Abstract
There has been a growing interest in the potential for plasticity-inducing pharmacological interventions to enhance post-stroke recovery. One group of drugs that continues to garner a great deal of attention in this regard is a class of antidepressants called the selective serotonin reuptake inhibitors. Here we propose a model for the mechanism by which these drugs may enhance plasticity after ischemic brain injury. First, we review the research in animal models demonstrating how selective serotonin reuptake inhibitors reopen the critical period for ocular dominance plasticity in adulthood. We then compare this period of heightened plasticity to the cellular and biochemical milieu of perilesional tissue after an ischemic event in the adult brain. We argue that selective serotonin reuptake inhibitors administered acutely after an ischemic stroke alter excitatory–inhibitory balance in perilesional tissue and reinstate a type of plasticity reminiscent of the critical period in development. Finally, we discuss opportunities for future research in this area in both the preclinical and clinical realms.
Stroke is a leading cause of long-term adult disability in the United States. While many stroke patients experience significant functional recovery in the first few months after a stroke, residual deficits persist beyond the first year in most patients [1, 2]. Given the attractiveness of a pharmacological approach for enhancing post-stroke recovery, many groups have explored the therapeutic potential of a class of anti-depressants called selective serotonin reuptake inhibitors (SSRIs). The first large-scale randomized clinical trial of SSRIs in acute stroke patients (FLAME) found that initiating fluoxetine acutely after an ischemic stroke improved motor outcomes at 90 days [3]. Furthermore, a meta-analysis of over 4000 stroke patients showed a similar benefit of SSRIs in recovery [4]. The improvement in motor outcomes appears to be specific to the SSRI class of antidepressants [5] and independent of the antidepressant effects of these drugs [3].
These findings raise an important question: What is the mechanism by which SSRIs enhance post-stroke recovery? To understand the effects of SSRIs at the molecular level, we discuss animal studies that have shown that SSRIs are capable of reopening the critical period for ocular dominance plasticity in adulthood by altering excitatory–inhibitory balance. Next, we extrapolate these findings to understand how SSRIs may improve recovery after stroke. We argue that, in the presence of rehabilitative therapy, SSRIs operate through the strengthening of unmasked connections and the creation of new ones in an experience-dependent fashion by decreasing inhibitory tone, which increases plasticity; the excitatory–inhibitory balance is then re-established afterwards. This proposed mechanism not only helps explain the effects of SSRIs on motor recovery after stroke, but also suggests that SSRIs may facilitate post-stroke recovery in other functional domains such as vision, language, and cognition.
Critical period in development
Critical periods in neural development occur in many functional domains and are characterized by the potential for large-scale synaptic plasticity and cortical reorganization. Understanding the cellular and molecular underpinnings of this process can help us find ways to reopen the critical period in adulthood, which could in turn facilitate post-stroke recovery. A commonly used system for the study of critical period plasticity is the visual cortex, where perturbations to visual experience cause changes in ocular dominance during a well-defined period of development [6], whereas similar perturbations fail to alter visual circuitry to the same degree in adulthood [7]. Recent animal studies show that SSRIs are capable of reopening the critical period in adulthood [8–10].
The balance between excitatory and inhibitory signaling governs the opening and closing of the critical period, as well as the changes in ocular dominance that result from monocular deprivation during the critical period. The beginning of the critical period is marked by an initial maturation of parvalbumin-positive gamma-aminobutyric acid (GABA)-ergic neurons. Increasing GABA type A signaling using diazepam causes a precocious opening of the critical period for ocular dominance plasticity, while the attenuation of synaptic GABA synthesis in glutamic acid decarboxylase-65 knockout mice prevents the onset of the critical period altogether [11, 12].
Monocular deprivation during the critical period shifts the excitatory–inhibitory balance of visually deprived cortex towards excitation to compensate for the sudden loss of activity from the deprived eye [11]. After monocular deprivation, pyramidal neurons become disinhibited due to a rapid loss of parvalbumin-positive inhibitory input [12]. Structural changes also promote the remodeling of cortical responsiveness to the two eyes: monocular deprivation induces the retraction or elongation of interneuron dendritic branch tips and axonal boutons [8], and the changes in eye-specific axonal and dendritic arborization of excitatory neurons [13]. The circuit refinement that normally occurs during the critical period does not happen, however, if the inhibitory tone is too high at the onset of the critical period [12].
At the end of the critical period, a second wave of inhibitory maturation stabilizes established cortical connections. This wave is triggered, in part, by brain-derived neurotrophic factor (BDNF). Transgenic overexpression of BDNF accelerates the maturation of inhibitory neurons in young mice and prematurely opens and closes the critical period [14].
SSRIs reopen the critical period in adulthood
Restoration of the juvenile form of ocular dominance plasticity in adulthood can be achieved using a variety of methods that reduce cortical inhibition, including treatment with SSRIs [9, 10]. In this section, we review the animal and human literature that shows that SSRIs promote a type of plasticity in adulthood reminiscent of the critical period for ocular dominance plasticity in the juvenile visual system. These studies suggest that the SSRI-induced acute increase in the local concentration of serotonin causes a decrease in long-range horizontal inhibition, facilitating synaptic plasticity.
Animal SSRI studies
Serotonin can modulate the homeostatic response of visual circuits by decreasing inhibition [15, 16], thereby tipping excitatory–inhibitory balance in favor of excitation. Similarly, SSRIs have been shown to decrease inhibitory tone in the adult rat visual cortex [10] in a serotonin-dependent manner [17], by decreasing extracellular GABA concentrations and reducing the number of parvalbumin-positive GABAergic interneurons [18–20]. In addition, fluoxetine facilitates the degradation of the perineuronal nets surrounding parvalbumin-positive interneurons [18, 19, 21], thus removing an important structural component of synaptic stabilization.
With SSRIs decreasing the inhibitory tone of the circuit, the cortex becomes hyperexcitable [22, 23], which creates a permissive environment for novel visual experiences, such as monocular deprivation, to affect circuit organization in a way that is not typically seen in adulthood. For example, this type of SSRI-mediated hyperexcitability facilitates LTP in adult rat hippocampal neurons [24] and promotes remodeling of pyramidal cell dendritic spines [22, 25].
Like the end of the critical period, inhibitory tone rises again in the cortex of fluoxetine-treated adult animals on the order of days after monocular deprivation. This increase in inhibition, which re-establishes excitatory–inhibitory balance, is mediated by an SSRI-dependent increase in BDNF [10] through either 5-HT1A receptors [17] or the activity-dependent expression of the immediate early gene Npas4 [26], a transcription factor that promotes BDNF-dependent [27] inhibitory synaptogenesis [14, 28]. The restoration of homeostatic balance in the visual cortex of fluoxetine-treated, monocularly-deprived adult animals allows for the persistence and consolidation of these synaptic changes.
Human SSRI studies
The consequences of SSRI treatment appear to be similar in humans and animals. In the motor system of non-depressed healthy adults, a single dose of an SSRI decreases intracortical inhibition and cortical excitability (reviewed by 29) and decreases functional connectivity of a variety of brain networks [30, 31]. In contrast, long-term administration of SSRIs seems to stabilize new circuits that perform a given task more efficiently. For instance, chronic SSRI treatment reduces the spread of motor cortex activation induced by a difficult finger tapping task in a manner that is proportional to how well the task is performed [32, 33] and increases sensitivity to repeated visual stimulation as evidenced by larger visual evoked potential amplitudes after treatment [34].
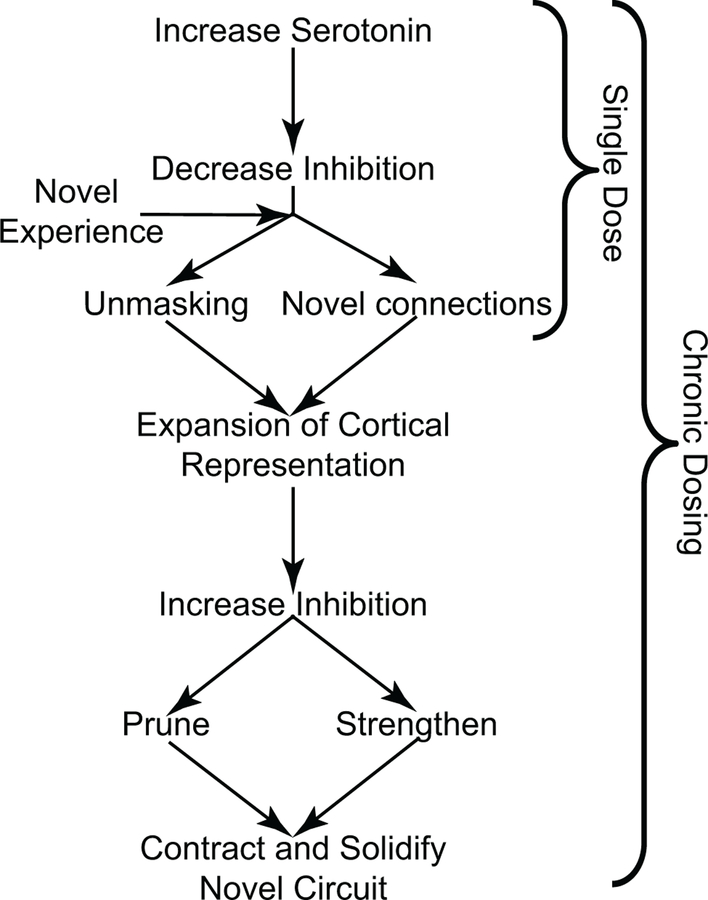
Taken together, animal and human studies suggest that a single dose of an SSRI decreases intracortical inhibition to unmask pre-existing connections and establish new ones in an experience-dependent fashion, leading to the expansion of the cortical representation. On the other hand, chronic treatment leads to an increase in inhibition, a contraction of the cortical representation, and persistence of the novel and more efficient pathway. These two phases of SSRI-induced plasticity (Fig. 1) may be driven by the divergent effects of SSRI-induced BDNF expression, which depend on whether BDNF is up-regulated transiently or chronically [35].
Fig. 1.
Single-dose vs. chronic SSRI administration has different effects on cortical plasticity. The first dose of an SSRI decreases inhibition which allows a novel experience to impose changes in the cortical circuit through unmasking of existing connections or establishing new connections. Chronic SSRI treatment then completes the rewiring process and restores excitatory–inhibitory balance, thereby consolidating an efficient novel circuit
SSRIs during monocular deprivation versus after stroke
In adult animal studies, SSRIs reopen the critical period by decreasing cortical inhibitory tone, which allows for an altered experience such as monocular deprivation to promote reorganization and reweighting of the cortical circuit. The type of plasticity characteristic of the critical period is also seen after ischemic injury in adulthood. Ischemia immediately reduces inhibitory synaptic transmission in perilesional tissue [36] through ipsilesional intracortical disinhibition [37, 38] and contralesional hyperactivation [39, 40]. These changes facilitate the unmasking and recruitment of preexisting cortical connections to rewire the circuit [41]. On top of the decrease in inhibitory tone produced by ischemia, SSRIs given to mice shortly after an ischemic event reduces the expression of inhibitory markers in perilesional tissue even further [20]. In addition, the extent of this reduction is associated with a prolongation of the sensitive period for successful forelimb rehabilitation [20]. Similarly, in subacute stroke patients, a single dose of fluoxetine causes hyper- activation of the ipsilesional primary motor cortex [42].
After rewiring and reweighting occurs in adult monocular deprivation SSRI experiments, chronic SSRI treatment re-establishes excitatory–inhibitory balance, which stabilizes the altered circuit. Similarly, in the months following the ischemic event in stroke patients with good recovery, excitatory–inhibitory balance of ipsilesional and contralesional tissue normalizes, with the return of normal levels of inhibition [39, 43, 44], and cortical recruitment disappears [41]. In contrast, stroke patients with poor recovery never show a restoration of excitatory–inhibitory balance, especially in the contralesional hemisphere [40]: it is as if the reopened critical period never closes and inhibition is chronically reduced. Other studies have found that restoration of excitatory–inhibitory balance, either through reducing inhibitory tone with GABA receptor agonists or increasing excitation with AMPA receptor modulators, can improve recovery after stroke [45]. Since chronic SSRI treatment increases BDNF levels after stroke [46] and post-stroke BDNF levels are positively correlated with good functional outcomes [47], SSRIs may provide another way to address the excitatory–inhibitory imbalance in stroke patients with poor recovery. We propose that a three-month course of SSRI therapy improves recovery after stroke [3] because it completes the rewiring process and restores excitatory–inhibitory balance in both hemispheres, thereby consolidating an efficient and effective altered circuit in the ipsilesional hemisphere. Evidence for this proposal comes largely from studying the visual and motor domains; however, it is possible that similar processes are at play in the recovery of more complex, distributed functions after stroke: there is some evidence to suggest that SSRIs can also improve stroke patients’ cognitive [48] and language [49] abilities.
A working model for SSRI-induced recovery after stroke
To summarize, we synthesize the following model to explain how SSRIs might enhance post-stroke recovery (Fig. 1). In the acute phase after a stroke, SSRIs enhance the excitability of perilesional cortex by decreasing inhibition, which allows for the reweighting of existing connections. In addition, SSRIs facilitate the development of new connections by removing the extracellular barriers to circuit remodeling (such as perineuronal nets) and boosting dendritic branch tip and axonal arborization dynamics. These changes, paired with rehabilitation therapies that challenge the damaged system, allow for greater experience-dependent plasticity, which facilitates functional recovery [50]. Finally, prolonged SSRI treatment leads to chronically elevated BDNF levels, which in turn increases local inhibition and restores the balance between excitation and inhibition. The restoration of the excitatory–inhibitory balance stabilizes the core of the novel circuit and prunes away redundant or inefficient components, leading to contraction of the cortical representation.
Future directions for studying SSRIs in post-stroke recovery
The current literature provides a rationale for using SSRIs to enhance recovery of neurologic function after an ischemic stroke. We see two opportunities for future research in this field: (1) investigation of the optimal dosing, timing and rehabilitation supplementation for this kind of treatment; and (2) exploration beyond the motor system to determine whether SSRIs can help enhance post-stroke recovery in other functional domains, such as vision and language.
SSRI dosing, timing and supplementation with rehabilitative therapies
To date, most studies investigating a role for fluoxetine in post-stroke recovery have used 20 mg of fluoxetine daily [3, 5, 51–54]. However, if a lower dose demonstrates similar efficacy, the likelihood of adverse effects with this treatment would diminish, especially in older patients [55]; this is an important open question that should be studied in animal stroke models and future clinical trials.
Our current understanding of the ideal timing for starting SSRI treatment after a stroke is “the sooner the better”. SSRIs are less effective at promoting recovery if started later than one week after a stroke, but one study suggests that they remain more effective than placebo for up to 6 months [52]. It is possible, however, that SSRIs may have beneficial effects even beyond six months given that chronic stroke patients who received a single dose of an SSRI had greater muscle activity in the paretic arm five hours later [56]. Whether SSRI treatment facilitates recovery in chronic stroke patients has yet to be investigated with a randomized double-blind placebo-controlled clinical trial. The question of optimal treatment duration also remains unanswered.
Another open question is whether patients already on an SSRI at the time of the stroke might show a different recovery trajectory compared to SSRI-naïve stroke patients. There are conflicting data on how SSRI use before an ischemic stroke affects functional recovery [57, 58]. The antiplatelet effects of SSRIs [59] may cause poor outcomes in hemorrhagic stroke patients or in ischemic stroke patients treated with intravenous thrombolysis when the patient was on an SSRI before the stroke [60, 61]. It is also possible that the benefit of SSRI treatment after stroke depends on the acute peak in synaptic serotonin levels upon commencing treatment [62], in which case there may be no SSRI-dependent boost in recovery, if the patient was taking an SSRI before the stroke.
Additionally, it is unclear whether SSRIs improve stroke recovery independent of a rehabilitation program. In the FLAME study, all patients, regardless of treatment group assignment, received rehabilitation therapies [3]. In contrast, the more recent negative clinical trial, FOCUS, did not require that all patients receive rehabilitative therapies, nor did it report the percentage of patients who did receive therapy [55]. The possibility that SSRIs need to be paired with rehabilitative therapy to be an effective treatment for stroke recovery is supported by the studies we have high- lighted here showing that rewiring occurs when SSRIs are paired with novel experience (such as monocular deprivation or physical therapy). Future studies with three arms (drug alone, therapy alone, drug plus therapy) could shed light on whether SSRIs need to be paired with rehabilitation to be maximally effective at promoting functional recovery.
Can SSRIs promote vision recovery after stroke?
While past [3–5, 48, 51–55, 63–66] and current clinical trials (AFFINITY, EFFECTS, FLOW, SELEIS, CISS, RECONISE, ELISA, and NCT02208466) studying the effects of SSRIs on stroke recovery have largely focused on strokes affecting motor function, we are currently conducting a randomized, placebo-controlled phase IIa exploratory clinical trial investigating a potential role for fluoxetine in promoting vision recovery in acute ischemic stroke patients with homonymous visual field deficits [67] (FLUORESCE: NCT02737930). Given that only 7.5–26% of stroke patients with homonymous visual field deficits completely recover vision [1, 68, 69] and that visual restoration therapy remains experimental in nature, with no currently proven rehabilitative treatment that enhances vision recovery in this patient population [70], the results from this and future pharmacological studies have the potential to expand the realm of possibilities for post-stroke vision restoration [71].
We have reason to believe that SSRIs will enhance vision recovery in hemianopic stroke patients for multiple reasons. First, as reviewed above, much of the basic research demonstrating the neuroplastic effects of SSRIs has been conducted in the visual system. Second, stroke patients with persistent hemianopia exhibit similar neural markers of poor recovery when compared to those with poor motor recovery, including persistent expansion of cortical activity [72, 73] and contralateral hemisphere activation [74–76]. Third, evidence of reorganization of the visual circuit has been detected in patients with spontaneous vision recovery after a stroke [73, 77]. Taken together, these studies suggest that SSRI administration in the first week after a stroke may push those with less potential for spontaneous vision recovery towards a more favorable outcome in the same way that it supports greater post-stroke motor recovery. In addition, since visual ability can be quantified with a high degree of spatial resolution using standard clinical measures (automated perimetry), and reorganization of the visual cortex can be probed using functional neuroimaging [78, 79], we believe future studies in the visual system have the potential to demonstrate not only the effectiveness of fluoxetine in post-stroke vision recovery, but also the neural substrates of fluoxetine-mediated plasticity after stroke.
Acknowledgements
Preparation of this manuscript was supported by NIH Grant F30 EY027988 to C.L.S., NIH Grants R21 NS099973 and R01 AA027111 and National Science Foundation Grant NSF 1557971 to A.K.M, NIH Grants T32 NS007338 to the University of Rochester and K12 HD093427 to support A.B., a grant from Research to Pre- vent Blindness to Z.R.W., NIH Grant R21 NS076176, R01 NS089609 and R01 EY028535 to B.Z.M., a grant from the Schmitt Program on Integrative Brain Research to B.S. and B.Z.M., and an NIH Institutional Grant P30 EY001319 to the Center for Visual Sciences at the University of Rochester. We thank Gregory DeAngelis for feedback on an earlier version of this manuscript and acknowledge the Seneca Nation of Indians and the Shawnee Tribe, on whose traditional territories the University of Rochester and Carnegie Mellon University reside, respectively.
Footnotes
Conflicts of interest The authors declare that they have no conflicts of interest.
Ethical standard The manuscript does not contain clinical studies or patient data.
References
- 1.Tiel K, Kolmel HW (1990) Patterns of recovery from homonymous hemianopia subsequent to infarction in the distribution of the posterior cerebral artery. Neuro-Ophthalmol 11:33–39 [Google Scholar]
- 2.Bonita R, Beaglehole R (1988) Recovery of motor function after stroke. Stroke 19:1497–1500 [DOI] [PubMed] [Google Scholar]
- 3.Chollet F, Tardy J, Albucher JF et al. (2011) Fluoxetine for motor recovery after acute ischaemic stroke (FLAME): a randomised placebo-controlled trial. Lancet Neurol 10:123–130 [DOI] [PubMed] [Google Scholar]
- 4.Mead G, Hsieh C, Lee R et al. (2012) Selective serotonin reuptake inhibitors (SSRIs) for stroke recovery (Review). Cochrane Data- base Syst Rev 11:1–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dam M, Tonin P, De Boni A et al. (1996) Effects of fluoxetine and maprotiline on functional recovery in poststroke hemiplegic patients undergoing rehabilitation therapy. Stroke 27:1211–1214 [DOI] [PubMed] [Google Scholar]
- 6.Wiesel TN, Hubel DH (1963) Single-cell responses in striate cortex of kittens deprived of vision in one eye. J Neurophysiol 26:1003–1017 [DOI] [PubMed] [Google Scholar]
- 7.Hubel DH, Wiesel TN (1970) The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol 206:419–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen JL, Lin WC, Cha JW et al. (2011) Structural basis for the role of inhibition in facilitating adult brain plasticity. Nat Neurosci 14:587–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guirado R, La Terra D, Bourguignon M et al. (2016) Effects of PSA removal from NCAM on the critical period plasticity triggered by the antidepressant fluoxetine in the visual cortex. Front Cell Neurosci 10:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maya Vetencourt JF, Sale A, Viegi A et al. (2008) The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science 320:385–388 [DOI] [PubMed] [Google Scholar]
- 11.Desai NS, Cudmore RH, Nelson SB, Turrigiano GG (2002) Critical periods for experience-dependent synaptic scaling in visual cortex. Nat Neurosci 5:783–789 [DOI] [PubMed] [Google Scholar]
- 12.Kuhlman SJ, Olivas ND, Tring E et al. (2013) A disinhibitory microcircuit initiates critical-period plasticity in the visual cortex. Nature 501:543–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oray S, Majewska A, Sur M (2004) Dendritic spine dynamics are regulated by monocular deprivation and extracellular matrix degradation. Neuron 44:1021–1030 [DOI] [PubMed] [Google Scholar]
- 14.Huang ZJ, Kirkwood A, Pizzorusso T et al. (1999) BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell 98:739–755 [DOI] [PubMed] [Google Scholar]
- 15.Baroncelli L, Sale A, Viegi A et al. (2010) Experience-dependent reactivation of ocular dominance plasticity in the adult visual cortex. Exp Neurol 226:100–109 [DOI] [PubMed] [Google Scholar]
- 16.Guidotti G, Calabrese F, Auletta F et al. (2012) Developmental influence of the serotonin transporter on the expression of Npas4 and GABAergic markers: modulation by antidepressant treatment. Neuropsychopharmacology 37:746–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maya Vetencourt JF, Tiraboschi E, Spolidoro M et al. (2011) Serotonin triggers a transient epigenetic mechanism that rein- states adult visual cortex plasticity in rats. Eur J Neurosci 33:49–57 [DOI] [PubMed] [Google Scholar]
- 18.Ohira K, Takeuchi R, Iwanaga T, Miyakawa T (2013) Chronic fluoxetine treatment reduces parvalbumin expression and peri-neuronal nets in gamma-aminobutyric acidergic interneurons of the frontal cortex in adult mice. Mol Brain 6:43–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guirado R, Perez-Rando M, Sanchez-Matarredona D et al. (2014) Chronic fluoxetine treatment alters the structure, connectivity and plasticity of cortical interneurons. Int J Neuropsychopharmacol 17:1635–1646 [DOI] [PubMed] [Google Scholar]
- 20.Ng KL, Gibson EM, Hubbard R et al. (2015) Fluoxetine maintains a state of heightened responsiveness to motor training early after stroke in a mouse model. Stroke 46:2951–2960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tiraboschi E, Guirado R, Greco D, et al. (2013) Gene expression patterns underlying the reinstatement of plasticity in the adult visual system. Neural Plast 2013:605079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guirado R, Varea E, Castillo-Gomez E et al. (2009) Effects of chronic fluoxetine treatment on the rat somatosensory cortex: activation and induction of neuronal structural plasticity. Neurosci Lett 457:12–15 [DOI] [PubMed] [Google Scholar]
- 23.Vialou V, Thibault M, Kaska S et al. (2015) Differential induction of FosB isoforms throughout the brain by fluoxetine and chronic stress. Neuropharmacology 99:28–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holderbach R, Clark K, Moreau J et al. (2007) Enhanced long- term synaptic depression in an animal model of depression. Biol Psychiatry 62:92–100 [DOI] [PubMed] [Google Scholar]
- 25.Hajszan T, MacLusky NJ, Leranth C (2005) Short-term treatment with the antidepressant fluoxetine triggers pyramidal dendritic spine synapse formation in rat hippocampus. Eur J Neurosci 21:1299–1303 [DOI] [PubMed] [Google Scholar]
- 26.Maya-Vetencourt JF, Tiraboschi E, Greco D et al. (2012) Experience-dependent expression of NPAS4 regulates plasticity in adult visual cortex. J Physiol 590:4777–4787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramamoorthi K, Fropf R, Belfort GM et al. (2011) Npas4 regulates a transcriptional program in CA3 required for contextual memory formation. Science 334:1669–1675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin Y, Bloodgood BL, Hauser JL et al. (2008) Activity-dependent regulation of inhibitory synapse development by Npas4. Nature 455:1198–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pinto CB, Velez FGS, Lopes F et al. (2017) SSRI and motor recovery in stroke: reestablishment of inhibitory neural network tonus. Front Neurosci 11:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schaefer A, Burmann I, Regenthal R et al. (2014) Serotonergic modulation of intrinsic functional connectivity. Curr Biol 24:2314–2318 [DOI] [PubMed] [Google Scholar]
- 31.Klaassens BL, van Gorsel HC, Khalili-Mahani N et al. (2015) Single-dose serotonergic stimulation shows widespread effects on functional brain connectivity. Neuroimage 122:440–450 [DOI] [PubMed] [Google Scholar]
- 32.Gerdelat-Mas A, Loubinoux I, Tombari D et al. (2005) Chronic administration of selective serotonin reuptake inhibitor (SSRI) paroxetine modulates human motor cortex excitability in healthy subjects. Neuroimage 27:314–322 [DOI] [PubMed] [Google Scholar]
- 33.Loubinoux I, Tombari D, Pariente J et al. (2005) Modulation of behavior and cortical motor activity in healthy subjects by a chronic administration of a serotonin enhancer. Neuroimage 27:299–313 [DOI] [PubMed] [Google Scholar]
- 34.Normann C, Schmitz D, Furmaier A et al. (2007) Long-term plasticity of visually evoked potentials in humans is altered in major depression. Biol Psychiatry 62:373–380 [DOI] [PubMed] [Google Scholar]
- 35.Turrigiano GG, Nelson SB (2000) Hebb and homeostasis in neuronal plasticity. Curr Opin Neurobiol 10:358–364 [DOI] [PubMed] [Google Scholar]
- 36.Schiene K, Bruehl C, Zilles K et al. (1996) Neuronal hyperexcitability and reduction of GABAa-receptor expression in the surround of cerebral photothrombosis. J Cereb Blood Flow Metab 16:906–914 [DOI] [PubMed] [Google Scholar]
- 37.Liepert J, Storch P, Fritsch A, Weiller C (2000) Motor cortex disinhibition in acute stroke. Clin Neurophysiol 111:671–676 [DOI] [PubMed] [Google Scholar]
- 38.Cicinelli P, Pasqualetti P, Zaccagnini M et al. (2003) Interhemispheric asymmetries of motor cortex excitability in the postacute stroke stage a paired-pulse transcranial magnetic stimulation study. Stroke 34:2653–2658 [DOI] [PubMed] [Google Scholar]
- 39.Bütefisch CM, Netz J, Weßling M et al. (2003) Remote changes in cortical excitability after stroke. Brain 126:470–481 [DOI] [PubMed] [Google Scholar]
- 40.Manganotti P, Acler M, Zanette GP et al. (2008) Motor cortical disinhibition during early and late recovery after stroke. Neurorehabil Neural Repair 22:396–403 [DOI] [PubMed] [Google Scholar]
- 41.Tombari D, Loubinoux I, Pariente J et al. (2004) A longitudinal fMRI study: in recovering and then in clinically stable sub-cortical stroke patients. Neuroimage 23:827–839 [DOI] [PubMed] [Google Scholar]
- 42.Pariente J, Loubinoux I, Carel C et al. (2001) Fluoxetine modulates motor performance and cerebral activation of patients recovering from stroke. Ann Neurol 50:718–729 [DOI] [PubMed] [Google Scholar]
- 43.Manganotti P, Patuzzo S, Cortese F et al. (2002) Motor disinhibition in affected and unaffected hemisphere in the early period of recovery after stroke. Clin Neurophysiol 113:936–943 [DOI] [PubMed] [Google Scholar]
- 44.Ward NS, Brown MM, Thompson AJ, Frackowiak RS (2003) Neural correlates of motor recovery after stroke: a longitudinal fMRI study. Brain 126:2476–2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carmichael ST (2012) Brain excitability in stroke: the yin and yang of stroke progression. Arch Neurol 69:161–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Espinera AR, Ogle ME, Gu X, Wei L (2013) Citalopram enhances neurovascular regeneration and sensorimotor functional recovery after ischemic stroke in mice. Neuroscience 247:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lasek-Bal A, Jedrzejowska-Szypulka H, Rozycka J et al. (2015) Low concentration of BDNF in the acute phase of ischemic stroke as a factor in poor prognosis in terms of functional status of patients. Med Sci Monit 21:3900–3905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jorge RE, Acion L, Moser D et al. (2013) Escitalopram and enhancement of cognitive recovery. Arch Gen Psychiatry 67:187–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hillis AE, Beh YY, Sebastian R et al. (2018) Predicting recovery in acute poststroke aphasia. Ann Neurol 83:612–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kerr AL, Cheng SY, Jones TA (2011) Experience-dependent neural plasticity in the adult damaged brain. J Commun Disord 44:538–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.He Y, Tang B, Cai Z, Zeng S (2016) Effects of fluoxetine on neural functional prognosis after ischemic stroke: a randomized controlled study in china. J Stroke Cerebrovasc Dis 25:761–770 [DOI] [PubMed] [Google Scholar]
- 52.Guo Y, He Y, Tang B et al. (2016) Effect of using fluoxetine at different time windows on neurological functional prognosis after ischemic stroke. Restor Neurol Neurosci 34:177–187 [DOI] [PubMed] [Google Scholar]
- 53.Miyai I, Reding MJ (1998) Effects of antidepressants functional recovery following stroke: a double-blind study. J Neurol Rehabil 12:5–13 [Google Scholar]
- 54.Kong Y, Dong W, Liu C (2007) Fluoxetine for poststroke depression: a randomized placebo controlled clinical trial. Neural Regen Res 2:162–165 [Google Scholar]
- 55.Dennis M, Mead G, Forbes J et al. (2018) Effects of fluoxetine on functional outcomes after acute stroke (FOCUS): a pragmatic, double-blind, randomised, controlled trial. Lancet 6736:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Berends HI, Nijlant JMM, van Putten MJAM et al. (2009) Single dose of fluoxetine increases muscle activation in chronic stroke patients. Clin Neuropharmacol 32:1–5 [PubMed] [Google Scholar]
- 57.Siepmann T, Kepplinger J, Zerna C et al. (2015) The effects of pretreatment versus de novo treatment with selective serotonin reuptake inhibitors on short-term outcome after acute ischemic stroke. J Stroke Cerebrovasc Dis 24:1886–1892 [DOI] [PubMed] [Google Scholar]
- 58.Etherton MR, Siddiqui KA, Schwamm LH (2018) Prestroke selective serotonin reuptake inhibitor use and functional outcomes after ischaemic stroke. Stroke Vasc Neurol 3:9–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Serebruany VL (2006) Selective serotonin reuptake inhibitors and increased bleeding risk: are we missing something? Am J Med 119:113–116 [DOI] [PubMed] [Google Scholar]
- 60.Mortensen JK, Larsson H, Johnsen SP, Andersen G (2014) Impact of prestroke selective serotonin reuptake inhibitor treatment on stroke severity and mortality. Stroke 45:2121–2123 [DOI] [PubMed] [Google Scholar]
- 61.Miedema I, Horvath KM, Uyttenboogaart M et al. (2010) Effect of selective serotonin re-uptake inhibitors (SSRIs) on functional outcome in patients with acute ischemic stroke treated with tPA. J Neurol Sci 293:65–67 [DOI] [PubMed] [Google Scholar]
- 62.Lange R, Weiller C, Liepert J (2007) Chronic dose effects of reboxetine on motor skill acquisition and cortical excitability. J Neural Transm 114:1085–1089 [DOI] [PubMed] [Google Scholar]
- 63.Acler M, Robol E, Fiaschi A, Manganotti P (2009) A double blind placebo RCT to investigate the effects of serotonergic modulation on brain excitability and motor recovery in stroke patients. J Neurol 256:1152–1158 [DOI] [PubMed] [Google Scholar]
- 64.Asadollahi M, Ramezani M, Khanmoradi Z, Karimialavijeh E (2018) The efficacy comparison of citalopram, fluoxetine, and placebo on motor recovery after ischemic stroke: a double-blind placebo-controlled randomized controlled trial. Clin Rehabil 32:1069–1075 [DOI] [PubMed] [Google Scholar]
- 65.Huang J, X L, X L et al. (2018) Effects of fluoxetine on poststroke dysphagia: a clinical retrospective study. J Stroke Cerebrovasc Dis 27:3320–3327 [DOI] [PubMed] [Google Scholar]
- 66.Mikami K, Ph D, Jorge RE et al. (2011) Effect of antidepressants on the course of disability following stroke. Am J Geriatr Psychiatry 19:1007–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schneider CL, Busza A, Prentiss EK et al. (2019) Fluoxetine may enhance visual recovery after acute ischemic stroke by cortical remapping of the blind visual field. Stroke 50(Suppl_1):ATMP43–ATMP43 [Google Scholar]
- 68.Rowe FJ, Wright D, Brand D et al. (2013) A prospective profile of visual field loss following stroke: prevalence, type, rehabilitation, and outcome. Biomed Res Int 2013:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Strbian D, Ahmed N, Wahlgren N et al. (2012) Intravenous thrombolysis in ischemic stroke patients with isolated homonymous hemianopia: analysis of safe implementation of thrombolysis in stroke-international stroke thrombolysis register (SITS-ISTR). Stroke 43:2695–2698 [DOI] [PubMed] [Google Scholar]
- 70.Horton JC, Fahle M, Mulder T, Trauzettel-Klosinski S (2017) Adaptation, perceptual learning, and plasticity of brain functions. Graefe’s Arch Clin Exp Ophthalmol 255:435–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Busza A, Schneider CL, Williams ZR et al. (2019) Using vision to study poststroke recovery and test hypotheses about neurorehabilitation. Neurorehabil Neural Repair 33:87–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dilks DD, Serences JT, Rosenau BJ et al. (2007) Human adult cortical reorganization and consequent visual distortion. J Neurosci 27:9585–9594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vaina LM, Soloviev S, Calabro FJ et al. (2014) Reorganization of retinotopic maps after occipital lobe infarction. J Cogn Neurosci 26:1266–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guo X, Jin Z, Feng X, Tong S (2014) Enhanced effective connectivity in mild occipital stroke patients with hemianopia. IEEE Trans Neural Syst Rehabil Eng 22:1210–1217 [DOI] [PubMed] [Google Scholar]
- 75.Nelles G, Widman G, de Greiff A et al. (2002) Brain representation of hemifield stimulation in poststroke visual field defects. Stroke 33:1286–1293 [DOI] [PubMed] [Google Scholar]
- 76.Raposo N, Cauquil AS, Albucher JF et al. (2011) Poststroke conscious visual deficit: clinical course and changes in cerebral activations. Neurorehabil Neural Repair 25:703–710 [DOI] [PubMed] [Google Scholar]
- 77.Brodtmann A, Puce A, Darby D, Donnan G (2009) Serial functional imaging poststroke reveals. Neurorehabil Neural Repair 23:150–159 [DOI] [PubMed] [Google Scholar]
- 78.Sereno MI, Dale AM, Reppas JB et al. (1995) Borders of multiple visual areas in humans revealed by functional magnetic resonance imaging. Science 268:889–893 [DOI] [PubMed] [Google Scholar]
- 79.Schneider CL, Prentiss EK, Busza A et al. (2019) Survival of retinal ganglion cells after damage to the occipital lobe in humans is activity-dependent. Proc R Soc B 286:20182733. [DOI] [PMC free article] [PubMed] [Google Scholar]