Abstract
Background
The objective of this study was to examine depressive symptoms overtime and quantify the variance in symptoms attributable to substance use among a cohort of HIV-positive and HIV-negative men.
Methods
Participants were enrolled in an NIH/NIDA funded cohort, with 534 men resulting in 1,888 visits between August 2014 and June 2018. Participants were between 18 and 45 years, and half were HIV-positive. At baseline and semi-annual visits, information was collected on depressive symptoms, sexual behaviors, and substance use. Changes overtime in symptom scores were evaluated using individual growth curve modeling.
Results
The average CES-D20 score was 19.5 (SD=12.7). Depressive symptoms were highest among daily/weekly methamphetamine users (56% vs. 39% occasional users and 27% non-users; p value<.01). Factors independently associated with depressive symptoms included methamphetamine use (adjusted OR=1.5; 95% CI 1.1–2.3) and transactional sex (adjusted OR=1.8; 95% CI 1.4–2.5). Based on growth curve modeling, methamphetamine was the most influential predictor of depressive symptoms, accounting for 10% of individual variance (p value<.01). Declines in depressive symptoms were noted for heavy users of a number of drugs, except for methamphetamine. For instance, those reporting daily/weekly heroin had a 3.38 point decline in CESD20 scores overtime (p value=0.01). However, heavy methamphetamine users had much higher CESD20 scores and their scores remained high overtime (p value for change=0.91).
Conclusions
The prevalence of depressive symptoms among this cohort of HIV-negative and HIV-positive MSM was high, especially among frequent methamphetamine users. These findings suggest that reducing methamphetamine use may have the potential to reduce depressive symptoms.
Keywords: Depressive symptoms, Substance use, Methamphetamine, HIV, MSM
1. Introduction
Health disparities and inequalities across a wide range of health outcomes, including mental health have been noted among sexual minorities (Buchmueller and Carpenter, 2010; Dilley et al., 2010; Lee et al., 2009; Medley et al., October 2016; NSDUH Data Review. Retrieved from http://www.samhsa.gov/data; Roberts et al., 2010; Struble et al., 2010). A recent population-based study found that compared to heterosexuals, sexual minorities were twice as likely to have a diagnosis of a mood disorder (including depression) even after adjusting for other factors such as age and education (Stinchcombe et al., 2018). Disparities in mental health and depression are often linked to experiences with stigma, discrimination, and substance use and further exaggerated among those who are younger, identify as bisexual, or are HIV-positive (Bing et al., 2001; Bjorkenstam et al., 2017; Dyer et al., 2012; la Roi et al., 2016; Medley et al., October 2016; NSDUH Data Review. Retrieved from http://www.samhsa.gov/data). Depression and substance use are often comorbid conditions with evidence indicating common genetic factors for both conditions (Kendler et al., 2003). However, disentangling the causal relationship between these conditions is challenging, especially given the bidirectional nature of substance use and depression. Data from the few studies that have explored this are mixed, with some studies finding that drug use precedes depression, while others note that drug use serves as a potential form of self-medication and follows the onset of depression (Grant et al., 2009; Grant et al., 2016).
Findings in the literature on the association between depressive symptoms and sexual behavior have been somewhat mixed among men who have sex with men (MSM). While some studies have found decreased interest in sex and sexual activity during times of increased depressive symptoms, others have found no association (Bradley et al., 2008; Crepaz and Marks, 2001; Dilley et al., 1998; Shiu et al., 2014). In studies where an association was noted, those with depressive symptoms had increases in the number of sexual partners, condomless sex, sexual encounters under the influence of drugs and alcohol, rates of exchange sex, and risk of sexually transmitted infections including HIV (Koblin et al., 2006; Meade and Sikkema, 2005; Reisner et al., 2009). Additionally, the co-occurrence of other issues such as substance use disorders along with depression increased the risk of HIV acquisition given the increased engagement in sexual transmission behaviors (Meade and Sikkema, 2005).
Depression is not only a risk factor for HIV acquisition, but evidence also indicates that among those who are HIV-positive, the prevalence of depression is high with up to half of HIV-positive patients living with depression (Choi et al., 2016; Ciesla and Roberts, 2001; Meade and Sikkema, 2005; Nacher et al., 2010). The co-occurrence of depression and HIV has been associated with negative outcomes including reduced quality of life and functionality, increases in medical comorbidities, as well as reduced rates of antiretroviral therapy (ART) prescriptions, decreases in ART adherence, and higher viral loads (independent of adherence behavior) (Ammassari et al., 2004; Mayston et al., 2012; Mitchell et al., 2012; Nanni et al., 2015). However, most studies on depression have used a cross sectional study design and less is known about changes in depressive symptomatology over time and patterns of persistence over time, particularly in populations with competing risks for depression including sexual minority status, HIV-positivity, and substance use. Given the potential fluctuating nature of depressive symptoms and its association with HIV transmission and acquisition, our study aimed to examine the prevalence, correlates, and changes in depressive symptoms over time, among a cohort of HIV-positive and high risk HIV-negative young men who have sex with men (MSM). Correlates of interest included sociodemographic characteristics, sexual risk behaviors, and substance use. Beyond the shape and direction of change in depressive symptoms (i.e., does it remain flat, increase, or decrease), we also aimed to quantify the extent to which substance use including the various patterns of drugs used contribute to the differences in depressive symptomatology. We recognize the complex and ongoing interaction between these two disorders and hypothesize that substance use impacts the trajectory of depressive symptoms and can vary by type of substance used. Understanding changes in depressive symptoms overtime and the extent to which various substance use patterns serve as a facilitator for these changes will contribute to our understanding of the intersection of substance use and mental health. Furthermore, unique to our study is our specific focus on a highly vulnerable population and our ability to explore differences by HIV-status.
2. Methods
2.1. Study population and design
Data for this study were based on those collected from participants in the mSTUDY – an NIH/NIDA funded cohort of racial/ethnically diverse, HIV-positive and high-risk HIV-negative MSM. The mSTUDY has been described elsewhere (Fulcher et al., 2018; Javanbakht et al., 2018; Okafor et al., 2017), but briefly, study enrollment started in August 2014 and participants were recruited from two different study sites in Los Angeles, CA including a community-based organization providing services for the lesbian, gay, bisexual, and transgender community and a community-based university research clinic. Participants enrolled in the mSTUDY between August 2014 and June 2018 were included in this analysis and were if they were: (1) between 18 and 45 years of age, (2) identified as male at birth, (3) if HIV-negative, reported condomless anal intercourse with a male partner in the past 6-months, (4) capable of providing informed consent, and (5) willing and able to return to the study every six months to complete study related activities. By design, half of the participants were HIV-positive and half were HIV-negative.
2.2. Study procedures and data collection
All participants provided written informed consent prior to study participation. At each study visit participants completed a self-administered, computer-based questionnaire. The questionnaire collected information on current depressive symptoms as well as demographics, substance use, and sexual risk behaviors. Current symptoms of depression were measured using the 20-item Center for Epidemiological Studies Depression Scale (CES-D20), first at baseline and again during follow-up visits. The scale was originally developed to measure depressive symptomatology in the general population and has since been used in other populations including those with HIV (Choi et al., 2015; Eaton et al., 2004; Meader et al., 2011; Natamba et al., 2014; Radloff, 1977). The CES-D20 measures depressive symptoms experienced in the past seven days as defined by the American Psychiatric Association Diagnostic and Statistical Manual (DSM-V) for a major depressive episode.
As part of the self-administered study questionnaire, participants were asked to report on the frequency of substance use in the past six months including the use of the following drugs: (1) cocaine powder; (2) crack cocaine; (3) ecstasy; (4) heroin; (5) marijuana; (6) methamphetamine; (7) ‘party drugs’ including GHB and ketamine; (8) poppers, and (9) illicit use of prescription medications. Frequent users for each drug was defined as those who reported weekly use or more often, occasional users were those who reported monthly use or less often, and non-users were those who reported no use. Questions on sexual behaviors relevant to this analysis focused on recent behaviors (past six months) and included information on number and gender of sex partners, reports of new sex partners, concurrent partnerships (i.e., sexual partnerships that overlap in time), and transactional sex defined as an exchange of money, drugs, shelter, or other goods for sex.
At each study visit samples were collected for STI/HIV testing. Urine samples as well as rectal and pharyngeal swabs were collected for chlamydia and gonorrhea testing using nucleic acid amplification testing (NAAT) technology (Aptima Combo 2®, GenProbe, San Diego, CA). Blood samples were collected for HIV testing among those who were HIV-negative and HIV-1 RNA levels for those who were HIV-positive. Blood samples were also collected for syphilis testing using the rapid plasma regain test (RPR), with confirmatory testing using the Treponema pallidum particle agglutination test (TPPA). All participants were scheduled to return every six months and the study procedures were repeated at each visit. The study was approved by the Institutional Review Board at the University of California Los Angeles.
2. 3. Analytic Strategy
The primary outcome of this analysis was depressive symptomatology. In order to create a dichotomous variable indicative of depressive symptoms we used a cut-point of 23 on the CES-D20 score. The cut-point was found to be more optimal for use among HIV-positive individuals (sensitivity: 1.0; specificity: 0.87) as compared to the cut-point of 16, which is more widely used in the general population (Choi et al., 2015). Univariate analyses provided descriptive statistics for the sample overall and by depressive symptomatology status (i.e., CES-D20 ≥ 23 vs. CES-D20 < 23). Comparisons of demographics, substance use, sexual risk behaviors, as well as clinical and laboratory characteristics between those who reported depressive symptoms compared to those who did not report depressive symptoms were based on chi-square methods adjusting for the effect of the subject (i.e., repeated measures). Factors associated with depressive symptomatology were assessed using regression analysis with generalized estimating equations (GEE) in order to account for the within subject correlations (Liang and Zeger, 1986; Zeger et al., 1988). We fit models with random intercepts and time effects to accommodate the repeated measures gathered from each participant and to allow participant-specific changes in the responses over time. Univariate analyses along with a priori knowledge informed variables for inclusion in the multivariable models.
Finally, we examined the unique trajectories and changes over time in depressive symptom scores using individual growth curve modeling (Goldstein et al., 1994; Willett JB, 1994). The individual growth model estimates both average and individual trajectories overtime thus allowing for an examination of inter- and intra-individual changes. Specifically, we used the unconditional growth model to express a mixed effects model in which the outcome (CES-D20 score) was modeled as a function of time allowing for a random intercept and slope. The conditional growth models were developed to consider substance use and HIV status as an explanatory variable. Separate conditional models were developed to consider substance use including a dichotomous substance use variable (i.e., yes, no), a three level substance use variable comparing methamphetamine, other drugs not including methamphetamine, and no drugs, as well as frequency of use. For the purposes of this analysis, the use of individual growth models allowed us to describe the overall trend in symptom scores over time – i.e., whether symptoms scores increased, decreased, or remained the same overtime both at the individual level and across individuals.
Additionally, we used the conditional linear growth models to determine whether, and to what extent HIV status and specific substance use patterns were associated with differences in changes in depressive symptom scores. All analyses were conducted using SAS version 9.4 (SAS Inc., Cary, NC).
3. Results
3.1. Characteristics of study population
Between August 2014 and June 2018, a total of 534 participants were enrolled in the mSTUDY with as much as 3.7 years of follow-up data. These participants accumulated a total of 1,888 visits, with 86% of participants having two or more visits, and 66% having 3 or more visits (median visits=3; range (1–7 visits). At baseline the average age of participants was 31.4 years (SD 7.0) with 43% identifying as African American, followed by 37% Hispanic/Latino and 14% white (Table 1). Nearly half reported being unemployed and 35% reported experiencing unstable housing in the 6 months prior to study enrollment. By design, half of the study participants (n=267) were HIV-positive. At baseline, HIV-positive patients were slightly older, were more likely to report being unemployed, and more likely to have a history of incarceration (Table 1).
Table 1.
Baseline characteristics among mSTUDY participants, by HIV status (8/2014 – 6/2018)
| Total (n=534)^ | HIV-positive (n=267)^ | HIV-negative (n=267)^ | P value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |||||||
| Socio-demographic characteristics | ||||||||||||
| Age, mean(SD) | 31.4 (7.0) | 33.8 (6.6) | 29.1 (6.6) | <.01 | ||||||||
| Race/ethnicity | 0.13 | |||||||||||
| African American | 228 | 42.7 | 110 | 41.3 | 118 | 44.1 | ||||||
| Hispanic/Latino | 196 | 36.7 | 97 | 36.3 | 99 | 37.1 | ||||||
| Other | 37 | 6.9 | 15 | 40.5 | 22 | 8.2 | ||||||
| White | 73 | 13.7 | 45 | 16.9 | 28 | 10.5 | ||||||
| Education | 0.06 | |||||||||||
| < High School | 66 | 12.5 | 41 | 15.7 | 25 | 9.4 | ||||||
| High School Graduate | 193 | 36.5 | 97 | 37.2 | 96 | 36.0 | ||||||
| > High School Graduate | 269 | 51.0 | 123 | 47.1 | 146 | 54.6 | ||||||
| Unemployed | 237 | 45.8 | 145 | 56.4 | 92 | 35.4 | <.01 | |||||
| Unstable Housing, past 6 months* | 189 | 35.4 | 94 | 35.2 | 95 | 35.6 | 0.93 | |||||
| Ever Incarcerated | 208 | 39.1 | 117 | 44.1 | 91 | 34.1 | 0.02 | |||||
Abbreviations. SD=Standard deviations
Sum may not equal total due to missing information
Defined as not having a regular place to stay in the past 6 months
3.2. Prevalence of depressive symptomatology
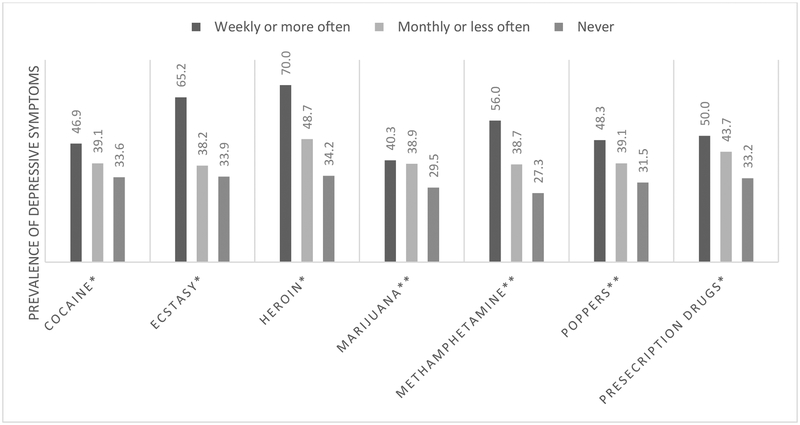
The median CES-D20 score was 19 (interquartile range: 9–26) and prevalence of depressive symptoms (i.e., CES-D20 ≥ 23) as reported across all study visits was 35% (n=656)(Table 2). Nearly half of those with a current prescription for antidepressants had depressive symptoms as compared to 29% of those without a prescription. The prevalence of depressive symptoms was higher in visits where participants reported unemployment (49% vs. 24%; p value<.01) as well as unstable housing (48% vs. 30%; p value <.01) and slightly higher among those who were HIV-positive (38% vs. 31%; p value=0.02). Additionally, differences in the prevalence were noted by both substance use and sexual behaviors. Among visits where participants reported methamphetamine use, nearly half reported depressive symptoms, as compared to 31% in visits where other drugs were reported (but not methamphetamine) and lowest in visits with no substance use (23%; p value<.01). Further exploration of substance use reveals a dose response relationship between amount of substance use and prevalence of depressive symptoms, with those in the highest use category (i.e., weekly or more often) having the highest prevalence of depressive symptoms as compared to those in the monthly or less often, and no drug use categories (Figure 1).
Table 2.
Prevalence of depressive symptoms across study visits among mSTUDY participants, by HIV-status (8/2014 – 6/2018)
| Total (n=1,888 visits) | HIV-positive (n=964 visits) | HIV-negative (n=924 visits) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CES-D Score ≥ 23 | P value~ | CES-D Score ≥ 23 | P value~ | CES-D Score ≥ 23 | P value~ | |||||||||||||||||
| n | % | n | % | n | % | |||||||||||||||||
| Total | 656 | 34.8 | -- | 366 | 37.8 | -- | 290 | 31.4 | -- | |||||||||||||
| Socio-demographic characteristics | ||||||||||||||||||||||
| Age at study visit, mean (SD) | 0.76 | 0.45 | 0.46 | |||||||||||||||||||
| CES-D Score ≥ 23 | 32.4 (6.8) | 34.6(6.3) | 29.9(6.6) | |||||||||||||||||||
| CES-D Score < 23 | 32.5 (7.1) | 35.0(6.7) | 30.5(6.6) | |||||||||||||||||||
| Race/ethnicity | 0.95 | 0.95 | 0.87 | |||||||||||||||||||
| African American | 279 | 35.1 | 143 | 38.0 | 136 | 32.5 | ||||||||||||||||
| Hispanic/Latino | 218 | 32.7 | 133 | 35.6 | 85 | 28.9 | ||||||||||||||||
| Other | 61 | 37.9 | 26 | 42.6 | 35 | 35.0 | ||||||||||||||||
| White | 98 | 37.0 | 64 | 41.6 | 34 | 30.6 | ||||||||||||||||
| Unemployed | <.01 | <.01 | <.01 | |||||||||||||||||||
| Yes | 385 | 49.2 | 242 | 50.2 | 143 | 47.5 | ||||||||||||||||
| No | 248 | 23.9 | 106 | 24.0 | 142 | 23.8 | ||||||||||||||||
| Unstable Housing, past 6 months* | <.01 | 0.08 | <.01 | |||||||||||||||||||
| Yes | 252 | 48.2 | 125 | 50.8 | 127 | 45.9 | ||||||||||||||||
| No | 404 | 29.6 | 241 | 33.6 | 163 | 25.2 | ||||||||||||||||
| Substance use behaviors | ||||||||||||||||||||||
| Smoker, current (cigarettes) | <.01 | 0.03 | <.01 | |||||||||||||||||||
| Yes | 266 | 49.3 | 152 | 46.9 | 114 | 52.8 | ||||||||||||||||
| No | 325 | 29.1 | 172 | 33.5 | 153 | 25.3 | ||||||||||||||||
| Binge drinking, past 6 months | 0.73 | 0.28 | 0.82 | |||||||||||||||||||
| Yes | 329 | 35.5 | 155 | 39.1 | 174 | 32.7 | ||||||||||||||||
| No | 326 | 34.2 | 211 | 37.4 | 115 | 29.7 | ||||||||||||||||
| Substance use (mutually exclusive categories), past 6 months | <.01 | <.01 | <.01 | |||||||||||||||||||
| Methamphetamine use | 338 | 47.1 | 230 | 47.4 | 108 | 46.6 | ||||||||||||||||
| Other substance use (excluding methamphetamine) | 204 | 30.6 | 78 | 34.4 | 126 | 28.6 | ||||||||||||||||
| No substance use | 113 | 22.8 | 58 | 23.3 | 55 | 22.3 | ||||||||||||||||
| Specific drugs used, past 6 months | ||||||||||||||||||||||
| Cocaine | 0.03 | 0.32 | 0.02 | |||||||||||||||||||
| Yes | 143 | 40.2 | 64 | 41.8 | 79 | 38.9 | ||||||||||||||||
| No | 512 | 33.6 | 302 | 37.8 | 210 | 29.3 | ||||||||||||||||
| Ecstasy | 0.05 | 0.09 | 0.20 | |||||||||||||||||||
| Yes | 104 | 40.6 | 57 | 45.6 | 47 | 35.9 | ||||||||||||||||
| No | 551 | 33.9 | 309 | 37.0 | 242 | 30.7 | ||||||||||||||||
| Heroin | 0.02 | 0.33 | 0.02 | |||||||||||||||||||
| Yes | 32 | 56.1 | 12 | 57.1 | 20 | 55.6 | ||||||||||||||||
| No | 623 | 34.2 | 354 | 37.7 | 269 | 30.5 | ||||||||||||||||
| Marijuana | <.01 | <.01 | 0.01 | |||||||||||||||||||
| Yes | 388 | 39.8 | 203 | 45.1 | 185 | 35.2 | ||||||||||||||||
| No | 267 | 29.5 | 163 | 31.9 | 104 | 26.5 | ||||||||||||||||
| Methamphetamine | <.01 | <.01 | <.01 | |||||||||||||||||||
| Yes | 338 | 47.1 | 230 | 47.4 | 108 | 46.6 | ||||||||||||||||
| No | 317 | 27.3 | 136 | 28.6 | 181 | 26.4 | ||||||||||||||||
| Poppers | <.01 | <.01 | 0.71 | |||||||||||||||||||
| Yes | 253 | 41.8 | 167 | 49.4 | 86 | 32.2 | ||||||||||||||||
| No | 402 | 31.5 | 199 | 31.9 | 203 | 31.1 | ||||||||||||||||
| Prescription drugs | 0.01 | 0.02 | 0.18 | |||||||||||||||||||
| Yes | 115 | 45.6 | 62 | 54.9 | 53 | 38.1 | ||||||||||||||||
| No | 540 | 33.2 | 304 | 35.6 | 236 | 30.3 | ||||||||||||||||
| Sexual behaviors | ||||||||||||||||||||||
| Gender of Sex Partners, past 6 months | 0.07 | 0.50 | 0.04 | |||||||||||||||||||
| Male only | 598 | 34.0 | 354 | 37.8 | 244 | 29.7 | ||||||||||||||||
| Male and Female | 58 | 45.0 | 12 | 44.4 | 46 | 45.1 | ||||||||||||||||
| New Sex Partner, past 6 months | 0.01 | 0.03 | 0.08 | |||||||||||||||||||
| Yes | 461 | 37.4 | 246 | 42.3 | 215 | 33.0 | ||||||||||||||||
| No | 195 | 29.8 | 120 | 31.4 | 75 | 27.6 | ||||||||||||||||
| Transgender Anal Sex Partner, past 6 months | 0.01 | 0.22 | <.01 | |||||||||||||||||||
| Yes | 66 | 49.3 | 22 | 56.4 | 44 | 46.3 | ||||||||||||||||
| No | 590 | 33.7 | 344 | 37.2 | 246 | 29.7 | ||||||||||||||||
| Intimate Partner Violence, past 12 months** | <.01 | <.01 | <.01 | |||||||||||||||||||
| Yes | 158 | 54.5 | 91 | 55.8 | 67 | 52.8 | ||||||||||||||||
| No | 478 | 30.9 | 266 | 34.2 | 212 | 27.5 | ||||||||||||||||
| Concurrent Sexual Partnership, past 6 months | 0.98 | 0.66 | 0.84 | |||||||||||||||||||
| Yes | 248 | 34.4 | 132 | 40.9 | 116 | 29.2 | ||||||||||||||||
| No | 323 | 32.8 | 186 | 34.3 | 137 | 30.9 | ||||||||||||||||
| Received $/drugs/shelter for sex, past 3 months | <.01 | <.01 | <.01 | |||||||||||||||||||
| Yes | 172 | 57.5 | 99 | 62.7 | 73 | 51.8 | ||||||||||||||||
| No | 449 | 29.7 | 252 | 32.6 | 197 | 26.6 | ||||||||||||||||
| Clinical and Laboratory Factors | ||||||||||||||||||||||
| HIV-serostatus | 0.02 | |||||||||||||||||||||
| HIV-positive | 366 | 37.8 | -- | -- | -- | -- | ||||||||||||||||
| HIV-negative | 290 | 31.4 | -- | -- | -- | -- | ||||||||||||||||
| HIV-1 RNA ≤ 20 copies/mL^ | 0.09 | |||||||||||||||||||||
| Yes | -- | -- | 188 | 34.8 | -- | -- | ||||||||||||||||
| No | -- | -- | 174 | 41.6 | -- | -- | ||||||||||||||||
| Any STI (Chlamydia, Gonorrhea, or Infectious Syphilis) | 0.39 | 0.35 | 0.96 | |||||||||||||||||||
| Yes | 131 | 38.5 | 81 | 39.5 | 50 | 37.0 | ||||||||||||||||
| No | 521 | 33.9 | 284 | 37.5 | 237 | 30.5 | ||||||||||||||||
| Prescription for Antidepressant# | <.01 | <.01 | <.01 | |||||||||||||||||||
| Yes | 270 | 49.4 | 168 | 48.3 | 102 | 51.3 | ||||||||||||||||
| No | 386 | 28.8 | 198 | 32.1 | 188 | 26.0 | ||||||||||||||||
Abbreviations. SD=Standard Deviation; PrEP=Pre-exposure Prophylaxis
p value adjusts for the effect of the subject (i.e. multiple observations for the same participant)
Defined as not having a regular place to stay in the past 6 months
Defined as being hit, kicked, or slapped by a lover, boyfriend/girlfriend when that person meant to hurt you physically
Among HIV-positive participants
Includes prescription for antidepressants including SSRIs, SNRIs, and TCAs
Figure 1.
Prevalence of depressive symptoms by type and amount of substance use (past 6 months) among mSTUDY participants (8/2014–6/2018)
Analyses stratified by HIV-status showed differences in sexual risk behaviors associated with depressive symptoms by HIV-status. HIV-positive participants who reported having new sex partners in the past 6 months had a higher prevalence of depressive symptoms as compared to those without a new sex partner (42% vs. 31%; p value <.01). Among HIV-negative participants a higher prevalence of depressive symptoms was noted during visits in which both male and female sex partners were reported as compared to male only partners, as well as visits in which transgender sex partners were reported (45% vs. 30%; p value=0.04; 46% vs. 30%; p value <.01 respectively).
Based on multivariable analyses, adjusting for unemployment, antidepressant medication use, depressive symptoms at prior visit, and HIV-status, methamphetamine use was independently associated with CES-D20 scores ≥ 23. Specifically, we found that methamphetamine users were 1.5 times as likely to have high depressive symptoms scores as compared to non-substance users (adjusted odds ratio (AOR)=1.5; 95% confidence interval (CI) 1.1–2.3), which was also higher than those who reported substance use not including methamphetamine (AOR=1.4; 95% CI 1.0–2.1). Additionally, transactional sex was independently associated with high depressive symptom scores (past 3 months) (AOR=1.8; 95% 1.4–2.5).
3.3. Trajectory of depressive symptomatology
Results of the growth model analyses are presented in Table 3. Based on the unadjusted linear growth model, we find that the estimated variance of the intercept and slope (80.72 and 1.99, respectively) are statistically meaningful (p<.01), suggesting that individuals varied in depressive symptoms scores and that the rate and direction of change overtime also varied by individual. Looking across all participants, the average CES-D20 score of 19.08 decreased slightly over time (i.e., across visits), though this change was neither clinically or statistically meaningful (0.33 points; p value=0.06). In our multivariable analyses (as presented above), we found that substance use was an important factor associated with depressive symptoms with the individual growth model analyses further demonstrating that substance use – methamphetamine in particular – was an influential predictor of the rate of change in depressive symptoms. When examining substance use overall (i.e., as a dichotomous variable) we found that the variance of the intercept changed from 80.72 to 78.47, meaning substance use over time accounted for 2.8% change in the individual difference in CES-D20 scores (Table 3). However, when we further delineated the impact of methamphetamine use, including the frequency of use, we found that methamphetamine use accounted for 10.2% of the individual differences in depressive symptoms (80.72–72.51)/80.72], more than any of the other substance we examined including cocaine, ecstasy, heroin, marijuana, party drugs, poppers, and prescription drugs which only accounted for anywhere between <1 to 1.5% of the individual variance in depressive symptoms (Table 4). Additionally, we found that frequent methamphetamine users had a 5.37 point increase in depressive symptom scores compared to non-users (p value<.01), reduced to a 2.51 point increase among occasional methamphetamine user (compared to non-users; p value<.01) and this remained even after controlling for HIV-status (Table 3). With inclusion of HIV status in the model, we find that variance in mean depressive symptoms remained the same (72.51 vs. 72.56) – in other words, the temporal impact of HIV on depressive symptoms scores was negligible. Likewise the unexplained individual variance over time did not change; HIV positive participants had CES-D20 scores that were 0.90 points higher and a 0.43 point faster increase in scores per visit when compared to those without HIV, though this difference was not statistically significant (p value=0.38 and 0.18, respectively).
Table 3.
Individual growth models for longitudinal changes in depressive symptoms among mSTUDY participants (8/2014–6/2018)
| Unconditional growth model | Conditional growth model: Substance Use (yes vs. no) | Conditional growth model: Substance Use (meth vs. other drugs vs. no drugs) | Conditional growth model: Meth Use Frequency | Conditional growth model: Meth Use Frequency and HIV status | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | (SE) | P value | Estimate | (SE) | P value | Estimate | (SE) | P value | Estimate | (SE) | P value | Estimate | (SE) | P value | |||||
| Random Variance | |||||||||||||||||||
| Intercept | 80.72 | 6.61 | <.01 | 78.47 | 6.51 | <.01 | 73.67 | 6.25 | <.01 | 72.51 | 6.16 | <.01 | 72.56 | 6.14 | <.01 | ||||
| Linear slope | 1.99 | 0.55 | <.01 | 1.93 | 0.55 | <.01 | 1.88 | 0.54 | <.01 | 1.85 | 0.52 | <.01 | 1.82 | 0.52 | <.01 | ||||
| Residual | 61.22 | 2.60 | <.01 | 61.48 | 2.63 | <.01 | 61.79 | 2.63 | <.01 | 61.26 | 2.60 | <.01 | 61.22 | 2.60 | <.01 | ||||
| Fixed Effects | |||||||||||||||||||
| Intercept | 19.08 | 0.48 | <.01 | 17.29 | 0.82 | <.01 | 17.14 | 0.81 | <.01 | 17.36 | 0.56 | <.01 | 16.93 | 0.68 | <.01 | ||||
| Visit | −0.33 | 0.16 | 0.06 | −0.26 | 0.29 | 0.36 | −0.26 | 0.29 | 0.36 | −0.18 | 0.19 | 0.36 | −0.28 | 0.23 | 0.11 | ||||
| Meth use | |||||||||||||||||||
| Weekly or more often | 5.37 | 0.95 | <.01 | 5.33 | 0.97 | <.01 | |||||||||||||
| Monthly or less often | 2.51 | 0.89 | <.01 | 2.47 | 0.91 | <.01 | |||||||||||||
| Never | 0.00 | 0.00 | |||||||||||||||||
| Meth Use*Time | |||||||||||||||||||
| Weekly or more often | 0.13 | 0.39 | 0.73 | 0.04 | 0.39 | 0.76 | |||||||||||||
| Monthly or less often | −0.04 | 0.36 | 0.91 | −0.11 | 0.37 | 0.91 | |||||||||||||
| Never | 0.00 | 0.00 | |||||||||||||||||
| HIV-positive | 0.90 | 0.92 | 0.36 | ||||||||||||||||
| HIV-positive*Time | 0.43 | 0.32 | 0.18 | ||||||||||||||||
Abbreviations. SE=standard error; meth=methamphetamine
Note. Unconditional growth model only includes the temporal predictor of time without other explanatory variables; the conditional growth models include random effects (i.e., slope and intercept) and is conditioned on the specific predictor variable noted in the column
Table 4.
Individual growth models examining association with type and amount of substance use and variation and change in depressive symptoms scores overtime among mSTUDY participants (8/2014 – 6/2018)
| % Difference in individual variation explained in CES-D20 scores* | Change in CES-D20 Score overtime (95% CI) | P value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cocaine | 0.7% | ||||||||||
| Weekly or more often | −2.42 | (−3.24 to −1.60) | <.01 | ||||||||
| Monthly or less often | −0.21 | (−0.60 to 0.18) | 0.59 | ||||||||
| Never | 0 | ||||||||||
| Ecstasy | 0.3% | ||||||||||
| Weekly or more often | −3.23 | (−4.54 to −1.92) | 0.01 | ||||||||
| Monthly or less often | 0.23 | (−0.21 to 0.67) | 0.61 | ||||||||
| Never | 0 | ||||||||||
| Heroin | 0.9% | ||||||||||
| Weekly or more often | −3.38 | (−4.69 to −2.07) | 0.01 | ||||||||
| Monthly or less often | −2.70 | (−4.04 to −1.36) | 0.03 | ||||||||
| Never | 0 | ||||||||||
| Marijuana | 1.2% | ||||||||||
| Weekly or more often | −0.29 | (−0.60 to 0.02) | 0.32 | ||||||||
| Monthly or less often | −0.33 | (−0.73 to 0.07) | 0.40 | ||||||||
| Never | 0 | ||||||||||
| Methamphetamine | 10.2% | ||||||||||
| Weekly or more often | 0.13 | (−0.23 to 0.49) | 0.91 | ||||||||
| Monthly or less often | −0.04 | (−0.42 to 0.34) | 0.73 | ||||||||
| Never | 0 | ||||||||||
| Party drugs | 1.5% | ||||||||||
| Weekly or more often | −1.86 | (−2.75 to −0.97) | 0.04 | ||||||||
| Monthly or less often | −0.09 | (−0.53 to 0.35) | 0.84 | ||||||||
| Never | 0 | ||||||||||
| Poppers | 0.7% | ||||||||||
| Weekly or more often | −0.83 | (−1.34 to −0.32) | 0.10 | ||||||||
| Monthly or less often | 0.16 | (−0.18 to 0.50) | 0.63 | ||||||||
| Never | 0 | ||||||||||
| Prescription drugs | 1.4% | ||||||||||
| Weekly or more often | −0.86 | (−1.51 to −0.21) | 0.19 | ||||||||
| Monthly or less often | 0.69 | (0.16 to 1.22) | 0.19 | ||||||||
| Never | 0 | ||||||||||
differences are measured as a change in the variance of the intercept of linear mixed model accounting for substance use and time
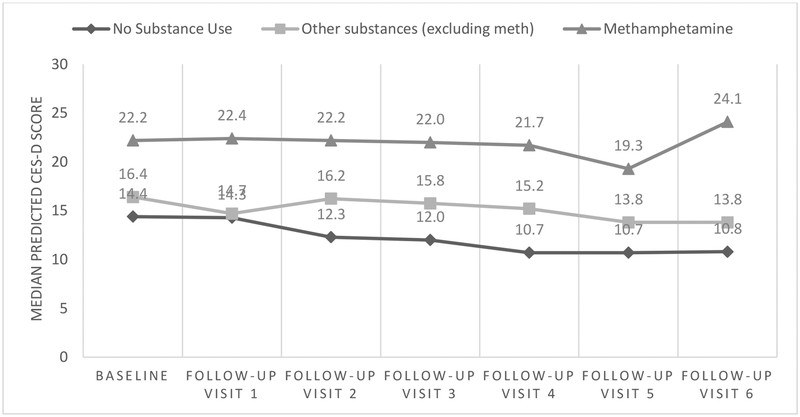
Across study visits we see clinically and statistically meaningful differences between depressive symptom scores when comparing methamphetamine users to other substance users and non-substance users (Figure 2). However, given that the interaction between methamphetamine use and time was not statistically significant, the modest changes in depression scores overtime are not different for methamphetamine users compared to non-users (Table 3). In fact, we find significant declines in depressive symptoms for heavy users of a number of drugs, though this is not the case for methamphetamine (Table 4). For instance, participants who reported using heroin weekly or more often had a 3.38 point decline in CESD20 scores overtime (p value=0.01), with similar patterns noted for heavy users of cocaine, ecstasy, and party drugs). However, methamphetamine users, especially heavy methamphetamine users on average had much higher CESD20 scores (Figure 2, Table 3) and their scores remained high overtime (non-significant change of 0.13; p value=0.91)(Table 3).
Figure 2.
Predicted CESD scores across study visit, by substance use status among mSTUDY participants (8/2014–6/2018)
4. Discussion
Findings from this study indicate that more than a third of this cohort of younger MSM reported symptoms consistent with depression. The longitudinal nature of our study and our extensive data on the various types and frequency of substances used allowed us to quantify the contribution of each substance to differences in depressive symptoms overall and with time. We found that while substance use was associated with symptoms of depression, methamphetamine use more than any other drug accounted for increases in depressive symptoms and was a more influential determinant of depressive symptom scores than HIV-status. Furthermore, we found that frequent methamphetamine use was associated with persistence of depressive symptoms even though frequent users of other drugs showed declines in depressive symptoms overtime. Finally, the equal distribution of HIV-positive and negative participants allowed us to identify sexual risk behaviors associated with depressive symptoms while considering the specific risk profiles for both HIV transmission and acquisition.
Our finding that non-substance users had the lowest depression scores was not unexpected, though our result showing that those who used substances less frequently had lower depression scores suggests that reductions in the amount of substance use even in the absence of abstinence may be associated with improvements in symptoms of depression. Additionally, relative to other substances, reduced use of methamphetamine had the greatest impact on depressive symptoms. While the cessation of drug use is the principal objective of any drug abuse treatment program, substance use disorders are often described as chronic relapsing conditions with relapse rates as high as 60% (Connors et al., 1996; McLellan et al., 2000). Consequently, consideration to ‘harm reduction strategies’ that lead to a decrease in methamphetamine use (not just abstinence) may still be helpful in this context. In fact, a randomized study of a 16-week substance use treatment program among methamphetamine dependent men found that reductions in negative outcomes (including depression) were linearly correlated with methamphetamine use (Jaffe et al., 2007). While the greatest benefit was seen for those who achieved methamphetamine abstinence, there were still benefits gained from reduced methamphetamine use. Likewise, another study specifically among methamphetamine using MSM found that those who reported a decrease in stimulant use showed reductions in sexual risk behaviors and improvements in indicators of HIV care (Carrico et al., 2014).
Management of depression has its own treatment cascade and despite the fact that depression is highly prevalent among sexual minorities, including those who are HIV-positive, it is widely underdiagnosed, untreated, and undertreated (Pence et al., 2012; Weaver et al., 2008). In fact, a recent analysis of the depression treatment cascade in the context of HIV care found that less than half of patients with a major depressive disorder were diagnosed clinically, 18% were receiving any treatment with only 7% receiving adequate treatment (Pence et al., 2012). Findings from our study support this in that nearly one-third of our participants who reported symptoms consistent with depression were not receiving pharmacological treatment. Additionally, our finding that half of those who reported depressive symptoms were in fact receiving medication for depression lends support to results that suggest that substance use may in fact affect medications used in the treatment of depression resulting in less benefit from these treatments (Grelotti et al., 2017). Although it has been suggested that strategies are needed to treat depression in parallel with substance use (Delaney et al., 2018), our data suggest that interventions that initially target reductions in substance use (methamphetamine in particular) may be useful in evaluating the extent to which co-morbid conditions such as depression decline before starting treatment regimen that target depression.
The relationship between substance use, depression, and risky sexual behaviors is likely complex and may be difficult to disentangle the effect of one factor on the other. For instance, our finding that those who reported transactional sex had a two fold increase in the odds of depressive symptoms may not only suggest a direct path from sex work to depression but may likely be indicative of an indirect path where sex work becomes an economic necessity for people who use drugs regularly. Other studies along with our previous work demonstrate that not only is transactional sex associated with substance use, but that a majority of the transactions may be occurring for the purpose of obtaining drugs (Bauermeister et al., 2017; Javanbakht et al., 2018; Weber et al., 2001). The intertwined nature of substance use, depression, and sexual risk behaviors suggests that this “syndemic” of conditions should be addressed concurrently particularly relevant to those who are dually effected.
There are several limitations to this study. First, the majority of our data elements including sexual risk behaviors and substance use were based on self-report which can result in an underestimation of these behaviors. Our use of computer assisted self-interviews may help to minimize reluctance in reporting behaviors that are socially stigmatized or illegal and may help to minimize the potential response bias (Catania et al., 1990; Fendrich et al., 1999). While the CES-D20 is a widely used validated tool for measuring depressive symptoms, it is not intended as a diagnostic tool for major depressive disorders. However, we hope that the use of a more conservative cut-point, validated among HIV-positive populations will help to increase the construct validity of the scale and allow for measurement of symptoms that are more closely aligned with a clinical diagnosis of depression (Choi et al., 2015). Disentangling the temporal ordering of substance use and depressive symptoms is challenging given our data and it’s difficult to say whether substance use led to depressive symptoms or whether the symptoms led to substance use. Our data measure depressive symptoms in the past seven days and substance use in the past six months so in that sense our measure of substance use precedes symptoms. Additionally, when we lagged the substance use by one visit (i.e., substance use reported 12 months prior to visit when depressive symptoms were reported) the results remained the same (data not shown). However, the potential chronicity and persistence of the behaviors and our outcome of interest makes it difficult to determine which is the cause and which is the effect. Finally, this study was based on participants recruited from a community based sexual health clinic and a university based research clinic and may not be generalizable to other populations.
The prevalence of depressive symptoms among this cohort of high risk HIV-negative and HIV-positive MSM was relatively high, especially among substance users. Additionally, frequent methamphetamine use was associated with persistence of depressive symptoms even though frequent users of other drugs showed declines in depressive symptoms overtime. Our findings reinforce the importance of providing effective substance use treatment and suggest that interventions targeted at reducing substance use and methamphetamine in particular may reduce depressive symptoms and impact other co-occurring issues such as sexual risk behaviors.
Highlights.
Frequent heroin and methamphetamine users had the highest prevalence of depressive symptoms
Compared to other substances, methamphetamine was the most influential predictor of depressive symptoms accounting for 10% of individual variance in symptoms
Declines in depressive symptoms overtime noted for most drugs, except for methamphetamine users who showed no change overtime
Role of Funding Source
This work was supported by NIH/NIDA grant number U01DA036267 and NIH/NIMH grant number P30MH058107
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
No conflict declared
References
- Ammassari A, Antinori A, Aloisi MS, Trotta MP, Murri R, Bartoli L, Monforte AD, Wu AW, Starace F, 2004. Depressive symptoms, neurocognitive impairment, and adherence to highly active antiretroviral therapy among HIV-infected persons. Psychosomatics 45(5), 394–402. [DOI] [PubMed] [Google Scholar]
- Bauermeister JA, Eaton L, Meanley S, Pingel ES, Partnership U, 2017. Transactional Sex With Regular and Casual Partners Among Young Men Who Have Sex With Men in the Detroit Metro Area. American journal of men’s health 11(3), 498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bing EG, Burnam MA, Longshore D, Fleishman JA, Sherbourne CD, London AS, Turner BJ, Eggan F, Beckman R, Vitiello B, Morton SC, Orlando M, Bozzette SA, Ortiz-Barron L, Shapiro M, 2001. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Arch Gen Psychiatry 58(8), 721–728. [DOI] [PubMed] [Google Scholar]
- Bjorkenstam C, Bjorkenstam E, Andersson G, Cochran S, Kosidou K, 2017. Anxiety and Depression Among Sexual Minority Women and Men in Sweden: Is the Risk Equally Spread Within the Sexual Minority Population? J Sex Med 14(3), 396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MV, Remien RH, Dolezal C, 2008. Depression symptoms and sexual HIV risk behavior among serodiscordant couples. Psychosom Med 70(2), 186–191. [DOI] [PubMed] [Google Scholar]
- Buchmueller T, Carpenter CS, 2010. Disparities in health insurance coverage, access, and outcomes for individuals in same-sex versus different-sex relationships, 2000–2007. Am J Public Health 100(3), 489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrico AW, Flentje A, Gruber VA, Woods WJ, Discepola MV, Dilworth SE, Neilands TB, Jain J, Siever MD, 2014. Community-based harm reduction substance abuse treatment with methamphetamine-using men who have sex with men. J Urban Health 91(3), 555–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catania JA, Gibson DR, Chitwood DD, Coates TJ, 1990. Methodological problems in AIDS behavioral research: influences on measurement error and participation bias in studies of sexual behavior. Psychol Bull 108(3), 339–362. [DOI] [PubMed] [Google Scholar]
- Choi SK, Boyle E, Burchell AN, Gardner S, Collins E, Grootendorst P, Rourke SB, Group OCS, 2015. Validation of Six Short and Ultra-short Screening Instruments for Depression for People Living with HIV in Ontario: Results from the Ontario HIV Treatment Network Cohort Study. PLoS One 10(11), e0142706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SK, Boyle E, Cairney J, Collins EJ, Gardner S, Bacon J, Rourke SB, 2016. Prevalence, Recurrence, and Incidence of Current Depressive Symptoms among People Living with HIV in Ontario, Canada: Results from the Ontario HIV Treatment Network Cohort Study. PLoS One 11(11), e0165816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciesla JA, Roberts JE, 2001. Meta-analysis of the relationship between HIV infection and risk for depressive disorders. Am J Psychiatry 158(5), 725–730. [DOI] [PubMed] [Google Scholar]
- Connors GJ, Maisto SA, Donovan DM, 1996. Conceptualizations of relapse: a summary of psychological and psychobiological models. Addiction 91 Suppl, S5–13. [PubMed] [Google Scholar]
- Crepaz N, Marks G, 2001. Are negative affective states associated with HIV sexual risk behaviors? A meta-analytic review. Health Psychol 20(4), 291–299. [DOI] [PubMed] [Google Scholar]
- Delaney JA, Nance RM, Whitney BM, Altice FL, Dong X, Trejo MEP, Matsuzaki M, Taxman FS, Chander G, Kuo I, Fredericksen R, Strand LN, Eron JJ, Geng E, Kitahata MM, Mathews WC, Mayer K, Moore RD, Saag MS, Springer S, Chandler R, Kahana S, Crane HM, 2018. Brief Report: Reduced Use of Illicit Substances, Even Without Abstinence, Is Associated With Improved Depressive Symptoms Among People Living With HIV. J Acquir Immune Defic Syndr 79(3), 283–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilley JA, Simmons KW, Boysun MJ, Pizacani BA, Stark MJ, 2010. Demonstrating the importance and feasibility of including sexual orientation in public health surveys: health disparities in the Pacific Northwest. Am J Public Health 100(3), 460–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilley JW, McFarland W, Sullivan P, Discepola M, 1998. Psychosocial correlates of unprotected anal sex in a cohort of gay men attending an HIV-negative support group. AIDS Educ Prev 10(4), 317–326. [PubMed] [Google Scholar]
- Dyer TP, Shoptaw S, Guadamuz TE, Plankey M, Kao U, Ostrow D, Chmiel JS, Herrick A, Stall R, 2012. Application of Syndemic Theory to Black Men Who Have Sex with Men in the Multicenter AIDS Cohort Study. Journal of Urban Health 89(4), 697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton WW, Smith C, Ybarra M, Muntaner C, Tien A, 2004. Center for Epidemiological Studies Depression Scale: Review and Revision (CESD and CESD-R), in: Maruish ME (Ed.) The use of psychological testing for treatment planning and outcomes assessment: Instruemtns for adults. Lawrence Erlbaum Associates, Mahwah, NJ, US, pp. 363–377. [Google Scholar]
- Fendrich M, Johnson TP, Sudman S, Wislar JS, Spiehler V, 1999. Validity of drug use reporting in a high-risk community sample: a comparison of cocaine and heroin survey reports with hair tests. Am J Epidemiol 149(10), 955–962. [DOI] [PubMed] [Google Scholar]
- Fulcher JA, Shoptaw S, Makgoeng SB, Elliott J, Ibarrondo FJ, Ragsdale A, Brookmeyer R, Anton PA, Gorbach PM, 2018. Brief Report: Recent Methamphetamine Use Is Associated With Increased Rectal Mucosal Inflammatory Cytokines, Regardless of HIV-1 Serostatus. J Acquir Immune Defic Syndr 78(1), 119–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein H, Healy MJ, Rasbash J, 1994. Multilevel time series models with applications to repeated measures data. Stat Med 13(16), 1643–1655. [DOI] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Chou SP, Huang B, Stinson FS, Dawson DA, Saha TD, Smith SM, Pulay AJ, Pickering RP, Ruan WJ, Compton WM, 2009. Sociodemographic and psychopathologic predictors of first incidence of DSM-IV substance use, mood and anxiety disorders: results from the Wave 2 National Epidemiologic Survey on Alcohol and Related Conditions. Mol Psychiatry 14(11), 1051–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Saha TD, Ruan WJ, Goldstein RB, Chou SP, Jung J, Zhang H, Smith SM, Pickering RP, Huang B, Hasin DS, 2016. Epidemiology of DSM-5 Drug Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions–III. JAMA Psychiatry 73(1), 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grelotti DJ, Hammer GP, Dilley JW, Karasic DH, Sorensen JL, Bangsberg DR, Tsai AC, 2017. Does substance use compromise depression treatment in persons with HIV? Findings from a randomized controlled trial. AIDS Care 29(3), 273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe A, Shoptaw S, Stein J, Reback CJ, Rotheram-Fuller E, 2007. Depression ratings, reported sexual risk behaviors, and methamphetamine use: latent growth curve models of positive change among gay and bisexual men in an outpatient treatment program. Exp Clin Psychopharmacol 15(3), 301–307. [DOI] [PubMed] [Google Scholar]
- Javanbakht M, Ragsdale A, Shoptaw S, Gorbach PM, 2018. Transactional Sex among Men Who Have Sex with Men: Differences by Substance Use and HIV Status. J Urban Health. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC, 2003. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch Gen Psychiatry 60(9), 929–937. [DOI] [PubMed] [Google Scholar]
- Koblin BA, Husnik MJ, Colfax G, Huang Y, Madison M, Mayer K, Barresi PJ, Coates TJ, Chesney MA, Buchbinder S, 2006. Risk factors for HIV infection among men who have sex with men. AIDS 20(5), 731–739. [DOI] [PubMed] [Google Scholar]
- la Roi C, Kretschmer T, Dijkstra JK, Veenstra R, Oldehinkel AJ, 2016. Disparities in Depressive Symptoms Between Heterosexual and Lesbian, Gay, and Bisexual Youth in a Dutch Cohort: The TRAILS Study. J Youth Adolesc 45(3), 440–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GL, Griffin GK, Melivin CL, 2009. Tobacco use among Sexual Minorities in the USA: 1987 to May 2007: A systematic Review. Tobacco Control 18, 275–282. [DOI] [PubMed] [Google Scholar]
- Liang KY, Zeger SL, 1986. Longitudinal data-analysis using generalized linear-models Biometrika 73(1), 13–22. [Google Scholar]
- Mayston R, Kinyanda E, Chishinga N, Prince M, Patel V, 2012. Mental disorder and the outcome of HIV/AIDS in low-income and middle-income countries: a systematic review. AIDS 26 Suppl 2, S117–135. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Lewis DC, O’Brien CP, Kleber HD, 2000. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA 284(13), 1689–1695. [DOI] [PubMed] [Google Scholar]
- Meade CS, Sikkema KJ, 2005. HIV risk behavior among adults with severe mental illness: a systematic review. Clin Psychol Rev 25(4), 433–457. [DOI] [PubMed] [Google Scholar]
- Meader N, Mitchell AJ, Chew-Graham C, Goldberg D, Rizzo M, Bird V, Kessler D, Packham J, Haddad M, Pilling S, 2011. Case identification of depression in patients with chronic physical health problems: a diagnostic accuracy meta-analysis of 113 studies. Br J Gen Pract 61(593), e808–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medley G, Lipari RN, Bose J, Cribb DS, Kroutil LA, McHentry G, October 2016; NSDUH Data Review. Retrieved from http://www.samhsa.gov/data. Sexual Orientation and Estimates of Adult Substance Use and Mental Health: Results from the 2015 National Survey on Drug Use and Health. [Google Scholar]
- Mitchell AJ, Lord O, Malone D, 2012. Differences in the prescribing of medication for physical disorders in individuals with v. without mental illness: meta-analysis. Br J Psychiatry 201(6), 435–443. [DOI] [PubMed] [Google Scholar]
- Nacher M, Adriouch L, Godard Sebillotte C, Hanf M, Vantilcke V, El Guedj M, Vaz T, Leconte C, Simart G, Djossou ML, Couppie P, 2010. Predictive factors and incidence of anxiety and depression in a cohort of HIV-positive patients in French Guiana. AIDS Care 22(9), 1086–1092. [DOI] [PubMed] [Google Scholar]
- Nanni MG, Caruso R, Mitchell AJ, Meggiolaro E, Grassi L, 2015. Depression in HIV infected patients: a review. Curr Psychiatry Rep 17(1), 530. [DOI] [PubMed] [Google Scholar]
- Natamba BK, Achan J, Arbach A, Oyok TO, Ghosh S, Mehta S, Stoltzfus RJ, Griffiths JK, Young SL, 2014. Reliability and validity of the center for epidemiologic studies-depression scale in screening for depression among HIV-infected and -uninfected pregnant women attending antenatal services in northern Uganda: a cross-sectional study. BMC Psychiatry 14, 303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okafor CN, Gorbach PM, Ragsdale A, Quinn B, Shoptaw S, 2017. Correlates of Preexposure Prophylaxis (PrEP) Use among Men Who Have Sex with Men (MSM) in Los Angeles, California. J Urban Health 94(5), 710–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pence BW, O’Donnell JK, Gaynes BN, 2012. Falling through the cracks: the gaps between depression prevalence, diagnosis, treatment, and response in HIV care. AIDS 26(5), 656–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS, 1977. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement 1(3), 385–401. [Google Scholar]
- Reisner SL, Mimiaga MJ, Skeer M, Bright D, Cranston K, Isenberg D, Bland S, Barker TA, Mayer KH, 2009. Clinically significant depressive symptoms as a risk factor for HIV infection among black MSM in Massachusetts. AIDS Behav 13(4), 798–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AL, Austin SB, Corliss HL, Vandermorris AK, Koenen KC, 2010. Pervasive trauma exposure among US sexual orientation minority adults and risk of posttraumatic stress disorder. Am J Public Health 100(12), 2433–2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiu CS, Chen YC, Tseng PC, Chung AC, Wu MT, Hsu ST, Ko NY, 2014. Curvilinear relationship between depression and unprotected sexual behaviors among men who have sex with men. J Sex Med 11(10), 2466–2473. [DOI] [PubMed] [Google Scholar]
- Stinchcombe A, Wilson K, Kortes-Miller K, Chambers L, Weaver B, 2018. Physical and mental health inequalities among aging lesbian, gay, and bisexual Canadians: cross-sectional results from the Canadian Longitudinal Study on Aging (CLSA). Can J Public Health. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struble CB, Lindley LL, Montgomery K, Hardin J, Burcin M, 2010. Overweight and obesity in lesbian and bisexual college women. J Am Coll Health 59(1), 51–56. [DOI] [PubMed] [Google Scholar]
- Weaver MR, Conover CJ, Proescholdbell RJ, Arno PS, Ang A, Ettner SL, Cost Subcommittee of the Hiv/Aids Treatment Adherence, H.O., Cost Study G, 2008. Utilization of mental health and substance abuse care for people living with HIV/AIDS, chronic mental illness, and substance abuse disorders. J Acquir Immune Defic Syndr 47(4), 449–458. [DOI] [PubMed] [Google Scholar]
- Weber AE, Craib KJ, Chan K, Martindale S, Miller ML, Schechter MT, Hogg RS, 2001. Sex trade involvement and rates of human immunodeficiency virus positivity among young gay and bisexual men. International journal of epidemiology 30(6), 1449–1454; discussion 1455–1446. [DOI] [PubMed] [Google Scholar]
- Willett JB, 1994. Chapter 11: Measuring Change: What Individual Growth Modeling Buys you, Change and Development: Issues of Theory, Method, and Application. Lawrence Erlbaum Associates, Inc., Publishers, NJ, pp. 213–243. [Google Scholar]
- Zeger SL, Liang KY, Albert PS, 1988. Models for longitudinal data - a generalized estimating equation approach Biometrics 44(4), 1049–1060. [PubMed] [Google Scholar]