Abstract
Aim |
Acute myocardial infarction and subsequent post-infarction heart failure are among the leading causes of mortality worldwide. The endocannabinoid system has emerged as an important modulator of cardiovascular disease, however the role of endocannabinoid metabolic enzymes in heart failure is still elusive. Herein, we investigated the endocannabinoids and their metabolic enzymes in ischemic end-stage failing human hearts and non-failing controls.
Methods and Results |
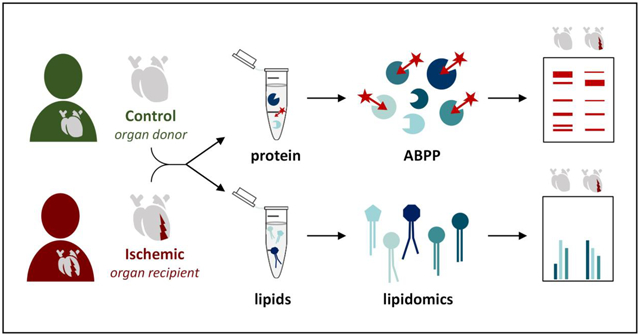
Quantitative real-time PCR, targeted lipidomics, and activity-based protein profiling (ABPP) enabled assessment of the endocannabinoids and their metabolic enzymes in ischemic end-stage failing human hearts and non-failing controls. Based on lipidomic analysis, two subgroups were identified within the ischemic heart failure group; the first similar to control hearts and the second with decreased levels of the endocannabinoid 2-arachidonoyl-glycerol (2-AG) and drastically increased levels of the endocannabinoid anandamide (AEA), other N-acylethanolamines (NAEs) and free fatty acids. The altered lipid profile was accompanied by strong reductions in the activity of 13 hydrolases, including the 2-AG hydrolytic enzyme monoacylglycerol lipase (MGLL).
Conclusions|
Our findings suggest the presence of different biological states within the ischemic heart failure group, based on alterations in the lipid and hydrolase activity profiles. In addition, this study demonstrates that ABPP is a valuable tool to rapidly analyze enzyme activity in clinical samples with potential for novel drug and biomarker discovery.
Keywords: Cardiac ischemia, endocannabinoid system, chemical proteomics, lipidomics
Graphical abstract
3. Introduction
Ischemic heart disease, involving acute myocardial infarction and subsequent heart failure development, was responsible for 12.7% of the total global mortality (2008), thereby making it the leading cause of mortality worldwide [1]. In the advanced stages of heart failure, a significant number of patients needs hospitalization and possible admission to intensive care units. These critically ill patients usually suffer from life threatening clinical syndromes such as pulmonary edema, associated with respiratory distress and low oxygen saturation; cardiogenic shock, defined as tissue hypoperfusion induced by ineffective cardiac contractility; or from cardio-renal syndrome.
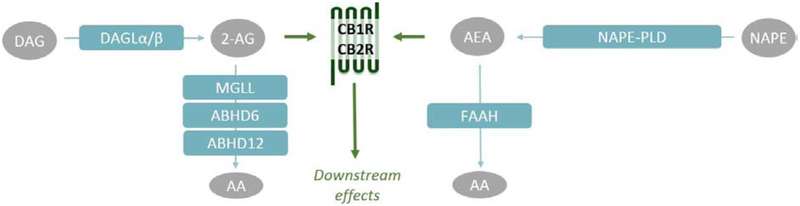
Recently, the endocannabinoid system (ECS) has emerged as a modulator of the cardiovascular system under diseased conditions [2–4]. The ECS is comprised of G-protein coupled receptors, the cannabinoid receptor type 1 and 2 (CB1R, CB2R), their endogenous lipid-derived ligands, the endocannabinoids 2-arachidonoylglycerol (2-AG) and anandamide (AEA), and the enzymes responsible for their synthesis and degradation (Figure 1) [5]. Diacylglycerol lipase α and β (DAGLα, DAGLβ) are the two main 2-AG biosynthetic enzymes [6]. The majority of 2-AG is hydrolyzed to arachidonic acid (AA) by monoacylglycerol lipase (MGLL) [7,8], but α,β-hydrolase domain containing proteins 6 and 12 (ABHD6, ABHD12) hydrolyze 2-AG as well [8–10]. The existence of multiple N-acylethanolamine (NAE) metabolic pathways makes AEA biosynthesis more complex. Direct hydrolysis of N-acylphospatidylethanolamines (NAPEs) to NAEs by NAPE phospholipase D (NAPEPLD), is considered the canonical pathway, but alternative multi-step pathways exist as well [11]. The NAEs are hydrolyzed to ethanolamines and free fatty acids by fatty acid amide hydrolase (FAAH) [12,13]. Of note, several other NAE species have been reported as bioactive lipids, suggesting an important modulatory role for this lipid class. For example, oleoylethanolamide (OEA) and stearoylethanolamide (SEA) have anorexic effects in the periphery [14,15] and palmitoylethanolamide (PEA) was reported to enhance antinociception [16,17].
Figure 1 |. The endocannabinoid system.
The endocannabinoid system comprises of cannabinoid receptor 1 and 2 (CB1R, CB2R), their endogenous ligands 2-arachidonoylglycerol (2-AG) and anandamide (AEA) and their metabolic enzymes: diacylglycerol lipase α and β (DAGLα, DAGLβ), monoacylglycerol lipase (MGLL), α,β-hydrolase domain containing proteins 6 and 12 (ABHD6, ABHD12), N-acylphosphatidylethanolamine phospholipase D (NAPE-PLD) and fatty acid amide hydrolase (FAAH). Alternative multi-step pathways for AEA biosynthesis are not shown.
The ECS regulates a broad spectrum of physiological and pathological processes, including energy balance, obesity, pain, inflammation, neurological and immunological disorders [18–20]. In the cardiovascular system the endocannabinoids have been implicated in vasodilation and/or vasoconstriction, cardiac protection against atherogenic inflammation, depression of cardiac function, and cell death of cardiomyocytes and endothelial cells in CB1/2R-dependent or -independent manner [3,21–23]. In vivo cardiovascular effects of the endocannabinoids may be exerted through the central and peripheral nervous system as well, or through direct effects on the myocardium and vasculature [24,25]. The two cannabinoid receptors have been shown to have opposing effects, e.g. CB1R facilitates the development of cardiometabolic disease and cardiac dysfunction [3,26], whereas CB2R mainly exerts anti-inflammatory effects [3,27,28].
Despite extensive investigation of the endocannabinoids and their receptors in cardiac function and dysfunction, only little is known about the endocannabinoid metabolic enzymes [3]. Most endocannabinoid metabolic enzymes, with the exception of NAPE-PLD, belong to the serine hydrolase family. This protein class can be targeted by activity-based probes covalently interacting with their catalytic serine residue. Activity-based probes are used in chemical proteomics to assess the functional state of an entire enzyme classes in complex biological samples [29,30]. In this study, the endocannabinoid metabolic enzymes were evaluated in ischemic end-stage failing human hearts and non-failing controls by chemical proteomics, lipidomics, and gene expression analysis to gain insight in the role of this complex system in post-ischemic chronic heart failure.
4. Experimental procedures
4.1. Materials and probes
Activity-based fluorophosphonate-based probes FP-TAMRA and FP-biotin were purchased from ThermoFisher and Santa Cruz biotechnology respectively. MB064 and MB108 were synthesized in-house as previously described [31]. All synthesized compounds were at least 95% pure and were analyzed by LC-MS, NMR, and HRMS. Other chemicals, reagents, and primers were purchased from Sigma Aldrich unless indicated otherwise.
4.2. Ethical statement
All experimental procedures were done in accordance with the ethical standards of the responsible institutional and national committee on human experimentation, adhering to the Helsinki Declaration (1975). Written informed consent was obtained from all patients involved in the study according to the protocol approved by the Local Ethics Committees of the Institute of Cardiology, Warszawa, Poland (IK-NP-0021–24/1426/14).
4.3. Sample collection and preparation
Healthy (control) human hearts were obtained from organ donor patients (n=6 for qRT-PCR, Set A and n=9 for ABPP, Set B, please see Table 1 for details) whose hearts were not used for transplantation due to technical reasons (e.g., donor/recipient incompatibility). The donors did not have any relevant previous cardiological history or any abnormalities in ECG and echocardiography (left ventricle dimensions/contractility within normal ranges). Explanted failing hearts were obtained from patients suffering from end-stage, advanced heart failure of ischemic etiology (n=6 for qRT-PCR, Set A and n=9 for ABPP, Set B, please see Table 1 for details).
Table 1 |. Clinical characteristics of study populations.
Values are given as mean ± SEM. Set A: Figure 2. Set B: Figures 3–5. BMI: body mass index, NYHA: New York Heart Association (3.5 is included in class IV), CO: cardiac output, EF: ejection fraction, LVED: left ventricular end-diastolic diameter, LVSD: left ventricular end-systolic diameter, PW: posterior wall-thickness, IVS: interventricular septum thickness; SVR: systemic vascular resistance, AST: aspartate transaminase, ALT: alanine transaminase, NT-proBNP: N-terminal prohormone of brain natriuretic peptide, LDL: low density lipoprotein, HDL: high density lipoprotein, n.a.: not available. Subgroups Ischemic 1 and 2 (set B) were compared by two-tailed t-test: * p < 0.05 *** p < 0.001.
| Control Set A | Ischemic Set A | Control Set B H1–9 | Ischemic Set B I1–9 | Ischemic 1 Set B I1–3, 8–9 | Ischemic 2 Set B 14–7 | |
|---|---|---|---|---|---|---|
| Samples (n) | 6 | 6 | 9 | 9 | 5 | 4 |
| Gender (female/male) | 1 / 5 | 1 / 5 | 2 / 7 | 0/9 | 0/5 | 0/4 |
| Age (year) | 34.7 ± 4.5 | 56.2 ± 4.1 | 37.8 ± 3.8 | 58.0 ± 2.7 | 60.4 ± 2.5 | 55.0 ± 5.5 |
| BMI (kg/m2) | 26.0 ± 5.0 | 26.1 ± 2.3 | 25.2 ± 1.3 | 26.6 ± 1.1 | 28.7 ± 1.2 | 24.0 ± 1.0 * |
| Cardiac functional parameters | ||||||
| NYHA functional class I/II/III/IV (n) | n.a. | 0/1/2/3 | n.a. | 0/1/5/3 | 0/1/2/2 | 0/0/3/1 |
| CO (L/min) | n.a. | 3.9 ± 0.6 | n.a. | 4.0 ± 0.21 | 4.5 ± 0.1 | 3.4 ± 0.2 *** |
| EF (%) | n.a. | 20.1 ± 3.2 | n.a. | 21.4 ±2.5 | 21.4 ± 3.7 | 21.5 ± 4.1 |
| LVED (mm) | n.a. | 69.2 ± 2.5 | n.a. | 75.0 ± 3.2 | 0.2 ± 3.6 | 81.0 ± 4.4 |
| LVSD (mm) | n.a. | 63.0 ± 3.2 | n.a. | 67.8 ± 4.5 | 62.3 ± 6.7 | 73.3 ± 5.5 |
| PW (mm) | n.a. | 9.1 ± 0.7 | n.a. | 8.4 ± 0.8 | 9.8 ± 1.0 | 6.7 ± 0.5 * |
| IVS (mm) | n.a. | 8.4 ± 1.1 | n.a. | 9.5 ± 0.7 | 10.2 ± 1.2 | 8.7 ± 0.6 |
| SVR (mmHg·min/L) | n.a. | 18.3 ± 1.8 | n.a. | 19.0 ± 2.2 | 15.1 ± 2.5 | 23.8 ± 2.2 * |
| Laboratory parameters | ||||||
| AST (U/L) | 79.6 ± 24.4 | 27.6 ± 3.1 | 76 ± 17.1 | 43.3 ± 10.8 | 54.8 ± 17.9 | 29.0 ± 6.5 |
| ALT (U/L) | 55.5 ± 19.2 | 27.6 ± 7.9 | 79 ± 13.8 | 34.8 ± 11.3 | 45 ± 19.9 | 22.2 ± 3.2 |
| Creatinine (μM) | n.a. | 107.6 ± 10.9 | n.a. | 115.8 ± 8.8 | 104.6 ± 13.8 | 130 ± 5.8 |
| NT-proBNP (pg/mL) | n.a. | 3605 ± 1267 | n.a. | 4031 ± 1322 | 2687 ± 1399 | 5711 ± 2352 |
| Total cholesterol (mM) | n.a. | 3.7 ± 0.3 | n.a. | 4.8 ± 0.4 | 4.3 ± 0.3 | 5.4 ± 0.8 |
| LDL cholesterol (mM) | n.a. | 1.9 ± 0.2 | n.a. | 3 ± 0.3 | 2.6 ± 0.2 | 3.5 ± 0.7 |
| HDL cholesterol (mM) | n.a. | 1.1 ± 0.1 | n.a. | 1 ± 0.1 | 1.1 ± 0.1 | 0.8 ±0.2 |
| Triacylglycerol (mM) | n.a. | 1.2 ± 0.1 | n.a. | 1.9 ± 0.3 | 1.5 ± 0.1 | 2.6 ± 0.6 |
| Glucose (mM) | n.a. | 6.5 ± 0.5 | n.a. | 5.7 ± 0.2 | 5.8 ± 0.3 | 5.6 ± 0.2 |
Sample collection was performed as previously described [32]. In brief, human tissue samples were taken at the time of heart explanation (avoiding scarred, fibrotic, or adipose tissue, endocardium, epicardium, or coronary vessels). The samples were rinsed immediately in saline, blotted dry, frozen in liquid nitrogen, powdered with a pestle and mortar in liquid nitrogen and stored in cryovials at −80 °C until further analysis. Healthy control samples were stored in cold cardioplegic solution and once it was decided that there is no compatible recipient the samples were handled and stored as described above.
4.4. Quantitative real-time PCR
4.4.1. RNA isolation
Total RNA was isolated from left ventricular samples (n = 6) with a chloroform/isopropanol precipitation method. In brief, Qiazol® (Qiagen) was added to each sample and homogenized with Tissue Lyser (Qiagen). Homogenates were centrifuged and DNA and protein was precipitated from the clean upper phase with chloroform. Total RNA was precipitated with isopropanol and pellets were washed twice with ethanol (vWR). Finally, total RNA was resuspended in nuclease-free water, and RNA concentration was determined by spectrophotometry (NanoDrop, Thermo Fischer Scientific).
4.4.2. cDNA synthesis
cDNA was synthesized from 1 μg total RNA by Sensifast cDNA synthesis kit (Bioline) according to the manufacturers protocol. cDNA was diluted 20 times with RNAse-free water. qRT-PCR reactions were performed on a LightCycler® 480 II instrument (Roche) by using SensiFAST SYBR Green master mix (Bioline). Polymerase was heat-activated for 2 min at 95 °C and targets were amplified and quantified in 40 cycles (denaturation: 5 s at 93 °C; annealing: 10 s at 60 °C; synthesis: 20 s at 72 °C). Forward and reverse primers for the fatty acid amide hydrolase (FAAH), cannabinoid receptor 1 (CNR1), cannabinoid receptor 2 (CNR2), diacylglycerol lipase α (DAGLα), diacylglycerol lipase β (DAGLβ), monoacylglycerol lipase (MGLL), N-acylphosphatidylethanolamine phospholipase D (NAPEPLD), α/β-hydrolase domain-containing 6 (ABHD6), α/β-hydrolase domain-containing 12 (Abhd12) were used for analysis. Hypoxanthine-guanine phosphoribosyltransferase (HPRT) was used as a housekeeping gene. Results were calculated with 2−ΔΔCp evaluation method. Primer sequences are shown in Supplementary Table S1.
4.5. Targeted lipidomics
4.5.1. Lipid extraction
Lipid extraction was performed as previously described [33], with minor adaptations. In brief, ~50 mg tissue was weighed into a pre-cooled 1.5 mL Eppendorf tube and reconstituted in ice cold ammonium acetate buffer (0.1 M, adjusted to pH 4 with acetic acid) (4 μL/mg tissue). The tissue was finely cut using chirurgical scissors and subsequently homogenized by a tissue homogenizer (M.P. Biomedicals, LLC, USA) and probe sonication (3 cycles, 10 s, 30% amplitude) while kept on ice. Samples were spiked with 10 μL of deuterated internal standard mix (Supplementary Table S2). After extraction with 1000 μL methyl tert-butyl ether (MTBE), the tubes were thoroughly mixed for 5 min using a bullet blender (Next Advance) at medium speed, followed by a centrifugation step (16,000 g, 5 min, 4 °C). Next, 850 μL of the upper MTBE layer was transferred to clean 1.5 mL Eppendorf tube. Samples were dried in a speedvac (Eppendorf) followed by reconstitution in acetonitrile:water (50 μL, 90:10, v/v). The reconstituted samples were centrifuged (16,000 g, 5 min, 4 °C) before transferring into LC-MS vials. 5 μL of each sample was injected into the LC-MS/MS system.
4.5.2. LC-MS/MS analysis
LC-MS/MS analysis was performed as previously described [33], with minor adaptations. A targeted analysis of 31 compounds, including endocannabinoids and related N-acylethanolamines (NAEs) along with the fatty acids (Supplementary Table S2), was measured using an Acquity UPLC I class Binary solvent manager pump (Waters) in conjugation with AB SCIEX 6500 quadrupole-ion trap (AB Sciex). The separation was performed with an Acquity HSS T3 column (2.1 × 100 mm, 1.8 μm) maintained at 45 °C. The aqueous mobile phase A consisted of 2 mM ammonium formate and 10 mM formic acid, and the organic mobile phase B was acetonitrile. The flow rate was set to 0.55 mL/min; initial gradient conditions were 55% B held for 2 min and linearly ramped to 100% B over 6 min and held for 2 min; after 10 s the system returned to initial conditions and held 2 min next injection. Electrospray ionization-MS and a selective Multiple Reaction Mode (MRM) was used for endocannabinoid quantification. Individually optimized MRM transitions using their synthetic standards for target compounds and internal standards are described in Supplementary Table 2. For each sample the normalized lipid abundance was calculated by dividing lipid abundance by total weight of tissue used for lipid extraction (49–52 mg tissue/sample). Subsequently, the normalized lipid abundances were averaged and the averaged control value was set at ratio 1 or at 100%.
4.6. Activity-based protein profiling
4.6.1. Sample preparation
Cardiac tissue was dounce homogenized in ice-cold lysis buffer (250 mM sucrose, 20 mM HEPES pH 7.2, 2 mM DTT, 1 mM MgCl2, 2 U/mL benzonase) and incubated on ice (15 min). Clear lysate was obtained as the supernatant fraction after two low-speed centrifugation steps (2500 g, 5 min, 4 °C). After dilution to 2 mg/mL in storage buffer (20 mM HEPES pH 7.2, 2 mM DTT), samples were used or flash frozen in liquid nitrogen and stored at −80 °C until further use.
4.6.2. Gel-based ABPP
Clear lysates (2 mg/mL) were incubated with activity-based probes MB064 (2 uM), FP-TAMRA (500 nM), or DH379 (1 μM) (20 min, rt). The reaction was quenched with Laemmli buffer (30 min, rt) and 20 μg protein was resolved by SDS-PAGE (10% acrylamide gel, ~80 min, 180 V) along with protein marker (PageRuler™ Plus, Thermo Fisher). In-gel fluorescence was measured in the Cy2, Cy3, and Cy5 channels (ChemiDoc™ MP, Bio-Rad) and gels were stained with Coomassie after scanning. Fluorescence was quantified and normalized to Coomassie staining using ImageLab™ software (Bio-Rad). No significant differences in Coomassie signal were detected, indicating similar protein content and gel loading.
4.6.3. Chemical proteomics with label-free quantification
The chemical proteomics workflow was modified from a previously published protocol [34]. In short, for general profiling of the serine hydrolases the whole lysates (250 μg protein, 1 mg/mL) were incubated with serine hydrolase probe cocktail (10 μM MB108, 10 μM FP-Biotin, 30 min, 37 °C, 300 rpm). A denatured protein sample (1% SDS, 5 min, 100 °C) was taken along as a negative control. Precipitation, alkylation, avidin enrichment, on-bead digestion and sample preparation was performed according to protocol. Dried peptides were stored at −20 °C until LC-MS analysis. Prior to measurement, samples were reconstituted in 50 μL LC-MS solution and transferred to LC-MS vials. LC-MS data was analyzed by ProteinLynx Global SERVER™ (PLGS, Waters) and IsoQuant software [35] (www.proteomeumb.org/MZw.html) (minimal peptide score 6, false discovery rate 1%). Excel was used for further analysis, with the following cut-offs: false discovery rate 1%, unique peptides ≥ 1, identified peptides ≥ 2, ratio positive over negative control ≥ 2, part of putative hydrolase target list. Graphs were created using GraphPad Prism 7 (GraphPad).
4.7. Western blot
Clear lysates (2 mg/mL) were denatured with Laemmli buffer (5 min, 100 °C) and 45 μg lysate was resolved by SDS-PAGE (10% acrylamide gel, 65 min, 200V) along with PageRuler™ Plus Protein Marker (Thermo Scientific). Proteins were transferred to 0.2 μm polyvinylidene difluoride membranes by Trans-Blot Turbo™ Transfer system (Bio-Rad). Membranes were washed with TBS (50 mM Tris, 150 mM NaCl) and blocked with 5% milk in TBS-T (50 mM Tris, 150 mM NaCl, 0.05% Tween 20) (1 h, rt). Membranes were then incubated with primary antibody rabbit-anti-MGLL (ab24701, Abcam, 1:200 in 5% milk in TBS-T, O/N, 4 °C) washed with TBS-T, incubated with secondary donkey-anti-rabbit Alexa647 (A-31573, Thermo Fisher; 1:10000 in 5% milk TBS-T, 1 h, rt), and washed with TBS-T and TBS. Fluorescence was detected on the ChemiDoc™ MP (Bio-Rad) in the Alexa647 channel, and Cy3/Cy5-channels for the protein marker.
4.8. Statistical methods
Statistical significance was determined by a Student’s t-test (two-tailed, unpaired, p-values) with Benjamini-Hochberg false discovery rate (FDR 10%, q-values) for lipidomics and proteomics data using GraphPad Prism 7 (GraphPad) software. Samples were compared to (mean) healthy controls and significance is indicated as * < 0.05, ** < 0.01, *** < 0.001.
5. Results
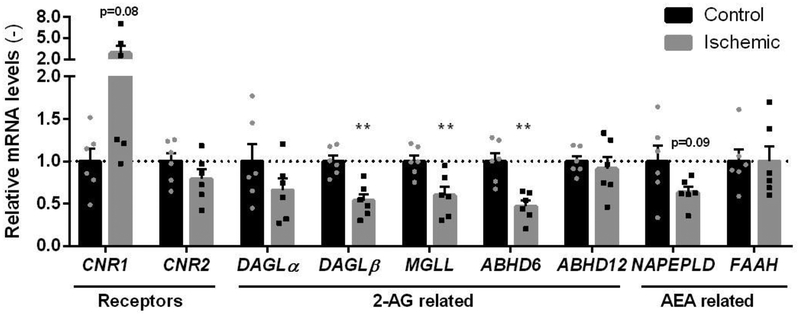
To investigate the involvement of the ECS in cardiac ischemia, tissue from the left ventricle was obtained from patients with terminal-stage heart failure (due to previous ischemic pathology) indicated for heart transplantation, as well as from non-failing control hearts (n=6, Table 1). There was no major difference in the general characteristics (Table 1) of patients (no overt diabetes, normal liver and kidney function). All of the heart failure patients received standard medication for chronic heart failure (ACE inhibitors, beta receptor antagonists, and mineralocorticoid receptor antagonists), as well as statins for ischemic heart disease. There was an increased level of NT-proBNP in all heart failure groups, that is a well-known marker of disease state. Considering the reported unreliability of cannabinoid receptor specific antibodies [26,36], we choose quantitative real-time polymerase chain reaction (qRT-PCR) to measure the expression levels of ECS-related genes in control and ischemic failing hearts (Figure 2). The expression levels of ECS-related proteins in control and ischemic failing hearts were determined by quantitative real-time polymerase chain reaction (qRT-PCR)(Figure 2). CB1R (CNR1) expression strongly increased in half of the ischemic samples, however the overall increase was not significant (p=0.08). Reduced expression of 2-AG biosynthetic enzyme DAGLβ and the 2-AG hydrolytic enzymes MGLL and ABHD6 was observed in the ischemic tissue. The AEA metabolic enzymes were not significantly altered, nor was CB2R (CNR2) expression.
Figure 2 |. Quantitative PCR on ECS-related genes in cardiac ischemia.
mRNA levels of endocannabinoid related genes were normalized to house-keeping gene hypoxanthine-guanine phosphoribosyltransferase (HPRT) expression and expressed relative to control (mean ± SEM, two-tailed t-test: ** p < 0.01).
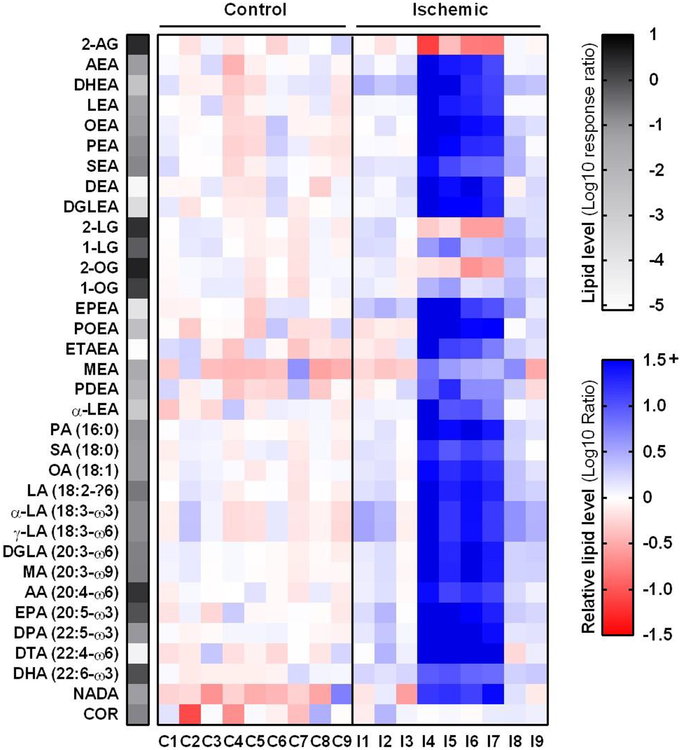
In light of the altered mRNA expression of ECS-related enzymes, the endocannabinoid levels in control and ischemic failing cardiac tissues were compared. Lipids were extracted from a second set of cardiac tissues (n=9, Table 1) and were analyzed by liquid chromatography coupled to mass spectrometry (LC-MS) (Figure 3, Supplementary Figure S1A). In addition, levels of NAEs, free fatty acids (FFA) and cortisol (COR) were measured in the same assay. The NAEs and their related FFA levels had strongly increased in the ischemic failing tissues (Figure 3, Supplementary Figure S1B). However, based on their lipid profile, the ischemic samples could be categorized into two subgroups. The first (Ischemic 1: I1-I3, I8-I9) had a lipid profile similar to control for most lipids including the endocannabinoids. Only several lipids were increased, including N-docosahexa-enoylethanolamide (DHEA), eicosapentaenoyl-ethanolamide (EPEA), α- and γ- linoleic acid (α-LA and γ-LA) (maximum fold-change: 2.8). In contrast, all NAE and FFA levels were increased with a fold change ranging from 4 to 120 in the second subgroup (Ischemic 2: I4-I7) as compared to controls. In addition, the endocannabinoid AEA was increased by a 31 ± 13-fold, while 2-AG was significantly reduced by a 5-fold in this subgroup.
Figure 3 |. Ischemic heart tissues can be categorized into subgroups based on divergent lipid profiles.
Heatmap summary of lipid analysis of healthy (control) and ischemic cardiac tissue. Lipid levels were normalized to tissue weight and are expressed as mean response ratio of controls (grayscale, log10) or relative to mean control (red-blue scale, log10 ratio). Detailed lipid characteristics in Supplementary Table S2.
Increased AEA levels have been reported in the past e.g. due to various forms of ischemia/reperfusion (I/R) (e.g. hepatic, brain). In the liver, I/R increased 2-AG and AEA levels positively correlated with tissue damage markers such as tumor necrosis factor α (TNF-α), but inflammatory stimuli per se only increased AEA levels [37]. NAE levels have also been shown to drastically increase post-mortem [38] and in infarcted myocardium [39–42], thus suggesting the observed effects may be related to the extent of tissue injury. Of note, there were no obvious differences in general clinical characteristics (gender, age, co-morbidity, recent ischemic events, etc.) between the two ischemic subgroups. Nonetheless, cardiac function in the second subgroup was more severely affected based on significantly decreased cardiac output (CO) and significantly increased systemic vascular resistance (SVR) (Table 1). In addition, the body mass index (BMI) of the second subgroup was significantly lower than that of the first subgroup; however, it was not significantly different from controls.
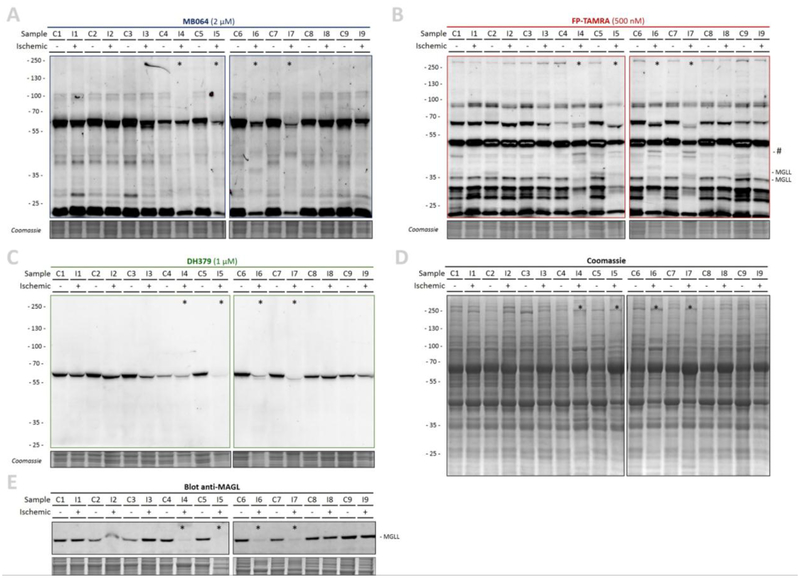
Next, the activity of the ECS metabolic enzymes was investigated by activity-based protein profiling (ABPP). The tissue was lysed by dounce-homogenization and clear lysates were labeled with fluorescent activity-based probes (Figure 4), which enabled visualization of probe targets by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and in-gel fluorescence scanning. The tailored lipase probe MB064 (Figure 4A) preferentially reacts with the DAGLα, DAGLβ, ABHD6, and ABHD12 [31]. FP-TAMRA (Figure 4B), a broad spectrum serine hydrolase probe fluorophosphonaterhodamine (FP-TAMRA), labels ABHD6, MGLL and FAAH amongst many other hydrolases [29,30]. Probe DH379 [43] enabled more selective the labeling of DAGL/ABHD6 (Figure 4C).
Figure 4 |. Activity-based protein profiling of healthy and ischemic human hearts.
(A-D) Gel-based ABPP analysis on healthy (control) and ischemic cardiac tissue. Whole lysates were labeled with activity-based probes (20 min, rt), resolved by SDS-PAGE and in-gel fluorescence was detected. Coomassie served as a protein loading control. (A) β-lactone probe MB064 (2 μM). (B) Broad-spectrum hydrolase probe FP-TAMRA (500 nM). (C) DAGL-probe DH379 (1 μM). (D) Coomassie staining. (E) Western-blot using anti-MGLL (1:200, O/N, 4 °C) verified MGLL expression. * Denotes samples with overall reduced serine hydrolase labeling.
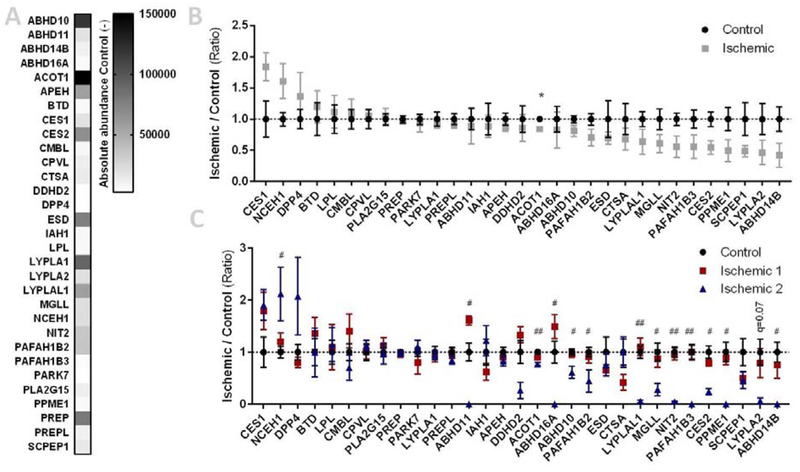
In total, more than 20 hydrolases were labeled, including MGLL (33 and 35 kDa) (Figure 4). The overall hydrolase activity in ischemic samples I4-I7 (subgroup 2 based on lipid profile, indicated with *,) was reduced as compared to the remaining ischemic samples and controls for which only limited deviations in labeling were observed. In addition, MGLL activity and expression were nearly abolished in samples I4–7 (Figure 4B, E). However, there were no obvious differences in the overall protein staining (Figure 4D) and general post-mortem degradation by autolysis thus appears unlikely. Interestingly, an additional band was observed in the activity profile of samples I4-I7 (Figure 4B, indicated with #). Of note, the other ECS metabolic hydrolases, including DAGLα (~120 kDa), DAGLβ (~70 kDa), ABHD6 (~35 kDa), and FAAH (~60 kDa) were not detected (Figure 4A–C), even though ABHD6 activity was detected in murine myocardium in the past [44].
The biotinylated counterparts of FP-TAMRA and MB064, FP-biotin and MB108 respectively, were then used for target identification by mass spectrometry-based chemical proteomics (Figure 5). In total, 31 hydrolases were identified, including MGLL as the only ECS-related hydrolase (Figure 5A, Supplementary Table S3). A slight, but non-significant, upregulation of CES1, NCEH1, and DPP4 was observed in the ischemic group, as well as downregulation of several hydrolases, including MGLL (Figure 5B). This downregulation of MGLL was further confirmed by Western blot analysis (Figure 4E). Strikingly, separation of the ischemic samples into two subgroups (based on lipid profile, Figure 3) revealed that 13 hydrolase activities, including MGLL, were drastically and significantly reduced in the subgroup with an altered lipid profile (Ischemic 2, Figure 5C) and worse cardiac function (Table 1). Hydrolase activities from the first subgroup, however, were not significantly altered. Notably, not all hydrolase activities were affected in the second subgroup and the extent of activity reduction is different between the altered hydrolases. This reinforces the hypothesis that the observed alterations in these samples are indeed not artefacts resulting from a general process such as postmortem autolysis.
Figure 5 |. Activity-based proteomics on healthy and ischemic heart tissues.
(A-C) Lysates from healthy (control) and ischemic cardiac tissues were labeled with MB108 and FP-biotin (10 μM each, 30 min, 37 °C) and analyzed by mass-spectrometry. A pre-boiled sample (10% SDS, 100 °C, 5 min) served as negative control. (A) Heatmap summary of mean abundance of hydrolases from control tissues. (B) Hydrolase activity relative to mean control. Data is expressed as mean ± SEM (n=9), t-test with Benjamini-Hochberg correction: * q < 0.05. (C) Hydrolase activity relative to mean control, ischemic samples categorized in subgroups. Data is expressed as mean ± SEM (control n=9, ischemic 1 n=5, ischemic 2 n=4), t-test with Benjamini-Hochberg correction. Control versus ischemic 1: not significant; control versus ischemic 2: # q < 0.05, ## q < 0.01.
Taken together, lipidomics and ABPP enabled the identification of a subgroup within the ischemic sample set, which showed drastic increases in NAE and FFA levels, whereas many hydrolase activities had decreased. Aside from MGLL, no endocannabinoid metabolic enzymes were detected, possibly due to instability or inactivity. Despite accurate sample handling, differences in the sample collection procedure cannot be excluded as a potential cause for the observed subgroups. In addition, therapeutic interventions or unknown clinical factors may separate the ischemic tissues from one another. However, these data demonstrated that ABPP could be used for the rapid analysis of serine hydrolases in clinical samples. In the future, this technique may aid in the discovery of drug targets or biomarkers.
6. Discussion
The role of cannabinoid receptors, as potential drug targets, has been extensively studied in various forms of cardiovascular diseases [3]. In addition, there are continuous efforts to develop new drugs either to target the cannabinoid receptors, or the metabolic pathways that are involved in endocannabinoid production and degradation [45]. Therefore, to better understand endocannabinoid metabolism and cannabinoid receptor signaling in critically ill patients, and to characterize the endocannabinoid system in clinically relevant human samples is of high importance, in order to find the most suitable patient groups for the drugs that are currently under development.
CB1R-mediated endocannabinoid signaling has been implicated in the pathogenesis of shock, atherosclerosis, and numerous forms of cardiomyopathies (ischemic, diabetic, doxorubicin-induced [3,20]. The in vivo effects of CB2R modulation have mainly been studied in models of myocardial infarction and stroke. The observed effects have primarily been attributed to limiting inflammatory cell infiltration to the injured tissue [27,46] and to inhibition on endothelial activation fibroblastmyofibroblast transformation [3,47].
The knowledge on the role of endocannabinoid metabolic enzymes in cardiovascular diseases is much more limited. FAAH knockout mice, having a threefold increase in myocardial AEA, displayed increased mortality, myocardial injury, and neutrophil infiltration in an experimental model of doxorubicin-induced cardiomyopathy in a CB1R-dependent manner [48]. FAAH deficiency also enhanced intra-plaque neutrophil recruitment in atherosclerosis-prone mice [49]. In obese humans, increased plasma levels of AEA and 2-AG strongly correlated with impaired coronary endothelial function and adverse cardiovascular events [50,51]. In epicardial fat from ischemic human hearts, CB1R was upregulated accompanied by downregulation of CB2R and FAAH [52]. Similar upregulation of CB1R was observed in atherosclerotic coronary artery sections from patients with unstable angina and in obese human subjects [26,53]. AEA or synthetic CB1R agonists also decrease cardiomyocyte contractility both in rodents and humans by interfering with excitation-contraction coupling [54,55]. Furthermore, endocannabinoids (through CB1R) promote p38 and JNK MAPKs activation in human and/or mouse cardiomyocytes and coronary artery endothelial cells facilitating apoptosis, as well as vascular inflammation both in vitro and/or in vivo [56–59]. These findings strongly suggest that the primary cardiovascular effects of endocannabinoids (particularly of AEA), similarly to synthetic CB1R agonists, are deleterious and in many cases CB1R-mediated [3]. Thus, in the present study, the markedly increased tissue levels of AEA in the more severe ischemic heart failure subgroup (having significantly decreased cardiac output (CO) and increased systemic vascular resistance (SVR)) might imply a potential disease modifying effect of AEA in chronic heart failure or reflect the extent of cardiovascular injury/dysfunction. This is also consistent with correlation of increased plasma levels of AEA with impaired coronary endothelial function and adverse cardiovascular events in obese human subjects [50,51]. Nonetheless, endocannabinoids have also been reported to exert protective effects in the heart via receptor-independent mechanisms (e.g. proposed to be involved in preconditioning mechanisms of the heart). However, these earlier studies are primarily based on ex vivo experiments and use descriptive and indirect approaches, or the protective effects are time- and disease-state-dependent [27,60].
In line with our results, Weiss et al. have reported that endocannabinoids are increased in the serum of patients suffering from heart failure due to dilated cardiomyopathy [61]. They have also shown a switch towards CB2R upregulation, without changes in the expression of CB1R. Although, our present gene expression analysis revealed differing trends in CB1R and CB2R expression, this can be explained by the differing etiology of dilated versus ischemic cardiomyopathies. Dilated cardiomyopathy development is commonly a result of viral myocarditis as well as less-understood autoinflammatory processes. Therefore, the upregulation of CB2R in that disease setting is likely a result of proinflammatory mechanisms. Notably, the results of prior studies relying on use of CB2R antibodies are also questionable in light of well-known problem of the specificity of CB2R antibodies in tissues.
Despite extensive drug development efforts of MGLL inhibitors [62,63], the role of MGLL and its substrate 2-AG in ischemic as well as in metabolic cardiac derangements is unclear and controversial In a very recent paper by Schloss et al. exogenous administration of 2-AG was reported to be detrimental in myocardial infarction, by promoting leukocyte recruitment to the damaged tissue [64]. In addition, the authors found a markedly decreased expression of MGLL in infarcted tissue, which is in line with the observed decrease in MGLL activity in the present study. Accordingly, treatment with MGLL inhibitor JZL-184 negatively affected post-infarction cardiac remodeling with extensive fibrotic scar formation, and impaired cardiac function [64]. On the other hand, MGLL seems to play a protective role in the stabilization of atherosclerotic plaques [65]. Vujic et al. described that, in spite of increased plaque formation in apolipoprotein E (ApoE)-MGLL double knockout mice, plaques contained less lipids and inflammatory cells, and more collagen as compared to wildtype controls, suggesting increased plaque stability. Based on these findings, it appears likely that MGLL plays a multifactorial role in ischemic heart failure as well as in other cardiometabolic conditions. Therefore, to understand the complex role of MGLL in lipid signaling and metabolism (related to the synthesis of the endocannabinoid 2AG, to the cleavage of monoacylglycerols, and providing free fatty acids for beta oxidation, as well as the production of arachidonic acid, a precursor of complex lipid mediators), there is a need for further studies that integrate these various aspects of MGLL-dependent signaling and metabolic pathways.
In addition, our ABPP-approach revealed other interesting changes, although these changes do not necessarily relate those in endocannabinoid metabolism. We found increased activity of carboxylesterase 1 (CES1), neutral cholesterol ester hydrolase 1 (NCEH1) and dipeptidyl peptidase 4 (DPP4) in the cardiac tissues from the ischemic heart failure group. Carboxylesterases are believed to be involved in the formation of toxic fatty acid ethyl ethers [66,67] and therefore their increased activity in heart failure might underlie the increased susceptibility to heavy alcohol consumption-induced toxicity after infarction [68]. NCEH1 is a critical enzyme in reverse cholesterol transport, by cleaving intracellular cholesterol-esters and allowing cholesterol to leave the cells and incorporate into high-density-lipoprotein (HDL) [69,70]. Possibly, the increased activity of this enzyme represents an adaptive mechanism, to tackle increased cholesterol and triacylglycerol load of the heart. The increased activity of DPP4 is also interesting in the failing heart in light of the revealed latent cardiotoxicity of saxagliptin, a selective DPP4 inhibitor, for the treatment of type 2 diabetes[71].
Conclusion
In summary, a MS-based approach was used to investigate the endocannabinoids and their metabolic enzymes in cardiac ischemia using ischemic end-stage failing hearts and non-failing controls. Targeted lipidomics analysis revealed the existence of two subgroups within the ischemic samples; the first largely similar to controls and the second with decreased 2-AG and increased AEA, NAE and FFA levels. The altered lipid profile was accompanied by a strong reduction in the activity of multiple hydrolases, including the 2-AG hydrolytic enzyme MGLL. These data suggested the presence of different biological states within the ischemic group, possibly the extent of tissue injury, despite the lack of basic clinical characteristics separating the patients other than impaired cardiac function. In addition, this study demonstrated ABPP as a tool to rapidly assess clinical samples which may be valuable in drug-target and biomarker discovery.
Supplementary Material
8. Acknowledgements / Funding
This study was supported by intramural funds of NIAAA (to PP and ZVV), as well as from grants of the National Research, Development and Innovation Office of Hungary, grant numbers NVKP_16-1-2016-0017; OTKA KH 125570; and the Higher Education Institutional Excellence Program of the Ministry of Human Capacities in Hungary, within the framework of the Therapeutic Development thematic program of the Semmelweis University (ZVV, VET, ZO, and PF). This project has received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement No 739593 (ZVV, VET, ZO). MS thanks the Netherlands Organisation for Scientific Research (NWO) for financial support (VICI-scheme).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest
The authors of this manuscript declare to have no conflict of interest.
- Supplementary information
- Figure S1
- Table S1–S3
- Peptide list of proteomics data (xlsx).
References
- [1].Finegold JA, Asaria P, Francis DP, Mortality from ischaemic heart disease by country, region, and age: Statistics from World Health Organisation and United Nations, Int. J. Cardiol 168 (2013) 934–945. 10.1016/j.ijcard.2012.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].O’Sullivan SE, Endocannabinoids and the cardiovascular system in health and disease, in: Endocannabinoids, Springer, Cham, 2015: pp. 393–422. 10.1007/978-3-319-20825-1_14. [DOI] [PubMed] [Google Scholar]
- [3].Pacher P, Steffens S, Haskó G, Schindler TH, Kunos G, Cardiovascular effects of marijuana and synthetic cannabinoids: The good, the bad, and the ugly, Nature Publishing Group, 2018. 10.1038/nrcardio.2017.130. [DOI] [PubMed] [Google Scholar]
- [4].Pacher P, Mukhopadhyay P, Mohanraj R, Godlewski G, Bátkai S, Kunos G, Modulation of the Endocannabinoid System in Cardiovascular Disease, Hypertension. 52 (2008) 601–607. 10.1161/hypertensionaha.105.063651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Di Marzo V, Endocannabinoid signaling in the brain: biosynthetic mechanisms in the limelight, Nat. Neurosci 14 (2011) 9–15. 10.1038/nn.2720. [DOI] [PubMed] [Google Scholar]
- [6].Bisogno T, Howell F, Williams G, Minassi A, Cascio MG, Ligresti A, Matias I, Schiano-Moriello A, Paul P, Williams EJ, Gangadbaran U, Hobbs C, Di Marzo V, Doherty P, Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain, J. Cell Biol 163 (2003) 463–468. 10.1083/jcb.200305129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Dinh TP, Freund TF, Piomelli D, A role for monoglyceride lipase in 2-arachidonoylglycerol inactivation, in: Chem. Phys. Lipids, 2002: pp. 149–158. 10.1016/S0009-3084(02)00150-0. [DOI] [PubMed] [Google Scholar]
- [8].Savinainen JR, Saario SM, Laitinen JT, The serine hydrolases MAGL, ABHD6 and ABHD12 as guardians of 2-arachidonoylglycerol signalling through cannabinoid receptors, Acta Physiol. 204 (2012) 267–276. 10.1111/j.1748-1716.2011.02280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Marrs WR, Blankman JL, Horne EA, Thomazeau A, Lin YH, Coy J, Bodor AL, Muccioli GG, Hu SS-J, Woodruff G, Fung S, Lafourcade M, Alexander JP, Long JZ, Li W, Xu C, Möller T, Mackie K, Manzoni OJ, Cravatt BF, Stella N, Hsing Lin Y, Coy J, Bodor AL, Muccioli GG, Shu-Jung Hu S, Woodruff G, Fung S, Lafourcade M, Alexander JP, Long JZ, Li W, Xu C, Möller T, Mackie K, Manzoni OJ, Cravatt BF, Stella N, Lin YH, Coy J, Bodor AL, Muccioli GG, Hu SS-J, Woodruff G, Fung S, Lafourcade M, Alexander JP, Long JZ, Li W, Xu C, Möller T, Mackie K, Manzoni OJ, Cravatt BF, Stella N, The serine hydrolase ABHD6 controls the accumulation and efficacy of 2-AG at cannabinoid receptors., Nat. Neurosci 13 (2010) 951–7. 10.1038/nn.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Blankman JL, Simon GM, Cravatt BF, A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol., Chem. Biol 14 (2007) 1347–1356. 10.1016/j.chembiol.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ueda N, Tsuboi K, Uyama T, Metabolism of endocannabinoids and related N-acylethanolamines: Canonical and alternative pathways, FEBS J. 280 (2013) 1874–1894. 10.1111/febs.12152. [DOI] [PubMed] [Google Scholar]
- [12].Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB, Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides, Nature. 384 (1996) 83–87. 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- [13].Mckinney MK, Cravatt BF, Structure and function of Fatty Acid Amide Hydrolase, Annu. Rev. Biochem 74 (2005) 411–432. 10.1146/annurev.biochem.74.082803.133450. [DOI] [PubMed] [Google Scholar]
- [14].Rodríguez De Fonseca F, Navarro M, Gómez R, Escuredo L, Nava F, Fu J, Murillo-Rodríguez E, Giuffrida A, Loverme J, Gaetani S, Kathuria S, Gall C, Piomelli D, An anorexic lipid mediator regulated by feeding, Nature. 414 (2001) 209–212. 10.1007/s10773-014-2266-7. [DOI] [PubMed] [Google Scholar]
- [15].Terrazzino S, Berto F, Carbonare MD, Fabris M, Guiotto A, Bernardini D, Leon A, Stearoylethanolamide exerts anorexic effects in mice via downregulation of liver stearoylcoenzyme A desaturase-1 mRNA expression, Faseb J. 18 (2004) 1580–1582. 10.1096/fj.03-1080fje. [DOI] [PubMed] [Google Scholar]
- [16].Calignano A, La Rana G, Giuffrida A, Piomelli D, Control of pain initiation by endogenous cannabinoids, Nature. 394 (1998) 277–281. 10.1038/28393. [DOI] [PubMed] [Google Scholar]
- [17].Jaggar SI, Hasnie FS, Sellaturay S, Rice AS , The anti-hyperalgesic actions of the cannabinoid anandamide and the putative CB2 receptor agonist palmitoylethanolamide in visceral and somatic inflammatory pain, Pain. 76 (1998) 189–199. 10.1016/S0304-3959(98)00041-4. [DOI] [PubMed] [Google Scholar]
- [18].Mechoulam R, Parker LA, The Endocannabinoid System and the Brain, Annu. Rev. Psychol 64 (2012) 21–47. 10.1146/annurev-psych-113011-143739. [DOI] [PubMed] [Google Scholar]
- [19].Di Marzo V, Bifulco M, De Petrocellis L, The endocannabinoid system and its therapeutic exploitation, Nat. Rev. Drug Discov 3 (2004) 771–784. 10.1038/nrd1495. [DOI] [PubMed] [Google Scholar]
- [20].Pacher P, Bátkai S, Kunos G, The Endocannabinoid System as an Emerging Target of Pharmacotherapy, Pharmacol. Rev 58 (2006) 389–462. 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hiley CR, Endocannabinoids and the heart, J. Cardiovasc. Pharmacol 53 (2009) 267–276. 10.1097/FJC.0b013e318192671d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Montecucco F, Di Marzo V, At the heart of the matter: The endocannabinoid system in cardiovascular function and dysfunction, Trends Pharmacol. Sci 33 (2012) 331–340. 10.1016/j.tips.2012.03.002. [DOI] [PubMed] [Google Scholar]
- [23].Pacher P, Kunos G, Modulating the endocannabinoid system in human health and disease -Successes and failures, FEBS J. 280 (2013) 1918–1943. 10.1111/febs.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bátkai S, Pacher P, Endocannabinoids and cardiac contractile function: Pathophysiological implications, Pharmacol. Res 60 (2009) 99–106. 10.1016/j.phrs.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Pacher P, Bátkai S, Kunos G, Cardiovascular pharmacology of cannabinoids., Handb. Exp. Pharmacol (2005) 599–625. http://www.ncbi.nlm.nih.gov/pubmed/16596789 (accessed December 14, 2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Valenta I, Varga ZV, Valentine H, Cinar R, Horti A, Mathews WB, Dannals RF, Steele K, Kunos G, Wahl RL, Pomper MG, Wong DF, Pacher P, Schindler TH, Feasibility Evaluation of Myocardial Cannabinoid Type 1 Receptor Imaging in Obesity: A Translational Approach, JACC Cardiovasc. Imaging 11 (2018) 320–332. 10.1016/j.jcmg.2017.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Steffens S, Pacher P, Targeting cannabinoid receptor CB2 in cardiovascular disorders: Promises and controversies, Br. J. Pharmacol 167 (2012) 313–323. 10.1111/j.1476-5381.2012.02042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Pacher P, Mechoulam R, Is lipid signaling through cannabinoid 2 receptors part of a protective system?, NIH Public Access, 2011. 10.1016/j.plipres.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Niphakis MJ, Cravatt BF, Enzyme Inhibitor Discovery by Activity-Based Protein Profiling, Annu. Rev. Biochem 83 (2014) 341–377. 10.1146/annurev-biochem-060713-035708. [DOI] [PubMed] [Google Scholar]
- [30].Liu Y, Patricelli MP, Cravatt BF, Activity-based protein profiling: The serine hydrolases, Proc. Natl. Acad. Sci 96 (1999) 14694–14699. 10.1073/pnas.96.26.14694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Baggelaar MP, Janssen FJ, van Esbroeck ACM, den Dulk H, Allarà M, Hoogendoorn S, McGuire R, Florea BI, Meeuwenoord N, Vandenelst H, Vandermarel GA, Brouwer J, Dimarzo V, Overkleeft HS, Vanderstelt M, Development of an activity-based probe and in silico design reveal highly selective inhibitors for diacylglycerol lipase-α in brain, Angew. Chemie - Int. Ed 52 (2013) 12081–12085. 10.1002/anie.201306295. [DOI] [PubMed] [Google Scholar]
- [32].Varga ZV, Pipicz M, Baán JA, Baranyai T, Koncsos G, Leszek P, Kusmierczyk M, Sánchez-Cabo F, García-Pavía P, Brenner GJ, Giricz Z, Csont T, Mendler L, Lara-Pezzi E, Pacher P, Ferdinandy P, Alternative splicing of NOX4 in the failing human heart, Front. Physiol 8 (2017) 935 10.3389/fphys.2017.00935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kantae V, Nahon KJ, Straat ME, Bakker LEH, Harms AC, Van Der Stelt M, Hankemeier T, Jazet IM, Boon MR, Rensen PCN, Endocannabinoid tone is higher in healthy lean South Asian than white Caucasian men, Sci. Rep 7 (2017). 10.1038/s41598-017-07980-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].van Rooden EJ, Florea BI, Deng H, Baggelaar MP, Van Esbroeck ACM, Zhou J, Overkleeft HS, Van Der Stelt M, Mapping in vivo target interaction profiles of covalent inhibitors using chemical proteomics with label-free quantification, Nat. Protoc 13 (2018) 752–767. 10.1038/nprot.2017.159. [DOI] [PubMed] [Google Scholar]
- [35].Liao Z, Wan Y, Thomas SN, Yang AJ, IsoQuant: A software tool for stable isotope labeling by amino acids in cell culture-based mass spectrometry quantitation, Anal. Chem 84 (2012) 4535–4543. 10.1021/ac300510t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Marchalant Y, Brownjohn PW, Bonnet A, Kleffmann T, Ashton JC, Validating Antibodies to the Cannabinoid CB2 Receptor: Antibody Sensitivity Is Not Evidence of Antibody Specificity, J. Histochem. Cytochem 62 (2014) 395–404. 10.1369/0022155414530995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Bátkai S, Osei-Hyiaman D, Pan H, El-Assal O, Rajesh M, Mukhopadhyay P, Hong F, Harvey-White J, Jafri A, Haskó G, Huffman JW, Gao B, Kunos G, Pacher P, Cannabinoid-2 receptor mediates protection against hepatic ischemia/reperfusion injury, FASEB J. 21 (2007) 1788–1800. 10.1096/fj.06-7451com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Schmid PC, Krebsbach RJ, Perry SR, Dettmer TM, Maasson JL, Schmid HHO, Occurrence and postmortem generation of anandamide and other long-chain N-acylethanolamines in mammalian brain, FEBS Lett. 375 (1995) 117–120. 10.1016/0014-5793(95)01194-J. [DOI] [PubMed] [Google Scholar]
- [39].Epps DE, Schmid PC, Natarajan V, Schmid HHO, N-Acylethanolamine accumulation in infarcted myocardium., Biochem. Biophys. Res. Commun 90 (1979) 628–33. 10.1016/0006-291X(79)91281-6. [DOI] [PubMed] [Google Scholar]
- [40].Epps DE, Natarajan V, Schmid PC, Schmid HHO, Accumulation of N-acylethanolamine glycerophospholipids in infarcted myocardium, Biochim. Biophys. Acta (BBA)/Lipids Lipid Metab 618 (1980) 420–430. 10.1016/0005-2760(80)90260-X. [DOI] [PubMed] [Google Scholar]
- [41].Natarajan V, Reddy PV, Schmid PC, Schmid HHO, On the biosynthesis and metabolism of N-acylethanolamine phospholipids in infarcted dog heart, Biochim. Biophys. Acta (BBA)/Lipids Lipid Metab 664 (1981) 445–448. 10.1016/0005-2760(81)90067-9. [DOI] [PubMed] [Google Scholar]
- [42].Natarajan V, Reddy PV, Schmid PC, Schmid HHO, N-acylation of ethanolamine phospholipids in canine myocardium, Biochim. Biophys. Acta (BBA)/Lipids Lipid Metab 712 (1982) 342–355. 10.1016/0005-2760(82)90352-6. [DOI] [PubMed] [Google Scholar]
- [43].Ogasawara D, Deng H, Viader A, Baggelaar MP, Breman A, den Dulk H, van den Nieuwendijk AMCH, Soethoudt M, van der Wel T, Zhou J, Overkleeft HS, Sanchez-Alavez M, Mo S, Nguyen W, Conti B, Liu X, Chen Y, Liu QS, Cravatt BF, van der Stelt M, Mori S, Nguyen W, Conti B, Liu X, Chen Y, Liu QS, Cravatt BF, van der Stelt M, Rapid and profound rewiring of brain lipid signaling networks by acute diacylglycerol lipase inhibition, Proc. Natl. Acad. Sci 113 (2016) 26–33. 10.1073/pnas.1522364112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Janssen APA, Van Der Vliet D, Bakker AT, Jiang M, Grimm SH, Campiani G, Butini S, Van Der Stelt M, Development of a Multiplexed Activity-Based Protein Profiling Assay to Evaluate Activity of Endocannabinoid Hydrolase Inhibitors, ACS Chem. Biol 13 (2018) 2406–2413. http://pubs.acs.org/doi/10.1021/acschembio.8b00534 (accessed September 14, 2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Blankman JL, Cravatt BF, Chemical Probes of Endocannabinoid Metabolism, Pharmacol. Rev 65 (2013) 849–871. 10.1124/pr.112.006387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Duerr GD, Heinemann JC, Gestrich C, Heuft T, Klaas T, Keppel K, Roell W, Klein A, Zimmer A, Velten M, Kilic A, Bindila L, Lutz B, Dewald O, Impaired border zone formation and adverse remodeling after reperfused myocardial infarction in cannabinoid CB2 receptor deficient mice, Life Sci. 138 (2015) 8–17. 10.1016/j.lfs.2014.11.005. [DOI] [PubMed] [Google Scholar]
- [47].Perier M, Souktani R, Defer N, Zimmer A, Manin S, Caramelle P, Escoubet B, Pavoine C, Lotersztajn S, Bourin M-C, Wan J, Pecker F, Deveaux V, The cannabinoid receptor type 2 promotes cardiac myocyte and fibroblast survival and protects against ischemia/reperfusion-induced cardiomyopathy, FASEB J. 23 (2009) 2120–2130. 10.1096/fj.09-129478. [DOI] [PubMed] [Google Scholar]
- [48].Mukhopadhyay P, Horváth B, Rajesh M, Matsumoto S, Saito K, Bátkai S, Patel V, Tanchian G, Gao RY, Cravatt BF, Haskó G, Pacher P, Fatty acid amide hydrolase is a key regulator of endocannabinoid-induced myocardial tissue injury, Free Radic. Biol. Med 50 (2011) 179–195. 10.1016/j.freeradbiomed.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Lenglet S, Thomas A, Soehnlein O, Montecucco F, Burger F, Pelli G, Galan K, Cravatt B, Staub C, Steffens S, Fatty acid amide hydrolase deficiency enhances intraplaque neutrophil recruitment in atherosclerotic mice, Arterioscler. Thromb. Vasc. Biol 33 (2013) 215–223. 10.1161/ATVBAHA.112.300275. [DOI] [PubMed] [Google Scholar]
- [50].Quercioli A, Pataky Z, Montecucco F, Carballo S, Thomas A, Staub C, Di Marzo V, Vincenti G, Ambrosio G, Ratib O, Golay A, MacH F, Harsch E, Schindler TH, Coronary vasomotor control in obesity and morbid obesity: Contrasting flow responses with endocannabinoids, leptin, and inflammation, JACC Cardiovasc. Imaging 5 (2012) 805–815. 10.1016/j.jcmg.2012.01.020. [DOI] [PubMed] [Google Scholar]
- [51].Quercioli A, Pataky Z, Vincenti G, Makoundou V, Di Marzo V, Montecucco F, Carballo S, Thomas A, Staub C, Steffens S, Seimbille Y, Golay A, Ratib O, Harsch E, MacH F, Schindler TH, Elevated endocannabinoid plasma levels are associated with coronary circulatory dysfunction in obesity, Eur. Heart J 32 (2011) 1369–1378. 10.1093/eurheartj/ehr029. [DOI] [PubMed] [Google Scholar]
- [52].Cappellano G, Uberti F, Caimmi PP, Pietronave S, Mary DASG, Dianzani C, Micalizzi E, Melensi M, Boldorini R, Nicosia G, Crosio E, Chiocchetti A, Aina F, Prat M, Dianzani U, Vacca G, Ariatti C, Grossini E, Different Expression and Function of the Endocannabinoid System in Human Epicardial Adipose Tissue in Relation to Heart Disease, Can. J. Cardiol 29 (2013) 499–509. 10.1016/j.cjca.2012.06.003. [DOI] [PubMed] [Google Scholar]
- [53].Sugamura K, Sugiyama S, Nozaki T, Matsuzawa Y, Izumiya Y, Miyata K, Nakayama M, Kaikita K, Obata T, Takeya M, Ogawa H, Activated endocannabinoid system in coronary artery disease and antiinflammatory effects of cannabinoid 1 receptor blockade on macrophages, Circulation. 119 (2009) 28–36. 10.1161/CIRCULATIONAHA.108.811992. [DOI] [PubMed] [Google Scholar]
- [54].Al Kury LT, Voitychuk OI, Ali RM, Galadari S, Yang KHS, Howarth FC, Shuba YM, Oz M, Effects of endogenous cannabinoid anandamide on excitation-contraction coupling in rat ventricular myocytes, Cell Calcium. 55 (2014) 104–118. 10.1016/j.ceca.2013.12.005. [DOI] [PubMed] [Google Scholar]
- [55].Bonz A, Laser M, Küllmer S, Kniesch S, Babin-Ebell J, Popp V, Ertl G, Wagner JA, Cannabinoids Acting on CB1 Receptors Decrease Contractile Performance in Human Atrial Muscle, J. Cardiovasc. Pharmacol 41 (2003) 657–664. 10.1097/00005344-200304000-00020. [DOI] [PubMed] [Google Scholar]
- [56].Rajesh M, Mukhopadhyay P, Haskó G, Liaudet L, Mackie K, Pacher P, Cannabinoid-1 receptor activation induces reactive oxygen species-dependent and -independent mitogen-activated protein kinase activation and cell death in human coronary artery endothelial cells, Br. J. Pharmacol 160 (2010) 688–700. 10.1111/j.1476-5381.2010.00712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Mukhopadhyay P, Rajesh M, Bátkai S, Patel V, Kashiwaya Y, Liaudet L, Evgenov OV, Mackie K, Haskó G, Pacher P, CB1 cannabinoid receptors promote oxidative stress and cell death in murine models of doxorubicin-induced cardiomyopathy and in human cardiomyocytes, Cardiovasc. Res 85 (2010) 773–784. 10.1093/cvr/cvp369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].El-Remessy AB, Rajesh M, Mukhopadhyay P, Horváth B, Patel V, Al-Gayyar MMH, Pillai BA, Pacher P, Cannabinoid 1 receptor activation contributes to vascular inflammation and cell death in a mouse model of diabetic retinopathy and a human retinal cell line, Diabetologia. 54 (2011) 1567–1578. 10.1007/s00125-011-2061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Rajesh M, Batkai S, Kechrid M, Mukhopadhyay P, Lee W-S, Horvath B, Holovac E, Cinar R, Liaudet L, Mackie K, Hasko G, Pacher P, Cannabinoid 1 Receptor Promotes Cardiac Dysfunction, Oxidative Stress, Inflammation, and Fibrosis in Diabetic Cardiomyopathy, Diabetes. 61 (2012) 716–727. 10.2337/db11-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Duerr GD, Heinemann JC, Suchan G, Kolobara E, Wenzel D, Geisen C, Matthey M, Passe-Tietjen K, Mahmud W, Ghanem A, Tiemann K, Alferink J, Burgdorf S, Buchalla R, Zimmer A, Lutz B, Welz A, Fleischmann BK, Dewald O, The endocannabinoid-CB2 receptor axis protects the ischemic heart at the early stage of cardiomyopathy, Basic Res. Cardiol 109 (2014) 425 10.1007/s00395-014-0425-x. [DOI] [PubMed] [Google Scholar]
- [61].Weis F, Beiras-Fernandez A, Sodian R, Kaczmarek I, Reichart B, Beiras A, Schelling G, Kreth S, Substantially altered expression pattern of cannabinoid receptor 2 and activated endocannabinoid system in patients with severe heart failure., J. Mol. Cell. Cardiol 48 (2010) 1187–1193. 10.1016/j.yjmcc.2009.10.025. [DOI] [PubMed] [Google Scholar]
- [62].Mulvihill MM, Nomura DK, Therapeutic potential of monoacylglycerol lipase inhibitors, in: Life Sci., Pergamon, 2013: pp. 492–497. 10.1016/j.lfs.2012.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Grabner GF, Zimmermann R, Schicho R, Taschler U, Monoglyceride lipase as a drug target: At the crossroads of arachidonic acid metabolism and endocannabinoid signaling, Pharmacol. Ther 175 (2017) 35–46. 10.1016/j.pharmthera.2017.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Steffens S, Lauer E, Guillamat-Prats R, Schloss MJ, Horckmans M, Lenglet S, Hering D, Thomas A, Weber C, 2-Arachidonoylglycerol mobilizes myeloid cells and worsens heart function after acute myocardial infarction, Cardiovasc. Res (2018). 10.1093/cvr/cvy242. [DOI] [PubMed] [Google Scholar]
- [65].Schauer S, Radovic B, Hoefler G, Rainer S, Eichmann TO, Lass A, Vujic N, Goeritzer M, Madreiter-Sokolowski CT, Rosenberger A, Woelfler A, Zimmermann R, Kratky D, Graier WF, Doddapattar P, Schlager S, Monoglyceride lipase deficiency modulates endocannabinoid signaling and improves plaque stability in ApoE-knockout mice, Atherosclerosis. 244 (2015) 9–21. 10.1016/j.atherosclerosis.2015.10.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Bora PS, Guruge BL, Miller DD, Chaitman BR, Ruyle MS, Purification and Characterization of Human Heart Fatty Acid Ethyl Ester Synthase/Carboxylesterase, J. Mol. Cell. Cardiol 28 (1996) 2027–2032. 10.1006/JMCC.1996.0195. [DOI] [PubMed] [Google Scholar]
- [67].Beckemeier ME, Bora PS, Fatty Acid Ethyl Esters: Potentially Toxic Products of Myocardial Ethanol Metabolism, J. Mol. Cell. Cardiol 30 (1998) 2487–2494. 10.1006/JMCC.1998.0812. [DOI] [PubMed] [Google Scholar]
- [68].Djoussé L, Gaziano JM, Alcohol consumption and heart failure: a systematic review., Curr. Atheroscler. Rep 10 (2008) 117–20. http://www.ncbi.nlm.nih.gov/pubmed/18417065 (accessed February 19, 2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Kumagai M, Hosokawa M, Jacobsen P, Uozaki H, Ohta K, Nagai R, Nagashima S, Nishi M, Li Y, Osuga J, Fukayama M, Kadowaki T, Sekiya M, Takahashi M, Fledelius C, Takanashi M, Takase S, Ishibashi S, Igarashi M, Yagyu H, Ohashi K, The Critical Role of Neutral Cholesterol Ester Hydrolase 1 in Cholesterol Removal From Human Macrophages, Circ. Res 107 (2010) 1387–1395. 10.1161/circresaha.110.226613. [DOI] [PubMed] [Google Scholar]
- [70].Sekiya M, ichi Osuga J, Nagashima S, Ohshiro T, Igarashi M, Okazaki H, Takahashi M, Tazoe F, Wada T, Ohta K, Takanashi M, Kumagai M, Nishi M, Takase S, Yahagi N, Yagyu H, Ohashi K, Nagai R, Kadowaki T, Furukawa Y, Ishibashi S, Ablation of Neutral Cholesterol Ester Hydrolase 1 Accelerates Atherosclerosis, Cell Metab. 10 (2009) 219–228. 10.1016/j.cmet.2009.08.004. [DOI] [PubMed] [Google Scholar]
- [71].Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, Ohman P, Frederich R, Wiviott SD, Hoffman EB, Cavender MA, Udell JA, Desai NR, Mosenzon O, McGuire DK, Ray KK, Leiter LA, Raz I, Saxagliptin and Cardiovascular Outcomes in Patients with Type 2 Diabetes Mellitus, N. Engl. J. Med 369 (2013) 1317–1326. 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.