Abstract
Due to a high density of negative charges on its surface, DNA condenses cations as counterions, forming the so-called ‘ion atmosphere’. Although the release of counterions upon DNA-protein association has been postulated to have a major contribution to the binding thermodynamics, this release remains to be confirmed through a direct observation of the ions. Here, using NMR spectroscopy, we characterize the ion atmosphere around DNA and directly detect the release of counterions upon DNA-protein association. NMR-based diffusion data reveal the highly dynamic nature of counterions within the ion atmosphere around DNA. Counterion release is observed as an increase in the apparent ionic diffusion coefficient, which directly provides the number of counterions released upon DNA-protein association.
Keywords: ion atmosphere, DNA, dynamics, NMR, protein
Graphical Abstract
COMMUNICATION
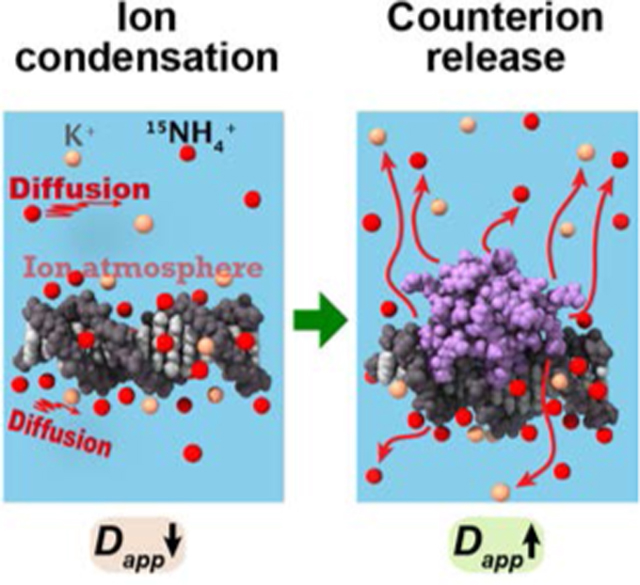
Through NMR-based diffusion measurements, we investigated condensation of cations around DNA and their release upon DNA-protein association. Ion condensation around DNA causes a decrease in the apparent diffusion coefficient Dapp of cations, whereas counterion release causes an increase in the apparent diffusion coefficient Dapp.
Counterions are important constituents of the structure and function of nucleic acids.[1] Due to their substantial negative charge, DNA and RNA electrostatically attract many cations as counterions. These ions surround the nucleic acids in a zone called the ion atmosphere.[2] The vast majority of counterions are invisible in crystal structures, even at a high resolution. It is not well understood how counterions behave. Four decades ago, 23Na nuclear magnetic resonance (NMR) was used to study interactions between DNA and Na+ ions,[3] although rapid 23Na quadrupole relaxation hampers detailed analyses. More recently, anomalous small-angle X-ray scattering enabled the physical detection of the ion atmosphere involving heavy alkali metal ions around DNA.[4] The ‘ion-counting’ method using atomic emission spectroscopy allowed a quantitative analysis of the ion atmosphere.[5] Computational studies of ion atmosphere have also been conducted, but do not necessarily yield good agreement with experimental data.[1] The dynamic aspects of counterions remain elusive, despite a long history of research.
An important dynamic process involving counterions is their release from the ion atmosphere upon DNA-protein association. This release has been considered to play a major role in thermodynamics and kinetics of DNA-protein interactions.[6] Ion pairing of DNA phosphates and protein basic side chains should force some counterions to leave as free ions, causing a significant increase in entropy. It was proposed that counterion release can explain the dependence of the equilibrium constants for DNA-protein association on salt concentration.[6] However, the strong dependence on salt concentration is also observed for protein-protein interactions, which do not appear to involve significant condensation and release of counterions.[7] The release process remains to be confirmed through a direct observation of the ions.
Here, using NMR spectroscopy, we investigate the dynamic properties of the ion atmosphere around DNA and directly observe counterion release upon DNA-protein association. For this purpose, we use 15N-labeled ammonium (15NH4+) ions and measure their diffusion in the presence of K+ ions. NH4+ and K+ ions have similar ionic radii and exert similar effects on activities of proteins and nucleic acids.[8] The slow 15N NMR relaxation of NH4+ ions allows us to precisely measure ionic diffusion that is influenced by the ion atmosphere and molecular association.
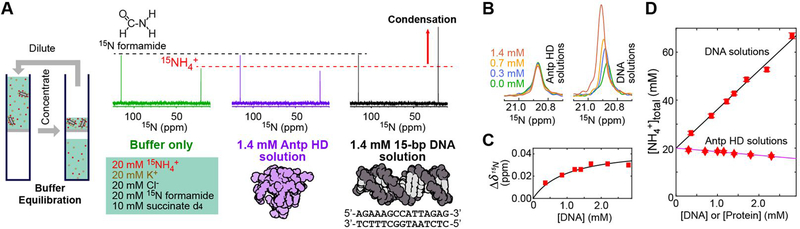
In this study, we used the fruit-fly Antennapedia homeodomain (Antp HD) and a 15-bp DNA duplex recognized by this protein. The thermodynamics of the Antp HD–DNA interactions have been investigated under various solution conditions in previous studies.[9] The dissociation constants for their specific DNA- protein complexes are 10−9–10−8 M at physiological ionic strengths.[9–10] Using centrifugal filters with a molecular weight cutoff of 3 kDa, solutions of the 15-bp DNA and solutions of the Antp HD were equilibrated with the pH 5.8 buffer shown in Figure 1A. Since counterions that are bound to DNA are unable to pass through the filter membrane, the total concentrations of cations in DNA solutions become higher compared with the buffer alone and protein solutions. This effect was clearly observed in the 15N NMR spectra (Figure 1A). The 15N resonance of NH4+ ions depended on the DNA concentration, indicating that the resonances of the free- and DNA-bound states were averaged due to fast exchange (Figure 1B–C). For the Antp HD solutions, the 15NH4+ signal exhibited little dependence on the protein concentration. As an internal control, 20 mM 15N formamide was used in each sample. This neutral compound is not condensed. Using the ratio of signal intensities of 15NH4+ to formamide, we measured the total concentration of 15NH4+ ions in each solution of the DNA or the protein (Figure 1D). These data show that 15NH4+ ions are condensed around the DNA, but not around the protein, and that the total concentration of 15NH4+ ions is a linear function of the DNA concentration, as predicted from Manning’s theory on counterion condensation.[6b]
Figure 1.
Condensation of 15NH4+ ions around DNA. (A) 1D 15N spectra recorded at 25˚C for solutions of the Antp HD (magenta) and the 15-bp DNA (black), each of which was equilibrated with the buffer shown in the green box. A 15N spectrum recorded for the buffer alone is also shown (green). (B) 15N NMR signals from 15NH4+ ions at different concentrations of the Antp HD and the 15-bp DNA. (C) Differences in the 15N chemical shift of 15NH4+ ions in the presence of various concentrations of the 15-bp DNA. (D) Total concentrations of 15NH4+ ions in the DNA or protein solutions after buffer equilibration. Each concentration was determined using the ratio of 15N NMR signal intensity of 15NH4+ to the intensity of the 15N formamide signal.
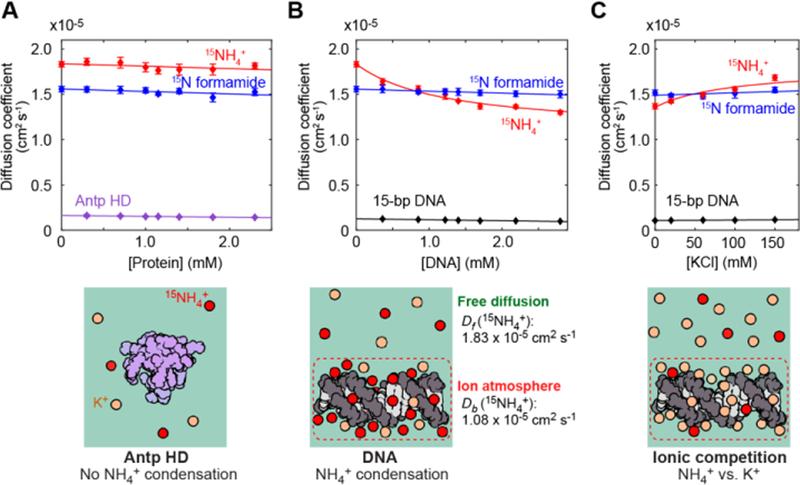
We measured the diffusion of 15NH4+ ions at 25˚C to investigate the dynamics of counterions around DNA using NMR. Since the 1H resonances of NH4+ ions were too broad to detect due to rapid hydrogen exchange with water, we used a 15N detection-based method for the diffusion measurements (Figures S1–3 in Supporting Information). Unfortunately, the small gyromagnetic ratio of 15N nuclei causes a lower sensitivity of detection. We circumvented this problem using nuclear Overhauser enhancement through 1H saturation, which drastically enhanced the ammonium 15N signal (Figure S3), allowing us to precisely determine the diffusion coefficient of 15NH4+ ions in each sample. The methodological details, including the effect of convection, are described in the Supporting Information. Figure 2A–B shows the diffusion coefficients of 15NH4+ ions measured for solutions of the 15-bp DNA or the Antp HD. Diffusion coefficients of 15NH4+ ions in the DNA solutions clearly depended on the DNA concentration, while those of formamide remained virtually constant (Figure 2B). In contrast, the 15NH4+ diffusion in the Antp HD solutions was not dependent on the protein concentration (Figure 2A). We also conducted diffusion measurements after adding KCl to the solution of 1.7 mM 15-bp DNA equilibrated with the same buffer (Figure 2C). The additional K+ ions should cause a decrease in the population of NH4+ ions within the ion atmosphere around DNA due to ionic competition. In fact, faster NH4+ diffusion was observed at higher concentrations of KCl. These results indicate that condensation of NH4+ ions around DNA slows NH4+ diffusion.
Figure 2.
Analysis of the behavior of NH4+ ions within the ion atmosphere. (A, B) Diffusion coefficients of 15NH4+ ions and 15N formamide in solutions of the 15-bp DNA (Panel B) and in solutions of the Antp HD (Panel A). Diffusion coefficients of the DNA and the protein are also plotted. (C) Competition between 15NH4+ and K+ ions for the ion atmosphere around DNA. The DNA concentration was 1.7 mM in this experiment on the ionic competition. Error bars represent the standard errors of the means for at least 3 replicates.
Using these data, we analyzed the diffusional properties of NH4+ ions within the ion atmosphere around DNA. Because NH4+ ions undergo rapid exchange between the free and DNA-bound states (Figure 1B), an apparent diffusion coefficient (Dapp) is given by Dapp = pfDf + pbDb,[11] where pf and pb are the populations of free and DNA-bound states, respectively, and Df and Db are their diffusion coefficients. Using this equation to fit to the DNA-concentration dependence data (see Supporting Information), we determined the Df and Db coefficients to be 1.83 × 10−5 cm2 s−1 and 1.08 × 10−5 cm2 s−1, respectively. If counterions are tightly bound to DNA, Db should be equal to the diffusion coefficient of DNA. However, the determined Db was 10-fold higher than the diffusion coefficient for the 15-bp DNA (0.10 × 10−5 cm2 s−1), suggesting that the 15NH4+ ions dynamically shifted their locations within the ion atmosphere. From the values of Df and Db coefficients along with Seki-Bagchi theory on the relationship between entropy and diffusion,[12] the increase in entropy due to the release of counterions is estimated to be 1.0 e.u. per ion. Although counterion release has been postulated to make a major entropic contribution to the binding free energy for DNA-protein association,[6c] the high mobility of counterions within the ion atmosphere may reduce the entropic increase. Our previous NMR studies revealed the highly dynamic nature of intermolecular ion pairs of Lys side-chain NH3+ groups and DNA phosphates.[13] Thus, the ionic interactions of DNA are highly dynamic.
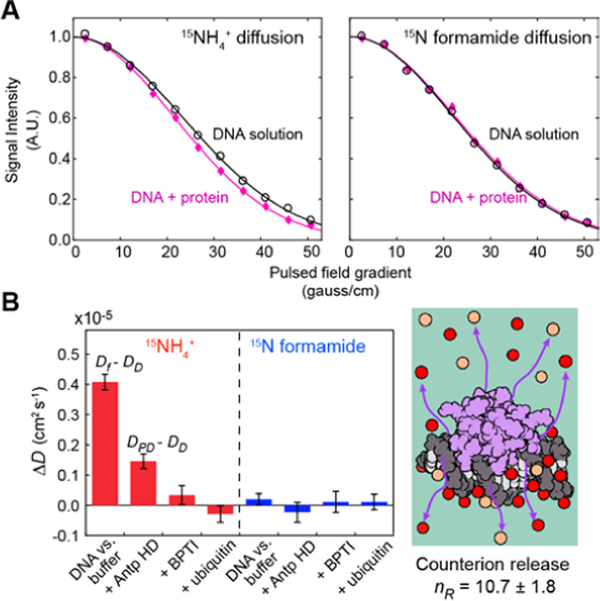
Because ionic diffusion is faster in the free state, the release of counterions upon DNA-protein association should cause an increase in the apparent diffusion coefficient of 15NH4+ ions. To examine this change for the molecular association of the Antp HD and the 15-bp DNA, we compared the diffusion of NH4+ ions in two samples. One was prepared by mixing a 185-μl solution of 2.8 mM 15-bp DNA with another 185-μl solution of 2.3 mM Antp HD equilibrated with the aforementioned buffer; the other was a control DNA solution prepared by mixing the DNA with the same volume of the buffer instead of the protein solution. Figure 3A shows the NMR data for the diffusion of 15NH4+ ions and 15N formamide. When DNA was mixed with the Antp HD and allowed to form the DNA-protein complex, 15NH4+ diffusion became significantly faster than that in the control sample of the 15-bp DNA mixed with the buffer alone, whereas formamide diffusion remained unchanged. We also conducted a corresponding experiment using 2.3 mM bovine pancreatic trypsin inhibitor (BPTI) or ubiquitin instead of the Antp HD. These proteins are similar in size. BPTI is positively charged and can nonspecifically interact with DNA,[14] which was confirmed through observation of NMR chemical shift perturbation. However, the increase in the NH4+ diffusion coefficient upon the addition of BPTI was insignificant (Figure 3B), suggesting that the counterions released upon the nonspecific BPTI-DNA association are not as many as those released upon the specific Antp HD-DNA association. Ubiquitin does not interact with DNA. In fact, the mixture of DNA with ubiquitin did not result in a significant difference in 15NH4+ diffusion, compared to the control (Figure 3B). Thus, the increase in the apparent diffusion coefficient of NH4+ ions observed for the Antp-DNA complex represents direct evidence of counterion release upon formation of the specific complex involving a large number of intermolecular ion pairs.
Figure 3.
The release of 15NH4+ ions upon association of Antp HD to the 15-bp DNA duplex. (A) NMR signal intensity modulation as a function of the strengths of the magnetic field gradient for solutions containing 1.4 mM 15-bp DNA in the presence (magenta) and absence (black) of 1.15 mM Antp HD. Uncertainty in signal intensity is smaller than each symbol. (B) Differences in measured diffusion coefficients. Data are compared for 1) the 1.4-mM DNA solution vs. the buffer, 2) the 1.4-mM DNA solution vs. the 1.4-mM DNA + 1.15-mM Antp HD solution; 3) the 1.4-mM DNA solution vs. the 1.4-mM DNA + 1.15-mM BPTI solution and 4) the 1.4-mM DNA solution vs. the 1.4-mM DNA + 1.15-mM ubiquitin solution.
As described in the Supporting Information, the total number of released counterions (nR) can be estimated using nR = nPφpc−1(DPD - DD) / (Df - DD), where nP is the number of phosphate groups per DNA (nP = 28 for the 15-bp DNA); φ, the binding occupancy of cation per DNA phosphate; pc, the fraction of DNA in the protein-bound state; and DD and DPD, the apparent diffusion coefficients of cations in the DNA and DNA-protein complex solutions, respectively. Values of DPD - DD and Df - DD are shown in Figure 3B. Using φ = 0.88 for double-stranded DNA,[6d] our data yield nR = 10.7 ± 1.8. As explained in the Supporting Information, this value appears to be consistent with the structural and dynamic properties of the Antp HD-DNA complex, although highly mobile regions involved in the DNA-protein association are invisible in the crystal structure. Thus, the NMR data on the ionic diffusion provide accurate information about counterion release upon the DNA-protein association.
In conclusion, we have demonstrated the NMR-based quantitative investigations of the ion atmosphere and the dynamics of counterions involved in macromolecular interactions. Although previous studies implicated counterion release using indirect data of equilibrium constants for DNA-protein association, there had been no direct evidence of counterion release. Our NMR-based method reveals the dynamic behavior of counterions around DNA and provides explicit evidence of counterion release through the direct observation of ions. Experimental data on the counterion dynamics will facilitate computational and theoretical studies to better understand the ion atmosphere and counterion release.
Experimental Section
All NMR experiments were conducted at 25˚C using a Bruker Avance III spectrometer equipped with a QCI cryogenic probe operated at the 1H frequency of 600 MHz. Other details are described in the Supporting Information.
Supplementary Material
Acknowledgements
We thank Dr. Montgomery Pettitt for useful discussion. This work was supported by Grant R35-GM130326 from the National Institutes of Health (to J.I.), and in part by Grant CHE-1608866 from National Science Foundation (to J.I.).
Footnotes
Supporting information for this article is given via a link at the end of the document.
References
- [1].Lipfert J, Doniach S, Das R, Herschlag D, Annu Rev Biochem 2014, 83, 813–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Chu VB, Bai Y, Lipfert J, Herschlag D, Doniach S, Curr Opin Chem Biol 2008, 12, 619–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].a) Bleam ML, Anderson CF, Record MT, Proc Natl Acad Sci U S A 1980, 77, 3085–3089; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Reuben J, Shporer M, Gabbay EJ, Proc Natl Acad Sci U S A 1975, 72, 245–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Das R, Mills TT, Kwok LW, Maskel GS, Millett IS, Doniach S, Finkelstein KD, Herschlag D, Pollack L, Phys Rev Lett 2003, 90. [DOI] [PubMed] [Google Scholar]
- [5].a) Bai Y, Greenfeld M, Travers KJ, Chu VB, Lipfert J, Doniach S, Herschlag D, J Am Chem Soc 2007, 129, 14981–14988; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Gebala M, Giambasu GM, Lipfert J, Bisaria N, Bonilla S, Li G, York DM, Herschlag D, J Am Chem Soc 2015, 137, 14705–14715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].a) Lohman TM, DeHaseth PL, Record MT Jr., Biophys Chem 1978, 8, 281–294; [DOI] [PubMed] [Google Scholar]; b) Manning GS, Acc Chem Res 1979, 12, 443–449; [Google Scholar]; c) Privalov PL, Dragan AI, Crane-Robinson C, Nucleic Acids Res 2011, 39, 2483–2491; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Record MT Jr., Lohman ML, De Haseth P, J Mol Biol 1976, 107, 145–158. [DOI] [PubMed] [Google Scholar]
- [7].a) Schreiber G, Fersht AR, Nat Struct Biol 1996, 3, 427–431; [DOI] [PubMed] [Google Scholar]; b) Vindigni A, White CE, Komives EA, Di Cera E, Biochemistry 1997, 36, 6674–6681. [DOI] [PubMed] [Google Scholar]
- [8].a) Ha JH, Capp MW, Hohenwalter MD, Baskerville M, Record MT Jr., J Mol Biol 1992, 228, 252–264; [DOI] [PubMed] [Google Scholar]; b) Hud NV, Schultze P, Sklenar V, Feigon J, J Mol Biol 1999, 285, 233–243; [DOI] [PubMed] [Google Scholar]; c) O’Brien MC, McKay DB, J Biol Chem 1995, 270, 2247–2250. [DOI] [PubMed] [Google Scholar]
- [9].a) Dragan AI, Li Z, Makeyeva EN, Milgotina EI, Liu Y, Crane-Robinson C, Privalov PL, Biochemistry 2006, 45, 141–151; [DOI] [PubMed] [Google Scholar]; b) Zandarashvili L, Nguyen D, Anderson KM, White MA, Gorenstein DG, Iwahara J, Biophys J 2015, 109, 1026–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Muller M, Affolter M, Leupin W, Otting G, Wüthrich K, Gehring WJ, EMBO J 1988, 7, 4299–4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Johnson CSJ, J Magn Reson Ser A 1993, 102, 214–218. [Google Scholar]
- [12].Seki K, Bagchi B, J Chem Phys 2015, 143, 194110. [DOI] [PubMed] [Google Scholar]
- [13].a) Anderson KM, Esadze A, Manoharan M, Brüschweiler R, Gorenstein DG, Iwahara J, J Am Chem Soc 2013, 135, 3613–3619; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Chen CY, Esadze A, Zandarashvili L, Nguyen D, Pettitt BM, Iwahara J, J Phys Chem Lett 2015, 6, 2733–2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Szopa J, Arch Immunol Ther Exp 1979, 27, 131–140. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.