Abstract
Tacrolimus exhibits unpredictable pharmacokinetics after lung transplant, partly explained by CYP-enzyme polymorphisms. However, whether exposure variability during the immediate post-operative period affects outcomes is unknown, and pharmacogenetic dosing may be limited by residual pharmacokinetic variability. We estimated adjusted associations between early post-operative tacrolimus concentrations and acute kidney injury (AKI) and acute cellular rejection (ACR), and identified clinical and pharmacogenetic factors that explain post-operative tacrolimus concentration variability in 484 lung transplant patients. Increasing tacrolimus concentration was associated with higher AKI risk: HR 1.54, (95%CI 1.20–1.96) per 5-mg/dL; and increasing AKI severity (OR 1.29 (1.04–1.60) per 5-mg/dL; but not ACR: HR 1.02 (95%CI 0.73–1.42). A model with clinical and pharmacogenetic factors explained 42% of concentration variance compared to 19% for pharmacogenetic factors only. Early tacrolimus exposure was independently associated with AKI after lung transplantation, but not ACR. Clinical factors accounted for substantial residual tacrolimus concentration variability not explained by CYP-enzyme polymorphisms.
Keywords: transplantation, toxicity, therapeutic index, therapeutic drug monitoring, adverse events, immunosuppressants, pharmacogenetics, pharmacokinetics-pharmacodynamics, precision medicine, CYP
Introduction
Tacrolimus is a potent calcineurin inhibitor that is a cornerstone of immunosuppression after lung transplantation.1 Tacrolimus therapy is complicated by highly variable pharmacokinetic (PK) parameters and a narrow therapeutic index that necessitates concentration monitoring.2 Many centers use a “trial and error” approach to initial dosing and titration, resulting in highly variable exposure in the early period after transplant.3 This variability may impact two clinically significant phenomena: acute kidney injury (AKI), a rapid loss of renal function that may be potentiated by high tacrolimus levels;4,5 and acute cellular rejection (ACR), immune-mediated allograft injury that may result from low tacrolimus levels.6,7 Both AKI and ACR have been linked to worse post-transplant clinical outcomes.8,9 The extent to which tacrolimus concentration variability in the early post-lung transplant period affects AKI and ACR rates, however, remains poorly understood.10
Tacrolimus is primarily metabolized by cytochrome P450 3A5 (CYP3A5) and 3A4 (CYP3A4) enzymes. Allelic variation in CYP3A5 and CYP3A4 genes, therefore, could explain a substantial proportion of tacrolimus pharmacokinetic variability.11 In kidney transplantation, patients who express functional CYP3A5 enzyme require approximately 50% higher tacrolimus doses.12 Recent kidney transplant trials, however, showed limited effectiveness of CYP3A5-based dosing for achieving target concentrations.13 These findings suggest that additional factors play a significant role in tacrolimus pharmacokinetic variability.13 Novel alleles in genes that determine tacrolimus metabolism (P450 oxidoreductase (POR) and peroxisome proliferator–activated receptor-α (PPARA))11,14,15 and numerous clinical factors2,3 may account for such residual variability. These novel alleles have not been examined in the lung transplant population. In addition, prior studies of pharmacogenetic and clinical predictors of tacrolimus exposure variability have focused on broad timeframes, from months to years post-lung transplant.16 Tacrolimus pharmacokinetic variability is likely to be more pronounced during the immediate post-transplant period, when clinical status and myriad interacting medications are often rapidly changing. Patients may also be particularly vulnerable to adverse effects during this time. Understanding predictors of early tacrolimus variability may therefore be key to informing effective precision dosing strategies. Further, in order to elucidate clinical utility and justify the expense and effort of pharmacogenetic dosing, it remains important to determine if early tacrolimus levels impact subsequent AKI and ACR rates.
This study was thus designed to answer two key questions: First, which clinical and pharmacogenetic factors explain early tacrolimus exposure variability? Second, what is the association between early tacrolimus exposure, AKI, and ACR during the immediate post-operative period? To address these questions, we conducted a retrospective analysis of patients from one center of the multicenter Lung Transplant Outcomes Group (LTOG) cohort study.17 We modeled tacrolimus concentration:dose ratio (CDR) using mixed effects linear regression; and we estimated adjusted associations between tacrolimus concentration, AKI, and ACR using Cox regression.
Results
Patient characteristics
During the study period, 494 patients were enrolled in the LTOG study at our center. From this population, 6 were excluded due to missing data, and 4 were excluded due to cyclosporine crossover, leaving 484 for the primary analysis (353 were genotyped). Median inpatient follow-up was 14 days (IQR, 11–14), and the median number of tacrolimus trough concentrations was 12 (IQR, 9–13). The median initial tacrolimus daily dose was 4 mg (IQR, 2–4). The average age was 60 years, most patients were White or Black race, and baseline renal dysfunction was uncommon (Table 1). Median allograft ischemic time was 4 hours and there was a roughly even split of single lung vs. bilateral lung transplant procedures.
Table 1.
Patient Characteristics
| Recipient pre-operative variables | |
| Age, years, median (IQR) | 60 (53, 64) |
| Female sex, n (%) | 193 (40) |
| Body mass index, mean (SD) | 26.4 (4.1) |
| Race, n (%) | |
| White | 421 (87) |
| African American | 46 (9) |
| Other | 17 (4) |
| Pulmonary diagnosis, n (%) | |
| Chronic obstructive pulmonary disease | 210 (43) |
| Idiopathic pulmonary fibrosis | 185 (38) |
| Cystic fibrosis | 38 (8) |
| Othera | 51 (11) |
| mPAP, median, (IQR) | 29.3 (23.3, 35.0) |
| Serum creatinine, mg/dl, median (IQR) | 0.8 (0.7, 1.0) |
| eGFR < 60 ml/min/1.73m2, n (%) | 33 (6.8) |
| Hematocrit, (%), mean (SD) | 34 (5) |
| Platelets, x1011 cells/L, median (IQR) | 180 (123, 251) |
| Donor Variables | |
| Age, years, median (IQR) | 35 (23, 48) |
| Female sex, n (%) | 200 (41) |
| Race, n (%) | |
| White | 330 (68) |
| African American | 98 (20) |
| Other | 56 (12) |
| Operative Variables | |
| Ischemic time, minutes, median (IQR) | 286 (227, 345) |
| Transplant type, single, n (%) | 215 (45) |
| Cardiopulmonary bypass use, n (%) | 236 (49) |
| Intra-operative transfusions, n (%) | |
| Red cells | 217 (45) |
| Plasma | 141 (29) |
| Cryoprecipitate | 25 (5) |
| Immunosuppression regimens | |
| Induction agent, n (%) | |
| Basiliximab | 419 (86.5) |
| Daclizumab | 62 (13) |
| Anti-thymocyte globulin | 3 (0.5) |
| Antiproliferative agent, n (%) | |
| Mycophenolate | 240 (50) |
| Azathioprine | 244 (50) |
| Corticosteroid dosea, mg, median (IQR) | 38 (32–44) |
| Antiviral prophylaxis, n (%) | |
| Ganciclovir | 374 (77) |
| Acyclovir | 110 (23) |
| CYP3A5 activity classificationb (n=353 genotyped patients) | |
| Number of CYP3A5 loss of function alleles | |
| None (extensive metabolizer, CYP3A5*1*1) | 12 (3) |
| One (intermediate metabolizer, CYP3A5*1*3, *1*6, *1*7) | 52 (15) |
| Two (poor metabolizer, CYP3A5*3*3, *6*6, *7*7, *3*6, *3*7, *6*7) | 289 (82) |
average dose during the first post-operative week;
Clinical Pharmacogenetics Implementation Consortium (CPIC) consensus guidelines12 recommend to classify CYP3A5 functional status using three single nucleotide polymorphisms: rs776746 (CYP3A5*3), rs10264272 (CYP3A5*6), and rs41303343 (CYP3A5*7). IQR – interquartile range; SD- standard deviation; mPAP- mean pulmonary artery pressure at the time of transplant; eGFR- estimated glomerular filtration rate;
Pharmacogenetic analysis
The individual trend-lines of tacrolimus trough concentration (Figure 1) showed substantial variability over time, with wide deviation from the target range of 8–12 ng/ml. The median percentage of time within target range was 27% (14–40%) for the two-week postoperative period. Exposure to CYP enzyme inhibitors was common, particularly in the second week (Figure S1). Nearly one in five patients had a CYP3A5 genotype associated with intermediate or extensive tacrolimus metabolism according to the Clinical Pharmacogenetics Implementation Consortium (CPIC) classification (Table 1; Table S1 shows other allele frequencies). CYP3A5 genotype showed clear associations with tacrolimus CDR. Patients who were CPIC-defined poor metabolizers had higher CDR values throughout the study period (Figure 2a), consistent with reduced tacrolimus metabolism. Absolute tacrolimus concentrations were higher in these patients as well (Figure 2b), though this was only evident through postoperative day six. Significantly higher CDR was observed in patients with the CYP3A4*22 loss of function allele (Figure S2a), while no association was apparent between CDR and either POR*28 or PPARA alleles (Figure S2b, S2c).
Figure 1. Individual Trend Lines of Tacrolimus Concentration.
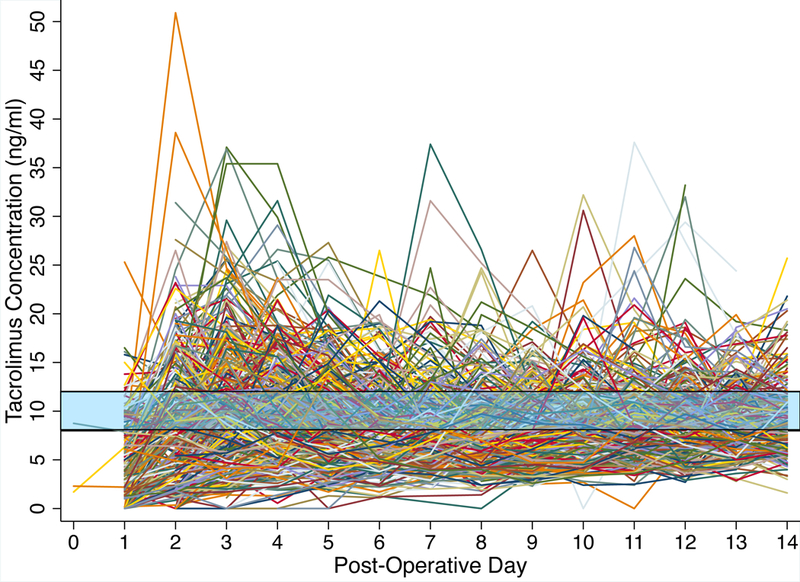
Trends of tacrolimus concentrations over time (n=5446 concentrations). The shaded area represents the target tacrolimus concentration range (8–12 ng/ml). Date and time of lab value reporting were used to determine post-operative day of the result. Of the 3408 (63%) values that also had available data on precise specimen draw time, 95% were considered “true troughs”, defined as being drawn between 9 & 13 hours after the preceding tacrolimus dose and before the next dose.
Figure 2. Tacrolimus concentration / dose ratio (a.) and concentrations (b.) over time by CYP3A5 genotype.

a.- Tacrolimus concentration / dose ratio over time, stratified by CYP3A5 genotype: patients with two loss of function alleles (solid line) vs. at least one normal allele (dashed line); b.-Tacrolimus concentration over time, stratified by CYP3A5 genotype: patients with two loss of function alleles (solid line) vs. at least one normal allele (dashed line). Trend lines depict means and error bars depict 95% confidence intervals
In the multivariable mixed effects model (Table 2), the strong association between CYP3A5 genotype and CDR remained. The CYP3A4*22 loss of function allele was associated with a 14.6% increase in CDR, but this estimate was non-significant. Several clinical variables exhibited strong associations with tacrolimus CDR, including CYP450 inhibitors (azole antifungals, amiodarone), transplant type, cystic fibrosis diagnosis, and hematocrit, which is a marker of tacrolimus binding to red blood cells. A model with both clinical factors and CYP3A5 genotype explained 42% of CDR variance (R2 = 0.42, 95% CI 0.38–0.48) compared to 31% for a model with only clinical variables (R2 = 0.31, 95% CI 0.27–0.37) and 19% for a model with only CYP3A5 genotype status (R2 = 0.19, 95% CI 0.14–0.27).
Table 2.
Multivariable mixed effects model for tacrolimus CDR
| Variable | Percent Change in CDRa |
|---|---|
| Post-operative day | −4.5 (−5.6, −3.4) |
| CYP3A5 Genotypeb | |
| Poor metabolizers | ref. |
| Intermediate metabolizers | −51.7 (−59.9, −41.7) |
| Extensive metabolizers | −60.7 (−72.8, −43.4) |
| CYP3A4*22 LoF Allele | 14.7 (−7.4, 42.2) |
| PPARAc | 5.3 (−7.2, 16.4) |
| POR*28d | −2.5 (−15.9, 9.4) |
| ABCB1 3435C>Te | −1.6 (−14.8, 13.8) |
| Hematocrit (5 % increase) | 10.0 (5.9, 14.4) |
| Weight (10 kg increase) | −8.3 (−11.7, −4.8) |
| Primary graft dysfunctionf | 18.5 (1.6, 38.1) |
| Procedure type | |
| Single lung | ref. |
| Bilateral lung | 40.7 (23.4, 60.5) |
| Cystic fibrosis | −28.9 (−44.6, −8.6) |
| Azole antifungal exposureg | |
| None | ref. |
| Fluconazole | 21.3 (7.6, 36.8) |
| Voriconazole | 79.7 (65.1, 95.5) |
| Amiodarone exposure | 18.6 (11.0, 26.7) |
analysis based on log-transformed CDRs. Model coefficients were exponentiated to provide the percentage change in CDR for a one unit change in each covariate, unless otherwise specified. Increases in CDR signify decreases in tacrolimus clearance
Likelihood ratio test, p<0.0001 for a model with CYP3A5 genotype vs. without genotype. The effects of CPIC defined intermediate vs. extensive metabolizer status on the CDR were similar (p=0.273)
At least one variant allele (GA, GG)
At least one variant allele (CT, TT)
At least one variant allele (CT, TT)
Grade 3 primary graft dysfunction at 48 or 72 hours after transplantation
Likelihood ratio test, p<0.0001
Acute Kidney Injury analysis
AKI occurred in 290/484 (60%) of patients, with most (89%) occurring during the first postoperative week. AKI rate increased with greater tacrolimus exposure intensity (Figure 3), though the absolute rates of AKI were higher during postoperative days 0–3 (Figure 3a) than days 4–14 (Figure 3b). In the multivariable adjusted time-varying Cox model, AKI rate increased 54% for each five ng/ml increase in average tacrolimus trough concentrations over the preceding three days (HR 1.54, (95%CI 1.20, 1.96). Results were similar in an analysis censored at postoperative day seven and across variations of the tacrolimus exposure definition (Table S2).
Figure 3. Incidence rate of acute kidney injury by tacrolimus exposure intensity.

Tacrolimus time-lagged average concentration on each day was classified into bins of concentration as follows: <8 ng/ml, 8–12 ng/ml, > 12 ng/ml. The rate of AKI was then calculated within each bin of person time as the number of AKI events / total person time within that range. Because baseline AKI rate varied substantially over time, analysis was stratified by time. a- AKI rate during post-operative days 0–3. b. AKI rate during post-operative days 4–14.
AKI stages 1, 2, and 3 occurred in 32%, 16%, and 12% of patients, respectively, with higher tacrolimus concentration on the day of AKI diagnosis associated with increasing severity: OR 1.29 (1.04–1.60) for a one-stage increase per 5-ng/ml increase. The predicted distribution of AKI severity at various concentrations is shown in Figure 4, which depicts a shift towards higher stage as tacrolimus exposure increases. Time to AKI resolution was somewhat longer with higher tacrolimus level on the day of AKI onset but the association was non-significant (adjusted HR: 0.91 (0.81–1.02) per five-ng/ml increase. We observed no significant direct associations between SNPs of interest and AKI (Table S2). The strength of the association between time varying tacrolimus concentration and AKI hazard varied across ABCB1 3435C>T genotype, but the test for interaction was not significant. The HR (per 5 ng/ml increase) in those homozygous for the wildtype ABCB1 allele was 1.23 (0.78–1.93), compared to 1.69 (1.28–2.23) in those with at least one ABCB1 3435C>T variant allele (p-value for interaction 0.107).
Figure 4. Effect of tacrolimus exposure intensity on acute kidney injury severity.
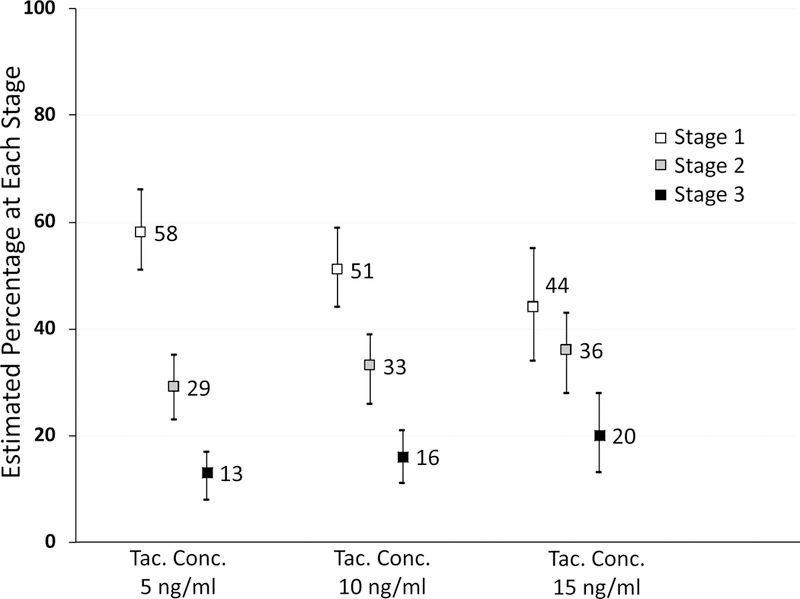
Analysis restricted to n=290 patients that experienced an acute kidney injury episode. Figure depicts the predicted distribution of acute kidney injury severity at various tacrolimus exposure intensities. Estimates are derived from an ordinal logistic regression model adjusted for diagnosis, total ischemic time, primary graft dysfunction, loop diuretic exposure on the day of AKI diagnosis, vasopressor support on the day of AKI diagnosis, and the post-operative day of AKI diagnosis. Adjusted OR from the model for a one stage increase: 1.29 (1.04–1.60) for a five-ng/ml increase in concentration
Acute Cellular Rejection and mortality
Patients received a median of three surveillance bronchoscopies during the first postoperative year (IQR 2–4). There was no clear association between average tacrolimus levels during the first postoperative week and ACR in the crude analysis (Figure 5), or in a fully adjusted cause-specific hazards model that accounted for the competing risk of death, HR 1.02 (95% CI 0.73–1.42) per 5 ng/ml increase in average week one tacrolimus concentration. Mortality increased significantly at higher tacrolimus levels in crude analysis; but this association was attenuated after adjustment for potential confounders, HR 1.32 (95% CI 0.87–1.99) per 5 ng/ml. The association between average week one tacrolimus concentration and the composite outcome of ACR or mortality, HR 1.12 (95% CI 0.85–1.47) was also non-significant. Similar results were seen in sensitivity analyses that varied the tacrolimus exposure definition (Table S2). We observed no significant associations between SNPs of interest and ACR or mortality (Table S2), nor any significant interaction between ABCB1 genotype and average tacrolimus concentration during the first week.
Figure 5. Acute cellular rejection and mortality.

Figure depicts the cumulative incidence of acute cellular rejection and mortality at one year after transplant.
Discussion
The standard approach to tacrolimus dosing after lung transplantation is largely reactive, where “one size fits all” initial doses are individualized based on therapeutic drug monitoring. In our population, this strategy led to widely varying exposure during the early postoperative period. An alternative, proactive strategy, would leverage information on the determinants of tacrolimus pharmacokinetics to provide individualized initial dosing and titration. The clinical utility of such an approach depends on two factors: 1) that valid predictors of dose response exist; and 2) that exposure variability impacts clinically meaningful outcomes. In this study, we found that SNPs determining CYP3A5 function had a strong impact on tacrolimus CDR. We also identified clinical variables that explained substantial residual pharmacokinetic variability. Further, tacrolimus concentrations in this early time period were significantly associated with AKI while demonstrating no association with ACR. These findings support the potential clinical utility of precision tacrolimus dosing algorithms that combine clinical and pharmacogenetic information.18 We hypothesize that such an approach may avoid excessive tacrolimus exposure and reduce early AKI and its associated morbidity.
Consistent with prior literature in lung and other transplant populations, we observed a strong effect of CYP3A5 polymorphisms on tacrolimus CDR values.11,12,16 Patients categorized as poor metabolizers had roughly 50% higher CDR values compared to intermediate and extensive metabolizers. We also observed that patients with the CYP3A4*22 variant allele had roughly 15% higher CDR values. While the confidence interval around this estimate included the null value, the magnitude of the point estimate for CYP3A4*22 is similar to previous studies in kidney transplant recipients.11,14 The CYP3A4*22 allele therefore may ultimately prove a useful predictor of CDR, but should be tested in larger lung transplant studies. The reason for negative findings with the additional SNPs investigated (POR*28, PPARA, ABCB1) is unclear.
Though CYP3A5 genotype predicts tacrolimus metabolism, a recent trial of CYP3A5 guided dosing in kidney transplantation did not improve time to therapeutic concentrations or clinical outcomes.13 Residual pharmacokinetic variability was proposed as a reason for this finding.11,13 Our study is the first to quantify and explain this residual variability in the first two weeks after lung transplantation, when a precision dosing algorithm may be most useful. The CDR analysis showed that a model with CYP3A5 genotype alone explained only 19% of CDR variability. The model that combining clinical factors with CYP3A5, however, explained 42% of CDR variability – a clinically meaningful increase. Notably, genotype effects on actual tacrolimus concentrations became insignificant after the first week, likely due to the effect of daily monitoring and titration. The capacity of pharmacogenetic dosing algorithms to improve concentration targeting, therefore, may be limited to this very early time frame.
Our data suggest a lower relative contribution of genetics to tacrolimus exposure variability compared to previous studies, where CYP3A5 status alone has accounted for up to 50% of tacrolimus variability.12,16 However, these previous estimates may not be directly comparable to our results. We studied the immediate post-operative period after lung transplantation, when hemodynamic instability and critical illness pathophysiology are common and can have strong effects on tacrolimus PK.2,3,19 In contrast, Birdwell et al reported data largely for kidney transplant recipients, who generally experience a more stable post-operative course.12 Calabrese et al analyzed tacrolimus concentrations obtained months to years after lung transplant,16 when the effects of post-operative critical illness are no longer apparent.3,19 We identified clinical predictors of tacrolimus CDR that may only be relevant during the early post-operative period. Specifically, transplant type and primary graft dysfunction are drivers of early inflammation and hemodynamic instability;3,19 and early fluctuations of hematocrit are often related to critical illness and fluid resuscitation.19 Our results thus suggest that effective dosing algorithms for this unique time period will need to leverage both genotype and population-specific clinical variables.
The clinical utility of any tacrolimus dosing algorithm for the early postoperative period depends on whether exposure variability during this time period negatively impacts outcomes. While the nephrotoxicity of long-term tacrolimus exposure is well characterized,4,5 tacrolimus may be also be acutely nephrotoxic at high levels due to afferent arteriolar vasoconstriction in the glomerulus.5 Only a single prior study has examined the relation between tacrolimus exposure and AKI during the early post-lung transplant period, showing an association of at least one tacrolimus level >15 ng/ml with AKI in the first two weeks.10 It is unclear, however, whether the analysis differentiated levels drawn before vs. after AKI episodes. Moreover, effects on AKI severity and duration were not evaluated.10 Further, dichotomizing tacrolimus exposure makes it difficult to extrapolate the results to other centers that use different concentration targets.
In our study, we observed a strong association between increasing time-lagged tacrolimus exposure and the hazard of developing AKI during the first two postoperative weeks. To our knowledge, this is the first study to determine the time-varying impact of tacrolimus exposure on AKI risk during this early time period. Moreover, we are the first to show that increasing tacrolimus concentrations on the day of AKI diagnosis are associated with worse AKI severity. This latter finding is particularly important, showing that tacrolimus toxicity may exacerbate AKI precipitated by other postoperative factors.
While direct nephrotoxicity is one explanation for the link between tacrolimus and AKI, it is also possible that the association is non-causal. Specifically, hepatic metabolism of tacrolimus is known to slow in the setting of critical illness19 such that high tacrolimus levels could be a non-causal bystander reflecting severity of illness. We found, however, that the tacrolimus-AKI association was robust to adjustment for severity of illness variables including primary graft dysfunction, diagnosis leading to transplant, vasopressor exposure, and other AKI risk factors. It therefore remains plausible that tacrolimus exposure optimization during the immediate postoperative period could reduce AKI rates and severity, as well as consequent CKD and its associated 4-fold increase in long-term mortality.20
Reduced tacrolimus concentrations during the early postoperative period may increase allograft rejection rates. The lung transplant population is at high rejection risk due to variable HLA matching, allograft exposure to the external environment, and substantial donor driven immune activation.9 Although it is biologically plausible that high tacrolimus exposure during the immediate post-transplant period is needed to prevent rejection, no prior studies have examined this question.21 In contrast to our AKI analyses, differential tacrolimus exposure during the first post-transplant week was not associated with ACR. While tacrolimus is a critical component of maintenance immunosuppression, therapeutic levels in the first week may be less important in the setting of concomitant induction therapy, such as our center’s use of basiliximab and high dose steroids. If true, early tacrolimus minimization may reduce AKI rates without sacrificing immunosuppression effectiveness. Renal-sparing strategies (e.g., lower concentration targets, delayed tacrolimus initiation) have been shown to reduce the rate of acute and chronic kidney disease in heart, kidney, and liver transplant populations.22–24 Such strategies would need more robust testing to consider after lung transplantation.25 In addition, some caution is warranted in interpreting our results. As noted, post-transplant severity of illness may impair hepatic metabolism (leading to higher tacrolimus concentrations) while also increasing ACR risk.9 A spurious association between high tacrolimus levels and ACR risk may therefore be seen in this patient subset, which may dilute any association of low tacrolimus levels with ACR among patients with a more routine post-transplant course. Notably, tacrolimus levels remained unassociated with ACR after adjustment for diagnosis leading to transplant, immunosuppression regimen, transplant type, primary graft dysfunction, and other risk factors.
We did not observe significant associations between our selected set of CYP polymorphisms and clinical outcomes. When the analysis of tacrolimus concentration and AKI was stratified by ABCB1 genotype, the magnitude of the hazard ratio was somewhat larger in those with a low activity genotype compared to those with high activity genotype (1.69 vs. 1.23, respectively), but the interaction was not significant. These results are in contrast to Calabrese, who observed higher AKI rates in patients with high activity ABCB1 genotypes, and lower ACR rates in patients with low activity ABCB1 genotypes, although interactions between genotype and tacrolimus concentration were not examined.16 The mechanism underlying the Calabrese AKI result is unclear, as the high activity genotype would be expected to reduce tacrolimus concentrations and lower AKI risk,11 Given the potential utility of using ABCB1 genotype to evaluate tacrolimus nephrotoxicity risk, additional larger studies of this association are warranted.
Our study has limitations. First, this was a single center study. Multicenter studies would better account for center effects such as case mix and heterogeneous induction protocols. Second, we studied only SNPs previously described to affect tacrolimus metabolism. It is possible that genome wide studies may identify additional predictive SNPs. Third, because the early post-transplant period is characterized by multiple potential renal insults it is difficult for us to rule out residual confounding of the tacrolimus-AKI relationship. While validation of our results in multicenter studies may help, ultimately the best proof of this relationship may be testing the impact of early tacrolimus-sparing strategies in clinical trials.
Conclusion
Tacrolimus concentrations during the first two weeks after lung transplantation are influenced by CYPA5 genotype, drug-drug interactions, and lung transplant specific clinical factors. Early tacrolimus concentrations are associated with incident postoperative AKI, but not ACR. Our data warrant multicenter validation, and ultimately may support studies to examine the impact of early tacrolimus minimization and precision-dosing strategies to reduce post-transplant renal dysfunction.
Concise Methods
The full study protocol can be found in the online supplementary material. In brief here, we conducted a retrospective analysis of lung transplant recipients at the University of Pennsylvania who were enrolled in the Lung Transplant Outcomes Group (LTOG) study, a multicenter prospective cohort of primary graft dysfunction (PGD).17 LTOG data were merged with electronic health record data (medications, laboratory values, dialysis orders) for the current analysis. Included subjects underwent lung transplantation between November 2003 and August 2015. The University of Pennsylvania institutional review board approved the study (IRB #806468), and patients provided informed consent.
Genotypes
Genotyping was performed on peripheral blood samples using an Affymetrix Axiom genotyping array with Applied Biosystems Axiom 2.0 Reagents.26 We selected SNPs that have been shown to significantly affect tacrolimus dose response in other solid organ transplant populations, including both established SNPs (those affecting CYP3A5), and novel candidate SNPs that may explain residual pharmacokinetic variability.11,12,14,15
CYP3A5 is the predominant tacrolimus metabolic pathway in patients that express the enzyme and previous research has shown this enzyme to be the most important source of pharmacogenetic variation in tacrolimus dose response.12 CYP3A5 variants included in the analysis were rs776746 (CYP3A5*3); rs10264272 (CYP3A5*6); and rs41303343 (CYP3A5*7). Prior research has shown that the *6 and *7 variants produce similar loss of CYP3A5 activity compared to the *3 variant, but are more common in the African American population.12 We classified CYPA5 with these SNPs according to the Clinical Pharmacogenetics Implementation Consortium (CPIC) recommenndations12: extensive metabolizers (CYP3A5*1*1), intermediate metabolizers (CYP3A5*1*3, *1*6, *1*7), poor metabolizers (CYP3A5*3*3, *6*6, *7*7, *3*6, *3*7, *6*7).
In addition, CYP3A4*22 (rs35599367), POR*28 (rs1057868), PPARA (c.208+3819A>G), and ABCB1 3435C>T (rs1045642) were genotyped based on kidney transplant studies that suggests these SNPs may explain additional tacrolimus concentration variability beyond the effects of CYP3A5.11–14,15 CYP3A4*22 is a loss of function allele that is found in 2–10% patients.11–14,15 POR*28 codes for a variant of P450 oxidoreductase (POR).11,15 Prior studies suggest that POR*28 increases CYP3A5 metabolism of tacrolimus.11,15 PPARA encodes for the nuclear receptor peroxisome proliferator-activated receptor alpha (PPAR-a).11,15 Prior research suggests that the variant allele results in reduced PPAR-α expression, which may lower CYP3A4 protein expression, enzymatic activity, and tacrolimus metabolism.11,15 ABCB1 encodes for P-glycoprotein (PGP), an ATP-dependent drug efflux pump present in the intestine, liver, and kidneys that may mediate tacrolimus uptake across cellular membranes.11 In addition to potentially affecting tacrolimus absorption, ABCB1 activity may modify tacrolimus pharmacodynamics by altering the uptake of tacrolimus into renal tubular cells and lymphocytes.11
Outcome definitions
Tacrolimus concentration:dose ratios (CDR) were calculated as a measure of tacrolimus pharmacokinetics.27 Daily CDRs were calculated by dividing each trough by the average dose over the preceding 48-hours (approximately four tacrolimus half-lives).2
We defined AKI during the first fourteen post-transplant days according to the Kidney Disease: Improving Global Outcomes (KDIGO) creatinine and dialysis criteria.28 We used the last creatinine obtained before surgery as the baseline value. We staged AKI over seven days following AKI onset. AKI resolution was defined as freedom from renal replacement therapy (RRT) for >14 days and a return of creatinine concentration to ≤25% above baseline at hospital discharge.29
We defined ACR based on transbronchial biopsy data according to the International Society for Heart and Lung Transplantation guidelines.30 The primary outcome was the occurrence of Grade A2 or higher rejection during the first postoperative year.31
Statistical Analysis
Linear mixed effects regression was used to model the effect of pharmacogenetic and clinical variables on tacrolimus CDR, adjusting for population stratification using the top four principle components derived from whole genome SNPs.32 Time to AKI was modeled using Cox proportional hazards regression, with daily tacrolimus trough concentrations included as a time-varying covariate. Specifically, for each day di, tacrolimus exposure history (AVG72) was defined as the average of troughs from the previous three days (di-4 through di-1). For days 0–2, we averaged troughs up to that time point. Ordinal logistic regression was used to model the effect of tacrolimus exposure on AKI severity stage. Time to AKI resolution was modeled with Cox-proportional hazards regression, with time zero set to the day of AKI diagnosis. In the AKI severity and resolution models, tacrolimus exposure was defined as the trough concentration measured on the day of AKI diagnosis. Time to ACR was modeled with cause-specific hazards regression to account for the competing risk of mortality,33 with tacrolimus exposure defined as the average trough concentration during the first postoperative week. Additional models were created for time to death, and a composite of ACR and death. In addition, separate models were constructed that examined the direct association between genotypes and AKI, ACR, and mortality. Lastly, we tested for interaction between tacrolimus concentration and the ABCB1 3435C>T genotype in the primary AKI and ACR analyses.
Each multivariable outcome model evaluated an extensive list of potential confounding variables, including factors associated with pre-operative health status (age, body mass index, race, sex, baseline glomerular filtration rate, diagnosis leading to transplant); donor characteristics (donor age, donor, sex, donor race); variables associated with post-operative severity of illness (total ischemic time, transplant type (single vs. bilateral lung), cardiopulmonary bypass use, intra-operative transfusions, primary graft dysfunction, vasopressors, post-operative platelet count, post-operative hematocrit count); potential nephrotoxins (AKI model); immunosuppressive medications (ACR and mortality models); and CYP enzyme inhibitors and inducers (CDR model). Each outcome model included covariates that changed the point estimated for tacrolimus exposure by ≥ 10%, and any covariates significantly associated with the outcome.34 Statistical inference was based on point estimates and 95% confidence intervals.35 Additional details of statistical modeling, including the multivariable model specifications and covariate definitions can be found in the full protocol in the online supplementary material.
Supplementary Material
The heat map shows the patterns of drug exposures over time in the study population. Columns represent specific drugs and each row represents a post-operative day of follow-up. Cell shading represents the percentage of patients exposed to a particular drug on each day.
Figures show concentration / dose ratio over time stratified by allele status. a-CYP3A4*22, Normal allele CC (dashed line), variant allele CT/TT solid line; b- POR*28, Normal allele CC (dashed line), variant allele CT/TT (solid line); c- PPARA (c.208+3819A>G), Normal allele AA (dashed line), variant allele GA/GG (solid line); d- ABCB1 (3435C>T), Normal allele CC (dashed line), variant allele CT/TT (solid line)
Allele frequencies by self-identified race
Sensitivity analyses and secondary analyses of SNP-outcome associations
Study Highlights.
What is the current knowledge on the topic?
Tacrolimus has an unpredictable dose response in the early period after lung transplant, but whether early exposure variability affects clinical outcomes is unknown. CYP-polymorphisms may be responsible for a substantial portion of tacrolimus exposure variability, but the relative importance of genetic variation during the early period after lung transplant is unclear.
What question did this study address?
First, what is the association between early tacrolimus exposure variability and outcomes after lung transplantation? Second, which clinical and pharmacogenetic factors explain early tacrolimus exposure variability after lung transplantation?
What does this study add to our knowledge?
Increasing tacrolimus exposure during the first two weeks after transplant was associated with increased acute kidney injury risk and worse injury severity, but did not impact rejection. Early tacrolimus exposure variability is explained in part by CYP3A5 genotype, but clinical factors accounted for substantial residual variability.
How might this change clinical pharmacology or translational science?
Our results suggest that effective precision dosing algorithms for tacrolimus will need to leverage both genotype and population-specific clinical variables. Such an approach may avoid excessive tacrolimus exposure and reduce early acute kidney injury after lung transplantation.
Acknowledgments
We would like to thank the Penn Data Store analysts for assistance with electronic health record data query and cleaning.
Funding
This work was funded by National Institutes of Health (K23DK097307, R01DK111638, to MGSS; K24HL115354 and R01HL087115 to JDC; and HL116656, HL135227, to EC); the Robert Wood Johnson Foundation AMFDP70640 to EC; and the University of Pennsylvania University Research Foundation.
Role of sponsors: The sponsors had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Footnotes
Conflict of Interest
Dr. Flesch’s spouse owns shares in the following pharmaceutical companies: Gilead Sciences, Allergan, Johnson & Johnson, Glaxo Smith Klein, Pfizer, and Teva Pharmaceutical Industries. All other authors declared no competing interests for this work.
Statement of Integrity: Dr. Miano had full access to study data and takes responsibility for its integrity and that of the data analysis.
References
- 1. 2016 Annual Data Report. Scientific Registry of Transplant Recipients http://srtr.transplant.hrsa.gov/annual_reports/Default.aspx Accessed [September 2017]
- 2.Staatz CE, Tett SE. Clinical Pharmacokinetics and Pharmacodynamics of Tacrolimus in Solid Organ Transplantation. Clin Pharmacokinet 2004; 43: 623–653 [DOI] [PubMed] [Google Scholar]
- 3.Sikma MA, van Maarseveen EM, van de Graaf EA, Kirkels JH, Verhaar MC, Donker DW et al. Pharmacokinetics and toxicity of tacrolimus early after heart and lung transplantation. Am J Transplant 2015; 15(9):2301–2313 [DOI] [PubMed] [Google Scholar]
- 4.Bloom RD, Doyle AM. Kidney disease after heart and lung transplantation. Am J Transplant. 2006. April;6(4):671–9. [DOI] [PubMed] [Google Scholar]
- 5.Naesens M, Kuypers DR, Sarwal M. Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol. 2009. February;4(2):481–508. [DOI] [PubMed] [Google Scholar]
- 6.Gallagher HM, Sarwar G, Tse T, et al. Erratic tacrolimus exposure, assessed using the standard deviation of trough blood levels, predicts chronic lung allograft dysfunction and survival. J Heart Lung Transplant. 2015;34(11):1442–1448 [DOI] [PubMed] [Google Scholar]
- 7.Ensor CR, Iasella CJ, Harrigan KM, Morrell MR, Moore CA, Shigemura N, Zeevi A, McDyer JF, Venkataramanan R. Increasing tacrolimus time-in-therapeutic range is associated with superior one-year outcomes in lung transplant recipients. Am J Transplant. 2018. June;18(6):1527–1533. [DOI] [PubMed] [Google Scholar]
- 8.Wehbe E, Duncan AE, Dar G, Budev M, Stephany B. Recovery from AKI and short- and long-term outcomes after lung transplatation. Clinical Journal of the American Society of Nephrology American Society of Nephrology 2013;8(1):19–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McManigle W, Pavlisko EN, Martinu T. Acute cellular and antibody-mediated allograft rejection. Semin Respir Crit Care Med. 2013. June;34(3):320–35. [DOI] [PubMed] [Google Scholar]
- 10.Sikma MA, Hunault CC, van de Graaf EA, Verhaar MC, Kesecioglu J, de Lange DW, Meulenbelt J. High tacrolimus blood concentrations early after lung transplantation and the risk of kidney injury. Eur J Clin Pharmacol. 2017. May;73(5):573–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang JT, Andrews LM, van Gelder T, Shi YY, van Schaik RH, Wang LL, Hesselink DA. Pharmacogenetic aspects of the use of tacrolimus in renal transplantation: recent developments and ethnic considerations. Expert Opin Drug Metab Toxicol. 2016. May;12(5):555–65. [DOI] [PubMed] [Google Scholar]
- 12.Birdwell KA, Decker B, Barbarino JM, Peterson JF2, Stein CM, Sadee W, Wang D, Vinks AA, He Y, Swen JJ, Leeder JS, van Schaik R, Thummel KE, Klein TE, Caudle KE, MacPhee IA. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guidelines for CYP3A5 Genotype and Tacrolimus Dosing. Clin Pharmacol Ther. 2015l;98(1):19–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shuker N, Bouamar R, van Schaik RH, Clahsen-van Groningen MC, Damman J, Baan CC, van de Wetering J, Rowshani AT, Weimar W, van Gelder T, Hesselink DA. A Randomized Controlled Trial Comparing the Efficacy of Cyp3a5 Genotype-Based With Body-Weight-Based Tacrolimus Dosing After Living Donor Kidney Transplantation. Am J Transplant. 2016. July;16(7):2085–96 [DOI] [PubMed] [Google Scholar]
- 14.Elens L, Capron A, van Schaik RH, et al. Impact of CYP3A4*22 allele on tacrolimus pharmacokinetics in early period after renal transplantation: toward updated genotype-based dosage guidelines. Ther Drug Monit. 2013;35(5):608–616. [DOI] [PubMed] [Google Scholar]
- 15.Lunde I, Bremer S, Midtvedt K, et al. The influence of CYP3A, PPARA, and POR genetic variants on the pharmacokinetics of tacrolimus and cyclosporine in renal transplant recipients. Eur J Clin Pharmacol. 2014;70(6):685–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calabrese DR, Florez R, Dewey K, Hui C, Torgerson D, Chong T, Faust H, Rajalingam R, Hays SR, Golden JA, Kukreja J, Singer JP, Greenland JR. Genotypes associated with tacrolimus pharmacokinetics impact clinical outcomes in lung transplant recipients. Clin Transplant. 2018. August;32(8):e13332. doi: 10.1111/ctr.13332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diamond JM, Lee JC, Kawut SM, Shah RJ, Localio AR, Bellamy SL, Lederer DJ, Cantu E, Kohl BA, Lama VN, Bhorade SM, Crespo M, Demissie E, Sonett J, Wille K, Orens J, Shah AS, Weinacker A, Arcasoy S, Shah PD, Wilkes DS, Ware LB, Palmer SM, Christie JD; Lung Transplant Outcomes Group. Clinical risk factors for primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med. 2013. March 1;187(5):527–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Passey C, Birnbaum AK, Brundage RC, Oetting WS, Israni AK, Jacobson PA. Dosing equation for tacrolimus using genetic variants and clinical factors. Br J Clin Pharmacol. 2011. December;72(6):948–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Czosnowski Q, Miano TA: Principles of Drug Dosing in Critically Ill Patients In: Critical Care Medicine, Principles of Diagnosis and Management in the Adult. 4th edition. Parrillo JE, Dellinger RP (Eds). Philadelphia, Mosby, 2014 [Google Scholar]
- 20.Ojo AO, Held PJ, Port FK et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med 2003; 349: 931–940 [DOI] [PubMed] [Google Scholar]
- 21.Ekberg H, van Gelder T, Kaplan B, Bernasconi C. Relationship of tacrolimus exposure and mycophenolate mofetil dose with renal function after renal transplantation. Transplantation. 2011. July 15;92(1):82–7. [DOI] [PubMed] [Google Scholar]
- 22.Zuckermann AO, Aliabadi AZ. Calcineurin-inhibitor minimization protocols in heart transplantation. Transpl Int. 2009;22(1):78–89. [DOI] [PubMed] [Google Scholar]
- 23.Sharif A, Shabir S, Chand S, Cockwell P, Ball S, Borrows R. Meta-analysis of calcineurin-inhibitor-sparing regimens in kidney transplantation. J Am Soc Nephrol. 2011. November;22(11):2107–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neuberger JM, Mamelok RD, Neuhaus P, Pirenne J, Samuel D, Isoniemi H et al. Delayed introduction of reduced-dose tacrolimus, and renal function in liver transplantation: the ‘ReSpECT’ study. Am J Transplant 2009; 9(2):327–336 [DOI] [PubMed] [Google Scholar]
- 25.Cippà PE, Schiesser M, Ekberg H, van Gelder T, Mueller NJ, Cao CA, Fehr T, Bernasconi C. Risk Stratification for Rejection and Infection after Kidney Transplantation. Clin J Am Soc Nephrol. 2015. December 7;10(12):2213–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Applied Biosystems Axiom 2.0 Reagents: https://www.thermofisher.com/order/catalog/product/901758
- 27.Ganetsky A, Miano TA, Hughes ME, Vonderheide RH, Porter DL, Reshef R. Lack of a significant pharmacokinetic interaction between Maraviroc and tacrolimus in allogeneic HSCT recipients. Journal of Antimicrobial Chemotherapy. 2015; 70: 2078–2083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.KDIGO Clinical Practice Guidelines for Acute Kidney Injury 2012. Available at: http://www.kdigo.org/clinical_practice_guidelines/AKI.php. Accessed May 13, 2017 [DOI] [PubMed]
- 29.Pannu N, James M, Hemmelgarn B, Klarenbach S; Alberta Kidney Disease Network: Association between AKI, recovery of renal function, and long-term outcomes after hospital discharge. Clin J Am Soc Nephrol 8: 194–202, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stewart S, Fishbein MC, Snell GI, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant. 2007;26(12): 1229–1242. [DOI] [PubMed] [Google Scholar]
- 31.Burton CM, Andersen CB, Jensen AS, Iversen M, Milman N, Boesgaard S, Arendrup H, Eliasen K, Carlsen J. The incidence of acute cellular rejection after lung transplantation: a comparative study of anti-thymocyte globulin and daclizumab. J Heart Lung Transplant. 2006. June;25(6):638–47. [DOI] [PubMed] [Google Scholar]
- 32.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007; 81:559–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lau B, Cole SR, Gange SJ. Competing Risk Regression Models for Epidemiologic Data. Am J Epidemiol. 2009. July 15; 170(2): 244–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol 1993; 138: 923–36 [DOI] [PubMed] [Google Scholar]
- 35.Wasserstein RL, Lazar NA. The ASA’s statement on p-values: context, process, and purpose. Am Stat. 2016;70(2): 129–133. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The heat map shows the patterns of drug exposures over time in the study population. Columns represent specific drugs and each row represents a post-operative day of follow-up. Cell shading represents the percentage of patients exposed to a particular drug on each day.
Figures show concentration / dose ratio over time stratified by allele status. a-CYP3A4*22, Normal allele CC (dashed line), variant allele CT/TT solid line; b- POR*28, Normal allele CC (dashed line), variant allele CT/TT (solid line); c- PPARA (c.208+3819A>G), Normal allele AA (dashed line), variant allele GA/GG (solid line); d- ABCB1 (3435C>T), Normal allele CC (dashed line), variant allele CT/TT (solid line)
Allele frequencies by self-identified race
Sensitivity analyses and secondary analyses of SNP-outcome associations


