Abstract
Introduction:
Infant mortality rates are higher in nonmetropolitan areas versus large metropolitan areas. Variation by race/ethnicity and cause of death has not been assessed. Urban–rural infant mortality rate differences were quantified by race/ethnicity and cause of death.
Methods:
National Vital Statistics System linked birth/infant death data (2014–2016) were analyzed in 2019 by 3 urban–rural county classifications: large metropolitan, medium/small metropolitan, and nonmetropolitan. Excess infant mortality rates (rate differences) by urban–rural classification were calculated relative to large metropolitan areas overall and for each racial/ethnic group. The number of excess deaths, population attributable fraction, and proportion of excess deaths attributable to underlying causes of death was calculated.
Results:
Nonmetropolitan areas had the highest excess infant mortality rate overall. Excess infant mortality rates were substantially lower for Hispanic infants than other races/ethnicities. Overall, 7.4% of infant deaths would be prevented if all areas had the infant mortality rate of large metropolitan areas. With more than half of births occurring outside of large metropolitan areas, the population attributable fraction was highest for American Indian/Alaska Natives (20.3%) and whites, non-Hispanic (14.3%). Excess infant mortality rates in both nonmetropolitan and medium/small metropolitan areas were primarily attributable to sudden unexpected infant deaths (42.3% and 31.9%) and congenital anomalies (30.1% and 26.8%). This pattern was consistent for all racial/ethnic groups except black, non-Hispanic infants, for whom preterm-related and sudden unexpected infant deaths accounted for the largest share of excess infant mortality rates.
Conclusions:
Infant mortality increases with rurality, and excess infant mortality rates are predominantly attributable to sudden unexpected infant deaths and congenital anomalies, with differences by race/ethnicity regarding magnitude and cause of death.
INTRODUCTION
Residents of nonmetropolitan areas tend to have worse health outcomes than metropolitan areas, including disproportionally high infant mortality rates (IMRs).1–5 Higher IMRs are associated with risk factors that are more common in nonmetropolitan areas, including smoking, obesity, and poverty, which are more common in rural areas across all racial/ethnic groups.6–14 Nonmetropolitan areas also have fewer healthcare providers, and residents often live farther away from healthcare resources, making it difficult to access care.12,15
Although previous studies indicate that IMRs from certain causes are higher in rural areas,2 the proportion of infant mortality attributable to urban–rural residence has not been quantified. Moreover, patterns may vary by race/ethnicity, as there are differences in the leading causes of infant mortality, as well as in urban–rural residence by race/ethnicity.11,16 The study objective is to quantify urban–rural infant mortality differences in the U.S. by race/ethnicity and cause of death.
METHODS
Study Sample
In 2019, U.S. resident infant death data from combined 2014–2016 National Vital Statistics System Period Linked Birth/Infant Death Files were analyzed. More than 99% of all infant (<1 year) death certificates were linked to their corresponding birth certificate.17–19 Linked death records were weighted to account for unlinked records.17–19 This study involved the secondary analysis of existing data and did not involve human subjects; therefore, no IRB approval was required.
Measures
The urbanization level was identified using the 2013 National Center for Health Statistics Urban–Rural Classification Scheme for Counties.20 Categories were grouped based on the maternal county of residence at birth: large metropolitan (in a metropolitan statistical area of ≥1 million population), medium/small metropolitan (in a metropolitan statistical area of <1 million population), and nonmetropolitan. Although the county classification is not completely contemporaneous with the outcome, urban–rural designations do not change rapidly.20
Maternal race/ethnicity was classified into 5 bridged single-race categories: white, non-Hispanic; black, non-Hispanic; Hispanic; Asian or Pacific Islander (API); and American Indian or Alaskan Native (AIAN).17 Maternal race/ethnicity information from the birth certificate is considered more reliable than information from the death certificate.16
Summary categories of infant cause of death were assigned using the underlying cause of death (ICD-10 codes) (Appendix Table 1, available online).21 Categories included congenital anomalies, preterm-related, other perinatal conditions, sudden unexpected infant death (SUID), infection, injury, and all other causes. Preterm-related deaths were defined using a previously developed classification scheme.22,23 The SUID category includes 3 causes with common sleep-related risk factors: sudden infant death syndrome, unknown cause, and accidental suffocation and strangulation in bed.24–26 These groupings summarize related causes that are relevant for prevention.16,22,24,27
Statistical Analysis
The IMRs (number of infant deaths per 1,000 live births) were calculated by urbanization level and racial/ethnic category. Excess IMRs were calculated relative to large metropolitan areas, which had the lowest IMR overall and for each racial/ethnic group. The numbers of excess deaths were calculated by multiplying the excess IMR by the number of births for each group. The proportions of deaths that were attributable to differences in urbanization (population attributable fraction) were estimated by dividing the number of excess deaths by the total infant deaths, and 95% CIs were calculated using the formulas proposed by Walter.28 This is the estimated proportion of infant deaths that could be avoided if all areas had the same IMR as large metropolitan areas. Finally, the proportions of the excess deaths attributable to each summary cause of death were calculated by dividing the cause-specific excess deaths from the total excess deaths by urbanization level and racial/ethnic category. Cause-specific analyses are not shown for API and AIAN infants because there were <20 deaths when stratified by urbanization level. Differences in IMRs were compared using z-tests,29 and discussed in the results if statistically significant (p<0.05).
RESULTS
IMRs ranged from a low of 5.43 deaths per 1,000 live births in counties in large metropolitan areas to 6.67 in counties in nonmetropolitan areas (Table 1). Although nonmetropolitan areas had a higher overall excess IMR, medium/small metropolitan areas had the highest number of excess deaths owing to a larger number of births occurring in those areas. Overall, 7.4% of infant deaths were attributable to differences in the urbanization level (i.e., could be prevented if all areas had the IMR of large metropolitan areas). Excess IMR in both nonmetropolitan and medium/small metropolitan areas was primarily attributable to SUID (42.3% and 31.9%) and congenital anomalies (30.1% and 26.8%) (Table 2).
Table 1.
Excess Infant Mortality by Urbanization Level and Race/Ethnicity
| Urbanization level and race/ethnicity |
Deaths, n | Births, n | Percent of total births |
IMRa | Excess IMRa |
Excess deaths |
Population attributable fraction, % (95% CI) |
|---|---|---|---|---|---|---|---|
| All races and ethnicities | |||||||
| Large metropolitan | 36,526 | 6,728,668 | 56 | 5.43 | Ref | – | – |
| Medium and small metropolitan | 22,533 | 3,570,488 | 30 | 6.31 | 0.88 | 3,151 | 4.5 (3.8, 5.2) |
| Nonmetropolitan | 10,766 | 1,613,292 | 14 | 6.67 | 1.24 | 2,008 | 2.9 (2.2, 3.5) |
| Total | 69,825 | 11,912,448 | 100 | 5.86 | – | 5,159 | 7.4 (6.7, 8.0) |
| White, non-Hispanic | |||||||
| Large metropolitan | 13,213 | 13,213 | 48 | 4.26 | Ref | – | – |
| Medium and small metropolitan | 11,569 | 2,146,116 | 33 | 5.39 | 1.14 | 2,437 | 7.6 (6.3, 8.9) |
| Nonmetropolitan | 7,201 | 1,191,436 | 18 | 6.04 | 1.79 | 2,131 | 6.7 (5.3, 8.0) |
| Total | 31,983 | 6,442,645 | 100 | 4.96 | – | 4,568 | 14.3 (13.2, 15.4) |
| Black, non-Hispanic | |||||||
| Large metropolitan | 12,267 | 1,160,095 | 65 | 10.57 | Ref | – | – |
| Medium and small metropolitan | 5,758 | 467,746 | 26 | 12.31 | 1.74 | 812 | 4.1 (3.0, 5.1) |
| Nonmetropolitan | 1,866 | 148,809 | 8 | 12.54 | 1.96 | 292 | 1.5 (0.6, 2.3) |
| Total | 19,891 | 1,776,650 | 100 | 11.20 | – | 1,104 | 5.6 (4.5, 6.6) |
| Hispanic | |||||||
| Large metropolitan | 8,593 | 1,795,606 | 65 | 4.79 | Ref | – | – |
| Medium and small metropolitan | 4,069 | 765,536 | 28 | 5.31 | 0.53 | 405 | 3.0 (1.7, 4.2) |
| Nonmetropolitan | 1,055 | 195,418 | 7 | 5.40 | 0.61 | 120 | 0.9 (–0.1, 1.8) |
| Total | 13,717 | 2,756,560 | 100 | 4.98 | – | 525 | 3.8 (2.6, 5.1) |
| Asian and pacific islander | |||||||
| Large metropolitan | 2,296 | 644,653 | 78 | 3.56 | Ref | – | – |
| Medium and small metropolitan | 840 | 155,804 | 19 | 5.39 | 1.83 | 285 | 8.7 (6.7, 10.6) |
| Nonmetropolitan | 151 | 24,321 | 3 | 6.23 | 2.67 | 64 | 2.0 (0.8, 3.2) |
| Total | 3,287 | 824,778 | 100 | 3.98 | – | 349 | 10.6 (8.6, 12.6) |
| American Indian and Alaskan native | |||||||
| Large metropolitan | 157 | 23,221 | 21 | 6.75 | Ref | – | – |
| Medium and small metropolitan | 298 | 35,286 | 32 | 8.44 | 1.69 | 59 | 6.3 (−14.7, 27.2) |
| Nonmetropolitan | 493 | 53,308 | 48 | 9.25 | 2.49 | 133 | 14.0 (−1.8, 29.8) |
| Total | 948 | 111,815 | 100 | 8.47 | – | 192 | 20.3 (8.9, 31.7) |
Note: Boldface indicates that the rate is significantly higher than the large metropolitan rate within each racial and ethnic category, p<0.05.
Infant mortality rates are defined as the number of infant deaths per 1,000 live births.
IMR, infant mortality rate.
Table 2.
Percent of Total Excess Infant Mortality by Cause of Death, Urbanization Level, and Race/Ethnicity
| Urbanization level and race/ ethnicity |
Preterm-related |
Congenital anomalies |
SUID |
Other perinatal conditions |
Infectious |
Injury |
Other causes |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IMRa (excess IMRa) |
% of total excessb |
IMRa (excess IMRa) |
% of total excessb |
IMRa (excess IMRa |
% of total excessb |
IMRa (excess IMRa) |
% of total excessb |
IMRa (excess IMRa) |
% of total excessb |
IMRa (excess IMRa) |
% of total excessb |
IMRa (excess IMRa) |
% of total excessb |
|
| All races and ethnicities | ||||||||||||||
| Large metro (ref) | 2.06 | – | 1.09 | – | 0.75 | – | 0.81 | – | 0.20 | – | 0.13 | – | 0.39 | – |
| Medium and small metro | 2.14 (0.08) | 9.4 | 1.33 (0.24) | 26.8 | 1.03 (0.28) | 31.9 | 0.91 (0.10) | 11.4 | 0.23 (0.03) | 3.9 | 0.22 (0.09) | 10.0 | 0.45 (0.06) | 6.5 |
| Nonmetro | 1.94 (−0.12)c | −9.3c | 1.46 (0.38) | 30.1 | 1.27 (0.53) | 42.3 | 0.94 (0.13) | 10.7 | 0.28 (0.08) | 6.4 | 0.28 (0.15) | 12.2 | 0.49 (0.10) | 7.7 |
| White, non-Hispanic | ||||||||||||||
| Large metro (ref) | 1.42 | – | 0.96 | – | 0.63 | – | 0.65 | – | 0.13 | – | 0.12 | – | 0.34 | – |
| Medium and small metro | 1.59 (0.17) | 15.1 | 1.22 (0.26) | 22.9 | 0.97 (0.34) | 29.7 | 0.79 (0.14) | 12.7 | 0.18 (0.05) | 4.1 | 0.22 (0.10) | 8.8 | 0.41 (0.08) | 6.7 |
| Nonmetro | 1.57 (0.15) | 8.2 | 1.45 (0.49) | 27.4 | 1.20 (0.57) | 31.8 | 0.88 (0.23) | 12.6 | 0.22 (0.09) | 4.9 | 0.27 (0.15) | 8.2 | 0.46 (0.12) | 6.8 |
| Black, non-Hispanic | ||||||||||||||
| Large metro (ref) | 4.65 | – | 1.41 | – | 1.70 | – | 1.41 | – | 0.45 | – | 0.28 | – | 0.69 | – |
| Medium and small metro | 5.34 (0.70) | 40.3 | 1.73 (0.32) | 18.4 | 2.08 (0.38) | 21.7 | 1.52 (0.11) | 6.5 | 0.52 (0.07) | 4.1 | 0.36 (0.08) | 4.8 | 0.76 (0.07) | 4.2 |
| Nonmetro | 5.13 (0.49) | 24.9 | 1.53 (0.12) | 6.1 | 2.22 (0.52) | 26.3 | 1.70 (0.29) | 15.0 | 0.63 (0.19) | 9.4 | 0.47 (0.19) | 9.8 | 0.85 (0.16) | 8.3 |
| Hispanic | ||||||||||||||
| Large metro (ref) | 1.74 | – | 1.23 | – | 0.48 | – | 0.75 | – | 0.17 | – | 0.09 | – | 0.32 | – |
| Medium and small metro | 1.76 (0.02) | 3.7 | 1.40 (0.18) | 33.4 | 0.60 (0.11) | 21.7 | 0.84 (0.09) | 17.4 | 0.21 (0.04) | 7.3 | 0.14 (0.06) | 10.6 | 0.36 (0.03) | 5.9 |
| Nonmetro | 1.76 (0.01) | 2.1 | 1.44 (0.22) | 35.2 | 0.71 (0.22) | 36.3 | 0.71 (−0.04)c | −6.0c | 0.25 (0.08) | 12.5 | 0.21 (0.12) | 20.1 | 0.32 (0.00) | −0.3c |
Notes: Boldface indicates that the cause-specific rate is significantly higher than the large metropolitan rate within each racial and ethnic category, p<0.05. Rates by urbanization for Asian Pacific Islander (API) and American Indian and Alaskan Native (AIAN) infants do not meet the standards of reliability or precision, based on fewer than 20 deaths in the numerator.
Infant mortality rates are defined as the number of infant deaths per 1,000 live births.
Percentage of excess is calculated by dividing the cause-specific excess IMRs from the total excess IMRs by urbanization level and racial/ethnic category.
Negative excess rates and percentages indicate lower cause-specific IMRs in nonmetropolitan areas relative to large metropolitan areas. Therefore, these causes do not help explain the overall disadvantage in nonmetropolitan areas and may have offset the excess deaths from other causes (and would serve to increase disparities if equalized).
IMR, infant mortality rate; SUID, sudden unexpected infant deaths.
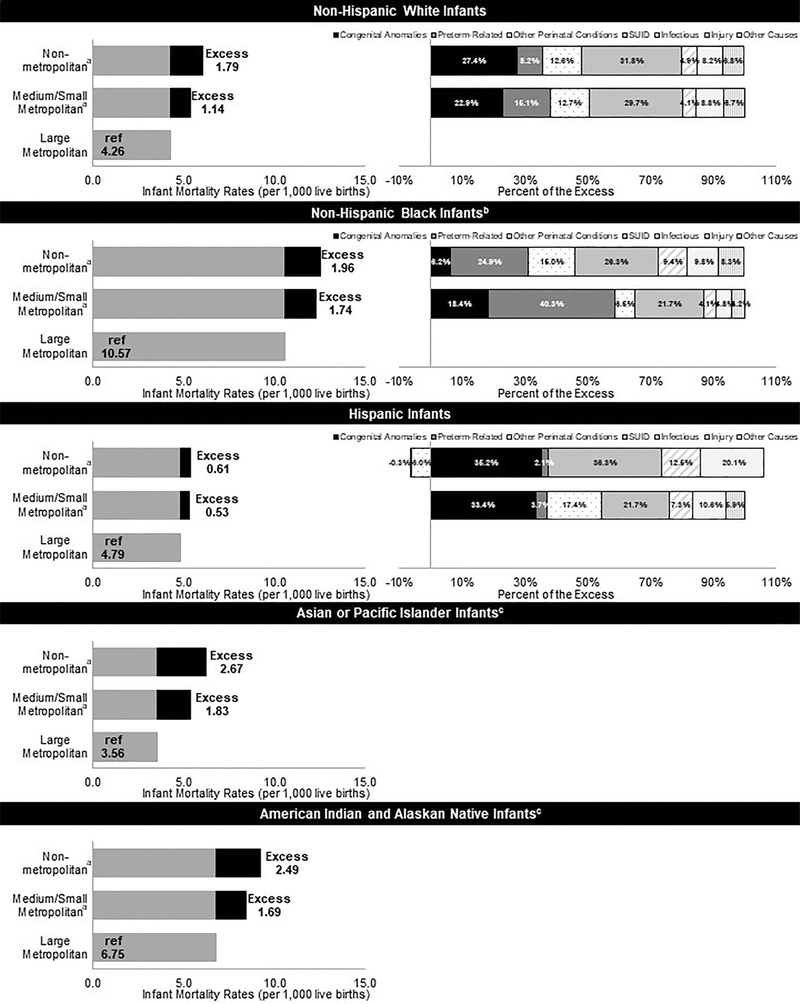
For all racial/ethnic groups, the IMRs were lowest in counties in large metropolitan areas (Figure 1). Black, non-Hispanic infants had the highest IMRs in every urbanization level (Table 1). Excess IMRs were substantially lower for Hispanic infants than other races/ethnicities. Medium/small metropolitan areas had the highest number of excess deaths for all racial/ethnic groups except for AIAN infants. The number of excess deaths was higher in nonmetropolitan areas for AIAN infants owing to nearly half of the AIAN births occurring in these areas. Because of different distributions in where births occur, a greater proportion of deaths were attributable to the urbanization level for AIAN (20.3%); white, non-Hispanic (14.3%); and API (10.6%) infants. For white, non-Hispanic and Hispanic infants, the excess deaths in nonmetropolitan areas and medium/small metropolitan areas compared with large metropolitan areas were mostly owing to congenital anomalies and SUID (Table 2). For black, non-Hispanic infants, the excess deaths were mostly attributable to preterm birth and SUID.
Figure 1.
Excess infant mortality by urbanization level, race/ethnicity, and summary cause of death.
aInfant mortality rate is statistically significantly higher than large metropolitan areas for all racial/ethnic categories, p<0.05.
bInfant mortality rate is statistically significantly higher in black, non-Hispanic infants compared with all racial/ethnic categories for every urbanization level, p<0.05.
cRates by urbanization do not meet standards of reliability or precision; based on fewer than 20 deaths in the numerator.
SUID, sudden unexpected infant deaths.
DISCUSSION
Infant mortality is higher in more rural areas, with some noted racial/ethnic differences in the magnitude of excess deaths by urbanization and the causes of death that contribute to excess deaths. Overall, excess deaths in nonmetropolitan and medium/small metropolitan areas were primarily attributable to SUID and congenital anomalies. Risk factors associated with both SUID and congenital anomalies may be more common in rural areas. For instance, maternal smoking is more common in rural areas13 and is a risk factor for both SUID and congenital anomalies.30,31 Additionally, maternal obesity is associated with congenital anomalies32 and occurs more frequently in rural areas.13 Reduced access to health care may also potentially contribute to excess infant deaths in rural areas through the delayed detection of congenital anomalies.12,15,33–35 For black, non-Hispanic infants, excess deaths in less urban areas were mostly attributable to preterm-related causes, which are also the largest source of black–white IMR disparities.16,36,37 Future research might determine whether excess preterm-related deaths for black, non-Hispanic infants in rural areas are due to greater rates of preterm birth or preterm-specific mortality. Although this analysis describes the impact of urbanization on infant mortality, notably, black, non-Hispanic and AIAN infants experience substantially higher IMRs in all areas compared with other racial/ethnic groups. A better understanding of the differences in IMRs by urbanization and race/ethnicity can inform more-targeted interventions to reduce preventable infant deaths in rural areas.
Limitations
A limitation of this study is that analyses did not account for individual- and area-level factors such as poverty level. The total disparity, without adjustment, was examined as an initial descriptive analysis to inform preventive efforts. However, other studies have shown a persistent rural IMR disadvantage after adjustment for individual and contextual socioeconomic factors.3–5 Additionally, cause-specific excess infant mortality for API and AIAN infants was not examined given the small numbers of deaths among these groups by cause.
CONCLUSIONS
Infant mortality increases with rurality, and excess deaths are predominantly attributable to SUID and congenital anomalies, with differences by race/ethnicity regarding the magnitude and cause of death.
Supplementary Material
ACKNOWLEDGMENTS
This work was performed under employment by the U.S. federal government; the authors did not receive any outside funding.
The findings and conclusions in this paper are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the Health Resources and Services Administration.
Footnotes
No financial disclosures were reported by the authors of this paper.
REFERENCES
- 1.Ely DM, Driscoll AK, Matthews TJ. Infant mortality rates in rural and urban areas in the United States, 2014. NCHS Data Brief. 2017;285:1–8.https://www.cdc.gov/nchs/data/databriefs/db285.pdf. Accessed October 24, 2019. [PubMed] [Google Scholar]
- 2.Ely DM, Hoyert DL. Differences between rural and urban areas in mortality rates for the leading causes of infant death: United States, 2013–2015. NCHS Data Brief. 2018;300:1–8. https://www.cdc.gov/nchs/data/databriefs/db300.pdf Accessed October 24, 2019. [PubMed] [Google Scholar]
- 3.Mohamoud YA, Kirby RS, Ehrenthal DB. Poverty, urban–rural classification and term infant mortality: a population-based multilevel analysis. BMC Preg Childbirth. 2019;19(1):40 10.1186/s12884-019-2190-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sparks PJ, McLaughlin DK, Stokes CS. Differential neonatal and post-neonatal infant mortality rates across US counties: the role of socioeconomic conditions and rurality. J Rural Health. 2009;25(4):332–341. 10.1111/j.1748-0361.2009.00241.x. [DOI] [PubMed] [Google Scholar]
- 5.Huynh M, Parker JD, Harper S, Pamuk E, Schoendorf KC. Contextual effect of income inequality on birth outcomes. Int J Epidemiol. 2005;34(4):888–895. 10.1093/ije/dyi092. [DOI] [PubMed] [Google Scholar]
- 6.El-Sayed AM, Finkton DW Jr, Paczkowski M, Keyes KM, Galea S. Socioeconomic position, health behaviors, and racial disparities in cause-specific infant mortality in Michigan, USA. Prev Med. 2015;76:8–13. 10.1016/j.ypmed.2015.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alberg AJ, Shopland DR, Cummings KM. The 2014 Surgeon General’s report: commemorating the 50th Anniversary of the 1964 Report of the Advisory Committee to the US Surgeon General and updating the evidence on the health consequences of cigarette smoking. Am J Epidemiol. 2014;179(4):403–412. 10.1093/aje/kwt335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Committee on Underserved Women, Committee on Obstetric Practice. Committee Opinion No. 721: Smoking cessation during pregnancy. Obstet Gynecol. 2017;130(4):e200–e204. 10.1097/AOG.0000000000002353. [DOI] [PubMed] [Google Scholar]
- 9.American College of Obstetricians and Gynecologists. Committee on Health Care for Underserved Women. Committee opinion no. 496: At-risk drinking and alcohol dependence: obstetric and gynecologic implications. Obstet Gynecol. 2011;118(2 Pt 1):383–388. 10.1097/AOG.0b013e31822c9906. [DOI] [PubMed] [Google Scholar]
- 10.U.S. Preventive Services Task Force. Folic acid for the prevention of neural tube defects: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;150(9):626–631. 10.7326/0003-4819-150-9-200905050-00009. [DOI] [PubMed] [Google Scholar]
- 11.Cromartie J. Rural America at a Glance, 2018 Edition. Washington, DC: U.S. Department of Agriculture; 2018. www.ers.usda.gov/publications/pub-details/?pubid=90555 Accessed September 26, 2019. [Google Scholar]
- 12.Meit M, Knudson A, Gilbert T, et al. The 2014 update of the rural–urban chartbook. https://ruralhealth.und.edu/projects/health-reform-policy-research-center/pdf/2014-rural-urban-chartbook-update.pdf. Published 2014. Accessed September 26, 2019.
- 13.Shaw KM, Theis KA, Self-Brown S, Roblin DW, Barker L. Chronic disease disparities by county economic status and metropolitan classification, Behavioral Risk Factor Surveillance System, 2013. Prev Chronic Dis. 2016;13:E119. 10.5888/pcd13.160088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Economic Research Service. Rural poverty & well-being. www.ers.usda.gov/topics/rural-economy-population/rural-poverty-well-being/ Published 2019. Accessed July 11, 2019.
- 15.Agency for Healthcare Research and Quality. National Healthcare Quality and Disparities Report chartbook on rural health care. www.ahrq.gov/sites/default/files/wysiwyg/research/findings/nhqrdr/chartbooks/qdr-ruralhealthchartbook-update.pdf Published 2017. Accessed September 26, 2019.
- 16.Matthews TJ, MacDorman MF, Thoma ME. Infant mortality statistics from the 2013 period linked birth/infant death data set. Natl Vital Stat Rep. 2015;64(9):1–30. https://www.cdc.gov/nchs/data/nvsr/nvsr64/nvsr_09.pdfAccessed October 24, 2019. [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. User guide to the 2016 period linked birth/infant death public use file.ftp://ftp.cdc.gov/pub/Health_Statistics/NCHS/Dataset_Documentation/DVS/periodlinked/LinkPE16Guide.pd Accessed October 22, 2018.
- 18.Centers for Disease Control and Prevention. User guide to the 2015 period linked birth/infant death public use file.ftp://ftp.cdc.gov/pub/Health_Statistics/NCHS/Dataset_Documentation/DVS/periodlinked/LinkPE15Guide.pdf Accessed October 22, 2018.
- 19.Centers for Disease Control and Prevention. User guide to the 2014 period linked birth/infant death public use file.ftp://ftp.cdc.gov/pub/Health_Statistics/NCHS/Dataset_Documentation/DVS/periodlinked/LinkPE14Guide.pdf Accessed October 22, 2018.
- 20.Ingram DD, Franco SJ. NCHS urban–rural classification scheme for counties. Vital Health Stat 2. 2012;166(154):1–65. https://www.cdcgov/nchs/data/series/sr_02/sr02_166.pdf Accessed October 24, 2019. [PubMed] [Google Scholar]
- 21.Hirai AH, Sappenfield WM, Kogan MD, et al. Contributors to excess infant mortality in the U.S. South. Am J Prev Med. 2014;46(3):219–227. 10.1016/j.amepre.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Callaghan WM, MacDorman MF, Rasmussen SA, Qin C, Lackritz EM. The contribution of preterm birth to infant mortality rates in the United States. Pediatrics. 2006;118(4):1566–1573. 10.1542/peds.2006-0860. [DOI] [PubMed] [Google Scholar]
- 23.MacDorman MF, Callaghan WM, Mathews TJ, Hoyert DL, Kochanek KD. Trends in preterm-related infant mortality by race and ethnicity, United States, 1999–2004. Int J Health Serv. 2007;37(4):635–641. 10.2190/HS.37.4.c. [DOI] [PubMed] [Google Scholar]
- 24.Shapiro-Mendoza CK, Tomashek KM, Anderson RN, Wingo J. Recent national trends in sudden, unexpected infant deaths: more evidence supporting a change in classification or reporting. Am J Epidemiol. 2006;163(8):762–769. 10.1093/aje/kwj117. [DOI] [PubMed] [Google Scholar]
- 25.Nashelsky MB, Pinckard JK. The death of SIDS. Acad Forensic Pathol. 2011;1(1):92–99. 10.23907/2011.010. [DOI] [Google Scholar]
- 26.Moon RY, Task Force On Sudden Infant Death Syndrome. SIDS and other sleep-related infant deaths: evidence base for 2016 updated recommendations for a safe infant sleeping environment. Pediatrics. 2016;138(5):e20162940. 10.1542/peds.2016-2940. [DOI] [PubMed] [Google Scholar]
- 27.Erck Lambert AB, Parks SE, Shapiro-Mendoza CK. National and state trends in sudden unexpected infant death: 1990–2015. Pediatrics. 2018;141(3):e20173519. 10.1542/peds.2017-3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walter SD. Calculation of attributable risks from epidemiological data. Int J Epidemiol. 1978;7(2):175–182. 10.1093/ije/7.2.175. [DOI] [PubMed] [Google Scholar]
- 29.Xu J, Murphy SL, Kochanek KD, Bastian B, Arias E. Deaths: final data for 2016. Natl Vital Stat Rep. 2018;67(5):1–76. https://www.cdc.gov/nchs/data/nvsr/nvsr67/nvsr67_05.pdf Accessed October 24, 2019. [PubMed] [Google Scholar]
- 30.Carlin RF, Moon RY. Risk factors, protective factors, and current recommendations to reduce sudden infant death syndrome: a review. JAMA Pediatr. 2017;171(2):175–180. 10.1001/jamapediatrics.2016.3345. [DOI] [PubMed] [Google Scholar]
- 31.Shiono PH, Klebanoff MA, Berendes HW. Congenital malformations and maternal smoking during pregnancy. Teratology. 1986;34(1):65–71. 10.1002/tera.1420340109. [DOI] [PubMed] [Google Scholar]
- 32.Stothard KJ, Tennant PW, Bell R, Rankin J. Maternal overweight and obesity and the risk of congenital anomalies: a systematic review and meta-analysis. JAMA. 2009;301(6):636–650. 10.1001/jama.2009.113. [DOI] [PubMed] [Google Scholar]
- 33.Hill GD, Block JR, Tanem JB, Frommelt MA. Disparities in the prenatal detection of critical congenital heart disease. Prenat Diagn. 2015; 35(9):859–863. 10.1002/pd.4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.March of Dimes. Nowhere to go: maternity care deserts across the U.S. www.marchofdimes.org/materials/Nowhere_to_Go_Final.pdf. Published 2018. Accessed September 26, 2019.
- 35.Hung P, Henning-Smith CE, Casey MM, Kozhimannil KB. Access to obstetric services in rural counties still declining, with 9 percent losing services, 2004–14. Health Aff (Millwood). 2017;36(9):1663–1671. 10.1377/hlthaff.2017.0338. [DOI] [PubMed] [Google Scholar]
- 36.Schempf AH, Branum AM, Lukacs SL, Schoendorf KC. The contribution of preterm birth to the black–white infant mortality gap, 1990 and 2000. Am J Public Health. 2007;97(7):1255–1260. 10.2105/AJPH.2006.093708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rossen LM, Schoendorf KC. Trends in racial and ethnic disparities in infant mortality rates in the United States, 1989–2006. Am J Public Health. 2014;104(8):1549–1556. 10.2105/AJPH.2013.301272. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.