Abstract
Objective
Previous studies have reported that obese individuals have reduced taste perception, but the relationship between obesity and taste is poorly understood. Earlier work has demonstrated that diet-induced obesity (DIO) directly impairs taste. Currently, it is not clear if these changes to taste are due to obesity or to the high fat diet exposure. The goal of the current study was to determine if diet or excess weight is responsible for the DIO induced taste deficits.
Methods
C57BL/6 mice were placed on either high fat or standard chow in the presence or absence of captopril. Mice on captopril did not gain weight when exposed to a high fat diet. Changes in the responses to different taste stimuli were evaluated using live cell imaging, brief-access licking, immunohistochemistry and real-time PCR.
Results
Diet and weight gain each affected taste responses but their effects varied by stimulus. Two key signaling proteins, gustducin and phospholipase Cβ2, were significantly reduced in the mice on the high fat diet with and without weight gain, identifying a potential mechanism for the reduced taste responsiveness to some stimuli.
Conclusions
Our data indicate that for some stimuli, diet alone can cause taste deficits, even without the onset of obesity.
Keywords: diet, weight, taste
Introduction
Studies have suggested that perception of taste stimuli is reduced in obese populations [1–8] which leads to increases in intake [9–11]. Despite this link between taste and obesity, little work has focused on identifying the mechanisms that are responsible for this relationship. We previously reported that diet-induced obesity (DIO) inhibits the responsiveness of peripheral taste receptor cells (TRCs) to taste stimuli, particularly sweet stimuli, and that these changes translate into behavioral effects [12]. There is strong support for the idea that a reduction in taste signaling drives increases in food intake [9, 10, 13] and our earlier study [12] demonstrated that DIO affects multiple aspects of taste starting with the initial signaling event.
There are two aspects of DIO that can potentially affect taste responses: excess weight or diet. We explored the respective roles of these factors for the identified DIO- dependent taste deficits. We focused on sweet taste because it was the most affected by DIO [12] and is reported to be affected by obesity [9–11, 14–16]. The goal of this study was to identify if there are independent effects of diet or excess weight on taste function.
To separate weight gain from high fat diet exposure, we added a low dose of captopril to the water of mice on a high fat (HF) diet. Previous work has shown that captopril (CAP), an angiotensin converting-enzyme inhibitor, protects against the development of diet-induced obesity [17] because animals on CAP voluntarily reduce their caloric consumption. Exposing DIO animals to CAP causes weight loss and reverses associated metabolic issues [17–19]. This approach avoids the stress that using food restriction and single housing might impose on the mouse. Using this pharmacological method, we compared DIO mice to mice on the same HF diet that did not become obese.
Materials
Mice
Husbandry practices were in compliance with the University at Buffalo Institutional Animal Care and Use Committee. All experiments used C57BL/6 mice (1– 6 months). At weaning, mice were placed on either standard (Harlan labs: calories are 18% from fat, 58% from carbohydrates, 24% from protein) or high fat (HF) chow (60% high fat Kcal feed, Harlan Labs, Inc., Madison, WI, USA; calories are 60% from fat, 22% from carbohydrates, 18% from protein). Mice were placed on the HF diet, or standard chow, in the presence and absence of captopril (CAP, 0.05mg/mL water). Initially CAP was replaced weekly but at week 2, CAP water was replaced every other day. Experiments were performed approximately 6–8 weeks after presentation of the HF diet. Omentum and retroperitoneal fat weights were collected and were calculated as either [(OM g/total weight g)*100] or [(RM g/total weight g)*100].
Taste cell isolation
TRCs were harvested from taste papillae of adult mice as previously described [12, 20–25]. Briefly, tongues were removed and injected beneath the lingual epithelium with 0.2 mL enzyme solution [3 mg Dispase II, 0.7 mg collagenase B (Roche diagnostics, Indianapolis, IN, USA) and 1 mg Trypsin Inhibitor in 1mL Tyrode’s]. The epithelial layer of the tongue was placed in Ca2+-Mg2+-free solution (140mM NaCl, 5mM KCl, 10mM Hepes, 2mM EGTA, 2mM BAPTA, pH 7.4) after being incubated at room temperature in oxygenated Tyrode’s. Cells were removed from circumvallate (CV) taste papillae via gentle suction and placed on a slide coated in Cell Tak (Discovery Labware, Bedford, MA USA).
Calcium Imaging
Isolated TRCs were incubated for 20 minutes in 2 μM Fura 2-AM and nonionic dispersing agent, Pluronic F-127 (Invitrogen, Eugene, OR, USA) and then washed for 20 minutes. Isolated cells were visualized using an Olympus IX71 scope and 40x oil immersion objective. TRCs were stimulated and Ca2+ changes were recorded every 4 seconds using a Sensicam QE camera at 340/380 nm excitation, 510 nm emission. Data was collected using Imaging Workbench 6.0 (Indec Biosystems, Santa Clara, CA, USA) and analyzed using Origin 8.6 (Origin Lab Corporation, Northampton, MA, USA). Response amplitude was calculated as: [(peak value-baseline value)/baseline value]*100. Only isolated TRCs with a resting Ca2+ baseline between 40–150nM were analyzed and an evoked response was defined as measurable if the increase in fluorescence was greater than two standard deviations above baseline.
Analysis of Licking Behavior
Unconditioned licking responses were recorded to varying concentrations of taste stimuli in a Davis Rig chamber (Davis MS80 Rig; Dilog Instruments and Systems, Tallahassee, FL) which has been described elsewhere [26]. Each individual lick was detected and recorded by a contact lickometer via DavisPro collection software (Dilog Instruments and Systems).
Mice were adapted to the Rig and trained to drink from the sipper tubes for 8 consecutive days [26]. During testing, animals were allowed to take as many trials as possible in 30 min. Mice were tested on varying concentrations of sucrose (0,3,10,30,60,100,300,1000 mM), saccharin (0,0.1,0.5,1,3,5,10,50 mM), acesulfame K (0,0.5,2,6,8,16,20,32 mM), KCl (0,12.5,25,50,100,200,300,400 mM), and denatonium benzoate (0,0.1,0.3,1,3,5,10,20 mM), in that order. Each stimulus was presented in randomized blocks on Monday, Wednesday and Friday in a single week. Animals were tested on sucrose and acesulfame K while water replete, however, trial initiation was lower than expected so animals were 22-h water deprived for all of the remaining stimuli. Once the mouse began licking the tube, they were allowed 10 seconds of access before the shutter closed.
For stimuli tested in the water deprived condition, lick ratios were calculated by dividing the average number of licks at each concentration by the average number of licks to water. For sucrose and acesulfame K, lick scores were calculated by subtracting the average number of licks at each concentration by the average number of licks to water. Lick scores were compared by ANOVA with treatment and solution concentration as factors. Significant interactions between treatment and concentration, were followed with sub-ANOVAs. Comparisons between CTL ± CAP ruled out any potential effects of CAP. CTL treated animals were collapsed and then compared to either the HF+CAP group (to determine an effect of diet alone) or to HF-CAP (to determine the effects of weight). Bonferoni corrected t-tests were conducted to identify concentrations that differed between groups.
Immunohistochemistry
Tongues were removed and placed in 4% paraformaldehyde/0.1M Phosphate buffer (PB, pH 7.2) overnight at 4oC. After cryoprotection, 40μm sections of the CV papillae were cut, washed in PBS and incubated in blocking solution (0.3% Triton X-100, 1% normal goat serum and 1% bovine serum albumin in 0.1M PBS). Samples were incubated in primary antibody overnight at 4oC. All primary antibodies were diluted in blocking solution and controls with no primary antibody were included in each experiment. After washing, sections were incubated in secondary antibody for 2 hours, washed, mounted in Fluoromount media with DAPI staining (Southern Biotechnology Associates, Birmingham, AL, USA) and visualized using confocal microscopy (Zeiss Axioimager Z1 and Axiovert 200M). Primary antibodies tested: α-gustducin (1:50, Santa Cruz Biotechnology, Santa Cruz, CA, USA) and PLCβ2 (1:1000, Santa Cruz). Primary antibody labeling was visualized using goat-anti-rabbit CY5 secondary antibody (1:500, Jackson ImmunoResearch Laboratories Inc., West Grove, PA, USA).
Cell count analysis
Cells in individual CV taste buds were analyzed using Zen 2012 Blue Edition with the analysis done blind to experimental condition. Images were taken at every micron and the nuclei in every 5th slice were counted as an estimate of the number of taste cells within each bud. Cells expressing the target protein were then counted at every 5th slice. Data is reported as the ratio of protein-expressing cells to total cells in the buds. Taste buds from at least 3 mice were analyzed for each experimental condition.
cDNA synthesis
Isolated TRCs (see above) were pelleted for RNA extraction. RNA was isolated using the Nucleospin RNA XS kit (Macherey-Nagel, Dϋren, Germany), treated with DNAase (Fermentas Life Sciences), and then used as a template to produce cDNA using SuperScript III Reverse Transcriptase (Invitrogen). PCR analysis was performed for GAPDH to ensure sample quality and check for genomic contamination. Contaminated samples were discarded. Primers are listed in Table S1.
Real-Time PCR
Real-Time PCR was performed using a BioRad MiniOpticon system (Bio-Rad Laboratories, Hercules, CA), with BioRad SYBR Green reagents (Bio-Rad Laboratories). cDNA from three mice for each experimental condition were run in triplicate. If there was more than 5% difference between the replicates, the data were discarded. Data was normalized to GAPDH expression for each sample to correct for any loading differences and reported as fold differences.
Solutions
All chemicals were purchased from Sigma Aldrich (St. Louis, MO, USA) unless otherwise noted. Tyrode’s solution contained: 140mM NaCl, 5mM KCl, 1mM MgCl2, 3mM CaCl2, 10mM HEPES, 10mM glucose and 1mM pyruvic acid; pH 7.4. The following chemicals are diluted into Tyrode’s and used for cellular stimulation during experiments: Sweet- 2mM saccharin (sac), 50mM sucrose (suc-50mM NaCl was replaced with 50mM sucrose) and 20mM acesulfame potassium (Ace K); Bitter- 5mM denatonium benzoate (den); Salt: 50mM NaCl replaced with 50mM KCl.
Results
High fat diet causes significant weight gain in the absence of captopril
Mice fed a high-fat (HF) diet rapidly gained weight. After one week, mice on HF food were significantly heavier than mice on standard chow (CTL). To determine if exposure to CAP reduces weight gain in mice on HF food, we measured the weights of a subset of mice that were on HF or standard chow, with or without CAP exposure. Mice on HF food with CAP in their water (HF+CAP), and mice on standard chow with captopril (CTL+CAP) were not significantly different from CTLs (Figure 1A). During the initial two weeks, when water was replaced weekly, mice on HF+CAP gained weight at a slightly higher rate than mice on CTL chow (+/− CAP). Providing fresh CAP water every other day resulted in the weights of the mice on HF+CAP being comparable to CTL mice.
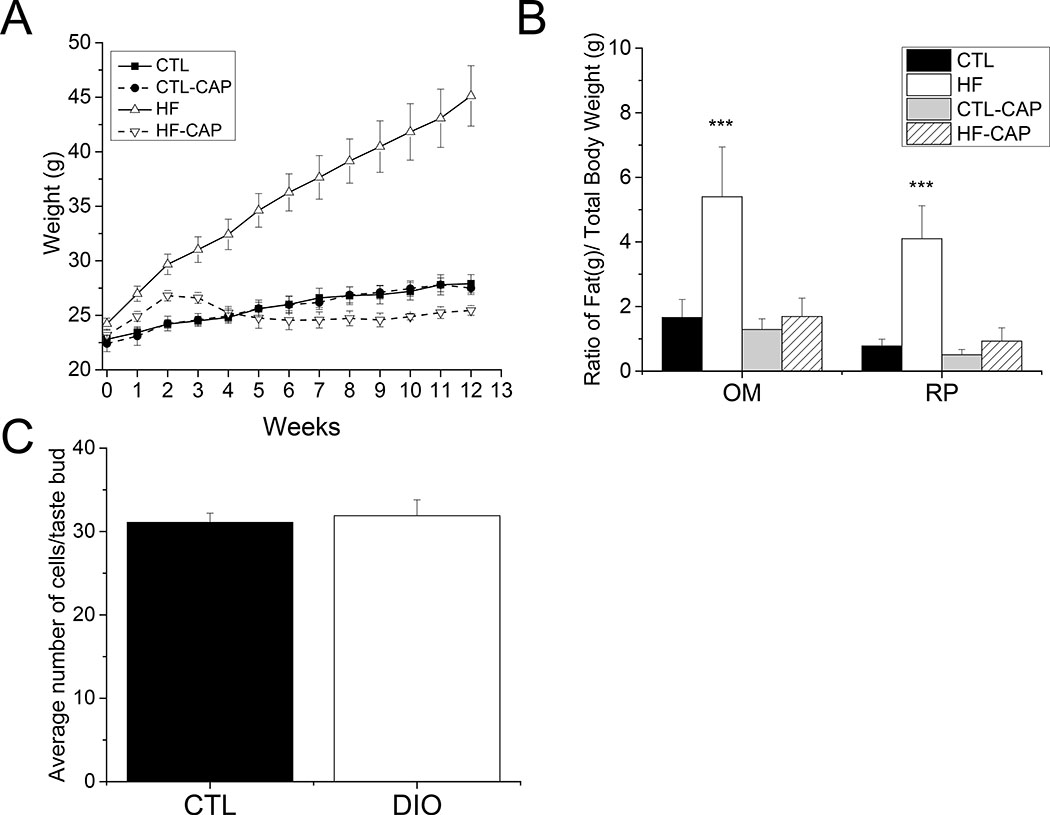
Figure 1: Mice on a HF diet become obese but do not gain excessive weight in they are exposed to captopril.
A) Diet induced obesity (DIO) was defined by measuring total body weight and adipose tissue weight for mice on HF (+/− CAP) compared to CTLs. There was a significant increase in the total body weight of the HF-CAP mice by 1 week on the HF diet. (p=0.01). No other significant differences were found for the other groups (n=4 for each group). B) The HF-CAP mice (white bar, n=10) had significantly more omentum and retroperitoneal fat as a ratio of total body weight compared to controls (***p<0.001) while mice on the HF diet with CAP (hatched bar, n=10) were not different from either control (black or gray bar, n=5 for each). Data are presented as a percentage of total weight. C) TRCs in control and DIO mice were counted using DAPI staining to label individual TRCs. The average number of TRCs was not reduced in DIO mice (+/− SEM, n=13 buds, 3 mice for each, p=0.71).
Greater omentum (OM) and retroperitoneal (RP) adipose tissues (Figure 1B) were larger for both the ratio of OM/total body weight (one way ANOVA, p<0.001) and the RP/total body weight in mice on the HF diet compared to mice in the other experimental conditions (one way ANOVA, p<0.001). No other significant differences were found. Based on these criteria, mice on the HF (−CAP) diet were identified as obese.
Diet induced obesity does not cause loss of taste receptor cells
To determine if diet-induced obesity (DIO) caused a significant loss of the TRCs, we analyzed taste buds from mice (n=3) that were on the HF diet for 10 weeks and had OM>3, RP>3. The number of TRCs per bud in these mice were compared to age matched littermates on CTL diet (n=3). Taste buds from the CV papillae were fixed and stained with DAPI to identify the nuclei (Figure S1) for cell counting. A total of 13 taste buds were analyzed for each condition and no significant differences were found between the CTL or DIO mice (Figure 1C, bar graph).
Diet and weight differentially affect sweet taste
We evaluated the effects of excess weight and exposure to HF diet for three sweet stimuli: acesulfame K (AceK, 20mM), saccharin (sac, 2mM) and sucrose (suc, 50mM), a bitter stimulus, denatonium benzoate (5mM), and a salt stimulus (50mM KCl). Three different measures were taken: 1) the percentage of TRCs that respond to a given stimulus, 2) the amplitude of the taste-evoked calcium signal in the responding cells, and 3) the unconditioned licking response in the behaving animal for each stimuli (Figures 2 and 3). To ensure that CAP does not independently affect taste, we included a group of mice kept on standard chow with CAP exposure for all experiments. No significant differences for any of the experimental conditions were found. The percentage of responsive TRCs were compared using Chi-square analyses [27] with Yates correction. Other analyses were compared using ANOVA followed by student t- tests or sub ANOVA, as specified.
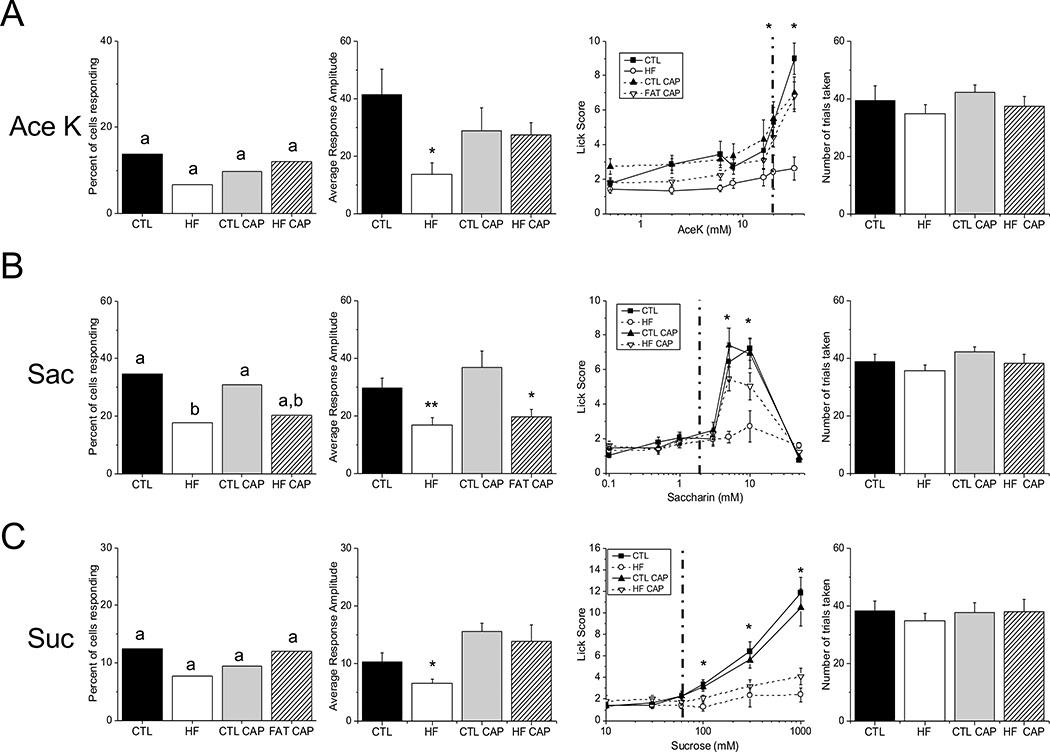
Figure 2: Differential effects of diet and weight on sweet stimuli.
A combination of live cell imaging and unconditioned brief-access licking assays were used to assess the relative effects of DIO and HF diet on the ability of the taste system to respond to sweet stimuli. The left column is the total number of taste cells that responded to each stimulus. Significant differences are indicated by different letters. The second column is the response amplitude of the taste-evoked calcium responses for cells isolated from mice under each experimental condition (*, p<0.05; **, p<0.01) and the third column is brief-access licking behavior for each stimulus (see Table 1).
The dotted line in the third column indicates the stimulus concentration used in the live cell imaging experiments. The fourth column is the average, cumulative number of trials taken in the brief-access study for each experimental group. There were no significant differences in the number of trials taken for any of the experimental groups for any of the sweet stimuli tested. A) No significant differences were found in the number of taste cells that responded to AceK, however response amplitudes from the HF mice were significantly lower than CTL (p=0.012) and HF+CAP (p=0.03). Consistent with the amplitude data, HF mice licked significantly less than the other treatments in the brief- access test. B) Treatment with a HF diet reduced the number of cells responding to saccharin (p=0.04), as well as the average response amplitude (p=0.001). In the brief- access taste test HF mice licked significantly less than other groups and the HF+CAP group trended to lower licking behavior (p=0.053) compared to CTLs. C) There was no effect of weight or diet on the percentage of cells responding to sucrose, but there was a reduction in response amplitude in HF animals (p=0.02). The behavioral data demonstrate that exposure to the HF diet is sufficient to alter licking behavior. Representative traces for each experimental condition are shown in Figure S2.
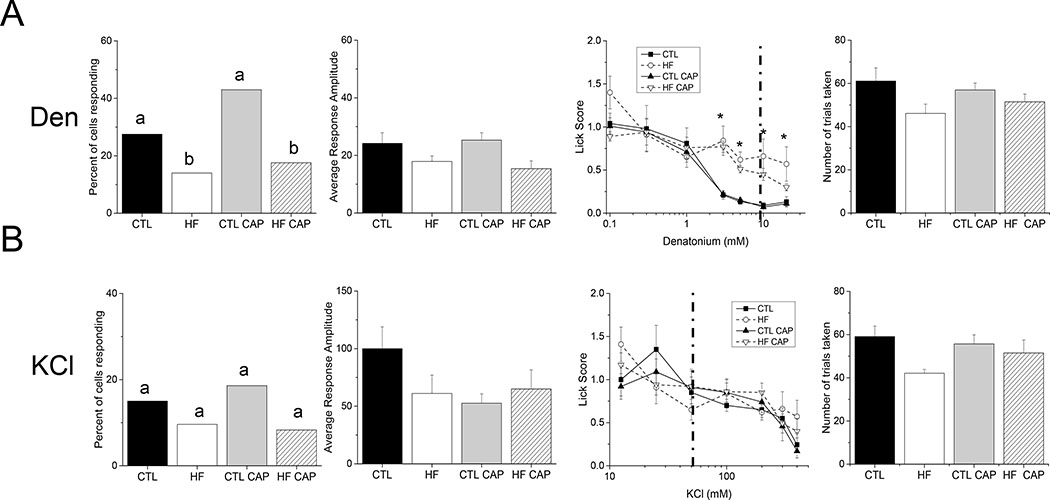
Figure 3: Effects of diet and weight on bitter and salt stimuli.
A combination of live cell imaging and unconditioned brief-access licking assays were used to assess the relative effects of DIO and HF diet on the ability of the taste system to respond to bitter (5mM denatonium, A) and salt (50mM KCl, B) stimuli. Individual data values for each stimulus is shown in Table S2. The left column is the total number of taste cells that responded to each stimulus. Significant differences are indicated by different letters. The second column is the response amplitude of the evoked responses from isolated taste cells from each experimental condition and the third column is brief-access licking behavior for each stimulus (*, p<0.05). The dotted line in the third column indicates the stimulus concentration used in the live cell imaging experiments. The fourth column is the average, cumulative number of trials taken in the brief-access study for each experimental group. There were no significant differences in the number of trials taken for any of the experimental groups. A) Exposure to the HF diet reduced the percentage of cells responding to Den (p=0.029 for HF-CAP and p=0.007 for HF+CAP), and decreased the behavioral sensitivity to Den, but did not affect the amplitudes of the responses. B) There was no significant effect of diet or weight on any measure of responding to KCl. Representative traces for each experimental condition are shown in Figure S3.
TRC responsiveness is defined as the number of TRCs that respond to a specific stimulus divided by the total number of TRCs that were exposed to that stimulus. These data are presented as a percentage on a bar graph and are shown in the first column of Figure 2 for Ace K (Figure 2A), saccharin (Figure 2B) and sucrose (Figure 2C). Individual values are shown in Table S2. No significant differences were found for the number of TRCs that responded to AceK (Figure 2A). While the average amplitude of the TRC responses to AceK was significantly reduced in the mice on the HF diet (−CAP) compared to the other groups (Figure 2A, second panel, p=0.03), no other significant differences were found. Unconditioned licking was only significantly reduced in the HF- CAP mice compared other treatments (specific values shown in Table 1), and there was no significant difference in the number of trials initiated (p=0.56) (Figure 2A, right two panels) or the number of licks to water (p=0.8). These data indicate that weight is likely the primary factor resulting in the reduced taste response to the artificial sweetener AceK and that the significantly reduced licking by the HF mice was not due to low motivation in the task that would be reflected in trial initiation.
Table 1.
| Hypothesis Group | all groups comparison all groups | effect of CAP in LF CTL ± CAP | effect of HF diet CTL vs HF+CAP | effect of weight CTL vs HF-CAP | effect of cap in HF CTL ± CAP | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| effect | F | df | p | F | df | p | F | df | p | F | df | p | F | df | p | |
| ace k | treatment | 15.613 | (3,28) | <0.001 | 0.004 | (1,14) | 0.947 | 2.777 | (1,22) | 0.11 | 19.14 | (1,22) | <0.001 | |||
| concentration | 23.848 | (6,168) | <0.001 | 25.786 | (6,84) | <0.001 | 33.83 | (6,132) | <0.001 | 32.449 | (6,132) | <0.001 | ||||
| interaction | 6.928 | (18,168) | <0.001 | 1.587 | (6,84) | 0.161 | 0.36 | (6,132) | 0.903 | 5.873 | (6,132) | <0.001 | ||||
| saccharin | treatment | 5.663 | (3,28) | 0.004 | 0.214 | (1,14) | 0.651 | 1.498 | (1,22) | 0.234 | 15.968 | (1,22) | 0.001 | |||
| concentration | 81.404 | (6,168) | <0.001 | 55.981 | (6,84) | <0.001 | 62.198 | (6,132) | <0.001 | 38.013 | (6,132) | <0.001 | ||||
| interaction | 5.244 | (18,168) | <0.001 | 0.368 | (6,84) | 0.898 | 2.142 | (6,132) | 0.053 | 14.854 | (6,132) | <0.001 | ||||
| sucrose | treatment | 20.71 | (3,28) | <0.001 | 1.587 | (1,14) | 0.228 | 22.587 | (1,22) | <0.001 | 47.005 | (1,22) | <0.001 | 4.622 | (1,14) | 0.50 |
| concentration | 73.212 | (6,168) | <0.001 | 86.372 | (6,84) | <0.001 | 49.475 | (6,132) | <0.001 | 40.186 | (6,132) | <0.001 | 5.24 | (6,84) | <0.001 | |
| interaction | 12.452 | (18,168) | <0.001 | 1.287 | (6,84) | 0.272 | 18.742 | (6,132) | <0.001 | 24.715 | (6,132) | <0.001 | 0.5 | (6,84) | 0.806 | |
| DB | treatment | 9.922 | (3,28) | <0.001 | 0.17 | (1,14) | 0.686 | 12.574 | (1,22) | 0.002 | 27.208 | (1,22) | <0.001 | 3.525 | (1,14) | 0.081 |
| concentration | 39.811 | (6,168) | <0.001 | 28.4 | (6,84) | <0.001 | 30.064 | (6,132) | <0.001 | 20.973 | (6,132) | <0.001 | 13.649 | (6,84) | <0.001 | |
| interaction | 2.516 | (18,168) | 0.001 | 0.055 | (6,84) | 0.999 | 3.918 | (6,132) | 0.001 | 5.068 | (6,132) | <0.001 | 0.86 | (6,84) | 0.528 | |
| KCl | treatment | 0.144 | (3,28) | 0.933 | 0.048 | (1,14) | 0.830 | |||||||||
| concentration | 20.648 | (6,168) | <0.001 | 13.587 | (6,84) | <0.001 | ||||||||||
| interaction | 1.529 | (18,168) | 0.108 | 0.609 | (6,84) | 0.723 | ||||||||||
Analyses of the saccharin responses revealed that TRC responsiveness was significantly reduced in the obese mice compared to controls (Figure 2B, left panel, p=0.04); however, it was not significantly different from number of responsive cells in the HF+CAP mice. While the number of TRCs from the HF+CAP mice that were responsive to saccharin was lower than the CTL−/+CAP mice, there were no significant differences identified. Evaluation of the response amplitudes identified significant differences in the mice on the HF diet (Figure 2B, second panel, p=0.001). Student’s t tests determined that the saccharin responses in the obese mice were significantly smaller than CTL (p=0.003) with comparable reductions in the HF+CAP mice compared to the CTL+CAP mice (p=0.017). There were no significant differences between the mice on the HF diet (+/− CAP) or the CTLs (+/− CAP). Unconditioned licking was significantly reduced in the obese mice (HF-CAP) compared to CTL and there was strong trend (p=0.053) for a reduced response in the HF+CAP mice compared to CTL+CAP (Figure 2B, third panel) with no differences in the number of trials taken (Figure 2B, right panel, p=0.35) or number of licks to water (p=0.2). Unlike the AceK results, these data suggest that diet is driving the reduction in saccharin responses which is magnified when the animal is also obese.
We found no differences in the percentage of sucrose responsive cells (p=0.79), but the amplitudes of the sucrose responses were reduced in the obese mice (p=0.02). Interestingly, in the behavioral analysis, both animals on the HF diet (+/−CAP) showed reduced unconditioned licking compared to the CTL mice (+/−CAP) as sucrose concentration increases (Table 1). There was no difference between groups in the number of trials initiated (p=0.97) or number of water licks (p=0.5). This suggests that the decrease in licking is due to the exposure to the HF diet, not changes in body mass. Representative live cell imaging traces are shown in Figure S2.
Diet affects bitter but not KCl taste
We also tested bitter (denatonium, 5mM) and salt stimuli (50mM KCl). Exposure to HF diet regardless of weight, significantly reduced the number of denatonium responsive TRCs (Figure 3A, left panel, p=0.029 for HF+CAP and p=0.007 for HF-CAP) but did not have a significant effect on the amplitude of the denatonium evoked signals (Figure 3A, second panel, p=0.05). The behavior data demonstrates that exposure to the HF diet reduced their sensitivity (increased unconditioned licking) to denatonium (Figure 3A, third panel and Table 1) with no change in the number of trials taken (Figure 3A, right panel, p=0.27) or water licks (p=0.9). In agreement with our earlier study [12], 50mM KCl responses were not affected by weight gain or diet exposure (Figure 3B, p=0.13). Representative cell imaging traces are shown in Figure S3.
Taste signaling proteins change in obese mice
To investigate why taste-evoked calcium responses were inhibited, we measured the expression of α-gustducin and phospholipase Cβ2 (PLCβ2), two proteins in the signaling pathway that transmits sweet and bitter taste signals in TRCs [28]. Immunohistochemical analysis suggested that there was a reduction in the expression of both of these signaling proteins, so we quantitated the number of TRCs expressing α- gustducin and PLCβ2 in the CV taste buds for mice from each experimental condition (n=at least 3 mice for each). Gustducin and PLCβ2 were analyzed separately.
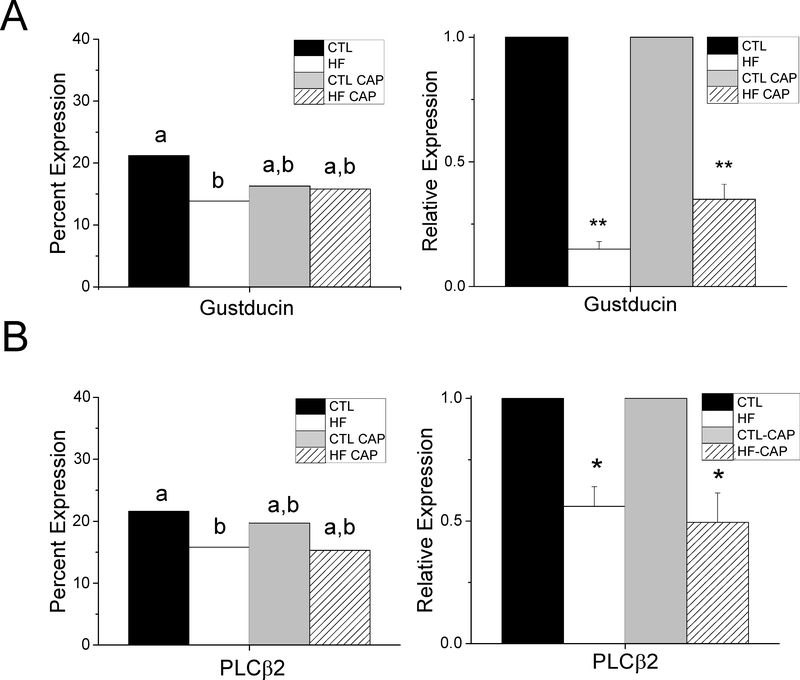
The relative expression of gustducin for each experimental condition is shown in Figure S4A. Chi-square analysis with Yates correction identified a significant reduction in the number of cells expressing α-gustducin in taste buds of obese mice (HF) compared to controls (Figure 4A, left panel, p=0.02). No other significant differences were found. Real-time PCR was used to quantitate the mRNA levels for gustducin from isolated taste buds (n=3/group). There was a significant reduction in gustducin transcript levels in both the HF +/− CAP mice (Figure 4A, right panel, p<0.01) compared to CTLs.
Figure 4: α-Gustducin and PLCβ2 expression are affected by DIO.
A) Quantitative analysis determined that there is a significant decrease in the number of α-gustducin-expressing cells in obese mice compared to controls (left panel, p=0.02). No other significant differences were found. Significant differences are indicated by different letters. qPCR analysis identified a significant reduction in gustducin mRNA levels in both HF (**, p=001) and HF+CAP mice (**, p=0.009) compared to CTL conditions. B) There is a significant reduction in the expression of PLCβ2 in cells from obese mice compared to CTL mice (left panel, p=0.04). Significant differences are indicated by different letters. No other significant differences were found. There is a significant reduction in the mRNA expression of PLCI32 in obese taste cells (right panel, *, p=0.035) as well as the mice on the HF +CAP (right panel,*, p=0.013).
PLCβ2 expression appears to be reduced in the CV taste buds of obese mice (Figure S4B). Analysis of PLCβ2 expression identified a significant reduction in the number of CV TRCs expressing PLCβ2 in the obese mice compared to CTL (Figure 4B, left panel, p=0.04). The number of PLCβ2 expressing cells in the HF+CAP mice was not significantly different from CTLs however, PLCβ2 transcript levels were significantly reduced in the HF +/− CAP mice (Figure 4B, right panel, p<0.05) compared to CTLs.
Discussion
Obesity is a complex disease and its relationship with the taste system is still not well understood. While multiple studies have suggested that perception of taste stimuli is reduced in obese populations [1–8] and that this leads to increased consumption [9–11], the mechanisms underlying these effects have not been identified. We have now demonstrated that a HF diet and excess weight can each inhibit taste responses and that these effects vary depending on the stimulus.
We chose to treat a subset of mice on the HF diet with a low dose of CAP as a way to expose them to a HF diet while preventing weight gain. Since these mice were only exposed to the HF diet, we were able to isolate the effect of diet exposure on taste. CAP, an angiotensin converting-enzyme inhibitor, commonly used to regulate blood pressure, inhibits the production of the angiotensin II hormone. Consequently, CAP can cause weight loss in DIO mice as well as a reversal of obesity associated metabolic issues [17–19]. DIO has been associated with an overexpression of angiotensin II which regulates fluid balance and plays a role in regulating body weight. It has been hypothesized that CAP leads to weight loss by reducing angiotensin II levels [29, 30].
We observed the effectiveness of CAP in the initial weight analyses (Figure 1A). When water+CAP was replaced weekly, the HF+CAP mice began to gain weight. When the water+CAP was exchanged more frequently, that weight was subsequently lost and the mice were comparable to mice on standard chow. While previous work reported angiotensin II enhanced sweet and suppressed salt responses [31], we found no difference in any measure between CTLs+/−CAP, suggesting that the CAP concentration used in our study was not sufficient to alter taste.
We measured the effects of DIO and diet on both TRC activity which initiates taste, and behavior, the final output response to taste stimuli. For each stimulus, we ensured that the behavioral experiments tested a range of concentrations that encompassed the stimulus concentration used in the imaging experiments (shown as a dotted line on the behavior graphs). For some stimuli (AceK and denatonium), the largest separation in the behavior data correlated with the concentrations used in the imaging experiments. However, for other stimuli (saccharin and sucrose), the concentrations used in the imaging experiments were lower than the stimulus concentrations where behavioral differences were recorded. While we used these concentrations in the imaging to avoid any non-specific effects on the cells, it is possible there would be more significant differences in the cell responses if a higher stimulus concentration could be used. Our results for denatonium differ from our previous findings [12]. Both studies identified differences in denatonium driven behaviors; however, our previous work reported a non-significant reduction in TRC responsiveness for DIO mice. We may have uncovered a significant effect in this study due to the larger sample size.
To identify the potential cellular mechanisms underlying the changes in taste cell activity, we evaluated the expression levels of two proteins with known roles in the transduction of bitter and sweet stimuli. α-Gustducin, a G-protein, is expressed in 20–30% of TRCs and is required for the normal transduction [32]. The number of gustducin- expressing cells was reduced in the obese mice but not in the mice on the HF+CAP (Figure 4A, S4A). Conversely, qPCR for α-gustducin identified a significant reduction in mRNA in TRCs from both groups of HF (+/− CAP) mice. Since there was not a reduction in the number of gustducin-expressing cells in the HF+CAP mice, and loss of gustducin significantly decreases the ability to detect bitter and sweet stimuli [33], we predict that the level of gustducin expression within individual TRCs of these mice may be reduced. Since gustducin is not solely responsible for the transduction of all sweet stimuli [34], the reduction in its expression may contribute to the selective diet effect on the sweet taste responses.
We then evaluated the expression of PLCβ2 which is also required for the transduction of bitter and sweet stimuli [35]. PLCβ2 expression was significantly reduced in the obese mice at both the protein and mRNA levels (Figure 4B, S4B). While there appeared to be fewer cells expressing PLCβ2 in the HF+CAP mice, no significant differences were identified at the protein level (p=0.09) even though there was a significant reduction in the mRNA levels (p=0.013). The modest reduction of PLCβ2 expression in these taste cells may be a contributor to the selective effects of diet in inhibiting taste responses. These data also suggest that the obesity and diet dependent inhibition of TRC activity are not specific to one protein, but to many components of the taste pathway.
The strong agreement between our cellular and behavioral data supports the premise that the diet-dependent inhibition of TRC activity is significant enough to impair the animal’s behavior. In the behavior trials, the HF-CAP animals were minimally responsive to all sweet and bitter stimuli tested, licking them similarly to water at all concentrations. The HF+CAP animals were minimally responsive to sucrose, and denatonium but responded to the artificial sweeteners with the same concentration specific responding that we saw in lean animals. Because we see the effects in the both the TRC and the behavioral assay, we do not believe that these differences are due to the more complex taste profile of the artificial sweeteners or postingestive feedback because both of these effects are absent in the cell imaging at the chosen concentration. We are left with the conclusion that diet and obesity alter the taste pathway in complicated ways that remain to be uncovered.
This complexity is further highlighted in the literature. Hajnal et al. (2005) reported that obesity developed on a standard chow (in rats lacking of the CCK-1 receptor) led to increased responding to sucrose [5]. Dando and colleagues reported a decrease in taste bud number in obese mice compared to controls, a finding we failed to replicate [36]. However, our work is in agreement with Dando’s in other ways, as we both found a decrease in PLCβ2 expression [37] and no change in taste bud size [36] in DIO mice. Diet, even in the absence of obesity, and perhaps other measures such as the length of the treatment, may all contribute independently to alterations in the taste system.
Our study highlights that the peripheral taste system can be significantly inhibited by diet, without the onset of obesity suggesting that diet exposure alone, may impair taste responses sufficiently to alter consumption which can then subsequently induce or maintain obesity.
Supplementary Material
Acknowledgments
We wish to thank Kristen E. Kay for technical assistance and Alan Siegel/UB North Campus Imaging Facility funded by NSF-MRI Grant DBI 0923133 for confocal images.
Funding: This work was supported by NSF funding 1256950 to Kathryn Medler, NIH DC006358 to Stefan Roberts and Kathryn Medler and DC016869 to A-M Torregrossa.
Footnotes
Disclosure: The authors declare no conflict of interest.
References
- 1.Bartoshuk LM, Duffy VB, Hayes JE, Moskowitz HR, and Snyder DJ: ‘Psychophysics of sweet and fat perception in obesity: problems, solutions and new perspectives’, Philos Trans R Soc Lond B Biol Sci, 2006, 361, (1471), pp. 1137–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bi S, Chen J, Behles RR, Hyun J, Kopin AS, and Moran TH: ‘Differential body weight and feeding responses to high-fat diets in rats and mice lacking cholecystokinin 1 receptors’, Am J Physiol Regul Integr Comp Physiol, 2007, 293, (1), pp. R55–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duffy VB: ‘Associations between oral sensation, dietary behaviors and risk of cardiovascular disease (CVD)’, Appetite, 2004, 43, (1), pp. 5–9 [DOI] [PubMed] [Google Scholar]
- 4.Duffy VB, Lanier SA, Hutchins HL, Pescatello LS, Johnson MK, and Bartoshuk LM: ‘Food preference questionnaire as a screening tool for assessing dietary risk of cardiovascular disease within health risk appraisals’, J Am Diet Assoc, 2007, 107, (2), pp. 237–245 [DOI] [PubMed] [Google Scholar]
- 5.Hajnal A, Covasa M, and Bello NT: ‘Altered taste sensitivity in obese, prediabetic OLETF rats lacking CCK-1 receptors’, Am J Physiol Regul Integr Comp Physiol, 2005, 289, (6), pp. R1675–1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hellstrom PM, Geliebter A, Naslund E, Schmidt PT, Yahav EK, Hashim SA, and Yeomans MR: ‘Peripheral and central signals in the control of eating in normal, obese and binge-eating human subjects’, Br J Nutr, 2004, 92 Suppl 1, pp. S47–57 [DOI] [PubMed] [Google Scholar]
- 7.Kovacs P, and Hajnal A: ‘Altered pontine taste processing in a rat model of obesity’, J Neurophysiol, 2008, 100, (4), pp. 2145–2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moran TH, Katz LF, Plata-Salaman CR, and Schwartz GJ: ‘Disordered food intake and obesity in rats lacking cholecystokinin A receptors’, Am J Physiol, 1998, 274, (3 Pt 2), pp. R618–625 [DOI] [PubMed] [Google Scholar]
- 9.Johnson AW: ‘Dietary manipulations influence sucrose acceptance in diet induced obese mice’, Appetite, 2012, 58, (1), pp. 215–221 [DOI] [PubMed] [Google Scholar]
- 10.Robinson MJ, Burghardt PR, Patterson CM, Nobile CW, Akil H, Watson SJ, Berridge KC, and Ferrario CR: ‘Individual Differences in Cue-Induced Motivation and Striatal Systems in Rats Susceptible to Diet-Induced Obesity’, Neuropsychopharmacology, 2015, 40, (9), pp. 2113–2123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun H, Yan J, Sun B, Song L, and Yan J: ‘Taste sensitivity to sucrose is lower in outbred Sprague-Dawley phenotypic obesity-prone rats than obesity-resistant rats’, Biochem Biophys Res Commun, 2017, 489, (2), pp. 155–163 [DOI] [PubMed] [Google Scholar]
- 12.Maliphol AB, Garth DJ, and Medler KF: ‘Diet-induced obesity reduces the responsiveness of the peripheral taste receptor cells’, PLoS One, 2013, 8, (11), pp. e79403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shin AC, Townsend RL, Patterson LM, and Berthoud HR: ‘“Liking” and “wanting” of sweet and oily food stimuli as affected by high-fat diet-induced obesity, weight loss, leptin, and genetic predisposition’, Am J Physiol Regul Integr Comp Physiol, 2011, 301, (5), pp. R1267–1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grinker J: ‘Obesity and sweet taste’, Am J Clin Nutr, 1978, 31, (6), pp. 1078–1087 [DOI] [PubMed] [Google Scholar]
- 15.Kawai K, Sugimoto K, Nakashima K, Miura H, and Ninomiya Y: ‘Leptin as a modulator of sweet taste sensitivities in mice’, Proc Natl Acad Sci U S A, 2000, 97, (20), pp. 11044–11049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ninomiya Y, Shigemura N, Yasumatsu K, Ohta R, Sugimoto K, Nakashima K, and Lindemann B: ‘Leptin and sweet taste’, Vitam Horm, 2002, 64, pp. 221–248 [DOI] [PubMed] [Google Scholar]
- 17.de Kloet AD, Krause EG, Kim DH, Sakai RR, Seeley RJ, and Woods SC: ‘The effect of angiotensin-converting enzyme inhibition using captopril on energy balance and glucose homeostasis’, Endocrinology, 2009, 150, (9), pp. 4114–4123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Premaratna SD, Manickam E, Begg DP, Rayment DJ, Hafandi A, Jois M, Cameron-Smith D, and Weisinger RS: ‘Angiotensin-converting enzyme inhibition reverses diet-induced obesity, insulin resistance and inflammation in C57BL/6J mice’, International journal of obesity, 2012, 36, (2), pp. 233–243 [DOI] [PubMed] [Google Scholar]
- 19.Weisinger RS, Stanley TK, Begg DP, Weisinger HS, Spark KJ, and Jois M: ‘Angiotensin converting enzyme inhibition lowers body weight and improves glucose tolerance in C57BL/6J mice maintained on a high fat diet’, Physiol Behav, 2009, 98, (1–2), pp. 192–197 [DOI] [PubMed] [Google Scholar]
- 20.Hacker K, Laskowski A, Feng L, Restrepo D, and Medler K: ‘Evidence for two populations of bitter responsive taste cells in mice’, J Neurophysiol, 2008, 99, (3), pp. 1503–1514 [DOI] [PubMed] [Google Scholar]
- 21.Hacker K, and Medler KF: ‘Mitochondrial calcium buffering contributes to the maintenance of Basal calcium levels in mouse taste cells’, J Neurophysiol, 2008, 100, (4), pp. 2177–2191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laskowski AI, and Medler KF: ‘Sodium-calcium exchangers contribute to the regulation of cytosolic calcium levels in mouse taste cells’, J Physiol, 2009, 587, (Pt 16), pp. 4077–4089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rebello MR, Maliphol AB, and Medler KF: ‘Ryanodine Receptors Selectively Interact with L Type Calcium Channels in Mouse Taste Cells’, PLoS One, 2013, 8, (6), pp. e68174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rebello MR, and Medler KF: ‘Ryanodine receptors selectively contribute to the formation of taste-evoked calcium signals in mouse taste cells’, Eur J Neurosci, 2010, 32, (11), pp. 1825–1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szebenyi SA, Laskowski AI, and Medler KF: ‘Sodium/calcium exchangers selectively regulate calcium signaling in mouse taste receptor cells’, J Neurophysiol, 2010, 104, (1), pp. 529–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dutta Banik D, Martin LE, Freichel M, Torregrossa AM, and Medler KF: ‘TRPM4 and TRPM5 are both required for normal signaling in taste receptor cells’, Proc Natl Acad Sci U S A, 2018, 115, (4), pp. E772–E781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sukumaran SK, Lewandowski BC, Qin Y, Kotha R, Bachmanov AA, and Margolskee RF: ‘Whole transcriptome profiling of taste bud cells’, Scientific reports, 2017, 7, (1), pp. 7595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Graus-Nunes F, Santos FO, Marinho TS, Miranda CS, Barbosa-da-Silva S, and Souza-Mello V: ‘Beneficial effects of losartan or telmisartan on the local hepatic renin-angiotensin system to counter obesity in an experimental model’, World J Hepatol, 2019, 11, (4), pp. 359–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winkler M, Schuchard J, Stolting I, Vogt FM, Barkhausen J, Thorns C, Bader M, and Raasch W: ‘The brain renin-angiotensin system plays a crucial role in regulating body weight in diet-induced obesity in rats’, Br J Pharmacol, 2016, 173, (10), pp. 1602–1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shigemura N, Iwata S, Yasumatsu K, Ohkuri T, Horio N, Sanematsu K, Yoshida R, Margolskee RF, and Ninomiya Y: ‘Angiotensin II modulates salty and sweet taste sensitivities’, J Neurosci, 2013, 33, (15), pp. 6267–6277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gilbertson TA, Damak S, and Margolskee RF: ‘The molecular physiology of taste transduction’, Curr Opin Neurobiol, 2000, 10, (4), pp. 519–527 [DOI] [PubMed] [Google Scholar]
- 32.Wong GT, Gannon KS, and Margolskee RF: ‘Transduction of bitter and sweet taste by gustducin’, Nature, 1996, 381, (6585), pp. 796–800 [DOI] [PubMed] [Google Scholar]
- 33.Glendinning JI, Bloom LD, Onishi M, Zheng KH, Damak S, Margolskee RF, and Spector AC: ‘Contribution of alpha-gustducin to taste-guided licking responses of mice’, Chem Senses, 2005, 30, (4), pp. 299–316 [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y, Hoon MA, Chandrashekar J, Mueller KL, Cook B, Wu D, Zuker CS, and Ryba NJ: ‘Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways’, Cell, 2003, 112, (3), pp. 293–301 [DOI] [PubMed] [Google Scholar]
- 35.Kaufman A, Choo E, Koh A, and Dando R: ‘Inflammation arising from obesity reduces taste bud abundance and inhibits renewal’, PLoS Biol, 2018, 16, (3), pp. e2001959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaufman A, Kim J, Noel C, and Dando R: ‘Taste loss with obesity in mice and men’, International journal of obesity, 2019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.