Abstract
Background:
During inflammation, stressed or infected cells can release adenosine tri-phosphate (ATP) to the extracellular medium, which can be hydrolyzed to adenosine by ectonucleotidases such as ectonucleoside triphosphate diphosphohydrolase 1 (CD39) and 5’ –nucleotidase (CD73). The role of CD73 in the modulation of cytokine release by human gingival fibroblasts (HGF) remains underexplored. Here, we investigated whether CD73-mediated hydrolysis of extracellular ATP (eATP) could affect Interleukin (IL)-1β-induced CXCL8 secretion.
Methods:
The levels of mRNA expression of adenosine receptors, CD39, and CD73 of periodontitis samples were retrieved from a public database. Moreover, HGF mRNA levels were measured by RT-qPCR after 3h, 6h or 24 h of IL-1β stimulation. IL-1β-induced CXCL8 protein levels were measured after pretreatment with 100 μM eATP in the presence of absence of CD73 inhibitor. The effect of eATP degradation to adenosine on CXCL8 levels was investigated using agonist and antagonist of adenosine receptors.
Results:
Levels of CD39, CD73 and adenosine receptor mRNA were differentially modulated by IL-1β. ATP pretreatment impaired IL-1β-induced CXCL8 secretion and required activation of heme-oxygenase 1 (HO-1) and phosphorylated adenosine monophosphate–activated protein kinase (pAMPK). The inhibition of CD73 or the inhibition of adenosine receptors abrogated the ATP effect on CXCL8 secretion.
Conclusion:
CD73-generated adenosine dampens IL-1β-induced CXCL8 in HGF and involves HO-1 and pAMPK signaling. These results imply that CD73 is a negative regulator of the inflammatory microenvironment, suggesting that this ectoenzyme could be involved in the generation of deficient CXCL8 gradient in chronic inflammation.
Keywords: Adenosine Triphosphate, fibroblasts, gingiva, adenosine, Interleukin-1beta, 5’-Nucleotidase
Summary Statement:
ATP degradation to adenosine decreases IL-1β-induced CXCL8 and involves upregulation of HO-1 and pAMPK in human gingival fibroblasts.
Introduction
Adenosine tri-phosphate (ATP) is traditionally associated with cellular energy metabolism in all eukaryotic and prokaryotic cells, but there is evidence that ATP and other nucleotides are released from cells following stress or injury during infection or inflammation1. Mechanistically, extracellular ATP (eATP) exerts its effects via purinergic receptors located on the cell surface2. eATP is not stable in aqueous medium but its half-life is also shortened dramatically in the presence of most cells due to degradation by ectonucleotidases, which are expressed on the surface of most eukaryotic cells3. Thus, adenosine is produced as an ATP breakdown product via spontaneous hydrolysis or by the serial dephosphorylation by ectonucleoside triphosphate diphosphohydrolase 1 (ENTPD1 or CD39) and 5′-nucleotidase (NT5E or CD73)4.
Recent awareness of the role of adenosine in the control of immune and inflammatory responses has prompted an interest in the potential use of adenosine-receptor-based therapies, especially in the treatment of chronic inflammatory conditions5. In this context, ATP has been shown to inhibit Interleukin (IL)-1-induced matrix metalloproteinases (MMPs) through the action of CD39 in primary human gingival fibroblasts (HGF)6.
Adenosine monophosphate (AMP)-activated protein kinase (AMPK) is a key regulator of cell metabolism with anti-inflammatory effects7. AMPK has been shown to have pleiotropic functions in inflammatory responses and tissue fibrosis in various models such as renal fibrosis8, cancer9 and osteoarthritis10. Activation of AMPK has been linked to reduced systemic oxidative stress through antioxidant defense pathways, preventing alveolar bone loss in the ligature-induced model of periodontitis11.
During cellular stress, resulting from a highly inflammatory microenvironment that is rich in IL-1β, the production of antioxidants such as Heme-oxygenase-1 (HO-1) can be induced12. HO-1 is a cytoprotective enzyme with anti-inflammatory properties that is required in the catabolism of free heme. HO-1 has been linked to many physiological and pathological processes through its ability to regulate the host inflammatory response13.
In previous studies, we have examined the macrophage response to eATP following ligation of the purinergic receptor, P2X7. We found that processing and secretion IL-1β is impaired by the periodontal pathogen Porphyromonas gingivalis through a P2X7 signaling-dependent pathway3, 14. Of note, the cytokine IL-1β plays a large role in development of periodontal disease15. Recently, we also demonstrated that autocrine IL-1 receptor activation is necessary for P. gingivalis clearance and for leukocyte recruitment16.
Despite the growing interest in understanding the role of adenosine during infection-driven inflammation or bone-related diseases, it is still unclear whether adenosine affects mesenchymal cell differentiation in an inflammatory environment and whether eATP contributes to the effective concentrations of adenosine. Specifically, it also remains unknown if adenosine produced from eATP degradation contributes to CXCL8 release by fibroblasts and if HO-1 or AMPK could modulate this process.
Given the central role played by the chemokine CXCL8 in development of the inflammatory response to periodontal infection, the overall goal of this study is therefore to investigate whether adenosine produced from CD73-mediated hydrolysis of eATP influences IL-1β-induced CXCL8 secretion by primary HGF, and to investigate the role that HO-1 and AMPK may play in modulating this response.
Materials and Methods
Gene Expression Profiling
Gene expression profile data were obtained from the National Center for Biotechnology Information Gene Expression Omnibus (NCBI GEO) database (accession no. GSE10334, http://www.ncbi.nlm.nih.gov/geo/). Analysis of the mRNA expression of the adenosine receptors A1, A2a, A2b and A3 and ectonucleotidases CD39 and CD73 from the NCBI GEO public database comparing healthy and diseased gingival tissues [dataset GSE10334]17 was performed in 64 samples per group. We selected 64 gingival tissue samples from diseased sites from the same patients that had a healthy gingival sample collected from an unaffected site17. Statistical analysis of microarray expression data was performed using the GEO2R web application (http://www.ncbi.nlm.nih.gov/geo/geo2r/). Additionally, a principal component analysis was performed to complement and reinforce the tendency of expression of each gene between the two groups analyzed through conventional GEO analysis. The principal component analysis was performed using the visualization from BioVinci version 1.1.5 (www.bioturing.com)¶.
Cell Culture
Human gingival fibroblasts (HGF) were purchased from ATCC (HGF-1) and also isolated from gingival biopsies as previously described18–20. All donors signed a written informed consent to participate. This study was approved by the Institutional Review Board at the University of the Pacific (protocol #17–66) and was conducted in accordance with the Helsinki Declaration of 1975, as revised in 2000. Briefly, gingival biopsies from clinically non-inflamed tissue were retrieved from the gingival margin. Primary cells were obtained by explants cultivated in Dulbecco’s Modified Eagle’s Medium# supplemented with 10% fetal bovine serum# and antibiotics (100 UI/mL penicillin/streptomycin)#. HGF were maintained at 37°C in a humidified 5% CO2 incubator and were used for experiments between the 4th and 8th passages.
Cell stimulation
HGF were used for experiments at ~75% confluence and were cultured for 24 hours before exposure to other test reagents. Cells were pre-treated with 100 μM ATP**, 0.1 μM CGS-15943**, 50 nM NECA†† or 100 μM α,β-Methyleneadenosine 5′-diphosphate sodium salt (AMP-CP) ** 1 hour prior to the 1 ng/mL IL-1β stimulation as shown in each figure legend. HGF were incubated with the respective reagents in a serum-free medium# for the time indicated in each figure legend.
RT-qPCR
Total RNAs‡‡ and cDNA§§ were obtained using Kits according to the manufacturer’s instructions. The qPCR was performed using the TaqMan Gene Expression PCR Master MixIIII and proprietary primersIIII targeting mRNA of ADORA1 (Hs00181231_m1), ADORA2A (Hs00169123_m1), ADORA2B (Hs00386497_m1), ADORA3 (Hs00181232_m1), NTPD1 (Hs009b9559_m1), NT5E (Hs00159686_m1) and GAPDH (Hs02758991_g1). All experiments were performed in a system¶¶ using the comparative cycle threshold (Ct) method (ΔΔCt). The human GAPDH gene was used as the reference gene for all the reactions.
ELISA
IL-1β-induced CXCL8 levels were quantified in supernatants from HGF after 24 h of stimulation according to the manufacturer’s instructions## and as previously described19. The cytokine concentration was determined by interpolation from a standard curve and presented as pg/mL (± one standard deviation of the mean) for duplicate samples of each of the tested conditions.
Western Blotting
Western blotting was conducted as previously described21. Cells were resuspended and collected in cell-lytic solution** containing 1% of a complete protease and phosphatase inhibitors**. The protein concentration was obtained using the BCA method ***, and 10 μg of protein was subjected to 10% SDS-PAGE gel electrophoresis. The protein was transferred to Immobilon polyvinylidene difluoride membranes** by electroblotting†††, blocked in 5% dried milk solution diluted in Tris-buffered saline (TBS) containing 0.01% Tween 20, incubated with the respective primary antibodies at a dilution of 1:1000 (see figure legends), and treated with horseradish peroxidase (HRP)-conjugated secondary antibodies. Protein levels for HO-1‡‡‡, pAMPK§§§ and total AMPK§§§ were measured after 24h of IL-1β stimulation.
The same membranes were stripped and re-probed with antibodies against β-actin§§§ as a loading control (dilution 1:1000). Protein bands were detected using a chemiluminescent substrateIIIIII, visualized and analyzed in chemiluminescense equipment¶¶¶. Densitometric analyses were performed with a software###.
Statistical analyses
Statistical analyses were performed with a software****. Data were compared using one-way ANOVA between groups and differences were identified using the Tukey’s post-test, with significance set at p < 0.05.
Results
Adenosine receptors and ectonucleotidases are differentially expressed in samples from healthy and periodontitis tissues.
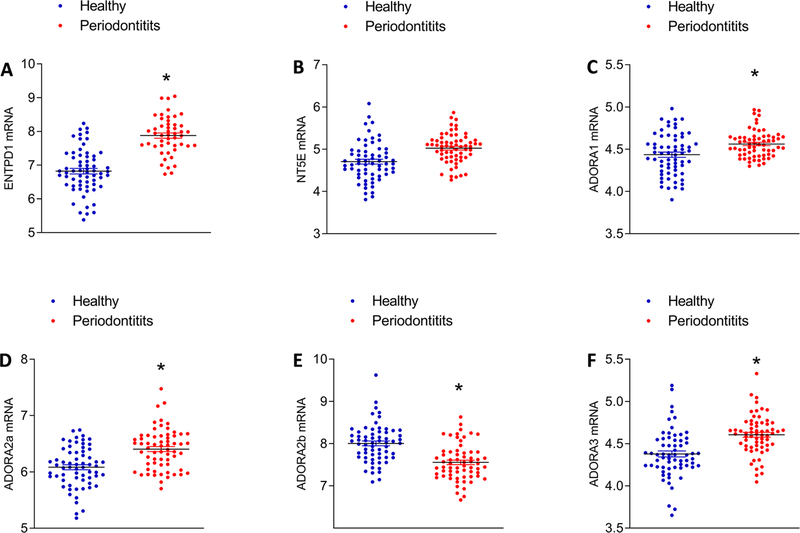
To determine whether ectonucleotidases and adenosine receptors are expressed in gingival tissues, we first analyzed the mRNA expression of the adenosine receptors A1, A2a, A2b and A3 and ectonucleotidases CD39 and CD73 from the NCBI GEO public database, comparing mRNA expression in healthy and disease gingival tissues [dataset GSE10334]17 as shown in Figure 1. Data from GEO dataset GSE1033417 of 64 samples per group revealed that mRNA expression of ENTPD1/CD39 (Figure 1A), NT5E/CD73 (Figure 1B) and adenosine receptors ADORA1 (Figure 1C), ADORA 2a (Figure 1D), ADORA 2b (Figure 1E) and ADORA 3 (Figure 1F) was differentially expressed in periodontitis samples, compared with healthy controls. ENTPD1/CD39, ADORA 1, ADORA 2a and ADORA3 mRNA were upregulated in periodontitis samples compared with healthy samples. To gain more insight into the possible association between the genes investigated here, we also performed a functional component analysis (PCA) (see supplemental Figure 1 in online Journal of Periodontology). The PCA confirmed the same profile that we observed in the conventional GEO analysis. All genes investigated in this study were positively associated with the presence of periodontitis except ADORA 2b.
Figure 1.
Ectonucleotidases (A, B) and adenosine receptor (C-F) mRNA expression in samples from healthy and periodontitis tissues from public expression database NCBI GEO by microarray analysis [dataset GSE10334] of 64 samples per group. *Statistically significant differences, p < 0.05.
Adenosine receptor and ectonucleotidase expression by IL-1β-stimulated human gingival fibroblasts
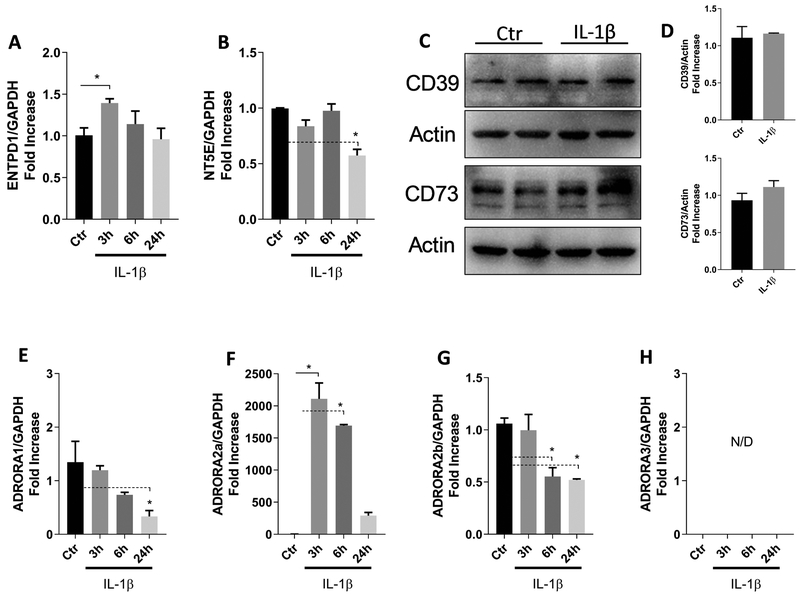
A large body of evidence demonstrates the relevance of IL-1β as a key inflammatory mediator in the pathogenesis of periodontitis22–25, so our next step was to challenge human gingival fibroblasts with IL-1β and check if the ectoenzymes and/or adenosine receptors would be modulated. We confirmed the mRNA expression of the ATP-metabolizing enzymes ENTPD1/CD39 (Figure 2A) and NT5E/CD73 (Figure 2B) as well as the adenosine receptors (Figures 2E through 2H) by human gingival fibroblasts stimulated with IL-1β for 3h, 6h or 24 h. Protein levels for CD39 and CD73 were relatively unchanged after 24 hours of IL-1β stimulation (Figures 2C and 2D). Among the adenosine receptors, ADORA 2a was highly expressed and upregulated after 3 h or 6 h (up to 2000-fold increase) (Figure 2F). ADORA 1 (Figure 2E) and ADORA 2b (Figure 2G) were slightly down-regulated and ADORA 3 was not detectable (Figure 2H). These results are consistent with previous reports showing that both ectoenzymes and all adenosine receptors are expressed by HGF6.
Figure 2.
Expression of adenosine receptors and ectonucleotidase by IL-1β-stimulated human gingival fibroblasts (HGF). HGF were stimulated with 1 ng/mL IL-1β, and mRNA expression of ENTPD1 (CD39) (A) and NT5E (CD73) (B) was evaluated after 3, 6, or 24 h. Protein levels of the ectonucleotidases were evaluated after 24 h of IL-1β challenge (C). Quantification of protein expression was performed by densitometric analysis and is presented as fold increase compared with control (non-stimulated cells) (D). Adenosine receptor mRNA expression for ADORA 1 (E), ADORA 2a (F), ADORA2b (G) and ADORA 3 (H). The GAPDH gene was used as the reference gene for RT-qPCR and actin protein was used as a loading control for Western Blot. Data are representative of three independent experiments. *Statistically significant differences, p < 0.05 compared with control.
Extracellular ATP attenuates IL-1β-induced CXCL8 secretion and increases HO-1 expression and pAMPK activation in human gingival fibroblasts
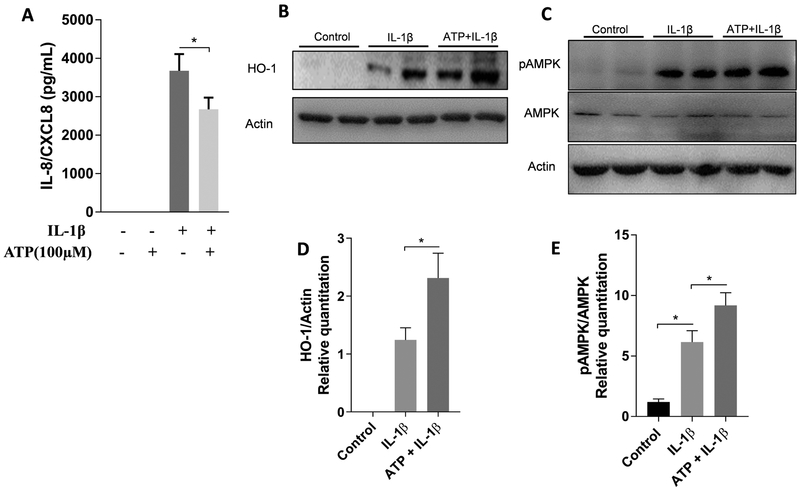
Since adenosine can be generated as a byproduct of eATP degradation through the action of CD39 and CD73, we examined the effect of eATP in IL-1β-induced CXCL8 secretion by gingival fibroblasts. Thus, cells were pretreated with 100 μM ATP 1 h prior to IL-1β stimulation. Extracellular ATP attenuated IL-1β-induced CXCL8 levels (Figure 3A). CXCL8 is a widely studied pro-inflammatory chemokine that acts as a chemoattractant for neutrophils26,27, 28, and gingival fibroblasts actively participate in the production of this chemokine19. Recently, decreased expression of CXCL8 was associated with the upregulation of heme-oxigenase-1(HO-1) expression and p-AMPK activation in human airway epithelial cells29. Thus, we investigated if HO-1 and p-AMPK are affected by IL-1β in HGF. As shown in Figure 3, increased levels of both HO-1 (Figures 3B and 3D) and pAMPK (Figures 3C and 3E) were detected after 24 h of IL-1β stimulation, and expression was even higher with eATP pre-treatment. These results suggested that production of adenosine from eATP could activate HO-1 and pAMPK, which in turn could decrease IL-1β-induced CXCL8 expression.
Figure 3.
Extracellular ATP attenuates IL-1β-induced CXCL8 secretion and increases the levels of HO-1 and pAMPK in HGF. Cells were pre-treated with 100 μM ATP for 1 hour prior to the 1 ng/mL IL-1β stimulation, and were incubated for a total of 24 h. Protein levels of CXCL8 (A) were measured by ELISA in cell supernatants. HO-1 (B), pAMPK and total AMPK (C) production were measured by Western blot. Protein quantification of HO-1 (D) and pAMPK (E) was performed by densitometric analysis and is presented as fold increase compared with control (non-stimulated cells). Data are representative of three independent experiments. *Statistically significant differences, p < 0.05.
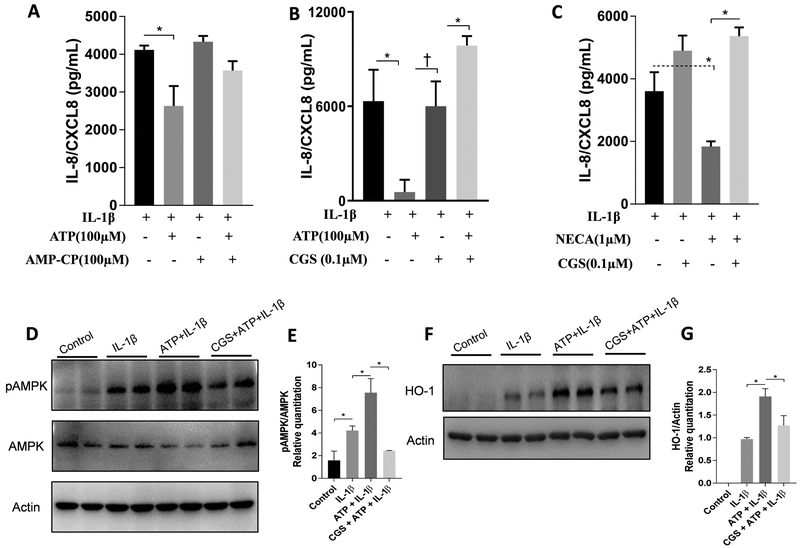
CD73-generated adenosine impairs IL-1β-induced CXCL8 secretion and involves HO-1 and p-AMPK
To examine whether adenosine generated from eATP degradation could impair CXCL8 secretion induced by IL-1β, we investigated CXCL8 secretion after CD73 inhibition and also after adenosine receptor blockage with a non-selective antagonist (CGS-15943). Both CD73 inhibition (Figure 4A) and adenosine receptor blockage (Figure 4B) abrogated the effect of ATP in IL-1β-induced CXCL8. To evaluate whether this effect was due to adenosine generation, we repeated the same experiment treating the cells with a potent agonist of adenosine receptors (an analog of adenosine, NECA). NECA also decreased IL-1β-induced CXCL8 secretion and CGS restored the NECA effect (Figure 4C). Finally, to determine if the adenosine inhibition of IL-1β-induced CXCL8 secretion was dependent on HO-1 and p-AMPK expression, we checked expression of both proteins after blocking adenosine receptors with CGS-15943. As expected, adenosine receptor blockage blocked the ATP-induced upregulation due to adenosine generation for both pAMPK (Figures 4D and 4E) and HO-1 (Figures 4F and 4G).
Figure 4.
ATP impairment of IL-1β-induced CXCL8 secretion is via CD73-generated adenosine and involves upregulation of HO-1 and pAMPK. Cells were pre-treated with (+) or without (−) 100 μM ATP, 0.1 μM CGS-15943, 50 nM NECA or 100 μM α,β Methyleneadenosine 5′-diphosphate sodium salt (AMP-CP) , as indicated in each graph, 1 hour prior to stimulation with1 ng/mL IL-1β, and were incubated for a total of 24 h. CXCL8 levels (A,B and C) were measured in cell supernatants by ELISA. Protein levels of pAMPK, total AMPK (D) and HO-1 (F) were measured by Western blot. Protein quantification of p-AMPK (E) and HO-1 (G) was performed by densitometric analysis and is presented as fold increase compared with control (non-stimulated cells). Data are representative of three independent experiments. *Statistically significant differences, p < 0.05. †p <0.01.
Discussion
In the present study, we demonstrated for the first time that adenosine generated from eATP breakdown through CD73 ectonucleotidase decreases IL-1β-induced CXCL8 secretion by gingival fibroblasts. We also showed that IL-1β followed by adenosine receptor activation leads to an increase in HO-1 and pAMPK levels, since both were dampened after adenosine receptor blockage, unveiling a potential mechanism for the lower CXCL8 gradient generated by eATP-derived adenosine.
We first investigated the expression of the mRNA levels of the adenosine receptors and ectonucleotidases ENTPD1 and NT5E, known as CD39 and CD73, respectively in human samples derived from healthy or periodontitis samples. We observed that there was an upregulation of almost all adenosine receptors, except for ADORA 2b which was downregulated compared to healthy condition. ENTPD1 was upregulated and NT5E showed a tendency of upregulation but not significantly. One possible explanation for not observing differences in the NT5E expression is because we evaluated gene expression data only. It is possible that the mRNA expression was not significantly upregulated but the ectoenzyme activity on those samples could actually be higher. A principal component analysis of the data also confirmed the positive correlations of all the genes - except for ADORA 2b - with the presence of periodontitis.
As described in previous studies30, 31, professional immune cells such as T regulatory (Treg) cells produce adenosine following sequential degradation of ATP and ADP via CD39 and CD73. Also, A2a receptor stimulation was reported to decrease T helper (Th)1/Th2 development and decrease Th17 generation, as reviewed elsewhere5. The ectoenzyme CD73 has also been shown to be involved in adenosine generation by human gingival fibroblasts32. In addition, primary gingival epithelial cells (GECs) express functional adenosine A2a receptor and P. gingivalis exploits it to successfully survive within the host cells by downregulation of the pro-inflammatory response33. Thus, adenosine signaling stimulates intracellular growth of P. gingivalis in GECs by enhancing the downstream anti-inflammatory effect of cyclic-AMP33. A role for adenosine in bone development was also strongly suggested by observation that adenosine A2b receptor-deficient mice reveal a decrease in bone volume34.
Since gingival fibroblasts are the most abundant resident and non-professional immune cells in the periodontal microenvironment, it was reasonable to investigate the effect of CD73-dependent degradation of eATP and adenosinergic stimulation in controlling the release of CXCL8, a well-known chemoattractant relevant in inflammatory conditions. The expression of adenosine receptors, CD39 and CD73 in HGF stimulated with IL-1α was previously shown6. This work showed that adenosine generated from CD73-dependent degradation of eATP impaired MMP expression by HGF. Here, we report the effect of adenosine derived from eATP degradation in CXCL8 release in HGF stimulated by IL-1β. We used a potent agonist of adenosine receptors to confirm that the dampening in CXCL8 secretion in the presence of eATP was due to eATP degradation to adenosine. We also confirmed the adenosine-dependent effect by using an antagonist of adenosine receptors. In addition, our study reveals new insights into the participation of HO-1 and pAMPK in this process.
Recent studies have shown a connection between adenosine and the enzyme HO-129, 35 which is known to be involved in the suppression of inflammatory response13. In a murine model of pulmonary inflammation, the activation of HO-1 significantly decreased polymorphonuclear leukocyte (PMN) migration into the lung, which was abolished in A2A- and A2B-deficient mice35. Therefore, the anti-inflammatory effects of HO-1 seem to be associated with A2A/A2B-receptor signaling. HO-1 has also shown to have a protective role in the attenuation of NF-κB activation in in vivo and in vitro models of acute lung injury29Interestingly, HO-1 controls the expression of immunoregulatory cytokines during infection, favoring pathogen survival in the host36. Indeed, HO-1 is a stress-responsive enzyme important for defense against oxidant-induced injury during inflammatory processes and is highly inducible by various stimuli, such as LPS, cytokines, heat shock, heavy metals, oxidants, and its substrate heme36. Our study shows for the first time the protective effects of adenosine produced as a byproduct of eATP degradation in gingival fibroblasts, and that these anti-inflammatory effects were accompanied by HO-1 induction and AMPK activation.
AMPK is a key regulator of cellular metabolism, and it has been reported that AMPK regulates multiple pathways promoting anti-inflammatory activity in endothelial cells37. AMPK senses the intracellular energy status and regulates rapidly energy-demanding metabolic pathways. It has been previously demonstrated that extracellular adenosine activates AMPK in the epithelial intestinal cell line IEC-638. The effect of AMPK activation on cytokine-stimulated proinflammatory signaling was also previously assessed in cultured adipocytes. Interestingly, it has been reported that direct activation of AMPK inhibits IL-1β signaling in murine 3T3-L1 adipocytes and embryonic fibroblasts39. Moreover, the AMPK activator AICAR can inhibit CXCL8 secretion in normal human thyroid cells40, reinforcing the view that AMPK is a key anti-inflammatory mediator.
Finally, we would like to highlight the relevance of these observations for the CXCL8 gradient during periodontal inflammation. The CXCL8 gradient for PMN migration could be disrupted in part by the in vitro observations described here. This is particularly significant since the effect on CXCL8 secretion by fibroblasts could have relevant effects on neutrophil migration if expressed locally in gingival tissue. This would provide a potential explanation for the CXCL8 gradient demonstrated by Tonetti et al41. This could also be a potential mechanism contributing to the “chronification” of the periodontal inflammation due to a potential dysfunctional CXCL8 gradient which was already described in chronic periodontitis patients26. We suggest this may happen as a consequence of the anti-inflammatory effects of adenosine generation as a byproduct of eATP in the inflammatory microenvironment.
Conclusions
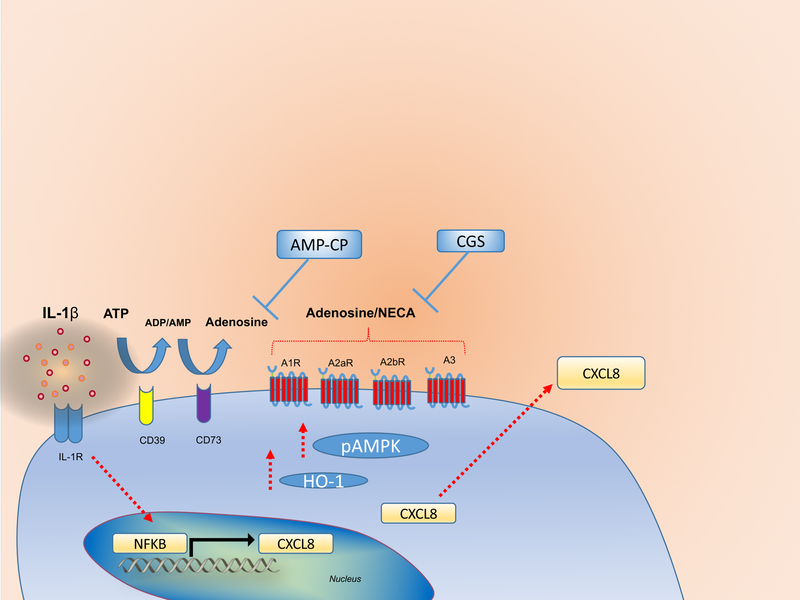
Taken together, our results demonstrate that adenosine generated from eATP breakdown through the action of CD73 can dampen IL-1β-induced CXCL8 secretion, which involves the upregulation of HO-1 and pAMPK as summarized in Figure 5. Hence, this study unveils the role of CD73-dependent generation of adenosine as a potential mechanism for the anti-inflammatory effects resulting of the deficient CXCL8 gradient which in turn contributes to the chronic inflammation.
Figure 5.
Adenosine generated from eATP breakdown through the action of CD73 can dampen IL-1β-induced CXCL8 secretion. This process involves the upregulation of HO-1 and pAMPK. Cell stimulation with IL-1β promotes CXCL8 expression and protein synthesis. Extracellular ATP, which has been shown to be released during cellular stress can be hydrolyzed to ADP/AMP by CD39. AMP can be further degraded by CD73, resulting in adenosine generation. Interaction of extracellular adenosine or its agonist NECA with adenosine receptors (A1, A2a, A2b or A3) dampens IL-1β-induced CXCL8 secretion via upregulation of HO-1 and pAMPK. CGS-15943 (Adenosine receptor blocker); AMP-CP (CD73 inhibitor).
Supplementary Material
Acknowledgments
There are no conflicts of interest associated with this study. This study was supported by Start-up Funds and Research Enhancement Award [DRES 03- Activity 117, 2017 to ACM] from the University of the Pacific, Arthur A. Dugoni School of Dentistry, and funding from the NIDCR grant R01DE016593.
Footnotes
BioTuring Inc., San Diego, CA
Gibco, Thermo Fisher Scientific, Waltham, MA
Millipore Sigma, St. Louis, MO.
Calbiochem, Sigma-Aldrich, St. Louis, MO.
Pure Link RNA mini Kit, Thermo Fisher Scientific, Waltham, MA
High Capacity cDNA Rev. Transcription Kit, Thermo Fisher Scientific, Waltham, MA
Applied Biosystems, Thermo Fisher Scientific Waltham, MA
StepOnePlus Real-Time PCR System, Applied Biosystems, Thermo Fisher Scientific
R&D Systems, Minneapolis, MN.
Pierce, Thermo Fisher Scientific, Waltham, MA
Bio-Rad Laboratories, Hercules, CA.
Abcam, Cambridge, MA.
Cell Signaling, Danvers, MA.
IIIIII: Clarity Max, BioRad, Hercules, CA.
ChemiDoc, BioRad, Hercules, CA.
Image Lab, BioRad, Hercules, CA.
GraphPad prism 7.0, GraphPad Software, San Diego, CA.
References
- 1.Faas MM, Saez T, de Vos P. Extracellular ATP and adenosine: The Yin and Yang in immune responses? Mol Aspects Med 2017;55:9–19. [DOI] [PubMed] [Google Scholar]
- 2.Idzko M, Ferrari D, Eltzschig HK. Nucleotide signalling during inflammation. Nature 2014;509:310–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morandini AC, Savio LE, Coutinho-Silva R. The role of P2X7 receptor in infectious inflammatory diseases and the influence of ectonucleotidases. Biomed J 2014;37:169–177. [DOI] [PubMed] [Google Scholar]
- 4.Sitkovsky MV, Lukashev D, Apasov S, et al. Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annu Rev Immunol 2004;22:657–682. [DOI] [PubMed] [Google Scholar]
- 5.Hasko G, Linden J, Cronstein B, Pacher P. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov 2008;7:759–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nemoto E, Gotoh K, Tsuchiya M, Sakisaka Y, Shimauchi H. Extracellular ATP inhibits IL-1-induced MMP-1 expression through the action of CD39/nucleotidase triphosphate dephosphorylase-1 on human gingival fibroblasts. Int Immunopharmacol 2013;17:513–518. [DOI] [PubMed] [Google Scholar]
- 7.Yan Y, Zhou XE, Xu HE, Melcher K. Structure and Physiological Regulation of AMPK. Int J Mol Sci 2018;19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu J, Shi J, Li M, et al. Activation of AMPK by metformin inhibits TGF-beta-induced collagen production in mouse renal fibroblasts. Life Sci 2015;127:59–65. [DOI] [PubMed] [Google Scholar]
- 9.Kim I, He YY. Targeting the AMP-Activated Protein Kinase for Cancer Prevention and Therapy. Front Oncol 2013;3:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Terkeltaub R, Yang B, Lotz M, Liu-Bryan R. Chondrocyte AMP-activated protein kinase activity suppresses matrix degradation responses to proinflammatory cytokines interleukin-1beta and tumor necrosis factor alpha. Arthritis Rheum 2011;63:1928–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tamaki N, Cristina Orihuela-Campos R, Inagaki Y, Fukui M, Nagata T, Ito HO. Resveratrol improves oxidative stress and prevents the progression of periodontitis via the activation of the Sirt1/AMPK and the Nrf2/antioxidant defense pathways in a rat periodontitis model. Free Radic Biol Med 2014;75:222–229. [DOI] [PubMed] [Google Scholar]
- 12.Araujo JA, Zhang M, Yin F. Heme oxygenase-1, oxidation, inflammation, and atherosclerosis. Front Pharmacol 2012;3:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung SW, Hall SR, Perrella MA. Role of haem oxygenase-1 in microbial host defence. Cell Microbiol 2009;11:199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramos-Junior ES, Morandini AC, Almeida-da-Silva CL, et al. A Dual Role for P2X7 Receptor during Porphyromonas gingivalis Infection. J Dent Res 2015;94:1233–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hou LT, Liu CM, Rossomando EF. Crevicular interleukin-1 beta in moderate and severe periodontitis patients and the effect of phase I periodontal treatment. J Clin Periodontol 1995;22:162–167. [DOI] [PubMed] [Google Scholar]
- 16.Almeida-da-Silva CLC, Ramos-Junior ES, Morandini AC, et al. P2X7 receptor-mediated leukocyte recruitment and Porphyromonas gingivalis clearance requires IL-1beta production and autocrine IL-1 receptor activation. Immunobiology 2018. [DOI] [PubMed] [Google Scholar]
- 17.Demmer RT, Behle JH, Wolf DL, et al. Transcriptomes in healthy and diseased gingival tissues. J Periodontol 2008;79:2112–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morandini AC, Sipert CR, Gasparoto TH, et al. Differential production of macrophage inflammatory protein-1alpha, stromal-derived factor-1, and IL-6 by human cultured periodontal ligament and gingival fibroblasts challenged with lipopolysaccharide from P. gingivalis. J Periodontol 2010;81:310–317. [DOI] [PubMed] [Google Scholar]
- 19.Morandini AC, Sipert CR, Ramos-Junior ES, Brozoski DT, Santos CF. Periodontal ligament and gingival fibroblasts participate in the production of TGF-beta, interleukin (IL)-8 and IL-10. Braz Oral Res 2011;25:157–162. [DOI] [PubMed] [Google Scholar]
- 20.Sipert CR, Morandini AC, Dionisio TJ, et al. In vitro regulation of CCL3 and CXCL12 by bacterial by-products is dependent on site of origin of human oral fibroblasts. J Endod 2014;40:95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morandini AC, Ramos-Junior ES, Potempa J, et al. Porphyromonas gingivalis fimbriae dampen P2X7-dependent interleukin-1beta secretion. J Innate Immun 2014;6:831–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu X, Offenbacher S, Lomicronpez NJ, et al. Association of interleukin-1 gene variations with moderate to severe chronic periodontitis in multiple ethnicities. J Periodontal Res 2015;50:52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDevitt MJ, Wang HY, Knobelman C, et al. Interleukin-1 genetic association with periodontitis in clinical practice. J Periodontol 2000;71:156–163. [DOI] [PubMed] [Google Scholar]
- 24.Jandinski JJ, Stashenko P, Feder LS, et al. Localization of interleukin-1 beta in human periodontal tissue. J Periodontol 1991;62:36–43. [DOI] [PubMed] [Google Scholar]
- 25.Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 2011;117:3720–3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roberts HM, Ling MR, Insall R, et al. Impaired neutrophil directional chemotactic accuracy in chronic periodontitis patients. J Clin Periodontol 2015;42:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martins-Green M, Petreaca M, Wang L. Chemokines and Their Receptors Are Key Players in the Orchestra That Regulates Wound Healing. Adv Wound Care (New Rochelle) 2013;2:327–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Damgaard C, Kantarci A, Holmstrup P, Hasturk H, Nielsen CH, Van Dyke TE. Porphyromonas gingivalis-induced production of reactive oxygen species, tumor necrosis factor-alpha, interleukin-6, CXCL8 and CCL2 by neutrophils from localized aggressive periodontitis and healthy donors: modulating actions of red blood cells and resolvin E1. J Periodontal Res 2017;52:246–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee JW, Chun W, Kwon OK, et al. 3,4,5-Trihydroxycinnamic acid attenuates lipopolysaccharide (LPS)-induced acute lung injury via downregulating inflammatory molecules and upregulating HO-1/AMPK activation. Int Immunopharmacol 2018;64:123–130. [DOI] [PubMed] [Google Scholar]
- 30.Antonioli L, Pacher P, Vizi ES, Hasko G. CD39 and CD73 in immunity and inflammation. Trends Mol Med 2013;19:355–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ernst PB, Garrison JC, Thompson LF. Much ado about adenosine: adenosine synthesis and function in regulatory T cell biology. J Immunol 2010;185:1993–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hashikawa T, Takedachi M, Terakura M, et al. Involvement of CD73 (ecto-5’-nucleotidase) in adenosine generation by human gingival fibroblasts. J Dent Res 2003;82:888–892. [DOI] [PubMed] [Google Scholar]
- 33.Spooner R, DeGuzman J, Lee KL, Yilmaz O. Danger signal adenosine via adenosine 2a receptor stimulates growth of Porphyromonas gingivalis in primary gingival epithelial cells. Mol Oral Microbiol 2014;29:67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corciulo C, Wilder T, Cronstein BN. Adenosine A2B receptors play an important role in bone homeostasis. Purinergic Signal 2016;12:537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Konrad FM, Zwergel C, Ngamsri KC, Reutershan J. Anti-inflammatory Effects of Heme Oxygenase-1 Depend on Adenosine A2A- and A2B-Receptor Signaling in Acute Pulmonary Inflammation. Front Immunol 2017;8:1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carasi P, Rodriguez E, da Costa V, et al. Heme-Oxygenase-1 Expression Contributes to the Immunoregulation Induced by Fasciola hepatica and Promotes Infection. Front Immunol 2017;8:883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mancini SJ, Boyd D, Katwan OJ, et al. Canagliflozin inhibits interleukin-1beta-stimulated cytokine and chemokine secretion in vascular endothelial cells by AMP-activated protein kinase-dependent and -independent mechanisms. Sci Rep 2018;8:5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aymerich I, Foufelle F, Ferre P, Casado FJ, Pastor-Anglada M. Extracellular adenosine activates AMP-dependent protein kinase (AMPK). J Cell Sci 2006;119:1612–1621. [DOI] [PubMed] [Google Scholar]
- 39.Mancini SJ, White AD, Bijland S, et al. Activation of AMP-activated protein kinase rapidly suppresses multiple pro-inflammatory pathways in adipocytes including IL-1 receptor-associated kinase-4 phosphorylation. Mol Cell Endocrinol 2017;440:44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Awwad O, Coperchini F, Pignatti P, et al. The AMPK-activator AICAR in thyroid cancer: effects on CXCL8 secretion and on CXCL8-induced neoplastic cell migration. J Endocrinol Invest 2018;41:1275–1282. [DOI] [PubMed] [Google Scholar]
- 41.Tonetti MS, Imboden MA, Gerber L, Lang NP, Laissue J, Mueller C. Localized expression of mRNA for phagocyte-specific chemotactic cytokines in human periodontal infections. Infect Immun 1994;62:4005–4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.