Abstract
Genetic discovery in psychiatry and clinical psychology is hindered by suboptimal phenotypic definitions. We argue that the hierarchical, dimensional, and data-driven classification system proposed by the Hierarchical Taxonomy of Psychopathology (HiTOP) consortium provides a more effective approach to identifying genes that underlie mental disorders, and to studying psychiatric etiology, than current diagnostic categories. Specifically, genes are expected to operate at different levels of the HiTOP hierarchy, with some highly pleiotropic genes influencing higher-order psychopathology (e.g. the general factor), whereas other genes conferring more specific risk for individual spectra (e.g. internalizing), subfactors (e.g. fear disorders), or narrow symptoms (e.g. mood instability). We propose that the HiTOP model aligns well with the current understanding of the higher-order genetic structure of psychopathology that has emerged from a large body of family and twin studies. We also discuss the convergence between the HiTOP model and findings from recent molecular studies of psychopathology indicating broad genetic pleiotropy, such as cross-disorder SNP-based shared genetic covariance and polygenic risk scores, and we highlight molecular genetic studies that have successfully redefined phenotypes to enhance precision and statistical power. Finally, we suggest how to integrate a HiTOP approach into future molecular genetic research, including quantitative and hierarchical assessment tools for future data-collection and recommendations concerning phenotypic analyses.
Keywords: behavior genetics, comorbidity, general factor, molecular genetics, nosology, pleiotropy
General Scientific Summary
This study articulates a testable model that aligns the current understanding of genetic influences on psychopathology with observed patterns of co-occurrence among mental disorders. Specifically, it discusses the evidence that genetic influences do not fit traditional psychiatric diagnoses, and demonstrates that alternative classification approaches, such as the HiTOP model, can maximize precision and statistical power in the search for molecular genetic variants linked to mental illness.
Psychiatric genetics promises to revolutionize our understanding of the neurobiology of mental illness and to inform drug development and personalized medicine approaches (Gandal, Leppa, Won, Parikshak, & Geschwind, 2016; Geschwind & Flint, 2015; Lester & Eley, 2013; Nelson et al., 2016). However, despite immense progress (Sullivan et al., 2017), genetic discovery in psychiatry remains hindered by shortcomings of phenotypic definitions, including diagnostic unreliability, comorbidity among disorders, and heterogeneity within them (Helzer et al., 2009; Kotov et al., 2017). The Hierarchical Taxonomy of Psychopathology (HiTOP) consortium proposed a data-driven classification system for a wide range of psychiatric disorders—based on a comprehensive review of existing nosologic and psychometric research—that addresses many of the shortcomings of traditional categorical diagnostic systems (Conway et al., 2018; Kotov, Krueger, & Watson, 2018; Kotov et al., 2017; Krueger et al., 2018). The HiTOP system promises to be a useful framework for psychiatric geneticists, who require valid and reliable phenotypes to maximize precision and statistical power in the search for genetic vulnerabilities to mental illness. Conversely, genetic findings are a crucial external validator of psychiatric nosology. The current paper discusses both issues: how the HiTOP model can inform future psychiatric genetic research by providing quantitative, hierarchically organized and easily implementable phenotypes, and how the HiTOP model dovetails with our existing understanding of the genetic architecture of psychopathology.
The HiTOP phenotypes – hierarchical and dimensional psychopathology
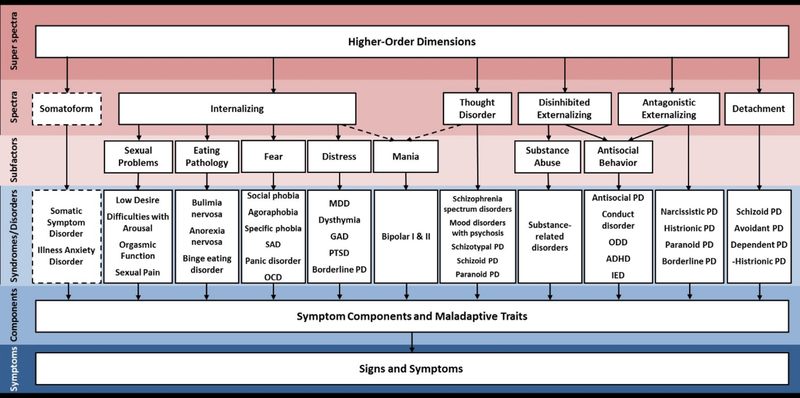
The HiTOP model organizes psychopathology into a hierarchy, in which each level captures a different degree of specificity with which mental illness is described (Figure 1). At the lowest level, the classification consists of individual maladaptive behaviors, symptoms and signs of illness. These can be aggregated into traits, such as compulsive checking or distractibility. At the next level, symptom components form dimensional syndromes, many of which are similar to existing diagnostic categories, such as obsessive-compulsive syndrome. The dimensional syndromes, in turn, form seven lower-order subfactors: distress, fear, sexual problems, eating pathology, mania, substance abuse and antisocial behavior. The subfactors, in turn, are organized into six higher-order spectra: internalizing, thought disorder, disinhibited externalizing, antagonistic externalizing, detachment, and somatoform. Finally, at the top of the hierarchy, there is a super spectrum, akin to a general factor of psychopathology (“p-factor”) (Caspi et al., 2014; Lahey et al., 2012; Lahey, Krueger, Rathouz, Waldman, & Zald, 2017). The proposed system is dynamic and flexible, as it accommodates updates as more structural data become available.
Figure 1 – The phenotypic HiTOP model.
Note: The figure is reprinted from Kotov et al. (2017). Constructs higher in the figure are broader and more general, whereas constructs lower in the figure are narrower and more specific.
SAD – social anxiety disorder, OCD – obsessive-compulsive disorder, MDD – major depressive disorder, GAD – generalized anxiety disorder, PTSD – post-traumatic stress disorder, PD – personality disorder, ODD – oppositional defiant disorder, ADHA – attention-deficit/hyperactivity disorder, IED – intermittent explosive disorder
As detailed elsewhere (Kotov et al., 2017), HiTOP addresses two cardinal limitations of traditional classification systems, such as the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) (American Psychiatric Association, 2013). First, the hierarchical approach provides a way to flexibly accommodate heterogeneity by grouping related symptoms together and assigning unrelated symptoms to different components, while also making comorbidity an explicit and predictable feature of the model by classifying related components together. For example, the model posits that a syndrome (e.g. obsessive-compulsive disorder, OCD) will consist of homogenous components (e.g. checking, cleaning, and ritual compulsions), that it will be most closely associated with other syndromes from the subfactor to which it is assigned (i.e., fear subfactor), be less closely associated with syndromes from other subfactors that belong to the same spectrum (e.g. distress, eating pathology, and sexual problems subfactors of the internalizing spectrum), and be even less closely associated with psychopathology from other spectra (e.g. substance abuse and antisocial behavior subfactors of the externalizing spectrum). The model also predicts that the proximity of these associations will be reflected in common risk factors, pathological processes, treatment responses, and illness course (Conway et al., 2018).
Second, the dimensional approach embodied in HiTOP frames mental health problems as continua, addressing many limitations of categorical classification, which include arbitrary boundaries between psychopathology and normality, diagnostic instability and inability to account for subthreshold cases. For example, the DSM–5 Field Trials have reported that 40% of diagnoses have not met even a relaxed cutoff for acceptable interrater reliability (Regier et al., 2013), although the same disorders often have evidenced excellent reliability when conceptualized dimensionally (Markon, Chmielewski, & Miller, 2011; Shea et al., 2002). Empirically-derived thresholds can be applied to dimensions in the HiTOP model to tailor them to specific clinical needs, such as screening or treatment decisions.
Hierarchical and quantitative genetic architecture – evidence from family and twin studies
There is ample empirical phenotypic evidence that psychopathology reflects the severe end of continuously distributed phenotypes (Krueger et al., 2018; Markon et al., 2011), indicating that quantitative phenotypes can be used to characterize clinical disorders in psychiatric genetic studies. First, a comprehensive review of taxometric research concluded that there is little support for discrete groups within the continuously distributed internalizing and externalizing spectra, as well as normal and maladaptive personality, although the evidence was less conclusive for schizotypy and substance use (Haslam, Holland, & Kuppens, 2012). Second, studies using latent variable modelling approaches also generally find that dimensional models fit data better than categorical models, in particular for disorders from the internalizing and externalizing spectra (Aslinger, Manuck, Pilkonis, Simms, & Wright, 2018; Conway, Hammen, & Brennan, 2012; Eaton et al., 2013; Krueger, Markon, Patrick, & Iacono, 2005; Luo, Donnellan, Burt, & Klump, 2016; Wright et al., 2013), although there is also some evidence of discontinuity (Forbes, Baillie, & Schniering, 2016; Forbush & Wildes, 2017; Klein & Kotov, 2016). Individual symptoms have typically also been found to be continuous rather than binary (Flett, Vredenburg, & Krames, 1997; Van Os, Linscott, Myin-Germeys, Delespaul, & Krabbendam, 2009).
In line with the phenotypic literature, biometrical studies have long demonstrated that genetic influences on psychiatric conditions operate in a dimensional fashion, reinforcing the conclusion that mental illness is better conceptualized in quantitative rather than categorical terms (Martin, Taylor, & Lichtenstein, 2017; Plomin, Haworth, & Davis, 2009). For example, Zavos et al. (2014) found that the same genetic factors influence severe and mild psychotic experiences in adolescents, indicating that quantitative genetic liability underpins a wide spectrum of psychotic symptoms. However, due to their traditional reliance on community samples, family and twin studies rarely encompass a sufficient number of participants with ascertained clinical diagnoses to directly test the genetic overlap between diagnoses and corresponding severity scores on a trait. Furthermore, some symptoms and diagnoses are too rare to study in typically-powered community samples. One important exception is a recent study that demonstrated a common, highly heritable broad depression factor underpinning major depressive disorder (MDD) diagnosis, depression symptoms, and neuroticism trait, although MDD and neuroticism also showed substantial unique genetic effects (Kendler et al., 2018). Additional evidence of the dimensional nature of psychopathology comes from molecular genetic studies, as described in more detail in the next section.
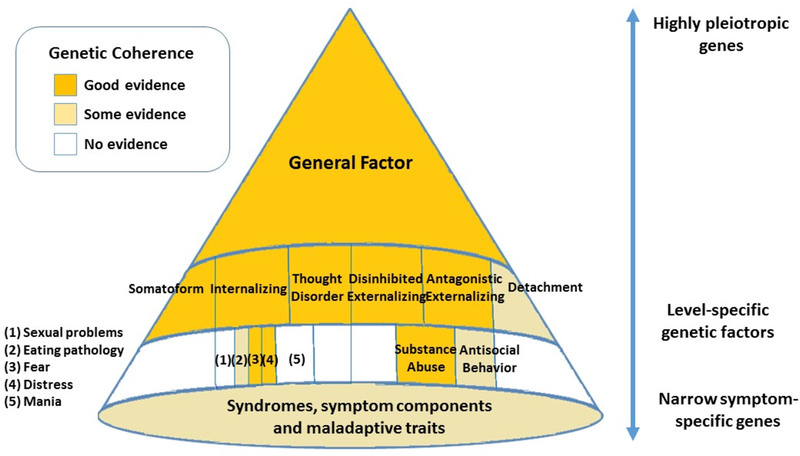
Moreover, biometric models have provided compelling evidence of genetic overlap across traditional psychiatric phenotypes, informing psychiatric taxonomy (Lahey, Krueger, Rathouz, Waldman, & Zald, 2016; Lahey, Van Hulle, Singh, Waldman, & Rathouz, 2011; Martin et al., 2017; Rhee, Lahey, & Waldman, 2015; Smoller et al., 2018; South & DeYoung, 2013; Waldman & Slutske, 2000). The current paper builds on this body of evidence by explicitly relating it to a multi-tiered hierarchical organization that emerged from research on phenotypic structure. Specifically, in HiTOP, the super spectrum is hypothesized to capture genetic influences common across most psychiatric disorders (i.e. pleiotropy). At the next highest level, the genetic variance can either be retained or divided among different levels of specificity, depending on the analytic framework taken, see Footnote 1 and Markon (2019). If the genetic variance is retained across the levels of the hierarchy, the six spectra reflect both common genetic influences, and influences specific to that spectrum. Additional genetic contributors emerge as one progresses down the hierarchy toward narrower dimensions, including specific traits and symptom clusters. If total genetic variance is divided into transdiagnostic and symptom-specific influences, e.g. using a bifactor approach, the genetic architecture of psychopathology is represented by independent sets of genes operating at different levels of specificity. Based on biometric modelling, the current review operationalizes etiological influences in a bifactor manner, unless stated otherwise. Accordingly, Figure 2 summarizes the level of empirical evidence for shared genetic influences at each level of the HiTOP hierarchy, herein referred to as genetic coherence. The key findings are described below, and a literature review is presented in Supplement 1.
Figure 2 -. Genetic influences at different levels of the HiTOP hierarchy.
Note:Genetic variants are expected to operate at different levels of the HiTOP hierarchy, with some highly pleiotropic genes influencing higher-order psychopathology, and others conferring risk for specific spectra, subfactors, or symptom components. For the full literature review supporting genetic coherence at different levels of the HiTOP hierarchy, see Supplement 1.
Several twin and family studies provide evidence relevant to the super spectrum. Collective results from studies investigating shared genetic influences on symptoms from three or more higher-order spectra jointly indicate that a single common genetic factor plausibly contributes to all six spectra (Kendler, Aggen, et al., 2011; Pettersson, Larsson, & Lichtenstein, 2016). For example, a Swedish national study of >1.5 million siblings identified a general genetic factor influencing diagnoses from the thought disorder, externalizing, and internalizing spectra, accounting for between 10% (for ADHD) to 36% (for drug abuse) of the total observed phenotypic variance in these diagnoses (Pettersson et al., 2016). Findings also included two sets of genetic influences independent of the general factor, one specific to thought disorder (accounting for 31% of the phenotypic variance in schizophrenia, 45% in schizoaffective disorder, and 16% in bipolar disorders), the other to the internalizing and externalizing spectra (ranging from 6% of the phenotypic variance explained for MDD to 42% for drug abuse), suggesting that the latter two dimensions were genetically more similar to each other than to the thought disorder spectrum. A second study of 2,111 Norwegian twins identified moderately correlated (r=.16-.49) internalizing, disinhibited externalizing, antagonistic externalizing, and detachment genetic dimensions (Kendler, Aggen, et al., 2011). Although an overall higher-order general factor was not explicitly examined, the pattern of genetic correlations among the extracted spectra suggests that a degree of common genetic vulnerability to all disorders is likely.
When several disorders or symptoms belonging to a particular HiTOP spectrum (e.g. internalizing, disinhibited externalizing) are modeled jointly, a common genetic factor underpinning all of them typically emerges. For example, one study assessed antisocial behavior, conduct disorder, and drug, nicotine, and alcohol dependence in a sample of 1,999 biological and adoptive families, and identified a highly heritable disinhibited externalizing spectrum that captured a high proportion of the genetic influences on the five disorders (i.e. 61% of phenotypic variance in the latent externalizing factor was due to genetic influences), indicating genetic coherence of this spectrum (Hicks, Foster, Iacono, & McGue, 2013). The spectra are also genetically distinct, in that conditions belonging to the same spectrum share more genetic variance than conditions from different spectra. Nonetheless, spectra that are most frequently studied together, internalizing and externalizing, show a substantial genetic overlap (Cosgrove et al., 2011; Hink et al., 2013; Kendler & Myers, 2014; Kendler, Prescott, Myers, & Neale, 2003; Wolf et al., 2010), and developmental studies have found evidence for a single genetic factor influencing both internalizing and externalizing disorders (Ehringer, Rhee, Young, Corley, & Hewitt, 2006; Spatola et al., 2007). For example, Spatola et al. (2007) found that a common genetic factor accounted for 36–45% of total phenotypic variance of individual Child Behavior Checklist scales measuring internalizing and externalizing symptoms. Moreover, bifactor analyses that modeled an overarching general factor alongside specific internalizing and disinhibited externalizing dimensions have found that although the general factor explains most of the heritability of internalizing and externalizing problems, a significant proportion of genetic variance remained that was specific to the internalizing and externalizing spectra (Lahey et al., 2011; Mikolajewski, Allan, Hart, Lonigan, & Taylor, 2013; Tackett et al., 2013; Waldman, Poore, van Hulle, Rathouz, & Lahey, 2016).
At the level of subfactors, studies support independent genetic influences on fear, distress and substance abuse subfactors that emerge alongside higher-order factors (Hettema, Prescott, Myers, Neale, & Kendler, 2005; Kendler et al., 2003; Kendler et al., 1995; Waszczuk, Zavos, Gregory, & Eley, 2014). For example, in a sample of 1,549 young adult twins, a genetic fear subfactor influencing panic disorder, separation anxiety and social phobia symptoms was derived, accounting for 6–15% of total heritability of these symptoms dimensions, and was independent of overarching internalizing genetic influences shared with depression and generalized anxiety symptoms, that accounted for over half (63–85%) of the total heritability of each symptom dimension (Waszczuk et al., 2014). Finally, both eating pathology and antisocial behavior subfactors demonstrate subfactor-specific genetic influences (Bornovalova, Hicks, Iacono, & McGue, 2010; Bulik et al., 2010; Cosgrove et al., 2011; Hink et al., 2013; Lahey et al., 2011; Mikolajewski et al., 2013; O’Connor et al., 2016; Tuvblad, Zheng, Raine, & Baker, 2009), but it remains unclear whether genetic factors underpinning these subfactors are independent of influences on higher-order spectra because in many of these studies higher levels of the hierarchy have not been modelled.
Twin studies have begun to investigate genetic influences on individual syndromes, symptom components, and maladaptive traits. Previous studies investigated genetic influences underpinning narrow symptom components of depression (Kendler, Aggen, & Neale, 2013), OCD (Iervolino, Rijsdijk, Cherkas, Fullana, & Mataix-Cols, 2011), ADHD (McLoughlin, Ronald, Kuntsi, Asherson, & Plomin, 2007; Nikolas & Burt, 2010), aggression (Coccaro, Bergeman, Kavoussi, & Seroczynski, 1997; Vernon, McCarthy, Johnson, Jang, & Harris, 1999) and antisocial personality (Kendler, Aggen, & Patrick, 2012; Rosenström et al., 2017), finding that some genetic influences operate at the level of the overarching syndrome, whereas other genetic influences are specific to individual symptom components. For example, while there was a common genetic factor influencing five symptom components of OCD - checking, hoarding, obsessing, ordering, and washing, accounting for 20–35% of total phenotypic variance – the first four OCD components also showed independent genetic influences that accounted for 11–23% of total phenotypic variance (Iervolino et al., 2011).
Finally, non-shared environmental influences (i.e., those uniquely experienced by only one co-twin or sibling) can be modeled using family and twin data. Non-shared environmental influences are typically disorder-specific and contribute to the distinction among psychiatric conditions, as described in more detail in Supplement 2. Nonetheless, some environmental influences contribute to the coherence of higher-order HiTOP spectra, such as internalizing (Hettema, Neale, Myers, Prescott, & Kendler, 2006; Hettema et al., 2005; Mosing et al., 2009), externalizing (Bornovalova et al., 2010; Krueger et al., 2002; Tuvblad et al., 2009), thought disorder (Cardno et al., 2012) and somatoform (Kato, Sullivan, Evengård, & Pedersen, 2009) spectra, although they account for considerably less common variance in those phenotypes than higher-order genetic factors. For example, in a study of >30,000 Swedish twins, non-shared environmental influences constituted about one-third of influences on a general factor underpinning symptoms of depression, generalized anxiety, and four somatoform syndromes (chronic widespread pain, chronic fatigue, irritable bowel syndrome, and recurrent headache), and about one-half of influences on an independent somatoform spectrum (Kato et al., 2009).
Hypotheses and future directions for quantitative genetics
Taken together, the existing behavior genetic literature indicates that the HiTOP model may be well aligned with the genetic architecture of psychopathology observed by family and twin studies. It suggests that key features of the HiTOP hierarchy, which were largely derived from psychometric modeling, could be genetically coherent (Figure 2). Nonetheless, the phenotypic structure hypothesized by the HiTOP model should be tested directly using confirmatory structural twin modelling and adjudicated where possible using fit statistics, in order to rigorously assess the degree of alignment between the phenotypic and genetic architecture of psychopathology. In particular, genetically informed structural analyses are needed to confirm the hypothesized genetic structure of several understudied aspects of psychopathology, such as the detachment spectrum and sexual problems subfactor. Furthermore, using identified or hypothesized phenotypic structures as a starting point for comparing different models of higher-order genetic influences in a confirmatory manner might bias phenotypic and genetic literatures towards convergence. Therefore, hypothesis-free, exploratory analyses of genetic correlations should also be conducted, akin to the approach taken by Kendler, Aggen, et al. (2011). In case genetic models derived from HiTOP organization are not confirmed, alternative structural models derived directly from genetic data would be able to inform future genetic research as well as influencing future revision of phenotypic models. Indeed, such findings could challenge relevant aspects of the HiTOP model and accordingly lead to revisions of the model.
From a study design perspective, achieving these goals would be facilitated by the inclusion of more comprehensive, transdiagnostic assessments across the full spectrum of severity, ranging from relevant personality traits to severe clinical problems. From an analytic perspective, it will be useful to focus on measuring and modeling the genetic structure of the lower-order dimensions (symptom components and maladaptive traits) to build a truly bottom-up model. Ultimately, a twin study of the full HiTOP model is needed to test the genetic architecture of psychopathology comprehensively, ideally at multiple levels of the hierarchy simultaneously. Finally, the nature of the phenotypic general factor remains debated, with some suggestions that it reflects traits that nonspecifically increase the general risk for psychopathology and others raising the possibility that it captures distress or impairment common to all psychiatric illness (Caspi & Moffitt, 2018; Oltmanns, Smith, Oltmanns, & Widiger, 2018; Waldman et al., 2016). Etiologic hypotheses regarding the general factor can be evaluated empirically, for example by testing whether there are significant genetic and environmental correlations between the general factor and measured negative affect, functional impairment, and other psychosocial and environmental variables (Tackett et al., 2013; Waldman et al., 2016).
Molecular genetic studies – evidence for transdiagnostic genetic pleiotropy
The field of molecular psychiatric genetics has made enormous advances in the last decade. For example, case-control genome-wide association study (GWAS) meta-analyses conducted by the Psychiatric Genomics Consortium (PGC) led to identification of >150 independent genetic associations reaching genome-wide significance for schizophrenia, 30 for bipolar disorder and 12 for ADHD (Stahl et al., 2017; Sullivan et al., 2017), with findings even higher when combined with other datasets, e.g. a total of 102 significant hits for depression (Howard et al., 2018). These studies have also demonstrated a quantitatively distributed polygenetic liability to mental illness, on a continuum from trait variation in the general population to corresponding clinical diagnoses (Martin et al., 2017; Sullivan et al., 2017). Beyond notable disorder-specific findings, molecular genetic studies have also informed the transdiagnostic genetic architecture of psychopathology (Gizer, 2016; Santoro et al., 2016; Smoller, 2013; Smoller et al., 2018; Wray et al., 2014). The overarching pattern of findings from GWAS indicates widespread pleiotropy, with many genes and individual variants influencing more than one disorder (Cross-Disorder Group of the Psychiatric Genomics Consortium, 2013b; Serretti & Fabbri, 2013; Sivakumaran et al., 2011).
Use of polygenic risk score (PRS) methodology allows for an examination of the prediction of phenotypes using millions of genome-wide common variants, and also shared common variant genetic covariance across disorders and traits. This has perhaps been the most informative methodology for understanding latent genetic architecture and real-world prediction of traits, though like with other molecular genetic studies, this work has been limited by the depth and breadth of the phenotypes being studied. Several reports on PRSs have informed etiology in the last decade. For example, a PRS for smoking has been observed to be associated with alcohol and cannabis use (R2=0.4–1.5%) (Vink et al., 2014), in line with common genetic influences on various forms of substance abuse. Other work has found a PRS for ADHD to be significantly associated with comorbid conduct disorder symptoms, with R2= 1.1% higher in comorbid than pure ADHD cases (Hamshere et al., 2013), consistent with a broader externalizing spectrum. In the internalizing domain, a PRS for depression was also associated with anxiety, explaining about 2.1% of variance in anxiety (Demirkan et al., 2011). Finally, a PRS for schizophrenia was associated with nicotine use, depression and anxiety symptoms, and family history of depression, anxiety, alcohol use disorder and drug use (Docherty et al., 2017), which is in line with broad genetic risk for psychopathology. Consistent with broad influences of PRS, a recent study found that PRSs for schizophrenia and neuroticism were associated with a general factor of psychopathology in adolescence (β=.06 and 07, respectively), with little evidence for disorder-specific associations (Jones et al., 2018). In sum, PRSs appear to capture largely transdiagnostic genetic influences on phenotypic presentation, with uncertain specificity of the polygenic signal when applied to categorical phenotypes in isolation.
Studies investigating common variant SNP-based bivariate genetic correlations have also revealed patterns of genetic overlap that inform our understanding of the genetic architecture of psychopathology and are consistent with the HiTOP organization. For example, one PGC study found that bipolar disorder showed the highest SNP-based genetic correlation with schizophrenia (rg=.68), and a considerable correlation with depression (rg=.47) (Cross-Disorder Group of the Psychiatric Genomics Consortium, 2013a), and another study reported a genetic correlation between bipolar disorder and anorexia nervosa (rg=.16) (Lo et al., 2016), which accords with bipolar disorder’s position within both the thought disorder and internalizing spectra in HiTOP (Figure 1). This pattern of results has been reported in data from non-PGC sources (Wang, Gaitsch, Poon, Cox, & Rzhetsky, 2017), as well as in a recent gene expression investigation (Gandal et al., 2018). The latter study found the highest transcriptome correlation between bipolar disorder and schizophrenia (rt=.70), with both disorders also showing significant, albeit smaller, transcriptome overlap with depression (rt=.25 and .30, respectively). Recent work has also identified very high SNP-based genetic overlap between depression, mood and anxiety disorders (Wang et al., 2017), supporting genetic coherence of the internalizing spectrum. Another study created a SNP-based genetic correlation matrix comprising a number of phenotypes related to substance use and found a pattern of correlations indicating the presence of substance specific genetic effects, as well as high genetic overlap between use of different types of substances (e.g. rg=.83 between cannabis initiation and smoking initiation, rg=.44 between nicotine and alcohol consumption) (Nivard et al., 2016), which supports genetic coherence of the substance abuse subfactor. Finally, several studies have revealed genetic correlations between disorders from different higher-order spectra, which is in line with the overarching genetic pleiotropy that may indicate a general factor of psychopathology (Anttila et al., 2018; Bulik-Sullivan et al., 2015; Cross-Disorder Group of the Psychiatric Genomics Consortium, 2013a; Lo et al., 2016; Selzam, Coleman, Moffitt, Caspi, & Plomin, 2018; Wang et al., 2017). Nonetheless, it is also important to note that not all phenotypes showed significant PRS or SNP-based genetic correlations, and it is unclear whether these results point to low genetic overlap, or are a result of low discovery GWAS sample sizes for some conditions (i.e. PTSD).
How HiTOP phenotypes can facilitate genetic discovery
Despite enormous progress, molecular genetic discovery in psychiatry has been dependent on categorical, case-versus-control analyses embedded within traditional diagnostic classification systems. The HiTOP model endeavors to refine this phenotypic framework and accelerate genetic discovery in two major ways. First, the hierarchical approach provides alternative, empirically-validated phenotypic targets of genetic inquiry. Specifically, the model allows genetic studies to address problems associated with comorbidity by focusing on major dimensions underlying numerous psychiatric conditions, and to reduce issues associated with within-disorder heterogeneity, by focusing on well-characterized tight-knit lower-order dimensions (Hodgson, McGuffin, & Lewis, 2017; Mullins & Lewis, 2017; Van Der Sluis, Verhage, Posthuma, & Dolan, 2010). Currently, when a new genetic variant is significantly associated with (or PRS is created for) a particular disorder (e.g. MDD), it is uncertain whether it indicates risk for a particular symptom within this condition (e.g. anhedonia), or for a higher-order spectrum to which that condition belongs more broadly (e.g. the internalizing spectrum) (Gatt, Burton, Williams, & Schofield, 2015; Hettema, Chen, Sun, & Brown, 2015; Serretti & Fabbri, 2013). Furthermore, extensive heterogeneity within traditional diagnoses likely obscures links with symptom-specific genetic variants and therefore large samples are needed to find relations between these variants and diagnoses (Manchia et al., 2013; Wray & Maier, 2014). Using HiTOP phenotypes with a known placement within the hierarchy can resolve these concerns, and enable genetic studies to choose phenotypic targets of the specific breadth that the study aims to investigate, e.g. an internalizing spectrum, distress subfactor, or anhedonia component. Indeed, differential discovery expected to emerge at each level of the phenotypic structure, and the HiTOP model provides tools for explicating this architecture systematically. As such, the HiTOP model can increase the statistical power and precision of genetic research by providing internally consistent phenotypic targets at every level of the hierarchy. Specifically, power analyses demonstrate that optimized phenotypic modelling will appreciably increase power to detect genetic effects over the use of total scores (Van Der Sluis et al., 2010).
Second, the quantitative approach supported by the HiTOP model may increase statistical power for genetic discovery. As discussed above, quantitative phenotypes better capture illness severity and characterize subthreshold cases than categorical diagnoses (Markon et al., 2011; Shea et al., 2002). The loss of this information when using categorical diagnoses can weaken the genetic signal, for example when subthreshold cases are included in the control group or when diagnosis changes over time (as misclassification between cases and non-cases and between cases of different disorders is quite common even in well-designed studies) (Bromet et al., 2011; Moffitt et al., 2010). The HiTOP model allows for thresholding when it is pragmatically useful, but does not require it. Furthermore, HiTOP avoids concerns regarding the selection of healthy controls. Contrasting healthy controls and cases with a clinical diagnosis has significant limitations (Preacher, Rucker, MacCallum, & Nicewander, 2005; Sher & Trull, 1996; Uher & Rutter, 2012). In particular, healthy controls and clinical cases typically differ on characteristics unrelated to the psychopathology of interest, such as intelligence, socio-economic status, and co-occurring mental health symptoms, which can conflate estimates of genetic influences and genetic associations among disorders. In addition, diagnostic misclassification inflates genetic correlations (Kendler, Chatzinakos, & Bacanu, 2019; Wray, Lee, & Kendler, 2012). Instead, samples from a single population with a dimensional assessment of symptom severity (e.g., a representative community sample or unselected group of patients seeking mental health services) are generally easier to obtain and allow for more precise and clinically useful estimates of effect size.
Quantitative phenotypes enhance statistical power, with power analyses demonstrating that continuous phenotypes yield more power over categorical diagnoses in GWAS under many conditions (Van der Sluis, Posthuma, Nivard, Verhage, & Dolan, 2013; Van Der Sluis et al., 2010; Yang, Wray, & Visscher, 2010). Specifically, case-control designs have advantageous power when as many cases as controls are recruited under the condition of low disease prevalence, but if the sample is representative of the population, quantitative phenotypes yield higher power. Notably, it is often difficult to recruit sufficiently large samples with “rare” disorders to meet power requirements of case-control design. Finally, quantitative approaches can identify non-linearity in genetic influences, and thus can indicate whether certain aspects of psychopathology are indeed better represented as categories (rather than assuming that to be the case), and can inform optimal thresholds for such classifications (Plomin & Kovas, 2005). This has been empirically tested in twin studies, with overwhelming evidence for continuity of etiological influences across symptom severity (Martin et al., 2017; Plomin et al., 2009). Nonetheless, discontinuities in etiology have also been demonstrated, for example, one twin study found that the extreme low end of the ADHD spectrum has a different genetic etiology from higher levels of ADHD symptoms, suggesting that low ADHD might reflect different genetic influences (Greven et al., 2016).
Hypotheses and novel phenotypic approaches in molecular genetic studies
Hypotheses and molecular evidence relating to the hierarchical structure.
Although the existing literature suggests that it will be possible to identify genetic vulnerabilities associated with different levels of the HiTOP hierarchy, predictions of this model should be directly tested in molecular genetic studies. Specifically, the model posits a set of testable hypotheses that would organize and encourage the exploration of the interface between phenotypic and molecular genetic studies. First, different genetic findings are hypothesized to emerge at different levels of the HiTOP hierarchy. For example, if the phenotype was first partitioned using a bifactor model into variance specific to the general factor, spectrum, subfactor etc., and GWASs were conducted to identify genetic hits associated with each level, the model predicts (1) that genetic variants will be identified at each level, and (2) that different, non-overlapping genes will be identified at each level. The same two predictions hold for PRS built for these phenotypes, and for associated downstream biological pathways. As such, a higher-order approach to phenotypes may increase the precision of genetic findings, differentiating between genetic liability for broad psychopathology and dimension-specific genetic risk factors. One implication of such genetic findings is that they might inform Mendelian randomization studies (Pingault et al., 2018) by providing more precise and less pleiotropic instrumental variables (e.g. PRS scores) for psychiatric predictors.
Furthermore, the HiTOP model makes specific predictions about the pattern of genetic correlations. Specifically, the HiTOP model hypothesizes that dimensions within the same spectrum (or subfactor) will show stronger common variant SNP-based genetic correlations than dimensions assigned to different spectra or subfactors. For example, the HiTOP model predicts that worry may have higher genetic correlations with depression and traumatic re-experiencing than with interaction anxiety, which in turn will be larger than the genetic correlation of worry with callousness.
Predictions about general and specific genetic vulnerabilities have been supported by molecular genetic evidence. A recent study found that a genetic general factor of psychopathology, derived using principal component analysis of genetic correlation matrices from three different molecular methods (Genome-wide Complex Trait Analysis, Linkage-Disequilibrium Score Regression, and PRS correlations), accounted for 19–57% of all genetic variance for a range of psychiatric traits (Selzam et al., 2018). While this finding demonstrates that a significant proportion of genetic variance is captured by the general factor, the remaining, in some cases, even larger proportion of genetic variance is captured at lower levels of the hierarchy. This has been tested more formally by applications of genomic structural equation modeling (genomic SEM), which extracts common genetic dimensions from bivariate genetic associations (Grotzinger et al., 2018). The first study to use this approach suggested that while many genes broadly influence liability to numerous psychiatric disorders, other genetic factors remain disorder-specific, mirroring the hierarchical structure from phenotypic and twin modelling literatures (Grotzinger et al., 2018). Another study using the genomic SEM method has modelled the intermediate level of the hierarchy and found support for correlated internalizing, externalizing, and thought disorder spectra (Luningham, Poore, Yang, & Waldman, 2018). As the matrix of genetic correlations expands, genomic SEM will allow further evaluation of the alignment between genetic architecture and the HiTOP model.
Hypotheses and molecular evidence relating to redefined phenotypes.
Direct comparison of the number of SNPs identified using dimensional and hierarchical phenotypes vs. DSM-based disorders serves as a more direct test of the hypothesis that the model has incremental utility for genetic studies. We hypothesize that HiTOP phenotypes will help to identify a higher number of genetic variant effects. Already, some molecular genetic studies have begun using the approach of combining individual phenotypes to form higher-order spectra (Lee et al., 2016; McGue et al., 2013; Neumann et al., 2016; Otowa et al., 2016; Xu et al., 2015). For example, a meta-analysis of GWAS of generalized anxiety disorder, panic, agoraphobia, social and specific phobias identified common variants associated with an overarching factor (i.e., the Internalizing spectrum), which revealed novel genes (Otowa et al., 2016).
Moreover, the HiTOP model predicts that specific symptom dimensions will have a degree of unique genetic influences, but this assumption remains to be tested. It is plausible that at the lowest level of the hierarchy, unique influences might be largely environmental, in line with the generalist genes hypothesis (Eley, 1997; Plomin & Kovas, 2005). Recent, molecular genetic studies tested this by examining narrow components of disorders (Hodgson et al., 2017). For example, depression subtypes were found to be characterized by partially distinct polygenic liabilities (Milaneschi et al., 2016) and different genetic influences were found for different depression symptoms (Nagel et al., 2017; Thorp et al., 2019). Similarly, a recent GWAS focused on anhedonia among patients with MDD identified 18 variants specific to this symptom dimension, with anhedonia-specific PRS predicting antidepressant treatment efficacy (Ren et al., 2018). Furthermore, when GWAS targeted another very narrow phenotype - mood instability- four new genetic variants were discovered (Ward et al., 2017). Overall, this provides initial support for the hypothesis of unique genetic effects on lower-order dimensions.
How to HiTOP: a practical guide for genetic studies
There are several practical ways in which psychiatric geneticists can incorporate the HiTOP phenotypic definitions into their research and test HiTOP hypotheses further, as summarized in Box 1. Studies in planning stages could consider including measures of dimensional phenotypes that capture transdiagnostic phenotypes, as well as the full dimension of liability to and severity of psychopathology from low to moderate to high, and not just its maladaptive ends captured by case vs. control status (Conway et al., 2010; Greven, Buitelaar, & Salum, 2018; Van Der Sluis et al., 2010). Some of the HiTOP-compatible scales (interview as well as self-report) that allow for higher-order and bifactor modeling are listed in Kotov et al. (2017). Many of these assessments can be administered remotely (e.g. via online surveys accessible on mobile devices) and have been validated in short versions. For example, the Patient Health Questionnaire assesses depression symptoms using nine items (Kroenke, Spitzer, & Williams, 2001). Moreover, abbreviated measures can still capture symptom dimensions, for example the Personality Inventory for DSM-5 consists of 25 items that cover five domains of personality psychopathology (Krueger, Derringer, Markon, Watson, & Skodol, 2013). These measures are suitable for large data collection efforts often required in genetic studies. Furthermore, if diagnostic interviews are used, the studies could aim to assess all symptoms without applying hierarchical exclusion (“skip out”) rules. This may require substantial modifications to interview measures and longer interviewing times, but will allow researchers to quantify dimensions and assess their hierarchical organization, neither of which is advisable for variables affected by exclusion rules, as these rules usually introduce serious distortions in the data (Kotov, Ruggero, Krueger, Watson, & Zimmerman, 2018). Moreover, skip-out free psychopathology severity dimensions have shown superior validity and reliability over categorical diagnoses derived from the same interview (Shankman et al., 2018).
Box 1 -. How to HiTOP: a practical guide for genetic studies.
For studies that are in planning stages:
Instead of relying exclusively on categorical phenotypes, studies could consider measuring empirically established spectra, and well-defined, validated dimensions within them.
When diagnostic interviews are included as part of the assessment battery, consider eliminating “skip-out” rules in order to assess a wide range of symptoms even when the person cannot meet diagnostic criteria for given disorder.
Even studies that aim to focus on a particular disorder would benefit from collecting measures of related forms of psychopathology, including personality, to better capture stable traits underlying disorder risk.
When using existing datasets:
In instances where phenotypic information is available from diagnostic interviews, it is still often possible to create HiTOP-informed higher-order variables (e.g., internalizing score).
Likewise, if symptom-level data are available from interview or self-report measures, lower-order quantitative variables can be created.
When working with both binary/ordinal and continuous data, researchers can employ statistical techniques (e.g. latent variable, “bass-ackwards” modeling (Goldberg, 2006)) to derive phenotypes at a desired level of generality within the hierarchy.
Higher-order spectra can be created by pooling items across different measures.
When harmonizing phenotypes from different datasets, quantitative measures can be pooled following standardization with respect to published population norms; factors scores can be harmonized by anchoring them to a marker common between datasets.
Another way to capture numerous different traits and the full spectrum of severity is to include measures of personality alongside measures of psychopathology (Krueger et al., 2002; Widiger et al., 2018). Genetic studies of clinical disorders (e.g. alcohol abuse) could assess related traits such as personality pathology (e.g., disinhibition), which are known to precede and contribute to the development of different forms of psychopathology (Hur, Stockbridge, Fox, & Shackman, 2018; Krueger & Tackett, 2006; Shackman et al., 2016; Widiger, 2011) and have been mapped onto the HiTOP model (Widiger et al., 2018). Personality traits may constitute a more stable and thus reliable target for genetic studies than diagnoses, as those are often quite unstable over time (Chmielewski, Clark, Bagby, & Watson, 2015; Markon et al., 2011; Shea et al., 2002), primarily due to unreliability of diagnostic ratings (Regier et al., 2013). In contrast, meta-analyses report that rank-order stability of personality traits reaches .70 in mid to late adulthood (Caspi, Roberts, & Shiner, 2005; Roberts & DelVecchio, 2000). Rank-order stability of normal personality traits is also higher than that reported for psychopathology symptoms (Ormel et al., 2013; Prenoveau et al., 2011).
It is also possible to apply the HiTOP approach when analyzing existing genomic datasets. Many such datasets, for example those collected by the PGC or UK Biobank, are open source or available for secondary analyses upon request, with detailed instructions on how to access these data posted on Consortia websites. However, such data often contain minimal or categorical phenotyping, with different measures used across studies. We therefore propose a range of approaches for pooling and analyzing these data.
First, pooling of items can be done across different measures and datasets to create higher-order spectra, as long as they are standardized with respect to published population norms, and factor scores can be harmonized by anchoring them to a marker common between datasets. When pooling data, it is recommended to test measurement invariance to ensure that the same construct is captured by different measures or in populations studied across datasets (Vandenberg & Lance, 2000). If invariance is not evident, the data should not be pooled. Second, phenotypes at the desired level of generality within the hierarchy can be derived from both continuous and binary/ordinal variables by employing statistical techniques such as structural equation modelling to derive latent variables, and “bass-ackwards” modelling (Goldberg, 2006).
Third, traditional diagnoses can be scored into dimensional variables at different levels of the hierarchy (e.g. depression severity, internalizing spectrum, general factor) using structural equation modelling, factor analysis or simple counts of diagnoses (Forbes, Tackett, Markon, & Krueger, 2016). If symptom-level data are available, for example because no “skip out” rules were applied in diagnostic interviews or from self-report questionnaires, lower-order quantitative variables can be created, such as using symptom counts or factor analyses to derive empirically coherent syndromes. Often such validated, lower-order components and syndromes can simply be computed by scoring previously-derived subscales within measures. Notably, these techniques flexibly allow for the phenotypes to be derived either within higher-order (variance retained across levels) or hierarchical (variance divided between levels) frameworks (Footnote 1). Finally, this guidance extends beyond analyzing genotypic datasets, as the same principles can be applied to phenotypes included in downstream molecular studies (e.g. methylome and transcriptome data).
Future challenges
A number of outstanding issues remain to be noted. First, shared methods variance could affect the phenotypic structure by inflating loadings on the higher-order factors, or exaggerating associations between symptoms from the same scale. One implication of such potential bias for genetic studies is that GWAS conducted for higher-order latent factors might capture genetic signal that reflects shared methods variance (e.g. constructs related to the response style), alongside the psychopathology. However, bifactor decomposition removes both the variance due to higher-order factors and due to common method biases, leaving specific factors free of both influences. In analyses where shared methods variance is a concern, statistical approaches to remedy measured and unmeasured sources of this confound can be applied to phenotypes prior to conducting genetic analyses (Podsakoff, MacKenzie, Lee, & Podsakoff, 2003). Furthermore, where feasible, future phenotypic and genetic studies could broaden the measurements to include different raters and instruments, something that has been successfully accomplished in very large-scale twin studies (Rimfeld et al., 2019). Notably, genetic structure obtained from twin studies is less affected by this limitation because error is captured by non-shared environmental effects, and common rater effects are usually resolved by relying on correlations across twins’ independent reports.
Second, studies that derive structure by analysis of covariation among disorders can be affected by symptom overlap between diagnoses, which also can affect the genetic structure by inflating the genetic overlap between different scales. This issue can be addressed by analysis of symptom-level data. Third, there are alternative theoretical approaches to interpreting associations among symptoms of psychopathology, most notably network analysis, which posits that covariance of symptoms arise because some symptoms (or their functional consequences) exert causal influences on other symptoms (Borsboom & Cramer, 2013). Consequently, in the network modelling framework, genetic influences are thought to influence individual differences in the strength of the causal relations between the symptoms (i.e. the edges) (Cramer, Kendler, & Borsboom, 2011). Emerging evidence demonstrates that genetic relatedness may indeed moderate associations between individual symptoms (Hasmi et al., 2017; Smeets, Lataster, Viechtbauer, Delespaul, & GROUP, 2014). In the future, generalized network psychometrics that combines network and latent variable models might provide a novel, unified approach to investigating the genetic structure underpinning psychopathology (Epskamp, Rhemtulla, & Borsboom, 2017).
Fourth, although developmental twin and family studies provide support for the alignment of phenotypic and genetic structures within internalizing and disinhibited externalizing spectra, (e.g. Lahey et al. (2011); (Mikolajewski et al., 2013), less is known about other spectra, and age differences and developmental trajectories of the higher-order genetic structure have been explicitly modeled in only a few twin studies (Waszczuk, Waaktaar, Eley, & Torgersen, 2019; Waszczuk et al., 2014; Waszczuk, Zavos, Gregory, & Eley, 2016). A better understanding of genetic influences on psychopathology in young people would inform whether different phenotyping definitions are required when conducting molecular genetic research with developmental samples.
Fifth, while the internalizing, externalizing, and thought disorder spectra, as well as many dimensions within them, have shown cross-cultural generalizability (de Jonge et al., 2018; Ivanova et al., 2007; Ivanova et al., 2015; Krueger, Chentsova-Dutton, Markon, Goldberg, & Ormel, 2003), other parts of the HiTOP model need to be similarly studied. Importantly, structural family and twin studies in non-Western populations are too rare to draw conclusions about the cross-cultural generalizability of higher-order genetic influences on psychopathology. Nonetheless, to date emerging evidence suggests that there may be cross-cultural generalizability (Ball et al., 2011), and genetic influences on normal personality have been shown to be invariant across cultures (Yamagata et al., 2006). Likewise, molecular psychiatric genetic research is limited by its heavy focus on populations of European ancestry (Martin et al., 2018; Torkamani, Wineinger, & Topol, 2018). Until other ancestry-specific GWAS are conducted for a wide range of psychopathology, the cross-cultural generalizability of genetic contributions to mental health cannot be addressed (Docherty et al., 2017).
Sixth, other current limitations of the HiTOP model that may impact its ability to inform genetic research, include no consideration of features such as the age of onset or chronicity, and uncertain placement of developmental disorders, due to paucity of structural data available on this topic (Kotov et al., 2017), and lack of a current validated comprehensive measure of all aspects of the model. Finally, concerning feasibility, expanding phenotypic assessments may increase participant burden and resources needed to conduct the genetic studies, in particular when large sample sizes are required. However, in some cases, such studies may need to add only a small number of psychometrically-sound measures to supplement the existing datasets, and the burden can be reduced by remote data collection, abbreviated instruments, or integration of information from electronic health records and behavioral tracking via phones or wearable devices. Overall, the extra effort required to obtain comprehensive phenotyping is a worthwhile investment to enable novel research in the field of psychiatric genetics, and a necessary translational step for connecting genotype data to the diverse clinical manifestation of mental illness.
Conclusions
Genetic discovery in psychiatry is hindered by suboptimal phenotypic definitions. We argue that the hierarchical, quantitative, and data-driven classification system proposed by the HiTOP consortium may provide a more effective approach to identifying genetic markers of mental illness than traditional diagnostic categories. The HiTOP approach promises to resolve problems of comorbidity, heterogeneity, and arbitrary diagnostic thresholds that impede progress in psychiatric genetics. In particular, genetic variants are expected to operate at different levels of the HiTOP hierarchy, with some highly pleiotropic genes influencing higher-order psychopathology, and others conferring risk for specific spectra, subfactors, or symptom components. We also demonstrated that the HiTOP model aligns well with our current understanding of the higher-order genetic structure of psychopathology, emerging from biometrical and molecular genetic findings of broad genetic pleiotropy.
Supplementary Material
Acknowledgements
We would like to thank Drs. Terrie Moffitt, Avshalom Caspi, and Douglas Samuel for providing comments on the manuscript.
Alexander Shackman is supported by the National Institutes of Health (DA040717, MH107444) and University of Maryland. Eiko Fried is supported by the ERC Consolidator Grant no. 647209.
Footnotes
None of the authors of this study report any conflicts of interest.
The current paper is a literature review and for this reason no ethics committee approval was required to conduct the project.
Some of the ideas included in the manuscript were presented at scientific meetings:
Waszczuk, M. A. (2017, October). Hierarchical Taxonomy of Psychopathology Model and Psychiatric Genetics. Paper presented at the second Hierarchical Taxonomy of Psychopathology Meeting, Baltimore, Maryland, USA.
Waszczuk, M. A. et al. (2018, June). Redefining phenotypes to advance psychiatric genetics: implications from the Hierarchical Taxonomy of Psychopathology. Paper presented at the annual meeting of the Behavior Genetics Association, Boston, Massachusetts, USA.
Footnote 1: There are two broad, complimentary classes of approaches to modeling the structure of psychopathology: higher-order and hierarchical/bifactor models (Markon, 2019). In higher-order models, general factors explain specific factors, with the latter nested in the former, and general variance is retained as one considers lower levels. The phenotypic HiTOP model is conceptualized as such higher-order model. In hierarchical/bifactor models, general and specific factors are orthogonal and uncorrelated with one another, explaining distinct nonnested components of shared variance among indicators. Higher-order structural biometric models usually consist of hierarchical approach. Thus, when discussing etiological influences, the total genetic variance in psychopathology is often divided into different levels of specificity. Consequently, an intermediate dimension such as the fear subfactor, when conceptualized in the higher-order framework, is influenced by genes operating at different levels of specificity, including general psychopathology genes, genes specific to the internalizing spectrum, and genes specific to the fear subfactor itself. In the hierarchical framework, if the unique phenotypic variance of the fear subfactor is extracted, for example using bifactor modelling (Reise, 2012), it is only influenced by the genes specific to the fear subfactor. The current review operationalizes etiological influences in the hierarchical framework due to this approach being used in biometric studies.
Supplementary Materials
Waszczuk, M. A., Eaton, N. R., Krueger, R. F., Shackman, A. J., Waldman, I. D., Zald, D. H., Lahey, B. B., Patrick, C. J., Conway, C. C., Ormel, J., Hyman, S. E., Fried, E. I., Forbes, M. K., Docherty, A., Althoff, R. R., Bach, B., Chmielewski, M., DeYoung, C. G., Forbush, K. T., Hallquist, M., Hopwood, C. J., Ivanova, M., Jonas, K. G., Latzman, R. D., Markon, K. E., Mullins-Sweatt, S. N., Pincus, A. L., Reininghaus, U., South, S. C., Tackett, J. L., Watson, D., Wright, A. G. C., Kotov, R. Redefining phenotypes to advance psychiatric genetics: Implications from the Hierarchical Taxonomy of Psychopathology.
References
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders, DSM-5 (Fifth ed.). Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- Anda RF, Felitti VJ, Bremner JD, Walker JD, Whitfield C, Perry BD, … Giles WH (2006). The enduring effects of abuse and related adverse experiences in childhood. European Archives of Psychiatry and Clinical Neuroscience, 256(3), 174–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anttila V, Bulik-Sullivan B, Finucane HK, Bras J, Duncan L, Escott-Price V, … Patsopoulos N (2018). Analysis of shared heritability in common disorders of the brain. science, 360(6395), eaap8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ask H, Waaktaar T, Seglem KB, & Torgersen S (2016). Common etiological sources of anxiety, depression, and somatic complaints in adolescents: a multiple rater twin study. Journal of Abnormal Child Psychology, 44(1), 101–114. [DOI] [PubMed] [Google Scholar]
- Aslinger EN, Manuck SB, Pilkonis PA, Simms LJ, & Wright AG (2018). Narcissist or narcissistic? Evaluation of the latent structure of narcissistic personality disorder. Journal of Abnormal Psychology, 127(5), 496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball HA, Siribaddana SH, Sumathipala A, Kovas Y, Glozier N, Rijsdijk F, … Hotopf M (2011). Genetic and environmental contributions to the overlap between psychological, fatigue and somatic symptoms: a twin study in Sri Lanka. Twin Research and Human Genetics, 14(01), 53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornovalova MA, Hicks BM, Iacono WG, & McGue M (2010). Familial transmission and heritability of childhood disruptive disorders. American Journal of Psychiatry, 167(9), 1066–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsboom D, & Cramer AO (2013). Network analysis: an integrative approach to the structure of psychopathology. Annual Review of Clinical Psychology, 9, 91–121. [DOI] [PubMed] [Google Scholar]
- Bramon E, & Sham PC (2001). The common genetic liability between schizophrenia and bipolar disorder: a review. Current psychiatry reports, 3(4), 332–337. [DOI] [PubMed] [Google Scholar]
- Bromet EJ, Kotov R, Fochtmann LJ, Carlson GA, Tanenberg-Karant M, Ruggero C, & Chang S. w. (2011). Diagnostic shifts during the decade following first admission for psychosis. American Journal of Psychiatry, 168(11), 1186–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulik-Sullivan B, Finucane HK, Anttila V, Gusev A, Day FR, Loh P-R, … Robinson EB (2015). An atlas of genetic correlations across human diseases and traits. Nature Genetics, 47(11), 1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulik CM, Thornton LM, Root TL, Pisetsky EM, Lichtenstein P, & Pedersen NL (2010). Understanding the relation between anorexia nervosa and bulimia nervosa in a Swedish national twin sample. Biological Psychiatry, 67(1), 71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt SA, Krueger RF, McGue M, & Iacono WG (2001). Sources of covariation among attention-deficit/hyperactivity disorder, oppositional defiant disorder, and conduct disorder: the importance of shared environment. Journal of Abnormal Psychology, 110(4), 516. [DOI] [PubMed] [Google Scholar]
- Calkins ME, Curtis CE, Grove WM, & Iacono WG (2004). Multiple dimensions of schizotypy in first degree biological relatives of schizophrenia patients. Schizophrenia Bulletin, 30(2), 317–325. [DOI] [PubMed] [Google Scholar]
- Cardno AG, Rijsdijk FV, Sham PC, Murray RM, & McGuffin P (2002). A twin study of genetic relationships between psychotic symptoms. American Journal of Psychiatry, 159(4), 539–545. [DOI] [PubMed] [Google Scholar]
- Cardno AG, Rijsdijk FV, West RM, Gottesman II, Craddock N, Murray RM, & McGuffin P (2012). A twin study of schizoaffective-mania, schizoaffective-depression, and other psychotic syndromes. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 159(2), 172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Houts RM, Belsky DW, Goldman-Mellor SJ, Harrington H, Israel S, … Poulton R (2014). The p factor one general psychopathology factor in the structure of psychiatric disorders? Clinical Psychological Science, 2(2), 119–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, & Moffitt TE (2018). All for one and one for all: Mental disorders in one dimension. American Journal of Psychiatry, appi. ajp. 2018.17121383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Roberts BW, & Shiner RL (2005). Personality development: Stability and change. Annual Review of Psychology, 56, 453–484. [DOI] [PubMed] [Google Scholar]
- Chmielewski M, Clark LA, Bagby RM, & Watson D (2015). Method matters: Understanding diagnostic reliability in DSM-IV and DSM-5. Journal of Abnormal Psychology, 124(3), 764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccaro EF, Bergeman CS, Kavoussi RJ, & Seroczynski AD (1997). Heritability of aggression and irritability: a twin study of the Buss—Durkee Aggression Scales in adult male subjects. Biological Psychiatry, 41(3), 273–284. [DOI] [PubMed] [Google Scholar]
- Conway C, Hammen C, & Brennan P (2012). A comparison of latent class, latent trait, and factor mixture models of DSM-IV borderline personality disorder criteria in a community setting: Implications for DSM-5. Journal of Personality Disorders, 26(5), 793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway CC, Forbes MK, Forbush KT, Fried EI, Hallquist MN, Kotov R, … South SC (2018). A hierarchical taxonomy of psychopathology can transform mental health research. Perspectives on Psychological Science, 14(3), 419–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway KP, Levy J, Vanyukov M, Chandler R, Rutter J, Swan GE, & Neale M (2010). Measuring addiction propensity and severity: the need for a new instrument. Drug and Alcohol Dependence, 111(1–2), 4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove VE, Rhee SH, Gelhorn HL, Boeldt D, Corley RC, Ehringer MA, … Hewitt JK (2011). Structure and etiology of co-occurring internalizing and externalizing disorders in adolescents. Journal of Abnormal Child Psychology, 39(1), 109–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer AO, Kendler KS, & Borsboom D (2011). Where are the genes? The implications of a network perspective on gene hunting in psychopathology.[A commentary on Johnson et al.]. European Journal of Personality, 25. [Google Scholar]
- Cross-Disorder Group of the Psychiatric Genomics Consortium. (2013a). Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nature Genetics, 45(9), 984–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross-Disorder Group of the Psychiatric Genomics Consortium. (2013b). Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. The Lancet, 381(9875), 1371–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jonge P, Wardenaar KJ, Lim CC, Aguilar-Gaxiola S, Alonso J, Andrade LH, … Gureje O (2018). The cross-national structure of mental disorders: results from the World Mental Health Surveys. Psychological Medicine, 48(12), 2073–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirkan A, Penninx BW, Hek K, Wray NR, Amin N, Aulchenko YS, … Uitterlinden AG (2011). Genetic risk profiles for depression and anxiety in adult and elderly cohorts. Molecular Psychiatry, 16(7), 773–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty AR, Moscati A, Dick D, Savage JE, Salvatore JE, Cooke M, … Riley BP (2017). Polygenic prediction of the phenome, across ancestry, in emerging adulthood. bioRxiv, 124651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton NR (2014). Transdiagnostic psychopathology factors and sexual minority mental health: Evidence of disparities and associations with minority stressors. Psychology of sexual orientation and gender diversity, 1(3), 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton NR, Krueger RF, Markon KE, Keyes KM, Skodol AE, Wall M, … Grant BF (2013). The structure and predictive validity of the internalizing disorders. Journal of Abnormal Psychology, 122(1), 86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehringer MA, Rhee SH, Young S, Corley R, & Hewitt JK (2006). Genetic and environmental contributions to common psychopathologies of childhood and adolescence: a study of twins and their siblings. Journal of abnormal child psychology, 34(1), 1–17. [DOI] [PubMed] [Google Scholar]
- Eley TC (1997). General genes: A new theme in developmental psychopathology. Current Directions in Psychological Science, 6, 90–95. [Google Scholar]
- Eley TC, Bolton D, O’Connor TG, Perrin S, Smith P, & Plomin R (2003). A twin study of anxiety-related behaviours in pre-school children. Journal of child psychology and psychiatry, 44(7), 945–960. [DOI] [PubMed] [Google Scholar]
- Epskamp S, Rhemtulla M, & Borsboom D (2017). Generalized network pschometrics: Combining network and latent variable models. Psychometrika, 82(4), 904–927. [DOI] [PubMed] [Google Scholar]
- Ettinger U, Meyhöfer I, Steffens M, Wagner M, & Koutsouleris N (2014). Genetics, cognition, and neurobiology of schizotypal personality: a review of the overlap with schizophrenia. Frontiers in psychiatry, 5, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flett GL, Vredenburg K, & Krames L (1997). The continuity of depression in clinical and nonclinical samples. Psychological Bulletin, 121(3), 395. [DOI] [PubMed] [Google Scholar]
- Forbes MK, Baillie AJ, Eaton NR, & Krueger RF (2017). A place for sexual dysfunctions in an empirical taxonomy of psychopathology. The Journal of Sex Research, 54(4–5), 465–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes MK, Baillie AJ, & Schniering CA (2016). Where do sexual dysfunctions fit into the meta-structure of psychopathology? A factor mixture analysis. Archives of Sexual Behavior, 45(8), 1883–1896. [DOI] [PubMed] [Google Scholar]
- Forbes MK, Tackett JL, Markon KE, & Krueger RF (2016). Beyond comorbidity: Toward a dimensional and hierarchical approach to understanding psychopathology across the life span. Development and Psychopathology, 28(4pt1), 971–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbush KT, & Wildes JE (2017). Application of structural equation mixture modeling to characterize the latent structure of eating pathology. International Journal of Eating Disorders, 50(5), 542–550. [DOI] [PubMed] [Google Scholar]
- Gandal MJ, Haney JR, Parikshak NN, Leppa V, Ramaswami G, Hartl C, … Werge TM (2018). Shared molecular neuropathology across major psychiatric disorders parallels polygenic overlap. science, 359(6376), 693–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandal MJ, Leppa V, Won H, Parikshak NN, & Geschwind DH (2016). The road to precision psychiatry: translating genetics into disease mechanisms. Nature Neuroscience, 19(11), 1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatt JM, Burton KL, Williams LM, & Schofield PR (2015). Specific and common genes implicated across major mental disorders: a review of meta-analysis studies. Journal of Psychiatric Research, 60, 1–13. [DOI] [PubMed] [Google Scholar]
- Geschwind DH, & Flint J (2015). Genetics and genomics of psychiatric disease. science, 349(6255), 1489–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie N, Zhu G, Heath A, Hickie I, & Martin N (2000). The genetic aetiology of somatic distress. Psychological Medicine, 30(05), 1051–1061. [DOI] [PubMed] [Google Scholar]
- Gizer IR (2016). Molecular genetic approaches to understanding the comorbidity of psychiatric disorders. Development and Psychopathology, 28(4pt1), 1089–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg LR (2006). Doing it all bass-ackwards: The development of hierarchical factor structures from the top down. Journal of Research in Personality, 40(4), 347–358. [Google Scholar]
- Greven CU, Buitelaar JK, & Salum GA (2018). From positive psychology to psychopathology: the continuum of attention-deficit hyperactivity disorder. Journal of child psychology and psychiatry, 59(3), 203–212. [DOI] [PubMed] [Google Scholar]
- Greven CU, Merwood A, van der Meer JM, Haworth CM, Rommelse N, & Buitelaar JK (2016). The opposite end of the attention deficit hyperactivity disorder continuum: genetic and environmental aetiologies of extremely low ADHD traits. Journal of child psychology and psychiatry, 57(4), 523–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotzinger AD, Rhemtulla M, de Vlaming R, Ritchie SJ, Mallard TT, Hill WD, … Koellinger PD (2018). Genomic SEM Provides Insights into the Multivariate Genetic Architecture of Complex Traits. bioRxiv, 305029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamshere ML, Langley K, Martin J, Agha SS, Stergiakouli E, Anney RJ, … Neale BM (2013). High loading of polygenic risk for ADHD in children with comorbid aggression. American Journal of Psychiatry, 170(8), 909–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansell N, Wright M, Medland S, Davenport T, Wray N, Martin N, & Hickie I (2012). Genetic co-morbidity between neuroticism, anxiety/depression and somatic distress in a population sample of adolescent and young adult twins. Psychological Medicine, 42(06), 1249–1260. [DOI] [PubMed] [Google Scholar]
- Haslam N, Holland E, & Kuppens P (2012). Categories versus dimensions in personality and psychopathology: a quantitative review of taxometric research. Psychological Medicine, 42(5), 903–920. [DOI] [PubMed] [Google Scholar]
- Hasmi L, Drukker M, Guloksuz S, Menne-Lothmann C, Decoster J, van Winkel R, … Derom C (2017). Network approach to understanding emotion dynamics in relation to childhood trauma and genetic liability to psychopathology: replication of a prospective experience sampling analysis. Frontiers in Psychology, 8, 1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helzer JE, Kraemer HC, Krueger RF, Wittchen H-U, Sirovatka PJ, & Regier DA (2009). Dimensional approaches in diagnostic classification: Refining the research agenda for DSM-V: American Psychiatric Pub. [Google Scholar]
- Hettema JM, Chen X, Sun C, & Brown T (2015). Direct, indirect and pleiotropic effects of candidate genes on internalizing disorder psychopathology. Psychological Medicine, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettema JM, Neale MC, Myers JM, Prescott CA, & Kendler KS (2006). A population-based twin study of the relationship between neuroticism and internalizing disorders. American Journal of Psychiatry, 163(5), 857–864. Retrieved from http://ajp.psychiatryonline.org/cgi/reprint/163/5/857 [DOI] [PubMed] [Google Scholar]
- Hettema JM, Prescott CA, Myers JM, Neale MC, & Kendler KS (2005). The structure of genetic and environmental risk factors for anxiety disorders in men and women. Archives of General Psychiatry, 62(2), 182–189. Retrieved from http://archpsyc.ama-assn.org/cgi/reprint/62/2/182 [DOI] [PubMed] [Google Scholar]
- Hicks BM, Foster KT, Iacono WG, & McGue M (2013). Genetic and environmental influences on the familial transmission of externalizing disorders in adoptive and twin offspring. JAMA Psychiatry, 70(10), 1076–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks BM, Krueger RF, Iacono WG, McGue M, & Patrick CJ (2004). Family transmission and heritability of externalizing disorders: a twin-family study. Archives of General Psychiatry, 61(9), 922–928. [DOI] [PubMed] [Google Scholar]
- Hicks BM, Schalet BD, Malone SM, Iacono WG, & McGue M (2011). Psychometric and genetic architecture of substance use disorder and behavioral disinhibition measures for gene association studies. Behavior Genetics, 41(4), 459–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hink LK, Rhee SH, Corley RP, Cosgrove VE, Hewitt JK, Schulz-Heik RJ, … Waldman ID (2013). Personality dimensions as common and broadband-specific features for internalizing and externalizing disorders. Journal of Abnormal Child Psychology, 41(6), 939–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson K, McGuffin P, & Lewis CM (2017). Advancing psychiatric genetics through dissecting heterogeneity. Human Molecular Genetics, 27(R2), R160–R165. [DOI] [PubMed] [Google Scholar]
- Howard DM, Adams MJ, Clarke T-K, Hafferty JD, Gibson J, Shirali M, … McIntosh AM (2018). Genome-wide meta-analysis of depression in 807,553 individuals identifies 102 independent variants with replication in a further 1,507,153 individuals. bioRxiv. Retrieved from http://biorxiv.org/content/early/2018/10/09/433367.abstract
- Hur J, Stockbridge MD, Fox AS, & Shackman AJ (2018). Dispositional negativity, cognition, and anxiety disorders: An integrative translational neuroscience framework. Progress in Brain Research, 247, 375–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iervolino AC, Rijsdijk FV, Cherkas L, Fullana MA, & Mataix-Cols D (2011). A multivariate twin study of obsessive-compulsive symptom dimensions. Archives of General Psychiatry, 68(6), 637–644. [DOI] [PubMed] [Google Scholar]
- Ivanova MY, Achenbach TM, Dumenci L, Rescorla LA, Almqvist F, Weintraub S, … Dobrean A (2007). Testing the 8-syndrome structure of the child behavior checklist in 30 societies. Journal of Clinical Child and Adolescent Psychology, 36(3), 405–417. [DOI] [PubMed] [Google Scholar]
- Ivanova MY, Achenbach TM, Rescorla LA, Turner LV, Ahmeti-Pronaj A, Au A, … Chen Y-C (2015). Syndromes of Self-Reported Psychopathology for Ages 18–59 in 29 Societies. Journal of Psychopathology and Behavioral Assessment, 37(2), 171–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobi C, Hayward C, de Zwaan M, Kraemer HC, & Agras WS (2004). Coming to terms with risk factors for eating disorders: application of risk terminology and suggestions for a general taxonomy. Psychological bulletin, 130(1), 19. [DOI] [PubMed] [Google Scholar]
- Jones HJ, Heron J, Hammerton G, Stochl J, Jones PB, Cannon M, … Linden DE (2018). Investigating the genetic architecture of general and specific psychopathology in adolescence. Translational psychiatry, 8(1), 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Sullivan PF, Evengård B, & Pedersen NL (2009). A population-based twin study of functional somatic syndromes. Psychological Medicine, 39(03), 497–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Aggen SH, Czajkowski N, Roysamb E, Tambs K, Torgersen S, … Reichborn-Kjennerud T (2008). The structure of genetic and environmental risk factors for DSM-IV personality disorders: a multivariate twin study. Archives of General Psychiatry, 65(12), 1438–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Aggen SH, Knudsen GP, Røysamb E, Neale MC, & Reichborn-Kjennerud T (2011). The structure of genetic and environmental risk factors for syndromal and subsyndromal common DSM-IV axis I and all axis II disorders. The American journal of psychiatry, 168(1), 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Aggen SH, & Neale MC (2013). Evidence for multiple genetic factors underlying DSM-IV criteria for major depression. JAMA Psychiatry, 70(6), 599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Aggen SH, & Patrick CJ (2012). A multivariate twin study of the DSM-IV criteria for antisocial personality disorder. Biological Psychiatry, 71(3), 247–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Chatzinakos C, & Bacanu S-A (2019). The Impact on Estimations of Genetic Correlations of the Use, in Genome Wide Case-Control Studies, of Super-Normal, Unscreened and Family-History Screened Controls. bioRxiv, 693614. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Czajkowski N, Tambs K, Torgersen S, Aggen SH, Neal MS, & Reichborn-Kjennerud T (2006). Dimensional representation of DSM-IV Cluster A personality disorders in a population-based sample of Norweigen twins: A multivariate study. Psychological Medicine, 36, 1583–1591. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Czajkowski N, Tambs K, Torgersen S, Aggen SH, Neale MC, & Reichborn-Kjennerud T (2006). Dimensional representations of DSM-IV cluster A personality disorders in a population-based sample of Norwegian twins: a multivariate study. Psychological Medicine, 36(11), 1583–1591. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Davis CG, & Kessler RC (1997). The familial aggregation of common psychiatric and substance use disorders in the National Comorbidity Survey: a family history study. The British Journal of Psychiatry, 170(6), 541–548. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Eaves LJ, Loken EK, Pedersen NL, Middeldorp CM, Reynolds C, … Gardner CO (2011). The impact of environmental experiences on symptoms of anxiety and depression across the life span. Psychological Science, 22(10), 1343–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, & Gardner CO (1997). The risk for psychiatric disorders in relatives of schizophrenic and control probands: a comparison of three independent studies. Psychological Medicine, 27(02), 411–419. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, Neale MC, Aggen S, Heath A, Colodro-Conde L, … Gillespie NA (2018). Shared and specific genetic risk factors for lifetime major depression, depressive symptoms and neuroticism in three population-based twin samples. Psychological Medicine, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, & Myers J (2014). The boundaries of the internalizing and externalizing genetic spectra in men and women. Psychological Medicine, 44(03), 647–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Kessler RC, Heath AC, & Eaves LJ (1993). A longitudinal twin study of personality and major depression in women. Archives of General Psychiatry, 50, 853–862. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Myers J, & Neale MC (2003). The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Archives of General Psychiatry, 60(9), 929–937. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Walters EE, Neale MC, Kessler RC, Heath AC, & Eaves LJ (1995). The structure of the genetic and environmental risk factors for six major psychiatric disorders in women: Phobia, generalized anxiety disorder, panic disorder, bulimia, major depression, and alcoholism. Archives of General Psychiatry, 52, 374–383. [DOI] [PubMed] [Google Scholar]
- Keyes KM, Eaton NR, Krueger RF, McLaughlin KA, Wall MM, Grant BF, & Hasin DS (2012). Childhood maltreatment and the structure of common psychiatric disorders. The British Journal of Psychiatry, 200(2), 107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kläning U, Trumbetta SL, Gottesman II, Skytthe A, Kyvik KO, & Bertelsen A (2016). A danish twin study of schizophrenia liability: investigation from interviewed twins for genetic links to affective psychoses and for cross-cohort comparisons. Behavior Genetics, 46(2), 193–204. [DOI] [PubMed] [Google Scholar]
- Klein DN, & Kotov R (2016). Course of depression in a 10-year prospective study: Evidence for qualitatively distinct subgroups. Journal of Abnormal Psychology, 125(3), 337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotov R, Krueger RF, & Watson D (2018). A paradigm shift in psychiatric classification: the Hierarchical Taxonomy Of Psychopathology (HiTOP). World Psychiatry, 17(1), 24–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotov R, Krueger RF, Watson D, Achenbach TM, Althoff RR, Bagby M, … Zimmerman M (2017). The Hierarchical Taxonomy Of Psychopathology (HiTOP): A dimensional alternative to traditional nosologies. Journal of abnormal psychology, 126(4), 454. [DOI] [PubMed] [Google Scholar]
- Kotov R, Ruggero CJ, Krueger RF, Watson D, & Zimmerman M (2018). The perils of hierarchical exclusion rules: A further word of caution. Depression and anxiety, 35(9), 903–904. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, & Williams JB (2001). The Phq-9. Journal of General Internal Medicine, 16(9), 606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, Chentsova-Dutton YE, Markon KE, Goldberg D, & Ormel J (2003). A cross-cultural study of the structure of comorbidity among common psychopathological syndromes in the general health care setting. Journal of abnormal psychology, 112(3), 437. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Derringer J, Markon K, Watson D, & Skodol A (2013). The personality inventory for DSM-5—brief form (PID-5-BF)—adult. Washington, DC: American Psychiatric Association. [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, & McGue M (2002). Etiologic connections among substance dependence, antisocial behavior, and personality: modeling the externalizing spectrum. Journal of Abnormal Psychology, 111(3), 411–424. [PubMed] [Google Scholar]
- Krueger RF, Kotov R, Watson D, Forbes MK, Eaton NR, Ruggero CJ, … Zimmerman J (2018). Progress in achieving empirical classification of psychopathology. World Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, Markon KE, Patrick CJ, & Iacono WG (2005). Externalizing psychopathology in adulthood: A dimensional-spectrum conceptualization and its implications for DSM-V. Journal of abnormal psychology, 114(4), 537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, & Tackett JL (2006). Personality and psychopathology: Guilford Press. [Google Scholar]
- Lahey BB, Applegate B, Hakes JK, Zald DH, Hariri AR, & Rathouz PJ (2012). Is there a general factor of prevalent psychopathology during adulthood? Journal of abnormal psychology, 121(4), 971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey BB, Krueger RF, Rathouz PJ, Waldman ID, & Zald DH (2016). A Hierarchical Causal Taxonomy of Psychopathology Across the Life Span. Psychological Bulletin, 143(2), 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey BB, Krueger RF, Rathouz PJ, Waldman ID, & Zald DH (2017). Validity and utility of the general factor of psychopathology. World Psychiatry, 16(2), 142–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey BB, Van Hulle CA, Singh AL, Waldman ID, & Rathouz PJ (2011). Higher-order genetic and environmental structure of prevalent forms of child and adolescent psychopathology. Archives of General Psychiatry, 68(2), 181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb DJ, Middeldorp CM, Van Beijsterveldt CE, & Boomsma DI (2012). Gene–environment interaction in teacher-rated internalizing and externalizing problem behavior in 7-to 12-year-old twins. Journal of child psychology and psychiatry, 53(8), 818–825. [DOI] [PubMed] [Google Scholar]
- Lee M, Aggen SH, Otowa T, Castelao E, Preisig M, Grabe HJ, … Tiemeier H (2016). Assessment and characterization of phenotypic heterogeneity of anxiety disorders across five large cohorts. International Journal of Methods in Psychiatric Research, 25(4), 255–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester KJ, & Eley TC (2013). Therapygenetics: Using genetic markers to predict response to psychological treatment for mood and anxiety disorders. Biology of mood & anxiety disorders, 3(4), 1–16. doi: 10.1186/2045-5380-3-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein P, Yip BH, Björk C, Pawitan Y, Cannon TD, Sullivan PF, & Hultman CM (2009). Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. The Lancet, 373(9659), 234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livesley WJ, Jang KL, & Vernon PA (1998). Phenotypic and genetic structure of traits delineating personality disorder. Archives of General Psychiatry, 55(10), 941–948. [DOI] [PubMed] [Google Scholar]
- Lo M-T, Hinds DA, Tung JY, Franz C, Fan C-C, Wang Y, … Kauppi K (2016). Genome-wide analyses for personality traits identify six genomic loci and show correlations with psychiatric disorders. Nature Genetics, 49(1), 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luningham JM, Poore HE, Yang J, & Waldman ID (2018). Testing Structural Models of Psychopathology at the Genomic Level. bioRxiv, 502039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, Donnellan MB, Burt SA, & Klump KL (2016). The dimensional nature of eating pathology: Evidence from a direct comparison of categorical, dimensional, and hybrid models. Journal of Abnormal Psychology, 125(5), 715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manchia M, Cullis J, Turecki G, Rouleau GA, Uher R, & Alda M (2013). The impact of phenotypic and genetic heterogeneity on results of genome wide association studies of complex diseases. PloS one, 8(10), e76295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markon KE (2019). Bifactor and hierarchical models: Specification, inference, and interpretation. Annual Review of Clinical Psychology, 15. [DOI] [PubMed] [Google Scholar]
- Markon KE, Chmielewski M, & Miller CJ (2011). The reliability and validity of discrete and continuous measures of psychopathology: a quantitative review. Psychological Bulletin, 137(5), 856. [DOI] [PubMed] [Google Scholar]
- Martin AR, Kanai M, Kamatani Y, Okada Y, Neale BM, & Daly MJ (2018). Hidden’risk’in polygenic scores: clinical use today could exacerbate health disparities. bioRxiv, 441261. [DOI] [PubMed] [Google Scholar]
- Martin J, Taylor MJ, & Lichtenstein P (2017). Assessing the evidence for shared genetic risks across psychiatric disorders and traits. Psychological Medicine, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGue M, Zhang Y, Miller MB, Basu S, Vrieze S, Hicks B, … Iacono WG (2013). A genome-wide association study of behavioral disinhibition. Behavior Genetics, 43(5), 363–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuffin P, Rijsdijk F, Andrew M, Sham P, Katz R, & Cardno AG (2003). The heritability of bipolar affective disorder and the genetic relationship to unipolar depression. Archives of General Psychiatry, 60(5), 497–502. [DOI] [PubMed] [Google Scholar]
- McLoughlin G, Ronald A, Kuntsi J, Asherson P, & Plomin R (2007). Genetic support for the dual nature of attention deficit hyperactivity disorder: substantial genetic overlap between the inattentive and hyperactive–impulsive components. Journal of Abnormal Child Psychology, 35(6), 999–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikolajewski AJ, Allan NP, Hart SA, Lonigan CJ, & Taylor J (2013). Negative affect shares genetic and environmental influences with symptoms of childhood internalizing and externalizing disorders. Journal of Abnormal Child Psychology, 41(3), 411–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milaneschi Y, Lamers F, Peyrot W, Abdellaoui A, Willemsen G, Hottenga JJ, … Lu C (2016). Polygenic dissection of major depression clinical heterogeneity. Molecular Psychiatry, 21(4), 516–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt TE, Caspi A, Taylor A, Kokaua J, Milne BJ, Polanczyk G, & Poulton R (2010). How common are common mental disorders? Evidence that lifetime prevalence rates are doubled by prospective versus retrospective ascertainment. Psychological Medicine, 40(6), 899–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosing MA, Gordon SD, Medland SE, Statham DJ, Nelson EC, Heath AC, … Wray NR (2009). Genetic and environmental influences on the co-morbidity between depression, panic disorder, agoraphobia, and social phobia: a twin study. Depression and anxiety, 26(11), 1004–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins N, & Lewis CM (2017). Genetics of Depression: Progress at Last. Current Psychiatry Reports, 19(8), 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel M, Jansen PR, Stringer S, Watanabe K, de Leeuw CA, Bryois J, … Munoz-Manchado AB (2017). GWAS Meta-Analysis of Neuroticism (N= 449,484) Identifies Novel Genetic Loci and Pathways. bioRxiv, 184820. [DOI] [PubMed] [Google Scholar]
- Nelson MR, Johnson T, Warren L, Hughes AR, Chissoe SL, Xu C-F, & Waterworth DM (2016). The genetics of drug efficacy: opportunities and challenges. Nature Reviews Genetics, 17(4), 197. [DOI] [PubMed] [Google Scholar]
- Neumann A, Pappa I, Lahey BB, Verhulst FC, Medina-Gomez C, Jaddoe VW, … Tiemeier H (2016). Single nucleotide polymorphism heritability of a general psychopathology factor in children. Journal of the American Academy of Child and Adolescent Psychiatry, 55(12), 1038–1045. e1034. [DOI] [PubMed] [Google Scholar]
- Nikolas MA, & Burt SA (2010). Genetic and environmental influences on ADHD symptom dimensions of inattention and hyperactivity: A meta-analysis: American Psychological Association. [DOI] [PubMed] [Google Scholar]
- Nivard M, Verweij K, Minică C, Treur J, Derks EM, Stringer S, … Minică CC (2016). Connecting the dots, genome-wide association studies in substance use. Molecular Psychiatry, 21(8), 1155. [DOI] [PubMed] [Google Scholar]
- O’Connor SM, Beam CR, Luo X, Cohen LA, VanHuysse JL, Emery RE, … Neale M (2016). Genetic and environmental associations between body dissatisfaction, weight preoccupation, and binge eating: Evidence for a common factor with differential loadings across symptom type. International Journal of Eating Disorders, 50(2), 157–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oltmanns JR, Smith GT, Oltmanns TF, & Widiger TA (2018). General factors of psychopathology, personality, and personality disorder: Across domain comparisons. Clinical Psychological Science, 2167702617750150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ormel J, Jeronimus BF, Kotov R, Riese H, Bos EH, Hankin B, … Oldehinkel AJ (2013). Neuroticism and common mental disorders: Meaning and utility of a complex relationship. Clinical psychology review, 33(5), 686–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ørstavik RE, Kendler KS, Røysamb E, Czajkowski N, Tambs K, & Reichborn-Kjennerud T (2012). Genetic and environmental contributions to the co-occurrence of depressive personality disorder and DSM-IV personality disorders. Journal of Personality Disorders, 26(3), 435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otowa T, Hek K, Lee M, Byrne EM, Mirza SS, Nivard MG, … Wolen A (2016). Meta-analysis of genome-wide association studies of anxiety disorders. Molecular Psychiatry, 21(10), 1391–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson E, Larsson H, & Lichtenstein P (2016). Common psychiatric disorders share the same genetic origin: a multivariate sibling study of the Swedish population. Molecular Psychiatry, 21(5), 717–721. [DOI] [PubMed] [Google Scholar]
- Pingault J-B, O’Reilly PF, Schoeler T, Ploubidis GB, Rijsdijk F, & Dudbridge F (2018). Using genetic data to strengthen causal inference in observational research. Nature Reviews Genetics, 1. [DOI] [PubMed] [Google Scholar]
- Plomin R, Haworth CM, & Davis OS (2009). Common disorders are quantitative traits. Nature Reviews: Genetics, 10(12), 872–878. [DOI] [PubMed] [Google Scholar]
- Plomin R, & Kovas Y (2005). Generalist genes and learning disabilities. Psychological Bulletin, 131(4), 592. [DOI] [PubMed] [Google Scholar]
- Podsakoff PM, MacKenzie SB, Lee J-Y, & Podsakoff NP (2003). Common method biases in behavioral research: A critical review of the literature and recommended remedies. Journal of Applied Psychology, 88(5), 879. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Rucker DD, MacCallum RC, & Nicewander WA (2005). Use of the extreme groups approach: a critical reexamination and new recommendations. Psychological methods, 10(2), 178. [DOI] [PubMed] [Google Scholar]
- Prenoveau JM, Craske MG, Zinbarg RE, Mineka S, Rose RD, & Griffith JW (2011). Are anxiety and depression just as stable as personality during late adolescence? Results from a three-year longitudinal latent variable study. Journal of Abnormal Psychology, 120(4), 832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regier DA, Narrow WE, Clarke DE, Kraemer HC, Kuramoto SJ, Kuhl EA, & Kupfer DJ (2013). DSM-5 field trials in the United States and Canada, Part II: test-retest reliability of selected categorical diagnoses. American Journal of Psychiatry, 170(1), 59–70. [DOI] [PubMed] [Google Scholar]
- Reise SP (2012). The rediscovery of bifactor measurement models. Multivariate Behavioral Research, 47(5), 667–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren H, Fabbri C, Uher R, Rietschel M, Mors O, Henigsberg N, … Dernovsek MZ (2018). Genes associated with anhedonia: a new analysis in a large clinical trial (GENDEP). Translational psychiatry, 8(1), 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SH, Lahey BB, & Waldman ID (2015). Comorbidity among dimensions of childhood psychopathology: Converging evidence from behavior genetics. Child development perspectives, 9(1), 26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimfeld K, Malanchini M, Spargo T, Spickernell G, Selzam S, McMillan A, … Plomin R (2019). Twins Early Development Study: a genetically sensitive investigation into behavioural and cognitive development from infancy to emerging adulthood. [DOI] [PMC free article] [PubMed]
- Roberts BW, & DelVecchio W (2000). The rank-order consistency of personality traits from childhood to old age: A quantitative review of longitudinal studies. Psychological Bulletin, 126, 3–25. [DOI] [PubMed] [Google Scholar]
- Rosenström T, Ystrom E, Torvik FA, Czajkowski NO, Gillespie NA, Aggen SH, … Reichborn-Kjennerud T (2017). Genetic and Environmental Structure of DSM-IV Criteria for Antisocial Personality Disorder: A Twin Study. Behavior Genetics, 47(3), 265–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro ML, Moretti PN, Pellegrino R, Gadelha A, Abílio VC, Hayashi MA, … Hakonarson H (2016). A current snapshot of common genomic variants contribution in psychiatric disorders. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 171(8), 997–1005. [DOI] [PubMed] [Google Scholar]
- Seglem KB, Torgersen S, Ask H, & Waaktaar T (2015). Weak etiologic links between control and the externalizing behaviors delinquency and substance abuse in adolescence. Personality and Individual Differences, 75, 179–184. [Google Scholar]
- Selzam S, Coleman JR, Moffitt TE, Caspi A, & Plomin R (2018). A polygenic p factor for major psychiatric disorders. bioRxiv, 287987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serretti A, & Fabbri C (2013). Shared genetics among major psychiatric disorders. The Lancet, 381(9875), 1339–1341. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Tromp DP, Stockbridge MD, Kaplan CM, Tillman RM, & Fox AS (2016). Dispositional negativity: An integrative psychological and neurobiological perspective. Psychological Bulletin, 142(12), 1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankman SA, Funkhouser CJ, Klein DN, Davila J, Lerner D, & Hee D (2018). Reliability and validity of severity dimensions of psychopathology assessed using the Structured Clinical Interview for DSM-5 (SCID). International Journal of Methods in Psychiatric Research, 27(1), e1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea MT, Stout R, Gunderson J, Morey LC, Grilo CM, McGlashan T, … Zanarini MC (2002). Short-term diagnostic stability of schizotypal, borderline, avoidant, and obsessive-compulsive personality disorders. American Journal of Psychiatry, 159(12), 2036–2041. [DOI] [PubMed] [Google Scholar]
- Sher KJ, & Trull TJ (1996). Methodological issues in psychopathology research. Annual Review of Psychology, 47(1), 371–400. [DOI] [PubMed] [Google Scholar]
- Silberg JL, & Bulik CM (2005). The developmental association between eating disorders symptoms and symptoms of depression and anxiety in juvenile twin girls. Journal of child psychology and psychiatry, 46(12), 1317–1326. [DOI] [PubMed] [Google Scholar]
- Silberg JL, Rutter M, & Eaves L (2001). Genetic and environmental influences on the temporal association between earlier anxiety and later depression in girls. [erratum appears in Biol Psychiatry 2001 Sep 1;50(5):393.]. Biological psychiatry, 49(12), 1040–1049. [DOI] [PubMed] [Google Scholar]
- Sivakumaran S, Agakov F, Theodoratou E, Prendergast JG, Zgaga L, Manolio T, … Campbell H (2011). Abundant pleiotropy in human complex diseases and traits. The American Journal of Human Genetics, 89(5), 607–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeets F, Lataster T, Viechtbauer W, Delespaul P, & GROUP. (2014). Evidence that environmental and genetic risks for psychotic disorder may operate by impacting on connections between core symptoms of perceptual alteration and delusional ideation. Schizophrenia Bulletin, 41(3), 687–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoller JW (2013). Disorders and borders: psychiatric genetics and nosology. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 162(7), 559–578. [DOI] [PubMed] [Google Scholar]
- Smoller JW, Andreassen OA, Edenberg HJ, Faraone SV, Glatt SJ, & Kendler KS (2018). Psychiatric genetics and the structure of psychopathology. Molecular Psychiatry, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoller JW, & Finn CT (2003). Family, twin, and adoption studies of bipolar disorder. Paper presented at the American Journal of Medical Genetics Part C: Seminars in Medical Genetics. [DOI] [PubMed] [Google Scholar]
- Song J, Bergen SE, Kuja-Halkola R, Larsson H, Landén M, & Lichtenstein P (2015). Bipolar disorder and its relation to major psychiatric disorders: a family-based study in the Swedish population. Bipolar Disorders, 17(2), 184–193. [DOI] [PubMed] [Google Scholar]
- South SC, & DeYoung NJ (2013). Behavior genetics of personality disorders: Informing classification and conceptualization in DSM-5. Personality Disorders: Theory, Research, and Treatment, 4(3), 270. [DOI] [PubMed] [Google Scholar]
- South SC, & Krueger R (2011). Genetic and environmental influences on internalizing psychopathology vary as a function of economic status. Psychological medicine, 41(1), 107–117. [DOI] [PubMed] [Google Scholar]
- Spatola CA, Fagnani C, Pesenti-Gritti P, Ogliari A, Stazi MA, & Battaglia M (2007). A general population twin study of the CBCL/6–18 DSM-oriented scales. Journal of the American Academy of Child and Adolescent Psychiatry, 46(5), 619–627. [DOI] [PubMed] [Google Scholar]
- Stahl E, Breen G, Forstner A, McQuillin A, Ripke S, Cichon S, … Kelsoe J (2017). Genomewide association study identifies 30 loci associated with bipolar disorder. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr L, Conway C, Hammen C, & Brennan P (2013). Transdiagnostic and disorder-specific models of intergenerational transmission of internalizing pathology. Psychological Medicine, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strober M, Freeman R, Lampert C, Diamond J, & Kaye W (2000). Controlled family study of anorexia nervosa and bulimia nervosa: evidence of shared liability and transmission of partial syndromes. American Journal of Psychiatry, 157(3), 393–401. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Agrawal A, Bulik CM, Andreassen OA, Børglum AD, Breen G, … Gelernter J (2017). Psychiatric genomics: an update and an agenda. American Journal of Psychiatry, 175(1), 15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tackett JL, Lahey BB, Van Hulle C, Waldman I, Krueger RF, & Rathouz PJ (2013). Common genetic influences on negative emotionality and a general psychopathology factor in childhood and adolescence. Journal of Abnormal Psychology, 122(4), 1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarbox SI, & Pogue-Geile MF (2011). A multivariate perspective on schizotypy and familial association with schizophrenia: a review. Clinical psychology review, 31(7), 1169–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton LM, Mazzeo SE, & Bulik CM (2010). The heritability of eating disorders: methods and current findings Behavioral neurobiology of eating disorders (pp. 141–156): Springer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton LM, Welch E, Munn-Chernoff MA, Lichtenstein P, & Bulik CM (2016). Anorexia nervosa, major depression, and suicide attempts: shared genetic factors. Suicide and Life-Threatening Behavior. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorp JG, Marees A, Ong J-S, An J, MacGregor S, & Derks E (2019). Investigating Genetic Heterogeneity in Major Depression Through Item-level Genetic Analyses of the PHQ-9. bioRxiv, 528067. [Google Scholar]
- Torgersen S, Czajkowski N, Jacobson K, Reichborn-Kjennerud T, Røysamb E, Neale M, & Kendler K (2008). Dimensional representations of DSM-IV cluster B personality disorders in a population-based sample of Norwegian twins: a multivariate study. Psychological Medicine, 38(11), 1617–1625. [DOI] [PubMed] [Google Scholar]
- Torkamani A, Wineinger NE, & Topol EJ (2018). The personal and clinical utility of polygenic risk scores. Nature Reviews Genetics, 1. [DOI] [PubMed] [Google Scholar]
- Tuvblad C, Zheng M, Raine A, & Baker LA (2009). A common genetic factor explains the covariation among ADHD ODD and CD symptoms in 9–10 year old boys and girls. Journal of Abnormal Child Psychology, 37(2), 153–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uher R, & Rutter M (2012). Basing psychiatric classification on scientific foundation: problems and prospects. International review of psychiatry, 24(6), 591–605. [DOI] [PubMed] [Google Scholar]
- Vachon DD, Krueger RF, Rogosch FA, & Cicchetti D (2015). Assessment of the Harmful Psychiatric and Behavioral Effects of Different Forms of Child Maltreatment. JAMA Psychiatry, 1135–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Sluis S, Posthuma D, Nivard M, Verhage M, & Dolan C (2013). Power in GWAS: lifting the curse of the clinical cut-off. Molecular Psychiatry, 18(1), 2. [DOI] [PubMed] [Google Scholar]
- Van Der Sluis S, Verhage M, Posthuma D, & Dolan CV (2010). Phenotypic complexity, measurement bias, and poor phenotypic resolution contribute to the missing heritability problem in genetic association studies. PloS one, 5(11), e13929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Os J, Linscott RJ, Myin-Germeys I, Delespaul P, & Krabbendam L (2009). A systematic review and meta-analysis of the psychosis continuum: evidence for a psychosis proneness–persistence–impairment model of psychotic disorder. Psychological Medicine, 39(2), 179–195. [DOI] [PubMed] [Google Scholar]
- Vandenberg RJ, & Lance CE (2000). A review and synthesis of the measurement invariance literature: Suggestions, practices, and recommendations for organizational research. Organizational research methods, 3(1), 4–70. [Google Scholar]
- Vernon PA, McCarthy JM, Johnson AM, Jang KL, & Harris JA (1999). Individual differences in multiple dimensions of aggression: a univariate and multivariate genetic analysis. Twin Research, 2(01), 16–21. [DOI] [PubMed] [Google Scholar]
- Vink JM, Hottenga JJ, Geus EJ, Willemsen G, Neale MC, Furberg H, & Boomsma DI (2014). Polygenic risk scores for smoking: predictors for alcohol and cannabis use? Addiction, 109(7), 1141–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade TD, Fairweather-Schmidt AK, Zhu G, & Martin NG (2015). Does shared genetic risk contribute to the co-occurrence of eating disorders and suicidality? International Journal of Eating Disorders, 48(6), 684–691. [DOI] [PubMed] [Google Scholar]
- Waldman ID, Poore HE, van Hulle C, Rathouz PJ, & Lahey BB (2016). External validity of a hierarchical dimensional model of child and adolescent psychopathology: Tests using confirmatory factor analyses and multivariate behavior genetic analyses. Journal of Abnormal Psychology, 125(8), 1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman ID, & Slutske WS (2000). Antisocial behavior and alcoholism: A behavioral genetic perspective on comorbidity. Clinical psychology review, 20(2), 255–287. [DOI] [PubMed] [Google Scholar]
- Wang K, Gaitsch H, Poon H, Cox NJ, & Rzhetsky A (2017). Classification of common human diseases derived from shared genetic and environmental determinants. Nature Genetics, 49(9), 1319–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J, Strawbridge R, Graham N, Bailey M, Freguson A, Lyall D, … Smith DJ (2017). Genome-Wide Analysis Of 113,968 Individuals In UK Biobank Identifies Four Loci Associated With Mood Instability. bioRxiv. doi: 10.1101/117796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waszczuk MA, Waaktaar T, Eley TC, & Torgersen S (2019). Aetiological influences on continuity and co-occurrence of eating disorders symptoms across adolescence and emerging adulthood. International Journal of Eating Disorders. [DOI] [PubMed] [Google Scholar]
- Waszczuk MA, Zavos HMS, Gregory AM, & Eley TC (2014). The phenotypic and etiological structure of depression and anxiety disorder symptoms in childhood, adolescence and young adulthood. JAMA Psychiatry, 71(8), 905–916. doi: 10.1001/jamapsychiatry.2014.655 [DOI] [PubMed] [Google Scholar]
- Waszczuk MA, Zavos HMS, Gregory AM, & Eley TC (2016). The stability and change of etiological influences on depression, anxiety symptoms and their co-occurrence across adolescence and young adulthood. Psychological medicine, 46(1), 161–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widiger TA (2011). Personality and psychopathology. World Psychiatry, 10(2), 103–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widiger TA, Sellbom M, Chmielewski M, Clark LA, DeYoung CG, Kotov R, … Mullins-Sweatt S (2018). Personality in a Hierarchical Model of Psychopathology. Clinical Psychological Science, 2167702618797105. [Google Scholar]
- Wolf EJ, Miller MW, Krueger RF, Lyons MJ, Tsuang MT, & Koenen KC (2010). Posttraumatic stress disorder and the genetic structure of comorbidity. Journal of Abnormal Psychology, 119(2), 320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray NR, Lee SH, & Kendler KS (2012). Impact of diagnostic misclassification on estimation of genetic correlations using genome-wide genotypes. European Journal of Human Genetics, 20(6), 668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray NR, Lee SH, Mehta D, Vinkhuyzen AA, Dudbridge F, & Middeldorp CM (2014). Research review: polygenic methods and their application to psychiatric traits. Journal of child psychology and psychiatry, 55(10), 1068–1087. [DOI] [PubMed] [Google Scholar]
- Wray NR, & Maier R (2014). Genetic basis of complex genetic disease: the contribution of disease heterogeneity to missing heritability. Current Epidemiology Reports, 1(4), 220–227. [Google Scholar]
- Wright AG, Krueger RF, Hobbs MJ, Markon KE, Eaton NR, & Slade T (2013). The structure of psychopathology: toward an expanded quantitative empirical model. Journal of Abnormal Psychology, 122(1), 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu MK, Gaysina D, Barnett JH, Scoriels L, van de Lagemaat LN, Wong A, … Group LG (2015). Psychometric precision in phenotype definition is a useful step in molecular genetic investigation of psychiatric disorders. Translational psychiatry, 5(6), e593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata S, Suzuki A, Ando J, Ono Y, Kijima N, Yoshimura K, … Spinath FM (2006). Is the genetic structure of human personality universal? A cross-cultural twin study from North America, Europe, and Asia. Journal of Personality and Social Psychology, 90(6), 987. [DOI] [PubMed] [Google Scholar]
- Yang J, Wray NR, & Visscher PM (2010). Comparing apples and oranges: equating the power of case-control and quantitative trait association studies. Genetic Epidemiology, 34(3), 254–257. [DOI] [PubMed] [Google Scholar]
- Young SE, Stallings MC, Corley RP, Krauter KS, & Hewitt JK (2000). Genetic and environmental influences on behavioral disinhibition. American Journal of Medical Genetics, 96(5), 684–695. [PubMed] [Google Scholar]
- Zavos HM, Freeman D, Haworth CM, McGuire P, Plomin R, Cardno AG, & Ronald A (2014). Consistent etiology of severe, frequent psychotic experiences and milder, less frequent manifestations: a twin study of specific psychotic experiences in adolescence. JAMA psychiatry, 71(9), 1049–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.