Abstract
Transcranial direct current stimulation (tDCS) is a promising method for altering the function of neural systems, cognition, and behavior. Evidence is emerging that it can also influence psychiatric symptomatology, including major depression and schizophrenia. However, there are many open questions regarding how the method might have such an effect, and uncertainties surrounding its influence on neural activity, and human cognition and functioning. In the present critical review, we identify key priorities for future research into major depression and schizophrenia, including studies of the mechanism(s) of action of tDCS at the neuronal and systems levels, the establishment of the cognitive impact of tDCS, as well as investigations of the potential clinical efficacy of tDCS. We highlight areas of progress in each of these domains, including data which appears to favor an effect of tDCS on neural oscillations rather than spiking, and findings that tDCS administration to the prefrontal cortex during task training may be an effective way to enhance behavioral performance. Finally, we provide suggestions for further empirical study that will elucidate the impact of tDCS on brain and behavior, and may pave the way for efficacious clinical treatments for psychiatric disorders.
Keywords: Transcranial direct current stimulation, neural mechanism, moderators, psychiatric treatment
Transcranial direct current stimulation is a method of brain stimulation involving passing a weak current (1–2mA) across the cortex using at least two electrodes. The method has a substantial history1, but has more recently undergone intensive evaluation for use as a tool for modulating cognitive function and the symptoms of psychiatric and neurological disorders2. tDCS has considerable potential as a treatment due to its relative cost, portability, safety and ease of use compared to other methods of neuromodulation3. Side effects, such as itching, burning sensation or headache are common but generally mild and without long term impact4, 5. Thus tDCS compares favorably with other therapeutic approaches such as antidepressants6 or transcranial magnetic simulation (TMS)3. However, there is still much work to be done to determine the full potential of this method as a scientific and clinical tool7. As the number of studies has grown exponentially in recent years1, a number of key unresolved questions has emerged as high priority research topics that need to be addressed for the field to continue to make progress.
In this critical review, we highlight three areas of investigation that we consider to be at the top of the research wish list for understanding the use of tDCS in experimental and clinical contexts. The first concerns the mechanism of action. The second concerns the establishment of the cognitive impact of tDCS and expected effect size, given the variability of the available data and ongoing exploration of tDCS parameter space. We then turn to an overview of clinical mental health research that has used tDCS. Finally, we suggest directions for future study to clarify the neural and behavioral impact of tDCS, and further enhance its value as a scientific and clinical tool.
1. Mechanism(s) of action
A key challenge for tDCS research, as with other forms of neuromodulation as well as for many pharmacotherapies, is the elucidation of its mechanism of action. Much of the information we have about the effects of tDCS on cognition and behavior has been obtained in the context of a limited understanding of the neural basis for tDCS effects on brain circuitry. This situation places constraints on the kinds of experiments we can design and hypotheses that can be tested using this method. Increased understanding of the impact of tDCS on neural activity would greatly expand our ability to design and interpret new experiments. For example, if it were known that a certain electric field applied to a particular region reduced neural activity by 10%, and behavioral output were linearly related to the activity in this region, we might expect behavioral output to be reduced by 10% compared with baseline. Such a relationship would afford opportunities to calibrate a particular protocol to maximize the desired effect, and perform control experiments in cases where the resulting data are ambiguous. A better understanding of the underlying mechanisms of action would also be greatly informative toward building theoretical models and linking findings across different outcome measures, which would enable the field to better connect brain to behavior.
1.1. Evidence for indirect, rather than direct, effect on neural spiking
Although this is still an ongoing topic of research, extant data suggests that tDCS is unlikely to have a straightforward linear effect on neural firing rates8. An important demonstration of this was performed by Voroslakos and colleagues9, who reported the effects of a range of administered currents to rodents during the recording of neural spiking and membrane potentials, both at the skull and on the scalp (skin). Crucially, no impact on neural spiking or membrane potential was observed, unless voltage gradients of around 1mV/mm or higher – the equivalent of a dose of about 5–6mA for human tDCS – were used. Such currents are generally not used for human tDCS: higher-than-typical currents (greater than 4.5mA) in humans have been associated with perceptual abnormalities, consistent with a direct impact on neural transmission9. To the extent that the impact of tDCS on behavior is determined primarily by changes in neural firing rates, these findings suggest that lower doses would be ineffective. It seems more likely, however, that tDCS impacts network-level neural functioning such as oscillatory dynamics, which are critical to cognition and behavior; we discuss this in the section below.
1.2. tDCS-induced electric fields
Direct measurements of the electrical field elicited by tDCS in humans have been obtained by Huang and colleagues10 (see also11). Intracranial electrode recordings were obtained during transcranial (alternating current) stimulation in individuals who were undergoing surgical treatment for epilepsy. A key contribution of this study was to provide validation of the models which are typically used to estimate the electric fields evoked by tDCS in the brain, which are up to 0.4mV/mm for 1mA stimulation and 0.8mV/mm for 2mA stimulation. Computational models were able to account for around 75% of the variance in electric field recordings10. While at low current strengths tDCS may not have an impact on neural spiking, this study demonstrated in human participants that current strengths in the range of most human tDCS studies (1–2mA) do have a substantial impact on cortical electric fields. Moreover, in vitro studies have observed detectable effects on neural recordings with electric fields as weak as 0.2mV/mm12. Specifically, Reato et al.13 reported parametric changes in electric fields in response to stimulation in rats, finding a variety of significant relationships between field strength and oscillatory power across different metrics.
This study suggests a possible lower bound by which tDCS might be expected to have an effect on neural firing properties. This is highly informative regarding understanding of the mechanism(s) of action, as it opens the possibility for 1–2mA tDCS to have an impact on a range of neural phenomena, including those which may be susceptible to weak currents. Of the various potential candidates, Liu and colleagues argue that stochastic and rhythm resonance are the most plausible neural mechanisms by which weak modulation of an electric field may affect neural information coding14. These effects would emerge from small changes in spike predictability and timing, and may exert an effect of cognition via an influence on neural (population) coding15. At a cellular level, these changes may arise from an impact of tDCS on membrane potentials, and thus spike probability and timing13, 14, and/or neural plasticity (i.e. long-term potentiation/depression: LTP/LTD16).
To the extent that tDCS has a modulatory role on neural activity (rather than eliciting spiking directly), the nature of the “baseline” neural activity to be modulated is thus crucial. This has been articulated within Bikson and colleague’s activity/selectivity hypothesis17. For example, a difficult working memory paradigm might elicit increases in theta power18 compared with a baseline task. The neural state created by this task might be differentially susceptible to modulation by tDCS compared with another state, and thus might yield different outcomes in behavior. Alternatively, an effect of tDCS on plasticity would naturally explain differential effects of tDCS on task training versus performance: tasks trained during tDCS would show enhanced performance at test via improved task encoding, but tDCS would not influence task performance directly19. Further work examining the effect of weak electrical currents focused on testing such hypotheses might provide further insights into tDCS’s effect in humans.
1.3. In vivo measures of neurophysiological impact of tDCS in humans
Given that in vivo studies with animals may only be indirectly applicable to humans, and that behavioral measures can reflect a complex variety of factors, human neuroimaging studies might play a valuable role in uncovering intervening variables which underlie the neural mechanism of tDCS. Techniques such as functional magnetic resonance imaging (fMRI), magnetoencephalography (MEG) and electroencephalography (EEG) are well-suited to examine neural phenomena that may be relevant to tDCS’s mechanism, as these methods do not assess neural spiking directly20, 21. Rather, these methods reflect a more global measure of the function of a neural region or system.
Regardless of the suggested neural mechanisms relating to oscillations or plasticity described above, it is worth noting that anodal and cathodal stimulation influence the directions of an induced electrical field rather than simply having an ‘excitatory’ or ‘inhibitory’ impact on brain function22. Accordingly, direct effects on the blood oxygenation level dependent (BOLD) signal, as a proxy of synaptic input to a given brain region21, have rarely been reported in response to anodal or cathodal stimulation. In one experiment, tDCS administered to the visual cortex had a relatively small but significant impact on visually evoked BOLD in this region23, perhaps somewhat obscured by ceiling effects. Local decreases in BOLD responses caused by anodal stimulation have also been reported (e.g.24), as well as decreases in resting perfusion and associated alterations in BOLD responses caused by prefrontal anodal/cathodal stimulation25. By contrast, there is a larger literature on changes in oscillatory activity, including changes in whole brain spectral properties at rest using EEG26, and more selective changes to gamma oscillations in visual cortex using MEG27, 28 (but see29). A number of studies have reported changes to theta oscillations during a variety of cognitive tasks following tDCS30–32. We have shown modulation of gamma oscillations over frontal cortex using anodal tDCS during a proactive cognitive control task33. We discuss studies that have found tDCS-induced changes in neural oscillations in more detail in Section 2 below. Given the crucial role that neural oscillations play in cognition, both in “local” regional cortical dynamics and in long-distance communication across regions in a neural network34–37, the impact of tDCS on neural oscillations may make this method uniquely poised to influence cognition (as opposed to methods that influence spiking directly such as TMS).
1.4. Importance of montage
One experimental variable that exerts a strong effect on the behavioral or physiological impact of tDCS is the arrangement (montage) and the size of the electrodes employed. A recent study of Fischer and others addressed several of these complexities38. This study involved administering tDCS before evoking motor responses (motor evoked potentials: MEPs) using transcranial magnetic stimulation (TMS), and then measuring the magnitude of these responses using electromyography (EMG). A crucial manipulation involved the montage of electrodes used for tDCS stimulation. Anodal stimulation was applied using a conventional montage (i.e. anodal stimulation on the left motor cortex with supraorbital cathodal stimulation), which elicited a small increase in excitability. A novel montage was also developed, in which bilateral motor cortex received anodal stimulation using an array of electrodes, while cathodal stimulation was applied to non-primary motor cortex regions including frontal and parietal regions. This novel montage produced a much larger and long-lasting increase in excitability. Despite conventional electrical field modeling showing that both montages should produce a similar current dose to motor cortex, the novel montage was physiologically more efficacious. Recurrent connections within the motor cortex network may amplify of the administered dose (see also13), leading to the observed enhancement. Overall, the results imply that the global, network-level impact of stimulation needs to be considered and not simply its local impact.
This argument is echoed by a recent meta-analysis of the impact of prefrontal cortical-administered tDCS on cognitive function39. This study examined the impact of methodological factors, such as the use of an extra-cranial (e.g. shoulder) cathode. Such studies yielded a larger and more consistent effect size than studies using a cranial cathode. This finding is compatible with the Fischer study38, insofar as it illustrates the importance of cathode location. For example, in studies of prefrontal cortical-targeted stimulation, a cathode on the opposite hemisphere of the prefrontal cortex could interfere with the effect of the anode (see also40). Likewise, smaller electrodes were also more efficacious, perhaps due to a more specific focal impact and less cross-network influence. Recent work has increased the range of montage options, such that high-definition (HD) montages are now available in addition to traditional anode-cathode pair montages41. HD montages may allow more focal stimulation to be administered, although further validation of the impact of different montages is necessary. The variety of montages available allows flexibility in future research as, depending on the region or network under investigation, it may be possible to implement relatively focal or diffuse tDCS as necessary for the intended manipulation.
Different neural regions might respond differently to tDCS, due to functional as well as anatomical differences between the regions. For example, recent evidence suggests that motion discrimination thresholds can be modulated by both anodal and cathodal stimulation via different psychophysiological mechanisms42, 43. Such studies imply that the effect of tDCS will be mediated by the manner in which the electric field interacts information representation in a particular region and how it subserves a specific psychological process.
1.5. Interim summary
Studies of humans using intracranial electrodes suggest that electrical field intensities elicited by tDCS are far below that which would be required to cause spiking. An influence of weak currents – within the range realistically administered by tDCS - can be observed on the entrainment of oscillations, however13. Moreover, certain montages appear to exert more powerful or otherwise different effects than might be easily captured by computational models38, suggesting that there might be amplifying effects generated by stimulating in a consistent way across a network. These experimental considerations may interact with the brain region examined and the type of behavioral output used to measure the effect of tDCS. Overall, the complexity of the extant literature seems consistent with the potential complexities of the effect of tDCS on neural function (Table 1).
Table 1:
Areas of progress in tDCS research, and questions to be addressed in future studies. References reflect examples of relevant investigations or discussions.
| Areas of Progress | Open Questions | |
|---|---|---|
| Mechanism(s) of Action | • Validated models of in vivo electric field10. • Neurophysiological effects of tDCS as measured by neuroimaging techniques105. |
• Precise effect of tDCS on neural information transmission9, 13. • Differential impact of tDCS on particular neuron types106, with implications for function. • Reliable neurophysiological assays of tDCS’s functional effect105. • Capacity for system-level compensation in response to modulation67. |
| Impact on Cognition | • Reproducible impact of tDCS on behavior19, 39. • Identification of parameter dimensions which may impact behavioral outcomes e.g. extracranial return electrodes, training versus testing, electrode size19, 39. |
• Trait-level moderators which may determine impact of tDCS e.g. anatomical, functional differences19, 39. • Precise prediction of all potential permutations of experimental variables including timing, dose and montage on cognitive process of interest19, 39. |
| Clinical Impact | • Small but reliable impact of tDCS on clinical symptoms68–70. • Identification of parameter dimensions which may impact behavioral outcomes e.g. current dose/duration, treatment resistance68, 69. |
• Trait-level moderators which may determine impact of tDCS e.g. behavioral, anatomical, functional differences108, 109. • Precise prediction of all potential permutations of experimental variables including timing, dose and montage on clinical outcome of interest68, 69. |
2. Determining typical cognitive impact
As the number of tDCS studies rises, an important question concerns the typical impact on cognition and behavior that can be expected with stimulation. There are several recent examples in the literature of an unsatisfactory ratio between meta-analytic estimate of effect size and the power of individual studies. Specifically, the relatively modest sample size of most studies in the tDCS literature may be problematic, given that estimates of behavioral effect sizes are often moderate or small44. Type I and type II error rates are not well calibrated if studies are relatively underpowered45. This concern has been identified in analyses of the effects of tDCS on cognitive function46, 47, but applies across other domains too. This scenario presents a challenge for establishing tDCS parameters that can have reliable behavioral impact, mostly because the types of studies that are being routinely conducted are often not of a sufficient power to provide adequate evidence against the null hypothesis. Nevertheless, as Meron and colleagues point out in their meta-analysis of the treatment impact of tDCS48, the low power of many tDCS experiments, given the modest meta-analytically derived effect sizes, can be addressed if reasonable and effective steps are taken to increase the effect size by eliminating irrelevant sources of variability. Moreover, meta-analyses can be hindered by methodological variation in the literature, which either restricts their focus, or forces them to combine heterogeneous studies that may not be straightforwardly comparable and thus underestimate a true effect size49.
2.1. Influence of PFC stimulation on cognitive control (goal maintenance; adaptive control; inhibition)
While studies of the impact of tDCS on sensorimotor systems can provide a valuable window into its neurophysiological effect, there is substantial interest in determining tDCS’s effect on more complex psychological processes – including cognitive50, 51 and emotional domains. Here, we focus particularly on modulation of the prefrontal cortex (PFC). The PFC has been the target of considerable experimental investigation, with numerous studies demonstrating potentially functionally meaningful modulation of cognitive and emotional processes by tDCS. For example, a number of studies have shown that anodal tDCS to dorsolateral prefrontal cortex (DLPFC) leads to improved cognitive performance in healthy adults52. Moreover, improved performance on working memory tasks53, 54, probabilistic learning tasks (for a subset of patients)55, adaptive control tasks30, and attention-vigilance tasks53 have all been demonstrated following anodal DLPFC tDCS in patients with schizophrenia. A common thread of these disparate tasks is that they all depend, in part, on DLPFC-mediated cognitive control functions such as goal maintenance.
Current evidence suggests that tDCS may have a more selective influence than a simple boosting of performance on difficult cognitive tasks. Simonsmeier and colleagues performed a meta-analysis of 35 studies examining a variety of cognitive tasks involving study and recall phases in mathematical and language domains19. The majority (n=29) of these studies employed tDCS, while most targeted frontal or parietal cortex. Here, greater effect sizes were observed when stimulation was administered before or during the study phase (d=0.71) than the performance phase (d=0.21). This pattern suggests that tDCS may assist with updating and maintaining aspects of the task representation in relevant neural circuitries (goal maintenance)56, 57, rather than a simple boosting of neural function to improve performance during test. Another meta-analysis by Imburgio and Orr39, more focused on the frontal lobe, reached a similar conclusion regarding the impact of tDCS on goal maintenance-related functions rather than inhibition or switching, as did another meta-analysis more focused on working memory training58.
Evidence is emerging that links tDCS-related improvement in task performance to neural oscillations. For example, Reinhart and colleagues have shown that prefrontal cortex-directed tDCS enhances behavioral performance on adaptive control tasks30, specifically on-demand changes in executive processes following increased cognitive demands (e.g. post-error adjustments in processing), as well as associated neural oscillatory measures in the theta frequency band (~4–7 Hz). Building on this finding in a recent study, Reinhart59 used high-definition transcranial alternating current stimulation (HD-tACS) to administer in-phase, anti-phase, and sham stimulation in the theta frequency band to the prefrontal cortex. In-phase theta stimulation to the prefrontal cortex synchronized theta oscillations between two prefrontal cortical regions, and improved behavioral correlates of adaptive control, compared with anti-phase stimulation or sham. In addition to an effect on adaptive control, prefrontal cortically-targeted tDCS may also have an impact on proactive control processes, which include goal and context maintenance over time. For example, we have recently shown an increase in oscillatory activity in the gamma band following anodal prefrontal cortical tDCS stimulation compared with sham, as well as a corresponding behavioral effect33.
There is also evidence of anatomical selectivity: montages focusing on inferior rather than dorsolateral regions of the prefrontal cortex, particularly on the right hemisphere, can influence inhibitory function60, 61. Moreover, other domains such as emotion regulation62, 63 and risk taking64, which may also depend on inhibitory processes, can also be modulated by right prefrontal cortical tDCS.
2.2. Interim summary
Evidence is emerging that tDCS can impact executive function in a fairly specific manner, both in terms of the impact on behavior and the anatomical selectivity. Moreover, an impact of tDCS on neural oscillations, rather than spiking, may reflect the underlying mechanism. It may be that the heterogeneity described in the literature is a result of using tDCS to evaluate cognitive models without an appropriate mapping onto the underlying neural mechanism, and a change of emphasis may assist future studies. For example, working memory models which emphasize oscillatory mechanisms of information storage65 may yield clearer predictions and better account for the resulting data than those that emphasize neural spiking66. Moreover, adapting a network-led rather than region-led approach67 may represent a more productive approach (Table 1).
3. Determining typical clinical impact: findings from tDCS studies of psychiatric disorders
3.1. Mood disorders
Observations of modulation of cognition produced by tDCS imply that cognitive or affective abnormalities in individuals with psychiatric conditions might be ameliorated by tDCS. Perhaps the most extensive work evaluating this proposal has been conducted in major depressive disorder (MDD). There have been numerous clinical trials examining the effect of tDCS on MDD, which generally have involved repeated sessions of stimulation to the left DLPFC17, 68. Such a procedure can yield a modest improvement in symptoms compared to sham tDCS68. As the literature grows, it has been possible to identify tDCS parameters and aspects of the trial that are associated with clinical efficacy, and there is a suggestion that effect sizes are increasing as the relevant parameters are identified. These parameters may include stimulation of greater current (trend-level69) for longer periods68. In addition, as might be expected, tDCS is unfortunately less effective for treatment-resistant patients17, 68. A recent clinical trial performed a controlled comparison of tDCS and sertraline70, finding a significant improvement in clinical symptoms compared to sham/placebo using tDCS, but a smaller effect size than sertraline. Likewise, a combination of sertraline and tDCS might provide quantitatively greater efficacy than tDCS alone71. However, another recent clinical trial72 failed to yield a significant effect of anodal tDCS on depression symptoms. This trial had a number of interesting features, including 2.5mA stimulation (1–2mA typically employed68), data collection across several sites and a relatively substantial placebo (sham) effect. Well-powered null trials of this sort are essential for providing further insight into relevant parameters which determine treatment magnitude and reliability.
An important question arising from these demonstrations of efficacy is whether tDCS would act on the mood-related or cognitive symptoms73 of depression, given that somatic symptoms such as weight gain/loss or sleep might be less likely to be directly impacted by prefrontal tDCS. Studies which have evaluated whether an impact on cognition or mood mediates the antidepressant effect of tDCS reported mixed results, with some finding no effect on cognition but some effect on emotion recognition74, and others suggesting a potential role for cognitive control75–79. A meta-analysis indicated no effect of cognition independent of mood improvement80, suggesting that identifying a specific effect of tDCS on cognition is difficult within a clinical trial design given general cognitive improvements in patients through a trial. More recent experimental approaches have sought to identify the effect of tDCS effect on cognitive processes important for mood regulation to shed further light on this question81.
3.2. Schizophrenia
Cognitive dysfunction across a wide variety of difficult cognitive tasks is a core feature of schizophrenia, and similar efforts have been made to improve cognitive performance in schizophrenia using tDCS. As noted above, cognitive control, including adaptive control, proactive control and inhibition have been among those targeted by a number of tDCS studies, and these functions are prominent among the cognitive impairments that are observed in schizophrenia and related psychotic disorders82. Thus, there have been several studies that examined the impact of tDCS on cognitive control functioning in schizophrenia, with some success. For example, Reinhart et al.30 found evidence that mid-frontal stimulation improved behavioral and neural oscillatory measures of adaptive control in participants with schizophrenia (see also53–55).
Evidence regarding auditory hallucinations is mixed, with some positive findings. For example, Mondino et al.83 found an effect of tDCS on auditory verbal hallucinations and resting state fronto-temporal connectivity following twice daily stimulation sessions of 20 minutes at 2 mA, administered with the cathode over the left temporoparietal junction and the anode over the left DLPFC. Brunelin et al.84 found an effect on auditory hallucinations lasting up to 3 months with a similar stimulation protocol. Although Fitzgerald et al.85 were unable to replicate the finding, this may have resulted from the procedure (stimulation once rather than twice daily) employed by the authors. The number of stimulations per day is a further example of a potentially relevant experimental parameter whose theoretical status is uncertain, but requires further examination.
Research on the effect of tDCS on both cognitive and psychotic symptoms in schizophrenia is a rapidly growing area of research, but there is not yet a large literature available.
3.3. Interim summary
In terms of the clinical areas discussed, there is some optimism that tDCS may be efficacious as a treatment strategy for psychiatric disorders. We have used schizophrenia and depression as examples, but note that tDCS may have more general applicability in psychiatric disorders (e.g.86). For example, it may alter compulsive behaviors in anxiety disorders87 and drug cue-elicited cravings and risk-taking behavior in substance abuse disorders88–90. Importantly, tDCS may induce clinically deleterious outcomes91–94, and such findings may also assist in defining an effective montage and dose. While identifying effective parameters can proceed by empirical, trial-and-error approaches alone, it is likely that greater understanding of the underlying mechanism of tDCS might yield wider benefits for designing clinical treatments. For example, insights obtained from cognitive neuroscience studies, with regard to oscillations and networks already mentioned, might impact the design of treatment studies (Table 1).
4. Recommendations for future research and conclusions
While the data we have reviewed reveal important uncertainties around tDCS’s neurophysiological mechanism and impact on cognition and behavior (see also95), they also reflect a growing confidence in this method. A reliable impact on neural activity and behavior, including motor thresholds, prefrontal cortex-related cognition and depression, has been described. We provide suggestions for future research that might increase such confidence further.
First, there have been recent calls for a sharper distinction between exploratory and confirmatory work in experimental neuroscientific and psychological research96. Certainly, tDCS research would benefit both from more exploratory work into the influence of the many relevant experimental parameters, as well as confirmatory work estimating the effect size of a particular parameter set. The latter might include replications (e.g.97), relatively large sample sizes and preregistration (e.g.98) and careful dissemination of inconsistent or null effects (e.g.99). Furthermore, it is essential to review current best practices regarding tDCS protocol design (e.g.100), which may enable more consistent stimulation to be administered across individuals and studies. Finally, given our discussion of plausible tDCS mechanisms, it is likely that the behavioral context in which the stimulation is administered may be important (i.e. cognitive, emotional, arousal states). Efforts to control these factors may be crucial in determining the impact of tDCS.
Second, much of the work into the effects of tDCS in humans has used conventional convenience samples68. As the development of tDCS extends to clinical cohorts, modelling of individual differences in response to tDCS may become increasingly important. Moreover, many clinical cohorts themselves are highly heterogeneous (e.g.101), and potential moderators of tDCS efficacy have already been identified, as described above (e.g.68, 102). Other self-report, behavioral or neuroimaging and electrophysiological measures may also provide similar benefit to determining the outcome of tDCS treatment as they can do for pharmacotherapy102. A key open question is whether some patients would benefit from tDCS over pharmacotherapy or vice versa.
Third, one reason for the complexity of tDCS findings is the potential for compensation by other networks. Many neural regions that are often targeted using tDCS, such as the prefrontal cortex, are argued to have flexible coding properties103, and therefore may have the capacity to adapt in the face of neural interventions104. One strategy to address this possibility is to simultaneously alter the function of two or more key nodes in a given network (e.g.67), reducing the likelihood for compensation of one node by a second or third. The simple empirical prediction is that modulating multiple nodes within a network would be more effective in modulating a given behavior than the modulation of a single node. Proximal measures of neurophysiology105 such as fMRI or MEG/EEG may have particular value in anticipating the potential for compensation by mapping out a network, as well as demonstrations of such compensation.
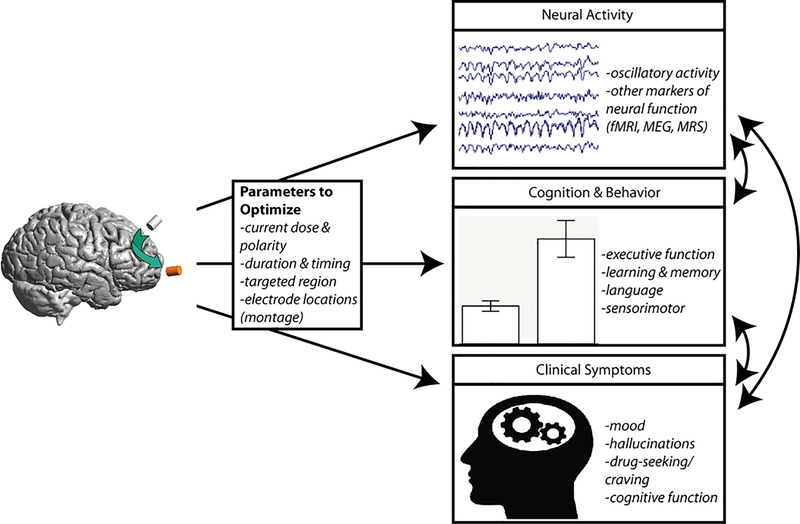
Fourth, in vivo basic science work to clarify neural mechanism and effective parameters will provide an essential role in uncovering the mechanism of tDCS. As describe above, such experiments have already provided key constraints over the potential neural mechanism of tDCS, ruling out several plausible accounts. There have been relatively few studies employing in vivo animal models, and such studies offer a unique potential to uncover core mechanistic relationships (Figure 1). For example, assumptions about the selective impact of tDCS on L5 pyramidal neurons106 could be tested rigorously.
Figure 1:
A schematic depicting the interactions between mechanistic, cognitive and clinical studies in tDCS research that can facilitate the development of mechanistically-informed interventions for psychiatric disorders.
Finally, a central challenge of tDCS research can be summarized in the following way: currently, in many areas of tDCS research, there are more parameter settings than there have been studies conducted. For example, the number of combinations of montages, current amplitude and duration, and timing of dose relative to behavioral or neural data collection, as well as other methodological factors (e.g. composition of the participant cohorts) will usually surpass the number of studies examining a particular phenomenon. A broadly analogous situation presents itself in neuroimaging research, where the number of potential, independent data analysis pipelines is extremely large107. Meta-analyses can provide some benefit in terms of defining experimental parameters that might lead to different experimental outcomes39. For meta-analyses to be maximally informative, however, there first must be a relatively large literature to meta-analyze. The heterogeneity of the emerging cognitive and clinical tDCS literatures has presented some challenges for recent meta-analytical efforts, requiring the combining of a limited amount of available data from studies with extremely different parameters (e.g. tDCS montages targeting different cortical regions). Computational models may play a crucial role in focusing future tDCS research by locating most informative parameters to resolve a particular question. As current flow modeling becomes more sophisticated, it might better incorporate neurophysiological constraints (e.g. from structural or resting state functional MRI or EEG), or constraints obtained from behavioral data (e.g. working memory capacity).
The goal of this critical review was to review the current state of tDCS research, both acknowledging areas of progress and identifying key open questions for future research to prioritize. tDCS has promise for modulating cognition and the symptoms of psychiatric disorders, but much remains to be understood about this method: how it works, and how it might be applied to study and/or enhance human cognition and functioning. Available data from studies in animals and humans suggests that the current strengths typically administered to humans modulate neural activity by way of changes in electrical fields and neural oscillations, rather than by eliciting neuronal spiking. Future research efforts to further elucidate the mechanism(s) of action can be expected to help shape experimental design and the kinds of hypotheses that it will be possible to test. Available data on the cognitive and clinical impact of tDCS suggests that while average effect sizes are currently moderate, it will become increasingly possible to fine-tune methodological factors and theoretical models to better target specific neural circuits and behavior. In summary, while there is much work still to be done, there is also an increasing amount of empirically-supported optimism that these future research directions will develop tDCS not only as a tool to examine the neural basis of behavior in translational research, but also as a neurobiologically-informed intervention to treat debilitating psychiatric disorders (Figure 1).
Acknowledgments
Funding: The present work was supported by NIMH grant 1R21MH108421-01A1 (to MLP and HWC)
Footnotes
Conflicts of interest: None of the authors declares any financial or other conflicts of interest that might have biased the work.
References
- 1.Yavari F, Jamil A, Mosayebi Samani M, Vidor LP, Nitsche MA. Basic and functional effects of transcranial Electrical Stimulation (tES)-An introduction. Neurosci Biobehav Rev 2018; 85: 81–92. [DOI] [PubMed] [Google Scholar]
- 2.Lefaucheur JP, Antal A, Ayache SS, Benninger DH, Brunelin J, Cogiamanian F et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin Neurophysiol 2017; 128(1): 56–92. [DOI] [PubMed] [Google Scholar]
- 3.Mutz J, Edgcumbe DR, Brunoni AR, Fu CHY. Efficacy and acceptability of non-invasive brain stimulation for the treatment of adult unipolar and bipolar depression: A systematic review and meta-analysis of randomised sham-controlled trials. Neurosci Biobehav Rev 2018; 92: 291–303. [DOI] [PubMed] [Google Scholar]
- 4.Moffa AH, Brunoni AR, Fregni F, Palm U, Padberg F, Blumberger DM et al. Safety and acceptability of transcranial direct current stimulation for the acute treatment of major depressive episodes: Analysis of individual patient data. J Affect Disord 2017; 221: 1–5. [DOI] [PubMed] [Google Scholar]
- 5.Aparicio LVM, Guarienti F, Razza LB, Carvalho AF, Fregni F, Brunoni AR. A Systematic Review on the Acceptability and Tolerability of Transcranial Direct Current Stimulation Treatment in Neuropsychiatry Trials. Brain Stimul 2016; 9(5): 671–681. [DOI] [PubMed] [Google Scholar]
- 6.Ferguson JM. SSRI Antidepressant Medications: Adverse Effects and Tolerability. Prim Care Companion J Clin Psychiatry 2001; 3(1): 22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunoni AR, Nitsche MA, Bolognini N, Bikson M, Wagner T, Merabet L et al. Clinical research with transcranial direct current stimulation (tDCS): challenges and future directions. Brain Stimul 2012; 5(3): 175–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esmaeilpour Z, Marangolo P, Hampstead BM, Bestmann S, Galletta E, Knotkova H et al. Incomplete evidence that increasing current intensity of tDCS boosts outcomes. Brain Stimul 2018; 11(2): 310–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Voroslakos M, Takeuchi Y, Brinyiczki K, Zombori T, Oliva A, Fernandez-Ruiz A et al. Direct effects of transcranial electric stimulation on brain circuits in rats and humans. Nat Commun 2018; 9(1): 483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang Y, Liu AA, Lafon B, Friedman D, Dayan M, Wang X et al. Measurements and models of electric fields in the in vivo human brain during transcranial electric stimulation. Elife 2017; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Opitz A, Falchier A, Yan CG, Yeagle EM, Linn GS, Megevand P et al. Spatiotemporal structure of intracranial electric fields induced by transcranial electric stimulation in humans and nonhuman primates. Sci Rep 2016; 6: 31236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jackson MP, Rahman A, Lafon B, Kronberg G, Ling D, Parra LC et al. Animal models of transcranial direct current stimulation: Methods and mechanisms. Clin Neurophysiol 2016; 127(11): 3425–3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reato D, Rahman A, Bikson M, Parra LC. Low-intensity electrical stimulation affects network dynamics by modulating population rate and spike timing. The Journal of neuroscience : the official journal of the Society for Neuroscience 2010; 30(45): 15067–15079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu A, Voroslakos M, Kronberg G, Henin S, Krause MR, Huang Y et al. Immediate neurophysiological effects of transcranial electrical stimulation. Nat Commun 2018; 9(1): 5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDonnell MD, Abbott D. What is stochastic resonance? Definitions, misconceptions, debates, and its relevance to biology. PLoS Comput Biol 2009; 5(5): e1000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kronberg G, Bridi M, Abel T, Bikson M, Parra LC. Direct Current Stimulation Modulates LTP and LTD: Activity Dependence and Dendritic Effects. Brain Stimul 2017; 10(1): 51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bikson M, Name A, Rahman A. Origins of specificity during tDCS: anatomical, activity-selective, and input-bias mechanisms. Front Hum Neurosci 2013; 7: 688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Onton J, Delorme A, Makeig S. Frontal midline EEG dynamics during working memory. Neuroimage 2005; 27(2): 341–356. [DOI] [PubMed] [Google Scholar]
- 19.Simonsmeier BA, Grabner RH, Hein J, Krenz U, Schneider M. Electrical brain stimulation (tES) improves learning more than performance: A meta-analysis. Neurosci Biobehav Rev 2018; 84: 171–181. [DOI] [PubMed] [Google Scholar]
- 20.Cohen MX. Where Does EEG Come From and What Does It Mean? Trends Neurosci 2017; 40(4): 208–218. [DOI] [PubMed] [Google Scholar]
- 21.Logothetis NK, Wandell BA. Interpreting the BOLD signal. Annu Rev Physiol 2004; 66: 735–769. [DOI] [PubMed] [Google Scholar]
- 22.Rawji V, Ciocca M, Zacharia A, Soares D, Truong D, Bikson M et al. tDCS changes in motor excitability are specific to orientation of current flow. Brain Stimul 2018; 11(2): 289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alekseichuk I, Diers K, Paulus W, Antal A. Transcranial electrical stimulation of the occipital cortex during visual perception modifies the magnitude of BOLD activity: A combined tES-fMRI approach. Neuroimage 2016; 140: 110–117. [DOI] [PubMed] [Google Scholar]
- 24.Fiori V, Kunz L, Kuhnke P, Marangolo P, Hartwigsen G. Transcranial direct current stimulation (tDCS) facilitates verb learning by altering effective connectivity in the healthy brain. Neuroimage 2018; 181: 550–559. [DOI] [PubMed] [Google Scholar]
- 25.Weber MJ, Messing SB, Rao H, Detre JA, Thompson-Schill SL. Prefrontal transcranial direct current stimulation alters activation and connectivity in cortical and subcortical reward systems: a tDCS-fMRI study. Hum Brain Mapp 2014; 35(8): 3673–3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boonstra TW, Nikolin S, Meisener AC, Martin DM, Loo CK. Change in Mean Frequency of Resting-State Electroencephalography after Transcranial Direct Current Stimulation. Front Hum Neurosci 2016; 10: 270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson TW, McDermott TJ, Mills MS, Coolidge NM, Heinrichs-Graham E. tDCS Modulates Visual Gamma Oscillations and Basal Alpha Activity in Occipital Cortices: Evidence from MEG. Cereb Cortex 2018; 28(5): 1597–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanley CJ, Singh KD, McGonigle DJ. Transcranial modulation of brain oscillatory responses: A concurrent tDCS-MEG investigation. Neuroimage 2016; 140: 20–32. [DOI] [PubMed] [Google Scholar]
- 29.Marshall TR, Esterer S, Herring JD, Bergmann TO, Jensen O. On the relationship between cortical excitability and visual oscillatory responses - A concurrent tDCS-MEG study. Neuroimage 2016; 140: 41–49. [DOI] [PubMed] [Google Scholar]
- 30.Reinhart RM, Zhu J, Park S, Woodman GF. Synchronizing theta oscillations with direct-current stimulation strengthens adaptive control in the human brain. Proc Natl Acad Sci U S A 2015; 112(30): 9448–9453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDermott TJ, Wiesman AI, Mills MS, Spooner RK, Coolidge NM, Proskovec AL et al. tDCS modulates behavioral performance and the neural oscillatory dynamics serving visual selective attention. Hum Brain Mapp 2019; 40(3): 729–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choe J, Coffman BA, Bergstedt DT, Ziegler MD, Phillips ME. Transcranial Direct Current Stimulation Modulates Neuronal Activity and Learning in Pilot Training. Front Hum Neurosci 2016; 10: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boudewyn M, Roberts BM, Mizrak E, Ranganath C, Carter CS. Prefrontal transcranial direct current stimulation (tDCS) enhances behavioral and EEG markers of proactive control. Cogn Neurosci. 2019. Jan-Apr;10(2):57–65. doi: 10.1080/17588928.2018.1551869. Epub 2018 Dec 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fries P A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn Sci 2005; 9(10): 474–480. [DOI] [PubMed] [Google Scholar]
- 35.Siegel M, Donner TH, Engel AK. Spectral fingerprints of large-scale neuronal interactions. Nat Rev Neurosci 2012; 13(2): 121–134. [DOI] [PubMed] [Google Scholar]
- 36.Hyafil A, Giraud AL, Fontolan L, Gutkin B. Neural Cross-Frequency Coupling: Connecting Architectures, Mechanisms, and Functions. Trends Neurosci 2015; 38(11): 725–740. [DOI] [PubMed] [Google Scholar]
- 37.Buzsaki G, Schomburg EW. What does gamma coherence tell us about inter-regional neural communication? Nat Neurosci 2015; 18(4): 484–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fischer DB, Fried PJ, Ruffini G, Ripolles O, Salvador R, Banus J et al. Multifocal tDCS targeting the resting state motor network increases cortical excitability beyond traditional tDCS targeting unilateral motor cortex. Neuroimage 2017; 157: 34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Imburgio MJ, Orr JM. Effects of prefrontal tDCS on executive function: Methodological considerations revealed by meta-analysis. Neuropsychologia 2018; 117: 156–166. [DOI] [PubMed] [Google Scholar]
- 40.Reinhart RM, Cosman JD, Fukuda K, Woodman GF. Using transcranial direct-current stimulation (tDCS) to understand cognitive processing. Atten Percept Psychophys 2017; 79(1): 3–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dmochowski JP, Datta A, Bikson M, Su Y, Parra LC. Optimized multi-electrode stimulation increases focality and intensity at target. J Neural Eng 2011; 8(4): 046011. [DOI] [PubMed] [Google Scholar]
- 42.Battaglini L, Noventa S, Casco C. Anodal and cathodal electrical stimulation over V5 improves motion perception by signal enhancement and noise reduction. Brain Stimul 2017; 10(4): 773–779. [DOI] [PubMed] [Google Scholar]
- 43.Zito GA, Senti T, Cazzoli D, Muri RM, Mosimann UP, Nyffeler T et al. Cathodal HD-tDCS on the right V5 improves motion perception in humans. Front Behav Neurosci 2015; 9: 257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Szucs D, Ioannidis JP. Empirical assessment of published effect sizes and power in the recent cognitive neuroscience and psychology literature. PLoS Biol 2017; 15(3): e2000797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Colling LJ, Szucs D. Statisical Inference and the Repliation Crisis. Review of Philosophy and Psychology 2018. [Google Scholar]
- 46.Medina J, Cason S. No evidential value in samples of transcranial direct current stimulation (tDCS) studies of cognition and working memory in healthy populations. Cortex 2017; 94: 131–141. [DOI] [PubMed] [Google Scholar]
- 47.Horvath JC, Forte JD, Carter O. Quantitative Review Finds No Evidence of Cognitive Effects in Healthy Populations From Single-session Transcranial Direct Current Stimulation (tDCS). Brain Stimul 2015; 8(3): 535–550. [DOI] [PubMed] [Google Scholar]
- 48.Meron D, Hedger N, Garner M, Baldwin DS. Transcranial direct current stimulation (tDCS) in the treatment of depression: Systematic review and meta-analysis of efficacy and tolerability. Neurosci Biobehav Rev 2015; 57: 46–62. [DOI] [PubMed] [Google Scholar]
- 49.Nitsche MA, Bikson M, Bestmann S. On the Use of Meta-analysis in Neuromodulatory Non-invasive Brain Stimulation. Brain Stimul 2015; 8(3): 666–667. [DOI] [PubMed] [Google Scholar]
- 50.Santarnecchi E, Brem AK, Levenbaum E, Thompson T, Kadosh RC, Pascual-Leone A. Enhancing cognition using transcranial electrical stimulation. Current Opinion in Behavioral Sciences 2015; 4: 171–178. [Google Scholar]
- 51.Filmer HL, Dux PE, Mattingley JB. Applications of transcranial direct current stimulation for understanding brain function. Trends Neurosci 2014; 37(12): 742–753. [DOI] [PubMed] [Google Scholar]
- 52.Filmer HL, Varghese E, Hawkins GE, Mattingley JB, Dux PE. Improvements in Attention and Decision-Making Following Combined Behavioral Training and Brain Stimulation. Cereb Cortex 2017; 27(7): 3675–3682. [DOI] [PubMed] [Google Scholar]
- 53.Smith RC, Boules S, Mattiuz S, Youssef M, Tobe RH, Sershen H et al. Effects of transcranial direct current stimulation (tDCS) on cognition, symptoms, and smoking in schizophrenia: A randomized controlled study. Schizophr Res 2015; 168(1–2): 260–266. [DOI] [PubMed] [Google Scholar]
- 54.Hoy KE, Bailey NW, Arnold SL, Fitzgerald PB. The effect of transcranial Direct Current Stimulation on gamma activity and working memory in schizophrenia. Psychiatry Res 2015; 228(2): 191–196. [DOI] [PubMed] [Google Scholar]
- 55.Vercammen A, Rushby JA, Loo C, Short B, Weickert CS, Weickert TW. Transcranial direct current stimulation influences probabilistic association learning in schizophrenia. Schizophr Res 2011; 131(1–3): 198–205. [DOI] [PubMed] [Google Scholar]
- 56.Friedman NP, Miyake A, Corley RP, Young SE, Defries JC, Hewitt JK. Not all executive functions are related to intelligence. Psychol Sci 2006; 17(2): 172–179. [DOI] [PubMed] [Google Scholar]
- 57.Barch DM, Moore H, Nee DE, Manoach DS, Luck SJ. CNTRICS imaging biomarkers selection: Working memory. Schizophr Bull 2012; 38(1): 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mancuso LE, Ilieva IP, Hamilton RH, Farah MJ. Does Transcranial Direct Current Stimulation Improve Healthy Working Memory?: A Meta-analytic Review. J Cogn Neurosci 2016; 28(8): 1063–1089. [DOI] [PubMed] [Google Scholar]
- 59.Reinhart RMG. Disruption and rescue of interareal theta phase coupling and adaptive behavior. Proc Natl Acad Sci U S A 2017; 114(43): 11542–11547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jacobson L, Javitt DC, Lavidor M. Activation of inhibition: diminishing impulsive behavior by direct current stimulation over the inferior frontal gyrus. J Cogn Neurosci 2011; 23(11): 3380–3387. [DOI] [PubMed] [Google Scholar]
- 61.Li LM, Violante IR, Leech R, Hampshire A, Opitz A, McArthur D et al. Cognitive enhancement with Salience Network electrical stimulation is influenced by network structural connectivity. Neuroimage 2019; 185: 425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vergallito A, Riva P, Pisoni A, Romero Lauro LJ. Modulation of negative emotions through anodal tDCS over the right ventrolateral prefrontal cortex. Neuropsychologia 2018; 119: 128–135. [DOI] [PubMed] [Google Scholar]
- 63.Ironside M, Browning M, Ansari TL, Harvey CJ, Sekyi-Djan MN, Bishop SJ et al. Effect of Prefrontal Cortex Stimulation on Regulation of Amygdala Response to Threat in Individuals With Trait Anxiety: A Randomized Clinical Trial. JAMA Psychiatry 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brevet-Aeby C, Brunelin J, Iceta S, Padovan C, Poulet E. Prefrontal cortex and impulsivity: Interest of noninvasive brain stimulation. Neurosci Biobehav Rev 2016; 71: 112–134. [DOI] [PubMed] [Google Scholar]
- 65.Lundqvist M, Herman P, Lansner A. Theta and gamma power increases and alpha/beta power decreases with memory load in an attractor network model. J Cogn Neurosci 2011; 23(10): 3008–3020. [DOI] [PubMed] [Google Scholar]
- 66.Compte A, Brunel N, Goldman-Rakic PS, Wang XJ. Synaptic mechanisms and network dynamics underlying spatial working memory in a cortical network model. Cereb Cortex 2000; 10(9): 910–923. [DOI] [PubMed] [Google Scholar]
- 67.Hartwigsen G Flexible Redistribution in Cognitive Networks. Trends Cogn Sci 2018; 22(8): 687–698. [DOI] [PubMed] [Google Scholar]
- 68.Brunoni AR, Moffa AH, Fregni F, Palm U, Padberg F, Blumberger DM et al. Transcranial direct current stimulation for acute major depressive episodes: meta-analysis of individual patient data. Br J Psychiatry 2016; 208(6): 522–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shiozawa P, Fregni F, Bensenor IM, Lotufo PA, Berlim MT, Daskalakis JZ et al. Transcranial direct current stimulation for major depression: an updated systematic review and meta-analysis. Int J Neuropsychopharmacol 2014; 17(9): 1443–1452. [DOI] [PubMed] [Google Scholar]
- 70.Brunoni AR, Moffa AH, Sampaio-Junior B, Borrione L, Moreno ML, Fernandes RA et al. Trial of Electrical Direct-Current Therapy versus Escitalopram for Depression. N Engl J Med 2017; 376(26): 2523–2533. [DOI] [PubMed] [Google Scholar]
- 71.Brunoni AR, Valiengo L, Baccaro A, Zanao TA, de Oliveira JF, Goulart A et al. The sertraline vs. electrical current therapy for treating depression clinical study: results from a factorial, randomized, controlled trial. JAMA Psychiatry 2013; 70(4): 383–391. [DOI] [PubMed] [Google Scholar]
- 72.Loo CK, Husain MM, McDonald WM, Aaronson S, O’Reardon JP, Alonzo A et al. International randomized-controlled trial of transcranial Direct Current Stimulation in depression. Brain Stimul 2018; 11(1): 125–133. [DOI] [PubMed] [Google Scholar]
- 73.Rock PL, Roiser JP, Riedel WJ, Blackwell AD. Cognitive impairment in depression: a systematic review and meta-analysis. Psychol Med 2014; 44(10): 2029–2040. [DOI] [PubMed] [Google Scholar]
- 74.Martin DM, Teng JZ, Lo TY, Alonzo A, Goh T, Iacoviello BM et al. Clinical pilot study of transcranial direct current stimulation combined with Cognitive Emotional Training for medication resistant depression. J Affect Disord 2018; 232: 89–95. [DOI] [PubMed] [Google Scholar]
- 75.Wolkenstein L, Plewnia C. Amelioration of cognitive control in depression by transcranial direct current stimulation. Biol Psychiatry 2013; 73(7): 646–651. [DOI] [PubMed] [Google Scholar]
- 76.Brunoni AR, Boggio PS, De Raedt R, Bensenor IM, Lotufo PA, Namur V et al. Cognitive control therapy and transcranial direct current stimulation for depression: a randomized, double-blinded, controlled trial. J Affect Disord 2014; 162: 43–49. [DOI] [PubMed] [Google Scholar]
- 77.Segrave RA, Arnold S, Hoy K, Fitzgerald PB. Concurrent cognitive control training augments the antidepressant efficacy of tDCS: a pilot study. Brain Stimul 2014; 7(2): 325–331. [DOI] [PubMed] [Google Scholar]
- 78.Oliveira JF, Zanao TA, Valiengo L, Lotufo PA, Bensenor IM, Fregni F et al. Acute working memory improvement after tDCS in antidepressant-free patients with major depressive disorder. Neurosci Lett 2013; 537: 60–64. [DOI] [PubMed] [Google Scholar]
- 79.Powell TY, Boonstra TW, Martin DM, Loo CK, Breakspear M. Modulation of cortical activity by transcranial direct current stimulation in patients with affective disorder. PLoS One 2014; 9(6): e98503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Martin DM, Moffa A, Nikolin S, Bennabi D, Brunoni AR, Flannery W et al. Cognitive effects of transcranial direct current stimulation treatment in patients with major depressive disorder: An individual patient data meta-analysis of randomised, sham-controlled trials. Neurosci Biobehav Rev 2018; 90: 137–145. [DOI] [PubMed] [Google Scholar]
- 81.Vanderhasselt MA, Brunoni AR, Loeys T, Boggio PS, De Raedt R. Nosce te ipsum--Socrates revisited? Controlling momentary ruminative self-referent thoughts by neuromodulation of emotional working memory. Neuropsychologia 2013; 51(13): 2581–2589. [DOI] [PubMed] [Google Scholar]
- 82.Lesh TA, Niendam TA, Minzenberg MJ, Carter CS. Cognitive control deficits in schizophrenia: mechanisms and meaning. Neuropsychopharmacology 2011; 36(1): 316–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mondino M, Jardri R, Suaud-Chagny MF, Saoud M, Poulet E, Brunelin J. Effects of Fronto-Temporal Transcranial Direct Current Stimulation on Auditory Verbal Hallucinations and Resting-State Functional Connectivity of the Left Temporo-Parietal Junction in Patients With Schizophrenia. Schizophr Bull 2016; 42(2): 318–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Brunelin J, Mondino M, Gassab L, Haesebaert F, Gaha L, Suaud-Chagny MF et al. Examining transcranial direct-current stimulation (tDCS) as a treatment for hallucinations in schizophrenia. Am J Psychiatry 2012; 169(7): 719–724. [DOI] [PubMed] [Google Scholar]
- 85.Fitzgerald PB, McQueen S, Daskalakis ZJ, Hoy KE. A negative pilot study of daily bimodal transcranial direct current stimulation in schizophrenia. Brain Stimul 2014; 7(6): 813–816. [DOI] [PubMed] [Google Scholar]
- 86.Kekic M, Boysen E, Campbell IC, Schmidt U. A systematic review of the clinical efficacy of transcranial direct current stimulation (tDCS) in psychiatric disorders. J Psychiatr Res 2016; 74: 70–86. [DOI] [PubMed] [Google Scholar]
- 87.Moffa AH, Brunoni AR, Nikolin S, Loo CK. Transcranial Direct Current Stimulation in Psychiatric Disorders: A Comprehensive Review. Psychiatr Clin North Am 2018; 41(3): 447–463. [DOI] [PubMed] [Google Scholar]
- 88.Fregni F, Liguori P, Fecteau S, Nitsche MA, Pascual-Leone A, Boggio PS. Cortical stimulation of the prefrontal cortex with transcranial direct current stimulation reduces cue-provoked smoking craving: a randomized, sham-controlled study. J Clin Psychiatry 2008; 69(1): 32–40. [DOI] [PubMed] [Google Scholar]
- 89.Boggio PS, Sultani N, Fecteau S, Merabet L, Mecca T, Pascual-Leone A et al. Prefrontal cortex modulation using transcranial DC stimulation reduces alcohol craving: a double-blind, sham-controlled study. Drug Alcohol Depend 2008; 92(1–3): 55–60. [DOI] [PubMed] [Google Scholar]
- 90.Batista EK, Klauss J, Fregni F, Nitsche MA, Nakamura-Palacios EM. A Randomized Placebo-Controlled Trial of Targeted Prefrontal Cortex Modulation with Bilateral tDCS in Patients with Crack-Cocaine Dependence. Int J Neuropsychopharmacol 2015; 18(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Boggio PS, Zaghi S, Villani AB, Fecteau S, Pascual-Leone A, Fregni F. Modulation of risk-taking in marijuana users by transcranial direct current stimulation (tDCS) of the dorsolateral prefrontal cortex (DLPFC). Drug Alcohol Depend 2010; 112(3): 220–225. [DOI] [PubMed] [Google Scholar]
- 92.Shahbabaie A, Golesorkhi M, Zamanian B, Ebrahimpoor M, Keshvari F, Nejati V et al. State dependent effect of transcranial direct current stimulation (tDCS) on methamphetamine craving. Int J Neuropsychopharmacol 2014; 17(10): 1591–1598. [DOI] [PubMed] [Google Scholar]
- 93.da Silva MC, Conti CL, Klauss J, Alves LG, do Nascimento Cavalcante HM, Fregni F et al. Behavioral effects of transcranial direct current stimulation (tDCS) induced dorsolateral prefrontal cortex plasticity in alcohol dependence. J Physiol Paris 2013; 107(6): 493–502. [DOI] [PubMed] [Google Scholar]
- 94.Berlow YA, Zandvakili A, Carpenter LL, Philip NS. Transcranial direct current stimulation for unipolar depression and risk of treatment emergent mania: An updated meta-analysis. Brain Stimul 2019; 12(4): 1066–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Horvath JC, Carter O, Forte JD. Transcranial direct current stimulation: five important issues we aren’t discussing (but probably should be). Front Syst Neurosci 2014; 8: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McIntosh RD. Exploratory reports: A new article type for Cortex. Cortex 2017; 96: A1–A4. [DOI] [PubMed] [Google Scholar]
- 97.Civile C, McLaren R, McLaren IPL. How we can change your mind: Anodal tDCS to Fp3 alters human stimulus representation and learning. Neuropsychologia 2018; 119: 241–246. [DOI] [PubMed] [Google Scholar]
- 98.Minarik T, Berger B, Althaus L, Bader V, Biebl B, Brotzeller F et al. The Importance of Sample Size for Reproducibility of tDCS Effects. Front Hum Neurosci 2016; 10: 453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jalali R, Miall RC, Galea JM. No consistent effect of cerebellar transcranial direct current stimulation on visuomotor adaptation. J Neurophysiol 2017; 118(2): 655–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Woods AJ, Antal A, Bikson M, Boggio PS, Brunoni AR, Celnik P et al. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin Neurophysiol 2016; 127(2): 1031–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fried EI. The 52 symptoms of major depression: Lack of content overlap among seven common depression scales. J Affect Disord 2017; 208: 191–197. [DOI] [PubMed] [Google Scholar]
- 102.Phillips ML, Chase HW, Sheline YI, Etkin A, Almeida JR, Deckersbach T et al. Identifying predictors, moderators, and mediators of antidepressant response in major depressive disorder: neuroimaging approaches. Am J Psychiatry 2015; 172(2): 124–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Duncan J An adaptive coding model of neural function in prefrontal cortex. Nat Rev Neurosci 2001; 2(11): 820–829. [DOI] [PubMed] [Google Scholar]
- 104.Tononi G, Sporns O, Edelman GM. Measures of degeneracy and redundancy in biological networks. Proc Natl Acad Sci U S A 1999; 96(6): 3257–3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Harty S, Sella F, Cohen Kadosh R. Mind the Brain: The Mediating and Moderating Role of Neurophysiology. Trends Cogn Sci 2017; 21(1): 2–5. [DOI] [PubMed] [Google Scholar]
- 106.Radman T, Ramos RL, Brumberg JC, Bikson M. Role of cortical cell type and morphology in subthreshold and suprathreshold uniform electric field stimulation in vitro. Brain Stimul 2009; 2(4): 215–228, 228 e211–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Carp J On the plurality of (methodological) worlds: estimating the analytic flexibility of FMRI experiments. Front Neurosci 2012; 6: 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Al-Kaysi AM, Al-Ani A, Loo CK, Powell TY, Martin DM, Breakspear M et al. Predicting tDCS treatment outcomes of patients with major depressive disorder using automated EEG classification. J Affect Disord 2017; 208: 597–603. [DOI] [PubMed] [Google Scholar]
- 109.D’Urso G, Dell’Osso B, Rossi R, Brunoni AR, Bortolomasi M, Ferrucci R et al. Clinical predictors of acute response to transcranial direct current stimulation (tDCS) in major depression. J Affect Disord 2017; 219: 25–30. [DOI] [PubMed] [Google Scholar]