Abstract
Metabolic programs are rewired in cancer cells to support survival and tumor growth. Among these, recent studies have demonstrated that glutamate-oxaloacetate transaminase 1 (GOT1) plays key roles in maintaining redox homeostasis and proliferation of pancreatic ductal adenocarcinomas (PDA). This suggests that small molecule inhibitors of GOT1 could have utility for the treatment of PDA. However, the development of GOT1 inhibitors has been challenging, and no compound has yet demonstrated selectivity for GOT1-dependent cell metabolism or selective growth inhibition of PDA cell lines. In contrast, potent inhibitors that covalently bind to the transaminase cofactor pyridoxal-5’-phosphate (PLP), within the active site of the enzyme, have been reported for kynurenine aminotransferase (KAT) and gamma-aminobutyric acid aminotransferase (GABA-AT). Given the drug discovery successes with these transaminases, we aimed to identify PLP-dependent suicide substrate-type GOT1 inhibitors. Here, we demonstrate that PF-04859989, a known KAT2 inhibitor, has PLP-dependent inhibitory activity against GOT1 and shows selective growth inhibition of PDA cell lines.
Keywords: GOT1, transaminase, aminotransferase, PLP, pancreatic cancer, metabolism
1. INTRODUCTION
Glutamate-oxaloacetate transaminase 1 (GOT1) plays an essential role in amino acid metabolism, the malate-aspartate shuttle, and, as a result, the tricarboxylic acid (TCA) cycle. Under normal physiological circumstances, these pathways maintain energy homeostasis and coordinate catabolic and anabolic metabolic programs. Recent reports have detailed how GOT1-associated pathways are rewired in cancer cells to support biosynthesis, redox balance, and proliferation. And, moreover, in certain contexts, like KRAS mutant pancreas ductal adenocarcinoma (PDA) [1,2], the tumor cells become dependent on these activities [3,4,5]. The results from these studies indicate that GOT1 small molecule inhibitors may have the potential to be effective therapeutics in PDA and other cancers. However, the discovery of selective and potent GOT1 inhibitors remains a challenge. While some studies have identified small molecule GOT1 inhibitors using high throughput screening, the effects of these compounds were limited to biochemical assays and showed neither recovery from changes in GOT1-dependent cell metabolism nor selective growth inhibition of PDA cell lines [6,7].
Given this difficulty developing potent GOT1 inhibitors, we focused on the success of drug discovery among other pyridoxal-5’-phosphate (PLP)-dependent transaminases. Vigabatrin is a selective catalytic suicide inhibitor of the enzyme gamma-aminobutyric acid aminotransaminase (GABA-AT), which irreversibly binds to the active site together with the PLP cofactor. Vigabatrin has remarkable selectivity in vivo, exhibiting no effect toward other neurotransmitter systems, and it was approved as an anticonvulsant twenty years ago [9]. Similarly, gabaculine, a compound derived from plants, is also a known and selective GABA-AT inhibitor that acts by covalently binding to PLP in the active site [10,11]. Treatment with gabaculine increased endogenous GABA levels in the brain and spinal cord in vivo [12]. For kynurenine aminotransaminase II (KAT2), a brain-specific KAT, PF-04859989 and BFF-122 act as inhibitors via covalent binding to PLP [13,14]. Both compounds also exhibit in vivo activity upon subcutaneous administration or intrastriatal injection, leading to reduced kynurenate levels in the rat brain.
Based on the previous successes with covalent inhibitors of KAT2 and GABA-AT, which form a covalent adduct with PLP, we tested these drugs for GOT1 inhibitory activity as a starting point for the drug design of novel GOT1 inhibitors. Indeed, we found that PF-04859989, a known potent, irreversible inhibitor of KAT2, shows GOT1 inhibitory activity. Further, we demonstrate that PF-04859989 is a covalent inhibitor of GOT1 and that it exhibits GOT1-dependent impairment of cell metabolism and selective growth inhibition in PDA cell lines.
2. MATERIALS AND METHODS
1.1. Cell lines and reagents
PATU-8988T (ACC-162) and PATU-8902 (ACC-179) PDA cell lines were purchased from DSMZ. IMR-90 (CCL-186) was purchased from ATCC. All cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum at 37°C and 5% CO2. PF-04859989, vigabatrin, gabaculine, DMEM, oxaloacetate (OAA), PLP, NADH, L-aspartate, amino-oxyacetate (AOA), and α-ketoglutarate were purchased from Sigma-Aldrich. WST-9 and 1-methoxy PMS were purchased from Dojindo. Recombinant GOT1, GOT2, and MDH1 recombinant proteins were purchased from ATGEN. KAT2 recombinant protein was purchased from Adipogen. Kynurenic acid sodium salt was purchased from Tocris. BFF-122 was purchased from Axon Medchem. H2DCF-DA dyes were purchased from Invitrogen.
2.2. GOT1/GOT2 enzyme assay
GOT1 or GOT2 enzymatic activity was measured by utilizing the coupling reaction of malate dehydrogenase 1 (MDH1), based on a protocol described previously [7]. MDH1 catalyzes the conversion of oxaloacetate (OAA) to malate using NADH, and the total enzymatic activity of GOT1 or GOT2 and MDH1 can be monitored by measuring the decrease in NADH. The coupling enzymatic reactions were performed in 100 mM HEPES (pH 8.0) supplemented with 100 mM KCl, 1 mM DTT, and 0.1% Triton X-100. The reaction was initiated by addition of 4 mM aspartate, 0.5 mM of α-ketoglutarate and 0.25 mM NADH. Each enzymatic reaction was performed for 60 min at room temperature in a 40-μL volume. The decrease of NADH was monitored by WST-based colorimetric assay by measuring the absorbance of WST-9 formazan at 600 nm. PF-04859989 inhibitory activity against GOT1 or GOT2 was confirmed by comparing with reactions containing only MDH1 enzymatic activity, as a counter assay. The MDH1 enzymatic assay was performed using the same experimental protocol as that for GOT1 with 0.5 mM OAA and 0.25 mM NADH as substrates. In experiments using apo-GOT1 recombinant protein, GOT1 enzymatic activity was directly detected by measuring the amount of product, glutamate, by liquid chromatography–mass spectrometry (LC-MS).
2.3. KAT2 enzymatic assay
KAT2 enzymatic activity was determined by measuring the fluorescence of kynurenate, a product of the enzymatic reaction (excitation 250 nm/emission 385 nm). The assay contained 50 mM Tris-HCl (pH 8.0), 0.5 mM EDTA, 1 mM DTT and 0.03% Briji-35. The reaction was initiated by the addition of 1 mM kynurenine, 0.5 mM α-ketoglutarate, and 20 μM PLP. The enzymatic reaction was performed for 60 min at room temperature in a 20-μL volume.
2.4. Apo-GOT1 purification
Human GOT1 (Genbank accession no. NP_002070) was expressed in E. coli as a C-terminal His-tagged protein. One liter of E.coli was cultured overnight at 18°C with 1 mM of IPTG. Affinity purification was performed using Ni-NTA Superflow (QIAGEN), and further purification was performed by ion-exchange chromatography and gel filtration. The absence of PLP from the purified GOT1 protein was confirmed using UV spectral analysis and an enzyme reaction of this protein without supplementation with PLP.
2.5. Cell proliferation assay
PATU8902, PATU8988T, and IMR-90 cells were plated overnight in 384-well white-walled plates at a density of 125 cells/well. The next day, cells were treated with different concentrations of PF-04859989 for 3 days at 37°C at 5% CO2. After treatment, cell viability was measured using the Cell Titer-Glo assay (Promega).
2.6. Metabolite supplement experiment
In the growth experiment in which media were supplemented with OAA or kynurenate, PATU-8902 cells were plated in 96-well plates at a density of 1,000 cells/well. The next day, cells were treated with 25 μM PF-04859989 diluted in growth medium supplemented with vehicle (H2O), 2 mM OAA or 2 mM kynurenate. Cells were grown for 2–6 days and media were not changed throughout the experiment. Cell Titer-Glo assays were used to measure cell viability.
2.7. Reactive oxygen species (ROS) quantification using DCFDA
The DCFDA assay was performed 24 h after treatment with PF-04859989 in 12-well plates. Cells were incubated with 5 μM DCFDA at 37°C at 5% CO2 for 60 min. Excess DCFDA was removed by washing the cells twice with PBS, and the labeled cells were then trypsinized, rinsed, and resuspended in BD FACS Flow solution. DCFDA fluorescence intensity in the cells was measured and analyzed by flow cytometry.
2.8. Measurement of cellular aspartate and malate
PATU-8902 cells were seeded in 24-well Ultra-Low Attachment Plates (Corning) with different concentrations of PF-04859989. After treatment for 1 day at 37°C at 5% CO2, cells were washed five times with PBS and collected. Samples were resuspended in a mixture of MeCN/MeOH (30:70, v/v) for the protein precipitation. After shaking, they were centrifuged at 14000rpm for 10 min at 4°C. The divided supernatants were dried under N2 and reconstituted in two different derivatization reagents. Dansyl chloride derivatization for the measurement of aspartate was performed at 70°C for 10 min, and O-benzylhydroxylamine (O-BHA) derivatization for the measurement of malate was performed at room temperature for 60 min. After the reaction, Aliquots (1 μL) were analyzed by LC-MS. An ACQUITY UPLC (Waters corporation) coupled with a SYNAPT G2-S (Waters corporation) was used to perform the LC-MS analysis. MassLynx software Version 4.1 (Waters corporation) was used for data analysis.
2.9. Statistical analysis
Data are expressed as the mean ± standard deviation (S.D.). Statistical significance was examined using one-way ANOVA with Prism software. P<0.05 was considered statistically significant.
3. RESULTS
PF-04859989 inhibition of GOT1 is dependent on pre-incubation time
We first examined whether known PLP-dependent irreversible-type inhibitors of KAT2 (PF-04859989, BFF-122) or GABA-AT (Vigabatrin, gabaculine) possess GOT1 inhibitory activity (Fig.1a). The irreversible inhibition process is often slow because of a chemical reaction between the enzyme or co-factor and inhibitor [16, 17]. Based on this, we evaluated inhibitory activity using 15 min and 24 h pre-incubation times. We found that only PF-04859989 inhibited the enzymatic activity of GOT1 (Fig.1b). We also confirmed that PF-04859989 did not inhibit MDH1 alone (Fig.1c). Furthermore, inhibition of GOT1 by PF-04859989 was slow, with the inhibitory activity only observed during the 24 h pre-incubation (Fig.1b). The IC50 value was 8.0 μM for the 24 h pre-incubation (Fig.1d).
Fig.1. Time-dependent inhibition of GOT1 enzyme by PF-04859989.
(a) Chemical structure of tested aminotransferase inhibitors that covalently bind PLP. (b) Percent inhibition of GOT1 enzymatic activity by PF-04859989, BFF-122, Vigabatrin and gabaculine. The pan-transaminase inhibitor amino-oxyacetate (AOA) was used as a positive control (c) Dose-dependency of MDH1 inhibitory activity by PF-04859989 for 24h pre-incubation time. (d) Dose-dependency of GOT1 inhibitory activity by PF-04859989 for each pre-incubation time. All data indicate mean of n=3 determinations.
Inhibition of GOT1 by PF-04859989 requires PLP
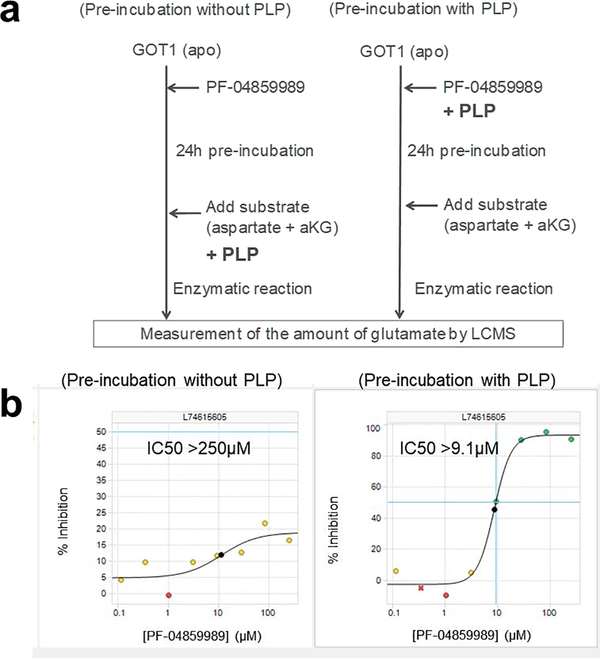
PF-04859989 was originally developed as a KAT2-selective inhibitor, and the inhibitory mechanism involved formation of a covalent adduct with the enzyme cofactor PLP [13]. We hypothesized that PF-04859989 also bound to PLP in the active site of GOT1, where the covalent adduct inhibits the enzymatic activity. To investigate this, we generated a recombinant apo-GOT1 protein without PLP in the active site. We purified the recombinant GOT1 protein and confirmed the absence of PLP in an enzymatic reaction of this protein without supplementation with PLP. Next, we evaluated the inhibitory activity of PF-04859989 against the recombinant apo-GOT1 with and without pre-incubation with PLP (Fig.2a). PF-04859989 was only able to inhibit GOT1 when PLP was included in the pre-incubation (Fig.2b). This result suggests that PF-04859989 forms an adduct with PLP and that this is required for GOT1 inhibition.
Fig.2. PLP-dependent inhibition of GOT1 by PF-04859989.
(a) Schema of the enzymatic protocol using the apo-form of GOT1 and PLP. (b) Inhibition of GOT1 by PF-04859989 with or without PLP. Graphs show the dose-dependency of the GOT1 inhibitory activity of PF-04859989 at 24 h of pre-incubation with or without PLP.
PF-04859989 impairs the growth of PDA cell lines in a GOT1 inhibition-dependent manner
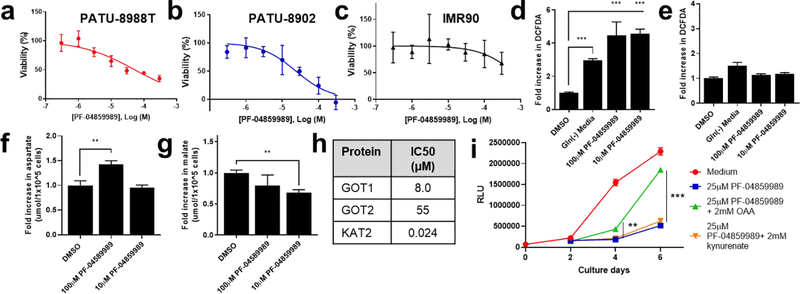
In our previous work, we demonstrated that PDA cells are dependent on GOT1 activity for proliferation using short hairpin (sh)RNA-mediated target knockdown [1,7]. Given the results of GOT1 inhibition by PF-04859989 described above, we tested the effect of PF-04859989 on PDA cell proliferation. We treated GOT1-dependent PATU-8902 or PATU-8988T cells [1] with PF-04859989 for 3 days. The growth of both cell lines was inhibited by treatment with PF-04859989 in a dose-dependent manner (Fig.3a,b). The IC50 was 24 μM and 54 μM, respectively. In contrast, PF-04859989 had lower effects at concentrations >300μM on the growth of IMR90 (Fig.3c), a human diploid fibroblast cell line that we previously found was insensitive to GOT1 inhibition [1,7]. These results suggest that PF-04859989 has growth inhibition selectively for these PDA cell lines, relative to normal fibroblasts.
Fig.3. Selective effects of PF-04859989 on PDA cells.
Viability dose-response curves for PF-04859989 on (a) PATU-8988T, (b) PATU-8902, and (c) IMR-90. Relative ROS levels in (d) PATU-8902 or (e) IMR-90 cells in the presence of Gln-free media or PF-04859989. Relative (f) aspartate or (g) malate levels in PATU-8902 cells in the presence of PF-04859989. (h) IC50 (μM) of PF-04859989 against purified GOT1, GOT2 or KAT2 in enzymatic assays with 24 hours of pre-incubation with PF-04859989. (i) Relative cell viability of PATU-8902 cells treated with PF-04859989 (25 μM) (blue line), with supplementation of OAA (2 mM) (green line) or kynurenate (2 mM) (orange line). Data are mean of n=3 determinations +/− S.D.
In our earlier work using GOT1 shRNA, we defined a role for GOT1 in the regulation of ROS [1]. This occurred through the GOT1-mediated conversion of glutamine-derived aspartate into oxaloacetate (OAA), which was subsequently converted into malate by MDH1 and then pyruvate by malic enzyme 1 (ME1). The ME1 step concomitantly generates reducing equivalents that mitigate redox stress. To determine if PF-04859989 was inhibiting GOT1 in cells, we first examined whether PF-04859989 caused an increase in ROS levels. We found that treatment of PATU-8902 cells with PF-04859989 increased ROS levels using the DCFDA assay (Fig.3d) in a manner greater than that observed with the positive control glutamine (Gln) withdrawal. In line with the defects observed in proliferation, these ROS effects were not observed in IMR90 cells at equivalent doses (Fig.3e). Additionally, we measured the levels of the GOT1 substrate, aspartate, and the downstream product of the GOT1 pathway in PDA, malate. PF-04859989 increased cellular aspartate levels following treatment at 100 μM but not 10 μM, and decreased malate levels at both 100 μM and 10 μM (Fig.3f,g). These results indicate that the activity of PF-04859989 is consistent with GOT1 inhibitory function in PDA cells.
Next, we evaluated the selectivity of PF-04859989. Consistent with previously reported results, we found that PF-04859989 showed lower inhibitory activity against the mitochondrial aspartate aminotransferase isoform GOT2, and strong inhibition of KAT2 [13] (Fig.3h). To investigate whether enzymatic inhibition of GOT1 or KAT2 affects the growth of PDA cell lines, we supplemented PF-4859989 treated PDA cells with OAA, a product of the GOT1 reaction that we previously illustrated could partially reverse the effects of genetic GOT1 inhibition [1], or kynurenate, a product of KAT2. We found that growth inhibition by PF-04859959 was partially reversed in the presence of OAA, while kynurenate had no effect on the inhibition (Fig.3i). This result supports our hypothesis that the selective growth inhibition of PDA cell lines by PF-04859989 occurs via GOT1 inhibition.
4. DISCUSSION
Pancreatic cancer cells depend on the GOT1 metabolic pathway to support their proliferation, suggesting that it may be a good drug target [1,2]. However, previous efforts by our groups have had difficulty identifying GOT1 inhibitors that display the desired selectivity in cell based models of pancreatic cancer [6,7]. Herein, we took an alternative approach to identify GOT1 inhibitors based on successes targeting other PLP-dependent transaminases. On the premise that some irreversible inhibitors are slow-acting, indicating the need for a long pre-incubation time, we showed that PF-04859989 exhibited inhibitory activity against GOT1 in a time-dependent manner (Fig.1c). Moreover, we demonstrated that PF-04859989 inhibited the growth of PDA cell lines and increased their cellular ROS levels but had a weaker effect on IMR-90, a normal fibroblast cell line (Fig.3). Unfortunately, PF-04859989 has poor pharmacokinetic properties due to rapid O-glucuronidation of the hydroxamate group, as described previously, and making it difficult to test the compound in xenograft models [18]. As such, an in vivo study will require further optimization of the pharmacokinetic profile, such as replacement of the hydroxylamine motif.
PF-04859989 showed greater inhibition of GOT1 than GOT2 in an enzymatic assay (Fig.3h). GOT1 and GOT2 exist in the cytosol and inner-membrane of the mitochondria, respectively, and the glutamate-oxaloacetate conversion reaction by the two enzymes is complementary. Therefore, it is unclear whether dual inhibition of GOT1/GOT2 activity correctly reflects the effects in cells. A previous report hypothesized that the dual inhibition of GOT1 and GOT2 by a small molecule would lead to a different profile to that obtained when a single isoform was knocked down by shRNA [1,7]. Based on this, we propose that the main reason for the selective inhibition of the proliferation of PDA cell lines is the selectivity of PF-04859989 for GOT1 versus GOT2. We also evaluated the influence of the strong KAT2 inhibitory activity by PF-04859989 on the PDA cell line PA-TU8902. Indeed, KAT2 is expressed not only in liver or brain but also in pancreas, although at relatively low levels [19]. We supplemented PF-04859989 treatment with OAA or kynurenate in the proliferation experiment with PDA cell lines and demonstrated that only supplementation with OAA was able to reverse the growth inhibition by PF-04859989, albeit partially (Fig.3i). These results suggest that the growth inhibition of PDA cell lines by PF-04859989 does not occur via inhibition of its more potent target KAT2.
In summary, we demonstrated that PF-04859989 inhibits GOT1 in a time- and PLP-dependent manner and shows selective growth inhibition of PDA cell lines, relative to a normal fibroblast line. Further discovery of PLP-dependent-type GOT1 inhibitors is expected to improve the therapeutic options as new drugs for PDA and other cancers.
HIGHLIGHTS.
A suicide inhibitor of glutamate-oxaloacetate transaminase 1 (GOT1) is identified
The GOT1 inhibitor acts by covalently modifying the pyridoxal phosphate co-factor
Pancreatic cancer cells are selectively sensitive to the GOT1 inhibitor
Proliferative defects of the GOT1 inhibitor are reversed by the GOT1 reaction product
Acknowledgements
C.A.L. is supported by a Pancreatic Cancer Action Network/AACR Pathway to Leadership award (13–70-25-LYSS); a Junior Scholar Award from The V Foundation for Cancer Research (V2016–009); a Kimmel Scholar Award from the Sidney Kimmel Foundation for Cancer Research (SKF-16–005); a 2017 AACR NextGen Grant for Transformative Cancer Research (17–20-01-LYSS); and an ACS Research Scholar Grant (RSG-18–186-01). L. C. C. is funded by Grant R35-CA197588.
Footnotes
Conflicts of Interest
T.Y., S.Y., O.K., N.T., Y.T., I.N., T.Y., and S.K. are employees of Astellas Pharma Inc., and the research reported in this manuscript was funded by Astellas Pharma Inc. L.C.C. and C.A.L. received financial support from Astellas Pharma Inc. to run these studies. C.A.L. and L.C.C. are inventors on patents pertaining to Kras regulated metabolic pathways, redox control pathways in pancreatic cancer, and targeting GOT1 as a therapeutic approach. L.C.C. owns equity in, receives compensation from, and serves on the Scientific Advisory Boards of Agios Pharmaceuticals and Petra Pharmaceuticals. L.C.C.’s laboratory also receives financial support from Petra Pharmaceuticals. Agios Pharmaceuticals is identifying metabolic pathways of cancer cells and developing drugs to inhibit such enzymes to disrupt tumor cell growth and survival.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5. REFERENCES
- [1].Son J, Lyssiotis CA, Ying H, Wang X, Hu S, Ligorio M, Perera RM, Ferrone CR, Mullarky E, Shyh-Chang N, Kang Y, Fleming JB, Bardeesy N, Asara JM, Haigis MC, DePinho RA, Cantley LC, Kimmelman AC, Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway, Nature (2013) 496 (2013) 101–105. 10.1038/nature12040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Abrego J, Gunda V, Enza V, K, Shukla SK, King RJ, Dasgupta A, Goode G, Murthy D, Yu F, Singh PK, GOT1-mediated anaplerotic glutamine metabolism regulates chronic acidosis stress in pancreatic cancer cells, Cancer Lett. 400 (2017) 37–46. 10.1016/j.canlet.2017.04.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Feld FM, Nagel PD, Weissinger SE, Welke C, Stenzinger A, Möller P, Lennerz JK, GOT1/AST1 expression status as a prognostic biomarker in pancreatic ductal adenocarcinoma, Oncotarget 6 (2015) 4516–4526. 10.18632/oncotarget.2799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zhou X, Curbo S, Li F, Krishnan S, Karlsson A, Inhibition of glutamate oxaloacetate transaminase 1 in cancer cell lines results in altered metabolism with increased dependency of glucose, BMC Cancer. 18 (2018) 559 10.1186/s12885-018-4443-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wong CC, Qian Y, Li X, Xu J, Kang W, Tong JH, To KF, Jin Y, Li W, Chen H, Go MY, Wu JL, Cheng KW, Ng SS, Sung JJ, Cai Z, Yu J, SLC25A22 Promotes Proliferation and Survival of Colorectal Cancer Cells With KRAS Mutations and Xenograft Tumor Progression in Mice via Intracellular Synthesis of Aspartate, Gastroenterology 151 (2016) 945–960. 10.1053/j.gastro.2016.07.011. [DOI] [PubMed] [Google Scholar]
- [6].Anglin J, Zavareh BB, Sander PN, Haldar D, Mullarky E, Cantley LC, Kimmelman AC, Lyssiotis CA, Lairson LL, Discovery and optimization of aspartate aminotransferase 1 inhibitors to target redox balance in pancreatic ductal adenocarcinoma, Bioorg Med Chem Lett. 28 (2018) 2675–2678. 10.1016/j.bmcl.2018.04.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Holt MC, Assar Z, Beheshti Zavareh R, Lin L, Anglin J, Mashadova O, Haldar D, Mullarky E, Kremer DM, Cantley LC, Kimmelman AC, Stein AJ, Lairson LL, Lyssiotis CA, Biochemical Characterization and Structure-Based Mutational Analysis Provide Insight into the Binding and Mechanism of Action of Novel Aspartate Aminotransferase Inhibitors, Biochemistry. 57 (2018) 6604–6614. 10.1021/acs.biochem.8b00914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sun W, Luan S, Qi C, Tong Q, Yan S, Li H, Zhang Y, Aspulvinone O, a natural inhibitor of GOT1 suppresses pancreatic ductal adenocarcinoma cells growth by interfering glutamine metabolism, Cell Commun Signal. 17 (2019) 111 10.1186/s12964-019-0425-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Tolman JA, Faulkner MA, Vigabatrin: a comprehensive review of drug properties including clinical updates following recent FDA approval, Expert Opin Pharmacother. 18 (2009) 3077–3089. 10.1517/14656560903451690. [DOI] [PubMed] [Google Scholar]
- [10].Rando RR, Bangerter FW, The Irreversible Inhibition of Mouse Brain gamma-Aminobutyric Acid (GABA)-a-Ketoglutaric Acid Transaminase by Gabaculine, J Am Chem Soc. 98 (1976) 6762–6764. 10.1021/ja00437a090 [DOI] [PubMed] [Google Scholar]
- [11].Walsh CT, Suicide substrates: mechanism-based enzyme inactivators with therapeutic potential, Trends in Biochemical Sciences 8 (1983) 254–257. 10.1016/0968-0004(83)90352-3 [DOI] [Google Scholar]
- [12].Katayama S, Irifune M, Kikuchi N, Takarada T, Shimizu Y, Endo C, Takata T, Dohi T, Sato T, Kawahara M, Increased gamma-aminobutyric acid levels in mouse brain induce loss of righting reflex, but not immobility, in response to noxious stimulation, Anesth Analg. 104 (2007) 1422–1429.doi: 10.1213/01.ane.0000261519.04083.3e [DOI] [PubMed] [Google Scholar]
- [13].Dounay AB, Anderson M, Bechle BM, Campbell BM, Claffey MM, Evdokimov A, Evrard E, Fonseca KR, Gan X, Ghosh S, Hayward MM, Horner W, Kim JY, McAllister LA, Pandit J, Paradis V, Parikh VD, Reese MR, Rong S, Salafia MA, Schuyten K, Strick CA, Tuttle JB, Valentine J, Wang H, Zawadzke LE, Verhoest PR, Discovery of Brain-Penetrant, Irreversible Kynurenine Aminotransferase II Inhibitors for Schizophrenia. ACS Med Chem Lett. 3 (2012) 187–192. 10.1021/ml200204m [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Rossi F, Valentina C, Garavaglia S, Sathyasaikumar KV, Schwarcz R, Kojima S, Okuwaki K, Ono S, Kajii Y, Rizzi M, Crystal structure-based selective targeting of the pyridoxal 5’-phosphate dependent enzyme kynurenine aminotransferase II for cognitive enhancement, J Med Chem. 53 (2010) 5684–5689. 10.1021/jm100464k [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Amori L, Guidetti P, Pellicciari R, Kajii Y, Schwarcz R, On the relationship between the two branches of the kynurenine pathway in the rat brain in vivo, J Neurochem. 109 (2009) 316325 10.1111/j.1471-4159.2009.05893.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Copeland RA, Evaluation of Enzyme Inhibitors in Drug Discovery: A Guide for Medicinal Chemists and Pharmacologists, 2nd Edition, Wiley Reviews in Hoboken, NJ: (2013) pp.1–572 ISBN: 978–1-118–48813-3 [PubMed] [Google Scholar]
- [17].McDonald AG, Tipton KF, Enzymes: Irreversible inhibition, eLS (2012) 10.1002/9780470015902.a0000601.pub2 [DOI] [Google Scholar]
- [18].Henderson JL, Sawant-Basak A, Tuttle JB, Dounay AB, McAllister LA, Pandit J, Rong S, Hou X, Bechle BM, Kim J, Parikh V, Ghosh S, Evrard E, Zawadzke LE, Salafia MA, Rago B, Obach RS, Clark A, Fonseca KR, Chang C, Verhoest PR Discovery of hydroxamate bioisosteres as KAT II inhibitors with improved oral bioavailability and pharmacokinetics. Med. Chem. Commun. 4 (2013) 125–129. 10.1039/c2md20166f [DOI] [Google Scholar]
- [19].Goh DL, Patel A, Thomas GH, Salomons GS, Schor DS, Jakobs C, Geraghty MT, Characterization of the human gene encoding alpha-aminoadipate aminotransferase (AADAT), Mol Genet Metab. 76 (2002) 172–180. 10.1016/S1096-7192(02)00037-9 [DOI] [PubMed] [Google Scholar]