Abstract
Neurological diseases and disorders are leading causes of death and disability worldwide. Many of these pathologies are associated with high levels of neuroinflammation and irreparable tissue damage. As the global burden of these pathologies continues to rise there is a significant need for the development of novel therapeutics. Due to their multipotent properties, stem cells have broad applications for tissue repair; additionally, stem cells have been shown to possess both immunomodulatory and neuroprotective properties. It is now believed that paracrine factors, such as extracellular vesicles (EVs), play a critical role in the functionality associated with stem cells. The diverse biological cargo contained within EVs are proposed to mediate these effects and, to date, the reparative and regenerative effects of stem cell EVs have been demonstrated in a wide range of cell types. While a high potential for their therapeutic use exists, there is a gap of knowledge surrounding their characterization, mechanisms of action, and how they may regulate cells of the CNS. Here, we report the isolation, characterization, and functional assessment of EVs from two sources of human stem cells, mesenchymal stem cells and induced pluripotent stem cells. We demonstrate the ability of these EVs to enhance the processes of cellular migration and angiogenesis, which are critical for both normal cellular development as well as cellular repair. Furthermore, we investigate their reparative effects on damaged cells, specifically those with relevance to the central nervous system. Collectively, our data highlight the similarities and differences among these EV populations and support the view that stem cells EV can be used to repair or partially reverse cellular damage.
Keywords: Induced pluripotent stem cells, mesenchymal stem cells, extracellular vesicles, CNS, astrocytes
Graphical Abstract

Introduction
The Central Nervous System (CNS), due to the complex nature of its structure and physiology, remains to be one of the least understood systems of the human body. Accordingly, neurological pathologies are considerably more complicated compared to other diseases and this has severely impaired the development of CNS-related therapeutics (DiNunzio and Williams 2008; de Lange et al. 2017). While neurological diseases and disorders are comprised of a wide range of conditions, some of the most of common include Alzheimer’s Disease (AD) and dementia, Parkinson’s Disease (PD), epilepsy, multiple sclerosis, and various cancers of the brain and nervous system (Nussbaum and Ellis 2003; Banerjee et al. 2009; Goldenberg 2012; Shah and Kochar 2018). Collectively, these pathologies represent leading causes of death and disability worldwide and place a substantial burden on the healthcare industry (GBD 2015; Gooch et al. 2017). Additionally, many types of viruses such as alpha herpesviruses, measles virus, Epstein-Barr virus, Influenza A, human T-cell leukemia virus (HTLV), and human immunodeficiency virus (HIV), can infect the nervous system and contribute to the development of neurological pathologies leading to microglia, astrocyte, and neuronal death (Koyuncu et al. 2013; Swanson and McGavern 2015; Dahm et al. 2016).
Despite the wide range of etiologies, neurodegeneration is a common feature shared among many CNS pathologies. Neurodegeneration, characterized by the structural and functional loss of neurons, often results in chronic mental decline which manifests as impairments in cognition, memory, and movement (Gao and Hong 2008; Chen et al. 2016). Immune activation and neuroinflamamation are major contributors to neurodegeneration, and it is believed that the improper regulation of inflammatory signaling cascades may lead to the irreparable tissue damage that is associated with various CNS pathologies (Amor et al. 2014; Stephenson et al. 2018). The mechanisms responsible for these properties have yet to be fully defined and therefore highlight the need for further study.
To date, the lack of effective therapeutics, coupled with the difficulties associated with CNS accessibility owing to the blood-brain-barrier (BBB), poses a major challenge for the treatment of CNS disorders. In recent years, however, there has been growing interest in the use of stem cell-based therapy for various pathologies, including those associated with the CNS (Uccelli et al. 2008; Martínez-Morales et al. 2013; Song et al. 2018). Stem cells, owing to their self-renewal and differentiation potential, have broad applications in the fields of tissue repair, recovery, and restoration (Wei et al. 2013; Marote et al. 2016; Yin et al. 2016; Phinney and Pittenger 2017). Specifically, mesenchymal stem cells (MSCs) and induced pluripotent stem cells (iPSCs) represent two sources of stem cells which are believed to offer great therapeutic potential.
MSCs represent a subset of non-hematopoietic adult stem cells that can be isolated from numerous sources of donor tissue including bone marrow, adipose, amniotic fluid, dental tissue, peripheral blood, lung, and skin (Aust et al. 2004; Seo et al. 2004; Griffiths et al. 2005; He et al. 2007; Marquez-Curtis et al. 2015; Ullah et al. 2015). Although cultures of MSCs represent a mixed population of cells that phenotypically display expression of many non-specific markers, The International Society for Cellular Therapy has specified the minimal defining criteria for MSCs to be: ability to adhere to plastic substrate, positive expression of CD73, CD90, CD105 surface markers, lack of expression of CD14, CD34, CD45 surface markers, and capability to differentiate into bone, fat, and cartilage under the appropriate culture conditions (Dominici et al. 2006). It has also been reported that MSCs can differentiate into endothelial-like cells, epithelial-like cells, and neuronal-like cells (Pittenger et al. 1999; Black and Woodbury 2001; Oswald et al. 2004; Paunescu et al. 2007). In addition to their differentiation potential, MSCs also possess several other properties which make them favorable candidates for therapeutic use. These properties include the ability to proliferate in culture, migrate to areas of tissue injury, and regulate inflammation (Prockop and Oh 2012; Dittmar and Entschladen 2013; Marquez-Curtis et al. 2015). Perhaps most importantly, their non-immunogenic phenotype has allowed for their widespread use in allogeneic transplants (Chamberlain et al. 2007; Samsonraj et al. 2017).
iPSCs, on the other hand, are pluripotent cells that are generated through the genetic reprogramming of adult somatic cells. This technology was developed and demonstrated using mouse cells in 2006, and the first successful reprograming of human somatic cells was reported in 2007 (Takahashi and Yamanaka 2006; Takahashi et al. 2007). Through the introduction of key transcription factors that are responsible for maintaining pluripotency, recipient cells can be successfully reverted into an embryonic stem cell-like state. While Oct¾, Sox2, Klf4, and c-Myc were regarded as the original “Yamanaka factors” responsible for inducing pluripotency, there have been successful variations and substitutions on their use (de Lazaro et al. 2014; Yu et al. 2014; Omole and Fakoya 2018). Once established, iPSCs can be continuously maintained in culture and have the capacity to differentiate into multiple cell-specific lineages from all three germ layers. Thus, iPSCs may act as possible replacements for embryonic stem cells and thereby eliminate the ethical concerns often associated their use (Robinton and Daley 2013). For these reasons, iPSC technology holds great promise for the fields of personalized medicine, disease modeling, and drug screening (Hirschi et al. 2014; Sayed et al. 2016).
To date, the regenerative potential of both MSCs and iPSCs has been widely studied in a variety of conditions ranging from cartilage repair and wound healing to cardiovascular, liver, immune, kidney, and neurological diseases (Braccioli et al. 2014; Trounson and McDonald 2015; Cefalo et al. 2016; Castro-Vinuelas et al. 2018; Song et al. 2018). The underlying mechanisms owing to these functional properties remain unknown; however, there is increasing evidence that paracrine factors, such as exosomes, may be primarily responsible for these effects (Baraniak and McDevitt 2010). Exosomes belong to a heterogeneous group of extracellular vesicles (EVs) that also include microvesicles and apoptotic bodies. The three subtypes of EVs are broadly categorized by their biogenesis, size distribution, and protein expression (Hessvik and Llorente 2018). EVs have attracted significant interest over the last decade as they have emerged as critical mediators of cellular communication. Through the transfer of their biological cargo (nucleic acids, proteins, and lipids), EVs released from donor cells have the potential to directly affect numerous biological processes in recipient cells (Abels and Breakefield 2016). For these reasons, EVs are now being considered as replacements to traditional stem cell therapies. Relative to cell-based strategies, the use of EVs offers several potential advantages such as increased stability, higher potency, lower immunogenicity, and importantly for potential CNS therapeutic application, the ability to cross the BBB (Koniusz et al. 2016; Riazifar et al. 2017).
In recent years, the MSC secretome has been extensively reviewed and it has been well established that MSC EVs play critical roles in such biological processes as bone regeneration, tissue repair, angiogenesis, and immunomodulation (Yu et al. 2014; Lai et al. 2015; Basu and Ludlow 2016; Burrello et al. 2016; Börger et al. 2017). While the exact mechanisms are not well understood, it is believed that miRNAs and proteins are largely responsible for the reparative and immunomodulatory functions associated with MSC EVs (Katsuda and Ochiya 2015; Fatima et al. 2017; Phinney and Pittenger 2017; Toh et al. 2018). Accordingly, there is increasing interest to evaluate the functional properties of stem cell-derived EVs, especially in the context of CNS repair. In animal models, MSC EVs have demonstrated the ability to increase formation of neuroblasts, induce neurite formation, and to promote repair and recovery after injury (Xin et al. 2012; Xin et al. 2013). Additionally, it has been suggested that MSC EVs may contribute to neuronal regeneration, potentially through the activation of neural stem cells, as well as reduce the inflammation commonly associated with various neurological disorders and brain injury (Braccioli et al. 2013; Koniusz et al. 2016).
iPSC EVs and EVs from iPSC derivatives (i.e. iPSC-derived MSCs and other iPSC-derived lineage specific cell types) are also beginning to be extensively evaluated. Like MSC EVs, these EVs have also been shown to exert protective effects on recipient cells. Recent studies have indicated that iPSC EVs and EVs from iPSC-derived MSCs may promote cellular proliferation and restore viability in response to glucose-induced injury in vitro (Ding et al. 2018), enhance angiogenesis to protect against ischemic injury in vivo (Hu et al. 2015), contribute to repair of bone damage and defects in vivo (Qi et al. 2016), and enhance wound healing in vivo (Kobayashi et al. 2018). Furthermore, there is evidence that EVs from iPSC-derived cardiomyocytes may regulate cell proliferation, inflammation, fibrosis, and apoptosis in a cardio protective manner (Wang et al. 2015; Jung et al. 2017).
Although the studies described above are promising, the field of EV research is still in early development and further studies are needed to better characterize the reparative and immunomodulatory mechanisms by which stem cell EVs act in damaged tissue, especially with respect to the CNS. Here, we evaluate the physical properties and functionality of EVs derived from human adult primary bone-marrow MSCs (BM-MSCs) and human iPSCs. Through the utilization of several robust cell-based assays we compare the effects of MSC and iPSC EVs on various recipient cell types that are relevant to the CNS including astrocytes, neurons, and monocyte-derived macrophages (MDMs). Results from our in vitro functional assays highlight the potential therapeutic applications of stem cell EVs for rescue and repair of these damaged cells.
Methods
Cell Culture and Reagents
Human BM-MSCs (PCS-500–012™), human iPSCs (ACS-1019™), human bronchial epithelial cells (HBECs) (PCS-300–010™), the human lung carcinoma cell line A549 (CCL-185™), the human astrocytoma cell line CCF-STTG1 (CRL-1718™), and the human neuroblastoma cell line SH-SY5Y (ATCC CRL-2266™), were obtained from the American Type Culture Collection (ATCC). BM-MSCs were cultured in Mesenchymal Stem Cell Basal Medium (PCS-500–030™) supplemented with Mesenchymal Stem Cell Growth Kit for Bone Marrow-derived MSCs (PCS-500–041™). iPSCs were cultured in Pluripotent Stem Cell SFM XF/FF (ACS-3002™). HBECs were cultured in Airway Epithelial Cell Basal Medium (PCS-300–030™) supplemented with Bronchial/Tracheal Epithelial Cell Growth Kit (PCS-300–040™). A549 cells were cultured in F-12 media (30–2004™) supplemented with fetal bovine serum (FBS) (30–2020™), CCF-STTG1 cells were cultured in RPMI media (30–2001™) supplemented with FBS, and SH-SY5Y cells were cultured in a 1:1 mixture of EMEM (30–2003™) and F12 medium supplemented with FBS. All media and reagents listed above were obtained from the ATCC.
HIV-1 infected monocytes (U1) and THP-1 cells were seeded and activated using phorbol 12-myristate 13-acetate (PMA) (10 μM) for 5 days to generate monocyte derived macrophages (MDMs). MDMs were grown in RPMI media supplemented with exosome-depleted FBS, L-glutamine, and Penicillin-Streptomycin (PS) (Quality Biological). All cell cultures were incubated at 37°C with 5% CO2.
Isolation of EVs
BM-MSCs and A549 cells were cultured in the presence of exosome-depleted FBS (Thermo Fisher Scientific) prior to the collection of conditioned medium. For iPSCs, which were grown in serum-free medium, conditioned medium was collected and pooled throughout the expansion. Batches of conditioned culture medium were spun and then filtered via dead-end filtration to clarify the medium. Each filtrate was separately concentrated via tangential flow filtration (TFF) following an in-house proprietary protocol (ATCC). Samples were immediately aliquotted and stored at −20°C for downstream analysis.
U1-MDM cells were pelleted via centrifugation. Briefly, the culture supernatant was filtered through a 0.22 μm filter and then centrifuged for 30 minutes at 10,000 × g at 4°C to remove cellular debris. The supernatant was ultracentrifuged twice more at 100,000 × g for 90 minutes at 4°C to pellet EVs. The EV pellet was washed once, resuspended in phosphate buffered saline (PBS), and then stored at −20°C. U1-MDM EV concentration was assessed using ZetaView® Z-NTA (Particle Metrix). The equipment was calibrated according to the manufacturer’s protocol using 100 nm polystyrene NanoStandards™ (Applied Microspheres). The EV sample was diluted in deionized water prior to analysis. The mean concentration (particles/mL) and size (nm) were calculated by the instrument’s built-in ZetaView software.
Characterization of Stem Cell EVs
Nanoparticle tracking analysis (NTA) was performed on stem cell EVs using the NanoSight®NS300 (Malvern). Prior to sample analysis the machine was calibrated following the manufacturers’ protocol. Samples were diluted in PBS prior to analysis. The mean concentration (particles/mL) and size (nm) were calculated by the instrument’s built-in NanoSight NTA software.
For western blot analysis, samples were mixed with Laemmli buffer, heated at 95°C, and run on a 4–20% Tris/glycine gel (Invitrogen). Gels were transferred to an Immobilon PVDF membrane (Millipore) overnight at 50 milliamps. Membranes were blocked with 5% milk in PBS 5 with 0.1% Tween®−20 and then incubated overnight at 4°C with the appropriate primary antibody: α-CD9 (EXOAB-CD9A-1), α-CD63 (EXOAB-CD63A-1), α-CD81 (EXOAB-CD81A-1) (Systems Biosciences) and α-β-Actin (49900) (Abcam). Membranes were washed and then incubated at 4°C with the appropriate secondary antibody. Membranes were developed with either Clarity™ or Clarity Max™ Western ECL Substrate (Bio-Rad) and images were captured with the ChemiDoc™ Touch Imaging System (Bio-Rad). Band intensity was quantified using ImageJ Software.
Proteomic Analysis
EVs were incubated overnight with a 30% slurry of NT80/82 Nanotrap® particles (Ceres Biosciences) and then treated with 8M urea. Samples were reduced with 10 mM DTT, alkylated with 50 mM iodoacetamid, diluted with water and NH4HCO3, and then digested with trypsin at 37°C for 4 hours. Samples were centrifuged at 12,000 × g for 10 minutes and supernatants were collected. ZipTip® was used to collect and dry peptide samples prior to loading into an Orbitrap Fusion™ mass spectrometer. A label-free precursor ion detection method (Proteome Discoverer™; Thermo Fisher Scientific) was used to identify peptides from Swiss-Prot. Search Tool for the Retrieval of Interacting Genes (STRING) was used to assess the protein-protein interaction among proteins retrieved from each EV prep. Biological pathway analysis was performed using Kyoto Encyclopedia of Genes and Genomes (KEGG) and KOBAS 3.0. Protein Fasta files were retrieved from the NCBI protein database using the UniProt accession numbers provided with the mass spectrometry data.
RNA Analysis
Total RNA was extracted from EVs using a phenol chloroform-based method. RNA sequencing and sequence analysis were performed by LC Sciences, LLC. Briefly, total RNA was analyzed using the 2100 Bioanalyzer System (Agilent) to ensure sample integrity. Ribosomal RNA was removed from samples using the Ribo-Zero Gold Kit (Illumina). The remaining RNA fractions were then used to construct the cDNA library; the average insert size for the libraries was 300 ± 50 bp. Pair-end sequencing was performed using the Hiseq 4000 (Illumina). Transcript quality was verified using FastQC, mapped reads were assembled, and expression levels were estimated using StringTie and Ballgown (Frazee et al. 2015; Pertea et al. 2015).
Cytokine Expression
EVs were simultaneously assayed for multiple cytokine/chemokine expression using a magnetic bead-based multiplex assay (Millipore Sigma). The assay was performed according to the manufacturer’s protocol with the following modifications: Triton X™−100 was added to each sample at a final concentration of 1%. Human cytokine standards were prepared in a solution of 1% Triton X-100 in PBS, and the manufacturer’s recommended assay buffer was substituted with 1% Triton X-100 in PBS. Each sample was analyzed in duplicate and measurement was performed using the FLEXMAP 3D® (Luminex). Prior to measurement the equipment was automatically calibrated following the manufacturer’s protocol.
EV Uptake
A549, BM-MSC, and iPSC EVs were labeled with SYTO® RNASelect Green Fluorescent Stain (Thermo Fisher) following the manufacturer’s protocol. To remove excess dye, labeled EVs were added to a Sephadex® G-10 (Sigma-Aldrich) column with a glass wool bottom in a 1 mL syringe packed with 0.75 mL beads, equilibrated with PBS, spun at 2000 × g for 2 minutes, and then stored overnight at 4°C. Labeled EVs were added directly to semi-confluent cultures of recipient cells and incubated at 37°C. After 6 hours the cells were fixed with 4% paraformaldehyde and mounted in ProLong® Gold Antifade Reagent with DAPI (Thermo Fisher). Cells were imaged via fluorescent microscopy using an inverted microscope fitted with the appropriate filters.
Cell Migration Assay
HBECs were seeded into cell culture inserts affixed within 6 well tissue culture plates and grown to confluence in their fully supplemented medium (day 0). On day 1 the inserts were removed, creating an artificial gap of approximately 500 μm. Monolayers were gently rinsed with PBS and basal medium was added to each well, followed by the indicated treatment. Untreated cells received PBS. EV treatments were based upon an approximate ratio of recipient cells to EVs. Each sample was performed in duplicate. Cells were incubated for an additional 48 hours post-treatment. Representative images were captured at different time points and gap width was quantified using a NIST-certified stage micrometer.
Angiogenesis Assay
The Angio-Ready™Angiogenesis Assay System (ACS-2001–2™; ATCC) was used to determine the effect of EVs on vascular-like tubular formation. The assay was initiated following the manufacturer’s protocol and each sample was performed in duplicate. On day 0 cells were seeded in complete Angio-Ready™ Angiogenesis Medium supplemented with VEGF (ACS-2008™; ATCC). On day 2 the medium was removed and the indicated treatment was introduced. Untreated cells received PBS. Positive control cells received fully supplemented, undiluted Angio-Ready™ Angiogenesis Medium. EV treatments were based upon an approximate ratio of recipient cells to EVs. Cells received an additional treatment on days 4 and 6. On day 7 cells were imaged via fluorescent microscopy using an inverted microscope fitted with the appropriate filters. Image analysis was performed using ImageJ software.
Cell Viability Assay (IR)
Cells were exposed to ionizing radiation (IR) using an RS 2000 X-ray Irradiator (Rad Source Technologies) as follows: CCF-STTG1 (15Gy), SH-SY5Y (5 Gy), THP-1 (25 Gy). Cells were immediately seeded and treated with EVs (day 0). Untreated cells received PBS. Each EV treatment was based on an approximate ratio of recipient cells to EVs. Each sample was performed in triplicate. Cells incubated for a period of 3–5 days depending on cell type. Representative images were taken and cellular viability was assessed with the CellTiter-Glo® Luminescent Cell Viability kit (Promega) following the manufacturer’s protocol. The GloMax®-Multi Detection System (Promega) was used to measure luminescence.
MDM (U1) EV Treatment
CCF-STTG1 cells were seeded into 96 well plates and pre-treated with stem cell EVs (day 0). Untreated cells received PBS. Each EV treatment was based on an approximate ratio of recipient cells to EVs. On day 1 cellular damage was induced by exposing cells to MDM (U1) EVs at an approximate ratio of 1:10,000 (recipient cells to EVs). Each sample was performed in triplicate. Representative images were taken on day 3.
Cellular Activation
CCF-STTG1 cells were seeded on day 0. After a period of 24–48 hours cells were exposed to IR (15 Gy) and then immediately treated with EVs. Untreated cells received PBS. EV treatments were based on an approximate ratio of recipient cells to EVs. On day 5 cells were harvested and pelleted. Cell pellets were resuspended in lysis buffer, incubated on ice for 20 minutes, then spun at 10,000 × g for 10 minutes to remove cellular debris. For western blot analysis lysates were added to Laemmli buffer, heated at 95°C, and run on a 4–20% Tris/glycine gel (Invitrogen). Gels were transferred to an Immobilon PVDF membrane (Millipore) overnight at 50 milliamps. Membranes were blocked with 5% milk in PBS with 0.1% Tween-20 and then incubated overnight at 4°C with the appropriate primary antibody: α-PKR(SC-136352), α-ADAR-1 (SC-271854), α-IFNβ (SC-53936) (Santa Cruz Biotechnology), α-P65 (7970) and α-β-Actin (49900) (Abcam). Membranes were washed and then incubated at 4°C with the appropriate secondary antibody. Membranes were developed with either Clarity™ or Clarity Max™ Western ECL Substrate (Bio-Rad) and images were captured with the ChemiDoc™ Touch Imaging System (Bio-Rad). Band intensity was quantified using ImageJ Software.
Statistical Analysis
All quantitative data was analyzed using Graph Pad Prism software. P values were determined using one-way analysis of variance (ANOVA) with multiple comparisons. Use of one-way ANOVA was justified under the assumption that experimental data approximated a normal distribution. P values were assessed as: statistically significant (<0.05), of greater significance (<0.005), and of greatest significance (<0.0001).
Results
Isolation and Characterization of Stem cell EVs
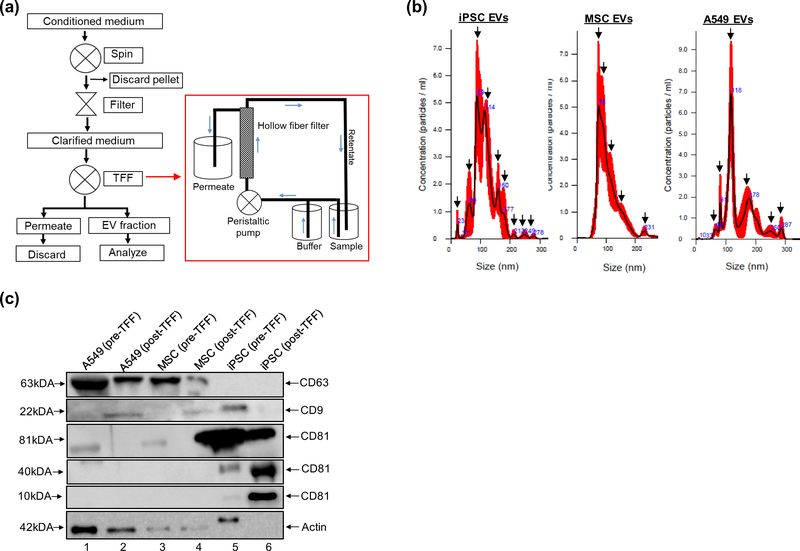
One of the major challenges facing the field of EV research is the lack of scalable methodology for EV isolation. Recently, the use of advanced filtration systems based on cross-flow filtration have been reported for the efficient isolation of EVs from large volumes (Heinemann et al. 2014; Heinemann and Vykoukal 2017; Andriolo et al. 2018; Busatto et al. 2018; Haraszti et al. 2018; McNamara et al. 2018; Watson et al. 2018). Here, we use EVs that have been isolated using similar protocols that have been optimized in our laboratory. This process combines the use of centrifugation, dead-end filtration, and TFF (Fig. 1a). Briefly, the TFF system consists of a sterile flow path fitted with a hollow fiber filter which selectively retains EV-like vesicles (retentate) and excludes smaller vesicles as waste (permeate) (Fig. 1a box). The sample and wash buffer are continuously pushed through the flow path by a peristaltic pump and the sample is concentrated to obtain the final product.
Fig. 1. EV isolation and characterization.
(a) Cells are scaled up to the desired surface area and volume. Conditioned medium is collected, spun, and filtered to remove debris. Clarified medium is serially diafiltrated via TFF to concentrate EVs. During TFF the sample and wash buffer are pushed through a hollow fiber filter by a peristaltic pump. Smaller molecules and proteins are excluded as waste. The retentate fraction, which contains EV-like vesicles, is directed back to the sample reservoir where it is recirculated and then concentrated. (b) NTA analysis of TFF EVs. Representative histograms of three independent measurements show the size distribution of iPSC, MSC, and A549 EVs. Arrows indicate prominent peaks associated with each EV preparation. (c) Western blot analysis was performed to evaluate the expression of selected EV markers (CD9, CD63, CD81) in pre- and post-TFF material
We have isolated EVs from iPSCs, BM-MSCs (hereafter referred to as MSC EVs), and A549 cells and have assessed their basic characteristics. As a control for EV characterization, we have included data from the human lung carcinoma cell line A549, from which we have reproducibly isolated multiple batches of EVs utilizing the process outlined above. Size distribution of each EV prep has been assessed via NTA. This data shows that iPSC EVs have a mode (peak) diameter of 89 nm, MSC EVs have a mode diameter of 75 nm, and A549 EVs have a mode diameter of 118 nm (Fig. 1b). While the highest concentrations of EVs fall between 70–120 nm in each preparation, iPSC EVs appeared to display a much more heterogeneous size distribution. Relative to MSC and A549 EVs, there were several peaks on both the left and right shoulder of the iPSC EV histogram, indicative of multiple populations of varying size.
Western blot (WB) has also been performed to evaluate the expression of well-known exosomal tetraspanin markers (CD63, CD9, CD81) in each EV prep. This data indicates that both MSC EVs and A549 EVs express CD63 and CD9, while CD81 appears undetectable in these samples. On the other hand, iPSC EVs appear to be enriched with CD81 while showing minimal to undetectable expression of CD63, CD9, and actin. Interestingly, iPSC EVs were found to express multiple forms of CD81 corresponding to approximately 81kDa, 40 kDa, and 10kDa in size and this expression was found to be significant relative to A549 and MSC EVs (Fig. 1c and Online Resource 1). Collectively, these data highlight our ability to perform large-scale EV isolation and suggest that EVs derived from different donor cells display unique phenotypes with respect to size distribution and tetraspanin expression.
Proteomic Analysis of Stem Cell EVs and their Associated Cytokines
Determination of the biological cargo contained within stem cell EVs is essential not only for characterization purposes, but also to aid in the interpretation of data generated from downstream assays. The RNA and proteomic composition of stem cell EVs has recently been the focus of several manuscripts; however, studies of this nature are still in their infancy (Fatima et al. 2017; Ferguson et al. 2018; Kaur et al. 2018; Toh et al. 2018). Additionally, a search of the available literature suggests that relative to MSC EVs, the biological content of human iPSC EVs is not yet well characterized. For these reasons we were interested in performing mass spectrometry-based proteomic analysis to evaluate and compare the protein content between MSC and iPSC EVs.
Data from mass spectrometry revealed 296, 273, 1697 proteins unique to iPSC, MSC, and A549 EVs, respectively. Examples of proteins unique to each EV preparation include: fibroblast growth factor-2 (FGF-2), creatine kinase, minichromosome maintenance complex component 4 (MCM4), protein DJ-1, and attractin (iPSC EVs); Janus kinase 2 (JAK2), Wnt-5B, S100A11, and galectin-1 (MSC EVs); matrix metallopeptidase 9 (MMP9), numerous Ras-related proteins, Src family kinases (Yes, Blk, Lck), and 14–3-3 proteins (A549 EVs). When the proteomic data from each EV preparation was crossed-referenced it was found that 306 proteins were common among these EVs (Fig. 2a). Among these common proteins were actin, fibronectin, thrombospondin, tubulin, and heat shock protein 90 (HSP90) (Online Resource 2).
Fig. 2. EV protein and cytokine analysis.
(a) Venn diagram showing the overlap and number of proteins associated with iPSC, MSC, and A549 EVs. The number of unique proteins as well as the number of proteins shared among all three EV preparations are shown. The proteome of each EV preparation was identified by mass spectrometry. (b) Predicted PPI networks associated with each EV preparation were generated using the STRING database. In the resulting networks proteins are presented as nodes which are connected by lines where thickness represents confidence level (0.7–0.9) and colors designate proteins associated with specific functions. Multiplex cytokine analysis was performed and the percentage of EV-associated cytokine (either internal or membrane associated) was calculated. (c) % EV association of FGF-2, VEGF, and IL-4 from all three EVs. Data is separated into 2 graphs to adjust for Y-axis scaling. ** p <0.005
These results were further illustrated by the protein-protein interaction (PPI) networks generated via the STRING database. Proteins are presented as nodes that are connected by lines, where line thickness represents the confidence level (0.7 – 0.9) and colors designate proteins associated with specific functions (Fig. 2b). The PPI network of iPSC EVs displayed 253 nodes and 1127 edges and consisted of tubulins, ribosomal KEGG pathway proteins, serpins (i.e. serine protease inhibitors), and proteins with globin domains. The PPI network of MSC EVs displayed 296 nodes and 1171 edges and mainly consisted of tubulins, peptidase regulator proteins, and proteins with globin domains. In contrast, the A549 EV protein network contained 716 nodes and 4542 edges and was comprised mainly of tubulins, Ras family proteins, 14–3-3 proteins, and ribosomal KEGG pathway proteins.
KEGG analysis also highlighted the involvement of common and unique EV-associated proteins in various biological pathways. Common among all three EV preps were proteins found to be heavily involved in extracellular matrix (ECM) receptor interactions and actin/tight junction assembly, while iPSC EVs had proteins involved in epidermal growth factor receptor (EGFR) interactions and receptor tyrosine kinase (RTK) signaling, MSC EVs had proteins involved in insulin-like growth factor (IGF), JAK-STAT, and Rap1 pathways, and A549 EVs had proteins associated with cancer-related pathways and Hippo signaling (i.e. MMPs, CDK4, 14–3-3) (Online Resources 3–6, respectively).
As a follow up to mass spectrometry, we simultaneously screened pre- and post- concentrated material to measure the levels of common human cytokine/chemokine targets utilizing a magnetic bead-based multiplex assay. As controls, we included culture medium specific to the donor cell type from which each EV sample was derived. The relative amount of EV-associated cytokines (either internal or membrane associated) was calculated by dividing the post-TFF values by a factor of 50. This was based on previous data that shows post-TFF samples are, on average, 50X more concentrated that pre-TFF samples (data not shown). The adjusted post-TFF cytokine values were then divided by the corresponding pre-TFF cytokine values and multiplied by 100 to determine the % EV-associated value. From this experiment, we identified three cytokines (FGF-2, VEGF, and IL-4) that displayed significantly higher association with iPSC EVs relative to MSC EVs (Fig. 2c). Interestingly, each of these cytokines were also found to be associated with A549 EVs. Taken together, this data indicates that EVs derived from different sources of stem cells have distinctly unique protein compositions and are differentially associated with various cytokines.
Functional Assessment of the Effect of Stem Cell EVs on Cellular Migration
We next evaluated the effects of EVs on cellular migration in vitro. For this assay and all subsequent assays, we include data only from iPSC and MSC EVs since we were interested in comparing the functional effects of EVs derived from non-cancerous cells. First, to confirm EV uptake within recipient cells, we co-incubated fluorescently labeled EVs with normal primary HBECs. Image analysis of fixed cells confirmed the intracellular localization of labeled EVs after six hours of incubation. Fluorescent microscopy revealed both a strong cytoplasmic and nuclear staining, corresponding to the presence of labeled EV material in HBECs (Online Resource 7a).
Utilizing HBECs as the recipient cell type we then performed a cellular migration assay which is expected to mimic an in vivo wound healing assay. Cellular migration, which directly correlates to gap closure, was assessed via microscopy and multiple measurements of gap width were taken for each condition at different time points. We observed both a time and dose-dependent decrease in gap width in response to each EV treatment (Fig. 3a and 3c). After 48 hours complete gap closure was observed in cells treated with the highest concentration of EVs, while a widely visible gap still remained in untreated cells. Quantification of this data revealed that treatment with iPSC EVs (1:5,000 and 1:50,000) significantly increased (p <0.0001) HBEC migration at both 24 and 48 hours post-treatment relative to untreated cells at the same time points (Fig. 3b). Similarly, treatment with MSC EVs (1:50,000) significantly increased (p <0.0001) HBEC migration after 2, 24, and 48 hours relative to untreated cells at the same time points (Fig. 3d). Additionally, our data indicate that lower concentrations of iPSC EVs were more efficient in promoting cellular migration relative to the same concentrations of MSC EVs at each time point. Importantly, we did not visually observe any adverse effects in EV-treated cells with respect to cellular morphology or attachment properties during this assay. Collectively, the data from these assays demonstrate the ability of both iPSC and MSC EVs to promote cellular migration in primary cells.
Fig. 3. Cellular migration assay.
HBEC recipient cells were seeded into cell culture inserts and grown to confluence. Upon removal of inserts, cells received the indicated treatment. Untreated cells received PBS. Representative images taken over 48 hours show the gap closure in response to (a) iPSC EVs and (c) MSC EVs. Scale bar = 100 μm. Gap width was quantified from multiple measurements taken at each time point with a NIST-certified micrometer. The average gap width in response to (b) iPSC EVs and (d) MSC EVs over time is shown. *** p <0.0001 relative to untreated at the corresponding time point; ** p <0.005 relative to untreated at the corresponding time point
Functional Analysis of Stem Cell EVs on Tubular Formation in Vitro
To further assess the functional effects of stem cell EVs we performed an in vitro angiogenesis assay. Since angiogenesis is a critical component in both normal development and tissue repair, we were interested in examining the effects of iPSC EVs and MSC EVs on the formation of vascular structures. We utilized a relatively novel and robust angiogenesis co-culture system which combines a proprietary mixture of human aortic endothelial cells (AECs) and human mesenchymal stem cells which are capable of forming vascular-like tubules under the proper culture conditions. The AECs used in this system were previously engineered to constitutively express GFP and therefore provided a fluorescent readout that allowed for the measurement and quantification of tubular length (see Methods section).
Since this assay is optimized to promote angiogenesis, we carried out our experiments under two different dilutions to reduce the concentrations of pro-angiogenic factors already present in the culture system. In each scenario, we observed that treatment with stem cell EVs resulted in the formation of extensive tubular networks relative to untreated cells as visualized by fluorescent microscopy. As a positive control cells received fully supplemented (undiluted) medium. After 7 days in culture the untreated cells displayed an overall fragmented and disjointed appearance. Both MSC and iPSC-EV treated cells, as well as the positive control sample, displayed well-defined branches that were capable of forming interconnected networks. These effects were observed in both the low (1:1) and high (1:10) dilutions of the assay system (Fig. 4a and 4c). Image analysis and tubular quantification performed with ImageJ software confirmed that after a period of 7 days EV treated cells displayed significantly longer tubules relative to untreated cells (Fig. 4b and 4d). When compared to iPSC EV treated cells, MSC EV treated cells displayed a slightly higher increase in tubular length. However, the differences between the average tubular lengths of both iPSC and MSC-EV treated cells as compared to the positive control were not statistically significant. We observed similar results in both 1:1 and 1:10 dilutions. Therefore, these data highlight the ability of stem cell EVs to contribute to the processes of tubular formation and angiogenesis.
Fig. 4. Angiogenesis assay.
The effect of iPSC and MSC EVs on tubular formation in vitro. Untreated and EV-treated cells received either 1:1 or 1:10 dilutions of the fully supplemented medium plus the indicated treatment. EV treatments were based upon an approximate ratio of recipient cells to EVs (1:1000). Untreated cells received PBS. Positive control cells received fully supplemented, undiluted medium. Representative images taken on day 7 of the assay show tubular formation in response to each treatment in (a) 1:1 and (c) 1:10 dilutions of the assay system. Scale bar = 200 μm. Three different fields of view per treatment were selected for analysis and tubular quantification was performed using ImageJ Software. The corresponding average tubular length in response to each treatment in (b) 1:1 and (d) 1:10 dilutions of the assay system are shown. * p <0.05 relative to untreated
Stem Cell EVs Rescue Cellular Viability Following Exposure to Ionizing Radiation
Based on our data showing increased levels of cellular migration and the capacity to promote tubular formation in primary cell types, we next sought to investigate the effects of stem cell EVs in damaged cells. Since exposure to IR is associated with numerous adverse effects in living tissue, including DNA breakage, chromosomal abnormalities, carcinogenesis, and cellular death, we chose to induce cellular damage by exposing recipient cells to IR (Desouky et al. 2015; Burgio et al. 2018).
First, we performed an uptake assay to assess the intracellular localization of labeled EVs in recipient astrocytes. As in HBECs, we observed strong cytoplasmic staining after treatment with fluorescently labeled EVs. We also observed nuclear staining that appeared to be more distinct in cells treated with labeled iPSC EVs as compared to cells treated with labeled MSC EVs (Online Resource 7b). To examine the effects of stem cell EVs on damaged cells, we then irradiated astrocytes and immediately treated them with stem cell EVs. Cells were observed daily and representative images in Fig. 5a (left panel) show the appearance of the cultures on day 5 of the assay. Untreated cells appeared healthy, well attached, and displayed characteristic spindle-like morphology. On the other hand, cells exposed to IR appeared unhealthy, granular, and had completely detached from the substrate, forming clumps of aggregated cells. Treatment of irradiated cells with stem cell EVs permitted cellular adherence; however, we observed slightly different appearances when comparing their respective morphologies. iPSC EV treated cells appeared slightly more rounded with fewer projections/spindles, while the morphology of MSC EV treated cells appeared to more closely resemble that of the untreated cells. Interestingly, we have also performed a similar assay using EVs derived from HIV-1 infected cells (U1-MDMs) to induce damage in recipient astrocytes and have observed similar phenotypes in response to treatment with stem cell EVs (Online Resource 8). Astrocytes were next assayed for viability using a luminescence-based assay. As shown in Fig. 5a (right panel), exposure to IR resulted in a dramatic decrease in viability relative to untreated cells. Treatment of irradiated cells with stem cell EVs, however, resulted in a significant increase in viability. Notably, the viability of cells treated with MSC EVs was similar to that of non-irradiated cells.
Fig. 5. The effect of stem cell EVs on recipient cells following IR.
Cells were exposed to IR and were immediately treated with stem cell EVs (day 0). EV treatments were based upon an approximate ratio of recipient cells to EVs (1:1000). Untreated cells received PBS. Representative images show the overall appearance and morphology of (a, left panel) astrocytes, (b, left panel) neurons, and (c, left panel) MDMs after a period of 3–5 days in culture. Scale bar = 100 μm. Cell viability was assessed and quantified for (a, right panel) astrocytes, (b, right panel) neurons, and (c, right panel). *** p <0.0001; ** p <0.005; * p <0.05 relative to irradiated cells
Based upon these results we next sought to determine if this effect would be consistent among other CNS-related cells, namely neurons and MDMs. After exposing cells to pre-determined doses of IR, stem cell EVs were immediately added and cultures were maintained for a period of 3–5 days. Representative images show that treatment with stem cell EVs permitted reattachment of irradiated cells and induced morphological changes in both neurons (Fig. 5b, left panel) and MDMs (Fig. 5c, left panel). When assayed for viability it was found, again, that treatment of both irradiated neurons (Fig. 5b, right panel) and MDMs (Fig. 5c, right panel) with stem cell EVs resulted in a significant increase in viability. Interestingly, stem cell EVs appear to have the strongest effect on MDMs, as treatment with both iPSC and MSC EVs rescued cellular viability to a level that was consistent with non-irradiated cells. Collectively, these data suggest that stem cell EVs are capable of partially reversing the phenotypes induced by IR and rescuing cellular viability in response to DNA damage.
Stem Cell EVs Exert Differential Effects on the Innate Immune Response
Lastly, to further expand upon the results outlined above and to explore possible mechanistic actions of stem cell EVs on damaged astrocytes, we studied the expression levels of several proteins that are involved in innate immunity. For these experiments we chose to focus on astrocytes since they represent the most prominent glial cell in the brain and, furthermore, are critical not only for neuronal survival and protection but also for actively maintaining homeostasis within the brain (Brambilla et al. 2013; Cekanaviciute and Buckwalter 2016; Colombo and Farina 2016). We rationalized that upon internalization and through the subsequent transfer of their biological cargo, such as RNA, EVs may mobilize a non-specific immune response in recipient cells. To examine this, we treated both irradiated and non-irradiated astrocytes with stem cell EVs and then performed WB to analyze the relative expression levels PKR, p65, IFNβ, and ADAR1. These targets were chosen based on their well-known involvement in mediating host immunity.
Exposure of astrocytes to ionizing radiation resulted in the increased expression of PKR, IFN, p65, and ADAR1 and, interestingly, treatment of non-irradiated cells with stem cell EVs also resulted in the increased expression of these proteins with iPSC EVs appearing to elicit a stronger increase in expression (Online Resource 9). The treatment of irradiated cells with iPSC EVs further increased expression of PKR, IFN, and p65, while treatment with MSC EVs lowered their expression (panel A). When this data was quantified, the effect of iPSC EVs on PKR expression in non-irradiated cells was found to be significantly increased (panel B). RNA sequencing was next performed and subsequent analysis of the long non-coding RNAs present in stem cell EVs identified one secondary structure, containing two continuous double-stranded regions composed of > 30 base pairs each, that was present in only iPSC EVs. Taken together, these data demonstrate the differential effects of iPSC and MSC EVs on common targets involved in the innate immune response in damaged cells. Furthermore, since a stretch of double-stranded RNA 30 base pairs in length or longer has been previously found to activate PKR (Cole 2007; Husain et al. 2012), our data is suggestive that these effects may be mediated, in part, by specific long non-coding RNAs.
Discussion
Neurological diseases and disorders represent a significant cause of death and disability, and it has been reported that their global burden has been rising over recent decades (GBD 2015). As the life expectancy for aging populations continues to rise, it stands to reason that the number of people at risk for developing CNS pathologies will also increase. Further compounding these issues are the numerous difficulties associated with CNS drug design and development (Rankovic 2014). Not only has there been an overall decline in the entry of CNS drugs into clinical trials but it has also been noted that these drugs are more likely than non-CNS drugs to experience failure (Kesselheim et al. 2015). Therefore, there is a considerable need to better understand the pathophysiology underlying these conditions and to further explore novel therapeutic approaches for their treatment.
Although stem cell therapy has been widely evaluated in the context of various pathologies, their mechanisms of action are not yet fully understood. In recent years there has been growing evidence that EVs are the paracrine mediators by which stem cells exert their functional effects in recipient tissues (Baraniak and McDevitt 2010). Consequently, there is high interest in studying EVs derived from stem cells, such as MSCs and iPSCs, as these vesicles may represent natural therapeutic agents. However, scalability of EV isolation protocols are challenging to accomplish. While traditional methods, such as ultra-centrifugation and density gradients, are suitable for small scale studies there is a great need for scalable methods to meet the growing needs of the scientific community (Furi et al. 2017; Konoshenko et al. 2018). Using an optimized EV isolation protocol, we have consistently been able to isolate high yields of EVs from batches of conditioned culture medium. In our recent experiments we have isolated EVs from both iPSCs and MSCs for characterization and functional analysis, with special emphasis placed on cells related to the CNS.
Due to the heterogeneity that exists among EV populations, it is critical to assess both the physical and biochemical properties of EVs isolated from different donor cell types. Our characterization of iPSC, MSC, and A549 EVs underscores this importance. Not only do these EVs display heterogeneity in their size distribution, but also their phenotypic expression of EV marker proteins, such as tetraspanins, and actin are variable. Additionally, we have identified clear differences in the biological cargo of stem cell EVs through our proteomic analysis and cytokine screening. For example, we found a number of proteins, both common and unique to each EV preparation, that share properties relating to replication, cell growth, and migration (MCM4, JAK2, fibronectin, FGF-2), differentiation (FGF-2, galectin-1), structural support (tubulin, actin), angiogenesis (thrombospondin, FGF-2), and immunomodulation (attractin, S100A11, galectin-1, protein DJ-1). As expected, A549 EVs contained many proteins that are associated with cancer and oncogenesis (MMP9, Rab proteins, SRC kinases, semaphorin 3C). Additionally, analysis of biological pathway interactions showed that proteins common among each EV preps interact with membrane-associated proteins involved in tight junction and actin assembly. iPSC and MSC EVs had proteins that were involved in multiple pathways associated with cell migration, survival, and angiogenesis, and A549 EVs had proteins that interacted with pathways associated with carcinogenesis.
The PPI networks also show clear differences between EVs derived from stem cells and cancer cells, with the A549 PPI network displaying high levels of redundancy correlating to the multiple layers of dysregulation in its proteome. The PPI networks of iPSCs EVs and MSC EVs, on the other hand, were better defined and contained much fewer proteins relative to A549 EVs. Interestingly, iPSC EVs possessed a PPI network of serine protease inhibitors (serpins), which are responsible for the regulation of numerous biological processes including inflammation (Fei et al. 2017; Soualmia and El Amri 2018).
Multiplex analysis also identified several EV-associated cytokines including fractalkine, MCP-1, MCP-3, PDGF, IL-8, and IL-6, among others (data not shown). However, there were clear differences with some cytokines having high association with specific EVs. Our analysis revealed three cytokines that were more highly associated with iPSC EVs relative to MSC and A549 EVs. These cytokines are known to possess immunomodulatory (IL-4), pro-angiogenic (VEGF, FGF-2), and pro-growth and differentiation (FGF-2) functions (Nugent and Iozzo 2000; Ferrara 2004; Dvorak 2005; Yun et al. 2010; Gadani et al. 2012; Luzina et al. 2012). It is important to note that while these and other cytokines are present in the conditioned medium of donor cultures, others have previously reported that the effects of recombinant proteins alone, as well as EV-depleted medium, are not as sufficient or potent as those elicited by EVs (Wu et al. 1999; Shabbir et al. 2015). Collectively, we believe that the presence of these proteins and cytokines within stem cell EVs may enhance their overall functional effects and may contribute to reparative mechanisms in damaged cells.
Cellular growth as well as angiogenesis are critical biological processes in both normal development and the repair of diseased and/or damage tissue. We have shown that stem cell EVs clearly promote these processes in a dose-dependent manner. Our experiments also found that stem cell EVs were able to enhance tubular formation even when culture conditions were sub-optimal. Dilutions of the angiogenesis medium suggested that EVs are able to compensate for the reduction of critical components, such as VEGF and FGF-2, which are known to induce angiogenesis (Seghezzi et al. 1998; Murakami and Simons 2008; Przybylski 2009; Hoeben et al. 2004). Our cytokine data suggest that iPSC EVs are associated with VEGF and FGF-2; however, we did not detect either of these cytokines in MSC EVs. Therefore, it is possible that iPSC EVs and MSC EVs target angiogenesis pathways via different mechanisms. Consistent with this, others have already found pro-angiogenic miRNAs to be associated with MSC EVs (Gong et al. 2017; Wang et al. 2017; Ma et al. 2018; Yang et al. 2018). With respect to the CNS, the pro-angiogenic functions of stem cell EVs may hold great promise for future studies relating to the BBB.
Equally significant are the immunomodulatory properties associated with stem cell EVs. It has been widely reported that MSC EVs can modulate the immune response in different cell types; however, to the best of our knowledge, direct comparisons between MSC and iPSC EVs are lacking. For our initial repair assays we chose a human astrocytoma cell line as the recipient cell type. Our justification for this was based on the conception that astrocytes act as the central regulators of the CNS and that their dysregulation is associated with the progression of many neurological pathologies (Phatnani and Maniatis 2015; Pekny et al. 2016). Therefore, these cells may represent potential therapeutic targets. We found that treatment with stem cell EVs had a significant impact on rescuing the viability of damaged astrocytes. Follow-up studies also confirmed that this effect was consistent when both neurons and MDMs were the recipient cells.
Our data also suggests that stem cell EVs may exert different immunomodulatory effects on irradiation-damaged astrocytes. This is most apparent in results showing that MSC EVs more quickly rescued viability of irradiated astrocytes relative to iPSC EVs. When we probed further by looking at the expression of common proteins involved in the immune response pathway, we found differential expression of these targets that were dependent on the EV treatment. As expected, exposure of astrocytes to IR resulted in increased PKR, p65, and IFNβ. This is consistent with the role of PKR as acellular stress response protein which, upon sensing of double stranded RNA (dsRNA), mediates the activation of NFKB and interferon production (García et al. 2006; Olejniczak et al. 2010). Treatment of irradiated cells with MSC EVs reduced the levels of each of these proteins with the most notable reduction observed in PKR. These results support the idea of an overall anti-inflammatory effect of MSC EVs in damaged cells. In contrast, the levels of PKR, p65, and IFNβ remained relatively high in irradiated cells after treatment with iPSC EVs. These results suggest that iPSC EVs may temporarily perpetuate an inflammatory state through the continued activation of PKR.
Adenosine deaminase acting on RNA (ADAR1) mediates one of the most common forms of RNA editing and is known to be expressed in high levels in the brain (O’Connell et al. 1995; Pfaller et al. 2011). ADAR1 is interferon-inducible and activated in response to dsRNA and, furthermore, has been associated with the inhibition of PKR (Slotkin and Nishikura 2013; Hung et al. 2018; Radetskyy et al. 2018). Our data show relatively high and stable levels of ADAR1 in most conditions, with a slight increase in iPSC EV treated cells. Since both PKR and ADAR1 recognize dsRNA, this led us to believe that iPSC EVs may be enriched in long non-coding RNAs which may non-specifically bind to these receptors in recipient cells. We hypothesize that the delivery of this cargo into recipient cells activates an immune response that is mediated, in part, through PKR and ADAR1. While the exact mechanisms of this transfer are largely unknown it most likely occurs via the fusion of EVs with the plasma membrane and the subsequent release of intraluminal content or, alternatively, through the fusion of EVs with the endosomal membrane following endocytosis (Mass et al. 2017; van Neil 2018).
Previous studies have reported that double-stranded RNA greater than 30 bp in length is required for the optimal activation of PKR (Cole 2007; Husain et al. 2012). Our finding of a long non-coding RNA in iPSC EVs, designated AC120498.9, meets this criterion. While this structure represents an uncharacterized transcript, it was nonetheless identified from >15,000 long non-coding RNAs and, furthermore, was not detected in MSC EVs. This finding supports our hypothesis of a specific PKR activation. Therefore, we propose that that exposure to iPSC EVs may initially, and temporarily, slow down growth in damaged cells as a result of interferon production. This could potentially explain why iPSC EVs appeared to exhibit a more delayed response than MSC EVs in improving cellular viability following IR exposure. Also, in consensus with this hypothesis, are the decreased levels of actin that were observed in iPSC EV treated cells, which is indicative of reduced cellular replication. Elevated ADAR1 could, over time, serve to reduce PKR activation which could potentially mitigate the pro-inflammatory state. EV-associated cytokines and proteins could then contribute to reparative mechanisms. In this manner, iPSC EVs may exert a multi-functional effect on damaged cells, acting upon multiple targets which ultimately allow cellular growth to slow down prior to initiating repair and regeneration of damaged tissue. This is in contrast to MSC EVs, which appear to exert a more direct and fast-acting effect on damaged cells. Our future experiments will examine whether this effect is consistent among other cell types of the CNS.
While our manuscript was under review, Liu et al. published a study comparing the functional effects of stem cell EVs using a model of cellular senescence (Liu et al. 2019). Importantly, this study employed enhanced purification methods to evaluate EVs isolated from both iPSCs and MSCs among multiple donors as well as EVs from a matched pair. Results from these studies demonstrated that both populations of EVs could significantly enhance growth of senescent cells in two different models, and through extensive proteomic analysis this effect was proposed to be mediated by members of the peroxiredoxin family (Liu et al. 2019). Consistent with our experiments, these data highlight the potential reparative effects of stem cell EVs. Relative to our studies; however, we note differences in the media formulations used to culture donor cells, the scalability of our EV isolation methodology, and the model of cellular repair. For instance, we utilize assays comprised of a single cell type (cellular migration; Fig. 3), two cell types (angiogenesis; Fig. 4), and assays using multiple CNS related cell types for repair (Fig. 5). Nonetheless, studies of this nature are imperative to drive the field further and serve to further support the holistic effect of stem cell EVs in the context of cellular repair and regeneration.
In conclusion, we have shown that iPSC and MSC EVs can exhibit similar functional effects in undamaged cells. The differences in their functionality, however, become most apparent in the context of damaged cells. Although both stem cell EV populations display immunomodulatory properties we have highlighted disparities in their potential mechanisms. Future experiments will include a more comprehensive analysis of the total RNA landscape of stem cell EVs to better understand their impact on recipient cells and the effects on intracellular targets. In addition, we will further evaluate the long non-coding RNA AC120498.9 to determine its PKR binding specificity (domain A, domain B, or both) and its associated function(s) in various recipient cell types. Furthermore, we will utilize advanced 3D neuronal models generated from iPSC-derived neuronal precursor cells for repair assays. These models will more accurately represent the in vivo phenotype and will allow us to examine the effect of EVs on multiple CNS-specific cell types in response to different sources of insult, including HIV-1 infection. As the EV field is still in early development, the completion of these studies will be imperative in moving the field towards the future therapeutic application of EVs, not only for the CNS but for other pathologies associated with inflammation and tissue damage.
Supplementary Material
Acknowledgements
We would like to thank all members of the Kashanchi lab, especially Catherine DeMarino, Michelle Pleet, and Gwen Cox for their contributions, as well as former ATCC colleague Alexei Miagkov for his contributions. We also would like express gratitude to members of ATCC senior management, especially Drs. Mindy Goldsborough and James Kramer for supporting this work. This work was further supported by National Institutes of Health (NIH) Grants (AI078859, AI074410, AI127351-01, AI043894, and NS099029 to FK) and (R33 CA206937 and R01AR068436 to LAL). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Competing Interests
HB, SP, and DY are employed by ATCC and LAL is affiliated with Ceres Nanosciences, Inc. All other authors declare no potential conflicts of interest
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- Abels ER and Breakefield XO (2016) Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cellular and Molecular Neurobiology 36:301–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amor S, Peferoen LA, Vogel DY, Breur M, van der Valk P, Baker D, van Noort JM (2014) Inflammation in neurodegenerative diseases--an update. Immunology 142:151–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andriolo G, Provasi E, Lo Cicero V, Brambilla A, Soncin S, Torre T, Milano G, Biemmi V, Vassalli G, Turchetto L, Barile L, Radrizzani M (2018) Exosomes From Human Cardiac Progenitor Cells for Therapeutic Applications: Development of a GMP-Grade Manufacturing Method. Frontiers in Physiology 9:1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aust L, Devlin B, Foster SJ, Halvorsen YD, Hicok K, du Laney T, Sen A, Willingmyre GD, Gimble JM (2004) Yield of human adipose-derived adult stem cells from liposuction aspirates. Cytotherapy 6:7–14 [DOI] [PubMed] [Google Scholar]
- Banerjee PN, Filippi D, Allen Hauser W (2009) The descriptive epidemiology of epilepsy-a review. Epilepsy Research 85:31–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraniak PR and McDevitt TC (2010) Stem cell paracrine actions and tissue regeneration. Regenerative Medicine 5:121–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu J and Ludlow JW (2016) Exosomes for repair, regeneration and rejuvenation. Expert Opinion on Biological Therapy 16:489–506 [DOI] [PubMed] [Google Scholar]
- Black IB and Woodbury D (2001) Adult rat and human bone marrow stromal stem cells differentiate into neurons. Blood Cells, Molecules, and Diseases 27:632–636 [DOI] [PubMed] [Google Scholar]
- Börger V, Bremer M, Ferrer-Tur R, Gockeln L, Stambouli O, Becic A, Giebel B (2017) Mesenchymal Stem/Stromal Cell-Derived Extracellular Vesicles and Their Potential as Novel Immunomodulatory Therapeutic Agents. International Journal of Molecular Sciences 18:1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braccioli L, van Velthoven C, Heijnen CJ (2014) Exosomes: a new weapon to treat the central nervous system. Molecular Neurobiology 49:113–119 [DOI] [PubMed] [Google Scholar]
- Brambilla L, Martorana F, Rossi D (2013) Astrocyte signaling and neurodegeneration: new insights into CNS disorders. Prion 7:28–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgio E, Piscitelli P, Migliore M (2018) Ionizing Radiation and Human Health: Reviewing Models of Exposure and Mechanisms of Cellular Damage An Epigenetic Perspective. International Journal of Environmental Research and Public Health 15:1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrello J, Monticone S, Gai C, Gomez Y, Kholia S, Camussi G (2016) Stem Cell-Derived Extracellular Vesicles and Immune-Modulation. Frontiers in Cell and Developmental Biology 4:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busatto S, Vilanilam G, Ticer T, Lin WL, Dickson DW, Shapiro S, Bergese P, Wolfram J (2018) Tangential Flow Filtration for Highly Efficient Concentration of Extracellular Vesicles from Large Volumes of Fluid. Cells 7:273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Viñuelas R, Sanjurjo-Rodríguez C, Piñeiro-Ramil M, Hermida-Gómez T, Fuentes-Boquete IM, de Toro-Santos FJ, Blanco-García FJ, Díaz-Prado SM (2018) Induced pluripotent stem cells for cartilage repair: current status and future perspectives. European Cells and Materials 36:96–109 [DOI] [PubMed] [Google Scholar]
- Cefalo MG, Carai A, Miele E, Po A, Ferretti E, Mastronuzzi A, Germano IM (2016) Human iPSC for Therapeutic Approaches to the Nervous System: Present and Future Applications. Stem Cell International 2016:4869071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cekanaviciute E and Buckwalter M (2016) Astrocytes: Integrative Regulators of Neuroinflammation in Stroke and Other Neurological Diseases. Neurotherapeutics 13:685–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain G, Fox J, Ashton B, Middleton J (2007) Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 25:2739–2749 [DOI] [PubMed] [Google Scholar]
- Chen WW, Zhang X, Huang WJ (2016) Role of neuroinflammation in neurodegenerative diseases (Review). Molecular Medicine Reports 13:3391–3396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole JL (2007) Activation of PKR: An open and shut case? Trends in Biomedical Sciences 32:57–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo E and Farina C (2016) Astrocytes: Key Regulators of Neuroinflammation. Trends in Immunology 37:608–620 [DOI] [PubMed] [Google Scholar]
- Dahm T, Rudolph H, Schwerk C, Schroten H, Tenenbaum T (2016) Neuroinvasion and Inflammation in Viral Central Nervous System Infections. Mediators of Inflammation 2016:8562805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange ECM, van den Brink W, Yamamoto Y, de Witte WEA, Wong YC (2017) Novel CNS drug discovery and development approach: model-based integration to predict neuro-pharmacokinetics and pharmacodynamics. Expert Opinion on Drug Discovery 12:1207–1218 [DOI] [PubMed] [Google Scholar]
- deLazaro I, Yilmazer A, Kostarelos K (2014) Induced pluripotent stem (iPS) cells: A new source for cell-based therapeutics? Journal of Controlled Release 185:37–44 [DOI] [PubMed] [Google Scholar]
- Desouky O, Ding N, Zhou G (2015) Targeted and non-targeted effects of ionizing radiation. Journal of Radiation Research and Applied Sciences 8:247–254 [Google Scholar]
- Ding Q, Sun R, Wang P, Zhang H, Xiang M, Meng D, Sun N, Chen AF, Chen S (2018) Protective effects of human induced pluripotent stem cell-derived exosomes on high glucose-induced injury in human endothelial cells. Experimental and Therapeutic Medicine 15:4791–4797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNunzio JC and Williams RO 3rd (2008) CNS disorders--current treatment options and the prospects for advanced therapies. Drug Development and Industrial Pharmacy 34:1141–1167 [DOI] [PubMed] [Google Scholar]
- Dittmar T and Entschladen F (2013) Migratory properties of mesenchymal stem cells. Advances in Biochemical Engineering/Biotechnology 129:117–136 [DOI] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement Cytotherapy 8:315–317 [DOI] [PubMed] [Google Scholar]
- Dvorak HF (2005) Angiogenesis: update 2005. Journal of Thrombosis and Haemostasis 3:1835–1842 [DOI] [PubMed] [Google Scholar]
- Fatima F, Ekstrom K, Nazarenko I, Maugen M, Valadi H, Hill AF, Camussi G, Nawaz M (2017) Non-coding RNAs in Mesenchymal Stem Cell-Derived Extracellular Vesicles: Deciphering Regulatory Roles in Stem Cell Potency, Inflammatory Resolve, and Tissue Regeneration. Frontiers in Genetics 8:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei R, Zhang H, Zhong S, Xue B, Gao Y, Zhou X (2017) Anti-inflammatory activity of a thermophilic serine protease inhibitor from extremophile Pyrobaculumneutrophilum. European Journal of Inflammation 15:143–151 [Google Scholar]
- Ferguson SW, Wang J, Lee CJ, Liu M, Neelamegham S, Canty JM, Nguyen J (2018) The microRNA regulatory landscape of MSC-derived exosomes: a systems view. Scientific Reports 8:1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N (2004) Vascular Endothelial Growth Factor: Basic Science and Clinical Progress. Endocrine Review 25:581–611 [DOI] [PubMed] [Google Scholar]
- Frazee A, Pertea G, Jaffe AE, Langmead B, Salzberg SL, Leek JT (2015) Ballgown bridges the gap between transcriptome assembly and expression analysis. Nature Biotechnology 33:243–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furi I, Momen-Heravi F, Szabo G (2017) Extracellular vesicle isolation: present and future. Annals of Translational Medicine 5:263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadani SP, Cronk JC, Norris GT, Kipnis J (2012) Interleukin-4: A Cytokine to Remember. Journal of Immunology 189:4213–4219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao HM and Hong JS (2008) Why neurodegenerative diseases are progressive: uncontrolled inflammation drives disease progression. Trends in Immunology 29:357–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García MA, Gil J, Ventoso I, Guerra S, Domingo E, Rivas C, Esteban M (2006) Impact of protein kinase PKR in cell biology: from antiviral to antiproliferative action. Microbiology and Molecular Biology Reviews 70:1032–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD 2015 Neurological Disorders Collaborator Group (2017) Global, regional, and national burden of neurological disorders during 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. The Lancet Neurology 16:877–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg MM (2012) Multiple Sclerosis Review. Pharmacy & Therapeutics 37:175–184 [PMC free article] [PubMed] [Google Scholar]
- Gong M, Yu B, Wang J, Wang Y, Liu M, Paul C, Millard RW, Xiao DS, Ashraf M, Xu M (2017) Mesenchymal stem cells release exosomes that transfer miRNAs to endothelial cells and promote angiogenesis. Oncotarget 8:45200–45212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooch CL, Pracht E, Borenstein AR (2017) The Burden of Neurological Disease in the United States: A Summary Report and Call to Action. Annals of Neurology 81:479–484 [DOI] [PubMed] [Google Scholar]
- Griffiths MJ, Bonnet D, Janes SM (2005) Stem cells of the alveolar epithelium. Lancet 366:249–260 [DOI] [PubMed] [Google Scholar]
- Haraszti RA, Miller R, Stoppato M, Sere YY, Coles A, Didiot MC, Wollacott R, Sapp E, Dubuke ML, Li X, Shaffer SA, DiFiglia M, Wang Y, Aronin N, Khvorova A (2018) Exosomes Produced from 3D Cultures of MSCs by Tangential Flow Filtration Show Higher Yield and Improved Activity. Molecular Therapy 26:2838–2847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q, Wan C, Li G (2007) Concise review: multipotent mesenchymal stromal cells in blood. Stem Cells 25:69–77 [DOI] [PubMed] [Google Scholar]
- Heinemann ML, Ilmer M, Silva LP, Hawke DH, Recio A, Vorontsova MA, Alt E, Vykoukal J (2014) Benchtop isolation and characterization of functional exosomes by sequential filtration. Journal of Chromatography A 1371:125–135 [DOI] [PubMed] [Google Scholar]
- Heinemann ML and Vykoukal J (2017) Sequential Filtration: A Gentle Method for the Isolation of Functional Extracellular Vesicles. Methods in Molecular Biology 1660:33–41 [DOI] [PubMed] [Google Scholar]
- Hessvik NP and Llorente A (2018) Current knowledge on exosome biogenesis and release. Cellular and Molecular Life Sciences 75:193–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschi KK, Li S, Roy K (2014) Induced Pluripotent Stem Cells for Regenerative Medicine. Annual Review of Biomedical Engineering 16:277–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA (2004) Vascular endothelial growth factor and angiogenesis. Pharmacological Reviews 56:549–580 [DOI] [PubMed] [Google Scholar]
- Hu GW, Li Q, Niu X, Hu B, Liu J, Zhou SM, Guo SC, Lang HL, Zhang CQ, Wang Y, Deng ZF (2015) Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells attenuate limb ischemia by promoting angiogenesis in mice. Stem Cell Research and Therapy 6:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung LY, Chen YJ, Mai TL, Chen CY, Yang MY, Chiang TW, Wang YD, Chuang TJ (2018) An Evolutionary Landscape of A-to-I RNA Editome across Metazoan Species. Genome Biology and Evolution 10:521–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain B, Mukerji I, Cole JL (2012) Analysis of high affinity binding of PKR to dsRNA. Biochemistry 51:8764–8770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung JH, Fu X, Yang PC (2017) Exosomes Generated From iPSC-Derivatives: New Direction for Stem Cell Therapy in Human Heart Diseases. Circulation Research 120:407–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuda T and Ochiya T (2015) Molecular signatures of mesenchymal stem cell-derived extracellular vesicle-mediated tissue repair. Stem Cell Research and Therapy 6:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S, Abu-Shahba AG, Paananen RO, Hongisto H, Hiidenmaa H, Skottman H, Seppanen-Kaijansinkko R, Mannerstrom B (2018) Small non-coding RNA landscape of extracellular vesicles from human stem cells. Scientific Reports 8:15503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesselheim AS, Hwang TJ, Franklin JM (2015) Two decades of new drug development for central nervous system disorders. Nature Reviews Drug Discovery 14:815–816 [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Ebisawa K, Kambe M, Kasai T, Suga H, Nakamura K, Narita Y, Ogata A, Kamei Y (2018) Effects of exosomes derived from the induced pluripotent stem cells on skin wound healing. Nagoya Journal of Medical Science 80:141–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koniusz S, Andrzejewska A, Muraca M, Sriwastava AK, Janowski M, Lukomska B (2016) Extracellular Vesicles in Physiology, Pathology, and Therapy of the Immune and Central Nervous System, with Focus on Extracellular Vesicles Derived from Mesenchymal Stem Cells as Therapeutic Tools. Frontiers in Cellular Neuroscience 10:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konoshenko MY, Lekchnov EA, Vlassov AV, Laktionov PP (2018) Isolation of Extracellular Vesicles: General Methodologies and Latest Trends. Biomed Research International 2018: 8545347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyuncu OO, Hogue IB, Enquist LW (2013) Virus Infections in the Nervous System. Cell Host & Microbe 13:379–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai RC, Yeo WWY, Lim SK (2015) Mesenchymal stem cell exosomes. Seminars in Cell & Developmental Biology 40:82–88 [DOI] [PubMed] [Google Scholar]
- Liu S, Mahairaki V, Bai H, Ding Z, Li J, Witwer KW, Cheng L (2019) Highly Purified Human Extracellular Vesicles Produced by Stem Cells Alleviate Aging Cellular Phenotypes of Senescent Human Cells. Stem Cells 37:779–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzina IG, Keegan AD, Heller NM, Rook GA, Shea-Donohue T, Atamas SP (2012) Regulation of inflammation by interleukin-4: a review of “alternatives”. Journal of Leukocyte Biology 92:753–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T, Chen Y, Chen Y, Meng Q, Sun J, Shao L, Yu Y, Huang H, Hu Y, Yang Z, Yang J, Shen Z (2018) MicroRNA-132, Delivered by Mesenchymal Stem Cell-Derived Exosomes, Promote Angiogenesis in Myocardial Infarction. Stem Cells International 2018:3290372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas SLN, Breakefield XO, Weaver AM (2017) Extracellular vesicles: unique intercellular delivery vehicles. Trends in Cell Biology 27:172–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marote A, Teixeira FG, Mendes-Pinheiro B, Salgado AJ (2016) MSCs-Derived Exosomes: Cell-Secreted Nanovesicles with Regenerative Potential. Frontiers in Pharmacology 7:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez-Curtis LA, Janowska-Wieczorek A, McGann LE, Elliott JA (2015) Mesenchymal stromal cells derived from various tissues: Biological, clinical and cryopreservation aspects. Cryobiology 71:181–197 [DOI] [PubMed] [Google Scholar]
- Martínez-Morales PL, Revilla A, Ocaña I, González C, Sainz P, McGuire D, Liste I (2013) Progress in stem cell therapy for major human neurological disorders. Stem Cell Reviews 9:685–699 [DOI] [PubMed] [Google Scholar]
- McNamara RP, Caro-Vegas CP, Costantini LM, Landis JT, Griffith JD, Damania BA, Dittmer DP (2018) Large-scale, cross-flow based isolation of highly pure and endocytosis-competent extracellular vesicles. Journal of Extracellular Vesicles 7:1541396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M and Simons M (2008) Fibroblast growth factor regulation of neovascularization. Current Opinion in Hematology 15:215–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent MA and Iozzo RV (2000) Fibroblast growth factor-2. The International Journal of Biochemistry & Cell Biology 32:115–120 [DOI] [PubMed] [Google Scholar]
- Nussbaum RL and Ellis CE (2003) Alzheimer’s disease and Parkinson’s disease. The New England Journal of Medicine 348:1356–1364 [DOI] [PubMed] [Google Scholar]
- O’Connell MA, Krause S, Higuchi M, Hsuan JJ, Totty NF, Jenny A, Keller W (1995) Cloning of cDNAs encoding mammalian double-stranded RNA-specific adenosine deaminase. Molecular and Cellular Biology 15:1389–1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olejniczak M, Galka P, Krzyzosiak WJ (2010) Sequence-non-specific effects of RNA interference triggers and microRNA regulators. Nucleic Acids Research 38:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omole AE and Fakoya AOJ (2018) Ten years of progress and promise of induced pluripotent stem cells: historical origins, characteristics, mechanisms, limitations, and potential applications. PeerJ 6:e4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald J, Boxberger S, Jørgensen B, Feldmann S, Ehninger G, Bornhäuser M, Werner C (2004) Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells 22:277–384 [DOI] [PubMed] [Google Scholar]
- Păunescu V, Deak E, Herman D, Siska IR, Tănasie G, Bunu C, Anghel S, Tatu CA, Oprea TI, Henschler R, Rüster B, Bistrian R, Seifried E (2007) In vitro differentiation of human mesenchymal stem cells to epithelial lineage. Journal of Cellular and Molecular Medicine 11:502–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekny M, Pekna M, Messing A, Steinhäuser C, Lee JM, Parpura V, Hol EM, Sofroniew MV, Verkhratsky A (2016) Astrocytes: a central element in neurological diseases. Acta Neuropathologica 131:323–345 [DOI] [PubMed] [Google Scholar]
- Pertea M, Pertea GM, Antonescu CM, Chang TC, Mendell JT, Salzberg SL (2015) StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nature Biotechnology 33:290–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaller CK, Li Z, George CX, Samuel CE (2011) Protein Kinase PKR and RNA Adenosine Deaminase ADAR1: New Roles for Old Players as Modulators of the Interferon Response. Current Opinion in Immunology 23:573–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phatnani H and Maniatis T (2015) Astrocytes in neurodegenerative disease. Cold Spring Harbor Perspectives in Biology 7:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phinney DG and Pittenger MF (2017) Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells 35:851–858 [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147 [DOI] [PubMed] [Google Scholar]
- Prockop DJ and Oh JY (2012) Mesenchymal stem/stromal cells (MSCs): role as guardians of inflammation. Molecular Therapy 20:14–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybylski M (2009) A review of the current research on the role of bFGF and VEGF in angiogenesis. Journal of Wound Care 18:516–519 [DOI] [PubMed] [Google Scholar]
- Qi X, Zhang J, Yuan H, Xu Z, Li Q, Niu X, Hu B, Wang Y, Li X (2016) Exosomes Secreted by Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Repair Critical-Sized Bone Defects through Enhanced Angiogenesis and Osteogenesis in Osteoporotic Rats. International Journal of Biological Sciences 12:836–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radetskyy R, Daher A, Gatignol (2018) ADAR1 and PKR, interferon stimulated genes with clashing effects on HIV-1 replication. Cytokine and Growth Factor Reviews 40:48–58 [DOI] [PubMed] [Google Scholar]
- Rankovic Z (2014) CNS Drug Design: Balancing Physicochemical Properties for Optimal Brain Exposure. Journal of Medicinal Chemistry 58:2584–2608 [DOI] [PubMed] [Google Scholar]
- Riazifar M, Pone EJ, Lötvall J, Zhao W (2017) Stem Cell Extracellular Vesicles: Extended Messages of Regeneration. Annual Review of Pharmacology and Toxicology 57:125–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinton DA and Daley GQ (2013) The promise of induced pluripotent stem cells in research and therapy. Nature 481:295–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samsonraj RM, Raghunath M, Nurcombe V, Hui JH, van Wijnen AJ, Cool SM (2017) Concise Review: Multifaceted Characterization of Human Mesenchymal Stem Cells for Use in Regenerative Medicine. Stem Cells Translational Medicine 6:2173–2185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraiva M and O’Garra A (2010) The regulation of IL-10 production by immune cells. Nature Reviews Immunology 10:170–181 [DOI] [PubMed] [Google Scholar]
- Sayed N, Liu C, Wu JC (2016) Translation of Human iPSCs: From Clinical Trial in a Dish to Precision Medicine. Journal of the American College of Cardiology 67:2161–2176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghezzi G, Patel S, Ren CJ, Gualandris A, Pintucci G, Robbins ES, Shapiro RL, Galloway AC, Rifkin DB, Mignatti P (1998) Fibroblast growth factor-2 (FGF-2) induces vascular endothelial growth factor (VEGF) expression in the endothelial cells of forming capillaries: an autocrine mechanism contributing to angiogenesis. The Journal of Cell Biology 141:1659–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, Young M, Robey PG, Wang CY, Shi S (2004) Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 364:149–155 [DOI] [PubMed] [Google Scholar]
- Shabbir A, Cox A, Rodriguez-Menocal L, Salgado M, Van Badiavas E (2015) Mesenchymal Stem Cell Exosomes Induce Proliferation and Migration of Normal and Chronic Wound Fibroblasts, and Enhance Angiogenesis In Vitro. Stem Cells and Development 24:1635–1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah V and Kochar P (2018) Brain Cancer: Implication to Disease, Therapeutic Strategies and Tumor Targeted Drug Delivery Approaches. Recent Patents on Anti-cancer Drug Discovery 13:70–85 [DOI] [PubMed] [Google Scholar]
- Singh S and Joshi N (2017) Astrocytes: inexplicable cells in neurodegeneration. The International Journal of Neuroscience 127:204–209 [DOI] [PubMed] [Google Scholar]
- Slotkin W and Nishikura K (2013) Adenosine-to-inosine RNA editing and human disease. Genome Medicine 5:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song CG, Zhang YZ, Wu HN, Cao XL, Guo CJ, Li YQ, Zheng MH, Han H (2018) Stem cells: a promising candidate to treat neurological disorders. Neural Regeneration Research 13:1294–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soualmia F and El Amri C (2018) Serine protease inhibitors to treat inflammation: a patent review (2011–2016). Expert Opinion on Therapeutic Patents 28:93–110 [DOI] [PubMed] [Google Scholar]
- Stephenson J, Nutma E, van der Valk P, Amor S (2018) Inflammation in CNS neurodegenerative diseases. Immunology 154:204–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson PA 2nd and McGavern DB (2015) Viral diseases of the central nervous system. Current Opinion in Virology 11:44–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K and Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126:663–676 [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131:861–872 [DOI] [PubMed] [Google Scholar]
- Toh WS, Lai RC, Zhang B, Lim SK (2018) MSC exosome works through a protein-based mechanism of action. Biochemical Society Transactions 46:843–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trounson A and McDonald C (2015) Stem Cell Therapies in Clinical Trials: Progress and Challenges. Cell Stem Cell 17:11–22 [DOI] [PubMed] [Google Scholar]
- Uccelli A, Moretta L, Pistoia V (2008) Mesenchymal stem cells in health and disease. Nature Reviews Immunology 8:726–736 [DOI] [PubMed] [Google Scholar]
- Ullah I, Subbarao RB, Rho GJ (2015) Human mesenchymal stem cells - current trends and future prospective. Bioscience Reports 35:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Niel G, D’Angelo G, Raposo G (2018) Shedding light on the cell biology of extracellular vesicles. Nature Reviews Molecular Cell Biology 19:213–228 [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang L, Li Y, Chen L, Wang X, Guo W, Zhang X, Qin G, He SH, Zimmerman A, Liu Y, Kim IM, Weintraub NL, Tang Y (2015) Exosomes/microvesicles from induced pluripotent stem cells deliver cardioprotective miRNAs and prevent cardiomyocyte apoptosis in the ischemic myocardium. International Journal of Cardiology 192:61–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Chen C, Yang D, Liao Q, Luo H, Wang X, Zhou F, Yang X, Yang J, Zeng C, Wang WE (2017) Mesenchymal stem cells-derived extracellular vesicles, via miR-210, improve infarcted cardiac function by promotion of angiogenesis. Biochimica et Biophysica Acta - Molecular Basis of Disease 1863:2085–2092 [DOI] [PubMed] [Google Scholar]
- Watson DC, Yung BC, Bergamaschi C, Chowdhury B, Bear J, Stellas D, Morales-Kastresana A, Jones JC, Felber BK, Chen X, Pavlakis GN (2018) Scalable, cGMP-compatible purification of extracellular vesicles carrying bioactive human heterodimeric IL-15/lactadherin complexes. Journal of Extracellular Vesicles 7:144208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Yang X, Han ZP, Qu FF, Shao L, Shi YF (2013) Mesenchymal stem cells: a new trend for cell therapy. Acta Pharmacologica Sinica 34:747–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Xia YP, Roth SI, Gruskin E and Mustoe TA (1999) Transforming growth factor-beta1 fails to stimulate wound healing and impairs its signal transduction in an aged ischemic ulcer model: importance of oxygen and age. The American Journal of Pathology 154:301–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin H, Li Y, Buller B, Katakowski M, Zhang Y, Wang X, Shang X, Zhang ZG, Chopp M (2012) Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells 30:1556–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin H, Li Y, Cui Y, Yang JJ, Zhang ZG, Chopp M (2013) Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. Journal of Cerebral Blood Flow and Metabolism 33:1711–1715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Cai Y, Zhang Y, Liu J, Xu Z (2018) Exosomes Secreted by Adipose-Derived Stem Cells Contribute to Angiogenesis of Brain Microvascular Endothelial Cells Following Oxygen-Glucose Deprivation In Vitro Through MicroRNA-181b/TRPM7 Axis. Journal of Molecular Neuroscience 65:74–83 [DOI] [PubMed] [Google Scholar]
- Yin PT, Han E, Lee KB (2016) Engineering Stem Cells for Biomedical Applications. Advanced Healthcare Materials 5:10–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B, Zhang X, Li X (2014) Exosomes Derived from Mesenchymal Stem Cells. International Journal of Molecular Sciences 15:4142–4157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun YR, Won JE, Jeon E, Lee S, Kang W, Jo H, Jang JH, Shin US, Kim HW (2010) Fibroblast growth factors: biology, function, and application for tissue regeneration. Journal of Tissue Engineering 2010:218142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.