Abstract
Understanding how patients and physicians discuss screening barriers may illuminate reasons for non-adherence to recommended CRC screening. The goal of the present study was to describe patients’ reporting of and physicians’ responses to CRC screening barriers and examine their associations with patients’ CRC screening behaviors. Audio-recorded primary care consultations (N=413) with patients due for CRC screening were used to identify CRC screening-related barrier talk and physician responses. Presence of barrier talk was associated with less patient adherence to CRC screening (OR=.568, p=.007). Neither CRC screening talk (n=413) nor physician responses (n=151) were associated with patients’ CRC screening. Among the consultations in which barrier talk occurred (n=151), patients most often reported test-related (28.9%) and psychological (26.1%) barriers. Barriers were most often reported in the context of CRC screening discussions (45.7%) or in direct response to a physician’s question about CRC screening (48.6%). Results indicated that patients rarely raised CRC screening barriers unprompted and that presence of barrier talk was predictive of CRC screening behavior. These findings may help improve future clinical practice by highlighting that patients may benefit from physicians initiating and facilitating discussions of CRC screening barriers and directly helping patients overcome known barriers to CRC screening.
Keywords: physician-patient communication, barriers to colorectal cancer screening, CRC adherence
Introduction
Multiple factors influence whether individuals engage in colorectal cancer (CRC) screening behavior (Beydoun & Beydoun, 2008), including discussing CRC screening, awareness of screening, and availability of screening. For instance, a lack of provider recommendation, patients not knowing that they are due for screening, financial burden, embarrassment regarding the test, and fear that the test will be painful are each associated with a reduced likelihood that patients will engage in CRC screening (Bynum, Davis, Green, & Katz, 2012; Jones, Devers, Kuzel, & Woolf, 2010; Jones, Woolf, et al., 2010). Across multiple studies, CRC screening has been shown to contribute to a reduction in CRC mortality (Kronborg, Fenger, Olsen, Jørgensen, & Søndergaard, 1996; Newcomb, Norfleet, Storer, Surawicz, & Marcus, 1992), especially when offered to persons who will likely obtain a net benefit and when the screening is done effectively (Holden, Jonas, Porterfield, Reuland, & Harris, 2010). Although overuse (screening persons with little potential for net benefit) and misuse (screening in ways that reduce net benefit) are growing concerns, underuse of CRC screening still remains a large problem (Holden et al., 2010). Less than 65% of eligible adults nationwide were screened according to recent Center for Disease Control (CDC) statistics (Center for Disease Control and Prevention (CDC), 2013).
A 2010 systematic review examining how to enhance the use and quality of CRC screening notes that it is critical both to discuss CRC screening with eligible persons and offer a range of screening options (Holden et al., 2010). Perhaps due in part to the important role CRC screening discussions play in predicting engagement in CRC screening, recent research focuses on how CRC screenings are discussed and recommended to patients. Prior research (Brenes & Paskett, 2000; Cairns & Viswanath, 2006; Khankari et al., 2007; Laiyemo et al., 2014; Modiri, Makipour, Gomez, & Friedenberg, 2013; Thompson, Lander, Xu, & Shyu, 2014; Wolf et al., 2006) and a recent systematic review (Peterson et al., 2016) reported overwhelming evidence that a physician simply recommending CRC screening is a major contributor to screening behavior. However, multiple studies have found that a significant number of patients are not adherent to CRC screening, even with a recommendation from physicians (Khankari et al., 2007; Lafata, Cooper, Divine, Oja-Tebbe, & Flocke, 2014). For instance, one study found that 53.0% of patients seen in a primary care clinic were non-adherent to CRC screening even though 93.0% had received a recommendation for screening (Lafata et al., 2014). In fact, CRC screening rates still remain low for a substantial proportion of all age-eligible adults (Centers for Disease Control and Prevention, 2013; Zapka, Puleo, Vickers-Lahti, & Luckmann, 2002). Thus, the data clearly indicate that physician recommendations for CRC screening are critical but not sufficient for CRC screening.
One possible source influencing the effectiveness of physician recommendations is how CRC screening is discussed. Namely, the quality and content of the physician-patient communication that surrounds the recommendation could explain some of the variance in predicting CRC screening that physician recommendation does not. This hypothesis is grounded in Goffman’s Facework Theory (Goffman, 1955). Facework Theory posits that in all social encounters, individuals communicate verbally and nonverbally in ways that display their evaluation of both the situation and the individuals they are encountering within that situation (e.g., the patient and provider). This theory has been applied to communication within healthcare settings (Bylund, Peterson, & Cameron, 2012) to highlight how both patients and providers communicate in ways that relate to their “face” or sense of self. Within this context and framework, communication that establishes a better face than one assumes for one’s self is associated with positive feelings whereas disconfirming information might lead to negative feelings as it can damage one’s face. Thus, in short, facework represent the actions that patients and providers may take to maintain or save face in the context of a clinical interaction (Bylund et al., 2012).
In the context of Facework Theory, how physicians bring up and discuss CRC screening may matter more than simply bringing up recommendations for screening itself. There may be barriers patients experience to engaging in CRC screening that are not brought up in clinical encounters due to patients attempting to “save face” or protect their sense of self. Thus, discussions of CRC screening barriers (“barrier talk”) could emerge in ways that damage, maintain, or save face. How these barriers are brought up and addressed by physicians may be critically important to determining if patients ultimately engage in CRC screening. For example, if patients are motivated to maintain or save their face, the way in which they bring up and discuss barriers may be heavily reliant upon how physicians introduce (or fail to introduce) this topic and respond to expressed barriers or concerns. In short, if physicians address and respond to patients’ barriers in a way that is face-saving, it may lead to better adherence to CRC screening recommendations than if they ignore or respond in dismissive ways that damage patients’ face.
Prior communication studies have highlighted this phenomenon by demonstrating the importance of how physicians discuss and recommend treatments in predicting adherence to recommendations. For instance, one study found that parental compliance with a recommendation to engage in “watchful waiting” of antibiotic use was enhanced by providers giving a higher quality explanation and instruction around this recommendation (MacGeorge, Caldes, Smith, Hackman, & San Jose, 2017). Furthermore, key aspects of communication features in recommending watchful waiting, such as perceptions of advantages of this approach and the tact of the provider, also positively influence parents’ compliance (MacGeorge, Smith, Caldes, & Hackman, 2017). These findings, along with the general framework of Advice Response Theory (ART) (MacGeorge, Guntzviller, Hanasono, & Feng, 2016), highlight that how advice or recommendations are given is critical to the likelihood that patients will be compliant to those recommendations. These findings fit in line with Facework Theory.
Based on this prior work, ia recommendation without sufficient dialogue about the screening test may be ineffective (Denberg et al., 2005). Furthermore, studies examining the role of the quality of communication in physician-patient discussions about cancer screening report a clear connection between quality and adherence (Lafata et al., 2014; Ling et al., 2008; Mosen et al., 2013; Nápoles et al., 2015). For instance, patients who report more positive communication (i.e., patient-rated communication as “good”) with their provider have been shown to be more likely to have been screened than those with less positive communication (i.e., patient-rated communication as “poor”) (Katz et al., 2004). This “good” communication could represent communication that helps patients “save” or “maintain” face. Furthermore, assessing patient understanding (Ling et al., 2008), comprehensiveness of discussion (Lafata et al., 2014; Mosen et al., 2013; Nápoles et al., 2015) as well as responsiveness to concerns and patients’ perceived level of encouragement (Nápoles et al., 2015) have also been shown to be associated with higher screening rates.
Despite research identifying patient-reported barriers to CRC screening and the impact of physician-patient communication on screening adherence, little research has focused on the role that physician-patient conversations may play in identifying and helping alleviate barriers to CRC screening. Understanding how patients raise and physicians respond to discussions around barriers to CRC screening may illuminate communication patterns for best clinical practice in discussing patients’ barriers to CRC screening. As a first step towards understanding how the discussion of CRC screening barriers may impact patient adherence to recommended screening processes, the primary aim of this study is to describe physician-patient communication regarding patient-reported screening barriers, in naturally occurring clinical visits. Specifically, the present study describes how barrier discussions arise during primary care office visits, the types of barriers discussed by patients, and the responses physicians have to these barriers. The secondary aim is to determine if discussion of barriers or physicians’ varying responses to barrier discussion are associated with patient adherence to physician-recommended CRC screening. These objectives aim to highlight real world examples of best patient-physician communication around CRC screening barriers to inform future clinical practice.
Methods
Study eligible primary care physicians and patients were affiliated with a large, integrated health system in southeast Michigan. All family and internal medicine physicians on staff with the health system’s medical group practice were invited to participate in an observational study of preventive health and physician-patient decision making. Physician recruitment was achieved via multiple approaches. First, the study PI attended a staff meeting at each of the primary care clinics to introduce the study. Some physicians agreed to study participation at these meetings. All providers who did not sign up at a meeting received an email inviting them to participate in the study. Because not all physicians routinely used their email accounts, those not responding to the email were approached via a telephone call. There was no selection process among eligible doctors and multiple attempts were made to contact all eligible providers.
The health system of participants in the present study had a large managed care population, to which each study participant was a member. Routine cancer screening, including screening for CRC, was monitored among this patient population for reporting. While reports were made available and pushed to the primary care providers, there were no meaningful or substantial financial incentives to either the physician or medical group at the time of the study. A total of 77 of the 163 study eligible physicians (47%) agreed to participate in this study. This rate is similar to previous studies in which physicians agree to participate in communication research wherein their clinical consultations are recorded [e.g., 52% (Cooper, Roter, Johnson, & Ford, 2003)]. These physicians had a total of n=413 patients participate in the study.
Among physicians agreeing to study participation, research staff monitored the appointment scheduling system to identify study eligible patients. Eligible patients included those who were insured, aged 50 to 80 years old, and due for CRC screening at the time of a scheduled routine annual physical examination with a study participating physician. Patients with EHR-documented colonoscopy in the past 10 years, sigmoidoscopy in the past 5 years or faecal occult blood test (FOBT) or faecal immunochemical test (FIT) in the past 12 months were excluded as were patients known to be above average risk for CRC (i.e., those with a personal or family history of CRC, those with prior polyps or a history of inflammatory bowel disease, familial adenomatous polyposis or hereditary non-polyposis). Thus, all patients recruited and enrolled in the study were at or beyond the recommended age to receive CRC screening (age 50 years or older), and thus would be in the category of individuals who are more at risk and most likely to benefit from CRC screening (Holden et al., 2010). Physician and patient participants were informed that this was a study about physician-patient communication and preventive care services; however, they were not informed of the main study questions or specific hypotheses.
Study eligible patients were recruited sequentially in the order of appointment scheduling between February 2007 and June 2009 via a letter of study introduction followed by a telephone contact. Study participation included completion of a pre-visit telephone survey, audio recording of the scheduled office visit, and completion of a brief post-visit survey. Patients who indicated verbal agreement to participate in the study at the time of the pre-visit survey were asked to arrive to their scheduled clinic appointment 15 minutes early to enable completion of informed consent. All aspects of the study were approved by the institution’s Institutional Review Board.
The pre-visit telephone survey was used to obtain relevant background information on patients. Specifically, patients’ demographic characteristics (age, gender, race, education, household income, and employment status) were assessed. Office visits were recorded using a small digital audio-recording device placed in the examination room by a trained research assistant. All recordings were transcribed prior to coding. Six months after the office visit, patients’ adherence to physician-recommended CRC screening was assessed (adherent, non-adherent) by examining the patients’ electronic health record for evidence of colonoscopy, fecal occult blood testing (FOBT), sigmoidoscopy, or barium enema use.
Analytic Approach
Office visits transcripts were coded for presence of barrier talk. An office visit was considered to have barrier-related talk when patients reported barriers in response to physicians’ recommendation regarding any form of CRC screening (colonoscopy, FOBT, both, or “other” which refers to sigmoidoscopy or barium enema). We used a content analysis approach to further code the barrier talk (Baxter & Babbie, 2004) with two team members (CLB and TAD) serving as coders. This process of content analysis is utilized to evaluate text systematically in order to convert qualitative data into quantitative data (Auer-Srnka & Koeszegi, 2007). Thus, all quotes presented in the results section represent examples of how content was coded rather than thematic qualitative data.
First, transcripts that contained barrier talk were coded to develop a detailed coding system. Second, the two coders independently coded the remaining transcripts using the developed content analysis coding system. The following categories, as noted, were identified and coded: (1) how the barrier was reported in discussion by patients, (2) the content of the barrier (e.g., specific patient-reported barriers reported in clinical encounter) and (3) the physician response(s) to the barriers (what they said/did in response to a patient raising a barrier). Finally, barriers and physician response(s) to barriers were coded into descriptive, specific categories (e.g., time/too busy to have CRC screening, fear of test, etc.). It should be noted that for each office visit, barriers and the corresponding physician responses to them were coded separately such that there could be multiple barriers within a single office visit and multiple responses to that barrier.
To understand the main domains of barriers reported by patients, five categories were created from the original 19 types of barriers reported.1 These categories included: (1) system failure barriers, meaning that there was a failure at a system level such as not calling back for appointment, etc.; (2) logistics barriers, in which a practical or logistical barrier such as a lack of transportation, lack of finances, or a lack of time interfered with completing a CRC screening; (3) test-related barriers, which refers to concerns about the preparation of, risks of, or issues associated with the test itself; (4) psychological barriers, which refer to psychological concerns (fear, avoidance, forgetting, embarrassment) related to receiving CRC screening; and (5) other/unknown, which refer to barriers that are either outside of the aforementioned categories or in which the patient noted a barrier but was unable to point to anything specific. Systems failure remained its own category due to both it being the most common barrier and its unique properties when examined alongside the remaining barriers. Because there was a high volume of categories, codes were collapsed into broader categories (e.g., logistics or psychological barriers) that allowed for analyses of how these barriers and responses related to one another and to adherence to CRC screening recommendations. These broad categories were developed by the first and senior authors, with content expertise in communication. Through a process of independent creation of categories and reconciliation, a final set of five categories was agreed upon.
Physician responses were collapsed into three main categories, indicating whether physicians provided: (1) a specific response to the barrier, meaning that the response focused on the content of the barrier; (2) a general response to the barrier, not focusing specifically on the content of the barrier but still responsive in some way to the patient-reported barrier; or (3) non-response. To examine the type of physician responses associated with each type of barrier category, a new variable was created that indicated whether or not a specific physician response category (specific, general, or none) occurred in response to a barrier (0 = not present; 1 = present). It should be noted that, in some cases, the same category of response occurred more than once. Additionally, most of the time physicians responded to a barrier with more than one type of response.
To understand those clinical encounters in which barriers were reported, descriptive statistics were run among these visits (n=151). Next, a series of logistic regressions and chi-square analyses were run to examine: (1) the influence of the presence or absence of barrier talk on patient adherence across all study eligible consultations (N=413) and (2) physician responses to barrier talk, among those for whom barriers were presented, on patient adherence to CRC screening (N=151). Descriptive statistics (means and frequency distributions) were used to examine the type and frequency of barriers reported by patients as well as physician responses to these barriers. Among the 151 consultations in which barrier talk occurred, consultations were further coded for barrier talk and responses to barrier talk. Initial coding of 54 of the 151 transcripts (35%) allowed us to first develop the detailed coding system noted in the analytic section. We then established inter-rater reliability by each coder independently coding 10% of the transcripts (Cohen’s Kappa = .80). After this inter-rater reliability was established, the coding system was utilized by one of the coding team members (CLB) to code the remaining transcripts.
To determine if the type of physician response(s) to barriers were associated with patients’ adherence to the physician’s recommendation for CRC screening, a series of chi-square tests were run to examine if each of the barrier response types (presence or absence of specific, general, no response) was significantly associated with patients’ being adherent or non-adherent to CRC screening.
To determine if physician responses to barriers were associated with patients’ adherence to CRC screening at the consultation level (n=151), we used logistic regression to examine the proportion of physician response types per consultation (of specific, general, and none) as a predictor of CRC screening adherence. First, a set of variables was created using the consultation as the unit of analysis (n=151 consultations) which indicated what proportion of the barriers discussed within that consultation were responded to specifically, generally, or with no response within a single consultation. Proportions were calculated because the number of barriers varied across consultations, so this provided a relative measure across all consultations. Second, a logistic regression was run in which all of these proportion variables were entered as predictors of adherence.
Results
Sample Characteristics
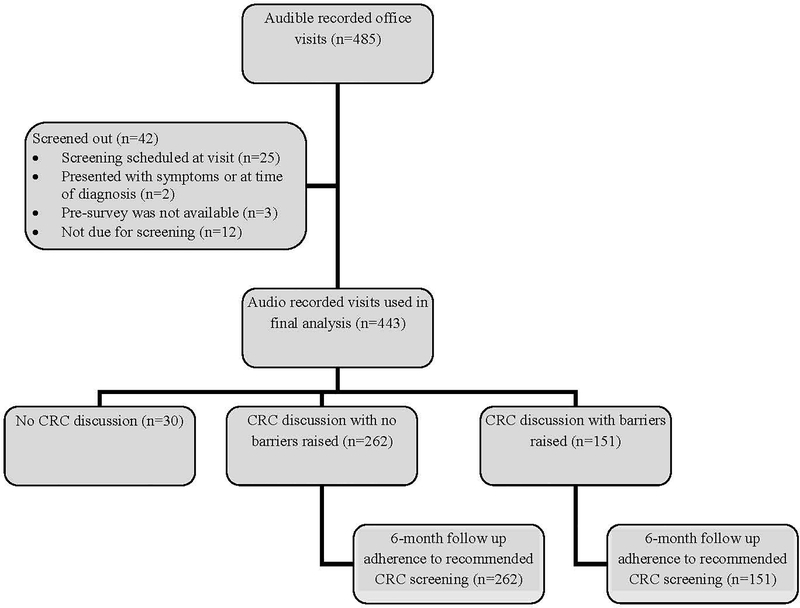
Data were collected for 500 office visits, resulting in 485 audible recordings (see Figure 1). Forty-two cases were excluded from the analyses for the following reasons: the patient had screening scheduled at the time of the office visit (n=25), talk indicated they were not due for CRC screening (n=12), presented with symptoms (n=1), presented in the midst of a diagnostic work up (n=1), or the pre-survey was not available (n=3). Ninety-three percent of the remaining visits (413 of 443) included talk related to CRC screening. Patients’ adherence to physicians’ recommended CRC screening from all of these consultations (n=413) were assessed in a 6-month follow up of patients’ EHRs. Among the 413 visits in which talk related to CRC screening was present, barrier-related talk was present in 37% (n=151).
FIGURE 1.
Flow diagram of patients by barrier discussion.
Reliability of Coding Scale
Approximately one-third of the transcripts were used in the initial process of developing and refining the content analysis system. First, we coded for the barrier, content and how it was introduced. Following, we coded the responses of the barriers. Inter-rater reliability was established on 10% of the data as follows: (1) Identifying barriers: 88% agreement; (2) Coding content of the barriers, kappa=.79. (3) Coding how the barrier was initiated, kappa=.80; (4) Coding doctors’ responses to barriers = .68. After achieving inter-rater reliability, the senior author coded the remainder of the barriers and responses, which resulted in the categories outlined in the methods section.
Consultations with Barrier-related Talk
As noted, descriptive statistics were run among visits with barrier talk present (n=151). Visits that contained any barrier-related discussion were made in consultation with a total of 51 physicians (n=51; see Table 1). On average, those physicians were 48.5 years old (SD=8.4), and 60.8% were female, 45.1% were white, and 70.6% were general internists. As shown in Table 1, n=151 patients were involved in these visits. Patients were an average of 60 years old (SD=7.9), and 78.8% were female, 70.9% were white, and 62.9% were married. All patients were above the age of 51 years old, indicating they were all of a recommended age to receive CRC screening at the time the study was conducted. Twenty-four percent of patients had a high school degree or less, and 42.4% had some college or a 2-year degree.
Table 1.
Physician (N=51) and patient characteristics (N=151).
| Physician characteristics | N (%) |
| Age | M= 48.50 yrs. (SD=8.41) |
| Gender, female | 31 (60.8%) |
| Race | |
| Caucasian | 23 (45.1%) |
| African American | 8 (15.7%) |
| Other | 20 (39.2%) |
| Patient characteristics | N (%) |
| Age | M= 60.00 yrs. (SD=7.87) |
| Gender, female | 119 (78.8%) |
| Race | |
| Caucasian | 107 (70.9%) |
| African American | 33 (21.9%) |
| Other | 11 (7.3%) |
| Education level | |
| Less than high school degree | 6 (4.0%) |
| High school degree/GED | 30 (19.9%) |
| Some college or 2-yr degree | 64 (42.4%) |
| 4-year college degree | 29 (19.2%) |
| More than 4-yr college degree | 22 (14.6%) |
| Marital Status, married | 95 (62.9%) |
Patient Reported Barriers
Overall, patients and doctors discussed a total of 19 different types of barriers to CRC screening. The most common of the 19 barriers were systems failure, concerns regarding the colonoscopy preparation for the colonoscopy, fear, time/too busy and competing health needs (see Table 2). The most common types of barrier was test-related barriers (28.8%); this was followed in frequency by psychological barriers (26.0%), logistics (20.2%), system failure (15.4%), and other/unknown (9.6%).
Table 2.
Codes used for analyzing the patient-reported barriers in the audio-recorded office visits consultations and prevalence of occurrence (n=208 barriers).
| Category | Patient-Reported Barriers | Prevalence |
|---|---|---|
| System failure barriers | Systems failure | 15.4% |
| Logistics barriers | Time/too busy | 6.7% |
| Competing health need/prioritization | 6.7% | |
| Transportation | 6.3% | |
| Cost (losing health insurance) | 0.5% | |
| Test-related barriers | Preparation | 12.5% |
| Risk of injury | 5.8% | |
| Need for sedation | 3.8% | |
| Pain associated with procedure | 3.4% | |
| Invasiveness of procedure | 3.4% | |
| Psychological barriers | Doesn’t see a need/not having trouble | 9.1% |
| Fear | 7.2% | |
| Doesn’t want it | 4.3% | |
| Forgot/slipped my mind | 1.9% | |
| Procrastinated | 1.4% | |
| Doesn’t want to know | 1.0% | |
| Embarrassing | 1.0% | |
| Other/unknown | Unknown | 5.8% |
| Other | 3.8% | |
Note: Data analyzed at the initial barrier level; multiple barriers could be presented per office visit (from n=1 to n=4 barriers per consultation). Multiple responses could exist for a single barrier
The vast majority (94.3%) of all barriers discussed were reported in the context of a CRC discussion or in response to a question about CRC screening. Thus, only a minority of barriers were raised entirely by the patient, without physician prompting (5.8%). Table 3 provides a summary of the prevalence of how barriers were introduced by patients within the office visit discussion. In some cases, patients introduced barriers by being prompted. Specifically, patients introduced barriers as a fairly immediate response to either a question asked regarding whether screening was done or if a referral was made (28.4%) (e.g., “Did you ever have a colonoscopy done?”), or in response to the doctor asking why they had not had a CRC screening or how they feel about a future screening (e.g., “We talk about it every year. How come you didn’t have it done?”) (20.2%). In other cases, patients introduced barriers unprompted by either raising them within the context of an ongoing discussion of CRC screening (45.7%) (e.g., the doctor talks about different options for colon cancer screening and about the prep before the patient brings up the barrier) or by bringing it up without any prompt (5.8%) (e.g., “You know, I never, that’s another thing, I’m such a chicken I never went for my colonoscopy.”)
Table 3.
Codes used for analyzing how patients introduced barriers in the audio-recorded office visits and prevalence of occurrence (n=208 barriers).
| Category | How Barrier was Introduced | Prevalence |
|---|---|---|
| Patient response to a question whether screening was done or if referral can be made. | 28.4% | |
| Patient response to the doctor asking why not or how they feel about a future one. | 20.2% | |
| Patient raises within context of the discussion. | 45.7% | |
| Patient raises entirely on own. | 5.8% |
Note: Data analyzed at the initial barrier level; multiple barriers could be presented per office visit (from n=1 to n=4 barriers per consultation). Multiple responses could exist for a single barrier.
Of the consultations (n=151), 68.2% contained talk about one barrier, 27.2% contained talk about two barriers, 3.3% contained talk about three barriers, and 1.3% contained talk about four barriers. Thus, the vast majority of the consultations contained talk about only one barrier. A mean of 1.37 barriers were discussed per consultation (SD=0.6). Among these consultations, the overwhelming majority (90.7%) contained a physician recommendation specifically for a colonoscopy (either alone [57.6%] or in combination with another modality [33.1%]). Most barrier talk, therefore, referred specifically to colonoscopies.
Physician Responses to Patient Reported Barriers
Physicians responded to barriers in a total of 12 different ways (see Table 4) that were combined into three main categories: (1) specific response, (2) general response, and (3) non-response (details below regarding the categories created). The most common physician responses to barriers included giving advice/suggestions on how to overcome the barrier (e.g., “When you call to schedule it, make sure you do it in the morning”), stating the benefit of getting a colonoscopy (e.g., “not for ten more years” or “find cancer early”); recommending a colonoscopy (e.g., “You really need to get the colonoscopy”); and asking if the patient will get a colonoscopy (e.g., “Can I refer you?”).
Table 4.
Codes used for analyzing the physician responses to patient-reported barriers in the audio-recorded office visits and prevalence of occurrence (n=208 barriers).
| Category | Physician Response to Patient-Reported Barrier | Prevalence |
|---|---|---|
| Specific response | Gives advice/suggestions on how to address the barrier | 32.7% |
| Probes for additional information about barrier | 18.3% | |
| Empathizes through normalizing, acknowledging, or validating | 13.9% | |
| Offers alternative to colonoscopy | 13.0% | |
| Offers help | 1.0% | |
| General response | States benefits of screening | 27.9% |
| Recommends screening | 25.0% | |
| Asks if patient will do screening | 22.6% | |
| Humor | 3.8% | |
| Refers to specialist | 2.4% | |
| Non-response | No substantive response | 6.7% |
| Orders only | 5.8% | |
Note: Data analyzed at the initial barrier level; multiple barriers could be presented per office visit (from n=1 to n=4 barriers per consultation). Multiple responses could exist for a single barrier
Among all barriers presented (n=208), the physician response most commonly present was a specific response to barriers (62.0%), followed by a general response to barriers (56.3%) and no response to barriers (12.5%). In the specific response category, physicians gave some form of guidance (e.g., “Actually, you won’t need to do that”), probed about the barrier (e.g., “Why are you scared?”); empathized through normalizing, acknowledging, or validating (e.g., “The prep is awful, I know, I did it.”); or offered an alternative or to help (e.g., “Do you need help getting the appointment?”). In the general response category, physicians responded more generally rather than on the content of the barrier by stating the benefits of it (e.g., “Find cancer early”); recommending it (e.g., “I recommend you do this”); asking if the patient will do it (e.g., “Can I refer you?”); using humor (e.g., “Tell them that was a different patient” when patient expresses being turned down for too many refusals); or referring to a specialist. Finally, in the non-response category, physicians simply ordered the CRC screening (without responding to the barrier) or gave no response.
Presence of Physician Response Type by Barrier Content
Table 5 provides a full breakdown of the presence (versus absence) of physician response categories (specific to barrier, general to barrier, or no response) for each of the barrier content types (system failure, logistics, test-related, psychological, and other/unknown). With the exception of psychological barriers, physicians responded to all barrier types most commonly with a specific response. For psychological barriers, physicians responded most commonly with a general response.
Table 5.
Frequency of the presence of physician response type (specifically to barrier, general to barrier, or no response) by barrier content.
| Barrier Content | Specific Response Present | General Response Present | No Response Present |
|---|---|---|---|
| 1. Systems failure (n=32) | 56.3% | 43.8% | 12.5% |
| 2. Logistics (n=42) | 61.9% | 50.0% | 19.0% |
| 3. Test-related (n=60) | 78.3% | 55.0% | 5.0% |
| 4. Psychological (n=54) | 48.1% | 68.5% | 15.1% |
| 5. Other/unknown (n=20) | 60.0% | 60.0% | 15.0% |
Note: Physician response (specific, general, and no response) was coded as present or absent in response to a specific barrier category type, regardless of the frequency of that response. In most cases, physicians responded to barrier types with multiple responses. As such, more than one response per barrier type can exist, totaling to over 100%.
Association between Physician Barriers and Patient Adherence to CRC Screening
All chi-square tests were non-significant, indicating that physician response types to barriers were not significantly associated with adherence to CRC screening. Results also indicate that no physician response types significantly predicted adherence to CRC screening recommendations.
Association between Presence of Barrier Talk and Patient Adherence to CRC Screening
Among the consultations in which CRC screening was discussed (n=413), a total of 193 (46.7%) were adherent and 220 (53.3%) were not adherent to their physician’s recommendation for CRC. Among this same sample of consultations (n=413), 37% (n=151) contained barrier talk and 63% (n=262) contained no barrier talk. Results from the simple logistic regression indicated that the presence of barrier talk (vs. no barrier talk) was significantly associated with patient adherence to physician recommended CRC screening such that those in which a barrier was discussed were approximately half as likely to adhere to a physician recommended CRC screening (odds ratio = 0.568, 95% CI: 0.377, 0.855, p=.007, see Table 6).
Table 6.
Logistic regression of adherence to CRC screening regressed on to presence of barrier talk.
| Variable | B | SE | df | Odds Ratio | 95% CI | p value |
|---|---|---|---|---|---|---|
| Barriers raised 0 = no barrier, 1 = barrier raised | −.566 | .209 | 1 | .568 | 0.377, 0.855 | .007 |
CRC = colorectal cancer; SE = standard error; df = degrees of freedom; CI = confidence interval 6
Discussion
The goal of the present study was to examine patients’ reporting of and physicians’ responses to CRC screening barriers and to examine the associations between these factors and patients’ adherence to recommended CRC screening behaviors. Results indicated that physician responses to barriers were not associated with patients’ CRC screening, but the presence of barrier talk was significantly associated with less patient adherence to CRC screening. Among the consultations in which barrier talk did occur, the majority of patients’ barrier were test-related and psychological. The majority of physicians responded to the barriers that were raised. Nearly all barriers were brought up in the context of an ongoing CRC screening discussion or as a direct response to a physician’s question, highlighting the important role of physicians in initiating these conversations so that patients can express their barriers to screening. This may reflect the importance of face-saving and face-maintaining communication techniques in CRC screening discussions.
Patients rarely initiated a conversation about the barrier themselves. Other research on doctor-patient communication describes how patients are reluctant to threaten the doctor’s face and fear “treading on the doctor’s turf,” (Imes, Bylund, Sabee, Routsong, & Sanford, 2008) which may help explain the reluctance of the patient to initiate the conversation about barriers. Future research should specifically measure how influences such as needing to save the doctor’s face may influence CRC screening and barriers discussions.
These findings are in line with prior research that has clearly established that several patient-reported barriers are associated with a reduced likelihood of patients engaging in CRC screening (Beydoun & Beydoun, 2008; Bynum et al., 2012; Jones, Devers, et al., 2010; Jones, Woolf, et al., 2010). Moreover, our findings build off prior research that has demonstrated physician recommendation for CRC screening is a major contributor to screening behavior (Brenes & Paskett, 2000; Cairns & Viswanath, 2006; Khankari et al., 2007; Laiyemo et al., 2014; Modiri et al., 2013; Thompson et al., 2014; Wolf et al., 2006).
Our findings that the barriers most commonly reported by patients included test-related barriers (preparation, risk of injury, etc.) and psychological barriers (fear, doesn’t want it, etc.) are consistent with prior research and suggest that modality-specific barriers (e.g., prep; stool card preparation) may be more critical intervention targets than general barriers (e.g., lack of knowledge, cost) (Jones, Devers, et al., 2010; Jones, Woolf, et al., 2010). These categories are ones that may be more likely to threaten a patient’s “face.” Moreover, our results indicate that common patient barriers may be related to concerns about the process of having CRC screening (e.g., test-related and psychological barriers).
Most barriers were reported within the context of an ongoing CRC screening discussion or in response to a direct question from a physician in regards to whether or not screening had been completed. This indicates that communication and connection with one’s physician may be critical to being able to bring up topics that may threaten one’s “face.” Less frequently, barriers were introduced when a physician asked the patient directly why he or she had not or was not willing to have screening. Patients rarely raised a barrier completely on their own. One implication of these findings is that it may take time during a discussion about CRC screening for a patient to be ready to introduce a barrier to CRC screening, which could be tied to concerns about losing “face.” This finding is in line with prior research which indicates that delivery of preventive services increases with visit length (Shires et al., 2012), indicating that time spent with one’s physician may be linked to better screening rates. Based on results from the present study, this could be linked to patients’ ability or willingness to bring up their concerns or barriers about CRC screening.
Prior research has indicated that CRC screening discussions do not often include consideration of patient preferences, choices, and concerns (Holden et al., 2010). The danger of not prompting patients to discuss their concerns regarding CRC screening is that patients may not be allowed to engage in informed decision-making through discussion of concerns, risks, benefits, and patients’ preferences (Ling et al., 2008; Wackerbarth, Tarasenko, Joyce, & Haist, 2007). Our data illustrate that physician prompting may be a key influencer on whether patients end up discussing a barrier, given that most barriers were reported in response to physician prompts. Similar to the importance of physician recommendations in patients adhering to CRC screening (Beydoun & Beydoun, 2008), it could also be important to address any barriers that may exist. Thus, it is recommended for physicians to ask their patients directly about potential concerns or barriers to CRC.
Very few barriers were unacknowledged by physicians. Instead, physicians responded to patients’ barriers with specific responses (e.g., offer help, give advice/suggestions) and general responses (e.g., state benefits of screening, recommend screening). This presence of response indicates that the majority of the time, physicians are attending to positive face. Despite this general trend of responding to barriers, results indicate that the type of physician response did not significantly predict patients’ engagement in CRC screening. One limit of our data is that they are not able to address CRC screening adherence rates among patients who may have barriers they choose not to discuss with their physician. Discussions regarding CRC screening may be critical to patients bringing up their concerns regarding CRC screening. Prior research has indicated that merely eliciting CRC screening barriers has been associated with screening (Nápoles et al., 2015), highlighting the importance of physicians having these discussions with their patients regarding CRC screening and potential barriers. Furthermore, prior research indicates that discussing not only barriers but patients’ understanding, preferences, and risks and benefits may be critical to both empowering patients to engage in informed decision making and to improve the rates of CRC screening among patients for whom it is appropriate (Holden et al., 2010). Thus, any discussion and response on the physician’s part may help improve patients’ adherence to CRC screening recommendations.
Interestingly, physicians responded the majority of the time to test-related barriers by giving a specific response, such as offering an alternative screening measure or probing for additional information. However, in responding to psychological barriers reported, physicians responded with general responses the majority of the time, such as using humor or asking the patient if they will do the screening. With the exception of psychological barriers, physicians responded to other barrier types (systems failure, logistics, other) with specific responses the majority of the time. These findings suggest that for psychological barriers, physicians may feel less equipped to provide specific recommendations or suggestions for how patient-reported barriers might be overcome. Further research is needed to examine whether this is due to a lack of comfort in responding to psychological barriers or a potential lack of understanding how to respond specifically. Regardless, there are established counseling methods such as motivational interviewing (Wahab, Menon, & Szalacha, 2008) that could be used by physicians in response to patient-reported barriers to CRC screening. In this context, physicians use interviewing techniques to elicit from patients their own reasons for not getting screened and then eliciting preferred ways to motivate themselves to engage in the behavior.
In the present study, 37.7% of patients (n=151) were adherent to CRC screening recommendations compared to 53.0% adherence among the larger sample (n=443) as previously reported (Lafata et al., 2014). Among patients in which a CRC screening discussion occurs (n=413), patients are half as likely to adhere to CRC screening recommendations in consultations in which barrier talk is present (vs. not present). This finding indicates that this lower rate of CRC screening adherence may be due to patients who reported barriers to CRC screening being at higher risk of not adhering to a CRC recommendation than the general patient population. Because only a third of the consultations had barriers present, however, these results should be interpreted with caution as it is possible this group difference is due to other factors. Nevertheless, among the studied primary care patients, the presence of discussing barriers in a clinical consultation does seem to be associated with a potentially higher risk of being less adherent. The physician response(s) to patient-reported CRC screening barriers observed in our study were not significantly associated with CRC screening adherence.
Despite the strengths of this study, there are limitations to our study that may have accounted for this lack of association and should be considered in interpreting results of the present study. First, because this was a study of naturally occurring conversations, we had varying numbers of barriers and responses in each visit. This data structuring limitation made it impossible to examine how physician responses to each specific barrier predicted CRC screening adherence. Rather, physician responses across consultations were collapsed and proportions of responses were examined. To study the potential effect of physician responses to individual barriers more clearly, future research could train physicians to use motivational interviewing or another counseling approach when barriers are reported and then compare the effectiveness of motivational interviewing training versus no motivational interviewing training on CRC screening adherence. This would allow for a more rigorous test of both the effect of responses on specific barrier types as well as the causality of physician responses to patient-reported barriers on CRC screening adherence.
Second, we did not examine patients’ preferred responses to barrier types. Future research could do this, which would help better guide recommendations for physicians regarding communication with and responding to the specific types of barriers reported by patients. Third, we examined all types of CRC screening (colonoscopy, fecal occult blood test, etc.), yet most discussions were centered around barriers to colonoscopy. As such, it is less clear which barriers were reported by patients regarding other forms of CRC screening. Additionally, because screening adherence was coded as a dichotomous variable (yes/no), it is also less clear how barrier discussions related to patients’ adherence to any form of CRC screening or to the form specifically recommended by their physicians.
Third, many of the recorded consultations analyzed in the present study are older and thus do not reflect consultations occurring in the present climate of CRC screening discussions. Namely, there has been a lot more public awareness raised in the past decade around the importance of CRC screening. As such, these consultations may reflect patient engagement with discussions around CRC screening in an environment in which it was not commonly discussed.
Fourth, patients’ intentionality to get screened prior to meeting with the physician was not assessed or accounted for in the present study. As such, responses to the screening rates does not fully reflect rates among only patients who did not express not having any intention of getting screened, regardless of their physician’s response. Future studies should examine patients’ intentionality and future plans before and after consultations to understanding more clearly how these discussions differentially influence CRC screening rates.
Fifth, the analyses conducted on adherence in the present study are limited in nature, as they do not control for demographic and sociodemographic factors that are known to be associated with health disparities in CRC screening rates. As such, it could be that certain sociodemographic factors are related to being more or less likely to raise barriers and this could in turn be related to the differences in adherence rates seen in the present study.
Sixth, the present study’s participants were predominately female, thus the present results may not be as generalizable to a male patient population. Because prior research indicates that gender is a predictor of differences in communication within health care settings (Street Jr, 2002), it is possible that the number of barriers raised and discussed in the present study is reflective of a more predominately female sample. Specifically, women tend to be more highly communicative and inclusive in their communication styles; thus, men may be less likely to raise barriers or to discuss them with their treating physician.
Finally, our study examined the effect of physician responses to patient-reported barriers in clinical consultations in which barriers were reported, but it did not consider encounters in which no barriers were reported. Future studies could examine the effect of not discussing barriers at all, not responding to barriers when discussed, and responding to barriers when discussed to determine which component is critical to improving CRC screening adherence (discussing barriers or responding to them). Results from the present study highlight the low rate of adherence among patients who discussed barriers (37.7%), which is much lower than the national average of approximately 62% (Center for Disease Control and Prevention (CDC), 2013) and of 53.0% among the larger sample from this study (Lafata et al., 2014). Additionally, results highlight that patients may often only be raising barriers in contexts in which they are asked directly about CRC screening or have a discussion surrounding CRC screening. This highlights the potential importance of considering Facework Theory in the application of communication training around CRC screening.
Conclusion
These findings can be applied to physicians’ clinical practice by demonstrating that patient communication around CRC screening barriers should include the physician probing for or asking about barriers to CRC screening in order to elicit patients’ concerns. By doing so, physicians may be better able to help patients reach informed and preference-concordant decisions about CRC screening. This is in line with Facework Theory (Bylund et al., 2012; Goffman, 1955), which would indicate that physicians bringing up and responding positively to patients’ concerns about barriers to CRC could help patients “save face” and thus be more willing to openly discuss their concerns and barriers to CRC screening.
To apply these findings, communication skills training could help physicians learn how to respond productively to patient-reported barriers in order to patients to achieve the morbidity and mortality benefits of using CRC screening when appropriate. Moreover, physicians may help empower patients to overcome barriers to CRC screening by engaging in motivational interviewing (Markland, Ryan, Tobin, & Rollnick, 2005; Miller & Rollnick, 2012), which is designed to elicit goals for behavioral change from the patients themselves. This method has been effective at improving engagement in healthy behaviors. In summary, it is critical to train physicians to elicit barriers from patients and respond appropriately in a way that empowers patients to overcome barriers and make informed decisions regarding CRC screening.
Acknowledgments
Funding support: This work was supported by grants from the National Cancer Institute (R01-CA112379, R01-CA197205, K07-CA140778; and K07-CA207580).
Footnotes
Conflicts of interest: The authors have not conflicts of interest to report.
Ethics statement: All study procedures were approved at each Institution’s IRB. All procedures were conducted in accordance with required ethical guidelines for protection of human subjects in research. Informed consent was obtained from each participant in the present study.
Note original 19 categories are listed in Table 2.
References
- Auer-Srnka KJ, & Koeszegi S (2007). From words to numbers: How to transform qualitative data into meaningful quantitative results. Schmalenbach Business Review, 59, 29–59. [Google Scholar]
- Baxter LA, & Babbie ER (2004). The basics of communication research. Belmont, CA: Wadsworth. [Google Scholar]
- Beydoun HA, & Beydoun MA (2008). Predictors of colorectal cancer screening behaviors among average-risk older adults in the United States. Cancer Causes and Control, 19(4), 339–359. Retrieved from http://download.springer.com/static/pdf/527/art%253A10.1007%252Fs10552-007-9100-y.pdf?originUrl=http%3A%2F%2Flink.springer.com%2Farticle%2F10.1007%2Fs10552-007-9100-y&token2=exp=1463577067~acl=%2Fstatic%2Fpdf%2F527%2Fart%25253A10.1007%25252Fs10552-007-9100-y.pdf%3ForiginUrl%3Dhttp%253A%252F%252Flink.springer.com%252Farticle%252F10.1007%252Fs10552-007-9100-y*~hmac=1528604cc3bd17b910593152b9f8015b34f670ccf915edf8bf81d40dd0e80ebc. [DOI] [PubMed] [Google Scholar]
- Brenes GA, & Paskett ED (2000). Predictors of stage of adoption for colorectal cancer screening. Preventive Medicine, 31(4), 410–416. [DOI] [PubMed] [Google Scholar]
- Bylund CL, Peterson EB, & Cameron KA (2012). A practitioner’s guide to interpersonal communication theory: An overview and exploration of selected theories. Patient education and counseling, 87(3), 261–267. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3297682/pdf/nihms340567.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bynum SA, Davis JL, Green BL, & Katz RV (2012). Unwillingness to participate in colorectal cancer screening: Examining fears, attitudes, and medical mistrust in an ethnically diverse sample of adults 50 years and older. American Journal of Health Promotion, 26(5), 295–300. Retrieved from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3807238/pdf/nihms509041.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns CP, & Viswanath K (2006). Communication and colorectal cancer screening among the uninsured: Data from the Health Information National Trends Survey (United States). Cancer Causes and Control, 17(9), 1115–1125. Retrieved from http://download.springer.com/static/pdf/395/art%253A10.1007%252Fs10552-006-0046-2.pdf?originUrl=http%3A%2F%2Flink.springer.com%2Farticle%2F10.1007%2Fs10552-006-0046-2&token2=exp=1462819093~acl=%2Fstatic%2Fpdf%2F395%2Fart%25253A10.1007%25252Fs10552-006-0046-2.pdf%3ForiginUrl%3Dhttp%253A%252F%252Flink.springer.com%252Farticle%252F10.1007%252Fs10552-006-0046-2*~hmac=47678bc430812515f952bfcc99b1a36948e0d1902a4f16e999594c246b4f1df3. [DOI] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention (CDC). (2013). Colorectal cancer screening rates remain low. Retrieved from https://www.cdc.gov/media/releases/2013/p1105-colorectal-cancer-screening.html
- Centers for Disease Control and Prevention. (2013). Vital signs: Colorectal cancer screening test use--United States, 2012. Morbidity and Mortality Weekly Report, 62(44), 881. [PMC free article] [PubMed] [Google Scholar]
- Cooper LE, Roter DL, Johnson RL, & Ford DE (2003). Patient-centered communication, ratings of care, and concordance of patient and physician race. Annals of internal medicine, 139(11), 907 Retrieved from http://annals.org/pdfaccess.ashx?url=/data/journals/aim/20054/0000605-200312020-00009.pdf. [DOI] [PubMed] [Google Scholar]
- Denberg TD, Melhado TV, Coombes JM, Beaty BL, Berman K, Byers TE, … Ahnen DJ (2005). Predictors of nonadherence to screening colonoscopy. Journal of General Internal Medicine, 20(11), 989–995. Retrieved from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1490266/pdf/jgi_164.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffman E (1955). On face-work: An analysis of ritual elements in social interaction. Psychiatry, 18(3), 213–231. [DOI] [PubMed] [Google Scholar]
- Holden DJ, Jonas DE, Porterfield DS, Reuland D, & Harris R (2010). Systematic review: Enhancing the use and quality of colorectal cancer screening. Annals of internal medicine, 152(10), 668–676. Retrieved from http://annals.org/pdfaccess.ashx?url=/data/journals/aim/20206/0000605-201005180-00008.pdf. [DOI] [PubMed] [Google Scholar]
- Imes RS, Bylund CL, Sabee CM, Routsong TR, & Sanford AA (2008). Patients’ reasons for refraining from discussing internet health information with their healthcare providers. Health communication, 23(6), 538–547. [DOI] [PubMed] [Google Scholar]
- Jones RM, Devers KJ, Kuzel AJ, & Woolf SH (2010). Patient-reported barriers to colorectal cancer screening: A mixed-methods analysis. American journal of preventive medicine, 38(5), 508–516. Retrieved from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2946825/pdf/nihms-191428.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RM, Woolf SH, Cunningham TD, Johnson RE, Krist AH, Rothemich SF, & Vernon SW (2010). The relative importance of patient-reported barriers to colorectal cancer screening. American journal of preventive medicine, 38(5), 499–507. Retrieved from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2946819/pdf/nihms191427.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz ML, James AS, Pignone MP, Hudson MA, Jackson E, Oates V, & Campbell MK (2004). Colorectal cancer screening among African American church members: A qualitative and quantitative study of patient-provider communication. BMC Public Health, 4(62), 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khankari K, Eder M, Osborn CY, Makoul G, Clayman M, Skripkauskas S, … Wolf MS (2007). Improving colorectal cancer screening among the medically underserved: A pilot study within a federally qualified health center. Journal of General Internal Medicine, 22(10), 1410–1414. Retrieved from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2305844/pdf/11606_2007_Article_295.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronborg O, Fenger C, Olsen J, Jørgensen OD, & Søndergaard O (1996). Randomised study of screening for colorectal cancer with faecal-occult-blood test. The Lancet, 348(9040), 1467–1471. [DOI] [PubMed] [Google Scholar]
- Lafata JE, Cooper G, Divine G, Oja-Tebbe N, & Flocke SA (2014). Patient–physician colorectal cancer screening discussion content and patients’ use of colorectal cancer screening. Patient education and counseling, 94(1), 76–82. Retrieved from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3865022/pdf/nihms-525996.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laiyemo AO, Adebogun AO, Doubeni CA, Ricks-Santi L, McDonald-Pinkett S, Young PE, … Klabunde CN (2014). Influence of provider discussion and specific recommendation on colorectal cancer screening uptake among US adults. Preventive Medicine, 67, 1–5. Retrieved from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4167462/pdf/nihms608003.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling BS, Trauth JM, Fine MJ, Mor MK, Resnick A, Braddock CH, … Ricci EM (2008). Informed decision-making and colorectal cancer screening: Is it occurring in primary care? Medical care, 46(9), S23–S29. Retrieved from http://ovidsp.tx.ovid.com/ovftpdfs/FPDDNCDCMEBHAI00/fs047/ovft/live/gv024/00005650/00005650-200809001-00005.pdf. [DOI] [PubMed] [Google Scholar]
- MacGeorge EL, Caldes EP, Smith RA, Hackman NM, & San Jose A (2017). Reducing unwarranted antibiotic use for pediatric acute otitis media: the influence of physicians’ explanation and instruction on parent compliance with ‘watchful waiting’. Journal of Applied Communication Research, 45(3), 333–345. [Google Scholar]
- MacGeorge EL, Guntzviller LM, Hanasono LK, & Feng B (2016). Testing advice response theory in interactions with friends. Communication Research, 43(2), 211–231. [Google Scholar]
- MacGeorge EL, Smith RA, Caldes EP, & Hackman NM (2017). Toward reduction in antibiotic use for pediatric otitis media: Predicting parental compliance with “watchful waiting” advice. Journal of health communication, 22(11), 867–875. Retrieved from https://www.tandfonline.com/doi/pdf/10.1080/10810730.2017.1367337?needAccess=true. [DOI] [PubMed] [Google Scholar]
- Markland D, Ryan RM, Tobin VJ, & Rollnick S (2005). Motivational interviewing and self-determination theory. Journal of Social and Clinical Psychology, 24(6), 811. [Google Scholar]
- Miller WR, & Rollnick S (2012). Motivational interviewing: Helping people change: Guilford press. [Google Scholar]
- Modiri A, Makipour K, Gomez J, & Friedenberg F (2013). Predictors of colorectal cancer testing using the California Health Inventory Survey. World Journal of Gastroenterology, 19(8), 1247–1255. Retrieved from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3587481/pdf/WJG-19-1247.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosen DM, Feldstein AC, Perrin N, Rosales AG, Smith DH, Liles EG, … Elston-Lafata J (2013). More comprehensive discussion of CRC screening associated with higher screening. The AMerican Journal of Managed Care, 19(4), 265–271. Retrieved from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3891849/pdf/nihms534796.pdf. [PMC free article] [PubMed] [Google Scholar]
- Nápoles AM, Santoyo-Olsson J, Stewart AL, Olmstead J, Gregorich SE, Farren G, … Pérez-Stable EJ (2015). Physician counseling on colorectal cancer screening and receipt of screening among Latino patients. Journal of General Internal Medicine, 30(4), 483–489. Retrieved from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4370980/pdf/11606_2014_Article_3126.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb PA, Norfleet RG, Storer BE, Surawicz TS, & Marcus PM (1992). Screening sigmoidoscopy and colorectal cancer mortality. Journal of the National Cancer Institute, 84(20), 1572–1575. [DOI] [PubMed] [Google Scholar]
- Peterson EB, Ostroff JS, DuHamel KN, D’Agostino TA, Hernandez M, Canzona MR, & Bylund CL (2016). Impact of provider-patient communication on cancer screening adherence: A systematic review. Preventive Medicine, 93, 96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shires DA, Stange KC, Divine G, Ratliff S, Vashi R, Tai-Seale M, & Lafata JE (2012). Prioritization of evidence-based preventive health services during periodic health examinations. American journal of preventive medicine, 42(2), 164–173. Retrieved from http://ac.els-cdn.com/S0749379711008385/1-s2.0-S0749379711008385-main.pdf?_tid=7b18d43a-e963-11e6-9477-00000aacb362&acdnat=1486052595_627ee054bd5268b06d6dad54db1bd567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street RL Jr (2002). Gender differences in health care provider–patient communication: are they due to style, stereotypes, or accommodation? Patient education and counseling, 48(3), 201–206. [DOI] [PubMed] [Google Scholar]
- Thompson VLS, Lander S, Xu S, & Shyu C-R (2014). Identifying key variables in African American adherence to colorectal cancer screening: The application of data mining. BMC Public Health, 14, 1173 Retrieved from http://download.springer.com/static/pdf/651/art%253A10.1186%252F1471-2458-14-1.pdf?originUrl=http%3A%2F%2Fhttp%3A%2F%2Fbmcpublichealth.biomedcentral.com%2Farticle%2F10.1186%2F1471-2458-14-1&token2=exp=1462818044~acl=%2Fstatic%2Fpdf%2F651%2Fart%25253A10.1186%25252F1471-2458-14-1.pdf*~hmac=437775317c7fa534bb25ff115d3760038b36909a719402aa5ce452447aea9f74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wackerbarth SB, Tarasenko YN, Joyce JM, & Haist SA (2007). Physician colorectal cancer screening recommendations: An examination based on informed decision making. Patient education and counseling, 66(1), 43–50. Retrieved from http://ac.els-cdn.com/S0738399106003417/1-s2.0-S0738399106003417-main.pdf?_tid=4fb89b82-e4c6-11e6-919a-00000aacb35e&acdnat=1485545287_8223c130e07b50df5d9e8f8894becbff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahab S, Menon U, & Szalacha L (2008). Motivational interviewing and colorectal cancer screening: A peek from the inside out. Patient education and counseling, 72(2), 210–217. Retrieved from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2562583/pdf/nihms-60084.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf MS, Satterlee M, Calhoun E, Skripkauskas S, Fulwiler D, Diamond-Shapiro L, … Mukundan P (2006). Colorectal cancer screening among the medically underserved. Journal of health care for the poor and underserved, 17(1), 47–54. [DOI] [PubMed] [Google Scholar]
- Zapka JG, Puleo E, Vickers-Lahti M, & Luckmann R (2002). Healthcare system factors and colorectal cancer screening. American journal of preventive medicine, 23(1), 28–35. [DOI] [PubMed] [Google Scholar]