Abstract
Aim of the Study:
During type 2 diabetes (T2D) and hypertension there is stimulation of renal proximal tubule angiotensinogen (AGT), but whether urinary excretion of AGT (uAGT) is an indicator of glomerular damage or intrarenal RAS activation is unclear. We tested the hypothesis that elevations in uAGT can be detected in the absence of albuminuria in a mouse model of T2D.
Methods:
Male C57BL/6 mice (N=10) were fed a high fat (HFD; 45% Kcal from fat) for 28 weeks, and the metabolic phenotype including body weight, blood pressures, glucose, insulin, ippGTT, HOMA-IR, and cholesterol was examined. In addition, kidney Ang II content and reactive oxygen species (ROS) was measured along with urinary albumin, creatinine, Ang II, and AGT.
Results:
All parameters consistent with T2D were present in mice after 12–14 weeks on the HFD. Systolic BP increased after 18 weeks in HFD but not NFD mice. Intrarenal ROS and Ang II concentrations were also increased in HFD mice. Remarkably, these changes paralleled the augmentation uAGT excretion (3.66 ± 0.50 vs. 0.92 ± 0.13 ng/mg by week 29; P<0.01), which occurred in the absence of overt albuminuria.
Conclusions:
In HFD-induced T2D mice, increases in uAGT occur in the absence of overt renal injury, indicating that this biomarker accurately detects early intrarenal RAS activation.
Keywords: Intrarenal Renin-Angiotensin System, Albuminuria, Kidney, Hyperglycemia, Diabetes Mellitus, Blood pressure
1. INTRODUCTION
The systemic renin-angiotensin system (RAS) is a key regulator of blood pressure (BP) and fluid and electrolyte homeostasis. The intrarenal RAS also plays a critical role in the regulation of kidney function, but when stimulated inappropriately promotes hypertension and renal disease. During angiotensin (Ang) II-dependent hypertension, higher levels of renal versus circulating Ang II indicate activation of the intrarenal RAS1. In contrast, RAS blockade reduces intrarenal RAS activation2, 3 in hypertensive as well as diabetic humans and rodents4, 5. Angiotensinogen (AGT), the key renin substrate in the formation of Ang II, is primarily formed and secreted by the liver but is also synthesized and secreted by the proximal tubule6, 7. Hypertension8, 9 and type 2 diabetes10, 11 increase intrarenal AGT transcription and protein in both humans and rodents. Indeed, urinary AGT levels (uAGT) are used as biomarker of intrarenal RAS activation and positively correlates with renal Ang II in both, humans8, 12 and experimental animal models13. However, in subjects with renal damage14, uAGT highly correlates with albuminuria15, suggesting that it may be an indicator of glomerular damage rather than intrarenal RAS activation16. Nevertheless, the lack of evidence of temporal changes of uAGT during the development of type-2 diabetes (T2D) has contributed to the uncertainness about the relationship between intrarenal RAS activation and the development of renal disease during T2D.
This study aimed to discriminate whether increases in uAGT could be dissociated from albuminuria and to understand the ability of uAGT to predict early intrarenal RAS activation in a mouse model of high fat diet (HFD)-induced T2D with slow development of renal disease. We tested the hypothesis that intrarenal RAS activation as reflected by increased uAGT excretion could be detected in the absence of the development of albuminuria in mice with HFD-induced T2D.
2. MATERIALS AND METHODS
2.1. Development of a high fat diet induced-type 2 diabetes (HFD-T2D) mouse model
All animal procedures were approved by the Tulane University Animal Care and Use Committee. Male C57BL/6 mice 3 weeks-old, were weaned and kept under standard conditions (12-h light/dark cycles and 21 ± 2°C). All the mice were 4 ± 1–2 weeks of age (N=20) where the study started. Samples of blood and urine were collected to measure baseline parameters (week 0). Due to the young age of mice to withstand the stress while in metabolic caging, we placed each mouse on hydrophobic sand (LabSand, Coastline Global Inc, Palo Alto, CA) using a single cage to collect urine samples according to the manufacturer’s instructions. At 5 weeks of age, mice (N=20) were randomly divided into 2 groups, based on dietary regimen: 1) Normal fat diet (NFD; N=10) and 2) High fat diet (HFD; N=10). The NFD (PicoLab® Rodent Diet 20 EXT IRR 5R53, St. Louis, MO) consisted of 25% Kcal from protein, 13% from fat, and 62% from carbohydrate. The HFD (TestDiet, DIO 58V8–45, St. Louis, MO) contained 18% Kcal from protein, 45% from fat, and 36% from carbohydrate. Two separate sets of mice were used: one for metabolic purposes (N=6 mice per group) and other for blood pressure recordings using radiotelemetry (N=4 mice per group). Food and water were ad libitum during the following 28 weeks, until the study was completed.
2.2. General Experimental Protocols
After 12 hours of fasting, blood samples were collected by submandibular plexus bleeding in EDTA (5mmol/L) chilled tubes. Plasma was obtained by centrifugation at 3500 × g at 4°C for 15 min. Urine samples were collected from mice individually housed in metabolic cages and centrifuged at 3500 × g, 4°C for 30 min to remove particulate. Urine and blood samples were collected every four weeks. At the study endpoint, mice were euthanized under deep isoflurane inhalation anesthesia and tissues were harvested, weighed, snap frozen in liquid nitrogen and stored at −80°C.
2.3. Assessment of the HFD-induced type 2 diabetes mouse model:
2.3.1. Body weight and metabolic measurements:
Body weights (BW) from mice fed either a NFD or HFD were measured every 4 weeks with accuracy within 0.1 g. To obtain the daily energy intake per mouse, food intake was multiplied by the number of Kcal provided per gram of food (NFD: 4.07 Kcal/g of food and HFD 4.60 Kcal/g of food)17. Blood glucose levels were monitored monthly, from a drop of blood obtained by milking tail and measured with a hand-held glucometer (ONETOUCH Ultra glucometer, LifeScan, catalog #ZJZ8158JT, Milpitas, CA). Intraperitoneal glucose tolerance tests (ipGTT) were performed monthly. Blood glucose concentrations were measured before (0 min) and after (15, 30, 60 and 120 min) a glucose overload (2 g/kg BW, i.p.)18. Plasma cholesterol and triglyceride concentrations were measured using a Wako Kit (Wako Diagnostics, catalog #439–17501 and 464–016001, respectively, Richmond, VA). Plasma insulin concentrations were quantified by ELISA (Rat/Mouse Insulin assay Kit, EMD Millipore, catalog #EZRMI-13K, Billerica, MA). Insulin resistance (IR) was calculated by the homeostasis model assessment index as HOMA-IR (Insulin X Glucose/22.5).
2.3.2. Diastolic, Systolic and Mean Arterial Pressures:
Mice were anesthetized by isoflurane inhalation and a left carotid catheter connected to a radiotelemetry transmitter was implanted (TA11-PAC10, Data Sciences Inc., Palo Alto, CA). Pressures were recorded in conscious mice after 2 weeks of recovery for one 24 h period each week for four weeks using Ponemah 6.0 software.
2.4. Angiotensinogen excretion in urine
Angiotensinogen concentration in urine (uAGT) was measured by ELISA (Mouse Total Angiotensinogen Assay Kit, IBL, catalog #27413, Japan). This assay was developed and optimized previously in our department19. Urinary excretion rates of uAGT were calculated from the 12 h volumes collected.
2.5. Albumin and creatinine in urine
Mouse albumin in urine was measured by ELISA (Abcam, catalog #108792, Billerica, MA)19. Creatinine concentrations, measured by colorimetric assay (QuantiChromTM Creatinine Assay Kit, BioAssay Systems, Hayward, CA), were used to calculate the urinary albumin/creatinine ratio (UACR) in order to correct for variations in urine concentration due to the degree of hydration.
2.6. Angiotensin II in kidney and urine
At the end of the study, urine samples were collected into tubes containing an inhibitor cocktail (5 mmol/L of EDTA, 20 μmol/L of pepstatin A, 10 μmol/L of PMSF, 20 μmol/L of enalaprilat, and 1.25 mmol/L of 1,10-phenanthroline). Left kidneys were decapsulated and immersed in cold 100% methanol. After homogenization, centrifugation and evaporation the samples were extracted and evaporated as described previously20. Angiotensin II concentrations were measured by EIA (Angiotensin II EIA Kit, Cayman, catalog #A05880, Montigny-le-Bretonneux, France) and expressed per gram of wet weight. Urinary excretion rates were calculated from the 12 h volumes collected.
2.7. Fibrotic factors protein levels in the kidney
Protein expression of several fibrotic factors including ALPHA-smooth muscle actin (α-SMA), fibronectin, collagen I, and transforming growth factor-β (TGF-β) were measured by Western blot of whole kidney lysates and normalized to β-actin, as previously reported21.
2.8. Reactive oxygen species (ROS) in the kidney
Superoxide production was quantitatively measured by electron spin resonance (ESR) spectroscopy using thee modified method previously reported22. The superoxide-specific spin probe 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethyl-pyrrolidine (CMH) was used to identify and quantitate superoxide levels (Noxygen GmbH, Elzach, Germany). Diethyldithiocarbamate (DETC; 2.5 μmol/l) and desferoxamine (DF; 25 μmol/l) were dissolved under nitrogen gas bubbling in ice-cold modified Krebs-Hepes (KH) buffer containing (in mmol/l) 99.01 NaCl, 4.69 KCl, 1.87 CaCl2, 1.20 MgSO4, 25 NaHCO3, 1.03 K2HPO4, 20 Na-HEPES, and 11.1 D-glucose, at pH 7.35. Kidney tissue was cut into 3–5 mm pieces and placed into wells of A 96-well plate containing KH buffer (200 μl/well) with freshly made CMH (200 μmol/l), DETC, and DF for 60 min at 37°C. First, 300 μl KH buffer was transferred to 1 ml syringe, snap frozen in liquid nitrogen. Second, 200 μl of KH Buffer containing the tissue sample was transferred to the same syringe before snap freezing again. The two-layered samples were transferred to a finger dewar filled with liquid nitrogen and inserted into the EMX ESR eScan BenchTop spectrometer (Bruker, Germany). ESR spectra of samples were acquired using the following ESR settings: field sweep, 80 G; microwave frequency, 9.39 GHz; microwave power, 2 mW; modulation amplitude, 5 G; conversion time, 327.68 ms; time constant, 5242.88 ms; 512 points resolution and receiver gain, 1×103. The amplitude measurements of the ESR spectra in arbitrary units were normalized to the dry weight of the corresponding tissue samples.
2.9. Statistical analyses
Results are expressed as mean ± SEM. All data were analyzed using GraphPad Prism software, and statistical significance was defined as P<0.05. Two-way repeated measures ANOVA were used to compare time course data between experimental groups, with Sidak’s multiple comparisons test to evaluate the effect of diet at each time point. Comparisons between groups obtained at the end of the study were analyzed by T-test. Statistical outliers were identified using the Grubbs’ test with α=0.05.
3. RESULTS
3.1. Phenotype characterization of the HFD-induced type 2 diabetes mouse model
3.1.1. The development of obesity and T2D in mice fed a HFD:
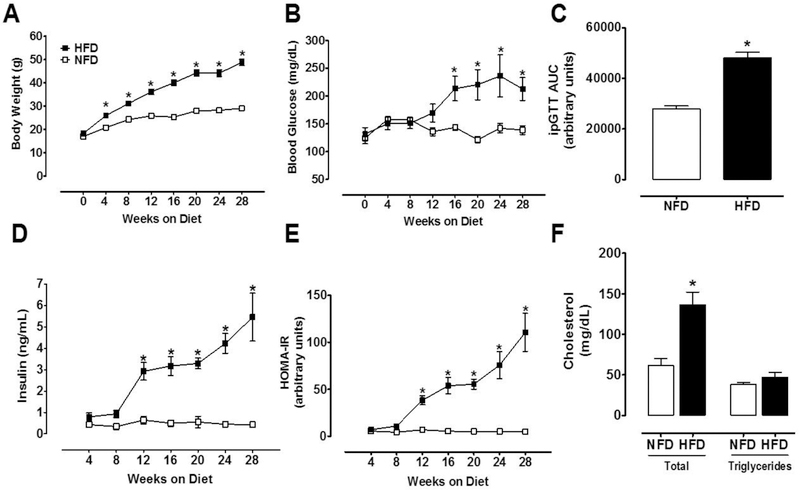
Mice under the HFD regimen ingested 20% less food daily (3.28 ± 0.08 vs. 4.17 ± 0.14; P<0.001) than NFD mice, but daily energy intake was equal due to caloric differences (15.56 ± 0.40 vs. 16.60 ± 0.58 Kcal/day per mouse, P=0.1483). Body weights were similar at baseline but became significantly higher in HFD mice starting at 4 weeks (Fig.1A). Both experimental groups continued to gain BW during the course of the study, and after 28 weeks HFD mice showed clear signs of obesity (49.1 ± 1.1 vs. 29.1 ± 0.7 g; P<0.001). Fasting blood glucose increased significantly by 16 weeks on HFD and remained elevated until the end of the experiment (Fig.1B). At 28 weeks, the area under curve for ipGTT was higher in HFD than NFD mice (P<0.001; Fig.1C). HFD mice developed hyperinsulinemia after 8 weeks on diet (Fig.1D) and IR after 12 weeks as evidenced by a considerable increase in HOMA-IR (Fig.1E). After 28 weeks on HFD, total cholesterol (136.5 ± 15 vs. 62 ± 8.2 mg/dL; P=0.003) but not triglycerides (47.3 ± 5.8 vs. 38.2 ± 2.6 mg/dL; P=0.24) were augmented (Fig. 1F).
Figure 1. Metabolic phenotype of mice fed a HFD.
After 28 weeks, HFD-fed mice showed marked signs of obesity and T2D as evidenced by (A) Body weight (2-way ANOVA, diet effect: P<0.001), (B) Blood glucose (2-way ANOVA, diet effect: P<0.05), (C) ipGTT (t-test, P<0.001), (D) Plasma insulin (2-way ANOVA, diet effect: P<0.001), (E) HOMA-IR (2-way ANOVA, diet effect: P<0.001), and (F) Plasma total cholesterol (t test P<0.003). NFD, normal fat diet (withe); HFD, high fat diet (black); Data are mean ± SEM.
3.1.2. Systolic, diastolic, and mean arterial pressures were elevated in HFD mice:
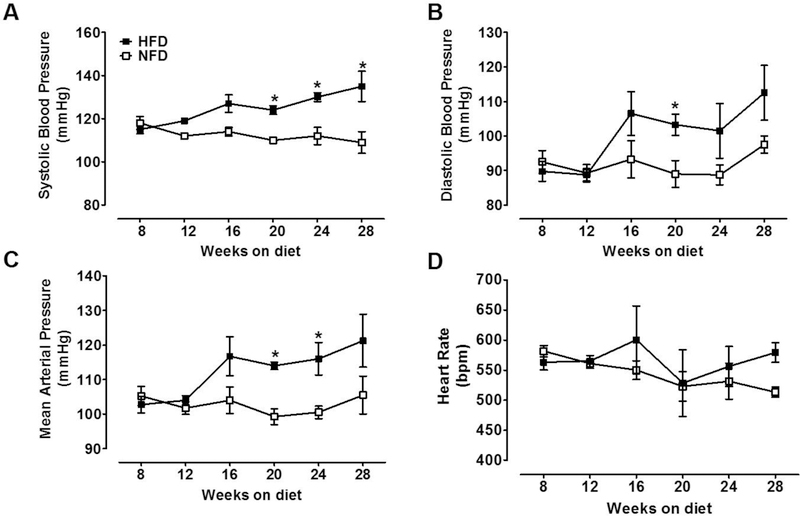
Systolic and diastolic BP and mean arterial pressure (MAP) values were similar until 18 weeks on the dietary regimen. Systolic BP started to increase in HFD mice but not in NFD mice (Fig. 2A). At 28 weeks, systolic BP in HFD mice reached the highest value compared with baseline (135 ± 7 vs. 115 ± 2 mmHg; P<0.001) and compared with age matched NFD mice (109 ± 1 mmHg; P<0.001). Similar effects were observed for diastolic BP (103.2 ± 3.0 vs. 89.4 ± 3.8 mm Hg; P=0.02; Fig. 2B) and MAP (114 ± 1 vs. 99.2 ± 2.2; P=0.001) at 20 weeks on diet. However, at 24 weeks on diet only MAP continued to increase (116 ± 4.6 vs. 100.5 ± 1.8; P=0.02; Fig. 2C). Heart rate (Fig. 2D) did not change whatsoever during the dietary regimen in any of the mouse groups.
Figure 2. Systolic BP in HFD-induced T2D mice and NFD mice.
Radiotelemetry measurements are representative of 24 h recordings. (A) Systolic BP was higher than in age-matched NFD mice after 20 weeks on the diet (2-way ANOVA, diet effect: P<0.001). (B) Diastolic BP was higher among the groups at 20 weeks (2-way ANOVA, diet effect; P<0.04). Mean arterial pressure (MAP) was elevated at 20 and 24 weeks (2-way ANOVA, diet effect; P<0.001). (D) Heart rate did not change between experimental groups. NFD, normal fat diet (withe); HFD, high fat diet (black); Data are mean ± SEM.
3.1.3. Albuminuria, Alb/Creat ratio in urine (UACR), and fibrotic markers were not increased in HFD mice:
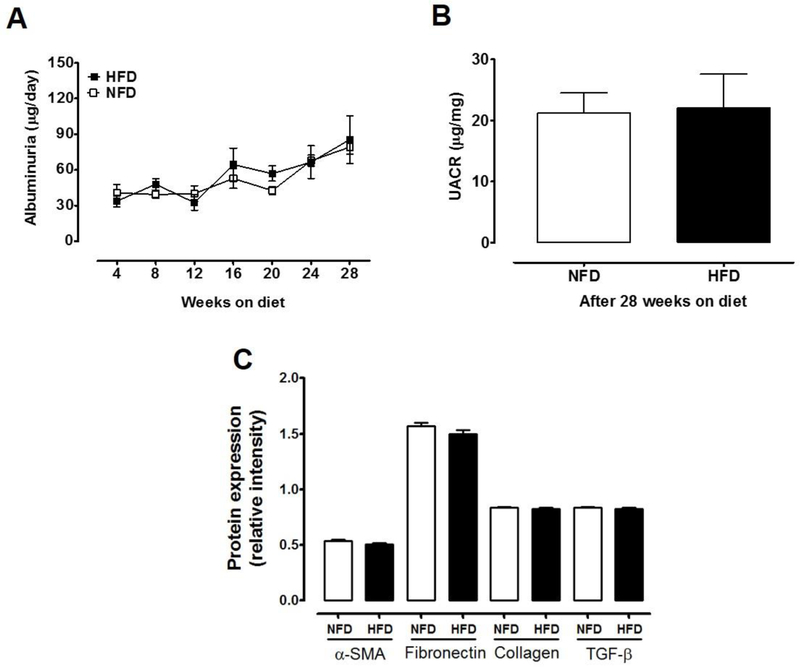
In NFD mice, albuminuria remained constant during the study (Fig.3A). Likewise, HFD did not alter the concentration of albumin in urine (85.5 ± 20 vs. 79 ± 6.1 µg/dL; P=0.43) neither UACR (21.2 ± 3.3 vs. 22.1 ± 5.5 µg/mg; P=0.90, Fig.3B) after 28 weeks on diet. The renal expression protein levels of α-SMA, fibronectin, collagen I and TGF-β, did not differ between HFD and NFD (Fig.3C), suggesting that in this model of HFD-T2D, even at 28 weeks under the dietary regimen, kidney injury was not yet apparent.
Figure 3. Albuminuria and UACR in HFD-induced T2D mice and NFD mice.
(A) Albuminuria was not impacted by diet (2-way ANOVA, diet effect: P=0.43) but similarly increased in both groups over time (time effect: P< 0.0001; Sidak’s test of week 28 vs. week 4: P<0.05). (B) UACR was not different after 28 weeks on diet (t-test, P=0.90). (C) Expression levels of TGF-β, fibronectin, collagen I, and α-SMA measured by Western blot in whole kidney samples were not different between experimental groups (t-test, P>0.05). NFD, normal fat diet (withe); HFD, high fat diet (black); Data are mean ± SEM.
3.2. Urinary excretion of angiotensinogen (uAGT) was augmented in HFD-T2D mice:
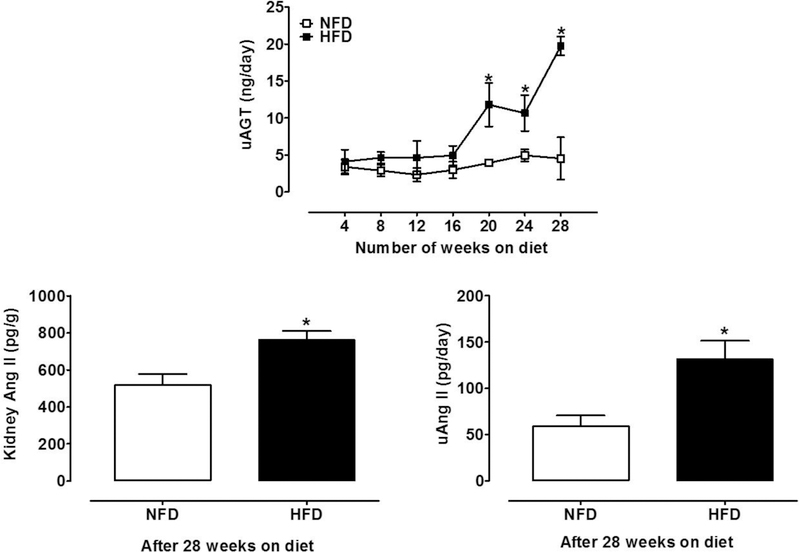
Starting at 20 weeks, uAGT was significantly higher in HFD mice compared to NFD mice. High fat-induced T2D mice exhibited 4-fold higher excretion of uAGT than controls by 28 weeks (19.7 ± 1.4 vs. 4.9 ± 0.8 ng/day; P<0.001; Fig.4A).
Figure 4. Urinary AGT, and kidney and urine Ang II levels in the HFD-induced T2D mouse model.
After 28 weeks on the diet, HFD mice showed a significant elevation in (A) urinary excretion rate of uAGT (2-way ANOVA, diet effect: P<0.001; time effect: P<0.001; Sidak’s test of week 28 vs. week P<0.001). In addition, HFD increased (B) kidney Ang II levels (t-test, P=0.01) and (C) urine Ang II (t-test, P=0.02). NFD, normal fat diet (withe); HFD, high fat diet (black); Data are mean ± SEM.
3.3. Angiotensin II increased in kidney and urine from HFD-T2D mice:
Ang II levels were elevated in the kidney (518 ± 60 vs. 762 ± 48 pg/g; P=0.01; Fig.4B) and urine (59 ±12 vs. 131 ± 20 pg/day; P=0.02; Fig. 4C) and of HFD mice compared with NFD mice.
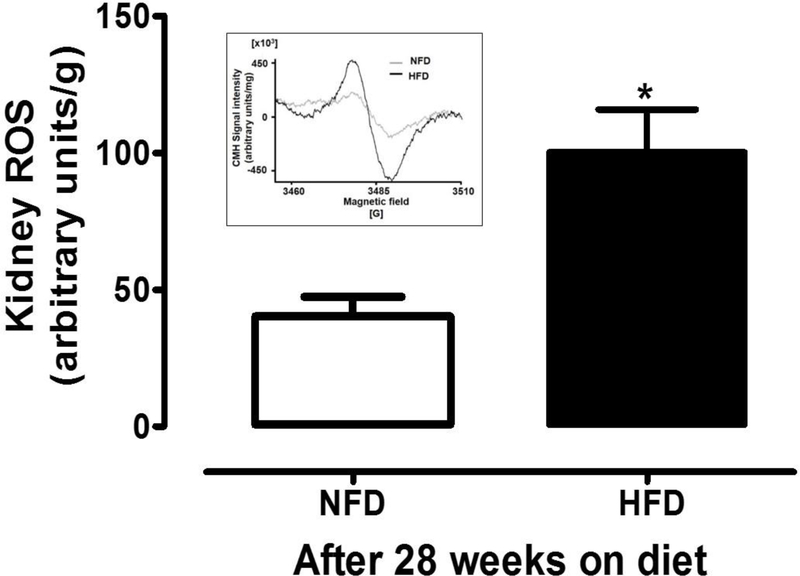
3.4. Kidney ROS production augmented in HFD-T2D mice:
Renal ROS production was significantly increased in HFD mice compared to NFD mice (40 ± 7 vs. 100 ± 16 arbitrary units/g; P<0.05; Fig. 5).
Figure 5. Kidney ROS production measured by ESR spectroscopy in whole kidneys from NFD and HFD mice.
Characteristic ESR spectra of CMH representing samples from HFD and NFD fed animals were shown in upper left insert and the cumulative data represented in the bar graphs. The HFD-T2D mice showed 2.5-fold elevation in ROS production compared to NFD mice. ROS production was normalized to the dry weight of the corresponding kidney samples in grams. NFD: normal fat diet (withe); HFD: high fat diet (black); ROS: reactive oxygen species; ESR: electron spin resonance. Data are mean ± SEM.
4. DISCUSSION
In the present study, we demonstrate that C57BL/6 mice fed a HFD for 28 weeks develop obesity and T2D. This mouse model of HFD-induced T2D exhibit body weight increases, hyperglycemia, hyperinsulinemia, insulin resistance, and lipid alterations. Importantly, HFD-induced T2D mice exhibit augmentation of AGT excretion in urine in the absence of albuminuria and renal fibrosis, indicating that uAGT is an early and accurate indicator of intrarenal RAS activation and not a consequence of glomerular damage.
After 28 weeks on HFD, C57BL/6 mice showed clear obesity and T2D phenotypes. Increased body weight gain was detected after 4 weeks, regardless of equal energy intake per day, which is likely due to differences in dietary-induced thermogenesis23. This outcome supports the concept that the source of caloric intake is more important than amount. The HFD mice also displayed hyperglycemia, glucose intolerance, insulin resistance, and hypercholesterolemia without significant increases in TG levels 17.
In this study, emphasis was given to evaluating uAGT as an indicator of intrarenal RAS activation in the absence of overt renal damage. Using a HFD-T2D mouse model, we demonstrated that uAGT increased at 20 weeks and remained elevated until 28 weeks on the diet. The magnitude of increases in uAGT agrees with previous studies using other experimental rodent models9, 24. Here, we report lower absolute uAGT values than previously described25, 26, which might be attributable to the collection of urine samples after 12 hours of fasting. AGT transcript levels decrease after fasting and are normalized after refeeding, indicating that nutrients, including glucose influence AGT 27.
Despite augmentation of uAGT excretion, albuminuria was not increased in mice fed a HFD for 28 weeks, even though T2D was established. In contrast, Deji et al. show significant increases in albuminuria of C57BL/6 mice on a HFD (60% of Kcal from fat) for 12 weeks indicating diet-induced kidney injury28. In the present study, albuminuria did not increase over time. Previous studies report that proteinuria increases with aging in rodents29 and humans30. However, rats fed a normal protein diet exhibit higher UACR than those fed a low protein diet31. The lack of difference in the levels of protein expression of kidney fibrotic factors, including α-SMA, fibronectin, collagen I, and TGF-β21, 32 between HFD versus NFD mice, indicate HFD does not stimulate kidney injury in C57BL/6 male mice, at least under the dietary regimen described in the present study. C57BL/6 mice seem to be resistant to the development of glomerulosclerosis, proteinuria, and hypertension as compared to other stains33. However, previous studies have shown that C57BL/6 mice may develop alterations, including increases in systolic BP, uAGT, and renal injury in response to either high dietary salt34, chronic intermittent hypoxia35 or during endogenous intrarenal AGT production36. The extent to which C57BL6 mice develop renal alterations upon experimental manipulations may help to explain the existing variability in the renal phenotype to the progression of tissue injury.
Because albuminuria and the protein expression of fibrotic factors in the kidney did not change over time with HFD, even in the presence of a slow progression of the development of T2D phenotype, we suggest that the administration of HFD (45% Kcal from fat) for 28 weeks in C57BL/6 male mice constitute a suitable model of T2D without a priori renal damage. The molecular weight of AGT (48–50 KDa) is slightly lower than albumin (66.5 KDa). Thereby, the absence of any sign of apparent glomerular damage in HFD mice argues against the enhancement of uAGT due to increased filtration of systemic AGT37. However, since the reabsorption of albumin in the proximal tube significantly impacts the amount that is excreted38, we cannot exclude the possibility that filtered albumin is recovered to a greater extent than AGT in the proximal tubule in this mouse model. In the mRen2 rat model, an increase in uAGT is detected without alterations in AGT mRNA or secretion, indicating an extrarenal source39. Nevertheless, intratubular AGT delivery to the distal nephron segments provides a substrate for the renin released by the collecting duct, and Ang I and Ang II is subsequently formed in the presence of tubular angiotensin converting enzyme1. Indeed, uAGT is considered an index of intrarenal RAS activation in Ang II-infused rats13, 25. In the present study, augmentation of uAGT was accompanied by increased Ang II levels in kidney and urine, similar to other rodent experimental models of Ang II-dependent hypertension13.
Increased intrarenal AGT contributes to the development and progression of hypertension and kidney injury in different models of experimental hypertension1. During T2D, the expression of AGT in renal proximal tubule is stimulated by elevated glucose, which drives the overproduction of ROS40. Indeed, treatment with canagliflozin, a sodium glucose transporter inhibitor (SGLT2) inhibits intrarenal oxidative stress in New Zealand Obese T2D mice41. Inhibition of SGLT2 may lead to attenuation of ROS generation and subsequent AGT augmentation in PTC. We also found that 28 weeks of the HFD increased ROS production in the kidney. ROS generation occurs in response to hyperglycemia via non-enzymatic glycation of proteins, glucose auto-oxidation42, and/or inhibition of anti-oxidative mechanisms through glycosylation of anti-oxidant enzymes43. Moreover, in Zucker diabetic fatty rats, ROS increases glomerular AGT expression in a dose- and time-dependent manner by activating extracellular-regulated kinase (ERK)/JNK pathway24. Although in the present study, we did not measure the time dependent changes of intrarenal Ang II, ROS, collagen, or fibronectin; collected evidence supports that the increases in uAGT in mice subjected to long-term HFD is mediated, at least in part, via increased ROS generation in response to hyperglycemia40, since both appeared in the absence of overt renal injury.
In the present study, the elevation in systolic BP was concomitant with the augmentation of uAGT, indicating that increased uAGT could contribute to the regulation of BP. Studies in transgenic mice support the concept that kidney-specific enhancement of the RAS contributes to BP regulation44. Sachetelli et al. reported that renal overproduction of AGT increases systolic BP in mice45. Proximal tubule-derived AGT regulates BP through luminal Ang II generation1, 46 which stimulates Na+ reabsorption by increasing proximal sodium/hydrogen exchanger 3 activity47 and distal epithelial sodium channels48, 49. Moreover, in the presence of ROS, the AGT molecule undergoes a subtle conformational change and releases Ang I more effectively when exposed to renin, thus facilitating Ang II generation50.
One caveat of the current study is that we did not continue the study past 28 weeks of the HFD to observe overt markers of renal damage. The use of a diet with lower fat content was intentional, to avoid quick induction of renal injury and allow us to discern whether increased uAGT occurs in response to intrarenal RAS activation or glomerular damage. By examining RAS expression in kidney tissues along with ROS, we were we able to show that uAGT appears in the absence of other markers of renal injury. Additional experiments should compare this novel biomarker with other clinical assays for oxidative stress including urinary 8-Isosprostane and TGF-beta. These experiments would determine whether uAGT increases are detected before other accepted markers for oxidative stress.
In conclusion, the data indicate that uAGT is an early marker of intrarenal RAS activation in mice with HFD-induced T2D, in the absence of signs of kidney injury such as albuminuria. Our data support a recent clinical study showing that in T2D patients, increases in uAGT precede the onset of albuminuria51. Increased uAGT excretion could be mediated, at least in part, by augmented kidney ROS production. Type 2 diabetes is a long-term disease and renal injury is an inevitable complication. In perspective, our results suggest that clinical evaluation emphasis should be placed on the detection of increased urinary excretion of biomarkers as early indicators of intrarenal RAS activation, rather than renal injury markers only, which are the consequence of the disease but not the cause. The complex interactions between the intrarenal RAS and the development of renal injury associated with T2D induced by HFD is critical to optimize current therapeutic strategies to improve precision medicine.
Highlights.
Whether the augmented excretion urinary AGT (uAGT), locally generated in the proximal tubule of the kidney, is an indicator of glomerular damage rather than intrarenal RAS activation, remains unclear.
Using a mouse model of slow progression of T2D phenotype induced by high fat diet (HFD), we demonstrated that augmentation uAGT occurred in the absence of overt albuminuria. These changes were accompanied by intrarenal increases in ROS and Ang II concentrations.
In HFD-induced T2D mice, increased uAGT precedes glomerular damage and kidney tissue injury and indicates intrarenal RAS activation.
Acknowledgements
We thank the Molecular and Analytical Core and the Mouse Phenotyping Core of the Tulane Renal Hypertension Center of Excellence for providing the physical infrastructure to perform some of the experiments. The authors also acknowledge the technical experience and support of Alexander Castillo (Physiology, Tulane University SOM) for radiotelemetry blood pressure approach.
Funding
This work was supported by the National Institutes of Health (MCP: DK104375 and P30GM103337; SHL: HL133619; PVK: NS094834) and the American Heart Association (PVK: 14SDG20490359; VNS: 16PRE31450006).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None
REFERENCES
- 1.Navar LG, Kobori H, Prieto MC, Gonzalez-Villalobos RA. Intratubular renin-angiotensin system in hypertension. Hypertension. 2011;57(3): 355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Navar LG, Harrison-Bernard LM. Intrarenal angiotensin II augmentation in angiotensin II dependent hypertension. Hypertens Res. 2000;23(4): 291–301. [DOI] [PubMed] [Google Scholar]
- 3.Nagai Y, Yao L, Kobori H, et al. Temporary angiotensin II blockade at the prediabetic stage attenuates the development of renal injury in type 2 diabetic rats. J Am Soc Nephrol. 2005;16(3): 703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parving HH, Lehnert H, Brochner-Mortensen J, et al. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345(12): 870–878. [DOI] [PubMed] [Google Scholar]
- 5.Fan YY, Kobori H, Nakano D, et al. Aberrant activation of the intrarenal renin-angiotensin system in the developing kidneys of type 2 diabetic rats. Horm Metab Res. 2013;45(5): 338–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ingelfinger JR, Zuo WM, Fon EA, Ellison KE, Dzau VJ. In situ hybridization evidence for angiotensinogen messenger RNA in the rat proximal tubule. An hypothesis for the intrarenal renin angiotensin system. J Clin Invest. 1990;85(2): 417–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kobori H, Harrison-Bernard LM, Navar LG. Expression of angiotensinogen mRNA and protein in angiotensin II-dependent hypertension. J Am Soc Nephrol. 2001;12(3): 431–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Michel FS, Norton GR, Maseko MJ, Majane OH, Sareli P, Woodiwiss AJ. Urinary angiotensinogen excretion is associated with blood pressure independent of the circulating renin-angiotensin system in a group of african ancestry. Hypertension. 2014;64(1): 149–156. [DOI] [PubMed] [Google Scholar]
- 9.Kobori H, Prieto-Carrasquero MC, Ozawa Y, Navar LG. AT1 receptor mediated augmentation of intrarenal angiotensinogen in angiotensin II-dependent hypertension. Hypertension. 2004;43(5): 1126–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyata K, Ohashi N, Suzaki Y, Katsurada A, Kobori H. Sequential activation of the reactive oxygen species/angiotensinogen/renin-angiotensin system axis in renal injury of type 2 diabetic rats. Clin Exp Pharmacol Physiol. 2008;35(8): 922–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogawa S, Kobori H, Ohashi N, et al. Angiotensin II Type 1 Receptor Blockers Reduce Urinary Angiotensinogen Excretion and the Levels of Urinary Markers of Oxidative Stress and Inflammation in Patients with Type 2 Diabetic Nephropathy. Biomark Insights. 2009;4: 97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobori H, Alper AB Jr., Shenava R, et al. Urinary angiotensinogen as a novel biomarker of the intrarenal renin-angiotensin system status in hypertensive patients. Hypertension. 2009;53(2): 344–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kobori H, Harrison-Bernard LM, Navar LG. Urinary excretion of angiotensinogen reflects intrarenal angiotensinogen production. Kidney Int. 2002;61(2): 579–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsusaka T, Niimura F, Pastan I, Shintani A, Nishiyama A, Ichikawa I. Podocyte injury enhances filtration of liver-derived angiotensinogen and renal angiotensin II generation. Kidney Int. 2014;85(5): 1068–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roksnoer LC, Verdonk K, van den Meiracker AH, Hoorn EJ, Zietse R, Danser AH. Urinary markers of intrarenal renin-angiotensin system activity in vivo. Curr Hypertens Rep. 2013;15(2): 81–88. [DOI] [PubMed] [Google Scholar]
- 16.Persson F, Lu X, Rossing P, Garrelds IM, Danser AH, Parving HH. Urinary renin and angiotensinogen in type 2 diabetes: added value beyond urinary albumin? J Hypertens. 2013;31(8): 1646–1652. [DOI] [PubMed] [Google Scholar]
- 17.Fernandes-Santos C, Carneiro RE, de Souza Mendonca L, Aguila MB, Mandarim-de-Lacerda CA. Pan-PPAR agonist beneficial effects in overweight mice fed a high-fat high-sucrose diet. Nutrition. 2009;25(7–8): 818–827. [DOI] [PubMed] [Google Scholar]
- 18.Mauvais-Jarvis F, Kahn CR. Understanding the pathogenesis and treatment of insulin resistance and type 2 diabetes mellitus: what can we learn from transgenic and knockout mice? Diabetes Metab. 2000;26(6): 433–448. [PubMed] [Google Scholar]
- 19.Kobori H, Katsurada A, Miyata K, et al. Determination of plasma and urinary angiotensinogen levels in rodents by newly developed ELISA. Am J Physiol Renal Physiol. 2008;294(5): F1257–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shao W, Seth DM, Prieto MC, Kobori H, Navar LG. Activation of the renin-angiotensin system by a low-salt diet does not augment intratubular angiotensinogen and angiotensin II in rats. AmJPhysiol Renal Physiol. 2013;304(5): F505–F514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cuevas CA, Gonzalez AA, Inestrosa NC, Vio CP, Prieto MC. Angiotensin II increases fibronectin and collagen I through the beta-catenin-dependent signaling in mouse collecting duct cells. Am J Physiol Renal Physiol. 2015;308(4): F358–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katakam PV, Gordon AO, Sure VN, Rutkai I, Busija DW. Diversity of mitochondria-dependent dilator mechanisms in vascular smooth muscle of cerebral arteries from normal and insulin-resistant rats. Am J Physiol Heart Circ Physiol. 2014;307(4): H493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crescenzo R, Bianco F, Mazzoli A, et al. Fat Quality Influences the Obesogenic Effect of High Fat Diets. Nutrients. 2015;7(11): 9475–9491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohashi N, Urushihara M, Satou R, Kobori H. Glomerular angiotensinogen is induced in mesangial cells in diabetic rats via reactive oxygen species--ERK/JNK pathways. Hypertens Res. 2010;33(11): 1174–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59(3): 251–287. [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez-Villalobos RA, Seth DM, Satou R, et al. Intrarenal angiotensin II and angiotensinogen augmentation in chronic angiotensin II-infused mice. Am J Physiol Renal Physiol. 2008;295(3): F772–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gabriely I, Yang XM, Cases JA, Ma XH, Rossetti L, Barzilai N. Hyperglycemia modulates angiotensinogen gene expression. Am J Physiol Regul Integr Comp Physiol. 2001;281(3): R795–802. [DOI] [PubMed] [Google Scholar]
- 28.Deji N, Kume S, Araki S, et al. Structural and functional changes in the kidneys of high-fat diet-induced obese mice. Am J Physiol Renal Physiol. 2009;296(1): F118–126. [DOI] [PubMed] [Google Scholar]
- 29.Barsha G, Denton KM, Mirabito Colafella KM. Sex- and age-related differences in arterial pressure and albuminuria in mice. Biol Sex Differ. 2016;7: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Epstein M. Aging and the kidney. J Am Soc Nephrol. 1996;7(8): 1106–1122. [DOI] [PubMed] [Google Scholar]
- 31.De Miguel C, Lund H, Mattson DL. High dietary protein exacerbates hypertension and renal damage in Dahl SS rats by increasing infiltrating immune cells in the kidney. Hypertension. 2011;57(2): 269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ito Y, Aten J, Bende RJ, et al. Expression of connective tissue growth factor in human renal fibrosis. Kidney Int. 1998;53(4): 853–861. [DOI] [PubMed] [Google Scholar]
- 33.Rabe M, Schaefer F. Non-Transgenic Mouse Models of Kidney Disease. Nephron. 2016;133(1): 53–61. [DOI] [PubMed] [Google Scholar]
- 34.Lantelme P, Rohrwasser A, Gociman B, et al. Effects of dietary sodium and genetic background on angiotensinogen and Renin in mouse. Hypertension. 2002;39(5): 1007–1014. [DOI] [PubMed] [Google Scholar]
- 35.Takahashi K, Ueda S, Kobayashi T, et al. Chronic intermittent hypoxia-mediated renal sympathetic nerve activation in hypertension and cardiovascular disease. Sci Rep. 2018;8(1): 17926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kobori H, Ozawa Y, Satou R, et al. Kidney-specific enhancement of ANG II stimulates endogenous intrarenal angiotensinogen in gene-targeted mice. Am J Physiol Renal Physiol. 2007;293(3): F938–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kobori H, Harrison-Bernard LM, Navar LG. Enhancement of angiotensinogen expression in angiotensin II-dependent hypertension. Hypertension. 2001;37(5): 1329–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wagner MC, Campos-Bilderback SB, Chowdhury M, et al. Proximal Tubules Have the Capacity to Regulate Uptake of Albumin. J Am Soc Nephrol. 2016;27(2): 482–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cohen JA, Lindsey SH, Pirro NT, Brosnihan KB, Gallagher PE, Chappell MC. Influence of estrogen depletion and salt loading on renal angiotensinogen expression in the mRen(2).Lewis strain. Am J Physiol Renal Physiol. 2010;299(1): F35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hsieh TJ, Fustier P, Wei CC, et al. Reactive oxygen species blockade and action of insulin on expression of angiotensinogen gene in proximal tubular cells. J Endocrinol. 2004;183(3): 535–550. [DOI] [PubMed] [Google Scholar]
- 41.Woods TC, Satou R, Miyata K, et al. Canagliflozin Prevents Intrarenal Angiotensinogen Augmentation and Mitigates Kidney Injury and Hypertension in Mouse Model of Type 2 Diabetes Mellitus. Am J Nephrol. 2019;49(4): 331–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ha H, Lee HB. Reactive oxygen species as glucose signaling molecules in mesangial cells cultured under high glucose. Kidney Int Suppl. 2000;77: S19–25. [DOI] [PubMed] [Google Scholar]
- 43.Wolff SP, Jiang ZY, Hunt JV. Protein glycation and oxidative stress in diabetes mellitus and ageing. Free Radic Biol Med. 1991;10(5): 339–352. [DOI] [PubMed] [Google Scholar]
- 44.Davisson RL, Ding Y, Stec DE, Catterall JF, Sigmund CD. Novel mechanism of hypertension revealed by cell-specific targeting of human angiotensinogen in transgenic mice. Physiol Genomics. 1999;1(1): 3–9. [DOI] [PubMed] [Google Scholar]
- 45.Sachetelli S, Liu Q, Zhang SL, et al. RAS blockade decreases blood pressure and proteinuria in transgenic mice overexpressing rat angiotensinogen gene in the kidney. Kidney Int. 2006;69(6): 1016–1023. [DOI] [PubMed] [Google Scholar]
- 46.Ichihara A, Kobori H, Nishiyama A, Navar LG. Renal renin-angiotensin system. Contrib Nephrol. 2004;143: 117–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saccomani G, Mitchell KD, Navar LG. Angiotensin II stimulation of Na(+)-H+ exchange in proximal tubule cells. Am J Physiol. 1990;258(5 Pt 2): F1188–1195. [DOI] [PubMed] [Google Scholar]
- 48.Peti-Peterdi J, Warnock DG, Bell PD. Angiotensin II directly stimulates ENaC activity in the cortical collecting duct via AT(1) receptors. J Am Soc Nephrol. 2002;13(5): 1131–1135. [DOI] [PubMed] [Google Scholar]
- 49.Mamenko M, Zaika O, Ilatovskaya DV, Staruschenko A, Pochynyuk O. Angiotensin II increases activity of the epithelial Na+ channel (ENaC) in distal nephron additively to aldosterone. J BiolChem. 2012;287(1): 660–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou A, Carrell RW, Murphy MP, et al. A redox switch in angiotensinogen modulates angiotensin release. Nature. 2010;468(7320): 108–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhuang Z, Bai Q, A L, Liang Y, Zheng D, Wang Y. Increased urinary angiotensinogen precedes the onset of albuminuria in normotensive type 2 diabetic patients. Int J Clin Exp Pathol. 2015;8(9): 11464–11469. [PMC free article] [PubMed] [Google Scholar]