Abstract
Background.
Preclinical and emerging clinical data show that glibenclamide reduces space occupying edema and brain swelling following cerebral ischemia. Glibenclamide is a potent inhibitor of numerous sulfonylurea receptor (SUR)-regulated channels, including KATP (SUR1-KIR6.2, SUR2A-KIR6.2, SUR2B-KIR6.2, SUR2B-KIR6.1) and SUR1-TRPM4. Here, we used molecularly specific oligodeoxynucleotides (ODNs) to investigate the role of various SUR-regulated ion channel subunits in post-ischemic brain swelling.
Methods.
Focal cerebral ischemia was induced in adult male rats by permanent middle cerebral artery occlusion (pMCAo). We used this model to study the effects of antisense-ODNs (AS-ODNs) directed against Abcc8/SUR1, Trpm4/TRPM4, Kcnj8/KIR6.1 and Kcnj11/KIR6.2 on hemispheric swelling, with sense or scrambled ODNs used as controls. We used antibodybased Förster resonance energy transfer (immuno-FRET) and co-immunoprecipitation to study the co-assembly of SUR1-TRPM4 heteromers.
Results.
In the combined control groups administered sense or scrambled ODNs, pMCAo resulted in uniformly large infarct volumes (mean±SD: 57.4±8.8%; n=34) at 24 hours after onset of ischemia, with no effect of AS-ODNs on infarct size. In controls, hemispheric swelling was 23.9±4.1% (n=34), and swelling was linearly related to infarct volume (P<0.02). In the groups administered anti-Abcc8/SUR1 or anti-Trpm4/TRPM4 AS-ODN, hemispheric swelling was significantly less, 11.6±3.9% and 12.8±5.8% respectively (P<0.0001), and the relationship between infarct volume and swelling was reduced and not significant. AS-ODNs directed against Kcnj8/KIR6.1 and Kcnj11/KIR6.2 had no significant effect on hemispheric swelling (23.3±5.4% and 22.9±5.8% respectively). Post-ischemic tissues showed co-assembly of SUR1-TRPM4 heteromers.
Conclusions.
Post-ischemic hemispheric swelling can be decoupled from infarct volume. SUR1-TRPM4 channels, not KATP, mediate post-ischemic brain swelling.
Keywords: SUR1-TRPM4 channel, Sulfonylurea receptor 1 (SUR1), Transient receptor potential melastatin 4 (TRPM4), K(ATP) channel, cerebral ischemia, hemispheric swelling, cerebral edema
INTRODUCTION
Brain swelling due to cerebral edema is a widely recognized complication of cerebral ischemia. The harmful, often fatal effects of brain swelling are well known in patients with large hemispheric (malignant) infarction (LHI) [2, 5], but swelling also may complicate smaller strokes. Recent evidence indicates that swelling is an independent predictor of poor outcome in nonmalignant strokes, with a swelling volume of only 11 mL being the threshold with the greatest sensitivity and specificity for predicting poor outcome [4].
Glibenclamide was first shown to reduce cerebral edema (excess tissue water) in a rodent model of LHI [27]. Subsequent work with different models of LHI confirmed that glibenclamide reduces ischemic brain swelling and its associated high mortality [28–30]. In rodent models of both fatal (malignant) and non-fatal stroke, reducing edema by treatment with glibenclamide is accompanied by improved neurological scores [28–30, 38]. Data from two phase 2 clinical trials showed that intravenous glibenclamide reduces edema and brain swelling in patients with LHI [12, 13, 25, 26, 35].
Glibenclamide is a potent inhibitor of numerous ion channels that are regulated by sulfonylurea receptor (SUR), including four molecularly distinct KATP channels (SUR1-KIR6.2, SUR2A-KIR6.2, SUR2B-KIR6.2, SUR2B-KIR6.1) [24, 33, 34], as well as SUR1-TRPM4 channels [27, 40]. Here, we sought to determine which of these ion channels might be the target of glibenclamide in post-ischemic brain swelling. We employed a strategy of gene suppression of the various ion channel subunits using intravenous antisense oligodeoxynucleotides (AS-ODNs), which exhibit augmented efficacy at low pH [10], as found in ischemic brain [18]. We studied a permanent middle cerebral artery occlusion (pMCAo) model, in which swelling is robust but generally non-lethal [32, 37]. Here, we report that suppressing Abcc8/SUR1 and Trmp4/TRPM4, but not Kcnj8/KIR6.1 or Kcnj11/KIR6.2, was highly effective in reducing hemispheric swelling, consistent with involvement of SUR1-TRPM4, not KATP.
METHODS
Ethics statement.
We certify that all applicable institutional and governmental regulations concerning the ethical use of animals were followed during the course of this research. Animal experiments were performed under a protocol approved by the Institutional Animal Care and Use Committee of the University of Maryland School of Medicine, and in accordance with the relevant guidelines and regulations as stipulated in the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize the number of animals used and their suffering.
pMCAo.
Male Wistar rats, aged 11–12 weeks (300–350 gm) (Envigo, Indianapolis, IN) underwent pMCAo using intra-arterial occluders (0.39 mm; catalogue #4039PK5Re; Doccol Corp, Redlands CA) and laser Doppler flowmetry to assure >75% reduction in relative cerebral blood flow, as we described [30, 31].
Treatments.
In three series of experiments, pMCAo rats were randomly assigned to receive either AS-ODN targeting a channel subunit, or scrambled ODN (Scr-ODN) or sense ODN (SE-ODN) as control, including series 1: AS-ODN targeting Abcc8/SUR1 (12 rats, with 13 Scr-ODN controls); series 2: Trpm4/TRPM4 (13 rats, with 16 SE-ODN controls); series 3: Kcnj8/KIR6.1 (7 rats) or Kcnj11/KIR6.2 (7 rats), with 5 Scr-ODN controls. Rat anti-Abcc8/SUR1 (5′-GGCCGAGTGGTTCTCGGT-3′), anti-Trpm4/TRPM4 (5’-TTGTGGTAACACTCTCCAAA-3’) and anti-Kcnj11/KIR6.2 (5’-CCTTTCGGGACAGCATGGCT-3’) AS-ODNs were described previously [9]. Rat anti-Kcnj8/KIR6.1 AS-ODN (5’-TCTTCCTGGCCAGCATCTTC-3’), which targets the translation initiation codon [7], was newly designed for the present study. ODNs, which were phosphorothioated at 4 distal bonds at both the 5’ and 3’ ends to protect against endogenous nucleases [8], were obtained commercially (Sigma-Aldrich). Prior to surgery, miniosmotic pumps (Alzet 2001D, 8 μL/hour; Durect Corp.) fitted with jugular vein catheters were loaded with ODN and primed. ODN solutions were prepared in sterile normal saline (NS) (4 mg/mL). Immediately after pMCAo, the jugular vein was catheterized, a loading dose of ODN (400 μg in 100 μL) was administered intravenously (IV), and the pump catheter was inserted into the jugular vein with the pump implanted subcutaneously in the dorsal thorax, to obtain IV delivery of ODN at a rate of 32 μg / hour until euthanasia, yielding a total dose delivered of ~1.2 mg / 24 hours.
Neurofunction.
At 24 hours, neurological function was evaluated by investigators blinded to treatment group using an 8-point neuroscore system (8, stroke-related death; 7, no response to stimulation; 6, ambulate only when stimulated; 5, circle or ambulate spontaneously only to the left; 4, spontaneous movements in all directions with circling to the left exhibited only if pulled by the tail; 3, reduced resistance to lateral push toward the left side; 2, left shoulder adduction when suspended by the tail; 1, left forelimb flexion when suspended by the tail, or failure to extend the right forepaw fully; 0, no neurological deficit), as we described [29]. Here, for analysis, scores were dichotomized at 5/6 based on the presence or absence of spontaneous movements. This threshold has high interobserver reliability, and was found previously to distinguish between treatment groups in the same pMCAo model [38].
Infarct volume and hemispheric swelling.
After evaluation of neurological function, rats were euthanized. Six coronal sections, 2 mm thick, were used to determine infarct volumes using 2,3,5-triphenyltetrazolium chloride (TTC), corrected for swelling, as described [16], and to determine hemispheric swelling, as described [16].
Exclusions.
A total of 86 rats underwent pMCAo. Of these, 3 were excluded prior to randomization for LDF <75%; 3 rats died after randomization, prior to planned euthanasia at 24 hours; 7 rats were excluded for TTC-infarct volumes <40%. These exclusions left 73 rats as the basis for this report, with group assignments detailed above (see “Treatments”).
Immunohistochemistry, immuno-FRET and co-immunoprecipitation.
These procedures were performed using custom anti-SUR1 and anti-TRPM4 antibodies, as we described [9, 40].
Data analysis.
Data are presented as mean ± S.D., or as box plots (small box, mean; - -, median; large box, 25th and 75th percentile; ×, 1st and 99th percentile; -, max and min). Infarct volumes and hemispheric swelling were analyzed using ANOVA with post-hoc Bonferroni comparisons. The relationship between infarct volume and hemispheric swelling was analyzed using linear regression. Dichotomized neurobehavioral scores were analyzed using Fisher’s exact test (2-tailed). Statistical analyses were performed using GraphPad Prism version 8. P <0.05 was considered to be statistically significant.
RESULTS
Infarct volume
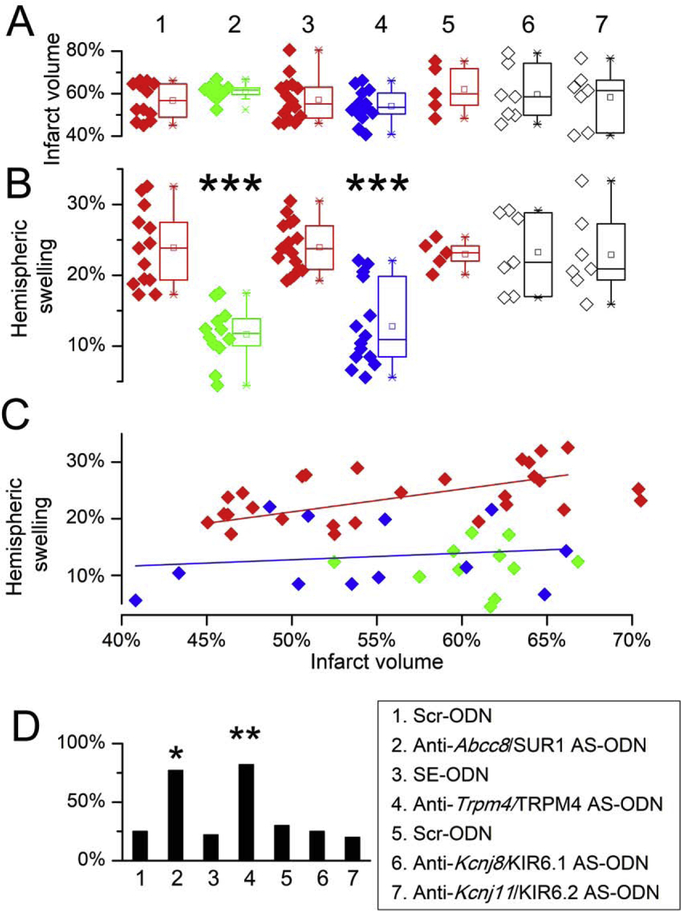
Rats underwent pMCAo and were randomly assigned to receive AS-ODNs directed against Abcc8/SUR1, Trpm4/TRPM4, Kcnj8/KIR6.1 or Kcnj11/KIR6.2, with sense or scrambled ODNs used as controls. In the combined control groups, TTC infarct volumes at 24 hours were 57.4±8.8% (n=34) of the hemisphere (Fig. 1) [38], and were not different between individual control groups (P=1) (Fig. 2A). In the four groups treated with AS-ODNs, infarct volumes were unchanged compared to controls (P=1) (Fig. 1, 2A).
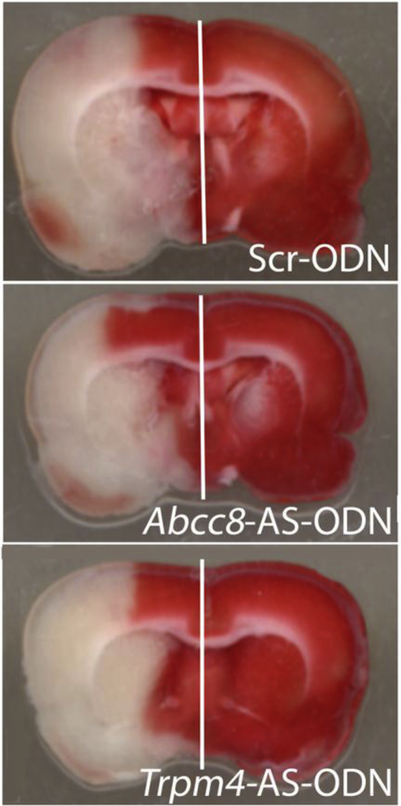
Figure 1. Coronal sections from the pMCAo model.
TTC-stained coronal sections show equivalently large infarcts, but reduced hemispheric swelling in rats administered AS-ODN targeting Abcc8/SUR1 and Trpm4/TRPM4, compared to control Scr-ODN.
Figure 2. In the pMCAo model, post-ischemic hemispheric swelling is reduced by AS-ODN targeting Abcc8/SUR1 and Trpm4/TRPM4.
A: Infarct volumes at 24 hours in groups 1–7. B: Hemispheric swelling at 24 hours in groups 1–7; ***, P <0. 0.0001. C: Plot of hemispheric swelling vs. infarct volume for pMCAo rats administered control Scr-ODN or SE-ODN (red diamonds) or anti-Abcc8/SUR1 AS-ODN (green diamonds) or anti-Trpm4/TRPM4 AS-ODN (blue diamonds); linear regression for CTR data indicated a slope that was significantly different from zero (P<0.02); linear regression for AS-ODN data indicated a slope that was shifted downward and was not significantly different from zero (P=0.35). D: Percent or rats in each group that exhibited spontaneous (non-coerced) movements at 24 hours after pMCAo in groups 1–7; *, P <0.05 for group 2 vs. 1; **, P <0.01 and for group 4 vs. 3.
Hemispheric swelling
In the combined control groups, hemispheric swelling at 24 hours was 23.9±4.1% (n=34) [38], and was not different between individual control groups (P=1) (Fig. 2B). Of the four groups treated with AS-ODNs, compared to controls, hemispheric swelling was significantly decreased by anti-Abcc8/SUR1 (P<0.0001) and anti-Trmp4/TRPM4 AS-ODN (P<0.0001) (Fig. 1, 2B), but not by anti-Kcnj8/KIR6.1 (P=1) or anti-Kcnj11/KIR6.2 (P=1) AS-ODN (Fig. 2B).
In the combined control groups, linear regression showed that hemispheric swelling was significantly related to infarct volume (P<0.02) (Fig. 2C). By contrast, in rats administered anti-Abcc8/SUR1 and anti-Trmp4/TRPM4 AS-ODN, the regression line was shifted downward, and the slope was not significantly different from zero.
Neuroscores, dichotomized according to the presence or absence of spontaneous movements, reflected hemispheric swelling. Significantly greater proportions of rats exhibited spontaneous movements that had been administered anti-Abcc8/SUR1 or anti-Trmp4/TRPM4 AS-ODN, compared to Scr-ODN, SE-ODN, anti-Kcnj8/KIR6.1 AS-ODN, or anti-Kcnj11/KIR6.2 AS-ODN (Fig. 2D).
SUR1-TRPM4 heterodimers
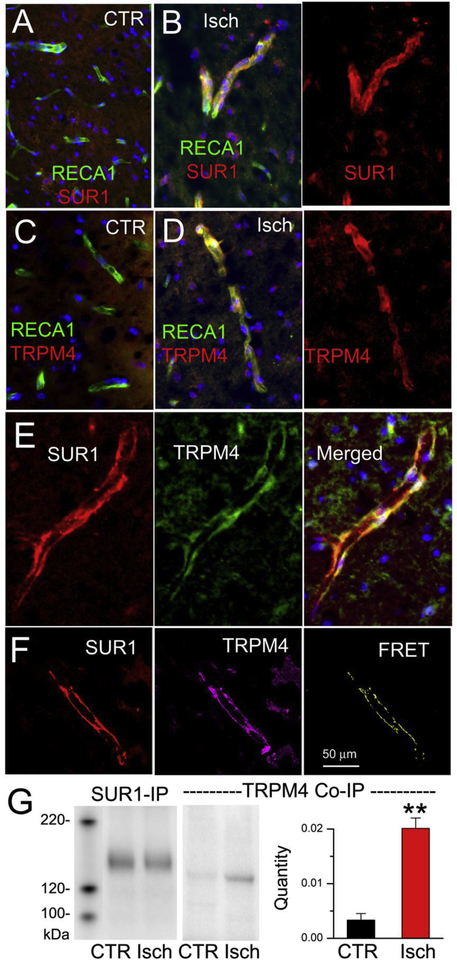
Analysis of post-ischemic brain tissues showed de novo upregulation of SUR1 and TRPM4, which colocalized in microvessels, and which co-assembled into heteromers, as indicated by immuno-FRET (Fig. 3A–F). Co-immunoprecipitation confirmed the co-assembly of SUR1-TRPM4 heteromers, with quantification showing a >10-fold increase post-ischemia (Fig. 3G).
Figure 3. SUR1-TRPM4 is upregulated in microvessels post-ischemia.
A–D: Double immunolabeling of contralateral control (CTR) (A,C) and ipsilateral post-ischemic (Isch) (B,D) tissues for the endothelial marker, RECA1 (green), and SUR1 (red) (A,B), or RECA1 and TRPM4 (red) (C,D); superimposed images are shown in A; B, left panel; C; D, left panel. E: Double immunolabeling of ipsilateral post-ischemic tissues for SUR1 (red) and TRPM4 (green); superimposed images are shown at right. F: Double immunolabeling of ipsilateral post-ischemic tissues for SUR1 (red) and TRPM4 (magenta); FRET signal shown at right. G: Contralateral control (CTR) and ipsilateral post-ischemic (Isch) tissues underwent immunoisolation using anti-SUR1 antibody; the resultant isolate was immunoblotted using anti-SUR1 antibody (SUR1-IP) or using anti-TRPM4 antibody (TRPM4 Co-IP); bar graph: quantity of SUR1-TRPM4 Co-IP normalized to HSC70; 3 replicates; **, P <0.01.
DISCUSSION
Glibenclamide is a potent inhibitor of four molecularly distinct KATP channels, SUR1-KIR6.2, SUR2A-KIR6.2, SUR2B-KIR6.2 and SUR2B-KIR6.1, some of which are expressed constitutively by various cell types in the brain [24, 33, 34]. Glibenclamide also is a potent inhibitor of SUR1-TRPM4 channels, which are not constitutively expressed, but are transcriptionally upregulated in all members of the neurovascular unit following cerebral ischemia [27, 39, 40]. In the case of these 5 ion channels, glibenclamide inhibits channel function by targeting the regulatory subunit, SUR. In addition, glibenclamide inhibits the NOD-like receptor pyrin domain containing 3 (NLRP3) inflammasome, an effect that is independent of KATP or SUR [43].
Historically, glibenclamide was considered to be a specific inhibitor of KATP, and so effects of glibenclamide post-ischemia, both favorable and potentially unfavorable, often were assumed to be due to blockade of KATP. Because KATP channels are reported to protect neurons from ischemia [33, 34], and to play an important role in ischemic preconditioning [3, 6], questions arose about potentially harmful effects of administering glibenclamide post-ischemia.
However, multiple reports have appeared demonstrating that post-ischemic administration of glibenclamide is beneficial. Glibenclamide inhibition of KATP, at least in theory, is said to reduce ATP consumption that would be required to replenish intracellular K+ extruded via KATP in post-ischemic neurons [19]. Glibenclamide favorably influences microglial function, enhances neurogenesis, and improves neurological outcome post-ischemia, with these effects attributed to KATP blockade [20–22]. Glibenclamide ameliorates ischemia-reperfusion injury via modulating oxidative stress and inflammation [1]. In non-lethal rat models of stroke, glibenclamide reduces infarct volume [31, 38], in lethal rat models, glibenclamide reduces brain swelling and death [27–30, 44], and in both non-lethal and lethal models, glibenclamide improves neurological outcome [28–30, 38, 42, 44]. Moreover, data from two phase 2 clinical trials show that intravenous glibenclamide reduces edema and brain swelling in patients with LHI [12, 13, 25, 26, 35].
In the last series of studies, the effects of glibenclamide were attributed not to KATP, but to SUR1-TRPM4. The initial work reporting involvement of SUR1-TRPM4 channels (at the time, named SUR1-regulated NCCa-ATP channels) in cerebral ischemia used patch clamp techniques to show that post-ischemic neurons express ion channels with the biophysical properties of SUR1-TRPM4, not KATP [27]. Support for involvement of SUR1-TRPM4 came from subsequent work on post-mortem tissues from humans with stroke, in which immuno-FRET was used to demonstrate co-assembly of SUR1-TRPM4 heteromers in all cell types of the neurovascular unit [17]. Support for involvement of TRPM4 came from work with a murine model of stroke, in which global gene deletion of Trpm4 yielded a 2-fold reduction in infarct volume and substantial recovery of motor function [15].
Here, we provide evidence that SUR1-TRPM4 is a critical target of glibenclamide in cerebral ischemia. The outcome measure that we studied – hemispheric swelling – was shown to be linked to SUR1-TRPM4, not KATP, based on experiments with molecularly specific AS-ODNs that targeted four channel subunits individually. Our experiments showed that hemispheric swelling was significantly reduced by AS-ODNs directed against Abcc8/SUR1 and Trpm4/TRPM4, but not Kcnj8/KIR6.1 or Kcnj11/KIR6.2, consistent with involvement of SUR1-TRPM4, not KATP, in post-ischemic brain swelling. Moreover, we showed here, as previously [17], that SUR1-TRPM4 heteromers co-assemble in post-ischemic tissues, consistent with the formation of functional SUR1-TRPM4 channels. The present results are arguably the strongest to date to distinguish between SUR1-TRPM4 and KATP, and to implicate SUR1-TRPM4 in post-ischemic cerebral edema.
The effect on hemispheric swelling of anti-Abcc8/SUR1 AS-ODN found here resembles closely the effect of glibenclamide in the same pMCAo model reported by an independent group, Wali et al. [38]. In both studies, control pMCAo rats showed 50–60% infarct volumes and 20–25% hemispheric swelling, and in both studies, swelling was reduced by approximately half, to 10–15% by treatments that targeted SUR1, either glibenclamide or anti-Abcc8/SUR1 AS-ODN. The major difference between the two studies was on infarct volume. Whereas the previous study by Wali et al. [38], as well as a report by us with the same model [31], showed significant reductions in infarct volumes with glibenclamide, here we found no effect of anti-Abcc8/SUR1 AS-ODN on infarct volume. Whether the lack of effect on infarct size with ODN reflects differential blood-brain barrier (BBB) penetration of ODN vs. drug, or some other difference, remains to be resolved.
It is generally accepted that infarct volume predicts hemispheric swelling [14, 31]. Most studies on treatments found to reduce post-ischemic edema are difficult to interpret regrading edema per se, since in most cases, the treatment also reduced infarct volume. By contrast, in the present study, the AS-ODNs that reduced hemispheric swelling had no effect on infarct volume, obviating this important confounder. In pMCAo rats administered control ODNs, we found a statistically significant positive correlation between infarct volume and hemispheric swelling, whereas in rats treated with AS-ODNs targeting Abcc8/SUR1 and Trpm4/TRPM4, this relationship was lost, with the regression line shifted downward and the slope no longer significantly different from zero. To our knowledge, this is the first demonstration of frank decoupling of brain swelling from infarct volume.
Apart from hemispheric swelling [16], which we studied here, measures of edema reported in preclinical studies include excess tissue water, extravasation of endogenous or exogenous blood-borne molecules, tissue density by computed tomography, and relative diffusivity of water by magnetic resonance imaging. Excess tissue water has been criticized as potentially misleading, since small changes in percent water content actually reflect much larger changes in brain swelling [11]. Midline shift, which is used frequently in human studies [23], is used only rarely in preclinical studies, although it correlates well with excess tissue water [36]. Our preference for hemispheric swelling is based on the simplicity and reliability of this measurement, and its ability to convey the clinically relevant concepts of space-occupying edema and mass effect that give rise to neurological dysfunction.
This study has limitations. Although AS-ODNs offer a high degree of molecular specificity, they have limited penetration into the brain, owing to the negligible transport of these highly polar molecules across the BBB [41]. AS-ODNs are devoid of cellular specificity, and thus our data cannot address which element of the microvascular BBB complex – endothelium, pericyte, or astrocyte endfoot – is involved in SUR1-mediated post-ischemic brain swelling. Our experiments focused on brain swelling and its neurofunctional consequence, but did not examine microglial function, neurogenesis, oxidative stress, or inflammation, which also are reported to benefit from glibenclamide treatment post-ischemia. Thus, our conclusion on involvement of SUR1-TRPM4 in brain swelling cannot, without further study, be extended to these other domains.
In summary, the data presented here show that post-ischemic hemispheric swelling can be decoupled from infarct volume, and that SUR1-TRPM4 channels, not KATP, mediate post-ischemic brain swelling.
Highlights.
Glibenclamide reduces space occupying edema and brain swelling following cerebral ischemia.
Glibenclamide inhibits a variety of SUR-regulated channels, including four distinct KATP channels and SUR1-TRPM4 channels.
We used antisense oligodeoxynucleotides to investigate the role of KATP vs. SUR1-TRPM4 in post-ischemic brain swelling.
We found that SUR1-TRPM4 channels, not KATP, mediate post-ischemic brain swelling.
ACKNOWLEDGEMENTS
J.M.S is supported by grants from the Department of Veterans Affairs (I01BX002889), the Department of Defense (SCI170199), the National Heart, Lung and Blood Institute (R01HL082517) and the National Institute of Neurological Disorders and Stroke (NINDS) (R01NS060801; R01NS102589; R01NS105633); V.G. is supported by grants from NINDS (R01NS061934; R01NS107262).
Abbreviations:
- AS-ODN
antisense oligodeoxynucleotide
- ATP
adenosine triphosphate
- BBB
blood-brain barrier
- FRET
Förster resonance energy transfer
- IV
intravenous
- LHI
large hemispheric infarction
- ODN
oligodeoxynucleotide
- NLRP3
NOD-like receptor pyrin domain containing 3
- NS
normal saline
- pMCAo
permanent middle cerebral artery occlusion
- Scr-ODN
scrambled oligodeoxynucleotide
- SE-ODN
sense oligodeoxynucleotide
- SUR
sulfonylurea receptor
- TRP
transient receptor potential
- TTC
2,3,5-triphenyltetrazolium chloride
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR DISCLOSURE STATEMENT
J.M.S. holds a US patent (7,285,574), “A novel non-selective cation channel in neural cells and methods for treating brain swelling.” J.M.S. is a member of the Board of Directors and holds shares in Remedy Pharmaceuticals, and is a paid consultant for Biogen. No support, direct or indirect, was provided to J.M.S., or for this project, by Remedy Pharmaceuticals or by Biogen. All other authors declare no competing financial interests.
References
- [1].Abdallah DM, Nassar NN, Abd-El-Salam RM, Glibenclamide ameliorates ischemia-reperfusion injury via modulating oxidative stress and inflammatory mediators in the rat hippocampus, Brain Res 1385 (2011) 257–262. [DOI] [PubMed] [Google Scholar]
- [2].Arch AE, Sheth KN, Malignant cerebral edema after large anterior circulation infarction: a review, Curr. Treat. Options. Cardiovasc. Med 16 (2014) 275. [DOI] [PubMed] [Google Scholar]
- [3].Bajgar R, Seetharaman S, Kowaltowski AJ, Garlid KD, Paucek P, Identification and properties of a novel intracellular (mitochondrial) ATP-sensitive potassium channel in brain, J Biol Chem 276 (2001) 33369–33374. [DOI] [PubMed] [Google Scholar]
- [4].Battey TW, Karki M, Singhal AB, Wu O, Sadaghiani S, Campbell BC, Davis SM, Donnan GA, Sheth KN, Kimberly WT, Brain edema predicts outcome after nonlacunar ischemic stroke, Stroke 45 (2014) 3643–3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bevers MB, Kimberly WT, Critical Care Management of Acute Ischemic Stroke, Curr Treat Options Cardiovasc Med 19 (2017) 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Busija DW, Katakam PV, Mitochondrial mechanisms in cerebral vascular control: shared signaling pathways with preconditioning, J Vasc Res 51 (2014) 175–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Dias N, Stein CA, Antisense oligonucleotides: basic concepts and mechanisms, Mol Cancer Ther 1 (2002) 347–355. [PubMed] [Google Scholar]
- [8].Galderisi U, Cascino A, Giordano A, Antisense oligonucleotides as therapeutic agents, Journal of cellular physiology 181 (1999) 251–257. [DOI] [PubMed] [Google Scholar]
- [9].Gerzanich V, Stokum JA, Ivanova S, Woo SK, Tsymbalyuk O, Sharma A, Akkentli F, Imran Z, Aarabi B, Sahuquillo J, Simard JM, Sulfonylurea Receptor 1, Transient Receptor Potential Cation Channel Subfamily M Member 4, and KIR6.2: Role in Hemorrhagic Progression of Contusion, J Neurotrauma 36 (2019) 1060–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Goodarzi G, Watabe M, Watabe K, Binding of oligonucleotides to cell membranes at acidic pH, Biochem. Biophys. Res. Commun 181 (1991) 1343–1351. [DOI] [PubMed] [Google Scholar]
- [11].Keep RF, Hua Y, Xi G, Brain water content. A misunderstood measurement?, Translational stroke research 3 (2012) 263–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kimberly WT, Battey TW, Pham L, Wu O, Yoo AJ, Furie KL, Singhal AB, Elm JJ, Stern BJ, Sheth KN, Glyburide is associated with attenuated vasogenic edema in stroke patients, Neurocrit. Care 20 (2014) 193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kimberly WT, Bevers MB, von Kummer R, Demchuk AM, Romero JM, Elm JJ, Hinson HE, Molyneaux BJ, Simard JM, Sheth KN, Effect of IV glyburide on adjudicated edema endpoints in the GAMES-RP Trial, Neurology 91 (2018) e2163–e2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kondo T, Reaume AG, Huang TT, Carlson E, Murakami K, Chen SF, Hoffman EK, Scott RW, Epstein CJ, Chan PH, Reduction of CuZn-superoxide dismutase activity exacerbates neuronal cell injury and edema formation after transient focal cerebral ischemia, J Neurosci 17 (1997) 4180–4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Loh KP, Ng G, Yu CY, Fhu CK, Yu D, Vennekens R, Nilius B, Soong TW, Liao P, TRPM4 inhibition promotes angiogenesis after ischemic stroke, Pflugers Arch 466 (2014) 563–576. [DOI] [PubMed] [Google Scholar]
- [16].McBride DW, Klebe D, Tang J, Zhang JH, Correcting for Brain Swelling’s Effects on Infarct Volume Calculation After Middle Cerebral Artery Occlusion in Rats, Translational stroke research 6 (2015) 323–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mehta RI, Tosun C, Ivanova S, Tsymbalyuk N, Famakin BM, Kwon MS, Castellani RJ, Gerzanich V, Simard JM, Sur1-Trpm4 Cation Channel Expression in Human Cerebral Infarcts, J Neuropathol Exp Neurol 74 (2015) 835–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Nedergaard M, Kraig RP, Tanabe J, Pulsinelli WA, Dynamics of interstitial and intracellular pH in evolving brain infarct, Am J Physiol 260 (1991) R581–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Nistico R, Piccirilli S, Sebastianelli L, Nistico G, Bernardi G, Mercuri NB, The blockade of K(+)-ATP channels has neuroprotective effects in an in vitro model of brain ischemia, Int. Rev. Neurobiol 82 (2007) 383–395. [DOI] [PubMed] [Google Scholar]
- [20].Ortega FJ, Gimeno-Bayon J, Espinosa-Parrilla JF, Carrasco JL, Batlle M, Pugliese M, Mahy N, Rodriguez MJ, ATP-dependent potassium channel blockade strengthens microglial neuroprotection after hypoxia-ischemia in rats, Exp Neurol 235 (2012) 282–296. [DOI] [PubMed] [Google Scholar]
- [21].Ortega FJ, Jolkkonen J, Mahy N, Rodriguez MJ, Glibenclamide enhances neurogenesis and improves long-term functional recovery after transient focal cerebral ischemia, J. Cereb. Blood Flow Metab 33 (2013) 356–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ortega FJ, Jolkkonen J, Rodriguez MJ, Microglia is an active player in how glibenclamide improves stroke outcome, Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 33 (2013) 1138–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ostwaldt AC, Battey TWK, Irvine HJ, Campbell BCV, Davis SM, Donnan GA, Kimberly WT, Comparative Analysis of Markers of Mass Effect after Ischemic Stroke, J Neuroimaging 28 (2018) 530–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Seino S, Physiology and pathophysiology of K(ATP) channels in the pancreas and cardiovascular system: a review, J Diabetes Complications 17 (2003) 2–5. [DOI] [PubMed] [Google Scholar]
- [25].Sheth KN, Elm JJ, Molyneaux BJ, Hinson H, Beslow LA, Sze GK, Ostwaldt AC, Del Zoppo GJ, Simard JM, Jacobson S, Kimberly WT, Safety and efficacy of intravenous glyburide on brain swelling after large hemispheric infarction (GAMES-RP): a randomised, double-blind, placebo-controlled phase 2 trial, Lancet Neurol 15 (2016) 1160–1169. [DOI] [PubMed] [Google Scholar]
- [26].Sheth KN, Petersen NH, Cheung K, Elm JJ, Hinson HE, Molyneaux BJ, Beslow LA, Sze GK, Simard JM, Kimberly WT, Long-Term Outcomes in Patients Aged </=70 Years With Intravenous Glyburide From the Phase II GAMES-RP Study of Large Hemispheric Infarction: An Exploratory Analysis, Stroke 49 (2018) 1457–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Simard JM, Chen M, Tarasov KV, Bhatta S, Ivanova S, Melnitchenko L, Tsymbalyuk N, West GA, Gerzanich V, Newly expressed SUR1-regulated NC(Ca-ATP) channel mediates cerebral edema after ischemic stroke, Nat. Med 12 (2006) 433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Simard JM, Geng Z, Silver FL, Sheth KN, Kimberly WT, Stern BJ, Colucci M, Gerzanich V, Does inhibiting Sur1 complement rt-PA in cerebral ischemia?, Ann. N. Y. Acad. Sci 1268 (2012) 95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Simard JM, Tsymbalyuk N, Tsymbalyuk O, Ivanova S, Yurovsky V, Gerzanich V, Glibenclamide is superior to decompressive craniectomy in a rat model of malignant stroke, Stroke 41 (2010) 531–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Simard JM, Woo SK, Tsymbalyuk N, Voloshyn O, Yurovsky V, Ivanova S, Lee R, Gerzanich V, Glibenclamide-10-h Treatment Window in a Clinically Relevant Model of Stroke, Transl. Stroke Res 3 (2012) 286–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Simard JM, Yurovsky V, Tsymbalyuk N, Melnichenko L, Ivanova S, Gerzanich V, Protective effect of delayed treatment with low-dose glibenclamide in three models of ischemic stroke, Stroke 40 (2009) 604–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Slivka A, Murphy E, Horrocks L, Cerebral edema after temporary and permanent middle cerebral artery occlusion in the rat, Stroke 26 (1995) 1061–1065; discussion 1065–1066. [DOI] [PubMed] [Google Scholar]
- [33].Szeto V, Chen NH, Sun HS, Feng ZP, The role of KATP channels in cerebral ischemic stroke and diabetes, Acta Pharmacol Sin 39 (2018) 683–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Tinker A, Aziz Q, Li Y, Specterman M, ATP-Sensitive Potassium Channels and Their Physiological and Pathophysiological Roles, Compr Physiol 8 (2018) 1463–1511. [DOI] [PubMed] [Google Scholar]
- [35].Vorasayan P, Bevers MB, Beslow LA, Sze GK, Molyneaux BJ, Hinson HE, Simard JM, Von Kummer R, Sheth KN, Kimberly WT, Intravenous glibenclamide reduces lesional water uptake in large hemispheric infarction, Stroke in press (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Walberer M, Blaes F, Stolz E, Muller C, Schoenburg M, Tschernatsch M, Bachmann G, Gerriets T, Midline-shift corresponds to the amount of brain edema early after hemispheric stroke--an MRI study in rats, J Neurosurg Anesthesiol 19 (2007) 105–110. [DOI] [PubMed] [Google Scholar]
- [37].Walberer M, Stolz E, Muller C, Friedrich C, Rottger C, Blaes F, Kaps M, Fisher M, Bachmann G, Gerriets T, Experimental stroke: ischaemic lesion volume and oedema formation differ among rat strains (a comparison between Wistar and Sprague-Dawley rats using MRI), Lab Anim 40 (2006) 1–8. [DOI] [PubMed] [Google Scholar]
- [38].Wali B, Ishrat T, Atif F, Hua F, Stein DG, Sayeed I, Glibenclamide Administration Attenuates Infarct Volume, Hemispheric Swelling, and Functional Impairments following Permanent Focal Cerebral Ischemia in Rats, Stroke Res. Treat 2012 (2012) 460909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Woo SK, Kwon MS, Geng Z, Chen Z, Ivanov A, Bhatta S, Gerzanich V, Simard JM, Sequential activation of hypoxia-inducible factor 1 and specificity protein 1 is required for hypoxia-induced transcriptional stimulation of Abcc8, J. Cereb. Blood Flow Metab 32 (2012) 525–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Woo SK, Kwon MS, Ivanov A, Gerzanich V, Simard JM, The sulfonylurea receptor 1 (Sur1)-transient receptor potential melastatin 4 (Trpm4) channel, J. Biol. Chem 288 (2013) 3655–3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Wu D, Boado RJ, Pardridge WM, Pharmacokinetics and blood-brain barrier transport of [3H]-biotinylated phosphorothioate oligodeoxynucleotide conjugated to a vectormediated drug delivery system, J Pharmacol Exp Ther 276 (1996) 206–211. [PubMed] [Google Scholar]
- [42].Wu Z, Zhu SZ, Hu YF, Gu Y, Wang SN, Lin ZZ, Xie ZS, Pan SY, Glibenclamide enhances the effects of delayed hypothermia after experimental stroke in rats, Brain Res 1643 (2016) 113–122. [DOI] [PubMed] [Google Scholar]
- [43].Zhang G, Lin X, Zhang S, Xiu H, Pan C, Cui W, A Protective Role of Glibenclamide in Inflammation-Associated Injury, Mediators Inflamm 2017 (2017) 3578702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Zhu S, Gao X, Huang K, Gu Y, Hu Y, Wu Y, Ji Z, Wang Q, Pan S, Glibenclamide Enhances the Therapeutic Benefits of Early Hypothermia after Severe Stroke in Rats, Aging Dis 9 (2018) 685–695. [DOI] [PMC free article] [PubMed] [Google Scholar]