Abstract
Background:
Immuno-suppressive cytokines have the potential to promote prostate cancer progression. Assessing their longitudinal changes may implicate mechanisms of progression, treatment resistance, and suggest new therapeutic targets.
Methods:
Thirty-seven men with biochemically recurrent (BCR) prostate cancer who received 6 months of androgen deprivation therapy (ADT) and were monitored until the time to PSA progression (TTPP) were identified from a completed phase III trial (). Serum samples were archived at baseline, three months after ADT, and at TTPP. Cytokine concentrations were quantified using a 36-parameter electrochemiluminescence assay. The Wilcoxon signed-rank sum test was used to compare observations between time points. Kaplan-Meier analysis was used to calculate TTPP dichotomized by cytokine values above or below the median. Pearson’s rank correlation coefficient was used to compare continuous variables.
Results:
Median TTPP was 399 days (range, 114 – 1641). Median PSA at baseline and progression were 8.5 ng/mL and 5.3 ng/mL, respectively. Twenty-three patients (62%) achieved undetectable PSA with ADT. Castrate levels of testosterone (< 50 ng/dL) after three months of ADT occurred in thirty-five patients (95%). TNF-α (P = 0.002), IL-23 (P = 0.002), and CXCL10 (P = 0.001) significantly increased from baseline to post-ADT. Certain cytokines correlated longitudinally: TNF-α correlated with IL-23 (r = 0.72, P < 0.001) and IL-8 (r = 0.59, P < 0.001) from baseline to post-ADT and to PSA progression. NLR (Neutrophil to Lymphocyte Ratio) correlated with IL-27 (r = 0.57, P < 0.001) and MIP-3α (r = 0.56, P < 0.001). Patients with a detectable PSA after ADT had elevated levels of IL-6 (P = 0.049) and IL-8 (P = 0.013) at PSA progression as compared to those with an undetectable PSA. There was a trend toward shorter TTPP in patients with TNF-α levels above the median (P = 0.042).
Conclusions:
Several innate cytokines were associated with biochemical recurrent prostate cancer.
Keywords: Biochemical recurrence, IL-8, TNF-α, IL-23
Introduction
Biochemical recurrence (BCR) in prostate cancer (PCa) is defined as a rise in prostate specific antigen (PSA), in the absence of radiographically detectable metastatic disease after definitive local therapy (e.g. radical prostatectomy or radiation) for primary disease. Approximately 30–50% of patients will develop BCR at 10 years following surgery or radiation.1 Treatment options for BCR include surveillance, salvage radiation, androgen deprivation therapy (either intermittent or continuous), and clinical trials. Patient-specific factors such as PSA doubling time, Gleason score, and time to BCR can assist with risk-stratification.2 However, predicting the duration of response to initial treatment remains challenging and there are few clinical parameters to guide treatment in this setting.
Proinflammatory and immunosuppressive cytokines mediate intracellular communication, regulate downstream gene transcription, and have been implicated in promoting carcinogenesis in a variety of tumor types including prostate cancer.3–5 In addition to those deleterious effects, certain cytokines can facilitate anti-tumor immunity by promoting effector T cell function. In that regard, a prospective, nested-case control study demonstrated an inverse relationship between the risk of developing prostate cancer and baseline levels of TH1 cytokines (IFN-γ and TNF-α), suggesting a potential role for cytotoxic T cells in tumor cell elimination at early stages of disease, consistent with the cancer immunoediting hypothesis.6 Conversely, cytokines secreted by myeloid and other immunosuppressive cellular compartments (e.g. IL-6 IL-23, IL-8, TNF-α) have been associated with progression to androgen-independent prostate cancer and shorter survival.7,8
Emerging data in a range of solid tumor types suggest that cytokine levels at treatment initiation, or changes during treatment, may be prognostic and/or predictive of outcomes with certain therapies.9 Additionally, elevated levels of cytokines, such as IL-8 and TNF-α, have been correlated with decreased survival in advanced prostate cancer.7,10–14 Cytokine data in intermediate stages of the disease, such as in men with BCR treated with ADT, are comparatively sparse. To address that issue, we performed longitudinal analyses of cytokine levels in men receiving a course of intermittent ADT as part of a clinical trial. Our initial hypothesis was that some of the cytokine changes described in later castration-resistant stages of disease might also be seen in earlier, hormone-sensitive stages. The overall goal of this study was to identify potential circulating biomarkers of response and / or resistance that might guide therapeutic decision-making.
Materials and Methods
Study Design and Patient Population
Patients were enrolled in a randomized, double-blinded trial examining the effect of adding thalidomide to a short-course (six months) of androgen deprivation therapy (ADT) in prostate cancer patients with biochemical recurrence (). Detailed methods and findings of the clinical trial have been previously published.15 Samples from the ADT + placebo group (prior to thalidomide crossover) were selected for this analysis. This multi-institutional IRB-approved study accrued 159 patients at eight academic centers (National Cancer Institute, Louisiana State University, University of Washington, Columbia University, Wayne State University, University of Minnesota, University of Pittsburgh, and Holy Cross Hospital, Fort Lauderdale, Florida). Eligible patients had previously received local definitive therapy for prostatic adenocarcinoma with radical prostatectomy, radiation, or cryosurgery. Eligible patients had an increasing PSA above their post-primary therapy PSA-nadir on two separate occasions, two weeks apart, with an absolute value greater than 1.0 ng/ml. Prior ADT was allowed if given more than 1 year prior to enrollment. Patients were required to have adequate hematologic, hepatic, and renal function as well as an ECOG performance status ≤ 2. Patients were excluded if there was radiographic evidence of metastatic disease or significant medical comorbidities. All enrolled patients received ADT, either leuprolide (22.5mg every three months) or goserelin (10.8mg every 3 months), for 6 months, after which they were randomized to receive either placebo or thalidomide 200mg daily until PSA progression. Patients were followed with clinical and laboratory assessments monthly. At the time of PSA progression, defined by the protocol as a return to on-study PSA value (minimum 1.0 ng/ml) or increased greater than 5 ng/ml, subjects received an additional 6 months of ADT followed with crossover to placebo or thalidomide, depending on initial randomization. Study assessments were continued until subsequent PSA progression, development of metastatic disease, or intolerable toxicity.
Cytokine analyses
Sera from patients enrolled at the National Cancer Institute were available for analysis. Samples were prospectively collected at monthly clinic visits with appropriate permissions as part of the clinical trial protocol. Each specimen was collected in a red top tube and underwent serum separation per standard protocol. Specimens were stored in aliquoted cryogenic vials without preservative at −80°C at The Frederick Central Repository. The specimens were transported on dry ice and thawed just prior to cytokine analysis. The MesoScale Discovery (MSD) multiplex electrochemiluminescence (MesoScale Diagnostics, Rockville, MD) assay was used to simultaneously quantify concentrations of 36 cytokines at baseline, after three months of androgen deprivation therapy, and at the time of PSA progression. All specimens were run in duplicate, using the V-PLEX Human Cytokine 36-plex kit according to the manufacturer’s directions. Concentrations were determined based on standard curves generated per plate. Sixteen cytokines were excluded from statistical analysis due the majority of samples (>60%) being below the lower limit of detection (IL-12p70, IL-1β, IL-2, IL-4, IL-8 (HA), GM-CSF, IL-17A, IL-21, IL-22, IL-23, IL-31, IL-1α, IL-5, TNF-β, IL-13, and Eotaxin-3). The following twenty cytokines were included in the final analysis: IFN-γ, IL-10, IL-6, IL-8, TNF-α, IL-12/IL-23p40, IL-15, IL-16, IL-7, VEGF, MCP-1, MCP-4, Eotaxin, CXCL10, MDC, TARC, MIP-1α, MIP-1β, IL-27, and MIP-3α.
Statistical Analysis
The primary statistical endpoints analyzed were treatment-related changes in cytokine levels after a short-course of ADT and the association of cytokine level changes with PSA progression. Principal component analyses did not reveal significant batch effects. A rescaling approach was utilized for cytokines that contained one or more data points below the lower limit of detection (LLD), including IFN-γ, IL-10, IL-6, MIP-1α, and TARC. Concentrations that registered below the LLD were treated as zero. The LLD value was subtracted from remaining data points accordingly, such that the analyses reported here may underestimate the degree of change. To facilitate comparisons between patients, percent-change in concentration of each cytokine between time points was used in analyses (i.e. a correlation matrix) to account for variability.
Descriptive statistics were used to describe the study population (Table 1), cytokine concentrations, and the percent change in cytokines between time points (Supp. Table 1). The Wilcoxon signed-rank sum test was used to compare paired observations between time points. All outliers were included in the analysis due to our limited sample size. Pearson’s rank correlation coefficient was used to compare continuous variables. False discovery rate (FDR) adjustment was used for multiple comparisons. The Kaplan-Meier method was used to calculate PSA progression dichotomized by cytokine values above or below the median. Statistical analysis and figure preparation were performed with Rstudio statistical software package version 1.1.463 (Rstudio, Boston, MA) and GraphPad Prism version 8.0 (GraphPad Software, San Diego, CA).
Table 1.
Clinicopathologic characteristics of cohort (n = 37).
| Parameter | Number | |
|---|---|---|
| Age at enrollment, median (range) | 68 (65 – 70) years | |
| Caucasian | 33 | |
| African American | 4 | |
| Prior definitive therapy | ||
| Radical prostatectomy only | 8 | |
| Radiation only | 6 | |
| Radical prostatectomy and radiation | 13 | |
| Radiation and hormonal blockade | 5 | |
| Radical prostatectomy, radiation, and hormonal blockade | 5 | |
| Gleason score at diagnosis | ||
| 5 | 2 | |
| 6 | 10 | |
| 7 | 16 | |
| 8 | 6 | |
| 9 | 3 | |
| Mean PSA levels (SD) | ||
| PSA at baseline (ng/ml) | 8.5 (13.44) | |
| PSA after ADT | 0.4 (0.73) | |
| PSA at progression | 5.3 (3.71) | |
| Mean Testosterone levels (SD) | ||
| T at Baseline | 343.96 (187.97) | |
| T at PSA progression | 348.09 (142.25) | |
| Mean DHT levels (SD) | ||
| DHT at Basline | 272.06 (186.42) | |
| DHT at PSA progression | 224.20 (107.12) | |
| Number of Patients with T suppression after ADT | ||
| < 50 ng/dL | 35 | |
| > 50 ng/dL | 2 |
Results
Clinical characteristics
Clinical and pathologic characteristics of the study population are summarized in Table 1. The most common Gleason score was 7 (16/37 patients, 43%). Ten subjects received prior hormonal therapy (27%) as part of their primary therapy regimen. Median time from study enrollment to PSA progression was 399 days (range, 114–1641). Twenty-three (62%) subjects achieved an undetectable PSA after three months of ADT, the other fourteen did not. Castrate-levels of testosterone (< 50 ng/dL) after three months of treatment with ADT occurred the majority of patients (35/37 =95%).
ADT is associated with changes in cytokine concentrations
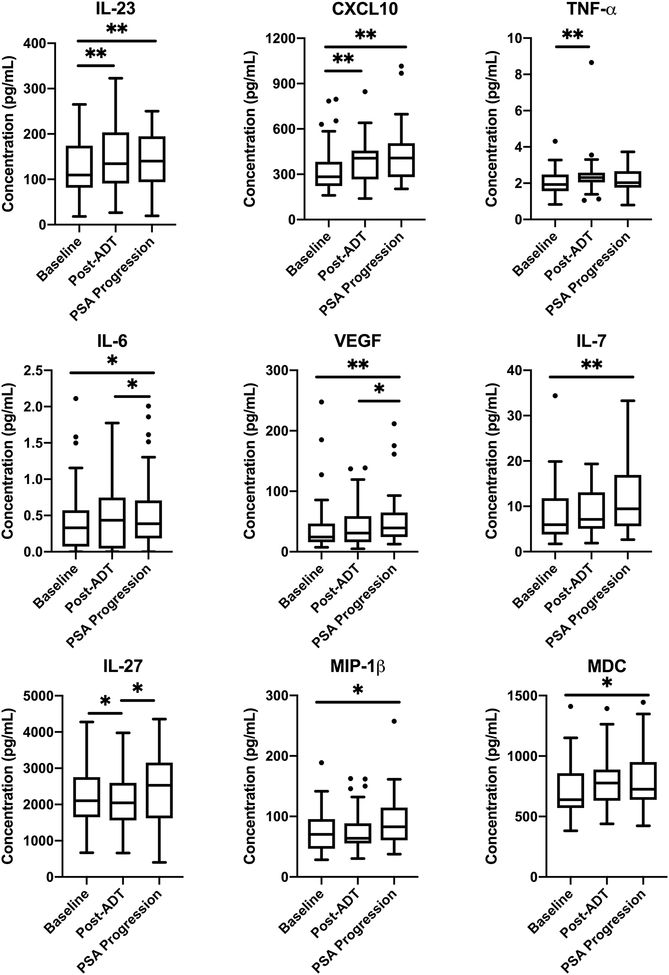
Nine cytokines demonstrated statistically significant changes between time points (Figure 1). Between baseline and 3 months after ADT, significant increases were seen in the tumor-promoting cytokines, TNF-α and IL-23, as well as in the anti-tumor cytokine, CXCL10. The pleiotropic cytokine IL-27 decreased over this time period (Supp. Table 1). From three months after ADT administration until PSA progression, increases in IL-27 and the angiogenic cytokine, VEGF, were observed. Curiously the pro-tumorigenic cytokine IL-6 was decreased at the time of PSA progression. Finally, in all cases, increases were observed in IL-23, IL-6, IL-7, CXCL10, VEGF, MDC, and MIP-1β from initial on-study baseline through 6 months of ADT until subsequent PSA progression. Although the potent and polyfunctional cytokine, IL-8, which mediates tumor cell proliferation and recruitment of increased inflammatory cells into the TME was numerically increased from baseline throughout all time points, those increases were not statistically significant (Supp. Table 1). The constellation of changes observed in circulating cytokines after ADT administration suggest that pro-tumorigenic changes may be occurring within the TME, ultimately tilting the balance in favor of tumor cell promotion. Further, these changes observed are unlikely to be result of generalized systemic inflammation as the NLR did not change significantly between time points and numerically decreased (1% change in median) from baseline to PSA progression (Supp. Table 1).
Figure 1. ADT is associated with changes in cytokine levels.
Cytokine concentrations at baseline, three months after ADT, and at time of PSA progression in all 37 analyzed patients. Horizontal lines represent the median, boxes the interquartile range (IQR), and whiskers the upper and lower quartiles. Wilcoxon signed-rank sum test was used to compare paired observations between different time points. Horizontal bars denote statistical significance between time points (P < 0.05 *, < 0.01 **). Outliers represented by black dots.
Innate cytokines correlate longitudinally in BCR
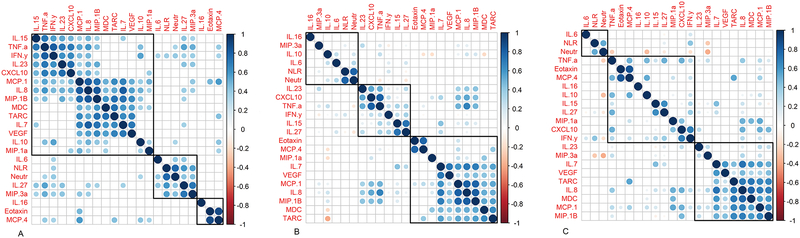
In the context of inflammation, cytokine and chemokine levels are orchestrated in a coordinated manner, i.e. in a TH1 response IL-2, TNF-α and IFN-γ are co-regulated.16 We therefore tested whether serum cytokine levels showed evidence of co-regulation over the course of ADT in BCR prostate cancer patients. For these analyses, we also included the neutrophil-to-lymphocyte ratio (NLR) and absolute neutrophil count given their demonstrated role as poor prognostic indicators in solid tumors, including prostate cancer.17–19 As shown in Figures 2A–2C, cluster analyses revealed a series of coordinately regulated cytokines, with clusters that varied over the time course of the study. For example, from baseline to 3 months post ADT, TNF-α was strongly correlated with IL-23 (r = 0.72, P < 0.001), CXCL10 (r = 0.70, P < 0.001), and IL-8 (r = 0.59, P < 0.001) (Figure 2A). At later time points (from 3 months post ADT to PSA progression), changes in TNF-α associated with those in the TH1 cytokine IFN-γ, the memory T cell promoting cytokine IL-15, and with IL-27 (Figure 2B). Between baseline and PSA progression, a dominant cluster of innate cytokines was consistent, this cluster includes IL-8, TARC, MDC and VEGF (Figure 2C). Interestingly, IL-8, which plays a major role in neutrophil recruitment and migration, was not significantly associated with the NLR in any of the clustering analyses performed. Taken together, these data support the notion that cytokines are coordinately regulated, and suggest it may be challenging to develop useful biomarkers based on measurements of a single cytokine or chemokine.
Figure 2. Innate immunosuppressive cytokines correlate longitudinally.
Correlation matrices of serum cytokine concentrations between time points, including A) from baseline to three months after ADT, B) from three months after ADT to time of PSA progression, and C) from baseline to time of PSA progression. Color intensity and the size of the circle are proportional to the strength of the correlation coefficient. Blue represents a positive correlation with darker colors approaching r = 1 and red represents a negative correlation with darker colors approaching r = −1. Only correlations with significance of ≤ 0.05 are shown. Empty spaces reflect correlations with p-value > 0.05. Clustering analysis was performed using H clust for 3 clusters. Pearson’s rank correlation coefficients were calculated and controlled for multiple comparisons using false discovery rates.
A Detectable PSA after ADT is associated with elevated levels of IL-8 and IL-6 at Progression
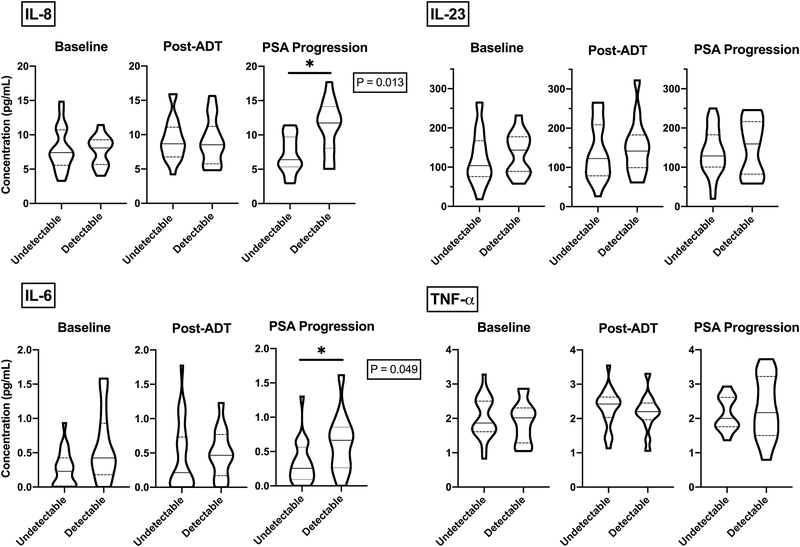
In prostate cancer, the depth of PSA response after ADT has been shown to be an independent predictor of survival.20–22 Corroborating those data, cytokines involved in disease progression and metastasis (IL-6, IL-8, IL-23, and TNF-α) were associated with shorter progression and survival.7,8,10,12,23,24 We hypothesized that levels of these innate cytokines would be relatively elevated in subjects who did not achieve a PSA response, i.e. patients who have a detectable PSA nadir after ADT. To test this hypothesis, median concentrations of IL-6, IL-8, IL-23, and TNF-α were compared between subjects with and without a PSA response (undetectable < 0.2 ng/mL vs. detectable > 0.2 ng/mL PSA) after 3 months of ADT. Pairwise comparisons between cytokine levels were performed at 3 time points: baseline, 3 months post ADT and at the time of PSA progression (Figure 3A–B). As per methods, adjustment for multiple comparisons was applied. Consistent with our hypothesis, IL-6 and IL-8 were both elevated at the time of PSA progression in patients who had a detectable PSA level after 3 months of ADT. These differences were significant at the time of PSA progression but not earlier. The other cytokines tested (IL-23 and TNF-α) did not show significant associations with PSA response to ADT at any of the three time points evaluated.
Figure 3. Lack of PSA Response to ADT is associated with elevations in immunosuppressive cytokines.
Violin plots depicting the frequency of distribution for IL-8, IL-6, IL-23, and TNF-α concentrations at baseline, three months after ADT, and at time of PSA progression in 37 patients dichotomized by PSA response after three months of ADT (undetectable, <0.2 ng/mL; detectable, > 0.2 ng/mL). Solid horizontal lines represent the median, dashed lines the upper and lower quartiles, width of the violin plot represents the proportion of the data located there. Wilcoxon signed-rank sum test was used to compare observations between different groups. Horizontal bars denote statistical significance between groups (P < 0.05 *).
Elevated TNF-α levels associated with shorter time to PSA progression
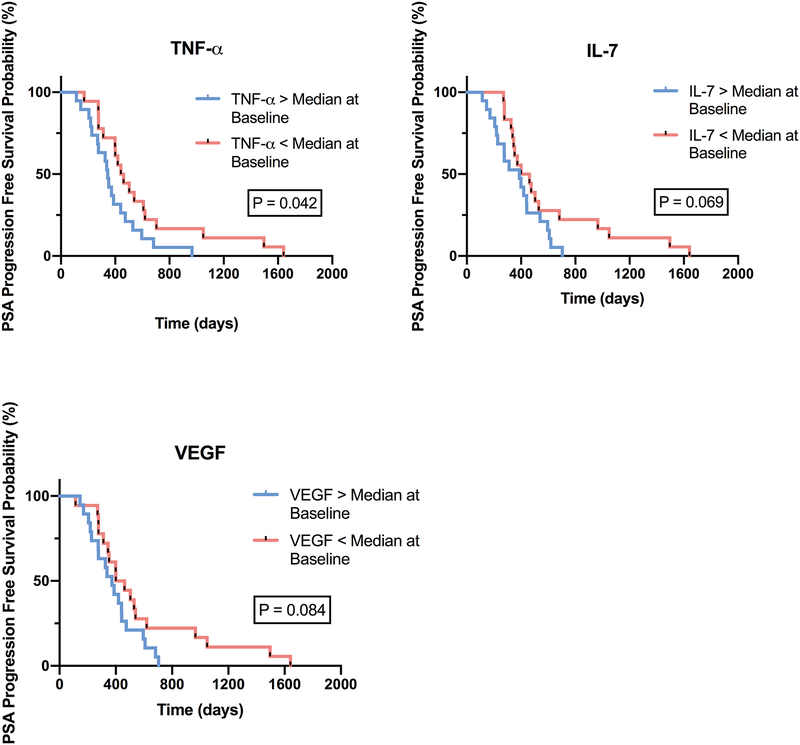
To determine whether levels of any of the cytokines studied have potential value as a positive or negative prognostic biomarker for disease progression (time to PSA progression), we performed Kaplan-Meier analyses for all 20 cytokines. As shown in Figure 4, time to PSA progression was shorter in patients with TNF-α levels above the median level prior to ADT administration (P = 0.042). There were also trends toward shorter time to PSA progression noted in subjects with VEGF and IL-7 above the median (P=0.084 and P=0.069, respectively) prior to ADT administration, but those were not statistically significant in relatively small sample set.
Figure 4. Elevated levels of immunosuppressive cytokines at baseline associated with shorter time to PSA progression.
Kaplan-Meier analysis of time to PSA progression-free survival in men with BCR treated with 6 months of ADT (n = 37) dichotomized by above or below median of TNF-α, IL-7, and VEGF concentrations at baseline.
Discussion
This analysis utilized archived serum samples from a previously conducted clinical trial to assess changes in circulating cytokines in men with BCR treated with a short course of ADT, using a state-of-the art analysis platform. These analyses were reasonably comprehensive, 20 analytes as well as NLR and absolute neutrophil count were included at three time points (baseline, post-ADT, and at PSA progression). As mentioned previously, immunosuppressive cytokines have been hypothesized to be causative in driving prostate cancer progression throughout all stages - from early tumor cell eradication, to driving benign prostatic hyperplasia through castration-resistant disease, and later to the development of resistance to cytotoxic treatments.8,13,14,25,26 Our initial hypothesis was that peripheral cytokine changes might be evident earlier, in the hormone-sensitive setting. And that similar to the CRPC setting, these changes might also correlate with survival. Our results support this hypothesis and add to this body of literature demonstrating that TNF-α may serve as a potential negative prognostic biomarker for PSA progression in patients treated with ADT, despite castrate-levels of testosterone. This finding requires validation in a second, likely larger, dataset.
We found that a number of serum cytokines significantly vary over a cycle of intermittent ADT. In particular, IL-23 was elevated post ADT, potentially consistent with a recently described role for this cytokine in mediating ADT resistance.7 IL-23 is generally perceived to be favorable to the growth of tumors as it drives the proliferation of IL-17 secreting T cells (TH17), and leads to downstream production of IL-6, TNF-α, GM-CSF, and IL-17.27 The innate cytokines TNF-α and CXCL10 were similarly elevated three months post ADT, potentially consistent with a model in which TNF-α plays a role at the forefront of a complex cytokine cascade mediated through nuclear factor-κB signaling.28 At the time of PSA progression, relative elevations (as compared to the three month time point) were noted in IL-6, VEGF and IL-27. These data are consistent with copious literature implicating IL-6 as a pro-inflammatory driver of prostate cancer progression.10,29–32 However these data show an association and do not prove causality.
We also found that the cytokines under study appear to be co-regulated to a large degree, for example IL-23 levels strongly correlated with those of CXCL10, TNF-α, IFN-γ. Although those data are in good agreement with the concept that orchestration of an immune response involves coordinated changes in multiple cytokine and chemokines, they highlight the notion that the implication of a single cytokine in inflammation-mediated prostate cancer progression is challenging. To our knowledge, this is the first report describing those correlations in the BCR setting.
In terms of clinical relevance, our data suggest TNF-α as a potential negative prognostic biomarker for patients receiving intermittent ADT. This finding suggests that patients with high TNF-α at baseline might require upfront treatment intensification or a continuous course of ADT. We were unable to identity a corresponding positive predictive biomarker, this may stem from the relatively small sample size, from inherent variability in cytokine levels, or from the relative heterogeneity of the subjects under study.
At the time of PSA progression, elevated levels of both IL-6 and IL-8 were significantly associated with a lack of PSA response. These findings were not seen at earlier time points. These data may reflect disease burden, or could implicate these cytokines as emerging inflammatory factors preventing an optimal response to ADT. In patients on ADT, the decision to initiate another cycle of therapy is frequently driven by a PSA “trigger” level, with a wide variation in the levels chosen in Phase III studies.33–35 From a patient perspective, a longer time off ADT is a favorable outcome, enabling the avoidance of side effects. In that regard, we found that TNF-α levels above the median were associated with a decreased time to PSA progression. Those data are broadly consistent with the notion that innate inflammation may play a role in PSA progression.
Weaknesses of this study include the relatively small sample size and the inherent heterogeneity in BCR PCa patients (Table 1). Strengths are that samples were archived at precise time points and carefully archived under controlled conditions. Another strength is the cytokine analysis platform utilized here, as well as the extensive spectrum of cytokines analyzed.
In future studies, we plan to validate these findings by a targeted interrogation of a larger sample set. Additionally, more in vitro and in vivo work focusing on the interaction between cytokines in the presence or absence of androgens might inform whether or not the changes observed were a result of androgen withdrawal or were reflective of underlying tumor biology. In pts with metastatic castration-sensitive recurrence, tumor lysates could be analyzed to assess the changes at the tissue and tumor microenvironment level and to determine the degree to which they correlate with those in the periphery.
Conclusions
In PCa patients on intermittent ADT, serum cytokines change over time in a coordinated manner. At the time of progression, elevated levels of the innate cytokines IL-6 and IL-8 are associated with failure to achieve a complete PSA response - despite castration-levels of testosterone. Further supporting a potential role for innate inflammation in prostate cancer progression, TNF-α levels above the median are associated with a foreshortened time to PSA recurrence.
Supplementary Material
Supplemental Table 1. Longitudinal assessment of serum cytokine concentrations at baseline, three months after ADT, and at time of PSA progression. (^ denotes those cytokines that were rescaled to account for values below LLD.)
Financial Support:
This project was made possible through a Prostate Cancer Foundation Challenge Award (PCF18CHAL07) to Dr. Charles Drake and a T32 Training Award (T32CA203703) to Dr. Jessica Hawley. This research was funded in part through the NIH/NCI Cancer Center Support Grant (P30CA013696).
Footnotes
Conflict of Interest Disclosure Statement: CGD is a co-inventor on patents licensed from JHU to BMS, has served as a paid consultant to for Bristol Myers Squibb (BMS), Compugen, Merck, Novartis, Roche-Genentech, Tizona and Werewolf pharmaceuticals, and has received sponsored research funding from the Bristol-Myers Squibb International Immuno-Oncology Network.
Supplementary information is available on The Prostate website.
References:
- 1.Kupelian PA, Mahadevan A, Reddy CA, Reuther AM, Klein EA. Use of different definitions of biochemical failure after external beam radiotherapy changes conclusions about relative treatment efficacy for localized prostate cancer. Urology. 2006;68(3):593–598. [DOI] [PubMed] [Google Scholar]
- 2.Paller CJ, Antonarakis ES. Management of biochemically recurrent prostate cancer after local therapy: evolving standards of care and new directions. Clin Adv Hematol Oncol. 2013;11(1):14–23. [PMC free article] [PubMed] [Google Scholar]
- 3.De Marzo AM, Platz EA, Sutcliffe S, et al. Inflammation in prostate carcinogenesis. Nat Rev Cancer. 2007;7(4):256–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hodge DR, Hurt EM, Farrar WL. The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer. 2005;41(16):2502–2512. [DOI] [PubMed] [Google Scholar]
- 5.Dranoff G Cytokines in cancer pathogenesis and cancer therapy. Nat Rev Cancer. 2004;4(1):11–22. [DOI] [PubMed] [Google Scholar]
- 6.Bhavsar NA, Bream JH, Meeker AK, et al. A peripheral circulating TH1 cytokine profile is inversely associated with prostate cancer risk in CLUE II. Cancer Epidemiol Biomarkers Prev. 2014;23(11):2561–2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calcinotto A, Spataro C, Zagato E, et al. IL-23 secreted by myeloid cells drives castration-resistant prostate cancer. Nature. 2018;559(7714):363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma J, Gray KP, Harshman LC, et al. Elevated IL-8, TNF-alpha, and MCP-1 in men with metastatic prostate cancer starting androgen-deprivation therapy (ADT) are associated with shorter time to castration-resistance and overall survival. Prostate. 2014;74(8):820–828. [DOI] [PubMed] [Google Scholar]
- 9.Sanmamed MF, Perez-Gracia JL, Schalper KA, et al. Changes in serum interleukin-8 (IL-8) levels reflect and predict response to anti-PD-1 treatment in melanoma and non-small-cell lung cancer patients. Ann Oncol. 2017;28(8):1988–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michalaki V, Syrigos K, Charles P, Waxman J. Serum levels of IL-6 and TNF-alpha correlate with clinicopathological features and patient survival in patients with prostate cancer. Br J Cancer. 2004;90(12):2312–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Torrealba N, Rodriguez-Berriguete G, Fraile B, et al. Expression of several cytokines in prostate cancer: Correlation with clinical variables of patients. Relationship with biochemical progression of the malignance. Cytokine. 2017;89:105–115. [DOI] [PubMed] [Google Scholar]
- 12.Lehrer S, Diamond EJ, Mamkine B, Stone NN, Stock RG. Serum interleukin-8 is elevated in men with prostate cancer and bone metastases. Technology in cancer research & treatment. 2004;3(5):411. [DOI] [PubMed] [Google Scholar]
- 13.Araki S, Omori Y, Lyn D, et al. Interleukin-8 is a molecular determinant of androgen independence and progression in prostate cancer. Cancer Res. 2007;67(14):6854–6862. [DOI] [PubMed] [Google Scholar]
- 14.Mahon KL, Lin HM, Castillo L, et al. Cytokine profiling of docetaxel-resistant castration-resistant prostate cancer. Br J Cancer. 2015;112(8):1340–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Figg WD, Hussain MH, Gulley JL, et al. A double-blind randomized crossover study of oral thalidomide versus placebo for androgen dependent prostate cancer treated with intermittent androgen ablation. J Urol. 2009;181(3):1104–1113; discussion 1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murphy KW, Casey Janeway’s Immunobiology. 9th edition ed. New York, NY: Garland Science; 2017. [Google Scholar]
- 17.Templeton AJ, McNamara MG, Seruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106(6):dju124. [DOI] [PubMed] [Google Scholar]
- 18.Ocana A, Nieto-Jimenez C, Pandiella A, Templeton AJ. Neutrophils in cancer: prognostic role and therapeutic strategies. Molecular cancer. 2017;16(1):137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yin X, Xiao Y, Li F, Qi S, Yin Z, Gao J. Prognostic Role of Neutrophil-to-Lymphocyte Ratio in Prostate Cancer: A Systematic Review and Meta-analysis. Medicine. 2016;95(3):e2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choueiri TK, Xie W, D’Amico AV, et al. Time to prostate-specific antigen nadir independently predicts overall survival in patients who have metastatic hormone-sensitive prostate cancer treated with androgen-deprivation therapy. Cancer. 2009;115(5):981–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hussain M, Tangen CM, Higano C, et al. Absolute prostate-specific antigen value after androgen deprivation is a strong independent predictor of survival in new metastatic prostate cancer: data from Southwest Oncology Group Trial 9346 (INT-0162). J Clin Oncol. 2006;24(24):3984–3990. [DOI] [PubMed] [Google Scholar]
- 22.Kwak C, Jeong SJ, Park MS, Lee E, Lee SE. Prognostic significance of the nadir prostate specific antigen level after hormone therapy for prostate cancer. J Urol. 2002;168(3):995–1000. [DOI] [PubMed] [Google Scholar]
- 23.Zabransky DJ, Smith HA, Thoburn CJ, et al. Lenalidomide modulates IL-8 and anti-prostate antibody levels in men with biochemically recurrent prostate cancer. Prostate. 2012;72(5):487–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopez-Bujanda Z, Drake CG. Myeloid-derived cells in prostate cancer progression: phenotype and prospective therapies. J Leukoc Biol. 2017;102(2):393–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Platz EA, Kulac I, Barber JR, et al. A Prospective Study of Chronic Inflammation in Benign Prostate Tissue and Risk of Prostate Cancer: Linked PCPT and SELECT Cohorts. Cancer Epidemiol Biomarkers Prev. 2017;26(10):1549–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu C, Zhu Y, Lou W, Cui Y, Evans CP, Gao AC. Inhibition of constitutively active Stat3 reverses enzalutamide resistance in LNCaP derivative prostate cancer cells. Prostate. 2014;74(2):201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tesmer LA, Lundy SK, Sarkar S, Fox DA. Th17 cells in human disease. Immunological reviews. 2008;223:87–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Balkwill F Tumour necrosis factor and cancer. Nat Rev Cancer. 2009;9(5):361–371. [DOI] [PubMed] [Google Scholar]
- 29.Yu SH, Zheng Q, Esopi D, et al. A Paracrine Role for IL6 in Prostate Cancer Patients: Lack of Production by Primary or Metastatic Tumor Cells. Cancer Immunol Res. 2015;3(10):1175–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu SH, Maynard JP, Vaghasia AM, De Marzo AM, Drake CG, Sfanos KS. A role for paracrine interleukin-6 signaling in the tumor microenvironment in prostate tumor growth. Prostate. 2019;79(2):215–222. [DOI] [PubMed] [Google Scholar]
- 31.Ishii K, Sasaki T, Iguchi K, et al. Interleukin-6 induces VEGF secretion from prostate cancer cells in a manner independent of androgen receptor activation. Prostate. 2018;78(11):849–856. [DOI] [PubMed] [Google Scholar]
- 32.Duscharla D, Reddy Kami Reddy K, Dasari C, Bhukya S, Ummanni R. Interleukin-6 induced overexpression of valosin-containing protein (VCP)/p97 is associated with androgen-independent prostate cancer (AIPC) progression. Journal of cellular physiology. 2018;233(10):7148–7164. [DOI] [PubMed] [Google Scholar]
- 33.Calais da Silva F, Calais da Silva FM, Goncalves F, et al. Locally advanced and metastatic prostate cancer treated with intermittent androgen monotherapy or maximal androgen blockade: results from a randomised phase 3 study by the South European Uroncological Group. Eur Urol. 2014;66(2):232–239. [DOI] [PubMed] [Google Scholar]
- 34.Crook JM, O’Callaghan CJ, Duncan G, et al. Intermittent androgen suppression for rising PSA level after radiotherapy. N Engl J Med. 2012;367(10):895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hussain M, Tangen CM, Berry DL, et al. Intermittent versus continuous androgen deprivation in prostate cancer. N Engl J Med. 2013;368(14):1314–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Longitudinal assessment of serum cytokine concentrations at baseline, three months after ADT, and at time of PSA progression. (^ denotes those cytokines that were rescaled to account for values below LLD.)