Abstract
CD4+ T cells mediate the pathogenesis of ischemic and nephrotoxic acute kidney injury (AKI). However, the underlying mechanisms of CD4+ T cell-mediated pathogenesis are largely unknown. We therefore conducted unbiased RNA-sequencing to discover novel mechanistic pathways of kidney CD4+ T cells post-ischemia compared to normal mouse kidney. Unexpectedly, lipocalin-2 (Lcn2) gene, which encodes neutrophil gelatinase-associated lipocalin (NGAL) had the highest (~60)-fold increase. The NGAL increase in CD4+ T cells during AKI was confirmed at the mRNA level with real-time PCR and at the protein level with ELISA. NGAL is a potential biomarker for the early detection of AKI and has multiple potential biological functions. However, the role of NGAL produced by CD4+ T cells is not known. We found that ischemic AKI in NGAL knockout (KO) mice had worse renal outcomes compared to wild type (WT) mice. Adoptive transfer of NGAL-deficient CD4+ T cells from NGAL KO mice into CD4 KO or WT mice led to worse renal function than transfer of WT CD4+ T cells. In vitro simulated ischemia/reperfusion showed that NGAL-deficient CD4+ T cells express higher levels of IFN-γ mRNA compared to WT CD4+ T cells. In vitro differentiation of naive CD4+ T cells to Th17, Th1 and Th2 cells led to significant increase in Lcn2 expression. Human kidney CD4+ T cell NGAL also increased significantly post-ischemia. These results demonstrate an important role for CD4+ T cell NGAL as a mechanism by which CD4+ T cells mediate AKI and extend the importance of NGAL in AKI beyond diagnostics.
Keywords: NGAL, Lcn2, acute kidney injury, ischemia reperfusion injury, CD4+ T cells, inflammation
INTRODUCTION
Acute kidney injury (AKI) remains a common and serious problem in hospitalized patients, and is associated with an overall 11% hospital mortality rate and as high as 50% in critically ill patients (1–4). Despite significant medical advancements, clinical outcomes of AKI still remain poor, thus there is an important need to discover novel pathophysiologic pathways with translational potential (5).
T cells have been demonstrated by many different groups to play an important role in the pathophysiology of ischemic and nephrotoxic AKI (6–11). This is consistent with a large body of work on the role for T cells in acute injury and repair in non-renal organs (12–16). However, little is known about the mechanisms by which T cells modulate the outcome of AKI. CD4+ T cells are an important T cell type that mediates AKI, thus an attractive target for further research in AKI. We therefore conducted an unbiased discovery search using next generation RNA-sequencing (RNA-seq) on kidney CD4+ T cells in a mouse model of ischemic AKI. We found that CD4+ T cell lipocalin2 (Lcn2), which encodes neutrophil gelatinase-associated lipocalin (NGAL), had the highest fold increase after ischemic AKI. This was unexpected given that NGAL is usually viewed in the renal community as a candidate early biomarker for AKI generated by tubular epithelial cells, and little is known about CD4+ T cell NGAL.
NGAL is a 21-kD protein of the lipocalin superfamily (17). The production of NGAL in kidney epithelial cells markedly increases during renal injury and the increase of both plasma and urine NGAL can be detected within 2 hours of injury (18). NGAL revealed its diagnostic and prognostic potential in the cardiac surgery-associated AKI as well as in adult AKI patients in emergency department (19, 20). In addition to its diagnostic efficiency, NGAL is known to possess multiple biological roles including bacteriostatic, iron trafficking, and chemotactic functions (21). NGAL is also involved in the development of kidney, inducing the differentiation of kidney progenitors in the metanephric mesenchyme into renal epithelia (22). NGAL can be produced in multiple organs including kidney, liver, heart, gut, and various populations of immune cells, such as macrophages or dendritic cells (23, 24). Systemic delivery of NGAL has been shown to have protective effects in ischemic AKI by inhibition of tubular cell death and induction of anti-oxidant genes (25, 26). A recent study found that macrophages overexpressing Lcn2 could induce intrinsic resistance to ischemia causing protection from kidney ischemia reperfusion injury (IRI) (27). However, little is known about CD4+ T lymphocyte-derived NGAL.
We confirmed the increase of Lcn2 mRNA expression discovered by RNA-seq through quantitative real-time PCR (qRT-PCR) in post-ischemic kidney CD4+ T cells. We also demonstrated an increase of kidney CD4+ T cell NGAL protein with ELISA. There was also an increase in systemic NGAL and spleen CD4+ T cell NGAL after AKI. We built on the discovery work to generate the hypothesis that CD4+ T cell-derived NGAL mediates ischemic AKI, and embarked on mechanistic studies of CD4+ T cell-derived NGAL in AKI. NGAL knockout (KO) mice were found to have worse kidney function after ischemic AKI than WT mice. Lcn2-deficient CD4+ T cells from NGAL KO mice, when adoptively transferred into wild type (WT) mice, worsened kidney function compared to transfer of WT CD4+ T cells. In vitro IRI demonstrated increased IFN-γ production in Lcn2-deficient kidney/splenic CD4+ T cells compared to WT, a potential mediator of the CD4+ T cell NGAL effect. Additional in vitro studies to identify major NGAL producing CD4 T cell subset showed significant Lcn2 expression in Th17 cells followed by Th1 and Th2 cells respectively compared to Th0 cells. To begin to assess human relevance, we studied kidney CD4+ T cells in humans and found a significant increase in CD4+ T cell NGAL from visually normal kidney sample of post-nephrectomy for renal cell carcinoma (RCC) compared to pre-clamp kidney tissue prior to nephrectomy. Thus, CD4+ T cell-derived NGAL is an important mechanism by which immune cells mediate AKI.
METHODS
Animals
Male C57BL/6J (WT), B6.129P2-Lcn2tm1Aade/AkiJ (NGAL KO), and B6.129S2-Cd4tm1Mak/J (CD4 KO) mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and bred under specific pathogen-free conditions at the central animal facility of the Johns Hopkins University. 8–14-week-old male mice were used and all animal experiments were performed using Johns Hopkins University Institutional Animal Care and Use Committee-approved protocols.
Isolation of kidney mononuclear cells and splenocytes from mice
Kidney mononuclear cells (KMNCs) and splenocytes were isolated according to an established protocol (28). Mice were anesthetized with intraperitoneal ketamine hydrochloride (125 mg/kg) and xylazine (7 mg/kg), and exsanguinated to reduce the number of circulating immune cells in kidneys. Both kidneys were removed, decapsulated, finely minced, and incubated in collagenase D (2 mg/ml; Sigma-Aldrich, St. Louis, MO) solution for 30 minutes at 37 °C. A single-cell suspension of the kidney digestion was achieved by mechanical disruption of the tissue using a 70 μm strainer (BD bioscience, San Jose, CA), and followed by centrifugation at room temperature using isotonic Percoll density gradient (GE Healthcare, Chicago, IL, USA) (1,500 x g for 30 minute in brake off mode) to collect mononuclear cell population as per the manufacturer’s instructions. Collected cells from each organ were washed and each pellet was re-suspended in RPMI media containing 5% FBS and counted. Spleens were filtered through 40 μm strainer (BD bioscience) to prepare single-cell suspensions and incubated with ACK lysis buffer (Quality Biological, Gaithersburg, MD) for 3 minutes to remove red blood cells.
FACS sorting of kidney CD4+ T cells
For FACS sorting, KMNCs were pre-incubated with anti-CD16/CD32 Fc block (2.4G, BD Bioscience) for 5 minutes on ice and stained with monoclonal Ab anti-TCRβ (H57–597) BV421, anti-CD4 (RM4–5) PerCP-Cy5.5 (BioLegend, San Diego, CA) for 30 minutes at 4 °C. TCR+CD4+ cells were sorted using a FACSAria II Cell Sorter (BD bioscience). After sorting, cells were used for the construction of RNA-seq libraries and for the transcriptomic verification using qRT-PCR.
Magnetic isolation of CD4+ T lymphocytes from kidneys and spleens
After isolation of KMNCs and splenocytes, cells were washed and resuspended with rinsing buffer (PBS containing 2% FBS and 1mM of EDTA) and CD4+ T cells were positively isolated following instruction provided with the CD4+ T cell isolation kit (Miltenyi Biotec, Gaithersburg, MD) with minor modification. Briefly, cells were pre-incubated with anti-mouse CD16/CD32 Fc block (2.4G) for 5 minutes on ice, followed by incubation with CD4 (L3T4) microbeads, mouse for 10 minutes at 4 °C, and then applied onto the pre-rinsed MACS column. Unlabeled cells were washed out from the column using the rinsing buffer, and the column was removed from the separator and the labeled CD4+ T cells were flushed out with the rinsing buffer by pushing the plunger into the column. These magnetically isolated CD4+ T cells were used for ELISA, adoptive cell transfer, and in vitro IRI experiments.
RNA sequencing
RNA samples were collected from flow-sorted renal CD4+ T cells of control C57BL6 WT male mice and 24 hr after IRI (n=3 per group with 5–6 mice used for each sample). Total RNA was isolated with RNeasy Mini kit (Qiagen, Valencia, CA), and the quality and quantity of the RNA were measured using Advanced Analytical Fragment Analyzer (Santa Clara, CA). The cDNA libraries were prepared from 1 ng of total RNA from each sample using SMARTer® Stranded Total RNA-Seq Kit v2 – Pico Input Mammalian kit (Takara, Mountain View, CA). RNA-seq was performed using Illumina NextSeq 500 (Illumina Inc., San Diego, CA) at the Deep Sequencing & Microarray Core in Johns Hopkins. Libraries were multiplexed and sequenced with 60 million 36 base-pair paired-end reads per sample using two flowcell lanes. Using Tophat version 2.1.0.and Cufflinks version 2.2.1 with default parameters (29), raw data were aligned to the GRCm38/mm10 mouse reference genome and Fragments Per Kilobase Of Exon Per Million (FPKM) values were obtained. The gene expression of IR-injured renal CD4+ T cells was compared with normal control using a cutoff of P < 0.05 and the minimum FPKM values of > 1.0 in log2 notation. The data discussed in this publication have been deposited in NCBI’s Gene Expression Omnibus (30) and are accessible through GEO Series accession number GSE139171. (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE139171).
Mouse renal ischemia-reperfusion model
An established model of renal ischemia-reperfusion in mice was used (31). Mice were anesthetized with an intraperitoneal injection of ketamine hydrochloride (125 mg/kg) and xylazine (7 mg/kg). Following an abdominal medial incision, the renal pedicle was dissected, and a microvascular clamp (Roboz Surgical Instrument, Gaithersburg, MD) was placed on each renal pedicle for 28 minutes. Animals were kept hydrated with 1 ml of warm saline and at a constant body temperature (37°C). After 28 minutes of ischemia, the clamps were removed and the kidneys were inspected for the restoration of blood flow. The wounds were sutured and the animals were allowed to recover with free access to food and water.
Adoptive cell transfer
Magnetically isolated splenic CD4+ T cells (~107) from NGAL KO mice or C57BL/6 WT mice were transferred into separate groups of 8–12 week-old CD4 KO mice or WT mice 24 hours before IRI. WT mice that did not receive any cell type (PBS only) were used as a control. The level of kidney injury in each group was measured based on the serum creatinine (SCr) level and histology.
Measurement of serum creatinine
Blood samples were collected at 0, 24, 48, and 72 hours after IRI, and the SCr was measured to assess renal function by Cobas Mira Plus automated analyzer system (Roche, Basel, Switzerland) using creatinine measurement reagents (Pointe Scientific Inc. Canton, MI).
Histologic Evaluation of Kidney Injury
The kidneys were harvested and cut into three equal transverse pieces. One piece from each kidney was fixed with 10% buffered formalin phosphate and embedded with paraffin for histologic evaluation. Tissue sections (5 μm) were stained with hematoxylin and eosin. A renal pathologist (L.J.A), who was blinded to the experimental groups, scored the percentage of necrotic tubules out of total tubules at least 10 high-power fields, and the average percentage of tubular necrosis in all fields was presented as the renal tubular injury score of each mouse.
Quantitative real-time PCR
Total RNA was isolated from renal or splenic CD4+ T cells with RNeasy Mini kit (Qiagen, Valencia, CA) and reverse transcribed using High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). Because of limited numbers of renal CD4+ T cells, 1.25 ng of cDNA from sorted renal CD4+ T cells was pre-amplified using TaqMan® PreAmp Master Mix (Applied Biosystems) for transcriptomic verification of Lcn2 over-expression in post-ischemic renal CD4+ T cells. Pre-designed TaqMan primer and probe sets (Applied Biosystems) were used to quantify Lcn2 (Mm01324470_m1), Ifn-γ (Mm01168134_m1), Tnf-α (Mm00443258_m1), Il-10 (Mm00439614_m1), and Il-17a (Mm00439618_m1) mRNA in CFX96 real-time PCR (Bio-Rad, Hercules, CA). The expression value for each gene was normalized to Rpl13a (Mm01612987_g1) and the relative fold expression values were calculated using a ΔΔ cycle threshold method (32).
ELISA
CD4+ T cells from mice kidneys and spleens were magnetically isolated from B6 WT mice. Collected cells from each organ were washed with PBS twice and the cell pellets were lysed with RIPA buffer supplemented with Protease Inhibitor Cocktail (Thermo Scientific, Waltham, MA) and EDTA (Thermo Scientific). The total protein of each cell lysates was quantified using Pierce™ BCA Protein Assay Kit (Thermo Scientific) and the levels of NGAL were assayed using the LEGEND MAX™ Mouse NGAL ELISA kit (Biolegend) according to the manufacturer’s protocol.
CD4 T cell differentiation
To identify major NGAL producing CD4 T cell subset, naïve CD4 T cells (CD62L+CD44-) were isolated from spleens of normal WT mice using EasySep Mouse Naïve CD4+ T Cell Isolation Kit (STEMCELL Technologies) and cultured in Th0, Th1, Th2, Th17 and iTreg differentiation conditions and Lcn2 mRNA quantified using real-time PCR. Briefly, purified (>90%) naïve CD4 T cells (1×106/ml) were activated with immobilized anti CD3 (5μg/mL) and soluble anti CD28 (2μg/mL) antibody in the presence of appropriate differentiation cytokines in complete RPMI 1640 supplemented with 10 % FBS, 100μM non-essential amino acid, 1% penicillin and streptomycin, 10mM HEPES, 55 μM β-mercaptoethanol and 2mM L-glutamine. The cells were cultured in Th1 (10 ng/mL IL-12 p40, 10 ng/ml IFN-γ, 5 μg/mL anti-IL-4 (11B11)), Th2 (10 ng/mL IL-4, 5μg/mL anti-IFN-γ (XMG1.2), 5μg/ml anti-IL12p40), Th17 (5 ng/mL TGF-β1, 20 ng/mL IL-6, 5 μg/mL anti-IFN-γ (XMG1.2), 5 μg/mL anti-IL-4 (11B11) or iTreg (10ng/mL TGF-β1, 10 ng/mL IL-2, 5μg/ml anti-IL12p40, 5μg/mL anti-IFN-γ (XMG1.2), 5μg/mL anti-IL-4 (11B11) differentiation condition for 48 hours as per established protocols (33, 34). Th0 cells were activated with CD3/CD28 but not provided any differentiating conditions. After 48 hours, Th0, Th1, Th2 and iTreg cells were expanded on fresh plates (without CD3/CD28) in the presence of IL2 (10ng/ml) whereas Th17 cells were expanded in the presence of IL23 (10ng/ml). The cells were harvested on day 4 and assessed for differentiation before quantifying Lcn2 mRNA expression.
In vitro hypoxia reoxygenation assay
Ischemia in kidney and splenic CD4+ T cells from NGAL KO and B6 WT mice was simulated by immersing the cell pellets in mineral oil (MiliporeSigma, St. Louis, MO) with minor modification of the protocol by Meldrum et al (35). Briefly, 106 magnetically isolated kidney and splenic CD4+ T cells were resuspended in complete RPMI media (supplemented with 10% FBS, 10 mM HEPES, and 100 U/ml penicillin and streptomycin), and cultured overnight in a 12-well plate coated with CD3/CD28 (5 ug/ml) and 50 U/ml of IL-2. Next day, cells were washed twice with PBS, and the cell pellet immersed in mineral oil for 10 minutes at 37 °C. After the ischemia phase, cells were washed with PBS twice and resuspended in complete RPMI media for 6 hours at 37 °C. CD4+ T cells with no IRI were used as the control. Cells were harvested and lysed in RLT buffer for the following qRT-PCR.
Human Samples
After informed consent, human kidney samples were collected from RCC patients undergoing nephrectomy surgery either before or after the clamping of renal pedicles. The kidney tissue was digested according to our protocol for isolation of KMNCs(36). Single-cell suspensions were pre-incubated with Human BD Fc Block (BD Biosciences) for 5 minutes and stained with monoclonal Ab anti-CD45 (HI30) BUV395 (BioLegend), TCRα/β (IP26) BV421, (BioLegend), CD4 (OKT4) FITC (eBioscience) for 30 minutes at 4 °C. Cells were fixed and permeabilized with fixation/permeabilization buffer (eBioscience) for 30 minutes at room temperature followed by another pre-incubation with Human BD Fc Block (BD Biosciences) for 5 minutes and intracellular staining with polyclonal Ab anti-NGAL APC (Assaypro, St. Charles, MO/Catalogue number: 31228–05161) at room temperature. APC-conjugated polyclonal Rabbit IgG was used as an isotype control (R&D systems, Minneapolis, MN) CD45+TCRα/β+CD4+ cells were analyzed for their intracellular NGAL expression using flow cytometry. The present study was conducted in accordance with the Declaration of Helsinki and approved by the Johns Hopkins Medicine Institutional Review Boards. No identifiable information was acquired during tissue collection.
Statistical analysis
Data were collected from at least three independent experiments and expressed as mean±SE. Statistical differences were analyzed using two-tailed Student’s t-test between two groups and one-way ANOVA test followed by the Newman-Keuls multiple comparisons test for more than three groups using Prism 6 (GraphPad Software, San Diego, CA). Statistical significance was determined as P < 0.05.
RESULTS
Lcn2/NGAL markedly increases in kidney CD4+ T cells following IR-induced AKI
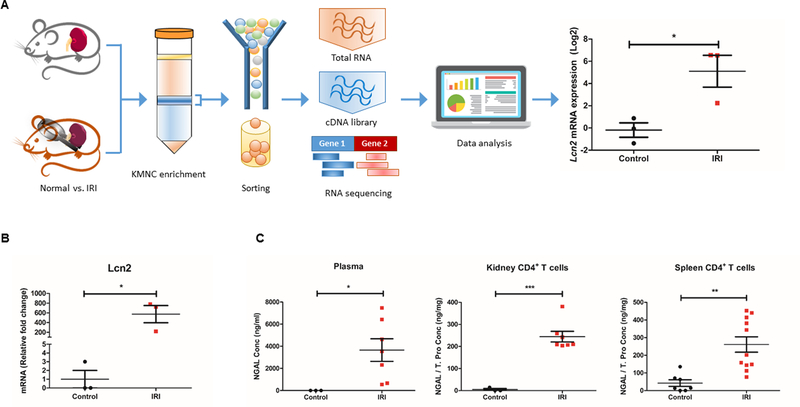
The RNA-seq data using flow-sorted kidney CD4+ T cells from post-ischemia as well as control WT mice showed that the expression of Lcn2 had the most robust increase (~60-fold) following IR-induced AKI among 52,633 transcripts (Fig. 1A). We then performed qRT-PCR to confirm Lcn2 expression in flow-sorted kidney CD4+ T cells from normal and post-ischemic WT mice. The mRNA expression of Lcn2 was significantly higher (~570 fold compared to normal, P < 0.05) in the kidney CD4+ T cells isolated from post-ischemic WT mice (Fig. 1B). We next verified whether this increased Lcn2 mRNA expression translates into NGAL protein expression in post-ischemic kidney CD4+ T cells using ELISA. We first confirmed the systemic increase of NGAL following kidney IRI by measuring the NGAL level in plasma, which showed approximately 5,500-fold increase compared to normal (P < 0.05) (Fig. 1C, left). We then performed ELISA on magnetically-isolated CD4+ T cells from kidney and spleen, which showed that kidney IRI significantly induced the expression of NGAL both in the kidney (244.3±24.1 ng/mg vs. 4.8±4.8 ng/mg, P < 0.001) and splenic CD4+ T cells (264.1±43.8 ng/mg vs. 42.4±18.9 ng/mg, P < 0.01) (Fig. 1C, middle and right). Together, these results indicate that IRI induces a marked increase of Lcn2/NGAL both at transcriptomic and translational level in WT murine CD4+ T cells.
Figure 1. The expression of Lcn2/NGAL is highly upregulated in kidney CD4+ T cells after ischemic AKI in mice.
(A) A schematic diagram representing the RNA-seq experiment. Kidney CD4+ T cells were flow-sorted from the control WT male mice and 24 hr after IRI, followed by RNA isolation, cDNA library construction, and RNA-seq. Data analysis of differential gene expression showed that Lcn2 had the highest fold change in post-ischemic CD4+ T cells compared to control (~60-fold increase) (n=3). (B) qRT-PCR showed that IRI significantly upregulated the expression of Lcn2 mRNA in kidney CD4+ T cells compared to the control kidney CD4+ T cells in WT male mice (~570-fold increase) (n=3). (c) NGAL protein levels increased in serum as well as in kidney and splenic CD4+ T cells after IRI. Ischemic AKI induced a robust increase of NGAL in plasma (~ 5,500-fold) (Left), in the cell lysates of both kidney CD4+ T cells (middle) and splenic CD4+ T cells (right) (n=3–11). Data presented as mean±SE. *P < 0.05, **P < 0.01, ***P < 0.001.
Lcn2/NGAL deficiency augments susceptibility to ischemic AKI in mice
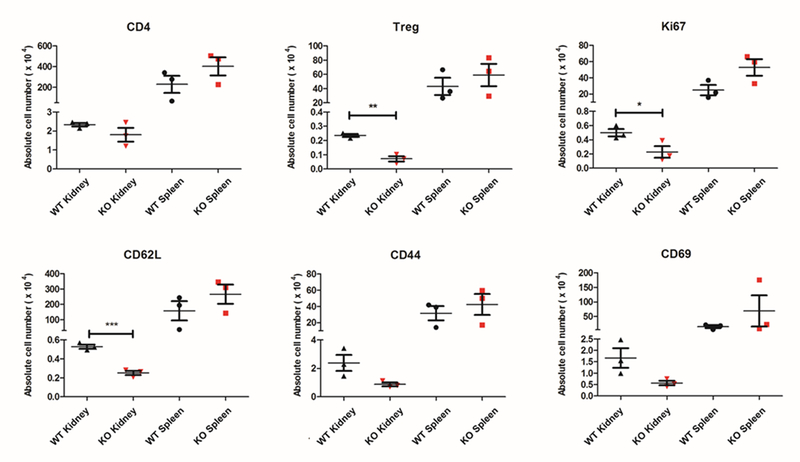
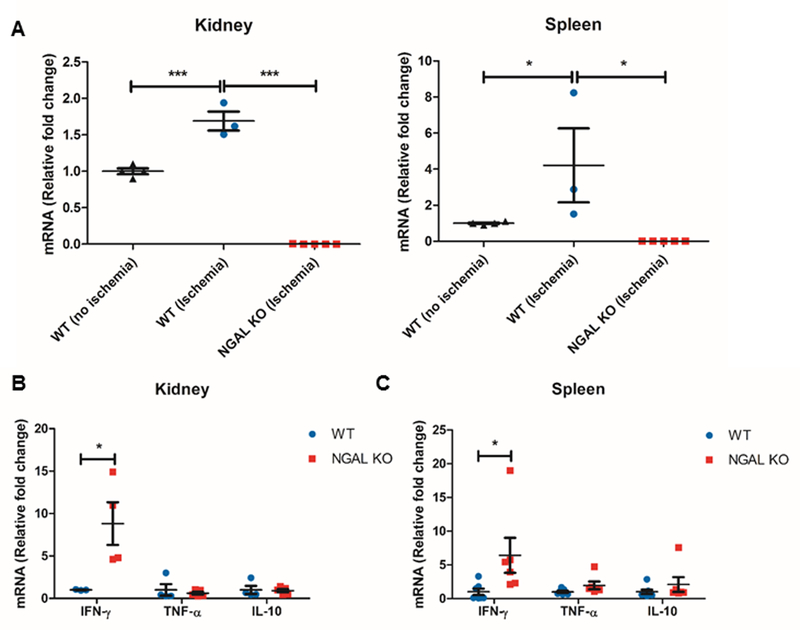
To begin to understand the role of NGAL in CD4+ T cell activation and function during AKI, we compared the baseline characteristics of the Lcn2-deficient CD4+ T cells from NGAL KO mice with the CD4+ T cells from WT mice. We isolated KMNCs from NGAL KO mice and WT mice and analyzed the number of CD4+ T cells (TCR+CD4+), regulatory T cells (Tregs, CD4+Foxp3+), proliferating CD4+ T cells (CD4+Ki67+) and activated CD4+ T cells using CD62L, CD44 and CD69 markers with flow cytometry (Fig. 2). There was no difference in the number of kidney CD4+ T cells between NGAL KO mice and WT mice, but NGAL KO mice had a significantly lower number of renal Tregs compared to the WT mice (P < 0.01). There were fewer numbers of Ki67+ proliferating kidney CD4+ T cells in the NGAL KO mice compared to the WT mice (P < 0.05). There was significantly lower number of CD62L+ cells in the Lcn2-deficient kidney CD4+ T lymphocytes (P < 0.001), but there was no statistical difference in the number of CD44+ or CD69+ CD4+ T cells. No significant difference was found on these parameters between the splenic CD4+ T cells from NGAL KO mice and WT mice.
Figure 2. Comparison of kidney & spleen CD4+ T cells from WT or NGAL KO mice.
Kidney mononuclear cells and splenocytes were obtained from WT and NGAL KO male mice and analyzed using flow cytometry. There was no difference in the absolute number of kidney/splenic CD4+ T cells between WT and NGAL KO groups. Kidney CD4+ T lymphocytes from NGAL KO mice had significantly lower number of Tregs and Ki-67+ proliferating cells compared to WT mice. Lcn2-deficient kidney CD4+ T cells also had reduced number of CD62L positive cells compared to kidney CD4+ T cells from WT mice. There was no difference in the number of CD69 or CD44 positive CD4+ T cells. There were no differences in any of these parameters when comparing splenic CD4+ T cells from WT and NGAL KO mice (n=3). Data presented as mean±SE. *P < 0.05, **P < 0.01, ***P < 0.001.
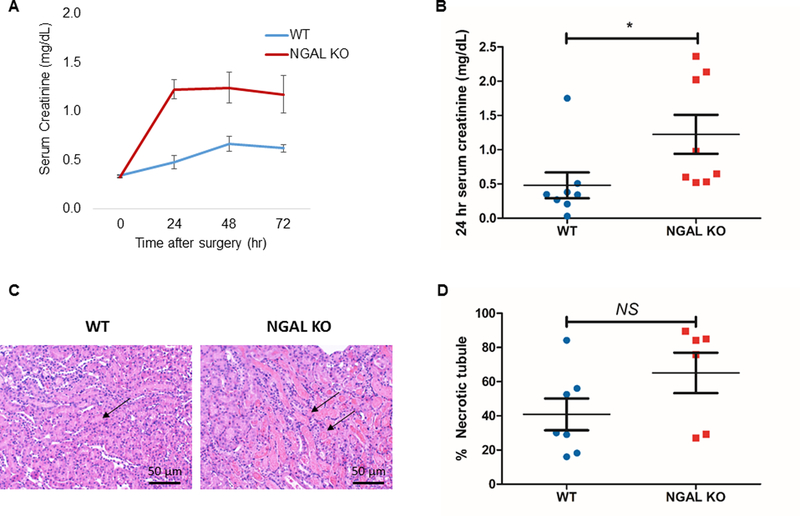
Prior to focusing on CD4+ T cell NGAL in AKI, we first tested the role of whole body NGAL during AKI. Kidney IR surgery was conducted in NGAL KO compared to WT mice. Each group of mice was followed up until 72 hours after 28-minute of ischemic injury (ischemia time was calibrated to best compare renal function outcomes with the anesthesia and temperatures used). We found that the deficiency of total body NGAL induced worse kidney function after IRI compared to WT controls. The SCr level 24 hours after IRI was significantly higher in the NGAL KO mice compared to WT mice (1.2±0.3 vs. 0.5±0.2, P < 0.05) (Fig. 3A, 3B). The kidneys were harvested for histologic evaluation after 72 hours of reperfusion and the kidney tissues were assessed by an expert pathologist (L.J.A) in a blinded manner. Histologic evaluation was mainly focused on the outer medulla where major tubular injury occurs during ischemic AKI. There was a trend for higher percentages of necrotic tubules found in the outer medulla of NGAL KO mice compared to the control. (Fig. 3C, 3D). Based on the robust increase of NGAL in post-ischemic CD4+ T cells from our study and the significant role of CD4+ T cells during ischemic AKI (6), these results suggest a potential role of CD4+ T cell-derived NGAL in ischemic AKI.
Figure 3. NGAL KO mice have worse renal function after kidney IRI.
(A) SCr levels were determined at baseline and 24, 48, and 72 hours after renal ischemia. (B) The level of SCr 24 hours after IRI was significantly higher in the NGAL KO male mice compared to WT male mice (n=8). (C) Representative images of hematoxylin and eosin-stained post-ischemic kidney sections of NGAL KO mice and WT mice. (D) NGAL KO mice showed a tendency for worse necrosis in outer medullary tubules compared to WT after IRI, but was not statistically significant (n=6–7). Data presented as mean±SE. *P < 0.05. Original magnification, x200 in C.
Adoptive transfer of Lcn2-deficient CD4+ T cells aggravates ischemic AKI
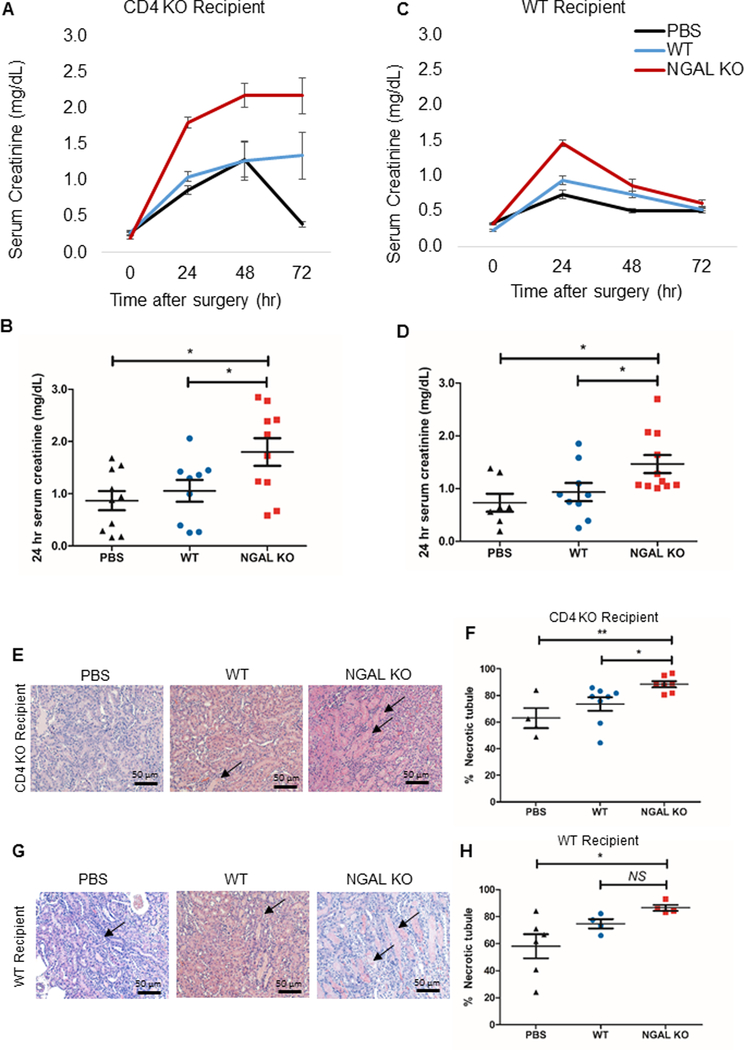
To directly test for the role of CD4+ T cell-derived NGAL in ischemic AKI, we adoptively transferred ~107 magnetically isolated splenic CD4+ T cells from NGAL KO mice and WT B6 mice. The cells were transferred either into WT or CD4 KO mice 24 hours before inducing AKI. Another control group was injected with PBS. All the mice were subjected to the same experimental conditions and were monitored for kidney function for 72 hours after IRI with SCr measured every 24 hours. The mice that received (a) Lcn2-deficient CD4+ T cells had a significantly higher SCr in comparison to those that received (b) WT CD4+ T cells or (c) PBS both into CD4 KO recipient (a, 1.8±0.3 vs. b, 1.1±0.2 vs. c, 1.0±0.2; P < 0.05) (Fig. 4A, 4B) and into WT recipient (a, 1.5±0.2 vs. b, 0.9±0.2 vs. c, 0.7±0.2; P < 0.05) at 24 hours after IRI (Fig. 4C, 4D).
Figure 4. Adoptive transfer of Lcn2-deficient CD4+ T cells aggravates the course of AKI.
Splenic CD4+ T cells were isolated from WT or NGAL KO male mice and adoptively transferred into either CD4 KO male mice (A, B) or WT male mice (C, D) 24 hours before IRI. Adoptive transfer of splenic CD4+ T cells from NGAL KO mice led to significantly worse renal function following IRI, compared to the mice that received WT splenic CD4+ T cells or no cells (PBS) in both CD4 KO and WT recipient mice. Panel B and D show individual data points for 24 hr serum creatinine (n=7–11). (E, G) Representative images of hematoxylin and eosin-stained kidney sections of each experimental groups either in CD4 KO recipient or WT recipient (F, H) Dot plots show the percent score of necrotic tubules in outer medulla of each experimental group either in CD4 KO recipient (F) or WT recipient (H) (n=7–11). There was significantly higher necrosis in outer medullary tubules in Lcn2-deficient CD4+ T cell transferred-group compared to WT CD4+ T cell transferred-group as well as PBS control both into the CD4 KO recipient and the WT recipient, except WT recipient transferred with WT CD4+ T cell. Data presented as mean±SE. *P < 0.05; **P < 0.01, NS, not significant. Original magnification, x200 in E and G.
The histologic evaluation on the kidney sections after 72 hours of reperfusion showed that there was significantly higher percentage of necrotic tubules in the outer medullary region (primary histologic target during ischemic AKI) of mice that received Lcn2-deficient CD4+ T cells compared to those that received WT CD4+ T cells (P < 0.05) or PBS (P < 0.01) in CD4 KO recipients (Fig. 4E, 4F). A similar trend was found in the WT recipients, but there was no statistical significance between the group transferred with Lcn2-deficient CD4+ T cells and the group with WT CD4+ T cells (Fig. 4G, 4H). Collectively, these data demonstrate that Lcn2 deficiency in CD4+ T cells directly exacerbate the course of ischemic AKI.
In vitro ischemia reperfusion induces IFN-γ in Lcn2-deficient CD4+ T cells
To better understand how Lcn2/NGAL influences the response of CD4+ T cells during IRI, we simulated in vitro IRI by immersing magnetically isolated kidney and splenic CD4+ T cells from NGAL KO mice and WT mice in mineral oil for 10 minutes, followed by normoxic condition for 6 hours using an established technique with modification (35). We observed ~1.7-fold increase of Lcn2 mRNA expression in WT kidney CD4+ T cells and ~4.2-fold increase in WT splenic CD4+ T cells after in vitro IRI compared to control (Fig. 5A). Lcn2 expression was not detected in the kidney or splenic CD4+ T cells from NGAL KO mice. Deficiency of Lcn2 resulted in 8.8-fold increase in IFN-γ mRNA expression in kidney CD4+ T cells from NGAL KO mice (P < 0.05) and 6.4-fold increase of IFN-γ mRNA in splenic CD4+ T cells from NGAL KO mice compared to CD4+ T cells from WT mice (P < 0.05) (Fig. 5B, 5C). We did not observe any significant differences in the expression of TNF-α or IL-10 mRNA. IL-17a mRNA was not detected in both conditions (data not shown). Thus, modulation of IFN-γ expression is a potential mechanism by which CD4+ T cell-derived NGAL modifies the course of AKI.
Figure 5. Lcn2-deficient CD4+ T cells upregulate the expression of IFN-γ after in vitro simulated ischemia reperfusion.
Ischemia reperfusion injury (IRI) was simulated in kidney and splenic CD4+ T cells by immersing the cells in mineral oil for 10 minutes, and followed by 6 hours of reperfusion at 37°C. (A) In vitro IRI significantly upregulated Lcn2 expression by ~1.7-fold in kidney CD4+ T cells, while there was ~4.2-fold increase in splenic CD4+ T cells compared to control (n=3). Lcn2 was not detected in cells from NGAL KO mice. (B, C) The levels of IFN-γ mRNA were significantly higher in Lcn2-deficient kidney/splenic CD4+ T cells compared to WT following in vitro IRI (n=3–11), but no significant differences in the expression of TNF-α or IL-10 mRNA. Data presented as mean±SE. *P ≤ 0.05, ***P ≤ 0.001.
In vitro differentiated CD4+ cells express increased Lcn2
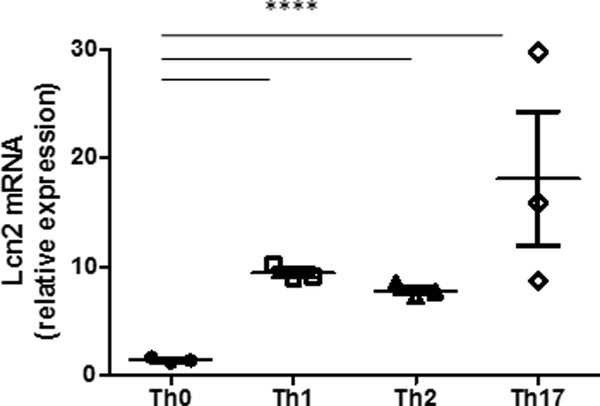
To evaluate the role of CD4 T cell differentiation on NGAL, naïve CD4 T cells (CD62L+CD44-) were cultured in Th0, Th1, Th2, Th17 and iTreg conditions, with Lcn2 expression quantified using real-time PCR. We found significantly (p< 0.001) higher expression of Lcn2 gene in Th1, Th2 and Th17 cells compared to Th0 cells. Lcn2 expression was highest (p< 0.05) in Th17 cells compared to Th1 and Th2 cells, while Th1 cells had higher (p< 0.05) Lcn2 expression compared to Th2 cells. We did not detect Lcn2 expression in iTreg cells under these in vitro differentiation conditions (Fig. 6).
Figure 6: Differentiated CD4 T cells express higher Lcn2 than Th0 cells.
Naïve CD4 T cell (CD62L+CD44-) were isolated from spleens of normal WT mice (10 week old), cultured in Th0, Th1, Th2, Th17 and iTreg conditions and Lcn2 mRNA quantified on day 4 using real-time PCR. Lcn2 mRNA expression in Th1, Th2 and Th17 cells was significantly (p< 0.001) higher compared to Th0 cells. Among differentiated CD4 T cells, Th17 cells had most significant increase in Lcn2 expression followed by Th1 and Th2 cells. Lcn2 expression in iTreg cells was not detectable. Data presented as mean±SE.***P < 0.001.
Human kidney CD4+ T cells increase expression of NGAL after ischemic injury
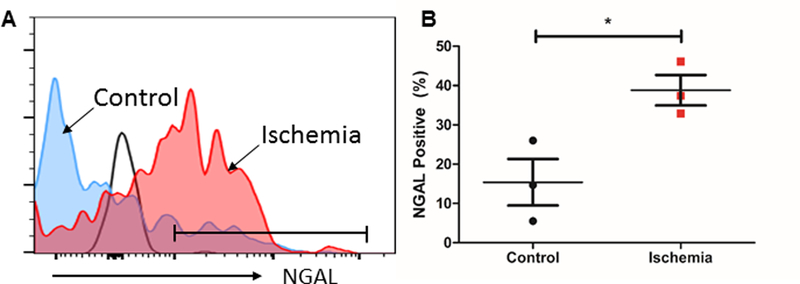
Many AKI studies in mice and in vitro have not translated to humans. We therefore performed a pilot study to evaluate whether human kidney CD4+ T cell NGAL also increases after ischemia. Kidney tissues were collected by carefully dissecting the non-malignant tissue from the patients undergoing nephrectomy due to RCC either before (control) or after renal pedicle clamping (post-ischemia). Human KMNCs were isolated using our previously established protocol (36), and then stained to measure the NGAL expression by flow cytometry. Flow cytometric analysis of CD45+TCR+CD4+ population revealed that post-ischemic kidney CD4+ T cells express higher level of NGAL compared to controls (38.8±3.9 % vs.15.4±5.9 %, P < 0.05) (Fig. 7). Thus, human kidney CD4+ T cells have a significant increase in NGAL post-ischemia as seen in murine kidney CD4+ T cells.
Figure 7. The expression of NGAL in CD4+ T cells significantly increases after human kidney ischemia.
(A) Representative histogram of NGAL expression in the human kidney CD4+ T cells after ischemic injury (red) and no ischemic injury (blue). Black line indicates isotype control. (B) Ischemia induced significantly higher CD4+ T cell NGAL expression compared to control human kidney CD4+ T cells (n=3). Data presented as mean±SE. *P ≤ 0.05
DISCUSSION
In order to understand mechanisms by which T cells mediate AKI, we performed discovery-driven next generation RNA-seq of mouse kidney CD4+ T cells during AKI. We found that Lcn2 gene, which encodes NGAL, had the highest fold increase post-ischemia compared to normal CD4+ T cells. This finding was verified both at the transcriptional and translational level with qRT-PCR and ELISA respectively. Subsequent hypothesis-driven studies demonstrated that adoptive transfer of Lcn2-deficient CD4+ T cells induced worse renal outcomes after AKI than transfer of WT CD4+ T cells into two different mouse strains. In vitro ischemia reperfusion studies demonstrated that Lcn2-deficient CD4+ T cells had higher IFN-γ mRNA expression compared to WT CD4+ T cells, identifying a potential mediator of CD4+ T cell-derived NGAL. Furthermore, in vitro CD4 T cell differentiation studies showed increased Lcn2 expression in Th1, Th2 and Th17 cells compared to Th0 cells with Th17 cells having the most significant increase compared to other CD4 T cell subsets. Translational data in human kidney ischemic injury also demonstrated upregulated NGAL in human kidney CD4+ T cells.
While exploring mechanisms of how CD4+ T cells mediate AKI, we unexpectedly found that NGAL is an important mediator. NGAL is usually viewed in the renal community as a promising early biomarker for AKI but there are a number of studies that have also demonstrated its biologic effects. Systemic delivery of NGAL around the time of IRI can rescue the kidney from ischemic AKI (25, 26). During IRI, large amounts of iron are released which induce significant oxidative stress and lead to tissue damage. Injected NGAL can behave as an iron-binding protein limiting the early damage from ischemic AKI, and also promote the recovery from acute tubular necrosis through anti-oxidant activity of heme oxygenase. A recent study demonstrated an important role of macrophage-derived Lcn2/NGAL in kidney-intrinsic cytoprotective pathways during IRI by injecting Lcn2-knockdown/overexpressing macrophages (27). They also found that IL-10-overexpressing macrophages could protect kidney from ischemic AKI and improve tissue repair through the induction of NGAL (37). Similar to this study, we also found that NGAL KO mice had worse renal damage after IR-induced AKI. However, it should be noted that another study found that NGAL KO mice did not have increased renal damage induced by IRI compared to WT mice (38). Differences in anesthesia, surgical techniques, timing of ischemia and other factors could account for these differences.
We found that NGAL KO mice had reduced kidney Tregs (CD4+CD25+FoxP3+), which could explain their worse outcome since Tregs have been shown to protect from AKI (7, 39, 40). It has also been shown that NGAL treatment could induce the stimulation and the expansion of the CD4+CD25+Foxp3+ cells in human peripheral blood mononuclear cells (41). Additionally, our study found elevated Lcn2 levels in Th17 cells under in vitro differentiation condition. Previous studies found that IL17 increases NGAL expression by Th17 cells (42, 43). These observations suggest that NGAL may also be involved in T cell differentiation process under steady state and during an ischemic event (Fig 8). Future studies about the potential role of Lcn2/NGAL on the expansion and migration of Tregs into kidney during IRI, cytokine production and role in T cell differentiation will help clarify the protective mechanism of T cell-derived NGAL in ischemic AKI.
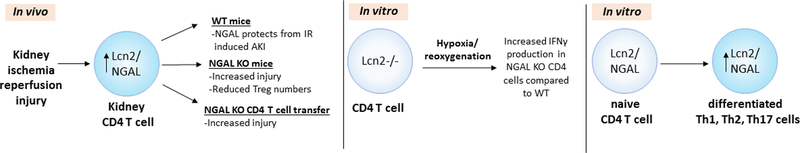
Figure 8: CD4 T cell NGAL in kidney ischemia reperfusion injury, hypoxia –reoxygenation and T cell differentiation.
NGAL expression increases in renal CD4 T cells following IR and provide functional and structural protection to kidneys. CD4 T cells that lack NGAL worsen kidney IRI due in part to increased production of pro-inflammatory IFNγ and possibly due to decreased Treg cell numbers. Furthermore, in vitro differentiation of naïve CD4 T cells leads to increased Lcn2/NGAL expression by differentiated Th1, Th2 and Th17 cells. These factors collectively lead to CD4 cell NGAL providing protection from kidney IRI.
The role of NGAL has been widely studied in other multiple disease models including myocardial infarction, heart failure, chronic inflammation, and inflammatory bowel disease (44–48). NGAL is likely to have a protective effect in the ischemic AKI in contrast to a role in worsening outcomes in cardiac injury or vascular inflammation. NGAL KO hearts showed a smaller infarct size and a preserved cardiac function compared to WT following cardiac IRI, and the increase of circulating NGAL level resulted in impaired recovery of cardiac function (45). Also, NGAL KO mice showed protection against the development of abdominal aortic aneurysm (46), and from mineralocorticoid-induced hypertension as well as vascular fibrosis (47). Recently, mice were generated with Lcn2-deficient immune cells by bone marrow transplantation, and showed the deleterious role of immune cell-derived NGAL in cardiac and renal inflammation induced by mineralocorticoid excess (49). NGAL is known to be involved in the maintenance of intestinal homeostasis due to its impact on the gut microbiota (50). NGAL deficiency resulted in expansion of facultative pathogenic Alistipes spp. in IL-10 KO mice, which exacerbated colitis. Lack of NGAL exhibited structural mucosal damage and barrier leakage in the intestine (48). NGAL KO mice have more colitogenic T cells and are more susceptible to dextran sodium sulfate-induced colitis, implying a regulatory role of NGAL in gut inflammation and gut bacterial dysbiosis (51).
Our study had a number of limitations. In the adoptive transfer experiment, restricted numbers of CD4+ T cells migrated to the recipient kidney, which made it unfeasible to analyze the subtypes of donor CD4+ T cells and their phenotypes. Further studies are also needed to conclude whether the pro-inflammatory effect of NGAL-deficiency is from the NGAL itself or from the effect of NGAL on CD4+ T cells. To obtain the maximum purity of renal CD4+ T cells, flow sorting was tried first for all the experiments. In the case when higher number of renal CD4+ T cells are required for ELISA and in vitro IRI, however, magnetic isolation substituted for the flow sorting, which reached ~85% of purity. We embarked on studies to genetically modify NGAL expression in adult mouse CD4+ T cells to complement our knockout mice studies, but met with technical obstacles. Furthermore, the “normal” kidney near RCC may not be fully normal tissue, but we had institutional and safety restrictions from obtaining human kidney samples in other clinical situations pre- and post-ischemia. There is at least one study that found single nucleotide polymorphism (rs13297265) upstream of LCN2 locus that was associated with increased cardiac hypertrophy and decreased cardiac function in humans suggesting important clinical relevance (52).
As the biological roles of NGAL continue to be studied, NGAL seems to work in various ways depending on the site of injury, the type of injury model, and the cells involved. Our current study sets the stage for further exploration into the biological role of CD4+ T cell-derived NGAL in renal injury, with therapeutic implications in humans.
Key points.
Kidney CD4 T cells significantly increase Lcn2/NGAL expression following IR injury
CD4 T cell Lcn2/NGAL plays a protects from IR induced acute kidney injury
Lcn2 expression increased in ischemic CD4 T cells from human kidney
ACKNOWLEDGMENTS
We appreciate a generous gift from Rogelio Miro of Panama. The authors also acknowledge Dr. Jonathan Powell and Rachel Helms for their guidance with CD4 T cell differentiation protocol, and Conover Talbot Jr. for help with submitting RNA-seq data to GEO database.
This work was supported by the National Institutes of Health (R01-DK111209 and R01-DK104662 to HR and AH respectively), and Dr. Werner Jackstädt-Foundation ( S134-10.117) grant to JTK.
Abbreviations used in this article
- AKI
acute kidney injury
- Lcn2
lipocalin-2
- NGAL
neutrophil gelatinase-associated lipocalin
- KO
knockout
- WT
wild type
- RNA-seq
RNA-sequencing
- IRI
ischemia-reperfusion injury
- qRT-PCR
quantitative real-time PCR
- RCC
renal cell carcinoma
- KMNCs
kidney mononuclear cells
- FPKM
fragments Per Kilobase Of Exon Per Million
- SCr
serum creatinine
Footnotes
DISCLOSURES
The authors declare no conflict of interest.
REFERENCES
- 1.Hoste EA, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, Edipidis K, Forni LG, Gomersall CD, Govil D, Honore PM, Joannes-Boyau O, Joannidis M, Korhonen AM, Lavrentieva A, Mehta RL, Palevsky P, Roessler E, Ronco C, Uchino S, Vazquez JA, Vidal Andrade E, Webb S, and Kellum JA 2015. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 41: 1411–1423. [DOI] [PubMed] [Google Scholar]
- 2.Rewa O, and Bagshaw SM 2014. Acute kidney injury-epidemiology, outcomes and economics. Nat Rev Nephrol 10: 193–207. [DOI] [PubMed] [Google Scholar]
- 3.Li PK, Burdmann EA, and Mehta RL 2013. Acute kidney injury: global health alert. Curr. Opin. Nephrol. Hypertens 22: 253–258. [DOI] [PubMed] [Google Scholar]
- 4.Campbell CA, Li L, Kotwal S, Georgiou A, Horvath AR, Westbrook J, and Endre Z 2019. Under-detection of Acute Kidney Injury in Hospitalised Patients - A Retrospective, multi-site, longitudinal study. Intern. Med. J [DOI] [PubMed] [Google Scholar]
- 5.Kellum JA 2015. Diagnostic Criteria for Acute Kidney Injury: Present and Future. Crit. Care Clin 31: 621–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burne MJ, Daniels F, El Ghandour A, Mauiyyedi S, Colvin RB, O’Donnell MP, and Rabb H 2001. Identification of the CD4+ T cell as a major pathogenic factor in ischemic acute renal failure. J. Clin. Invest 108: 1283–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gandolfo MT, Jang HR, Bagnasco SM, Ko GJ, Agreda P, Satpute SR, Crow MT, King LS, and Rabb H 2009. Foxp3+ regulatory T cells participate in repair of ischemic acute kidney injury. Kidney Int. 76: 717–729. [DOI] [PubMed] [Google Scholar]
- 8.Savransky V, Molls RR, Burne-Taney M, Chien CC, Racusen L, and Rabb H 2006. Role of the T-cell receptor in kidney ischemia–reperfusion injury. Kidney Int. 69: 233–238. [DOI] [PubMed] [Google Scholar]
- 9.Liu M, Chien CC, Burne-Taney M, Molls RR, Racusen LC, Colvin RB, and Rabb H 2006. A pathophysiologic role for T lymphocytes in murine acute cisplatin nephrotoxicity. J. Am. Soc. Nephrol 17: 765–774. [DOI] [PubMed] [Google Scholar]
- 10.Day YJ, Huang L, Ye H, Li L, Linden J, and Okusa MD 2006. Renal ischemia-reperfusion injury and adenosine 2A receptor-mediated tissue protection: the role of CD4+ T cells and IFN-gamma. J. Immunol 176: 3108–3114. [DOI] [PubMed] [Google Scholar]
- 11.Mehrotra P, Patel JB, Ivancic CM, Collett JA, and Basile DP 2015. Th-17 cell activation in response to high salt following acute kidney injury is associated with progressive fibrosis and attenuated by AT-1R antagonism. Kidney Int. 88: 776–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhong Q, Jenkins J, Moldobaeva A, D’Alessio F, and Wagner EM 2016. Effector T Cells and Ischemia-Induced Systemic Angiogenesis in the Lung. Am. J. Respir. Cell Mol. Biol 54: 394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang DJ, Zhu ZX, Fu HB, Yan RL, Chen J, Cheng YJ, Cai QP, and Wang Q 2014. Hypertonic saline activates CD4+ and CD8+ T-lymphocytes in the small intestine to alleviate intestinal ischemia-reperfusion injury. Eur. Rev. Med. Pharmacol. Sci 18: 3069–3075. [PubMed] [Google Scholar]
- 14.Caldwell CC, Okaya T, Martignoni A, Husted T, Schuster R, and Lentsch AB 2005. Divergent functions of CD4+ T lymphocytes in acute liver inflammation and injury after ischemia-reperfusion. Am. J. Physiol. Gastrointest. Liver Physiol 289: G969–976. [DOI] [PubMed] [Google Scholar]
- 15.Kuboki S, Sakai N, Tschop J, Edwards MJ, Lentsch AB, and Caldwell CC 2009. Distinct contributions of CD4+ T cell subsets in hepatic ischemia/reperfusion injury. Am. J. Physiol. Gastrointest. Liver Physiol 296: G1054–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D’Alessio FR, Kurzhagen JT, and Rabb H 2019. Reparative T lymphocytes in organ injury. J. Clin. Invest 129: 2608–2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parikh CR, and Devarajan P 2008. New biomarkers of acute kidney injury. Crit. Care Med 36: S159–165. [DOI] [PubMed] [Google Scholar]
- 18.Cruz DN, Ronco C, and Katz N 2010. Neutrophil gelatinase-associated lipocalin: a promising biomarker for detecting cardiac surgery-associated acute kidney injury. J. Thorac. Cardiovasc. Surg 139: 1101–1106. [DOI] [PubMed] [Google Scholar]
- 19.Zhou F, Wang L, Han L, and Luo Q 2015. Diagnostic value of neutrophil gelatinase-associated lipocalin for early diagnosis of cardiac surgery-associated acute kidney injury: a meta-analysis. Eur. J. Cardiothorac. Surg 49: 746–755. [DOI] [PubMed] [Google Scholar]
- 20.Nickolas TL, Schmidt-Ott KM, Canetta P, Forster C, Singer E, Sise M, Elger A, Maarouf O, Sola-Del Valle DA, O’Rourke M, Sherman E, Lee P, Geara A, Imus P, Guddati A, Polland A, Rahman W, Elitok S, Malik N, Giglio J, El-Sayegh S, Devarajan P, Hebbar S, Saggi SJ, Hahn B, Kettritz R, Luft FC, and Barasch J 2012. Diagnostic and prognostic stratification in the emergency department using urinary biomarkers of nephron damage: a multicenter prospective cohort study. J. Am. Coll. Cardiol 59: 246–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buonafine M, Martinez-Martinez E, and Jaisser F 2018. More than a simple biomarker: the role of NGAL in cardiovascular and renal diseases. Clin. Sci. (Lond.) 132: 909–923. [DOI] [PubMed] [Google Scholar]
- 22.Yang J, Goetz D, Li JY, Wang W, Mori K, Setlik D, Du T, Erdjument-Bromage H, Tempst P, Strong R, and Barasch J 2002. An iron delivery pathway mediated by a lipocalin. Mol. Cell 10: 1045–1056. [DOI] [PubMed] [Google Scholar]
- 23.Thorsvik S, Bakke I, van Beelen Granlund A, Royset ES, Damas JK, Ostvik AE, and Sandvik AK 2018. Expression of neutrophil gelatinase-associated lipocalin (NGAL) in the gut in Crohn’s disease. Cell Tissue Res. 374: 339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chakraborty S, Kaur S, Guha S, and Batra SK 2012. The multifaceted roles of neutrophil gelatinase associated lipocalin (NGAL) in inflammation and cancer. Biochim. Biophys. Acta 1826: 129–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mori K, Lee HT, Rapoport D, Drexler IR, Foster K, Yang J, Schmidt-Ott KM, Chen X, Li JY, Weiss S, Mishra J, Cheema FH, Markowitz G, Suganami T, Sawai K, Mukoyama M, Kunis C, D’Agati V, Devarajan P, and Barasch J 2005. Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J. Clin. Invest 115: 610–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mishra J, Mori K, Ma Q, Kelly C, Yang J, Mitsnefes M, Barasch J, and Devarajan P 2004. Amelioration of Ischemic Acute Renal Injury by Neutrophil Gelatinase-Associated Lipocalin. J. Am. Soc. Nephrol 15: 3073–3082. [DOI] [PubMed] [Google Scholar]
- 27.Jung M, Brune B, Hotter G, and Sola A 2016. Macrophage-derived Lipocalin-2 contributes to ischemic resistance mechanisms by protecting from renal injury. Sci. Rep. 6: 21950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee SA, Noel S, Sadasivam M, Allaf ME, Pierorazio PM, Hamad ARA, and Rabb H 2018. Characterization of kidney CD45intCD11bintF4/80+MHCII+CX3CR1+Ly6C- “intermediate mononuclear phagocytic cells”. PLoS One 13: e0198608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, and Pachter L 2012. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc 7: 562–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Edgar R, Domrachev M, and Lash AE 2002. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 30: 207–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ascon M, Ascon DB, Liu M, Cheadle C, Sarkar C, Racusen L, Hassoun HT, and Rabb H 2009. Renal ischemia-reperfusion leads to long term infiltration of activated and effector-memory T lymphocytes. Kidney Int. 75: 526–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Livak KJ, and Schmittgen TD 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 33.Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao B, Worley PF, Kozma SC, and Powell JD 2009. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity 30: 832–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jager A, Dardalhon V, Sobel RA, Bettelli E, and Kuchroo VK 2009. Th1, Th17, and Th9 effector cells induce experimental autoimmune encephalomyelitis with different pathological phenotypes. J. Immunol 183: 7169–7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meldrum KK, Meldrum DR, Hile KL, Burnett AL, and Harken AH 2001. A novel model of ischemia in renal tubular cells which closely parallels in vivo injury. J. Surg. Res 99: 288–293. [DOI] [PubMed] [Google Scholar]
- 36.Martina MN, Bandapalle S, Rabb H, and Hamad AR 2014. Isolation of double negative alphabeta T cells from the kidney. J Vis Exp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jung M, Sola A, Hughes J, Kluth DC, Vinuesa E, Viñas JL, Pérez-Ladaga A, and Hotter G 2012. Infusion of IL-10–expressing cells protects against renal ischemia through induction of lipocalin-2. Kidney Int. 81: 969–982. [DOI] [PubMed] [Google Scholar]
- 38.Berger T, Togawa A, Duncan GS, Elia AJ, You-Ten A, Wakeham A, Fong HE, Cheung CC, and Mak TW 2006. Lipocalin 2-deficient mice exhibit increased sensitivity to Escherichia coli infection but not to ischemia-reperfusion injury. Proc. Natl. Acad. Sci. U. S. A 103: 1834–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kinsey GR, Sharma R, Huang L, Li L, Vergis AL, Ye H, Ju S-T, and Okusa MD 2009. Regulatory T Cells Suppress Innate Immunity in Kidney Ischemia-Reperfusion Injury. J. Am. Soc. Nephrol 20: 1744–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jun C, Ke W, Qingshu L, Ping L, Jun D, Jie L, Bo C, and Su M 2014. Protective effect of CD4(+)CD25(high)CD127(low) regulatory T cells in renal ischemia-reperfusion injury. Cell. Immunol 289: 106–111. [DOI] [PubMed] [Google Scholar]
- 41.La Manna G, Ghinatti G, Tazzari PL, Alviano F, Ricci F, Capelli I, Cuna V, Todeschini P, Brunocilla E, Pagliaro P, Bonsi L, and Stefoni S 2014. Neutrophil gelatinase-associated lipocalin increases HLA-G(+)/FoxP3(+) T-regulatory cell population in an in vitro model of PBMC. PLoS One 9: e89497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karlsen JR, Borregaard N, and Cowland JB 2010. Induction of neutrophil gelatinase-associated lipocalin expression by co-stimulation with interleukin-17 and tumor necrosis factor-alpha is controlled by IkappaB-zeta but neither by C/EBP-beta nor C/EBP-delta. J. Biol. Chem 285: 14088–14100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shen F, Ruddy MJ, Plamondon P, and Gaffen SL 2005. Cytokines link osteoblasts and inflammation: microarray analysis of interleukin-17- and TNF-alpha-induced genes in bone cells. J. Leukoc. Biol. 77: 388–399. [DOI] [PubMed] [Google Scholar]
- 44.Martínez-Martínez E, Buonafine M, Boukhalfa I, Ibarrola J, Fernández-Celis A, Kolkhof P, Rossignol P, Girerd N, Mulder P, López-Andrés N, Ouvrard-Pascaud A, and Jaisser F 2017. Aldosterone Target NGAL (Neutrophil Gelatinase–Associated Lipocalin) Is Involved in Cardiac Remodeling After Myocardial Infarction Through NFκB Pathway. Hypertension 70: 1148–1156. [DOI] [PubMed] [Google Scholar]
- 45.Yang B, Fan P, Xu A, Lam KS, Berger T, Mak TW, Tse HF, Yue JW, Song E, Vanhoutte PM, Sweeney G, and Wang Y 2012. Improved functional recovery to I/R injury in hearts from lipocalin-2 deficiency mice: restoration of mitochondrial function and phospholipids remodeling. Am J Transl Res 4: 60–71. [PMC free article] [PubMed] [Google Scholar]
- 46.Tarín C, Fernandez-Garcia CE, Burillo E, Blanco-Colio LM, Torres-Fonseca MM, Llamas-Granda P, Ramos-Mozo P, Martín-Ventura JL, Egido J, Pastor-Vargas C, Castejón B, Mak TW, and Berger T 2016. Lipocalin-2 deficiency or blockade protects against aortic abdominal aneurysm development in mice. Cardiovasc. Res 111: 262–273. [DOI] [PubMed] [Google Scholar]
- 47.Tarjus A, Martinez-Martinez E, Amador C, Latouche C, El Moghrabi S, Berger T, Mak TW, Fay R, Farman N, Rossignol P, Zannad F, Lopez-Andres N, and Jaisser F 2015. Neutrophil Gelatinase-Associated Lipocalin, a Novel Mineralocorticoid Biotarget, Mediates Vascular Profibrotic Effects of Mineralocorticoids. Hypertension 66: 158–166. [DOI] [PubMed] [Google Scholar]
- 48.Moschen AR, Gerner RR, Wang J, Klepsch V, Adolph TE, Reider SJ, Hackl H, Pfister A, Schilling J, Moser PL, Kempster SL, Swidsinski A, Orth Holler D, Weiss G, Baines JF, Kaser A, and Tilg H 2016. Lipocalin 2 Protects from Inflammation and Tumorigenesis Associated with Gut Microbiota Alterations. Cell Host Microbe 19: 455–469. [DOI] [PubMed] [Google Scholar]
- 49.Buonafine M, Martinez-Martinez E, Amador C, Gravez B, Ibarrola J, Fernandez-Celis A, El Moghrabi S, Rossignol P, Lopez-Andres N, and Jaisser F 2018. Neutrophil Gelatinase-Associated Lipocalin from immune cells is mandatory for aldosterone-induced cardiac remodeling and inflammation. J. Mol. Cell. Cardiol 115: 32–38. [DOI] [PubMed] [Google Scholar]
- 50.Moschen AR, Adolph TE, Gerner RR, Wieser V, and Tilg H 2017. Lipocalin-2: A Master Mediator of Intestinal and Metabolic Inflammation. Trends Endocrinol. Metab 28: 388–397. [DOI] [PubMed] [Google Scholar]
- 51.Singh V, Yeoh BS, Chassaing B, Zhang B, Saha P, Xiao X, Awasthi D, Shashidharamurthy R, Dikshit M, Gewirtz A, and Vijay-Kumar M 2016. Microbiota-Inducible Innate Immune Siderophore Binding Protein Lipocalin 2 Is Critical for Intestinal Homeostasis. Cell. Mol. Gastroenterol. Hepatol 2: 482–498.e486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marques FZ, Prestes PR, Byars SG, Ritchie SC, Wurtz P, Patel SK, Booth SA, Rana I, Minoda Y, Berzins SP, Curl CL, Bell JR, Wai B, Srivastava PM, Kangas AJ, Soininen P, Ruohonen S, Kahonen M, Lehtimaki T, Raitoharju E, Havulinna A, Perola M, Raitakari O, Salomaa V, Ala-Korpela M, Kettunen J, McGlynn M, Kelly J, Wlodek ME, Lewandowski PA, Delbridge LM, Burrell LM, Inouye M, Harrap SB, and Charchar FJ 2017. Experimental and Human Evidence for Lipocalin-2 (Neutrophil Gelatinase-Associated Lipocalin [NGAL]) in the Development of Cardiac Hypertrophy and heart failure. J Am Heart Assoc 6. [DOI] [PMC free article] [PubMed] [Google Scholar]