gonadotropin hormone levels41. These data suggest that endogenous cortisol profile may be a useful indicator for rate of bone loss.
Glucocorticoids have both direct and indirect actions on bone. Glucocorticoids directly impair osteoblastic differentiation and survival42. Conversely, glucocorticoids promote mesenchymal precursor cells differentiation into adipocytes through inhibition of Wnt/beta-catenin signaling38,43. Furthermore, glucocorticoids inhibit osteoblast-driven synthesis of type I collagen, resulting in a decreased bone matrix available for mineralization. Modification of the elastic modulus surrounding the osteocyte lacunae and reduced mineral to matrix ratio can also account for increased lacunar size and disproportionate loss of bone strength in relation to bone mass44. Most importantly, glucocorticoids increase the expression of receptor activator of NF-kB ligand (RANKL) and decrease the expression of its soluble decoy receptor, osteoprotegerin (OPG) in stromal and osteoblastic cells, thus resulting increased bone resorption. In addition to osteoclastogenesis, glucocorticoids also enhance and prolong the lifespan of these bone resorbing cellular subunits45, 46. In addition to direct effects on bone homeostasis, glucocorticoids also have indirect effects through decreasing calcium absorption and reabsorption from the gastrointestinal tract and renal tubules, respectively47.
In recent years, significant effort has been made to characterize the oral microbiome that exists in health versus periodontal disease. The host-microbiome homeostatic profile is affected by many environmental factors, one of which is psychological stressors through suppression of the immune system. A recent study used a metatranscriptomic approach to delineate the effects of cortisol on the oral microbiome48. In fact, this group reported that cortisol directly induced shifts in gene expression profiles of the microbiome toward of disease and periodontal disease progression48. Thus, cortisol can also directly affect host susceptibility to periodontal disease and progression. This host susceptibility appears to be multifactorial, including factors of the microbial profile, host modulation, bone homeostasis, immune system regulation, inflammatory burden.
Salivary DHEA levels
Of the articles reporting DHEA or CgA plasma/serum levels, 3 articles followed the inclusion criteria. Of those articles there was significant heterogeneity among type of periodontal condition, stress scale used, and patient inclusion criteria, so statistical analysis could not be completed. In addition to DHEA levels, Cakmak (2016, 2014) evaluated cortisol in addition to DHEA in generalized chronic periodontitis, generalized aggressive periodontitis, localized chronic periodontitis, and healthy patients. While these articles did show an association between patients with aggressive periodontitis, it remains unclear if these factors are causative, due to the cross-sectional study design.
DHEA and cortisol are known adrenocorticotropic hormone-related steroids that are associated with periodontal disease severity49. Our findings echoed this report, but the connection between DHEA, stress-related diseases and the clinical outcomes of periodontitis progression are less clear. Interestingly, elevated salivary DHEA was reported to be a better indicator of depression compared to cortisol in medicated, but still clinically depressed, patients 50. In our analysis, higher levels of salivary DHEA levels were associated with increased periodontal destruction. These data were further supported by another study that suggested that plasma DHEA levels more adequately reflected hypothalamic-pituitary axis dysregulation than cortisol 51. Interestingly, Vittek (1984) found a correlation (r=0.88) of free circulating hormone levels of DHEA and its concentration in the saliva, but reported that it was unlikely that the increased levels of DHEA found in the saliva reflect leakage of protein-bound steroids into the mouth from the inflammation sites52. This study was repeated by other experts, who reported similar considerations53, 54
Proposed mechanisms for the observed salivary measurements included altered glandular and peripheral tissue production. Furthermore, biosynthesis of proteases in the submandibular gland were shown to be controlled by androgens and thyroid hormones 55. Moreover, specific receptors for androgens in the gingival epithelium suggest that proteases in epithelial cells may be controlled by these hormones 56. However, more work on these mechanisms and how they influence inflammatory mediators, host response to insult, and regenerative strategies requires more research for complete elucidation.
Inflammation/Interleukins
A range of evidence points to increased inflammation in stress-related disorders, including data from animal models and clinical research that discuss alterations in inflammatory markers and the use of anti-inflammatory agents as a psychiatric intervention treatment option3. A recent article by Franklin et al. (2018) demonstrated that stress induced inflammatory responses occurred via danger-associated molecular pattern (DAMP) pathways in microglial Neurons57. In fact, exposure to short-term stress was sufficient to increase receptor for advanced glycation end products (RAGE) messenger RNA as well as anhedonic behavior in rats. Interestingly, RAGE knock-out mice were resilient to stress-induced anhedonia. Furthermore, a recent human clinical study conducted by Holmes et al (2018) contributed novel results adding to the mounting evidence that suggests that elevated systemic inflammatory burden is clinically relevant and associated with stress-disorders including depression58. The authors used [11C](R)-PK11195 positron emission tomography to visualize neuroinflammation during depression episodes in 14 medication-free patients. These patterns were not present in the 13 matched healthy controls, and these data suggest that there is incentive to evaluate anti-inflammatory therapy in patients suffering from depressive disorders. Thus, these current and impactful psychoneuroimmunology studies suggest that there is a clinically relevant level of increased inflammatory burden among patients that have stress-disorders.
A variety of inflammatory molecules have been identified to monitor periodontal disease progression (i.e. IL-1B, IL-6, and MMPs) 59 Interestingly, four of the five articles identified in our literature search that correlated stress-disorders with increased inflammatory chemokines. The most recent report (Mousavijazi et al. 2013) described a study investigating the association between stress disorders and elevation of inflammatory mediators related to periodontal disease in adult patients and stratified their analysis by chronic periodontitis and aggressive periodontitis with treated and untreated subcategories19. The authors observed that stress-disorders had a pivotal role in the stimulation of inflammatory processes via increase of crevicular IL-1B in both aggressive and chronic periodontitis compared to matched controls.
Alternatively, one of the five articles that followed the inclusion criteria showed no correlation between inflammatory chemokines and registered stress values. Mengel et al. (2002) measured chemokines in the peripheral blood of patients with periodontal disease and recorded any interactions with psychosocial stress18. When discussing the results of this article, the authors stated that the stress evaluation was heterogeneous and non-standardized. Interestingly, the authors did note a more critical assessment of the questionnaire revealed family-induced stress correlated significantly with periodontal tissue loss. This correlation suggests that a relationship between stress and immunological mediators may be present and require rigor in understanding temporal and chronic psychosocial stress.
We know that systemic inflammatory burden is a well-established risk factor for periodontal disease progression, and are beginning to understand the complex connections that inflammatory-associated diseases are affiliated. In summary, stress-related disorders (i.e. depression, anxiety) increase the inflammatory burden of an individual’s system, which increases the risk of periodontal patient relapse or progression. As such, individuals with stress disorders should be monitored more closely and tighter hygiene recalls should be discussed.
Limitations and recommendations for future research
This review is not without limitation. Because there was limited collection of clinical attachment level measurements, the subsequent analysis could only infer clinical assessment of bone loss or gain from reported pocket depth measurements. In addition, systemic measurements must be taken to compare global health effects of stress-related disorders on global inflammatory markers, such as CRP, or global organ health such as kidney or liver functional testing.
Conclusion:
Whether periodontal disease severity follows, or stems from, stress/cortisol levels or other biomarkers discussed in this paper is still unknown; however, associations of the multi-factorial array of cortisol, DHEA, and inflammatory biomarkers in combination with observations of increased disease severity suggest that a relationship between psychological stress and periodontal disease warrants further investigation. As such, moving forward into the practice of personalized medicine and the clinical treatment planning for large guided bone regeneration procedures, sinus augmentation, or full mouth rehabilitation using implant therapy, individuals should be screened for systemic inflammatory burden to help optimize the successful outcomes of these procedures. Stress related disorders should be included in the list of globally screened diseases because it can change the biochemistry of both the local periodontal microenvironment as well as the global systemic inflammatory burden.
Supplementary Material
Supplementary Table 1: List of excluded articles.
Supplementary Table 2: Newcastle Ottawa Scale quality assessment.
Supplementary Checklist 1: PRISMA 2015 Checklist
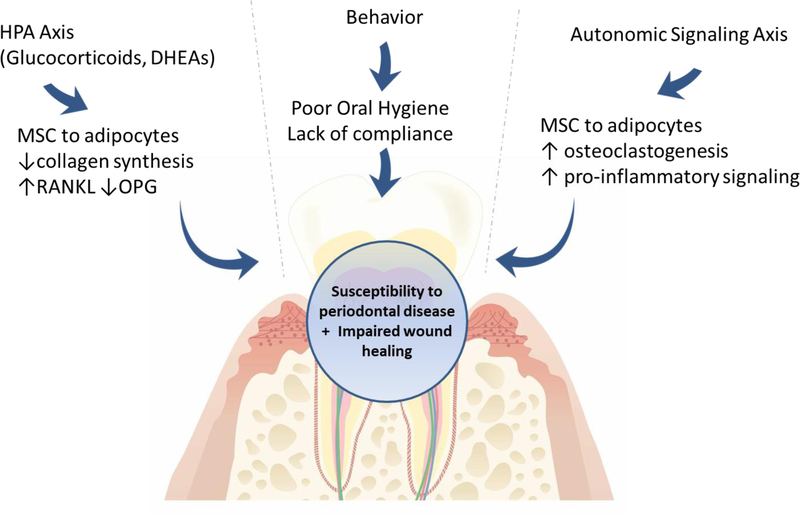
Figure 2:
Adrenergic control of bone remodeling in the progression of periodontal disease is complex and guided by many local and systemic factors. Stress-related disorders function through a two-fold process to affect bone mass and remodeling: 1) hypothalamic/adrenal cortex/cortisol signaling axis and 2) Direct adrenergic nerve signaling, which collectively affects immune capacity, alters inflammatory signaling, and disrupts the bone remodeling balance.
Acknowledgments
Funding:
This paper was partially supported by the University of Michigan Periodontal Graduate Student Research Fund. AMD is funded by the Tissue Engineering and Regenerative Medicine fellowship (5T32DE007057-42).
Footnotes
Compliance with Ethical Standards:
Conflict of interest:
The authors deny any conflicts of interest related to this study, we do not have any financial interests, either directly or indirectly from the information listed in the paper.
Ethical approval:
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent:
For this type of study, formal consent is not required. /Informed consent was obtained from all individual participants included in the study.
References:
- 1.Denes A, Boldogkoi Z, Uhereczky G, et al. Central autonomic control of the bone marrow: multisynaptic tract tracing by recombinant pseudorabies virus. Neuroscience 2005;134(3):947–63. [DOI] [PubMed] [Google Scholar]
- 2.Corr A, Smith J, Baldock P. Neuronal control of bone remodeling. Toxicologic pathology 2017;45(7):894–903. [DOI] [PubMed] [Google Scholar]
- 3.Stein DJ, Naudé PJ, Berk M. Stress, Depression, and Inflammation: Molecular and Microglial Mechanisms. Biological psychiatry 2018;83(1):5. [DOI] [PubMed] [Google Scholar]
- 4.Genco RJ, Borgnakke WS. Risk factors for periodontal disease. Periodontology 2000 2013;62(1):59–94. [DOI] [PubMed] [Google Scholar]
- 5.Stone PW. Popping the (PICO) question in research and evidence-based practice. Appl Nurs Res 2002;15(3):197–8. [DOI] [PubMed] [Google Scholar]
- 6.Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015;350:g7647. [DOI] [PubMed] [Google Scholar]
- 7.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009;62(10):1006–12. [DOI] [PubMed] [Google Scholar]
- 8.Jaiswal R, Shenoy N, Thomas B. Evaluation of association between psychological stress and serum cortisol levels in patients with chronic periodontitis - Estimation of relationship between psychological stress and periodontal status. J Indian Soc Periodontol 2016;20(4):381–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fenol A, Jebi S, Krishnan S, et al. Association of stress, salivary cortisol level, and periodontitis among the inmates of a central prison in Kerala. Dent Res J (Isfahan) 2017;14(4):288–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mesa F, Magan-Fernandez A, Munoz R, et al. Catecholamine metabolites in urine, as chronic stress biomarkers, are associated with higher risk of chronic periodontitis in adults. J Periodontol 2014;85(12):1755–62. [DOI] [PubMed] [Google Scholar]
- 11.Refulio Z, Rocafuerte M, de la Rosa M, Mendoza G, Chambrone L. Association among stress, salivary cortisol levels, and chronic periodontitis. J Periodontal Implant Sci 2013;43(2):96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mannem S, Chava VK. The effect of stress on periodontitis: A clinicobiochemical study. J Indian Soc Periodontol 2012;16(3):365–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seraphim AP, Chiba FY, Pereira RF, et al. Relationship among Periodontal Disease, Insulin Resistance, Salivary Cortisol, and Stress Levels during Pregnancy. Braz Dent J 2016;27(2):123–7. [DOI] [PubMed] [Google Scholar]
- 14.Haririan H, Andrukhov O, Bottcher M, et al. Salivary Neuropeptides, Stress and Periodontitis. J Periodontol 2017:1–15. [DOI] [PubMed] [Google Scholar]
- 15.Aurer A, Aurer-Kozelj J, Stavljenic-Rukavina A, et al. Inflammatory mediators in saliva of patients with rapidly progressive periodontitis during war stress induced incidence increase. Coll Antropol 1999;23(1):117–24. [PubMed] [Google Scholar]
- 16.Giannopoulou C, Kamma JJ, Mombelli A. Effect of inflammation, smoking and stress on gingival crevicular fluid cytokine level. J Clin Periodontol 2003;30(2):145–53. [DOI] [PubMed] [Google Scholar]
- 17.Kamma JJ, Giannopoulou C, Vasdekis VG, Mombelli A. Cytokine profile in gingival crevicular fluid of aggressive periodontitis: influence of smoking and stress. J Clin Periodontol 2004;31(10):894–902. [DOI] [PubMed] [Google Scholar]
- 18.Mengel R, Bacher M, Flores-De-Jacoby L. Interactions between stress, interleukin-1beta, interleukin-6 and cortisol in periodontally diseased patients. J Clin Periodontol 2002;29(11):1012–22. [DOI] [PubMed] [Google Scholar]
- 19.Mousavijazi M, Naderan A, Ebrahimpoor M, Sadeghipoor M. Association between psychological stress and stimulation of inflammatory responses in periodontal disease. J Dent (Tehran) 2013;10(1):103–11. [PMC free article] [PubMed] [Google Scholar]
- 20.Cakmak O, Tasdemir Z, Aral CA, Dundar S, Koca HB. Gingival crevicular fluid and saliva stress hormone levels in patients with chronic and aggressive periodontitis. J Clin Periodontol 2016;43(12):1024–31. [DOI] [PubMed] [Google Scholar]
- 21.Cakmak O, Alkan BA, Ozsoy S, Sen A, Abdulrezzak U. Association of gingival crevicular fluid cortisol/dehydroepiandrosterone levels with periodontal status. J Periodontol 2014;85(8):e287–94. [DOI] [PubMed] [Google Scholar]
- 22.Reshma AP, Arunachalam R, Pillai JK, et al. Chromogranin A: Novel biomarker between periodontal disease and psychosocial stress. J Indian Soc Periodontol 2013;17(2):214–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steer RA, Ranieri WF, Beck AT, Clark DA. Further Evidence for the Validity of the Beck Anxiety Inventory with Psychiatric Outpatients. Journal of Anxiety Disorders 1993;7(3):195–205. [Google Scholar]
- 24.Beck AT, Erbaugh J, Ward CH, Mock J, Mendelsohn M. An Inventory for Measuring Depression. Archives of General Psychiatry 1961;4(6):561-&. [DOI] [PubMed] [Google Scholar]
- 25.Beck AT, Weissman A, Lester D, Trexler L. The measurement of pessimism: the hopelessness scale. J Consult Clin Psychol 1974;42(6):861–5. [DOI] [PubMed] [Google Scholar]
- 26.Spielberger CD, Gorsuch RL, Lushene RE. Manual for the state-trait anxiety inventory. Paolo Alto: Consulting Psychologists Press; 1970. [Google Scholar]
- 27.Savoia MG. Relação entre eventos vitais adversos e mecanismos de ‘‘coping’’ no transtorno de pânico. [São Paulo, Brazil: Universidade de São Paulo; 1995. [Google Scholar]
- 28.Harding TW, de Arango MV, Baltazar J, et al. Mental disorders in primary health care: a study of their frequency and diagnosis in four developing countries. Psychol Med 1980;10(2):231–41. [DOI] [PubMed] [Google Scholar]
- 29.Lipp MEN, Guevara AJH. Validação empírica do Inventário de Sintomas de Stress (ISS). Estudos de Psicologia 1994;11:43–49. [Google Scholar]
- 30.Holmes TH, Rahe RH. The Social Readjustment Rating Scale. J Psychosom Res 1967;11(2):213–8. [DOI] [PubMed] [Google Scholar]
- 31.Janke W, Erdmann G, W K. The Stress Coping Questionnaire (SVF) Manual. Göttingen: Verlag für Psychologie, Dr. CJ Hogrefe; 1985:7–30. [Google Scholar]
- 32.Russell D, Peplau LA, Cutrona CE. The revised UCLA Loneliness Scale: concurrent and discriminant validity evidence. J Pers Soc Psychol 1980;39(3):472–80. [DOI] [PubMed] [Google Scholar]
- 33.Pearlin LI, Schooler C. The structure of coping. J Health Soc Behav 1978;19:2–21. [PubMed] [Google Scholar]
- 34.Derogatis LR, Melisaratos N. The Brief Symptom Inventory: an introductory report. Psychol Med 1983;13(3):595–605. [PubMed] [Google Scholar]
- 35.Carver CS, Scheier MF, Weintraub JK. Assessing Coping Strategies - a Theoretically Based Approach. Journal of Personality and Social Psychology 1989;56(2):267–83. [DOI] [PubMed] [Google Scholar]
- 36.Hilgert J, Hugo F, Bandeira D, Bozzetti M. Stress, cortisol, and periodontitis in a population aged 50 years and over. Journal of dental research 2006;85(4):324–28. [DOI] [PubMed] [Google Scholar]
- 37.Katuri KK, Dasari AB, Kurapati S, et al. Association of yoga practice and serum cortisol levels in chronic periodontitis patients with stress-related anxiety and depression. Journal of International Society of Preventive & Community Dentistry 2016;6(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Canalis E, Mazziotti G, Giustina A, Bilezikian J. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporosis International 2007;18(10):1319–28. [DOI] [PubMed] [Google Scholar]
- 39.Briot K, Roux C. Glucocorticoid-induced osteoporosis. RMD open 2015;1(1):e000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chiodini I, Mascia ML, Muscarella S, et al. Subclinical hypercortisolism among outpatients referred for osteoporosis. Annals of internal medicine 2007;147(8):541–48. [DOI] [PubMed] [Google Scholar]
- 41.Dennison E, Hindmarsh P, Fall C, et al. Profiles of endogenous circulating cortisol and bone mineral density in healthy elderly men. The Journal of Clinical Endocrinology & Metabolism 1999;84(9):3058–63. [DOI] [PubMed] [Google Scholar]
- 42.Canalis E, Giustina A. Glucocorticoid-induced osteoporosis: summary of a workshop. The Journal of Clinical Endocrinology & Metabolism 2001;86(12):5681–85. [DOI] [PubMed] [Google Scholar]
- 43.Westendorf JJ, Kahler RA, Schroeder TM. Wnt signaling in osteoblasts and bone diseases. Gene 2004;341:19–39. [DOI] [PubMed] [Google Scholar]
- 44.Lane NE, Yao W, Balooch M, et al. Glucocorticoid-treated mice have localized changes in trabecular bone material properties and osteocyte lacunar size that are not observed in placebo-treated or estrogen-deficient mice. Journal of bone and mineral research 2006;21(3):466–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yao W, Cheng Z, Busse C, et al. Glucocorticoid excess in mice results in early activation of osteoclastogenesis and adipogenesis and prolonged suppression of osteogenesis: a longitudinal study of gene expression in bone tissue from glucocorticoid-treated mice. Arthritis & Rheumatism: Official Journal of the American College of Rheumatology 2008;58(6):1674–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kondo T, Kitazawa R, Yamaguchi A, Kitazawa S. Dexamethasone promotes osteoclastogenesis by inhibiting osteoprotegerin through multiple levels. Journal of cellular biochemistry 2008;103(1):335–45. [DOI] [PubMed] [Google Scholar]
- 47.Canalis E, Giustina A, Bilezikian JP. Mechanisms of anabolic therapies for osteoporosis. New England Journal of Medicine 2007;357(9):905–16. [DOI] [PubMed] [Google Scholar]
- 48.Duran-Pinedo AE, Solbiati J, Frias-Lopez J. The effect of the stress hormone cortisol on the metatranscriptome of the oral microbiome. npj Biofilms and Microbiomes 2018;4(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ishisaka A, Ansai T, Soh I, et al. Association of salivary levels of cortisol and dehydroepiandrosterone with periodontitis in older Japanese adults. Journal of periodontology 2007;78(9):1767–73. [DOI] [PubMed] [Google Scholar]
- 50.Assies J, Visser I, Nicolson NA, et al. Elevated salivary dehydroepiandrosterone-sulfate but normal cortisol levels in medicated depressed patients: preliminary findings. Psychiatry research 2004;128(2):117–22. [DOI] [PubMed] [Google Scholar]
- 51.Fabian TJ, Dew MA, Pollock BG, et al. Endogenous concentrations of DHEA and DHEA-S decrease with remission of depression in older adults. Biological psychiatry 2001;50(10):767–74. [DOI] [PubMed] [Google Scholar]
- 52.Vittek J, Kirsch S, Rappaport S, Bergman M, Southren A. Salivary concentrations of steroid hormones in males and in cycling and postmenopausal females with and without periodontitis. Journal of periodontal research 1984;19(5):545–55. [DOI] [PubMed] [Google Scholar]
- 53.Mandel ID. The diagnostic uses of saliva. Journal of Oral Pathology & Medicine 1990;19(3):119–25. [DOI] [PubMed] [Google Scholar]
- 54.Kaufman E, Lamster IB. The diagnostic applications of saliva—a review. Critical Reviews in oral biology & medicine 2002;13(2):197–212. [DOI] [PubMed] [Google Scholar]
- 55.Takuma T, Tanemura T, Hosoda S, Kumegawa M. Effects of thyroxine and 5α-dihydrotesterone on the activities of various enzymes in the mouse submandibular gland. Biochimica et Biophysica Acta (BBA)-General Subjects 1978;541(2):143–49. [DOI] [PubMed] [Google Scholar]
- 56.Hernandez M, Wenk E, Southren A, Rappaport S, Vittek J. LOCALIZATION OF ANDROGENS-H-3 IN HUMAN GINGIVA BY AUTORADIOGRAPHY. Paper presented at: Journal of Dental Research, 1981. [Google Scholar]
- 57.Franklin TC, Wohleb ES, Zhang Y, et al. Persistent increase in microglial RAGE contributes to chronic stress-induced priming of depressive-like behavior. Biological psychiatry 2018;83(1):50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Holmes SE, Hinz R, Conen S, et al. Elevated translocator protein in anterior cingulate in major depression and a role for inflammation in suicidal thinking: a positron emission tomography study. Biological psychiatry 2018;83(1):61–69. [DOI] [PubMed] [Google Scholar]
- 59.Jaedicke KM, Preshaw PM, Taylor JJ. Salivary cytokines as biomarkers of periodontal diseases. Periodontology 2000 2016;70(1):164–83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: List of excluded articles.
Supplementary Table 2: Newcastle Ottawa Scale quality assessment.
Supplementary Checklist 1: PRISMA 2015 Checklist