Abstract
Studies investigating the associations between genetic or environmental factors and Parkinson’s disease have uncovered a number of factors shared with cardiovascular disease, either as risk factors or manifestations of cardiovascular disease itself. Older age, male sex and possibly type 2 diabetes are examples. On the other hand, coffee consumption and physical activity are each associated with a lower risk of both Parkinson’s disease and cardiovascular disease. This observation raises questions about the underlying pathophysiological links between cardiovascular disease and Parkinson’s disease. There is evidence for common mechanisms in the areas of glucose metabolism, cellular stress, lipid metabolism and inflammation. On the other hand, smoking and total/LDL cholesterol appear to have opposite associations with cardiovascular disease and Parkinson’s disease. Thus, it is uncertain whether or not treatment of cardiovascular risk factors will impact on the onset or progression of Parkinson’s disease. The available data suggest that a nuanced approach is necessary to manage risk factors such as cholesterol levels once the associations are better understood. Ultimately, the choice of therapy may be tailored to a patient’s comorbidity profile. This review presents the epidemiological evidence for both concordant and discordant associations between cardiovascular disease and PD, discusses the cellular and metabolic processes that may underlie these links, and explores the implications this has for patient care and future research.
Keywords: Parkinson’s disease, pathogenesis, cardiovascular, risk factors
Introduction
During the search to understand the etiology of Parkinson’s disease (PD), a vast array of studies has investigated the associations between genetic or environmental factors and PD. Many of the associations that have been found have links to cardiovascular (CV) disease, either as risk factors or manifestations of CV disease itself. These observations raise questions about the underlying pathophysiological links between CV disease and PD, and may have important implications for the appropriate clinical care of individuals with PD who have CV risk factors requiring treatment. Due to the limitations of observational research, definitive conclusions regarding the causal nature of associations cannot be drawn, but the epidemiological studies have occurred in parallel with substantial laboratory research that may provide clues. The aim of this viewpoint is to examine known or reported associations, and highlight potential interconnection as they relate to our current understanding of PD pathogenesis. One result of this effort may be suggestions for research that would improve our understanding of the pathophysiology of PD. In addition, it may help guide treatment for PD patients in which the competing risks of CV disease and PD need to be balanced.
Cardiovascular Risk Factors and Parkinson’s disease
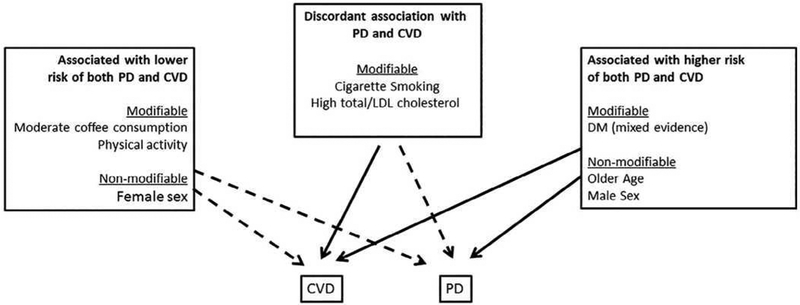
Important heart-brain connections are indisputable and multi-faceted.1 Potentially shared CV risk factors have been in the forefront of dementia research for the past several decades.2 The possibility of etiological connection is strengthened by well-designed longitudinal studies that found associations of midlife diabetes (DM), hypertension (HTN), obesity, and hypercholesterolemia with higher risk of late-life cognitive impairment and dementia.3 Potential links between heart disease and PD also have attracted attention in recent years, but have been largely limited to studying CV comorbidities4 and cardiac autonomic dysfunction in PD patients (e.g., orthostatic hypotension).5 This is despite the fact that many potential risk factors that have been investigated for PD are classic CV risk factors (e.g., DM, HTN, and obesity) and the fact that common mechanistic hypotheses (e.g., oxidative stress and chronic inflammation) have been proposed for both diseases.6 However, understanding these associations between these classic CV risk factors and PD is not straightforward, as they are often complex, sometimes controversial or even counterintuitive (Figure 1).
Figure 1: Associations between cardiovascular risk factors and Parkinson’s disease.
Green arrows indicate inverse associations between the risk factor and disease. Red arrows indicate positive associations between the risk factor and disease. CVD=cardiovascular disease; PD = Parkinson’s disease.
Factors that have concordant associations with cardiovascular risk and PD
Compared to a sedentary lifestyle, physical activities, even at modest levels, are associated with lower risks of CV diseases and stroke.7 The inverse association with leisure-time physical activity and PD is one of the most consistent epidemiological findings, supported by multiple longitudinal and well-designed case-control studies.8–11 There are, however, caveats. First, the risk reduction is most consistently observed for moderate to vigorous activities.8–11 Second, findings on early-life physical activity in relation to PD risk is much less consistent than that for late-life.9–11 Given the long prodromal stage of PD development, reverse causality cannot be excluded. Finally, the biological mechanisms underlying exercise and a lower PD risk are largely speculative, but may involve increased neuroplasticity and brain-derived neurotrophic factors12 and a reduction in neuroinflammation.13,10
Moderate coffee consumption (3–5 cups/day) is associated with lower risk of multiple CV outcomes.14 Coffee is a complex mixture of compounds that have diverse and sometimes antagonistic roles in CV health, which may explain the likely U-shaped associations of coffee consumption and CV risk.15 The association of coffee consumption and PD appears to be monotonically protective16, 17 and the hypothetical mechanism has been focused on caffeine as a nonspecific adenosine A2A receptor antagonist.18
At least for coffee consumption and physical activity, apparently disparate mechanisms appear to be involved, leaving no obvious unifying pathway or biology despite the concordant associations.
Factors that have discordant associations with cardiovascular versus PD risk
According to the Global Burden of Diseases study, approximately 25% of men and 5.4% of women worldwide are daily smokers, making cigarette smoking one of the most preventable causes of chronic diseases including CV disease.19 The association of smoking with PD, however, is inverse and understanding its basis is complicated. Smokers have about 50% lower risk of developing PD than non-smokers.20, 21 The relationship cannot be explained by higher mortality among smokers (i.e., the competing risk hypothesis), however, this does not necessarily imply causality. Given the prolonged and largely unknown prodromal stage of PD, alternative hypotheses such as reverse causation and confounding by personality are very difficult to exclude. Answering this causal inference question has significant public health implications. If smoking indeed reduces PD risk or delays its onset as indirectly suggested by some studies,22, 23 the decreasing trend in smoking may further increase the burden of PD upon our ever growing aging populations.24
Also discordant are the relationships with serum cholesterol. Although not entirely consistent, epidemiological studies have found that higher total or LDL cholesterol is associated with lower PD risk,25–28 and slower PD progression,29, 30 in clear contrast to their detrimental role in CV health. The evidence is reasonably robust, including support from multiple prospective cohorts25–27. If future studies show causality, there will be important clinical implications for statin use in PD patients or in individuals at risk for PD.27, 31, 32
Cardiovascular risk factors with mixed evidence or null associations with PD risk
There is controversy about whether DM is more prevalent in patients who are later diagnosed with PD. Interestingly, most cohort studies describe a modestly increased risk of PD after a diagnosis of DM (Table 1a) whereas most case-control studies observed no association or even a decreased risk of PD in patients with DM (Table 1b). The studies are heterogeneous regarding the demographics of the populations, the definitions of the outcome and exposure, and the time between the diagnosis of DM and the observation of PD. There have been three recent meta-analyses including cohort studies (pooled adjusted relative risk of 1.38, 95% CI 1.18–1.62),33 case-control studies (OR 0.75, 95% CI 0.58–0.98)34 or both types (confirming these discordant effect estimates).35 One possible explanation for the discrepancy between the results from cohort and case-control studies could be the introduction of survival bias in the latter study design because of an increased mortality among the diabetic patients leading to an inverse relationship between DM and PD in case-control studies. A prospective cohort study observed that the risk of PD was greater in patients with a short duration of DM than in longer-standing DM patients and this was not explained by a selective mortality in those with longer DM duration.36 In contrast, a higher risk of PD has been observed in those with DM duration >10 years in two much larger studies.37, 38 The results of the later studies support a causal relationship between DM and PD. A large cohort study including more than 14,000 patients with PD yielded a higher risk of PD in those with young onset DM.37 The authors explain their observation by genetic effects having more impact in the young onset DM population whereas the association of DM with PD is rather linked to lifestyle and environmental factors in the older population.
Table 1a:
Diabetes (Cohort studies)
| Author (Year) | Source population | Country | Sample size | Definition of Diabetes & PD, covariates | Results | Adjusted variables | Remarks |
|---|---|---|---|---|---|---|---|
| De Pablo-Fernandez et al. 201837 | Hospital-based cohort of Type 2 DM patients and diabetes-free controls | UK | 14’252 PD patients |
PD: 1st hospital admission for PD Exclusion of individuals with a coded diagnosis of cerebrovascular disease, vascular/drug-induced parkinsonism, or normal pressure hydrocephalus DM: Hospital admission for type 2 DM |
Overall: HR 1.32 (95% CI 1.29–1.35) Patients aged 25–44 y at the time of the 1st admission for DM: 3.81 (95% CI 2.84–5.11), result based on small numbers (58 PD patients) Women: 1.42 (95% CI 1.37–1.47) Men: 1.27 (95% CI 1.23–1.30) |
Age, sex, year of cohort entry, region of residence, socioeconomic status |
Sensitivity analyses: exclusion of patients with <1 yr between DM and PD: same results Limitations: Potential selection bias caused by restriction to hospitalized cases (i.e., more severe DM) |
| Yang et al. 2017159 | National Health Insurance claims database Mean follow-up 7.3 y |
Taiwan | 1’782 PD patients |
PD: ≥3 Dx in ambulatory care or ≥1 Dx in inpatient care DM: Same as for PD, Dx based on ADA criteria |
Overall : HR 1.19 (95% CI 1.08–1.32) Women 1.29 (95% CI 1.12–1.49) Men : 1.12 95% CI (0.97–1.30) |
Age, sex, insurance premium, residential area, occupation, CCI, schizophrenia and bipolar disorder, prescription of flunarizine, MCP or zolpidem | Exclusion of patients with <1 yr between DM and PD |
| Sun et al. 2012160 | National Health Insurance claims database | Taiwan | 2’422 PD patients |
PD: Outpatient claims or hospitalization records (≥1 year after cohort entry) DM: Prevalent Dx plus ≥1 DM Dx during follow-up |
Overall: HR 1.61 (95% CI 1.56–1.66) Women : HR 1.70 (95% CI 1.63–1.77) Men: HR 1.51 (95% CI 1.44–1.57) Men (21–40 y): HR 2.10 (95% CI 1.01–4.42) Additional adjustment for medical visits : Overall HR 1.37 (95% CI 1.32–1.41) |
Age, sex, geographic area, urbanization status, medical visits, hypertension, hyperlipidemia, cardiovascular disease | Results for men (21–40 ys) based on 6 PD cases |
| Xu et al. 201138 | National Institutes of Health-AARP Diet and Health Study | USA | 1’565 PD patients |
PD: Self-reported, validated by the treating physician DM: Self-reported |
OR 1.41 (95% CI 1.20–1.66) Only patients with DM duration at baseline ≥10 y: OR 1.75 (95% CI 1.36–2.25) |
Age, sex, race, education, smoking, coffee consumption, BMI, physical activity | Sensitivity analysis: exclusion of cases with stroke, heart disease, cancer, or poor/fair health: similar results |
| Palacios et al. 2011114 | Cancer Prevention Study II Nutrition Cohort Mean follow-up 6.4 y |
USA | 656 PD patients |
PD: Incident, confirmed by neurologist or medical record review DM: Self-reported at baseline |
HR 0.88 (95% CI 0.62–1.25) | Age, sex, smoking, diet, alcohol/coffee consumption, BMI, education, physical activity, pesticide exposure | Sensitivity analyses: exclusion of PD cases during first 5 y of follow-up: similar results |
| Driver et al. 200836 | Physicians Health Study (randomized trial) Median follow-up 23.1 y |
USA | 556 PD patients |
PD: Self–reported (90% accurate according to validation study) DM: Self–reported (Type 2 DM) |
Men:
RR 1.34 (95% CI 1.01–1.77) Association significantly modified by BMI (increased risk of PD with low BMI) |
Age, smoking, alcohol consumption, BMI, hypercholesterolemia, hypertension, physical activity | Increased risk with shorter DM duration -> no causal association |
| Simon et al. 200740 | Nurses’ Health Study and Health Professionals Follow-up Study Mean Follow-up: 22.9 y/12.6 y |
USA | 530 PD patients |
PD: Self-report, confirmed by treating physician (15%), neurologist (82%), or by review of medical records (3%) DM: Self-reported physician’s Dx (validated) PD and DM status assessed at baseline and every 2 ys thereafter |
With updated history of DM: RR 1.04 (95% CI 0.74–1.46) Only baseline info on DM: RR 1.12 (95% CI 0.69–1.81) |
Age, sex, smoking |
Patients with prevalent stroke excluded Additional adjustment for BMI, physical activity, hypertension, cholesterolemia, alcohol/coffee consumption, diet, NSAID use yielded similar results |
| Hu et al. 2007161 | Prospective study based on cross-sectional surveys in five geographic areas Mean follow-up 18 y |
Finland | 633 PD patients |
PD: Data from the National Insurance Institution register, confirmed by two neurologists DM: Self-report, hospital discharge diagnoses or drug claims Assessment of covariates by questionnaires |
Overall: HR 1.85 (95% CI 1.23–2.80) Men: HR 1. 08 (95% CI 1.03–3.15) Women: HR 1.93 (95% CI 1.05–3.53) |
Age, study year Additional adjustments for BMI, systolic blood pressure, cholesterol, education, physical activity smoking, alcohol/coffee/tea consumption yielded similar results |
Only baseline info on DM included Similar results in several sensitivity analyses |
| Grandinetti et al. 199439 | Honolulu Heart Program Follow-up: 26 ys |
USA | 58 PD patients |
PD: Hospital records, death certificates, or medical records of neurologists DM: Self-report plus physical examination at baseline |
RR 1.20 (95% CI 0.67–2.12) | Age | Main objective of the study: assessment of the impact of cigarette smoking on the risk of PD |
AARP = American Association of Retired Persons; ADA = American Diabetes Association; BMI = Body mass index; CCI = Charlson Comorbidity Index; CI = Confidence interval; DM = Diabetes mellitus; Dx = Diagnosis, HR = Hazard ratio; NSAID: Non-steroidal anti-inflammatory drugs; MCP = metoclopramide; OR = Odds ratio; PD = Parkinson disease; RR = Relative risk; Rx = Prescription; UK = United Kingdom; yr/ys = year(s)
Table 1b:
Diabetes (Case-control studies)
|
Author (Year) |
Source population | Country | Sample size | Definition of Diabetes & PD, covariates | Results | Adjusted variables | Remarks |
|---|---|---|---|---|---|---|---|
| Skeie et al. 201370 | Norwegian ParkWest Study from western and southern Norway | Norway | 212 PD patients |
PD: Incident PD cases, identified through neurology departments according to Gelb criteria162 DM: Medical and drug history by self-report, referral letters, medical records from hospital and GPs, medical examination |
OR 1.94 (95% CI 0.82–4.57) | Not stated | |
| Savica et al. 201243 | Records-linkage system of the Rochester Epidemiology Project All residents from Olmsted County |
USA | 196 PD patients |
PD: Incident PD cases, 2 of four cardinal signs, no other cause and responsive to L-Dopa (validated approach) DM: Review of medical records (physician’s diagnosis or use of antidiabetic drugs) |
OR 0.67 (95% CI 0.31–1.48) | Age, sex, smoking, coffee consumption | No recall bias possible since diagnoses of comorbidities were documented before the onset of PD |
| Schernhammer et al. 2011163 | Nationwide hospital records (Danish Hospital Register) | Denmark | 1’931 PD patients |
PD: Hospitalization or outpatient visit for PD plus ≥1 antiPD medication DM: Had to be present at least 2 ys before PD
|
Exposure: DM diagnosis: OR 1.36 (95% CI 1.08–1.71) OR 1.50 (95% CI 1.02–2.22) women OR 1.29 (95% CI 0.97–1.72) men Onset of PD <60 y: OR 2.68 (95% CI 1.04–6.91) Exposure: antidiabetic drug use >2ys prior to PD diagnosis: OR 1.35 (95% CI 1.10–1.65) Onset of PD <60 y: OR 3.07 (95% CI 1.65–5.70) |
Age, sex | Exclusion of PD patients with diagnosis of Alzheimer <2 y before PD and with prescription of PD inducing medication 180 days prior to PD: similar results |
| Miyake et al. 201044 | PD cases and controls recruited from hospitals in two regions | Japan | 249 PD patients |
PD: Cases included within 6 y of onset of PD, diagnosed by neurologists according to the UK PD Society Brain Bank clinical diagnostic criteria DM: Based on antidiabetic drug treatment (information from questionnaires) |
OR 0.38 (95% CI 0.17–0.79) Women: OR 0.39 (95% CI 0.11–1.20) Men: OR 0.34 (95% CI 0.11–0.91) |
Age, sex, region of residence, smoking, education, physical activity, BMI, alcohol/coffee consumption, dietary glycemic index | |
| D’Amelio et al. 2009164 | Outpatients consecutively recruited at the Neurological Department | Italy | 318 PD patients |
PD: 2 of four cardinal signs, exclusion criteria: 2nd-ary causes of parkinsonism, DIP 180 days prior to PD Dx, or cognitive decline within one yr after PD Dx DM: Self-report (questionnaire) validated by review of medical records (80% valid), plus antidiabetic drug use |
OR 0.4 (95% CI 0.2–0.8) | Age, sex, BMI, smoking, alcohol/coffee consumption, years of education | |
| Becker et al. 2008165 | Primary care database representative of UK population | UK | 3’637 PD patients |
PD: Dx recorded by GP plus ≥2 Rx for anti-PD drugs, no drugs inducing parkinsonism 6 months prior to PD Dx DM: Dx recorded by GP |
OR 0.95 (95% CI 0.80–1.14) | Age, sex, smoking, BMI, diabetes, Asthma/COPD, ischemic heart disease, heart failure, stroke, arrhythmia, hyperlipidemia, epilepsy, affective and neurotic disorders, schizophrenia, dementia | |
| Scigliano et al. 200647 | Hospitalized patients to neurology department | Italy | 178 PD patients |
PD: Bradykinesia plus tremor, rigidity, or postural instability + good response to L-Dopa 58% with PD duration ≤1yr 30% 1–4ys, 12% ≥4ys DM: Patients with antidiabetic medication in medical records |
OR 0.30 (95% CI 0.13–0.72) | Age, sex | Exclusion of patients with atypical parkinsonism and with DIP Control from the hospitalized population potentially more unwell than the general population |
| Powers et al. 200646 | From neurology and general practice clinics of the Group Health Cooperative HMO | USA | 362 PD patients |
PD: Incident cases, diagnosed by neurologist or GP (2 of 4 cardinal signs), no drugs causing PD within 12 months prior to PD Dx DM: Questionnaire + chart review |
Men:
OR 0.52 (95% CI 0.28–0.97) Women: OR 0.80 (95% CI 0.35–1.83) |
Age, sex, ethnicity, smoking, education | |
| Ho et al. 198951 | Individuals living at homes for the elderly | HongKong | 35 PD patients (not necessarily incident cases) |
PD: Clinical examination by 3 examiners plus assessment at geriatric clinic, positive response to L-Dopa Exclusion criteria: history of cerebrovascular disease, DIP DM: Self-reported |
OR 1.6 (95% CI 0.5–5.1) | Age, sex | Very low number of included/exposed cases (6 patients with DM) |
BMI = Body mass index; CI = Confidence interval; COPD = Chronic obstructive pulmonary disease; DIP = Drug induced parkinsonism; DM = Diabetes mellitus; Dx = Diagnosis, GP = General Practitioner; HMO = Health maintenance organization; OR = Odds ratio; PD = Parkinson disease; Rx = Prescription; UK = United Kingdom; yr/ys = year(s)
There have been fewer studies published focusing on the effect of a previous diagnosis of HTN on the risk of PD (Tables 2a and 2b) and no meta-analysis of the available data has yet been performed. In a small cohort study of 58 PD patients, no statistically significant increased risk of PD in association with prior HTN was found.39 More recent cohort studies found a null result in 530 PD patients,40 and a statistically significant increased risk of PD in patients with HTN only for women.41 Additionally, ten case-control studies examined the association between HTN and subsequent risk of PD42–51, five of them yielding a statistically significantly reduced risk of PD,44, 45, 47–49 whereas the other five studies (with small sample sizes) showing no effect.42, 43, 46, 50, 51 The judicious conclusion from these studies is that an effect size, if present, is small.
Table 2a:
Hypertension (HTN) (Cohort studies)
|
Author (Year) |
Source population | Country | Sample size | Definition of Diabetes & PD, covariates | Results | Adjusted variables | Remarks |
|---|---|---|---|---|---|---|---|
| Qiu et al. 201141 | Seven consecutive population surveys on representative samples from 6 geographic regions Mean follow-up 18.8.ys |
Finland | 794 PD patients |
PD: From National Social Insurance Institution register, Dx confirmed by 2 specialists, exclusion of patients with history of stroke at baseline Blood pressure: Measured at study sights according to WHO guidelines |
Reference: <130/80 mmHg Women: 130–139/80–89 mmHg: HR 1.63 (95% CI 1.07–2.47) >140/90 mmHg: HR 1.62 (95% CI 1.09–2.42) Men: 130–139/80–89 mmHg: HR 0.94 (95% CI 0.64–1.39) >140/90 mmHg: HR 0.90 (95% CI 0.63–1.28) |
Age, sex, study year, education, smoking, alcohol/tea/coffee consumption, BMI, physical activity, DM, cholesterol, use of antihypertensive agents | |
| Simon et al. 200740 | Nurses’ Health Study and Health Professionals Follow-up Study Mean Follow-up: 22.9 y/12.6 y |
USA | 530 PD patients |
PD: Self-report, confirmed by treating physician (15%), neurologist (82%), or by review of medical records (3%) HTN: Self-reported physician’s Dx(validated), SBP >160 mmHg or DBP >90 mmHg or use of antihypertensive agents |
RR 0.96 (95% CI 0.80–1.15) | Age, sex, smoking | |
| Grandinetti et al. 199439 | Honolulu Heart Program | 58 PD patients |
PD: Hospital records, death certificates, or medical records of neurologists HTN: self-report plus physical examination at baseline |
RR 1.25 (95% CI 0.68–2.28) | Age | Main objective of the study: assessment of the impact of cigarette smoking on the risk of PD |
BMI = Body mass index; CI = Confidence interval; DBP = Diastolic blood pressure; Dx = Diagnosis; HR = Hazard ratio; HTN Hypertension; PD = Parkinson disease; RR = Relative risk; SBP = Systolic blood pressure; WHO = World health Organization; yr/ys = year(s)
Table 2b:
Hypertension (HTN) (Case-control studies)
| Author (Year) | Source population | Country | Sample size | Definition of Diabetes & PD, covariates | Results | Adjusted variables | Remarks |
|---|---|---|---|---|---|---|---|
| Vikdahl et al. 201542 | Population from a catchment area in Northern Sweden | Sweden | 84 PD patients |
PD: Incident cases, diagnosed by 2 neurologists according to UK PD Society Brain Bank clinical diagnostic criteria HTN: From crosslink to The Northern Sweden Health and Disease Study database, via questionnaire |
HR 0.98 (95% CI 0.96–0.99) | Matching: age, sex, year of health survey, geographic area Adjustment: age, BMI, physical activity |
Comorbidities were diagnosed 2–8 y before onset of motor symptoms |
| Savica et al. 201243 | All residents from Olmsted County (Records-linkage system of the Rochester Epidemiology Project) |
USA | 196 PD patients |
PD: Incident PD cases, 2 of four cardinal signs, no other cause and responsive to L-Dopa (validated approach) HTN: Medical records (physicians’ diagnosis or use of antihypertensive drugs) |
OR 0.99 (95% CI 0.63–1.55) | Age, sex, smoking, coffee consumption | No difference between men and women No recall bias possible since Dx of comorbidities were documented before the onset of PD |
| Miyake et al. 201044 | PD cases and controls recruited from hospitals in two regions | Japan | 249 PD patients |
PD: Cases included within 6 y of onset of PD, diagnosed by neurologists according to the UK PD Society Brain Bank clinical diagnostic criteria HTN: Based on antihypertensive drug treatment (information from questionnaires) |
Overall: OR 0.43 (95% CI 0.29–0.64) Women: OR 0.47 95% CI (0.28–0.78) Men: OR 0.38 (95% CI 0.19–0.72) |
Age, sex, region of residence, smoking, education, physical activity, BMI, alcohol/coffee consumption, dietary glycemic index | |
| Becker et al. 200845 | Primary care database representative of UK population | UK | 3’637 PD patients |
PD: Dx recorded by GP plus ≥2 Rx for anti-PD drugs, no drugs inducing parkinsonism 6 months prior to PD Dx HTN: Dx recorded by GP |
OR 0.83 (95% CI 0.74–0.92) | Age, sex, smoking, BMI, diabetes, Asthma/COPD, ischemic heart disease, heart failure, stroke, arrhythmia, hyperlipidemia, epilepsy, affective and neurotic disorders, schizophrenia, dementia | |
| Powers et al. 200646 | From neurology and general practice clinics of the Group Health Cooperative HMO | US | 362 PD patients |
PD: Incident cases, diagnosed by neurologist or GP (2 of 4 cardinal signs), no drugs causing PD 12 months prior to PD Dx, no other causes of parkinsonism High blood pressure: Questionnaire + chart review |
Men:
OR 0.80 (95% CI 0.55–1.17) Women: OR 1.62 (95% CI 1.00–2.62) |
Age, sex, ethnicity, smoking, education | |
| Scigliano et al. 200647 | Hospitalized patients to neurology department | Italy | 178 PD patients |
PD: Bradykinesia plus tremor, rigidity, or postural instability + good response to levodopa 58% with PD duration ≤1yr 30% 1–4ys, 12% ≥4ys HTN: Patients on antihypertensive medication |
OR 0.59 (95% CI 0.37–0.92) | Age, sex | Exclusion of patients with atypical parkinsonism and with DIP Controls were drawn from the hospitalized population: potentially more unwell than the general population |
| Paganini-Hill et al. 200148 | Residents of retirement community | USA | 395 PD patients (not necessarily incident cases) |
PD: Review of hospital discharge diagnoses or death certificates or physicians’ Dx mentioned in follow-up questionnaire HTN: History of HTN or use of antihypertensive medication (both self-reported) |
Hypertension OR 0.71 (95% CI 0.56–0.89) Current use of antihypertensive medication OR 0.62 (95% CI 0.48–0.80) |
Age, sex, smoking, alcohol/coffee consumption, number of children, Vitamin A/C | |
| McCann et al. 199849 | Patients recruited from hospitals, residential care centres and community groups | Australia | 224 PD patients |
PD: According to diagnostic criteria of Calne et al166 HTN: self-reported |
OR 0.3 (95% CI 0.18–0.42) | Age, sex, rural residency, family history of PD, stroke, ingestion of well, spring or bore water | |
| Semchuk et al. 199350 | Population-based case register of Calgary residents with neurologist-confirmed idiopathic PD | Canada | 130 PD patients |
PD: Confirmed by neurologists HTN: Self-reported |
Cases and controls did not differ regarding history of hypertension | Age, sex | |
| Ho et al. 198951 | Individuals living at homes for the elderly | HongKong | 35 PD patients (not necessarily incident cases) |
PD: Clinical examination by 3 examiners plus assessment at geriatric clinic, positive response to L-Dopa HTN: Self-reported |
OR 0.9 (95% CI 0.3–2.4) | Age, sex | Very low number of included/exposed cases (7 patients with hypertension) |
BMI = Body mass index; CI = Confidence interval; DIP = Drug induced parkinsonism; Dx = Diagnosis, HMO = Health maintenance organization; HTN = Hypertension; OR = Odds ratio; PD = Parkinson disease; Rx = Prescription; UK = United Kingdom; yr/ys = year(s)
Many other CV risk factors have been studied in relation to PD risk, but the data are largely null or, at best, mixed. In general, epidemiological studies found limited evidence that obesity52, 53 was associated with the risk of developing PD. More studies have examined alcohol consumption in relation to PD risk. Although a meta-analysis of earlier case-control studies suggests a weak inverse relationship,54 recent prospective studies suggest an overall null relationship.55, 56 One recent cohort study analyzed the risk of PD associated with a previous diagnosis of metabolic syndrome and its components57 (Table 3). In this analysis, metabolic syndrome was associated with a statistically significant decreased risk of PD (RR 0.50, 95% CI 0.30–0.83), as was increased plasma fasting glucose (RR 0.56, 95% CI 0.32–0.98). Elevated blood pressure was not associated with a change in risk of PD (RR 1.07, 95% CI 0.55–2.07). However, the exposures were only measured at baseline of the 30 years of follow-up and the study included only 89 patients with PD. A Mediterranean dietary pattern has been associated with reduced risk of CV disease in a number of studies.58 Preliminary evidence from cross-sectional studies suggests that this dietary pattern may also be negatively associated with PD,59 as well as prodromal PD.60
Table 3:
Metabolic syndrome (Cohort study)
| Author (Year) | Source population | Country | Sample size | Definition of Diabetes & PD, covariates | Results | Adjusted variables | Remarks |
|---|---|---|---|---|---|---|---|
| Sääksjärvi et al. 201557 | Mini-Finland Health Survey (in 40 areas of Finland) Follow-up 30 y |
Finland | 89 PD patients |
PD: Data from the National Insurance Institution register, based on clinical diagnostic criteria, incident cases, Dx confirmed by two neurologists Metabolic syndrome: ≥3 of the following components:167
|
Metabolic syndrome: RR 0.50 (95% CI 0.30–0.83) Plasma fasting glucose: <5.6 mmol/L: reference ≥5.6 mmol/L: RR 0.56 (95% CI 0.32–0.98) Elevated blood pressure: RR 1.07 (95% CI 0.55–2.07) |
Age, sex, education, smoking, alcohol/coffee consumption, physical activity, serum Vit D |
Limitations: Lack of repeated measurements of exposure variables |
The relationship between hyperhomocysteinaemia and cardiovascular risk remains unclear, with many epidemiological studies suggesting an association, while interventional trials of homocysteine lowering have thus far failed to demonstrate any advantage (for review see61). There is, however, considerable interest in the potential role of B12 and folic acid supplementation to enhance the methylation of homocysteine to methionine, made particularly necessary in PD due to the impact of chronic levodopa use on elevated homocysteine levels. In the setting of low B12 levels it has been proposed that homocysteine metabolism to methionine exploits betaine as a cofactor which in consequence has negative effects on acetylcholine production, and potential negative effects on cholinergic process such as gait, balance and cognition (for a recent review see62).
As illustrated in the tables, the studies of DM and HTN and PD are very heterogeneous in terms of sample size, definition of comorbid diseases, and definition of PD. Furthermore, exclusion criteria and the kind and number of confounding variables included in the multivariate analyses also varied. Moreover, factors such as duration of comorbid disease, medication use, and additional biases (e.g. selection bias of included individuals, recall bias in case-control studies assessing exposure via self-report etc.) may have also influenced the results. A further methodological challenge in large database studies is the possibility of diagnostic misclassification. In the context of vascular risk factors and PD, the possibility of misclassifying vascular parkinsonism as PD must be considered.
Challenges in studying cardiovascular risk factors in PD etiology.
Like PD, DM, HTN, and the metabolic syndrome are predominantly diseases of the elderly. They also have in common a rather subtle onset that can obscure the observed occurrence sequence. The common co-occurrence of multiple CV risk factors make it difficult to disentangle their relationship with PD risk. In addition, most of the classic CV risk factors involve diet and lifestyle that constantly change throughout one’s lifetime, influenced by education, socioeconomics, religion, as well as overall health and the aging process. Late-onset sporadic PD, is a slowly progressive degenerative disease that takes years, if not decades, to develop before a clinical diagnosis becomes possible. Many factors may be active during this decades-long prodromal stage, affecting both the onset of PD and its progression. Furthermore, a range of nonmotor symptoms (e.g., cognitive and personality changes, hyposmia) and subtle motor signs may arise within the prodromal PD period. Although empirical data are limited, many of these symptoms and signs may potentially affect diet and lifestyle. A prospective study suggested that PD patients tend to lose weight about 2–4 years before clinical diagnosis, despite a decrease in physical activity and increase in calorie intake.63 This exceedingly long and dynamic PD prodromal development is critically important in studying PD etiology, but to date, poorly understood. Ideally, future etiological studies of PD should account for these complexities in PD prodromal development. Longitudinal and repeated evaluation from an early age will be an important methodologic feature.
Cardiovascular comorbidity and PD
Suggestive links between CV disease and PD are not only restricted to risk factors, but also manifest CV disease. Newly diagnosed PD patients may be at a statistically significant increased risk for a subsequent myocardial infarction (MI) based on a recent study using data from the National Health Insurance database in Taiwan, which found the hazard ratio of MI in incident PD patients to be 1.67 (95% CI 1.15–2.41).64 Studies assessing the cause of death in patients with PD have yielded inconsistent results with respect to the frequency of a diagnosis of ischemic heart disease (IHD) as the proximate cause of death in PD patients compared to the general population: an increased risk of (IHD-related) death in one earlier study using primary care data from the UK (HR 2.6, 95% CI 1.5–3.4),65 no changed risk compared to the general population (HR 1.1, 95% CI 0.6–2.0),66 as well as a lower proportion of IHD-related deaths in the PD population (13% vs 23% of deaths67 and 12% vs 19% of deaths).68 At the time of the PD diagnosis, IHD seems to be as frequent as in non-PD controls: an OR of 1.05, 95% CI 0.93–1.19 was reported from a study from the UK including 3,637 incident PD cases and the same number of PD-free controls.69
A previous diagnosis of stroke has been found to be more prevalent in PD patients than in non-PD controls in a smaller study from Norway (OR 5.00, 95% CI 1.44–17.35).70 An increased risk for a first-time PD diagnosis after a stroke may in part be explained by the vascular changes and by ischemic brain damage caused by a cerebrovascular accident. One larger study from the UK showed an increased risk of ischemic stroke after the PD diagnosis (OR 1.55, 95% CI 0.98–2.46).69
Taken together there is evidence, albeit not definitive, that the risk of incident MI and stroke may be increased following the diagnosis of PD. These data need to be interpreted with caution because of the possibility of ascertainment bias due to increased contact with the health care system after PD diagnosis.
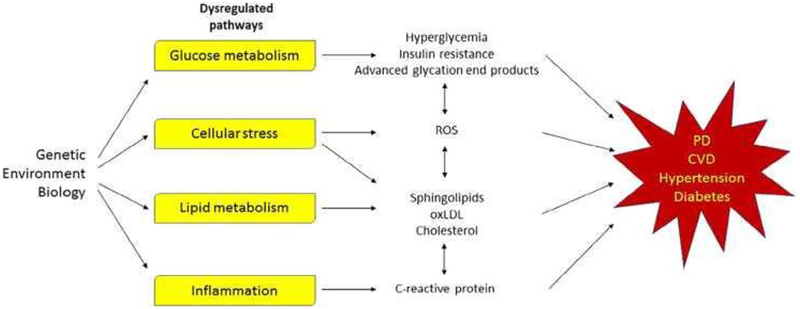
Contributions from the basic sciences
The pathophysiological explanation for the risk factors and comorbidities associated with CV disease and PD are presently unknown, but both chronic diseases share dysregulated pathways including inflammation and metabolism.71–73 Pathways and their potential relationships to CV disease, PD and their risk factors are summarized in Figure 2.
Figure 2: Pathways dysregulated in Parkinson’s disease and cardiovascular disease.
Yellow boxes represented pathways that are dysregulated in both conditions. CVD = cardiovascular disease; PD = Parkinson’s disease.
Glucose, lipid and cholesterol metabolism
Hyperglycemia and insulin resistance, low-grade inflammation and overproduction of reactive oxygen species and advanced glycation end products are thought to contribute to an elevated risk of both CV disease and PD. The implications of poor glucose regulation for CV health is well known; among DM patients, CV disease is the leading cause of death. The brain consumes about 25% of the body’s glucose to fuel oxidative metabolism. Hyperglycemia is particularly detrimental to nigrostriatal dopaminergic neurons that are rich in mitochondria, have high levels of iron ions that promote the production of highly reactive free hydroxyl radicals, and low levels of the antioxidant glutathione. This combination of characteristics may be a factor in the susceptibility of substantia nigra pars compacta dopamine neurons in patients with poor regulation of glucose metabolism. Consistent with this situation is the observation that in early stage PD patients in the De Novo Parkinson Cohort, disease progression was faster in participants who had CV disease risk factors, unregulated blood glucose, high uric acid levels and inflammation.74
Changes in lipid metabolism also play a role in both CV disease and PD. Oxidized low-density lipoproteins (oxLDL) are a major contributor to atherosclerotic plaque formation. OxLDL increase the expression of arginase, which competes with endothelial nitric oxide for arginine, reduces nitric oxide (NO) bioavailability and promotes atherosclerosis progression.75 Idiopathic PD patients have higher plasma oxLDL than controls, but it is not clear whether this is important in disease initiation and/or progression.76
Accumulation of the sphingolipid ceramide impairs insulin action, is a modulator of mitochondrial and ER stress, promotes apoptosis, and potentially links CV disease, insulin resistance, low-grade inflammation77 and PD. In a study of participants in the prospective PREDIMED (Prevención con Dieta Mediterránea) trial, plasma ceramide concentrations were linked to non-fatal acute MI, non-fatal stroke, and CV death.78 PD is also associated with altered sphingolipid metabolism.79 Ceramides and sphingomyelins are altered in postmortem PD brain tissue compared to the controls.80 Some forms of ceramide in the plasma of PD patients are higher in individuals with dementia compared to non-demented patients.81, 82 In addition, mutations in the SMPD1 gene that encode sphingomyelinase is correlated with an increased risk of PD.83–88 Mutations in the GBA gene that encodes glucocerebrosidase, which produces ceramide from glucocerebroside, are also associated with PD.89, 90 In the lysosome, sphingomyelinase and glucocerebrosidase hydrolyze sphingolipids to produce ceramide. Sphingomyelin can modify the expression of α-synuclein.91 Because the degradation of overproduced or pathological forms of α-synuclein depends on sphingomyelinase, changes in ceramide abundance may play a central role in PD pathology.92 An additional central role has been proposed for ceramide metabolism in the pathobiology of PD based on retromer dysfunction and mitochondrial defects.93 Together, these studies suggest that an imbalance of lipids may result in mitochondrial and endolysosomal dysfunction that leads to neuronal death in PD. Activating ceramidase, an enzyme that converts ceramide to sphingosine, would reduce ceramide levels and be potentially beneficial for treating CV disease, PD, insulin resistance and inflammation.94
One very interesting conundrum sometimes seen in medicine is when a given intervention may have opposite effects on different disorders. The relationship of cholesterol to the heart and PD is one excellent example. It is well-established that in people with elevated cholesterol, cholesterol-lowering drugs like statins have beneficial effects on CV health.95 As introduced above, significant literature has provided evidence that circulating cholesterol also may be related to PD, yet the interpretation of the evidence has not been straightforward. Early case-control studies found that higher plasma cholesterol was associated with lower PD prevalence47, 96–98 and later prospective studies showed that low cholesterol predated the diagnosis of PD.26, 27, 40, 99, 100 Moreover, higher baseline cholesterol has been linked to slower PD progression,101 better cognitive and motor performance,30 as well as delayed age of PD onset.102
Despite this trend, the observed cholesterol-PD relationship may not be causal. PD diagnosis may prime for adoption of a “healthier” lifestyle, thereby leading to lower cholesterol. Alternately, an unknown behavioral (e.g., smoking) or medical (e.g., use of statin) confounder may play a role or lower plasma cholesterol simply may reflect metabolic or non-motor changes that are associated with PD. Indeed, although one often thinks of cholesterol as being related to the CV system, the brain is the most cholesterol-rich organ in the body (accounting for ~25% of the total cholesterol). In the adult brain it is synthesized primarily by astrocytes and then transported to neurons via endocytosis and interaction with the LDL receptor (LDLR) and apolipoprotein E,103 thus the cholesterol in brain is made mainly de novo,104, 105 and there is limited exchange of cholesterol across the blood brain barrier (BBB).106 There is, however, evidence for the uptake of LDL particles and other apolipoproteins across the BBB, possibly via the LDLR and/or LDLR-related proteins, and oxysterols also may mediate peripheral-central cholesterol communication.107
Another fascinating association relates to the APOE gene. The least common ε2 allele is represented in only 8% of the population, but individuals with an ε2 allele have a propensity for lower plasma LDL-cholesterol levels, whereas the ε4 is linked to higher LDL-cholesterol levels.108 Yet while the ε2 allele is linked to a number of beneficial outcomes in terms of CV disease and lower risk of Alzheimer’s disease (AD), some studies,109, 110 but not all,111 have associated it with higher risk of PD whereas the more common ε4 allele is associated with poorer CV disease outcomes and a significantly increased risk of AD,112, 113 but is associated with lower PD risk.114 There are fascinating mechanisms that may be relevant, for example, a large clinical study provided evidence that lipids and lipoproteins may affect dopamine neuron-specific signaling cascades.115 Other studies show that cholesterol recycling may be linked to PD,109, 110, 114 and related genes are associated with increased PD risk116 or are affected in animal models of PD117 or PD itself.118
Despite the literature linking serum/plasma total- and LDL-cholesterol to PD,26, 27, 30, 40, 47, 96–102 the cause of the association is not known and further complicated by the compartmentalization of brain and peripheral cholesterol. An investigation of a potential causal relationship between circulating cholesterol levels and PD took into consideration age, gender, APOE polymorphisms, smoking history, statin, and several related gene single nucleotide polymorphisms. Based on propensity score methods, lower total- and LDL-cholesterol were inversely associated with PD suggesting that circulating total- and LDL-cholesterol levels may influence PD risk.119, 120 A recent study assessed whether brain cholesterol metabolism is related to PD by quantifying fasting plasma levels of both a brain and peripheral cholesterol metabolite. The data showed that the brain-derived cholesterol metabolite was inversely linked to PD and was relatively stable over time, suggesting that the numerous associations noted above may have a mechanistic basis.121
There are many possible mechanisms that may be involved. Cholesterol is essential for synaptogenesis,103 and there may be higher cholesterol turnover during the compensatory repair of injured neuronal pathways as higher levels of cholesterol metabolites are found in postmortem brain and more cholesterol catabolic metabolites in cerebrospinal fluid from PD patients.122, 123 In addition, the (S)24-OH cholesterol metabolite is known to be lower in PD patients121, is formed solely in the brain, and is reported to be a positive allosteric modulator of the N-methyl-D-aspartate glutamate receptor.124 Indeed, glutamate may activate the synthetic enzyme CYP46A1 allosterically, thereby increasing the production of (S)24-OH.125 The down-regulation of CYP46A1 leads to a compensatory decrease in cholesterol synthesis and consequent decreases in geranylgeraniol, a key metabolite in synaptic plasticity.126, 127 Cholesterol has, however, many cellular functions, and a great deal of additional research is necessary to elucidate the actual mechanisms that may be involved.
There is controversy over whether the cholesterol-PD association is actually a result of the effects of cholesterol lowering agents (specifically statins) as opposed to a biological factor related to disease etiology. Statins have been suggested to be neuroprotective for PD,128–130 yet a prospective study in the Atherosclerosis Risk in Community (ARIC) cohort found that statin usage was associated with increased future risk of PD.27 Most recently, an analysis of the large MarketScan national claims database in the US found that statins were positively associated with PD diagnosis.31 Although these data suggest caution to proposing statins as being neuroprotective for PD, it did not deter the launch of a trial of simvastatin as a neuroprotective agent.131 This and future research will hopefully settle these issues.
Inflammation
Inflammation plays a key role in the development and progression of CV disease,132 DM,133 and PD.134 Chronically elevated levels of C-reactive protein (CRP) are associated with all three diseases. In CV disease, inflammation is involved initially with the recruitment of leukocytes to the arterial wall and later with the rupture of unstable plaques. CRP is most likely involved with complement activation, apoptosis, endothelial NO synthase inhibition, vascular cell activation, monocyte recruitment, lipid accumulation, thrombosis, and pro-inflammatory cytokine formation.135 CRP may activate the mechanistic target of rapamycin (mTOR) signaling and TGF-α/Smad3 pathways, which could increase renal fibrosis and lead to DM136, 137 and increase risk of PD.
Brain and gut inflammation play a role in the development and progression of PD. Of note is the fact that autoreactive T lymphocytes, autoantigen presentation, and microglial activation are present in PD patients. The recent identification of α-synuclein-specific T cells in PD patients suggests that PD shares similarities with autoimmune disorders.138, 139 There is strong evidence to support the hypothesis that α-synuclein deposition in PD patients begins in the gut and travels through the vagus nerve into the central nervous system (CNS).140 It is possible that the adaptive immune system may be primed against a-synuclein deposition in the gut. PD patients also have an increased abundance of peripheral pro-inflammatory cytokines and chemokines that act on CNS endothelial cells that form the BBB, thus increasing vascular permeability141 and making the brain more susceptible to circulating immune cells, antibodies, and pro-inflammatory cytokines.
Next steps for research and translation to the clinic
Addressing CV risk
In the context of a neurodegenerative process like PD, patients can ill afford to have additional causes for neuronal dysfunction. Evidence indicating that PD symptom severity is worse in the presence of microvascular disease is consistent with this idea.142 High CV disease risk scores are associated with higher PD motor scores, and worse cognitive performance, but nevertheless PD patients with high CV risk scores are frequently not treated with statins,143, 144 despite some evidence of their beneficial effects in this subgroup without PD.145 As alluded to already, there is also evidence that suggest that the use of statins may increase PD risk, suggesting that this important issue should be approached cautiously.27, 31, 119
One theoretical approach to this would be to undertake randomized trials testing interventions using conventional CV primary prevention approaches to determine if they have an impact on the rate of development or progression of symptom severity in PD. Given the possible detrimental effects of some interventions (e.g., statins, see above, or aggressive treatment of HTN in PD patients with autonomic dysfunction) patients would have to be clearly informed of the possible risks, and close data safety monitoring employed. Personalized approaches based on CV risk score146, 147 could be designed, although long term follow-up and large sample sizes would likely be necessary to provide a clear test of any hypothesis. Whether equipoise exists for such a trial is critically important, especially given that some trial participants would be randomized not to receive routine CV disease treatments. Arguably, rather than embarking on a logistically and ethically challenging trial, we suggest that it might instead be best to devote greater efforts to ensure PD physicians consider their patients holistically and encourage them to treat patients’ CV risk along with PD symptoms.
Candidate neuroprotective therapeutics
There is considerable overlap between those agents that have been shown to be useful in addressing CV risk factors, and therapeutic candidates for neuroprotection in PD. For example, treatments for DM may be effective in reducing CV risk in DM patients,148 and are now also the subject of interest for neuroprotective trials in PD.
A key question that arises in considering how best to design trials to assess potential benefits in PD relates to the theoretical mechanism(s) of action of these drugs. It is possible that benefits from anti-diabetic drugs in PD might simply relate to their same peripheral mechanism of action (e.g., glucose lowering) that might cause reduced α-synuclein glycation,149, 150 and thus any beneficial effects could be extrapolated to all agents with peripheral glucose lowering actions. If so, this would have a major influence on a clinical trial design, as the intervention could be personalized in terms of both drug choice and dose, according to an individual’s baseline glucose/HbA1c levels and preference regarding mode of administration and/or idiosyncratic side effects.
If any of the CV drugs have “off target” (i.e., independent of their effects on glucose, blood pressure, cholesterol, etc.) beneficial effects on PD neurodegeneration, then trials must focus on single agents and/or drug classes that may share a common mechanism(s) of “neuroprotection.” The GLP-1 receptor agonists may be interesting because of their potential neuroprotective/neurorestorative properties in a range of animal models151–154 alongside data indicating potential mechanisms through anti-inflammatory effects on microglia/astrocytic processes154 or anti-apoptotic effects through the Akt/mTOR pathway.155 Whether these effects are distinct or if there is overlap between the neurodegenerative and microvascular disease processes is of clear interest but not yet known.
In a one-year phase 2 trial of 60 patients randomized to self-inject exenatide or placebo, PD patients using exenatide had smaller increase in the Movement Disorders Society Unified PD Rating Scale part 3 motor scores when assessed in the off-medication state.156 Despite the washout design of this trial (the primary outcome was evaluated 12 weeks after cessation of exenatide), it is still unclear whether these encouraging effects represent a disease-modifying effect or a prolonged symptomatic one. To evaluate this, a larger randomized two-year multicenter trial is being organized to test the impact of exenatide or placebo for two years.
Additional trials of exenatide, liraglutide, lixisenatide and semaglutide also are being planned or already in recruiting phases, reflecting the considerable academic and commercial interest in these GLP-1 receptor agonists for PD. For all of these trials, it will be of interest to perform subgroup analyses to compare whether effect sizes are greatest according to baseline glucose/HbA1c levels, or PD risk genotypes. This may help interpret whether effects of GLP-1 receptor agonists bear any relationship to CV disease risk or act via independent cellular mechanisms.
In parallel with the interest in GLP-1 receptor agonist approaches, there has been interest in the thiazolidinedione drugs, in particular pioglitazone as a PD neuroprotective agent based on epidemiological and laboratory evidence indicating its potential benefit. Unfortunately there was no advantage seen among patients treated with Pioglitazone for 44 weeks in a double blind randomised controlled trial.157 These results highlight the uncertainty regarding how strongly preclinical laboratory evidence predicts efficacy in people with PD, as well as questions regarding the stage of disease or duration of exposure that may be necessary for disease modifying effects to become detectable.
Similarly, simvastatin has been reported to have beneficial effects in the toxicant-based models of PD, with evidence indicating anti-inflammatory effects as well as beneficial effects on α-synuclein aggregation.158 Although observational studies have yielded conflicting results,32 the potential for disease-modifying effects of statins in PD progression has led to a clinical trial using double blind-trial methodology, although some have challenged whether this study is justified based on available evidence.27, 31, 121 The PD STAT trial has also used a parallel group design comparing 80 mg of simvastatin or placebo taken daily for two years. One of the challenges of this study has been to identify sufficient numbers of patients who were not already prescribed a statin, or not likely to be prescribed a statin over the two-year period based on their CV risk score.131
In the design of clinical trials studying the effects of CV drugs it will be helpful to plan a priori subgroup analyses or design the randomization strategy to help us to understand the profiles of individuals most likely to benefit and least likely to be harmed. It is perhaps sensible to hypothesize that the CV drugs are likely to have their greatest effects in patients with high CV risks, which will presumably be additive to any effects on the neurodegenerative processes of PD. However there is also the potential for detrimental effects (e.g., use of brain-permeable cholesterol lowering drugs in subgroups of PD patients with low preexisting cholesterol, without other CV risk), thus pre-defined subgroup analyses are likely to be helpful.
Conclusions
CV disease and PD share biological processes, particularly inflammation, insulin resistance, lipid metabolism, and oxidative stress. It is unclear, however, whether or not these processes are the consequence of shared risk factors. There are modifiable risk factors that are inversely associated with both CV disease and PD, particularly physical activity and moderate coffee consumption, but the mechanisms by which they are associated with PD are not established and research to date provides most evidence for disparate mechanisms. Nonetheless, these risk factors (or their underlying mechanisms) represent logical targets for primary or secondary prevention strategies regardless of diagnosis. Despite less clear epidemiological evidence in PD, good glycemic control and treatment of HTN are also health interventions with clear benefits for CV health that can be supported to optimize health in PD patients or individuals at risk for PD based on biological mechanisms and other benefits on brain health. CV risk factors with more obvious common mechanistic links to PD (such as DM, HTN and obesity sharing oxidative stress and inflammation as mechanisms) still are not established PD risk factors, probably indicating that their associations with PD are small in magnitude.
On the other hand there are associations, in particular with cholesterol and smoking, that have discordant relationships with PD and CVD. As with the concordant associations the mechanisms (at least with PD) are not well understood and addressing this knowledge gap should help to direct preventive therapies in a way that balances risks and benefits. It will be important to understand the degree of overlap in the disease-associated mechanisms in order to guide a nuanced approach to application depending on the individuals’ combination of risk factors and established disease.
Acknowledgements
The authors thank Professor Richard Mailman for helpful suggestions on the content of this manuscript.
Contributor Information
Judy Potashkin, The Cellular and Molecular Pharmacology Department, The Chicago Medical School, Rosalind Franklin University of Medicine and Science, North Chicago, IL, USA.
Xuemei Huang, Translational Brain Research Center and Department of Neurology, Penn State College of Medicine, Hershey PA.
Claudia Becker, Basel Pharmacoepidemiology Unit, Division of Clinical Pharmacy and Epidemiology, Department of Pharmaceutical Sciences, University of Basel, Switzerland.
Honglei Chen, Michigan State University, East Lansing MI.
Thomas Foltynie, Department of Clinical & Movement Neurosciences, UCL Institute of Neurology, Queen Square, London, UK. WC1N 3BG..
Connie Marras, The Edmond J Safra Program in Parkinson’s Research, Toronto Western Hospital, University of Toronto, Toronto, Canada.
References
- 1.Vicario A, Cerezo GH. At the Heart of Brain Disorders - Preventing Cognitive Decline and Dementia. Eur Cardiol 2015;10:60–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tolppanen AM, Solomon A, Soininen H, Kivipelto M. Midlife vascular risk factors and Alzheimer’s disease: evidence from epidemiological studies. J Alzheimers Dis 2012;32:531–540. [DOI] [PubMed] [Google Scholar]
- 3.Knopman DS, Gottesman RF, Sharrett AR, et al. Midlife vascular risk factors and midlife cognitive status in relation to prevalence of mild cognitive impairment and dementia in later life: The Atherosclerosis Risk in Communities Study. Alzheimers Dement 2018;14:1406–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scorza FA, Fiorini AC, Scorza CA, Finsterer J. Cardiac abnormalities in Parkinson’s disease and Parkinsonism. J Clin Neurosci 2018;53:1–5. [DOI] [PubMed] [Google Scholar]
- 5.Espay AJ, LeWitt PA, Hauser RA, Merola A, Masellis M, Lang AE. Neurogenic orthostatic hypotension and supine hypertension in Parkinson’s disease and related synucleinopathies: prioritisation of treatment targets. Lancet Neurol 2016;15:954–966. [DOI] [PubMed] [Google Scholar]
- 6.Lang AE, Espay AJ. Disease Modification in Parkinson’s Disease: Current Approaches, Challenges, and Future Considerations. Mov Disord 2018;33:660–677. [DOI] [PubMed] [Google Scholar]
- 7.Lavie CJ, Arena R, Swift DL, et al. Exercise and the cardiovascular system: clinical science and cardiovascular outcomes. Circ Res 2015;117:207–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thacker EL, Chen H, Patel AV, et al. Recreational physical activity and risk of Parkinson’s disease. Movement Disorders 2008;23:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shih IF, Starhof C, Lassen CF, Hansen J, Liew Z, Ritz B. Occupational and recreational physical activity and Parkinson’s disease in Denmark. Scand J Work Environ Health 2017;43:210–216. [DOI] [PubMed] [Google Scholar]
- 10.Chen H, Zhang SM, Schwarzschild MA, Hernan MA, Ascherio A. Physical activity and the risk of Parkinson disease. Neurology 2005;64:664–669. [DOI] [PubMed] [Google Scholar]
- 11.Xu Q, Park Y, Huang X, et al. Physical activities and future risk of Parkinson disease. Neurology 2010;75:341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mak MK, Wong-Yu IS, Shen X, Chung CL. Long-term effects of exercise and physical therapy in people with Parkinson disease. Nature reviews Neurology 2017;13:689–703. [DOI] [PubMed] [Google Scholar]
- 13.Real CC, Garcia PC, Britto LRG. Treadmill Exercise Prevents Increase of Neuroinflammation Markers Involved in the Dopaminergic Damage of the 6-OHDA Parkinson’s Disease Model. Journal of molecular neuroscience : MN 2017;63:36–49. [DOI] [PubMed] [Google Scholar]
- 14.Poole R, Kennedy OJ, Roderick P, Fallowfield JA, Hayes PC, Parkes J. Coffee consumption and health: umbrella review of meta-analyses of multiple health outcomes. Bmj 2017;359:j5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding M, Bhupathiraju SN, Satija A, van Dam RM, Hu FB. Long-term coffee consumption and risk of cardiovascular disease: a systematic review and a dose-response meta-analysis of prospective cohort studies. Circulation 2014;129:643–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ascherio A, Zhang SM, Hernán MA, et al. Prospective study of caffeine consumption and risk of Parkinson’s disease in men and women. Annals of neurology 2001;50:56–63. [DOI] [PubMed] [Google Scholar]
- 17.Liu R, Guo X, Park Y, et al. Caffeine intake, smoking, and risk of Parkinson disease in men and women. American journal of epidemiology 2012;175:1200–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwarzschild MA, Xu K, Oztas E, et al. Neuroprotection by caffeine and more specific A2A receptor antagonists in animal models of Parkinson’s disease. Neurology 2003;61:S55–61. [DOI] [PubMed] [Google Scholar]
- 19.GBD 2015 Tobacco Collaborators. Smoking prevalence and attributable disease burden in 195 countries and territories, 1990–2015: a systematic analysis from the Global Burden of Disease Study 2015. Lancet 2017;389:1885–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen H, Huang X, Guo X, et al. Smoking duration, intensity, and risk of Parkinson disease. Neurology 2010;74:878–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ritz B, Lee PC, Lassen CF, Arah OA. Parkinson disease and smoking revisited: ease of quitting is an early sign of the disease. Neurology 2014;83:1396–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Reilly EJ, Chen H, Gardener H, Gao X, Schwarzschild MA, Ascherio A. Smoking and Parkinson’s disease: using parental smoking as a proxy to explore causality. Am J Epidemiol 2009;169:678–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wirdefeldt K, Gatz M, Pawitan Y, Pedersen NL. Risk and protective factors for Parkinson’s disease: a study in Swedish twins. Annals of neurology 2005;57:27–33. [DOI] [PubMed] [Google Scholar]
- 24.Rossi A, Berger K, Chen H, Leslie D, Mailman RB, Huang X. Projection of the prevalence of Parkinson’s disease in the coming decades: Revisited. Mov Disord 2018;33:156–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Lau LML, Koudstaal PJ, Hofman A, Breteler MMB. Serum Cholesterol Levels and the Risk of Parkinson’s Disease. Am J Epidemiol 2006;164:998–1002. [DOI] [PubMed] [Google Scholar]
- 26.Huang X, Abbott RD, Petrovitch H, Mailman RB, Ross GW. Low LDL cholesterol and increased risk of Parkinson’s disease: Prospective results from Honolulu-Asia Aging Study. Movement Disorders 2008;23:1013–1018. [DOI] [PubMed] [Google Scholar]
- 27.Huang X, Alonso A, Guo X, et al. Statins, plasma cholesterol, and risk of Parkinson’s disease: A prospective study. Movement disorders : official journal of the Movement Disorder Society 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang X, Chen H, Miller WC, et al. Lower low-density lipoprotein cholesterol levels are associated with Parkinson’s disease. Movement Disorders 2006;22:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang X, Auinger P, Eberly S, et al. Serum cholesterol and the progression of Parkinson’s disease: results from DATATOP. PLoS One 2011;6:e22854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sterling NW, Lichtenstein M, Lee EY, et al. Higher Plasma LDL-Cholesterol is Associated with Preserved Executive and Fine Motor Functions in Parkinson’s Disease. Aging Dis 2016;7:237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu G, Sterling NW, Kong L, et al. Statins may facilitate Parkinson’s disease: Insight gained from a large, national claims database. Mov Disord 2017;32:913–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bykov K, Yoshida K, Weisskopf MG, Gagne JJ. Confounding of the association between statins and Parkinson disease: systematic review and meta-analysis. Pharmacoepidemiol Drug Saf 2017;26:294–300. [DOI] [PubMed] [Google Scholar]
- 33.Yue X, Li H, Yan H, Zhang P, Chang L, Li T. Risk of Parkinson Disease in Diabetes Mellitus: An Updated Meta-Analysis of Population-Based Cohort Studies. Medicine (Baltimore) 2016;95:e3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu L, Fu DL, Li HQ, Liu AJ, Li JH, Zheng GQ. Diabetes and risk of Parkinson’s disease: an updated meta-analysis of case-control studies. PLoS One 2014;9:e85781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cereda E, Barichella M, Pedrolli C, et al. Diabetes and risk of Parkinson’s disease: a systematic review and meta-analysis. Diabetes Care 2011;34:2614–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Driver JA, Smith A, Buring JE, Gaziano JM, Kurth T, Logroscino G. Prospective cohort study of type 2 diabetes and the risk of Parkinson’s disease. Diabetes Care 2008;31:2003–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Pablo-Fernandez E, Goldacre R, Pakpoor J, Noyce AJ, Warner TT. Association between diabetes and subsequent Parkinson disease: A record-linkage cohort study. Neurology 2018;91:e139–e142. [DOI] [PubMed] [Google Scholar]
- 38.Xu Q, Park Y, Huang X, et al. Diabetes and risk of Parkinson’s disease. Diabetes Care 2011;34:910–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grandinetti A, Morens DM, Reed D, MacEachern D. Prospective study of cigarette smoking and the risk of developing idiopathic Parkinson’s disease. Am J Epidemiol 1994;139:1129–1138. [DOI] [PubMed] [Google Scholar]
- 40.Simon KC, Chen H, Schwarzschild M, Ascherio A. Hypertension, hypercholesterolemia, diabetes, and risk of Parkinson disease. Neurology 2007;69:1688–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qiu C, Hu G, Kivipelto M, et al. Association of blood pressure and hypertension with the risk of Parkinson disease: the National FINRISK Study. Hypertension 2011;57:1094–1100. [DOI] [PubMed] [Google Scholar]
- 42.Vikdahl M, Backman L, Johansson I, Forsgren L, Haglin L. Cardiovascular risk factors and the risk of Parkinson’s disease. Eur J Clin Nutr 2015;69:729–733. [DOI] [PubMed] [Google Scholar]
- 43.Savica R, Grossardt BR, Ahlskog JE, Rocca WA. Metabolic markers or conditions preceding Parkinson’s disease: a case-control study. Mov Disord 2012;27:974–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miyake Y, Tanaka K, Fukushima W, et al. Case-control study of risk of Parkinson’s disease in relation to hypertension, hypercholesterolemia, and diabetes in Japan. J Neurol Sci 2010;293:82–86. [DOI] [PubMed] [Google Scholar]
- 45.Becker C, Jick SS, Meier CR. Use of antihypertensives and the risk of Parkinson disease. Neurology 2008;70:1438–1444. [DOI] [PubMed] [Google Scholar]
- 46.Powers KM, Smith-Weller T, Franklin GM, Longstreth WT Jr., Swanson PD, Checkoway H. Diabetes, smoking, and other medical conditions in relation to Parkinson’s disease risk. Parkinsonism Relat Disord 2006;12:185–189. [DOI] [PubMed] [Google Scholar]
- 47.Scigliano G, Musicco M, Soliveri P, Piccolo I, Ronchetti G, Girotti F. Reduced risk factors for vascular disorders in Parkinson disease patients: a case-control study. Stroke 2006;37:1184–1188. [DOI] [PubMed] [Google Scholar]
- 48.Paganini-Hill A Risk factors for parkinson’s disease: the leisure world cohort study. Neuroepidemiology 2001;20:118–124. [DOI] [PubMed] [Google Scholar]
- 49.McCann SJ, LeCouteur DG, Green AC, et al. The epidemiology of Parkinson’s disease in an Australian population. Neuroepidemiology 1998;17:310–317. [DOI] [PubMed] [Google Scholar]
- 50.Semchuk KM, Love EJ, Lee RG. Parkinson’s disease: a test of the multifactorial etiologic hypothesis. Neurology 1993;43:1173–1180. [DOI] [PubMed] [Google Scholar]
- 51.Ho SC, Woo J, Lee CM. Epidemiologic study of Parkinson’s disease in Hong Kong. Neurology 1989;39:1314–1318. [DOI] [PubMed] [Google Scholar]
- 52.Chen H, Zhang SM, Schwarzschild MA, Hernan MA, Willett WC, Ascherio A. Obesity and the risk of Parkinson’s disease. Am J Epidemiol 2004;159:547–555. [DOI] [PubMed] [Google Scholar]
- 53.Wang YL, Wang YT, Li JF, Zhang YZ, Yin HL, Han B. Body Mass Index and Risk of Parkinson’s Disease: A Dose-Response Meta-Analysis of Prospective Studies. PLoS One 2015;10:e0131778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jimenez-Jimenez FJ, Alonso-Navarro H, Garcia-Martin E, Agundez JAG. Alcohol consumption and risk for Parkinson’s disease: a systematic review and meta-analysis. J Neurol 2018. [DOI] [PubMed] [Google Scholar]
- 55.Liu R, Guo X, Park Y, et al. Alcohol Consumption, Types of Alcohol, and Parkinson’s Disease. PLoS One 2013;8:e66452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Palacios N, Gao X, O’Reilly E, et al. Alcohol and risk of Parkinson’s disease in a large, prospective cohort of men and women. Mov Disord 2012;27:980–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saaksjarvi K, Knekt P, Mannisto S, Lyytinen J, Heliovaara M. Prospective study on the components of metabolic syndrome and the incidence of Parkinson’s disease. Parkinsonism Relat Disord 2015;21:1148–1155. [DOI] [PubMed] [Google Scholar]
- 58.Becerra-Tomas N, Blanco Mejia S, Viguiliouk E, et al. Mediterranean diet, cardiovascular disease and mortality in diabetes: A systematic review and meta-analysis of prospective cohort studies and randomized clinical trials. Crit Rev Food Sci Nutr 2019:1–21. [DOI] [PubMed] [Google Scholar]
- 59.Alcalay RN, Gu Y, Mejia-Santana H, Cote L, Marder KS, Scarmeas N. The association between Mediterranean diet adherence and Parkinson’s disease. Mov Disord 2012;27:771–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maraki MI, Yannakoulia M, Stamelou M, et al. Mediterranean diet adherence is related to reduced probability of prodromal Parkinson’s disease. Mov Disord 2019;34:48–57. [DOI] [PubMed] [Google Scholar]
- 61.Chrysant SG, Chrysant GS. The current status of homocysteine as a risk factor for cardiovascular disease: a mini review. Expert Rev Cardiovasc Ther 2018;16:559–565. [DOI] [PubMed] [Google Scholar]
- 62.McCarter SJ, Teigen LM, McCarter AR, Benarroch EE, St Louis EK, Savica R. Low Vitamin B12 and Parkinson Disease: Potential Link to Reduced Cholinergic Transmission and Severity of Disease. Mayo Clin Proc 2019;94:757–762. [DOI] [PubMed] [Google Scholar]
- 63.Chen H, Zhang SM, Hernan MA, Willett WC, Ascherio A. Weight loss in Parkinson’s disease. Annals of neurology 2003;53:676–679. [DOI] [PubMed] [Google Scholar]
- 64.Liang HW, Huang YP, Pan SL. Parkinson disease and risk of acute myocardial infarction: A population-based, propensity score-matched, longitudinal follow-up study. Am Heart J 2015;169:508–514. [DOI] [PubMed] [Google Scholar]
- 65.Ben-Shlomo Y, Marmot MG. Survival and cause of death in a cohort of patients with parkinsonism: possible clues to aetiology? J Neurol Neurosurg Psychiatry 1995;58:293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fall PA, Saleh A, Fredrickson M, Olsson JE, Granerus AK. Survival time, mortality, and cause of death in elderly patients with Parkinson’s disease: a 9-year follow-up. Mov Disord 2003;18:1312–1316. [DOI] [PubMed] [Google Scholar]
- 67.Beyer MK, Herlofson K, Arsland D, Larsen JP. Causes of death in a community-based study of Parkinson’s disease. Acta Neurol Scand 2001;103:7–11. [DOI] [PubMed] [Google Scholar]
- 68.Pennington S, Snell K, Lee M, Walker R. The cause of death in idiopathic Parkinson’s disease. Parkinsonism Relat Disord 2010;16:434–437. [DOI] [PubMed] [Google Scholar]
- 69.Becker C, Jick SS, Meier CR. Risk of stroke in patients with idiopathic Parkinson disease. Parkinsonism Relat Disord 2010;16:31–35. [DOI] [PubMed] [Google Scholar]
- 70.Skeie GO, Muller B, Haugarvoll K, Larsen JP, Tysnes OB. Parkinson disease: associated disorders in the Norwegian population based incident ParkWest study. Parkinsonism Relat Disord 2013;19:53–55. [DOI] [PubMed] [Google Scholar]
- 71.Santiago JA, Potashkin JA. Shared dysregulated pathways lead to Parkinson’s disease and diabetes. Trends Mol Med 2013;19:176–186. [DOI] [PubMed] [Google Scholar]
- 72.Santiago JA, Potashkin JA. System-based approaches to decode the molecular links in Parkinson’s disease and diabetes. Neurobiol Dis 2014;72 Pt A:84–91. [DOI] [PubMed] [Google Scholar]
- 73.Balakumar P, Maung UK, Jagadeesh G. Prevalence and prevention of cardiovascular disease and diabetes mellitus. Pharmacol Res 2016;113:600–609. [DOI] [PubMed] [Google Scholar]
- 74.Mollenhauer B, Zimmermann J, Sixel-Doring F, et al. Baseline predictors for progression 4 years after Parkinson’s disease diagnosis in the De Novo Parkinson Cohort (DeNoPa). Mov Disord 2019;34:67–77. [DOI] [PubMed] [Google Scholar]
- 75.Rabelo LA, Ferreira FO, Nunes-Souza V, da Fonseca LJ, Goulart MO. Arginase as a Critical Prooxidant Mediator in the Binomial Endothelial Dysfunction-Atherosclerosis. Oxid Med Cell Longev 2015;2015:924860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Andican G, Konukoglu D, Bozluolcay M, Bayulkem K, Firtiina S, Burcak G. Plasma oxidative and inflammatory markers in patients with idiopathic Parkinson’s disease. Acta Neurol Belg 2012;112:155–159. [DOI] [PubMed] [Google Scholar]
- 77.Chavez JA, Summers SA. A ceramide-centric view of insulin resistance. Cell Metab 2012;15:585–594. [DOI] [PubMed] [Google Scholar]
- 78.Wang DD, Toledo E, Hruby A, et al. Plasma Ceramides, Mediterranean Diet, and Incident Cardiovascular Disease in the PREDIMED Trial (Prevencion con Dieta Mediterranea). Circulation 2017;135:2028–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Motyl J, Strosznajder JB. Sphingosine kinase 1/sphingosine-1-phosphate receptors dependent signalling in neurodegenerative diseases. The promising target for neuroprotection in Parkinson’s disease. Pharmacol Rep 2018;70:1010–1014. [DOI] [PubMed] [Google Scholar]
- 80.Abbott SK, Li H, Munoz SS, et al. Altered ceramide acyl chain length and ceramide synthase gene expression in Parkinson’s disease. Mov Disord 2014;29:518–526. [DOI] [PubMed] [Google Scholar]
- 81.Mielke MM, Maetzler W, Haughey NJ, et al. Plasma ceramide and glucosylceramide metabolism is altered in sporadic Parkinson’s disease and associated with cognitive impairment: a pilot study. PLoS One 2013;8:e73094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Xing Y, Tang Y, Zhao L, et al. Associations between plasma ceramides and cognitive and neuropsychiatric manifestations in Parkinson’s disease dementia. J Neurol Sci 2016;370:82–87. [DOI] [PubMed] [Google Scholar]
- 83.Mao CY, Yang J, Wang H, et al. SMPD1 variants in Chinese Han patients with sporadic Parkinson’s disease. Parkinsonism Relat Disord 2017;34:59–61. [DOI] [PubMed] [Google Scholar]
- 84.Gan-Or Z, Orr-Urtreger A, Alcalay RN, Bressman S, Giladi N, Rouleau GA. The emerging role of SMPD1 mutations in Parkinson’s disease: Implications for future studies. Parkinsonism Relat Disord 2015;21:1294–1295. [DOI] [PubMed] [Google Scholar]
- 85.Dagan E, Adir V, Schlesinger I, et al. SMPD1 mutations and Parkinson disease. Parkinsonism Relat Disord 2015;21:1296–1297. [DOI] [PubMed] [Google Scholar]
- 86.Foo JN, Liany H, Bei JX, et al. Rare lysosomal enzyme gene SMPD1 variant (p.R591C) associates with Parkinson’s disease. Neurobiol Aging 2013;34:2890 e2813–2895. [DOI] [PubMed] [Google Scholar]
- 87.Alcalay RN, Mallett V, Vanderperre B, et al. SMPD1 mutations, activity, and alpha-synuclein accumulation in Parkinson’s disease. Mov Disord 2019;34:526–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Deng S, Deng X, Song Z, et al. Systematic Genetic Analysis of the SMPD1 Gene in Chinese Patients with Parkinson’s Disease. Mol Neurobiol 2016;53:5025–5029. [DOI] [PubMed] [Google Scholar]
- 89.Jin H, Chen J, Li K, et al. A novel p.L216I mutation in the glucocerebrosidase gene is associated with Parkinson’s disease in Han Chinese patients. Neurosci Lett 2018;674:66–69. [DOI] [PubMed] [Google Scholar]
- 90.Arkadir D, Dinur T, Mullin S, et al. Trio approach reveals higher risk of PD in carriers of severe vs. mild GBA mutations. Blood Cells Mol Dis 2018;68:115–116. [DOI] [PubMed] [Google Scholar]
- 91.Kim WS, Halliday GM. Changes in sphingomyelin level affect alpha-synuclein and ABCA5 expression. J Parkinsons Dis 2012;2:41–46. [DOI] [PubMed] [Google Scholar]
- 92.Bienias K, Fiedorowicz A, Sadowska A, Prokopiuk S, Car H. Regulation of sphingomyelin metabolism. Pharmacol Rep 2016;68:570–581. [DOI] [PubMed] [Google Scholar]
- 93.Lin G, Wang L, Marcogliese PC, Bellen HJ. Sphingolipids in the Pathogenesis of Parkinson’s Disease and Parkinsonism. Trends Endocrinol Metab 2019;30:106–117. [DOI] [PubMed] [Google Scholar]
- 94.Xia JY, Holland WL, Kusminski CM, et al. Targeted Induction of Ceramide Degradation Leads to Improved Systemic Metabolism and Reduced Hepatic Steatosis. Cell Metab 2015;22:266–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2889–2934. [DOI] [PubMed] [Google Scholar]
- 96.Huang X, Miller WC, et al. Cardiovascularly “desirable” cholesterol levels associated with Parkinson’s disease. Ann Neurol 2005;58:S24. [Google Scholar]
- 97.Huang X, Chen H, Miller WC, et al. Lower low-density lipoprotein cholesterol levels are associated with Parkinson’s disease. Mov Disord 2007;22:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Miyake Y, Tanaka K, Fukushima W, et al. Case-control study of risk of Parkinson’s disease in relation to hypertension, hypercholesterolemia, and diabetes in Japan. J Neurol Sci 2010;293:82–86. [DOI] [PubMed] [Google Scholar]
- 99.de Lau LM, Koudstaal PJ, Hofman A, Breteler MM. Serum cholesterol levels and the risk of Parkinson’s disease. Am J Epidemiol 2006;164:998–1002. [DOI] [PubMed] [Google Scholar]
- 100.de Lau LM, Stricker BH, Breteler MM. Serum cholesterol, use of lipid-lowering drugs, and risk of Parkinson disease. Mov Disord 2007;22:1985. [DOI] [PubMed] [Google Scholar]
- 101.Huang X, Auinger P, Eberly S, et al. Serum cholesterol and the progression of Parkinson’s disease: results from DATATOP. PLoS One 2011;6:e22854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mahlknecht P, Sprenger F, Seppi K, Poewe W. Plasma fasting cholesterol profiles and age at onset in Parkinson’s disease. Mov Disord 2015. [DOI] [PubMed] [Google Scholar]
- 103.Mauch DH, Nagler K, Schumacher S, et al. CNS synaptogenesis promoted by glia-derived cholesterol. Science 2001;294:1354–1357. [DOI] [PubMed] [Google Scholar]
- 104.Orth M, Bellosta S. Cholesterol: its regulation and role in central nervous system disorders. Cholesterol 2012;2012:292598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vance JE, Karten B, Hayashi H. Lipid dynamics in neurons. Biochem Soc Trans 2006;34:399–403. [DOI] [PubMed] [Google Scholar]
- 106.Bjorkhem I, Meaney S. Brain cholesterol: long secret life behind a barrier. Arterioscler Thromb Vasc Biol 2004;24:806–815. [DOI] [PubMed] [Google Scholar]
- 107.Bjorkhem I Crossing the barrier: oxysterols as cholesterol transporters and metabolic modulators in the brain. J Intern Med 2006;260:493–508. [DOI] [PubMed] [Google Scholar]
- 108.Davies NM, Windmeijer F, Martin RM, et al. Use of genotype frequencies in medicated groups to investigate prescribing practice: APOE and statins as a proof of principle. Clin Chem 2011;57:502–510. [DOI] [PubMed] [Google Scholar]
- 109.Huang X, Chen PC, Poole C. APOE-[epsilon]2 allele associated with higher prevalence of sporadic Parkinson disease. Neurology 2004;62:2198–2202. [DOI] [PubMed] [Google Scholar]
- 110.Williams-Gray CH, Goris A, Saiki M, et al. Apolipoprotein E genotype as a risk factor for susceptibility to and dementia in Parkinson’s disease. J Neurol 2009;256:493–498. [DOI] [PubMed] [Google Scholar]
- 111.Federoff M, Jimenez-Rolando B, Nalls MA, Singleton AB. A large study reveals no association between APOE and Parkinson’s disease. Neurobiol Dis 2012;46:389–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 1993;261:921–923. [DOI] [PubMed] [Google Scholar]
- 113.Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nature reviews Neurology 2013;9:106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Palacios N, Gao X, McCullough ML, et al. Obesity, diabetes, and risk of Parkinson’s disease. Mov Disord 2011;26:2253–2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Klemann C, Martens GJM, Sharma M, et al. Integrated molecular landscape of Parkinson’s disease. NPJ Parkinsons Dis 2017;3:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Singh NK, Banerjee BD, Bala K, Mitrabasu, Dung Dung AA, Chhillar N. APOE and LRPAP1 gene polymorphism and risk of Parkinson’s disease. Neurol Sci 2014;35:1075–1081. [DOI] [PubMed] [Google Scholar]
- 117.Domenger D, Dea D, Theroux L, Moquin L, Gratton A, Poirier J. The MPTP neurotoxic lesion model of Parkinson’s disease activates the apolipoprotein E cascade in the mouse brain. Exp Neurol 2012;233:513–522. [DOI] [PubMed] [Google Scholar]
- 118.Wilhelmus MMM, Bol JG, Van Haastert ES, et al. Apolipoprotein E and LRP1 Increase Early in Parkinson’s Disease Pathogenesis. The American journal of pathology 2011;179:2152–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang L, Wang X, Wang M, et al. Circulating Cholesterol Levels May Link to the Factors Influencing Parkinson’s Risk. Front Neurol 2017;8:501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gao J, Huang X, Park Y, Hollenbeck A, Chen H. An exploratory study on CLU, CR1 and PICALM and Parkinson disease. PLoS One 2011;6:e24211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Huang X, Sterling NW, Du G, et al. Brain cholesterol metabolism and Parkinson’s disease. Mov Disord 2019;34:386–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bjorkhem I, Lovgren-Sandblom A, Leoni V, et al. Oxysterols and Parkinson’s disease: evidence that levels of 24S-hydroxycholesterol in cerebrospinal fluid correlates with the duration of the disease. Neurosci Lett 2013;555:102–105. [DOI] [PubMed] [Google Scholar]
- 123.Lee CY, Seet RC, Huang SH, Long LH, Halliwell B. Different patterns of oxidized lipid products in plasma and urine of dengue fever, stroke, and Parkinson’s disease patients: cautions in the use of biomarkers of oxidative stress. Antioxidants & redox signaling 2009;11:407–420. [DOI] [PubMed] [Google Scholar]
- 124.Sun MY, Linsenbardt AJ, Emnett CM, et al. 24(S)-Hydroxycholesterol as a Modulator of Neuronal Signaling and Survival. Neuroscientist 2016;22:132–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mast N, Anderson KW, Johnson KM, Phan TTN, Guengerich FP, Pikuleva IA. In vitro cytochrome P450 46A1 (CYP46A1) activation by neuroactive compounds. J Biol Chem 2017;292:12934–12946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kotti TJ, Ramirez DM, Pfeiffer BE, Huber KM, Russell DW. Brain cholesterol turnover required for geranylgeraniol production and learning in mice. Proc Natl Acad Sci U S A 2006;103:3869–3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kotti T, Head DD, McKenna CE, Russell DW. Biphasic requirement for geranylgeraniol in hippocampal long-term potentiation. Proc Natl Acad Sci U S A 2008;105:11394–11399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Venosa A Cholesterol Medication Simvastatin Tested As Potential Treatment For Parkinson’s Disease In New Clinical Trial. Medical Daily [serial online] 2016;http://www.medicaldaily.com/cholesterol-medication-simvastatin-tested-potential-treatment-parkinsons-disease-new-369008. [Google Scholar]
- 129.Tan EK, Tan LC. Holding on to statins in Parkinson disease. Neurology 2013;81:406–407. [DOI] [PubMed] [Google Scholar]
- 130.Uo Plymouth. Could a cholesterol-lowering drug be a potential treatment for Parkinson’s? Cholesterol-lowering drug Simvastatin trialled as a potential neuroprotective treatment for Parkinson’s. ScienceDaily 2016 11 January 2016. [Google Scholar]
- 131.Carroll CB, Wyse RKH. Simvastatin as a Potential Disease-Modifying Therapy for Patients with Parkinson’s Disease: Rationale for Clinical Trial, and Current Progress. J Parkinsons Dis 2017;7:545–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Anchah L, Hassali MA, Lim MS, Ibrahim MI, Sim KH, Ong TK. Health related quality of life assessment in acute coronary syndrome patients: the effectiveness of early phase I cardiac rehabilitation. Health Qual Life Outcomes 2017;15:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Azadbakht L, Atabak S, Esmaillzadeh A. Soy protein intake, cardiorenal indices, and C-reactive protein in type 2 diabetes with nephropathy: a longitudinal randomized clinical trial. Diabetes Care 2008;31:648–654. [DOI] [PubMed] [Google Scholar]
- 134.Prins BP, Abbasi A, Wong A, et al. Investigating the Causal Relationship of C-Reactive Protein with 32 Complex Somatic and Psychiatric Outcomes: A Large-Scale Cross-Consortium Mendelian Randomization Study. PLoS Med 2016;13:e1001976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pfutzner A, Schondorf T, Hanefeld M, Forst T. High-sensitivity C-reactive protein predicts cardiovascular risk in diabetic and nondiabetic patients: effects of insulin-sensitizing treatment with pioglitazone. J Diabetes Sci Technol 2010;4:706–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.You YK, Huang XR, Chen HY, Lyu XF, Liu HF, Lan HY. C-Reactive Protein Promotes Diabetic Kidney Disease in db/db Mice via the CD32b-Smad3-mTOR signaling Pathway. Sci Rep 2016;6:26740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lai W, Tang Y, Huang XR, et al. C-reactive protein promotes acute kidney injury via Smad3-dependent inhibition of CDK2/cyclin E. Kidney Int 2016;90:610–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sulzer D, Alcalay RN, Garretti F, et al. T cells from patients with Parkinson’s disease recognize alpha-synuclein peptides. Nature 2017;546:656–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Garretti F, Agalliu D, Lindestam Arlehamn CS, Sette A, Sulzer D. Autoimmunity in Parkinson’s Disease: The Role of alpha-Synuclein-Specific T Cells. Front Immunol 2019;10:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 2003;24:197–211. [DOI] [PubMed] [Google Scholar]
- 141.Liebner S, Dijkhuizen RM, Reiss Y, Plate KH, Agalliu D, Constantin G. Functional morphology of the blood-brain barrier in health and disease. Acta Neuropathol 2018;135:311–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Schwartz RS, Halliday GM, Soh D, Cordato DJ, Kril JJ. Impact of small vessel disease on severity of motor and cognitive impairment in Parkinson’s disease. J Clin Neurosci 2018;58:70–74. [DOI] [PubMed] [Google Scholar]
- 143.Swallow DM, Lawton MA, Grosset KA, et al. Statins are underused in recent-onset Parkinson’s disease with increased vascular risk: findings from the UK Tracking Parkinson’s and Oxford Parkinson’s Disease Centre (OPDC) discovery cohorts. J Neurol Neurosurg Psychiatry 2016;87:1183–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cheng KK, Swallow DM, Grosset KA, Grosset DG. Statin usage, vascular diagnosis and vascular risk factors in Parkinson’s disease. Scott Med J 2017;62:104–109. [DOI] [PubMed] [Google Scholar]
- 145.Zhu XC, Dai WZ, Ma T. Overview the effect of statin therapy on dementia risk, cognitive changes and its pathologic change: a systematic review and meta-analysis. Ann Transl Med 2018;6:435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hippisley-Cox J, Coupland C, Vinogradova Y, Robson J, Brindle P. Performance of the QRISK cardiovascular risk prediction algorithm in an independent UK sample of patients from general practice: a validation study. Heart 2008;94:34–39. [DOI] [PubMed] [Google Scholar]
- 147.Hippisley-Cox J, Coupland C, Vinogradova Y, Robson J, May M, Brindle P. Derivation and validation of QRISK, a new cardiovascular disease risk score for the United Kingdom: prospective open cohort study. BMJ 2007;335:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Knobler H, Chajek-Shaul T. Glycaemic control and cardiovascular disease: is there a light at the end of the tunnel? QJM 2017;110:421–423. [DOI] [PubMed] [Google Scholar]
- 149.Jiang X, Wang X, Tuo M, Ma J, Xie A. RAGE and its emerging role in the pathogenesis of Parkinson’s disease. Neurosci Lett 2018;672:65–69. [DOI] [PubMed] [Google Scholar]
- 150.Sadowska-Bartosz I, Bartosz G. Effect of glycation inhibitors on aging and age-related diseases. Mech Ageing Dev 2016;160:1–18. [DOI] [PubMed] [Google Scholar]
- 151.Harkavyi A, Abuirmeileh A, Lever R, Kingsbury AE, Biggs CS, Whitton PS. Glucagon-like peptide 1 receptor stimulation reverses key deficits in distinct rodent models of Parkinson’s disease. J Neuroinflammation 2008;5:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bertilsson G, Patrone C, Zachrisson O, et al. Peptide hormone exendin-4 stimulates subventricular zone neurogenesis in the adult rodent brain and induces recovery in an animal model of Parkinson’s disease. J Neurosci Res 2008;86:326–338. [DOI] [PubMed] [Google Scholar]
- 153.Kim S, Moon M, Park S. Exendin-4 protects dopaminergic neurons by inhibition of microglial activation and matrix metalloproteinase-3 expression in an animal model of Parkinson’s disease. J Endocrinol 2009;202:431–439. [DOI] [PubMed] [Google Scholar]
- 154.Yun SP, Kam TI, Panicker N, et al. Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson’s disease. Nat Med 2018;24:931–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Athauda D, Gulyani S, Karnati H, et al. Utility of Neuronal-Derived Exosomes to Examine Molecular Mechanisms That Affect Motor Function in Patients With Parkinson Disease: A Secondary Analysis of the Exenatide-PD Trial. JAMA Neurol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Athauda D, Maclagan K, Skene SS, et al. Exenatide once weekly versus placebo in Parkinson’s disease: a randomised, double-blind, placebo-controlled trial. Lancet 2017;390:1664–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Investigators NETiPDF-Z. Pioglitazone in early Parkinson’s disease: a phase 2, multicentre, double-blind, randomised trial. The Lancet Neurology 2015;14:795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Roy A, Pahan K. Prospects of statins in Parkinson disease. Neuroscientist 2011;17:244–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yang YW, Hsieh TF, Li CI, et al. Increased risk of Parkinson disease with diabetes mellitus in a population-based study. Medicine (Baltimore) 2017;96:e5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sun Y, Chang YH, Chen HF, Su YH, Su HF, Li CY. Risk of Parkinson disease onset in patients with diabetes: a 9-year population-based cohort study with age and sex stratifications. Diabetes Care 2012;35:1047–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hu G, Jousilahti P, Bidel S, Antikainen R, Tuomilehto J. Type 2 diabetes and the risk of Parkinson’s disease. Diabetes Care 2007;30:842–847. [DOI] [PubMed] [Google Scholar]
- 162.Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol 1999;56:33–39. [DOI] [PubMed] [Google Scholar]
- 163.Schernhammer E, Hansen J, Rugbjerg K, Wermuth L, Ritz B. Diabetes and the risk of developing Parkinson’s disease in Denmark. Diabetes Care 2011;34:1102–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.D’Amelio M, Ragonese P, Callari G, et al. Diabetes preceding Parkinson’s disease onset. A case-control study. Parkinsonism Relat Disord 2009;15:660–664. [DOI] [PubMed] [Google Scholar]
- 165.Becker C, Brobert GP, Johansson S, Jick SS, Meier CR. Diabetes in patients with idiopathic Parkinson’s disease. Diabetes Care 2008;31:1808–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Calne DB, Snow BJ, Lee C. Criteria for diagnosing Parkinson’s disease. Annals of neurology 1992;32 Suppl:S125–127. [DOI] [PubMed] [Google Scholar]
- 167.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009;120:1640–1645. [DOI] [PubMed] [Google Scholar]