Abstract
The scientific literature on links among alcohol use, total energy intake, cardiometabolic disease, and obesity is conflicting. To clarify the link between alcohol use and cardiometabolic health, this systematic review (PROSPERO CRD42016039308A) uses PRISMA guidelines to synthesize how alcohol use affects dietary intake (carbohydrate, fat, and protein intake) in humans. A search of Google Scholar, PsycINFO, and PubMed from June 2016-March 2019 yielded 30 qualified studies. Experimental and observational studies allowed for inferences about effects of a single drinking occasion and of frequent drinking, respectively. Alcohol quantities were standardized according to the 2015–2020 Dietary Guidelines for Americans. On average, methodological quality of the studies was medium strength. Results indicated that a single occasion of light and moderate drinking as well as frequent light and moderate drinking were linked to greater fat and protein intake, albeit the majority of studies did not detect differences in dietary intake due to these drinking behaviors. Frequent heavy drinking, on the other hand, was linked to less carbohydrate intake in the majority of studies. Overall, alcohol use does not appear to uniformly affect diet but instead appears to affect intake of specific macronutrients in a dose-dependent manner, most consistently decreasing carbohydrate intake with heavier use.
Keywords: alcohol, dietary intake, systematic review, PROSPERO CRD42016039308
Introduction
The scientific literature on alcohol use and cardiometabolic health is conflicting. On one hand, any alcohol use causes greater total energy intake, which may harm cardiometabolic health (1, 2). On the other hand, frequent moderate drinking—about 1–2 U.S. standard drinks/day for women and about 2–3 U.S. standard drinks/day for men—is associated with reduced risk of clinical events including atherosclerosis, myocardial infarction, ischemic stroke, and type II diabetes (3). Moreover, the association between alcohol use and obesity [Body Mass Index (BMI) ≥ 30] is complex. Greater alcohol use is associated with higher BMI in some studies but lower BMI in other studies (4). Higher versus lower BMI is generally associated with poorer cardiometabolic health (5), although BMI categories can misclassify cardiometabolic health status (6). In sum, the scientific literature points to potential cardiometabolic health benefits and negative consequences of alcohol use, leaving clinicians and the public unclear as to how this behavior fits into the prevention and treatment of cardiometabolic diseases and obesity.
One novel approach to clarifying the link between alcohol use and cardiometabolic health is to evaluate how alcohol use changes diet, or the foods someone eats. Indeed, a growing movement in cardiometabolic disease and obesity research emphasizes the importance of investigating the amount of different macronutrients (carbohydrates, fat, and protein) in an individual’s diet, not just total energy intake, to understand disease risk (7). Carefully controlled experimental studies show that diets with different macronutrient compositions differentially affect energy expenditure even when calorically equivalent (8, 9). Moreover, experimental and observational research indicates that diets consisting of greater intake of refined carbohydrates (e.g., breads, pastas) and fat heighten risk for cardiometabolic disease and obesity irrespective of total energy intake (7, 10). Experimental research also indicates that greater intake of protein facilitates weight loss (10), and weight loss can predict reduced risk for cardiometabolic disease (11; c.f. 12). Systematically evaluating the current evidence on whether alcohol use shifts dietary intake will therefore be clinically informative. For instance, does alcohol use confer cardiometabolic risk through increasing intake of refined carbohydrates and fat and decreasing protein intake? Or might the cardiometabolic consequences of alcohol use be attenuated through increased protein intake and decreased intake of refined carbohydrates and fat? Additionally, this evaluation will improve upon existing clinical and public guidelines on alcohol use and dietary intake (13), which is important because a high prevalence of alcohol use exists globally (14).
No prior paper has synthesized and critiqued the scientific literature on whether alcohol use shifts dietary intake. The current paper therefore fills this gap by including a systematic review of the empirical literature investigating the effect of alcohol use on dietary intake in humans. Systematic reviews have strength because they use explicit methods to identify, select, and appraise studies unlike narrative reviews, which can be affected by bias (15). Additionally, a systematic review can include studies that utilize different designs (15). This is particularly important for investigating how alcohol use affects dietary intake because, although experimental studies with alcohol administration allow for causal conclusions, these studies are practically constrained in multiple ways: to lighter alcohol quantities, to one drinking and eating occasion, and to a laboratory setting (2). Observational studies, on the other hand, are well suited to capture naturalistic patterns of drinking including frequent intake of heavier alcohol quantities, and to test how these drinking behaviors are related to patterns of dietary intake. Given that cardiometabolic diseases and obesity develop after long-term shifts in dietary intake (16), evaluating associations between frequent drinking and dietary intake patterns has especially strong clinical implication. Moreover, evaluating the specific alcohol quantities and frequencies linked with changes in dietary intake will improve precision in clinical and public discourse on the topic.
In order for associations between alcohol use and dietary intake to be interpreted in the context of specific alcohol quantities and frequencies, alcohol use was standardized across studies into light drinking, moderate drinking, and heavy drinking based on the 2015–2020 Dietary Guidelines for Americans (13). This standardization accounts for sex differences in alcohol pharmacokinetics. Also, the drinking behaviors tested in experimental studies were subsequently referenced to as single drinking occasions, given that experimental studies administered an alcoholic beverage at one drinking occasion in a short time period. In contrast, the drinking behaviors evaluated in observational studies were subsequently referenced to as frequent drinking behaviors, given that observational studies measured typical patterns of drinking engaged in a longer time period (e.g., past 12 months). Changes in dietary intake were assessed by parsing out the influence of alcohol use on intake of carbohydrates versus fat versus protein. Given that greater proportional intake of refined carbohydrates not unrefined carbohydrates (e.g., fruits, vegetables) heightens risk for cardiometabolic diseases and obesity (10), the influence of alcohol use on intake of refined carbohydrates versus unrefined carbohydrates was further delineated. Finally, where possible, results were compared across male and female participants to identify potential moderation by biological sex.
Method
Search Strategy
The systematic review was pre-registered at PROSPERO (CRD42016039308A) and conducted in accordance to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (15). The search for eligible studies occurred from June 1st, 2016 through February 28th, 2018, and was updated in March 2019. Databases included: (a) Google Scholar, PsycINFO, and PubMed, (b) reference sections of relevant articles, and (c) tables of contents of Appetite, Eating Behaviors, Health Psychology, Journal of Health Psychology, and Journal of Studies on Alcohol and Drugs. Search strings included “alcohol” AND “eating”; “alcohol” AND “food” AND “intake”; “alcohol” AND “nutrition”; and “alcohol” AND “diet”.
Inclusion Criteria
Inclusionary study characteristics were: (a) included at least one measure of alcohol use (e.g., alcohol preload, 24-hour dietary recall), (b) included at least one measure of dietary intake (e.g., ad libitum food intake, 24-hour dietary recall), (c) included an explicit a priori investigation of how alcohol use impacts dietary intake, and (d) conducted in humans. Inclusionary report characteristics were: (a) reported in English. Studies were not excluded based on publication year (publication years of included studies ranged from 1985–2017).
Study Selection
Five trained research assistants conducted blind literature reviews. They examined titles and abstracts from the search, recorded citations, checked for duplicate inclusion, obtained relevant articles in full, reviewed the content of the full article, and labeled each citation as eligible or ineligible. One lead research assistant verified eligibility. In the case of discrepancies, the lead research assistant and the first author adjudicated the disagreement.
Data Extraction
The lead research assistant extracted data on: (a) sample characteristics including information on sample size, biological sex, age, and BMI, (b) study methods including study design and the alcohol use and dietary intake measures, and (c) study results. The first author reviewed these data and fully read each eligible study in alphabetic order by citation.
Alcohol use was standardized as light, moderate, or heavy drinking based on the 2015–2020 Dietary Guidelines for Americans (13). For female participants, light drinking was defined as <1 standard drink/day (operationally 0–0.49 standard drinks/day), moderate drinking was defined as 1 standard drink/day (operationally 0.5–1.49 standard drinks/day), and heavy drinking was defined as ≥2 standard drinks/day (operationally ≥1.5 standard drinks/day) (17). For male participants, light drinking was defined as <1 standard drink/day (operationally 0–0.49 standard drinks/day), moderate drinking was defined as 1–2 standard drinks/day (operationally 0.5–2.49 standard drinks/day), and heavy drinking was defined as ≥3 standard drinks/day (operationally ≥2.5 standard drinks/day) (17). In studies wherein female and male participants were not separated for analysis, alcohol use was standardized based on the guidelines for female participants to provide the most conservative estimation. Alcohol use was also referenced to as a single drinking occasion or frequent drinking based on study design (experimental = a single drinking occasion, observational = frequent drinking).
A U.S. standard drink contains roughly 14 g of alcohol, which is found in about 12 oz. of regular beer, 5 oz. of wine, and 1.5 oz. of distilled spirits. However, internationally there is variation in the alcohol grams that determine a “standard” drink. For example, most European and non-U.S., English-speaking nations identify standard drinks to contain roughly 10 g of alcohol. Therefore, when generalizing and applying these results in non-U.S. countries, this difference should be acknowledged.
Assessment of Methodological Quality
The first author assessed risk of bias in individual studies according to the Downs and Black Quality Index scoring system (18) with external review from the lead research assistant. In this system, authors use a validated checklist to assess the quality of randomized and nonrandomized studies. The system consists of five subscales that address bias from reporting, external validity, internal validity, confounding, or power. For the current review, the power subscale was scored dichotomously as “0” for no report of a power analysis or “1” for a reported power analysis. Thus, total scores could range from 0–27. In the case of discrepancies, the first author and lead research assistant adjudicated the disagreement. One study (19) involved a subset of the authors of the current paper, thus an impartial Ph.D.-level individual with no conflict of interest assessed the risk of bias for that study.
Results
Study Characteristics
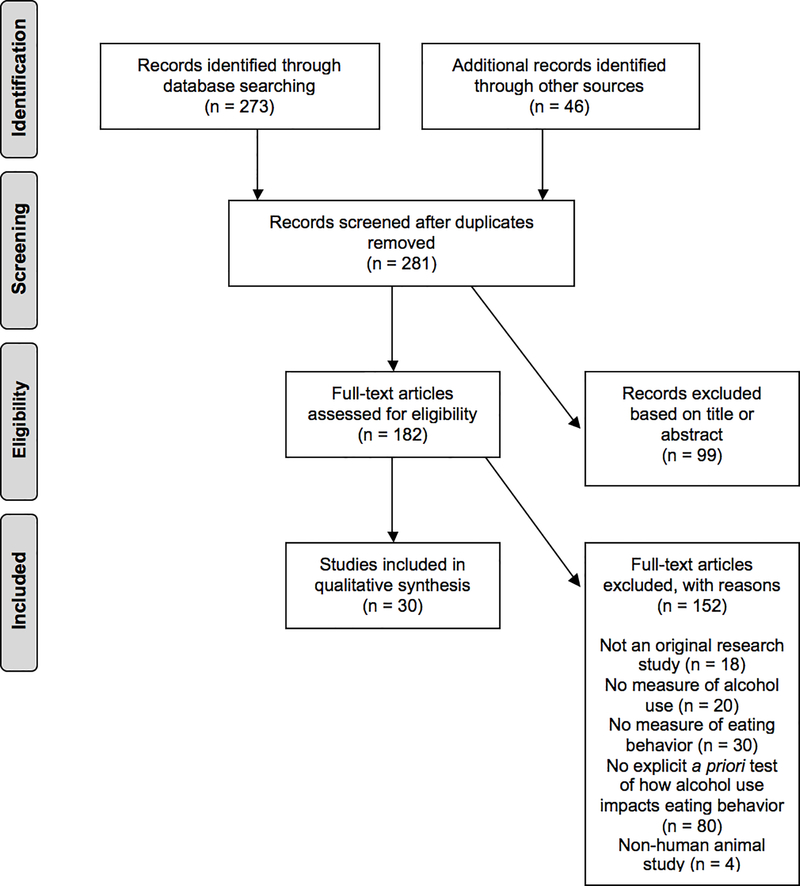
Numbers of studies screened, assessed for eligibility, and included in the review (with reasons for exclusions) are provided in Figure 1. The search yielded a final sample of 30 studies (17, 19–48). Of these, 18 (60%) were experimental and 12 (40%) were observational studies. A total of 273,751 participants were included across studies, of which 195,389 (71%) were women. Twelve (40%) study samples were drawn from the U.S., 9 (30%) from England, 2 (7%) from France, 2 (7%) from Germany, 2 (7%) from The Netherlands, 1 (3%) from Scotland, 1 (3%) from Denmark, and 1 (3%) from Japan. Average unweighted age across samples was 24.93 (SD = 5.79) ranging from 15 to 85+. Average unweighted BMI was 22.37 (SD = 0.72) ranging from 16.20 to 29.90.
Figure 1:
PRISMA Flow Chart for Qualified Studies
Experimental studies administered alcohol at one drinking occasion, allowing participants to drink for 5–30 minutes. Then, to assess alcohol-induced changes in dietary intake, researchers observed ad libitum food intake 10 minutes to 5 hours later. One experiment differed by using 24 hour dietary recall to capture dietary intake in the 24 hours after alcohol administration (35). Observational studies investigated cross-sectional associations between frequent drinking behaviors and dietary intake in large, nationally representative datasets. However, one study instead assessed longitudinal associations between frequent drinking behaviors and dietary intake (19), and another aggregated daily diary data (24). Measures of alcohol use and dietary intake in observational studies generally included 24-hour dietary recall (49) and food frequency questionnaires (including questions about alcohol) (50).
Methodological Quality
Table 1 presents Downs and Black Quality Index scores for each study. On average, the methodological quality of included studies was medium strength (M = 18.10, SD = 2.18, Range = 13–22). Bias from reporting was generally low but several studies did not report one or a few of the following: testing for covariates, estimates of random variability in outcomes, characteristics of participants lost to follow-up, and/or actual probability values (19–21, 24, 27, 29, 31, 35–43, 45, 46, 51, 52). Bias from lack of internal validity was also generally low but the majority of experiments did not report an attempt to blind the participants and/or the experimenters measuring outcomes (20–22, 25, 28, 30, 35–41, 44, 46–48). Moreover, bias from confounding was low and only evident for observational studies because of no randomization. In contrast, bias from lack of external validity was generally high because the experimental study samples were not from representative sources and the representativeness of the study samples in a few observational studies was unable to be determined (19, 24). Bias from lack of power was also generally high because only one study in the review reported a power analysis to determine sample size (35).
Table 1:
Summary of Results for Included Studies
| Study | Quality Index Score | Sample Size | BMI M (SD) | Sex | Alcohol Use | Refined Carbohydrates Intake | Fat Intake | Protein Intake | Unrefined Carbohydrates Intake |
|---|---|---|---|---|---|---|---|---|---|
| Experimental Studies (Single Drinking Occasion) | |||||||||
| Caton et al. (2004) | 17 | 12 | Not reported; Inclusion criterion < 26 | 100% men | None | Referent | Referent | Referent | Referent |
| Light | ns | ns | ns | ns | |||||
| Moderate | ns | ns | ns | ns | |||||
| Caton et al. (2005) | 19 | 12 | Not reported; Inclusion criterion < 27 | 100% men | None | Referent | Referent | Referent | |
| Moderate | + | + | |||||||
| Christiansen et al. (2016) | 21 | 60 | 20.82 (2.82) | 100% women | None | Referent | Referent | ||
| Heavy | + | + | |||||||
| Eiler et al. (2015) | 19 | 35 | 21.70 (1.80) | 100% women | None | Referent | Referent | Referent | |
| Heavy | + | + | + | ||||||
| Hetherington et al. (2001) | 18 | 26 | Not reported; Inclusion criterion 20–27 | 100% men | None | Referent | Referent | Referent | Referent |
| Moderate | + | + | + | + | |||||
| Hofmann et al. (2008) | 18 | 63 | 21.80 (2.18) | 100% women | None | Referent | Referent | ||
| Light | + | + | |||||||
| Hollister (1971) | 13 | 24 | Not reported | 95.83% men; 4.17% women |
None | Referent | Referent | Referent | |
| Heavy | ns | ns | ns | ||||||
| Mattes (1996) | 18 | 16 | Not reported | 50% men; 50% women |
None | Referent | Referent | Referent | Referent |
| Light | ns | + | + | ns | |||||
| Moderate | ns | + | + | ns | |||||
| Ouwens et al. (2003) | 16 | 116 | 23.10 (2.90) | 100% women | None | Referent | Referent | ||
| Heavy | ns | ns | |||||||
| Polivy et al. (1976a) | 15 | 40 | Not reported | 100% women | None | Referent | Referent | ||
| Heavy | ns | ns | |||||||
| Polivy et al. (1976b) | 15 | 55 | Not reported | 100% women | None | Referent | Referent | ||
| Heavy | ns | ns | |||||||
| Poppit et al. (1996) | 18 | 20 | 23.00 (2.80) | 100% women | None | Referent | Referent | Referent | |
| Heavy | ns | ns | ns | ||||||
| Raben et al. (2003) | 18 | 19 | 22.10 (0.40) | 52.63% men; 47.37% women |
None | Referent | Referent | Referent | Referent |
| Moderate | ns | ns | ns | ns | |||||
| Rose et al. (2015) | 17 | 114 | Not reported | 57.89% women; 42.11% men |
None | Referent | Referent | Referent | |
| Heavy | ns | ns | ns | ||||||
| Schrieks et al. (2015) | 18 | 23 | 23.00 (0.10) | 100% men | None | Referent | Referent | Referent | Referent |
| Moderate | ns | + | ns | ||||||
| Yeomans et al. (1999) | 15 | 22 | 23.20 (0.80) | 100% men | None | Referent | Referent | Referent | |
| Moderate | ns | ns | ns | ||||||
| Yeomans et al. (2002) | 18 | 18 | 22.64 (SD not reported) | 100% men | None | Referent | Referent | Referent | |
| Moderate | ns | ns | ns | ||||||
| Yeomans (2010) | 17 | 40 | 22.80 (SD not reported) | 100% women | None | Referent | Referent | Referent | Referent |
| Moderate | + | + | + | + | |||||
| Observational Studies (Frequent Drinking) | |||||||||
| Butler et al. (2017) | 21 | 14,375 | Not reported | 48.27% women 51.73% men |
Men | ||||
| None | + | ns | ns | + | |||||
| Light | + | + | ns | + | |||||
| Moderate | Referent | Referent | Referent | Referent | |||||
| Heavy | − | − | − | − | |||||
| Women | |||||||||
| None | + | + | ns | + | |||||
| Light | + | + | ns | + | |||||
| Moderate | Referent | Referent | Referent | Referent | |||||
| Heavy | − | − | ns | − | |||||
| Colditz et al. (1991) | 20 | 138,031 | Not reported | 64.87% women 35.13% men |
Men | ||||
| None | Referent | Referent | Referent | Referent | |||||
| Light | ns | ns | ns | ns | |||||
| Moderate | ns | ns | ns | ns | |||||
| Heavy | − | + | ns | − | |||||
| Women | |||||||||
| None | Referent | Referent | Referent | Referent | |||||
| Light | ns | ns | ns | ns | |||||
| Moderate | ns | ns | ns | ns | |||||
| Heavy | − | ns | ns | − | |||||
| Cummings et al. (2017) | 17 | 2,379 | 23.41 (5.39) | 100% women | None | Referent | Referent | ||
| Light | − | ns | |||||||
| Moderate | − | ns | |||||||
| Heavy | − | ns | |||||||
| de Castro et al. (1990) | 15 | 92 | 21.90 (SD not reported) | 75% women 25% men |
None | Referent | Referent | Referent | Referent |
| Light | ns | ns | ns | ns | |||||
| Moderate | ns | ns | + | ns | |||||
| Gruchow et al. (1985) | 19 | 10,428 | Not reported | 59% women 41% men |
Men | ||||
| None | Referent | Referent | Referent | Referent | |||||
| Light | − | + | + | - | |||||
| Moderate | − | ns | ns | − | |||||
| Heavy | − | ns | ns | − | |||||
| Women | |||||||||
| None | Referent | Referent | Referent | Referent | |||||
| Light | ns | + | + | ns | |||||
| Moderate | − | ns | ns | − | |||||
| Heavy | − | ns | ns | − | |||||
| Hillers et al. (1985) | 17 | 179 | Not reported | 100% men | None | Referent | Referent | Referent | Referent |
| Light | ns | ns | ns | ns | |||||
| Moderate | ns | ns | ns | ns | |||||
| Heavy | − | − | − | − | |||||
| Ishikawa et al. (2017) | 21 | 711 | Not reported | 100% men | Moderate | Referent | Referent | Referent | Referent |
| Heavy | − | − | − | − | |||||
| Kesse et al. (2001) | 19 | 72,904 | Not reported | 100% women | None | Referent | Referent | Referent | Referent |
| Light | − | + | ns | − | |||||
| Moderate | − | + | ns | − | |||||
| Heavy | − | + | + | − | |||||
| Ma et al. (2000) | 22 | 6,745 | Not reported | 54.93% men 45.07% women |
Men | ||||
| None | Referent | Referent | Referent | Referent | |||||
| Light | ns | ns | ns | ns | |||||
| Moderate | ns | − | ns | ns | |||||
| Heavy | − | − | − | − | |||||
| Women | |||||||||
| None | Referent | Referent | Referent | Referent | |||||
| Light | ns | ns | − | ns | |||||
| Moderate | ns | ns | − | ns | |||||
| Heavy | − | − | − | − | |||||
| Ruf et al. (2005) | 21 | 24,894 | Not reported | 53.33% women 46.67% men |
Men | ||||
| None | Referent | Referent | Referent | Referent | |||||
| Light | − | − | − | − | |||||
| Moderate | − | − | − | − | |||||
| Heavy | − | − | − | − | |||||
| Women | |||||||||
| None | Referent | Referent | Referent | Referent | |||||
| Light | − | + | ns | − | |||||
| Moderate | − | + | ns | − | |||||
| Heavy | − | + | ns | − | |||||
| Ruidavets et al. (2004) | 21 | 1,100 | Not reported | 100% men | None | Referent | Referent | Referent | Referent |
| Light | ns | ns | ns | ns | |||||
| Moderate | − | + | + | − | |||||
| Heavy | − | + | + | − | |||||
| Walmsley et al. (1998) | 20 | 1,198 | Not reported | 52% men 48% women |
None | Referent | Referent | Referent | |
| Light | + | + | + | ||||||
| Moderate | + | + | + | ||||||
| Heavy | − | + | − | ||||||
Notes: For each study, the drinking quantities that were tested are listed. If a drinking quantity (compared to the referent group) was significantly linked with greater intake of a macronutrient, the correspondent table cell is labeled with a plus sign; if significantly linked with less intake, it is labeled with a minus sign; and if a significant difference in intake was not detected, it is labeled “ns” to indicate non-significance. Significance was determined where p < .05. Table cells are blank if the correspondent macronutrient was not tested. For some observational studies, analyses were separated by biological sex; this was noted with the text “men” and “women” above those results.
Alcohol Use & Dietary Intake
Table 1 presents a summary of the findings from included studies separated by study design/drinking frequency. For each study, the drinking quantities that were tested are listed. If a drinking quantity (compared to the referent group) was significantly linked with greater intake of a macronutrient, the correspondent table cell is labeled with a plus sign; if significantly linked with less intake, it is labeled with a minus sign; and if a significant difference in intake was not detected, it is labeled “ns” to indicate non-significance. Significance was determined where p < .05. Table cells are blank if the correspondent macronutrient was not tested. For some observational studies, analyses were separated by biological sex; this was noted with the text “men” and “women” above those results.
Alcohol use & refined carbohydrate intake
Eighteen experimental studies tested whether a single occasion of drinking affected refined carbohydrate intake from foods such as bread, breadsticks, cake, chips, chocolate, chocolate chip cookies, chocolate mini-rolls, cookies, crackers, fruit loaf, ice cream, milkshakes, M&Ms, noodles, pasta, and tortilla chips. The majority (66.7%) did not detect differences in refined carbohydrate intake after a single occasion of drinking. The minority (33.3%) found that a single occasion of drinking increased refined carbohydrate intake. Experiments that tested a single occasion of light or moderate drinking were more likely to find an increase in refined carbohydrate intake (effect detected in 40% of those experiments) relative to experiments that tested a single occasion of heavy drinking (effect detected in 25% of those experiments). Additionally, 11 observational studies tested whether frequent drinking was associated with refined carbohydrate intake from foods such as added sugars, candies, cereal, and chocolate. Some (45.5%) found that frequent light and moderate drinking were associated with less refined carbohydrate intake, some (27.3%) found these behaviors were not associated with refined carbohydrate intake, and a few (18.2%) found these behaviors were associated with greater refined carbohydrate intake. The majority (90.9%) found that frequent heavy drinking was associated with less refined carbohydrate intake with the minority (9.1%) finding it was not associated with refined carbohydrate intake.
Alcohol use & fat intake
Eighteen experimental studies tested whether a single occasion of drinking affected fat intake from foods such as beef, cheese, chips, chocolate, crackers, cream cheese, ham, ice cream, milkshakes, M&Ms, paté, salami, and tortilla chips. Most (55.6%) did not detect differences in fat intake after a single occasion of drinking. Some (44.4%) found that a single occasion of drinking increased fat intake. Experiments that tested a single occasion of light or moderate drinking were more likely to find an increase in fat intake (effect detected in 60% of those experiments) relative to experiments that tested a single occasion of heavy drinking (effect detected in 25% of those experiments). In addition, 11 observational studies tested whether frequent drinking was associated with fat intake including intake of eggs, cheese, chocolate, cottage cheese, lamb, milk, processed meat, poultry, unspecified meat, unspecified dairy products, vegetable oil, and yogurt. Most (54.5%) found that frequent light and moderate drinking had no association with fat intake and some (45.5%) found these behaviors were associated with greater fat intake. Some (36.4%) found that frequent heavy drinking was associated with less fat intake, some (36.4%) found that it was associated with greater fat intake, and a few (27.3%) found it had no association with fat intake
Alcohol use & protein intake
Twelve experimental studies tested whether a single occasion of drinking affected protein intake from foods such as beef, cheese, lean ham, paté, salami, smoked beef, tuna, unspecified meat, and yogurt. The majority (58.3%) did not detect differences in protein intake after a single occasion of drinking. The minority (41.7%) found that a single occasion of drinking increased protein intake. Experiments that tested a single occasion of light or moderate drinking were more likely to find an increase in protein intake (effect detected in 44.4% of those experiments) relative to experiments that tested a single occasion of heavy drinking (effect detected in 33.3% of those experiments). Also, 9 observational studies tested whether frequent drinking was associated with protein intake including intake of eggs, cheese, fish, lamb, processed meat, poultry, seafood, unspecified meat, and yogurt. The majority (55.6%) found that frequent light and moderate drinking had no association with protein intake whereas some (33.3%) found that these behaviors were associated with greater protein intake, and one study (11.1%) found that these behaviors were associated with less protein intake. Some (44.4%) found that frequent heavy drinking was associated with less protein intake whereas a few (33.3%) found it was not associated with protein intake, and a few (22.2%) found it was associated with greater protein intake.
Alcohol use & unrefined carbohydrate intake
Six experimental studies tested whether a single occasion of drinking affected unrefined carbohydrate intake from foods such as cucumber, grapes, tomatoes, and unspecified vegetables. The majority (83.3%) did not detect differences in unrefined carbohydrate intake after a single occasion of drinking. The minority (16.7%) indicated that a single occasion of drinking increased unrefined carbohydrate intake. Specifically, an increase in unrefined carbohydrate intake was only found in an experiment that tested the effect of a single occasion of moderate drinking. Also, 10 observational studies tested whether frequent drinking was associated with unrefined carbohydrate intake from foods such as unspecified fruits, grains, and vegetables. Some (40%) indicated that frequent light and moderate drinking were associated with less unrefined carbohydrate intake, others (40%) found these drinking behaviors were not associated with unrefined carbohydrate intake, and a few (20%) indicated that these drinking behaviors were associated with greater unrefined carbohydrate intake. The majority (90%) found that frequent heavy drinking was associated with less unrefined carbohydrate intake with the minority (10%) finding it was not associated with unrefined carbohydrate intake
Biological Sex Differences
There was no strong evidence of differences in effects due to biological sex. The likelihood of detecting that a single occasion of drinking stimulated intake of refined carbohydrates, fat, protein, or unrefined carbohydrates was equivalent between studies with samples including exclusively female participants (effects detected in 50% of those experiments) and studies with samples including exclusively male participants (effects detected in 50% of those experiments). Of the studies that tested whether frequent drinking was associated with dietary intake and separated results by biological sex, the majority (60%) found consistent results between male and female participants. However, one of these studies (20%) found that heavy drinking was associated with greater fat intake only in male participants, and another (20%) found that light, moderate, and heavy drinking were associated with less fat intake in male participants but greater fat intake in female participants.
Potential Confounds
Since observational studies provided results on how frequent drinking related to dietary intake, it is possible that associations between alcohol use and dietary intake may be in part explained by confounding variables. For instance, a widely held belief is that smoking cigarettes can suppress eating [albeit empirical evidence does not support this belief (53)], and those who engage in frequent heavy drinking sometimes also smoke cigarettes (34). Thus, cigarette smoking may explain why, for instance, frequent heavy drinking was associated with less carbohydrate intake. Yet, several of the observational studies ruled out this confound by adjusting for the effect of cigarette smoking in analyses (17, 23, 42, 43, 45). In one case, frequent light and heavy drinking were associated with greater cigarette smoking but only frequent heavy drinking was associated with less carbohydrate intake (34); this is inconsistent with the notion that smoking explains the association between frequent heavy drinking and less carbohydrate intake.
Another possible confounding variable is BMI because BMI can impact the percentage of alcohol in someone’s bloodstream, which may alter the influence of certain alcohol quantities on dietary intake (54). Three observational studies adjusted for BMI in analyses and found results consistent with studies that did not adjust for BMI (23, 33, 43). However, since the majority of observational studies did not adjust for BMI, it will be important for future research to address this confound. In addition to calculating standard alcoholic drinks, researchers could calculate Blood Alcohol Content (BAC) based on participant’s biological sex, body weight, and time course of their drinking (54). Lastly, results were found above and beyond the adjustment of many other variables including age (17, 23, 32–34, 43, 45), age of menarche (19), chronic disease status (17), education (17, 34, 43), employment status (34), income (19, 34), liking of foods (40), living area (43, 45), physical activity (17, 43), race/ethnicity (17, 19, 34), self-reported health (45), and sex (45). This inspires confidence that the documented associations between alcohol use and dietary intake are not largely explained by confounding variables.
Discussion
To help clarify the link between alcohol use and cardiometabolic health, this paper offers the first systematic review of experimental and observational studies investigating how alcohol use affects dietary intake (carbohydrate, protein, and fat intake) in humans. The inclusion of experimental and observational studies allowed for inferences about the effects of a single drinking occasion versus frequent drinking. Moreover, alcohol use was standardized into light drinking, moderate drinking, and heavy drinking based on the 2015–2020 Dietary Guidelines for Americans to specify alcohol quantities implicated in effects, and this standardization accounts for sex differences in alcohol pharmacokinetics (13, 17). This review adhered to PRISMA (15) and Downs and Black Quality Index (18) guidelines to synthesize and critique the existing literature, yielding insights into associations among alcohol use and dietary intake that may guide clinicians and the public.
Although some assume that any alcohol use uniformly affects diet by increasing intake of all foods (55), synthesized results from this review do not support this claim. To begin, a single occasion of drinking increased intake of foods but studies did not consistently detect this effect. Counterintuitively, the effect was more likely occur in response to a single occasion of light or moderate drinking compared to a single occasion of heavy drinking. Moreover, when an effect was detected, a single occasion of drinking most often increased intake of fat and protein, and to a lesser extent increased intake of refined and unrefined carbohydrates. This pattern was observed in female and male participants, which suggests there was no moderation by biological sex.
Next, the majority of studies testing the link between frequent drinking and dietary intake observed associations but these differed by alcohol dose. Similar to the observed effects of a single occasion of light and moderate drinking, frequent light and moderate drinking were linked with greater intake of fat and protein, and to a lesser extent with greater intake of unrefined and refined carbohydrates. In contrast, frequent heavy drinking was linked with intake of fewer refined and unrefined carbohydrates, and to a lesser extent with lower protein and fat intake. This pattern was observed in female and male participants, further suggesting of no moderation by biological sex.
Overall, these synthesized results suggest four important conclusions regarding how alcohol use affects dietary intake. The first is that a single occasion of drinking does not reliably shift diet but frequent drinking appears to more consistently influence diet. This is likely because the effect of a single occasion of drinking on dietary intake is small (2) and only through frequent recurrence does it become larger and more consistently observable. Since cardiometabolic diseases and obesity develop over time (16), the distinction between transient versus enduring alcohol-induced dietary changes is an important one. Future studies on the effects of alcohol use on dietary intake should prioritize repeated measures or hybrid designs that use experimental paradigms repeated several times across an individual’s lifespan.
The second conclusion these results suggest is that alcohol use affects dietary intake in a dose-dependent manner. Most often, alcohol use was linked with greater dietary intake at light and moderate quantities; however, it was not linked with changes in dietary intake or was linked with less dietary intake at heavier quantities. This finding corroborates with a prior meta-analysis that shows that a single occasion of light/moderate drinking (<2 standard drinks) increased total nonalcoholic energy intake whereas a single occasion of “heavy” drinking (2–4 standard drinks) had no effect (2). Why might alcohol use counterintuitively stimulate dietary intake at light and moderate doses but fail to stimulate or even decrease dietary intake at heavier doses? One potential explanation is that the dose-dependent effects on dietary intake are mediated through changes in dopaminergic activity in the brain (19, 54). At low doses, alcohol stimulates dopamine release, which may increase motivation for several rewarding substances including food (56). At high doses, however, alcohol saturates neural pathways with dopamine (57), which may decrease this motivation. Another potential explanation is that dose-dependent effects on dietary intake coincide with biphasic alcohol effects. At low doses, alcohol causes stimulatory effects (e.g., positive mood) (58, 59), which may increase the motivation for food. At high doses, alcohol causes sedative effects (e.g., nausea, central nervous system depression), which may decrease this motivation (58, 59). At high doses, alcohol may also cause gastric expansion and satiation (26), which could decrease food intake. Moreover, it is plausible that the different aforementioned mechanisms collectively cause alcohol use to affect dietary intake in a dose-dependent manner. Future research should directly test these dose-dependent mechanisms with advanced measurement methods. For instance, researchers could intravenously administer alcohol to observe subjective and dietary effects across different quantities of alcohol dose (60). Also, paradigms are available that would allow for the effect of alcohol use on dietary intake to be observed while using neural imaging methods (61).
The third conclusion these results suggest is that alcohol use differentially affects intake of specific macronutrients. In detail, when alcohol use was linked with greater dietary intake, it was most often intake of fat and protein and least often intake of carbohydrates. In contrast, when alcohol use was linked with less dietary intake, it was most often intake of carbohydrates and least often intake of fat and protein. An evident explanation for the macronutrient-specific pattern of findings is that alcohol itself is a refined carbohydrate (i.e., alcohol is fermented sugar); ingesting it may decrease motivation for carbohydrates and increase motivation for different macronutrients (e.g., fat, protein) (62). Non-human animal models suggest that this could be mediated by gut-brain processing in response to carbohydrate intake and regulated by several hormones and neurotransmitters including insulin, serotonin, and dopamine (62). Future research should directly test how these physiological systems are integrated into the seemingly dose-dependent effect of alcohol use on dietary intake in humans.
The fourth conclusion these results suggest is that alcohol use similarly influences dietary intake in female and male individuals. One might have expected biological sex to moderate effects because male and female individuals tend to show different patterns of alcohol (14) and dietary intake [albeit sex differences in dietary intake are inconsistently observed (63)]. In the current review, biological sex differences in alcohol pharmacokinetics were accounted for by sex-specific definitions of light, moderate, and heavy drinking, which might explain the lack of moderation. Furthermore, the mechanisms by which alcohol use influences dietary intake appear to be distinct from those explaining biological sex differences in alcohol (64) and dietary intake (63) more generally. Future research might consider testing this explicitly.
The current results should be interpreted in light of limitations. In terms of study-level limitations, on average, methodological quality of the studies was medium strength. Researchers can improve methodological quality by fully disclosing method and statistical choices, using the Downs and Black Quality Index checklist to determine what needs to be reported, and pre-registering studies to increase accountability. In addition, researchers can avoid risk of bias by invoking double-blind procedures or reporting what study information was disclosed to experimenters/participants. It is challenging to achieve experimenter blindness when an experimenter is serving alcoholic beverages/food. However, one approach is having researchers other than the experimenter serve beverages/food. To achieve participant blindness, researchers can deceive participants as to the true nature of the study by providing cover stories. A commonly used and validated cover story is that participants are invited to the lab to taste and rate new drink and food products (65). Also, although most studies thoroughly quantified dietary intake by providing the percentage of total energy intake that macronutrients contributed to and/or the number of calories and/or grams of foods eaten, some did not. To reduce variability across studies, researchers should quantify dietary intake in each of the aforementioned ways and/or publicly share data so that other quantifications could be derived in the future. Lastly, this review yielded many studies that were more than a decade old, some more than two decades old, and some more than three decades old. Even though there exists a large enough literature to warrant this review, there are opportunities for future scientific growth and improvement.
Regarding review-level limitations, the current review did not summarize information on effect sizes for results. This was because, as mentioned above, studies were inconsistent in how they quantified dietary intake and also because some studies only presented inferential statistical estimates without effect size information. Thorough quantifications of dietary intake and reporting of effect sizes will allow for meta-analytic methods to be applied in this domain in the future. Another review-level limitation was that three of the current paper’s authors were authors of one study included in the review. To reduce the possibility that this biased the review, a Ph.D. individual with no conflict of interest assessed the methodological quality of that study. Also, the selected review eligibility requirements may have limited the scope of included studies. For example, the initial search yielded two studies on dietary intake of those with alcohol use disorder (66, 67). These studies were ineligible for the review because a diagnosis of alcohol use disorder confounds drinking with other behaviors (e.g., withdrawal symptoms, quit attempts) and because in some dietary intake was measured when individuals were undergoing treatment. Understanding how features of alcohol use disorder influence dietary intake—and how this may change during treatment—might nonetheless be of interest to clinicians.
Dietary intake was operationalized as the macronutrient composition of foods because—irrespective of total energy intake—diets consisting of greater proportional intake of refined carbohydrates and fat heighten risk for cardiometabolic disease and obesity, and diets consisting of greater protein intake may facilitate weight loss (10). However, it may have been beneficial to also provide information on how alcohol use was associated with overall diet quality (e.g., summary measure of variety in nutrient intake). Also, greater intake of certain types of fat (i.e., industrial trans fats) may carry the most risk for cardiometabolic disease and obesity (68), so it may have benefited the review to further parse fat intake into groups based on type of fat. Likewise, greater proportional intake of ultra-processed foods (i.e., food-derived substances including sugar-sweetened beverages; sweet/savory packaged snacks; mass-produced breads; “instant” meals; and reconstituted meats) compared to minimally processed foods (i.e., foods obtained directly from plants/animals including fresh fruits and vegetables, eggs, milk) increases risk of cardiometabolic disease and obesity irrespective of total energy intake (69, 70). Unfortunately the majority of included studies did not provide enough detail so that the association of alcohol use and overall diet quality could be summarized, so that fat intake could be parsed into different groups based on fat type, and so that dietary intake could be operationalized based on processing level. Future research on how alcohol use affects diet should provide detail on the type of fat measured and should consider using standardized classification procedures to categorize overall diet quality (71) and food processing levels (72).
Overall, alcohol use does not reliably increase intake of food. When alcohol stimulates food intake, the scientific literature suggests it is most often at low doses—whether at a single occasion or frequently—and is specific to stimulation of fat and protein intake. In contrast, the scientific literature suggests that frequent heavy drinking is consistently linked with intake of fewer carbohydrates. Understanding these nuanced effects of alcohol use on dietary intake may improve the precision of clinical and public discourse on the topic, and may improve existing cardiometabolic disease and obesity prevention and treatment efforts (13). Indeed, findings from this review suggest that alcohol use may confer some risk for cardiometabolic disease and obesity through dietary change. Specifically, a single occasion of and frequent light/moderate drinking may confer risk by increasing fat intake; however, this may be partially offset by increased protein intake. Also, the cardiometabolic consequences of heavy drinking may be diminished due to decreases in refined carbohydrate intake. The latter finding might even explain why greater alcohol use is associated with a lower BMI in several studies (4). Future research might test this explicitly by testing refined carbohydrate intake as a mediator or moderator of the link between alcohol use and obesity. It is important, however, that any findings suggesting that alcohol use mitigates risk for cardiometabolic disease and obesity through dietary changes be interpreted in the context of the broader literature on the consequences of drinking. Alcohol use increases risk for addiction (73), depression (74), interpersonal violence (75), several cancers (76), liver cirrhosis (77), pancreatitis (78), and unintentional injuries (e.g., vehicle accidents, falls, drowning) (78).
In sum, there are many questions to be answered about the links among alcohol use, dietary intake, cardiometabolic disease, and obesity but the scientific evidence challenges the lay assumption that any alcohol use increases intake of all foods (55). Research replicating the observed findings will strengthen the scientific literature and shed light on precise behavioral targets (e.g., light/moderate drinking facilitating a high-fat diet) that may be relevant to those seeking guidance on the cardiometabolic health effects of alcohol use.
Supplementary Material
Acknowledgments
Jenna R. Cummings was supported by Award Number DGE-1144087 from the National Science Foundation and Award Number T32HD079350 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Science Foundation or the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The authors would like to acknowledge the hard work of Cristina Gonzalez, Lindsay Hong, Kara Hoover, Tanvi Mamtora, and Dorothy Nguyen in conducting blind literature reviews for this project. The authors would also like to acknowledge the work of Jacqueline Kim in assisting with quality assessment of research included in this review.
Footnotes
Potential conflicts of interest: None.
Most included studies quantified dietary intake by (1) calculating the percentage of total energy intake that macronutrients contributed to and (2) calculating the number of calories and/or grams of high-carbohydrate, high-fat, and high-protein foods eaten. However, some included studies quantified dietary intake by only calculating one of these. For consistency, we refer to all quantifications as “carbohydrate,” “fat,” and “protein” intake.
References
- 1.Yeomans MR, Caton S, Hetherington MM: Alcohol and food intake. Curr Opin Clin Nutr Metab Care. 2003, 6:639–644. [DOI] [PubMed] [Google Scholar]
- 2.Kwok A, Dordevic AL, Paton G, Page MJ, Truby H: Effect of alcohol consumption on food energy intake: a systematic review and meta-analysis. British Journal of Nutrition. 2019, 121:481–495. [DOI] [PubMed] [Google Scholar]
- 3.Poli A, Marangoni F, Avogaro A, et al. : Moderate alcohol use and health: a consensus document. Nutrition, Metabolism, & Cardiovascular Diseases. 2013, 23:487–504. [DOI] [PubMed] [Google Scholar]
- 4.Sayon-Orea C, Martinez-Gonzalez MA, Bes-Rastrollo M: Alcohol consumption and body weight: a systematic review. Nutr Rev. 2011, 69:419–431. [DOI] [PubMed] [Google Scholar]
- 5.Guh DP, Zhang W, Bansback N, et al. : The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009, 9:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tomiyama AJ, Hunger JM, Nguyen-Cuu J, Wells C: Misclassification of cardiometabolic health when using body mass index categories in NHANES 2005–2012. International Journal of Obesity. 2016, 40:883–886. [DOI] [PubMed] [Google Scholar]
- 7.Stanhope KL, Goran MI, Bosy-Westphal A, et al. : Pathways and mechanisms linking dietary components to cardiometabolic disease: thinking beyond calories. Obesity Reviews. 2018, 19:1205–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ebbeling CB, Swain JF, Feldman HA, et al. : Effects of dietary composition on energy expenditure during weight-loss maintenance. JAMA. 2012, 307:2627–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall KD, Chen KY, Guo J, et al. : Energy expenditure and body composition changes after an isocaloric ketogenic diet in overweight and obese men. The American Journal of Clinical Nutrition. 2016, 104:324–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madsen L, Liaset B, Kristiansen K: Macronutrients and obesity: views, news and reviews. Future Lipidology. 2008, 3:43–74. [Google Scholar]
- 11.Neter JE, Stam BE, Kok FJ, Grobbee DE, Geleijnse JM: Influence of weight reduction on blood pressure: a meta-analysis of randomized controlled trials. Hypertension. 2003, 42:878–884. [DOI] [PubMed] [Google Scholar]
- 12.Aucott L, Poobalan A, Smith WC, et al. : Effects of weight loss in overweight/obese individuals and long-term hypertension outcomes: a systematic review. Hypertension. 2005, 45:1035–1041. [DOI] [PubMed] [Google Scholar]
- 13.Services USDoHaH, Agriculture USDo: 2015–2020 Dietary Guidelines for Americans. Retrieved May 3rd, 2019 from https://health.gov/dietaryguidelines/2015/guidelines/
- 14.Peacock A, Leung J, Larney S, et al. : Global statistics on alcohol, tobacco and illicit drug use: 2017 status report. Addiction. 2018, 113:1905–1926. [DOI] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG, Group TP: Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLOS Medicine. 2009, 6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chow CC, Hall KD: Short and long-term energy intake patterns and their implications for human body weight regulation. Physiology & Behavior. 2014, 134:60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Butler L, Popkin BM, Poti JM: Associations of alcoholic beverage consumption with dietary intake, waist circumference, and body mass index in US adults: National Health and Nutrition Examination Survey 2003–2012. Journal of the Academy of Nutrition and Dietetics. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Downs SH, Black N: The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. Journal of Epidemiology of Community Health. 1998, 52:377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cummings JR, Ray LA, Tomiyama AJ: Food-alcohol competition: As young females eat more food, do they drink less alcohol? Journal of Health Psychology. 2017, 22:674–683. [DOI] [PubMed] [Google Scholar]
- 20.Caton SJ, Ball M, Ahern A, Hetherington MM: Dose-dependent effects of alcohol on appetite and food intake. Physiol Behav. 2004, 81:51–58. [DOI] [PubMed] [Google Scholar]
- 21.Caton SJ, Marks JE, Hetherington MM: Pleasure and alcohol: manipulating pleasantness and the acute effects of alcohol on food intake. Physiol Behav. 2005, 84:371–377. [DOI] [PubMed] [Google Scholar]
- 22.Christiansen P, Rose A, Randall-Smith L, Hardman CA: Alcohol’s acute effect on food intake is mediated by inhibitory control impairments. Health Psychol. 2016, 35:518–522. [DOI] [PubMed] [Google Scholar]
- 23.Colditz GA, Giovannucci E, Rimm EB, et al. : Alcohol intake in relation to diet and obesity in women and men. American Journal of Clinical Nutrition. 1991, 54:49–55. [DOI] [PubMed] [Google Scholar]
- 24.de Castro JM, Orozco S: Moderate alcohol intake and spontaneous eating patterns of humans: Evidence of unregulated supplementation. American Journal of Clinical Nutrition. 1990, 52:246–253. [DOI] [PubMed] [Google Scholar]
- 25.Eiler WJ 2nd, Dzemidzic M, Case KR, et al. : The aperitif effect: Alcohol’s effects on the brain’s response to food aromas in women. Obesity (Silver Spring). 2015, 23:1386–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gruchow HW, Sobocinski KA, Barboriak JJ, Scheller JG: Alcohol consumption, nutrient intake and relative body weight among US adults. The American Journal of Clinical Nutrition. 1985, 42:289–295. [DOI] [PubMed] [Google Scholar]
- 27.Hausmann MF: The behavior of albino rats in choosing food and stimulants. Journal of Comparative Psychology. 1932, 13:279–309. [Google Scholar]
- 28.Hetherington MM, Cameron F, Wallis DJ, Pirie LM: Stimulation of appetite by alcohol. Physiology & Behavior. 2001, 74:283–289. [DOI] [PubMed] [Google Scholar]
- 29.Hillers VN, Massey LK: Interrelationships of moderate and high alcohol consumption with diet and health status. The American Journal of Clinical Nutrition. 1985, 41:356–362. [DOI] [PubMed] [Google Scholar]
- 30.Hofmann W, Friese M: Impulses got the better of me: alcohol moderates the influence of implicit attitudes toward food cues on eating behavior. J Abnorm Psychol. 2008, 117:420–427. [DOI] [PubMed] [Google Scholar]
- 31.Hollister L: Hunger and appetite after single doses of marihuana, alcohol, and dextroamphetamine. Clinical Pharmacology & Therapeutics. 1971, 12:44–49. [DOI] [PubMed] [Google Scholar]
- 32.Ishikawa M, Yokoyama T, Murayama N: Alcohol energy intake is related to low body mass index in Japanese older adults: Data from the 2010–2011 national health and nutrition survey. Journal of Nutrition Health and Aging. 2017, 21:1095–1101. [DOI] [PubMed] [Google Scholar]
- 33.Kesse E, Clavel-Chapelon F, Slimani N, van Liere M, Group TEN: Do eating habits differ according to alcohol consumption? Results of a study of the French cohort of the European Prospective Investigation into Cancer and Nutrition (E3N-EPIC). The American Journal of Clinical Nutrition. 2001, 74:322–327. [DOI] [PubMed] [Google Scholar]
- 34.Ma J, Betts NM, Hampl JS: Clustering of Lifestyle Behaviors: The Relationship between Cigarette Smoking, Alcohol Consumption, and Dietary Intake. American Journal of Health Promotion. 2000, 15:107–117. [DOI] [PubMed] [Google Scholar]
- 35.Mattes RD: Dietary compensation by humans for supplemental energy provided as ethanol or carbohydrate in fluids. Physiology & Behavior. 1996, 59:179–187. [DOI] [PubMed] [Google Scholar]
- 36.Ouwens MA, van Strien T, van der Staak CPF: Absence of a disinhibition effect of alcohol on food consumption. Eating Behaviors. 2003, 4:323–332. [DOI] [PubMed] [Google Scholar]
- 37.Polivy J, Herman CP: The effects of alcohol on eating behavior: Disinhibition or sedation? Addictive Behaviors. 1976, 1:121–125. [Google Scholar]
- 38.Polivy J, Herman CP: Effects of alcohol on eating behavior: Influence of mood and perceived intoxication. Journal of Abnormal Psychology. 1976, 85:601–606. [PubMed] [Google Scholar]
- 39.Poppitt SD, Eckhardt JW, McGonagle J, Murgatroyd PR, Prentice AM: Short term effects of alcohol consumption on appetite and energy intake. Physiology & Behavior. 1996, 60:1063–1070. [DOI] [PubMed] [Google Scholar]
- 40.Raben A, Agerholm-Larsen L, Flint A, Holst JJ, Astrup A: Meals with similar energy densities but rich in protein, fat, carbohydrate, or alcohol have different effects on energy expenditure and substrate metabolism but not on appetite and energy intake. The American Journal of Clinical Nutrition. 2003, 77:91–100. [DOI] [PubMed] [Google Scholar]
- 41.Rose AK, Hardman CA, Christiansen P: The effects of a priming dose of alcohol and drinking environment on snack food intake. Appetite. 2015, 95:341–348. [DOI] [PubMed] [Google Scholar]
- 42.Ruf T, Nagel G, Altenburg HP, Miller AB, Thorand B: Food and nutrient intake, anthropometric measurements and smoking according to alcohol consumption in the EPIC Heidelberg study. Ann Nutr Metab. 2005, 49:16–25. [DOI] [PubMed] [Google Scholar]
- 43.Ruidavets JB, Bataille V, Dallongeville J, et al. : Alcohol intake and diet in France, the prominent role of lifestyle. Eur Heart J. 2004, 25:1153–1162. [DOI] [PubMed] [Google Scholar]
- 44.Schrieks IC, Stafleu A, Griffioen-Roose S, et al. : Moderate alcohol consumption stimulates food intake and food reward of savoury foods. Appetite. 2015, 89:77–83. [DOI] [PubMed] [Google Scholar]
- 45.Walmsley CM, Bates CJ, Prentice A, Cole TJ: Relationship between alcohol and nutrient intakes and blood status indices of older people living in the UK: Further analysis of data from the National Diet and Nutrition Survey of people aged 65 years and over, 1994/5. Public Health Nutrition. 1998, 1:157–167. [DOI] [PubMed] [Google Scholar]
- 46.Yeomans MR, Hails NJ, Nesic JS: Alcohol and the appetizer effect. Behavioral Pharmacology. 1999, 10:151–161. [DOI] [PubMed] [Google Scholar]
- 47.Yeomans MR, Phillips MF: Failure to Reduce Short-term Appetite Following Alcohol is Independent of Beliefs about the Presence of Alcohol. Nutritional Neuroscience. 2002, 5:131–139. [DOI] [PubMed] [Google Scholar]
- 48.Yeomans MR: Short term effects of alcohol on appetite in humans. Effects of context and restrained eating. Appetite. 2010, 55:565–573. [DOI] [PubMed] [Google Scholar]
- 49.Karvetti RL, Knuts LR: Validity of the 24-hour dietary recall. Journal of the American Dietetic Association. 1985, 85:1437–1442. [PubMed] [Google Scholar]
- 50.Rimm EB, Giovannucci E, Stampfer MJ, et al. : Reproducibility and validity of an expanded self-administered semiquantitative Food Frequency Questionnaire among male health professionals. American Journal of Epidemiology. 1992, 135:1114–1126. [DOI] [PubMed] [Google Scholar]
- 51.Forsander OA: The interaction between voluntary alcohol consumption and dietary choice. Alcohol & Alcoholism. 1988, 23:143–149. [PubMed] [Google Scholar]
- 52.Forsander OA, Sinclair JD: Protein, carbohydrate, and ethanol consumption: Interactions in AA and ANA rats. Alcohol. 1988, 5:233–238. [DOI] [PubMed] [Google Scholar]
- 53.Perkins KA, Sexton JE, Di Marco A, Fonte C: Acute effects of tobacco smoking on hunger and eating in male and female smokers. Appetite. 1994, 22:149–158. [DOI] [PubMed] [Google Scholar]
- 54.Gearhardt AN, Corbin WR: Body mass index and alcohol consumption: family history of alcoholism as a moderator. Psychol Addict Behav. 2009, 23:216–225. [DOI] [PubMed] [Google Scholar]
- 55.Alcohol Think Again: Alcohol and Nutrition. 2019. from https://alcoholthinkagain.com.au/Alcohol-Your-Health/Alcohol-and-Your-Long-Term-Health/Alcohol-and-Nutrition
- 56.Volkow ND, Wang GJ, Tomasi D, Baler RD: Obesity and addiction: neurobiological overlaps. Obes Rev. 2013, 14:2–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weiss F, Lorang MT, Bloom FE, Koob GF: Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. The Journal of Pharmacology and Experimental Therapeutics. 1993, 267:250–258. [PubMed] [Google Scholar]
- 58.Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM: Development and validation of the Biphasic Alcohol Effects Scale. Alcoholism: Clinical & Experimental Research. 1993, 17:140–146. [DOI] [PubMed] [Google Scholar]
- 59.Pohorecky LA: Biphasic action of ethanol. Biobehavioral Reviews. 1977, 1:231–240. [Google Scholar]
- 60.Ray LA, Hutchison KE: A Polymorphism of the μ-Opioid Receptor Gene (OPRM1) and Sensitivity to the Effects of Alcohol in Humans. Alcoholism: Clinical & Experimental Research. 2004, 28:1789–1795. [DOI] [PubMed] [Google Scholar]
- 61.Gearhardt AN, Yokum S, Orr PT, et al. : Neural correlates of food addiction. Arch Gen Psychiatry. 2011, 68:808–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wurtman JJ, Moses PL, Wurtman RJ: Prior carbohydrate consumption affects the amount of carbohydrate that rats choose to eat. Journal of Nutrition. 1983, 113:70–78. [DOI] [PubMed] [Google Scholar]
- 63.Northstone K: Dietary patterns: the importance of sex differences. British Journal of Nutrition. 2012, 108:393–394. [DOI] [PubMed] [Google Scholar]
- 64.Becker JB, Hu M: Sex differences in drug abuse. Fronteirs in Neuroendocrinology. 2008, 29:36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jones A, Button E, Rose AK, et al. : The ad-libitum alcohol ‘taste test’: secondary analyses of potential confounds and construct validity. Psychopharmacology. 2016, 233:917–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barboriak JJ, Rooney CB, Leitschuh TH, Anderson AJ: Alcohol and nutrient intake of elderly men. Journal of the American Dietetic Association. 1978, 72:493–495. [PubMed] [Google Scholar]
- 67.Yung L, Gordis E, Holt J: Dietary choices and likelihood of abstinence among alcoholic patients in an outpatient clinic. Drug and Alcohol Dependence. 1983, 12:355–362. [DOI] [PubMed] [Google Scholar]
- 68.de Souza RJ, Mente A, Maroleanu A, et al. : Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: systematic review and meta-analysis of observational studies. BMJ. 2015, 351:h3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schnabel L, Kesse-Guyot E, Alles B, et al. : Association between ultraprocessed food consumption and risk of mortality among middle-aged adults in France. JAMA Internal Medicine. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hall KD, Ayuketah A, Brychta R, et al. : Ultra-processed diets cause excess calorie intake and weight gain: an inpatient randomized controlled trial of ad libitum food intake. Cell Metabolism. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kennedy ET, Ohls J, Ma SC, Fleming K: The Healthy Eating Index: Design and applications. Journal of the American Dietetic Association. 1995, 95:1103–1108. [DOI] [PubMed] [Google Scholar]
- 72.Monteiro CA, Cannon G, Moubarac JC, et al. : The UN Decade of Nutrition, the NOVA food classification and the trouble with ultra-processing. Public Health Nutrition. 2018, 21:5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Koob GF: Theoretical frameworks and mechanistic aspects of alcohol addiction: alcohol addiction as a reward deficit disorder. Curr Top Behav Neurosci. 2013, 13:3–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Merikangas KR, Mehta RL, Molnar BE, et al. : Comorbidity of substance use disorders with mood and anxiety disorders: Results of the international consortium in psychiatric epidemiology. Addictive Behaviors. 1998, 23:893–907. [DOI] [PubMed] [Google Scholar]
- 75.Lipsey MW, Wilson DB, Cohen MA, Derzon JH: Is there a causal relationship between alcohol use and violence? Recent Developments in Alcoholism. 2002:245–282. [DOI] [PubMed] [Google Scholar]
- 76.Boffetta P, Hashibe M: Alcohol and cancer. The Lancet Oncology. 2006, 7:149–156. [DOI] [PubMed] [Google Scholar]
- 77.Corrao G, Bagnardi V, Zambon A, Torchio P: Meta-analysis of alcohol intake in relation to risk of liver cirrhosis. Alcohol & Alcoholism. 1998, 33:381–392. [DOI] [PubMed] [Google Scholar]
- 78.Thakker KD: An overview of health risks and benefits of alcohol consumption. Alcoholism: Clinical & Experimental Research. 1998, 22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.