Abstract
Alcohol addiction causes major health problems throughout the world, causing numerous deaths and incurring a huge economic burden to society. To develop an intervention for alcohol addiction, it is necessary to identify molecular target(s) of alcohol and associated molecular mechanisms of alcohol action. The functions of many central and peripheral synapses are impacted by low concentrations of ethanol. While the postsynaptic targets and mechanisms are studied extensively, there are limited studies on the presynaptic targets and mechanisms. This article is an endeavor in this direction, focusing on the effect of ethanol on the presynaptic proteins associated with the neurotransmitter release machinery. Studies on the effects of ethanol at the levels of gene, protein, and behavior are highlighted in this article.
Keywords: ethanol, alcoholism, neurotransmitter, presynaptic, postsynaptic, neuron, volatile anesthetics, exocytosis, SNARE complex
Introduction
Alcohol is one of the most harmful drugs of abuse (Nutt et al., 2010) and alcohol addiction is a major health problem throughout the world, causing numerous deaths and incurring a huge economic burden to society (Sacks et al., 2015). Defining the molecular mechanism of alcohol (ethanol) action is key to developing an intervention to alcohol addiction. To this end, it is important to understand ethanol’s role in the changes of behavior and the brain during the descent into addiction. At relatively low concentrations, ethanol affects the function of many central and peripheral synapses (Liu and Hunt, 1999) and modulates synaptic plasticity (McCool, 2011, Lovinger and Roberto, 2013, Roberto and Varodayan, 2017). Although clear effects of ethanol have been identified in postsynaptic compartments, its effects on the presynaptic compartments is less studied. Ethanol has multiple targets (Harris et al., 2008, Howard et al., 2011) and affects many neuronal circuitries in the brain (Abrahao et al., 2017). While ethanol’s effects on postsynaptic receptors, such as GABAA (Olsen and Liang, 2017), glycine (Soderpalm et al., 2017) and glutamate (Rao et al., 2015) are well-established, there has been increasing evidence of a significant effect of ethanol on presynaptic function at concentrations well below 100 mM, but above 17 mM, which is the legal threshold for intoxication in human (Siggins et al., 1987, Roberto et al., 2003, Nie et al., 2004, Diamond and Gordon, 1997). Recent studies suggest that ethanol may be directly affecting synaptic transmission by altering vesicle fusion and neurotransmitter release (Barclay et al., 2010), possibly by interacting with the proteins associated with the neurotransmitter release machinery. The objective of the present article is to critically review relevant data from the literature and provide a future perspective of the current research. The effects of ethanol described here are at the level of gene, protein or behavioral changes. Effects of volatile anesthetics on these proteins, wherever available, are also included, as there is a notion that alcohol and volatile anesthetics may share a common mechanism of action (Franks and Lieb, 1984, Franks and Lieb, 1985, Homanics et al., 2002, Stubbs and Rubin, 1993) and are believed to share the same binding site in ligand-gated ion channels, such as glycine and GABAA receptors (Sauguet et al., 2013).
Neurotransmitter release machinery
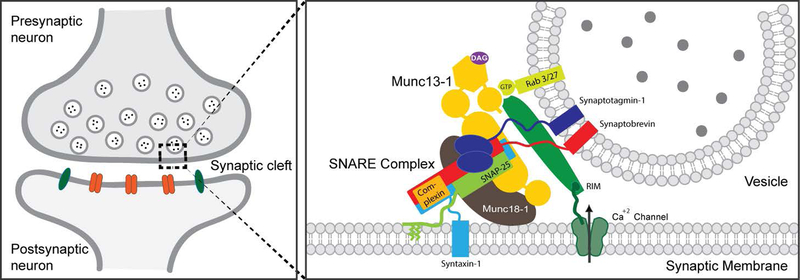
The neurotransmitters are released by Ca2+-triggered synaptic vesicle exocytosis. Involvement of the central component proteins of the release machinery leads to membrane fusion and neurotransmitter release (Rizo, 2018, Ramakrishnan et al., 2012). Major proteins that constitute this machinery are syntaxin, Munc18, synaptobrevin, SNAP-25, synaptotagmin, Munc13, and complexin (Sudhof, 2013). First, vesicles are attached to the protein complexes at the presynaptic membrane, facilitating the contact between v-SNARE (soluble N-ethyl maleimide sensitive factor attachment protein receptor) and t-SNARE proteins. Synaptobrevin is a v-SNARE because of its vesicle localization, while syntaxin 1 and SNAP-25 are called t-SNAREs because they reside on the target membrane. Syntaxin 1, SNAP-25, and synaptobrevin-2 bind tightly with each other through their α-helices to form a core structure and bring the vesicle and plasma membrane together. Synaptotagmin 1 (Hui et al., 2011) acts as the major Ca2+ sensor by triggering neurotransmitter release upon its binding to membrane phospholipids and to the SNAREs and causing conformational changes within the SNARE proteins. Recycling of the SNARE and another round of vesicle fusion occurs by the dissociation of N-ethyl maleimide sensitive factor (NSF) and soluble NSF attachment proteins (SNAPs) from the SNARE complex. The roles of Munc18–1 and Munc13–1 are to orchestrate the SNARE complex formation. Munc18–1 binds to synaptobrevin and to syntaxin 1 in its self-inhibited “closed” conformation. Munc13–1 facilitates the assembly formation by bridging the vesicle and plasma membranes. Complexins (Yang et al., 2010) play major roles in regulating this interactions. In addition, DoC2 proteins (Groffen et al., 2010) and synapsin (Pan et al., 2009) are also recognized for their involvement in the regulation of Ca2+-triggered exocytosis. A simplified picture showing the involvement of several proteins are shown in Figure 1 (Maximov et al., 2009, Kaeser et al., 2011).
Figure 1:
A simplified model showing the active zone proteins involved in vesicle priming and fusion in mammals. Some of the proteins shown are RIM, Munc13, Ca2+ channels, SNAP-25, syntaxin 1, Munc18–1, complexin, Rab3 and synaptotagmin 1. Munc13 is mammalian homolog of Unc13 in nematode worm C. elegans and Dunc13 in Drosophila. Munc18 is the mammalian homolog of Unc18 in C. elegans and Drosophila.
Syntaxin 1A
Syntaxin 1A is a member of the nervous system-specific syntaxin superfamily of proteins consisting of fifteen members in human (Teng et al., 2001). The proteins vary in their cellular localization, tissue distributions and functions. For example, syntaxin 1(A/B) is located at the presynaptic plasma membrane of neuronal and secretory cells, but syntaxin 12 is localized in endosomes and has ubiquitous tissue distribution. While syntaxin 1(A/B) is involved in neuronal exocytosis and regulates secretion, syntaxin 12 is involved in the recycling of surface protein and early endosome fusion (Teng et al., 2001). Syntaxin is a key protein in the synaptic exocytosis process and in ion channel regulation (Vardar et al., 2016).
Syntaxin consists of a single C-terminal transmembrane domain (TMD), an α-helical SNARE domain (known as H3), a short linker region, and the N-terminal Habc domain. The SNARE domain plays an important role as both SNAP-25 and synaptobrevin dock at this domain in order to form the SNARE complex. The Habc domain, which consists of three α-helical regions, serves as an autoinhibitory domain. It associates with the SNARE domain of syntaxin inducing a “closed” state, thereby creating a physical barrier to the formation of the SNARE motif (Fernandez et al., 1998, Khvotchev et al., 2007). These domains are conserved in Drosophila and C. elegans and their sequence identity is about 66–70% with rat syntaxin 1A.
Previous studies suggest that syntaxin proteins are involved in mechanisms of alcohol action. At the gene level, a mutation in the Syntaxin 1A gene disrupts the capacity to acquire ethanol tolerance in Drosophila (Krishnan et al., 2012). Syntaxin 12, another member of the syntaxin family, is a potential contributor to ethanol preference in mice (Treadwell et al., 2004, Weng et al., 2009). The conclusion was based upon the expression and ethanol preference studies in C57BL/6J and DBA/2J inbred mouse strains using a two-bottle choice drinking paradigm. It was shown that Stx12 mRNA levels were elevated in the brains of D2 mice as compared to B6 strain mice. Upon acute alcohol treatment, expression was reduced in D2, but not in B6. Further, Stx12 mRNA expression was higher in the ethanol-avoiding F2 mice than the ethanol-preferring F2 mice. In a subsequent study, the authors showed that Syntaxin 12 c.*1370G>A polymorphism segregated with alcohol preference in the B6D2F2 population and syntaxin 12 expression and alcohol preference in the selected B6D2F2 males were correlated (Weng et al., 2009). Syntaxin 12 is also responsible for the recycling of AMPA receptors that mediate fast excitatory synaptic neurotransmission in the brain (Lee et al., 2001), suggesting a connection between AMPA receptor and ethanol preference. While studying the expression levels in the prefrontal cortex of human chronic alcoholics, elevated expression was observed for a synaptic vesicle protein, synaptophysin 1, but there were no significant changes in the expression levels of syntaxin 1A, SNAP-25 or VAMP (Henriksson et al., 2008).
The role of syntaxin 1 in mediating the action of general anesthetics is well-studied in vivo and in vitro (Zalucki et al., 2015, Nagele et al., 2005, Hawasli et al., 2004). The expression of a syntaxin 1A truncation mutant in the H3 domain, md130A, blocked isoflurane-mediated inhibition of neurotransmitter release in permeabilized PC12 cells (Herring et al., 2009). This was also reflected in behavioral sensitivity tests where there was significant reduction in the locomotion of C. elegans md130A heterozygotes upon isoflurane treatment (van Swinderen et al., 1999). This suggests that the isoflurane-syntaxin interaction influences the inhibition of the release machinery. In Drosophila, similar, but not identical, syntaxin mutations in the H3 domain result in strong resistance to the effects of isoflurane (Zalucki et al., 2015). The binding of volatile anesthetics with syntaxin 1A has been characterized by NMR and CD spectroscopy and some of these anesthetics have been shown to cause structural alterations of the protein (Nagele et al., 2005).
In summary, Syntaxin 1A in Drosophila and Stx12 gene in mice are associated with ethanol preference. In Drosophila, C. elegans and PC12 cells, syntaxin 1A is shown to mediate actions of general anesthetics.
SNAP-25
Synaptosomal-associated protein 25 kDa (SNAP-25) is a major protein of the neural SNARE complexes. Its role has been implicated in the pathology of various neurological disorders, such as Alzheimer’s disease, schizophrenia, attention deficient hyperactivity disorder and epilepsy (Corradini et al., 2009, Noor and Zahid, 2017). In forming the SNARE complex, synaptobrevin, syntaxin 1 and SNAP-25 wrap around each other and form a coiled-coil quaternary structure with their α-helices.
A recent proteomic analysis of orbitofrontal cortical samples from adult male monkeys following long-term alcohol drinking showed significant increases in SNAP-25 protein expression (Nimitvilai et al., 2017). In mouse cortical neurons, treatment of 60 mM ethanol for 1 h and heat shock up-regulate Snap25 gene expression (Varodayan et al., 2011). A more recent study found a significant decrease in SNAP-25 protein in the lateral amygdala of a male macaque with a history of heavy drinking (Alexander et al., 2018). No significant changes were observed in females. A long-term ethanol drinking paradigm (Grant et al., 2008) was used in this study.
In studying the effects of general anesthetic isoflurane on SNAP-25, it was found that isoflurane’s ability to inhibit neurotransmitter release in PC12 cells was dependent on the levels of SNAP-25 and SNAP-23 (Xie et al., 2013). NMR studies show that isoflurane binds to SNAP-25 at clinical concentrations (Nagele et al., 2005).
In summary, ethanol affects the expression of SNAP-25 at the mRNA and protein levels. Proteomic analysis data indicate that protein expression is brain region- and gender-specific. Cellular experiments suggest that the effect of general anesthetics is dependent on SNAP-23/25.
VAMP
Synaptobrevins belong to the vesicle-associated membrane protein (VAMP) family of proteins. There are eight members in this family in human, VAMP 1–8. VAMP1 and VAMP2 are known as synaptobrevins and are expressed in the brain. VAMP8 is known as endobrevin and is expressed in pancreatic acinar cells. Synaptobrevins are small integral membrane proteins of secretory vesicles. Out of four α-helices of the core SNARE complex, synaptobrevin contributes one, syntaxin contributes one and the remaining two are contributed by SNAP-25.
Using culture of mouse cortical neurons treated with 10–150 mM ethanol, it was found that ethanol activated the transcription factor heat shock factor 1 (HSF1) to induce Vamp2 mRNA expression, while Vamp1 mRNA levels remained unaffected (Varodayan and Harrison, 2013). Ethanol and heat shock also increased VAMP2 protein levels, without affecting VAMP1 expression, which is consistent with observations at the mRNA levels (Varodayan et al., 2011). The authors suggested that these differences could be due to different location of the alcohol response element (ARE) sequence in the Vamp1 and Vamp2 genes and differential expression of the corresponding proteins in the CNS. Vamp2 is expressed throughout the mouse brain, particularly in the cortex, whereas Vamp1 predominates in regions of the diencephalon and midbrain (Varodayan and Harrison, 2013).
Using a mouse model of alcoholic pancreatitis, it has been shown that VAMP8 mediates basolateral exocytosis and its deletion induces alcoholic pancreatitis (Cosen-Binker et al., 2008).
Rab3
Rab3 is the major isoform of the Rab family of proteins known to regulate presynaptic exocytosis (Fukuda, 2008). There are seventy different Rab proteins identified in human to date. These proteins vary in their localization, membrane trafficking pathways and effector proteins (Hutagalung and Novick, 2011). For example, while Rab3A is localized in secretory vesicles and plasma membrane, Rab6 is localized in the Golgi and Rab11 is localized in the Golgi, recycling endosomes and early endosomes. While Rab3A is involved in exocytosis and neurotransmitter release, Rab6 is involved in intra-Golgi transport, endosome to Golgi and Golgi to ER trafficking. Rabs are GTPases and act as molecular switches. In the GTP-bound state, they produce docking/fusion-competent vesicles by associating with the membrane. However, upon GTP hydrolysis, the production of the competent vesicles are switched off (Grosshans et al., 2006). Rab3-null mice show increased synaptic depression, implicating its role in vesicle recruitment (Geppert et al., 1994a).
In C. elegans, rab-3-null mutants show decreased ethanol sensitivity (Kapfhamer et al., 2008, Davies et al., 2012). In the dispersion assay, in the presence of 400 mM exogenous ethanol (internal concentration, 15–20 mM), the mutant worms dispersed to a greater extent, as compared to wild-type. Also, multiple rab-3 loss-of-function mutants moved significantly faster than wild-types. This phenotype is replicated in the loss-of-function mutant of its GTP exchange factor, aex-3 (Kapfhamer et al., 2008). These results indicate that the reduction in ethanol sensitivity was specific to the GTP-bound state of the Rab3 protein and not just due to the loss of Rab3. Further, the role of Rab3 in ethanol resistance was confirmed by measuring the voluntary ethanol consumption of the knockout mice (Kapfhamer et al., 2008). In a two-bottle choice drinking paradigm, Rab3A+/−mice voluntarily consumed significantly more ethanol than wild-type or Rab3A−/−mice and ethanol preference was not dependent on ethanol concentration in Rab3A+/−mice, establishing the role of this protein in modulating the drinking behavior. The authors concluded that this resistance to ethanol sensitivity may reduce recruitment of vesicles from the reserve to the releasable pool. This study also showed that reduction of Rab3 in both C. elegans and mice altered the behavioral response to ethanol.
Recent proteomic analysis studies found a significant decrease in Rab3c protein in the lateral amygdala of a female macaque with a history of heavy drinking (Alexander et al., 2018) using a long-term drinking paradigm (Grant et al., 2008). No significant changes were observed in males.
To test if ethanol-impaired secretion of pituitary FSH and LH is associated with Rab proteins, Ren et al (Ren et al., 2005) studied the effect of ethanol in diet on the expression of Rab6, Rab3B, Rab11 and Rab1B in rats. It was shown that the decrease in the expression of these proteins were dependent on the time of ethanol exposure (5–60 days). However, mRNA levels of these Rab proteins were unaffected by such ethanol exposure. The authors concluded that this reduction in key Rab proteins may cause alteration in vesicle trafficking and ethanol-induced disruption of pituitary FSH and LH secretion (Ren et al., 2005).
In rat ethanol-damaged livers, Rab2’s association with a Golgi compartment was significantly reduced as compared with controls, but no changes with Rab6 was observed (Larkin et al., 1996).
The effect of volatile anesthetic halothane on rab-3 gene was studied in C. elegans. The results show that the worms with mutants of the rab-3 displayed resistance to halothane (Davies et al., 2012).
In summary, Rab3 modulates drinking behaviors in mice and ethanol sensitivity in C. elegans. Ethanol alters expression of several Rab isoforms in rodents at the protein level. In C. elegans, rab-3 regulates the actions of general anesthetics.
Munc18
Munc18–1 is a member of the Sec1/Munc18 (SM) protein family, which is critically involved in most types of intracellular membrane trafficking (Carr and Rizo, 2010, Sudhof and Rothman, 2009). There are six members in this family of proteins in humans. Total abrogation of neurotransmitter release observed in Munc18–1 knockout mice illustrates the importance of Munc18–1 in this process (Verhage et al., 2000). Munc18–1 binds tightly to syntaxin 1 in its closed conformation (Misura et al., 2000), to the syntaxin 1 N-terminal region through both the N-peptide and the Habc domain, and to the four-helix bundle of the SNARE complex (Dulubova et al., 2007, Shen et al., 2007, Xu et al., 2010).
The syntaxin binding protein 1 gene (Stxbp1) encodes the Sec1/Munc18-type protein and is a candidate for an ethanol preference drinking locus on mouse chromosome 2 (Fehr et al., 2005). The genetic study of two mouse strains, C57BL/6J and DBA/2J, with the former having higher ethanol preference than the latter in a two-bottle choice assays indicated a correlation with a polymorphism (D216N) in Munc18–1 (Fehr et al., 2005). The orthologous mutation (D214N) in the C. elegans Unc18 resulted in resistance to both the stimulatory (21 mM) and sedative effects (400 mM) of ethanol (Graham et al., 2009). These unc-18-null transgenics are rescued by both D214N and wild-type, producing phenotypically similar locomotion, suggesting no significant effect of this mutation in the vesicle fusion. Amperometric recording revealed that this missense mutation lengthened the duration of quantum release and slowed the frequency of release, suggesting ethanol’s interference in synaptic vesicle exocytosis (Graham et al., 2009).
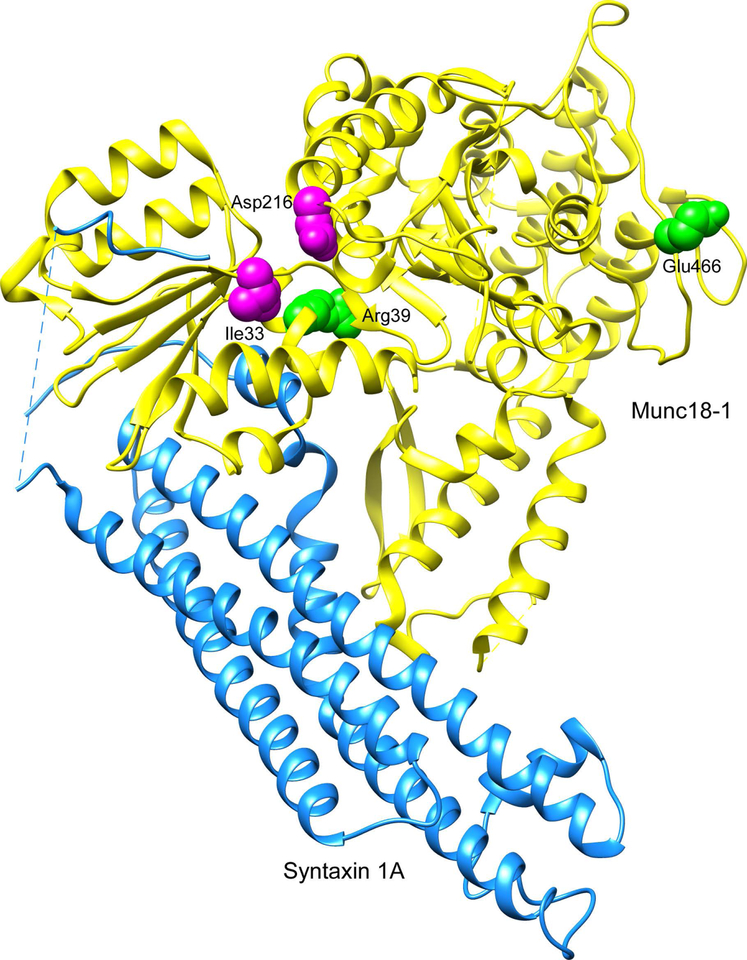
D216N mutation in Munc18 protein affects its binding to syntaxin. This is neither through a closed conformation mode of interaction nor through binding to the syntaxin N-terminus. Rather, this mutant has a specific impairment in binding the assembled SNARE complex. Analysis of an alternative Munc18–1 mutation (I133V), which also affects syntaxin binding and shows similar ethanol sensitivity to the D216N, revealed the link between reduced SNARE complex binding and ethanol resistance (Figure 2). Transcriptome analysis of human brain tissue identified STXBP1 as a hub gene in a co-expression module corresponding to lifetime ethanol consumption in humans (Farris et al., 2015).
Figure 2:
Location of Munc18–1 residues responsible for ethanol sensitivity. Asp216 and Ile133 shown in magenta interact with syntaxin and mutations at these sites in C. elegans make them resistant to the stimulatory and sedative effects of acute ethanol. Arg39 and Glu466 (465 in Unc18) shown in green interact with Rab3. In comparison to wild-type Unc18, both the R39C and E465K mutants show increased sensitivity to acute ethanol. The residues between Arg9 and Arg27 are represented as a dashed line to reflect the disorder in the crystal structure. The model was built using the crystal structure of the Munc18–1/syntaxin 1A complex (PDB ID: 3C98).
The Rab3 binding residues in Munc18 were also studied for their alcohol sensitivity in C. elegans (Johnson et al., 2013). Expressing the orthologous E466K mutation (Unc-18 E465K) enhanced alcohol sensitivity, which is independent of Rab3. On the other hand, Unc-18 R39C, which decreases syntaxin binding, enhanced sensitivity to alcohol in a Rab3-dependent manner. It was also shown that the overexpression of R39C could partially suppress the reduction in neurotransmitter release in Rab3 mutant worms, whereas wild-type or E465K mutants did not show such rescue (Figure 2). The conclusion was that the epistatic interactions between Unc18 and Rab3 in modulating sensitivity to alcohol are not similar to their interactions that affect neurotransmitter release (Johnson et al., 2013).
A significant increase in the levels of Munc18 was observed in the orbitofrontal cortical samples of adult male monkeys following long-term ethanol drinking (Nimitvilai et al., 2017).
In alcoholic pancreatitis, pre-incubation with ethanol enabled low-dose cholecystokinin to displace Munc18c from basolateral plasma membrane, leading to SNARE complex assembly in the basolateral plasma membrane (Lam et al., 2007). Further, it was shown that 20 mM ethanol or submaximal or supramaximal cholecystokinin stimulation caused PKCα-mediated activation of Munc18c and triggered pathologic basolateral exocytosis in pancreatic acinar cells (Cosen-Binker et al., 2007).
In summary, the D214N polymorphism in Munc18–1 is correlated with differential drinking behavior in B6 and D2 mice. The orthologous mutation in Unc-18 develops resistance to both stimulatory and sedative effects of ethanol in C. elegans. Several sites of interaction between Munc18 and Rab3 and between Munc18 and syntaxin that related to neurotransmitter release are not correlated with ethanol sensitivity. Ethanol drinking increases Munc18 protein levels in the orbitofrontal cortical region of monkey brain.
Munc13–1 and Munc13–2
Munc13–1 belongs to a family of evolutionarily-conserved presynaptic active zone proteins that are essential for vesicle fusion (Betz et al., 1997, Sassa et al., 1999) and neurotransmitter release (Betz et al., 1998, Brose et al., 2000, Varoqueaux et al., 2002) in mammals. Its orthologs in Drosophila and C. elegans are called Dunc13 and Unc13, respectively. Munc13–1 is essential for fusion competence of predominantly glutamatergic synaptic vesicles (Augustin et al., 1999). Glutamatergic neurons from Munc13–1 knockout mice show a 90% reduction in the readily releasable vesicle pool (RRP) and evoked transmitter release, even though they form an ultrastructurally normal number of synapses. This deficit of transmitter release competence in Munc13–1 knockout mice was due to a complete shutdown of the majority of glutamatergic synapses.
Munc13–1 protein interacts with both syntaxin and Munc18 proteins during synaptic vesicle fusion (Betz et al., 1997, Sassa et al., 1999). In addition to helping to open syntaxin 1, Munc13–1 also bridges synaptic vesicles and plasma membranes. Recent structure and function studies on Munc13–1 suggests that it is a master regulator of neurotransmitter release (Dittman, 2019).
Munc13–1 is a member of the Munc13 family of proteins. Munc13–2, Munc13–3 and Munc13–4 are other members known to date (Chen et al., 2013). Munc13–1 is expressed predominantly in the hippocampus, cerebellum, cortex, and striatum regions of rat brain (Augustin et al., 1999a) and modulates short-term presynaptic plasticity (Das et al., 2013, Lipstein et al., 2013) and long-term potentiation through its interactions with an active zone protein, RIM. On the other hand, Munc13–2 is expressed in rostral brain regions, including cerebral cortex and the CA region of the hippocampus. Munc13–3 is expressed exclusively in the cerebellum (Augustin et al., 1999a). Munc13–1 and 13–2 double- knockout mice result in complete abolishment of neurotransmitter release (Augustin et al., 1999, Varoqueaux et al., 2002, Aravamudan et al., 1999, Richmond et al., 1999).
Munc13–1 is a large peripheral membrane protein with a molecular weight of ~200 kDa. It consists of three C2 domains, one C1 domain, and a MUN domain. The high-affinity diacylglycerol (DAG)/phorbol ester-binding C1 domain is in between the N-terminal C2A domain and a Ca2+ binding C2 domain (C2B). The characteristic MUN domain connects the C2B and the C-terminal C2 domain (C2C) (Aravamudan et al., 1999, Sturm et al., 2013, Basu et al., 2005). DAG is the endogenous ligand for Munc13–1 and its binding to the C1 domain lowers the energy barrier for vesicle fusion, facilitating neurotransmitter release (Basu et al., 2007, Rhee et al., 2002). The H567K point mutation in the C1 domain abolishes the augmentation in vesicle fusion in mice and leads to death of the pups within hours after birth, despite no alteration in Ca2+-evoked neurotransmitter release. These observations illustrate the importance of the C1 domain in the regulation of release and survival (Rhee et al., 2002). DAG/phorbol ester binding to the Munc13–1 C1 domain is hindered by a tryptophan side chain that blocks the phorbol ester-binding site. This tryptophan residue modulates both phorbol ester and membrane binding (Das et al., 2018).
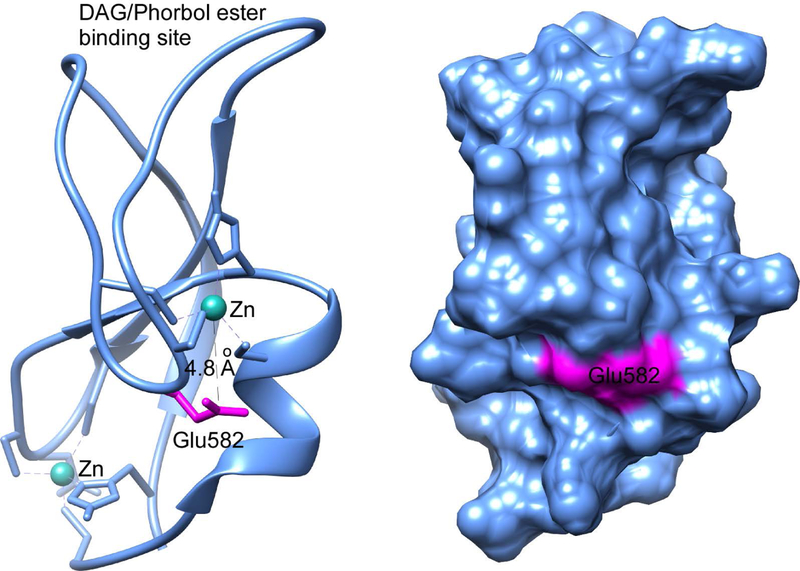
Munc13–1 binds to ethanol and deficits in Drosophila ortholog Dunc13 lead to defects in alcohol sensitivity, tolerance and self-administration in Drosophila (Das et al., 2013, Xu et al., 2018). Using a two-choice CAFÉ assay, it was shown that Dunc13P84200/+ heterozygotes display increased ethanol preference compared to the wild type. Synaptic vesicle release in excitatory neurons downstream of Ca2+ influx into the active zone in Drosophila is impaired by ethanol, and a reduction in Dunc13 in Dunc13P84200/+ produces resistance to the sedative effects of ethanol both behaviorally and physiologically. It was shown that the binding of ethanol to the C1 domain of Munc13–1 at intoxicating concentrations reduces the binding of its endogenous ligand, diacylglycerol (DAG), which will reduce the activity of Munc13–1 and should lead to presynaptic inhibition. The authors argued that this reduction of Munc13–1/Dunc13 activity will be similar to the genetic reduction of Dunc13, suggesting a possible role of Dunc13 in the development of ethanol tolerance (Xu et al., 2018). However, these effects were not observed in mice that were treated with ethanol using the drinking-in-the-dark paradigm (Wooden et al., 2019), suggesting that the effects of ethanol on Munc13–1/Dunc13 were species-dependent. Alcohols bind to the Glu-582 residue of the C1 domain of Munc13–1 (Figure 3). Glutamic acid is also known to bind alcohol in proteins, such as Glu-262 in the acetylcholine receptor (Pratt et al., 2000); Glu-33 in the L1 cell adhesion molecule (Arevalo et al., 2008); Glu-163 and Glu-193 in the Rho GDP dissociation inhibitor (Ho et al., 2008); Glu-146 in lignin peroxidase (Ambert-Balay et al., 1998); and Glu-13 in pepsin (Andreeva et al., 1984). The glutamate residue is likely to form a hydrogen bond with the ethanol molecule and mutating this residue with alanine reduces the alcohol binding (Das et al., 2013, Xu et al., 2018). Structural studies of the LUSH protein–alcohol complex highlighted the importance of hydrogen bonding between the alcohol molecule and the alcohol binding residue. In LUSH, the odorant-binding protein in Drosophila, the hydroxyl group of alcohol forms hydrogen bonds with Thr-57, the replacement of which by alanine completely abolished alcohol binding (Kruse et al., 2003, Thode et al., 2008). The alcohol-binding residue in PKCδ C1B, a structural homolog of Munc13–1 C1 is Tyr-236 (Das et al., 2004, Shanmugasundararaj et al., 2012).
Figure 3:
Location of the alcohol binding residue in Munc13–1 C1. Photolabeling and mass spectrometric analysis identified Glu582 as the alcohol-binding residue, which is shown in magenta. The ribbon (left) and surface (right) diagrams were generated using the NMR structure of Munc13–1 C1 (PDB ID: 1Y8F).
In Munc13–1 C1, the Glu-582 residue is about 4.8Å from the nearest Zn2+ that coordinates with three cysteine and one histidine residues, indicating a possible role of Zn2+ in the alcohol binding to this protein (Figure 3). It has been demonstrated that 25 mM ethanol inhibited binding of DAG to the Munc13–1 C1 domain in the presence of 5 mM ZnSO4. A similar role of Zn2+ has also been implicated in the alcohol binding for GABA (Aguayo and Alarcon, 1993, Frye et al., 1996) and Glycine receptors (Laube et al., 2000, McCracken et al., 2010, McCracken et al., 2013b, McCracken et al., 2013a).
The McCool group studied the role of Munc13–2 on ethanol-anxiety interaction by measuring glutamate release in the basolateral amygdala, which contributes to both the anxiolytic effect of ethanol intoxication and the anxiogenic effects of ethanol withdrawal. In comparing two different strains of mice, first they found that C57BL/6J mice expressed substantially higher levels of Munc13–2 as compared with the DBA/2J strain, whereas expression of several release-related proteins, including Munc13–1, was equivalent (Gioia et al., 2016). Using shRNA, they knocked down the expression of Munc13–2 in the mPFC terminal within the BLA of C57BL/6J, whose glutamate terminals are normally ethanol-insensitive. This manipulation made the glutamate terminals ethanol-sensitive, which is generally seen for the glutamate terminals of DBA/2J mice. Ethanol inhibition of vesicle recycling, releasable pool recovery and post-tetanic potentiation following high-frequency stimulation were dependent on the levels of Munc13–2, suggesting that Munc13–2 is involved in the ethanol-anxiety interactions (Gioia et al., 2017).
In a drinking-in-the-dark paradigm, Munc13–1 was found to be upregulated in the hippocampus, which is associated with memory (Ghosh et al., 2017). Recent proteomic analysis studies found a significant decrease in Munc13–2 in the lateral amygdala of macaque with a history of heavy drinking (Alexander et al., 2018).
The effect of general anesthetic, isoflurane on Unc13 was studied using locomotion assays and global level of neurotransmitter assays on adult C. elegans. It was found that unc-13 loss-of-function mutants were highly resistant to general anesthetic isoflurane. It was also found that isoflurane decreased DAG-mediated synaptic localization of Unc13 (Metz et al., 2007).
In summary, alcohol binds to the C1 domain of Munc13–1. Reduction of Dunc13 increases ethanol self-administration and develops tolerance in Drosophila. Reduction of munc13–1 in mice, however did not affect these behaviors. In C. elegans, Unc13 mediates the action of general anesthetics.
Complexin 2
Complexins are small, soluble proteins that play major roles in neurotransmitter release (McMahon et al., 1995). A marked decrease in evoked release was observed upon reducing the levels of complexin 1 and complexin 2, the two major mammalian isoforms (Huntwork and Littleton, 2007, Martin et al., 2011, Maximov et al., 2009, Reim et al., 2001). Also, a decrease in vesicle priming was observed upon knockout or knockdown of these two isoforms (Xue et al., 2010, Yang et al., 2010). Complexin’s role has also been implicated in the etiology or pathogenesis of several CNS diseases, such as schizophrenia, Huntington’s disease, depression, bipolar disorder, Parkinson’s disease, Alzheimer’s disease, and also in fetal alcohol syndrome disorders (Brose, 2008).
Proteomic analysis suggests a significant decrease in complexin 2 in the lateral amygdala of a male macaque with a history of very heavy drinking (Alexander et al., 2018). A long-term ethanol self-administration method of drinking was used in this study (Grant et al., 2008).
Synaptotagmin 1
Synaptotagmin 1 is a member of the synaptotagmin family of proteins and acts as a Ca2+ sensor in synaptic exocytosis and other types of Ca2+-evoked secretion (Brose et al., 1992). There are fifteen members in this family. Synaptotagmin 1 is expressed in the forebrain, midbrain, and in most brainstem and spinal cord neurons (Xu et al., 2007). Impairment in the fast synchronous component of evoked excitatory postsynaptic currents (EPSCs) was observed in hippocampal neurons of synaptotagmin 1-deficient mice (Geppert et al., 1994b). However, the reduction of inhibitory post synaptic currents (IPSCs) was observed in cortical neurons of the same animals (Xu et al., 2007). In mouse hippocampal cultures, overexpression of synaptotagmin 1 increases the probability of evoked vesicle release (Han et al., 2004). Synaptotagmin has been shown to be a novel biomarker for Alzheimer’s disease (Ohrfelt et al., 2016).
In a microarray screen, Syt1, encoding synaptotagmin 1 protein, was identified as an alcohol-responsive gene (Pignataro et al., 2007). Ethanol induces synaptotagmin 1 expression in mouse neurons via activation of heat shock factor 1 (Varodayan et al., 2011). Cortical neurons were exposed to varying concentrations of ethanol for a specified time period (15 min-24 h) and upregulation of both mRNA and protein was observed in a rapid and robust manner. Ethanol also altered the distribution of the synaptotagmin 1 protein along neuronal processes as shown by the increase in the number and size of the synaptotagmin 1-positive puncta per 100 μm of neurite length as compared to the control. The authors concluded that this may be a mechanism by which ethanol could affect neurotransmitter release.
Synapsin
Synapsins are phosphoproteins that bind to synaptic vesicles and to actin. They also bind to ATP, and show structural similarity to ATP-utilizing enzymes (Song and Augustine, 2015, Hilfiker et al., 1999). The mammalian genome contains three synapsin genes encoding synapsin I, synapsin II and synapsin III, each having two different isoforms. Drosophila, however, have a single copy of the gene. Numerous in vitro studies suggest synapsins’ involvements in several neuronal processes, such as neurite elongation, synaptogenesis, synaptic maturation, regulation of synaptic plasticity and neurotransmitter release (Ferreira et al., 1995, Han and Greengard, 1994).
Using a Drosophila knockout for all synapsin isoforms, the role of these proteins have been implicated in the development of tolerance to ethanol (Godenschwege et al., 2004). In this study, flies were exposed to 50% ethanol vapor in a vertical tube until the loss of postural control was observed. Initially, both the knock-outs and the wild-type flies displayed similar sensitivity to ethanol. However, upon a second exposure to intoxicating levels of ethanol, the knock-outs of synapsins took longer time for the loss of postural control, suggesting the role of synapsins in ethanol tolerance.
Dynamin
Dynamin is a ~100 kDa GTPase responsible for endocytosis in the eukaryotic cell (Jimah and Hinshaw, 2018) and is implicated in membrane vesicle scission including synaptic vesicle recycling (Praefcke and McMahon, 2004). During clathrin-mediated endocytosis, dynamin binds to and assembles around the neck of the endocytic vesicle (Morlot and Roux, 2013). In mammals, three different dynamins are known to date. While Dynamin-2 is ubiquitous, Dynamin-1 is predominantly expressed in the presynaptic compartment of the neurons. Dynamin-3, on the other hand, is expressed in the postsynaptic compartment and in testicular tissue.
Microarray analysis of 5000 genes in the dorsal hippocampus of rats treated with 12% ethanol (v/v) for fifteen months showed down-regulation of the dynamin-1 gene (Saito et al., 2002). In Drosophila, it has been found that functional dynamin protein activity is required for acquisition of tolerance during ethanol intoxication, although ethanol did not alter the expression level of the single copy of the dynamin gene, shibire (Krishnan et al., 2012).
Summary and future perspectives
Ethanol’s impact on the proteins involved in the neurotransmitter release machinery has been summarized in Table 1 and the key points are: (1) syntaxin 1A, Dunc13, synapsins and Dynamin 1 contribute to the development of tolerance. (2) protein-protein interactions involving syntaxin, Unc18 and Rab3 are important for ethanol sensitivity. (3) Syntaxin 1A, Dunc13, Rab3 and Munc18 mediate alcohol drinking behavior. (4) Ethanol upregulates expression of syntaxin 1b, synaptophysin 1, SNAP-25, synaptotagmin 1, Munc13–1, Vamp-2 and Munc18 either at the mRNA or protein level. (5) Ethanol downregulates the expression of Rab3b, Rab3c, Rab6, Rab11, Munc13–2 and complexin 2 either at the mRNA or protein level. (6) Ethanol’s effect on protein expression are brain region- and gender-specific. (7) Ethanol sensitivity is species-dependent. (8) Ethanol’s effects are protein isoform-specific, as in Munc13–1/2 and VAMP-1/2.
Table 1:
Effect of ethanol/anesthetics on the SNARE complex associated proteins
| SNARE Protein | Gene | Alcohol/anesthetic | Mode of action | Reference |
|---|---|---|---|---|
| Syntaxin 1A/Syntaxin 12 | Syntaxin 1A | Ethanol | Tolerance to ethanol in Drosophila | Krishnan et al., 2012 |
| Stx12 | Ethanol | Ethanol preference in mice | Treadwell et al., 2004; Weng et al., 2009 | |
| Stx12 | Ethanol | Protein expression level in mice | Weng et al., 2009 | |
| Unc-64 | Isoflurane and halothane | Resistance to anesthesia in C. elegans | van Swinderen et al., 1999 | |
| Syntaxin 1A | Isoflurane | Resistance to anesthesia in Drosophila | Zalucki et al., 2015 | |
| Stx1a | Isoflurane and halothane | In vitro protein binding | Nagele et al., 2005 | |
| SNAP-25 | Snap25 | Ethanol | Protein expression in monkey brain | Nimitvilai et al., 2017; Alexander et al., 2018 |
| Snap25 | Ethanol | Gene expression in mice neuron | Varodayan et al., 2011 | |
| Snap25 | Isoflurane | Protein expression and neurotransmitter release in PC12 cells | Xie et al., 2013 | |
| Isoflurane | In vitro protein binding | Nagele et al., 2005 | ||
| VAMP2 (Synaptobrevin) | Vamp2 | Ethanol | Protein and gene expression level in mice cortical neuron | Varodayan et al., 2011; Varodayan and Harrison, 2013 |
| Rab3 | RAB3C | Ethanol | Protein expression in monkey brain | Alexander et al., 2018 |
| rab-3 | Ethanol | Changes in locomotion in C. elegans | Kapfhamer et al., 2008; Johnson et al., 2013; Davies et al., 2012 | |
| RAB3A | Ethanol | Drinking behavior in mice | Kapfhamer et al., 2008 | |
| rab-3 | Halothane | Changes in locomotion in C. elegans | Davies et al., 2012 | |
| Unc18/Munc18 | unc-18 | Ethanol | Protein-protein interaction in behavioral changes in C. elegans | Graham et al., 2009; Johnson et al., 2013 |
| Stxbp1 | Ethanol | Changes in gene expression in mice | Fehr et al., 2005 | |
| Munc-18 | Ethanol | Changes in protein expression in monkey brain | Nimitvilai et al., 2017 | |
| Unc13/Dunc13/Munc13 | unc-13 | Isoflurane | Resistance to anesthesia and synaptic localization in C. elegans | Metz et al., 2007 |
| Dunc13 | Ethanol | Changes in ethanol preference and tolerance in Drosophila | Das et al., 2013; Xu et al., 2018 | |
| munc13–1 | Ethanol | Protein expression in mice brain | Ghosh et al., 2017 | |
| munc13–1 | Ethanol, butanol, octanol | In vitro protein binding | Das et al., 2013; Xu et al., 2018 | |
| munc13–2 | Ethanol | Protein expression in monkey brain | Alexander et al., 2018 | |
| Complexin 2 | CPLX2 | Ethanol | Protein expression in monkey brain | Alexander et al., 2018 |
| Synaptotagmin 1 | Syt1 | Ethanol | Changes in gene expression in mice | Pignataro et al., 2007 |
| Syt1 | Ethanol | Change in gene and protein expression in mice | Varodayan et al., 2011 | |
| Synapsin | Syn | Ethanol | Tolerance to ethanol in Drosophila | Godenschwege et al., 2004 |
| Dynamin 1 | shibire | Ethanol | Tolerance to ethanol in Drosophila | Krishnan et al., 2012 |
Now, based on these findings, will it be possible to draw some sort of general principles on ethanol’s effect on presynaptic function? Clearly, ethanol has effects (expression, localization and function) on several proteins involved in vesicle priming, release and recycling. However, the major issue here is the heterogeneity of the data, meaning that some effects are species-specific, gender-specific, brain region-specific or protein isoform-specific. For example, while Dunc13 heterozygous flies showed higher ethanol preference as compared to wild-type, there were no differences in drinking preference between Munc13–1 heterozygous and wild-type mice. For Dynamin-1, ethanol down-regulated the gene in rats, but it did not do so in flies. For Rab3, reduction of the protein increased ethanol sensitivity in rab-3 heterozygous C. elegans and increased ethanol preference in heterozygous mice, but not in homozygous mice. At the level of protein-protein interactions, both E466K and R39C mutants of Unc18 that affect Rab3 binding are ethanol-sensitive. Whereas ethanol sensitivity in E466K is independent of Rab3, ethanol sensitivity in the R39C requires Rab3. Therefore, ethanol’s effect on presynaptic function at an organismal level is complex and will reflect the time-dependent integration of all these effects. In mammals, added complexities arise from gender-differences and the presence of multiple isoforms of a single protein having differential effects. However, the identification of genes important for ethanol responses should be the focus of newer studies, rather than the directionality or magnitude of an ethanol phenotype.
Studies on the structure and function of the exocytosis machinery proteins by itself is a fascinating area of current research, wherein the exact role of some of these proteins are still under investigation. Elucidating the impact of ethanol on these proteins bears additional significance because it may provide a mechanism by which a relatively low affinity drug molecule like ethanol could bring about changes in the neurotransmitter concentrations in the presynaptic compartment, leading to the changes in the brain and behavior. The microarray analysis identified some of the alcohol-sensitive genes; proteomic analysis identified expression levels of some of these proteins; electrophysiology measurements quantified the changes in neurotransmitter release; mutational analysis provided important information on the protein-protein interactions; and knockouts were used for studying the behavioral changes. While these studies establish ethanol’s role in the neurotransmitter release in the presynaptic compartments, literature data are very diverse, sketchy and piecemeal. Therefore, more systematic studies are required to understand the mechanism of ethanol’s action in a region-specific, neuron-specific, and neuro-circuitry-specific manner in the brain. Most of the studies reported in this article are based on invertebrate model systems, such as Drosophila and C. elegans. It is critical to conduct these experiments in the vertebrate system, in a gender-specific manner, to correlate the findings from model systems to human alcoholism. The binding affinity of ethanol for the proteins that directly bind it, should be measured in isolation and in the milieu (in association with other components of the SNARE complex), which will provide insights as to how protein-protein interaction can modulate alcohol affinity. Some of these proteins are shown to exist in different isoforms and each isoform should be studied for its role in ethanol action. Furthermore, elucidating the structure of the ethanol-protein complex can provide useful knowledge on the microenvironment of the alcohol binding site that could be useful in designing drugs for alcohol addiction.
Acknowledgements
This research has been supported by funding from National Institutes of Health Grant 1R01 AA022414–01A1 to JD. JD thanks Youngki You for assistance with Figure 1 and Courtney Hunt, Ph.D. for editing the manuscript.
Abbreviations:
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- ATP
adenosine triphosphate
- BLA
basolateral amygdala
- CA
Cornu Ammonis
- CD
circular dichroism
- DAG
diacylglycerol
- DoC2
double C2 domain protein
- EPSC
excitatory postsynaptic current
- FRET
fluorescence resonance energy transfer
- FSH
follicle stimulating hormone
- GABA
gamma amino butyric acid
- GTP
guanosine triphosphate
- H3
syntaxin SNARE domain
- Habc
syntaxin regulatory domain
- Hsc70
heat shock 70 kDa protein 8
- IPSC
inhibitory postsynaptic current
- LH
luteinizing hormone
- NMR
nuclear magnetic resonance
- PKC
protein kinase C
- PFC
prefrontal cortex
- SNARE
soluble N-ethylmaleimide-sensitive fusion protein attachment protein receptor
- v-SNARE
vesicle SNARE
- t-SNARE
target SNARE
- NSF
N-ethylmaleimide sensitive fusion protein
- SNAP
soluble N-ethylmaleimide sensitive fusion protein attachment protein
- SNAP-25
synaptosomal-associated protein of 25 kDa molecular mass
- SNAP-23
synaptosomal-associated protein of 23 kDa molecular mass
- SM
Sec1/Munc-18 proteins
- RRP
readily-releasable pool of vesicles
- TMD
trans membrane domain
Footnotes
Conflict of interest statement
Nothing declared.
References
- Abrahao KP, Salinas AG, Lovinger DM (2017) Alcohol and the Brain: Neuronal Molecular Targets, Synapses, and Circuits. Neuron 96:1223–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguayo LG, Alarcon JM (1993) Modulation of the developing rat sympathetic GABAA receptor by Zn++, benzodiazepines, barbiturates and ethanol. J Pharmacol Exp Ther 267:1414–1422. [PubMed] [Google Scholar]
- Alexander NJ, Rau AR, Jimenez VA, Daunais JB, Grant KA, McCool BA (2018) SNARE Complex-Associated Proteins in the Lateral Amygdala of Macaca mulatta Following Long-Term Ethanol Drinking. Alcohol Clin Exp Res 42:1661–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambert-Balay K, Fuchs SM, Tien M (1998) Identification of the veratryl alcohol binding site in lignin peroxidase by site-directed mutagenesis. Biochem Biophys Res Commun 251:283–286. [DOI] [PubMed] [Google Scholar]
- Andreeva NS, Zdanov AS, Gustchina AE, Fedorov AA (1984) Structure of ethanol-inhibited porcine pepsin at 2-A resolution and binding of the methyl ester of phenylalanyl-diiodotyrosine to the enzyme. J Biol Chem 259:11353–11365. [PubMed] [Google Scholar]
- Aravamudan B, Fergestad T, Davis WS, Rodesch CK, Broadie K (1999) Drosophila UNC-13 is essential for synaptic transmission. Nat Neurosci 2:965–971. [DOI] [PubMed] [Google Scholar]
- Arevalo E, Shanmugasundararaj S, Wilkemeyer MF, Dou X, Chen S, Charness ME, Miller KW (2008) An alcohol binding site on the neural cell adhesion molecule L1. Proc Natl Acad Sci USA 105:371–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustin I, Rosenmund C, Sudhof TC, Brose N (1999) Munc13–1 is essential for fusion competence of glutamatergic synaptic vesicles. Nature 400:457–461. [DOI] [PubMed] [Google Scholar]
- Augustin I, Andrea B, Herrmann C, Tobias J, Brose N (1999a) Differential expression of two novel Munc13 proteins in rat brain. Biochem J 337:363–371. [PMC free article] [PubMed] [Google Scholar]
- Barclay JW, Graham ME, Edwards MR, Johnson JR, Morgan A, Burgoyne RD (2010) Presynaptic targets for acute ethanol sensitivity. Biochem Soc Trans 38:172–176. [DOI] [PubMed] [Google Scholar]
- Basu J, Betz A, Brose N, Rosenmund C (2007) Munc13–1 C1 domain activation lowers the energy barrier for synaptic vesicle fusion. J Neurosci 27:1200–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu J, Shen N, Dulubova I, Lu J, Guan R, Guryev O, Grishin NV, Rosenmund C, Rizo J (2005) A minimal domain responsible for Munc13 activity. Nat Struct Mol Biol 12:1017. [DOI] [PubMed] [Google Scholar]
- Betz A, Ashery U, Rickmann M, Augustin I, Neher E, Sudhof TC, Rettig J, Brose N (1998) Munc13–1 is a presynaptic phorbol ester receptor that enhances neurotransmitter release. Neuron 21:123–136. [DOI] [PubMed] [Google Scholar]
- Betz A, Okamoto M, Benseler F, Brose N (1997) Direct interaction of the rat unc-13 homologue Munc13–1 with the N terminus of syntaxin. The Journal of biological chemistry 272:2520–2526. [DOI] [PubMed] [Google Scholar]
- Brose N (2008) Altered complexin expression in psychiatric and neurological disorders: cause or consequence? Mol Cells 25:7–19. [PubMed] [Google Scholar]
- Brose N, Petrenko AG, Sudhof TC, Jahn R (1992) Synaptotagmin: a calcium sensor on the synaptic vesicle surface. Science 256:1021–1025. [DOI] [PubMed] [Google Scholar]
- Brose N, Rosenmund C, Rettig J (2000) Regulation of transmitter release by Unc-13 and its homologues. Curr Opin Neurobiol 10:303–311. [DOI] [PubMed] [Google Scholar]
- Carr CM, Rizo J (2010) At the junction of SNARE and SM protein function. Curr Opin iCell Biol 22:488–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Cooper B, Kalla S, Varoqueaux F, Young SM Jr. (2013) The Munc13 proteins differentially regulate readily releasable pool dynamics and calcium-dependent recovery at a central synapse. J Neurosci 33:8336–8351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corradini I, Verderio C, Sala M, Wilson MC, Matteoli M (2009) SNAP-25 in neuropsychiatric disorders. Ann N Y Acad Sci 1152:93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosen-Binker LI, Binker MG, Wang CC, Hong W, Gaisano HY (2008) VAMP8 is the v-SNARE that mediates basolateral exocytosis in a mouse model of alcoholic pancreatitis. J Clin Invest 118:2535–2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosen-Binker LI, Lam PP, Binker MG, Reeve J, Pandol S, Gaisano HY (2007) Alcohol/cholecystokinin-evoked pancreatic acinar basolateral exocytosis is mediated by protein kinase C alpha phosphorylation of Munc18c. J Biol Chem 282:13047–13058. [DOI] [PubMed] [Google Scholar]
- Das J, Addona GH, Sandberg WS, Husain SS, Stehle T, Miller KW (2004) Identification of a general anesthetic binding site in the diacylglycerol-binding domain of protein kinase Cdelta. J Biol Chem 279:37964–37972. [DOI] [PubMed] [Google Scholar]
- Das J, Kedei N, Kelsey JS, You Y, Pany S, Mitchell GA, Lewin NE, Blumberg PM (2018) Critical Role of Trp-588 of Presynaptic Munc13–1 for Ligand Binding and Membrane Translocation. Biochemistry 57:732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das J, Xu S, Pany S, Guillory A, Shah V, Roman GW (2013) The pre-synaptic Munc13–1 binds alcohol and modulates alcohol self-administration in Drosophila. J Neurochem. 126:715–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies AG, Friedberg RI, Gupta H, Chan CL, Shelton KL, Bettinger JC (2012) Different genes influence toluene- and ethanol-induced locomotor impairment in C. elegans. Drug Alcohol Depend 122:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond I, Gordon AS (1997) Cellular and molecular neuroscience of alcoholism. Physiol Rev 77:1–20. [DOI] [PubMed] [Google Scholar]
- Dittman JS (2019) Unc13: a multifunctional synaptic marvel. Current opinion in neurobiology 57:17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulubova I, Khvotchev M, Liu S, Huryeva I, Sudhof TC, Rizo J (2007) Munc18–1 binds directly to the neuronal SNARE complex. Proc Natl Acad Sci U S A 104:2697–2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris SP, Arasappan D, Hunicke-Smith S, Harris RA, Mayfield RD (2015) Transcriptome organization for chronic alcohol abuse in human brain. Mol Psychiatry 20:1438–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr C, Shirley RL, Crabbe JC, Belknap JK, Buck KJ, Phillips TJ (2005) The syntaxin binding protein 1 gene (Stxbp1) is a candidate for an ethanol preference drinking locus on mouse chromosome 2. Alcohol Clin Exp Res 29:708–720. [DOI] [PubMed] [Google Scholar]
- Fernandez I, Ubach J, Dulubova I, Zhang X, Sudhof TC, Rizo J (1998) Three-dimensional structure of an evolutionarily conserved N-terminal domain of syntaxin 1A. Cell 94:841–849. [DOI] [PubMed] [Google Scholar]
- Ferreira A, Han HQ, Greengard P, Kosik KS (1995) Suppression of synapsin II inhibits the formation and maintenance of synapses in hippocampal culture. Proc Natl Acad Sci U S A 92:9225–9229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks NP, Lieb WR (1984) Do general anaesthetics act by competitive binding to specific receptors? Nature 310:599–601. [DOI] [PubMed] [Google Scholar]
- Franks NP, Lieb WR (1985) Mapping of general anaesthetic target sites provides a molecular basis for cutoff effects. Nature 316:349–351. [DOI] [PubMed] [Google Scholar]
- Frye GD, Fincher AS, Grover CA, Jayaprabhu S (1996) Lanthanum and zinc sensitivity of GABAA-activated currents in adult medial septum/diagonal band neurons from ethanol dependent rats. Brain Res 720:101–110. [DOI] [PubMed] [Google Scholar]
- Fukuda M (2008) Regulation of secretory vesicle traffic by Rab small GTPases. Cell Mol Life Sci 65:2801–2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geppert M, Bolshakov VY, Siegelbaum SA, Takei K, De Camilli P, Hammer RE, Sudhof TC (1994a) The role of Rab3A in neurotransmitter release. Nature 369:493–497. [DOI] [PubMed] [Google Scholar]
- Geppert M, Goda Y, Hammer RE, Li C, Rosahl TW, Stevens CF, Sudhof TC (1994b) Synaptotagmin I: a major Ca2+ sensor for transmitter release at a central synapse. Cell 79:717–727. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Wooden J, Leasure J, Das J (2017) Ethanol upregulates active zone protein Munc13–1: A possible implication in presynaptic physiology and alcoholism Alcohol Clin Ex Res 41S, pp 24A–24A. [Google Scholar]
- Gioia DA, Alexander N, McCool BA (2017) Ethanol Mediated Inhibition of Synaptic Vesicle Recycling at Amygdala Glutamate Synapses Is Dependent upon Munc13–2. Frontiers in neuroscience 11:424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioia DA, Alexander NJ, McCool BA (2016) Differential Expression of Munc13–2 Produces Unique Synaptic Phenotypes in the Basolateral Amygdala of C57BL/6J and DBA/2J Mice. J Neurosci 36:10964–10977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godenschwege TA, Reisch D, Diegelmann S, Eberle K, Funk N, Heisenberg M, Hoppe V, Hoppe J, Klagges BR, Martin JR, Nikitina EA, Putz G, Reifegerste R, Reisch N, Rister J, Schaupp M, Scholz H, Schwarzel M, Werner U, Zars TD, Buchner S, Buchner E (2004) Flies lacking all synapsins are unexpectedly healthy but are impaired in complex behaviour. Eur J Neurosci 20:611–622. [DOI] [PubMed] [Google Scholar]
- Graham ME, Edwards MR, Holden-Dye L, Morgan A, Burgoyne RD, Barclay JW (2009) UNC-18 modulates ethanol sensitivity in Caenorhabditis elegans. Molecular biology of the cell 20:43–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant KA, Leng X, Green HL, Szeliga KT, Rogers LS, Gonzales SW (2008) Drinking typography established by scheduled induction predicts chronic heavy drinking in a monkey model of ethanol self-administration. Alcohol Clin Exp Res 32:1824–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groffen AJ, Martens S, Diez Arazola R, Cornelisse LN, Lozovaya N, de Jong AP, Goriounova NA, Habets RL, Takai Y, Borst JG, Brose N, McMahon HT, Verhage M (2010) Doc2b is a high-affinity Ca2+ sensor for spontaneous neurotransmitter release. Science 327:1614–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosshans BL, Ortiz D, Novick P (2006) Rabs and their effectors: achieving specificity in membrane traffic. Proc Natl Acad Sci U S A 103:11821–11827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han HQ, Greengard P (1994) Remodeling of cytoskeletal architecture of nonneuronal cells induced by synapsin. Proc Natl Acad Sci U S A 91:8557–8561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han W, Rhee JS, Maximov A, Lao Y, Mashimo T, Rosenmund C, Sudhof TC (2004) N-glycosylation is essential for vesicular targeting of synaptotagmin 1. Neuron 41:85–99. [DOI] [PubMed] [Google Scholar]
- Harris RA, Trudell JR, Mihic SJ (2008) Ethanol’s molecular targets. Science signaling 1:re7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawasli AH, Saifee O, Liu C, Nonet ML, Crowder CM (2004) Resistance to volatile anesthetics by mutations enhancing excitatory neurotransmitter release in Caenorhabditis elegans. Genetics 168:831–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksson R, Kuzmin A, Okvist A, Harper C, Sheedy D, Garrick T, Yakovleva T, Bakalkin G (2008) Elevated synaptophysin I in the prefrontal cortex of human chronic alcoholics. Synapse 62:829–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring BE, Xie Z, Marks J, Fox AP (2009) Isoflurane inhibits the neurotransmitter release machinery. J Neurophysiol 102:1265–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilfiker S, Pieribone VA, Czernik AJ, Kao HT, Augustine GJ, Greengard P (1999) Synapsins as regulators of neurotransmitter release. Philos Trans R Soc Lond. B, Biol Sci 354:269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho C, Shanmugasundararaj S, Miller KW, Malinowski SA, Cook AC, Slater SJ (2008) Interaction of anesthetics with the Rho GTPase regulator Rho GDP dissociation inhibitor. Biochemistry 47:9540–9552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homanics GE, Xu Y, Tang P (2002) Integrated approaches to the action of general anesthetics and alcohol. Physiol Behav 77:495–499. [DOI] [PubMed] [Google Scholar]
- Howard RJ, Slesinger PA, Davies DL, Das J, Trudell JR, Harris RA (2011) Alcohol-binding sites in distinct brain proteins: the quest for atomic level resolution. Alcohol Clin Exp Res 35:1561–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui E, Gaffaney JD, Wang Z, Johnson CP, Evans CS, Chapman ER (2011) Mechanism and function of synaptotagmin-mediated membrane apposition. Nat Struct Mol Biol 18:813–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntwork S, Littleton JT (2007) A complexin fusion clamp regulates spontaneous neurotransmitter release and synaptic growth. Nat Neurosci 10:1235–1237. [DOI] [PubMed] [Google Scholar]
- Hutagalung AH, Novick PJ (2011) Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev 91:119–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimah JR, Hinshaw JE (2019) Structural insights into the mechanism of dynamin superfamily proteins. Trends Cell Biol 29:257–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JR, Kashyap S, Rankin K, Barclay JW (2013) Rab-3 and unc-18 interactions in alcohol sensitivity are distinct from synaptic transmission. PloS one 8:e81117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeser PS, Deng L, Wang Y, Dulubova I, Liu X, Rizo J, Sudhof TC (2011) RIM proteins tether Ca2+ channels to presynaptic active zones via a direct PDZ-domain interaction. Cell 144:282–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapfhamer D, Bettinger JC, Davies AG, Eastman CL, Smail EA, Heberlein U, McIntire SL (2008) Loss of RAB-3/A in Caenorhabditis elegans and the mouse affects behavioral response to ethanol. Gene Brain Behav 7:669–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khvotchev M, Dulubova I, Sun J, Dai H, Rizo J, Sudhof TC (2007) Dual modes of Munc18–1/SNARE interactions are coupled by functionally critical binding to syntaxin-1 N terminus. J Neurosci 27:12147–12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan HR, Al-Hasan YM, Pohl JB, Ghezzi A, Atkinson NS (2012) A role for dynamin in triggering ethanol tolerance. Alcoh Clin Exp Res 36:24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse SW, Zhao R, Smith DP, Jones DN (2003) Structure of a specific alcohol-binding site defined by the odorant binding protein LUSH from Drosophila melanogaster. Nat Struct Biol 10:694–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam PP, Cosen Binker LI, Lugea A, Pandol SJ, Gaisano HY (2007) Alcohol redirects CCK-mediated apical exocytosis to the acinar basolateral membrane in alcoholic pancreatitis. Traffic 8:605–617. [DOI] [PubMed] [Google Scholar]
- Larkin JM, Oswald B, McNiven MA (1996) Ethanol-induced retention of nascent proteins in rat hepatocytes is accompanied by altered distribution of the small GTP-binding protein rab2. J Clin Invest 98:2146–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laube B, Kuhse J, Betz H (2000) Kinetic and mutational analysis of Zn2+ modulation of recombinant human inhibitory glycine receptors. J Physiol 522 Pt 2:215–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Valtschanoff JG, Kharazia VN, Weinberg R, Sheng M (2001) Biochemical and morphological characterization of an intracellular membrane compartment containing AMPA receptors. Neuropharmacol 41:680–692. [DOI] [PubMed] [Google Scholar]
- Lipstein N, Sakaba T, Cooper BH, Lin KH, Strenzke N, Ashery U, Rhee JS, Taschenberger H, Neher E, Brose N (2013) Dynamic control of synaptic vesicle replenishment and short-term plasticity by Ca(2+)-calmodulin-Munc13–1 signaling. Neuron 79:82–96. [DOI] [PubMed] [Google Scholar]
- Liu Y, Hunt WA (1999) The Drunken Synapse: Studies of alcohol related disorders, in Series The Drunken Synapse: Studies of alcohol related disorders, Klewer Academic/Plenum Publishing Corp., New York. [Google Scholar]
- Lovinger DM, Roberto M (2013) Synaptic effects induced by alcohol. Curr Top Behav Neurosci 13:31–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JA, Hu Z, Fenz KM, Fernandez J, Dittman JS (2011) Complexin has opposite effects on two modes of synaptic vesicle fusion. Curr Biol 21:97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maximov A, Tang J, Yang X, Pang ZP, Sudhof TC (2009) Complexin controls the force transfer from SNARE complexes to membranes in fusion. Science 323:516–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCool BA (2011) Ethanol modulation of synaptic plasticity. Neuropharmacol 61:1097–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken LM, Blednov YA, Trudell JR, Benavidez JM, Betz H, Harris RA (2013a) Mutation of a zinc-binding residue in the glycine receptor alpha1 subunit changes ethanol sensitivity in vitro and alcohol consumption in vivo. J Pharmacol Exp Ther 344:489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken LM, Trudell JR, Goldstein BE, Harris RA, Mihic SJ (2010) Zinc enhances ethanol modulation of the alpha1 glycine receptor. Neuropharmacol 58:676–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken LM, Trudell JR, McCracken ML, Harris RA (2013b) Zinc-dependent modulation of alpha2- and alpha3-glycine receptor subunits by ethanol. Alcohol Clin Exp Res 37:2002–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon HT, Missler M, Li C, Sudhof TC (1995) Complexins: cytosolic proteins that regulate SNAP receptor function. Cell 83:111–119. [DOI] [PubMed] [Google Scholar]
- Metz LB, Dasgupta N, Liu C, Hunt SJ, Crowder CM (2007) An evolutionarily conserved presynaptic protein is required for isoflurane sensitivity in Caenorhabditis elegans. Anesthesiology 107:971–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misura KM, Scheller RH, Weis WI (2000) Three-dimensional structure of the neuronal-Sec1-syntaxin 1a complex. Nature 404:355–362. [DOI] [PubMed] [Google Scholar]
- Morlot S, Roux A (2013) Mechanics of dynamin-mediated membrane fission. Annu Rev Biophys 42:629–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagele P, Mendel JB, Placzek WJ, Scott BA, D’Avignon DA, Crowder CM (2005) Volatile anesthetics bind rat synaptic snare proteins. Anesthesiology 103:768–778. [DOI] [PubMed] [Google Scholar]
- Nie Z, Schweitzer P, Roberts AJ, Madamba SG, Moore SD, Siggins GR (2004) Ethanol augments GABAergic transmission in the central amygdala via CRF1 receptors. Science 303:1512–1514. [DOI] [PubMed] [Google Scholar]
- Nimitvilai S, Uys JD, Woodward JJ, Randall PK, Ball LE, Williams RW, Jones BC, Lu L, Grant KA, Mulholland PJ (2017) Orbitofrontal Neuroadaptations and Cross-Species Synaptic Biomarkers in Heavy-Drinking Macaques. J Neurosci 37:3646–3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noor A, Zahid S (2017) A review of the role of synaptosomal-associated protein 25 (SNAP-25) in neurological disorders. Int J Neurosci 127:805–811. [DOI] [PubMed] [Google Scholar]
- Nutt DJ, King LA, Phillips LD (2010) Drug harms in the UK: a multicriteria decision analysis. Lancet 376:1558–1565. [DOI] [PubMed] [Google Scholar]
- Ohrfelt A, Brinkmalm A, Dumurgier J, Brinkmalm G, Hansson O, Zetterberg H, Bouaziz-Amar E, Hugon J, Paquet C, Blennow K (2016) The pre-synaptic vesicle protein synaptotagmin is a novel biomarker for Alzheimer’s disease. Alzheimer’s Res Ther 8:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RW, Liang J (2017) Role of GABAA receptors in alcohol use disorders suggested by chronic intermittent ethanol (CIE) rodent model. Mol Brain 10:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan PY, Tian JH, Sheng ZH (2009) Snapin facilitates the synchronization of synaptic vesicle fusion. Neuron 61:412–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignataro L, Miller AN, Ma L, Midha S, Protiva P, Herrera DG, Harrison NL (2007) Alcohol regulates gene expression in neurons via activation of heat shock factor 1. J Neurosci 27:12957–12966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praefcke GJ, McMahon HT (2004) The dynamin superfamily: universal membrane tubulation and fission molecules? Nat Rev Mol Cell Biol 5:133–147. [DOI] [PubMed] [Google Scholar]
- Pratt MB, Husain SS, Miller KW, Cohen JB (2000) Identification of sites of incorporation in the nicotinic acetylcholine receptor of a photoactivatible general anesthetic. J Biol Chem 275:29441–29451. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan NA, Drescher MJ, Drescher DG (2012) The SNARE complex in neuronal and sensory cells. Mol Cell Neurosci 50:58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao PS, Bell RL, Engleman EA, Sari Y (2015) Targeting glutamate uptake to treat alcohol use disorders. Front Neurosci 9:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reim K, Mansour M, Varoqueaux F, McMahon HT, Sudhof TC, Brose N, Rosenmund C (2001) Complexins regulate a late step in Ca2+-dependent neurotransmitter release. Cell 104:71–81. [DOI] [PubMed] [Google Scholar]
- Ren JC, Zhu Q, Lapaglia N, Emanuele NV, Emanuele MA (2005) Ethanol-induced alterations in Rab proteins: possible implications for pituitary dysfunction. Alcohol 35:103–112. [DOI] [PubMed] [Google Scholar]
- Rhee JS, Betz A, Pyott S, Reim K, Varoqueaux F, Augustin I, Hesse D, Sudhof TC, Takahashi M, Rosenmund C, Brose N (2002) Beta phorbol ester- and diacylglycerol-induced augmentation of transmitter release is mediated by Munc13s and not by PKCs. Cell 108:121–133. [DOI] [PubMed] [Google Scholar]
- Richmond JE, Davis WS, Jorgensen EM (1999) UNC-13 is required for synaptic vesicle fusion in C. elegans. Nat Neurosci 2:959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizo J (2018) Mechanism of neurotransmitter release coming into focus. Protein Sci 27:1364–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Moore SD, Tallent MK, Siggins GR (2003) Ethanol increases GABAergic transmission at both pre- and postsynaptic sites in rat central amygdala neurons. Proc Natl Acad Sci U S A 100:2053–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Varodayan FP (2017) Synaptic targets: Chronic alcohol actions. Neuropharmacol 122:85–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks JJ, Gonzales KR, Bouchery EE, Tomedi LE, Brewer RD (2015) 2010 National and State Costs of Excessive Alcohol Consumption. American journal of preventive medicine 49:e73–e79. [DOI] [PubMed] [Google Scholar]
- Saito M, Smiley J, Toth R, Vadasz C (2002) Microarray analysis of gene expression in rat hippocampus after chronic ethanol treatment. Neurochem Res 27:1221–1229. [DOI] [PubMed] [Google Scholar]
- Sassa T, Harada S, Ogawa H, Rand JB, Maruyama IN, Hosono R (1999) Regulation of the UNC-18-Caenorhabditis elegans syntaxin complex by UNC-13. J Neurosc 19:4772–4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauguet L, Howard RJ, Malherbe L, Lee US, Corringer PJ, Harris RA, Delarue M (2013) Structural basis for potentiation by alcohols and anaesthetics in a ligand-gated ion channel. Nat Commun 4:1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugasundararaj S, Das J, Sandberg WS, Zhou X, Wang D, Messing RO, Bruzik KS, Stehle T, Miller KW (2012) Structural and functional characterization of an anesthetic binding site in the second cysteine-rich domain of protein kinase Cdelta*. Biophys J 103:2331–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Tareste DC, Paumet F, Rothman JE, Melia TJ (2007) Selective activation of cognate SNAREpins by Sec1/Munc18 proteins. Cell 128:183–195. [DOI] [PubMed] [Google Scholar]
- Siggins GR, Bloom FE, French ED, Madamba SG, Mancillas J, Pittman QJ, Rogers J (1987) Electrophysiology of ethanol on central neurons. Ann N Y Acad Sci 492:350–366. [DOI] [PubMed] [Google Scholar]
- Soderpalm B, Lido HH, Ericson M (2017) The Glycine Receptor-A Functionally Important Primary Brain Target of Ethanol. Alcohol Clin Exp Res 41:1816–1830. [DOI] [PubMed] [Google Scholar]
- Song SH, Augustine GJ (2015) Synapsin Isoforms and Synaptic Vesicle Trafficking. Mol Cells 38:936–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs CD, Rubin E (1993) Molecular mechanisms of ethanol and anesthetic actions: Lipid-and protein-based theories, in Alcohol, Cell Membranes, and Signal Transduction in Brain, Alcohol, Cell Membranes, and Signal Transduction in Brain, pp 1–11, Springer. [Google Scholar]
- Sturm VE, Sollberger M, Seeley WW, Rankin KP, Ascher EA, Rosen HJ, Miller BL, Levenson RW (2013) Role of right pregenual anterior cingulate cortex in self-conscious emotional reactivity. Soc Cogn Affect Neurosci 8:468–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhof TC (2013) Neurotransmitter release: the last millisecond in the life of a synaptic vesicle. Neuron 80:675–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhof TC, Rothman JE (2009) Membrane fusion: grappling with SNARE and SM proteins. Science 323:474–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thode AB, Kruse SW, Nix JC, Jones DN (2008) The role of multiple hydrogen-bonding groups in specific alcohol binding sites in proteins: insights from structural studies of LUSH. J Mol Biol 376:1360–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng FY, Wang Y, Tang BL (2001) The syntaxins. Genome Biol 2:REVIEWS3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadwell JA, Pagniello KB, Singh SM (2004) Genetic segregation of brain gene expression identifies retinaldehyde binding protein 1 and syntaxin 12 as potential contributors to ethanol preference in mice. Behav Genet 34:425–439. [DOI] [PubMed] [Google Scholar]
- van Swinderen B, Saifee O, Shebester L, Roberson R, Nonet ML, Crowder CM (1999) A neomorphic syntaxin mutation blocks volatile-anesthetic action in Caenorhabditis elegans. Proc Natl Acad Sci U S A 96:2479–2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardar G, Chang S, Arancillo M, Wu YJ, Trimbuch T, Rosenmund C (2016) Distinct Functions of Syntaxin-1 in Neuronal Maintenance, Synaptic Vesicle Docking, and Fusion in Mouse Neurons. J Neurosci 36:7911–7924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varodayan FP, Harrison NL (2013) HSF1 transcriptional activity mediates alcohol induction of Vamp2 expression and GABA release. Front Integr Neurosci 7:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varodayan FP, Pignataro L, Harrison NL (2011) Alcohol induces synaptotagmin 1 expression in neurons via activation of heat shock factor 1. Neurosci 193:63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varoqueaux F, Sigler A, Rhee JS, Brose N, Enk C, Reim K, Rosenmund C (2002) Total arrest of spontaneous and evoked synaptic transmission but normal synaptogenesis in the absence of Munc13-mediated vesicle priming. Proc Natl Acad Sci U S A 99:9037–9042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhage M, Maia AS, Plomp JJ, Brussaard AB, Heeroma JH, Vermeer H, Toonen RF, Hammer RE, van den Berg TK, Missler M, Geuze HJ, Sudhof TC (2000) Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science 287:864–869. [DOI] [PubMed] [Google Scholar]
- Weng J, Symons MN, Singh SM (2009) Studies on Syntaxin 12 and alcohol preference involving C57BL/6J and DBA/2J strains of mice. Behav Genet 39:183–191. [DOI] [PubMed] [Google Scholar]
- Wooden JI, Schuller K, Roman G, Das J, Leasure JL (2019) Munc 13–1 heterozygosity does not alterVoluntary ethanol consumption or sensitivity in mice. Alcohol. S0741–8329, 30013–30018. doi: 10.1016/j.alcohol.2019.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, McMillan K, Pike CM, Cahill AL, Herring BE, Wang Q, Fox AP (2013) Interaction of anesthetics with neurotransmitter release machinery proteins. J Neurophysiol 109:758–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Mashimo T, Sudhof TC (2007) Synaptotagmin-1, −2, and −9: Ca(2+) sensors for fast release that specify distinct presynaptic properties in subsets of neurons. Neuron 54:567–581. [DOI] [PubMed] [Google Scholar]
- Xu S, Pany S, Benny K, Tarique K, Al-Hatem O, Gajewski K, Leasure JL, Das J, Roman G (2018) Ethanol Regulates Presynaptic Activity and Sedation through Presynaptic Unc13 Proteins in Drosophila. eNeuro 5 ENEURO.0125–18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Su L, Rizo J (2010) Binding of Munc18–1 to synaptobrevin and to the SNARE four-helix bundle. Biochemistry 49:1568–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue M, Craig TK, Xu J, Chao HT, Rizo J, Rosenmund C (2010) Binding of the complexin N terminus to the SNARE complex potentiates synaptic-vesicle fusogenicity. Nat Struct Mol Biol 17:568–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Kaeser-Woo YJ, Pang ZP, Xu W, Sudhof TC (2010) Complexin clamps asynchronous release by blocking a secondary Ca(2+) sensor via its accessory alpha helix. Neuron 68:907–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalucki OH, Menon H, Kottler B, Faville R, Day R, Bademosi AT, Lavidis N, Karunanithi S, van Swinderen B (2015) Syntaxin1A-mediated Resistance and Hypersensitivity to Isoflurane in Drosophila melanogaster. Anesthesiology 122:1060–1074. [DOI] [PubMed] [Google Scholar]