Can polarity-inverted membranes self-assemble on Titan, and what does it mean for astrobiology under cryogenic conditions?
Abstract
The environmental and chemical limits of life are two of the most central questions in astrobiology. Our understanding of life’s boundaries has implications on the efficacy of biosignature identification in exoplanet atmospheres and in the solar system. The lipid bilayer membrane is one of the central prerequisites for life as we know it. Previous studies based on molecular dynamics simulations have suggested that polarity-inverted membranes, azotosomes, made up of small nitrogen-containing molecules, are kinetically persistent and may function on cryogenic liquid hydrocarbon worlds, such as Saturn’s moon Titan. We here take the next step and evaluate the thermodynamic viability of azotosome formation. Quantum mechanical calculations predict that azotosomes are not viable candidates for self-assembly akin to lipid bilayers in liquid water. We argue that cell membranes may be unnecessary for hypothetical astrobiology under stringent anhydrous and low-temperature conditions akin to those of Titan.
INTRODUCTION
Saturn’s moon Titan features rich atmospheric chemistry and a dynamic surface morphology that is driven by seasonal rainfall and cycling of predominately methane and ethane (1). Hydrocarbon lakes and seas have been observed near the polar regions of Titan (2), drawing comparisons with the hydrologic cycle of Earth and its presumed relevance for the origin of life (3). However, the surface conditions of Titan are a frigid 90 to 94 K. Moreover, in contrast to Earth, Titan’s outermost surface is covered by products of the atmospheric photochemistry that are likely to be essentially free of oxygen. A frozen water ice crust is suspected underneath the outermost organic layer. Proposed as a strict test case for the limits of life (3), Titan offers a unique opportunity to explore just how far toward chemical complexity nature can proceed without liquid water, at low temperature, provided time scales nearing the age of the solar system.
One bottleneck for chemical reactivity on Titan is the lack of thermal energy (kT = 0.75 kJ/mol at 90 K). Sunlight is one energy source (~0.4 W/m2) available on the surface of Titan that might allow for chemistry to occur (4). Several major products of the atmospheric photochemistry, such as acetylene, hydrogen cyanide, and molecular hydrogen (5, 6), also carry a substantial amount of chemical energy. At the surface, these chemicals might drive exothermic reactions and, it has been speculated, possibly support metabolism in methanogenic forms of life (7–9). Albeit singularly unlikely, the mere possibility of these processes raises questions regarding the factual limits of biology (10). We here address the likelihood of abiotic formation of cell membranes, one of several assumed prerequisites for the origin of life (11, 12), on worlds such as Titan.
Cell membranes are often considered an essential prerequisite for life as we know it (Fig. 1) (13, 14). Living systems on Earth rely on lipid bilayer membranes to protect the delicate molecular machinery needed for metabolism and replication. The cell membrane also has several other important functions ranging from selective uptake of nutrients and ions to intercellular communication and waste disposal (15). By extrapolation, it is reasonable to assume that some sort of compartmentalization should be important also for life outside of Earth. One of the earliest works on alternative compartmentalization is by Oparin, who suggested that coacervate colloidal droplets might be predecessors to the cell (16). This option for protocells is controversial but under active study (17, 18). The premise of compartmentalization as central to life led Stevenson et al. (19) to suggest the fascinating possibility of so-called azotosomes on Titan. Azotosomes are defined as membranes made up of small molecules with a nitrogen head group followed by a hydrocarbon end group. In contrast to normal lipid membranes in water, azotosome membranes display an inverted polarity, with hydrophobic groups on the outside (Fig. 1). Molecular dynamics simulations in cryogenic methane have been used to predict that these structures, if made from acrylonitrile (C2H3CN), would have a similar elasticity as normal lipid bilayer membranes in aqueous solution. Simulations have also indicated that the proposed structure would be kinetically persistent near 90 K, with respect to the removal of one acrylonitrile monomer (19).
Fig. 1. Membranes on different worlds?
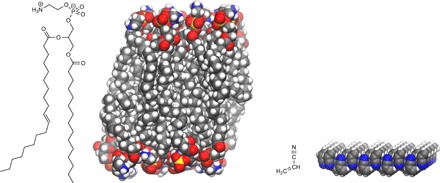
(Left) Model of a phosphatidylethanolamine bilayer, one main component of the inner bacterial membrane (42). (Right) An azotosome membrane, a theoretical structure made from acrylonitrile that exhibits an inverted polarity compared to normal lipid bilayers. Azotosome membranes have been suggested to allow for cell-like vesicles in cryogenic (90 K) hydrocarbon liquids that are present on Saturn’s moon Titan (19).
The possibility of azotosomes has spurred discussions regarding the limits of life (20) and even inspired the synthesis of different kinds of reversed surfactants operable under ambient Earth conditions (21). Only 2 years after the original prediction, an impressive detection of acrylonitrile on Titan using the Atacama Large Millimeter/submillimeter Array was announced (22). In the latter publication, it was suggested that “The corresponding production rate of vinyl cyanide and its saturation mole fraction imply the availability of sufficient dissolved material to form ~107 cell membranes/cm3 in Titan’s sea Ligeia Mare.” In light of the potential importance of these predictions and statements, it is important to return and scrutinize the likelihood of azotosomes on Titan.
Both abiotic and biological normal membranes and micelles, such as the one shown at the left in Fig. 1, form through spontaneous self-assembly processes that are driven by favorable thermodynamics (23, 24). The resulting supramolecular aggregates form spontaneously because they correspond to, or lie near, the thermodynamic ground state. That is, their formation proceeds from different rearrangements of the constituent molecules that have a higher Gibbs energy. The question we ask here is whether or not the proposed azotosome membrane is similarly viable on thermodynamic grounds, just like normal membranes and micelles are. The seminal work by Stevenson et al. (19) presented estimates to the kinetic persistence of azotosomes. To complement this picture, we here present thermodynamic stability estimates, derived from quantum chemical calculations. Following a discussion of our results, we address their implications for hypothetical exobiology under the stringent thermodynamic conditions on worlds such as Titan.
RESULTS AND DISCUSSION
One basis for the “lipid world” or “cells-first” hypothesis (13, 14, 25), in which abiotic formation of membranes contributed to the emergence of life, is that lipids in water spontaneously self-assemble into supramolecular structures, such as membranes and micelles, above a critical concentration. The Gibbs energy for transferring a lipid monomer from the aqueous phase into a micelle has been measured as ΔG ≈ −4.2 kJ/mol for each CH2 group in a lipid chain (23, 24). This means that, for a small lipid monomer with six CH2 groups, the energy stabilization is −25 kJ/mol monomer added to the membrane. For a longer chain with 16 CH2 groups, the micelle formation process in water is thermodynamically downhill with approximately −70 kJ/mol of added lipid monomer. For comparison, −70 kJ/mol corresponds to 20% of the energy of a carbon–carbon single bond or 30 kBT at 298 K.
For the self-assembly of azotosomes on Titan to occur spontaneously, the envisioned structure would need to be not only kinetically persistent, as was previously shown computationally (19), but also thermodynamically lower in energy than the corresponding molecular crystal (the molecular ice). The molecular ice is our contender for acrylonitrile self-assembly because (i) polar small molecules have very low solubility in cryogenic liquid methane (26) and (ii) the expected thermodynamic ground state for any molecule larger than ethane in these conditions is a crystalline solid. Yokoyama and Ohashi (27) have determined the experimental crystal structure of acrylonitrile using x-ray diffraction at 153 K. The reported crystal structure is disordered and corresponds to four well-defined structures in which acrylonitrile molecules have different orientations in the crystal lattice (these P212121, Pna21, P21/a, and P21/n phases are shown in the Supplementary Materials). This orientational disorder is not uncommon in crystals of small and weakly polar molecules, and often disappears at lower measurement temperatures. Attempts are ongoing to determine the crystal structure at lower temperatures (28).
We have applied quantum mechanics in the form of dispersion-corrected density functional theory (DFT) to calculate the energy of the four phases of acrylonitrile ice that are commensurate with the experimental diffraction data. The energy difference calculated between the possible ice structures is small, ~1 kJ/mol of acrylonitrile, and is in accord with a slight difference in molecular packing and the disorder observed in the structure determination. Of the four possibilities, the Pna21 phase shown in Fig. 2 is calculated to be of lowest energy.
Fig. 2. Quantum chemical predictions on membrane stability.
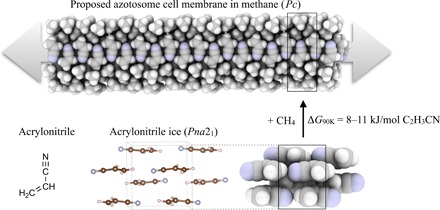
The relative free energy of the azotosome and acrylonitrile ice. Quantum mechanical calculations predict that the azotosome is not a thermodynamically viable candidate for self-assembly of cell-like membranes on Titan. The necessary building block acrylonitrile will preferentially form the molecular ice. Crystal symmetries of the considered phases are shown within parenthesis.
Also shown in Fig. 2 is the geometrically relaxed structure of the previously proposed azotosome membrane in methane. Our DFT calculations have confirmed the absence of imaginary phonon modes, which ensures the dynamic stability of the structure (see the Supplementary Materials). The dynamic stability of the membrane was additionally confirmed by a DFT-based quantum molecular dynamics simulation in liquid methane at 90 K (see Materials and Methods). The membrane in our DFT simulation closely resembles the structure optimized at 0 K and the structure presented by Stevenson et al. (19). That is, the azotosome does represent a local minimum on the potential energy surface. However, our calculations also predict the azotosome to be thermodynamically unstable with respect to the molecular Pna21 ice structure by 8 to 11 kJ/mol of acrylonitrile at 90 K. The range of this estimate is dependent on the type of DFT method used. Our calculations, which are detailed in Materials and Methods, account for thermal and entropic effects at Titan surface-relevant conditions and include an explicit consideration of dispersion interactions with the surrounding methane environment. Without the surrounding methane, the azotosome membrane would lie even higher in energy, 14 to 17 kJ/mol of acrylonitrile, compared to the molecular ice.
Whereas one could consider environments other than methane facilitating the self-assembly of azotosomes, the nonpolar vinyl groups on the membrane’s surface limit these interactions to weak dispersion effects. These interactions are unlikely to be of substantially greater strength than what is calculated here for methane. The problem of thermodynamics for life’s origin is not unique to Titan. For example, that the Gibbs energy requirements for macromolecular formation are reduced on surfaces is well known, and “surface life” has been considered one possible first step in life’s evolution on Earth (14, 29).
The low temperature on the surface of Titan ensures that entropic corrections, −TΔS contributions to the Gibbs energy, are small. Thermal and entropic effects are calculated to lower the energy of azotosome membrane relative to the ice by only 2 kJ/mol of acrylonitrile at 90 K and do not affect our conclusions. Because several thousands of acrylonitrile monomers will need to come together to form an azotosome, the energy difference of >8 kJ/mol of added monomer is cumulative and substantial. In our most conservative estimate, an azotosome consisting of only 1000 acrylonitrile monomers would lie >8 MJ/mol higher in energy compared to the corresponding molecular ice. Such a high energy conformational state is statistically impossible to reach in a Boltzmann distribution at 90 K. That is, whereas the kinetic or dynamic stability of azotosomes is not in question, our calculations demonstrate that the proposed structure cannot self-assemble for thermodynamic reasons.
Our calculations here are limited to the evaluation of acrylonitrile-based azotosome self-assembly under Titan relevant conditions. This focus is motivated by molecular dynamics simulations by Stevenson et al. (19) that have compared azotosomes built up from several different kinds of molecular building blocks. The acrylonitrile-based azotosome was predicted to have sufficient kinetic stability to ensure long-term persistence near 90 K. In comparison, hypothetical membrane structures made of other larger molecules such as acetonitrile, butanenitrile, hexanenitrile, aminopropane, aminobutane, propionitrile, pentanenitrile, aminopentane, and hexylamine were predicted to be considerably less kinetically stable or unstable. Combined, these results do not point to a plausible self-assembly route of cryogenic operable membranes. However, we cannot rule out the possibility that other polarity-inverted membranes, built from more strongly interacting constituents, may be relevant in warmer hydrocarbon environments.
Speculations on the implications for life’s chances on Titan-like worlds
Does the absence of azotosomes, or other cell membranes, mean that there can be no life-governing processes under cryogenic conditions? Of course, life under these stringent conditions is supremely unlikely. However, if we, for the sake of argument, consider life on cold hydrocarbon worlds such as Titan, then it is not necessarily so that this life would require cell membranes.
The role and evolution of the cell membrane for life on Earth is an active topic of research (13, 14, 18, 25). One reason for membranes in life as we know it is to ensure local entropy reduction and to safeguard the precious contents of the cell from being diluted and rendered inactive in a much larger body of warm liquid water. Focusing now on the presumed cell content, not membranes, it is reasonable to assume that polymeric chemistry and some kind of covalently bound macromolecules are an absolute requirement for life (12, 30). However, on Titan, any hypothetical life-bearing macromolecule or crucial machinery of a life form will exist in the solid state and never risk destruction by dissolution (10). The question is then whether these biomolecules would benefit from a cell membrane. Already rendered immobile by the low temperature, biological macromolecules on Titan would need to rely on the diffusion of small energetic molecules, such as H2, C2H2, or HCN, to reach them in order for growth or replication to ensue. Transport of these molecules might proceed in the atmosphere or through the surrounding methane/ethane environment. A membrane would likely hinder this beneficial diffusion. Similarly, a membrane would likely hinder necessary removal of waste products of metabolism, such as methane and nitrogen, in the opposite direction.
The role of a hypothetical cell membrane as protection against a harmful chemical surrounding can also be questioned under Titan’s conditions. This is because of the exponential dependence on chemical rates with temperature. Typical carbon–carbon and carbon–oxygen single bonds have strengths of approximately 330 kJ/mol, which is why life as we know it on Earth is reliant on efficient catalysts (enzymes) to lower barriers of activation to 55 to 110 kJ/mol and allow for biochemistry. In order for a chemical reaction to proceed at a reasonable rate (milliseconds → years) near Titan’s 90 to 94 K surface, barrier heights need to lie in the range of 17 to 35 kJ/mol (these estimates have been derived from transition state theory by assuming first-order rate kinetics and omit consideration of tunneling). Because of the narrower energetic range for thermally driven reaction pathways, there are arguably fewer options for reactivity that might damage macromolecules on Titan compared to on Earth (10).
CONCLUSIONS
The azotosome, proposed to allow for cryogenically operable membranes in liquid methane, is intriguing, as it challenges our principal understanding of the limits of biology. The azotosome proposal has brought to the fore the necessity in computational astrobiology of following up predictions of properties with identification of plausible formation routes, whenever possible. Arriving at specific predictions of chemistry that might support biological processes under the stringent kinetic, thermodynamic, and environmental constraints on worlds such as Titan is exceedingly difficult. The challenge of reliably modeling both properties and formation routes (kinetics and thermodynamics) naturally increases with the growing complexity of the material in question. We have here evaluated the thermodynamic stability of the azotosome membrane using DFT calculations. Our calculations reveal that, whereas the azotosome membrane might be kinetically persistent, the structure is not thermodynamically feasible. Hence, it is not a viable candidate for self-assembly akin to lipid bilayers in liquid water. Because of several factors related to Titan’s anhydrous and low-temperature environment, we argue that the need for cell membranes in hypothetical astrobiology under these conditions is unlikely. Whereas computational predictions on the existence or nonexistence of azotosome membranes can be subject to experimental testing, speculations on the factual environmental limits of prebiotic chemistry and biology will, for the time being, be just that—speculation. Nevertheless, careful computational exploration of proposed prebiotic and biological structures and processes, and their plausibility, can help to guide future in situ sampling of the surface chemistry of Titan. The advent of predictive computational astrobiology should prove particularly important given the Dragonfly mission’s scheduled landfall on Titan in 2034 (31, 32).
MATERIALS AND METHODS
Construction of the membrane
The azotosome unit cell was constructed of four acrylonitrile molecules with neighboring nitrile groups spaced ~3.5 Å apart in a close-packed hexagonal pattern (see the Supplementary Materials for optimized coordinates). The structure before optimization was chosen so to resemble the optimized geometry in the article by Stevenson et al. (19). Phonon band structures provided in the Supplementary Materials show that the membrane and the considered ice are both dynamically stable, i.e., they represent minima on the multidimensional potential energy surface. The dynamic stability of the membrane was additionally confirmed by dynamics simulations. Details of our phonon calculations and dynamics simulations are given below and in the Supplementary Materials.
Extended calculations
Extended DFT calculations were performed with the Vienna Ab Initio Simulation Package (VASP) version 5.4.1 (33, 34). Geometries were optimized using the Predew-Burke-Ernzerhof (PBE) (35) generalized gradient approximation functional, with dispersion correction calculated with the DFT-D3 method by Grimme et al. (36) with Becke-Johnson damping (37). In addition to the PBE-D3 method, extended calculations were also performed with the general-purpose nonlocal dispersion-corrected vdw-df-cx functional (38). The energy of the azotosome membrane calculates as 3 kJ/mol higher at the vdw-df-cx level of theory compared to with the PBE-D3 method. Standard projected augmented wave potentials (34, 39) were used together with a plane-wave kinetic energy cutoff of 700 eV. The reciprocal space resolution was at least 2π 0.07 Å−1 in all calculations. All energies and forces were converged to less than 1 meV/atom (<0.1 kJ/mol). Brillouin zone sampling was performed on Γ-centered k-meshes when calculating the azotosome membrane, and a Monkhorst-Pack k-mesh for calculations on the experimentally determined structures of acrylonitrile. The nonsolvated azotosome membrane was first optimized with a vacuum layer of 10 Å on each side. The optimized structure of this local minima provides the relative energy of the azotosome, in the absence of methane solvation, compared to the crystal structure of acrylonitrile (modeling of the effect of solvation is described below). Other conformations of acrylonitrile might give rise to other dynamically stable local minima that could still be considered azotosomes. However, given the small calculated differences in energy between crystal phases of acrylonitrile ice, less than 2 kJ/mol acrylonitrile, alternative azotosome structures are unlikely to be notably lower in energy compared to the structure we consider here. Crystal structures commensurate with diffraction data on acrylonitrile were extracted from work done by Yokoyama and Ohashi (27) (Cambridge Crystallographic Structure Database reference code POQMIR). Our calculated density of the acrylonitrile crystal at 0 K was 1.13 g/cm3. This is larger than the 1.03 g/cm3 measured experimentally at 153 K. This difference is expected and can be explained by thermal expansion effects that are not included in our calculations. As anticipated, the calculated crystal density was far above the density of liquid acrylonitrile at 298 K (0.81 g/cm3). Optimized geometries and convergence tests of the energy cutoff value and k-point densities are provided in the Supplementary Materials.
Methane solvation
To calculate the effect of methane solvation of the azotosome, 42 methane molecules were added uniformly above and below a 2 × 2 × 1 supercell of the membrane, and the entire structure was then relaxed. The solvation energy of the membrane was calculated by comparing the solvated structure with the membrane in vacuum and the experimentally determined crystal structure of methane (P4m2), taken from the Cambridge Crystallographic Structure Database (reference code ZZZWEQ14).
Thermal and entropic corrections
Thermal corrections were obtained through phonon calculations on optimized PBE-D3 geometries. Force constants were calculated through the direct method through displacements on a 2 × 2 × 2 supercell for the Pna21 ice structure and a 3 × 3 × 1 supercell for the azotosome membrane unit cell using the Phonopy v.1.12.6 code (40). Our computational approach predicts relative energies of the competing crystal phases of acetonitrile to within 0.6 kJ/mol of the experimentally determined phase transition enthalpy provided by the National Institute of Standards and Technology, where ΔHtrans is reported as 1.2 kJ/mol at 162.5 K.
Because acrylonitrile is a small and rigid molecule, it does not have an internal configurational space with an associated configurational entropy, like normal lipids do. Nevertheless, configurational entropy associated with the packing of acrylonitrile likely stabilizes both the membrane and the ice structure to a small degree. The accessible conformational space is likely smaller in the membrane. This is because acrylonitrile molecules can reorient in three dimensions in the crystal structure of acrylonitrile ice, whereas in the two-dimensional azotosome membrane, the nitrile groups need to be pointed inwards. We can estimate the configurational entropy in the ice as ~1 kJ/mol from the experimentally determined disorder [from kT ln(4)]. That is, whereas configurational entropy will affect the stability of both the crystal of acrylonitrile and the effectively two-dimensional azotosome, these effects are not large enough to affect our conclusions. Other entropic effects, such as those associated with the dynamics of the systems, are similarly small due to the low temperature. Our estimate of the Gibbs energy difference between the azotosome membrane and the ice includes the entropy associated with lattice and molecular vibration. This correction is 1.1 kJ/mol acrylonitrile, in favor of the membrane.
Molecular dynamics simulations
Stevenson et al. (19) have used umbrella sampling coupled to optimized potentials for liquid simulations (OPLS) force field–based molecular dynamics simulations to show that the azotosome membrane has dynamic stability in liquid methane. To further verify the structural integrity of the membrane, we have performed a Born-Oppenheimer ab initio molecular dynamics simulation of the solvated azotosome embedded in methane at 90 K in an NVT ensemble. The 3.40-nm3 simulation cell is the same as was obtained from the VASP optimization and contains 16 acrylonitrile molecules and 42 methane molecules (the cell parameters are available in the Supplementary Materials). The simulation was run at the PBE-D3 level of theory using CP2K v6.1 (41), a default DZVP-GTH basis set and Goedecker-Teter-Hutter (GTH) potentials with an energy cutoff of 280 rydberg. The system was equilibrated for 11 ps using the canonical sampling through velocity rescaling (CSVR) thermostat with a time constant of 1 fs and then allowed to evolve for 35 ps using the Nosé-Hoover chain thermostat of length 4 with a time constant of 500 fs. A time step of 0.5 fs was used for both the equilibration and the production run. The variation of the potential energy throughout the simulation and a snapshot of the equilibrated membrane are shown in the Supplementary Materials. The dynamics simulations confirm that the average structural and energetic fluctuations close to the considered minimum are smaller than 1 kJ/mol acrylonitrile.
Supplementary Material
Acknowledgments
We acknowledge Chalmers University of Technology and computational resources provided by the Swedish National Infrastructure for Computing (SNIC) at C3SE. We also thank J. Lunine and J. Stevenson for valuable discussions on this topic. M. Engqvist and F. Sessa are thanked for proofreading. Funding: This work was funded by the Swedish Research Council (2016-04127) and Chalmers University of Technology. Author contributions: M.R. designed the study. H.S. performed the calculations. H.S. and M.R. wrote the manuscript. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All optimized structures and the input files for the molecular dynamics simulations are available in the Supplementary Materials. Additional data available related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/4/eaax0272/DC1
Section S1. Phonon band structures of the azotosome membrane and the acrylonitrile ice
Section S2. Convergence tests
Section S3. Optimized structures
Section S4. The dynamics of the solvated membrane
Section S5. Coordinates and cell parameters
Fig. S1. Phonon band structure of the optimized azotosome membrane.
Fig. S2. Calculated phonon band structure of the Pna21 phase of acrylonitrile.
Fig. S3. Convergence of the energy with respect to plane-wave energy cutoff in calculations on the membrane structure.
Fig. S4. Convergence of the energy change with respect to plane-wave energy cutoff used in calculations on the Pna21 phase of the acrylonitrile ice.
Fig. S5. Structure of the acrylonitrile-based membrane following optimization in vacuum at the PBE-D3 level of theory.
Fig. S6. Crystal structures of different crystalline phases of acrylonitrile and their relative energies in kJ/mol.
Fig. S7. The optimized P4m2 crystal structure of methane.
Fig. S8 The optimized structure of the azotosome membrane solvated by methane.
Fig. S9. The dynamic stability of the azotosome.
Table S1. Total energies in eV/atom of the Pna21 phase of acrylonitrile and the membrane structure as a function of k-point density.
REFERENCES AND NOTES
- 1.Tokano T., McKay C. P., Neubauer F. M., Atreya S. K., Ferri F., Fulchignoni M., Niemann H. B., Methane drizzle on Titan. Nature 442, 432–435 (2006). [DOI] [PubMed] [Google Scholar]
- 2.Stofan E. R., Elachi C., Lunine J. I., Lorenz R. D., Stiles B., Mitchell K. L., Ostro S., Soderblom L., Wood C., Zebker H., Wall S., Janssen M., Kirk R., Lopes R., Paganelli F., Radebaugh J., Wye L., Anderson Y., Allison M., Boehmer R., Callahan P., Encrenaz P., Flamini E., Francescetti G., Gim Y., Hamilton G., Hensley S., Johnson W. T. K., Kelleher K., Muhleman D., Paillou P., Picardi G., Posa F., Roth L., Seu R., Shaffer S., Vetrella S., West R., The lakes of Titan. Nature 445, 61–64 (2007). [DOI] [PubMed] [Google Scholar]
- 3.Lunine J. I., Titan and habitable planets around M-dwarfs. Faraday Discuss. 147, 405–418 discussion 527–552 (2010). [DOI] [PubMed] [Google Scholar]
- 4.McKay C. P., Pollack J. B., Courtin R., The greenhouse and antigreenhouse effects on Titan. Science 253, 1118–1121 (1991). [DOI] [PubMed] [Google Scholar]
- 5.Teanby N. A., Irwin P. G. J., de Kok R., Vinatier S., Bézard B., Nixon C. A., Flasar F. M., Calcutt S. B., Bowles N. E., Fletcher L., Howett C., Taylor F. W., Vertical profiles of HCN, HC3N, and C2H2 in Titan’s atmosphere derived from Cassini/CIRS data. Icarus 186, 364–384 (2007). [Google Scholar]
- 6.Teanby N. A., Irwin P. G. J., de Kok R., Nixon C. A., Coustenis A., Bézard B., Calcutt S. B., Bowles N. E., Flasar F. M., Fletcher L., Howett C., Taylor F. W., Latitudinal variations of HCN, HC3N, and C2N2 in Titan’s stratosphere derived from Cassini CIRS data. Icarus 181, 243–255 (2006). [Google Scholar]
- 7.McKay C. P., Titan as the abode of life. Life 6, 8 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKay C. P., Smith H. D., Possibilities for methanogenic life in liquid methane on the surface of Titan. Icarus 178, 274–276 (2005). [Google Scholar]
- 9.Schulze-Makuch D., Grinspoon D. H., Biologically enhanced energy and carbon cycling on Titan? Astrobiology 5, 560–567 (2005). [DOI] [PubMed] [Google Scholar]
- 10.Rahm M., Lunine J. I., Usher D. A., Shalloway D., Polymorphism and electronic structure of polyimine and its potential significance for prebiotic chemistry on Titan. Proc. Natl. Acad. Sci. U.S.A. 113, 8121–8126 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benner S. A., Ricardo A., Carrigan M. A., Is there a common chemical model for life in the universe? Curr. Opin. Chem. Biol. 8, 672–689 (2004). [DOI] [PubMed] [Google Scholar]
- 12.Schulze-Makuch D., Irwin L. N., The prospect of alien life in exotic forms on other worlds. Naturwissenschaften 93, 155–172 (2006). [DOI] [PubMed] [Google Scholar]
- 13.Szostak J. W., Bartel D. P., Luisi P. L., Synthesizing life. Nature 409, 387–390 (2001). [DOI] [PubMed] [Google Scholar]
- 14.Norris V., Raine D. J., A fission-fusion origin for life. Orig. Life Evol. Biosph. 28, 523–537 (1998). [DOI] [PubMed] [Google Scholar]
- 15.Powell K., Biology from scratch: Built from the bottom up, synthetic cells could reveal the bounderies of life. Nature 563, 172–175 (2018).30405232 [Google Scholar]
- 16.A. I. Oparin, The Origin of Life on the Earth (Oliver & Boyd, 1957), 495 pp. [Google Scholar]
- 17.D. W. Deamer, Light Transducing Membranes: Structure, Function, and Evolution (Academic Press, 1977). [Google Scholar]
- 18.Drobot B., Iglesias-Artola J. M., Le Vay K., Mayr V., Kar M., Kreysing M., Mutschler H., Tang T.-Y. D., Compartmentalised RNA catalysis in membrane-free coacervate protocells. Nat. Commun. 9, 3643 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stevenson J., Clancy P., Lunine J., Membrane alternatives in worlds without oxygen: Creation of an azotosome. Sci. Adv. 1, e1400067 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schulze-Makuch D., Schulze-Makuch A., Houtkooper J. M., The physical, chemical and physiological limits of life. Life 5, 1472–1486 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Facchin M., Scarso A., Selva M., Perosa A., Riello P., Towards life in hydrocarbons: Aggregation behaviour of “reverse” surfactants in cyclohexane. RSC Adv. 7, 15337–15341 (2017). [Google Scholar]
- 22.Palmer M. Y., Cordiner M. A., Nixon C. A., Charnley S. B., Teanby N. A., Kisiel Z., Irwin P. G. J., Mumma M. J., ALMA detection and astrobiological potential of vinyl cyanide on Titan. Sci. Adv. 3, e1700022 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reynolds J. A., Tanford C., Stone W. L., Interaction of L-α-didecanoyl phosphatidylcholine with the AI polypeptide of high density lipoprotein. Proc. Natl. Acad. Sci. U.S.A. 74, 3796–3799 (1977). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.King M. D., Marsh D., Head group and chain length dependence of phospholipid self-assembly studied by spin-label electron spin resonance. Biochemistry 26, 1224–1231 (1987). [DOI] [PubMed] [Google Scholar]
- 25.Segré D., Ben-Eli D., Deamer D. W., Lancet D., The lipid world. Orig. Life Evol. Biosph. 31, 119–145 (2001). [DOI] [PubMed] [Google Scholar]
- 26.Stevenson J. M., Fouad W. A., Shalloway D., Usher D., Lunine J., Chapman W. G., Clancy P., Solvation of nitrogen compounds in Titan’s seas, precipitates, and atmosphere. Icarus 256, 1–12 (2015). [Google Scholar]
- 27.Yokoyama Y., Ohashi Y., Crystal structure of acrylonitrile. Bull. Chem. Soc. Jpn. 71, 345–348 (1998). [Google Scholar]
- 28.R. Hodyss, T. Vu, M.L. Cable, M. Choukroun, M. Malaska, H.E. Maynard-Casely, What rocks on Titan? New phases of acrylonitrile and prospects for mineralogy with organic molecules. EPSC Abstr. 13, EPSC-DPS2019-1044-1 (2019).
- 29.Wächtershäuser G., Before enzymes and templates: Theory of surface metabolism. Microbiol. Rev. 52, 452–484 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D. Schulze-Makuch, L. Irwin, Life in the Universe: Expectations and Constraints (Springer, 2004). [Google Scholar]
- 31.Voosen P., NASA to fly drone on Titan. Science 365, 15 (2019). [DOI] [PubMed] [Google Scholar]
- 32.E. P. Turtle, M. G. Trainer, J. W. Barnes, R. D. Lorenz, K. E. Hibbard, D. S. Adams, P. Bedini, W. B. Brinckerhoff, M. L. Cable, C. Ernst, C. Freissinet, K. Hand, A. G. Hayes, S. M. Hörst, J. R. Johnson, E. Karkoschka, J. W. Langelaan, D. J. Lawrence, A. Le Gall, J. M. Lora, S. M. MacKenzie, C. P. McKay, R. S. Miller, S. Murchie, C. D. Neish, C. E. Newman, J. Palacios, M. P. Panning, A. M. Parsons, P. N. Peplowski, L. C. Quick, J. Radebaugh, S. C. R. Rafkin, M. A. Ravine, S. Schmitz, J. M. Soderblom, K. S. Sotzen, A. M. Stickle, E. R. Stofan, C. Szopa, T. Tokano, C. Wilson, R. A. Yingst, K. Zacny, M. T. Burks, Dragonfly: In situ exploration of Titan’s organic chemistry and habitability, 50th Lunar and Planetary Science Conference, The Woodlands, TX, 18 to 22 March, 2019. [Google Scholar]
- 33.Kresse G., Furthmüeller J., Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996). [DOI] [PubMed] [Google Scholar]
- 34.Kresse G., Joubert D., From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999). [Google Scholar]
- 35.Perdew J. P., Burke K., Ernzerhof M., Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996). [DOI] [PubMed] [Google Scholar]
- 36.Grimme S., Antony J., Ehrlich S., Krieg H., A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 154104 (2010). [DOI] [PubMed] [Google Scholar]
- 37.Grimme S., Ehrlich S., Goerigk L., Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 32, 1456–1465 (2011). [DOI] [PubMed] [Google Scholar]
- 38.Berland K., Hyldgaard P., Exchange functional that tests the robustness of the plasmon description of the van der Waals density functional. Phys. Rev. B 89, 035412 (2014). [Google Scholar]
- 39.Bloechl P. E., Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994). [DOI] [PubMed] [Google Scholar]
- 40.Togo A., Tanaka I., First principles phonon calculations in materials science. Scr. Mater. 108, 1–5 (2015). [Google Scholar]
- 41.Hutter J., Iannuzzi M., Schiffmann F., VandeVondele J., cp2k: Atomistic simulations of condensed matter systems. Wiley Interdiscip. Rev. 4, 15–25 (2014). [Google Scholar]
- 42.Murzyn K., Róg T., Pasenkiewicz-Gierula M., Phosphatidylethanolamine-phosphatidylglycerol bilayer as a model of the inner bacterial membrane. Biophys. J. 88, 1091–1103 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/4/eaax0272/DC1
Section S1. Phonon band structures of the azotosome membrane and the acrylonitrile ice
Section S2. Convergence tests
Section S3. Optimized structures
Section S4. The dynamics of the solvated membrane
Section S5. Coordinates and cell parameters
Fig. S1. Phonon band structure of the optimized azotosome membrane.
Fig. S2. Calculated phonon band structure of the Pna21 phase of acrylonitrile.
Fig. S3. Convergence of the energy with respect to plane-wave energy cutoff in calculations on the membrane structure.
Fig. S4. Convergence of the energy change with respect to plane-wave energy cutoff used in calculations on the Pna21 phase of the acrylonitrile ice.
Fig. S5. Structure of the acrylonitrile-based membrane following optimization in vacuum at the PBE-D3 level of theory.
Fig. S6. Crystal structures of different crystalline phases of acrylonitrile and their relative energies in kJ/mol.
Fig. S7. The optimized P4m2 crystal structure of methane.
Fig. S8 The optimized structure of the azotosome membrane solvated by methane.
Fig. S9. The dynamic stability of the azotosome.
Table S1. Total energies in eV/atom of the Pna21 phase of acrylonitrile and the membrane structure as a function of k-point density.


