Abstract
Objective:
Melanoma preventive interventions for children with familial risk are critically needed, because ultraviolet radiation (UVR) exposure and sunburn occurrence early in life are the primary modifiable risk factors for melanoma. The current study examined the feasibility and acceptability of a new, family-focused telehealth intervention for children with familial risk for melanoma and their parents. The study also explored changes in child sun protection and risk behaviors, sunburn occurrence, and objectively measured UVR exposure.
Methods:
This was a prospective study with a single group design (n=21 parent-child dyads, children ages 8–17). Dyads were asked to participate in three in-person assessments and three live videoteleconference intervention sessions.
Results:
The intervention was feasibly delivered and the intervention content was acceptable to parents and children. The intervention was associated with improvements in child use of certain sun protection strategies over time and declines in child UVR exposure.
Conclusions:
A telehealth-delivered, family-focused melanoma preventive intervention was feasibly delivered and was acceptable to parent-child dyads. Future melanoma preventive interventions for this at-risk population could incorporate eHealth technologies to facilitate improvements in use of sun protection and monitoring of UVR exposure. This trial was registered with Clinicaltrials.gov, number .
Keywords: melanoma, prevention, children, family, eHealth, telehealth, intervention, cancer, oncology
Melanoma, the most lethal form of skin cancer, is the fifth most common cancer and was estimated to be diagnosed in more than 91,000 people in the United States in 2018.1 Prevention of melanoma is a high priority, especially among at-risk populations.2 Children who have a parent with melanoma have a 2-fold increased lifetime risk for developing the disease compared to the general population (2.3% lifetime risk).1,3 These children have a unique opportunity to engage in melanoma preventive behaviors early in life, in order to mitigate their familial risk for melanoma.
Ultraviolet radiation (UVR) exposure and sunburn occurrence, particularly during childhood, are the only well-established modifiable risk factors for melanoma.4,5 It is estimated that as many as 90% of melanomas are caused by UVR exposure, and sunburns early in life increase risk for melanoma.5,6 Children, with the help of their parents or caregivers, should be advised to consistently engage in a range of sun protection behaviors and to avoid intentional tanning.5,7 Daily recommended sun protection behaviors include wearing protective clothing, a wide-brimmed hat and sunglasses, seeking shade, avoiding outdoor activity during peak UVR exposure hours (10 am-4 pm), and applying and reapplying sunscreen every 2 hours and after sweating or swimming.7 Unfortunately, children, including those with familial risk, do not regularly employ these recommended behaviors and sustain sunburns, thus increasing lifetime melanoma risk.5,8–12 Children and parents from families affected by melanoma experience barriers to child sun protection use, including lack of melanoma prevention knowledge and family modeling of sun protection use, and logistical challenges.9,13 In these families, higher levels of perceived barriers to sun protection predict lower child use of sun protection.12 Perceived barriers are therefore important to address because they could further compound risk for developing melanoma.
The two published interventions for children with a familial history of melanoma have primarily focused on parents and children under age 12.14,15 The interventions improved sunscreen application and re-application, protective clothing use, and hat use.14,15 Less emphasis has been placed on sunburns as an outcome; however, sunburn is important to target because childhood sunburns tie to melanoma later in life.16 Given that child sun protection behaviors require children and parents to work together to plan for and implement sun protection, interventions should involve both parents and children.9,13 There have not yet been interventions for children with a familial risk for melanoma that have targeted parent-child dyads to foster collaboration on child sun protection.
Telehealth formats can be useful for delivering live interventions to parent-child dyads because they reach families in their own environment, could increase engagement among children,17 alleviate the burden on families of travelling to in-person programs, have potential for wider dissemination, and could be more cost-effective. Interventions that build on technology to support behavior change show promise,18,19 although have not yet been used with children with familial risk for melanoma.20
The current study was a single-group pilot with a small sample size, designed to determine the feasibility and acceptability of a new, family-focused telehealth intervention and to detect signals in changes in outcomes of interest among children at elevated risk for melanoma. The primary goals were to examine the feasibility and acceptability of the intervention for children with a parent with a history of melanoma and their parents. A secondary goal was to explore pre- to post-intervention changes in child sun protection, intentional tanning, sunburn occurrence, and objectively measured UVR exposure. We hypothesized that parents and children would report that child use of sun protection increased over time. The study included the collection of dyadic data from both parents and children in order to examine potential similarities and differences in their perspectives on intervention acceptability and child sun protection and tanning. The study also piloted an objective measure of UVR exposure among children to supplement self-reported measures and explored potential age differences in UVR exposure.
Methods
Participants
Parents and children from the same family were eligible to participate if the parent was the primary caregiver for a child between the ages of 8–17, and the child had a parent with a history of melanoma. A broad age range was selected in order to understand whether the intervention could be feasibly implemented among children of different ages. Families were recruited through a National Cancer Institute-designated Comprehensive Cancer Center in the Intermountain West of the United States via a registry of melanoma patients (n=1,383), participants in prior studies who agreed to be re-contacted (n=65), and social media (n=6). To maintain independence in analyses, only one parent, preferably the parent with a history of melanoma, was included. To facilitate family participation, if families had multiple eligible children, one or more children could participate. In families with multiple participating children, parents were asked to select which child had the most difficulty with implementing sun protection, and to maintain independence, only this child was included in analyses.
Procedures
Families were asked to participate in three in-person assessment visits (measures were completed via paper, computer, and via data download) and three video teleconference intervention sessions (see Online Supplemental Materials). The in-person visits consisted of a pre-intervention assessment (Visit 1; April-September), a post-intervention assessment two weeks after the last intervention session and 8 weeks after Visit 1 (Visit 2; June-October), and a 4 week post-intervention assessment to examine short-term effect maintenance (Visit 3; July-December). Intervention sessions were held April through September via live video teleconference between an interventionist and family every 2 weeks between in-person Visits 1 and 2. All study procedures were approved by the Institutional Review Board (IRB_00090292).
After parents provided informed consent and parental permission, and children provided written and verbal assent, each family received an intervention workbook. Teleconference sessions were held using videoconferencing software (WebEx™) which allowed dyads and the interventionist to see and hear one another in real-time and screen-share materials. The research team assisted families in setting up videoconferencing software and provided cameras to families if needed.
FLARE intervention.
The Family Lifestyles, Actions, and Risk Education (FLARE) intervention was developed using a patient-centered approach to address barriers to child sun protection.13,21 FLARE is grounded in Social Cognitive and Protection Motivation Theories.22,23 FLARE included content based on our prior melanoma education interventions, evidence-based behavioral strategies to improve parent-child collaboration, and work documenting common and unique barriers encountered by families.9,13,21,24–28
Intervention visits were each approximately 60 minutes long and families were able to simultaneously view screen-shared materials and the interventionist. During the first visit, content covered included education on melanoma risk and sun protection,24,25 flexibility in implementing sun protection to accommodate child preferences and activities, and societal standards for being tan. To encourage parent and child modeling of sun protection behaviors, interventionists guided families in completing a family skin protection plan whereby each family member details which sun protection strategies they plan to use for their outdoor activities. The second visit covered behavioral and organizational strategies for implementing sun protection. The third visit covered communication skills and tools (e.g., parent-child communication, parental monitoring, handling peer opposition). During each visit, families updated their skin protection plan, and applied the problem-solving framework (i.e., Bright Ideas)29 to a parent/child-reported challenge to implementing child sun protection which could vary each visit (see Online Supplemental Material).
Interventionist training and treatment fidelity.
Two master’s-level interventionists with a background in public health delivered the intervention. Both interventionists received training on delivering FLARE from the first author. Training included review of intervention manuals, didactic sessions on content, role-playing, and delivering FLARE to families not in the study. All sessions were videorecorded. To promote fidelity, a scripted intervention manual was employed. Interventionists completed post-session fidelity checklists and participated in weekly supervision meetings to discuss sessions and receive corrective feedback based on supervisor review of sessions. A trained research assistant reviewed 20% of the videorecordings and completed fidelity checklists (see Online Supplemental Material).
Measures
Feasibility.
Recruitment metrics summarizing the proportion of eligible families enrolled in the study and reasons for non-enrollment were recorded (≥80% recruitment rate was considered the threshold for feasibility). Child and parent attendance at intervention session and reasons for non-attendance were recorded (≥80% attendance was considered the threshold for feasibility). Parent- and child-reported barriers to study participation were assessed via an investigator-designed item at the first post-intervention assessment. For this item, participants were asked to check off any barriers that made study participation challenging (e.g., technological challenges, sessions interfering with other activities). The fidelity checklist completed by the research assistant assessed whether interventionists delivered each component of the intervention and process skills (e.g., use of open-ended questions; see Online Supplemental Materials).
Acceptability.
At the first post-intervention visit, parents and children were each asked to rate the intervention in terms of its acceptability on several domains, including intervention content (e.g., “The FLARE program included the information I wanted”), comprehension (e.g., “The FLARE program was confusing”), appeal (e.g., “The FLARE program information was interesting”), novelty of information learned, and perceived effect of content on their motivation to engage in melanoma prevention and control behaviors. Responses for the 13 items were on a 5-point Likert-type scale ranging from Strongly Disagree to Strongly Agree (several items were reverse scored; see Online Supplemental materials). Average ratings of “agree” or “strongly agree” were considered to indicate adequate acceptability. Items were adapted for use based on the literature and prior work developing educational interventions for at-risk families.11,30–32 Parents and children were also asked to rate how often (“not at all” to “often”) they used each intervention component (e.g., Information on child’s risk for melanoma, behavioral strategies), and how helpful each component was on a 4 point Likert-type scale from “Not at all helpful” to “Very helpful.”
Preliminary sun protection, tanning, and sunburn outcomes.
t all assessments, parents and children were asked to report on the child’s use of sun protection (e.g., sunscreen, long-sleeved shirt; rated on a 5-point Likert-type scale from “Never” to “Always”), intentional tanning (indoor and outdoor; same Likert-type scale), and sunburn frequency over the prior month using adapted items from the Sun Habits Survey.33 Frequency of child red or painful sunburns was measured on a scale from 0 to 5+ sunburns. To understand the context in which child sunburns occurred and reasons that sunburn occurred, daily diaries were completed by children and parents in which they recorded the activity children were participating in when the sunburn occurred and the reason the sunburn occurred.
UVR exposure.
As a biomarker of UVR exposure, children were asked to wear a UVR monitoring device on their wrist for one-week periods following the pre-intervention and immediate post-intervention visits, and the week prior to the follow-up . The device is a research-grade scientific instrument34 and communicates wirelessly with a docking cradle via a terminal communication program35 that allows for configuration of the devices and data download during in-person visits.34,36 UVR data were captured in 10-second intervals. Children were asked to wear the devices 6 am-9 pm, to wear the device on the same wrist outside clothing, and to remove them during activities involving water submersion and place the device face up nearby. Level of compliance to wearing the device was examined, to better understand the acceptability of the device in this population. Average UVR exposure for each week of monitoring was calculated using all available data from the week.
Analytic Approach
Descriptive statistics were used to summarize feasibility and acceptability. To explore potential changes in child sun protection, tanning, and sunburn over time, paired samples t-tests were conducted to compare pre-intervention (Visit 1) and post-intervention means (Visit 2), and to compare post-intervention (Visit 2) and one month follow-up means (Visit 3) from parent and child perspectives. Sunburn diaries were content analyzed to summarize the activities in which children were participating when they received a sunburn and frequencies were calculated to summarize reported reasons for sunburn. To explore potential effects of the intervention on children’s UVR exposure, we examined UVR monitoring data captured by the device using linear mixed models (LMMs), adjusted for season.
Results
Twenty-one parents (90% mothers, 10% fathers) and 21 children (62% female, Mage=11.3 years, SDage=2.7 years) participated. Almost all parents (95%) had a personal history of melanoma (Table 1).
Table 1.
Demographic characteristics of parents and children
| Parents (N=21) | N(%) Unless otherwise noted |
|---|---|
| Age of parent, M(SD) | 41.3 (5.04) |
| Sex | |
| Male (father) | 2 (10) |
| Female (mother) | 19 (90) |
| Race | |
| White Non-Hispanic | 20 (95) |
| White Hispanic | 1 (5) |
| Level of education | |
| High school graduate or GED | 3 (14) |
| Voc., Tech., or Some college (2 yr. degree) | 6 (29) |
| Bachelor’s Degree | 10 (47) |
| Doctoral Degree | 2 (9) |
| Marital status | |
| Married or partnered | 17 (81) |
| Divorced | 4 (19) |
| Personal melanoma history | 20 (95) |
| Family history of melanoma | 10 (48) |
| Household income (median) | $90,000–99,999 |
| Skin type (Fitzpatrick) | |
| I | 5 (24) |
| II | 6 (29) |
| III | 9 (43) |
| IV | 1 (5) |
| Children (n =21) | |
| Age, M(SD) | 11.3 (2.7) |
| 8–12 | 14 (67) |
| 3–17 | 7 (33) |
| Sex | |
| Male | 8 (38) |
| Female | 13 (62) |
| Race | |
| White Non-Hispanic | 19 (90) |
| White Hispanic | 1 (5) |
| Biracial | 1 (5) |
| Skin type (Fitzpatrick) | |
| II | 7 (33) |
| III | 9 (43) |
| IV | 4 (19) |
| Missing | 1 (5) |
Feasibility
In total, 57 adults were screened and 49 were eligible (ineligibility due to not having children 8–17 years). Of the 49 eligible, 21 (43%) participated (76% from registry, 24% from prior studies). Reasons for non-participation included time limitations (n=12; too busy), living too far away to attend in-person visits required for device data download (n=2), and non-response to calls (n=14). Among the participating families who had more than one child eligible to participate, three children from 2 families did not participate. The vast majority (86%) of enrolled families attended all three intervention sessions. Of the remaining families, 1 (5%) attended two of the three intervention sessions, and 2 (9%) did not attend any. All families attended the first post-intervention visit, and two families did not attend the follow-up.
In terms of barriers to participation, parents and children reported that the electronic format and time required to participate were not significant barriers (e.g., technological challenges associated with the teleconference system and UVR devices endorsed as barriers by 6% parents, 22% children). Participation in the study was generally not viewed as getting in the way of other activities (11% parents, 17% children reported it got in the way). Some participants reported that the in-person assessments were “a hassle” (44% parents, 17% children). Fidelity to the intervention manual was excellent at 100%.
Acceptability
Both parents and children reported that intervention content was relevant, helpful, and novel, that the materials were appealing or engaging, and that the intervention would motivate them to implement melanoma prevention and control behaviors (see Online Supplemental Materials). Of the parents who endorsed that they used FLARE topics or skills outside of the intervention session, the vast majority reported that the topics or skills were helpful. For instance, all (100%) parents reported that information on the child’s elevated risk for melanoma was somewhat or very helpful and 88% who reported using the structured problem-solving approach rated the approach as somewhat or very helpful. In general, children endorsed the intervention content as less helpful than parents did with 42–71% of children reporting that specific topics were somewhat or very helpful (see Online Supplemental Materials).
Preliminary Intervention Effects
Descriptive statistics for children’s sun protection, tanning, and sunburn outcomes are included in Online Supplemental Materials. From pre- (Visit 1) to post-intervention (Visit 2) there were several changes in outcomes. Parents reported that children’s frequency of wearing long pants or skirt and staying in the shade increased pre- to post-intervention (t(17)=−1.91, p=.07; t(17)=−2.03, p=.05, respectively). Children’s sunglasses use per parent-report decreased in frequency pre- to post-intervention (t(15)=2.15, p=.04). Children reported increased frequency in wearing long pants or skirt (t(16)= −2.87, p=.01) and shade-seeking when outdoors (t(15)=−3.57, p=.003) pre- to post-intervention. Children also reported declines in outdoor tanning pre- to post-intervention (t(17)=2.05, p=.05). Other child sun protection behaviors and sunburn occurrence did not change pre- to post-intervention. In terms of maintenance of effects from post-intervention (Visit 2) to one month post-intervention (Visit 3), the pre- to post-intervention changes noted above were sustained (p’s>.05). Parents reported that children’s use of long-sleeved shirts increased post-intervention to the one month follow-up (t(15)=−4.04, p=.001). Intentional indoor tanning was only endorsed by one participant at any assessment and thus was not examined.
Between the pre- to post-intervention assessments (Visits 1 and 2), increased child age was significantly associated with parent-reported increases in child sunscreen re-application (r=.522, p=.046), long-sleeved shirt use (r=.472, p=.048), and shade use (r=.517, p=.028) and with child-reported shade use (r=.553, p=.017). Between the post-intervention and one month follow-up assessments (Visits 2 and 3), increased child age was significantly associated with parent-reported increases in child shade use (r=.611, p=.015) and sunglasses use (r=.637, p=.011) as well as with child-reported long-sleeved shirt use (r=.521, p=.039).
Of the 13 child sunburn occurrences reported by 8 families via daily diary, 8 (62%) occurred during contact with water such as when swimming or at a water park, 3 (23%) occurred during organized sports, and 2 (15%) occurred during unstructured time playing outside. Participants reported 17 different reasons that sunburns occurred: Did not use sunscreen or protective clothing (6, 35%), sunscreen washed or sweated off (4, 24%), did not re-apply sunscreen (4, 24%), outside longer than expected (2, 12%), and other (i.e., sunscreen did not protect enough; 1, 6%).
UVR Outcomes
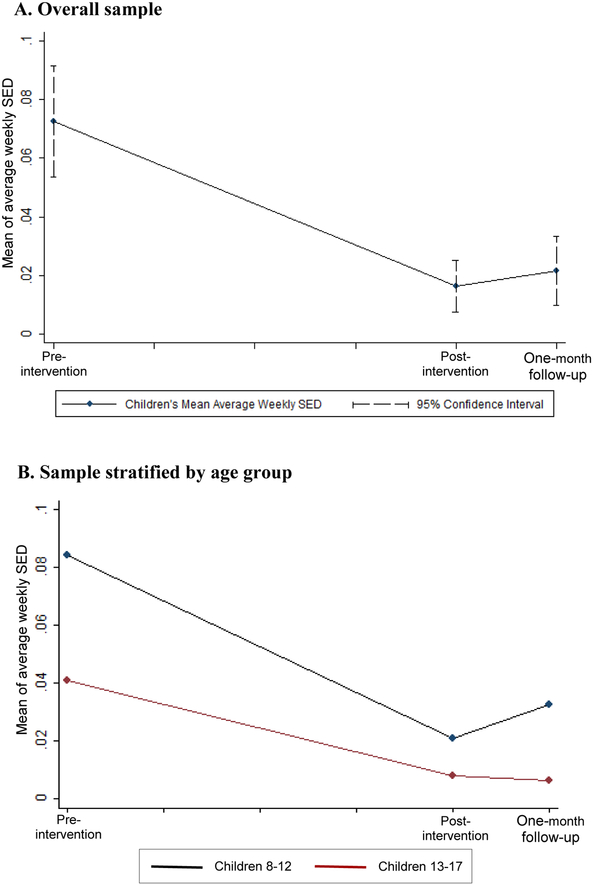
The devices captured children’s UVR exposure for 59% of the desired monitoring days. Missing data was due to: device not worn (21%), device not returned to the research team (11%), and device malfunction and/or corrupted data (9%). Across the entire sample, pre- to post-intervention, there was a decline in UVR exposure among children of 1.43 Standard Erythemal Doses (SEDs) per week (SE=0.34, p=0.0013, 95% CI: −2.09, −0.76). There was not a significant change in UVR exposure post-intervention to one month follow-up (estimate of per week SED change=0.34, SE=0.78, p=0.68, 95% CI: −1.86, 1.18). See Figure 1 for average UVR exposure trajectories for the entire sample and for children ages 8–12 versus ages 13–17.
Figure 1. Children’s trajectory of average weekly UVR exposure as measured by UVR monitors.
Note. There were 8 weeks between pre- and post-intervention, and 4 weeks between post-intervention and one-month follow-up.
Discussion
The primary goal of this pilot study was to examine the feasibility and acceptability of a new telehealth skin cancer preventive intervention for children with a familial risk for melanoma and their parents. The intervention demonstrated adequate feasibility in terms of its delivery via a live video teleconference format and high levels of family attendance. The intervention content and utility was acceptable to parents and children; however, childrens’ responses suggested that content could be modified to be more engaging for them. Consistent with hypotheses, the intervention was associated with improvements in children’s frequency of use of certain sun protection strategies, particularly protective clothing use and shade-seeking, and declines in objectively measured UVR exposure after adjusting for season, particularly among younger children. These improvements were maintained through the one-month follow-up. The decreased levels of UVR exposure are likely clinically significant, particularly for individuals with lighter skin types.37 Although the current study was a pilot, findings were consistent with those of prior skin cancer preventive interventions that demonstrated positive effects on certain sun protection outcomes.14,15 The current results also suggest that childrens’ sunburns most frequently occur during water activities, when sun protection strategies are not used at all or when sunscreen has worn off. Together, the findings indicate that future interventions may need to consider targeted strategies to address specific contexts leading to child sunburn. A larger randomized trial of the intervention is needed to definitively test effects on sun protection, tanning, and sunburn outcomes, as well as UVR exposure.
Study limitations.
Limitations were that the sample primarily included high socioeconomic status families and mothers residing in a geographic location with high incidence of melanoma38 and featured a modest recruitment rate. Future trials would benefit from actively recruiting socioeconomically, gender, and racially/ethnically diverse samples from a wider geographic area, and using remote data collection procedures.
Clinical implications.
The next generation of interventions for children with an elevated familial risk for melanoma could build on the current study and other interventions by using eHealth approaches such as the telehealth format used in FLARE. Interventions could employ text messaging or SmartPhone applications to reinforce behavioral skills and to support self-monitoring of sun protection use or UVR exposure.
Future trials of skin cancer preventive interventions for this at-risk pediatric population should ideally be well-powered to detect changes in sun protection, tanning, and sunburn occurrence,39 control for seasonality, and examine outcomes using self/parent-reported and objective methods and over longer-term periods. Studies could seek to understand whether reported use of certain sun protection methods may be most associated with reductions in UVR exposure. Efforts to prevent melanoma among children with an elevated familial risk for melanoma have the potential to reduce the incidence of and morbidity associated with a potentially deadly disease where prevention starts in childhood.
Supplementary Material
Acknowledgements:
We greatly appreciate the efforts of the following individuals in assisting with study procedures: Ayesha Patil, Heloisa Caputo, Kelsey Zaugg, Angela Zhu.
Funding: This work was supported by the National Cancer Institute (K07CA196985 to Y.P.W.; R01CA158322 to L.G.A. and S.A.L.), and the Office of Communications, Genetic Counseling Shared Resource, and Cancer Biostatistics Shared Resource supported by grant P30CA042014 to Huntsman Cancer Institute. Effort by Dr. Leachman was supported in part by the Oregon Health and Science University Knight Cancer Institute. This work was supported by funding from the Undergraduate Research Opportunities Program at the University of Utah to Angela Zhu. Dr. Tercyak was supported by P30CA051008 to Georgetown Lombardi Comprehensive Cancer Center. Dr. Hay was supported by P30CA008748 to Memorial Sloan Kettering Cancer Center. Data entry was completed using REDCap, supported by NIH (8UL1TR000105, formerly UL1RR025764). The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH.
Footnotes
Data Availability Statement: The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Surveillance Epidemiology and End Results Program. SEER Stat fact sheets: Melanoma of the skin. 2018; http://seer.cancer.gov/statfacts/html/melan.html. Accessed April 18, 2018. [Google Scholar]
- 2.US Department of Health, and Human Services. The Surgeon General’s call to action to prevent skin cancer. Washington, DC: Department of Health and Human Services; 2014. [Google Scholar]
- 3.Cho E, Rosner BA, Feskanich D, Colditz GA. Risk factors and individual probabilities of melanoma for whites. J Clin Oncol. 2005;23(12):2669–2675. [DOI] [PubMed] [Google Scholar]
- 4.PDQ Screening and Prevention Editorial Board Skin cancer prevention (PDQ): Health professional version. Bethesda, MD: 2017. [Google Scholar]
- 5.Balk SJ. Ultraviolet radiation: A hazard to children and adolescents. Pediatrics. 2011;127(3):e791–e817. [DOI] [PubMed] [Google Scholar]
- 6.Parkin DM, Mesher D, Sasieni P. Cancers attributable to solar (ultraviolet) radiation exposure in the UK in 2010. Br J Cancer. 2011;105 Suppl 2(Suppl 2):S66–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Academy of Dermatology. Prevent skin cancer. 2017; https://www.aad.org/public/spot-skin-cancer/learn-about-skin-cancer/prevent. Accessed July 11, 2017.
- 8.Geller AC, Brooks DR, Colditz GA, Koh HK, Frazier AL. Sun protection practices among offspring of women with personal or family history of skin cancer. Pediatrics. 2006;117(4):e688–694. [DOI] [PubMed] [Google Scholar]
- 9.Glenn BA, Lin T, Chang LC, et al. Sun protection practices and sun exposure among children with a parental history of melanoma. Cancer Epidemiol Biomarkers Prev. 2015;24(1):169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tripp MK, Peterson SK, Prokhorov AV, et al. Correlates of sun protection and sunburn in children of melanoma survivors. Am J Prev Med. 2016;51(3):e77–e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu YP, Nagelhout E, Aspinwall LG, et al. A novel educational intervention targeting melanoma risk and prevention knowledge among children with a familial risk for melanoma. Patient Educ Couns. 2017;101(3):452–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu YP, Parsons BG, Aspinwall LG, et al. Parent and child perspectives on perceived barriers to child sun protection and their association with sun protection strategies among children of melanoma survivors. Pediatr Dermatol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu YP, Parsons BG, Mooney R, et al. Barriers and facilitators to melanoma prevention and control behaviors among at-risk children. J Community Health. 2018;43(5):993–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gritz ER, Tripp MK, Peterson SK, et al. Randomized controlled trial of a sun protection intervention for children of melanoma survivors. Cancer Epidemiol Biomarkers Prev. 2013;22(10):1813–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glanz K, Steffen AD, Schoenfeld E, Tappe KA. Randomized trial of tailored skin cancer prevention for children: the Project SCAPE family study. J Health Commun. 2013;18(11):1368–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perna FM, Dwyer LA, Tesauro G, et al. Research on skin cancer-related behaviors and outcomes in the NIH grant portfolio, 2000–2014: Skin cancer intervention across the cancer control continuum (SCI-3C). JAMA Dermatol. 2017;153(5):398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Common Sense Media. The common sense census: Media use by tweens and teens. 2015; https://www.commonsensemedia.org/research/the-common-sense-census-media-use-by-tweens-and-teens. Accessed April 2, 2019. [Google Scholar]
- 18.Cushing CC, Steele RG. A meta-analytic review of eHealth interventions for pediatric health promoting and maintaining behaviors. J Pediatr Psychol. 2010:jsq023. [DOI] [PubMed] [Google Scholar]
- 19.Krishna S, Boren SA, Balas EA. Healthcare via cell phones: a systematic review. Telemed J E Health. 2009;15(3):231–240. [DOI] [PubMed] [Google Scholar]
- 20.Wu YP, Aspinwall LG, Conn BM, Stump T, Grahmann B, Leachman SA. A systematic review of interventions to improve adherence to melanoma preventive behaviors for individuals at elevated risk. Prev Med. 2016;88:153–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu YP, Parsons BG, Aspinwall LG, et al. Barriers to and facilitators of melanoma preventive behaviors predict reported sun protection in children with familial risk Paper presented at: Annual meeting of the Society for Behavioral Medicine 2018; New Orleans, LA. [Google Scholar]
- 22.Bandura A Health promotion from the perspective of social cognitive theory. Psychol Health. 1998;13(4):623–649. [Google Scholar]
- 23.Rogers RW. A protection motivation theory of fear appeals and attitude change. J Psychol. 1975;91(1):93–114. [DOI] [PubMed] [Google Scholar]
- 24.Wu YP, Aspinwall LG, Nagelhout E, et al. Development of an educational program integrating concepts of genetic risk and preventive strategies for children with a family history of melanoma. J Cancer Educ. 2016:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taber JM, Aspinwall LG, Kohlmann W, Dow R, Leachman SA. Parental preferences for CDKN2A/p16 testing of minors. Genet Med. 2010;12(12):823–838. [DOI] [PubMed] [Google Scholar]
- 26.Kahana S, Drotar D, Frazier T. Meta-analysis of psychological interventions to promote adherence to treatment in pediatric chronic health conditions. J Pediatr Psychol. 2008;33(6):590–611. [DOI] [PubMed] [Google Scholar]
- 27.D’Zurilla TJ, Goldfried MR. Problem solving and behavior modification. J Abnorm Psychol. 1971;78(1):107–126. [DOI] [PubMed] [Google Scholar]
- 28.Hamilton K, Cleary C, White KM, Hawkes AL. Keeping kids sun safe: exploring parents’ beliefs about their young child’s sun-protective behaviours. Psychooncology. 2016;25(2):158–163. [DOI] [PubMed] [Google Scholar]
- 29.Sahler OJ, Varni JW, Fairclough DL, et al. Problem-solving skills training for mothers of children with newly diagnosed cancer: a randomized trial. J Dev Behav Pediatr. 2002;23(2):77–86. [DOI] [PubMed] [Google Scholar]
- 30.Gwadry-Sridhar F, Guyatt GH, Arnold JMO, et al. Instruments to measure acceptability of information and acquisition of knowledge in patients with heart failure. Eur J Heart Fail. 2003;5(6):783–791. [DOI] [PubMed] [Google Scholar]
- 31.Kothe EJ, Mullan BA. Acceptability of a theory of planned behaviour email-based nutrition intervention. Health Promot Int. 2014;29(1):81–90. [DOI] [PubMed] [Google Scholar]
- 32.Gage H, Grainger L, Ting S, et al. Specialist rehabilitation for people with Parkinson’s disease in the community: a randomised controlled trial. HS&DR. 2014. [PubMed] [Google Scholar]
- 33.Glanz K, Yaroch AL, Dancel M, et al. Measures of sun exposure and sun protection practices for behavioral and epidemiologic research. Arch Dermatol. 2008;144(2):217–222. [DOI] [PubMed] [Google Scholar]
- 34.Sherman Z Developments in electronic UV dosimeters. 2014. [Google Scholar]
- 35.Campbell HS, Birdsell JM. Knowledge, Beliefs, and Sun Protection Behaviors of Alberta Adults. Prev Med. 1994;23(2):160–166. [DOI] [PubMed] [Google Scholar]
- 36.Dishman RK, Motl RW, Saunders R, et al. Self-efficacy partially mediates the effect of a school-based physical-activity intervention among adolescent girls. Prev med. 2004;38(5):628–636. [DOI] [PubMed] [Google Scholar]
- 37.Cassidy PB, Liu T, Florell SR, et al. A phase II randomized placebo-controlled trial of oral N-acetylcysteine for protection of melanocytic nevi against UV-induced oxidative stress in vivo. Cancer Prev Res. 2017;10(1):36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.U.S. Cancer Statistics Working Group. United States Cancer Statistics: 1999–2014 Incidence and Mortality Web-based Report. In: Department of Health and Human Services, Centers for Disease Control and Prevention, and National Cancer Institute; 2017. [Google Scholar]
- 39.Preventive US Services Task Force. Behavioral counseling to prevent skin cancer: U.S. Preventive Services Task Force recommendation statement. JAMA. 2018;319(11):1134–1142. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.