Abstract
Early initiation of antiretroviral therapy (ART) in vertically HIV infected children limits the size of the virus reservoir, but whether the time of treatment initiation (TI) can durably impact host immune responses associated with HIV infection is still unknown. This study was conducted in PBMC of 20 HIV-infected virally suppressed children on ART (mean age 9.4 years), classified as Early-Treated (ET, Age at ART initiation ≤0.5yrs, n=14) or Late-Treated (LT, Age at ART initiation 1-10yrs, n=6). Frequencies and functions of Ag-specific CD4 (CD40L+) and CD8 (CD69+) T cells were evaluated by intracellular IL-2, IFN-γ, TNF-α production with IL-21 in CD4 or CD107a, Granzyme-B and Perforin in CD8 T cells following stimulation with HIV gp140 protein (ENV) or GAG peptides by multiparameter flow cytometry.
ET showed a higher proportion of cytokine-producing ENV- and GAG-specific CD4 and CD8 T cells compared to LT. In particular, ET were enriched in polyfunctional T cells. RNAseq analysis showed upregulation of immune activation pathways in LT compared to ET.
Our results suggest that timing of TI in HIV infected children has a long-term and measurable impact on the quality of the HIV-specific T-cell immune responses and transcriptional profiles of PBMC, reinforcing the importance of early TI.
Introduction
Mother to child transmission (MTCT) is the main cause of HIV infection in children.
Implementation of guidelines for prevention of MTCT with ART has greatly reduced the incidence of perinatal HIV transmission, but a sizeable number of infants continue to be infected. WHO guidelines recommend that ART should be initiated within 1 year of age in all children diagnosed with HIV, regardless of clinical staging or CD4 cell count (1).
Timing of early treatment has varied greatly in clinical studies with data for treatment initiation in vertically HIV infected children spanning from few hours after birth (2) up to 1 year of age (3). Benefits of early ART in virally controlled HIV infected individuals have been described in both adults and children, and include reduction in HIV reservoir size and prevention of disease progression. It is now well established that the earlier the treatment is initiated after infection, the greater the reduction in the reservoir in HIV infected adults (4, 5), and children (6–9). Early treatment is also associated with longer time to viral rebound after ART interruption (2, 10, 11).
The impact of timing of treatment initiation on induction of HIV-specific immune responses or the durability of HIV-specific immunity after virologic suppression is less clear and even controversial. Early treatment, within 6 months of infection in HIV infected adults, has been associated with preservation of quantity and quality of both T and B cell immune function (12–14), and enhanced recovery of CD4 T cells (15). Positive effects of early ART initiation have been noted in children as well with evidence of preservation of memory B cell responses in children treated at age <1 year (3, 16). However, there is no conclusive evidence about the persistence and more importantly about the quality of the HIV-specific T cell responses in virally suppressed early treated children.
Early treatment can also be associated with loss of HIV-specific immunity. Virus suppression due to early treatment initiation results in shorter exposure of the immune system to HIV antigens with potential negative effects on the antigen-specific immune response. Indeed, it is reported that approximately 50% of individuals treated within 3 months of age lose HIV-specific circulating antibodies and become seronegative (8, 17) Poor detection of HIV-specific response in CD8 (18, 19) and CD4 (20) T cell was reported in HIV infected children under viral control in whom ART had been initiated within 6 months of age.
A clear understanding of the impact of timing of treatment initiation on the HIV-specific memory responses in situations of controlled viremia is important for developing strategies aimed at permanent HIV remission in HIV-infected children.
Here, we applied an in vitro stimulation protocol and multi-parameter flow-cytometry based approach in PBMC of virally suppressed HIV infected young individuals in whom ART was started early (≤6 months of age) or late (>1 year) after birth. Our goal was to ascertain quantity and quality of the HIV-specific CD4 and CD8 T cell responses to two different HIV antigens (GAG and ENV). We also evaluated the transcriptional signatures in unstimulated PBMC from these groups in order to identify persistent transcriptional effects associated with time of treatment initiation.
Methods
Study Design
Peripheral blood mononuclear cells (PBMC) were collected from 20 perinatally HIV infected children (age range 2.1–15.9yrs) with durable viral control (plasma HIV-RNA<50cp/mL) being followed at Bambino Gesù Children Hospital, Rome, Italy.
Characteristics of the participants are reported in Table 1. Briefly, children were divided based on their time of ART initiation into 14 early treated (ET), for those who initiated ART at ≤6 months of age and 6 late treated (LT) for those who initiated ART at >1 year of age. ET were statistically younger than LT and with more female than LT but without differences in time on suppressive ART that was an average of 5.3 years and 5.4 years respectively for ET and LT. CD4 absolute count in ET was higher than LT. For 1 LT we did not have the exact date of ART initiation nor the exact visit date and for such reason it was dropped for all the analysis where these variables were involved. A HIV-negative group (n=6), similar in age distribution (range 2.5–26.9 year) to the HIV infected, was included only for the RNAseq experiment.
Table 1:
Characteristics of study participants. ET: participants treated within 6 months; LT: participants treated after 1 year.
| PID | Group | Gender | Timing of ART initiation (wk) | Time on suppressive ART (y) | Age (y) | HIV DNA (Copies/106 PBMC) | CD4 count (cells/uL) |
|---|---|---|---|---|---|---|---|
| PSN 14 | ET | F | 1 | 5.2 | 6.3 | 0 | 1526.9 |
| PSN 18 | ET | F | 1 | 1 | 2.1 | 0 | 974.6 |
| PSN 25 | ET | M | 1 | 7.5 | 8.4 | 77 | 1304.5 |
| PSN 10 | ET | F | 5 | 7.5 | 8.8 | 28 | 906.6 |
| PSN 12 | ET | F | 8 | 2.5 | 3.6 | 27 | 964.9 |
| PSN 23 | ET | F | 9 | 5.4 | 6.2 | 314 | 1359.2 |
| PSN 19 | ET | F | 11 | 12.8 | 14.4 | 412 | 820.1 |
| PSN 6 | ET | F | 12 | 15.2 | 16.2 | 237 | NA |
| PSN 28 | ET | F | 17 | 12.7 | 13.6 | 0 | 432.7 |
| PSN 1 | ET | F | 19 | 2.1 | 3.9 | 679 | 1057.2 |
| PSN 20 | ET | F | 22 | 4.8 | 6.3 | 241 | 841.8 |
| PSN 4 | ET | M | 24 | 1.1 | 4.5 | 195 | 1474.8 |
| PSN 9 | ET | F | 24 | 1.8 | 2.5 | 188 | 2939.0 |
| PSN 22 | ET | M | 24 | 13.9 | 15.2 | 427 | 1336.1 |
| Median (Range) | 11.5 (1–24) | 5.3 (1–15.2) | 6.3 (2.1–15.2) | 191.5 (0–676) | 1057 (432.7–2939) | ||
| H002 | LT | M | 70 | 14.6 | 15.9 | NA | 545.0 |
| H019 | LT | F | 484 | 5.4 | 14.6 | 246.9 | 505.9 |
| H020 | LT | M | >96 | NA | 15.4 | 20.99 | 856.6 |
| H042 | LT | M | 520 | 0.7 | 10.6 | NA | 770.4 |
| H045 | LT | M | 303 | 3.6 | 9.4 | 59.3 | 507.0 |
| H084 | LT | M | 295 | 6.8 | 12.4 | 72.4 | 1114.3 |
| Median (Range) | 303 (70–520) | 5.4 (0.7–14.6) | 12.4 (9.4–15.9) | 72.4 (59.3–246.99) | 657.7 (505.9–1114.3) | ||
Monoclonal antibodies (mAb)
The following fluorochrome conjugated anti-human mAb were used for flow cytometry studies: PD-1 BV421, CD40L BV605, Perforin PE-Dazzle 594 and CD8 PerCP from BioLegend (San Diego, CA); CD3 BUV496, CD4 APC-Cy7, CD69 BV650, IL-2 BV711, CXCR5 Alexa647, IFN-γ PE-Cy7, TNF-α FITC, Granzyme-B and CD27 BV480 from BD Bioscience (San Jose, CA); IL-21 PE from e-Biosciences, (San Diego, CA); and CD45RO-PE-Cy5.5 from Beckman Coulter (Fullerton, CA). Live/Dead® Fixable Blue Dead Cell Stain Kit from ThermoFisher (Boston, MA) was used to detect and exclude dead cells. All the reagents were tested and titrated before usage.
PBMC stimulation and flow cytometry staining
PBMC were thawed, and rested over night at 37 ⁰C 5% CO2. Cells were stimulated at the concentration of 5 million per milliliter for 12 hours in presence of 2 μg/mL gp140 (a gift from Dr. Kalyanaraman, ABL, Maryland, US), 2 μg/mL GAG PTE peptides (AIDS Reagent Program, Division of AIDS, NIAID, NIH: HIV-1 PTE Gag Peptide Pool from NIAID, DAIDS), 1 μg/mL of staphylococcal enterotoxins B (SEB, List Biological Laboratories, Inc.) or Medium (Negative control). At the beginning of culture the following reagents were added: 1 μg/mL costimulatory molecule anti-CD28 mAb (BD Biosciences), 0.65 ul/mL protein transport inhibitor Monensin (BD GolgiStop™) and the degranulation marker (CD107a). After 6 hours of stimulation BFA (10 μg/mL) was added to the stimulation to permit the accumulation of cytokines within the PBMC (21).
Following the culture period, cells were stained for flow cytometry using previously titrated mAb. Briefly, live cells were stained with Fixable Blue live/dead and then incubated with appropriate surface antibody mixture (CD3, CD4, CD8, CD27, CD45RO, CXCR5). Cells were then fixed and permeabilized with Cytofix/Cytoperm Buffer (BD Biosciences) and stained for intracellular molecules CD69, CD40L, IL-2, IFN-γ, TNF-α, IL-21, Granzyme-B, Perforin.
Total CD4 and CD8 T cells and subsets were analyzed for the expression of activation induced molecule CD40L or CD69 to identify the Ag-specific T cells (Fig. S1A). Ag-specific cells were further evaluated for the stimulus-induced expression of intracellular cytokines IFNγ, IL-21, IL-2 and TNFα for CD4 T cells and IFNγ, IL-2, TNFα, Perforin and Granzyme-B for CD8 T cells (Fig. 2A and 4A). Stained cells were acquired on a BD LSRFortessa™ and analysis performed using FlowJo v10.0.8 (Treestar) software. Polyfunctionality of T cells was defined as the simultaneous detection of 2 or more of the analyzed cytokines
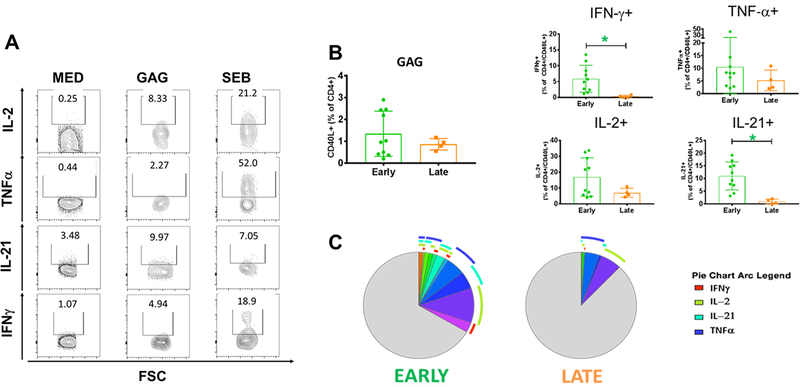
Fig. 2: HIV GAG-specific CD4T Cell Responses in Early and Late Treated Children.
The quality of the GAG-specific CD4 T cells was evaluated by the ability of these cells to produce cytokines after Ag-specific stimulation. A) Gating strategy for cytokines detection in HIV-specific CD4 T cell and total CD4 for the unstimulated control (Med). B) Frequency of total and CD40L+ CD4 T cells producing IFN-γ, TNF-α, IL-2 or IL-21 in early (green) and late (orange) treated. C) Positive and negative expression of the cytokines were combined by Boolean gating to generate all possible subsets. Each color in the pie corresponds to a specific combination of markers. The arcs surrounding the pie indicate the presence of IFN-γ (Red); IL-2 (Green); IL-21 (Azure) and TNF-α (Blue). Mann-Whitney test was used to compare the 2 groups. Statistical differences (pval≤0.05) are identified by *
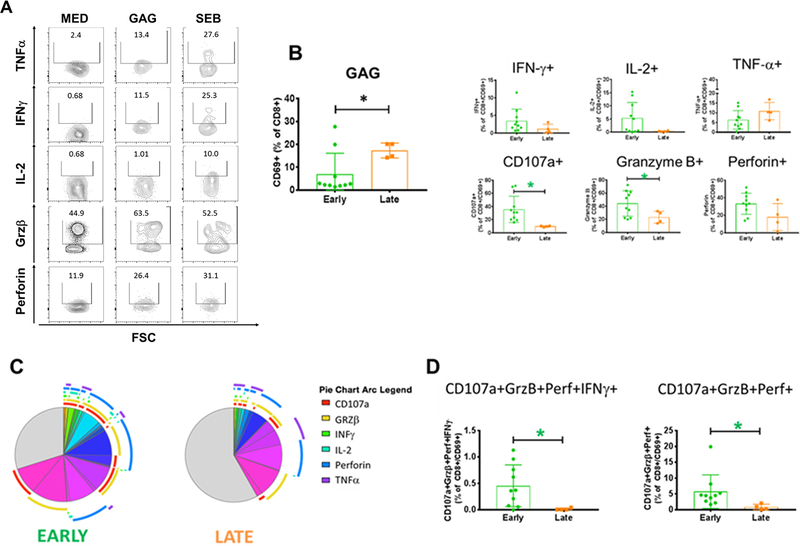
Fig. 4: HIV GAG-specific CD8T Cell Responses in Early and Late Treated Children.
The quality of the GAG-specific CD8 T cells was evaluated by the ability of these cells to produce cytokines after Ag-specific stimulation. A) Gating strategy for cytokines detection in HIV-specific CD8 T cell and total CD8 for the unstimulated control (Med). B) Frequency of total and CD69+ CD8 T cells producing IFN-γ, TNF-α, IL-2, Perforin, CD107a or Granzyme-B. in early (green) and late (orange) treated C) Positive and negative expression of the cytokines were combined by Boolean gating to generate all possible subsets. Each color in the pie corresponds to a specific combination of markers. The arcs surrounding the pie indicate the presence of CD107a (Red); Granzyme-B (Yellow); IFN-γ (Green); IL-2 (Azure); Perforin (Blue) and TNF-α (Violet). D) Cytotoxic Ag-specific CD8 T cells were identified by the simultaneous detection of CD107a, Perforin and Granzyme-B. Mann-Whitney test was used to compare the 2 groups. Statistical differences (pval≤0.05) are identified by *
HIV cell associated DNA
Total HIV-deoxyribonucleic acid (DNA) was quantified from PBMC at the enrollment time point for all patients using an in-house, real-time quantitative polymerase chain reaction assay as previously described (22). Briefly, Qiagen DNA mini blood kit was used for extraction of DNA. An ABI Prism 7500 real-time PCR instrument with Invitrogen RT-PCR reagents was used for amplification and detection of HIV-1 DNA. Results are reported as copies of HIV per million cells.
RNA sequencing
Total RNA was extracted (QIAGEN RNeasy plus mini kit) from cryopreserved PBMC and sequenced (Illumina NextSeq500; 75 bp, paired-end, 40 million reads/sample). Raw demultiplexed fastq paired-end read files were trimmed of adapters and filtered using the program skewer (23) to remove any with an average phred quality score of less than 30 or a length of less than 36 bp. Trimmed reads were aligned to the Homo Sapiens NCBI reference genome assembly version GRCh38 using the HISAT2 (24) and sorted using SAMtools (25). Aligned reads were counted and assigned to gene meta-features using the program featureCounts (26) as part of the Subread package. Count files were assessed for quality control, normalized and analyzed using an in-house pipeline utilizing the LIMMA-trend method (27) for differential gene expression testing and the GSVA (28) library for gene set variation analysis (GSVA). Accession numbers for upload to the Gene Expression Omnibus and Sequence Read Archive public databases is GSE138804 (https://www.ncbi.nlm.nih.gov/geo/).
Statistical analysis
For the flow cytometry data, all the correlations were evaluated using the Spearman test. Comparison between two groups was made using the Mann-Whitney test. Statistical tests were 2-sided and the tests were considered statistically significant with a p-val≤0.05 and performed using GraphPad Prism version 8.0.0 for Windows, GraphPad Software, La Jolla California USA, www.graphpad.com.
Boolean analysis and figures were produced using Simplified Presentation of Incredibly Complex Evaluations (SPICE, distributed by the National Institute of Allergy and Infectious Diseases, NIH, (http://exon.niaid.nih.gov/spice) ).
Differentially expressed genes (DEG) and pathways were determined by moderated t-statistics (pval≤0.05), with pathway enrichment being performed by gene set variation analysis (GSVA) in R Bioconductor.
Study Approval
Written informed consent was signed by a parent or a legal guardian.
The study was approved by the University of Miami Institutional Review Board and Bambino Gesù Children Hospital ethical committee.
Results
Early and Late treated children have similar frequencies of CD4 and CD8 T cell maturation subsets
We characterized the CD4 and CD8 T cell compartments by flow cytometry (Fig. 1A). ET and LT had similar frequencies of each of the major maturation subsets for CD4 T cells (Fig. 1B). In CD8 T cells, we observed a trend (pval 0.09) toward lower frequency of total CD8 T cells in ET. The frequency of the maturation CD8 subsets were similar between ET and LT with the exception of central memory CD8 T cells that were lower in ET (Fig. 1C).
Fig. 1: CD4 and CD8 maturational T cells subsets in ET and LT.
Flow cytometry was used to quantify the frequency of CD4 and CD8 T cells along with their maturational subsets. CD27 and CD45RO were used to identify the memory subsets. Tfh were defined as Central Memory CD4 T cells positive for CXCR5. A) Example of gating strategy for the identification of the Top) CD8 T cells maturational subsets: Naïve (CD27+CD45RO-); Central Memory (CD27+CD45RO+); Effector Memory (CD27-CD45RO+) and Effector (CD27-CD45RO-). Bottom) Gating strategy for the identification of the CD4 T cells maturational subsets: Naïve (CD27+CD45RO-); Central Memory (CD27+CD45RO+); Effector Memory (CD27-CD45RO+); Effector (CD27-CD45RO-) and Tfh (CD27+CD45RO+CXCR5+). B) Total CD4 T cells and subsets were evaluated in early (green) and late (orange) treated. C) Total CD8 T cells and subsets were evaluated in early (green) and late (orange) treated. Mann-Whitney test was used to compare the 2 groups. Statistical differences (pval≤0.05) are identified by *
Early treated children show better quality of the ENV- and GAG-specific CD4 T cells
After in vitro stimulation of PBMC in ET and LT with HIV peptides, the ENV- and GAG-responsive CD4 T cells were identified by upregulation of CD40L as marker of induced activation (Fig. S1A). The function of CD40L+ CD4 T cells was investigated by cytokine production in response to Ag-specific stimulation. We assessed IFN-γ, IL-2, and TNFα (Fig. 2A) by intracellular flow cytometry based on their key roles in achieving protective immune responses to viral infection, especially in combination (29, 30). We also included IL-21 to monitor the ability of HIV-specific Tfh-like cells to improve B cell responses (31–33). Despite similar frequency of GAG-specific CD4 T cells in both groups, total GAG-specific IL-21+ and IFN-γ+ CD4 T cells were higher in the ET. Frequencies of IL-2 or TNF-α producing GAG-specific CD4 T cells were also higher in ET but did not reach statistical significance (Fig. 2B).
In order to get a better understanding of the quality of the HIV-specific cells, we evaluated ‘polyfunctionality’ determined as the capacity of the cells to produce two or more cytokines simultaneously. Cells that showed no production of cytokines were defined as ‘paucifunctional’. First, we compared the cumulative frequencies of Ag-specific CD4 T cells producing 4, 3, 2, 1 or no cytokines in ET and LT to generate an overview of the proportions of polyfunctional and paucifunctional cells in each group. This analysis showed that GAG-specific polyfunctional CD4 T cells producing 3 or 2 cytokines were statistically higher in ET (Fig. S1B). Next, we characterized the composition of cytokine co-production using Boolean analysis to identify whether the differences between the 2 groups were attributable to the expansion of specific subsets of Ag-specific cells. We observed higher frequencies of the combination of 4, 3 or 2 cytokine producing GAG-specific CD4 T cells in ET, while LT showed higher frequency of paucifunctional GAG-specific CD4 T cells (Fig. 2C and S1B).
This analysis was repeated for ENV-specific CD4 T cells. Similar to the GAG-specific CD4 T cells observations, frequencies of ENV-specific CD4 T cells were comparable in ET and LT, but ET demonstrated higher frequency only for IL-21 producing cells with no statistical differences noted in the frequencies of IFN-γ, IL-2 or TNFα producing ENV-specific CD4 T cells (Fig. 3A). In ET, the cumulative analysis showed higher frequencies of 2 cytokine-producers in CD4 T cells responding to ENV stimulation. Boolean analysis showed a trend towards higher frequencies of combinations of 4 and 3 cytokine-producers, but single IL-21 producing ENV-specific CD4 T cell subset that was the only significantly higher in ET whereas paucifunctional CD4 T cells were again higher in LT (Fig3B and S1C).
Fig. 3: HIV ENV-specific CD4T Cell Responses in Early and Late Treated Children.
The quality of the ENV-specific CD4 T cells was evaluated by the ability of these cells to produce cytokines after Ag-specific stimulation. A) Frequency of total and CD40L+ CD4 T cells producing IFN-γ, TNF-α, IL-2 or IL-21 in early (green) and late (orange) treated. C) Positive and negative expression of the cytokines were combined by Boolean gating to generate all possible subsets. Each color in the pie corresponds to a specific combination of markers. The arcs surrounding the pie indicate the presence of IFN-γ (Red); IL-2 (Green); IL-21 (Azure) and TNF-α (Blue). Mann-Whitney test was used to compare the 2 groups. Statistical differences (pval≤0.05) are identified by *
To understand whether the differences in the HIV-specific CD4 T cell response observed between ET and LT were due to a generalized defect of the CD4 T cell compartment of the LT, we analyzed cytokine production following stimulation with the super-antigen, Staphylococcal enterotoxin B (SEB) (34). Frequencies of SEB-specific CD40L+ CD4 T cells were similar in ET and LT but the profile of the cytokines produced was different from the HIV antigen stimulation (Fig. S2A).
Finally, we evaluated the correlation between age, time under ART and HIV DNA with Total, ENV- and GAG- specific CD4 T cells but no significant association were noted (Table S1).
Overall, these results demonstrated that early treatment preserves function and quality of the ENV- and GAG-specific CD4 T cells and these differences are not due to a generalized impaired function of the CD4 T cells in LT.
Early treated children have a better quality of HIV-specific CD8 T cells despite lower frequency
The CD8 T cell compartment was also evaluated for HIV-specific cell quantity and quality. After in vitro stimulation, the ENV- and GAG-responsive CD8 T cells were identified by upregulation of CD69 as marker of induced activation (Fig. S1A). For assessment of CD8 T cell function, in addition to antiviral cytokines such as IFN-γ, IL-2, and TNF-α, we also evaluated molecules involved in CD8 T cell cytotoxic functions including the degranulation marker CD107a and the lytic molecules Perforin and Granzyme-B (Fig. 4A). Frequencies of GAG-specific CD8 T cells were higher in LT. However, ET showed statistically higher proportion of CD107a+ and Granzyme-B+ cells along with a trend of higher IL-2 (pval 0.08) and IFN-γ (pval 0.1) GAG-specific CD8 T cells (Fig. 4B). Cumulative frequency analysis showed a statistically higher proportion of 3 and 2 cytokine/marker+ cells as well as a trend of higher proportion of 5 (pval 0.1) and 4 (pval 0.054) producing GAG-specific CD8 T cells in ET (Fig. 4C and S1D). Interestingly, within the polyfunctional subsets that were higher in ET we found the cytotoxic GAG-specific CD8 T cells identified by the coexpression of CD107a, Perforin and Granzyme-B (Fig. 4D). LT showed higher frequency of paucifunctional GAG-specific CD8 T cells (Fig. 4C and S1D).
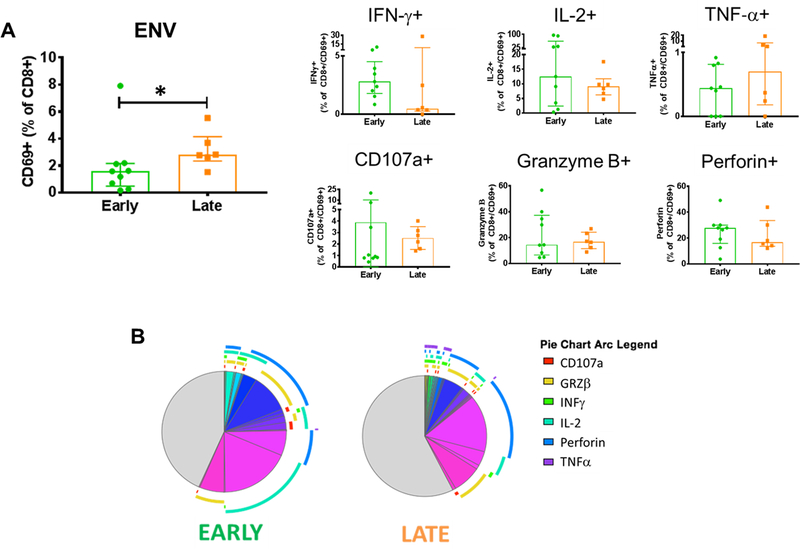
The frequency of ENV-specific CD8 T cells were also higher in LT. However, qualitative analysis showed very different results compared to the GAG-specific. In fact, only a trend toward higher IFN-γ production in ET was observed for the single producing ENV-specific CD8 T cells (Fig. 5A). Moreover, analysis of cumulative frequencies showed a trend toward more paucifunctional despite higher proportion of 5 cytokine/marker positive cells in LT (Fig. 5B and S1E). Frequencies of CD69+ CD8 T cells after SEB stimulation were not different between ET and LT, despite a trend (pval 0.07) towards higher frequencies in LT. We observed higher frequency of CD107a+CD69+ CD8 T cells in ET but no statistical differences were observed for the remaining 5 markers investigated (Fig. S2B). Total, ENV- and GAG- specific CD8 T cells show no association with age, time under ART and HIV DNA (Table S1).
Fig. 5: HIV ENV-specific CD8 T Cell Responses in Early and Late Treated Children.
The quality of the ENV-specific CD8 T cells was evaluated by the ability of these cells to produce cytokines after Ag-specific stimulation. A) Frequency of total and CD69+ CD8 T cells producing IFN-γ, TNF-α, IL-2, Perforin, CD107a or Granzyme-B in early (green) and late (orange) treated B) Positive and negative expression of the cytokines were combined by Boolean gating to generate all possible subsets. Each color in the pie corresponds to a specific combination of markers. The arcs surrounding the pie indicate the presence of CD107a (Red); Granzyme-B (Yellow); IFN-γ (Green); IL-2 (Azure); Perforin (Blue) and TNF-α (Violet). Mann-Whitney test was used to compare the 2 groups. Statistical differences (pval≤0.05) are identified by *
Overall, ET show a lower frequency but a better quality of HIV-specific CD8 T cells especially in GAG-specific CD8 T cells.
Late treated children show a unique PBMC transcriptional profile that is different from early treated and uninfected individuals
To understand the effect of timing of treatment initiation at transcriptional level, we performed RNASeq in PBMC. Sequencing was performed in 13 ET, 6 LT and 6 HIV-negative participants for comparison.
Pathway enrichment analysis of the differentially expressed genes between the participant groups revealed different pathway clusters that segregated HIV-negative from both HIV+ groups as well as ET from LT (Fig. 6). Only 4 HIV infected participants (3 ET and 1 LT) segregate separately from their groups but no peculiarity was seen in these individuals in terms of age distribution (range 62–14.6 years), time under ART (4.8–12.7 years), HIV-DNA (0–314 Copies/106 PBMC) and CD4 absolute count (432–1359 cells/uL). However, we noted that all of them are female.
Fig. 6: RNAseq shows differences into the transcriptional profile of Early and Late Treated Children.
RNA extracted from unstimulated PBMC from Early treated (azure), Late treated (pink) and HIV negative (yellow) individuals was sequenced. Top 50 differentially modulated pathways are presented here. Upregulated and down modulated pathways are shown in red and blue filled squares respectively. The 3 groups in analysis shown a very clear separation: from left to right we encounter the LT first (pink square) followed by HIV-negative (yellow square) and then ET (azure square). When we look at the top 50 differentially modulated pathways we can see 3 different clusters named: HIV up (Orange), ET down (Green) and LT up (Blue).
To have an overview of the differences in the transcriptional profiles for the 3 groups in analysis we evaluated the heatmap of the top 50 differentially expressed pathways.
LT exhibited an overall upregulation of almost all of the top 50 differentially modulated pathways. Cluster 1 (HIV up) contains pathways upregulated in ET and LT compared to HIV-negative. The pathways included in this cluster are known to be upregulated during viral infection (e.g. TCYTOTOXIC) or by HIV infection (e.g. TCAPOPTOSIS, CTLA-4, IL-17) (35, 36). Cluster 2 (ET down) is composed of pathways down modulated in ET individuals compared to LT and HIV-negative. Of interest is the presence of pathways associated with metabolism, particularly glycolysis (e.g. Leptin, PGC1A, Glycolysis, Feeder). Cluster 3 (LT up) is comprised of pathways upregulated only in LT in which the majority are strongly associated with proliferation (e.g. SHH), calcium signaling (e.g. PLCE and calcineurin) and T cell activation (e.g. NFAT). Several pathways identified in Clusters 2 and 3 (e.g. SHH, PLCE, calcineurin, NFAT, glycolysis, feeder) were also directly associated with the time of treatment initiation (Fig. S3A) and negatively with the frequency of the GAG-specific cytotoxic CD8 T cells (Fig. S3B).
Overall, these results indicate that, despite being under ART-mediated viral control for more than 5 years, vertically HIV infected children who started ART after 1 year of life maintain an activated transcriptional profile in PBMC with a distinct upregulation of pathways related to proliferation, calcium signaling and immune activation not seen in ET or HIV-negative individuals.
Discussion
Early diagnosis and ART initiation can limit the size of the HIV reservoir and preserve immune system in children with perinatal HIV infection (2, 3, 6, 7, 10, 11, 16). However, early treatment also limits viral exposure and can affect the development of an efficient Ag-specific immune response. HIV antibody in particular can be lost with early treatment but knowledge of cellular HIV-specific immunity is limited, and is largely restricted to CD8 T cells (13, 14, 18–20). The objectives of the present study were to characterize the immune and transcriptional profile along with quantitative and qualitative evaluation of ENV- and GAG-specific CD4 and CD8 T cells in ET and LT HIV infected children. To improve our ability to detect discrete differences, we selected two groups of children who had initiated ART at different ages (≤6 months or >1 year) and had maintained virus suppression for a period of 1–10 years on ART. The participants were a subset of children from the group recently reported showing relationship of HIV reservoir and seroreversion in ET children (8). Our findings indicate that ET children maintain HIV-specific CD4 and CD8 T cell responses that are qualitatively superior to LT children. Moreover, they exhibit a transcriptomic profile in PBMC that is strikingly different in comparison with the LT group. These results illustrate the long term impact of timing of ART initiation on the immune system that positions the ET perinatally HIV infected children favorably for interventions aimed at achieving a functional cure.
Viral suppression with ART has an overall restorative effect on the immune systems of HIV infected individuals (37, 38) in most instances. Indeed, our analysis showed that except for higher TCM in CD8 of LT, the ET and LT were similar in the distribution of T cell maturation subsets, and in responses to a non-HIV stimulus, SEB, suggesting that both groups have a similar potential for an effective T cell immune response. Recent data in HIV infected children, however, indicate that despite overall immune restoration after 1 year of ART and virus suppression, the extent of qualitative recovery of HIV-specific T-cell function remains uncertain (39). Using in vitro antigen stimulation followed by surface and intracellular staining, we identified CD4 and CD8 T cells that were activated by HIV and further evaluated their quality based on production of cytokines. We used HIV antigen induced upregulation of CD40L on CD4 T cells and CD69 on CD8 T cells as markers of Ag-specific activation (40, 41). This approach enhanced the dataset since we were able to detect cells that responded to stimulation but did not produce cytokines. Additionally, this approach allowed us to maximize our participant group and overall sample size by avoiding a pre-screening for specific HLA types needed for tetramer technology for the identification of Ag-specific cells (41).
Regardless of the antigen used, frequencies of HIV-specific CD4 T cells were similar in both groups, while those of CD8 T cells were lower in ET. This result is in agreement with previous observations that the frequency of HIV-specific CD8 T cells is reduced by early ART (13, 14, 18–20). Differences between ET and LT groups became evident in functional analysis of HIV-specific CD4 and CD8 T cells. Different antigens are known to elicit different responses, with ENV response arising earlier then GAG (42) but the latter is known to be more protective (43–45). In ET, both GAG-specific CD4 and CD8 T cells not only showed a higher frequency of the single cytokine producer cells, but also the frequencies of the polyfunctional cells producing combination of 2 or more cytokines were higher. Polyfunctionality of HIV-specific CD4, especially GAG-specific, and the frequency of cytotoxic HIV-specific CD8 T cells are characteristics observed in HIV viral controllers, and are associated with better protection and slower disease progression (29, 46). In addition to a lower frequency of polyfunctional HIV-specific cells, LT showed higher frequency of paucifunctional HIV-specific CD4 and CD8 T cells for both GAG and ENV stimulation. The presence of these cells in LT is of interest because despite their inability to produce any of the cytokines investigated, they were Ag-specific cells based on expression of CD40L or CD69. Although we consider these cells as paucifunctional, a more in depth investigation is required to understand whether these cells lack functionality, or whether they produced a different set of cytokines. The possibility of an exhausted phenotype also needs consideration since it was not examined. Overall, our data showed that early treatment initiation preserves HIV-specific CD4 and CD8 T cell functions that are important for control of infection.
We investigated potential links between HIV-specific CD4 and CD8 T cells and the viral reservoir in our participants but did not observe any correlation between HIV DNA and the frequency of HIV-specific CD4 or CD8 T cells. Since we also did not find any correlations between age, time of ART initiation or time on suppressive ART with frequency of HIV-specific CD4 or CD8 T cells (Table S1), we speculated that other factors could contribute in the preservation of these cells such as viral characteristics, or a specific immune profile at the time of treatment initiation. Altogether, these results suggest that maintenance of the HIV-specific T cell response is not solely related to age, HIV DNA copies or time under ART but may also be impacted by other factors.
Recently, it was shown in adults that starting ART during hyperacute infection also affects the immune transcriptome (14). To explore this in children we investigated the transcriptional profile of unstimulated PBMC from both groups and included a healthy HIV negative control group. Despite using PBMC and the absence of stimulation, we could clearly separate the 3 groups on the basis of 3 different pathways clusters. The LT up cluster of pathways showed upregulation of pathways associated with proliferation, calcium signaling and T cell immune activation in LT, suggesting that early ART initiation preserved not only the HIV-specific T cell response but the immune transcriptome as well. Interestingly, the ET down cluster showed down-modulation of pathways associated with the potential for immune activation (e.g. glycolysis pathways) compared to LT and HC. Activated cells have a higher energy demand and rely on glycolysis for sustenance (47). Thus, upregulation of pathways associated with glycolysis metabolism is considered a marker of immune activation. Additionally, HIV envelope antigens are reported to induce proliferation and a switch toward glycolysis (48, 49). The reason behind such differences in ET and LT is probably linked to a reduced systemic immune activation in ET due to lower viral replication and/or microbial translocation (50). Reduced immune activation is linked to lower exhaustion and better immune function (51). Accordingly, we observed that the majority of the pathways upregulated in LT compared to ET were also negatively associated with the frequency of the cytotoxic GAG-specific CD8 T cells. Further in depth analysis of the metabolic and molecular targets identified in this study is needed to elucidate additional relationships.
Our study had two main limitations: 1) the low number of study participants and 2) the cross-sectional study design that prevented us from gathering information about the kinetics of these cells. In fact, it is not known whether the beneficial effect of early ART initiation is in preventing the death of particular cell subsets or an indirect preservation of their function (e.g. by controlling systemic inflammation).
This study reinforces the concept that perinatally infected children treated early are potentially ideal candidates for evaluation of therapeutic interventions aimed at achieving a functional cure. Studies that further define early treatment in greater detail from birth onwards are needed for understanding long-term impact of time of treatment initiation on HIV specific immunity in virally controlled perinatally HIV infected children
Supplementary Material
Keypoints.
HIV-specific T-cell responses persist despite long term viral suppression
Timing of ART initiation impacts the quality of the HIV-specific T-cell responses
Baseline PBMC transcriptome is different in HIV infected children treated early
Acknowledgments
We acknowledge all patients and guardians who decided to participate to the study. We thank the Onco-Genomics Shared Resource at Sylvester Comprehensive Cancer Center for NGS services, Maria Pallin, Celeste M. Sanchez and Margaret Roach for technical help and Nadia Iavarone and Tamara Di Marco for clinical assistance. We thank Flow Cytometry Core Facility of the University of Miami for instrumentation and for facilitating conduct of the flow cytometry experiments. The following reagent was obtained through the AIDS Reagent Program, Division of AIDS, NIAID, NIH: HIV-1 PTE Gag Peptide Pool from NIAID, DAIDS.
“This work was supported by National Institutes of Health Grant: R01 AI127347 (to S.P.) and the EPIICAL project (http://www.epiical.org/), supported by PENTA-ID Foundation (http://penta-id.org/), funded through an independent grant by ViiV Healthcare UK. We thank the Laboratory Sciences Core of the Miami Center for AIDS Research (P30AI073961) for technical support”
Bibliography
- 1.WHO. 2016. CONSOLIDATED GUIDELINES ON THE USE OF ANTIRETROVIRAL DRUGS FOR TREATING AND PREVENTING HIV INFECTION 2016. [PubMed]
- 2.Persaud D, Gay H, Ziemniak C, Chen YH, Piatak M Jr., Chun TW, Strain M, Richman D, and Luzuriaga K 2013. Absence of detectable HIV-1 viremia after treatment cessation in an infant. N Engl J Med 369: 1828–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pensieroso S, Cagigi A, Palma P, Nilsson A, Capponi C, Freda E, Bernardi S, Thorstensson R, Chiodi F, and Rossi P 2009. Timing of HAART defines the integrity of memory B cells and the longevity of humoral responses in HIV-1 vertically-infected children. Proc Natl Acad Sci U S A 106: 7939–7944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheret A, Bacchus-Souffan C, Avettand-Fenoel V, Melard A, Nembot G, Blanc C, Samri A, Saez-Cirion A, Hocqueloux L, Lascoux-Combe C, Allavena C, Goujard C, Valantin MA, Leplatois A, Meyer L, Rouzioux C, Autran B, and Group OA-S 2015. Combined ART started during acute HIV infection protects central memory CD4+ T cells and can induce remission. J Antimicrob Chemother 70: 2108–2120. [DOI] [PubMed] [Google Scholar]
- 5.Ananworanich J, Chomont N, Eller LA, Kroon E, Tovanabutra S, Bose M, Nau M, Fletcher JLK, Tipsuk S, Vandergeeten C, O’Connell RJ, Pinyakorn S, Michael N, Phanuphak N, Robb ML, Rv, and R. S. s. groups. 2016. HIV DNA Set Point is Rapidly Established in Acute HIV Infection and Dramatically Reduced by Early ART. EBioMedicine 11: 68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rainwater-Lovett K, Ziemniak C, Watson D, Luzuriaga K, Siberry G, Petru A, Chen Y, Uprety P, McManus M, Ho YC, Lamers SL, and Persaud D 2017. Paucity of Intact Non-Induced Provirus with Early, Long-Term Antiretroviral Therapy of Perinatal HIV Infection. PLoS One 12: e0170548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinez-Bonet M, Puertas MC, Fortuny C, Ouchi D, Mellado MJ, Rojo P, Noguera-Julian A, Munoz-Fernandez MA, and Martinez-Picado J 2015. Establishment and Replenishment of the Viral Reservoir in Perinatally HIV-1-infected Children Initiating Very Early Antiretroviral Therapy. Clin Infect Dis 61: 1169–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rocca S, Zangari P, Cotugno N, De Rossi A, Ferns B, Petricone D, Rinaldi S, Giaquinto C, Bernardi S, Rojo P, Rossi P, Pahwa S, Nastouli E, Palma P, and Consortium E 2018. Human Immunodeficiency Virus (HIV)-Antibody Repertoire Estimates Reservoir Size and Time of Antiretroviral Therapy Initiation in Virally Suppressed Perinatally HIV-Infected Children. J Pediatric Infect Dis Soc. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuhn L, Paximadis M, Da Costa Dias B, Loubser S, Strehlau R, Patel F, Shiau S, Coovadia A, Abrams EJ, and Tiemessen CT 2018. Age at antiretroviral therapy initiation and cell-associated HIV-1 DNA levels in HIV-1-infected children. PLoS One 13: e0195514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cotton MF, Violari A, Otwombe K, Panchia R, Dobbels E, Rabie H, Josipovic D, Liberty A, Lazarus E, Innes S, van Rensburg AJ, Pelser W, Truter H, Madhi SA, Handelsman E, Jean-Philippe P, McIntyre JA, Gibb DM, Babiker AG, and Team CS 2013. Early time-limited antiretroviral therapy versus deferred therapy in South African infants infected with HIV: results from the children with HIV early antiretroviral (CHER) randomised trial. Lancet 382: 1555–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saez-Cirion A, Bacchus C, Hocqueloux L, Avettand-Fenoel V, Girault I, Lecuroux C, Potard V, Versmisse P, Melard A, Prazuck T, Descours B, Guergnon J, Viard JP, Boufassa F, Lambotte O, Goujard C, Meyer L, Costagliola D, Venet A, Pancino G, Autran B, Rouzioux C, and Group AVS 2013. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog 9: e1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moir S, Buckner CM, Ho J, Wang W, Chen J, Waldner AJ, Posada JG, Kardava L, O’Shea MA, Kottilil S, Chun TW, Proschan MA, and Fauci AS 2010. B cells in early and chronic HIV infection: evidence for preservation of immune function associated with early initiation of antiretroviral therapy. Blood 116: 5571–5579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Macatangay BJ, and Rinaldo CR 2015. Preserving HIV-specific T cell responses: does timing of antiretroviral therapy help? Curr Opin HIV AIDS 10: 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ndhlovu ZM, Kazer SW, Nkosi T, Ogunshola F, Muema DM, Anmole G, Swann SA, Moodley A, Dong K, Reddy T, Brockman MA, Shalek AK, Ndung’u T, and Walker BD 2019. Augmentation of HIV-specific T cell function by immediate treatment of hyperacute HIV-1 infection. Sci Transl Med 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Le T, Wright EJ, Smith DM, He W, Catano G, Okulicz JF, Young JA, Clark RA, Richman DD, Little SJ, and Ahuja SK 2013. Enhanced CD4+ T-cell recovery with earlier HIV-1 antiretroviral therapy. N Engl J Med 368: 218–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cagigi A, Rinaldi S, Cotugno N, Manno EC, Santilli V, Mora N, Zangari P, Aquilani A, Tchidjou KH, Giaquinto C, Bernardi S, Rossi P, and Palma P 2014. Early highly active antiretroviral therapy enhances B-cell longevity: a 5 year follow up. Pediatr Infect Dis J 33: e126–131. [DOI] [PubMed] [Google Scholar]
- 17.Persaud D, Patel K, Karalius B, Rainwater-Lovett K, Ziemniak C, Ellis A, Chen YH, Richman D, Siberry GK, Van Dyke RB, Burchett S, Seage GR 3rd, Luzuriaga K, and Pediatric HIVACS 2014. Influence of age at virologic control on peripheral blood human immunodeficiency virus reservoir size and serostatus in perinatally infected adolescents. JAMA Pediatr 168: 1138–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scott ZA, Chadwick EG, Gibson LL, Catalina MD, McManus MM, Yogev R, Palumbo P, Sullivan JL, Britto P, Gay H, and Luzuriaga K 2001. Infrequent Detection of HIV-1-Specific, But Not Cytomegalovirus-Specific, CD8+ T Cell Responses in Young HIV-1-Infected Infants. The Journal of Immunology 167: 7134–7140. [DOI] [PubMed] [Google Scholar]
- 19.Jones RB, and Walker BD 2016. HIV-specific CD8(+) T cells and HIV eradication. J Clin Invest 126: 455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ananworanich J, Puthanakit T, Suntarattiwong P, Chokephaibulkit K, Kerr SJ, Fromentin R, Bakeman W, Intasan J, Mahanontharit A, Sirivichayakul S, Chomont N, and Group H-NS 2014. Reduced markers of HIV persistence and restricted HIV-specific immune responses after early antiretroviral therapy in children. AIDS 28: 1015–1020. [DOI] [PubMed] [Google Scholar]
- 21.Lamoreaux L, Roederer M, and Koup R 2006. Intracellular cytokine optimization and standard operating procedure. Nat Protoc 1: 1507–1516. [DOI] [PubMed] [Google Scholar]
- 22.Butler K, Inshaw J, Ford D, Bernays S, Scott K, Kenny J, Klein N, Turkova A, Harper L, Nastouli E, Paparini S, Choudhury R, Rhodes T, Babiker A, and Gibb D 2016. BREATHER (PENTA 16) short-cycle therapy (SCT) (5 days on/2 days off) in young people with chronic human immunodeficiency virus infection: an open, randomised, parallel-group Phase II/III trial. Health Technol Assess 20: 1–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang H, Lei R, Ding SW, and Zhu S 2014. Skewer: a fast and accurate adapter trimmer for next-generation sequencing paired-end reads. BMC Bioinformatics 15: 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim D, Langmead B, and Salzberg SL 2015. HISAT: a fast spliced aligner with low memory requirements. Nat Methods 12: 357–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, and S. Genome Project Data Processing. 2009. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25: 2078–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liao Y, Smyth GK, and Shi W 2014. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30: 923–930. [DOI] [PubMed] [Google Scholar]
- 27.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, and Smyth GK 2015. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 43: e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanzelmann S, Castelo R, and Guinney J 2013. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinformatics 14: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Braeckel E, Desombere I, Clement F, Vandekerckhove L, Verhofstede C, Vogelaers D, and Leroux-Roels G 2013. Polyfunctional CD4(+) T cell responses in HIV-1-infected viral controllers compared with those in healthy recipients of an adjuvanted polyprotein HIV-1 vaccine. Vaccine 31: 3739–3746. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto T, Iwamoto N, Yamamoto H, Tsukamoto T, Kuwano T, Takeda A, Kawada M, Tsunetsugu-Yokota Y, and Matano T 2009. Polyfunctional CD4+ T-cell induction in neutralizing antibody-triggered control of simian immunodeficiency virus infection. J Virol 83: 5514–5524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yue FY, Lo C, Sakhdari A, Lee EY, Kovacs CM, Benko E, Liu J, Song H, Jones RB, Sheth P, Chege D, Kaul R, and Ostrowski MA 2010. HIV-specific IL-21 producing CD4+ T cells are induced in acute and chronic progressive HIV infection and are associated with relative viral control. J Immunol 185: 498–506. [DOI] [PubMed] [Google Scholar]
- 32.Strbo N, de Armas L, Liu H, Kolber MA, Lichtenheld M, and Pahwa S 2008. IL-21 augments natural killer effector functions in chronically HIV-infected individuals. AIDS 22: 1551–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adoro S, Cubillos-Ruiz JR, Chen X, Deruaz M, Vrbanac VD, Song M, Park S, Murooka TT, Dudek TE, Luster AD, Tager AM, Streeck H, Bowman B, Walker BD, Kwon DS, Lazarevic V, and Glimcher LH 2015. IL-21 induces antiviral microRNA-29 in CD4 T cells to limit HIV-1 infection. Nat Commun 6: 7562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Papageorgiou AC, Tranter HS, and Acharya KR 1998. Crystal structure of microbial superantigen staphylococcal enterotoxin B at 1.5 A resolution: implications for superantigen recognition by MHC class II molecules and T-cell receptors. J Mol Biol 277: 61–79. [DOI] [PubMed] [Google Scholar]
- 35.Kaufmann DE, Kavanagh DG, Pereyra F, Zaunders JJ, Mackey EW, Miura T, Palmer S, Brockman M, Rathod A, Piechocka-Trocha A, Baker B, Zhu B, Le Gall S, Waring MT, Ahern R, Moss K, Kelleher AD, Coffin JM, Freeman GJ, Rosenberg ES, and Walker BD 2007. Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nat Immunol 8: 1246–1254. [DOI] [PubMed] [Google Scholar]
- 36.Johnson TP, Patel K, Johnson KR, Maric D, Calabresi PA, Hasbun R, and Nath A 2013. Induction of IL-17 and nonclassical T-cell activation by HIV-Tat protein. Proc Natl Acad Sci U S A 110: 13588–13593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gibb DM, Newberry A, Klein N, de Rossi A, Grosch-Woerner I, and Babiker A 2000. Immune repopulation after HAART in previously untreated HIV-1-infected children. Paediatric European Network for Treatment of AIDS (PENTA) Steering Committee. Lancet 355: 1331–1332. [DOI] [PubMed] [Google Scholar]
- 38.Collaboration of Observational HIVERESG, Sabin CA, Smith CJ, d’Arminio Monforte A, Battegay M, Gabiano C, Galli L, Geelen S, Gibb D, Guiguet M, Judd A, Leport C, Dabis F, Pantazis N, Porter K, Raffi F, Thorne C, Torti C, Walker S, Warszawski J, Wintergerst U, Chene G, and Lundgren J 2008. Response to combination antiretroviral therapy: variation by age. AIDS 22: 1463–1473. [DOI] [PubMed] [Google Scholar]
- 39.Muenchhoff M, Adland E, Roider J, Kloverpris H, Leslie A, Boehm S, Keppler OT, Ndung’u T, and Goulder PJR 2019. Differential Pathogen-Specific Immune Reconstitution in Antiretroviral Therapy-Treated Human Immunodeficiency Virus-Infected Children. J Infect Dis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cohen GB, Kaur A, and Johnson RP 2005. Isolation of viable antigen-specific CD4 T cells by CD40L surface trapping. J Immunol Methods 302: 103–115. [DOI] [PubMed] [Google Scholar]
- 41.Frentsch M, Arbach O, Kirchhoff D, Moewes B, Worm M, Rothe M, Scheffold A, and Thiel A 2005. Direct access to CD4+ T cells specific for defined antigens according to CD154 expression. Nat Med 11: 1118–1124. [DOI] [PubMed] [Google Scholar]
- 42.Malhotra U, Holte S, Zhu T, Delpit E, Huntsberry C, Sette A, Shankarappa R, Maenza J, Corey L, and McElrath MJ 2003. Early Induction and Maintenance of Env-Specific T-Helper Cells following Human Immunodeficiency Virus Type 1 Infection. Journal of Virology 77: 2663–2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schieffer M, Jessen HK, Oster AF, Pissani F, Soghoian DZ, Lu R, Jessen AB, Zedlack C, Schultz BT, Davis I, Ranasinghe S, Rosenberg ES, Alter G, Schumann RR, and Streeck H 2014. Induction of Gag-specific CD4 T cell responses during acute HIV infection is associated with improved viral control. J Virol 88: 7357–7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ranasinghe S, Flanders M, Cutler S, Soghoian DZ, Ghebremichael M, Davis I, Lindqvist M, Pereyra F, Walker BD, Heckerman D, and Streeck H 2012. HIV-specific CD4 T cell responses to different viral proteins have discordant associations with viral load and clinical outcome. J Virol 86: 277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nabi G, Genannt Bonsmann MS, Tenbusch M, Gardt O, Barouch DH, Temchura V, and Uberla K 2013. GagPol-specific CD4(+) T-cells increase the antibody response to Env by intrastructural help. Retrovirology 10: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, Lederman MM, Benito JM, Goepfert PA, Connors M, Roederer M, and Koup RA 2006. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8(+) T cells. Blood 107: 4781–4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Menk AV, Scharping NE, Moreci RS, Zeng X, Guy C, Salvatore S, Bae H, Xie J, Young HA, Wendell SG, and Delgoffe GM 2018. Early TCR Signaling Induces Rapid Aerobic Glycolysis Enabling Distinct Acute T Cell Effector Functions. Cell Rep 22: 1509–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Valentin-Guillama G, Lopez S, Kucheryavykh YV, Chorna NE, Perez J, Ortiz-Rivera J, Inyushin M, Makarov V, Valentin-Acevedo A, Quinones-Hinojosa A, Boukli N, and Kucheryavykh LY 2018. HIV-1 Envelope Protein gp120 Promotes Proliferation and the Activation of Glycolysis in Glioma Cell. Cancers (Basel) 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ahmed D, Roy D, and Cassol E 2018. Examining Relationships between Metabolism and Persistent Inflammation in HIV Patients on Antiretroviral Therapy. Mediators Inflamm 2018: 6238978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paiardini M, and Muller-Trutwin M 2013. HIV-associated chronic immune activation. Immunol Rev 254: 78–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eckard AR, Rosebush JC, Lee ST, O’Riordan MA, Habib JG, Daniels JE, Labbato D, Uribe-Leitz M, Chahroudi A, and McComsey GA 2016. Increased Immune Activation and Exhaustion in HIV-infected Youth. Pediatr Infect Dis J 35: e370–e377. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.