Abstract
Background:
Prescription opioid abuse continues to be a public health concern of epidemic proportions. Notwithstanding the extensive literature regarding opioid action, there has been little systematic research regarding the effects of opioid dependence and withdrawal on aspects of cognition-related behavior in laboratory animals. The present studies examined the effects of the prescription opioid oxycodone on learning processes in nonhuman primates.
Methods:
The ability of subjects to repeatedly learn novel touchscreen-based visual discriminations was examined during three conditions of opioid exposure. Discrimination learning was examined, first, during oxycodone self-administration (3-hr sessions, 0.1 mg/kg/infusion) and, next, during non-contingent chronic treatment with oxycodone (10 mg/kg/day). Finally, discrimination learning was re-examined during antagonist-precipitated opioid withdrawal (0.001–0.1 mg/kg naltrexone) and, subsequently, following abrupt discontinuation of oxycodone treatment.
Results:
Although motoric behavior was disrupted by oxycodone, neither the development of discrimination learning nor steady-state performance were impaired following oxycodone self-administration or during non-contingent chronic oxycodone treatment. However, discrimination learning was substantially impaired during oxycodone withdrawal, whether elicited by naltrexone or by abrupt oxycodone discontinuation. Moreover, these learning impairments were concordant with autonomic signs of opioid withdrawal.
Conclusions:
Taken together, the present studies indicate that impairment of learning processes can accompany the unconditioned signs of opioid withdrawal.
Keywords: opioid, oxycodone, naltrexone, self-administration, withdrawal, cognition, nonhuman primate
1. Introduction
Prescription opioid abuse has risen dramatically during the last decade (Ahmad et al., 2018). Despite extensive research efforts committed to understanding opioid action, clinical investigations of the effects of opioid treatment on cognitive performance have reported highly variable effects. For example, Sjøgren et al. (2005) found that patients treated with opioids for chronic pain performed more poorly than drug-free comparison subjects in tasks measuring information processing and working memory, but not in sustained attention and psychomotor speed. However, in another study of patients receiving long-term (>6 months) morphine for pain management, deficits in psychomotor speed were observed, in addition to information processing and episodic memory, but not in sustained attention or verbal fluency (Kamboj et al., 2005). When verbal fluency was tested in healthy volunteers, the number of statements answered correctly was not affected following increasing doses of oxycodone, but the overall number of statements answered was significantly lower (Zacny and Gutierrez, 2003), suggesting that oxycodone may affect the ability of the subject to engage in the cognitive task rather than accuracy in performance. Although the conclusions from the above studies were derived from palliative care patients or healthy volunteers, opioid effects on cognitive function in subjects with opioid use disorder also appear to be complicated. For example, some research has documented deficits in cognitive flexibility, working memory, and executive function during or immediately following opioid use (Ornstein et al., 2000; Rogers et al., 1999; Strang et al., 1989; Zacny et al., 1995). On the other hand, cognitive function following abstinence has been reported to improve when compared to performance observed during periods of opioid abuse (Davis et al., 2002; Mintzer et al., 2005). Notably, there may be differences between the magnitude of deficits during early abstinence (days 1–21) and late abstinence (>21 days), perhaps as a consequence of the time course of withdrawal-induced neural dysregulation following discontinuation (Rapeli et al., 2006). However, even considering the time of measurement, data regarding the effects of prior opioid exposure on cognitive function are not in close agreement. That is, some studies report no cognitive deficits following 3 weeks of abstinence (Gerra et al., 1998; Davis et al., 2002) whereas others report persistent deficits in measures of executive function such as impulse control, visual attention, and cognitive flexibility (Lee and Pau, 2002; Pau et al., 2002; Mintzer et al., 2005).
Surprisingly, the effects of chronic opioid treatment, dependence, and withdrawal on aspects of cognitive function have not been systematically investigated in laboratory animals. Given inconsistencies such as those highlighted in the clinical studies above, the present studies were designed to examine, under controlled laboratory conditions, the effects of the prescription opioid oxycodone in a model of cognition-related behavior using nonhuman primates. A touchscreen-based repeated acquisition task was used to measure the development and maintenance of visual discrimination learning (Harlow, 1949; Kangas and Bergman, 2014), a cognitive process that previously has been shown to be sensitive to the adverse effects of several commonly abused drugs such as methamphetamine, Δ9-tetrahydrocannabinol, and cocaine (Kangas and Bergman, 2016; Kangas et al., 2016; 2019). The ability of subjects to repeatedly learn novel visual discriminations was examined during three conditions of opioid exposure. First, we studied the effects of self-administered oxycodone on discrimination learning by assessing performance immediately following daily sessions of i.v. self-administration. Opioid taking behavior was examined for its obvious translational value and, as well, because drug effects can vary depending on whether they are self-administered or experimenter-administered (e.g., Dworkin et al., 1995). Second, we studied the influence of non-contingent opioid administration on discrimination learning by conducting daily sessions during chronic treatment with a relatively high dosage of oxycodone administered via a subcutaneous drug pump. Finally, we compared the effects of antagonist (naltrexone)-precipitated withdrawal and abrupt discontinuation of oxycodone treatment on autonomic responses and discrimination learning to determine if opioid withdrawal modifies learning processes.
2. Methods
2.1. Subjects
Six adult male squirrel monkeys (Saimiri sciureus) were used in the present studies. Subjects had served previously in studies of dopamine-related drugs or cannabinoids, and had not received drug treatment for at least 6 months prior to the present studies. None of the subjects had previous experience with opioids, touchscreen tasks, or i.v. drug self-administration. Subjects were pair-housed in a temperature- and humidity-controlled vivarium (lights on at 7 a.m. and off at 7 p.m.). All subjects were given unlimited access to water in the home cage and were maintained at approximate free-feeding weights by post-session access to a nutritionally balanced diet of high protein biscuits (Purina Monkey Chow, St. Louis, MO). In addition, fresh fruit and environmental enrichment were provided daily. The protocol for the present studies was approved by the Institutional Animal Care and Use Committee at McLean Hospital in a facility licensed by the US Department of Agriculture and in accordance with guidelines provided by the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Animals Resources, Commission on Life Sciences, National Research Council (2011).
2.2. Procedures
2.2.1. Oxycodone Self-administration.
Three of the 6 subjects (oxycodone self-administration group) were prepared with intravenous (i.v.) catheters for drug delivery using procedures initially described by Herd et al., (1969). Under isoflurane anesthesia and in aseptic conditions, one end of a hydrophilically coated polyurethane catheter (inside diameter, 0.381 mm; outside diameter, 0.762 mm) was inserted into the right femoral vein, secured, and passed subcutaneously to exit the subject’s back in the mid-scapular region. Catheterized subjects wore nylon jackets at all times to protect the catheters. During self-administration sessions, subjects sat in a Plexiglas chair (Kangas and Bergman, 2016) in a ventilated light- and sound-attenuating enclosure. Subjects faced a panel containing two response levers, colored stimulus lights, and a food receptacle that was situated between the levers and could be accessed easily. Each press of the lever with a force greater than 0.25 N produced an audible click and was recorded as a response. Two pumps (PHM-100–10, Med Associates, St. Albans, VT) outside the enclosure were used to deliver response-contingent infusions during the session. One pump delivered 0.15 ml of a 30% sweetened condensed milk solution into the food receptacle during initial training; the second pump delivered i.v. infusions of vehicle or oxycodone. All experimental events and data collection were controlled by Med Associates (St. Albans, VT) interfacing equipment and operating software. Subjects initially were trained to respond on one lever under a 5-response fixed ratio (FR5) schedule of milk delivery. Responses on the other lever had no programmed consequences. Lever assignment was counterbalanced across subjects. The completion of the FR5 on the active lever turned off the stimulus lights, delivered the milk reinforcer, and initiated a timeout (TO) period of 60 s during which all stimulus lights were off and responding had no scheduled consequences.
Following the establishment of milk-maintained responding under the FR5 schedule, i.v. infusions of oxycodone (0.1 ml/infusion) gradually replaced milk deliveries as reinforcing events. Subjects initially self-administered oxycodone with simultaneous milk delivery during 1-hr sessions, 5 days a week (M-F), beginning with 0.15 mL of milk delivery paired with 0.01 mg/kg/inj of oxycodone. After 3 days of stable oxycodone intake (±20% of the 3-session mean), milk volumes were periodically decreased to 0.1 ml, 0.075 ml, 0.05 ml, 0.025 ml, and 0 ml, allowing for responding to stabilize between decreases in volume. Once oxycodone intake without milk delivery was stable, an oxycodone dose-response function (0.001–0.1 mg/kg/inj) was determined three times in each subject. Doses were studied in a mixed order and each unit dose was studied for a minimum of 5 days and until session intake across 3 consecutive sessions was within ±20% of the 3-session mean. Subsequently, the session length was increased to 2 hr and then to 3 hr in order to increase daily oxycodone intake. Oxycodone dose-response functions were redetermined under 2-hr and 3-hr session durations with otherwise identical conditions. The unit dose and session length that resulted in the maximum mean daily intake was identified (0.1 mg/kg/inj, 3-hr sessions), and daily self-administration sessions continued for 30 sessions under those conditions. Starting with session 31, subjects were transferred to an adjacent touchscreen chamber immediately following each self-administration session to evaluate the effects of self-administered oxycodone on discrimination learning. The remaining 3 subjects (drug-free comparison group) also were tested for discrimination learning in the manner described below, but without daily self-administration sessions.
2.2.2. Repeated Acquisition.
Details, schematics, and photographs of the chamber used for touchscreen-based repeated acquisition studies can be found in Kangas and Bergman (2012; 2017). All experimental events and data collection were programmed in E-Prime Professional 2.0 (Psychology Software Tools, Inc., Sharpsburg, PA). Subjects in both experimental groups were trained to touch the screen using previously described shaping methods (Kangas and Bergman, 2012). To avoid the possibility of opioid-related effects on initial training, the oxycodone self-administration group was trained to touch the screen prior to any oxycodone exposure (i.e., immediately prior to i.v. catheterization). Previously established methods were used to train subjects to discriminate repeatedly novel visual discriminations (Kangas and Bergman, 2014). Each session began with concurrent presentation of two 7×7 cm digital photographs, each in a different randomly selected quadrant of the screen. A touch response on one stimulus initiated the delivery of 0.15 ml of a 30% sweetened condensed milk solution into the reservoir (S+) paired with an 880-ms yellow screen flash, and followed by a 10-s intertrial interval (ITI) blackout; a touch response to the other stimulus immediately initiated the 10 s ITI (S−). The same two stimuli were presented during each of 200 trials comprising the day’s session. The two stimuli for each session were selected from a laboratory bank of >10,000 photographic images. Thus, the subject was required to repeatedly learn new S+/S− discriminations based on distinguishing features of two stimuli that had not been previously viewed. Discrimination mastery was defined as the number of trials into a session until a subject performed correctly in 9 out of 10 consecutive trials (i.e., 90% correct). If the subject failed to master the discrimination within the 200-trial session, the same stimuli were presented during the next day’s 200-trial session. Subjects in both the oxycodone self-administration and drug-free comparison group were exposed to the repeated acquisition task until 30 discriminations were mastered.
2.2.3. Chronic Oxycodone Infusion
Despite high levels of oxycodone intake during the self-administration sessions, no observable signs of withdrawal were noted in any subject before self-administration sessions or during other times of the day. To produce clear signs of physical dependence, each of the 3 subjects in the oxycodone self-administration group was implanted with a subcutaneous (s.c.) catheter that connected to a programmable iPRECIO® pump (Primetech Corp., Tokyo, Japan) residing in an inside pocket of the subject’s jacket and set to deliver oxycodone at a constant flow rate 24 hr/day, 7 days/week. Under isoflurane anesthesia and in aseptic conditions, one end of a hydrophilically coated polyurethane catheter (inside diameter, 0.025 in; outside diameter, 0.040 in) was inserted into the lower back and secured via a polyurethane cuff to the latissimus dorsi muscle approximately 2 cm lateral to the spine. The other end of the catheter was passed subcutaneously to a point below the right scapula where it exited the subject’s back and was connected to the pump. Oxycodone concentrations in the pump were periodically increased in order to increase daily dosages from 1 to 3, 5, 7, and 10 mg/kg/day over the course of approximately 1 month. Repeated acquisition sessions continued 7 days a week throughout these escalating oxycodone conditions to examine their effects on task performance.
2.2.4. Naltrexone-Precipitated Withdrawal
Following 10 consecutive days of continuous treatment with 10 mg/kg/day oxycodone, experiments were conducted to examine the effects of antagonist-precipitated withdrawal on repeated acquisition performance. Subjects received an i.m. injection of naltrexone (0.001–0.1 mg/kg) or saline 10 min prior to the repeated acquisition session, once weekly. Subjects were placed in a 25×25 cm observational chamber immediately after the injection and monitored using visual inspection by one of the present authors (RJD) for the following overt signs of withdrawal: wet dog shakes, tremor, diarrhea, and emesis. During this interval, the occurrence or nonoccurrence of each sign of withdrawal was noted; the observer was not blinded to the treatment condition. After 10 min, subjects were transferred to the touchscreen chamber for repeated acquisition assessment.
2.2.5. Abrupt Oxycodone Discontinuation
After acute naltrexone tests were completed, exposure to 10 mg/kg/day oxycodone was continued for an additional 7 days. Oxycodone administration was then abruptly terminated via deactivation of the drug pump. Subjects were observed for the occurrence or nonoccurrence of withdrawal signs as described above during 1-hr observation periods that preceded each daily repeated acquisition session. The first repeated acquisition session occurred 24 hr following discontinuation of oxycodone treatment and continued daily for 10 consecutive days following drug discontinuation.
2.2.6. Naltrexone Assessment during Untreated Conditions
To determine whether naltrexone had any effects on discrimination learning independent of its precipitation of opioid withdrawal, control studies were conducted when subjects were not dependent on oxycodone. These final assessments began 30 days after termination of chronic oxycodone treatment. Subjects were given an i.m. injection of naltrexone (0.001–0.1 mg/kg), once per week, 10 min prior to the repeated acquisition session.
2.3. Data analysis
In i.v. oxycodone self-administration studies, the primary dependent measures were number of injections per session and total session intake of oxycodone (mg/kg). Session intake was calculated by multiplying the total number of injections in a session by the self-administered unit dose of oxycodone (mg/kg/inj). Dose-response functions were constructed by averaging intake for each subject during the last 3 sessions of each unit dose tested and then presenting group averages for unit dose intake (±SEM). The primary dependent measures used to evaluate repeated acquisition performance were trials-to-mastery (see above for mastery criterion) and mean reaction time. Reaction time was defined as the time to make a response after presentation of each S+/S− stimulus pair and presented as the mean of the 200 trials that comprised each session.
2.4. Drugs
Oxycodone (oxycodone hydrochloride) and naltrexone (naltrexone hydrochloride) were purchased from Sigma Pharmaceuticals (St. Louis, MO), prepared for administration in 0.9% saline solution, refrigerated, and protected from light. For i.v. self-administration, oxycodone solutions were filtered through at 0.22 μm pore-size membrane filter and all infusions of oxycodone or its vehicle were delivered in 0.1 ml volumes. For i.m. treatment, vehicle or doses of naltrexone were administered in volumes of 0.3 ml/kg body weight or less into thigh muscle. All doses are expressed in terms of their free base weight.
3. Results
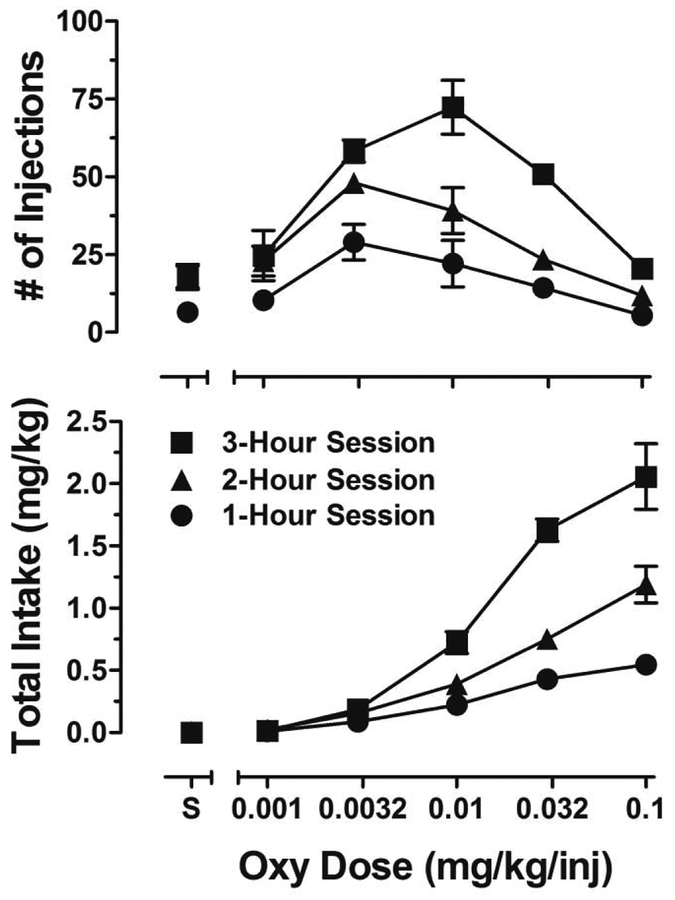
Figure 1 presents dose-response functions for the number of oxycodone infusions (top panel) and intake (bottom panel) during 1-hr (circles), 2-hr (triangles), and 3-hr (squares) self-administration sessions. The number of oxycodone infusions across unit doses describes an inverted U-shape function during each of the session-lengths tested. Total oxycodone intake increased in a dose- and session length-dependent manner, with a 2-fold increase from 1 to 2 hr and a 4-fold increase from 1 to 3 hr. The unit dose of 0.1 mg/kg/inj during 3-hr sessions produced maximum oxycodone intake in all subjects. Consequently, this unit dose and session length was chosen for subsequent self-administration sessions. Under these conditions, the subjects were given 30 sessions for intake to stabilize. Oxycodone intake increased during the 30 self-administration sessions between the determination of dose-response functions and the initiation of repeated acquisition testing (1.9 ±0.4 to 3.3 ±0.7 mg/kg). However, intake did not increase further during the 30 days of repeated acquisition testing (2.9 ±0.6 to 2.8 ±0.3 mg/kg).
Figure 1.
Mean (±SEM) oxycodone injections (top panel) and total oxycodone intake (bottom panel) during 1-hr (circles), 2-hr (triangles), and 3-hr (squares) sessions in which different unit doses of oxycodone (mg/kg/inj) and saline (S) were available to self-administer.
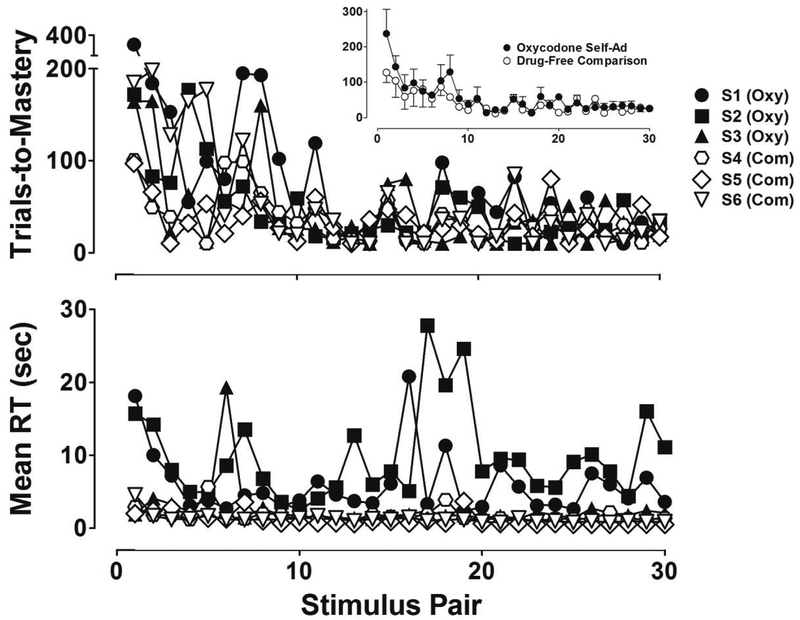
Figure 2 presents the number of trials to master 30 novel discriminations (top panel) and mean reaction time (bottom panel) for individual subjects that self-administered oxycodone under the peak intake conditions (filled symbols) and drug-free comparison subjects (unfilled symbols). The inset in the top panel shows the same data but presented as group means (±SEM). Although there was variability in trials-to-mastery, especially during the first 10 visual discriminations mastered, a downward trend across stimulus pairs was evident in all subjects in both groups. As highlighted in the group mean data in the Figure 2 inset, subjects that self-administered oxycodone before each repeated acquisition session required, on average, more trials to master the first 9 out of 10 discriminations compared to drug-free comparison subjects. However, this difference was small. Moreover, following the mastery of 10 novel discriminations, the number of trials to master subsequent discriminations was nearly indistinguishable between group means and, as well, the individual subject performances that comprised them. Notwithstanding the small differences observed in trials-to-mastery, a substantial group difference was evident in reaction time. As shown in the bottom panel of Figure 2, mean reaction times for individual subjects in the oxycodone group were highly variable across sessions and much longer than the mean reaction times of drug-free comparison subjects, which consistently ranged from 1–2 sec. Between-subject variability was evident in the oxycodone group – S1’s condition-wide reaction time averaged 2.5 sec, which was only slightly longer than the mean value for drug-free comparison subjects. However, reaction times for the other oxycodone subjects (S2 and S3) were considerably more disrupted, with condition-wide mean values of 9.8 and 5.9 sec.
Figure 2.
Number of trials-to-mastery (top panel) and mean reaction time (bottom panel) during repeated acquisition sessions in which 30 novel discriminations were learned in subjects that self-administered oxycodone (filled symbols) and drug-free comparison subjects (unfilled symbols). The inset in the top panel shows the same data presented as group means (±SEM).
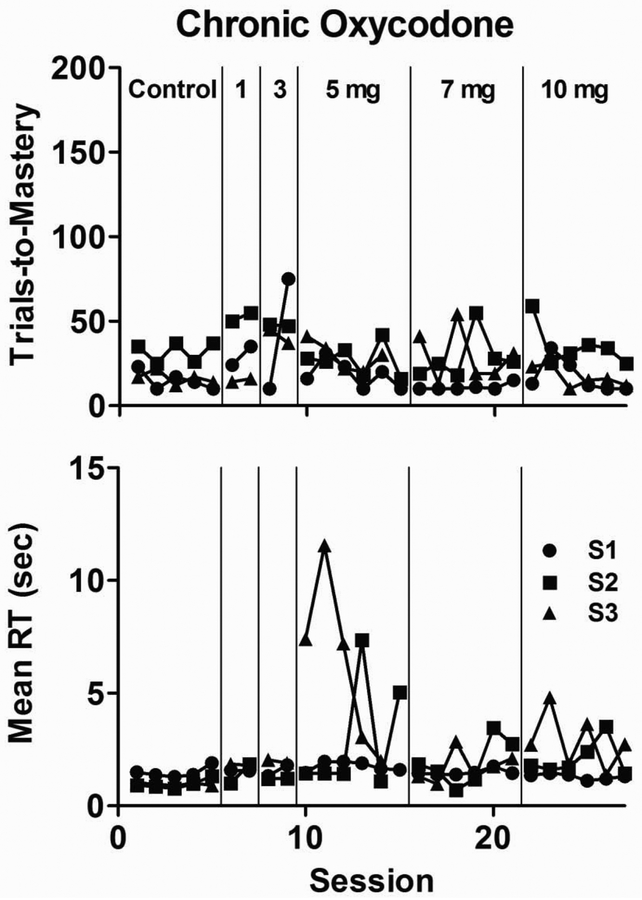
Despite high levels of oxycodone intake that led to disruptions in reaction time, no observable signs of opioid withdrawal were noted in any subject—either before self-administration sessions or during other times of the day. To further evaluate the effects of non-contingent chronic oxycodone delivery on repeated discrimination learning, s.c. pumps were implanted to deliver oxycodone continuously. As shown in Figure 3, trials-to-mastery remained, with few exceptions, similar to control values observed during the 5 sessions that immediately preceded chronic administration for all subjects during the period in which oxycodone dosage was incrementally increased from 1 to 10 mg/kg/day. Mean reaction time data also approximated control values for all subjects until oxycodone intake was increased to 5 mg/kg/day; markedly slowed reaction times were evident during select sessions at this dose in 2 of 3 subjects, but improved when daily intake of oxycodone was increased further to 7 and 10 mg/kg/day. After oxycodone intake was increased to 10 mg/kg/day, all subjects were observed to be noticeably less active in their home cage, and each lost approximately 10% of their body weight. These observations precluded further increases in daily oxycodone administration.
Figure 3.
Number of trials-to-mastery (top panel) and mean reaction time (bottom panel) during the 5 sessions that immediately preceded chronic exposure (Control) and escalating non-contingent chronic oxycodone treatment from 1–10 mg/kg/day.
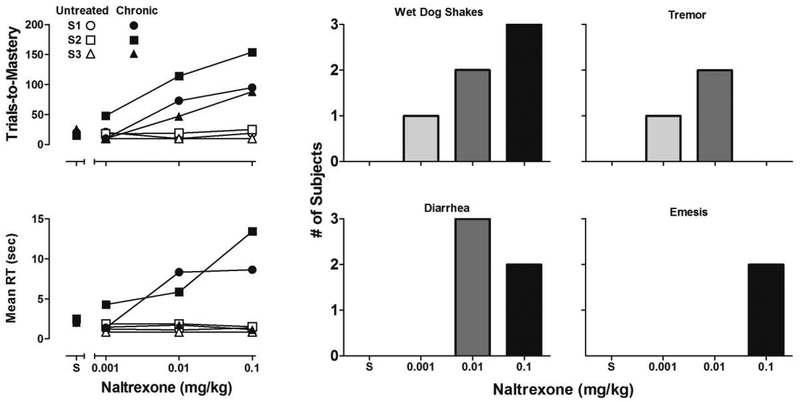
Following 10 consecutive days of continuous treatment with 10 mg/kg/day oxycodone, acute naltrexone tests on repeated acquisition performance were conducted. As the top-left panel in Figure 4 shows, naltrexone administration during chronic oxycodone exposure (filled symbols) produced dose-related increases in trials-to-mastery in all subjects at doses that had no effect in the same subjects when not chronically treated with oxycodone (unfilled symbols). As the bottom-left panel in Figure 4 indicates, acute naltrexone administration also produced similar dose-related increases in mean reaction time in 2 of 3 subjects during chronic oxycodone exposure but not when subjects were not chronically treated. As shown in the Figure 4 bar graphs, observable signs of withdrawal were also documented during the 10-min observation periods that immediately followed acute administration of naltrexone. The number of subjects that exhibited wet dog shakes increased in a dose-dependent manner. Tremor was observed in 1 subject following administration of 0.001 mg/kg and in 2 subjects following 0.01 mg/kg, but not following 0.1 mg/kg naltrexone. Diarrhea was not observed following administration of 0.001 mg/kg but was observed in all subjects following 0.01 mg/kg and in 2 subjects following 0.1 mg/kg naltrexone. Emesis was observed in 2 subjects following administration of 0.1 mg/kg naltrexone.
Figure 4.
Number of trials-to-mastery (top-left panel) and mean reaction time (bottom-left panel) during saline (S) and acute naltrexone tests in subjects chronically exposed to 10 mg/kg/day oxycodone (filled symbols) and in the same subjects untreated with oxycodone for 30 days (unfilled symbols). Bar graphs depict the number of subjects to exhibit withdrawal signs during the 10-min observation periods that immediately followed each acute administration of naltrexone.
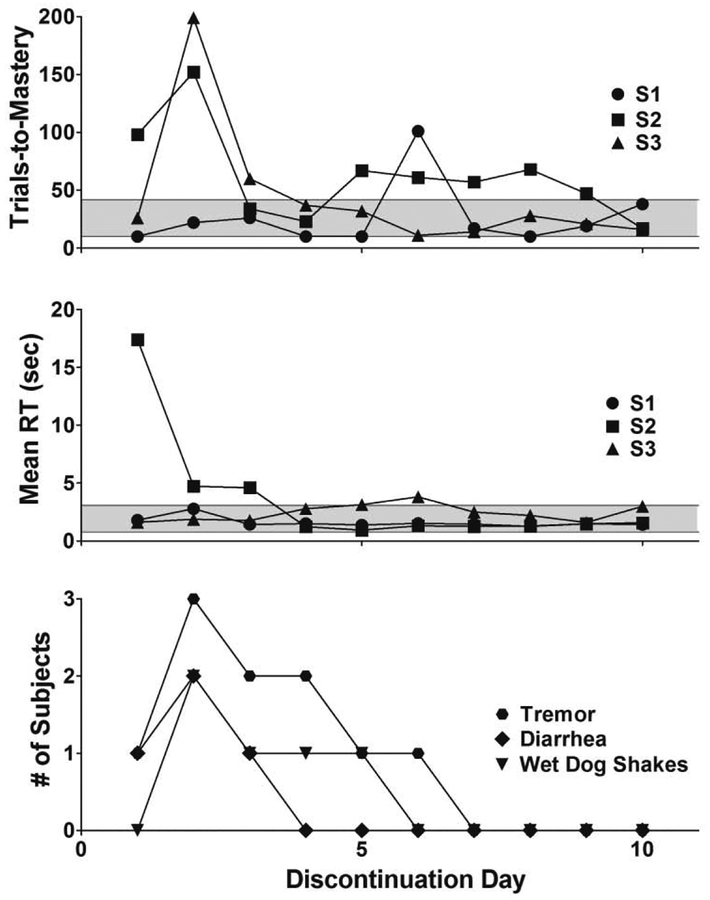
Figure 5 presents the effects of abrupt oxycodone discontinuation on trials-to-mastery (top panel), mean reaction time (middle panel), and observable signs of withdrawal (bottom panel) during the 10 consecutive days that followed drug pump deactivation. The gray area in the top and middle panel represents the range of each measure across individual subjects during the5 sessions prior to oxycodone discontinuation. Trials-to-mastery during the session conducted 24 hrs following oxycodone discontinuation remained within the previously observed range for 2 of 3 subjects. The largest disruption in trials-to-mastery was observed during the discrimination learning session conducted 48-hrs following drug discontinuation – S1 mastered the discrimination within the range of control values (22 trials-to-mastery), whereas S2 and S3 took a considerably greater number of trials-to-mastery (152 and 199 trials). S3’s discriminative performance returned to levels within the previously observed range on discontinuation day 4 and, excepting discontinuation day 6, S1’s discriminative performance remained within control range throughout the discontinuation condition. However, discriminative performance for S2 did not return to previously observed control range until discontinuation day 10. Perturbation of mean reaction time after oxycodone discontinuation followed a somewhat different time course than observed for trials-to-mastery. The largest increase was evident 24 hrs following drug pump deactivation in S2, and these increases remained outside of control ranges for 3 days. Mean reaction time measures for S1 and S3 remained unperturbed and largely within previously observed control ranges throughout the discontinuation condition. Observable signs including tremor, wet dog shakes, and diarrhea also were observed following discontinuation, with a time course that was similar to that noted above for the disruption in trials-to-mastery. Thus, withdrawal sign counts across individual subjects reached a peak during day 2 of oxycodone discontinuation and then decreased daily through discontinuation day 6, after which signs of withdrawal were no longer evident in any subject.
Figure 5.
Number of trials-to-mastery (top panel), mean reaction time (middle panel), and number of subjects to exhibit signs on withdrawal during 60-min observation periods preceding each repeated acquisition session (bottom panel) during the 10 days after oxycodone discontinuation. The gray area in the top and middle panels represent the range of trials-to-mastery and response time across subjects during the 5 sessions prior to oxycodone discontinuation.
4. Discussion
The present studies were conducted to systematically examine effects of oxycodone on discrimination learning in nonhuman primates during periods of opioid self-administration, dependence, and withdrawal. Oxycodone served as a positive reinforcer and, as shown before for self-administered drugs under fixed-ratio schedules, yielded an inverted U-shaped function relating unit dose to the number of infusions per session. These findings are consistent with previous studies of oxycodone self-administration in nonhuman primates and rodents (e.g., Altschuler et al., 2015; Beardsley et al., 2004; Mavrikaki et al., 2017; Withey et al., 2019). Increasing self-administration session length from 1 to 2 to 3 hr produced step-wise increases in daily intake and the parameters that were chosen for daily oxycodone self-administration during the examination of discrimination learning (i.e., 3-hr sessions of 0.1 mg/kg/inj) yielded total daily intakes (>2 mg/kg) comparable to those reported in a study of patients dependent on OxyContin® (2.63 mg/kg; see Hays, 2004).
Although the number of trials to master initial discriminations following oxycodone self-administration sessions was greater, on average, than for drug-free comparison subjects, differences in the development of repeated acquisition performance across individual subjects were temporary and small. These findings contrast with those from previous studies in nonhuman primates that self-administered or received experimenter-administered methamphetamine, Δ9-tetrahydrocannabinol, or cocaine. In those studies, repeated acquisition performance was significantly retarded (Kangas and Bergman, 2016; Kangas et al., 2016; 2019), indicating that the repeated acquisition task can be highly sensitive to the disruptive effects of abused drugs on learning processes. From this perspective, the small effects on the development of discrimination learning in the present studies are highly notable and suggest that self-administered opioids may not greatly disturb learning processes. The lack of persistent effects of oxycodone on learning here are not attributable to insufficient doses as oxycodone-induced disruptions in reaction time, while variable among subjects, were pronounced and persistent—likely reflecting the well-documented sedative and stuporific effects of opioid agonists (Beardsley et al., 2004; Dykstra et al., 1987; Jungquist et al., 2017; Withey et al., 2018). Indeed, the ability of other μ-opioids such as heroin and methadone to dose-dependently reduce response rates without affecting accuracy has been observed previously in studies of response chain acquisition in monkeys (Moerschbaecher et al., 1983).
Despite differences in onset (immediate vs. gradual), similar observable signs of opioid withdrawal were precipitated by naltrexone administration or emerged following abrupt discontinuation of oxycodone treatment. Presumably, these observable signs reflect, respectively, autonomic effects of immediate displacement of oxycodone from its target receptors and time-dependent reduction in occupation of μ-opioid receptors following agonist discontinuation. Unconditioned signs of opioid withdrawal were correlated with disruptions in the acquisition of discrimination learning. The mechanism responsible for the disruption in this learning set was not explicitly assessed in the current experiments but could be a result of changes in basic motoric or higher-level cognitive processes. Interestingly, the dose-related effects of naltrexone on trials-to-mastery, mean reaction time, and unconditioned signs of withdrawal were correlated during antagonist-precipitated withdrawal. However, following abrupt oxycodone discontinuation, perturbations in trials-to-mastery and reaction time differed in time course, suggesting that the effects of withdrawal on learning and motoric performance can occur independently. The present findings are consistent with a previous study in nonhuman primates showing that unconditioned opioid withdrawal signs (e.g., tremors, diarrhea, emesis) also typically peak within 1–3 days following discontinuation of chronic exposure to the μ-opioid agonist morphine (Becker et al., 2008). Moreover, in the present studies, the observable autonomic and cognition-related effects of withdrawal following oxycodone discontinuation had largely subsided by day 6, a time course that is consistent with data from previous preclinical studies of morphine withdrawal in nonhuman primates (Becker et al., 2008; Brandt and France, 1998). Interestingly, these data support the view that individuals should be drug-free for at least 7 days before starting naltrexone treatment protocols (e.g., Vivitrol®; Sigmon et al., 2012), so as to fully avoid any cognitive disruption associated with naltrexone-precipitated withdrawal.
In conclusion, although motoric behavior was disrupted following high-intake oxycodone self-administration sessions, the development of discrimination learning was not. Similarly, the development of oxycodone dependence via chronic exposure had no persistent effects on discrimination learning. However, withdrawal, either by naltrexone precipitation or abrupt oxycodone discontinuation, provoked considerable disruption of discrimination learning that was highly correlated with observable autonomic signs of opioid withdrawal. It is noteworthy that previous studies investigating the effects of psychoactive drugs in nonhuman primates (Kangas et al., 2016) and humans (Zacny and Gutierrez, 2003) have demonstrated varying degrees of cognitive disruption depending on the chosen task and underlying neural process, thus suggesting that the degree of cognitive impairment produced by oxycodone may vary with task selection. Therefore, it will be important for future preclinical studies to examine whether these findings extend to other types of cognition-related behavior (e.g., attention, short-term memory, cognitive flexibility). In the meantime, these studies provide confirmation that disruption of learning processes can accompany the well-documented autonomic signs of opioid withdrawal.
Highlights.
Discrimination learning was assessed during three conditions of opioid exposure.
Learning was not affected by self-administered oxycodone.
Learning was impaired during withdrawal via naltrexone and abrupt discontinuation.
Impairment of learning was concordant with autonomic signs of opioid withdrawal.
Acknowledgements
The authors thank Roger Spealman for comments on a previous version of this manuscript.
Role of Funding Source
This research was supported by grant K01-DA035974 (BDK) from the National Institute on Drug Abuse. The funding source had no involvement beyond financial support of this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
No conflict declared.
REFERENCES
- Ahmad FB, Rossen LM, Spencer MR, Warner M, Sutton P, 2018. Provisional drug overdose death counts. National Center for Health Statistics. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm. [Google Scholar]
- Altschuler JH, Niikura K, Butelman E, Kreek MJ, Zhang Y, 2015. Adolescent oxycodone self administration alters subsequent antinociceptive effect of oxycodone in C57BL/6J mice in adulthood. Drug Alcohol Depend. 156, E6. [Google Scholar]
- Beardsley P, Aceto M, Cook C, Bowman E, Newman J, Harris L, 2004. Discriminative Stimulus, Reinforcing, Physical Dependence, and Antinociceptive Effects of Oxycodone in Mice, Rats, and Rhesus Monkeys. Exp Clin Psychopharmacol. 12, 163–172. [DOI] [PubMed] [Google Scholar]
- Becker GL, Gerak LR, Koek W, France CP, 2008. Antagonist-precipitated and discontinuation-induced withdrawal in morphine-dependent rhesus monkeys. Psychopharmacology 201, 373–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt M, France C, 1998. Chronic l-alpha acetylmethadol in rhesus monkeys: Discriminative stimulus and other behavioral measures of dependence and withdrawal. J Pharmacol Exp Ther. 287, 1029–1037. [PubMed] [Google Scholar]
- Davis P, Liddiard H, McMillan T, 2002. Neuropsychological deficits and opiate abuse. Drug Alcohol Depend. 67, 105–108. [DOI] [PubMed] [Google Scholar]
- Dworkin SI, Mirkis S, Smith JE, 1995. Response-dependent versus response-independent presentation of cocaine: differences in the lethal effects of the drug. Psychopharmacology 1995, 117, 262–266. [DOI] [PubMed] [Google Scholar]
- Dykstra L, Gmerek D, Winger G, Woods J, 1987. Kappa opioids in rhesus monkeys. I. Diuresis, sedation, analgesia and discriminative stimulus effects. J Pharmacol Exp Ther. 242, 413–420. [PubMed] [Google Scholar]
- Gerra G, Calbiani B, Zaimovic A, Sartori R, Ugolotti G, Ippolito L, Delsignore R, Rustichelli P, Fontanesi B, 1998. Regional cerebral blood flow and comorbid diagnosis in abstinent opioid addicts. Psychiatry Res. 83,117–126. [DOI] [PubMed] [Google Scholar]
- Harlow HF, 1949. The formation of learning sets. Psychol Rev. 56, 51–65. [DOI] [PubMed] [Google Scholar]
- Hays L, 2004. A Profile of OxyContin Addiction. J Addict Dis. 23, 1–9. [DOI] [PubMed] [Google Scholar]
- Herd JA, Morse WH, Kelleher RT, Jones LG, 1969. Arterial hypertension in the squirrel monkey during behavioral experiments. Am J Physiol 217, 24–29. [DOI] [PubMed] [Google Scholar]
- Jungquist CR, Smith KW, Nicely KC, Polomano R, 2017. Monitoring Hospitalized Adult Patients for Opioid-Induced Sedation and Respiratory Depression. Am J Nurs. 117(3 Suppl 1), S27–S35. [DOI] [PubMed] [Google Scholar]
- Kamboj SK, Tookman AH, Jones L, Curran V, 2005. The effects of immediate-release morphine on cognitive functioning in patients receiving chronic opioid therapy in palliative care. Pain 117, 388–395. [DOI] [PubMed] [Google Scholar]
- Kangas BD, Bergman J, 2012. A novel touch-sensitive apparatus for behavioral studies in unrestrained squirrel monkeys. J. Neurosci. Meth 209, 331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kangas BD, Bergman J, 2014. Repeated acquisition and discrimination reversal in the squirrel monkey (Saimiri sciureus). Anim. Cogn 17, 221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kangas BD, Bergman J, 2016. Effects of self-administered methamphetamine on discrimination learning and reversal in nonhuman primates. Psychopharmacology 233, 373–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kangas BD, Bergman J, 2017. Touchscreen technology in the study of cognition-related behavior. Behav Pharmacol. 28, 623–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kangas BD, Doyle RD, Kohut SJ, Bergman J, Kaufman MJ, 2019. Effects of chronic cocaine self-administration and N-acetylcysteine on learning, cognitive flexibility, and reinstatement in nonhuman primates. Psychopharmacology 236, 2143–2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kangas BD, Leonard MZ, Shukla V, Alapafuja S, Nikas S, Makriyannis A, Bergman J, 2016. Comparisons of Δ9-Tetrahydrocannabinol and Anandamide on a Battery of Cognition-Related Behavior in Nonhuman Primates. J Pharmacol Exp Ther. 357, 125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TMC, Pau CWH, 2002. Impulse control differences between abstinent heroin users and matched controls. Brain Inj. 16, 885–889. [DOI] [PubMed] [Google Scholar]
- Mavrikaki M, Pravetoni M, Page S, Potter D, Chartoff E, 2017. Oxycodone self-administration in male and female rats. Psychopharmacology 234, 977–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintzer MZ, Copersino ML, Stitzer ML, 2005. Opioid abuse and cognitive performance. Drug Alcohol Depend. 78, 225–230. [DOI] [PubMed] [Google Scholar]
- Moerschbaecher JM, Thompson DM, Winsauer PJ, 1983. Effects of heroin, methadone, LAAM and cyclazocine on acquisition and performance of response sequences in monkeys. Pharmacol Biochem Behav. 19,701–710. [DOI] [PubMed] [Google Scholar]
- National Research Council, 2011. Guide for the care and use of laboratory animals: eighth edition National Academy Press, Washington DC. [Google Scholar]
- Ornstein TJ, Iddon JL, Baldacchino AM, Sahakian BJ, London M, Everitt BJ, et al. , 2000. Profiles of cognitive dysfunction in chronic amphetamine and heroin abusers. Neuropsychopharmacology 23, 113–126. [DOI] [PubMed] [Google Scholar]
- Pau CWH, Tatia MC, Lee TMC, Chan SF, 2002. The impact of heroin on frontal executive functions. Arch Clin Neuropsychol. 17, 663–670. [PubMed] [Google Scholar]
- Rapeli P, Kivisaari R, Autti T, Kähkönen S, Puuskari V, Jokela O, Kalska H, 2006. Cognitive function during early abstinence from opioid dependence: A comparison to age, gender, and verbal intelligence matched controls. BMC Psychiatry 6, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RD, Everitt BJ, Baldacchino A, Blackshaw AJ, Swainson R, Wynne K, et al. , 1999. Dissociable deficits in the decision-making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan-depleted normal volunteers: evidence for monoaminergic mechanisms. Neuropsychopharmacology 20, 322–339. [DOI] [PubMed] [Google Scholar]
- Sigmon SC, Bisaga A, Nunes EV, O’Connor PG, Kosten T, Woody G, 2012. Opioid Detoxification and Naltrexone Induction Strategies: Recommendations for Clinical Practice. Am J Drug Alcohol Abuse, 38, 187–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjøgren P, Christrup LL, Petersen MA, Højsted J, 2005. Neuropsychological assessment of chronic non-malignant pain patients treated in a multidisciplinary pain centre. European J Pain 9, 453–462. [DOI] [PubMed] [Google Scholar]
- Strang J, Gurling H, 1989. Computerized-Tomography and Neuropsychological Assessment in Long-Term High-Dose Heroin-Addicts. Brit. J. Addict 84, 1011–1019. [DOI] [PubMed] [Google Scholar]
- Withey SL, Paronis CA, Bergman J, 2018. Concurrent Assessment of the Antinociceptive and Behaviorally Disruptive Effects of Opioids in Squirrel Monkeys. J Pain 19, 728–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withey SL, Spealman RD, Bergman J, Paronis CA, 2019. Behavioral effects of Opioid Full and Partial Agonists During Chronic Buprenorphine Treatment. J Pharmacol Exp Ther 371, 544–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacny JP, 1995. A review of the effects of opioids on psychomotor and cognitive functioning in humans. Exp Clin Psychopharmacol. 3, 432–466. [Google Scholar]
- Zacny JP, Gutierrez S, 2003. Characterizing the subjective, psychomotor, and physiological effects of oral oxycodone in non-drug-abusing volunteers. Psychopharmacology. 170, 242–254. [DOI] [PubMed] [Google Scholar]