Abstract
Introduction
The acute respiratory distress syndrome (ARDS) is a devastating clinical condition common in patients with respiratory failure. Based largely on numerous preclinical studies and recent Phase I/II clinical trials, administration of stem cells, specifically mesenchymal stem or stromal cells (MSC), as a therapeutic for acute lung injury (ALI) holds great promise. However, concern for the use of stem cells, specifically the risk of iatrogenic tumor formation, remains unresolved. Accumulating evidence now suggest that stem cell-derived conditioned medium (CM) and/or extracellular vesicles (EV), might constitute compelling alternatives.
Areas covered
The current review focuses on the preclinical studies testing MSC CM and/or EV as treatment for ALI and other inflammatory lung diseases.
Expert opinion
Clinical application of MSC or their secreted CM may be limited by the cost of growing enough cells, the logistic of MSC storage, and the lack of standardization of what constitutes MSC CM. However, the clinical application of MSC EV remains promising, primarily due to the ability of EV to maintain the functional phenotype of the parent cell as a therapeutic. However, utilization of MSC EV will also require large-scale production, the cost of which may be prohibitive unless the potency of the EV can be increased.
Keywords: Acute Lung Injury, Acute Respiratory Distress Syndrome, Exosomes, Extracellular Vesicles, Mesenchymal Stem or Stromal Cells, Microvesicles
1. INTRODUCTION
Acute respiratory distress syndrome (ARDS) is a common and devastating clinical condition among patients with respiratory failure in the intensive care units with high morbidity and mortality rates. Even among patients who survive ARDS, physical and psychological dysfunctions remain, affecting their long-term quality of life[1]. The pathophysiology of ARDS is characterized by increased permeability of the alveolar-capillary membrane, influx of protein-rich pulmonary edema fluid, and surfactant dysfunction, which results in severe hypoxemia. Dysregulated immune activation also results in the release of proinflammatory cytokines/chemokines and neutrophilic influx into the alveolar space. Despite the development of supportive therapies such as lung protective ventilation, prone positioning, neuromuscular blockade and extracorporeal membrane oxygenation, mortality rates remain as high as 35% for mild, 40% for moderate and 46% for severe ARDS[2–6]. There are no effective pharmacotherapy for ARDS.
Stem cell based therapy using mesenchymal stem or stromal cells (MSC), endothelial progenitor cells (EPC), embryonic stem cells (ESC) and induced pluripotent stem cells (iPSC) has emerged as a possible novel treatment option for ALI[7]. For this review, we will focus on MSC due to the enormous published literature demonstrating therapeutic efficacy, including some early Phase I and II clinical trials, widespread bioavailability and limited ethical concerns[8,9]. MSC represent a class of adult stem cells, initially identified and isolated from the bone marrow in 1968 by Friedenstein et al., which are capable of multipotential differentiation and have significant immunomodulatory or anti-inflammatory effects[10]. MSC can be derived from a variety of tissues including the bone marrow, adipose tissue, placenta, umbilical cord and cord blood. More importantly, therapeutic application of MSC is effective in various preclinical ALI models induced by lipopolysaccharide (LPS), gram positive and negative bacteria, bleomycin, ischemia-reperfusion injury, acute pancreatitis or following cecal ligation and puncture (CLP). Recently, MSC have also been administered in Phase I and II clinical trials in patients with moderate to severe ARDS or sepsis with no significant side effects or adverse events[8,9,11]. In the START study, a prospective, randomized, multi-center Phase IIa clinical trial of allogeneic bone marrow derived MSC for the treatment of ARDS, one dose of intravenous MSC (10 million cells/kg predicted body weight) was safe in patients with moderate to severe ARDS. Interestingly, despite adequate cell viability at the time of manufacturing, there were significant variations in MSC viability at the time of administration (36% to 85%) which may impact efficacy[8]. In the Cell Immunotherapy for Septic Shock (CISS) Phase 1 dose escalation clinical trial, infusion of freshly cultured allogeneic bone marrow derived MSC, up to a dose of 3 million cells/kg (250 million cells), into patients with septic shock had no MSC associated adverse events[11]. Stratification of the interventional cohort by MSC dose revealed an early but transient dampening of pro-inflammatory cytokines[12].
The mechanisms underlying the therapeutic effects of MSC in ALI are multiple and have been extensively reviewed[7]. In summary, once considered for their regenerative properties, MSC have limited engraftment rates and trans-differentiation capability in damaged tissues[13]. More importantly, administration of the CM of MSC have resulted in a similar therapeutic effect as seen with the cells[14]. Therefore, it is now widely accepted that MSC are effective as a therapeutic mainly due to the secretion or release of paracrine soluble factors including growth factors, anti-inflammatory cytokines, antimicrobial peptides, organelles (i.e. mitochondria) and, more recently, extracellular vesicles (EV)[15]. In this review, we will summarize the biological rationale, available preclinical data, and the possible mechanisms for the therapeutic effects of MSC derived CM and EV for ALI and other lung diseases.
2. MSC SECRETOME OR CONDITIONED MEDIUM FOR ACUTE LUNG INJURY AND OTHER INFLAMMATORY LUNG DISEASES
The biological rationales for using MSC derived secretome or CM are largely based on three preclinical findings: 1) The majority of studies have demonstrated that the mechanisms underlying the therapeutic effects of MSC were due to secretion of soluble factors[15]. For example, in both sterile and infectious models of ALI, MSC secretion of keratinocyte growth factor (KGF) and angiopoietin-1have been shown to protect the alveolar epithelium and endothelium from injury in terms of lung protein permeability and loss of alveolar fluid clearance (AFC)[15–20]. Other MSC soluble factors such as interleukin-10, prostaglandin-E2, TSG-6 or, more recently, specialized pro-resolving lipid mediators such as Lipoxin A4 have also been found to have anti-inflammatory properties whereas other soluble factors such as Lipocalin 2 or LL-37 have antimicrobial activity[21–26]; 2) Most preclinical studies have shown low engraftment rates (<5%) following intravenous or intra-bronchial administration, demonstrating that MSC regeneration of injured cells was not significant[27–31]; 3) Finally, cell-free MSC CM recapitulated many of the therapeutic effects of MSC[32].
In a model of ALI induced by intra-tracheal (IT) LPS, MSC CM (concentrated 25x) administered IT 4 hours following injury decreased the alveolar influx of inflammatory cells and prevented pulmonary edema formation in part by promoting an anti-inflammatory M2 macrophage phenotype via insulin-like growth factor I secretion[33]. In LPS-induced ALI in an ex vivo perfused human lung, Lee et al. found that IT administration of MSC CM 1 hour following injury decreased inflammation, prevented the influx of neutrophils and prevented pulmonary edema formation by restoring lung protein permeability and increasing AFC in the injured alveolus[17]. Blocking KGF secretion by pretreating MSC with KGF siRNA abrogated the therapeutic properties of the MSC CM.
In bleomycin-induced ALI, investigators demonstrated that MSC CM attenuated the influx of inflammatory cells within the alveolar space and reversed histological evidence of lung fibrosis. Anti-inflammatory and anti-fibrotic effects were found to be driven by the restoration of lung-resident MSC which was accompanied by an inhibition of T cell proliferation[34]. Several investigators utilized hyperoxia-induced injury in a model of bronchopulmonary dysplasia (BPD) in mice or rat pups to study the therapeutic effects of MSC CM (concentrated 20–25x)[35–41]. Hyperoxic conditions were applied immediately following birth and lasted for up to 14 days, and MSC CM was given via the intraperitoneal (IP), intravenous (IV), or IT route once or daily[35–38,40,41]. These studies demonstrated that MSC CM reduced lung inflammation and histological injury, restored lung compliance, and prevented pulmonary hypertension, a cardinal feature of BPD. Several pathways were identified as responsible for the beneficial effects of MSC CM in BPD, such as inhibition of macrophage stimulating factor-1 and monocyte chemoattractant protein-1, increase in osteopontin expression, suppression of proinflammatory cytokines (interleukin-6, interleukin-1β), increase in stanniocalcin-1 and expression of other antioxidants, and increased angiogenesis[35,38,40]. Pierro et al. administered MSC CM either during the hyperoxic exposure or 14 days following the hyperoxic exposure, which enabled them to study respectively a preventive and treatment approach in rat pups[41]. Interestingly, in both studies, MSC CM was capable of decreasing lung inflammation while increasing lung compliance and improving lung histology. The authors also found that both pulmonary arterial remodeling and right ventricular hypertrophy (i.e., pulmonary hypertension) were prevented or fully reversed in the group of animals treated with MSC CM.
MSC CM have also showed promising results in asthma and chronic emphysema in terms of reducing inflammation and histological damage within the bronchoalveolar airspace and lung parenchyma[42–44]. In both acute and chronic ovalbumin-induced asthma models in mice, Ionescu et al. showed that MSC CM attenuated the infiltration of inflammatory cells into the alveolar or peri-bronchial space, restored the bronchodilator response to salbutamol, suppressed the increase in both dynamic lung resistance and elastance, and reduced the thickness of airway smooth muscle layers[42]. The beneficial effects of MSC CM were partially explained by the restoration of a regulatory T cell subset overexpressing IL-10 and the induction of an emerging subset of IL-10 secreting monocytes-macrophages. In a rat model of emphysema induced by cigarette smoke (CS) exposure, MSC CM improved lung histology, increased lung vasculature density, and lowered right ventricular systolic pressure[43].
These studies provided evidence that MSC CM was capable of recapitulating the therapeutic effects of MSC in ALI and other inflammatory lung diseases. However, using MSC CM as a therapeutic clinically has potential limitations: 1) Due to the lack of standardization in terms of the culture conditions used for MSC (i.e., preconditioning), it is difficult to assess the potency of MSC derived CM vs. MSC among the preclinical studies; 2) More importantly, this lack of understanding of the potency of the MSC CM makes it difficult to determine the optimal therapeutic dose, volume of instillate, timing, and route of administration.
3. MSC DERIVED EXTRACELLULAR VESICLES
MSC derived EV were isolated and characterized from human MSC CM as a therapeutic for multiple organ injury models prior to study in ALI[45,46]. Although their potency differed from the cells, MSC EV were found to have a similar phenotype and functional effect as their parent MSC which was dependent on their content (mRNA, microRNA, transfer RNA, proteins and organelle)[47]. MSC EV were selectively enriched in distinct class of RNAs. Eirin et al. found that there were at least 4 miRNAs and 255 mRNAs which were further enriched in EV compared with MSC[48]. EV preferentially expressed mRNA for transcription factors and genes involved in angiogenesis, adipogenesis, Golgi apparatus and TGF-β signaling whereas genes for mitochondrial function, calcium signaling and the cytoskeleton were selectively excluded from the EV. Among the 386 annotated miRNAs in MSC EV, the level of miR148a, miR532–5p, miR378, and let-7f were significantly higher in EV than in MSC[49]. Protein levels were also expressed differently in MSC and MSC EV. EV did express MSC surface markers including CD105, CD73, CD29, and CD44 as well as exosome specific markers such as CD9 and CD63[50,51]. However, MSC EV expressed 4,937 distinct proteins vs. 5,469 distinct proteins for MSC. Among these proteins, 128 proteins related to angiogenesis, blood coagulation, apoptosis, extracellular matrix remodeling, and regulation of inflammation were upregulated in EV vs. MSC, whereas 563 proteins were excluded from EV[48]. Further studies are warranted to determine if the differences in the biological content of EV vs. MSC are responsible for the different potency of the EV as a therapeutic. Specifically, do mRNA, microRNA or protein levels in the MSC vs. EV differ substantially enough to account for the different biological effects.
3.1. Extracellular Vesicles
Most eukaryotic cells release small membrane bound and anuclear microparticles into the extracellular space in both physiological or pathophysiological conditions often labelled “extracellular vesicles” by the International Society for Extracellular Vesicles[52]. EV are considered important mediators of intracellular communication in part due to their ability to transfer their bioactive contents to target cells. Recently, the potential roles of EV as biomarkers and vectors involved in the pathogenesis or as a therapeutic in multiple diseases have generated significant excitement[53]. EV comprise a large group of heterogeneous particles and are classified as exosomes, microvesicles (MV) (also referred to as ectosomes, shedding vesicles, microparticles, plasma membrane-derived vesicles or exovesicles in the literature) and apoptotic bodies (or apobodies) according to their size, biogenesis and secretion mechanisms (Table 1) [54].
Table 1.
Characteristics of Extracellular Vesicles
| Vesicles | Size (nm) | Origin | Markers | Cell surface markers |
|---|---|---|---|---|
| Exosomes | 20–200 | Intraluminal vesicles within multi-vesicular bodies are released into the extracellular space by fusion with the cell membrane. | CD63, CD81, CD9, Tsg101, Alix, Hsc70 | CD63, CD9 |
| Microvesicles | 100–1000 | Outward budding and fission of the plasma membrane. | Lipid raft-associated molecules (TF, flotillin), PS | ARF6, VCAMP3 |
| Apoptotic Bodies | >1000 | By blebbing of plasma membranes of apoptotic cells. | PS | TSP, C3b |
Hsc70, heat-shock cognate protein 70; PS, phosphatidylserine; TF, tissue factor; Tsg101, tumor susceptibility gene 101.
The smallest of the EV are exosomes with a diameter of 20 to 200 nm and formed during the maturation of early endosomes into late multi-vesicular bodies (MVB). Once the MVB fuse with the plasma membrane, the intraluminal vesicles within the MVB are released into the extracellular space and are termed exosomes[55,56]. Exosomes contain a series of evolutionarily conserved proteins including tetraspanins (CD63, CD81, CD9), heat-shock proteins (Hsp60, Hsp70 and Hsp90), ALG-2 interacting protein X, tumor susceptibility gene 101, and MHC classes I and II and express low amounts of phosphatidylserine[57]. Microvesicles (MV) are larger in size (100–1000 nm) and are generated from outward budding and fission of the plasma membrane. The budding is formed via the translocation of phosphatidylserine into the outer-membrane leaflet and is completed through contraction of cytoskeletal structures by actin-myosin interaction[58]. MV display high amounts of phosphatidylserine, cholesterol, and proteins associated with lipid rafts and are enriched in sphingomyelin and ceramide[59]. Apoptotic bodies, with a size >1000 nm, are released from the plasma membrane of apoptotic cells as blebs and are destined to be cleared locally through phagocytosis[60,61]. Apoptotic bodies are characterized by phosphatidylserine externalization and contain histones and fragmented DNA. Certain markers can be used to identify exosomes, MV, and apoptotic bodies. For example, CD63 and CD9 are specific markers of exosomes; ARF6 and VCAMP3 are recently proposed markers of MV; and TSP and C3b are generally accepted markers of apoptotic bodies (Table 1)[58].
Both exosomes and MV carry multiple cellular components specific to their parent cell in various physiological and pathological states[62,63]. Two web-based compendium of proteins and RNAs found in exosomes are freely accessible at Exocarta (http://www.exocarta.org) or Vesiclepedia (http://www.microvesicles.org) [64,65]. There are various ways EV interact with their target cells. EV can either directly stimulate the target cells with specific receptor-ligand interactions or transfer receptors, bioactive lipids as well as intracellular RNAs/proteins through endocytosis, fusion or phagocytosis of the EV by target cells[66–70]. The observation in multiple studies that pretreatment of EV with RNAase eliminated the biological effects of EV demonstrated the importance of the EV content[71].
3.2. Isolation and Characterization of Extracellular Vesicles
There are multiple techniques to isolate EV from cell culture or biological fluids including ultracentrifugation, filtration, immune-affinity isolation, and polymeric precipitation. These techniques have been described extensively, and we will only briefly highlight the commonly used ones[54]. In ultracentrifugation, EV are pelleted using high speed centrifugation (100,000 – 200,000×g) to remove cellular debris[72,73]. Unfortunately, ultracentrifugation cannot separate out MV from exosomes nor can it totally remove protein complexes or lipoprotein particles, although resuspending and re-centrifuging can decrease these contaminants[74]. Combining ultracentrifugation with density gradients may allow separation of exosomes, with relatively low density, from the remaining EV[75]. EV can also be isolated based on their size using size exclusion filters and/or column chromatography. Ultrafiltration is faster than ultracentrifugation and does not require special equipment. However, the use of excessive force with either technique may result in deformation and damage to the EV[76]. In immune-affinity isolation, specific surface proteins on EV are used to select a desired EV population via antigen-antibody interaction, ligand-receptor binding or immunoaffinity or magneto immunocapture which may be superior to ultracentrifugation in terms of exosome yield[77]. Various alternative techniques have also been described such as polymeric precipitation or microfluidics-based devices[78–80].
A consensus panel was convened by the International Society of Extracellular Vesicles in 2018 to characterize EV to allow better comparisons between preclinical studies[81]. The analyses of their recommendations on global quantification (i.e., protein concentration vs. cell count of the source), transmembrane/lipid bound or cystolic protein characterization (i.e., to differentiate MV from exosomes), and description of single vesicle (i.e., electron microscopy) vs. molecular size profile (i.e., nanoparticle analyses) are beyond the scope of this review. However, improved characterization of EV will be critical in understanding the potency of MSC EV between studies.
4. THERAPEUTIC EFFECT OF MSC EV IN PRECLINICAL MODELS OF ALI
Multiple recent studies have explored the therapeutic use of MSC derived EV in ALI models. Zhu et al. found that IT instillation of human MSC MV following LPS induced ALI reduced extravascular lung water, pulmonary edema and lung protein permeability compared to injured mice at 48 hours[82]. Elimination of angiopoietin-1 or KGF mRNA in the MSC MV by siRNA eliminated the protective effects in terms of reducing alveolar inflammation and lung protein permeability[83]. Monsel et al. demonstrated that the administration of human MSC MV improved survival and decreased lung inflammation, protein permeability, and bacterial growth in a mice model of severe Escherichia coli pneumonia[84]. Further investigation of the mechanisms suggested that MSC MV enhanced the phagocytosis of bacteria by monocytes/macrophages via transfer of cyclooxygenase 2 mRNA from the MV to the monocytes/macrophages resulting in a shift towards an anti-inflammatory M2 phenotype. Recently, Park et al. found similar therapeutic effects of MSC EV in an ex vivo perfused human lung model of severe bacterial pneumonia[85]. The investigators found that intravenous instillation of MSC EV restored AFC rate and lung protein permeability, reduced pulmonary edema, and, perhaps more importantly, decreased the bacterial load in the injured alveolus replicating the findings in mice. All the investigators were unable to duplicate the beneficial effects of MSC EV with EV isolated from human lung fibroblasts used as a control.
5. MECHANISMS UNDERLYING THE THERAPEUTIC EFFECTS OF MSC EV IN ALI
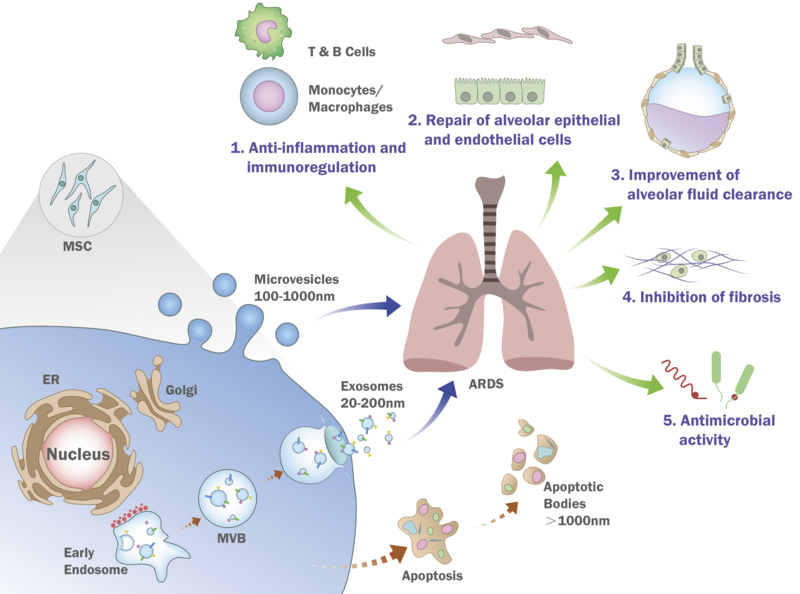
Studies on the mechanisms underlying the therapeutic effects of MSC EV in ALI are on-going, however, several pathways have been identified, many of which are similar to ones identified with MSC (Figure).
Figure. Schematic of the Possible Mechanisms Underlying the Therapeutic Effects of Mesenchymal Stem or Strom Cell Extracellular Vesicles.
Both exosomes and microvesicles released from MSC have shown reparative properties in pre-clinical lung injury models in terms of immunomodulation, repair of the alveolar epithelium and endothelium, suppression of fibrosis as well as reparative properties specific to ALI/ARDS (restoration of the alveolar fluid clearance and antimicrobial activity). Although MSC EV is being tested in an early Phase I clinical trial for preterm neonates with BPD, some major limitations to clinical use are whether the production and potency of the MSC can be scaled up or increased significantly to generate enough EV to treat larger size individuals such as adults.
5.1. Immunomodulation/Anti-inflammatory Properties
Dysregulated inflammation is a critical pathological feature of ALI/ARDS. With their immune-privileged status and ability to immunomodulate innate and adaptive immune responses, MSC have been found to have significant anti-inflammatory properties in preclinical models of ALI and/or sepsis[86–88]. Similar to MSC, MSC EV were also found to modulate immune cells. Budoni et al. found that MSC EV inhibited B cell proliferation and differentiation in a dose-dependent manner, an effect which was more pronounced with EV derived from cytokine primed MSC. MSC EV could also regulate the activation of T cells[89,90]. Human bone marrow derived MSC exosomes induced the conversion of T helper (Th) cells toward Th type 2 rather than Th type 1 phenotype, suppressed the differentiation of T cells into interleukin 17-producing effector T cells and increased the level of regulatory T cells[91]. Del Fattore et al. also demonstrated that MSC EV increased the ratio between regulatory T and effector T cells reducing the pro-inflammatory cytokines released from T cells including IFN-γ, TNFα, and IL-1β while increasing the anti-inflammatory cytokines such as TGF-β, IL-10, and PGE2[92,93]. Perhaps more importantly, multiple investigators found that MSC EV were able to polarize macrophages from an inflammatory M1 to an anti-inflammatory M2 phenotype[94]. Ti et al. showed that exosomes derived from LPS pretreated MSC promoted M2 macrophage polarization and upregulated the expression of anti-inflammatory cytokines, an effect which was mediated by shuttling let-7b and the subsequent activation of the TLR4/NFκ β/STAT3/AKT regulatory signaling pathway[95]. In addition, administration of MSC EV suppressed inflammation and promoted tissue repair in other immune related disorders such as acute Graft-Versus-Host Disease, pancreatitis, and burn injury[96–98].
5.2. Promoting the Repair of Alveolar Epithelium and Endothelium in ALI
Several studies have shown the potential reparative properties of MSC EV on microvascular endothelial and epithelial cells, which are often severely injured in the lung during ALI and associated with increased mortality in patients with ARDS[99]. Hu et al. found that MSC EV restored protein permeability across human lung microvascular endothelial cells injured with an inflammatory insult via transfer of Angiopoietin-1 mRNA from the EV to the cell, preventing “actin stress fiber” formation[100]. Bian et al. found that co-incubation of MSC EV with human umbilical vein endothelial cells increased the proliferation, migration, and tube formation of the endothelial cells in vitro[101]. MSC EV were also found to have pro-angiogenic properties in vivo by stimulating vessel formation in SCID mice[102]. Similar studies using an acute myocardial infarction model demonstrated MSC EV improved coronary blood flow, reduced infarct size and preserved cardiac function[101,103,104]. Wang et al. found that miR-210 enriched in MSC EV targeted angiogenesis-related gene Efna3 and was one of the key effectors in increased angiogenesis[103]. Although there are several recent studies exploring the therapeutic roles of MSC EV in pulmonary arterial hypertension in models of BPD (discussed below), studies on whether MSC or MSC EV can repair the pulmonary vascular dysfunction often seen in ARDS are limited and will require future study[105].
There have been only a few studies exploring the effect of MSC EV on the alveolar epithelium such as the one by Monsel et al. which demonstrated that MSC MV increased intracellular ATP levels and survival among human alveolar epithelial type II cells injured with cytomix, an inflammatory mixture of TNFα, IL-1β and IFNγ often used as a surrogate for ARDS pulmonary edema fluid[84]. However, MSC EV have been studied as a therapeutic using epithelial cells originating from other tissues/organs. Zhou et al. found that exosomes from human umbilical cord-derived MSC ameliorated oxidative stress induced injury and apoptosis in NRK-52E cells, a rat renal proximal epithelial cell line, and promoted cell proliferation[106]. Similarly, Lindoso et al. found that incorporation of MSC EV into injured proximal tubular epithelial cells following ischemia-reperfusion injury in vitro prevented cell death[107]. The proposed mechanisms were through the regulation of intracellular mRNA associated with apoptosis, cytoskeleton reorganization and hypoxia such as CASP3 and 7, SHC1 and SMAD4 via induction of HGF and ERK 1/2 MAPK pathways or through inhibition of mitochondrial fission through miRNA transferred from MSC EV[106–109]. The effect MSC EV on alveolar epithelium in the content of ALI needs further study.
5.3. Improvement of Alveolar Fluid Clearance
Impaired AFC resulting in pulmonary edema formation is a clinical hallmark of ARDS and is associated with increased mortality rates[110]. Using both in vitro and in vivo, multiple investigators have demonstrated that MSC instillation restored AFC rate following LPS-induced ALI and following severe bacterial pneumonia in part by secretion of KGF and angopoietin-1, which restored the critical sodium channel, ENaCα among alveolar epithelial type II cells and prevented “actin stress fiber” formation in the lung epithelium[17,18]. Li et al. also found that secretion of KGF by MSC up-regulated the expression of the α1 subunit of the Na+-K+-ATPase in LPS injured alveolar epithelial type II cells, restoring alveolar fluid transport[111]. Similar to MSC, administration of MSC EV restored AFC rate following Escherichia coli pneumonia in mice and in an ex vivo perfused human lung in part by transfer of KGF mRNA from the EV to the target cells with subsequent expression of the growth factor[84,85].
5.4. MSC EV Antimicrobial Activity
Bacterial pneumonia is the among the predominant cause of ARDS in critically ill patients. Prior concerns were raised whether MSC immunomodulation of immune cells would aggravate infection and lead to worse outcomes. However, multiple preclinical studies have demonstrated that MSC secreted antimicrobial factors such as Lipocalin 2 or LL-37 and increased the phagocytosis of bacteria by innate immune cells such as monocytes/macrophages. In addition, recent studies have showed that MSC can inhibit neutrophil apoptosis, prolong leukocyte survival, and enhance their function, possibly leading to enhanced antimicrobial activity and decreasing the trafficking of immune cells to the injured alveolus[112,113]. Similar to MSC, multiple investigators have found that administration of MSC EV decreased the bacterial load following severe bacterial pneumonia and/or sepsis[84,85,114]. Morrison et al. found that increased phagocytosis of bacteria by macrophages induced by MSC EV was in part through EV transfer of mitochondria to injured alveolar monocytes/macrophages, leading to increased oxidative phosphorylation[115]. Interestingly, the antimicrobial effect of the EV could be enhanced through MSC preconditioning prior to the isolation of the released vesicles by TLR-3 agonist or IFNγ[84,116]. MSC EV were also found to be beneficial with virus clearance. Qian et al. found that exosomes secreted from umbilical cord derived MSC inhibited HCV viral replication with low cell toxicity and enhanced the anti-HCV ability of IFNα and telaprevir[117]. Transfer of microRNAs including let-7f, miR-145, miR-199a, and miR-221 from MSC exosomes to target cells largely contributed to the suppression of HCV RNA replication.
5.5. MSC EV Inhibition of Lung Fibrosis Following ALI
Among a subset of patients with ARDS who survives the initial inflammatory insult, some patients develop severe, dysregulated fibroproliferation in the lung, characterized by abnormal and excessive deposition of extracellular matrix proteins[118]. These survivors develop chronic interstitial and intra-alveolar fibrosis leading to reduced pulmonary function and quality of life[119]. Prevention of lung fibrosis following ARDS may improve long-term prognosis among survivors. Despite the lack of studies on the effect MSC EV on lung fibrosis following ALI, multiple investigators have demonstrated the beneficial effects of MSC EV on lung fibrosis in the lung and other organ injury models. Choi et al. found that the administration of MSC derived MV reduced collagen deposition and inflammation in a model of idiopathic pulmonary fibrosis in mice[120]. In a wound healing model in mice, Fang et al. found that umbilical cord derived MSC reduced scar formation and myofibroblast accumulation which was predominantly dependent on inhibition of TGF-β2/SMAD2 pathway by MSC exosomes[121]. In a liver fibrosis model in mice, the upregulation and transfer of miRNA-181–5p in MSC exosomes to liver parenchymal cells prevented liver fibrosis via autophagy activation[122]. These preclinical studies and others formed the biological rationale for using intravenous human MSC in patients with idiopathic pulmonary fibrosis; a recent Phase I clinical trial was completed which demonstrated safety with no MSC related adverse events[123].
6. THERAPEUTIC USE OF MSC EV FOR OTHER LUNG DISEASES
In a model of hypoxia-induced pulmonary artery hypertension in mice, Lee et al. demonstrated that exosomes mediated the cytoprotective effects of bone marrow derived MSC[105]. Administration of MSC exosomes protected against the elevation of right ventricular systolic pressure and the development of right ventricular hypertrophy after three weeks of hypoxic exposure. Specifically, treatment with MSC exosomes interfered with the early hypoxic signal in the lung, suppressing inflammation, the up-regulation of hypoxia-induced mitogenic factor, and alveolar macrophage activation. Whereas, surprisingly, exosomes-depleted CM had no therapeutic effect, suggesting a limited role of soluble factors released by MSC. In a follow up study, these investigators found that an unique subset of MSC exosomes, flotillin 1+, improved lung morphology and pulmonary development, decreases lung fibrosis and ameliorated pulmonary vascular remodeling in a neonatal hyperoxia model of BPD[124]. Similarly, Chen et al. demonstrated that MSC MV decreased pulmonary pressure and right ventricular pressure and reduce right ventricular hypertrophy and pulmonary arteriole remodeling during the development of pulmonary arterial hypertension in rats[125].
Moreover, MSC exosomes were found to have reparative effects in a preclinical model of allergic airway inflammation provoked by mucosal sensitization and challenge with aspergillus hyphal extract (AHE)[126]. In AHE-induced ALI model, the authors found that the reduction in soluble Th2- (IL-4 and IL-5) and Th17- (IL-17) associated cytokines in bronchoalveolar lavage fluid was accompanied by an increase in IFNγ expression. This suggested that administration of MSC EV was as effective as the cells themselves in mitigating Th2/Th17-mediated airways hyper-responsiveness, by shifting the Th2/Th17 inflammatory response towards a counter-regulatory Th1 response and reducing lung inflammation.
Lastly, using MSC as a therapeutic to prevent silica-induced lung inflammation and fibrosis, Phinney et al. found that MSC shed exosomes that modulated toll-like receptor signaling and cytokine secretion in macrophages in part by transfer of regulatory microRNAs[127]; miR451, known to suppress TNF and macrophage migration inhibitory factor, was highly abundant in MSC exosomes, suggesting that the possible transfer of miR-451 to and increased expression in macrophages inhibited TNF secretion in response to silica. The authors also found that MSCs managed intracellular oxidative stress by the transfer of depolarized mitochondria by MSC. The vesicles were engulfed and re-utilized by macrophages, resulting in enhanced bioenergetics.
7. APPLICATION AND EFFICACY OF OTHER STEM OR PROGENITOR CELLS IN PRECLINICAL LUNG INJURY MODELS
Other stem or progenitor cells including EPC, bone marrow-derived mononuclear cells (BMDMC), iPSC, and ESC can also exert protective effects during ALI by promoting endothelial barrier integrity, ameliorating inflammation, and/or possibly by differentiation and regeneration into alveolar type I or II cells (Table 2)[128–134]. Asahara et al. first described circulating bone marrow-derived cells, termed EPC, that had the capacity to proliferate and differentiate into mature endothelial cells[135]. Yamada subsequently found that the release of EPC from the bone marrow into the circulation was involved in lung repair following LPS induced lung injury[130]. Clinically, the number of circulating EPC were significantly higher in patients with ARDS when compared with control patients and were associated with increased survival[136,137]. More studies subsequently found that exogenous administration EPC or EV derived from EPC could repair the pulmonary endothelium, maintain the lung alveolar barrier integrity, modulate the immune response and effectively attenuate LPS-induced ALI through direct or indirect mechanisms[129,138–142]. Multiple investigators have found that BMDMC, a mixture of different cell types including MSC and EPC, have therapeutic effects following pulmonary or extra-pulmonary induced ALI[143–147]. ESC are isolated from the inner cell mass at the blastocyst stage of embryonic development and have the ability to proliferate indefinitely in culture, without loss of differentiation potential, and can generate cells of all three germ layers. Because of the attendant ethical problems, studies using ESC are limited for ALI. However, efforts have been made to differentiate human ESC into lung cell lines to promote lung injury repair via regeneration[131,148]. More recently, iPSC have generated considerable interest due to development of induced embryonic cell lines from somatic cells, avoiding the ethical issue of using human embryos for research, and their ability to attenuate lung inflammation and injury following LPS-induced ALI, ventilator-induced lung injury (VILI) and cigarette smoke (CS)-induced lung injury[132–134,149,150].
Table 2.
Other Stem or Progenitor Cell Based Therapies For Preclinical Lung Injury Models (Representative Studies)
| Stem Cell | Animal Model | Effector Cell or EV | Proposed Target Cells | Potential Biological Mechanisms | Biological Effects | Reference |
|---|---|---|---|---|---|---|
| EPC | LPS induced ALI in mice | EPC-exosome shuttled miR-126 | Human small airway epithelial cells | MiR-126–3p and miR-126–5p, which are abundant in EPC derived exosomes, increased epithelial tight junction protein expression while decreasing ALI associated inflammatory genes such as phosphoinositide-3-kinase regulatory subunit 2 (PIK3R2), high mobility group box 1 (HMGB1), and VEGFα. | Maintained the lung alveolar epithelial barrier integrity and reduced lung neutrophil infiltration and the levels of cytokines/chemokines in the BALF, ameliorating alveolar edema and lung injury. | Zhou et al. 2019142 |
| EPC | LPS induced ALI in rats | EPC-exosome shuttled miR-126 | EC | MiR-126 transferred to target EC and downregulated SPRED1 and promoted RAF/ERK signaling pathways. | Enhanced the proliferation, migration and tube formation of the endothelial cells. Improved EC function in vitro and restored pulmonary integrity in vivo. | Wu et al. 2018141 |
| EPC | CLP-induced sepsis in mice | EPC-exosome shuttled miR-126 | EC | EPC exosome shuttled miR-126–3p and miR-126–5p suppressed lung and renal vascular leakage and reduced liver and kidney dysfunction. Attenuated sepsis-induced increases in plasma levels of cytokines and chemokines in part by decreasing LPS-induced HMGB1 and vascular cell adhesion molecule 1 (VCAM1) levels. | Attenuated lung, liver, and kidney injury and improved survival of septic mice. | Zhou et al. 2018140 |
| EPC | Hypoxia/ Reoxygena-tion induced injury in human brain micro-vascular EC | EPC MV | EC | Decreased ROS production and apoptosis. Increased eNOS and nitric oxide (NO) production as well as the ability to form an endothelium via the PI3K pathway. These beneficial effects were mediated in part through the transfer of miR-126 from the MV to the EC. | Restoration of endothelial function. | Wang et al. 2013139 |
| EPC | LPS induced ALI in rabbits | EPC | EC and other lung cells | Inhibited infiltration of poly-morphonuclear cells, reduced the extent of hyaline membrane formation and hemorrhage, and the wet-to-dry ratio in the injured lung. Decreased the concentrations of IL-1β, ICAM-1, ROS, NO and malondialdehyde and increased the levels of IL-10 and VEGF in the injured lung tissues. | Reparative effect on the pulmonary endothelium and attenuation of LPS induced ALI. | Cao et al. 2012138 |
| EPC | LPS induced ALI in rats | EPC | EC | Reduced pulmonary edema, inflammation, hemorrhage, and hyaline membrane formation in the injured alveolus. Decreased the expression of endothelin-1 and iNOS and increased anti-inflammatory cytokine IL-10. | Attenuated lung injury and improved survival in ALI. | Mao et al. 2010129 |
| MAPC | Ischemia/ reperfusion induced ALI in pigs | MAPC | Decreased the infiltration of neutrophils and the levels of TNF-α, IL-1β and IFN-γ in the BALF in the injured alveolus. | Suppressed the innate immune response during ALI. | Martens et al. 2017153 | |
| iPSC | LPS-induced ALI in mice | iPSC | PMN | Reduced neutrophil infiltration and decreased the expression of VCAM-1 and proinflammatory cytokines (MIP-2, TNF-α, IL-6, and IL-1β) by reducing the expression of TREM-1 and p38 mitogen-activated protein kinase signaling in the injured lung. | Attenuated the role of neutrophils in lung inflammation and injury induced by LPS. | Su et al. 2019132 |
| iPSC | LPS-induced ALI in mice | iPSC or iPSC CM | PMN | Reduced neutrophil chemotaxis via increase in GRK2 activity and reduction of CXCR2 expression. | Attenuated lung injury. | Su et al. 2017133 |
| iPSC-MSC | Cigarette smoke (CS)-induced lung injury in rats | Released Stem cell factor (SCF) | Bronchial epithelial cells | Decreased circulating 8-isoprostane and cytokine-induced neutrophil chemoattractant-1 and ameliorated CS-induced infiltration of macrophages and neutrophils and apoptosis/proliferation imbalance. | Reparative effect on lung airway cells. | Li et al. 2017149 |
| iPSC | VILI in mice | iPSC CM | Inhibited PI3K/Akt pathway and increased IFNγ-induced protein 10 (IP-10) expression. | Attenuated lung injury. | Li et al. 2013150 | |
| iPSC | LPS-induced ALI in mice | iPSC CM | Reduced the activity of NFκβ and neutrophils and decreased the levels of TNF-α, IL-6, and macrophage inflammatory peptide-2. | Attenuated lung injury. | Yang et al. 2011134 | |
| ESC-MSC | LPS-induced ALI in mice | ESC Derived MSC | Reduced the influx of white blood cells and neutrophils and decreased the secretion of MIP-2 and TNF-α in the injured alveolus. | Decreased LPS-induced inflammation and attenuated lung injury. | Hao et al. 2015152 | |
| ESC | Bleomycin-induced ALI in mice | ESC derived lung lineage specific cells | Lung epithelial cells | Transplantation of differentiated ESC homed to the injured lung and increased the migration of other progenitors of hematopoietic and nonhematopoietic origin and reversed fibrosis and lung inflammation. Mechanisms was in part via paracrine mechanisms (i.e., secretion of key growth factors such as TGFβ, FGF, and VEGF). | Attenuated lung fibrosis and inflammation. | Banerjee et al. 2012148 |
| ESC-AECII | Bleomycin-induced ALI in mice | ESC-Derived AECII | AEC I | Differentiated into AEC I, improved arterial blood oxygen saturation, and decreased collagen deposition. | Attenuated lung injury and increased survival. | Wang et al. 2010131 |
| BMDMC | Papain-induced emphysema in mice | BMDMC | Reduced the infiltration of neutrophils and decreased the levels of KC, MIP-2, and IFN-γ. Reversed the activity of DUOX and decreased apoptosis. Decreased alveolar diameter and increased lung functional residual capacity. These beneficial effects were mainly attributed to the paracrine effects of BMDMC (limited engraftment in the lung). | Suppressed inflammation, apoptosis and oxidative response to injury, improving pulmonary function. | Machado et al. 2018143 | |
| BMDMC | Hemorrhagic shock induced lung injury in rats | BMDMC | Attenuated lung inflammation and pulmonary congestion via activation of Akt and the inhibition of glycogen synthase kinase-3β and NFκβ. | Attenuated lung injury. | Nandra et al. 2012144 | |
| BMDMC | CLP induced sepsis in mice | BMDMC | Reduced caspase-3, IL-6 and IL-1β, VEGF, PDGF, HGF, and TGF-β, but increased IL-10 mRNA expression in the lung tissue. Promoted the repair of the endothelium and epithelium, improving lung mechanics, and prevented the increase in apoptotic cells in the lung, liver, and kidney. | Suppressed lung inflammation, promoted lung repair and reduced mortality. | Ornellas et al. 2011145 | |
| BMDMC | LPS induced pulmonary and extra-pulmonary ALI in mice | BMDMC | Reduced the levels of IL-6, KC (murine IL-8 homolog), and IL-10, decreased the expression of IGF, PDGF, and TGF-β, and increased VEGF level in both ALI models. Promoted the repair of the lung epithelium and endothelium, decreased collagen fiber content and cell apoptosis in lung, kidney, and liver (low lung engraftment rates). | Improved the survival and lung mechanics of injured mice. | Araujo et al. 2010146 | |
| BMDMC | LPS induced ALI in mice | BMDMC | Prevented LPS-induced lung inflammation, alveolar collapse, and interstitial edema. Promoted lung epithelial and endothelial repair. | Improved lung mechanics, alleviated lung injury, and reduced mortality. | Prota et al. 2010147 |
Bone marrow-derived mononuclear cell (BMDMC), cecal ligation and puncture (CLP), embryonic stem cells (ESC), endothelial cells (EC), endothelial progenitor cell (EPC), ESC derived alveolar type II epithelial cell (ESC-AECII), induced pluripotent stem cell-derived MSC (iPSC-MSC), multipotent adult progenitor cell (MAPC), ventilator-induced lung injury (VILI).
7.1. Secretome From Other Stem or Progenitor Cells for ALI
Unlike MSC, BMDMC, iPSC and ESC are multipotent and capable of differentiation into multiple lung cells including endothelial and epithelial cells or even MSC[151,152]; thus, early studies focused predominantly on the multi-lineage differentiation, migration and homing of these cells into the injured lung to promote repair[148]. For example, Wang et al. found that instillation of ESC derived alveolar epithelial II cells abrogated bleomycin induced lung injury; more importantly, these derived type II cells behaved in a similar fashion as primary alveolar epithelial type II cells and differentiated into cells expressing phenotypic markers of alveolar type I epithelial cells following transplantation[131]. Li et al. found that iPSC derived MSC possessed anti-apoptotic/pro-proliferative capacity in both in vivo and in vitro models of CS-induced lung injury, which was more effective than bone marrow derived MSC[149]. However, several groups subsequently found that the engraftment of these differentiated progenitor cells in the lung were very low. Because the therapeutic effects were similar to MSC, researchers speculated that these stem or progenitor cells also exerted their protective effects through paracrine mechanism[153]. Multiple studies have demonstrated that treatment with the CM of iPSC could modulate lung inflammation and attenuate lung injury following LPS induced ALI or VILI via suppression of NFκβ activity and inhibition of PI3K/Akt pathway and IFNγ-induced protein 10-dependent paracrine regulation[134,150]. More importantly, most subsequent studies demonstrated that the beneficial effects of the CM were similar to those of iPSC. Li et al. found that stem cell factor in the CM of iPSC derived MSC was responsible for their anti-apoptotic and proliferative effect[149].
7.2. EV Released From Other Stem or Progenitor Cells for ALI
EV from non-MSC stem or progenitor cells have exhibited a number of beneficial effects in animal models of ALI, sepsis, acute kidney injury following ischemia/reperfusion, etc.[140,154,155]. Ranghino et al. found that EPC MV improved neovascularization and promoted regeneration in a severe hindlimb ischemia model induced by ligation and resection of the left femoral artery. However, RNase-pretreatment of the MV or DICER-knock-down of EPC derived MV significantly reduced the therapeutic effects of the MV, suggesting that the RNA content of the MV was critical[154]. In ischemia/reperfusion injury model using human brain microvascular EC, Wang et al. found that EPC MV decreased ROS production and apoptosis and increased eNOS and NO production as well as the ability to form an endothelium via the PI3K pathway; the beneficial effects were through the transfer of miR126 from the MV to the EC[139]. Zhou et al. found that EPC derived exosomes suppressed lung and renal vascular leakage, decreased plasma levels of inflammatory cytokines/chemokines and improved the survival in a CLP-induced sepsis model through transfer of miR-126–3p and miR-126–5p from the exosomes to the target cells. Inhibition of microRNA-126–5p and 3p through transfection with inhibitors abrogated the beneficial effects of EPC exosome[140]. Other investigators came to a similar conclusion concerning the importance of miR-126 in the therapeutic effects of EPC derived EV. EPC EV ameliorated LPS induced ALI via transfer of miR-126 which downregulated SPRED1 and promoted RAF/ERK signaling pathways and decreased phosphoinositide-3-kinase regulatory subunit 2, HMGB1, and VEGFα[141,142]. Studies using EV collected from stem cells such as BMDMC, iPSC or ESC for ALI are currently limited.
8. CONCLUSION
MSC derived EV exert similar therapeutic properties in ALI models as their parent cells while potentially avoiding the limitation of using live cells such as the risk of pulmonary embolism and tumorigenesis. Studying the biological content of the EV have elucidated some of the mechanisms underlying the beneficial effects of the EV in terms of suppressing inflammation and promoting lung barrier integrity and the resolution of pulmonary edema. However, clinical application of MSC derived EV or even EV derived from BMDMC, iPSC, or ESC for ARDS is limited by the need to grow enough cells to generate the EV and the prohibitive cost of culturing the cells. Increasing either the potency of the EV by preconditioning or the trafficking of the EV to the injured alveolus may be possible solutions. Further studies on MSC EV are warranted given the potential of using MSC in ARDS in early Phase I and II clinical trials.
9. EXPERT OPINION
The use of MSC EV in lung diseases offer several advantages compared to MSC: 1) EV are non-self-replicating, reducing the risk of iatrogenic tumor formation. In a typical clinical trial using MSC, a patient may receive up to 700 million cells per dose for a 70 kg individual or 5 – 10 million cells/kg/dose, and screening for tumor formation will be followed by only a computed tomography of the chest, abdomen and pelvis; 2) EV can be stored at – 80°C and remain biologically active. For most clinical trials, MSC will be stored in DMSO in liquid nitrogen in a bone marrow transplant facility, potentially limiting the number of hospitals capable of storing MSC for clinical use; 3) Due to small size of EV compared to the parent MSC (approximately 100x smaller based on a MSC size of 17 μm), administration of MSC EV do not cause significant elevation in pulmonary arterial pressure in contrast to MSC which are large and sticky. This lack of hemodynamic effect allows multiple administration; 4) And lastly, MSC EV are considered immunopriviledged, although MSC can be induced to express higher levels of MHC II with inflammation potentially leading to an immune reaction in the recipient[156]. However, multiple questions remain which may diminish the enthusiasm of using MSC EV for clinical use.
9.1. Large Scale MSC Extracellular Vesicle Generation
Based on preclinical studies, the amount of MSC EV needed to generate an equivalent effect as MSC in lung injury is roughly 5–10x higher[84,85]. For a 70 kg patient receiving a dose of 10 × 106 MSC/kg, the number of MSC needed to generate enough EV may be as high as 7 billion cells per dose, making the production costs prohibitive. Although MSC are relatively easy to expand using conventional tissue flasks and bioreactors, their growth in culture is finite and their biological properties may become altered with repeated passages. One approach to scale up EV production could be the use of bioreactors to culture MSC, which has demonstrated significant increases in EV yield from cells compared with conventional tissue culture flasks[157,158]. However, whether the conditions used for culture in the bioreactor alters MSC EV phenotype and their therapeutic impact will need to be studied further[159–162]. To process large sample volume, a simple and effective technique termed hydrostatic filtration dialysis has recently been developed[163]. Although this would raise safety issues, another potential strategy is MSC immortalization by genetic modification and clonal isolation[164]. Chen et al. recently proposed a robust scalable manufacturing process for MSC EV through oncogenic immortalization of human ESC derived MSC by transfection with the MYC gene[165].
9.2. Issues of Potency
Multiple methods have been developed to precondition MSC (hypoxia, serum deprivation, oxidative stress, mechanical stretch, heat shock, inflammatory agonist or mediators), which may change the surface and intracellular content of the cell and/or the released vesicles towards a more immunomodulatory state[112,166–168]. Recent studies such as the one by Islam et al. have also demonstrated that the extracellular microenvironment of the infused MSC is critical for their properties in ALI models, which has significant ramifications for the phenotype of the released EV and for selecting patients who may benefit from the therapy[169,170]. In the study by Monsel et al., EV released from MSC pretreated with a TLR3 agonist exhibited both higher antimicrobial activity and the capacity to skew human monocytes towards a M2 anti-inflammatory phenotype[84]. Varkouhi et al. also found that EV released from IFNγ primed human umbilical cord MSC more effectively attenuated E.coli pneumonia induced lung injury compared with EV from naïve MSC via enhanced macrophage phagocytosis and killing of the bacteria[116]. Tsoyi et al. found that preconditioning MSC with carbon monoxide gas exposure increased the secretion of specialized pro-resolving lipid mediators which led to a survival advantage in a preclinical model of sepsis[23]. In addition, in other organ injury models such as myocardial ischemia or liver cirrhosis, investigators found that pretreatment of MSC with tadalafil, a long acting phosphodiesterase inhibitor, or SDF-1α increased MSC survival or trafficking to the site of injury[171,172]. How pre-treatment modulates the phenotype of the released vesicles will need to be determined. Another recent method to enhance the potency of MSC may be to over-express a mRNA or micoRNA of interest. For example, Huleihel et al. transduced miRNAs (let-7d) into human MSCs in an attempt to reduce fibrosis in mice injured with bleomycin[173]. Studies are on-going to determine whether such preconditioning steps can reduce the dose of MSC EV, reducing the cost of production and making EV therapy possibly feasible for clinical trial.
9.3. Risk of MSC EV Administration
Although MSC EV clearly lack the potential to directly form tumors, this does not imply that MSC EV administration is without any risk of promoting neoplasia[174]. Roccaro et al. found that MSC EV from multiple myeloma (MM) patients promoted tumor growth and induced cell dissemination and metastasis to distant MM niches[175]. Compared to normal MSC EV, MSC EV from MM patients had lower miR-15a expression; miR-15a is associated with tumor-suppressive properties[176]. In another study, MSC EV co-implanted with SGC-7901 (human gastric cancer) cells increased tumor growth and angiogenesis when compared with SGC-7901 cells alone[177].
9.4. Future Directions
Based on the strong preclinical demonstration of MSC EV efficacy in various organ injury models, there are now several Phase I safety clinical trials using MSC EV in stroke, diabetes, wound care and, more recently, for preterm neonates at risk for BPD (). However, until the issues of potency and large scale production of MSC EV are resolved, the widespread application of such a promising therapeutic may be limited to small size individuals.
ARTICLE HIGHLIGHTS.
Early Phase I and II clinical trials using human bone marrow derived MSC as a therapeutic for moderate to severe acute respiratory distress syndrome or sepsis have demonstrated that intravenous instillation of the cells is safe.
Although MSC derived CM can recapitulate the therapeutic effects of MSC in preclinical ALI models, their potential use as a therapeutic may be limited due to the lack of standardization among studies (i.e. preconditioning, volume and concentration of CM instillate) as well as the optimal therapeutic dose, timing and route of administration.
MSC derived EV may represent a more attractive area of research for treating inflammatory lung diseases, including ALI, in part due to the mechanisms underlying their therapeutic effects: the transfer of mRNA, microRNA, proteins, receptors, and possibly organelles from the EV to the injured tissue.
In preclinical models, MSC EV ameliorated most of the major pathologies associated with ALI: increase in lung protein permeability, pulmonary edema, alveolar inflammation, and overwhelming bacterial infection.
In anticipation for any clinical application, utilization of MSC EV will require some mechanisms to increase the potency of the EV due to the prohibitive cost of growing enough cells to generate the vesicles.
Acknowledgments
Funding
This work was supported by the National Institute of Health National Heart, Lung, and Blood Institute grant number HL 113022 (Dr. JW Lee).
Declaration of Interests
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Abbreviations Used
- AFC
Alveolar fluid clearance
- ALI
Acute lung injury
- ARDS
Acute respiratory distress syndrome
- BMDMC
Bone marrow-derived mononuclear cells
- BPD
Bronchopulmonary dysplasia
- CD
Cluster of differentiation
- CLP
Cecal ligation and puncture
- CM
Conditioned medium
- CS
Cigarette smoke
- DMSO
Dimethylsulfoxide
- EPC
Endothelial progenitor cells
- ESC
Embryonic stem cells
- EV
Extracellular vesicles
- IP
Intra-peritoneal
- iPSC
Induced pluripotent stem cells
- IT
Intra-tracheal
- IV
Intravenous
- KGF
Keratinocyte growth factor
- LPS
Lipopolysaccharide
- MHC
Major histocompatibility complex
- miRNA
MicroRNA
- mRNA
Messenger RNA
- MSC
Mesenchymal stem or stromal cells
- MV
Microvesicles
- MVB
Multi-vesicular bodies
- RNA
Ribonucleic acid
- siRNA
Small interfering ribonucleic acid
- TLR
Toll-like receptor
Footnotes
Reviewer Disclosures
Peer reviewers on this manuscript have no relevant financial relationships or otherwise to disclose.
REFERENCES
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Biehl M, Kashyap R, Ahmed AH, et al. Six-month quality-of-life and functional status of acute respiratory distress syndrome survivors compared to patients at risk: a population-based study. Crit Care. 2015. October 2;19:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amato MB, Barbas CS, Medeiros DM, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998. February 5;338(6):347–54. [DOI] [PubMed] [Google Scholar]
- 3.Guerin C, Reignier J, Richard JC. Prone positioning in the acute respiratory distress syndrome. N Engl J Med. 2013. September 5;369(10):980–1. [DOI] [PubMed] [Google Scholar]
- 4.Papazian L, Forel JM, Gacouin A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010. September 16;363(12):1107–16. [DOI] [PubMed] [Google Scholar]
- 5.Australia, New Zealand Extracorporeal Membrane Oxygenation Influenza I, Davies A, et al. Extracorporeal Membrane Oxygenation for 2009 Influenza A(H1N1) Acute Respiratory Distress Syndrome. JAMA. 2009. November 4;302(17):1888–95. [DOI] [PubMed] [Google Scholar]
- 6.Bellani G, Laffey JG, Pham T, et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA. 2016. February 23;315(8):788–800. [DOI] [PubMed] [Google Scholar]
- 7.Monsel A, Zhu YG, Gennai S, et al. Cell-based therapy for acute organ injury: preclinical evidence and ongoing clinical trials using mesenchymal stem cells. Anesthesiology. 2014. November;121(5):1099–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matthay MA, Calfee CS, Zhuo H, et al. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. Lancet Respir Med. 2019. February;7(2):154–162.** A clinical trial demonstrating stafety of administration of human MSCs as therapy for moderate to severe ARDS at a dose up to 10 million cells/kg predicted body weight.
- 9.Zheng G, Huang L, Tong H, et al. Treatment of acute respiratory distress syndrome with allogeneic adipose-derived mesenchymal stem cells: a randomized, placebo-controlled pilot study. Respir Res. 2014. April 4;15:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedenstein AJ, Petrakova KV, Kurolesova AI, et al. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968. March;6(2):230–47. [PubMed] [Google Scholar]
- 11.McIntyre LA, Stewart DJ, Mei SHJ, et al. Cellular Immunotherapy for Septic Shock. A Phase I Clinical Trial. Am J Respir Crit Care Med. 2018. February 1;197(3):337–347.** A clinical trial demonstrating stafety of administration of human MSCs as therapy for sepsis using freshlyt isolated human MSCs.
- 12.Schlosser K, Wang JP, Dos Santos C, et al. Effects of Mesenchymal Stem Cell Treatment on Systemic Cytokine Levels in a Phase 1 Dose Escalation Safety Trial of Septic Shock Patients. Crit Care Med. 2019. July;47(7):918–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kotton DN, Fabian AJ, Mulligan RC. Failure of bone marrow to reconstitute lung epithelium. Am J Respir Cell Mol Biol. 2005. October;33(4):328–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goolaerts A, Pellan-Randrianarison N, Larghero J, et al. Conditioned media from mesenchymal stromal cells restore sodium transport and preserve epithelial permeability in an in vitro model of acute alveolar injury. Am J Physiol Lung Cell Mol Physiol. 2014. June 1;306(11):L975–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee JW, Fang X, Krasnodembskaya A, et al. Concise review: Mesenchymal stem cells for acute lung injury: role of paracrine soluble factors. Stem Cells. 2011. June;29(6):913–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guery BP, Mason CM, Dobard EP, et al. Keratinocyte growth factor increases transalveolar sodium reabsorption in normal and injured rat lungs. Am J Respir Crit Care Med. 1997. May;155(5):1777–84. [DOI] [PubMed] [Google Scholar]
- 17.Lee JW, Fang X, Gupta N, et al. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci U S A. 2009. September 22;106(38):16357–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JW, Krasnodembskaya A, McKenna DH, et al. Therapeutic effects of human mesenchymal stem cells in ex vivo human lungs injured with live bacteria. Am J Respir Crit Care Med. 2013. April 1;187(7):751–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mei SH, McCarter SD, Deng Y, et al. Prevention of LPS-induced acute lung injury in mice by mesenchymal stem cells overexpressing angiopoietin 1. PLoS Med. 2007. September;4(9):e269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fang X, Neyrinck AP, Matthay MA, et al. Allogeneic human mesenchymal stem cells restore epithelial protein permeability in cultured human alveolar type II cells by secretion of angiopoietin-1. J Biol Chem. 2010. August 20;285(34):26211–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nemeth K, Leelahavanichkul A, Yuen PS, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009. January;15(1):42–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee RH, Yu JM, Foskett AM, et al. TSG-6 as a biomarker to predict efficacy of human mesenchymal stem/progenitor cells (hMSCs) in modulating sterile inflammation in vivo. Proc Natl Acad Sci U S A. 2014. November 25;111(47):16766–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsoyi K, Hall SR, Dalli J, et al. Carbon Monoxide Improves Efficacy of Mesenchymal Stromal Cells During Sepsis by Production of Specialized Proresolving Lipid Mediators. Crit Care Med. 2016. December;44(12):e1236–e1245.* Novel study using carbon monoxide as a pretreatment to improve the efficacy of MSC in a preclinical sepsis model.
- 24.Fang X, Abbott J, Cheng L, et al. Human Mesenchymal Stem (Stromal) Cells Promote the Resolution of Acute Lung Injury in Part through Lipoxin A4. J Immunol. 2015. August 1;195(3):875–81. [DOI] [PubMed] [Google Scholar]
- 25.Gupta N, Krasnodembskaya A, Kapetanaki M, et al. Mesenchymal stem cells enhance survival and bacterial clearance in murine Escherichia coli pneumonia. Thorax. 2012. June;67(6):533–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krasnodembskaya A, Song Y, Fang X, et al. Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37. Stem Cells. 2010. December;28(12):2229–38.* An early preclinical study demonstrating the antimicrobial properties of MSC. Largely reduced the concern of clinically giving an immunosuppressive (i.e., MSC) for patients with ARDS with overwhelming infection.
- 27.Gnecchi M, Zhang Z, Ni A, et al. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008. November 21;103(11):1204–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair--current views. Stem Cells. 2007. November;25(11):2896–902. [DOI] [PubMed] [Google Scholar]
- 29.Togel FE, Westenfelder C. Mesenchymal stem cells: a new therapeutic tool for AKI. Nat Rev Nephrol. 2010. March;6(3):179–83. [DOI] [PubMed] [Google Scholar]
- 30.Wagers AJ, Sherwood RI, Christensen JL, et al. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science. 2002. September 27;297(5590):2256–9. [DOI] [PubMed] [Google Scholar]
- 31.Togel F, Hu Z, Weiss K, et al. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005. July;289(1):F31–42. [DOI] [PubMed] [Google Scholar]
- 32.Fung ME, Thebaud B. Stem cell-based therapy for neonatal lung disease: it is in the juice. Pediatr Res. 2014. January;75(1–1):2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ionescu L, Byrne RN, van Haaften T, et al. Stem cell conditioned medium improves acute lung injury in mice: in vivo evidence for stem cell paracrine action. Am J Physiol Lung Cell Mol Physiol. 2012. December 1;303(11):L967–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jun D, Garat C, West J, et al. The pathology of bleomycin-induced fibrosis is associated with loss of resident lung mesenchymal stem cells that regulate effector T-cell proliferation. Stem Cells. 2011. April;29(4):725–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aslam M, Baveja R, Liang OD, et al. Bone marrow stromal cells attenuate lung injury in a murine model of neonatal chronic lung disease. Am J Respir Crit Care Med. 2009. December 1;180(11):1122–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Haaften T, Byrne R, Bonnet S, et al. Airway delivery of mesenchymal stem cells prevents arrested alveolar growth in neonatal lung injury in rats. Am J Respir Crit Care Med. 2009. December 1;180(11):1131–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hansmann G, Fernandez-Gonzalez A, Aslam M, et al. Mesenchymal stem cell-mediated reversal of bronchopulmonary dysplasia and associated pulmonary hypertension. Pulm Circ. 2012. Apr-Jun;2(2):170–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waszak P, Alphonse R, Vadivel A, et al. Preconditioning enhances the paracrine effect of mesenchymal stem cells in preventing oxygen-induced neonatal lung injury in rats. Stem Cells Dev. 2012. October 10;21(15):2789–97. [DOI] [PubMed] [Google Scholar]
- 39.Tropea KA, Leder E, Aslam M, et al. Bronchioalveolar stem cells increase after mesenchymal stromal cell treatment in a mouse model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2012. May 1;302(9):L829–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sutsko RP, Young KC, Ribeiro A, et al. Long-term reparative effects of mesenchymal stem cell therapy following neonatal hyperoxia-induced lung injury. Pediatr Res. 2013. January;73(1):46–53. [DOI] [PubMed] [Google Scholar]
- 41.Pierro M, Ionescu L, Montemurro T, et al. Short-term, long-term and paracrine effect of human umbilical cord-derived stem cells in lung injury prevention and repair in experimental bronchopulmonary dysplasia. Thorax. 2013. May;68(5):475–84. [DOI] [PubMed] [Google Scholar]
- 42.Ionescu LI, Alphonse RS, Arizmendi N, et al. Airway delivery of soluble factors from plastic-adherent bone marrow cells prevents murine asthma. Am J Respir Cell Mol Biol. 2012. February;46(2):207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huh JW, Kim SY, Lee JH, et al. Bone marrow cells repair cigarette smoke-induced emphysema in rats. Am J Physiol Lung Cell Mol Physiol. 2011. September;301(3):L255–66. [DOI] [PubMed] [Google Scholar]
- 44.Kim SY, Lee JH, Kim HJ, et al. Mesenchymal stem cell-conditioned media recovers lung fibroblasts from cigarette smoke-induced damage. Am J Physiol Lung Cell Mol Physiol. 2012. May 1;302(9):L891–908. [DOI] [PubMed] [Google Scholar]
- 45.Bruno S, Grange C, Deregibus MC, et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol. 2009. May;20(5):1053–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lai RC, Arslan F, Lee MM, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010. May;4(3):214–22. [DOI] [PubMed] [Google Scholar]
- 47.Baglio SR, Rooijers K, Koppers-Lalic D, et al. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res Ther. 2015. July 1;6:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eirin A, Zhu XY, Puranik AS, et al. Integrated transcriptomic and proteomic analysis of the molecular cargo of extracellular vesicles derived from porcine adipose tissue-derived mesenchymal stem cells. PLoS One. 2017;12(3):e0174303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eirin A, Riester SM, Zhu XY, et al. MicroRNA and mRNA cargo of extracellular vesicles from porcine adipose tissue-derived mesenchymal stem cells. Gene. 2014. November 1;551(1):55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eirin A, Zhu XY, Puranik AS, et al. Comparative proteomic analysis of extracellular vesicles isolated from porcine adipose tissue-derived mesenchymal stem/stromal cells. Sci Rep. 2016. October 27;6:36120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim HS, Choi DY, Yun SJ, et al. Proteomic analysis of microvesicles derived from human mesenchymal stem cells. J Proteome Res. 2012. February 3;11(2):839–49. [DOI] [PubMed] [Google Scholar]
- 52.Gould SJ, Raposo G. As we wait: coping with an imperfect nomenclature for extracellular vesicles. J Extracell Vesicles. 2013;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McVey M, Tabuchi A, Kuebler WM. Microparticles and acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2012. September;303(5):L364–81. [DOI] [PubMed] [Google Scholar]
- 54.Witwer KW, Buzas EI, Bemis LT, et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles. 2013;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Feng D, Zhao WL, Ye YY, et al. Cellular internalization of exosomes occurs through phagocytosis. Traffic. 2010. May;11(5):675–87. [DOI] [PubMed] [Google Scholar]
- 56.Lee Y, El Andaloussi S, Wood MJ. Exosomes and microvesicles: extracellular vesicles for genetic information transfer and gene therapy. Hum Mol Genet. 2012. October 15;21(R1):R125–34. [DOI] [PubMed] [Google Scholar]
- 57.Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009. August;9(8):581–93. [DOI] [PubMed] [Google Scholar]
- 58.Akers JC, Gonda D, Kim R, et al. Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J Neurooncol. 2013. May;113(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gyorgy B, Szabo TG, Pasztoi M, et al. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci. 2011. August;68(16):2667–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ihara T, Yamamoto T, Sugamata M, et al. The process of ultrastructural changes from nuclei to apoptotic body. Virchows Arch. 1998. November;433(5):443–7. [DOI] [PubMed] [Google Scholar]
- 61.Erwig LP, Henson PM. Clearance of apoptotic cells by phagocytes. Cell Death Differ. 2008. February;15(2):243–50. [DOI] [PubMed] [Google Scholar]
- 62.Ratajczak J, Miekus K, Kucia M, et al. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006. May;20(5):847–56. [DOI] [PubMed] [Google Scholar]
- 63.Collino F, Deregibus MC, Bruno S, et al. Microvesicles derived from adult human bone marrow and tissue specific mesenchymal stem cells shuttle selected pattern of miRNAs. PLoS One. 2010. July 27;5(7):e11803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mathivanan S, Simpson RJ. ExoCarta: A compendium of exosomal proteins and RNA. Proteomics. 2009. November;9(21):4997–5000. [DOI] [PubMed] [Google Scholar]
- 65.Kalra H, Simpson RJ, Ji H, et al. Vesiclepedia: a compendium for extracellular vesicles with continuous community annotation. PLoS Biol. 2012;10(12):e1001450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sheldon H, Heikamp E, Turley H, et al. New mechanism for Notch signaling to endothelium at a distance by Delta-like 4 incorporation into exosomes. Blood. 2010. September 30;116(13):2385–94. [DOI] [PubMed] [Google Scholar]
- 67.Nolte-’t Hoen EN, Buschow SI, Anderton SM, et al. Activated T cells recruit exosomes secreted by dendritic cells via LFA-1. Blood. 2009. February 26;113(9):1977–81. [DOI] [PubMed] [Google Scholar]
- 68.Rozmyslowicz T, Majka M, Kijowski J, et al. Platelet- and megakaryocyte-derived microparticles transfer CXCR4 receptor to CXCR4-null cells and make them susceptible to infection by X4-HIV. AIDS. 2003. January 3;17(1):33–42. [DOI] [PubMed] [Google Scholar]
- 69.Taraboletti G, D’Ascenzo S, Giusti I, et al. Bioavailability of VEGF in tumor-shed vesicles depends on vesicle burst induced by acidic pH. Neoplasia. 2006. February;8(2):96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Taraboletti G, D’Ascenzo S, Borsotti P, et al. Shedding of the matrix metalloproteinases MMP-2, MMP-9, and MT1-MMP as membrane vesicle-associated components by endothelial cells. Am J Pathol. 2002. February;160(2):673–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Deregibus MC, Cantaluppi V, Calogero R, et al. Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood. 2007. October 1;110(7):2440–8. [DOI] [PubMed] [Google Scholar]
- 72.Zarovni N, Corrado A, Guazzi P, et al. Integrated isolation and quantitative analysis of exosome shuttled proteins and nucleic acids using immunocapture approaches. Methods. 2015. October 1;87:46–58. [DOI] [PubMed] [Google Scholar]
- 73.Thery C, Amigorena S, Raposo G, et al. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006. April;Chapter 3:Unit 3 22. [DOI] [PubMed] [Google Scholar]
- 74.Kessler RJ, Fanestil DD. Interference by lipids in the determination of protein using bicinchoninic acid. Anal Biochem. 1986. November 15;159(1):138–42. [DOI] [PubMed] [Google Scholar]
- 75.Li P, Kaslan M, Lee SH, et al. Progress in Exosome Isolation Techniques. Theranostics. 2017;7(3):789–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zeringer E, Barta T, Li M, et al. Strategies for isolation of exosomes. Cold Spring Harb Protoc. 2015. April 1;2015(4):319–23. [DOI] [PubMed] [Google Scholar]
- 77.Tauro BJ, Greening DW, Mathias RA, et al. Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon cancer cell line LIM1863-derived exosomes. Methods. 2012. February;56(2):293–304. [DOI] [PubMed] [Google Scholar]
- 78.Kim DK, Nishida H, An SY, et al. Chromatographically isolated CD63+CD81+ extracellular vesicles from mesenchymal stromal cells rescue cognitive impairments after TBI. Proc Natl Acad Sci U S A. 2016. January 5;113(1):170–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Samsonov R, Shtam T, Burdakov V, et al. Lectin-induced agglutination method of urinary exosomes isolation followed by mi-RNA analysis: Application for prostate cancer diagnostic. Prostate. 2016. January;76(1):68–79. [DOI] [PubMed] [Google Scholar]
- 80.Taylor DD, Zacharias W, Gercel-Taylor C. Exosome isolation for proteomic analyses and RNA profiling. Methods Mol Biol. 2011;728:235–46. [DOI] [PubMed] [Google Scholar]
- 81.Thery C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhu YG, Feng XM, Abbott J, et al. Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin-induced acute lung injury in mice. Stem Cells. 2014. January;32(1):116–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tang XD, Shi L, Monsel A, et al. Mesenchymal Stem Cell Microvesicles Attenuate Acute Lung Injury in Mice Partly Mediated by Ang-1 mRNA. Stem Cells. 2017. July;35(7):1849–1859. [DOI] [PubMed] [Google Scholar]
- 84.Monsel A, Zhu YG, Gennai S, et al. Therapeutic Effects of Human Mesenchymal Stem Cell-derived Microvesicles in Severe Pneumonia in Mice. Am J Respir Crit Care Med. 2015. August 1;192(3):324–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Park J, Kim S, Lim H, et al. Therapeutic effects of human mesenchymal stem cell microvesicles in an ex vivo perfused human lung injured with severe E. coli pneumonia. Thorax. 2019. January;74(1):43–50.* Therapetuic use of MSC EV in an ex vivo perfused human lung injured with severe bacterial infection.
- 86.Chamberlain G, Fox J, Ashton B, et al. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007. November;25(11):2739–49. [DOI] [PubMed] [Google Scholar]
- 87.Keating A. Mesenchymal stromal cells: new directions. Cell Stem Cell. 2012. June 14;10(6):709–716. [DOI] [PubMed] [Google Scholar]
- 88.Walter J, Ware LB, Matthay MA. Mesenchymal stem cells: mechanisms of potential therapeutic benefit in ARDS and sepsis. Lancet Respir Med. 2014. December;2(12):1016–26. [DOI] [PubMed] [Google Scholar]
- 89.Budoni M, Fierabracci A, Luciano R, et al. The immunosuppressive effect of mesenchymal stromal cells on B lymphocytes is mediated by membrane vesicles. Cell Transplant. 2013;22(2):369–79. [DOI] [PubMed] [Google Scholar]
- 90.Di Trapani M, Bassi G, Midolo M, et al. Differential and transferable modulatory effects of mesenchymal stromal cell-derived extracellular vesicles on T, B and NK cell functions. Sci Rep. 2016. April 13;6:24120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen W, Huang Y, Han J, et al. Immunomodulatory effects of mesenchymal stromal cells-derived exosome. Immunol Res. 2016. August;64(4):831–40. [DOI] [PubMed] [Google Scholar]
- 92.Del Fattore A, Luciano R, Pascucci L, et al. Immunoregulatory Effects of Mesenchymal Stem Cell-Derived Extracellular Vesicles on T Lymphocytes. Cell Transplant. 2015;24(12):2615–27. [DOI] [PubMed] [Google Scholar]
- 93.Favaro E, Carpanetto A, Lamorte S, et al. Human mesenchymal stem cell-derived microvesicles modulate T cell response to islet antigen glutamic acid decarboxylase in patients with type 1 diabetes. Diabetologia. 2014. August;57(8):1664–73. [DOI] [PubMed] [Google Scholar]
- 94.Lo Sicco C, Reverberi D, Balbi C, et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles as Mediators of Anti-Inflammatory Effects: Endorsement of Macrophage Polarization. Stem Cells Transl Med. 2017. March;6(3):1018–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ti D, Hao H, Tong C, et al. LPS-preconditioned mesenchymal stromal cells modify macrophage polarization for resolution of chronic inflammation via exosome-shuttled let-7b. J Transl Med. 2015. September 19;13:308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang L, Gu Z, Zhao X, et al. Extracellular Vesicles Released from Human Umbilical Cord-Derived Mesenchymal Stromal Cells Prevent Life-Threatening Acute Graft-Versus-Host Disease in a Mouse Model of Allogeneic Hematopoietic Stem Cell Transplantation. Stem Cells Dev. 2016. December 15;25(24):1874–1883. [DOI] [PubMed] [Google Scholar]
- 97.Yin G, Hu G, Wan R, et al. Role of Microvesicles From Bone Marrow Mesenchymal Stem Cells in Acute Pancreatitis. Pancreas. 2016. October;45(9):1282–1293. [DOI] [PubMed] [Google Scholar]
- 98.Li X, Liu L, Yang J, et al. Exosome Derived From Human Umbilical Cord Mesenchymal Stem Cell Mediates MiR-181c Attenuating Burn-induced Excessive Inflammation. EBioMedicine. 2016. June;8:72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bull TM, Clark B, McFann K, et al. Pulmonary vascular dysfunction is associated with poor outcomes in patients with acute lung injury. Am J Respir Crit Care Med. 2010. November 1;182(9):1123–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hu S, Park J, Liu A, et al. Mesenchymal Stem Cell Microvesicles Restore Protein Permeability Across Primary Cultures of Injured Human Lung Microvascular Endothelial Cells. Stem Cells Transl Med. 2018. August;7(8):615–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bian S, Zhang L, Duan L, et al. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J Mol Med (Berl). 2014. April;92(4):387–97. [DOI] [PubMed] [Google Scholar]
- 102.Lopatina T, Bruno S, Tetta C, et al. Platelet-derived growth factor regulates the secretion of extracellular vesicles by adipose mesenchymal stem cells and enhances their angiogenic potential. Cell Commun Signal. 2014. April 11;12:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang N, Chen C, Yang D, et al. Mesenchymal stem cells-derived extracellular vesicles, via miR-210, improve infarcted cardiac function by promotion of angiogenesis. Biochim Biophys Acta Mol Basis Dis. 2017. August;1863(8):2085–2092. [DOI] [PubMed] [Google Scholar]
- 104.Shao L, Zhang Y, Lan B, et al. MiRNA-Sequence Indicates That Mesenchymal Stem Cells and Exosomes Have Similar Mechanism to Enhance Cardiac Repair. Biomed Res Int. 2017;2017:4150705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lee C, Mitsialis SA, Aslam M, et al. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation. 2012. November 27;126(22):2601–11.* Therapeutic use of MSC EVs in a preclinical model of bronchopulmonary dysplasia, demonstrating the importance of microRNA in the EVs for the biological effects.
- 106.Zhou Y, Xu H, Xu W, et al. Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro. Stem Cell Res Ther. 2013. April 25;4(2):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lindoso RS, Collino F, Bruno S, et al. Extracellular vesicles released from mesenchymal stromal cells modulate miRNA in renal tubular cells and inhibit ATP depletion injury. Stem Cells Dev. 2014. August 1;23(15):1809–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ju GQ, Cheng J, Zhong L, et al. Microvesicles derived from human umbilical cord mesenchymal stem cells facilitate tubular epithelial cell dedifferentiation and growth via hepatocyte growth factor induction. PLoS One. 2015;10(3):e0121534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gu D, Zou X, Ju G, et al. Mesenchymal Stromal Cells Derived Extracellular Vesicles Ameliorate Acute Renal Ischemia Reperfusion Injury by Inhibition of Mitochondrial Fission through miR-30. Stem Cells Int. 2016;2016:2093940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001. May;163(6):1376–83. [DOI] [PubMed] [Google Scholar]
- 111.Li JW, Wu X. Mesenchymal stem cells ameliorate LPS-induced acute lung injury through KGF promoting alveolar fluid clearance of alveolar type II cells. Eur Rev Med Pharmacol Sci. 2015. July;19(13):2368–78. [PubMed] [Google Scholar]
- 112.Cassatella MA, Mosna F, Micheletti A, et al. Toll-like receptor-3-activated human mesenchymal stromal cells significantly prolong the survival and function of neutrophils. Stem Cells. 2011. June;29(6):1001–11. [DOI] [PubMed] [Google Scholar]
- 113.Raffaghello L, Bianchi G, Bertolotto M, et al. Human mesenchymal stem cells inhibit neutrophil apoptosis: a model for neutrophil preservation in the bone marrow niche. Stem Cells. 2008. January;26(1):151–62. [DOI] [PubMed] [Google Scholar]
- 114.Zheng G, Huang R, Qiu G, et al. Mesenchymal stromal cell-derived extracellular vesicles: regenerative and immunomodulatory effects and potential applications in sepsis. Cell Tissue Res. 2018. October;374(1):1–15. [DOI] [PubMed] [Google Scholar]
- 115.Morrison TJ, Jackson MV, Cunningham EK, et al. Mesenchymal Stromal Cells Modulate Macrophages in Clinically Relevant Lung Injury Models by Extracellular Vesicle Mitochondrial Transfer. Am J Respir Crit Care Med. 2017. November 15;196(10):1275–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Varkouhi AK, Jerkic M, Ormesher L, et al. Extracellular Vesicles from Interferon-gamma-primed Human Umbilical Cord Mesenchymal Stromal Cells Reduce Escherichia coli-induced Acute Lung Injury in Rats. Anesthesiology. 2019. May;130(5):778–790. [DOI] [PubMed] [Google Scholar]
- 117.Qian X, Xu C, Fang S, et al. Exosomal MicroRNAs Derived From Umbilical Mesenchymal Stem Cells Inhibit Hepatitis C Virus Infection. Stem Cells Transl Med. 2016. September;5(9):1190–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Burnham EL, Janssen WJ, Riches DW, et al. The fibroproliferative response in acute respiratory distress syndrome: mechanisms and clinical significance. Eur Respir J. 2014. January;43(1):276–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Orme J Jr., Romney JS, Hopkins RO, et al. Pulmonary function and health-related quality of life in survivors of acute respiratory distress syndrome. Am J Respir Crit Care Med. 2003. March 1;167(5):690–4. [DOI] [PubMed] [Google Scholar]
- 120.Choi M, Ban T, Rhim T. Therapeutic use of stem cell transplantation for cell replacement or cytoprotective effect of microvesicle released from mesenchymal stem cell. Mol Cells. 2014. February;37(2):133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fang S, Xu C, Zhang Y, et al. Umbilical Cord-Derived Mesenchymal Stem Cell-Derived Exosomal MicroRNAs Suppress Myofibroblast Differentiation by Inhibiting the Transforming Growth Factor-beta/SMAD2 Pathway During Wound Healing. Stem Cells Transl Med. 2016. October;5(10):1425–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Qu Y, Zhang Q, Cai X, et al. Exosomes derived from miR-181–5p-modified adipose-derived mesenchymal stem cells prevent liver fibrosis via autophagy activation. J Cell Mol Med. 2017. October;21(10):2491–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Glassberg MK, Minkiewicz J, Toonkel RL, et al. Allogeneic Human Mesenchymal Stem Cells in Patients With Idiopathic Pulmonary Fibrosis via Intravenous Delivery (AETHER): A Phase I Safety Clinical Trial. Chest. 2017. May;151(5):971–981.** A Phase I clinical trail for the use of human MSCs in patients with pulmonary fibrosis demonstrating no adverse events associated with administration of the cells.
- 124.Willis GR, Fernandez-Gonzalez A, Anastas J, et al. Mesenchymal Stromal Cell Exosomes Ameliorate Experimental Bronchopulmonary Dysplasia and Restore Lung Function through Macrophage Immunomodulation. Am J Respir Crit Care Med. 2018. January 1;197(1):104–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chen JY, An R, Liu ZJ, et al. Therapeutic effects of mesenchymal stem cell-derived microvesicles on pulmonary arterial hypertension in rats. Acta Pharmacol Sin. 2014. September;35(9):1121–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cruz FF, Borg ZD, Goodwin M, et al. Systemic Administration of Human Bone Marrow-Derived Mesenchymal Stromal Cell Extracellular Vesicles Ameliorates Aspergillus Hyphal Extract-Induced Allergic Airway Inflammation in Immunocompetent Mice. Stem cells translational medicine. 2015. September 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Phinney DG, Di Giuseppe M, Njah J, et al. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat Commun. 2015;6:8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kawasaki T, Nishiwaki T, Sekine A, et al. Vascular Repair by Tissue-Resident Endothelial Progenitor Cells in Endotoxin-Induced Lung Injury. Am J Respir Cell Mol Biol. 2015. October;53(4):500–12. [DOI] [PubMed] [Google Scholar]
- 129.Mao M, Wang SN, Lv XJ, et al. Intravenous delivery of bone marrow-derived endothelial progenitor cells improves survival and attenuates lipopolysaccharide-induced lung injury in rats. Shock. 2010. August;34(2):196–204. [DOI] [PubMed] [Google Scholar]
- 130.Yamada M, Kubo H, Kobayashi S, et al. Bone marrow-derived progenitor cells are important for lung repair after lipopolysaccharide-induced lung injury. J Immunol. 2004. January 15;172(2):1266–72. [DOI] [PubMed] [Google Scholar]
- 131.Wang D, Morales JE, Calame DG, et al. Transplantation of human embryonic stem cell-derived alveolar epithelial type II cells abrogates acute lung injury in mice. Mol Ther. 2010. March;18(3):625–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Su VY, Yang KY, Chiou SH, et al. Induced Pluripotent Stem Cells Regulate Triggering Receptor Expressed on Myeloid Cell-1 Expression and the p38 Mitogen-Activated Protein Kinase Pathway in Endotoxin-Induced Acute Lung Injury. Stem Cells. 2019. May;37(5):631–639. [DOI] [PubMed] [Google Scholar]
- 133.Su VY, Chiou SH, Lin CS, et al. Induced pluripotent stem cells reduce neutrophil chemotaxis via activating GRK2 in endotoxin-induced acute lung injury. Respirology. 2017. August;22(6):1156–1164. [DOI] [PubMed] [Google Scholar]
- 134.Yang KY, Shih HC, How CK, et al. IV delivery of induced pluripotent stem cells attenuates endotoxin-induced acute lung injury in mice. Chest. 2011. November;140(5):1243–1253. [DOI] [PubMed] [Google Scholar]
- 135.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997. February 14;275(5302):964–7. [DOI] [PubMed] [Google Scholar]
- 136.Burnham EL, Taylor WR, Quyyumi AA, et al. Increased circulating endothelial progenitor cells are associated with survival in acute lung injury. Am J Respir Crit Care Med. 2005. October 1;172(7):854–60. [DOI] [PubMed] [Google Scholar]
- 137.Yamada M, Kubo H, Ishizawa K, et al. Increased circulating endothelial progenitor cells in patients with bacterial pneumonia: evidence that bone marrow derived cells contribute to lung repair. Thorax. 2005. May;60(5):410–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cao JP, He XY, Xu HT, et al. Autologous transplantation of peripheral blood-derived circulating endothelial progenitor cells attenuates endotoxin-induced acute lung injury in rabbits by direct endothelial repair and indirect immunomodulation. Anesthesiology. 2012. June;116(6):1278–87. [DOI] [PubMed] [Google Scholar]
- 139.Wang J, Chen S, Ma X, et al. Effects of endothelial progenitor cell-derived microvesicles on hypoxia/reoxygenation-induced endothelial dysfunction and apoptosis. Oxid Med Cell Longev. 2013;2013:572729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhou Y, Li P, Goodwin AJ, et al. Exosomes from Endothelial Progenitor Cells Improve the Outcome of a Murine Model of Sepsis. Mol Ther. 2018. May 2;26(5):1375–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wu X, Liu Z, Hu L, et al. Exosomes derived from endothelial progenitor cells ameliorate acute lung injury by transferring miR-126. Exp Cell Res. 2018. September 1;370(1):13–23. [DOI] [PubMed] [Google Scholar]
- 142.Zhou Y, Li P, Goodwin AJ, et al. Exosomes from endothelial progenitor cells improve outcomes of the lipopolysaccharide-induced acute lung injury. Crit Care. 2019. February 13;23(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Machado MN, Mazzoli-Rocha F, Casquilho NV, et al. Bone Marrow-Derived Mononuclear Cell Therapy in Papain-Induced Experimental Pulmonary Emphysema. Front Physiol. 2018;9:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nandra KK, Takahashi K, Collino M, et al. Acute treatment with bone marrow-derived mononuclear cells attenuates the organ injury/dysfunction induced by hemorrhagic shock in the rat. Shock. 2012. June;37(6):592–8. [DOI] [PubMed] [Google Scholar]
- 145.Ornellas DS, Maron-Gutierrez T, Ornellas FM, et al. Early and late effects of bone marrow-derived mononuclear cell therapy on lung and distal organs in experimental sepsis. Respir Physiol Neurobiol. 2011. September 15;178(2):304–14. [DOI] [PubMed] [Google Scholar]
- 146.Araujo IM, Abreu SC, Maron-Gutierrez T, et al. Bone marrow-derived mononuclear cell therapy in experimental pulmonary and extrapulmonary acute lung injury. Crit Care Med. 2010. August;38(8):1733–41. [DOI] [PubMed] [Google Scholar]
- 147.Prota LF, Lassance RM, Maron-Gutierrez T, et al. Bone marrow mononuclear cell therapy led to alveolar-capillary membrane repair, improving lung mechanics in endotoxin-induced acute lung injury. Cell Transplant. 2010;19(8):965–71. [DOI] [PubMed] [Google Scholar]
- 148.Banerjee ER, Laflamme MA, Papayannopoulou T, et al. Human embryonic stem cells differentiated to lung lineage-specific cells ameliorate pulmonary fibrosis in a xenograft transplant mouse model. PLoS One. 2012;7(3):e33165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Li X, Zhang Y, Liang Y, et al. iPSC-derived mesenchymal stem cells exert SCF-dependent recovery of cigarette smoke-induced apoptosis/proliferation imbalance in airway cells. J Cell Mol Med. 2017. February;21(2):265–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Li LF, Liu YY, Yang CT, et al. Improvement of ventilator-induced lung injury by IPS cell-derived conditioned medium via inhibition of PI3K/Akt pathway and IP-10-dependent paracrine regulation. Biomaterials. 2013. January;34(1):78–91. [DOI] [PubMed] [Google Scholar]
- 151.Conese M, Carbone A, Castellani S, et al. Paracrine effects and heterogeneity of marrow-derived stem/progenitor cells: relevance for the treatment of respiratory diseases. Cells Tissues Organs. 2013;197(6):445–73. [DOI] [PubMed] [Google Scholar]
- 152.Hao Q, Zhu YG, Monsel A, et al. Study of Bone Marrow and Embryonic Stem Cell-Derived Human Mesenchymal Stem Cells for Treatment of Escherichia coli Endotoxin-Induced Acute Lung Injury in Mice. Stem Cells Transl Med. 2015. July;4(7):832–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Martens A, Ordies S, Vanaudenaerde BM, et al. Immunoregulatory effects of multipotent adult progenitor cells in a porcine ex vivo lung perfusion model. Stem Cell Res Ther. 2017. July 5;8(1):159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ranghino A, Cantaluppi V, Grange C, et al. Endothelial progenitor cell-derived microvesicles improve neovascularization in a murine model of hindlimb ischemia. Int J Immunopathol Pharmacol. 2012. Jan-Mar;25(1):75–85. [DOI] [PubMed] [Google Scholar]
- 155.Cantaluppi V, Gatti S, Medica D, et al. Microvesicles derived from endothelial progenitor cells protect the kidney from ischemia-reperfusion injury by microRNA-dependent reprogramming of resident renal cells. Kidney Int. 2012. August;82(4):412–27. [DOI] [PubMed] [Google Scholar]
- 156.Romieu-Mourez R, Francois M, Boivin MN, et al. Regulation of MHC class II expression and antigen processing in murine and human mesenchymal stromal cells by IFN-gamma, TGF-beta, and cell density. J Immunol. 2007. August 1;179(3):1549–58. [DOI] [PubMed] [Google Scholar]
- 157.Hupfeld J, Gorr IH, Schwald C, et al. Modulation of mesenchymal stromal cell characteristics by microcarrier culture in bioreactors. Biotechnol Bioeng. 2014. November;111(11):2290–302. [DOI] [PubMed] [Google Scholar]
- 158.Mitchell JP, Court J, Mason MD, et al. Increased exosome production from tumour cell cultures using the Integra CELLine Culture System. J Immunol Methods. 2008. June 1;335(1–2):98–105. [DOI] [PubMed] [Google Scholar]
- 159.de Jong OG, Verhaar MC, Chen Y, et al. Cellular stress conditions are reflected in the protein and RNA content of endothelial cell-derived exosomes. J Extracell Vesicles. 2012;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lara AR, Galindo E, Ramirez OT, et al. Living with heterogeneities in bioreactors: understanding the effects of environmental gradients on cells. Mol Biotechnol. 2006. November;34(3):355–81. [DOI] [PubMed] [Google Scholar]
- 161.King JA, Miller WM. Bioreactor development for stem cell expansion and controlled differentiation. Curr Opin Chem Biol. 2007. August;11(4):394–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yeatts AB, Choquette DT, Fisher JP. Bioreactors to influence stem cell fate: augmentation of mesenchymal stem cell signaling pathways via dynamic culture systems. Biochim Biophys Acta. 2013. February;1830(2):2470–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Musante L, Tataruch D, Gu D, et al. A simplified method to recover urinary vesicles for clinical applications, and sample banking. Sci Rep. 2014. December 23;4:7532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yeo RW, Lai RC, Zhang B, et al. Mesenchymal stem cell: an efficient mass producer of exosomes for drug delivery. Adv Drug Deliv Rev. 2013. March;65(3):336–41. [DOI] [PubMed] [Google Scholar]
- 165.Chen TS, Arslan F, Yin Y, et al. Enabling a robust scalable manufacturing process for therapeutic exosomes through oncogenic immortalization of human ESC-derived MSCs. J Transl Med. 2011. April 25;9:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Waterman RS, Tomchuck SL, Henkle SL, et al. A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS One. 2010. April 26;5(4):e10088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Elabd C, Ichim TE, Miller K, et al. Comparing atmospheric and hypoxic cultured mesenchymal stem cell transcriptome: implication for stem cell therapies targeting intervertebral discs. J Transl Med. 2018. August 10;16(1):222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhu Z, Gan X, Fan H, et al. Mechanical stretch endows mesenchymal stem cells stronger angiogenic and anti-apoptotic capacities via NFkappaB activation. Biochem Biophys Res Commun. 2015. December 25;468(4):601–5. [DOI] [PubMed] [Google Scholar]
- 169.Islam D, Huang Y, Fanelli V, et al. Identification and Modulation of Microenvironment Is Crucial for Effective Mesenchymal Stromal Cell Therapy in Acute Lung Injury. Am J Respir Crit Care Med. 2019. May 15;199(10):1214–1224. [DOI] [PubMed] [Google Scholar]
- 170.Fergie N, Todd N, McClements L, et al. Hypercapnic acidosis induces mitochondrial dysfunction and impairs the ability of mesenchymal stem cells to promote distal lung epithelial repair. FASEB J. 2019. April;33(4):5585–5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Elmadbouh I, Ashraf M. Tadalafil, a long acting phosphodiesterase inhibitor, promotes bone marrow stem cell survival and their homing into ischemic myocardium for cardiac repair. Physiol Rep. 2017. November;5(21). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hajinejad M, Pasbakhsh P, Omidi A, et al. Resveratrol pretreatment enhanced homing of SDF-1alpha-preconditioned bone marrow-derived mesenchymal stem cells in a rat model of liver cirrhosis. J Cell Biochem. 2018. March;119(3):2939–2950. [DOI] [PubMed] [Google Scholar]
- 173.Huleihel L, Sellares J, Cardenes N, et al. Modified mesenchymal stem cells using miRNA transduction alter lung injury in a bleomycin model. Am J Physiol Lung Cell Mol Physiol. 2017. July 1;313(1):L92–L103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Rani S, Ryan AE, Griffin MD, et al. Mesenchymal Stem Cell-derived Extracellular Vesicles: Toward Cell-free Therapeutic Applications. Mol Ther. 2015. May;23(5):812–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Roccaro AM, Sacco A, Maiso P, et al. BM mesenchymal stromal cell-derived exosomes facilitate multiple myeloma progression. J Clin Invest. 2013. April;123(4):1542–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Roccaro AM, Sacco A, Thompson B, et al. MicroRNAs 15a and 16 regulate tumor proliferation in multiple myeloma. Blood. 2009. June 25;113(26):6669–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zhu W, Huang L, Li Y, et al. Exosomes derived from human bone marrow mesenchymal stem cells promote tumor growth in vivo. Cancer Lett. 2012. February 1;315(1):28–37. [DOI] [PubMed] [Google Scholar]