Abstract
Very little is known about how reward programs are implemented in real-world substance use treatment settings and whether training in contingency management (CM), an empirically supported rewards-based intervention, impacts their design quality. Providers (N = 214) completed surveys assessing CM beliefs, training, and practices related to use of tangible rewards in treatment. For providers reporting they had not used rewards in treatment previously (54%, n = 116), we assessed beliefs about and interest in adopting a reward-based program. For those endorsing prior reward experience (46%, n = 98), we assessed the features and delivery of rewards and the relation of reward-based intervention training to four parameters related to CM efficacy: reinforcement magnitude, immediacy, frequency, and escalation. Among providers without reward experience, endorsement of supportive statements about CM predicted interest in adopting a rewards-based program. Providers with reward experience were most often targeted treatment attendance and engaged in behaviors likely to decrease the effectiveness of the intervention, including use of low magnitudes (≤ $25/client), delayed reinforcement, failure to escalate reward values, and offering reward opportunities less than weekly. Providers with longer durations of training were more likely to engage in behaviors consistent with effective CM, including larger magnitude rewards and immediate delivery of rewards. Results indicate that real-world treatment clinics are using reward-based programs, but not in ways consistent with research protocols. Longer training exposure is associated with greater adherence to some aspects of CM protocol design. Other evidence-based design features are not being implemented as recommended even with training.
Keywords: motivational incentives, financial incentives, treatment providers, substance use disorder treatment
Contingency management (CM) is a treatment for substance use disorders that involves the use of tangible rewards to promote behavior change (Petry, 2000). CM is recognized as an evidence-based practice by both the National Institutes of Health and the Substance Abuse and Mental Health Services Administration (e.g., National Institute on Drug Abuse, 2018), yet its use in clinical practice remains rare. Multiple studies (Benishek et al., 2010; Herbeck et al., 2008; McGovern et al., 2004; Willenbring et al., 2004a) suggest that implementation of CM lags far behind other psychosocial evidence-based interventions such as cognitive behavioral therapy and relapse prevention. This underutilization occurs despite CM generating larger effects relative to these interventions (Dutra et al., 2008).
Several design features have been associated with CM’s efficacy. Magnitude of reinforcement appears to have clear lower boundaries below which effects on client outcomes are no better than standard treatment. In Petry et al. (2004), 120 clients with cocaine dependence entering intensive outpatient treatment (IOP) in community clinics were randomized to one of three conditions: standard IOP, standard IOP plus low-cost prize CM (i.e., clients earn about $80 maximum expected value), or standard IOP plus typical prize CM (i.e., clients earn about $240 maximum expected value). Prize CM offers opportunities to draw slips from a fishbowl each time the client demonstrates the targeted behavior(s). The slips vary in magnitude (i.e., small, large, jumbo) and only some (usually about half) earn tangible rewards. Participants in the $240 CM condition had significantly longer durations of consecutive abstinence and submitted a higher percentage of negative samples during treatment relative to IOP alone; however, the low- cost CM condition did not improve upon outcomes in the IOP alone condition.
Similar magnitude effects have been identified with voucher schedules (Businelle et al., 2009; Lussier et al., 2006; Silverman et al., 1999). In contrast to prize CM, voucher schedules do not have probabilistic and varying magnitude reinforcement. Instead, clients earn a fixed monetary amount in the form of vouchers each time they demonstrate the target behavior(s). In meta-analyses (Griffith et al., 2000; Lussier et al., 2006), immediacy of reward following demonstration of the target behavior is a significant moderator of outcomes. Frequency of reward opportunities may also be important, with more frequent opportunities linked to larger effects (Griffith et al., 2000). However, frequency is dependent on the target behavior and thus is quite variable (Petry, 2012). Progressive, escalating schedules in which the magnitude of reinforcement increases with consecutive demonstration of the target behavior also appear related to participant behavior in laboratory studies (Roll et al., 1996).
Though uptake of CM in real-world clinical settings is slow, an example of successful widespread implementation is the Department of Veterans Affairs’ (VA) national roll-out of CM in their outpatient substance use treatment programs (DePhilippis et al., 2018). In 2011, in recognition of the low rate of CM offered to veterans, the VA earmarked seed funds for CM programs and provided 1.5-day regional trainings led by CM experts (Petry et al., 2014; Rash et al., 2013). In addition to the initial training workshops, telephone-based pre-implementation planning support and post-implementation coaching was available. Participation in these calls was strongly encouraged and uptake was high, with programs participating in a mean 6.5 calls (DePhilippis et al., 2018) and since 2015, 75% or more programs participated in at least one call per year (Rash & DePhilippis, 2019). Of the 129 programs ultimately trained in CM, 126 (98%) implemented CM by the end of 2018 (Rash & DePhilippis, 2019). The majority (70%) implemented the protocol recommended in the trainings (DePhilippis et al., 2018), which incorporated the evidence-based design features noted above (i.e., sufficient magnitude, escalation, immediacy, frequency). The recommended protocol involved twice-weekly prize-based CM reinforcing stimulant abstinence over the course of 12 weeks with average maximum expected earnings of $364. Quality of CM delivery was generally high (67–96% indicated using practices ‘always’).
The VA rollout represents ideal conditions for implementation: strong support from leadership, expert involvement, funding, and provision of training and ongoing coaching. Other work (Hartzler et al., 2014, 2016; Henggeler et al., 2008, 2013; Petry et al., 2010, 2012a) suggests that community providers can implement CM protocols with good adherence, but these projects, similar to the VA effort, involved extensive training and/or support from experts. Importantly, quality of CM delivery is related to client outcomes (Hartzler et al., 2014, 2017; Petry et al., 2010, 2012a), suggesting maintaining quality in the transfer from research to clinical settings will be critical to the overall success of CM programs. Clinics without the robust support provided in the above demonstration projects may find it more difficult to implement reward- based interventions with good adherence, and without adherence to established protocols, these interventions may be less effective (Glasner-Edwards & Rawson, 2010). Unfortunately, implementation without intensive training or support is the normative experience in most clinical settings. Olmstead et al. (2012) surveyed 345 clinic directors of private-sector substance abuse treatment centers. Among centers reporting that they implement, only 55% provided formal initial and ongoing training in CM either in-house or off-site.
Little is known about how programs design and implement rewards-based programs, particularly in the absence of intensive training. A prior study (Rash et al., 2012) examined CM beliefs and practices in 617 substance use treatment providers. About half of the providers indicated that they had used CM with clients (most with very little training), and the limited data collected about the structure of the rewards programs indicated that most were not implementing CM consistent with behavioral principles associated with improved client outcomes. For example, 72% reported using no cost or very low magnitude reinforcement (< $25 total per client over the duration of the program). In comparison, typical vouchers schedules in research trials involve maximum possible reinforcement earnings ranging from $180 to $1,950 per client over 12 weeks (with average maximum voucher earnings of $894 across 19 conditions targeting drug abstinence and of 12 weeks duration from the Lussier et al., 2000 meta-analysis). Prize CM programs often range $250-$450 in average maximum expected earnings per client over 12 weeks (e.g., Peirce et al., 2006; Petry et al., 2004, 2005, 2012b; mean across 17 conditions targeting drug abstinence and providing prizes for 12 week durations reported in the Benishek et al., 2014 meta-analysis = $429, range $9 to $1,391).
Willenbring et al. (2004b) assessed concordance of clinic protocols with evidence-based guidelines in nine VA opioid agonist treatment clinics. Only one of nine used rewards in a manner consistent with CM principles. The other eight clinics included features counter to accepted CM practice, including poorly defined target behaviors; difficult-to-achieve behavioral targets; client-initiated report of goal achievement rather than staff monitoring; and, delayed reinforcement of behavior usually in combination with a high bar to earn reinforcement (e.g., 90 days of abstinence from all illicit substance and attendance to all scheduled visits in order to receive reinforcement).
The present study builds on those initial findings (Rash et al., 2012; Willenbring et al., 2004b) by investigating rewards program design and implementation practices in real-world clinical settings with attention to evidence-based design features. Identification of adherent and non-adherent practices may be useful in the tailoring of training and dissemination material toward those features most likely to deviate from recommended and evidence-based practices.Specifically, this study assessed the prevalence of tangible reward use as part of treatment among substance abuse treatment providers and compared providers with and without this experience. Among providers using rewards, we examined the characteristics of the reward program, including structure (e.g., voucher, prize), target behaviors, funding sources, and types of rewards.
We also examined whether extent of CM training was associated with adherence to four core behavioral principles associated with CM efficacy: magnitude of rewards available to clients, immediacy of reward delivery, frequency of reward opportunities, and use of an escalating schedule. We selected magnitude, immediacy, and frequency because these parameters have been identified as significant moderators of treatment effects in meta-analyses of CM studies (e.g., Griffith et al., 2000; Lussier et al., 2006). Escalating reinforcement schedules (and ones with reset conditions) sustain continuous abstinence for longer durations than fixed reinforcement schedules (Roll & Higgins, 2000; Roll et al., 2006; Romanowich & Lamb, 2015). Though no meta-analyses have examined escalation as a moderator, we elected to include it as a key parameter given that many CM schedules include escalation and based on evidence from laboratory studies cited above. We expected those with longer durations of CM training to demonstrate better adherence to CM design features associated with positive client outcomes.
Method
Participants/Procedure
Participants were eligible if aged 18 or older and self-identified as an addictions treatment provider or administrator involved in the delivery of substance use disorder treatment. Participation did not require prior experience with rewards or CM. The online survey hosted by SurveyMonkey was initiated by 251 individuals. We excluded 37 individuals who did not complete any questions related to rewards (e.g., only demographic information provided). The remaining 214 responses were retained for analysis.
We distributed the anonymous online survey link to substance use treatment providers through regional and national professional organizations related to addictions treatment using listservs and other web communication outlets (e.g., organizations’ social media pages, websites, message boards). We also contacted substance use treatment clinic directors and requested that they distribute the survey link to their staff. Study procedures were approved by the university’s Institutional Review Board (IRB). The IRB approved a waiver for signed written informed consent, but all participants read text describing the study and indicated consent to participate by entering the survey.
At the end of the survey, participants could opt to submit their contact information for entry in a random drawing for a $100 gift certificate. Contact information collected for the purpose of the random drawing was stored separately from the survey data with no link between the two sources.
The sample was predominantly female (72%), Caucasian (84%), and not Hispanic (91%). Most had bachelor’s or higher degrees (87%) and worked in an outpatient setting (52%). In terms of primary role within their organization, 56% identified as a clinician, 18% as a clinical supervisor, 9% as an administrator, and 17% as another role. Most (74%) had 6 or more years of experience in the addictions field. See Table 1.
Table 1.
Characteristics of Providers With and Without Reported Experience Using Rewards
| Characteristics | Reward Experience (n = 98) |
No Reward Experience (n = 116) |
Total Sample (N =214) |
|---|---|---|---|
| Female | 69 (70%) | 84 (72%) | 153 (72%) |
| Race | |||
| Caucasian | 79 (82%) | 96 (86%) | 175 (84%) |
| African American | 10 (10%) | 6 (5%) | 16 (8%) |
| Other | 7 (7%) | 10 (9%) | 17 (8%) |
| Not Hispanic | 94 (96%) | 101 (87%) | 195 (91%) |
| Age | 31.54 (10.76) | 31.48 (11.59) | 31.50 (11.19) |
| Education | |||
| PhD/MD/other | 15 (15%) | 14 (12%) | 29 (14%) |
| Master level | 54 (55%) | 58 (50%) | 112 (52%) |
| Bachelor level | 19 (19%) | 26 (22%) | 45 (21%) |
| Other | 10 (10%) | 18 (16%) | 28 (13%) |
| In recovery^ | 35 (36%) | 46 (40%) | 81 (38%) |
| Primary role in organization | |||
| Administrator | 13 (13%) | 7 (6%) | 20 (9%) |
| Clinical supervisor | 21 (22%) | 17 (15%) | 38 (18%) |
| Clinician | 50 (51%) | 69 (59%) | 119 (56%) |
| Other | 14 (14%) | 23 (20%) | 37(17%) |
| Work setting | |||
| Outpatient | 46 (47%) | 65 (56%) | 111 (52%) |
| Residential | 19 (19%) | 20 (17%) | 39 (18%) |
| Methadone | 4 (4%) | 13 (11%) | 17 (8%) |
| Corrections | 8 (8%) | 4 (4%) | 12 (6%) |
| Other | 21 (22%) | 14 (12%) | 35 (16%) |
| Years of experience in addictions field | |||
| 1 year or less | 4 (4%) | 9 (8%) | 13 (6%) |
| 2–5 years | 16 (16%) | 28 (24%) | 44 (20%) |
| 6–10 years | 25 (26%) | 22 (19%) | 47 (22%) |
| 11–20 years | 25 (26%) | 26 (22%) | 51 (24%) |
| More than 20 years | 28 (28%) | 31 (27%) | 59 (28%) |
| Extent of training in rewards-based interventions* | |||
| 6 or more hours | 35 (36%) | 12 (10%) | 47 (22%) |
| Less than 6 hours | 63 (64%) | 104 (90%) | 167 (78%) |
| CMBQ subscales | |||
| General barriers* | 2.13 (0.70) | 2.85 (0.66) | 2.53 (0.77) |
| Training-related barriers* | 2.37 (1.01) | 3.36 (0.86) | 2.92 (1.05) |
| Supportive* | 3.57 (0.89) | 3.28 (0.92) | 3.40 (0.91) |
Notes. Values represent n (%) except for age and CMBQ subscales [M (SD)].
indicates significant between group difference at p < .05.
CMBQ = Contingency Management Beliefs Questionnaire.
refers to whether the provider identified as being in recovery from an addiction.
Measures
For all participants, the survey asked about demographic and personal characteristics (e.g., whether the provider identified as in recovery from addictions, educational level), work- related characteristics (e.g., program type, primary role within organization), training experiences related to reward-based interventions (“also called contingency management or motivational incentives”), and perceived barriers to the adoption of reward-based programs (Contingency Management Beliefs Questionnaire, CMBQ; Rash et al., 2012). The CMBQ contains 35 statements regarding general and practical barriers related to CM (e.g., philosophical concerns, time/cost barriers, relapse when contingencies withdrawn, undermines internal motivation), training-related barriers (e.g., need/want more training, no supervision available, organizational support), and CM-supportive statements (e.g., good for the client-counselor relationship, helpful in keeping clients engaged in treatment). Participants rated each statement on a 5-point Likert scale (‘not important at all’ to ‘very important’) for its importance in decisions to use or continue using CM in clinical practice. The questionnaire demonstrated stable and reliable psychometric properties in a large national sample of substance abuse treatment providers (Rash et al., 2012).
Based on the participant’s response to a question about prior experience using tangible rewards in substance use treatment, s/he was directed to one of two sets of additional questions. Experience with rewards was defined broadly, such that individuals directly administering rewards, as well as those involved in the development, training, implementation, or supervision of reward programs, could participate. Individuals in the planning stages could also participate. Participants with no prior experience with reward-based programs were asked about their interest in adopting such a program as part of clinical practice. Adoption interest was measured on a 7- point Likert scale, with 1 indicating ‘extremely interested’ and 7 indicating ‘no interest at all.’ Participants with prior experience using rewards responded to a set of questions assessing implementation experiences, including design/structure, target behaviors, types of rewards, time to administer rewards, and funding sources.
Data Analysis
Prevalence of reward-based programs among providers was determined by endorsement of the question, “do you have personal experience using tangible rewards or reward-based interventions with your clients as part of substance abuse treatment?” We compared providers with and without reward experience on demographic, personal and organizational characteristics, and CMBQ beliefs using chi-square or independent t-tests depending on whether the variable of interest was categorical or continuous. For those without reward experience, we assessed whether beliefs predicted interest in adopting such a program using an ordinal multiple regression analysis with the three CMBQ subscale scores entered as continuous variables.
For providers endorsing use of rewards in treatment, we characterized the typical reward program, including program structure, target behaviors, reward types, and funding sources, using frequencies. We then compared providers with more (6+ hours) versus less (<6 hours) training related to reward-based programs on adherence to behavioral parameters associated with CM’s efficacy using chi-square analyses. These training durations were used because few had training consistent with most implementation efforts (i.e., 8 to 16 hours training time; Hartzler et al., 2014; Henggeler et al., 2008; Kellogg et al., 2005; Petry et al., 2012a; Rash et al., 2013). This demarcation represented more than a half-day training, which we considered a reasonable time to denote good quality coverage during a training.
Magnitude referred to the total amount of reinforcement available per client over the duration of the reward program, categorized as $25 or less, $26–100, or more than $100. Responses skewed toward lower values, and these values were clearly inconsistent with established CM protocol magnitudes. The highest magnitude category ‘more than $100’ would include amounts consistent with prize and (at the highest values) voucher protocols. The middle category ($26–100) captured the remaining reported magnitudes. Immediacy of reward following demonstration of the target behavior was categorized as same day or delayed. Frequency of reward opportunities was categorized as less than weekly, weekly, or twice weekly or more. Escalation in rewards with consistent performance was either present or not present in the reward schedule. Alpha was set at less than .05, and analyses were conducted in SPSS version 24.
Results
Comparison of providers with and without reward-based intervention experience
Of the 214 providers, 98 (46%) reported experience using reward-based programs with clients. See Table 1. No differences were present (ps > .05) between those with and without experience using rewards on demographics (sex, age, race, ethnicity, and education level), recovery status (i.e., identifies as in recovery from an addiction), or practice/organization characteristics (i.e., primary role within the organization, work setting, years of experience in the addictions field).
Providers endorsing use of reward-based programs were more likely to report 6 or more hours of reward-based intervention training (36%) compared to those who did not endorse use (10%), χ2 (1) = 19.95, p < .001. Providers with experience using reward-based programs had significantly lower mean endorsement of general and training-related barriers to implementation and higher mean endorsement of supportive/positive statements related to reward programs compared to their peers with no prior experience (ps <.05). In terms of experience with rewards, 45 providers (46%) had used rewards with 16 or more clients.
Prediction of adoption interest
Among those without prior reward experience (n = 116), the regression model with the three CMBQ subscales predicting adoption interest accounted for 38% of variance explained, χ2(3) = 42.12, p < .001. Only the supportive subscale significantly predicted adoption interest, Wald χ2(1) = 32.34, p < .001. Interest in adoption (1 = “extremely interested”, 7 = “no interest at all”) was stronger among those with higher mean supportive subscale scores [ordered log odds = −1.45, 95% CI = −1.95 to −0.95]. Neither the general barriers subscale (p = .07) nor the training- related barriers (p = .38) subscale were significant predictors of interest in adoption.
Examination of rewards programs
Among providers with experience using rewards (n = 98), we examined characteristics of the reward program itself. See Table 2. Of evidence-based reward program types, fishbowl or prize-based rewards had the highest endorsement (28%); however, the most commonly endorsed category overall was ‘other’ (32%) and another 14% were not familiar enough with these terms or with the program to be able to categorize it (i.e., ‘don’t know’). Attendance was the most commonly endorsed behavioral target (58%), with multi-drug abstinence the second most common behavior (37%). Nicotine abstinence (6%) and medication adherence (11%) targets were the least common target behaviors. Some programs (11%) indicated ‘other’ behaviors; examples from the narrative description of these other behaviors included attitude, engagement/participation, personal growth, and positive social behaviors.
Table 2.
Characteristics of Community Programs using Rewards by Extent of Training in Reward-based Interventions
| Program Features | 6+ Hours of Training (n = 35) |
<6 Hours Training (n = 63) |
Total Providers Using Rewards (N=98) |
|---|---|---|---|
| Program type | |||
| Fishbowl or prizes | 10 (34%) | 12 (25%) | 22 (28%) |
| Vouchers | 5 (17%) | 5 (10%) | 10 (13%) |
| Stars/tokens | 4 (13%) | 4 (8%) | 8 (10%) |
| Cash | 1 (3%) | 1 (2%) | 2 (3%) |
| Other | 9 (30%) | 16 (33%) | 25 (32%) |
| Don’t know | 1 (3%) | 11 (22%) | 12 (14%) |
| Types of rewards | |||
| Praise | 27 (77%) | 44 (70%) | 71 (72%) |
| Certificates (e.g., attendance, completion) | 22 (63%) | 35 (56%) | 57 (58%) |
| Food items in clinic | 14 (40%) | 25 (40%) | 39 (40%) |
| Small tangible items | 11 (31%) | 24 (38%) | 35 (36%) |
| Status recognition | 11 (31%) | 24 (38%) | 35 (36%) |
| Gift certificates | 14 (40%) | 18 (29%) | 32 (33%) |
| Activities, group outings | 13 (37%) | 14 (22%) | 27 (28%) |
| Medallions, buttons | 8 (23%) | 15 (24%) | 23 (24%) |
| Clinic privileges | 7 (20%) | 14 (22%) | 21 (21%) |
| Large tangible items | 7 (20%) | 8 (13%) | 15 (15%) |
| Home/community privileges | 4 (11%) | 11 (18%) | 15 (15%) |
| Modified treatment schedule | 2 (6%) | 8 (13%) | 10 (10%) |
| Behavioral target | |||
| Attendance to treatment sessions | 23 (66%) | 34 (54%) | 57 (58%) |
| Abstinence (multiple drug classes) | 16 (46%) | 20 (32%) | 36 (37%) |
| Treatment activities | 8 (23%) | 20 (32%) | 28 (29%) |
| Abstinence (alcohol) | 13 (37%) | 14 (22%) | 27 (28%) |
| 12-step attendance | 8 (23%) | 19 (30%) | 27 (28%) |
| Homework completion | 10 (29%) | 16 (25%) | 26 (27%) |
| Abstinence (single drug or drug class) | 9 (26%) | 12 (19%) | 21 (21%) |
| Medication adherence | 5 (14%) | 6 (10%) | 11 (11%) |
| Abstinence (nicotine) | 4 (11%) | 2 (3%) | 6 (6%) |
Notes. Values represent n (%). Program type was missing responses from 19 individuals. For the types of rewards offered and the behavioral targets, providers could select all that applied to their program.
Non-monetary rewards (e.g., praise [72%], certificates [58%], status recognition [36%]) were commonly integrated into reward programs. Large tangible items, as typically used in voucher or prize-CM, were rarely endorsed (15%), and this low rate of endorsement was consistent with the low maximum amount of rewards available over the duration of the program for each client (i.e., 39% reported maximum magnitudes of less than $25, of which 10% used non-monetary social rewards). In terms of the period of time during which rewards were available to a client, 24% reported less than 12 weeks in duration, 34% reported durations of 12 weeks or more, 20% reported undefined or unknown durations.
In terms of funding source for rewards, five providers received Veterans Administration funding through the nationwide roll-out of CM (DePhilippis et al., 2018; Petry et al., 2014; Rash et al., 2013; Rash & DePhilippis, 2019). Thirteen clinics received funds via federal or state grants or initiatives. Clinics provided funds for 21 respondents, and an additional 7 reported their clinic sought donations to fund the program. Many (n = 28) exclusively used non-monetary rewards for the program and did not need funding support. Eight respondents had not yet decided how to fund their future rewards-based program, and the remainder used ‘other’ means to support their program.
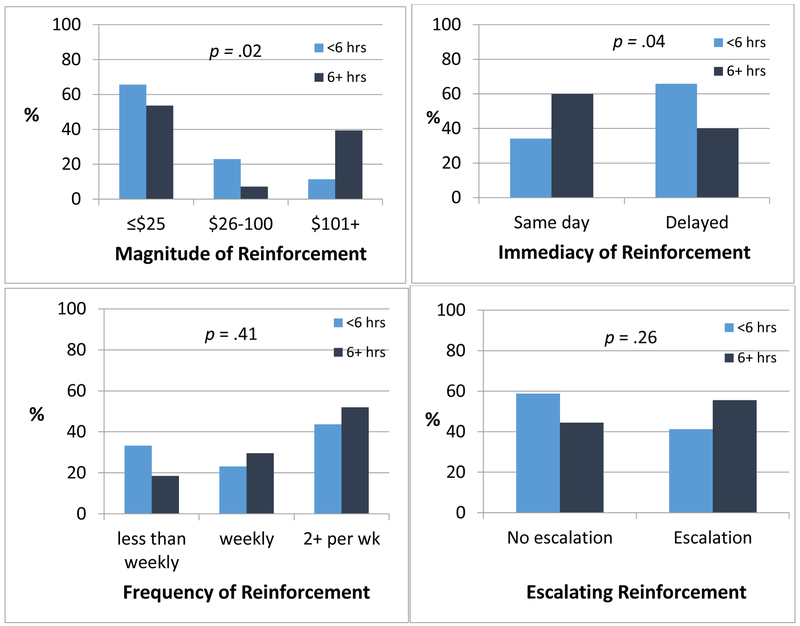
Extent of training and schedule parameters
Figure 1 presents four key CM schedule parameters by extent of reward-based intervention training. Training was significantly associated with maximum magnitude of reinforcement available across the duration of the program per client, χ2(2) = 7.87, p = .02, and with immediacy of reinforcement following the target behavior, χ2(1) = 4.21, p = .04. Individuals with 6 or more hours of reward-based intervention training were more likely to use higher magnitude rewards and to reinforce the target behavior on the same day. Extent of training was not associated with the use of escalation in the reinforcement schedule, χ2(1) = 1.25, p = .26, which was incorporated in only 53% of schedules, or with the frequency of reward opportunities, χ2(2) = 1.78, p = .41. Most providers offered weekly (26%) or twice weekly or more (47%) reward opportunities, and 27% offered rewards less than weekly.
Figure 1.
Key parameters associated with contingency management (CM) efficacy by level of CM-related training. Extent of training was not associated with frequency of reinforcement opportunities nor use of escalating incentives (bottom 2 panels), with 27% reinforcing less than weekly and 53% not using an escalating schedule. Extent of training was significantly associated with typical maximum reinforcement available across the duration of treatment and with the immediacy of providing reinforcement following demonstration of the target behavior (top 2 panels). Individuals with 6 or more hours of CM-related training were more likely to use larger magnitudes of total reinforcement and to reinforce target behaviors the same day they occur.
To assess whether providers with more extensive training were more likely to use higher magnitude reinforcement due to funding, we assessed whether training and source of funding were related. Specifically, we examined the relation of training category and whether the clinic received external support (i.e., VA, state or federal grants) or were funded by other means. Providers with more extensive training were more likely to have external support (33% vs 12%), χ2 (1) = 6.61, p < .01.
Discussion
A relatively large percentage of providers (46%) indicated that they use tangible rewards as part of treatment. Prior studies have consistently reported low rates of CM implementation as part of clinical practice ranging from 20% to 30% (Herbeck et al., 2008; Olmstead et al., 2012). Our estimate is larger possibly due to the framing of our question toward the use of tangible rewards rather than CM specifically. Clinicians in this survey favored use of rewards to encourage treatment attendance, prize CM (i.e., the ‘fishbowl’ approach), and low-cost rewards. Generally, reward programs did not adhere to accepted and research-tested standards. This divergence was most evident in terms of reward magnitudes, in that most providers reported use of rewards of $25 or less per client over the course of treatment, which falls well below the magnitudes used in efficacious research protocols. Longer CM training was associated with greater adherence to some aspects of CM protocol design. Other important design features (escalation, frequency of rewards) were not implemented as recommended even with training.
Implementation of CM faces obstacles such as negative perceptions (e.g., Benishek et al., 2010; Kirby et al., 2006; Rash et al., 2012). Poorly executed CM programs are unlikely to generate effects seen in research trials and may leave providers with the perception that CM is ineffective, thus reinforcing global negative beliefs about CM. Exposure and trialability (i.e., how easily an intervention can be experimented with on a limited basis) are important factors that promote adoption and implementation of evidence-based practices (Glasner-Edwards & Rawson, 2010), and these factors are important to CM as well (Hartzler, 2015b). A negative experience with CM might be equally compelling. Beyond impacts on clinician perceptions, Walker et al. (2010) noted that implementation problems can negatively impact client’s enthusiasm for and confidence in CM. Ultimately, clinics implementing poorly designed reward protocols may not experience improved client outcomes, and thus may be unlikely to sustain these programs.
We asked providers about their use of tangible rewards. It is possible that existing reward programs or ones in the planning stage were not intended to be CM. It is also possible that some providers thought they were using rewards consistent with CM principles when in fact they were not or failed to understand that modifications could impact efficacy. Narrative descriptions of the programs indicated that many had no specific structure, such as an undefined number of reward opportunities or infrequent reward opportunities (e.g., one-time reward for program completion), and poorly specified target behaviors (e.g., attitude). Training on effective use of rewards in clinical practice should emphasize practices that are and are not consistent with guidelines and why they may or may not lead to improved outcomes, using examples wherever possible.
Some providers in this study reported using a reward program that adhered to some principles (e.g., frequent opportunity) of CM, but not others (e.g., low magnitude, delayed reward). Deviating from established protocols is not unique to CM. In a sample of 510 clinical staff, those who perceived more barriers were more likely to make modifications to a number of evidence-based practices (Lundgren et al., 2013). More senior staff (i.e., decision makers, more years of experience) were more likely than junior staff to modify protocols, suggesting the importance of engaging senior staff early on in training and implementation efforts rather than focusing exclusively on front-line staff. An earlier study (Lundgren et al., 2011) evaluating modifications to evidence-based practices in substance use treatment organizations among such decision makers (i.e., program directors) found that nearly 50% of the evidence-based practices used in the clinic had been modified. Modifications are typically well-intentioned; 73% of modifications were attributed to the need to respond to client or clinic needs (Lundgren et al., 2011). However, most decision makers perceived their changes as minor, even in cases where these changes were likely to impact efficacy.
Anecdotally, as trainers and consultants, we see many modifications to CM protocols that are perceived as minor but are not consistent with evidence-based practices. Even well- intentioned changes to CM protocols have the potential to undermine a program’s efficacy, such as lowering the magnitude available per client in order to serve more clients in total. Expert involvement, as utilized in the VA effort, is associated with higher effect sizes in CM studies (Prendergast et al., 2006). Factors that may affect this association include thorough training and the opportunity for consultation as programs consider modifications. As with other evidence- based practices (Lundgren et al., 2011), those who foresee a need to modify CM schedules should seek expert input to ensure that modifications will not negatively impact treatment outcomes.
These results suggest that more thorough training is associated with adherence to some recommended features of CM schedules. The VA rollout offers one example of CM training that might serve as model for future implementation efforts. Providers attended a 1.5 day in-person workshop and had ongoing coaching calls subsequent to the trainings (DePhilippis et al., 2018; Petry et al., 2014; Rash & DePhilippis, in press; Rash et al., 2013). Others (Hartzler et al., 2014; Henggeler et al., 2008; Kellogg et al., 2005; Petry et al., 2012ab) provide similar duration (12–16 hours) trainings. Regardless of the training format and duration, it should be sufficient to promote good execution of CM sessions because clinician skill is related to patient outcomes (Hartzler et al., 2017; Petry et al., 2012a,b). Roleplays (Petry et al., 2012a,b), vignettes (Hartzler, 2015a), and adherence tools (Petry et al., 2010) can assist with skill assessment.
Another influencing factor may be funding for reward programs. Programs with sufficient and often external funding may have more flexibility to use higher magnitude and escalating reinforcement. Low magnitude rewards may improve some limited conditions (Kropp, Lewis, & Winhusen, 2016), but very low magnitude rewards generally results in no or low treatment effects (Lussier et al., 2006; Petry et al., 2004). Policy change, particularly as relates to insurance coverage, will be necessary to increase accessibility to funding and reimbursement of CM costs by private and public payers (Petry et al., 2017; Roll et al., 2009).With such resources, providers may have more flexibility to design and implement CM programs that reflect research protocols. As relates to frequency of monitoring and reinforcement, technologies, such as internet-connected computers, smartphones, wearable sensors, and other devices permit remote reinforcement of target behaviors with lower staff demand and thus may improve adherence with frequent reinforcement opportunities (e.g., Alessi & Petry, 2013; Alessi et al., 2017; Dallery & Raiff, 2011; Dallery et al., 2007, 2015; Rash et al., 2018; Stoops et al., 2009). Similarly, software solutions to accounting and administration logistics of implementing CM may reduce barriers and demands on staff time (https://www.dynamicarehealth.com/research).
Limitations of this study include a small sample size, which may impact representativeness and generalizability. Given the recruitment strategies which targeted multiple state provider organizations, as well as national organization, we expect participation was not geographically restricted and thus representative of a wide range of regions. However, we did not assess state of practice directly.
With a larger sample, future studies might assess and conduct sub-analyses by individual target behaviors. Importantly, the frequency of monitoring and reinforcement opportunities is heavily dependent on the substance or behavior targeted. For example, reinforcement schedules for nicotine assessed by carbon monoxide breathalyzers would generally have much higher frequency visits than cocaine or opioids. The descriptions in this report provide a broad overview of practices, and we have focused on delineating those practices that are not adherent to research standards regardless of the target behavior (i.e., less than weekly, delayed delivery, very low magnitudes).
We did not ask participants to report their primary clinics in order to preserve anonymity. As such, we do not know if many respondents came from a small number of clinics, nor were we able to control for clinic specific effects (i.e., nesting) in analyses. Given the anonymity of the survey, we could not verify participants’ report that they were clinicians or administrators involved in the treatment of substance use disorders. Last, we chose to ask about ‘rewards and reward-based interventions’ rather than CM programs exclusively because understanding how rewards are used in everyday clinical settings provides valuable information for trainers and consultants to bridge to recommended practices. Trainers can quickly identify and reinforce good practices and work toward moving inconsistent practices toward evidence- based guidelines.
The results of this study highlight the need for improved adherence to CM treatment guidelines and principles among rewards-based programs in use in clinical settings. Knowledge of the specific weaknesses in rewards programs implemented in these real-world clinics can inform training targets for future CM rollout efforts. Successful examples of implementation of CM in clinical settings may serve as models for clinics interested in offering CM to improve client outcomes.
Acknowledgments
This work was supported in part by National Institutes of Health grants: R01-HD075630, R01-AA023502, P50-DA009241, P60-AA003510, R01-AA021446, R01-MD013550, K23-DA034879. We would like to acknowledge the contributions of the late Nancy Petry, who contributed to the design of this study.
This work has been presented in part at the Research Society on Alcoholism, a special topics conference of the Association of Behavior Analysis, and the College of Problems on Drug Dependence.
Contributor Information
Carla J. Rash, Calhoun Cardiology Center – Behavioral Health, UConn Health School of Medicine;
Sheila M. Alessi, Calhoun Cardiology Center – Behavioral Health, UConn Health School of Medicine;
Kristyn Zajac, Calhoun Cardiology Center – Behavioral Health, UConn Health School of Medicine..
References
- Alessi SM, Barnett NP, Petry NM (2017). Experiences with SCRAMx alcohol monitoring technology in 100 alcohol treatment outpatients. Drug & Alcohol Dependence, 178, 417– 424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessi SM, Petry NM (2013). A randomized study of cellphone technology to reinforce alcohol abstinence in the natural environment. Addiction, 108, 900–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benishek LA, Dugosh KL, Kirby KC, Matejkowski J, Clements NT, Seymour BL, Festinger DS (2014). Prize-based contingency management for the treatment of substance abusers: A meta-analysis. Addiction, 109, 1426–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benishek LA, Kirby KC, Dugosh KL, Padovano A. (2010). Beliefs about the empirical support of drug abuse treatment interventions: a survey of outpatient treatment providers. Drug & Alcohol Dependence, 107(2–3), 202–8. [DOI] [PubMed] [Google Scholar]
- Businelle MS, Rash CJ, Burke RS, & Parker JD (2009). Using vouchers to increase continuing care participation in veterans: Does magnitude matter? The American Journal on Addictions, 18, 122–129. 10.1080/10550490802545125 [DOI] [PubMed] [Google Scholar]
- Dallery J, Raiff BR (2011). Contingency management in the 21st century: Technological innovations to promote smoking cessation. Substance Use & Misuse, 46, 10–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallery J, Glenn IM, Raiff BR (2011). An internet-based abstinence reinforcement treatment for cigarette smoking. Drug and Alcohol Dependence, 86, 230–238. [DOI] [PubMed] [Google Scholar]
- Dallery J, Meredith S, Jarvis B, Nuzzo PA (2015). Internet-based group contingency management to promote smoking abstinence. Experimental & Clinical Psychopharmacology, 23, 176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePhilippis D, Petry NM, Bonn-Miller MO, Rosenbach SB, & McKay JR (2018). The national implementation of Contingency Management (CM) in the Department of Veterans Affairs: Attendance at CM sessions and substance use outcomes. Drug and Alcohol Dependence, 185(December 2017), 367–373. 10.1016/j.drugalcdep.2017.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, & Otto MW (2008).A meta-analytic review of psychosocial interventions for substance use disorders. The American Journal of Psychiatry, 165(2), 179–87. 10.1176/appi.ajp.2007.06111851 [DOI] [PubMed] [Google Scholar]
- Festinger DS, Dugosh KL, Kirby KC, & Seymour BL (2014). Contingency management for cocaine treatment: Cash vs. vouchers. Journal of Substance Abuse Treatment, 47, 168–174. 10.1016/j.jsat.2014.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasner-Edwards S, & Rawson R (2010). Evidence-based practices in addiction treatment: review and recommendations for public policy. Health Policy (Amsterdam, Netherlands), 97(2–3), 93–104. 10.1016/j.healthpol.2010.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith JD, Rowan-szal GA, Roark RR, & Simpson DD (2000). Contingency management in outpatient methadone treatment: A meta-analysis. Drug and Alcohol Dependence, 58(1–2), 55–66. 10.1016/S0376-8716(99)00068-X [DOI] [PubMed] [Google Scholar]
- Hartzler B (2015a). Adapting the helpful responses questionnaire to assess communication skills involved in delivering contingency management: Preliminary psychometrics..Journal of Substance Abuse Treamtent, 55, 52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzler B (2015b). Building a bonfire that remains stoked: Sustainment of a contingency management intervention developed through collaborative design. Substance Abuse Treatment, Prevention, and Policy, 10(1), 30 10.1186/s13011-015-0027-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzler B, Beadnell B, & Donovan D (2017). Predictive validity of addiction treatment clinicians’ post-training contingency management skills for subsequent clinical outcomes. Journal of Substance Abuse Treatment, 72, 126–133. 10.1016/j.jsat.2015.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzler B, Jackson TR, Jones BE, Beadnell B, & Calsyn DA (2014). Disseminating contingency management: Impacts of staff training and implementation at an opiate treatment program. Journal of Substance Abuse Treatment, 46(4), 429–438. 10.1016/j.jsat.2013.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzler B, Peavy KM, Jackson TR, & Carney M (2016). Finding harmony so the music plays on: pragmatic trial design considerations to promote organizational sustainment of an empirically-supported behavior therapy. Addiction Science & Clinical Practice, 11(1), 2 10.1186/s13722-016-0049-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henggeler S. W. Ph, D., Chapman J. E. Ph, D., Rowland MD, Halliday-boykins C. A. Ph, D. (2007). If you build it, they will come: Statewide practitioner interest in contingency management for youths. Journal of Substance Abuse Treatment, 32, 121–131. 10.1016/j.jsat.2006.07.006 [DOI] [PubMed] [Google Scholar]
- Herbeck DM, Hser YI, & Teruya C (2008). Empirically supported substance abuse treatment approaches: A survey of treatment providers’ perspectives and practices.Addictive Behaviors, 33(5), 699–712. 10.1016/j.addbeh.2007.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Badger GJ, & Budney AJ (2000). Initial abstinence and success in achieving longer term cocaine abstinence. Experimental and Clinical Psychopharmacology, 8(3),377–386. 10.1037/1064-1297.8.3.377 [DOI] [PubMed] [Google Scholar]
- Higgins ST, Budney AJ, Bickel WK, Foerg FE, Donham R, & Badger GJ (1994). Incentives improve outcome in outpatient behavioral treatment of cocaine dependence. Archives of General Psychiatry, 51, 568–576. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Sigmon SC, Wong CJ, Heil SH, Badger GJ, Donham R, … Anthony S (2003). Community reinforcement therapy for cocaine-dependent outpatients. Archives of General Psychiatry, 60(10), 1043–1052. 10.1001/archpsyc.60.9.1043 [DOI] [PubMed] [Google Scholar]
- Kellogg SH, Burns M, Coleman P, Stitzer M, Wale JB, & Kreek MJ (2005). Something of value: The introduction of contingency management interventions into the New York City Health and Hospital Addiction Treatment Service. Journal of Substance Abuse Treatment, 28(1), 57–65. 10.1016/j.jsat.2004.10.007 [DOI] [PubMed] [Google Scholar]
- Kirby KC, Benishek LA, Dugosh KL, & Kerwin ME (2006). Substance abuse treatment providers’ beliefs and objections regarding contingency management: Implications for dissemination. Drug and Alcohol Dependence, 85(1), 19–27. 10.1016/j.drugalcdep.2006.03.010 [DOI] [PubMed] [Google Scholar]
- Kropp F, Lewis D, & Winhusen T (2017). The effectiveness of ultra-low magnitude reinforcers: Findings from a “real-world” application of contingency management. Journal of Substance Abuse Treatment, 72, 111–116. [DOI] [PubMed] [Google Scholar]
- Lundgren L, Amodeo M, Cohen A, Chassler D, & Horowitz A (2011). Modifications of evidence-based practices in community-based addiction treatment organizations: A qualitative research study. Addictive Behaviors, 36(6), 630–635. 10.1016/j.addbeh.2011.01.003 [DOI] [PubMed] [Google Scholar]
- Lundgren L. Ph, D., Amodeo M. Ph, D., Chassler D, Krull I. Ph, D. (2013). Organizational readiness for change in community-based addiction treatment programs and adherence in implementing evidence-based practices: A national study. Journal of Substance Abuse Treatment, 45(5), 457–465. 10.1016/j.jsat.2013.06.007 [DOI] [PubMed] [Google Scholar]
- Lussier JP, Heil SH, Mongeon JA, Badger GJ & Higgins ST (2006). A meta- analysis of voucher-based reinforcement therapy for substance use disorders. Addiction, 101(2), 192–203. 10.1111/j.1360-0443.2006.01311.x [DOI] [PubMed] [Google Scholar]
- McGovern MP, Fox TS, Xie H, & Drake RE (2004). A survey of clinical practices and readiness to adopt evidence-based practices: Dissemination research in an addiction treatment system. Journal of Substance Abuse Treatment, 26(4), 305–312. 10.1016/j.jsat.2004.03.003 [DOI] [PubMed] [Google Scholar]
- National Institute on Drug Abuse (2018). Principles of Drug Addiction Treatment: A research- based guide (3rd edition). National Institutes of Health, US Department of Health and Human Services; Bethesda, MD. [Google Scholar]
- Olmstead TA, Abraham AJ, Martino S, & Roman PM (2012). Counselor training in several evidence-based psychosocial addiction treatments in private US substance abuse treatment centers. Drug and Alcohol Dependence, 120(1–3), 149–154. 10.1016/j.drugalcdep.2011.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirce JM, Petry NM, Stitzer ML, Blaine J, Kellogg S, Satterfield F, … Kolodner K (2006). Effects of lower-cost incentives on stimulant abstinence in methadone maintenance treatment - A National Drug Abuse Treatment Clinical Trials Network study. Archives of General Psychiatry, 63(2), 201–208. 10.1001/archpsyc.63.2.201 [DOI] [PubMed] [Google Scholar]
- Petry NM (2000). A comprehensive guide to the application of contingency management procedures in clinical settings. Drug and Alcohol Dependence, 58(1–2), 9–25. 10.1016/S0376-8716(99)00071-X [DOI] [PubMed] [Google Scholar]
- Petry NM (2012). Contingency Management for Substance Abuse Treatment: A Guide to Implementing this Evidence-Based Practice. New York: Routledge. [Google Scholar]
- Petry NM, Alessi SM, Ledgerwood DM (2012a). Contingency management delivered by community therapists in outpatient settings. Drug & Alcohol Dependence, 122, 86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Alessi SM, Ledgerwood DM, Sierra S (2010). Psychometric properties of the contingency management competence scale. Drug & Alcohol Dependence, 109, 167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Alessi SM, Olmstead TA, Rash CJ, & Zajac K(2017). Contingency management treatment for substance use disorders: How far has it come, and where does it need to go? Psychology of Addictive Behaviors, 31, 897–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Barry D, Alessi SM, Rounsaville BJ, & Carroll KM (2012b). A randomized trial adapting contingency management targets based on initial abstinence status of cocaine-dependent clients. Journal of Consulting and Clinical Psychology, 80(2), 276–85. 10.1037/a0026883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Dephilippis D, Rash CJ, Drapkin M, & McKay JR (2014). Nationwide dissemination of contingency management: The Veterans Administration initiative. American Journal on Addictions, 23(3), 205–210. 10.1111/j.1521-0391.2014.12092.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Peirce JM, Stitzer ML, Blaine J, Roll JM, Cohen A, … Li R (2005). Effect of prize-based incentives on outcomes in stimulant abusers in outpatient psychosocial treatment programs: A National Drug Abuse Treatment Clinical Trials Network study. Archives of General Psychiatry, 62, 1148–1156. 10.1001/archpsyc.62.10.1148 [DOI] [PubMed] [Google Scholar]
- Petry NM, Tedford J, Austin M, Nich C, Carroll KM, & Rounsaville BJ (2004). Prize reinforcement contingency management for treating cocaine users: How low can we go, and with whom? Addiction, 99(3), 349–360. 10.1111/j.1360-0443.2003.00642.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast M, Podus D, Finney J, Greenwell L, & Roll J (2006). Contingency management for treatment of substance use disorders: A meta-analysis. Addiction, 101(11), 1546–1560. 10.1111/j.1360-0443.2006.01581.x [DOI] [PubMed] [Google Scholar]
- Rash CJ, DePhilippis D (in press). Considerations for Implementing Contingency Management in Substance Abuse Treatment Clinics: The Veterans Affairs Initiative as a Model. Perspectives on Behavior Science. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash CJ, DePhilippis D, McKay JR, Drapkin M, & Petry NM (2013). Training workshops positively impact beliefs about contingency management in a nationwide dissemination effort. Journal of Substance Abuse Treatment, 45(3), 306–312. 10.1016/j.jsat.2013.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash CJ, Petry NM, Kirby KC, Martino S, Roll J, Stitzer ML. (2012). Identifying provider beliefs related to contingency management adoption using the contingency management beliefs questionnaire. Drug & Alcohol Dependence, 121, 205–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash CJ, Petry NM, Alessi SM, Barnett NP (2018). Monitoring alcohol use in heavy drinking soup kitchen attendees. Alcohol, doi: 10.1016/j.alcohol.2018.10.001. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll JM, & Higgins ST (2000). A within-subject comparison of three different schedules of reinforcement of drug abstinence using cigarette smoking as an exemplar. Drug & Alcohol Dependence, 58(1–2), 103–109. 10.1016/S0376-8716(99)00073-3 [DOI] [PubMed] [Google Scholar]
- Roll JM, Higgins ST, & Badger GJ (1996). An experimental comparison of three different schedules of reinforcement of drug abstinence using cigarette smoking as an exemplar. Journal of Applied Behavior Analysis, 29(4), 495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll JM, Huber A, Sodano R, Chudzynski JE, Moynier E, & Shoptaw S (2006). A Comparison of Five Reinforcement Schedules for Use in Contingency Management- Based Treatment of Methamphetamine Abuse. The Psychological Record, 56(1), 67–81. [Google Scholar]
- Romanowich P, & Lamb RJ (2015). The effects of fixed versus escalating reinforcement schedules on smoking abstinence. Journal of Applied Behavior Analysis, 48(1), 25–37. 10.1002/jaba.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman K, Chutuape MA, Bigelow GE, & Stitzer ML (1999). Voucher-based reinforcement of cocaine abstinence in treatment-resistant methadone clients: Effects of reinforcement magnitude. Psychopharmacology, 146(2), 128–138. 10.1007/s002130051098 [DOI] [PubMed] [Google Scholar]
- Stoops WW, Dallery J, Fields NM, Nuzzo PA, Schoenberg NE, Martin CA, Casey B, Wong CJ (2009). An internet-based absitnence reinforemnet smoking cessaiton intervnetion in rurual smokers. Drug & Alcohol Dependence, 105, 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker R, Rosvall T, Field CA, Allen S, McDonald D, Salim Z, … Adinoff B (2010). Disseminating contingency management to increase attendance in two community substance abuse treatment centers: Lessons learned. Journal of Substance Abuse Treatment, 39(3), 202–209. 10.1016/j.jsat.2010.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willenbring ML, Hagedorn HJ, Postier AC, & Kenny M (2004b). Variations in evidence-based clinical practices in nine United States Veterans Administration opioid agonist therapy clinics. Drug and Alcohol Dependence, 75(1), 97–106. 10.1016/j.drugalcdep.2004.01.009 [DOI] [PubMed] [Google Scholar]
- Willenbring ML, Kivlahan D, Kenny M, Grillo M, Hagedorn H, & Postier A (2004a). Beliefs about evidence-based practices in addiction treatment: A survey of Veterans Administration program leaders. Journal of Substance Abuse Treatment, 26(2), 79–85. 10.1016/S0740-5472(03)00161-2 [DOI] [PubMed] [Google Scholar]