Abstract
In biparental species, each parent represents a semi-independent source of variable caregiving. The nature of care may differ between parents, and the type of care offspring seek is likely to change across development. We asked if caregiving differed between prairie vole (Microtus ochrogaster) mothers and fathers, and which parent pups prefer over development. We categorized parents as high- or low-contact based on daily recordings of grooming and brooding behavior. Pups were tested for their preferences between parents on postnatal days 10, 14, 18, 22, and 26. We expected individual parents would vary in the amount of care they gave, with pups preferring the parent that gives the most care and/or that most meets their needs at each developmental timepoint. Mothers spent more time in contact with pups than fathers. Mothers were consistent in the amount of care they gave, whereas fathers were variable caregivers. Pups never preferred fathers over mothers, but only demonstrated a preference for mothers before weaning. Lastly, the amount of contact did not influence pup preferences. Our data indicate that mothers are consistent sources of caregiving relative to fathers, and pups show little evidence of attachment to a specific parent beyond meeting its own immediate needs.
Keywords: Attachment, Developmental Needs, Parental Care, Paternal Care, Prairie Vole, Preference
1. INTRODUCTION
It is commonly believed that parent-offspring attachment is a strong bond formed early and persisting through life (Bowlby 1982). Such bonds serve an important life-sustaining purpose for offspring. For example, offspring derive several benefits from parental care including protection from the environment (e.g., extreme temperatures, predation), nourishment (e.g., food provisioning in the form of lactation and food delivery), and emotional support (i.e., social contact) (Clutton-Brock 1991; Alonso-Alvarez & Velando 2012). Indeed, parental care can have a profound impact on the development of young well into adulthood. Maternal care is well recognized for its important role in offspring physiological and behavioral development, and its influence on the epigenetic control of phenotype (Liu et al. 1997; Caldji et al. 1998; Francis et al. 1999; Weaver et al. 2004). Although there are comparatively fewer studies on the long-term impact of paternal care in mammals, paternal care is also known to impact physical development, behavior, cognition, and reproductive success (Cantoni & Brown 1997; Piovanotti & Vieira 2004; Cao et al. 2014; Bales & Saltzman 2016).
As with all behavior, some degree of individual variation in parental care should be expected across individuals and contexts (Royama 1996; Wright et al. 1998; Freeman-Gallant & Rothstein 1999; Schwagmeyer & Mock 2003; Maccooll & Hatchwell 2007). In uniparental species, the consequences of variable care are large because only one parent is responsible for the care, protection, and nourishment of the offspring. Biparental species, in contrast, have the opportunity to share the costs of care and can presumably compensate for parental deficiencies or variability in parental care of a partner (Whittingham & Roberstson 1993; McNamara et al. 1999; Nakagawa et al. 2007). At the same time, offspring are exposed to two sources of potential variability in caregiving. This sets up an interesting dynamic between the two caregivers in a biparental pair, and an even more interesting dynamic between the offspring and each parent. For example, will offspring develop preferences for a particular caregiver over another, and if so why?
Offspring needs change over the course of postnatal development. In altricial young, protection from the elements is completely in the hands of the parents. For example, the need for thermoregulation is greatest when pups are youngest and most vulnerable (Case 1978; Evans 1992). As offspring mature and become independently mobile, the reliance on parents decreases. However, parents still provide care, nourishment, and social contact (Russell 1982; Martin 1984). The extent to which offspring seek the contact of caregivers should reflect their immediate needs at a given particular point in development (Alberts 2008). By this logic, evaluating offspring preferences for parents at multiple stages of postnatal development has the potential to reveal current offspring needs. Furthermore, the degree to which offspring seek social contact with parents at all, and specifically with respect to relatively more responsive parents, could reveal the extent to which an offspring expresses social attachment with one or both parents, or if the basis of the offspring-parent relationship is purely utilitarian (i.e., pups seek care for survival from parents that incur personal costs to invest in them).
Prairie voles (Microtus ochrogaster) are socially monogamous, biparental rodents native to the Midwestern United States (Getz & Carter 1996; Wang & Insel 1996). Thomas and Birney (1979) argued that both mothers and fathers contribute similarly to parental care, with fathers providing all aspects of care short of lactation. Both male and female parents build and maintain the nest, cache food, and groom and brood the pups (Thomas & Birney 1979). Although male and female prairie voles contribute great effort to caregiving on balance, parental effort across individuals varies and parents can be classified as high or low contact pairs (Perkeybile et al. 2013).
The present study considered prairie vole parental care from the perspective of both parents and pups. We asked if mothers and fathers differ in the total amount of care they give pups. We also evaluated the individual variation in caregiving across parents. From an offspring perspective, we examined if pups have preferences for one parent over the other and, if so, whether preferences change over developmental time. Finally, we asked if pups would prefer high care parents to low care parents.
2. METHODS
2.1. Animal Welfare
All procedures used in this study were approved by the Institutional Animal Care and Use Committee of Cornell University (ACUP 2013-0102). The work conducted here was entirely behavioral and all animals fell under the USDA/AWA pain/distress category C. The work involved minimal transport of animals, and brief exposure to testing apparatus and conspecifics (see below). No animals in this experiment were raised in isolation.
2.2. Subjects
Thirty breeding pairs were created specifically for this experiment. The breeders were the F1 generation from our colony of voles, which were originally trapped in Champaign County, IL, USA. Before pairing, animals were weaned into same-sex sibling groups at postnatal day (PND) 21. The animals were housed in standard polycarbonate rodent cages (29 × 18 × 13 cm) with Sani-Chip bedding, provided with nesting material, and kept on a 14:10 L:D cycle, with lights on at 0800 h. Animals were provided Rodent Chow 5000 (Harlan Teklad, Madison, WI, USA) and water ad libitum. Ambient temperature was maintained at 20 ± 2°C. At the time of use, all animals were sexually naïve adults (between 50 and 150 days old).
Female prairie voles exhibit induced estrus and ovulation (Carter et al. 1980). Prior to pairing, we induced sexual receptivity in females by exposing them for 48 h to soiled bedding and nesting material from an unrelated and unfamiliar male with which they would ultimately be paired (Richmond & Stehn 1976; Carter et al. 1980; Dluzen et al. 1981). This method robustly and reliably induces estrus and sexual receptivity in female prairie voles. Males were also exposed to the soiled bedding from the female vole with which they would be paired. The day before pairs were created, males were outfitted with a light blue zip-tie around their neck. This allowed us to distinguish between fathers and mothers in our daily cage recordings. Pairs were closely monitored for pregnancy and parturition.
We have observed that the average number of prairie vole pups per litter is typically four. To standardize the litter size (and hence parental effort per pup) within a natural range, litters were culled to a maximum of four pups on the day birth occurred. Litters with three pups also remained in the study, whereas litters with two pups or less were excluded (N=12 breeding pairs). Mothers were outfitted with a purple zip-tie around their neck 18 days after the birth of pups in order to easily distinguish between mothers and pups of around the same size. Our animals habituated to zip-tie collaring quickly and showed no abnormal behaviors following a very brief (~2–5 min) period of acclimation, during which time some minor scratching at the collar was observed.
2.3. Daily home cage recordings
Home cages were recorded daily starting after parturition. Recordings were always conducted between 10:00 and 14:00. Home cages were placed under a video camera, giving us an aerial view of parental behaviors. Fifteen minutes of home cage activities were recorded and then analyzed using Observer XT v13 software (Noldus Information Technology, Leesburg, VA, USA). Both mothers and fathers were scored for the following behaviors: Brooding (parent is on top of at least one pup; pup may be either partially or completely underneath the parent), Grooming (parent is manipulating pup with its mouth and front paws), Locomotion (parent is moving around the cage and is not in contact with any pup), and Stillness (parent is not moving around the cage and is not in contact with any pup).
2.4. Parental Preference Tests
On PND 9, we identified the sex of each pup, and one male from each litter (N=18) was chosen as the subject animal for the series of Parental Preference Tests (PPTs). Because female offspring were being used for another experiment, we exclusively tested male pups in this study. This male was ear-tagged for easy identification and used as the subject animal for all five PPTs as it grew. Beginning on PND 10, PPTs were run every four days (PND 10, PND 14, PND 18, PND 22, and PND 26). As with cage recordings, PPT recordings were always conducted between 10:00 and 14:00. Pups at PND 10 can thermoregulate independently, have begun to locomote, and begin the transition to solid food (McGuire & Novak 1984). Typical weaning occurs on PND 21. Thus, we assessed parental preferences three times before weaning and two times after weaning. For the purpose of this study, pups were kept with their parents and siblings until PND 26. Because prairie voles reliably give birth every 21 days after the first litter, all subjects had been exposed to newborn siblings from PND 22 to PND 26.
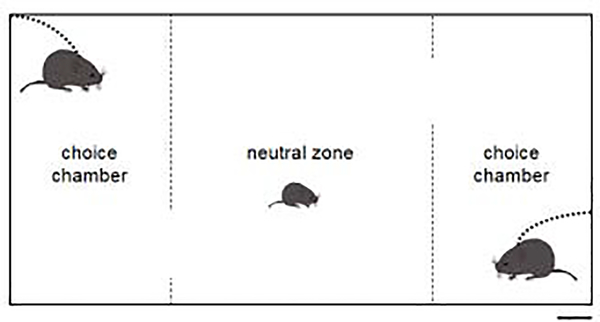
The Parental Preference Test in this study was based on a simple modification of the classic partner preference test frequently used in voles to assess whether animals demonstrate a pairbond (Williams et al. 1992). PPTs were run in a rectangular (106.7 × 50.8 × 30.5 cm), opaque, white acrylic apparatus (1.3 cm thick walls) with three chambers, including a central neutral zone (45.7 × 50.8 × 30.5 cm) with two choice chambers (27.9 × 50.8 × 30.5 cm) on either side (Figure 1). Two rectangular cutouts in the opposite walls of the middle chamber allowed passage between the neutral zone and the choice chambers. Before the PPT began, the mother and the father of the subject animal were tethered to the walls of the choice chambers (side was chosen with a coin flip for each trial). Tethering involves using a plastic zip-tie as a collar connected to a lightweight chain attached to the apparatus and does not inhibit animals from normal activities (e.g., moving, eating or mating; Wolff & Dunlap 2002; Ophir et al. 2007). We laced a zip-tie through the original collar on the parents and attached this to the lightweight chain. Parents were given a 20-min acclimation period to adjust to the tethers and were observed during this period for discomfort and distress (i.e., excessive or continued scratching at the collar, freezing lasting more than a minute at a time, absence of engaging in normal grooming behavior, locomotion or chamber exploration). All animals demonstrated normal behavior within 10 min or less.
Figure 1.
Overhead schematic of parent preference testing apparatus (to scale). The dashed lines represent the walls separating the neutral zone from the choice chambers. The dotted lines represent the tethers preventing parents from exiting their respective choice chambers. Scale bar = 10 cm
Neither parent could be seen from the neutral zone of the apparatus and parents were unable to move to the neutral zone. To initiate the PPT, the pup was placed in the center of the neutral zone and all behavior was video recorded from overhead. The PPT lasted one hour, after which both the pup and the parents were returned to their home cage. Videos were subsequently analyzed using Observer XT software. We quantified the amount of time pups spent in each chamber (neutral zone, father chamber, and mother chamber), and the amount of time pups spent in contact with a parent (either under the parent or side-by-side with the parent).
2.5. Analysis and Statistical methods
Data were analyzed using R (R Core Team 2016). Mothers and fathers were individually divided into either ‘high contact’ or ‘low contact’ parents based on the daily cage recordings. Because these cage recordings were taken from an aerial view, it was impossible to quantify detailed parental care behaviors. Instead, we used time parents spent in contact with pups as a proxy for parental care. The total amount of time a parent spent in contact with pups (either brooding or grooming) was first added together for each day (from PND 1 to 10) to create a measure of how much time parents were in contact with pups early in life. Contact scores for each day were averaged over PND 1 to PND 10 to create a total contact score for each parent. We then performed a median split to categorize high and low contact parents. We used a linear mixed model (LMM) to examine the relationship between ‘high contact’ and ‘low contact’ parents and the relative amount of time a pup spent with either parent during the PPT. The relative amount of time pups spent with each parent in the PPT was calculated by taking the amount of time a pup spent in contact with a parent and dividing that by the amount of time that pup spent in that parent’s chamber. Examining the relative amount of time a pup spends with a parent gives a more accurate description of a pup’s choice than total time spent in each chamber. A pup that spends the entire test exploring the chamber of a parent but never comes into contact with that parent has not shown a preference for the parent per se. To account for repeated measures in this study, all LLMs were run with the lme4 package (Bates et al. 2015). Random effects in the model were the pup identity (ID), the interaction between ID and the parent (mother vs. father), and the interaction between ID and the day of PPT (e.g., PND 10). Within the LMM, pairwise comparisons were made using the emmeans package (Lenth 2018). Significance threshold was set at P < 0.05 for all statistical tests. Finally, multiple helper packages were used to import and visualize the data (Wickham 2009; Fox & Weisberg 2011; R Core Team 2015).
3. RESULTS
3.1. Variation in parental contact
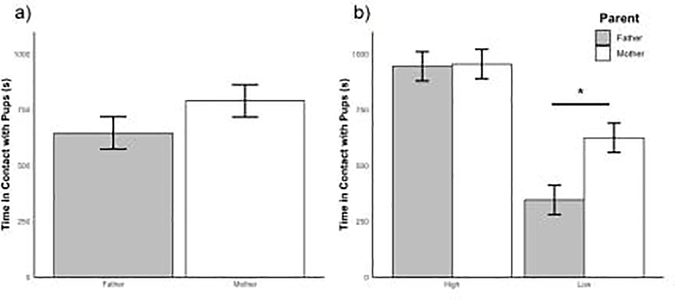
We first examined whether there was a difference between the amount of contact mothers and fathers gave to pups. Because Thomas and Birney (1979) found that both mothers and fathers contribute similarly to pup care, we expected both parents to spend similar amounts of time in contact with pups. As expected, in an LMM that looked only at parental contact over PND 1 to PND 10, both mothers and fathers spent similar amounts of time in contact with pups (mean difference (X) ± standard error (SE) = 144.02 ± 101.82, P = 0.18, Figure 2a). We next added parental contact score to the model because the first model glossed over any potential effects of high contact or low contact parents. Even with this more complex model, mothers and fathers spent the same amount of time in contact with pups, when controlling for contact score (X = 9.88 ± 85.95, P = 0.91). Not surprisingly, high contact parents spent more time in contact with pups than low contact parents when controlling for parent (X= 598.30 ± 89.56, P < 0.001). Importantly, even though overall mothers and fathers spent similar amounts of time in contact with pups, a significant interaction existed between contact score and parent (X = 268.28 ± 125.52, P = 0.04). This interaction appears to be driven by low contact parents; low contact fathers spent significantly less time in contact with pups than low contact mothers (Pairwise comparison: X = 278.16 ± 88.73, P = 0.02). In contrast, high contact mothers and high contact fathers spent the same amount of time in contact with pups (Pairwise comparison: X = 9.88 ± 88.73, P = 1.00, Figure 2b).
Figure 2.
(a) The average amount of time mothers (white) and fathers (grey) spent in contact with pups over the first ten days post-partum without taking into effect contact score. Mothers and fathers spent the same amount of time in contact with pups. (b) The average amount of time mothers (white) and fathers (grey) spent in contact with pups over the first ten days post-partum, separated by high and low contact parents. Error bars refer to ± SEM; (*) indicates p < 0.05.
Pups change considerably in the first 10 days post birth. For example, prairie vole pups grow fur, can open their eyes, and begin to leave the nest by PND 10, none of which is true at birth. Given this change in mobility and potential thermoregulatory ability, pups could need differing amounts of contact throughout these first 10 days of life. We next examined how parental contact varied across PND 1 to PND 10 to determine if both high and low contact parents engage in high amounts of contact early in life when pups are particularly altricial. We also examined how this potential variation interacted with parent contact scores to determine if high contact parents continue with more contact throughout development or if low contact parents reduce contact after the first few days.
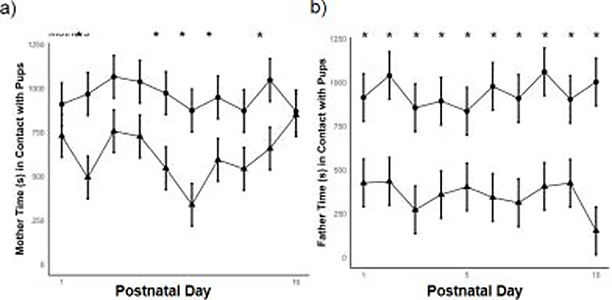
During this early stage in life, pups are attached to their mother’s nipples via milk teeth and must be forcibly removed if the mother wishes to forage without them. Given the potential difficulty in removing pups, we hypothesized that there should be less variability in contact time between high and low contact mothers across development. Indeed, when keeping PND constant, high contact and low contact mothers spent similar amounts of time in contact with their pups (X = 180.27 ± 171.95, P = 0.30). In other words, even though we characterized mothers based on contact score, there was no difference in the amount of time spent in contact with pups on average. Differences between high and low contact mothers only emerge on specific days. Pairwise comparisons between each PND age showed high contact mothers spent more time in contact with pups than low contact mothers on PND 2 (X = 474.50 ± 171.95, P < 0.01), PND 5 (X = 426.50 ± 171.95, P = 0.01), PND 6 (X = 535.10 ± 171.95, P < 0.01), PND 7 (X = 355.00 ± 171.95, P = 0.04), and PND 9 (X = 388.50 ± 171.94, P = 0.03). All other pairwise comparisons were not significantly different (all X’s ≤ 329.40; all P’s ≥ 0.06). See Figure 3a.
Figure 3.
(a) Comparison between the amount of contact high and low contact mothers (circles and triangles, respectively) spent with their pups over the first ten days post-partum. (b) Comparison between the amount of contact high and low contact fathers (circles and triangles, respectively) spent with their pups over the first ten days post-partum. Error bars refer to ± SEM; (*) indicates p < 0.05.
In contrast to mothers, fathers are not physically attached to pups at any point in early life, potentially enabling greater variability in paternal care. Indeed, fathers were easily separated into high and low contact fathers, unlike mothers. When PND was held constant, high contact fathers spent more time in contact with pups than low contact fathers (X = 488.55 ± 192.99, P = 0.01). This difference is even clearer when looking at pairwise comparisons between high and low contact fathers for each day, in which high contact fathers showed greater contact than low contact fathers at every age between PND 1 and PND 10 (all X’s ≥ 432.90 ± 192.99 and all P’s ≤ 0.03, see Figure 3b). Taken together with the parental contact data from mothers, these results suggest that fathers can be clearly classified into high or low contact types, while mothers show relatively less variability in parental contact.
3.2. Pup preferences for parents
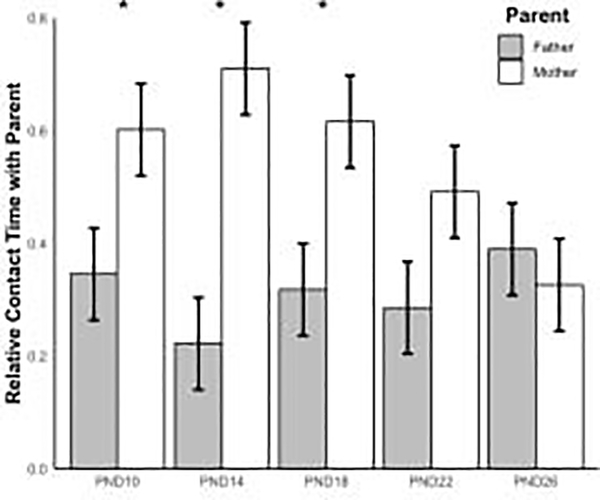
We next examined whether the variability in parental contact reported above influenced a pup’s preference for a parent. A preference for a parent was defined as a significant difference in the relative time spent in contact with each parent during the Parent Preference Test (PPT). Relative time spent in contact was calculated by taking the time a pup spent in contact with a parent and dividing by the total time the pup spent in that parent’s chamber. Our first model omitted parental contact score and included the age of the pups during the PPT (PND 10, PND 14, PND 18, PND 22, PND 26). Pups spent more time with mothers than fathers when test age was held constant (X = 0.26 ± 0.12, P = 0.03, Figure 4). Although there were no significant interactions between parent and test age, pairwise comparisons showed differences in relative time spent with each parent for only the first 3 test days. Specifically, pups spent more relative time with mothers than fathers on PND 10, PND 14, and PND 18 (X = 0.26 ± 0.12, P = 0.03; X = 0.49 ± 0.12, P < 0.001; X = 0.30 ± 0.12, P = 0.01, respectively). This difference was not seen on the last 2 testing days (PND 22: X = 0.21 ± 0.12, P = 0.08; PND 26: X = 0.063 ± 0.12, P = 0.59 see Figure 4).
Figure 4.
Comparison between the amount of time pups spent in relative contact with either their mother (white) or father (grey) on Parental Preference Test days. Error bars refer to ± SEM; (*) indicates p < 0.05.
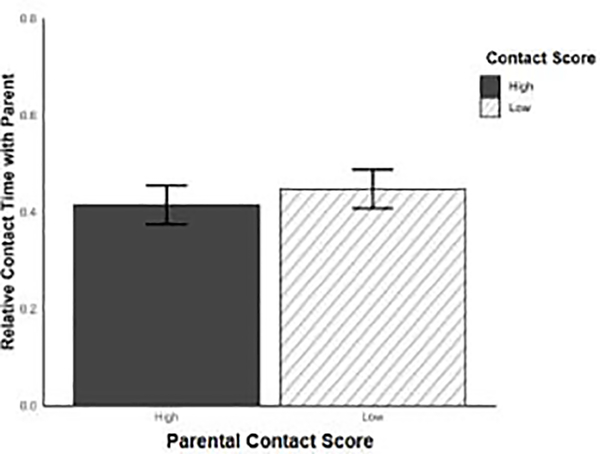
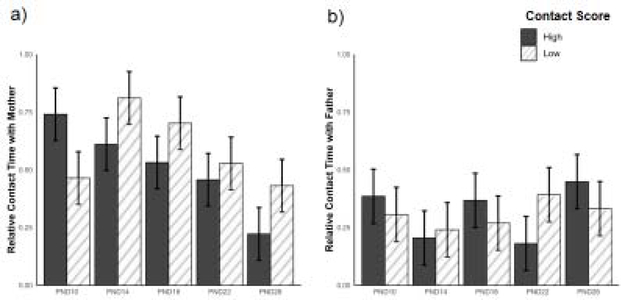
We then examined whether the amount of contact from parents influenced pup’s preference. When parent sex was not considered, pups spent the same amount of relative time in contact with high contact parents and low contact parents (X = 0.033 ± 0.056, P = 0.56, Figure 5). Because we found a consistent and significant difference between high and low contact fathers but not mothers, we examined pup preference for high or low contact parents within mothers and fathers separately. Using this approach, we found that pups spent the same relative amount of time with both high contact and low contact mothers across test age (X = 0.28 ± 0.16, P = 0.09). Although mother contact score did not affect pup preference, test age did; pups spent less relative time with both high and low contact mothers on PND 26 than PND 10 (X = 0.52 ± 0.16, P < 0.01, Figure 6a). Pup preference for fathers showed a similar pattern to pup preference for mothers. Across test age, pups spent the same amount of relative time with high and low contact fathers (X = 0.079 ± 0.17, P = 0.64). However, in contrast to pup preference for mothers, pup preference for fathers stayed consistent across all test ages (all X’s ≤ 0.20 ± 0.17, all P’s ≥ 0.2, Figure 6b).
Figure 5.
Comparison between the amount of relative contact time pups spend with high (black) or low (hatched) contact parents. Error bars represent ± SEM.
Figure 6.
(a) Comparison between the amount of time pups spent in contact with high (black) or low (hatched) contact mothers. (b) Comparison between the amount of time pups spent in contact with high (black) or low (hatched) contact fathers. Error bars represent ± SEM.
4. DISCUSSION
We have shown that biparental prairie vole parents spent similar amounts of time in contact with pups. However, the variation in paternal care was greater than it was for maternal care, indicating that fathers are the primary source for variation in the care that pups receive. Pups with high contact parents do not differ from pups with low contact parents when choosing the parent with which to spend time. Yet, when given a choice, pups preferred to spend relatively more time in contact with mothers than fathers, but only when they were very young (pre-weaning). Once pups were relatively mobile, exclusively eating solid food, and could independently thermoregulate, they no longer demonstrated a preference for one parent over the other. We elaborate on these results below.
4.1. Variation in Paternal, but not Maternal, Care
Prairie voles are socially monogamous and biparental (Getz et al. 1993; Ophir et al. 2008). Furthermore, Thomas and Birney (1979) reported that mothers and fathers contribute similarly to parental care. Our results are consistent with their report in that both mothers and fathers spent similar amounts of time in contact with pups. Notably, even though both parents showed similar amounts of contact time overall, they could be divided into high contact and low contact parents. Even more interesting is that there was greater variation among fathers, allowing them to be easily divided categorically into high and low contact fathers; less variation in mother contact made this categorization less definite. This distinction revealed an interesting outcome, demonstrating that although both high contact mothers and fathers had similar contact scores, low contact mothers and fathers were significantly different from each other. Importantly, high and low contact fathers also differed significantly from each other; this was not seen in high and low contact mothers. Together, this suggests that (low) paternal care is the primary source of variation in parental care for prairie vole offspring. Parental investment theory suggests that differences in the effort mothers and fathers dedicate to offspring care become increasingly asymmetric when one sex is relatively more energetically invested than the other (Trivers 1972). In mammals, females usually dedicate more effort to offspring care, a pattern often attributed to the energetic investments in gametes, gestation, and lactation. This asymmetry in offspring investment is thought to free males to pursue alternative (high mate search) strategies in lieu of providing care to offspring (Maynard Smith 1977; Clutton-Brock 1991; Szekely et al. 2000). Although many of these factors are largely still present in biparental and monogamous species, equity in offspring investment is more common among monogamous and biparental mammals (Kleiman 1977).
Our data indicate an interesting middle ground can be found in male prairie voles, in which some fathers appear to contribute more paternal care than other males. This is consistent with results from other studies showing that prairie vole males engage in alternative mating tactics (Getz et al. 1993; Solomon & Jaquot 2002; Ophir et al. 2008b). These studies have supported the hypothesis that some males remain single and attempt to mate multiply (wanderers) and other males form pairbonds (residents). Resident prairie voles can be further sub-divided into males that engage exclusively in intra-pair copulations (true residents) and others that engage in extra-pair copulations (roving residents) (Ophir 2017; Rice et al. 2018). The variation we report in male, but not female, parental care parallels the patterns of variation in mating tactics associated with different life-history strategies. It is currently unknown whether parental care and mating tactics are two parts of a suite of correlated behaviors that represent evolved or developed syndromes, or if these are dissociable and independent characteristics from one another. Interestingly, Ophir et al. (2008a) showed that females can potentially discriminate between ‘good’ and ‘bad’ fathers (i.e., they discriminate between males that provide relatively high or low alloparental care, and low or high infanticidal tendencies toward pups from other parents, respectively). Despite this ability to discriminate, females ironically preferred less alloparental and more infanticidal males. However, that study also showed that more alloparental and less infanticidal males were more aggressive to females upon first meeting them. It is interesting to consider the possibility that the most paternal males might also be highly aggressive with unfamiliar conspecifics (regardless of sex), reflecting a ‘protective phenotype’ that could potentially result in both pup care and protection, and mate guarding and partner fidelity.
4.2. Potential Long-term Impact of Natural Variation in Paternal Care on Offspring Development
Overall, our data suggest that pups only demonstrate a preference for a parent if it satisfies their current developmental needs. Even though pups did not show a preference between high and low contact parents, this variation could have a lasting impact on adult behaviors. This is particularly true given that it was fathers that showed the greatest variation between high and low contact. Previous studies have shown that the complete absence of a father affects pup development and behavior. Prairie vole pups raised by a single mother developed slower, were less likely to engage in alloparental behavior as juveniles, and take longer to form pairbonds as adults (Wang & Novak 1992; Wang & Novak 1994; Ahern & Young 2009). Studies with both parents present have shown an effect of the total amount of parental care on juvenile behavior, juvenile development, and neural immunoreactivity and structure (Perkeybile et al. 2013; Perkeybile & Bales 2015; Seelke et al. 2015). However, to our knowledge, no studies have investigated the developmental effects of having a low contact father specifically. Having a low contact father represents a less severe postnatal environment when compared to the complete absence of a father. Thus, the presence of a low contact father during development could result in pup behaviors that are comparable to those seen in pups raised by single mothers, so long as mothers do not compensate for the absence of, or reduction in care from fathers. Alternatively, the form or intensity of behavioral effects that result from low-care fathers could induce different consequences than those attributable to low-care mothers or instances when fathers are completely absent. Either way, raising pups with a high or low contact father represents a potentially powerful manipulation that will deepen and enrich the study of long-term effects of parental care on offspring development.
4.3. Does the Utilitarian Value of Parents to Pups Drive Parental Preferences?
For simplicity, we assumed that ‘high contact’ parents would be preferred parents from the pup’s perspective because these parents would have presumably spent more time grooming and assisting pup thermoregulation when the pup was young. Thus, increased contact and care could help create stronger preferences for high contact parents over low contact parents. To our surprise, pups did not show a preference for high contact parents over low contact parents.
Developing prairie vole pups did show a significant preference for mothers over fathers, but this was only found when pups were youngest (PND 10, PND 14, and PND 18). This is consistent with findings from another biparental species, the Mandarin vole (Lasiopodomys mandarinus), in which pups between 14 and 21 days old prefer mothers over fathers (He et al. 2017). Importantly, mothers and their milk are the sole source of nutrition for pups early in life. The fact that pups are literally attached via milk teeth to their mother not only decreases the amount of variability in parental contact possible, but milk represents the sole source (or primary source) of nutrition to which pups have access (depending on their age). Prairie vole pups are entirely dependent on their mother’s milk for the first ten days after birth and continue to nurse while fully transitioning to solid food around PND 20 (McGuire & Novak 1984). Although fathers provide important parental care such as thermoregulation, the lack of nursing and physical attachment might explain some of the variation in paternal care we observed between high and low contact fathers (see above). For pups, however, mothers are sources of at least two valuable resources (food and heat) whereas fathers can offer only heat (at least under laboratory conditions). Our first three PPT trials all fell within the developmental time range in which pups were dependent on nursing, and they were the only tests where we see a preference for mothers. Unfortunately, due to the overhead position of our cameras, it was impossible to definitively determine if pups were actively nursing when they were in contact with their mothers. Nevertheless, the fact that the preferences for one parent (mother) over the other (father) disappeared as pups became increasingly independent suggests that pups do not form social attachment with parents the way adult prairie voles bond with mating partners (Williams et al. 1992) or parents bond with offspring (Gubernick 1981). Indeed, the lack of pup preferences for high contact and low contact parents indicates that pups do not discriminate between parents based on parental responsiveness and instead seek the parent that most satisfies their immediate developmental needs (Alberts 2008). Similar results have recently been reported in Richardson’s ground squirrels; there is no effect of mother removal on offspring survival or offspring stress response, as long as the removal occurs after weaning (Freeman et al. 2019). In these respects, pups only appear to demonstrate social preferences for mothers when mothers can contribute to or serve offspring survival needs.
To further support this utilitarian view of pup preferences for mothers over fathers, we note that lactating mothers in many rodent species have an increased body temperature in comparison to non-lactating mothers (Leon et al. 1978; Scribner & Wynne-Edwards 1994; Speakerman 2007). This higher body temperature and the resulting increased thermoregulatory aid may be a part of the preference pups have for mothers over fathers. As pups begin to grow fur around PND 4 and become able to independently thermoregulate by PND 14 (McGuire & Novak 1984), the value of thermoregulatory superiority of mothers over fathers might dissipate. Interestingly, the preference for mothers over fathers persisted past this age, although the provisioning of milk could continue to drive the pup preferences that persisted through PND 18. The progressive decrease in preference for mothers over fathers as pups become increasingly independent of the unique resources that mothers offer supports classic theory on parent-offspring conflict in which pups exploit parental resources to maximize their own individual body condition without regard to the lifetime effort or energy available to parents (Trivers 1974). It is difficult to ascertain whether this reflects a purely utilitarian view of parents as sources for basic resources. Indeed, pups very well could reciprocate the social bonds that parents form with them. However, it is interesting to note that by the time pups were just past the age of weaning, they spent between about 30 and 40% of their time in contact with mothers and fathers (respectively). This suggests that offspring may continue to seek prosocial contact with parents independent of care-giving needs, but that the rate at which they seek social support is reduced and no longer biased toward a particular parent. Taken together, our study emphasizes the importance of considering offspring as active participants in the social dynamics that shape family unit interactions and how those dynamics develop as offspring mature.
HIGHLIGHTS.
Mother prairie voles spend more time in contact with pups than fathers
Mothers are relatively consistent in their care; fathers are more variable
Pups prefer mothers before weaning but show no preference after weaning
Pups show no preference for parents based on amount of individual parental care
5. ACKNOWLEDGEMENTS
We would like to acknowledge Jintana Cunningham and Hallie Malina for their assistance in behavioral scoring. We also wish to acknowledge the assistance of Stephen Parry from the Cornell statistical consulting unit during data analysis.
Funding: This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development [HD079573 to A.G.O.].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ahern TH & Young LJ (2009). The impact of early life family structure on adult social attachment, alloparental behavior, and the neuropeptide systems regulating affiliative behaviors in the monogamous prairie vole (Microtus ochrogaster). Frontiers in Behavioral Neuroscience, 3, 1–19. 10.3389/neuro.08.017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts JR (2008). The nature of nurturant niches in ontogeny. Philosophical Psychology, 21(3), 295–303. 10.1080/09515080802169814 [DOI] [Google Scholar]
- Alonso-Alvaraze C & Velando A (2012). Benefits and costs of parental care In Royle NJ, Smiseth PT, & Kölliker M (Eds.), The Evolution of Parental Care (pp. 40–59). Oxford, United Kingdom: Oxford Press. [Google Scholar]
- Bates D, Maechler M, Bolker B, & Walker S (2015). Fitting linear mixed-effects models using lme4. Journal of Statistical Software, 67, 1–48. [Google Scholar]
- Bowlby J (1982). Attachment and loss: Retrospect and prospect. American Journal of Orthopsychiatry, 52, 664–678. 10.1111/j.1939-0025.1982.tb01456.x. [DOI] [PubMed] [Google Scholar]
- Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, & Meaney MJ (1998). Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. PNAS, 95, 5335–5340. 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantoni D & Brown RE (1997). Paternal investment and reproductive success in the California mouse, Peromyscus californicus. Animal Behaviour, 54, 377–386. 10.1006/anbe.1996.0583. [DOI] [PubMed] [Google Scholar]
- Cao Y, Wu R, Tai F, Zhang X, Yu P, An X, Qiao X, & Hao P (2014). Neonatal paternal deprivation impairs social recognition and alters levels of oxytocin and estrogen receptor alpha mRNA expression in the MeA and NAcc, and serum oxytocin in mandarin voles. Hormones and Behavior, 65, 57–65. 10.1016/j.yhbeh.2013.11.005. [DOI] [PubMed] [Google Scholar]
- Carter CS, Getz LL, Gavish L, Mc Dermott JL, & Arnold P (1980). Male-related pheromones and the activation of female reproduction in the prairie vole (Microtus ochrogaster). Biology of Reproduction, 23, 1038–1045. 10.1095/biolreprod23.5.1038. [DOI] [PubMed] [Google Scholar]
- Case TJ (1978). On the evolution and adaptive significance of postnatal growth rates in the terrestrial vertebrates. The Quarterly Review of Biology, 53, 243–282. 10.1086/410622. [DOI] [PubMed] [Google Scholar]
- Clutton-Brock TH (1991). The evolution of parental care. Princeton University Press. [Google Scholar]
- Dluzen DE, Ramirez VD, Carter CS, & Getz LL (1981). Male vole urine changes luteinizing hormone-releasing hormone and norepinephrine in female olfactory bulb. Science, 212, 573–575. 10.1126/science.7010608. [DOI] [PubMed] [Google Scholar]
- Evans RM (1992). Embryonic and neonatal elicitation of parental brooding and feeding responses in American white pelicans. Animal Behaviour, 44, 667–675. 10.1016/S0003-3472(05)80294-5. [DOI] [Google Scholar]
- Fox J, Weisberg S (2011). An {R} companion to applied regression, second edition. Thousand Oaks CA: Sage; URL: http://socserv.socsci.mcmaster.ca/jfox/Books/Companion. [Google Scholar]
- Francis D, Diorio J, Liu D, & Meaney MJ (1999). Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science, 286, 1155–1158. 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- Freeman AR, Wood TJ, Bairos-Novak KR, Anderson WG, & Hare JF (2019). Gone girl: Richardson’s ground squirrel offspring and neighbors are resilient to female removal. Royal Society Open Science, 6, 190904 10.1098/rsos.190904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman-Gallant CR & Rothstein MD (1999). Apparent heritability of parental care in Savannah Sparrows. The Auk, 116, 1132–1136. 10.2307/4089694. [DOI] [Google Scholar]
- Getz LL & Carter CS (1996). Prairie-vole partnerships. American Scientist, 84, 56–62. [Google Scholar]
- Getz LL, McGuire B, Pizzuto T, Hofmann JE, & Frase B (1993). Social organization of the prairie vole (Microtus ochrogaster). Journal of Mammalogy, 74, 44–58. 10.2307/1381904. [DOI] [Google Scholar]
- Gubernick DJ (1981). Parent and Infant Attachment in Mammals In: Gubernick DJ & Klopfer PH (Eds.), Parental Care in Mammals (pp. 243–305). Boston, MA: Springer. [Google Scholar]
- He Z, Zhang S, Yu C, Li Y, Jia R, &Tai F. (2017). Emotional attachment of pre-weaning pups to mothers and fathers in mandarin voles. Behavioural Processes, 135, 87–94. 10.1016/j.beproc.2016.12.008. [DOI] [PubMed] [Google Scholar]
- Kleiman DG (1977). Monogamy in mammals. The Quarterly review of biology, 52, 39–69. 10.1086/409721. [DOI] [PubMed] [Google Scholar]
- Lenth R (2018). emmeans: Estimated Marginal Means, aka Least-Squares Means. R package version 1.2.2. https://CRAN.R-project.org/package=emmeans [Google Scholar]
- Leon M, Croskerry PG, & Smith GK (1978). Thermal control of mother-young contact in rats. Physiology and Behavior, 21, 793–811. 10.1016/0031-9384(78)90021-5. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, & Meaney MJ (1997). Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science, 277, 1659–1662. 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Maccoll ADC & Hatchwell BJ (2007). Heritability of parental effort in a passerine bird. Evolution, 57, 2191–2195. 10.1111/j.0014-3820.2003.tb00398.x. [DOI] [PubMed] [Google Scholar]
- Martin P (1984). The meaning of weaning. Animal Behaviour, 32, 1257–1259. 10.1016/S0003-3472(84)80245-6. [DOI] [Google Scholar]
- Maynard Smith J 1977. Parental investment: a prospective analysis. Animal Behaviour, 25, 1–9. 10.1016/0003-3472(77)90062-8. [DOI] [Google Scholar]
- McGuire B & Novak M (1984). A comparison of maternal behaviour in the meadow vole (Microtus pennsylvanicus), prairie vole (M. ochrogaster) and pine vole (M. pinetorum). Animal Behaviour, 32, 1132–1141. 10.1016/S0003-3472(84)80229-8. [DOI] [Google Scholar]
- McNamara JM, Gasson CE, & Houston AI (1999). Incorporating rules for responding into evolutionary games. Nature, 401, 368–371. [DOI] [PubMed] [Google Scholar]
- Miller J (1974). Energy requirements for growth in Microtus ochrogaster. Masters dissertation, Colorado State University. [Google Scholar]
- Nakagawa S, Gillespie DOS, Hatchwell BJ, & Burke T (2007). Predictable males and unpredictable females: sex difference in repeatability of parental care in a wild bird population. Journal of Evolutionary Biology, 20, 1674–1681. 10.1111/j.1420-9101.2007.01403.x. [DOI] [PubMed] [Google Scholar]
- Ophir AG, Phelps SM, Sorin AB, & Wolff JO (2007). Morphological, genetic, and behavioral comparisons of two prairie vole populations in the field and laboratory. Journal of Mammalogy, 88, 989–999. 10.1644/06-MAMM-A-250R.1. [DOI] [Google Scholar]
- Ophir AG, Crino OL, Wilkerson QC, Wolff JO, & Phelps SM (2008a). Female-directed aggression predicts paternal behavior, but female prairie voles prefer affiliative males to paternal males. Brain, Behavior and Evolution, 71, 32–40. 10.1159/000108609. [DOI] [PubMed] [Google Scholar]
- Ophir AG, Campbell P, Hanna K, & Phelps SM (2008). Field tests of cis-regulatory variation at the prairie vole avpr1a locus: Association with V1aR abundance but not sexual or social fidelity. Hormones and Behavior, 54, 694–702. 10.1016/j.yhbeh.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Ophir AG (2017). Navigating monogamy: nonapeptide sensitivity in a memory neural circuit may shape social behavior and mating decisions. Frontiers in neuroscience, 11, 397 10.3389/fnins.2017.00397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkeybile AM, Griffin LL, & Bales KL (2013). Natural variation in early parental care correlates with social behaviors in adolescent prairie voles (Microtus ochrogaster). Frontiers in Behavioral Neuroscience, 7, 1–16. 10.3389/fnbeh.2013.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkeybile AM & Bales KL (2015). Early rearing experience is associated with vasopressin immunoreactivity but not reactivity to an acute non-social stressor in the prairie vole. Physiology & Behavior, 147, 149–156. 10.1016/j.physbeh.2015.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piovanotti MRA & Vieira ML (2004). Presence of the father and parental experience have differentiated effects of pup development in Mongolian gerbils (Meriones unguiculatus). Behavioural Processes, 66, 107–117. 10.1016/j.beproc.2004.01.007. [DOI] [PubMed] [Google Scholar]
- R Core Team (2015). foreign: Read data stored by Minitab, S, SAS, SPSS, Stata, Systat, Weka, dBase, …. R package version 0.8–66. https://CRAN.R-project.org/package=foreign.
- R Core Team (2016). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: URL https://www.R-project.org/. [Google Scholar]
- Rice MA, Restrepo LF, & Ophir AG (2018). When to cheat: Modeling dynamics of paternity and promiscuity in socially monogamous prairie voles (Microtus ochrogaster). Frontiers in Ecology and Evolution, 6, 141 10.3389/fevo.2018.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond M & Stehn R (1976). Olfaction and reproductive behavior in microtine rodents In Mammalian olfaction, reproductive processes, and behavior (pp.197–217). Academic Press. [Google Scholar]
- Royama TR (1996). Factors governing feeding rate, food requirement and brood size of nestling Great Tits Parus major. Ibis, 108, 313–347. 10.1111/j.1474-919X.1966.tb07348.x. [DOI] [Google Scholar]
- Russell EM (1982). Patterns of parental care and parental investment in marsupials. Biological Reviews, 57, 423–486. 10.1111/j.1469-185X.1982.tb00704.x. [DOI] [PubMed] [Google Scholar]
- Schwagmeyer PL & Mock DW (2003). How consistently are good parents good parents? Repeatability of parental care in the House Sparrow, Passer domesticus. Ethology, 109, 303–313. 10.1046/j.1439-0310.2003.00868.x. [DOI] [Google Scholar]
- Scribner SJ & Wynne-Edwards KE (1994). Disruption of body temperature and behavior rhythms during reproduction in dwarf hamsters (Phodopus). Physiology and Behavior, 55, 361–369. 10.1016/0031-9384(94)90147-3. [DOI] [PubMed] [Google Scholar]
- Seelke AMH, Perkeybile AM, Grunewald G, & Bales KL, Krubitzer LA (2015). Individual differences in cortical connections of somatosensory cortex are associated with parental rearing style in prairie voles (Microtus ochrogaster). Journal of Comparative Neurology, 524, 564–577. 10.1002/cne.23837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon NG & Jacquot JJ (2002). Characteristics of resident and wandering prairie voles, Microtus ochrogaster. Canadian Journal of Zoology, 80, 951–955. 10.1139/z02-053. [DOI] [Google Scholar]
- Speakerman JR (2007). The physiological costs of reproduction in small mammals. Philosophical Transactions of the Royal Society B, 636, 375–398. 10.1098/rstb.2007.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szekely T, Webb JN & Cuthill IC (2000). Mating patterns, sexual selection and parental care: an integrative approach In Apollonio M, Festa-Bianchet M & Mainardi D (Eds.), Vertebrate mating systems (pp. 194–223). London: World Science Press. [Google Scholar]
- Trivers RL (1972). Parental Investment and Sexual Selection In Campbell B (Ed.), Sexual Selection and the Descent of Man (pp 1871–1971). Chicago: Aldine. [Google Scholar]
- Trivers RL (1974). Parent-offspring conflict. Integrative and Comparative Biology, 14, 249–264. 10.1093/icb/14.1.249. [DOI] [Google Scholar]
- Thomas JA & Birney EC (1979). Parental care and mating system of the prairie vole, Microtus ochrogaster. Behavioral Ecology and Sociobiology, 5, 171–186. [Google Scholar]
- Wang Z & Insel TR (1996). Parental behavior in voles. Advances in the study of behaviour, 25, 361–384. 10.1016/S0065-3454(08)60338-1. [DOI] [Google Scholar]
- Wang Z &Novak MA (1992). Influence of the social environment on parental behavior and pup development of meadow voles (Mictorus pennsylvanicus) and prairie voles (M. ochrogaster). Journal of Comparative Psychology, 106, 163–171. 10.1037/0735-7036.106.2.163. [DOI] [Google Scholar]
- Wang Z & Novak MA (1994). Alloparental care and the influence of father presence on juvenile prairie voles, Microtus ochrogaster. Animal Behaviour, 47, 281–288. 10.1006/anbe.1994.1040. [DOI] [Google Scholar]
- Weaver ICG, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seck JR, Dymov S, Szyf M, & Meaney MJ (2004). Epigenetic programming by maternal behavior. Nature Neuroscience, 7, 847–854. [DOI] [PubMed] [Google Scholar]
- Wickham H (2009). ggplot2: Elegant graphics for data analysis. Springer-Verlag; New York. [Google Scholar]
- Williams JR, Catania KC, & Carter CS (1992). Development of partner preferences in female prairie voles (Microtus ochrogaster): the role of social and sexual experience. Hormones and Behavior, 26, 339–349. 10.1016/0018-506X(92)90004-F. [DOI] [PubMed] [Google Scholar]
- Whittingham LA & Robertson RJ (1993). Nestling hunger and parental care in Red-winged Blackbirds. The Auk, 110, 240–246. 10.1093/auk/110.2.240. [DOI] [Google Scholar]
- Wolff JO & Dunlap ASF (2002). Multi-mating, probability of conception, and litter size in the prairie vole (Microtus ochrogaster). Behavioural Processes, 58, 105–110. 10.1016/S0376-6357(02)00022-0. [DOI] [PubMed] [Google Scholar]
- Wright J, Both C, Cotton PA, Bryant D (1998). Quality vs. quantity: energetic and nutritional trade-offs in parental provisioning strategies. Journal of Animal Ecology, 67, 620–634. [Google Scholar]