Abstract
Background:
Cerebellar atrophy (especially involving the superior anterior cerebellar vermis) is among the most salient and clinically significant effects of chronic hazardous alcohol consumption on brain structure. Smaller cerebellar volumes are also associated with chronic cigarette smoking. The present study investigated effects of both chronic alcohol consumption and cigarette smoking on cerebellar structure and its relation to performance on select cognitive/behavioral tasks.
Methods:
Using T1-weighted MRIs, the Cerebellar Analysis Tool Kit segmented the cerebellum into bilateral hemispheres and three vermis parcels from four participant groups: smoking (s) and non-smoking (ns) abstinent alcohol-dependent treatment seekers (ALC) and controls (CON) (i.e., sALC, nsALC, sCON, and nsCON). Cognitive and behavioral data were also obtained.
Results:
We found detrimental effects of chronic drinking on all cerebellar structural measures in ALC participants, with largest reductions seen in vermis areas. Furthermore, both smoking groups had smaller volumes of cerebellar hemispheres but not vermis areas compared to their non-smoking counterparts. In exploratory analyses, smaller cerebellar volumes were related to lower measures of intelligence. In sCON, but not sALC, greater smoking severity was related to smaller cerebellar volume and smaller superior anterior vermis area. In sALC, greater abstinence duration was associated with larger cerebellar and superior anterior vermis areas, suggesting some recovery with abstinence.
Conclusions:
Our results show that both smoking and alcohol status are associated with smaller cerebellar structural measurements, with vermal areas more vulnerable to chronic alcohol consumption and less affected by chronic smoking. These morphometric cerebellar deficits were also associated with lower intelligence and related to duration of abstinence in sALC only.
Keywords: cerebellum, alcohol, smoking, ataxia, cognition, MRI
Introduction
Consistent evidence demonstrates a co-occurrence of alcohol use disorder (AUD) and cerebellar pathology (Andersen, 2004, Baker et al., 1999, Harper, 1993, Torvik and Torp, 1986, Victor et al., 1959, Victor et al., 1989), including alcohol-related cerebellar atrophy e.g., (Sullivan et al., 2000a, Sullivan et al., 2000b, Davila et al., 1994), which contributes to the hallmark impairments of gait and balance in chronic alcohol abusers (Baker et al., 1999, Victor et al., 1959, Sullivan, 2003, Sullivan et al., 2000a, Sullivan et al., 2010, Sullivan et al., 2006, Sullivan et al., 2009, Currie et al., 2013, Vassar and Rose, 2014). With the neocerebellum also involved in neurocognition, alcohol-related cerebellar pathology has also been associated with impairments in non-motor and higher-order cognitive functions, such as attention shifting (Allen et al., 1997), working memory (Hayter et al., 2007, Chanraud et al., 2010, Chen and Desmond, 2005a, Chen and Desmond, 2005b), executive skills (Nakamura-Palacios et al., 2014) and language/verbal tasks (Booth et al., 2007). Moreover, patients with cerebellar pathology unrelated to excessive alcohol use demonstrate deficits in executive functioning, language skills, and affective behaviors (Schmahmann and Sherman, 1998). Such findings support the theory that cerebellar dysfunction may mechanistically contribute to cognitive dysfunction, motor impairment and postural instability in AUD.
Recent work has also linked smaller cerebellar volumes to chronic cigarette smoking. An early analysis found smaller cerebellar gray matter density in smokers versus non-smokers, but no difference in gray matter volume (Gallinat et al., 2006). A later voxel-wise analysis localized a cluster of significant gray matter volume reduction in Crus I, a region in the posterior lobe of the cerebellar hemispheres, in smokers compared to never-smokers (Kuhn et al., 2012), although these findings were not replicated in a similar study (Fritz et al., 2014). More recently, smaller bilateral cerebellar gray matter volumes have been reported in smokers (Franklin et al., 2014, Vnukova et al., 2017). Lastly, 3-week-abstinent alcohol dependent treatment seekers (ALC) who smoke (sALC; many of whom contributed to this cerebellar structural analysis) showed poorer performance on postural stability tasks compared to their non-smoking counterparts (nsALC) (Schmidt et al., 2014), an effect that could be mediated by cerebellar structural abnormalities (Vassar and Rose, 2014).
In addition to studies showing group differences between substance users and controls, research has suggested that substance use contributes to accelerated brain aging. Excessive alcohol use and smoking have both been reported to amplify age-related volume loss in the brain (Pfefferbaum et al., 1992, Durazzo et al., 2017, Durazzo et al., 2014b). A recent voxel-based structural analysis measured gray matter volumes in 110 brain regions in those with AUD and controls aged 25–65 years to examine for potential alcohol-related accelerated aging. Results indicated that, in the later decades of life, the brain age of the chronic drinkers was increased by an impressive 12 years, consistent with the accelerated brain aging theory in substance users (Guggenmos et al., 2017). Similarly, we recently compared cortical and subcortical volumes in non-smokers and smokers aged 22–70 years without any other substance use disorder and also found chronic smoking associated with accelerated age-related volume loss in subcortical white and gray matter regions, including the cerebellum (Durazzo et al., 2017).
In this study, we investigated effects of both chronic alcohol consumption and cigarette smoking on cerebellar structure and their relations to performance on select cognitive tasks, as their joint and separate effects on the cerebellum have not previously been reported. The Cerebellar Analysis Tool Kit (CATK; (Cardenas et al., 2014, Price et al., 2014) was used to segment the cerebellum as visible on T1-weighted MR images into left and right hemispheres and three vermis parcels. From two separate neuroimaging research studies conducted concurrently, we constructed four groups of participants: sALC, nsALC, smoking controls (sCON), and non-smoking CON (nsCON). We hypothesized that (i) all cerebellar measurements (area and volume) are smaller as a function of both chronic smoking and alcohol status, with the largest effect of alcohol consumption on the superior anterior vermis, (ii) smoking exacerbates the effects of alcohol on cerebellar morphometry, and (iii) smaller cerebellar measures are associated with worse performance on select cognitive/clinical tasks. We also predicted that greater smoking and alcohol consumption severity are associated with smaller cerebellar measures. In addition, we explored whether group differences in cerebellar measures related to smoking and alcohol were better explained by a fixed factor model or by a model of accelerated aging.
Materials and Methods
Participants
Participants were drawn from two different research projects on alcohol and tobacco use disorders conducted in the greater San Francisco area. Treatment-seeking ALC participants were recruited from substance abuse treatment programs at the San Francisco VA Medical Center and Kaiser Permanente. The treatment seekers participating in this research were clients of outpatient treatment programs at the local VA and Kaiser Permanente, with whom we have had longstanding research relationships. Importantly, clinical staff at these treatment centers distributed research study-related information to treatment clients and those who were interested to learn more about the study then contacted research study personnel for screening. As such, the study participants were fully self-referred into this research. CON, without histories of medical or psychiatric conditions known or suspected to influence brain structural outcome measures, were recruited from the local community. All participants were between the ages of 25 and 70 years (see Table 1 for demographics). Medical exclusion criteria were a current or past history of intrinsic cerebral tumors, human immunodeficiency virus or acquired immune deficiency syndrome, cerebrovascular accident, aneurysm, insulin dependent diabetes, chronic obstructive pulmonary disease, non-alcohol-related seizures, significant exposure to known neurotoxins, demyelinating and neurodegenerative diseases, Wernicke-Korsakoff Syndrome, alcohol-induced persisting dementia, and traumatic brain injury resulting in loss of consciousness for more than 15 minutes. Psychiatric exclusion criteria included schizophrenia or other thought disorders, bipolar disorder, dissociative disorders, posttraumatic stress disorder, obsessive compulsive disorder, and panic disorder (with or without agoraphobia), all according to DSM-IV-TR criteria. Hepatitis C, type-2 diabetes, hypertension, and unipolar mood disorders were not exclusionary given their high prevalence in substance use disorder.
Table 1:
Participant Demographics, drinking and smoking measures
| nsCON N=17 |
sCON N=31 |
nsALC N=21 |
sALC N=23 |
|
|---|---|---|---|---|
| % Male | 82 | 87 | 76 | 91 |
| Age (yrs) mean ± SD (min-max) | 48±12 (26–69) | 49±9 (33–64) | 51±12 (25–71) | 49±7 (33–60) |
| Education (yrs) | 16±2 | 15±2† | 15±2 | 13±2‡* |
| 1-yr avg drinks per month& | 12±13 | 22±19 | 291±152 | 387±194* |
| 3-yr avg drinks per month& | 14±13 | 22±19 | 248±135 | 378±202* |
| Lifetime avg drinks per month& | 18±14 | 26±13 | 171±93 | 256±129* |
| Total lifetime drinks˜ | 6759±5642 | 9540±6691 | 72403±53646 | 100697±60623 |
| Fagerstrom total score | NA | 4.8±1.6 | NA | 4.0±1.6 |
| Cigarettes per day | NA | 18.3±6.7 | NA | 15.1±7.1 |
| Years smoking at current level | NA | 16±12 | NA | 15±12 |
| Total years smoking | NA | 29±11 | NA | 26±9 |
Abbreviations: nsCON=non-smoking controls, sCON=smoking controls, nsALC=non-smoking abstinent alcohol-dependent treatment seekers, sALC= smoking abstinent alcohol-dependent treatment seekers.
1 standard alcoholic drink contains 13.6 g of ethanol
sCON < nsCON, uncorrected p≤0.05
sALC < nsCON, uncorrected p≤0.05
sALC < nsALC (edu) and sALC > nsALC (drinking severity measures)
All other pairwise comparisons, uncorrected p>0.05
At baseline, all ALC treatment-seekers met DSM-IV-TR criteria for alcohol dependence and were abstinent from all substances except tobacco for an average of 20±11 days (nsALC: 18±10; sALC: 21±11; p>0.05). At the time of assessment, all smoking participants were actively smoking at least 10 cigarettes per day, had been doing so for the past 5 years or more, had no periods of smoking cessation greater than 1 month in the 5 years prior to study, and were not concurrently using other tobacco or non-tobacco nicotine products. No smoker was engaged in any pharmacological/behavioral smoking cessation program. Non-smoking participants never smoked or smoked less than 40 cigarettes during their lifetime and used no cigarette/tobacco products for 10 years prior to study. Participants provided written informed consent according to the Declaration of Helsinki, and all procedures were approved by the institutional research review boards of the University of California San Francisco and the San Francisco VA Medical Center.
All participants with T1-weighted MRIs deemed usable by experienced imagers (i.e., full cerebellum in the field of view, no movement, good signal-to-noise ratio assessed by visual review), and accurate cerebellar segmentations were included in the analyses. This resulted in a final sample of 17 nsCON (48±12 yrs, 14 males), 31 sCON (49±9 yrs, 27 males), 21 nsALC (51±12 yrs, 16 males), and 23 sALC (49±7 yrs, 21 males).
Clinical and Neurocognitive Measures
Each participant completed the Structured Clinical Interview for DSM-IV Axis I Disorder Patient Edition, Version 2.0, as well as questionnaires assessing depressive (Beck Depressive Inventory, second edition (Beck et al., 1996)) and anxiety symptoms (State-Trait Anxiety Inventory, form Y-1 (state) and Y-2 (trait), STAI, (Spielberger, 1983)). Lifetime alcohol consumption was assessed with the Lifetime Drinking History semi-structured interview (Skinner and Sheu, 1982, Sobell et al., 1988). The average number of standard alcoholic drinks (containing 13.6 g of ethanol) consumed per month was derived for one and three years before enrollment and over lifetime. The Fagerstrom Tolerance Test for Nicotine Dependence (Heatherton et al., 1991) was used to assess level of nicotine dependence, years smoking at current level, total years of cigarette smoking, and average number of daily cigarettes currently smoked.
Participants completed a comprehensive neurocognitive battery (Durazzo et al., 2013b). We focused on cognitive measures that have been shown to be associated with cerebellar morphological integrity: Wisconsin Card Sorting Test-64 (WCST; shifting, self-monitoring and use of verbal feedback to guide decision making (Heaton and Staff, 1993)), Trail Making Test (TMT) (Reitan and Wolfson, 1985): Trails A (processing speed) and B (set-shifting, visuomotor scanning, and graphomotor speed), Grooved Pegboard (fine motor dexterity (Oldfield, 1971)), and the Sharpened-Romberg task with eyes-closed (static postural stability (Fregly et al., 1972)). Premorbid verbal intelligence was assessed with the American National Adult Reading Test (AMNART VIQ) (Grober and Sliwinski, 1991) and general intelligence with the Ward-7 Full Scale IQ (using a z-transformed composite score based on WAIS-III Arithmetic, Block Design, Digit Span, Digit Symbol, Information, Picture Completion, and Similarities subtests). All measures, with the exception of the Sharpened-Romberg, are well normed and commonly used in clinical and/or research settings (Strauss et al., 2006). In order to mitigate the potential for nicotine withdrawal effects on function, smokers were allowed to smoke ad libitum prior to all assessments and were allowed to take cigarette smoking breaks, if requested.
We obtained gross lab markers of recent drinking for most subjects (e.g., GGT, AST, ALT, mean corpuscular volume). These markers were approaching or were within normal limits at the time of assessment for all participants. The VA and Kaiser hospitals also routinely conducted breathalyzers and urine substance use assays on patients in their substance abuse treatment program, from which we drew our participants. Prior to assessment at our laboratory, participants’ urine was tested for five common substances (THC, opiates, PCP, cocaine, and amphetamines) and participants were breathalyzed for recent ethanol consumption. No participant was positive for the above common substances or ethanol at the time of assessment.
MRI Acquisition and Image Processing
MRI data were acquired on a 4.0 T Bruker MedSpec system using an 8-channel transmit-receive head coil (Siemens, Erlangen, Germany). A Magnetization Prepared Rapid Gradient Echo sequence (TR/TE/TI = 2300/3/950 ms, 7° flip angle, 1.0 mm isotropic resolution) was used to acquire 3D sagittal T1-weighted images for cerebellar segmentation.
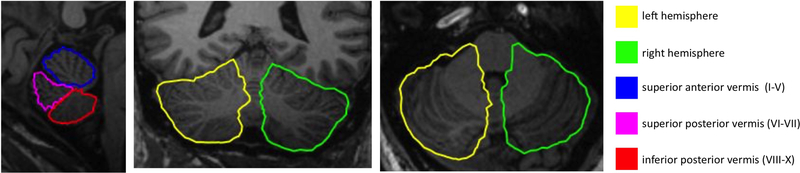
We used CATK (Cerebellar Analysis Tool Kit, (Cardenas et al., 2014, Price et al., 2014)) to segment T1-weighted MR images of the cerebellum into left and right hemispheres and three vermis parcels (superior anterior I-V, superior posterior VI-VII, and inferior posterior VIII-X); measures of cerebellar volume (total and hemispheric) or area of the three vermis parcels are the focus of this report. CATK functions as a fully-automated T1 MRI cerebellum delineation and parcellation tool. It uses an active profile-appearance modeling (AAM, (Patenaude et al., 2011, Cootes, 2000, Cootes, 2001)) framework, which combines surface-based registration with statistical models of shape and texture derived from high-resolution T1-weighted images acquired from 43 healthy participants (mean age 44 years, range 5–96 years, 49% male), providing delineation of the cerebellar hemispheres and three vermal lobes. The advantage of this is that strong prior knowledge about the cerebellum inherent in the data (such as the overall shape of the cerebellar vermal lobes) are taken into account during segmentation of new data, resulting in a segmentation method that enforces smoothness according to probable variations specific to the structures. Figure 1 shows an example segmentation output for CATK. All cerebellar segmentations were visually reviewed for accuracy by authors VAC and CMH, and poor segmentations were excluded.
Figure 1:
3D parcellation of: vermis superior anterior lobe (lobules I–V) blue; vermis superior posterior lobe (lobules VI–VII) magenta; vermis inferior posterior lobe (lobules VIII–X) red; hemispheres, yellow and green, from a representative participant in the study.
Statistics
Comparisons of demographic and clinical data between all four groups were conducted with univariate analysis of variance. Multivariate analyses of covariance (MANCOVA) using a 2 × 2 design (alcohol-by-smoking status) examined the effects of alcohol and smoking on three cerebellar volume measures (total, left cerebellar hemisphere, right cerebellar hemisphere) and three vermis cross-sectional areas (superior anterior, superior posterior, and inferior posterior), while controlling for age, sex, and intracranial vault volume (ICV; estimated using FreeSurfer). The non-significant sex term and non-significant alcohol-by-smoking status interaction terms were removed from final models. Age was not a significant covariate in the model for vermis areas and was removed in the final model. A sensitivity analysis conducted using G*Power (Faul et al., 2007) using our sample of 92 subjects and assuming equal group sizes, conventional levels of α = 0.05 and β = 0.20 (80% power), showed that an effect size of 0.08 could be detected. Using our smallest group size of 17 and assuming 4 equal groups (i.e., assuming a sample of only 68 subjects), an effect size 0.11 could be detected. These effect sizes are considered “small” according to Cohen (Cohen, 1988). Because of our a priori hypotheses and relatively small number of outcome measures, we did not correct for multiple comparisons.
To explore whether the accelerated aging hypothesis better explained our cerebellar data than the fixed factor statistical model, we employed an alternative model of the effects of smoking and alcohol on cerebellar volume using MANOVA with cerebellar measures as dependent variables and age, ICV, and alcohol-by-smoking-status-by-age interactions as independent variables; this allowed us to model potential age-related differences in cerebellar structures among the four groups.
General linear modeling was used to explore relationships of cerebellar measures (after correcting for the effects of ICV by regressing each cerebellar measure on ICV and using the residual predicted values as the independent variables) to measures of neurocognition. Measures of neurocognition included individual tests that focus on cerebellar function, as described above. Since the left, right, and total cerebellar measures were highly collinear (all r>0.93), a composite cerebellar measure was created (0.5(total+right+left)); the composite measure and all three vermis measures were included as independent variables in these models with education, age, smoking and alcohol status as covariates. In each group separately (sCON, nsALC, and sALC), Pearson correlations were computed using the predicted values derived from the general linear models to examine associations between cerebellar measures with measures of smoking severity in sCON, and with measures of drinking severity and abstinence in nsALC. In sALC, associations between cerebellar and smoking severity measures were adjusted for the monthly average of alcoholic drinks consumed over one year before study, and associations between cerebellar and drinking severity and abstinence measures were adjusted by the Fagerstrom total score. Given our a priori hypotheses and relatively small number of outcome measures, we did not correct the statistical significance of these relationships for multiple comparisons.
Results
Sample characterization
Sample characteristics are summarized in Table 1. Age and proportion of female participation did not differ between the four study groups (both p>0.55). sALC drank more than nsALC (3-yr monthly avg, p<0.01; 1-yr monthly avg, p=0.06; lifetime monthly avg, p<0.01). nsCON had more years of education than sCON (p=0.04) or sALC (p<0.01), who had fewer years of education than nsALC (p=0.03). sCON and sALC did not differ on measures of smoking severity (all p>0.10).
Main effects of smoking and alcohol on cerebellar measures
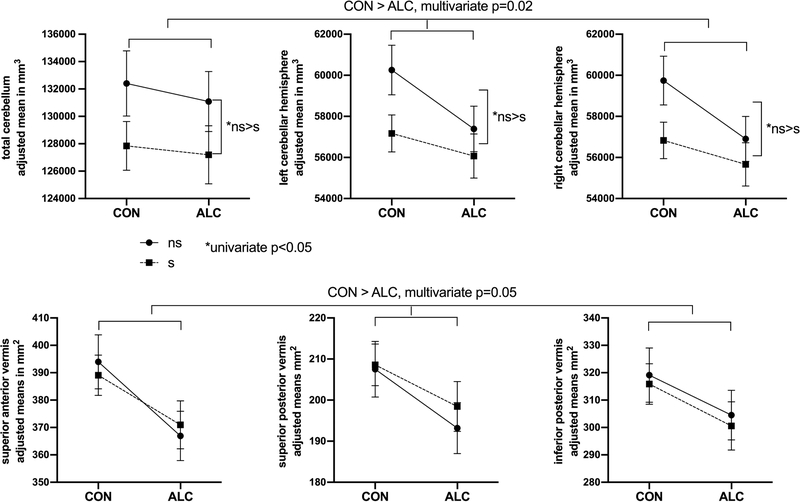
Cerebellar and intracranial volumes are summarized in Table 2. ICV was not significantly different among the four groups (p>0.47). Total, left, and right cerebellar volumes showed significant multivariate effects of alcohol status (F3,81=3.47, p=0.02), age (F3,81=5.22, p=0.002), and ICV (F3,81=15.04, p<<0.01), but not smoking status (p=0.21). The ALC groups had significantly smaller (approximately 3.2%) left and right hemisphere volumes relative to CON groups. Multivariate effects of age indicate a decrease of approximately 2 ml in cerebellar volume with each additional decade of age. Although the multivariate effect of smoking status was not significant, univariate models showed significant smoking effects (all p<0.05 uncorrected), and plots revealed 2% smaller cerebellar volumes in sCON and sALC relative to their non-smoking counterparts, as shown in Figure 2. Vermal cross-sectional areas showed significant multivariate effects of alcohol status (F3,82=2.67, p=0.05) and ICV (F3,82=2.89, p=0.04), with ALC groups showing 4.4–5.9% smaller vermal areas relative to CON. Age and smoking status showed no significant effects on vermis measures (both p>0.32), and plots comparing smoking to non-smoking groups showed no consistent effect of smoking (all univariate p>0.67), as shown in Figure 2.
Table 2:
Cerebellar Measures by Group
| nsCON N=17 |
sCON N=31 |
nsALC N=21 |
sALC N=23 |
|
|---|---|---|---|---|
| Total Cerebellum (mm3) | 131,106±12,559 | 128,595±11,969 | 131,162±13,036 | 126,285±11,043 |
| Left Cerebellar Hemisphere (mm3) | 59,848±6,518 | 57,509±5,837† | 57,443±5,813 | 55,885±4,788‡ |
| Right Cerebellar Hemisphere (mm3) | 59,301±6,335 | 57,168±5,785† | 56,922±5,564 | 55,459±4,871‡ |
| Superior Anterior Vermis (mm2) | 391±33 | 392±46 | 372±39& | 370±45 |
| Superior Posterior Vermis (mm2) | 208±28 | 209±28 | 193±25 | 198±26 |
| Inferior Posterior Vermis (mm2) | 316±33 | 317±50 | 307±34 | 297±39 |
| ICV (mm3) | 1,376,572±220,548 | 1,469,520±190,085 | 1,458,896±196,621 | 1,430,624±205,340 |
Abbreviations: nsCON=non-smoking controls, sCON=smoking controls, nsALC=non-smoking abstinent alcohol-dependent treatment seekers, sALC= smoking abstinent alcohol-dependent treatment seekers.
nsALC < nsCON, uncorrected p≤0.05
sCON < nsCON, uncorrected p≤0.05
sALC < nsCON, uncorrected p≤0.05
All other pairwise comparisons, uncorrected p>0.05
Figure 2:
Cerebellar measurements (mean ± SEM, adjusted for age and ICV) are shown for the four groups. All cerebellar parcels (total, left hemisphere, and right hemisphere volumes shown on top; superior anterior, superior posterior, and inferior posterior vermis areas on the bottom) were smaller in ALC; cerebellar volumes but not vermal areas were smaller in smokers.
Accelerated cerebellar aging model
Because age was not significantly associated with any cerebellar vermis measure, only the total, left and right hemisphere cerebellar volumes were examined with an accelerated aging model. All three cerebellar volumes showed significant multivariate effects of age (F3,80=5.17, p<0.02), ICV (F3,80=14.61, p<<0.01), and age-by-alcohol-by-smoking status interactions (F9,246=1.91, p=0.05). An examination of the parameter estimates from the univariate model showed that, compared to nsCON, significantly greater volume losses with increasing age were observed in both the left and right cerebellar hemispheres of sCON (p=0.04 and p=0.05, respectively) and sALC (both p=0.01), as illustrated in Supplement 1.
Neurocognitive Results
Performance on individual neurocognitive tests previously associated with cerebellar function is summarized in Table 3. There were no group differences due to alcohol or smoking status for the AMNART, Grooved Pegboard, time to complete the TMT Trails-A or TMT Trails-B tests, time to complete the WCST, and number of nonperseverative errors on the WCST (all p>0.11). However, smokers (sALC and sCON combined) made more perseverative errors and perseverative responses (both p<0.02) than all non-smokers, both alcohol groups had markedly shorter standing times on the Sharpened-Romberg eyes-closed tasks (p<<0.01) compared to all CON, and there were group effects on the WCST total number correct (smoking p=0.04) and total errors (smoking p=0.03, alcohol p=0.04). The significant pairwise differences on these measures reported in Table 3 are consistent with these overall smoking and alcohol effects.
Table 3:
Cognitive Measures by Group
| nsCON N=17 |
sCON N=31 |
nsALC N=21 |
sALC N=23 |
|
|---|---|---|---|---|
| AMNART (Verbal IQ estimate) | 120±8 | 117±6 | 119±6 | 113±9 |
| Ward-7 Full Scale IQ (general intelligence) | 1.25±0.63 | 0.31±0.85† | 0.49±0.97& | 0.34±0.81 |
| Grooved Pegboard – Dominant (sec) | 69±8 | 76±13 | 84±34 | 74±11 |
| Grooved Pegboard – Nondominant (sec) | 73±12 | 70±22 | 67±29 | 78±21 |
| Sharpened-Romberg – eyes closed (sec)** | 171±73 | 161±65§ | 101±99& | 101±99‡☆ |
| TMT Trails – A (sec) | 28±9 | 32±9 | 34±15 | 31±9 |
| TMT Trails – B (sec) | 56±21 | 71±20 | 76±28& | 63±27 |
| WCST – total time (sec) | 286±27 | 374±76† | 392±137& | 386±129 |
| WCST – total correct** | 52±5 | 42±13†§ | 50±6 | 47±11☆ |
| WCST – total errors**, * | 11±5 | 23±13†§ | 14±6 | 17±11☆ |
| WCST – perseverative errors* | 6±4 | 12±6†§ | 6±2 | 9±7☆ |
| WCST – perseverative responses* | 6±5 | 13±8†§ | 6±3 | 10±9☆ |
| WCST – nonperseverative errors | 5±2 | 10±8† | 8±5 | 8±5☆ |
Abbreviations: AMNART=American National Adult Reading Test, sec=seconds, TMT=Trail Making Test, WCST=Wisconsin Card Sorting Test, nsCON=non-smoking controls, sCON=smoking controls, nsALC=non-smoking abstinent alcohol-dependent treatment seekers, sALC= smoking abstinent alcohol-dependent treatment seekers.
overall smoking effect, uncorrected p≤ 0.05
overall alcohol effect, uncorrected p≤0.05
nsALC < nsCON (Sharpened-Romberg, IQ) or nsALC > nsCON (WCST–total time, TMT-Trails B), uncorrected p≤0.05
sCON < nsCON (WCST – total correct, IQ) or sCON > nsCON (WCST – all other measures), uncorrected p≤0.05
sALC < nsCON, uncorrected p≤0.05
sCON <nsALC (WCST – total correct), uncorrected p≤0.05
sCON < sALC (WCST – total correct) or sCON > sALC (Sharpened Romberg, other WCST measures), uncorrected p≤0.05
All other pairwise comparisons, uncorrected p>0.05
Associations between cerebellar and neurocognitive measures
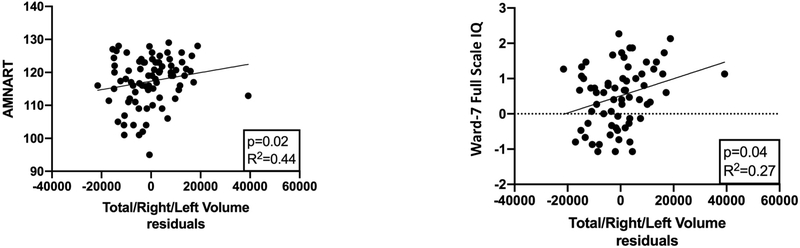
Since the 2 × 2 MANOVAs reported above showed that ICV was related to all cerebellar measures, we regressed each cerebellar measure on ICV and used the residual predicted values as independent variables in our cognitive model. As summarized in Figure 3, smaller cerebellar composite volume was associated with lower AMNART (parameter estimate β=0.001, p=0.02) and Ward-7 Full Scale IQ (parameter estimate β=2.3E-05, p=0.04) scores, over and above the effects of education. The interpretation is that each 1000 mm3 increase in residual cerebellar volume was associated with a 1 point increase in AMNART score and a 0.023 increase in the IQ z-score. None of the cerebellar measures significantly predicted the Grooved Pegboard, TMT-Trails A, time to complete the TMT Trails B, or any WCST measure (time to complete, total correct, total errors, or number of perseverative errors). Given the reports in the literature (Sullivan et al., 2010, Bernard et al., 2015, Bernard and Seidler, 2013, Medina et al., 2010), we were surprised to find that no cerebellar measures predicted Sharpened-Romberg performance over the entire group. However, when we examined groups separately, we found that the composite cerebellar volume measures was associated with performance on the Sharpened-Romberg task in nsALC (β=0.006, p<0.01), such that each 1000 mm3 increase in cerebellar volume corresponded to a 1 second increase in standing time.
Figure 3:
Scatterplots of AMNART scores (American National Adult Reading Test) and the Ward-7 Full Scale IQ versus total cerebellar volumes (residuals after regression on intracranial volume); solid line shows the best linear fit.
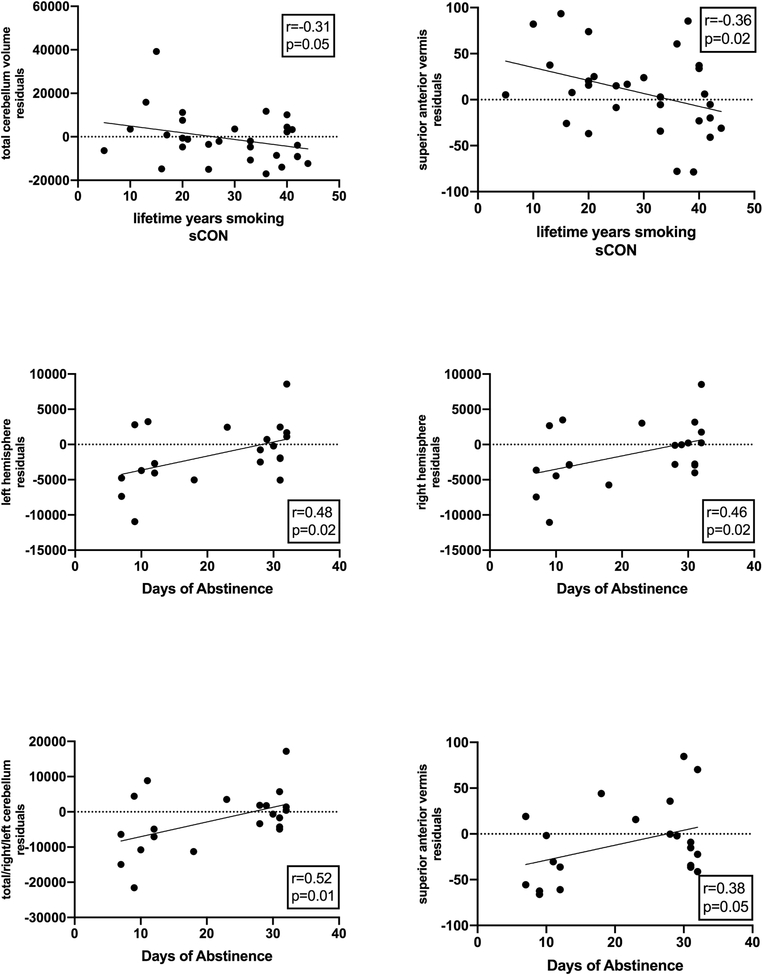
Associations between cerebellum and smoking/drinking severity and abstinence
Several measures of smoking and alcohol consumption showed correlations of moderate strengths with our cerebellar measures (see Figure 4). Among sCON, the number of years smoked over lifetime was associated with smaller total cerebellar volume (r=−0.31, p=0.05) and superior anterior vermis area (r=−0.36, p=0.02. Among sALC, smoking or drinking severity measures were not associated with cerebellar measures, but the number of days of alcohol abstinence were significantly correlated with total (r=0.52, p=0.01), left (r=0.48, p=0.02), and right (r=0.46, p=0.02) cerebellar volume, and superior anterior vermis area (r=0.38, p=0.05), with larger measures associated with longer abstinence. There were no significant associations between drinking severity and cerebellar measures in nsALC.
Figure 4:
Scatterplots of measures of smoking severity or days of alcohol abstinence versus cerebellar measurements (residuals after regression on intracranial volume); solid line shows the best linear fit.
Discussion
Cerebral atrophy is commonly reported in AUD and has also been described with chronic cigarette smoking e.g., (Durazzo and Meyerhoff, 2007). Here, we investigated the effects of alcohol dependence and chronic cigarette smoking on cerebellar function and morphometry using a previously described and validated MRI segmentation tool kit. As hypothesized, detrimental effects of alcohol dependence in 3-week-abstinent treatment seekers were found on volume measures of bilateral cerebellar hemispheres and on sagittal area measures of all three vermis sections segmented, with the largest reductions observed in vermis areas. Though our hypothesis that sALC would display the smallest cerebellar measurements was not confirmed, the results did support our hypothesis of smaller cerebellar volume as a function of smoking status. Both smoking groups (sCON and sALC) had smaller volumes of cerebellar hemispheres than their non-smoking counterparts. Vermis areas, however, did not differ significantly between smokers and nonsmokers. The morphometric deficits in these cross-sectional analyses were related to long-term drinking and smoking measures, suggesting that cerebellar structures may respond to chronic substance use rather than be premorbidly determined. Furthermore, the variance in cerebellar volumes is explained almost equally well by an accelerated aging model or one modeling group differences between nsCON and both smoking groups (sCON and sALC).
Our observation that alcohol status was related to 3% smaller cerebellar hemisphere volumes and up to 5.9% smaller vermis areas compared to controls agrees with prior studies that observed smaller vermis (Karhunen et al., 1994, Sullivan et al., 2000b, Sullivan et al., 2000a, Yokota et al., 2006) or volume loss in the cerebellar hemispheres (Sullivan et al., 2000b, Sullivan et al., 2000a, Sullivan, 2003, Chanraud et al., 2007, Anderson et al., 2010). The congruence of our findings with previous research provides compelling evidence that CATK, the automated cerebellar measurement software used here, is valid in clinical and non-clinical samples. The studies cited above counted Purkinje cells in autopsy patients, manually traced the cerebellum, or used voxel-based morphometry. Though cell counting and manual tracing are gold standards, their time-consuming nature limits their application to small samples, whereas voxel-based methods, although automatic, may not be sufficiently sensitive after necessary correction for multiple comparisons. CATK provides a straightforward, uncomplicated and convenient way of measuring cerebellar volumes and areas noninvasively, quickly, and automatically, being accurate and sensitive enough to confirm previously reported group differences related to chronic alcohol consumption in new samples.
In prior ataxia research involving largely the same ALC and CON participants of this study (89% of the ALC and 92% of the CON participants studied in this manuscript), we showed that chronic smoking was associated with reduced performance on the Sharpened-Romberg eyes-closed task in both groups (Schmidt et al., 2014). Since this postural stability task is a test of cerebellar integrity, we were not surprised to find cerebellar structural deficits related to chronic smoking. Other work had reported smoking-related volume deficits in ventrolateral and dorsolateral prefrontal cortices and cerebellum (Vnukova et al., 2017, Fritz et al., 2014, Franklin et al., 2014, Brody et al., 2004, Hanlon et al., 2016). Moreover, we have repeatedly demonstrated that smoking exacerbates neuropsychological and brain structural deficits in ALC and impedes their neurobiological and functional recovery during abstinence from alcohol (Durazzo et al., 2013a, Durazzo et al., 2014b, Durazzo et al., 2014c, Mon et al., 2009, Pennington et al., 2015, Pennington et al., 2013). Although the multivariate test for an overall effect of smoking in the present study was not significant at alpha=0.05, the results of our uncorrected univariate analyses were strongly suggestive of smaller cerebellar volumes in smokers, whereas cerebellar vermis areas were not related to smoking status (Figure 2). Although the vermis has the same cell types as the flocculonodular, anterior, and posterior lobes of the cerebellum, there is some evidence in mice that the morphology of Purkinje cells may be different in the vermis (Nedelescu et al., 2018), potentially offering some explanation as to why our vermis measures were not smaller in smokers. Another possibility is that smoking affects the lateral boundaries of the vermis which are extraordinarily difficult to measure reliably and are not reflected in our mid-sagittal vermis cross-sectional area.
In sCON, greater severity of smoking was related to smaller cerebellar volumes and smaller area of the superior anterior vermis, even though we did not observe a significant effect of smoking status on any vermis measure. One prior study found putamen volume in male smokers positively correlated with severity of smoking but did not report a similar relationship with cerebellar volume (Franklin et al., 2014); however, it is possible that the stringent multiple comparison corrections used in their voxel-based analyses may have obscured any such a relationship. In sALC but not nsALC, we found that greater abstinence duration was related to greater measures of total, left, right, and superior anterior cerebellum. If sALC had had smaller cerebellar measures upon treatment entry (corresponding to an additive effect of alcohol and smoking) that had partially recovered before they were imaged, it might explain why a significant multivariate effect of smoking was not observed in our main analyses. Further research with imaging at treatment entry and longitudinal follow-up is warranted to investigate whether this is the case.
In exploratory analyses across all subjects, we found that worse performance on the AMNART, a measure of premorbid intelligence, and the Ward-7 Full Scale IQ, a measure of general intelligence, were associated with smaller cerebellar volumes. General intelligence has been linked to distributed neocortical gray matter (Menary et al., 2013, Colom et al., 2006), raising the possibility that large corticocerebellar networks also contribute to intelligence. In a recent study, independent components analysis was used to identify brain networks based on similar gray matter patterns across 92 healthy individuals aged 17–48 years. The cerebello-parietal network identified in this analysis was associated with an estimate of IQ, where greater loading of this network (i.e., greater inferior parietal lobe and cerebellar crus II gray matter co-occurrence) predicted higher IQ (Yoon et al., 2017). Earlier work using voxel-based morphometry showed that bilateral cerebellar gray matter was associated with general cognitive ability in older adults with a mean age of 69 years (Hogan et al., 2011). Our results are consistent with these previous studies, and show that the relationship between cerebellar volume and IQ extend to a greater age range and clinical samples. Across all subjects, the number of perseverative responses on the WCST (a classic measure of executive functioning) was weakly associated with the superior anterior vermis area (trend level p=0.08), although smoking explained more of the variance in WCST performance than the vermis area. Previous reports related cerebellar structure to WCST performance in those with an AUD (Chanraud et al., 2007, Sullivan, 2003), but those studies did not account for any effects of smoking on WCST performance and cerebellar structure. Thus, it is possible that these previously reported relationships of cerebellar structure to WCST performance were mediated by potential effects of smoking in the samples. We did not replicate previously reported associations of cerebellar measures with Sharpened-Romberg eyes-closed (Sullivan et al., 2010, Bernard et al., 2015, Bernard and Seidler, 2013, Medina et al., 2010), or associations between trail-making tasks and cerebellum as previously hypothesized (Zahr et al., 2010) when examined over the entire sample. However, when the groups were examined separately, in nsALC we observed that larger cerebellar volumes were associated with longer times on the Sharpened-Romberg task. Pooling all subjects to increase our sample size limited our detection of this association, perhaps due to ceiling effects where more subjects obtained the maximum time on the Sharpened-Romberg tasks within the CON groups, and the standard deviation was also smaller. In sALC we did not find an association between cerebellar volumes and Sharpened-Romberg. Examination of scatterplots revealed that some sALC had maximal times despite small cerebellar volumes, suggesting that smoking may have compensated for the effects of alcohol in these participants.
We found a significant effect of age on cerebellar hemisphere volume measures consistent with previous work on the effects of normal aging on cerebellar volumes (Bernard et al., 2015, Bernard and Seidler, 2014). Using a model with the age-by-alcohol-by-smoking interaction, we found evidence for accelerated aging of the cerebellar hemispheres related to both alcohol and smoking status, where both sCON and sALC showed steeper negative slopes with aging than nsCON, suggesting accelerated aging for cerebellar volumes as a function of chronic smoking, with and without chronic drinking. The findings for greater age-related cerebellar hemisphere volume loss in sCON relative to nsCON is consistent with our findings in a larger sample, using FreeSurfer to quantitate cerebellar cortical volumes (Durazzo et al., 2017). As in the original model, the vermis area measures were not associated with age. A model comparison showed that this alternative model had almost exactly the same goodness of fit (adjusted R2=0.349) as our original fixed factor model (adjusted R2=0.354) and explained the same amount of variance in our data.
Amplified cerebral oxidative stress (OxS) has been proposed as a mechanism contributing to neurobiological abnormalities related to both heavy alcohol consumption and cigarette smoking in humans (Kim et al., 2004, Kim et al., 2003, Moriarty et al., 2003, Bloomer, 2007, Northrop-Clewes and Thurnham, 2007, Durazzo et al., 2014a) and animal models (Mendez-Alvarez et al., 1998, Panda et al., 2000, Kovacic, 2005, Anbarasi et al., 2006, Das et al., 2009, Valavanidis et al., 2009, Waly et al., 2011). It is well established that OxS is directly associated with damage to membrane lipids, proteins, carbohydrates, DNA and RNA of brain neuronal, glial, and vascular tissue [for review see (Durazzo et al., 2014a)]. Granular neurons of the cerebellar cortex are highly susceptible to OxS (Wang and Michaelis, 2010). While not universally accepted [see (Salmon et al., 2010)], increasing OxS burden with aging is also suggested to be a fundamental mechanism contributing to neurodegeneration in normal aging (Halliwell, 2006, Zimniak, 2011). Collectively, our findings of greater age-related cerebellar volume loss in sCON and sALC suggest the chronic OxS associated with alcohol dependence and/or chronic cigarette smoking may interact with the OxS associated with normal aging, thus amplifying degeneration of the cerebellar structures investigated here.
Prior research using measures of gray matter density in an atlas that segments the cerebellum into 28 parcels has shown sex differences in the human cerebellum (Fan et al., 2010). There have also been reports that smoking and alcohol have differential effects on women and men (Sung et al., 2015, Sawyer et al., 2017). In our study, we did not observe significant effects of sex on any cerebellar measure. However, our sample was overwhelmingly male (see Table 1), limiting our ability to detect sex effects. Moreover, we examined only a small number of cerebellar parcels and did not differentiate between gray and white matter, further limiting our ability to detect cerebellar sex effects previously reported. Future work should examine more women and explore differences among cerebellar lobules, potentially revealing more effects of smoking, alcohol, and sex.
Summary and Conclusions
Overall, our results demonstrate that alcohol dependence and chronic cigarette smoking are associated with smaller cerebellar structural measurements, with vermis areas more vulnerable to alcohol dependence and less affected by smoking. We observed some evidence that these cerebellar deficits were associated with lower intelligence. Further evidence within subgroups indicated that the severity of smoking or alcohol abstinence duration was related to cerebellar structure, reflecting injury related to chronic substance use rather than premorbid abnormalities. Although the CATK provides a quick, reliable, and automated method for cerebellar segmentation, our results are limited due to the relatively small number of cerebellar parcels examined; methods that segment the cerebellum into further anatomically/functionally defined subdivisions might reveal more specific cerebellar-cognitive associations. Our results are also limited by the modest sample sizes, especially our correlations within subgroups, although a power analysis demonstrated sufficient sample sizes to detect group alcohol and smoking differences with small effect sizes. Despite these limitations, CATK’s agreement with previous findings of alcohol effects in the cerebellum using gold standard manual tracing convince us that the method is valid and accurate and that our findings of smoking exacerbating cerebellar hemisphere deficits while relatively sparing the vermis are robust. Our findings showing associations between cerebellar structure and intelligence, smoking severity, and abstinence duration were more exploratory and hypothesis-generating, and suggest that further studies on the interaction of smoking, AUD, and age on brain structure, function, and recovery are warranted to help develop more effective AUD treatment interventions.
Supplementary Material
Acknowledgments:
This work was supported by R01 AA10788 (DJM) and K01 DA24136 (TCD). We thank Dr. George Fein and Mr. Mathew Price of Neurobehavioral Research, Inc., for providing the CATK cerebellar segmentation software. The authors have no conflicts of interest to declare.
References
- Allen G, Buxton R, Wong E, Courchesne E (1997) Attentional Activation of the Cerebellum Independent of Motor Involvement. Science 275:1940–1943. [DOI] [PubMed] [Google Scholar]
- Anbarasi K, Vani G, Balakrishna K, Devi CS (2006) Effect of bacoside A on brain antioxidant status in cigarette smoke exposed rats. Life Sci 78:1378–84. [DOI] [PubMed] [Google Scholar]
- Andersen BB (2004) Reduction of Purkinje cell volume in cerebellum of alcoholics. Brain Res 1007:10–8. [DOI] [PubMed] [Google Scholar]
- Anderson CM, Rabi K, Lukas SE, Teicher MH (2010) Cerebellar lingula size and experiential risk factors associated with high levels of alcohol and drug use in young adults. Cerebellum 9:198–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KG, Harding AJ, Halliday GM, Kril JJ, Harper CG (1999) Neuronal loss in functional zones of the cerebellum of chronic alcoholics with and without Wernicke’s encephalopathy. Neuroscience 91:429–38. [DOI] [PubMed] [Google Scholar]
- Beck A RS, Brown G (1996) Manual for the Beck Depression Inventory-II. Psychological Corporation. [Google Scholar]
- Bernard JA, Leopold DR, Calhoun VD, Mittal VA (2015) Regional cerebellar volume and cognitive function from adolescence to late middle age. Hum Brain Mapp 36:1102–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JA, Seidler RD (2013) Relationships between regional cerebellar volume and sensorimotor and cognitive function in young and older adults. Cerebellum 12:721–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JA, Seidler RD (2014) Moving forward: age effects on the cerebellum underlie cognitive and motor declines. Neurosci Biobehav Rev 42:193–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomer RJ (2007) Decreased blood antioxidant capacity and increased lipid peroxidation in young cigarette smokers compared to nonsmokers: Impact of dietary intake. Nutr J 6:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth J, Wood L, Lu D, Houk JC, Bitan T (2007) The role of the basal ganglia and cerebellin in language processing. Brain Res. 1133:136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Jarvik ME, Lee GS, Smith EC, Huang JC, Bota RG, Bartzokis G, London ED (2004) Differences between smokers and nonsmokers in regional gray matter volumes and densities. Biol Psychiatry 55:77–84. [DOI] [PubMed] [Google Scholar]
- Cardenas V, Price M, Infante M, Moore E, Mattson S, Riley E, Fein G (2014) Automated cerebellar segmentation: Validation and application to detect smaller volumes in children prenatally exposed to alcohol. NeuroImage: Clinical 4:295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanraud S, Martelli C, Delain F, Kostogianni N, Douaud G, Aubin H-J, Reynaud M, Martinot J-L (2007) Brain morphometry and cognitive performance in detoxified alcohol-dependents with preserved psychosocial functioning. Neuropsychopharmacology 32:429–438. [DOI] [PubMed] [Google Scholar]
- Chanraud S, Pitel AL, Rohlfing T, Pfefferbaum A, Sullivan EV (2010) Dual tasking and working memory in alcoholism: relation to frontocerebellar circuitry. Neuropsychopharmacology 35:1868–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Desmond JE (2005a) Temporal dynamics of cerebro-cerebellar network recruitment during a cognitive task. Neuropsychologia 43:1227–1237. [DOI] [PubMed] [Google Scholar]
- Chen SH, Desmond JE (2005b) Cerebrocerebellar networks during articulatory rehearsal and verbal working memory tasks. Neuroimage 24:332–8. [DOI] [PubMed] [Google Scholar]
- Cohen J (1988) Statistical Power Analysis for the Behavioral Sciences: Second Edition, Lawrence Erlbaum Associates, Inc., Hillsdale, NJ. [Google Scholar]
- Colom R, Jung RE, Haier RJ (2006) Distributed brain sites for the g-factor of intelligence. Neuroimage 31:1359–65. [DOI] [PubMed] [Google Scholar]
- Cootes T 2000. An Introduction to Active Shape Models Image Processing and Analysis. Oxford: Oxford University Press. [Google Scholar]
- Cootes TF, Edwards GJ, Taylor CJ (2001) Active Appearence Models. Transactions on Pattern Analysis and Machine Intelligence 23:681–685. [Google Scholar]
- Currie S, Hadjivassiliou M, Craven IJ, Wilkinson ID, Griffiths PD, Hoggard N (2013) Magnetic resonance imaging biomarkers in patients with progressive ataxia: current status and future direction. Cerebellum 12:245–66. [DOI] [PubMed] [Google Scholar]
- Das S, Gautam N, Dey SK, Maiti T, Roy S (2009) Oxidative stress in the brain of nicotine-induced toxicity: protective role of Andrographis paniculata Nees and vitamin E. Appl Physiol Nutr Metab 34:124–35. [DOI] [PubMed] [Google Scholar]
- Davila MD, Shear PK, Lane B, Sullivan EV, Pfefferbaum A (1994) Mammillary body and cerebellar shrinkage in chronic alcoholics: an MRI and neuropsychological study. Neuropsychology 8:433. [Google Scholar]
- Durazzo TC, Mattsson N, Weiner MW, Alzheimer’s Disease Neuroimaging I (2014a) Smoking and increased Alzheimer’s disease risk: a review of potential mechanisms. Alzheimers Dement 10:S122–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Meyerhoff DJ (2007) Neurobiological and neurocognitive effects of chronic cigarette smoking and alcoholism. Front Biosci 12:4079–100. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Meyerhoff DJ, Yoder KK, Murray DE (2017) Cigarette smoking is associated with amplified age-related volume loss in subcortical brain regions. Drug Alcohol Depend 177:228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Mon A, Gazdzinski S, Meyerhoff DJ (2013a) Chronic cigarette smoking in alcohol dependence: associations with cortical thickness and N-acetylaspartate levels in the extended brain reward system. Addict Biol 18:379–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Mon A, Pennington D, Abe C, Gazdzinski S, Meyerhoff DJ (2014b) Interactive effects of chronic cigarette smoking and age on brain volumes in controls and alcohol-dependent individuals in early abstinence. Addict Biol 19:132–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Pennington DL, Schmidt TP, Meyerhoff DJ (2014c) Effects of cigarette smoking history on neurocognitive recovery over 8 months of abstinence in alcohol-dependent individuals. Alcohol Clin Exp Res 38:2816–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Pennington DL, Schmidt TP, Mon A, Abe C, Meyerhoff DJ (2013b) Neurocognition in 1-month-abstinent treatment-seeking alcohol-dependent individuals: interactive effects of age and chronic cigarette smoking. Alcohol Clin Exp Res 37:1794–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L, Tang Y, Sun B, Gong G, Chen ZJ, Lin X, Yu T, Li Z, Evans AC, Liu S (2010) Sexual dimorphism and asymmetry in human cerebellum: an MRI-based morphometric study. Brain Res 1353:60–73. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A (2007) G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39:175–91. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Wetherill RR, Jagannathan K, Johnson B, Mumma J, Hager N, Rao H, Childress AR (2014) The effects of chronic cigarette smoking on gray matter volume: influence of sex. PLoS One 9:e104102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregly AR, Graybiel A, Smith MJ (1972) Walk on floor eyes closed (WOFEC): a new addition to an ataxia test battery. Alcoholism Clinical and Experimental Research 43:395–9. [PubMed] [Google Scholar]
- Fritz HC, Wittfeld K, Schmidt CO, Domin M, Grabe HJ, Hegenscheid K, Hosten N, Lotze M (2014) Current smoking and reduced gray matter volume-a voxel-based morphometry study. Neuropsychopharmacology 39:2594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallinat J, Meisenzahl E, Jacobsen LK, Kalus P, Bierbrauer J, Kienast T, Witthaus H, Leopold K, Seifert F, Schubert F, Staedtgen M (2006) Smoking and structural brain deficits: a volumetric MR investigation. Eur J Neurosci 24:1744–50. [DOI] [PubMed] [Google Scholar]
- Grober E, Sliwinski M (1991) Development and validation of a model for estimating premorbid verbal intelligence in the elderly. Clinical & Experimental Neuropsychology 13:933–49. [DOI] [PubMed] [Google Scholar]
- Guggenmos M, Schmack K, Sekutowicz M, Garbusow M, Sebold M, Sommer C, Smolka MN, Wittchen HU, Zimmermann US, Heinz A, Sterzer P (2017) Quantitative neurobiological evidence for accelerated brain aging in alcohol dependence. Transl Psychiatry 7:1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B (2006) Oxidative stress and neurodegeneration: where are we now? J Neurochem 97:1634–58. [DOI] [PubMed] [Google Scholar]
- Hanlon CA, Owens MM, Joseph JE, Zhu X, George MS, Brady KT, Hartwell KJ (2016) Lower subcortical gray matter volume in both younger smokers and established smokers relative to non-smokers. Addict Biol 21:185–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper CG, Kril JJ (ed.) 1993. Neuropathological changes in alcoholics, in alcohol-induced brain damage, Rockville, MD: U.S. Department of Health and Human Services. [Google Scholar]
- Hayter A, Langdon D, Ramnani N (2007) Cerebellar contributions to working memory. Neuroimage 36:943–954. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO (1991) The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict 86:1119–27. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Staff P (1993) Wisconsin Card Sorting Test: Computer Version-2, Research Edition, Psychological Assessment Resources Inc., Lutz, FL. [Google Scholar]
- Hogan MJ, Staff RT, Bunting BP, Murray AD, Ahearn TS, Deary IJ, Whalley LJ (2011) Cerebellar brain volume accounts for variance in cognitive performance in older adults. Cortex 47:441–50. [DOI] [PubMed] [Google Scholar]
- Karhunen PJ, Erkinjuntti T, Laippala P (1994) Moderate alcohol consumption and loss of cerebellar Purkinje cells. BMJ 308:1663–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Ensunsa JL, Zhu QY, Kim JS, Shin HS, Keen CL (2004) An 18-month follow-up study on the influence of smoking on blood antioxidant status of teenage girls in comparison with adult male smokers in Korea. Nutrition 20:437–44. [DOI] [PubMed] [Google Scholar]
- Kim SH, Kim JS, Shin HS, Keen CL (2003) Influence of smoking on markers of oxidative stress and serum mineral concentrations in teenage girls in Korea. Nutrition 19:240–3. [DOI] [PubMed] [Google Scholar]
- Kovacic P (2005) Unifying mechanism for addiction and toxicity of abused drugs with application to dopamine and glutamate mediators: electron transfer and reactive oxygen species. Med Hypotheses 65:90–6. [DOI] [PubMed] [Google Scholar]
- Kuhn S, Romanowski A, Schilling C, Mobascher A, Warbrick T, Winterer G, Gallinat J (2012) Brain grey matter deficits in smokers: focus on the cerebellum. Brain Struct Funct 217:517–22. [DOI] [PubMed] [Google Scholar]
- Medina KL, Nagel BJ, Tapert SFJPRN (2010) Abnormal cerebellar morphometry in abstinent adolescent marijuana users. 182:152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menary K, Collins PF, Porter JN, Muetzel R, Olson EA, Kumar V, Steinbach M, Lim KO, Luciana M (2013) Associations between cortical thickness and general intelligence in children, adolescents and young adults. Intelligence 41:597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez-Alvarez E, Soto-Otero R, Sanchez-Sellero I, Lopez-Rivadulla Lamas M (1998) In vitro inhibition of catalase activity by cigarette smoke: relevance for oxidative stress. J Appl Toxicol 18:443–8. [DOI] [PubMed] [Google Scholar]
- Mon A, Durazzo TC, Gazdzinski S, Meyerhoff DJ (2009) The impact of chronic cigarette smoking on recovery from cortical gray matter perfusion deficits in alcohol dependence: longitudinal arterial spin labeling MRI. Alcohol Clin Exp Res 33:1314–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarty SE, Shah JH, Lynn M, Jiang S, Openo K, Jones DP, Sternberg P (2003) Oxidation of glutathione and cysteine in human plasma associated with smoking. Free Radic Biol Med 35:1582–8. [DOI] [PubMed] [Google Scholar]
- Nakamura-Palacios EM, Souza RS, Zago-Gomes MP, De Melo AM, Braga FS, Kubo TT, Gasparetto EL (2014) Gray matter volume in left rostral middle frontal and left cerebellar cortices predicts frontal executive performance in alcoholic subjects. Alcohol Clin Exp Res 38:1126–33. [DOI] [PubMed] [Google Scholar]
- Nedelescu H, Abdelhack M, Pritchard AT (2018) Regional differences in Purkinje cell morphology in the cerebellar vermis of male mice. J Neurosci Res 96:1476–1489. [DOI] [PubMed] [Google Scholar]
- Northrop-Clewes CA, Thurnham DI (2007) Monitoring micronutrients in cigarette smokers. Clin Chim Acta 377:14–38. [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971) Grooved Pegboard Test. Neuropsychology 9:97–113. [Google Scholar]
- Panda K, Chattopadhyay R, Chattopadhyay DJ, Chatterjee IB (2000) Vitamin C prevents cigarette smoke-induced oxidative damage in vivo. Free Radic Biol Med 29:115–24. [DOI] [PubMed] [Google Scholar]
- Patenaude B, Smith SM, Kennedy DN, Jenkinson M (2011) A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage 56:907–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington DL, Durazzo TC, Schmidt TP, Abe C, Mon A, Meyerhoff DJ (2015) Alcohol use disorder with and without stimulant use: brain morphometry and its associations with cigarette smoking, cognition, and inhibitory control. PLoS One 10:e0122505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington DL, Durazzo TC, Schmidt TP, Mon A, Abe C, Meyerhoff DJ (2013) The effects of chronic cigarette smoking on cognitive recovery during early abstinence from alcohol. Alcohol Clin Exp Res 37:1220–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Lim KO, Zipursky RB, Mathalon DH, Rosenbloom MJ, Lane B, Ha CN, Sullivan EV (1992) Brain gray and white matter volume loss accelerates with aging in chronic alcoholics: a quantitative MRI study. Alcoholism Clinical and Experimental Research 16:1078–89. [DOI] [PubMed] [Google Scholar]
- Price M, Cardenas VA, Fein G (2014) Automated MRI cerebellar size measurements using active appearance modeling. Neuroimage 103:511–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan RM, Wolfson D (1985) The Halstead-Reitan Neuropsychological Test Battery, Neuropsychology Press, Tucson. [Google Scholar]
- Salmon AB, Richardson A, Perez VI (2010) Update on the oxidative stress theory of aging: does oxidative stress play a role in aging or healthy aging? Free Radic Biol Med 48:642–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer KS, Oscar-Berman M, Barthelemy OJ, Papadimitriou GM, Harris GJ, Makris N (2017) Gender dimorphism of brain reward system volumes in alcoholism. Psychiatry Res Neuroimaging 263:15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD, Sherman JC (1998) The cerebellar cognitive affective syndrome. Brain 121 (Pt 4):561–79. [DOI] [PubMed] [Google Scholar]
- Schmidt TP, Pennington DL, Durazzo TC, Meyerhoff DJ (2014) Postural stability in cigarette smokers and during abstinence from alcohol. Alcohol Clin Exp Res 38:1753–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner HA, Sheu WJ (1982) Reliability of alcohol use indices. The Lifetime Drinking History and the MAST. Journal of Studies on Alcohol 43:1157–70. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, Riley DM, Schuller R, Pavan DS, Cancilla A, Klajner F, Leo GI (1988) The reliability of alcohol abusers’ self-reports of drinking and life events that occurred in the distant past. Studies of Alcohol 49:225–32. [DOI] [PubMed] [Google Scholar]
- Spielberger CD (1983) State-Trait Anxiety Inventory for Adults: Form Y Review Set - Manual, Test, Scoring Key, Mind Garden, Inc., Redwood City, CA. [Google Scholar]
- Strauss E, Sherman EMS, Spreen O (2006) A compendium of neuropsychological tests: administration, norms, and commentary, Oxford University Press, Oxford; New York. [Google Scholar]
- Sullivan EV (2003) Compromised pontocerebellar and cerebellothalamocortical systems: speculations on their contributions to cognitive and motor impairment in nonamnesic alcoholism. Alcoholism Clinical and Experimental Research 27:1409–19. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Deshmukh A, Desmond JE, Lim KO, Pfefferbaum A (2000a) Cerebellar volume decline in normal aging, alcoholism, and Korsakoff’s syndrome: relation to ataxia. Neuropsychology 14:341–52. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Deshmukh A, Desmond JE, Mathalon DH, Rosenbloom MJ, Lim KO, Pfefferbaum A (2000b) Contribution of alcohol abuse to cerebellar volume deficits in men with schizophrenia. Alcoholism Clinical and Experimental Research 57:894–902. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rohlfing T, Pfefferbaum A (2010) Pontocerebellar volume deficits and ataxia in alcoholic men and women: no evidence for “telescoping”. Psychopharmacology (Berl) 208:279–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Rose J, Pfefferbaum A (2006) Effect of vision, touch and stance on cerebellar vermian-related sway and tremor: a quantitative physiological and MRI study. Cerebral Cortex 16:1077–86. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rose J, Rohlfing T, Pfefferbaum A (2009) Postural sway reduction in aging men and women: relation to brain structure, cognitive status, and stabilizing factors. Neurobiol Aging 30:793–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung YH, Yurgelun-Todd DA, Kondo DG, Shi XF, Lundberg KJ, Hellem TL, Huber RS, Mcglade EC, Jeong EK, Renshaw PF (2015) Gender differences in the effect of tobacco use on brain phosphocreatine levels in methamphetamine-dependent subjects. Am J Drug Alcohol Abuse 41:281–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torvik A, Torp S (1986) The prevalence of alcoholic cerebellar atrophy. A morphometric and histological study of an autopsy material. J Neurol Sci 75:43–51. [DOI] [PubMed] [Google Scholar]
- Valavanidis A, Vlachogianni T, Fiotakis K (2009) Tobacco smoke: involvement of reactive oxygen species and stable free radicals in mechanisms of oxidative damage, carcinogenesis and synergistic effects with other respirable particles. Int J Environ Res Public Health 6:445–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassar RL, Rose J (2014) Motor systems and postural instability. Handb Clin Neurol 125:237–51. [DOI] [PubMed] [Google Scholar]
- Victor M, Adams R, Mancall EL (1959) A restricted form of cerebellar cortical degeneration occurring in alcoholic patients.. Alcoholism Clinical and Experimental Research 1:579–688. [Google Scholar]
- Victor M, Adams RD, Collins GH (eds.) 1989. The Wernicke-Korsakoff syndrome and related neurological disorders due to alcoholism and malnutrition, Philadephia: F.A. Davis Co. [Google Scholar]
- Vnukova M, Ptacek R, Raboch J, Stefano GB (2017) Decreased Central Nervous System Grey Matter Volume (GMV) in Smokers Affects Cognitive Abilities: A Systematic Review. Med Sci Monit 23:1907–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waly MI, Al Moundhri MS, Ali BH (2011) Effect of curcumin on cisplatin- and oxaliplatin-induced oxidative stress in human embryonic kidney (HEK) 293 cells. Ren Fail 33:518–23. [DOI] [PubMed] [Google Scholar]
- Wang X, Michaelis EK (2010) Selective neuronal vulnerability to oxidative stress in the brain. Front Aging Neurosci 2:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota O, Tsuchiya K, Terada S, Oshima K, Ishizu H, Matsushita M, Kuroda S, Akiyama H (2006) Frequency and clinicopathological characteristics of alcoholic cerebellar degeneration in Japan: a cross-sectional study of 1,509 postmortems. Acta Neuropathol 112:43–51. [DOI] [PubMed] [Google Scholar]
- Yoon YB, Shin WG, Lee TY, Hur JW, Cho KIK, Sohn WS, Kim SG, Lee KH, Kwon JS (2017) Brain Structural Networks Associated with Intelligence and Visuomotor Ability. Sci Rep 7:2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr NM, Pitel AL, Chanraud S, Sullivan EV (2010) Contributions of studies on alcohol use disorders to understanding cerebellar function. Neuropsychol Rev 20:280–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimniak P (2011) Relationship of electrophilic stress to aging. Free Radic Biol Med 51:1087–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.