Abstract
Background
Aldehyde dehydrogenase 2 (ALDH2) protects against alcohol-evoked cardiac dysfunction in male rodents but its role in the estrogen (E2)-dependent hypersensitivity of female rats to alcohol-evoked myocardial oxidative stress and dysfunction is not known.
Study design
We addressed this question by studying the effect of cyanamide (ALDH2 inhibitor) on cardiac function, blood pressure, alcohol metabolizing enzymes (alcohol dehydrogenase, cytochrome P450 2E1, catalase and ALDH2) activities and cardiac redox status (reactive oxygen species, ROS; malondialdehyde, MDA) in the absence or presence of ethanol in female sham-operated (SO) and ovariectomized (OVX) rats.
Results
Cyanamide attenuated the ethanol-evoked myocardial dysfunction (reduced dP/dtmax and LVDP) in SO rats. Ethanol, cyanamide or their combination did not alter dP/dtmax or LVDP in OVX rats. Cyanamide induced cardiac oxidative stress and abrogated the subsequent alcohol-evoked increases in ROS and MDA levels in SO rats. Neither ethanol nor cyanamide influenced ROS or MDA levels in OVX rats. Importantly, cyanamide exaggerated ethanol-evoked hypotension in SO and uncovered this hypotensive response in OVX rats, which implicates ALDH2 in the vasodilating effect of ethanol.
Conclusions
Contrary to our hypothesis, cyanamide attenuated the E2-dependent cardiac dysfunction caused by alcohol, likely by preconditioning the heart to oxidative stress, while exacerbating the vasodilating effect of alcohol. The latter might predispose to syncope when cyanamide and alcohol are combined in females.
Keywords: Aldehyde dehydrogenase 2, Ethanol, Myocardial dysfunction, Blood pressure, Oxidative stress
Introduction
The role of aldehyde dehydrogenase 2 (ALDH2) in the sex/estrogen (E2)-dependent hypersensitivity to alcohol-induced cardiac dysfunction is poorly understood. ALDH2 detoxification of acetaldehyde and other alcohol-evoked reactive aldehydes such as malondialdehyde (MDA) (Zimatkin et al., 2006) supports its cardioprotective role as a redox enzyme (Chen et al., 2014). This premise is specifically supported by the enhanced (Choi et al., 2011) and attenuated (Li et al., 2006) cardiotoxic effects of alcohol in cardiac myocytes isolated from male mice lacking and overexpressing ALDH2, respectively. Further, the induction of cardiac ALDH2, which is expressed in the heart (Stewart et al., 1996), protects against cardiac dysfunction in male rats in vivo (El-Mas and Abdel-Rahman, 2015). However, cyanamide (ALDH2 inhibitor) mitigation of ethanol-evoked oxidative stress in hepatocytes from male rats infers a pro-oxidant role for ALDH2 (Bailey and Cunningham, 1998). On the other hand, cyanamide, as alcohol deterrent therapy, caused liver dysfunction in some studies (Tamai et al., 2000, Yokoyama et al., 1995). Collectively, the effects of ALDH2 inhibition alone and in the presence of alcohol are complex and might be confounded by sex/ovarian hormones.
Consistent with faster ethanol metabolism in females (Cole-Harding and Wilson, 1987, Thomasson, 1995), our studies implicated E2-dependent enhancement of ethanol metabolism to acetaldehyde in the exacerbated cardiac oxidative stress and dysfunction in female rats (Yao and Abdel-Rahman, 2016, Yao and Abdel-Rahman, 2017b). Similar findings along with higher HNE and MDA (Ibrahim et al., 2014) are puzzling because they occur in the presence of E2-dependent higher cardiac ALDH2 (Steagall et al., 2017). It is likely that the higher cardiac ALDH2 activity (faster acetaldehyde/MDA elimination) prevents further deterioration of cardiac function in ethanol-treated female rats. Alternatively, it is possible that the higher acetaldehyde-derived acetate levels contribute to the adverse cardiac effects of alcohol because acetate depresses cardiac function animal cohorts of both sexes (Kirkendol et al., 1978), but not in healthy men (Suokas et al., 1988). Currently, there are no studies on the consequences of ALDH2 inhibition (cyanamide) to understand its role in the E2-dependent hypersensitivity to alcohol-evoked cardiac dysfunction and hypotension. It will also be important to determine if cyanamide, which is used as alcohol deterrent (DeMaster et al., 1998), contributed to the hypotensive shock associated with combining cyanamide with alcohol (Kondo et al., 2013), and if this clinical problem is exacerbated in the presence of E2.
In present study, we discerned the effects of ALDH2 inhibition (cyanamide) on alcohol-induced myocardial dysfunction and hypotension in proestrous (highest endogenous E2 level) and ovariectomized (OVX) rats. Ex vivo measurements of the activities of alcohol metabolizing enzymes (alcohol dehydrogenase, ADH; cytochrome P450 2E1, CYP2E1; catalase and ALDH2) and the levels of oxidative stress mediators (ROS and MDA) were conducted to facilitate data interpretation.
Material and Methods
Animals
Sprague-Dawley female rats (170–200 g; 12–14 weeks old) were purchased from Charles River (Raleigh, NC). The rats were housed in the animal facility of East Carolina University. Surgical anesthesia was induced by i.p. ketamine (90 mg/kg) and xylazine (10 mg/kg) and s.c. buprenorphine (0.03 mg/kg) was administrated for pre- and post-operative analgesia. All surgical procedures were approved by the East Carolina University Institutional Animal Care and Use Committee and were consistent with the Guide for the Care and Use of Laboratory Animals (2011).
Ovariectomy
We followed the method described in our previous studies (El-Mas and Abdel-Rahman, 2000, Yao and Abdel-Rahman, 2017b). Briefly, the ovaries were isolated and removed via an incision in lower part of the back. The rats in the SO group were subjected to the same procedure without removing the ovaries.
Vascular and Left Ventricular Catheterizations
Two weeks after OVX or SO, femoral artery and left ventricular (via right carotid artery) catheters (Konigsberg Instruments Inc., Pasadena, CA) were inserted, under anesthesia and pre- and post-operative analgesia (as detailed above), for blood pressure and myocardial function measurements, respectively. A femoral venous catheter was inserted for i.v. administration as detailed in our previous studies (Yao and Abdel-Rahman, 2016, Yao and Abdel-Rahman, 2017b).
Hemodynamic Measurements
After one-day recovery from surgery, the hemodynamic measurements were conducted on conscious freely moving rats as detailed in our previous studies (Yao and Abdel-Rahman, 2016, Ibrahim et al., 2014). Blood pressure and left ventricular function were continuously recorded by ML870 (PowerLab 8/30, AD Instruments, Colorado Spring, CO) via Gould-Statham pressure transducers (Oxnard-CA).
Blood Ethanol Concentration
Blood ethanol concentration was measured by reaction with alcohol dehydrogenases in a 96-well plate as in our studies (Yao and Abdel-Rahman, 2016, El-Mas et al., 2008, Yao and Abdel-Rahman, 2017b). The absorbance was detected at 340 nm with a microplate reader (Tencan Group Ltd., Männedorf, Schweiz).
Measurement of ALDH2 and Catalase Activities
Cardiac ALDH2 and catalase activities were measured by commercial kits for ALDH2 activity assay kit (Abcam, Ann Arbor, MI) and colorimetric CAT assay kit (Sigma-Aldrich, St. Louis, MO), respectively. We followed the manufacturer’s protocols and our reported studies (El-Mas et al., 2012, Ibrahim et al., 2014, Yao and Abdel-Rahman, 2016, Yao and Abdel-Rahman, 2017b).
Measurement of ADH and CYP2E1 Activities
As detailed in our study (Yao and Abdel-Rahman, 2017b), 20 μl of the supernatant of heart tissue homogenate was used for measuring ADH activity. The reaction was conducted in a 96-well plate. Absorbance, measured at 340 nm, was used to calculate the ADH activity from the standard curve. Based on our (Yao and Abdel-Rahman, 2017b) and other (Zhang et al., 2011) studies, CYP2E1 activity was measured by the method of oxidation of p-nitrophenol (PNP) by CYP2E1 in the presence of NADPH in a 96-well plate. The optical density was read at 546 nm.
Measurement of ROS Level
Myocardial ROS level was measured by a fluorometric assay using DCFH-DA (Molecular Probes-Thermo Fisher Scientific Inc, Raleigh, NC) as in our previous studies (Yao and Abdel-Rahman, 2016, McGee and Abdel-Rahman, 2012, Yao and Abdel-Rahman, 2017b). Briefly, the supernatant of the left ventricular tissue homogenate was used to react with DCFH-DA. The fluorescence intensities were read at 485/530 nm (excitation/emission) at 37 °C.
Measurement of NADPH Oxidase (Nox) Activity
As described (La Favor et al., 2013), the left ventricular tissue was homogenized in ice-cold lysis buffer with cOmplete™ Protease Inhibitor Cocktail (Sigma Aldrich, St. Louis, MO) and centrifuged at 10,000 rpm for 10 min. The supernatant (100 μg protein) was incubated with 10 μM Amplex Red (Molecular Probes, OR; 120 μl), 2.0 U/ml horseradish peroxidase (Sigma Aldrich, St. Louis, MO), 30 U/ml superoxide dismutase (Sigma Aldrich, St. Louis, MO) and 100 μM NADPH (Sigma Aldrich, St. Louis, MO) in PBS for 90 min at 37°C. Fluorescence intensity (530 nM ex/590 nM em) was then measured with a microplate reader every 5 min with shaking. Total NADPH-dependent hydrogen peroxide generated in the sample was used as an index of Nox activity, which was normalized to the total protein content, as determined by Bradford assay (Bio-Rad).
Measurement of MDA Level
TBARS Assay Kit (Cayman Chemical, Ann Arbor, MI, USA) was used to measure myocardial and plasma MDA level following the manufacturer’s protocol and the method described in our studies (Yao and Abdel-Rahman, 2016, Yao and Abdel-Rahman, 2017b). The MDA-TBA adduct was detected colorimetrically at 530–540 nm.
Western Blot
ERK1/2 phosphorylation in myocardial tissue was measured by western blot as detailed in our studies (Yao and Abdel-Rahman, 2016, Ibrahim et al., 2014, Yao and Abdel-Rahman, 2017b). Briefly, 40 μg of protein was separated in a 4–12% gel electrophoresis (Novex Tis-Glycine gel, Life Technologies, CA), and transferred to nitrocellulose membrane (Bio-Rad, Hercules, CA). The membranes were incubated with mouse anti p-ERK1/2 and rabbit anti-ERK1/2 primary antibody (1:500, Cell Signaling, Danvers, MA) overnight at 4°C, and then incubated with IRDye680/800-conjugated anti-mouse/rabbit secondary antibody (1:15000, LI-COR Biosciences, Lincoln, NE). Odyssey Infrared Imager and Odyssey application software version 3 (LI-COR Biosciences, Lincoln, NE) were used to detect and quantify the protein bands.
Experimental Protocols
Effect of Cyanamide (ALDH2 Inhibition) on Ethanol-evoked Hemodynamic Responses
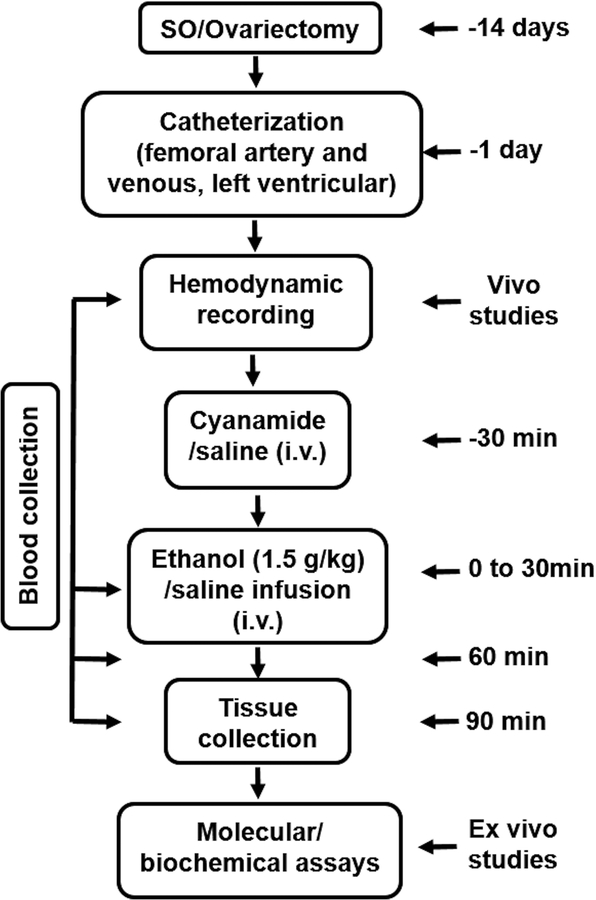
The experimental protocol is depicted in Fig. 1 and the number of rats per group is shown in Table 1. All rats were allowed at least 30 min for hemodynamic variables to stabilize at baseline in the conscious state. SO (n = 26) and OVX (n = 28) rats were each divided into 4 groups (n = 5–8) to receive cyanamide (10 mg/kg, i.v) or saline 30 min before the 30 min i.v. infusion of ethanol (0.05 g/kg/min) or saline as follows: saline-saline, saline-ethanol, cyanamide-saline and cyanamide-ethanol. The hemodynamic variables were monitored for additional 60 min after the completion of ethanol or saline infusion (Fig. 1). The doses of ethanol (El-Mas and Abdel-Rahman, 2014, El-Mas and Abdel-Rahman, 2015) and cyanamide (Kinoshita et al., 2001) were based on reported studies. Maximum rate of left ventricular pressure rise (dP/dtmax), left ventricular developed pressure (LVDP) and mean arterial pressure (MAP) were extracted from the cardiac function and blood pressure recordings, respectively, using LabChart software.
Figure 1.
The experimental protocol and schedule showing time of surgery, vivo and ex vivo studies employed to investigate the effect of cyanamide (ALDH2 inhibitor) on ethanol (1.5 g/kg)-evoked myocardial dysfunction in conscious female sham operated (SO) and ovariectomized (OVX) rats.
Table 1.
The effect of cyanamide pretreatment on maximum rate of left ventricular pressure rise (dP/dtmax), left ventricular developed pressure (LVDP) and mean arterial pressure (MAP) prior to ethanol or saline infusion. Values are mean ± SEM.
| Groups | N | Hemodynamic Variable | Baseline | ||
|---|---|---|---|---|---|
| dP/dtmax (mmHg/s) | 7870±1032 | ||||
| Saline | 5 | LVDP (mmHg) | 166.7±5.1 | ||
| Saline | MAP (mmHg) | 94.7±1.7 | |||
| dP/dtmax (mmHg/s) | 7827±1236 | ||||
| Ethanol | 6 | LVDP (mmHg) | 159.3±11.1 | ||
| SO | MAP (mmHg) | 95.4±8.2 | |||
| dP/dtmax (mmHg/s) | 7992±1323 | ||||
| Saline | 7 | LVDP (mmHg) | 161.0±13.7 | ||
| Cyanamide | MAP (mmHg) | 92.8±6.1 | |||
| dP/dtmax (mmHg/s) | 8667±774 | ||||
| Ethanol | 8 | LVDP (mmHg) | 175.2±9.5 | ||
| MAP (mmHg) | 100.8±5.6 | ||||
| dP/dtmax (mmHg/s) | 8808±598 | ||||
| Saline | 6 | LVDP (mmHg) | 174.1±3.6 | ||
| Saline | MAP (mmHg) | 103.5±7.4 | |||
| dP/dtmax (mmHg/s) | 8189±688 | ||||
| Ethanol | 7 | LVDP (mmHg) | 167.1±6.8 | ||
| OVX | MAP (mmHg) | 97.9±5.1 | |||
| dP/dtmax (mmHg/s) | 8768±312 | ||||
| Saline | 8 | LVDP (mmHg) | 172.7±4.0 | ||
| Cyanamide | MAP (mmHg) | 101.8±4.5 | |||
| dP/dtmax (mmHg/s) | 8160±750 | ||||
| Ethanol | 7 | LVDP (mmHg) | 163.0±9.1 | ||
| MAP (mmHg) | 96.5±4.3 | ||||
Effect of ALDH2 Inhibition (Cyanamide) on Cardiac Ethanol Metabolizing Enzymes, ROS and MDA in Presence or Absence of Ovarian Hormones
Blood was obtained before (baseline) and at 30, 60 and 90 min after starting ethanol or saline infusion. At the conclusion of the hemodynamic measurements in the 8 groups of SO and OVX rats, the rats were euthanized, hearts were collected and kept at −80°C until used for conducting the molecular studies. Blood and cardiac tissues were used for the measurements of blood alcohol level, ethanol metabolizing enzymes (ADH, catalase, CYP2E1 and ALDH2) activities and the levels of myocardial oxidative stress mediators (ROS, MDA, Nox activity, and ERK1/2 phosphorylation) in control and treatment groups.
Drugs
Ethanol (Midwest Grain Products Co. (Weston, MO) and cyanamide (Sigma Aldrich, St. Louis, MO) were diluted in saline.
Data Analysis and Statistics
The hemodynamic indices and biochemical data in ethanol vs. saline treatment were analyzed by one-way ANOVA followed by post-hoc Tukey’s t-test comparison (table 1). The hemodynamic responses to ethanol in the presence or absence of cyanamide were analyzed by t-test (table 1). All statistical analyses were run with Prism version 5 (GraphPad Software, Inc. La Jolla, CA). Values are presented as mean ± SEM. p < 0.05 was considered significant.
Results
Cyanamide Alleviated Ethanol-evoked Myocardial Dysfunction in SO rats
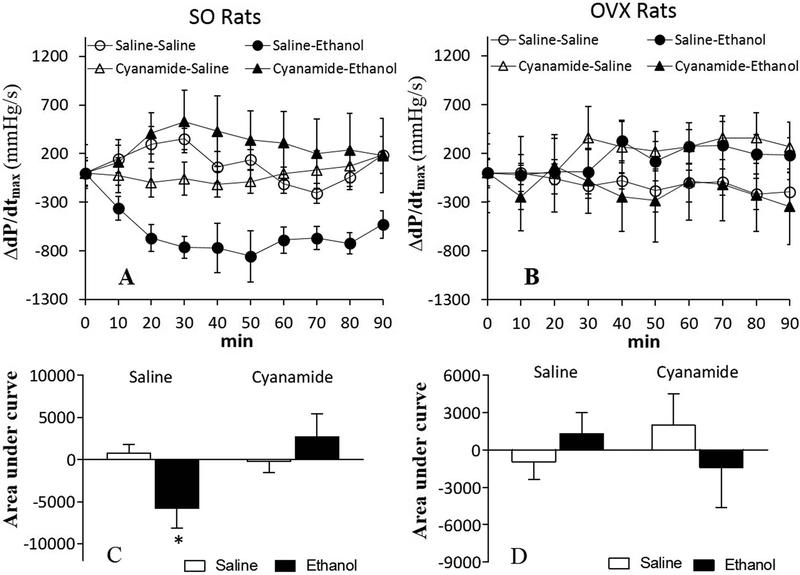
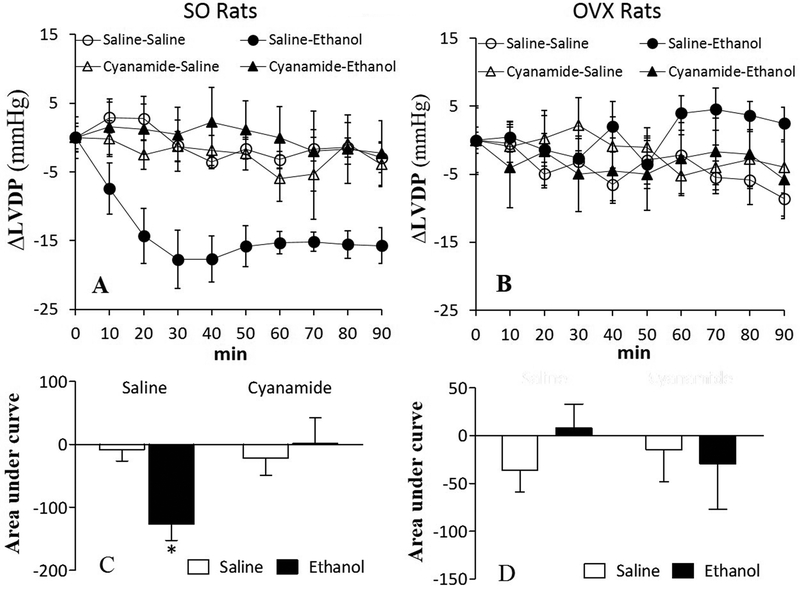
Compared with saline, ALDH2 inhibition (cyanamide; 10 mg/kg) had no effect on dP/dtmax or LVDP either in SO or OVX rats (Table 1; Figs. 2 and 3). Ethanol (1.5 g/kg) caused myocardial dysfunction, presented as reductions (p < 0.05) in dP/dtmax (Figs. 2A and 2C) and LVDP (Figs. 3A and 3C) in SO, but not in OVX (Figs. 2B and 2D, 3B and 3D), rats. Prior ALDH2 inhibition virtually abolished (p < 0.05) ethanol-evoked reductions in dP/dtmax (Figs. 2A and 2C) and LVDP (Figs. 3A and 3C) in SO rats, but its combination with ethanol remained without effect on dP/dtmax (Figs. 2B and 2D) and LVDP (Figs. 3B and 3D) in OVX rats.
Figure 2.
Prior ALDH2 inhibition (cyanamide; 10 mg/kg) prevented ethanol (1.5 g/kg)-evoked reduction in the maximum rate of left ventricular pressure rise (dP/dtmax) in sham operated (SO) rats, presented as time course responses (A) and area under the curve (C); alcohol had no effect on dP/dtmax in ovariectomized (OVX) rats (B and D). Values are presented as mean ± SEM. Data were analyzed by one-way ANOVA followed by post-hoc Tukey’s t-test comparison. *p < 0.05, versus saline-saline. #p < 0.05, versus saline-ethanol.
Figure 3.
Prior inhibition of ALDH2 (cyanamide; 10 mg/kg) prevented ethanol (1.5 g/kg)-evoked reduction in left ventricular developed pressure (LVDP) in sham operated (SO) rats, presented as time course responses (A) and area under the curve (C); alcohol had no effect on LVDP in ovariectomized (OVX) rats (B and D). Values are presented as mean ± SEM. Data were analyzed by one-way ANOVA followed by post-hoc Tukey’s t-test comparison. *p < 0.05, versus saline-saline. #p < 0.05, versus saline-ethanol.
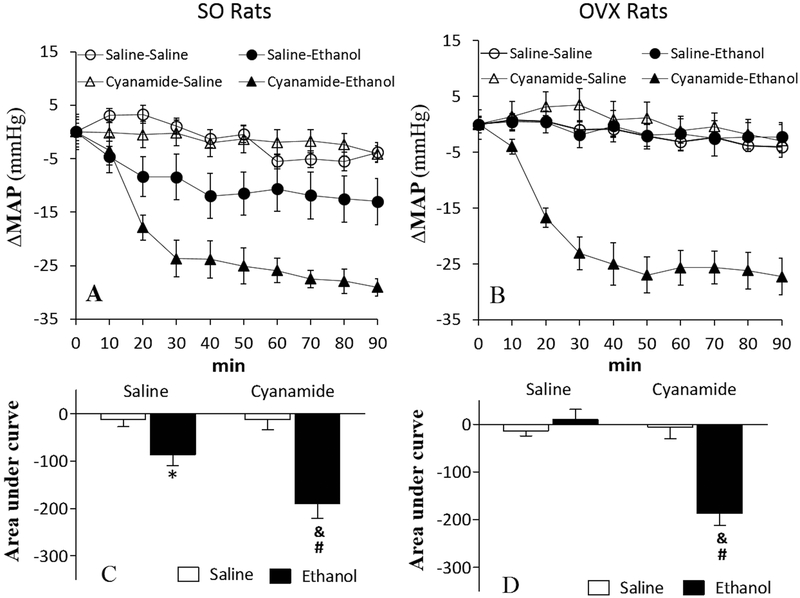
Cyanamide Exacerbated Ethanol-evoked Hypotension in SO Rats and Uncovered it in OVX Rats
Cyanamide alone had no effect on mean arterial pressure (MAP) either in SO or OVX rats (Table 1; Fig. 4). Consistent with our previous findings, ethanol reduced (p < 0.05) MAP in SO (Figs. 4A and 4C), but not in OVX (Figs. 4B and 4D), rats. In the presence of cyanamide, the hypotensive effect of ethanol was substantially (p < 0.05) enhanced in SO rats (Figs. 4A and 4C) and occurred (p < 0.05) in OVX rats (Figs. 4B and 4D). In the presence of cyanamide, the magnitude of ethanol-evoked hypotension was similar regardless of the hormonal status (Fig. 4).
Figure 4.
Prior inhibition of ALDH2 (cyanamide; 10 mg/kg) exacerbated the reduction in mean arterial pressure (MAP) caused by ethanol (1.5 g/kg) in sham operated (SO) rats, presented as time course responses (A) and area under the curve (C). Alcohol caused substantial reduction in MAP only in cyanamide-treated ovariectomized (OVX) rats (B and D). Values are presented as mean ± SEM. Data were analyzed by one-way ANOVA followed by post-hoc Tukey’s t-test comparison. *p < 0.05, versus saline-saline. #p < 0.05, versus saline-ethanol. &p < 0.05, versus cyanamide-saline.
Cyanamide Elicited Divergent Effects on Ethanol Metabolism in SO and OVX Rats
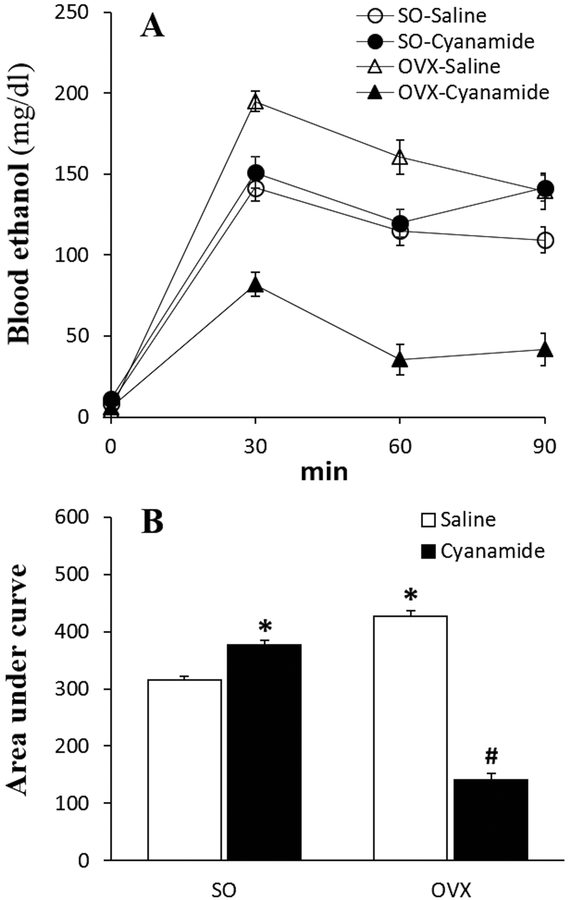
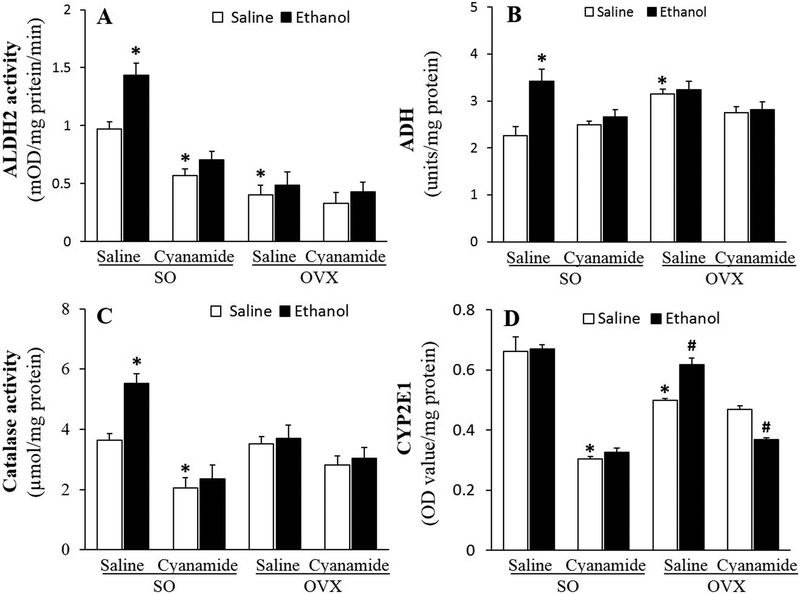
The same ethanol regimen (1.5 g/kg over 90 min) lead to a lower (p < 0.05) blood ethanol concentration in SO than in OVX rats (Fig. 5). This finding coincided with greater (p < 0.05) ethanol-induced activation of three of its metabolizing enzymes, ALDH2 (Fig. 6A), ADH (Fig. 6B) and catalase (Fig. 6C) in the hearts of SO, while in OVX rats, ethanol only increased (p < 0.05) CYP2E1 activity (Fig. 6D). Further, basal cardiac ALDH2 and CYP2E1 were higher (p < 0.05) in SO than in OVX rats (Figs. 6A and 6D).
Figure 5.
Prior inhibition of ALDH2 (cyanamide; 10 mg/kg) elevated and reduced blood ethanol concentration in sham operated (SO) and ovariectomized (OVX) rats, respectively, presented as time course (A) and area under the curve (B). Values are presented as mean ± SEM. Data were analyzed by one-way ANOVA followed by post-hoc Tukey’s t-test comparison. *p < 0.05, versus saline-saline in SO rats. #p < 0.05, versus saline-saline in OVX rats.
Figure 6.
In the absence of ethanol, cyanamide (10 mg/kg) inhibited ALDH2 (A), catalase (C) and CYP2E1 (D) activities and abrogated the ethanol (1.5 g/kg)-evoked increases in ALDH2, ADH and catalase (A-C) in the hearts of sham operated (SO) rats. Cyanamide had no effect on these enzymes in ovariectomized (OVX) rats (A-C) except for increasing and reducing CYP2E1 activity in the absence and presence of ethanol, respectively (D). Values are presented as mean ± SEM. Data were analyzed by one-way ANOVA followed by post-hoc Tukey’s t-test comparison. *p < 0.05 versus saline-saline in SO rats. #p < 0.05, versus saline-saline in OVX rats.
In addition to inhibiting (p < 0.05) ALDH2 activity (Fig. 6A), cyanamide inhibited (p < 0.05) catalase (Fig. 6C) and CYP2E1 (Fig. 6D) in the hearts of SO, but had no effect on any of these enzymes in OVX (Figs. 6A and 6D), rats. Compared to saline, ethanol only reduced (p < 0.05) cardiac CYP2E1 activity in cyanamide-treated OVX rats (Fig. 6D).
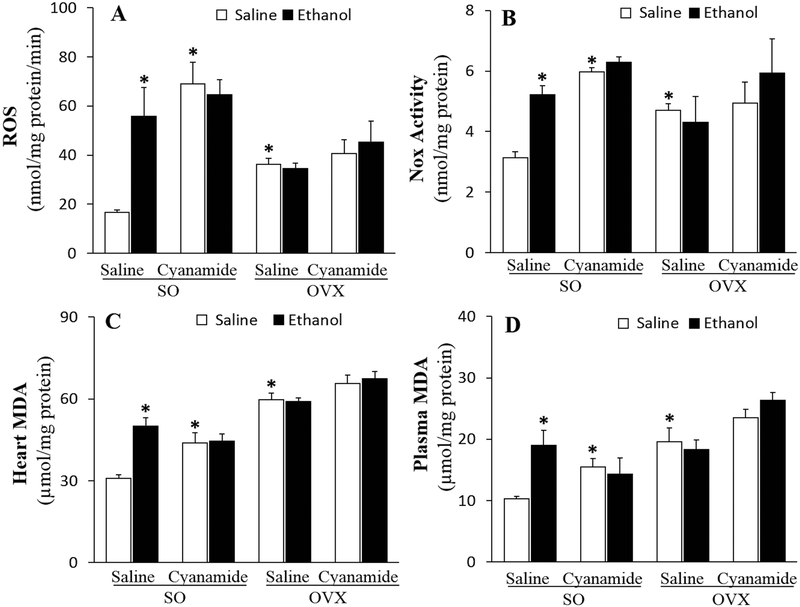
Cyanamide Abrogated the E2-dependent Ethanol-induced Myocardial Oxidative Stress
Ex vivo studies showed higher (p < 0.05) myocardial ROS level (Fig. 7A), Nox activity (Fig. 7B), MDA (Fig. 7C) and plasma MDA (Fig. 7D) in OVX rats than in SO rats, supporting a dampening effect of ovarian hormones/E2 on these mediators of oxidative stress. Cyanamide or ethanol increased (p < 0.05) these mediators of oxidative stress in SO, but not in OVX rats (Fig. 7). However, in cyanamide-treated SO rats, ethanol did not increase the level of any these mediators of oxidative stress (Fig. 7).
Figure 7.
Cyanamide (10 mg/kg) alone increased ROS (A), NADPH oxidase, Nox (B) and malondialdehyde, MDA (C) and prevented their increases by ethanol (1.5 g/kg) in the hearts and MDA in the plasma (D) of sham operated (SO) rats. Cyanamide had no effect on the higher levels of these mediators of oxidative stress in ovariectomized (OVX) rats in the absence or presence of ethanol (A-D). Values are presented as mean ± SEM. Data were analyzed by one-way ANOVA followed by post-hoc Tukey’s t-test comparison. *p < 0.05 versus saline-saline in SO rats.
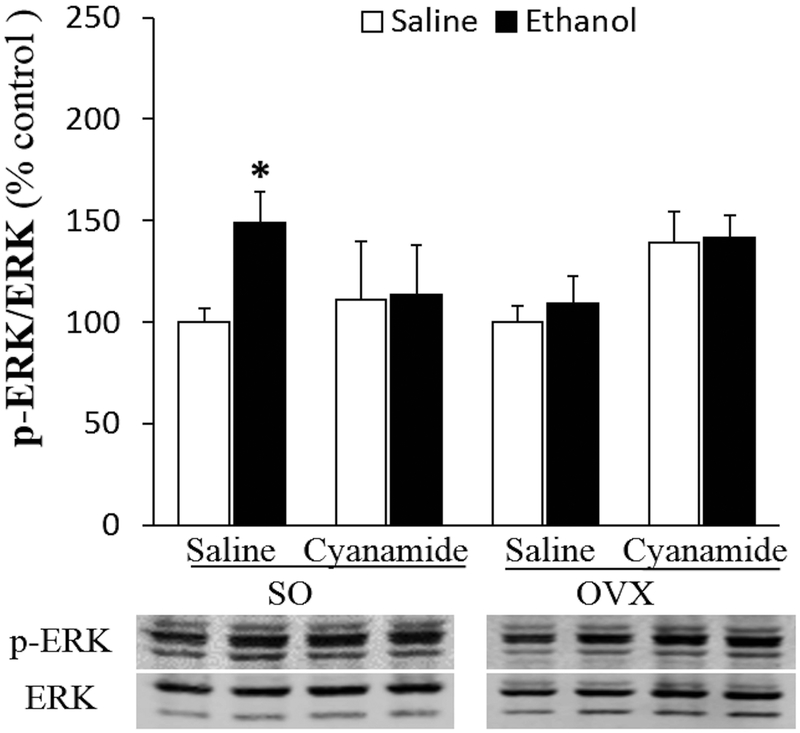
Cyanamide Abrogated Ethanol-induced Myocardial ERK1/2 Phosphorylation in SO Rats
Myocardial ERK1/2 phosphorylation level was similar in SO and OVX rats and was not influenced by cyanamide (Fig. 8). Ethanol enhanced (p < 0.05) ERK1/2 phosphorylation only in SO rats, and this effect was abrogated by cyanamide (Fig. 8).
Figure 8.
Cyanamide (10 mg/kg) alone had no effect on cardiac ERK1/2 phosphorylation in sham operated (SO) or ovariectomized (OVX) rats but attenuated the ethanol (1.5 g/kg)-evoked induction of cardiac ERK1/2 phosphorylation in SO rats. Values are presented as mean ± SEM. Data were analyzed by one-way ANOVA followed by post-hoc Tukey’s t-test comparison. *p < 0.05 versus saline-saline in SO rats.
Discussion
We showed that inhibition of metabolic oxidation to acetaldehyde mitigates the E2-dependent hypersensitivity to ethanol-evoked cardiac oxidative stress and dysfunction (Yao and Abdel-Rahman, 2016, Yao and Abdel-Rahman, 2017b). Here, we hypothesized that acetaldehyde accumulation (following ALDH2 inhibition with cyanamide) exacerbates this ovarian hormones/E2-dependent adverse cardiac effect of ethanol. The most important findings of the present study are: (i) Contrary to our hypothesis, cyanamide alleviated the ethanol-evoked cardiotoxicity in SO rats; (ii) When administered alone, cyanamide increased cardiac oxidative stress (Nox, ROS and MDA) in SO, but did not add to the higher oxidative stress mediators in OVX, rats; (iii) Cyanamide exacerbated, and uncovered, ethanol-evoked hypotension in SO and OVX rats, respectively; (iv) At the dose used, cyanamide caused greater suppression of cardiac ALDH2, catalase and CYP2E1 activities, along with higher blood alcohol levels in SO than in OVX rats. Collectively, ALDH2 inhibition with the alcohol deterrent drug cyanamide paradoxically mitigated the E2-dependent cardiotoxic effect of alcohol. However, the occurrence of severe hypotension, irrespective of the hormonal status, may have clinical ramifications in individuals who combine cyanamide with alcohol.
The ability of ALDH2 inhibition (cyanamide) to mitigate the E2-dependent cardiotoxic effect of ethanol contradicts current knowledge on ALDH2 biology. ALDH2 detoxifies ethanol-derived acetaldehyde (Stornetta et al., 2018) and confers cardio-protection (Budas et al., 2010) via reduction in oxidative stress (Ohsawa et al., 2003, Choi et al., 2011). Therefore, the higher myocardial ROS and MDA in saline-treated OVX rats and in cyanamide-treated SO rats (Fig. 7) are consistent with these reported studies and support the dependence of the anti-oxidant role of ALDH2 on ovarian hormones/E2. This premise is further supported by the higher ALDH2 activity in SO than in OVX rats (Fig. 6A), our previous preclinical findings (Steagall et al., 2017), the positive correlation between E2 and antioxidant status during the menstrual cycle (Massafra et al., 2000) as well as the reversal of the higher oxidative stress by E2 replacement after surgical menopause (Bellanti et al., 2013). It is possible that the effective dose of cyanamide was higher in OVX than in SO rats due to the lower ALDH2 activity in OVX rats. While this possibility might constitute a potential limitation, it is notable that the chosen cyanamide dose has been used in reported studies on male rats, which exhibit lower ALDH2 activity than SO rats (Kinoshita et al., 2001). It is also important to comment on the potential influence of the duration of the OVX as well as the age on the outcome of our study. To limit such potential impact, we allowed 2 weeks after conducting OVX, which is adequate for wash out of the most ovarian hormones based on reported studies including ours (Mohamed et al., 1999, Dougherty et al., 2017), and we used rats of similar age to those used in our previous studies to facilitate data interpretation.
ALDH2 catalysis of ethanol-derived acetaldehyde metabolism into acetate is associated with ROS production (Bosron and Li, 1986, Zimatkin et al., 2006). It is noteworthy that oxidative stress and other reactive molecules such as MDA contribute to alcohol-evoked cardiotoxicity (Richardson et al., 1998, Zhang et al., 2004), which along with hypotension, is exacerbated in female rats (Ibrahim et al., 2014, El-Mas and Abdel-Rahman, 2014, Yao and Abdel-Rahman, 2016) in E2-dependent manner (El-Mas and Abdel-Rahman, 2000, El-Mas and Abdel-Rahman, 2015). However, the role of ALDH2 in these E2-dependent cardiotoxic effect of ethanol is unknown. ALDH2 overexpression confers protection against alcohol-induced cardiomyocyte injury both in vitro (Li et al., 2006) and in vivo (Doser et al., 2009) while ALDH2 knockout exacerbates ethanol-induced cardiac dysfunction (Eom et al., 2007, Ma et al., 2010). Further, earlier preclinical and clinical findings suggest that pharmacological ALDH2 inhibition (cyanamide) leads to acetaldehyde accumulation and enhances ethanol-evoked cardiovascular toxicity, mostly observed as significant hypotension (Chen et al., 2014, Koppaka et al., 2012). However, the majority of these earlier studies were conducted in male rodents or men.
Based on ALDH2 function, we were puzzled by cyanamide mitigation of the ethanol-evoked myocardial dysfunction in SO rats (Figs. 2A and 3A). Interestingly, cyanamide also dampened ethanol-evoked increases in myocardial ROS and MDA, Nox activity and ERK1/2 phosphorylation (Figs. 6–8). The absence of the hemodynamic and molecular responses in OVX rats, under the same conditions (Figs. 2B, 2D; 3B, 3D, and 7-9), further implicates these molecular responses in ethanol-evoked myocardial dysfunction and their dependence on ovarian hormones/E2. While differences in ALDH2 activity in SO and OVX rats might explain the ovarian hormones/E2-hypersensitivity to alcohol-evoked cardiotoxicity, differences in other ethanol metabolizing enzymes cannot be ignored. For example, the higher activities of ADH (Fig. 6B) and CYP2E1 (Fig.6D) and the additional activation by ethanol of ALDH2 and catalase in SO, compared with OVX, rats suggest faster formation and elimination of acetaldehyde in SO rats. Further, it is important to consider the off-target effects of cyanamide on other ethanol metabolizing enzymes.
The demonstrated (Fig. 6), and reported (Koppaka et al., 2012), ALDH2 inhibition by cyanamide results in higher ethanol-derived acetaldehyde levels (Tambour et al., 2007). Importantly, cyanamide inhibition of cardiac catalase (Fig. 6) agrees with its ability to mimic 3-amino triazole (3-AT) as inhibitor of hepatic catalase in vivo (DeMaster et al., 1986). It is likely that this off-target effect and CYP2E1 inhibition, which were greater in SO rats (Fig. 6), contributed, at least partly, to cyanamide attenuation of ethanol-evoked myocardial dysfunction in SO rats. This premise is supported by our findings that 3-AT partly attenuated ethanol-evoked cardiac dysfunction in SO rats (Yao and Abdel-Rahman, 2017a). It is also possible that cyanamide-evoked elevations in ROS and MDA levels (Figs. 7A, C and D) and in reported studies (Yokoyama et al., 1995, Zhang et al., 2011) explain, at least partly, the lack of ethanol-evoked myocardial dysfunction following ALDH2 inhibition. It is noteworthy that oxidative stress plays a critical role in ethanol-evoked myocardial dysfunction in the presence of ovarian hormones or E2 (Ibrahim et al., 2014, El-Mas and Abdel-Rahman, 2014, Yao and Abdel-Rahman, 2016). This evidence is further supported by ethanol’s inability to cause myocardial dysfunction (Figs. 2 and 3) in the presence of higher cardiac oxidative stress in OVX and in cyanamide-treated SO rats (Fig. 7).
It is imperative to note that E2 exacerbates ethanol-evoked oxidative stress in OVX and in male rats (El-Mas and Abdel-Rahman, 2014, El-Mas and Abdel-Rahman, 2015). This counterintuitive exacerbation of oxidative stress is mediated, at least partly, via E2 enhancement of catalase activity, which catalyzes ethanol oxidation to acetaldehyde (Yao and Abdel-Rahman, 2017a). Further, pharmacological interventions that circumvented oxidative stress abrogated the E2-dependent myocardial dysfunction caused by ethanol in our model system (El-Mas and Abdel-Rahman, 2014, Ibrahim et al., 2014). It is also important to reconcile the paradoxical protective effect of cyanamide against ethanol-evoked cardiac dysfunction along with cyanamide-evoked cardiac oxidative stress. The latter effect observed in saline-treated SO, but not in OVX, rats (Fig. 7) is consistent with the physiological function of ALDH2 and its higher activity in SO than in OVX rats (Fig. 6A) in E2-dependent manner (Steagall et al., 2017). Notably, the higher oxidative stress levels following the loss of ovarian hormones/E2 (Fig. 7) was associated with attenuated ethanol-induced cardiac oxidative stress and dysfunction in OVX rats, compared with SO rats (Figs. 2 and 3). These findings suggest that a pre-existing higher oxidative stress might increase the tolerance to ethanol-evoked cardiac dysfunction. On this basis, it is likely that cyanamide-induced oxidative stress served a protective role against ethanol-evoked cardiac dysfunction.
An interesting finding is the cyanamide exacerbation, and uncovering of, ethanol-evoked hypotension in SO and OVX rats, respectively (Fig. 4). Ethanol-evoked hypotension occurs in female or male rats only in the presence of E2 as a consequence of depressed cardiac function (Ibrahim et al., 2014, El-Mas and Abdel-Rahman, 2014, Yao and Abdel-Rahman, 2016, El-Mas and Abdel-Rahman, 2000, El-Mas and Abdel-Rahman, 2015). This explanation, however, does not apply to the occurrence of ethanol-evoked hypotension in cyanamide treated OVX rats in the absence of any reduction in cardiac function (Figs. 2A, 2C and 3A, 3C). It is notable that in the presence of cyanamide, the accumulated ethanol-derived acetaldehyde, which elicits vasodilation leading to hypotension (Tottmar and Hellstrom, 1979), suggests that, in cyanamide-pretreated rats, ethanol-evoked hypotension occurs independent of ovarian hormones. This premise is supported by the findings that cyanamide-dependent effects of alcohol in female rats agree with increased cardiovascular toxicity in male rats (Hillbom et al., 1983) and humans (Kupari et al., 1983); both studies attribute these effects to acetaldehyde accumulation. Nonetheless, as discussed earlier, in the presence of ovarian hormones, or following E2 administration in OVX rats, ethanol causes greater cardiovascular toxicity under the same experimental conditions in our reported studies (El-Mas and Abdel-Rahman, 2014, El-Mas and Abdel-Rahman, 1999). It is likely, therefore, that ethanol-induced vascular relaxation, which involves redox-sensitive (ROS) (Rocha et al., 2012) and endothelial factors such as endothelial nitric oxide synthase (eNOS) (Duarte et al., 2004, Venkov et al., 1999), nitric oxide (NO) (Puddey et al., 2001, Rocha et al., 2012) and prostaglandin (Greenberg et al., 1993) contribute to the present findings.
In conclusion, the current study provides evidence that cyanamide alleviates ethanol-evoked myocardial dysfunction in female SO rats in ovarian hormones/E2 dependent manner. The findings implicate the inhibition of the targeted enzyme, ALDH2, as well as the other ethanol metabolizing enzymes, catalase and CYP2E1, in the unexpected protective effect of cyanamide against ethanol-evoked cardiotoxicity in E2-replete rats. Nonetheless, it is imperative to also note that, irrespective of the hormonal status, combining cyanamide with ethanol precipitated substantial hypotension. The latter, which likely leads to syncope, deserves further investigation to understand the roles of ALDH2 and the final oxidative ethanol metabolite, acetate, in this clinically serious problem.
Acknowledgements
This work was supported by NIH/NIAAA grant 2R01 AA014441-12 from the National Institute on Alcohol Abuse and Alcoholism.
The authors thank Ms. Kui Sun for her technical assistance.
Abbreviations
- ALDH2
aldehyde dehydrogenase 2
- E2
estrogen
- ROS
reactive oxygen species
- SO
sham-operated
- OVX
ovariectomized
- MDA
malondialdehyde
- ADH
alcohol dehydrogenase
- CYP2E1
cytochrome P450 2E1
- Nox
NADPH oxidase
- dP/dtmax
maximum rate of left ventricular pressure rise
- LVDP
left ventricular developed pressure
- MAP
mean arterial pressure
- ERK1/2
extracellular signal–regulated kinases 1/2
References
- Bailey SM, Cunningham CC (1998) Acute and chronic ethanol increases reactive oxygen species generation and decreases viability in fresh, isolated rat hepatocytes. Hepatology 28:1318–1326. [DOI] [PubMed] [Google Scholar]
- Bellanti F, Matteo M, Rollo T, De Rosario F, Greco P, Vendemiale G, Serviddio G (2013) Sex hormones modulate circulating antioxidant enzymes: impact of estrogen therapy. Redox Biol 1:340–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosron WF, Li TK (1986) Genetic polymorphism of human liver alcohol and aldehyde dehydrogenases, and their relationship to alcohol metabolism and alcoholism. Hepatology 6:502–510. [DOI] [PubMed] [Google Scholar]
- Budas GR, Disatnik MH, Chen CH, Mochly-Rosen D (2010) Activation of aldehyde dehydrogenase 2 (ALDH2) confers cardioprotection in protein kinase C epsilon (PKCvarepsilon) knockout mice. J Mol Cell Cardiol 48:757–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Ferreira JC, Gross ER, Mochly-Rosen D (2014) Targeting aldehyde dehydrogenase 2: new therapeutic opportunities. Physiol Rev 94:1–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H, Tostes RC, Webb RC (2011) Mitochondrial aldehyde dehydrogenase prevents ROS-induced vascular contraction in angiotensin-II hypertensive mice. J Am Soc Hypertens 5:154–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole-Harding S, Wilson JR (1987) Ethanol metabolism in men and women. Journal of studies on alcohol 48:380–387. [DOI] [PubMed] [Google Scholar]
- DeMaster EG, Redfern B, Nagasawa HT (1998) Mechanisms of inhibition of aldehyde dehydrogenase by nitroxyl, the active metabolite of the alcohol deterrent agent cyanamide. Biochem Pharmacol 55:2007–2015. [DOI] [PubMed] [Google Scholar]
- DeMaster EG, Redfern B, Shirota FN, Nagasawa HT (1986) Differential inhibition of rat tissue catalase by cyanamide. Biochem Pharmacol 35:2081–2085. [DOI] [PubMed] [Google Scholar]
- Doser TA, Turdi S, Thomas DP, Epstein PN, Li SY, Ren J (2009) Transgenic overexpression of aldehyde dehydrogenase-2 rescues chronic alcohol intake-induced myocardial hypertrophy and contractile dysfunction. Circulation 119:1941–1949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Dougherty BJ, Kopp ES, Watters JJ (2017) Nongenomic Actions of 17-beta Estradiol Restore Respiratory Neuroplasticity in Young Ovariectomized Female Rats. J Neurosci 37:6648–6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte J, Andriambeloson E, Diebolt M, Andriantsitohaina R (2004) Wine polyphenols stimulate superoxide anion production to promote calcium signaling and endothelial-dependent vasodilatation. Physiol Res 53:595–602. [PubMed] [Google Scholar]
- El-Mas MM, Abdel-Rahman AA (1999) Estrogen-dependent hypotensive effects of ethanol in conscious female rats. Alcohol Clin Exp Res 23:624–632. [PubMed] [Google Scholar]
- El-Mas MM, Abdel-Rahman AA (2000) Ovariectomy alters the chronic hemodynamic and sympathetic effects of ethanol in radiotelemetered female rats. Clin Exp Hypertens 22:109–126. [DOI] [PubMed] [Google Scholar]
- El-Mas MM, Abdel-Rahman AA (2014) Nongenomic effects of estrogen mediate the dose-related myocardial oxidative stress and dysfunction caused by acute ethanol in female rats. Am J Physiol Endocrinol Metab 306:E740–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Mas MM, Abdel-Rahman AA (2015) Estrogen modulation of the ethanol-evoked myocardial oxidative stress and dysfunction via DAPK3/Akt/ERK activation in male rats. Toxicol Appl Pharmacol 287:284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Mas MM, Fan M, Abdel-Rahman AA (2008) Endotoxemia-mediated induction of cardiac inducible nitric-oxide synthase expression accounts for the hypotensive effect of ethanol in female rats. J Pharmacol Exp Ther 324:368–375. [DOI] [PubMed] [Google Scholar]
- El-Mas MM, Fan M, Abdel-Rahman AA (2012) Differential modulation by vascular nitric oxide synthases of the ethanol-evoked hypotension and autonomic dysfunction in female rats. Alcohol 46:727–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eom SY, Zhang YW, Ogawa M, Oyama T, Isse T, Kang JW, Lee CJ, Kim YD, Kawamoto T, Kim H (2007) Activities of Antioxidant Enzymes Induced by Ethanol Exposure in Aldehyde Dehydrogenase 2 Knockout Mice. J Health Sci 53:378–381. [Google Scholar]
- Greenberg SS, Xie J, Wang Y, Kolls J, Shellito J, Nelson S, Summer WR (1993) Ethanol relaxes pulmonary artery by release of prostaglandin and nitric oxide. Alcohol 10:21–29. [DOI] [PubMed] [Google Scholar]
- Hillbom ME, Sarviharju MS, Lindros KO (1983) Potentiation of ethanol toxicity by cyanamide in relation to acetaldehyde accumulation. Toxicol Appl Pharmacol 70:133–139. [DOI] [PubMed] [Google Scholar]
- Ibrahim BM, Fan M, Abdel-Rahman AA (2014) Oxidative stress and autonomic dysregulation contribute to the acute time-dependent myocardial depressant effect of ethanol in conscious female rats. Alcohol Clin Exp Res 38:1205–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita H, Jessop DS, Finn DP, Coventry TL, Roberts DJ, Ameno K, Jiri I, Harbuz MS (2001) Acetaldehyde, a metabolite of ethanol, activates the hypothalamic-pituitary-adrenal axis in the rat. Alcohol Alcohol 36:59–64. [DOI] [PubMed] [Google Scholar]
- Kirkendol RL, Pearson JE, Bower JD, Holbert RD (1978) Myocardial depressant effects of sodium acetate. Cardiovasc Res 12:127–136. [DOI] [PubMed] [Google Scholar]
- Kondo Y, Fuke C, Higa A, Kukita I (2013) [Cyanamide-ethanol reaction induced shock: report of a case and literature review]. Chudoku Kenkyu 26:295–299. [PubMed] [Google Scholar]
- Koppaka V, Thompson DC, Chen Y, Ellermann M, Nicolaou KC, Juvonen RO, Petersen D, Deitrich RA, Hurley TD, Vasiliou V (2012) Aldehyde dehydrogenase inhibitors: a comprehensive review of the pharmacology, mechanism of action, substrate specificity, and clinical application. Pharmacol Rev 64:520–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupari M, Lindros K, Hillbom M, Heikkila J, Ylikahri R (1983) Cardiovascular effects of acetaldehyde accumulation after ethanol ingestion: their modification by beta-adrenergic blockade and alcohol dehydrogenase inhibition. Alcohol Clin Exp Res 7:283–288. [DOI] [PubMed] [Google Scholar]
- La Favor JD, Anderson EJ, Dawkins JT, Hickner RC, Wingard CJ (2013) Exercise prevents Western diet-associated erectile dysfunction and coronary artery endothelial dysfunction: response to acute apocynin and sepiapterin treatment. Am J Physiol Regul Integr Comp Physiol 305:R423–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SY, Li Q, Shen JJ, Dong F, Sigmon VK, Liu Y, Ren J (2006) Attenuation of acetaldehyde-induced cell injury by overexpression of aldehyde dehydrogenase-2 (ALDH2) transgene in human cardiac myocytes: role of MAP kinase signaling. J Mol Cell Cardiol 40:283–294. [DOI] [PubMed] [Google Scholar]
- Ma H, Yu L, Byra EA, Hu N, Kitagawa K, Nakayama KI, Kawamoto T, Ren J (2010) Aldehyde dehydrogenase 2 knockout accentuates ethanol-induced cardiac depression: role of protein phosphatases. J Mol Cell Cardiol 49:322–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massafra C, Gioia D, De Felice C, Picciolini E, De Leo V, Bonifazi M, Bernabei A (2000) Effects of estrogens and androgens on erythrocyte antioxidant superoxide dismutase, catalase and glutathione peroxidase activities during the menstrual cycle. J Endocrinol 167:447–452. [DOI] [PubMed] [Google Scholar]
- McGee MA, Abdel-Rahman AA (2012) Enhanced vascular neuronal nitric-oxide synthase-derived nitric-oxide production underlies the pressor response caused by peripheral N-methyl-D-aspartate receptor activation in conscious rats. J Pharmacol Exp Ther 342:461–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed MK, El-Mas MM, Abdel-Rahman AA (1999) Estrogen enhancement of baroreflex sensitivity is centrally mediated. Am J Physiol 276:R1030–1037. [DOI] [PubMed] [Google Scholar]
- Ohsawa I, Nishimaki K, Yasuda C, Kamino K, Ohta S (2003) Deficiency in a mitochondrial aldehyde dehydrogenase increases vulnerability to oxidative stress in PC12 cells. J Neurochem 84:1110–1117. [DOI] [PubMed] [Google Scholar]
- Richardson PJ, Patel VB, Preedy VR (1998) Alcohol and the myocardium. Novartis Found Symp 216:35–45; discussion 45–50. [DOI] [PubMed] [Google Scholar]
- Rocha JT, Hipolito UV, Callera GE, Yogi A, Neto Filho Mdos A, Bendhack LM, Touyz RM, Tirapelli CR (2012) Ethanol induces vascular relaxation via redox-sensitive and nitric oxide-dependent pathways. Vascul Pharmacol 56:74–83. [DOI] [PubMed] [Google Scholar]
- Steagall RJ, Yao F, Shaikh SR, Abdel-Rahman AA (2017) Estrogen receptor alpha activation enhances its cell surface localization and improves myocardial redox status in ovariectomized rats. Life Sci 182:41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart MJ, Malek K, Crabb DW (1996) Distribution of messenger RNAs for aldehyde dehydrogenase 1, aldehyde dehydrogenase 2, and aldehyde dehydrogenase 5 in human tissues. Journal of investigative medicine : the official publication of the American Federation for Clinical Research 44:42–46. [PubMed] [Google Scholar]
- Stornetta A, Guidolin V, Balbo S (2018) Alcohol-Derived Acetaldehyde Exposure in the Oral Cavity. Cancers (Basel) 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suokas A, Kupari M, Heikkila J, Lindros K, Ylikahri R (1988) Acute cardiovascular and metabolic effects of acetate in men. Alcohol Clin Exp Res 12:52–58. [DOI] [PubMed] [Google Scholar]
- Tamai H, Yokoyama A, Okuyama K, Takahashi H, Maruyama K, Suzuki Y, Ishii H (2000) Comparison of cyanamide and disulfiram in effects on liver function. Alcohol Clin Exp Res 24:97S–99S. [PubMed] [Google Scholar]
- Tambour S, Closon C, Tirelli E, Quertemont E (2007) Effects of cyanamide and acetaldehyde accumulation on the locomotor stimulant and sedative effects of ethanol in mice. Behav Pharmacol 18:777–784. [DOI] [PubMed] [Google Scholar]
- Thomasson HR (1995) Gender differences in alcohol metabolism. Physiological responses to ethanol. Recent developments in alcoholism : an official publication of the American Medical Society on Alcoholism, the Research Society on Alcoholism, and the National Council on Alcoholism 12:163–179. [DOI] [PubMed] [Google Scholar]
- Tottmar O, Hellstrom E (1979) Blood pressure response to ethanol in relation to acetaldehyde levels and dopamine-beta-hydroxylase activity in rats pretreated with disulfiram, cyanamide and coprine. Acta Pharmacol Toxicol (Copenh) 45:272–281. [DOI] [PubMed] [Google Scholar]
- Venkov CD, Myers PR, Tanner MA, Su M, Vaughan DE (1999) Ethanol increases endothelial nitric oxide production through modulation of nitric oxide synthase expression. Thromb Haemost 81:638–642. [PubMed] [Google Scholar]
- Yao F, Abdel-Rahman AA (2016) Estrogen receptor ERalpha plays a major role in ethanol-evoked myocardial oxidative stress and dysfunction in conscious female rats. Alcohol 50:27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao F, Abdel-Rahman AA (2017a) Combined Catalase and ADH Inhibition Ameliorates Ethanol-Induced Myocardial Dysfunction Despite Causing Oxidative Stress in Conscious Female Rats. Alcohol Clin Exp Res 41:1541–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao F, Abdel-Rahman AA (2017b) Estrogen Receptors alpha and beta Play Major Roles in Ethanol-Evoked Myocardial Oxidative Stress and Dysfunction in Conscious Ovariectomized Rats. Alcohol Clin Exp Res 41:279–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama A, Sato S, Maruyama K, Nakano M, Takahashi H, Okuyama K, Takagi S, Takagi T, Yokoyama T, Hayashida M, et al. (1995) Cyanamide-associated alcoholic liver disease: a sequential histological evaluation. Alcohol Clin Exp Res 19:1307–1311. [DOI] [PubMed] [Google Scholar]
- Zhang W, Lu D, Dong W, Zhang L, Zhang X, Quan X, Ma C, Lian H, Zhang L (2011) Expression of CYP2E1 increases oxidative stress and induces apoptosis of cardiomyocytes in transgenic mice. FEBS J 278:1484–1492. [DOI] [PubMed] [Google Scholar]
- Zhang X, Li SY, Brown RA, Ren J (2004) Ethanol and acetaldehyde in alcoholic cardiomyopathy: from bad to ugly en route to oxidative stress. Alcohol 32:175–186. [DOI] [PubMed] [Google Scholar]
- Zimatkin SM, Pronko SP, Vasiliou V, Gonzalez FJ, Deitrich RA (2006) Enzymatic mechanisms of ethanol oxidation in the brain. Alcohol Clin Exp Res 30:1500–1505. [DOI] [PubMed] [Google Scholar]