Abstract
BACKGROUND:
The prevalence of cardiovascular disease (CVD) and its mortality continues to increase. Various studies have shown aspirin can reduce CVD mortality but has adverse side effects. Research on a comparison between aspirin and honey has not been done, but both have antiplatelet effects.
AIM:
This study is aimed to prove the antiplatelet effects on honey and compare the antiplatelet effects of aspirin with honey based on the bleeding time in mice.
METHODS:
This study is a true experimental design with a post-test only control group using 32 male mice, Double Ditsch Webster, ± 3 months old, the weight of 20-30 g, divided into 4 groups. Consisting of a negative control group (placebo), aspirin and honey. The suspension has given orally for 12 days using the probe. The research was conducted at the Laboratory of Pharmacology Department of Pharmacology and Therapeutics Faculty of Medicine, the University of North Sumatra in September until December 2015. The data collected was bleeding time in mice. Data analysed by Shapiro Wilk test, Kruskal Wallis and Mann Whitney.
RESULTS:
The mean bleeding time was a placebo (102.88 seconds), aspirin (369.38 seconds) and honey (304.63 seconds). Mann Whitney test showed significant results in the aspirin and honey groups against the control group (placebo) with p = 0.001. There were no significant differences in the aspirin group against honey (p = 0.172). Honey has an antiplatelet effect in mice. The mean bleeding time in mice given honey is longer or closer to the mean bleeding time in the aspirin group.
CONCLUSION:
The results could be used as a basis for further research to determine its use in humans with cardiovascular disease.
Keywords: Honey, Bleeding time, Antiplatelet
Introduction
Hemostasis is a body reaction that occurs sequentially to stop the bleeding. When blood vessels are damaged or broken, then the hemostasis process must occur quickly in areas that have been damaged and carefully controlled to be effective. Three major mechanisms that occur to reduce blood loss are vascular spasm, platelet plug formation, and coagulation (blood clotting) [1].
The process of hemostasis can occur without any additional injury to the blood vessels of patients with a history of heart disease and abnormalities in blood vessels [1]. In other words, thrombosis is the formation of a pathological hemostatic plugin blood vessels that do not bleed [2]. For example, in patients with transient ischemic attacks (TIAs), cerebrovascular disease (stroke), myocardial infarction or peripheral artery blockage are classified as cardiovascular disease (CVD) [3].
Cardiovascular disease (CVD) is the leading cause of disability and premature death worldwide. The underlying pathology of CVD is atherosclerosis [4]. According to the World Health Organization (WHO) in 2012 [5]. Deaths caused by noncommunicable diseases (NCDs) account for 38 million of the 56 million global deaths, which is about 68%. The main cause of NCD death in 2012 is cardiovascular disease (CVD) that is as many as 17.5 million deaths, or 46% of all deaths due to NCD.
In the Asia / Pacific region, the main cause of death is CVD, which is estimated at 9.3 million deaths and accounts for about a third of all deaths in 2012 [3].
According to WHO-Noncommunicable Diseases (NCD), Country Profiles in 2014 [6]. Cardiovascular disease (CVD) has been the leading cause of death in Indonesia, which is about 37% of all deaths (1,551,000) in the country.
Administration of antiplatelet drugs such as aspirin can reduce mortality caused by CVD. This antiplatelet therapy is said to be effective in treating severe vascular disease with both short-term and long-term administration [7]. Aspirin (Acetylsalicylic acid-ASA) works by inhibiting the synthesis of thromboxane A2 (TXA2) in platelets and prostacyclin (PGI2) in blood vessels by irreversibly inhibiting cyclo-oxygenase enzymes [8]. However, the dose of aspirin used as an antiplatelet drug in clinical trials is not equivalent [9]. Excessive administration of single-dose aspirin, as well as long-term, can cause the risk of poisoning [10]. The severity of complications that occur with aspirin depends on the dose and duration of treatment. Complications can include bleeding and perforation of the gastrointestinal system [11]. It turns out that any pharmacological therapy has adverse side effects either directly or indirectly. Then safe, natural ingredients are needed as alternative pharmacological therapies or as supplements [12].
Honey is a sweet and thick liquid with a unique flavour generated by honeybees [13]. Honey is very efficacious in medical therapy because of the existence of various phenolic components that have many biological activities, including antioxidants and anti-inflammatory [14]. Research conducted by Ahmed et al., in 2011 [15], proved to have the effect of anti-platelet honey. Honey contains flavonoids, including hesperetin that serves as anti platelet aggregation [16].
With the effect of antiplatelet on honey, the researchers are interested in researching the comparison of the effectiveness of aspirin with honey as antiplatelet based on measurement of bleeding time on the tail of mice. This research is expected to be useful in preventing CVD both in primary and secondary.
Material and Methods
Types of Research
This research is a true experimental design because it performs randomisation (simple random sampling), control, and treatment. The study design is the posttest-only control group design.
P = Population (Mice);
R = Randomization;
K1 = Ex. Plasebo Control (-) without treatment;
K2 = Ex. Plasebo Control (+) with aspirin administration without treatment;
K3 = Ex. Treatment by giving oral Honey for 12 days.
Simple random sampling
Number labels are created on each animal that meets the inclusion criteria.
Then select as many as 32 mice from them to be sampled and divided into 4 groups at random.
Provision of intervention in experimental animals (mice) was made single-blind.
Time and Place of Study
This research has been conducted in Pharmacology Laboratory of Department of Pharmacology and Therapeutics Faculty of Medicine, University of Sumatera Utara. This research has been conducted from September to December 2015. Research has been conducted after obtaining approval from Ethical Clearance from the Ethics Commission of the Faculty of Medicine, University of North Sumatra.
Population and Sample Research
In this study have been used male mice (Mus musculus), Double Ditsch Webster strains age: ± 3 months (adult), weight 20-30 grams, healthy, has never been used for other studies. Mice obtained from Laboratorium FMIPA Biology University of North Sumatra Medan. The number of group animals is determined by the formula, according to Federer (1963), as follows:
(t-1) (n-1) ≥ 15
Explanation:
n = sample size
t = number of groups of experimental animals
Then the required sample size is:
(t-1) (n-1) ≥ 15
(4-1) (n-1) ≥ 15
(n-1) ≥ 5
n ≥ 6
It takes a sample of six animals for each group based on Federer’s formula. Added with an estimated drop out of 10%, then the minimum sample size required for each group are seven animals.
Based on the minimum number of samples allowed statistically and not violating the 3 R (Reduction, Replacement, Refinement) principles in the experimental animal studies, the sample size is taken to eight for each group. So, the total number of experimental animals used is 32 mice.
Criteria for Inclusion, Exclusion and Drop Out
Inclusion Criteria: 1. Mice (Mus musculus) strain Double Ditsch Webster, male, age: 2-3 months, weight: 20-30 grams and 2. Mice healthy, active moves and come from the same group
Exclusion Criteria: Previous mice have received drug intervention.
Drop Out Criteria: Mice die within the study period.
Material
In this study, the materials used are 1. High Desert® 75 mg Honey and 2. Aspirin 80 mg.
Preparing and Maintaining Animals Try
Before the study, the adaptation of animals at the site with a light 12-hour cycle of dark schedule, a standard diet that is eating and drinking ad libitum. The food consumed comes from Charoen Pokhpand. Try animals kept at 25 ± 10°C, 60% relative humidity. Mice were preserved during the study period; weight was weighed before and after the trial.
Procedures
Male mice (Mus musculus), DDW strain, healthy, weight: 20-30 grams, divided into 4 groups: 1. Normal mice as control (-); 2. Mice with aspirin but not given intervention (positive control); 3. Mice with oral honey for 12 days; and 4. Admission was performed on the mice from the first day of the study, for 12 days at doses according to the Conversion Dosage Table 1.
Table 1.
Table Dosing Calculation Calculations (Laurence & Bacharach, 1964) orally
| Mice 20 gram | Mouse 200 gr | Guinea Pig 400 gr | Rabbit 1,5 kg | Cat 2 kg | Monkey 4 kg | Dog 12 kg | Human 70 kg | |
|---|---|---|---|---|---|---|---|---|
| Mice 20 gr | 1.0 | 7.0 | 12.25 | 27.8 | 29.7 | 64.1 | 124.2 | 387.9 |
| Mouse 200 gr | 0.14 | 1.0 | 1.74 | 3.9 | 4.2 | 9.2 | 17.8 | 56.0 |
| Guinea Pig 400 gr | 0.08 | 0.57 | 1.0 | 2.25 | 2.4 | 5.2 | 10.2 | 31.5 |
| Rabbit 1,5 kg | 0.04 | 0.25 | 0.44 | 1.0 | 1.08 | 2.4 | 4.5 | 14.2 |
| Cat 2 kg | 0.03 | 0.23 | 0.41 | 0.92 | 1.0 | 2.2 | 4.1 | 13.0 |
| Monkey 4 kg | 0.016 | 0.11 | 0.19 | 0.42 | 0.45 | 1.0 | 1.9 | 6.1 |
| Dog 12 kg | 0.008 | 0.06 | 0.1 | 0.22 | 0.24 | 0.52 | 1.0 | 3.1 |
| Human 70 kg | 0.0026 | 0.018 | 0.031 | 0.07 | 0.076 | 0.16 | 0.32 | 1.0 |
Conversion Dosage Calculation: 1. Aspirin: 80 mg x 0.0026 x 1000: 20 = 10.40 mg / kg BW and 2. Honey: 75 mg x 0.0026 x 1000: 20 = 9.75 mg / kg BW;
After 12 days of intervention, all mice have been tested for measuring bleeding time by using Duke-Tail bleeding method as follows: 1. The area under investigation (tail of mice) has been cleaned with cotton alcohol; 2. First, the tail of the mice is cut (made wound) at 1 mm diameter using a scalpel and let the blood out freely, when the blood starts stopwatch is run; and 3. Exhausted blood is sucked with filter paper every 30 seconds until the blood stops flowing (do not let filter paper touch the wound), the stopwatch is stopped when the blood cannot be sucked again using filter paper, and the time is recorded.
Data Collection
Data collected in this study were primary data obtained from the measurement of bleeding time in mice tail after 12 days of intervention.
Data Analysis
The data obtained have been analysed using a computer program. The data have been tested for normality by using the Shapiro-Wilk test because of the small number of samples. Followed by a homogeneity test using the Levene test. The Kruskal-Wallis non-parametric statistical test is performed because the data distribution is normal but the data variant is not the same (not homogeneous), followed by post hoc analysis using the Mann Whitney test. A difference is significant when p < 0.05.
Results
Description of Research Location
This research was conducted in Pharmacology Laboratory of Department of Pharmacology and Therapeutics Faculty of Medicine, University of Sumatera Utara. The location is located on Jalan Dr T. Mansur, No. 5, Medan, North Sumatera.
Description of Research Sample
In this study used as many as 32 male mice (Mus musculus), Double Ditsch Webster strains age: ± 3 months (adult), healthy, weight 20-30 grams as a sample, which is divided into 4 groups each amounting to 8 mice, the control group (placebo), the aspirin group and the honey group.
Results of Data Analysis
The antiplatelet effect in mice counted from the time of bleeding was analysed using a computer program. From the research obtained data as follows:
Data in Table 2 and Figure 1 showed the meantime of bleeding at the highest mouse tail was in group of mice with aspirin (mean = 369.38 sec) then followed by group of mice with honey (mean = 304.63 seconds), and the lowest was the control group (placebo) with a mean value of 102.88 seconds.
Table 2.
Bleeding Time Taken for Mice (sec)
| Group | N | Mean | SD | Median | Minimum | Maximum |
|---|---|---|---|---|---|---|
| Control (Placebo) | 8 | 102.88 | 15.93 | 101.50 | 74.00 | 125.00 |
| Aspirin | 8 | 369.38 | 120.97 | 402.00 | 201.00 | 507.00 |
| Honey | 8 | 304.63 | 141.29 | 380.50 | 248.00 | 536.00 |
| 326.50 | 129.00 | 462.00 |
Figure 1.
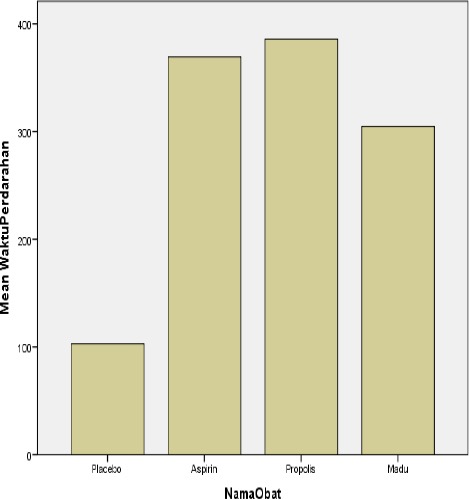
Mean Time Bleeding of Mice (sec)
Test Data Normality
Data on bleeding time in each group were tested for normality by using the Shapiro-Wilk test. The results show that the data is normally distributed (p > 0.05), presented in Table 3.
Table 3.
Normality Test Result of Bleeding Time
| Group | N | p | Explanation |
|---|---|---|---|
| Control (Placebo) | 8 | 0.847 | Normal |
| Aspirin | 8 | 0.188 | Normal |
| Honey | 8 | 0.133 | Normal |
Data Homogeneity Test
Data on bleeding time on mouse tail were tested homogeneity by Levene’s test. The results show a non-homogeneous data variant (p < 0.05), presented in Table 4.
Table 4.
Homogeneous Test of Time Bleeding between Groups
| Levene Statistic | df1 | df2 | Sig. | Explanation |
|---|---|---|---|---|
| 9,760 | 3 | 28 | 0.0001 | Not Homogeneous |
Comparability Test
The comparability test aims to compare the meantime of bleeding in the placebo, aspirin and honey groups. Based on the result of the normality and homogeneity test, the data in this research are normally distributed but do not have the same variant (not homogeneous). The comparative analysis used is a non-parametric test that is Kruskal-Wallis. Non-parametric Kruskal-Wallis statistical tests showed significant differences in at least two treatment groups (p = 0.0001), thus followed by a post hoc analysis with Mann Whitney test.
Advanced Test (Post Hoc Test)
The follow-up test aims to see which groups have significant differences. In this study, the follow-up test used was the Mann Whitney test. The results of the test analysis are presented in Table 5.
Table 5.
Data Analysis with Mann Whitney Test
| Group | Control (Placebo) | Aspirin | Honey |
|---|---|---|---|
| Control (Placebo) | - | 0.001* | 0.001* |
| Aspirin | 0.001* | - | 0.172 |
| Honey | 0.001* | 0.172 | - |
Data in Table 5 showed that there was a significant difference between aspirin and honey group to the control group (placebo), i.e., p = 0.001. There was no significant difference between aspirin group to honey (p = 0.172).
Discussion
This study is a true experimental design study to prove the effect of antiplatelet on honey and to know the comparison of effectiveness between aspirin with honey as antiplatelet based on the measurement of duration of bleeding in mice tail. The time of bleeding is the time to start bleeding on the tail of the mice that are cut until the blood stops. The presence of antiplatelet effect indicated by honey is characterised by the longer time of bleeding after the treatment of wound on the tail of mice.
The average bleeding time in the treatment group given honey was longer than the control group (placebo) and almost close to the average time of positive control group bleeding (aspirin). This is so because of flavonoids. One of the contents of honey has an antiplatelet activity that can be associated with the increased production of prostacyclin by endothelial cells. The prostacyclin inhibits the aggregation process through cAMP synthesis which will inhibit the expression of GPIIb / IIIa platelet receptors [17]. Inhibition of the aggregation process leads to an average time of bleeding in the tail of the mouse with prolonged honey and propolis. From the statistical test, there were significant differences in bleeding time in the control group (placebo) against the treatment group given honey. In the treatment group treated with honey showed no significant difference to the positive control group (aspirin). This proves that honey has antiplatelet effects in mice as do aspirin.
The mechanism of inhibition of platelet aggregation by honey depends on several factors. A study has shown that exposure to hydrogen peroxide (one of honey content) can lead to platelet activity inhibition of platelet aggregation [18]. Also, honey may affect platelet function by inhibiting LDL oxidation that indirectly inhibits platelet aggregation [19]. This is because research has shown that activated platelets after aggregation will produce some cytokines that activate phagocytes resulting in increased production of oxygen free radicals that will eventually lead to the oxidation of LDL [18]. The active component of propolis CAPE has been shown to have an antiplatelet effect based on the following mechanism by increasing the formation of cGMP which will activate the cyclic phosphorylation of GMP-dependent VASP Ser157 and then inhibit the activity of PKC (protein kinase C). In the end, there is inhibition of phosphorylation of P47 and triggers the inhibition of platelet aggregation [20].
From the results of this study, it turns out the average time of bleeding in the tail of mice with the provision of honey longer or closer to the positive control group (aspirin). It can be considered as an alternative antiplatelet therapy or as a supplement in the prevention of heart disease and blood vessels. Therefore, further research needs to be done on humans for more effective results as well as doing this research using a large sample.
In conclusion, based on data analysis obtained in this study, the conclusions are: 1) this study proves that honey can provide an antiplatelet effect in mice with the occurrence of lengthening of bleeding time on the tail and 2) the mean bleeding time in mice tail (304.63 sec) was close to the meantime of bleeding with aspirin (369.38 sec) so that honey could be considered as a supplement in treating heart disease and blood vessels.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Tortora GJ, Derrickson B. Dalam:Principles of Anatomy &Physiology:Maintenance and Continuity of the Human Body. 13th ed. Vol. 2. Asia: John Wiley &Sons. Inc; 2011. The Cardiovascular System:The Blood; pp. 728–756. [Google Scholar]
- 2.Rang HP, Dale MM, Ritter JM, Flower RJ, Henderson G. Dalam: Rang &Dale's Pharmacology. 7th ed. Spain: 2012. Hemostasis and thrombosis; pp. 294–308. https://doi.org/10.1016/B978-0-7020-3471-8.00024-X. [Google Scholar]
- 3.OECD/WHO. Mortality from cardiovascular disease. Health at a Glance:Asia/Pacific 2014: Measuring Progress towards Universal Health Coverage. Vol. 2014. OECD Publishing; pp. 22–23. [Google Scholar]
- 4.WHOPrevention of Cardiovascular Disease:Pocket Guidelines for Assessment and Management of Cardiovascular Risk:WHO/ISH Cardiovascular Risk Prediction Charts for the African Region. Diunduh dari. 2007. [Diakses pada 23 April 2015]. http://www.who.int/cardiovascular_diseases/guidelines/PocketGL.ENGLISH. AFR-D-E.rev1.pdf .
- 5.WHO. NCD mortality and morbidity. WHO. Diunduh dari. 2012. [Diakses pada 14 April 2015]. http://www.who.int/gho/ncd/mortality_morbidity/en/.
- 6.WHO-Noncommunicable Diseases (NCD) Country Profiles. Indonesia. Diunduh dari. 2014. [Diakses pada 24 April 2015]. www.who.int/nmh/countries/idn_en.pdf .
- 7.Trialists'Collaboration A. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death. myocardial infarction. and stroke in high risk patients. Bmj. 2002;324(7329):71–86. doi: 10.1136/bmj.324.7329.71. https://doi.org/10.1136/bmj.324.7329.71 PMid:117⇅1 PMCid:PMC64503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosmiati H, Gan VHS, Antikoagulan, Ganiswara S.G, Setiabudy R, Suyatna F.D, Purwantyastuti, Antitrombosit. Trombolitik dan Hemostatik. Dalam . Farmakologi dan Terapi. edisi ke-4. Vol. 1995. Jakarta: Gaya Baru; 1995. pp. 747–761. [Google Scholar]
- 9.Patrono C, Coller B, FitzGerald GA, Hirsh J, Roth G. Platelet-active drugs:the relationships among dose. effectiveness. and side effects:the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3):234S–64S. doi: 10.1378/chest.126.3_suppl.234S. https://doi.org/10.1378/chest.126.3_suppl.234S PMid:15383474. [DOI] [PubMed] [Google Scholar]
- 10.Litovitz TL. et al. Annual Report of the American Association of Poison Control Centers Toxic Exposure Surveillance System. American Journal of Emergency Medicine. 2001;20(5):337–395. doi: 10.1053/ajem.2001.25272. https://doi.org/10.1053/ajem.2001.25272 PMid:11555795. [DOI] [PubMed] [Google Scholar]
- 11.Farrugia C.A. The Antiplatelet Activity of Aspirin. The chronic ill. 1999;3:14–18. [Google Scholar]
- 12.Ndagu LF, Arjana AA, Berata IK. Madu Berefek Protektif Terhadap Infiltrasi Sel Radang dan Perdarahan Ginjal Akibat Induksi Aspirin. Indonesia Medicus Veterinus. 2013;2(1):102–114. [Google Scholar]
- 13.Farooqui T. Honey:An Anti-Aging Remedy to Keep you Healthy in a Natural Way. Diunduh dari. 2009. [Diakses pada 23 May 2015]. http://www.scienceboard.net/community/perspectives.228.html.
- 14.Farooqui T, Farooqui AA. Dalam:Boukraa. L. Honey in Traditional and Modern Medicine. Vol. 2014. USA: Taylor &Francis Group; Honey for Cardiovascular Disease; pp. 187–216. https://doi.org/10.1201/b15608-10. [Google Scholar]
- 15.Ahmed A, Khan RA, Azim MK, Saeed SA, Mesaik MA, Ahmed S, Imran I. Effect of natural honey on human platelets and blood coagulation proteins. Pakistan journal of pharmaceutical sciences. 2011;24(3):389–397. [PubMed] [Google Scholar]
- 16.Jin YR, Han XH, Zhang YH, Lee JJ, Lim Y, Chung JH, Yun YP. Antiplatelet activity of hesperetin. a bioflavonoid. is mainly mediated by inhibition of PLC-γ2 phosphorylation and cyclooxygenase-1 activity. Atherosclerosis. 2007;194(1):144–52. doi: 10.1016/j.atherosclerosis.2006.10.011. zhttps://doi.org/10.1016/j.atherosclerosis.2006.10.011 PMid:17092506 . [DOI] [PubMed] [Google Scholar]
- 17.Akhlaghi M. Bandy B. Mechanisms of flavonoid protection against myocardial ischemia-reperfusion injury. Journal of molecular and cellular cardiology. 2009;46(3):309–17. doi: 10.1016/j.yjmcc.2008.12.003. https://doi.org/10.1016/j.yjmcc.2008.12.003 PMid:19133271. [DOI] [PubMed] [Google Scholar]
- 18.Ferroni P, Basili S, Falco A, Davì G. Oxidant stress and platelet activation in hypercholesterolemia. Antioxidants and Redox Signaling. 2004;6(4):747–56. doi: 10.1089/1523086041361587. https://doi.org/10.1089/1523086041361587 PMid:15242556. [DOI] [PubMed] [Google Scholar]
- 19.Hegazi AG, Abd El-Hady FK. Influence of honey on the suppression of human low density lipoprotein (LDL) peroxidation (in vitro) eCAM. 2009;6(1):113–21. doi: 10.1093/ecam/nem071. https://doi.org/10.1093/ecam/nem071 PMid:18955249 PMCid:PMC2644272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen T, Lee J, Lin K, Shen C, Chou D, Sheu J. Antiplatelet activity of caffeic acid phenethyl ester is mediated through a cyclic GMP-dependent pathway in human platelets. Chinese Journal of Physiology. 2007;50(3):121–126. [PubMed] [Google Scholar]


