Abstract
Background
Patients with smaller, single hepatocellular carcinoma (HCC) tumors and cirrhosis awaiting liver transplantation (LT) can have their tumors successfully eradicated with thermal ablation (TA). Accurate surveillance magnetic resonance imaging (MRI) reporting is critical for evaluating treatment response and tumor recurrence. The purpose of this study is to assess the validity of the Liver Imaging Reporting and Data System (LI-RADS) Treatment Response (LR-TR) criteria.
Methods
Retrospective analysis of a single-center database of patients with small HCC tumors (<3 cm in diameter) (who underwent both laparoscopic TA and LT from 2006 – 2017. Post-ablation MRI were assigned LR-TR categories (Nonviable, Equivocal, and Viable) for ablated lesions and LI-RADS categories (probable or definite HCC) for untreated lesions. Interpretations were compared to the histopathology of the explanted liver after LT.
Results
Forty-five patients with 81 tumors (59 ablated and 22 untreated), mean size 2.2 cm, were included. Twenty-three (39%) of the ablated tumors had viable HCC on histopathology. The sensitivity/specificity of LR-TR categories (Nonviable/Equivocal vs Viable) of ablated tumors is 30%/99%, with a PPV/NPV of 93%/69%. The sensitivity varies with residual tumor size. The sensitivity/specificity of LI-RADS 4 and 5 diagnostic criteria at detecting new HCC is 65%/94% with a PPV/NPV of 85%/84%. The inter-rater reliability (IRR) is high for LR-TR categories (90% agreement, Cohen’s ĸ = 0.75) and LI-RADS LR-4 and LR-5 diagnostic categories (91% agreement, Cohen’s ĸ = 0.80).
Conclusions
In patients with HCC that are <3 cm in diameter, the LR-TR criteria after TA has high IRR but low sensitivity suggesting that the LR-TR categories are precise but inaccurate. The low sensitivity may be secondary to thermal ablation’s disruption in the local blood flow of the tissue which could affect the arterial enhancement phase on MRI. Additional investigation and new technologies may be necessary to improve imaging after ablation.
Keywords: Locoregional therapy, liver ablation, liver cancer
Introduction:
Liver transplantation offers patients with cirrhosis and hepatocellular carcinoma (HCC) the best opportunity for long-term cancer-free survival.1 However, transplantation is only available for patients who meet the Milan Criteria.2–4 Locoregional therapies are used to prevent the tumor burden from progressing while the patient awaits transplantation.2,3,5 Tumor ablation with either radiofrequency (RFA) or microwave ablation (MWA) is recommended as a locoregional bridging therapy by the American Association for the Study of Liver Diseases (AASLD) and European Association for the Study of the Liver (EASL) and is particularly effective for single smaller tumors (e.g. <3 cm).3,5 Ablation reduces dropout rates from liver transplantation waitlists by safely preventing tumor progression without causing hepatic decompensation.6,7 Ablation also has higher rates of complete tumor necrosis compared to alternative strategies to control tumor growth (e.g., transcatheter arterial chemoembolization (TACE)).3,5,8–11
Following treatment with ablation, patients must undergo serial surveillance imaging with high-field magnetic resonance imaging (MRI) or contrast-enhanced computed tomography (CT) to evaluate for lesion necrosis, an indicator of successful ablation, while simultaneously looking for evidence of local or distant HCC recurrence. Accurately measuring the extent of necrosis is important to confirm clinical response or detect tumor recurrence, but classification systems have struggled with assessing post-ablation response.12 Tumor response after locoregional therapy was initially based on assessment tools such as the Response Evaluation Criteria in Solid Tumors (RECIST), which was designed to evaluate tumor response to chemotherapy.13,14 These criteria suffered from poor inter-rater reliability (IRR) and were unsatisfactory for assessing response to ablation, given that post-treatment tumor size does not adequately account for tumor necrosis.15–19 These limitations culminated in the development of the modified RECIST (mRECIST) and EASL criteria, which consider the reduction in viable tissue instead of tumor size. These tools also demonstrate better correlation with survival and have improved interrater variability.20–24 These classification systems are used as reference standards for clinical trials but have not been widely adopted in clinical practice or in transplantation policies.25
In 2011, the American College of Radiology (ACR) launched the Liver Imaging Reporting and Data System (LI-RADS) in order to standardize interpretation and reporting of untreated liver lesions in cirrhotic patients.26 This diagnostic reporting system categorizes an untreated hepatic lesion based on its benign or malignant HCC appearance ranging from 1 (definitely benign) to 5 (definitely HCC).27 In 2014, the ACR added a classification for treated nodules (LR-Treated) and in 2017, added the LI-RADS Tumor Response (LR-TR) classification system to assess the response after lesions are treated with locoregional therapy. Using this system, lesions are classified as nonviable, equivocal, or viable based on their post-treatment appearance on CT or MRI.27 While evaluating the response of HCC lesions to locoregional therapy has improved, the true sensitivity of MRI surveillance after ablation remains unclear. No studies have evaluated the accuracy of the LR-TR criteria on treated lesions in this patient population.
Accurate post-treatment surveillance of HCC is vital for patients awaiting liver transplantation since treatment response/tumor stage impacts long-term survival. Among patients who achieve complete pathologic response with locoregional therapy, HCC recurrence after transplantation is low (0–8.6% over 5 years).6,28–31 Conversely, failure to respond to therapy is associated with waitlist dropout due to tumor progression or post-transplant recurrence, and decreased post-transplant survival.29,32–35 Given the importance of accurate post-treatment surveillance and uncertain test characteristics of post-ablation cross-sectional imaging, we evaluated the diagnostic accuracy and IRR of LR-TR categories using surveillance MRI obtained after thermal ablation of small HCC (<3 cm diameter) and compared this with histopathology of the native explanted liver after transplantation as the gold standard.
Materials and Methods
Data and Population
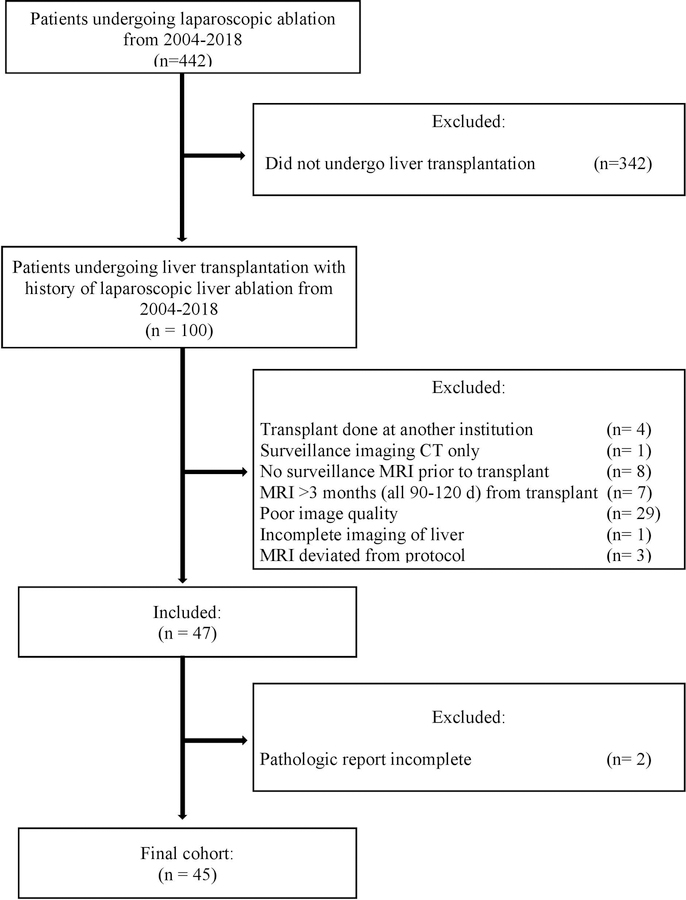
This study is a single center retrospective review of all hepatocellular carcinoma (HCC) patients undergoing liver transplantation who had previously undergone laparoscopic liver ablations between January 2006 and December 2017. Ablations during this timeframe included both RFA and MWA and were performed by a single surgeon with the assistance of laparoscopic ultrasound. Patients were included in the study if they had HCC, at least one laparoscopic hepatic ablation prior to liver transplantation, and had at least one surveillance MRI post-ablation and prior to transplantation. Patients were excluded if their transplant was performed at another institution, if they had no surveillance MRI within the 3 months prior to transplant, if the MRI was poor quality (motion artifact, greater than 5mm imaging slices, or incomplete imaging of the liver), if there were deviations from MRI protocol, or if there was no complete pathology report of the explanted liver (Figure 1). No organs from executed prisoners were used in transplantation of this cohort. Approval was obtained from the University of North Carolina Institutional Review Board.
Figure 1.
CONSORT Flow Diagram
MRI Protocol
MRIs were performed using either a 1.5 T (Avanto®/Aera®, Siemens Medical System; GE-Signa HDx®, GE Healthcare) or 3-T (Trio®/Skyra®, Siemens Medical System) MRI system with phased array torso coil used in all studies. LI-RADS is approved for use with both 1.5 and 3-T scanners as long as a phased array torso coil is used. We are not aware of any data that 3-T scanners have improved sensitivity for identifying HCC recurrence and there is no specific preference for 3-T scanners within current AASLD guidelines.3 Our standard abdominal MRI protocol included the following sequences: axial unenhanced T1-weighted dual-echo inphase/out-of-phase 2D gradient-echo sequence, coronal T2-weighted half-Fourier single-shot fast spin-echo sequence, axial T2-weighted half-Fourier single-shot fast spin-echo sequence with and without fat suppression, and axial fat-suppressed 3D gradient-echo sequences were used to perform dynamic contrast-enhanced study. The hepatobiliary phase images were acquired at 20 minutes after injection of Gadoxetate disodium and 90–150 minutes after injection of Gabobenate dimeglumine using fat-suppressed 3D gradient-echo sequences. Subtraction imaging was used when available but was not routinely performed on all MRI imaging.
The intravenous gadolinium-based contrast agents were administered at a dose of 0.1 mmol/kg using a power-injection system (Spectris Solaris® EP, Medrad, Pittsburgh, PA) at a rate of 2 mL/s followed by 20 mL saline flush.
Image Analysis
The last MRI before liver transplantation was reviewed independently by two board-certified radiologists with fellowship training in abdominal imaging. The radiologists were allowed to review prior MRIs if available but were blinded to pathologic outcome of the explanted liver. Each radiologist independently scored the lesions based on the LR-TR and LI-RADS diagnostic categories.25,26 At the time of review, both radiologists had more than 9 years experience in liver MRI interpretation and more than 5 years experience applying LI-RADS to MRI. The previously ablated tumors were assigned one of three LR-TR categories: LR-TR Nonviable (for probably or definitely not viable), LR-TR Equivocal (for equivocally viable), or LR-TR Viable (for probably or definitely viable). The LI-RADS diagnostic categories were used for non-ablated lesions seen on MRI. Only lesions that met LI-RADS criteria for LR-4 (probably HCC) or LR-5 (definitely HCC) were recorded, since these lesions would warrant further locoregional treatment.
Reference Standards
The gold standard for residual or new HCC involves histopathologic examination of the native explanted liver after liver transplantation. Each native liver underwent full histopathologic examination including gross and microscopic assessment. Pathology reports of the explanted liver were reviewed and matched with reader assignment of LR-TR or LI-RADS diagnostic categories based on Couinaud classification of hepatic segments. If no new lesions were identified on pathology, this was also noted. In cases where the lesion location was ambiguous or spanning more than one liver segment, pathology and imaging were concurrently reviewed to localize the lesion after LR-TR or LI-RADS diagnostics were assigned.
Variables and Outcomes
The main outcomes of this study are the determination of the sensitivity, specificity, and IRR of LR-TR in detecting residual viable tumor. The primary outcomes were evaluated by comparing the LR-TR categories to the histopathology of each tumor from the explanted liver after transplantation. In the initial analysis, detectable disease on MRI was defined as LR-TR Viable alone. A separate analysis was carried out defining detectable disease on MRI as LR-TR Viable or LR-TR Equivocal. The IRR was obtained by comparing the LR-TR categories assigned by each radiologist for each individual lesion.
Secondary outcomes include the sensitivity, specificity, and IRR of LI-RADS diagnostic categories at detecting untreated HCC. New disease is defined as the presence of a LI-RADS 4 or 5 lesion seen on MRI. For IRR, LI-RADS 4 and 5 categories assigned by each radiologist are treated as separate entities.
Ablation characteristics include lesion location (Couinaud classification), year of ablation, and use of imaging guidance for ablation (Pathfinder Navigation Guidance). Post-ablation tumor characteristics include viability of ablated tumor, size of residual tumor, differentiation of tumor, presence of lymphovascular invasion, and presence of perineural invasion. Additionally, the number of post-ablation surveillance MRIs obtained prior to transplant was collected.
Statistical Analysis
Overall and radiologist-specific sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy are calculated. LR-TR has three categories for classifying an ablated lesion they are: Nonviable, Equivocal, or Viable. LR-TR is initially evaluated as a dichotomous outcome, with Nonviable and Equivocal combined in the same category as not detecting residual disease versus Viable as detecting residual disease. Further analysis in this study classified LR-TR Equivocal and Viable as identification of residual disease for comparison to the initial analyses. LI-RADS diagnostic categories are evaluated as a dichotomous outcome (LR-4/5 vs no lesion identified), only tumors ≥1 cm are included in this analysis.
IRR for both the LR-TR categories (LR-TR nonviable, LR-TR equivocal, and LR-TR viable) and LI-RADS diagnostic categories (LI-RADS 4 and 5 only) are evaluated using the percent agreement and Cohen’s kappa coefficient. The kappa coefficient was assessed using the Altman benchmarked scale with a value of ≤0.20 corresponding to poor agreement, 0.21–0.40 corresponding to fair agreement, 0.41–0.60 corresponding to moderate agreement, 0.61–0.80 corresponding to good agreement, and ≥0.81 corresponding to very good agreement.
Patients’ demographics, underlying disease characteristics, and ablation characteristics are summarized using descriptive statistics. Bivariate analyses compared ablation characteristics, tumor characteristics, and post-ablation imaging follow-up based on correct classification (Correct Classification vs Incorrect Classification) of ablated or new tumor seen on liver explant. Post-ablation imaging follow-up was recorded as the number of MRIs performed after thermal ablation and prior to liver transplantation. Chi-square and Student’s t-tests are performed for categorical and continuous variables.
Logistic regression was used to evaluate the association between correct classification of both LR-TR categories and LI-RADS diagnostic categories to the number of post-ablation follow up images.
Statistical significance was set at p<0.05 and all tests were 2-sided. All statistical analyses were conducted using STATA 14.1 (StataCorp, Inc., College Station, TX).
Results
Patient population
A total of 442 patients were analyzed in the database and 45 patients met the inclusion criteria. The average age at the time of transplantation was 60 years old (SD ±6) and the majority of patients were male and non-Hispanic white (Table 1). The most common cause of cirrhosis was Hepatitis C virus. Patients had an average Child-Pugh Score of 7 (SD ±2) and MELD of 11 (SD ±4). The average time from ablation to liver transplant was 219 days (SD ±153) (Table 1) and patients had a median of 2.8 years of follow-up after LT. 22/59 patients had tumors larger than 2 cm, and the mean number of tumors per patient was 1.9 (SD ± 1.3) (Table 1).
Table 1:
Baseline Characteristics of Patients with Hepatocellular Carcinoma (HCC) who Underwent Liver Transplant between 2004–2017
| Overall, n | 45 |
|---|---|
| ŧAge, mean (SD) | 60 (6) |
| Male, n (%) | 38 (75) |
| Race/Ethnicity, n (%) | |
| NHW | 39 (77) |
| NHB | 10 (20) |
| Hispanic | 2 (4) |
| Cause of liver disease, n (%) | |
| Hepatitis C | 24 (53) |
| Hepatitis C + EtOH | 13 (29) |
| EtOH | 2 (4) |
| NASH/NAFLD | 2 (4) |
| Autoimmune Hepatitis | 1 (2) |
| MELD, mean (SD) | 11 (4) |
| Child-Pugh Score, mean (SD) | 7 (1.5) |
| Child-Pugh A, n (%) | 20 (44) |
| Child-Pugh B, n (%) | 23 (51) |
| Child-Pugh C, n (%) | 2 (4) |
| Pre-ablation AFP, median (IQR) | 21 (5–66) |
| Time from ablation to first MRI (days), mean (SD) | 34 (132) |
| Time from ablation to LT (days), mean (SD) | 219 (15) |
| Time from last pre-LT MRI to LT (days), mean (SD) | 51 (26) |
| Number of Tumors, mean (SD) | 1.9 (1.3) |
| Number of MRIs Prior to LT, mean (SD) | 2.3 (1.5) |
| 1 image, n (%) | 13 (29) |
| 2 images, n (%) | 19 (42) |
| 3 images, n (%) | 8 (18) |
| ≥ 4 images, n (%) | 5 (11) |
Age at the time of transplantation; NHW - non-Hispanic white; NHB - non-Hispanic black; EtOH - alcohol; NASH/NAFLD - non-alcoholic steatohepatitis/non-alcoholic fatty liver disease; MELD - Model for End-Stage Liver Disease; AFP - alpha fetoprotein; LT - liver transplant; SD - standard deviation; MRI - magnetic resonance imaging
A total of 81 tumors were identified on explanted liver histopathology, including 59 previously ablated tumors and 22 untreated tumors. Of the 59 ablated tumors, 23 (39%) had viable HCC on final pathology, with an average viable tumor size of 2.2 cm (SD ±1.4). Among the 23 tumors with viable HCC on final histopathology, the mean time from ablation to LT was 209 days (SD ±124), mean time until first post-treatment MRI was 35 days (SD ±18) and 22 of the tumors (95.7%) received their first post-treatment surveillance within 60 days. Of these 23 tumors, three had radiographic evidence of local recurrence. One had first surveillance imaging at 20 days post-ablation and went on to receive TACE. The second had first surveillance imaging at 109 days post-ablation and went on to receive LT four months after recurrence. The third had no evidence of residual disease on first surveillance imaging (27 days post-ablation), but had recurrence noted on a subsequent MRI (118 days post-ablation) and went on to receive LT one month after recurrence.
The majority of the ablations were performed in segments 7 and 8 (Table 2). The average size of untreated HCC is 1.3 cm (SD ±0.5), with the majority of these lesions located in segments 7 and 8 of the explanted liver (Table 3). Only three patients (7%) in this study were outside of Milan criteria based on final histopathology of their explanted liver and four patients (9%) developed an HCC recurrence, occurring on average 320 days after transplantation (range 146–684 days). Two of the 4 patients who had post-transplant recurrence of HCC were outside of Milan criteria.
Table 2:
Characteristics of Correctly Identified Ablation Site
| All N=59 |
Correctly Class. n=44 |
Incorrect Class. n=15 |
p-value | |
|---|---|---|---|---|
| Viable, n (%) | 23 (39) | 8 (18) | 15 (100) | <0.001 |
| Non-viable, n (%) | 36 (61) | 36 (82) | 0 | |
| ŧLocation | ||||
| II/III | 12 (20) | 10 (23) | 2 (13) | 0.31 |
| IVA/IVB | 5 (9) | 3 (7) | 2 (13) | |
| V/VI | 16 (27) | 14 (32) | 2 (13) | |
| VII/VIII | 26 (44) | 17 (39) | 9 (60) | |
| Year of Ablation, n (%) | ||||
| 2006–2010 | 12 (20.3) | 7 (15.91) | 5 (33.3) | 0.35 |
| 2011–2013 | 29 (49.2) | 23 (52.3) | 6 (40.0) | |
| 2014–2017 | 18 (30.5) | 14 (31.8) | 4 (26.7) | |
| Type of Ablation | ||||
| RFA | 4 (7) | 3 (7) | 1 (7) | 0.98 |
| MWA | 55 (93) | 41 (93) | 14 (93) | |
| Pathfinder | ||||
| Yes | 33 (56) | 26 (59) | 7 (47) | 0.40 |
| No | 26 (44) | 18 (41) | 8 (53) | |
| Number of MRIs Prior to LT, mean (SD) | 2.3 (1.5) | 2.1 (0.6) | 1.5 (0.5) | <0.01 |
| 1 image, n (%) | 13 (29) | 6 (14) | 7 (47) | 0.02 |
| 2 images, n (%) | 19 (42) | 16 (36) | 6 (40) | |
| 3 images, n (%) | 8 (18) | 14 (32) | 2 (13) | |
| ≥ 4 images, n (%) | 5 (11) | 8 (18) | 0 | |
| Size, mean (SD) | 2.2 (1.4) | 2.4 (0.3) | 2.1 (1.7) | 0.58 |
| <1 cm, n (%) | 5 (22) | 0 | 5 (33) | 0.09 |
| 1 cm - 2 cm, n(%) | 7 (30) | 3 (38) | 4 (27) | ref. |
| > 2 cm, n (%) | 11 (48) | 5 (62) | 6 (40) | 0.91 |
| Differentiation | ||||
| Well, n (%) | 3 (13) | 0 | 3 (20) | 0.38 |
| Moderate, n (%) | 18 (78) | 7 (87) | 11 (73) | |
| Poor, n (%) | 2 (9) | 1 (13) | 1 (7) | |
| LVI, n (%) | 3 (13) | 1 (13) | 2 (13) | 0.96 |
| PNI, n (%) | 0 | 0 | 0 | - |
Location is based on Couinaud classification of hepatic segments; RFA - radiofrequency ablation; MWA - microwave ablation; Pathfinder - Pathfinder Navigation Guidance; LT - Liver transplant; SD - standard deviation; LVI - lymphovascular invasion; PNI - perineural invasion
Table 3:
Characteristics of Correctly Identified New HCC
| All N=55 |
Correctly Class. n= 45 |
Incorrect Class. n= 10 |
p-value | |
|---|---|---|---|---|
| New HCC, n (%) | 22 (40) | 13 (29) | 9 (90) | <0.001 |
| No HCC, n (%) | 33 (60) | 32 (71) | 1 (10) | |
| ŧLocation, n (%) | ||||
| I | 1 (5) | 0 | 1 (11) | 0.32 |
| II/III | 4 (18) | 1 (8) | 3 (33) | |
| IVA/IVB | 2 (9) | 1 (8) | 1 (11) | |
| V/VI | 4 (18) | 3 (23) | 1 (11) | |
| VII/VIII | 11 (50) | 8 (62) | 3 (33) | |
| Year of Ablation, n (%) | ||||
| 2006–2010 | 15 (27) | 13 (29) | 2 (20) | 0.35 |
| 2011–2013 | 23 (42) | 20 (44) | 3 (30) | |
| 2014–2017 | 17 (31) | 12 (27) | 5 (50) | |
| Number of MRIs Prior to LT, mean (SD) | 2.2 (1.5) | 2.4 (1.5) | 1.6 (0.7) | 0.14 |
| 1 image, n (%) | 18 (33) | 13 (29) | 5 (50) | 0.44 |
| 2 images, n (%) | 22 (40) | 18 (40) | 4 (40) | |
| 3 images, n (%) | 10 (18) | 9 (20) | 1 (10) | |
| ≥ 4 images, n (%) | 5 (9) | 5 (11) | 0 | |
| Size, mean (SD) | 1.3 (0.5) | 1.4 (0.1) | 1.1 (0.4) | 0.22 |
| <1 cm, n (%) | 5 (23) | 2 (15) | 3 (33) | 0.47 |
| 1 cm - 2 cm, n(%) | 16 (73) | 10 (77) | 6 (67) | |
| > 2 cm, n (%) | 1 (5) | 1 (8) | 0 | |
| Differentiation | ||||
| Well, n (%) | 1 (5) | 0 | 1 (11) | 0.22 |
| Moderate, n (%) | 21 (95) | 13 (100) | 8 (89) | |
| Poor, n (%) | 0 | 0 | 0 | |
| LVI, n (%) | 3 (14) | 2 (15) | 1 (11) | 0.77 |
| PNI, n (%) | 0 | 0 | 0 | - |
Class. - Classification;
Location is based on Couinaud classification of hepatic segments; SD - Standard Deviation; LVI - lymphovascular invasion; PNI - perineural invasion
Accuracy of LI-RADS
A total of 12 patients had LR-viable or equivocal lesions on their last pre-transplant MRI and 33 had LR-nonviable lesions. For LR-viable/equivocal lesions, the mean time from ablation to pre-LT MRI was 156 days (SD ±142) and mean time between last MRI to LT was 58 days (SD ±29). For LR-nonviable lesions the mean times from ablation to pre-LT MRI and last MRI to LT were 171 days (SD ±158) and 50 days (SD ±27), respectively.
The overall sensitivity and specificity of LR-TR categories (Nonviable/Equivocal vs Viable) of ablated tumors is 30% and 99% respectively, with PPV and NPV of 93% and 69% respectively (Table 4). The sensitivity of LR-TR varied substantially by size- it was 0% for residual tumor size <1 cm, 36% for residual tumor 1–2 cm, and 41% for residual tumor >2 cm (Table 4). The PPV and NPV is 0% and 88% for residual tumor size <1 cm, 83% and 89% for size 1–2 cm, and 90% and 85% for size >2 cm (Table 4).
Table 4:
Detection of Residual HCC after Thermal Ablation using LI-RADS Treatment Response Categories (LR-TR)
| Observer 1 | Observer 2 | Combined | |
|---|---|---|---|
|
All Ablated Lesions (23/59 viable) | |||
| Sensitivity | 30% (7/23) | 30% (7/23) | 30% |
| Specificity | 97% (35/36) | 100% (36/36) | 99% |
| PPV | 88% (7/8) | 100% (7/7) | 93% |
| NPV | 69% (35/51) | 69% (36/52) | 69% |
| Accuracy | 71% (42/59) | 73% (43/59) | 72% |
|
Lesion < 1 cm (5/41 viable) | |||
| Sensitivity | 0% (0/5) | 0% (0/5) | 0% |
| Specificity | 97% (35/36) | 100% (36/36) | 99% |
| PPV | 0% (0/1) | 0% (0/0) | 0% |
| NPV | 88% (35/40) | 88% (36/41) | 88% |
| Accuracy | 85% (35/41) | 88% (36/41) | 87% |
|
Lesion 1–2 cm (7/43 viable) | |||
| Sensitivity | 29% (2/7) | 43% (3/7) | 36% |
| Specificity | 97% (35/36) | 100% (36/36) | 99% |
| PPV | 67% (2/3) | 100% (3/3) | 83% |
| NPV | 88% (35/40) | 90% (36/40) | 89% |
| Accuracy | 86% (37/43) | 91% (39/43) | 88% |
|
Lesion > 2 cm (11/47 viable) | |||
| Sensitivity | 46% (5/11) | 36% (4/11) | 41% |
| Specificity | 97% (35/36) | 100% (36/36) | 99% |
| PPV | 83% (5/6) | 100% (4/4) | 90% |
| NPV | 85% (35/41) | 84% (36/43) | 85% |
| Accuracy | 85% (40/47) | 85% (40/47) | 85% |
LI-RADS equivocal was treated as no residual tumor identified on MRI; PPV - positive predictive value; NPV - negative predictive value
After adjusting the cutoff point for detection of residual tumor to include LR-TR Equivocal with LR-TR Viable, the overall sensitivity and specificity is 44% and 86% respectively, with PPV and NPV of 67% and 71%, respectively (Table 6). The sensitivity is 20% for residual tumor <1 cm, 43% for residual tumor 1–2 cm, and 55% for residual tumor >2 cm. (Table 4). The PPV and NPV were 17% and 89% for residual tumor size <1 cm, 38% and 89% for size 1–2 cm, and 55% and 86% for size >2 cm (Table 6).
Table 6:
Detection of New HCC using LI-RADS diagnosis categories
| Observer 1 | Observer 2 | Combined | |
|---|---|---|---|
|
Lesions ≥ 1cm (17/49 new) | |||
| Sensitivity | 65% (11/17) | 59% (10/17) | 62% |
| Specificity | 94% (31/33) | 97% (32/33) | 96% |
| PPV | 85% (11/13) | 91% (10/11) | 88% |
| NPV | 84% (31/37) | 82% (32/39) | 83% |
| Accuracy | 84% (42/50) | 84% (42/50) | 84% |
|
Lesion 1–2 cm (16/49 new) | |||
| Sensitivity | 63% (10/16) | 56% (9/16) | 59% |
| Specificity | 94% (31/33) | 97% (32/33) | 96% |
| PPV | 83% (10/12) | 90% (9/10) | 86% |
| NPV | 84% (31/37) | 82% (32/39) | 83% |
| Accuracy | 84% (41/49) | 84% (41/49) | 84% |
|
Lesion > 2 cm (1/31 new) | |||
| Sensitivity | 100% (1/1) | 100% (1/1) | 100% |
| Specificity | 94% (31/33) | 97% (32/33) | 96% |
| PPV | 33% (1/3) | 50% (1/2) | 40% |
| NPV | 100% (31/31) | 100% (32/32) | 100% |
| Accuracy | 94% (32/34) | 97% (33/34) | 96% |
LI-RADS 4/5 were treated as new tumor identified on MRI; PPV - positive predictive value; NPV - negative predictive value;
The overall sensitivity and specificity of LI-RADS 4 and 5 diagnostic criteria at detecting new HCC is 65% and 94% respectively, with a PPV of 85% and NPV of 84% (Table 5). The sensitivity for new tumors 1–2 cm is 63%, and >2 cm is 100% (Table 5). The PPV and NPV are 83% and 84% for residual tumor size 1–2 cm and 33% and 100% for size >2 cm (Table 5).
Table 5:
Detection of Residual HCC after Thermal Ablation using LR-TR categories with Variation in Cutoff Points
| LR-TR Equivocal + Viable | LR-TR Viable Only | |||||
|---|---|---|---|---|---|---|
| Obs. 1 | Obs. 2 | Combined | Obs. 1 | Obs. 2 | Combined | |
|
All Ablated Lesions (23/59 viable) | ||||||
| Sensitivity | 44% (10/23) | 44% (10/23) | 44% | 30% (7/23) | 30% (7/23) | 30% |
| Specificity | 86% (31/36) | 86% (31/36) | 86% | 97% (35/36) | 100% (36/36) | 99% |
| PPV | 67% (10/15) | 67% (10/15) | 67% | 88% (7/8) | 100% (7/7) | 93% |
| NPV | 71% (31/44) | 71% (31/44) | 71% | 69% (35/51) | 69% (36/52) | 69% |
| Accuracy | 70% (41/59) | 70% (41/59) | 70% | 71% (42/59) | 73% (43/59) | 72% |
|
Lesion < 1 cm (5/41 viable) | ||||||
| Sensitivity | 20% (1/5) | 20% (1/5) | 20% | 0% (0/5) | 0% (0/5) | 0.0% |
| Specificity | 86% (31/36) | 86% (31/36) | 86% | 97% (35/36) | 100% (36/36) | 99% |
| PPV | 17% (1/6) | 17% (1/6) | 17% | 0% (0/1) | 0% (0/0) | 0% |
| NPV | 87% (31/35) | 89% (31/35) | 89% | 88% (35/40) | 88% (36/41) | 88% |
| Accuracy | 78% (32/41) | 78% (32/41) | 78% | 85% (35/41) | 88% (36/41) | 87% |
|
Lesion 1–2 cm (7/43 viable) | ||||||
| Sensitivity | 43% (3/7) | 43% (3/7) | 43% | 29% (2/7) | 43% (3/7) | 36% |
| Specificity | 86% (31/36) | 86% (31/36) | 86% | 97% (35/36) | 100% (36/36) | 99% |
| PPV | 38% (3/8) | 38% (3/8) | 38% | 67% (2/3) | 100% (3/3) | 83% |
| NPV | 89% (31/35) | 89% (31/35) | 89% | 88% (35/40) | 90% (36/40) | 89% |
| Accuracy | 79% (34/43) | 79% (34/43) | 79% | 86% (37/43) | 91% (39/43) | 88% |
|
Lesion > 2 cm (11/47 viable) | ||||||
| Sensitivity | 55% (6/11) | 55% (6/11) | 55% | 46% (5/11) | 36% (4/11) | 41% |
| Specificity | 86% (31/36) | 86% (31/36) | 86% | 97% (35/36) | 100% (36/36) | 99% |
| PPV | 55% (6/11) | 55% (6/11) | 55% | 83% (5/6) | 100% (4/4) | 90% |
| NPV | 86% (31/36) | 86% (31/36) | 86% | 85% (35/41) | 84% (36/43) | 85% |
| Accuracy | 79% (37/47) | 79% (37/47) | 79% | 85% (40/47) | 85% (40/47) | 85% |
LI-RADS Equivocal was treated as detecting residual tumor identified on MRI; Obs. – Observer; PPV - positive predictive value; NPV - negative predictive value;
Interrater reliability (IRR)
The IRR for LR-TR categories (Nonviable, Equivocal, and Viable) was good, with 90% agreement and Cohen’s kappa coefficient of 0.75 (SE ± 0.09). For the LI-RADS diagnostic categories (LR-4 and LR-5) the IRR was also good, with a 91% agreement and Cohen’s kappa coefficient of 0.80 (SE ± 0.08). The combined IRR for both LI-RADS diagnostic and treatment response categories had a 90% agreement and Cohen’s kappa coefficient of 0.87 (SE ± 0.04).
Number of surveillance MRIs and correct diagnosing
Having 3 or more surveillance MRIs performed between the time of ablation and time of transplantation is significantly associated with a higher odds of correct classification by LR-TR, compared to having only 1 MRI (OR 12.8, 95% CI 2.1, 78.6, p<0.01) (Table 7). No association is seen between the number of surveillance MRIs and correct identification of new lesions using the LI-RADS diagnostic categories, p=0.15 (Table 7).
Table 7.
Odds Ratios for correct LI-RADS categorization of ablated tumors
| LR-TR categories | LI-RADS diagnostic categories | |||
|---|---|---|---|---|
| Number of MRIs Prior to LT | OR (95% CI) | p-value | OR (95% CI) | p-value |
| 1 image | ref. | ref. | ref. | ref. |
| 2 images | 3.11 (0.74 – 13.11) | 0.12 | 1.73 (0.39 – 7.72) | 0.47 |
| ≥3 images | 12.83 (2.10 – 78.60) | <0.01 | 5.38 (0.55 – 52.43) | 0.15 |
LR-TR - LI-RADS Treatment Response Categories (LR-TR Viable vs LR-TR Equivocal/Non-Viable); OR - odds ratio; CI - confidence interval; LT - liver transplant
Discussion
This is the first study to evaluate the diagnostic accuracy and interrater reliability of the LR-TR categories after thermal ablation for small HCC. Our study finds a high IRR but a lower sensitivity (30%) than anticipated while maintaining a high IRR. The IRR is consistent with previous studies evaluating the interrater agreement of the LI-RADS diagnostic categories.36–38 After changing the cutoff point for detecting disease to include LR-TR Equivocal, sensitivity remains low (44%), with a decrease in specificity and PPV. As the size of the residual disease increases, the sensitivity of the LR-TR scale improves, though it remains below 50%. Additionally, we are unable to detect any residual viable tumor that is less than 1cm.
While this is the first study to evaluate the sensitivity of the LR-TR criteria, previous studies using different imaging criteria have also demonstrated low sensitivities.39–41 One study comparing resected or explanted liver histopathology of tumors that had previously undergone locoregional therapy (TACE or ablation) found a 38% sensitivity and 83% specificity for detecting residual HCC simply using nodular arterial enhancement and 28% sensitivity and 89% specificity for washout seen on cross-sectional imaging.39 Both arterial enhancement and washout are included in the LR-TR Viable category and help define residual disease. Though Ehman et al did not study the LR-TR categories, their sensitivity analysis is consistent with our results. A smaller study evaluating the resection specimen or explanted native liver of 22 hepatocellular carcinomas that had received locoregional therapy (TACE or ablation) found cross-sectional imaging was 40% sensitive and 100% specific at detecting residual viable tumor.40 Another study looking at only tumors that underwent radiofrequency ablation found surveillance cross-sectional imaging is 36% sensitive and 100% specific at identifying residual viable tumor.41 The applicability of these studies is limited by the use of either a combination of locoregional modalities (TACE and ablation) or the combination of cross-sectional imaging (CT and MRI) in their analyses. In contrast, our study examines a single locoregional therapy modality (thermal ablation) and a single imaging modality (gadolinium-enhanced MRI).
The sensitivity of the LI-RADS diagnostic criteria for identifying new tumors at least 1 cm in size (62%) is lower than previously reported. However, in the prior studies liver explant histopathology was not required to validate the diagnostic imaging. For example, a study looking at 240 patients who underwent MRI surveillance for HCC found the LI-RADS 4 and 5 criteria to be 86% sensitive and 84% specific at detecting HCC. However, only 12% of the cohort used surgical specimens for the diagnoses while the other 88% used core biopsy or radiologic follow-up as the referent standard for true HCC.37 Core needle biopsy has a high false negative rate due to the inability to distinguish well-differentiated HCC from cirrhotic liver and the inaccuracy of targeting specimens.42,43 Another study found the LI-RADS sensitivity and specificity to be 76% and 85%, but the true diagnosis of HCC was solely made by follow-up imaging in more than one-third of these patients.44 A more recent study using histopathology of the explanted liver reports a much lower sensitivity for LI-RADS 4 (43%) and LI-RADS 5 (57%) lesions.45 Future studies may need to incorporate histopathologic diagnosis as the gold standard, since imaging criteria may not be as sensitive or specific as previously thought.
In the current study, laparoscopic ablation was 61% effective at causing complete tumor necrosis. This is better than the previously reported 50% viability after locoregional therapy (thermal ablation and TACE) found on explanted native liver after transplant.39 Other reports evaluating tumor necrosis after ablation need to be interpreted cautiously as many of them rely on core needle biopsy or surveillance imaging to determine complete necrosis.46–49
With both the LR-TR and LI-RADS diagnostic categories, we find under-classification of true HCC tumor burden. Prior studies have also demonstrated significant under-staging of radiographic imaging of HCC compared to the actual histopathologic burden.40,50 The lower sensitivity with LR-TR compared to the LI-RADS diagnostic categories may be due to disruption in blood flow to the ablation cavity that is caused by ablation itself. This disruption in blood flow may lead to decreased arterial enhancement and washout on cross-sectional imaging, thus affecting the ability to detect residual disease. This has potential consequences in staging patients awaiting transplantation and could also impact survival after transplantation. Prior studies have found that having a complete or near complete pathologic response after locoregional therapy improves both overall survival and disease-free survival after liver transplantation.28,30 The largest of these studies, with over 500 patients, found that the disease-specific survival was 99% in patients who had a complete pathologic response after locoregional therapy compared to 86% for patients with partial or no response.28 Therefore, having accurate diagnostic tools to evaluate residual and new HCC is of the utmost importance. We did find improved diagnostic accuracy of LR-TR categorization in patients who had three or more MRIs from the time of ablation to the time of transplantation. The number of previous diagnostic images should be considered when discussing further treatment of HCC. Additionally, the interpretation of LR-TR categories should be undertaken in a multidisciplinary setting taking into account both clinical and radiographic data.
Our study had many strengths, including using complete explanted liver for histopathologic exam. Additionally, we are able to evaluate a single locoregional therapy (thermal ablation) with the LR-TR categories in comparison to other studies that often have mixed locoregional therapies. There are limitations to this study. With its retrospective design we are not able to fully re-review the histopathology of the native explanted liver after transplantation. While significant detail is obtained on initial evaluation of the gross liver at the time of initial examination, it is not possible to re-create this evaluation or obtain additional data after the tissue no longer exists. Accurate size of the residual/recurrent HCC after ablation is also limited as the pathology reports only documented the longest axis of tumor. Many cases may have a narrow short axis measurement contributing to the low sensitivity. Additionally, the LI-RADS diagnostic criteria detects HCC in patients with cirrhosis who are at risk for HCC. Our use of the LI-RADS diagnostic criteria in this sample may have led to a higher PPV since this cohort was limited to patients who were already treated for HCC and therefore at a higher baseline risk for HCC than patients with cirrhosis alone. In addition, it is important to interpret our reported sensitivities and specificities in the context of the population in which they were derived (i.e. patients with adequate post-ablation imaging who went on to transplant) and the unavailability of subtraction imaging on all reviewed MRIs. Lastly, a minority of patients in our cohort had tumors bigger than 2.5 cm and our findings may therefore not be generalizable to patients with larger lesions or to the general population of patients who receive LRT prior to transplantation.
In conclusion, we demonstrate that using the LR-TR categories after thermal ablation for HCC has high precision but low accuracy. This may be due to the disruption of blood flow in the tissue, which may affect the arterial enhancement and washout seen on MRI. The accuracy did improve with an increase in the number of MRIs available for comparison from the time of ablation to the time of transplant. Based on the findings in our study we feel that future studies should emphasize incorporating histopathology as a gold standard. The ultimate goal involves better identification of those patients who are at greater risk of disease progression and/or recurrence.
Acknowledgments
Grant Support: This work is supported by the Cancer Care Quality Research Training Program from a National Institutes of Health grant R25CA116339. This research was supported in part by NIH grant T32 DK007634 (AMM).
Abbreviations:
- AASLD
American Association for the Study of Liver Diseases
- ACR
American College of Radiology
- CT
computed tomography
- EASL
European Association for the Study of the Liver
- HCC
hepatocellular carcinoma
- LI-RADS
Liver Imaging Reporting and Data System
- LR-TR
Liver Imaging Reporting and Data System Treatment Response
- MELD
Model for End-Stage Liver Disease
- MRI
magnetic resonance imaging
- MWA
microwave ablation
- NPV
negative predictive value
- PPV
positive predictive value
- RECIST
Response Evaluation Criteria in Solid Tumors
- RFA
radiofrequency ablation
- TACE
transcatheter arterial chemoembolization
- WHO
World Health Organization
Footnotes
Conflict of Interest:
Katherine S. Cools – has no conflicts of interest
Andrew M. Moon – No conflicts of interest
Lauren M.B. Burke – No conflicts of interest.
Katrina A. McGinty – no conflicts of interest
Paula D. Strassle - has no conflicts of interest
David A. Gerber – Conflicts of Interest: Medtronic (consultant)
References
- 1.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334(11):693–699. [DOI] [PubMed] [Google Scholar]
- 2.Freeman RB Jr., Wiesner RH, Harper A, et al. The new liver allocation system: moving toward evidence-based transplantation policy. Liver Transpl. 2002;8(9):851–858. [DOI] [PubMed] [Google Scholar]
- 3.Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380. [DOI] [PubMed] [Google Scholar]
- 4.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334(11):693–699. [DOI] [PubMed] [Google Scholar]
- 5.EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56(4):908–943. [DOI] [PubMed] [Google Scholar]
- 6.Mazzaferro V, Battiston C, Perrone S, et al. Radiofrequency ablation of small hepatocellular carcinoma in cirrhotic patients awaiting liver transplantation: a prospective study. Ann Surg. 2004;240(5):900–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu DS, Yu NC, Raman SS, et al. Percutaneous radiofrequency ablation of hepatocellular carcinoma as a bridge to liver transplantation. Hepatology. 2005;41(5):1130–1137. [DOI] [PubMed] [Google Scholar]
- 8.Majno PE, Adam R, Bismuth H, et al. Influence of preoperative transarterial lipiodol chemoembolization on resection and transplantation for hepatocellular carcinoma in patients with cirrhosis. Ann Surg. 1997;226(6):688–701; discussion 701–683.s [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Decaens T, Roudot-Thoraval F, Bresson-Hadni S, et al. Impact of pretransplantation transarterial chemoembolization on survival and recurrence after liver transplantation for hepatocellular carcinoma. Liver Transpl. 2005;11(7):767–775. [DOI] [PubMed] [Google Scholar]
- 10.Porrett PM, Peterman H, Rosen M, et al. Lack of benefit of pre-transplant locoregional hepatic therapy for hepatocellular cancer in the current MELD era. Liver Transpl. 2006;12(4):665–673. [DOI] [PubMed] [Google Scholar]
- 11.Pompili M, Mirante VG, Rondinara G, et al. Percutaneous ablation procedures in cirrhotic patients with hepatocellular carcinoma submitted to liver transplantation: Assessment of efficacy at explant analysis and of safety for tumor recurrence. Liver Transpl. 2005;11(9):1117–1126. [DOI] [PubMed] [Google Scholar]
- 12.Bruix J, Reig M, Rimola J, et al. Clinical decision making and research in hepatocellular carcinoma: pivotal role of imaging techniques. Hepatology. 2011;54(6):2238–2244. [DOI] [PubMed] [Google Scholar]
- 13.Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47(1):207–214. [DOI] [PubMed] [Google Scholar]
- 14.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–216. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki C, Torkzad MR, Jacobsson H, et al. Interobserver and intraobserver variability in the response evaluation of cancer therapy according to RECIST and WHO-criteria. Acta Oncol. 2010;49(4):509–514. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki C, Jacobsson H, Hatschek T, et al. Radiologic measurements of tumor response to treatment: practical approaches and limitations. Radiographics. 2008;28(2):329–344. [DOI] [PubMed] [Google Scholar]
- 17.Bonekamp D, Bonekamp S, Halappa VG, et al. Interobserver agreement of semi-automated and manual measurements of functional MRI metrics of treatment response in hepatocellular carcinoma. Eur J Radiol. 2014;83(3):487–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Therasse P. Measuring the clinical response. What does it mean? Eur J Cancer. 2002;38(14):1817–1823. [DOI] [PubMed] [Google Scholar]
- 19.Forner A, Ayuso C, Varela M, et al. Evaluation of tumor response after locoregional therapies in hepatocellular carcinoma: are response evaluation criteria in solid tumors reliable? Cancer. 2009;115(3):616–623. [DOI] [PubMed] [Google Scholar]
- 20.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim BK, Kim SU, Kim MJ, et al. Number of target lesions for EASL and modified RECIST to predict survivals in hepatocellular carcinoma treated with chemoembolization. Clin Cancer Res. 2013;19(6):1503–1511. [DOI] [PubMed] [Google Scholar]
- 22.Shim JH, Lee HC, Kim SO, et al. Which response criteria best help predict survival of patients with hepatocellular carcinoma following chemoembolization? A validation study of old and new models. Radiology. 2012;262(2):708–718. [DOI] [PubMed] [Google Scholar]
- 23.Gillmore R, Stuart S, Kirkwood A, et al. EASL and mRECIST responses are independent prognostic factors for survival in hepatocellular cancer patients treated with transarterial embolization. J Hepatol. 2011;55(6):1309–1316. [DOI] [PubMed] [Google Scholar]
- 24.Memon K, Kulik L, Lewandowski RJ, et al. Radiographic response to locoregional therapy in hepatocellular carcinoma predicts patient survival times. Gastroenterology. 2011;141(2):526–535, 535 e521–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kielar A, Fowler KJ, Lewis S, et al. Locoregional therapies for hepatocellular carcinoma and the new LI-RADS treatment response algorithm. Abdom Radiol (NY). 2018;43(1):218–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitchell DG, Bruix J, Sherman M, Sirlin CB. LI-RADS (Liver Imaging Reporting and Data System): summary, discussion, and consensus of the LI-RADS Management Working Group and future directions. Hepatology. 2015;61(3):1056–1065. [DOI] [PubMed] [Google Scholar]
- 27.CT/MRI Liver Imaging Reporting and Data System (LI-RADS), version 2017. In: American Colleg of Radiology; 2017. [Google Scholar]
- 28.Agopian VG, Morshedi MM, McWilliams J, et al. Complete pathologic response to pretransplant locoregional therapy for hepatocellular carcinoma defines cancer cure after liver transplantation: analysis of 501 consecutively treated patients. Ann Surg. 2015;262(3):536–545; discussion 543–535. [DOI] [PubMed] [Google Scholar]
- 29.Cucchetti A, Cescon M, Bigonzi E, et al. Priority of candidates with hepatocellular carcinoma awaiting liver transplantation can be reduced after successful bridge therapy. Liver Transpl. 2011;17(11):1344–1354. [DOI] [PubMed] [Google Scholar]
- 30.Allard MA, Sebagh M, Ruiz A, et al. Does pathological response after transarterial chemoembolization for hepatocellular carcinoma in cirrhotic patients with cirrhosis predict outcome after liver resection or transplantation? J Hepatol. 2015;63(1):83–92. [DOI] [PubMed] [Google Scholar]
- 31.Pompili M, Francica G, Ponziani FR, Iezzi R, Avolio AW. Bridging and downstaging treatments for hepatocellular carcinoma in patients on the waiting list for liver transplantation. World J Gastroenterol. 2013;19(43):7515–7530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Millonig G, Graziadei IW, Freund MC, et al. Response to preoperative chemoembolization correlates with outcome after liver transplantation in patients with hepatocellular carcinoma. Liver Transpl. 2007;13(2):272–279. [DOI] [PubMed] [Google Scholar]
- 33.Otto G, Herber S, Heise M, et al. Response to transarterial chemoembolization as a biological selection criterion for liver transplantation in hepatocellular carcinoma. Liver Transpl. 2006;12(8):1260–1267. [DOI] [PubMed] [Google Scholar]
- 34.Lai Q, Avolio AW, Graziadei I, et al. Alpha-fetoprotein and modified response evaluation criteria in solid tumors progression after locoregional therapy as predictors of hepatocellular cancer recurrence and death after transplantation. Liver Transpl. 2013;19(10):1108–1118. [DOI] [PubMed] [Google Scholar]
- 35.Riaz A, Miller FH, Kulik LM, et al. Imaging response in the primary index lesion and clinical outcomes following transarterial locoregional therapy for hepatocellular carcinoma. Jama. 2010;303(11):1062–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Becker AS, Barth BK, Marquez PH, et al. Increased interreader agreement in diagnosis of hepatocellular carcinoma using an adapted LI-RADS algorithm. Eur J Radiol. 2017;86:33–40. [DOI] [PubMed] [Google Scholar]
- 37.Basha MAA, AlAzzazy MZ, Ahmed AF, et al. Does a combined CT and MRI protocol enhance the diagnostic efficacy of LI-RADS in the categorization of hepatic observations? A prospective comparative study. Eur Radiol. 2018. [DOI] [PubMed]
- 38.Fowler KJ, Tang A, Santillan C, et al. Interreader Reliability of LI-RADS Version 2014 Algorithm and Imaging Features for Diagnosis of Hepatocellular Carcinoma: A Large International Multireader Study. Radiology. 2018;286(1):173–185. [DOI] [PubMed] [Google Scholar]
- 39.Ehman EC, Umetsu SE, Ohliger MA, et al. Imaging prediction of residual hepatocellular carcinoma after locoregional therapy in patients undergoing liver transplantation or partial hepatectomy. Abdom Radiol (NY). 2016;41(11):2161–2168. [DOI] [PubMed] [Google Scholar]
- 40.Hanson JA, Ason R, Weinreb J, Van Dyke A, Mitchell KA. Radiology Estimates of Viable Tumor Percentage in Hepatocellular Carcinoma Ablation Cavities Correlate Poorly With Pathology Assessment. Archives of Pathology & Laboratory Medicine. 2013;137(3):392–399. [DOI] [PubMed] [Google Scholar]
- 41.Lu DS, Yu NC, Raman SS, et al. Radiofrequency ablation of hepatocellular carcinoma: treatment success as defined by histologic examination of the explanted liver. Radiology. 2005;234(3):954–960. [DOI] [PubMed] [Google Scholar]
- 42.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42(5):1208–1236. [DOI] [PubMed] [Google Scholar]
- 43.Pawlik TM, Gleisner AL, Anders RA, Assumpcao L, Maley W, Choti MA. Preoperative assessment of hepatocellular carcinoma tumor grade using needle biopsy: implications for transplant eligibility. Ann Surg. 2007;245(3):435–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim YY, An C, Kim S, Kim MJ. Diagnostic accuracy of prospective application of the Liver Imaging Reporting and Data System (LI-RADS) in gadoxetate-enhanced MRI. European Radiology. 2018;28(5):2038–2046. [DOI] [PubMed] [Google Scholar]
- 45.Choi SH, Byun JH, Kim SY, et al. Liver Imaging Reporting and Data System v2014 With Gadoxetate Disodium-Enhanced Magnetic Resonance Imaging Validation of LI-RADS Category 4 and 5 Criteria. Investigative Radiology. 2016;51(8):483–490. [DOI] [PubMed] [Google Scholar]
- 46.D’Onofrio M, Cardobi N, Ruzzenente A, et al. Unenhanced magnetic resonance imaging immediately after radiofrequency ablation of liver malignancy: preliminary results. Abdominal Radiology. 2018;43(6):1379–1385. [DOI] [PubMed] [Google Scholar]
- 47.Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Radiofrequency ablation improves prognosis compared with ethanol injection for hepatocellular carcinoma <= 4cm. Gastroenterology. 2004;127(6):1714–1723. [DOI] [PubMed] [Google Scholar]
- 48.Brunello F, Veltri A, Carucci P, et al. Radiofrequency ablation versus ethanol injection for early hepatocellular carcinoma: A randomized controlled trial. Scandinavian Journal of Gastroenterology. 2008;43(6):727–735. [DOI] [PubMed] [Google Scholar]
- 49.Martin RCG, Scoggins CR, McMasters KM. Microwave hepatic ablation: Initial experience of safety and efficacy. Journal of Surgical Oncology. 2007;96(6):481–486. [DOI] [PubMed] [Google Scholar]
- 50.El-Gazzaz G, Sourianarayanane A, Menon KV, et al. Radiologic-histological correlation of hepatocellular carcinoma treated via pre-liver transplant locoregional therapies. Hepatobiliary Pancreat Dis Int. 2013;12(1):34–41. [DOI] [PubMed] [Google Scholar]