Abstract
Enteral nutrition (EN) is effective in Crohn’s disease (CD) patients and has been shown to have an inhibitory effect on loss of response to anti-tumor necrosis factor (TNF)-alpha antibody therapy; however, the current level of evidence is not sufficient. The objective of this meta-analysis was to determine whether EN in combination anti-TNF-alpha antibody therapy is useful in maintaining remission. PubMed was used to identify all relevant studies. A total of nine articles were identified including one randomized control trial, two prospective cohort studies, and six retrospective cohort studies. We performed a meta-analysis on all these articles to assess the remission maintenance effect of EN (n = 857). The remission or response maintenance effect in the EN group was 203/288 (70.5%), which was higher than 306/569 (53.8%) in the non-EN group. The odds ratio for long-term remission or response using fixed effects model and random effects model were 2.23 (95% CI 1.60–3.10) and 2.19 (95% CI 1.49–3.22), respectively. The usefulness of EN was unclear in two prospective studies that were conducted immediately after remission induction with anti-TNF-alpha antibody therapy was detected. Differences in the definition of relapse and the observation period among articles were considered to be limitations. This analysis suggests that EN is effective for maintaining remission in patients already in remission or response as a result of anti-TNF-alpha antibody maintenance therapy.
Electronic supplementary material
The online version of this article (10.1007/s00535-019-01634-1) contains supplementary material, which is available to authorized users.
Keywords: Crohn’s disease, Enteral nutrition, Anti-TNF-alpha antibody, Meta-analysis
Introduction
Crohn’s disease (CD) is a chronic inflammatory bowel disease and tends to follow a progressive course with the development of bowel complications such as stricture and fistula over time [1–3]. The cause of CD remains unknown, but the involvement of dietary antigens has been known, and dietary habits such as excessive fat intake are suggested in both onset and relapse [4–7]. Therefore, implementing enteral nutrition (EN) consisting mainly of amino acids and peptides and limiting the total amount of oral intake and fat intake by patients with CD can, as a consequence, reduce dietary antigens and improve the pathology of CD [8, 9]. EN has also other mechanisms linked to the preventive effects such as decreasing mucosal cytokines [10], correction of gut permeability [11], and modification of gut flora [12]. Furthermore, obesity and visceral fat are regarded as risk factors for the onset and exacerbation of CD [13, 14], and well-balanced nutrition augmented by EN can assist in their reduction. Therefore, dietary restriction or EN is widely recognized as effective treatments in patients with CD, and also patients noticed it [15]. Exclusive enteral nutrition (EEN) with elemental, semi-elemental diets and polymeric formulations are used in pediatric patients as first-line therapy for remission induction and its use is recommended in many guidelines [16–18]. In contrast, the use of EN is usually limited in adult CD patients, including patients with complicated short bowel syndrome or intestinal dysfunction after extensive digestive tract resection [19]. However, in Asia, especially in Japan, it is considered that EEN is an effective treatment for patients with active CD as a remission induction therapy [20, 21]. Moreover, EN is established as a remission maintenance therapy and several papers reported the efficacy of EN in various situations for patients with CD [22–24]. Currently, the use of anti-tumor necrosis factor (TNF)-alpha antibodies has become mainstream because of their high efficacy in CD [25–29]. According to the recent nationwide cohort study, parallel to an increasing use of thiopurines and anti-TNF-alpha antibody in inflammatory bowel disease (IBD) over time, a persistent significant decrease in surgery rates was confirmed [30]. On the other hand, despite the use of anti-TNF-alpha antibody of IBD, surgery is still required in 30–40% of patients with CD during the maintenance therapy [31]. Hence, considering the long-term outcome of CD, loss of response (LOR) due to the lower blood trough level and the emergence of anti-drug antibody (ADA) are problematic [32–34]. These have been addressed by combination therapy with immunomodulators (IMMs) or dose increases, or switching to different classes of drugs [35, 36], but long-term safety and medical economic issues have been pointed out [37, 38]. In fact, the patients tend to accept elevated severe adverse effect (AE) risks in exchange for clinical efficacy; however, they are not able to accept even mild AE risks if the treatment efficacy is lower or uncertain [39]. In recent years, reports indicating that EN can enhance the therapeutic effect and suppress LOR of anti-TNF-alpha antibody agents have been attracting attention [40–45]. Implementation of EN itself is inherently extremely safe, although there are minor concerns such as osmotic diarrhea. Since EN is not a drug, it is not necessary to consider the possible exacerbation of side effects due to interactions with concomitantly administered drugs. In addition, since the mechanism of action is different from the other treatments mentioned above, an add-on effect can be expected [46]. This meta-analysis was performed to determine whether EN in combination with anti-TNF-alpha antibody therapy is useful.
Materials and methods
Literature search
A literature search on PubMed was conducted for articles published by October 31, 2018, using the following search formula: (Crohn’s disease OR Crohn disease) AND (elemental diet OR enteral nutrition OR polymeric diet) AND (infliximab OR adalimumab OR certolizumab pegol OR golimumab OR TNF-alpha inhibitor).
Study selection and exclusion
Two authors (F.H and T.T) reviewed the results of the literature search independently. Inclusion criteria were defined as follows: (1) anti-TNF-alpha antibody is used as maintenance therapy (at least 16 weeks) in adult CD patients, (2) clinical remission or response maintenance effect is compared between patients who received EN and patients who did not receive EN (the dose of EN was not taken into consideration) and (3) the number of event occurrences is clearly described both for the EN and non-EN groups. Exclusion criteria were defined as follows: studies of diseases other than CD (e.g., ulcerative colitis and inflammatory bowel disease unclassified), abstracts without full texts, case reports, reviews and pediatric studies.
Statistical analysis
Point estimates of the odds ratio for long-term remission (EN group/non-EN group) and their 95% confidence intervals were determined for each article and overall for all articles. Two models were applied as statistical models for the common odds ratio of all articles with respect to long-term remission: a fixed effects model, which is a model where literature effects are not considered as variables, and a random effects model, which is a model where literature effects are considered as variables. The methods used to estimate the common odds ratio were the Mantel–Haenszel method for the fixed effects model and the DerSimonian–Laird method for the random effects model [47, 48]. The Breslow–Day test was performed as a test of heterogeneity between articles in assessing the odds ratio for long-term remission [49]. The null hypothesis is “The true value of the odds ratio for each article is the same among all articles”. Statistically significant heterogeneity between articles was defined as P < 0.05. In addition, the Higgins and Thompson's I2 statistic was calculated as a measure of heterogeneity [50]. The statistical software SAS ver.9.4 (SAS Institute, Cary, NC, USA) was used for analysis. Statistical analyses were performed by an independent third party, AC Medical Inc. (Tokyo, Japan).
Results
Study selection
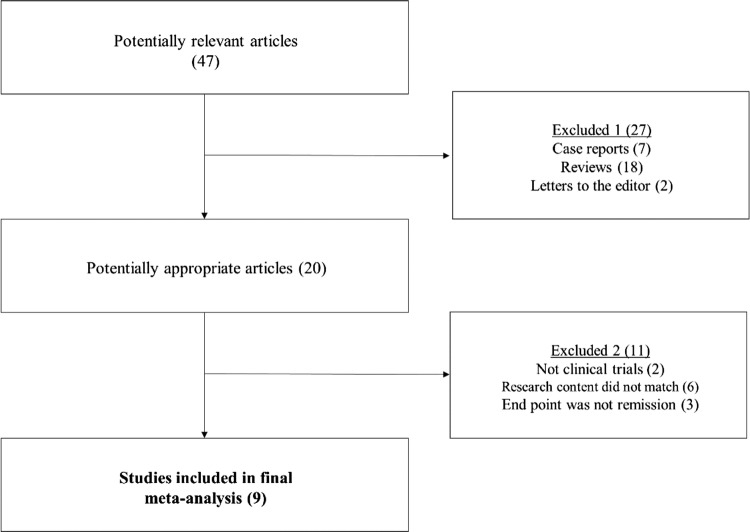
The initial literature search identified a total of 47 articles. From the articles, seven case reports, 18 reviews, and two letters to the editor were excluded. Of the remaining 20 articles, two more were excluded as they were not clinical trials, six because the research content did not match (infliximab vs enteral nutrition monotherapy: 1, infliximab without enteral nutrition: 4, infliximab + enteral nutrition vs conventional therapy: 1), and three articles where the end point was not clinical remission or response. Based on the results of these exclusions, nine articles meeting all criteria were included in this meta-analysis [40–45, 51–53] (Fig. 1). In two of the articles [52, 53], the number of relapse events was not accurately described, but shown only in a graph. We, therefore, confirmed the number of events by communicating with the author via email.
Fig. 1.
Algorithm demonstrating article search of this meta-analysis. Finally, nine studies were included in this study
Details of selected studies
The selected articles included one randomized control trial (RCT), two prospective observational cohort studies and six retrospective observational cohort studies. After all the total number of this meta-analysis contained 857 patients. The type of enteral formulation used was elemental diet (ELENTAL®, EA Pharma Co., Ltd, Tokyo, Japan) only in seven articles and semi-elemental diet and polymeric formulations also included in two articles. The type of anti-TNF-alpha antibody was infliximab (IFX) alone in six articles, one of which included patients treated at 10 mg/kg. In addition, adalimumab (ADA) alone was used in one article, and both IFX and ADA were used in two articles. The definition of EN group and relapse or LOR, and the review period varied depending on the article (Table 1). Only 3 out of 9 studies mentioned dose of EN intake in detail (Table 1). All selected studies were from Japan. The dose escalation of IFX (10 mg/kg every 8 week) and the shortening of administration of IFX (5 mg/kg every 4–7 week) were approved in 2011 and 2017, respectively, in Japan. The dose escalation of ADA (80 mg every other week) was approved in 2016. The shortening of administration of ADA is not approved yet. Therefore, indication and treatment strategy on dose escalation and shortening of anti-TNF-α antibody were different among the selected articles depending on the time periods conducted the study (shown in Supplementary Table 1).
Table 1.
Summary of studies including EN in meta-analysis
| Ref. no. | Author | Study design | Group | Therapy for Crohn's disease | Average dose of EN | Total number | Long-term remission or response | Definition of clinical remission or response | (%) Duration of follow-up period | |
|---|---|---|---|---|---|---|---|---|---|---|
| Year | Randomization | Number | Percent | |||||||
| [40] | Tanaka et al. | Retrospective | EN + | IFX (5 mg/kg) + EN (> 3762 kJ/day) | NA | 51 | 30 | 58.8 | Reduction in HBI score, or number of fistula | 16 weeks after IFX last dose |
| 2006 | Observational | EN − | IFX (5 mg/kg) without EN | NA | 59 | 22 | 37.3 | Increase in HBI score, or number of fistula | 16 weeks from IFX last dose | |
| [41] | Yamamoto et al. | Prospective | EN + | IFX (5 mg/kg) + EN (≥1200 kcal/day) | NA | 32 | 25 | 78.1 | CDAI score < 150 | 56 weeks after remission |
| 2010 | Observational | EN − | IFX (5 mg/kg) without EN | NA | 24 | 16 | 66.7 | CDAI score ≥ 150 | 56 weeks from remission | |
| [42] | Sazuka et al. | Retrospective | EN + | IFX (5 mg/kg) + EN (≥ 600 kcal/day) | NA | 29 | 23 | 79.3 | CDAI score < 150 | Last visit in follow-up |
| 2012 | Observational | EN − | IFX (5 mg/kg) + EN (<600 kcal/day) | NA | 45 | 22 | 48.9 | CDAI score ≥ 150, etc | Median follow-up from remission: 85 weeks | |
| [43] | Hirai et al. | Retrospective | EN + | IFX (5 mg/kg) + EN (≥ 900 kcal/day) | 1233 ± 62 (mean ± SE) | 45 | 31 | 68.9 | CRP < 0.3 mg/dL | Last visit in follow-up |
| 2013 | Observational | EN − | IFX (5 mg/kg) + EN (< 900 kcal/day) | 535 ± 32 (mean ± SE) | 57 | 24 | 42.1 | CRP ≥ 1.5 mg/dL, etc |
Mean follow-up from remission EN + group: 525.3 days (75.0 weeks) EN− group: 558.9 days (79.8 weeks) |
|
| [44] | Kamata et al. | Retrospective | EN + | IFX (5 mg/kg) + EN (≥ 900 kcal/day) | 1246 ± 350 (mean ± SE) | 28 | 27 | 96.4 | Clinical remission | Last visit in follow-up |
| 2015 | Observational | EN − | IFX (5 mg/kg) + EN (< 900 kcal/day) | 142 ± 238 (mean ± SE) | 97 | 77 | 79.4 | Clinical symptoms, etc |
Mean follow-up from remission EN + group: 799 days (114 weeks) EN- group: 771 days (110 weeks) |
|
| [45] | Hirai et al. | Prospective | EN + | IFX (5 mg/kg)/ADA (40 mg) + EN (≥ 900 kcal/day prescribEN) | NA | 37 | 24 | 64.9 | CDAI score < 200 | Last visit in follow-up |
| 2018 | Observational | EN − | IFX (5 mg/kg)/ADA (40 mg) only | NA | 35 | 22 | 62.9 | CDAI score ≥ 200 | 2 years (104 weeks) from IFX/ADA first dose | |
| [51] | Hisamatsu et al. | Prospective | EN + | IFX (10 mg/kg) + EN (900–1200 kcal/day) | NA | 14 | 11 | 78.6 | CDAI score < 150 | Last visit in follow-up |
| 2018 | Randomized | EN − | IFX (10 mg/kg) without EN | NA | 6 | 3 | 50.0 | Termination of maintenance therapy | 56 weeks form IFX 10 mg/kg dose | |
| [52] | Moroi et al. | Retrospective | EN + | IFX (5 mg/kg)/ADA (40 mg) + EN (≥ 900 kcal/day) | NA | 27 | 13 | 48.1 | Clinical remission | Last visit in follow-up |
| 2018 | Observational | EN − | IFX (5 mg/kg)/ADA (40 ng) + EN (< 900 kcal/day) | NA | 154 | 75 | 48.7 | Clinical relapse | At most 10 years (521 weeks) from IFX first dose | |
| [53] | Sugita et al. | Retrospective | EN + | ADA (40 mg) + EN (≥ 900 kcal/day) | 1044 ± 192 (mean ± SD) | 25 | 19 | 76.0 | HBI score < 5 | Last visit in follow-up |
| 2018 | Observational | EN − | ADA(40 mg) + EN (< 900 kcal/day) | 88 ± 200 (mean ± SD) | 92 | 45 | 48.9 | HBI score ≥ 5, etc |
Mean follow-up from remission EN + group: 1282 days (183 weeks) EN − group: 1340 days (191 weeks) |
|
IFX infliximab, NA not applicable, HBI Harvey–Bradshaw Index, ADA adalimumab, CDAI Crohn's Disease Activity Index, EN elemental nutrition, CRP C-reactive protein
Enteral nutrition during anti-TNF-alpha inhibitor and LOR risk
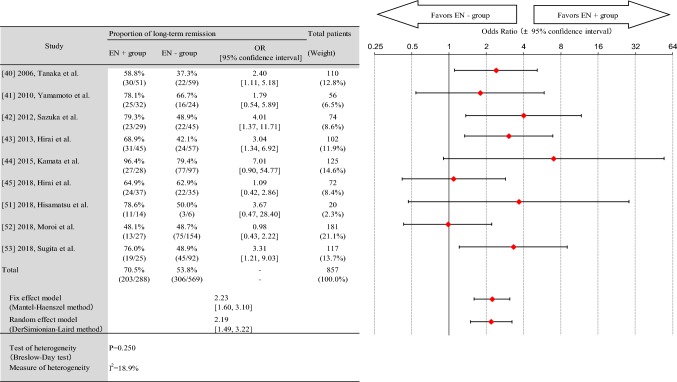
The remission maintenance effect in the EN group was 203/288 (70.5%), which was higher than 306/569 (53.8%) in the non-EN group. Figure 2 presents a forest plot of the odds ratios (OR) for long-term remission. The pooled OR of EN for clinical remission or response maintenance was 2.23 [95% confidence interval (CI) 1.60–3.10] in the fixed effects model and 2.19 [95% CI 1.49–3.22] in the random effects model. The results of the heterogeneity test showed no statistically significant heterogeneity (P = 0.250). The measure of heterogeneity was at a relatively low level (I2 = 18.9%).
Fig. 2.
Forest plot of odds ratio for long-term remission. The odds ratio for long-term remission using fixed effects model and random effects model was 223 (95% CI 160–310) and 219 (95% CI 149–322), respectively
Publication bias
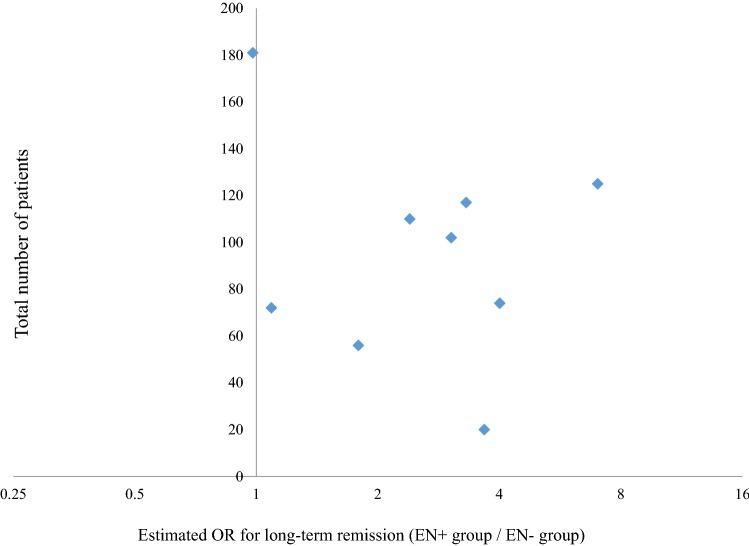
A high publication bias was suggested by a funnel plot representing the article-by-article point estimates of the odds ratio for long-term remission on the horizontal axis and the number of patients (total for both the EN group and non-EN group) for each article on the vertical axis (Fig. 3).
Fig. 3.
Funnel plot of odds ratio for long-term remission
Discussion
Many cases of Crohn’s disease (CD) eventually require surgery and relapse after surgery is common. CD is regarded as a disease that causes progressive disability [2]. However, recently, it has been reported that this disease course can be changed by introducing effective medical treatment with an anti-TNF-alpha antibody at an appropriate time [30, 54, 55]. While anti-TNF-alpha antibody therapy not only improves symptoms but also induces mucosal healing in many cases, LOR often occurs during the maintenance therapy [32, 33]. If LOR occurs, measures that take blood concentration and anti-drug antibodies into account are recommended [35, 36]. In addition, in IFX, which is highly immunogenic, combination with IMMs is known to improve the therapeutic effect and reduce the risk of LOR [56, 57]. EN does not directly induce an increase in anti-TNF-alpha antibody blood concentration or reduce antibody production. However, an increasing number of recent reports have shown how the use of EN in combination with an anti-TNF-alpha antibody is clinically useful due to the add-on effect of EN itself [40–45, 51–53]. In the present review, in which these studies were meta-analyzed, the pooled OR for maintenance of remission or relapse with EN was 2.23 in the fixed effects model and 2.19 in the random effects model. In other words, the meta-analysis suggested that combination with EN improves the remission or response maintenance effect of treatment with an anti-TNF-alpha antibody. However, it remains unclear on the dose of EN, adherence to EN, the timing of starting EN for getting more combination efficacy. Hisamatsu et al. concluded that combination with EN at over 1200 kcal was useful in patients with LOR among those patients where the IFX dose had already been doubled [51]. Except for this limited indication, it was not possible to identify the patients who would benefit from EN in combination with medical therapy or to clarify the required dose in this review. This was due to variations in the clinical backgrounds of the target patients and the dose of nutritional therapy despite the lowest dose being set at 600 kcal. Since it is difficult to continue EN over a prolonged period, it is practically impossible to conduct RCTs to examine the therapeutic effect of EN. One RCT was included and only three prospective studies were included in this meta-analysis. In retrospective studies, patients who were able to continue receiving EN for a prolonged period are analyzed as the EN group, enabling assessment of the effect of EN combination therapy; however, recruitment bias cannot be ruled out. According to the report by Hirai et al., although patient adherence was confirmed in the EN group prior to the enrollment in accordance with the protocol for the prospective cohort, only 29.7% were able to continue with EN at the targeted calorific content of 900 kcal over a 2-year period [45]. The authors considered that the low adherence in the EN group was the main reason why the efficacy of combination therapy with EN was not demonstrated. Measures to improve adherence, such as alleviating amino acid odor, are needed. In the two prospective trials, the subjects were patients who achieved clinical remission by anti-TNF-alpha antibodies in early treatment phase and were subsequently divided into the EN group and the non-EN group. These trials did not include patients who were in clinical remission by means of maintenance phase of anti-TNF-alpha antibodies. Therefore, the administration period of TNF-alpha antibodies was relatively short and the majority of the subjects were naive to biologics. These factors might be influenced on the result of failing to confirm the usefulness of concomitant EN. On the contrary, in the other trials examined mainly for the patients with maintenance therapy of anti-TNF-alpha antibodies as the subjects, it was suggested that concomitant EN with anti-TNF-alpha antibody was effective for preventing relapse.
In fact, there is another meta-analysis, which was published in Nguyen et al. in 2015, on current topic [58]. They reported that specialized EN therapy with IFX resulted in 109 of 157 (69.4%) patients reaching clinical remission compared with 84 of 185 (45.4%) with IFX monotherapy [OR 2.73; 95% CI 1.73–4.31, p < 0.01]. The slight difference between the results of these two meta-analyses was presumed to be related to the type of anti-TNF-α antibodies (subjects of meta-analysis by Nguyen et al. were administered only IFX), the number of included papers and the timing of publications.
This review has several limitations. First, as mentioned above, the backgrounds of target patients and the definitions of relapse are different. Many of the studies reviewed used a retrospective cohort as the study design and there are only a few high quality studies. Second, all the studies adopted for meta-analysis were conducted in Japan and it cannot be confirmed if the results of these studies can be extrapolated to other regions. Third, publication bias exists. The remission maintenance rate is generally high because the target in all articles was limited to patients who used anti-TNF-alpha antibody therapy. The articles are associated with the interventional use of EN in combination with medical therapy, and this could lead to the conclusion that EN has an add-on effect or at the very least is comparable. And last but least, the issue on the therapeutic drug monitoring and anti-drug antibody were not analyzed because all studies did not mention these topics.
In conclusion, EN in combination with anti-TNF-alpha antibody therapy can help to prevent the incidence of clinical relapse including LOR in maintenance therapy. The combined therapy may affect the better course, for example, to extend clinical remission or response. Although the required dose of EN is unknown, doses of at least 600–900 kcal have been cited in reports in which efficacy was demonstrated. There is a possibility that EN appears to be more strongly indicated in CD patients with non-colonic type whose dose of an IFX has already been doubled due to LOR. Prospective studies with a high level of evidence need to be conducted worldwide in the future.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This study was funded by EA Pharma Co., Ltd.
Author contributions
FH: conception and design of the study; analysis and interpretation of data; drafting of the manuscript. TT, YT, MK, TB, NT, MM, TH: analysis and interpretation of data, KY, TU: interpretation of data. All authors discussed the results and commented on the manuscript.
Compliance with ethical standards
Conflict of interest
Fumihito Hirai received lecture fees from Abbvie GK, EA Pharma Co., Ltd, Janssen Pharmaceutical K.K, Mochida Pharmaceutical Co., Ltd and Mitsubishi Tanabe Pharma Co.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cosnes J, Cattan S, Blain A, et al. Long-term evolution of disease behavior of Crohn’s disease. Inflamm Bowel Dis. 2002;8:244–250. doi: 10.1097/00054725-200207000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Beaugerie Laurent, Seksik Philippe, Nion–Larmurier Isabelle, Gendre Jean–Pierre, Cosnes Jacques. Predictors of Crohn’s Disease. Gastroenterology. 2006;130(3):650–656. doi: 10.1053/j.gastro.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 3.Solberg IC, Vatn MH, Høie O, et al. Clinical course in Crohn’s disease: results of a Norwegian population-based ten-year follow-up study. Clin Gastroenterol Hepatol; 2007: 5 1430–8. [DOI] [PubMed]
- 4.Racine A, Carbonnel F, Chan SS, et al. Dietary patterns and risk of inflammatory bowel disease in Europe: results from the EPIC study. Inflamm Bowel Dis. 2016;22:345–354. doi: 10.1097/MIB.0000000000000638. [DOI] [PubMed] [Google Scholar]
- 5.Ananthakrishnan AN, Khalili H, Konijeti GG, et al. Longterm intake of dietary fat and risk of ulcerative colitis and Crohn’s disease. Gut. 2014;63:776–784. doi: 10.1136/gutjnl-2013-305304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanaka M, Iwao Y, Sasaki S, et al. Moderate dietary temperance effectively prevents relapse of Crohn disease: a prospective study of patients in remission. Gastroenterol Nurs. 2007;30:202–210. doi: 10.1097/01.SGA.0000278169.35930.f8. [DOI] [PubMed] [Google Scholar]
- 7.Ferreira P, Cravo M, Guerreiro CS, et al. Fat intake interacts with polymorphisms of caspase9 Fas ligand and PPARgamma apoptotic genes in modulating Crohn’s disease activity. Clin Nutr. 2010;29:819–823. doi: 10.1016/j.clnu.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 8.Massironi S, Rossi RE, Cavalcoli FA, et al. Nutritional deficiencies in inflammatory bowel disease: therapeutic approaches. Clin Nutr. 2013;32:904–910. doi: 10.1016/j.clnu.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 9.Zachos M, Tondeur M, Griffiths AM. Enteral nutritional therapy for induction of remission in Crohn’s disease. Cochrane Database Syst Rev 2007; 1:CD000542. [DOI] [PubMed]
- 10.Yamamoto T, Nakahigashi M, Saniabadi AR, et al. Impacts of long-term enteral nutrition on clinical and endoscopic disease activities and mucosal cytokines during remission in patients with Crohn’s disease: a prospective study. Inflamm Bowel Dis. 2007;13:1493–1501. doi: 10.1002/ibd.20238. [DOI] [PubMed] [Google Scholar]
- 11.Guzy C, Schirbel A, Paclik D, et al. Enteral and parenteral nutrition distinctively modulate intestinal permeability and T cell function in vitro. Eur J Nutr. 2009;48:12–21. doi: 10.1007/s00394-008-0754-3. [DOI] [PubMed] [Google Scholar]
- 12.Leach ST, Mitchell HM, Eng WR, et al. Sustained modulation of intestinal bacteria by exclusive enteral nutrition used to treat children with Crohn’s disease. Aliment Pharmacol Ther. 2008;28:724–733. doi: 10.1111/j.1365-2036.2008.03796.x. [DOI] [PubMed] [Google Scholar]
- 13.Holt DQ, Moore GT, Strauss BJ, et al. Visceral adiposity predicts post-operative Crohn's disease recurrence. Aliment Pharmacol Ther. 2017;45:1255–1264. doi: 10.1111/apt.14018. [DOI] [PubMed] [Google Scholar]
- 14.Li Y, Zhu W, Gong J, et al. Influence of exclusive enteral nutrition therapy on visceral fat in patients with Crohn's disease. Inflamm Bowel Dis. 2014;20:1568–1574. doi: 10.1097/MIB.0000000000000114. [DOI] [PubMed] [Google Scholar]
- 15.Limdi JK, Aggarwal D, McLaughlin JT. Dietary practices and beliefs in patients with inflammatory Bowel disease. Inflamm Bowel Dis. 2016;22:164–170. doi: 10.1097/MIB.0000000000000585. [DOI] [PubMed] [Google Scholar]
- 16.Lochs H, Dejong C, Hammarqvist F, et al. ESPEN guidelines on enteral nutrition: gastroenterology. Clin Nutr. 2006;25:260–274. doi: 10.1016/j.clnu.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 17.Ruemmele FM, Veres G, Kolho KL, et al. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn's disease. J Crohn's Colitis. 2014;8:1179–1207. doi: 10.1016/j.crohns.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Sandhu BK, Fell JM, Beattie RM, et al. Guidelines for the management of inflammatory bowel disease in children in the United Kingdom. J Pediatr Gastroenterol Nutr. 2010;50:S1–S13. doi: 10.1097/MPG.0b013e3181c92c53. [DOI] [PubMed] [Google Scholar]
- 19.Dignass A, Van Assche G, Lindsay JO, et al. The second European evidence-based Consensus on the diagnosis and management of Crohn's disease: current management. J Crohns Colitis. 2010;4:28–62. doi: 10.1016/j.crohns.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Matsuoka K, Kobayashi T, Ueno F, et al. Evidence-based clinical practice guidelines for inflammatory bowel disease. J Gastroenterol. 2018;53:305–353. doi: 10.1007/s00535-018-1439-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okada M, Yao T, Yamamoto T, et al. Controlled trial comparing an elemental diet with prednisolone in the treatment of active Crohn’s disease. Hepatogastroenterology. 1990;37:72–80. [PubMed] [Google Scholar]
- 22.Takagi S, Utsunomiya K, Kuriyama S, et al. Effectiveness of an ‘half elemental diet’ as maintenance therapy for Crohn’s disease: a randomized-controlled trial. Aliment Pharmacol Ther. 2006;24:1333–1340. doi: 10.1111/j.1365-2036.2006.03120.x. [DOI] [PubMed] [Google Scholar]
- 23.Esaki M, Matsumoto T, Hizawa K, et al. Preventive effect of nutritional therapy against postoperative recurrence of Crohn disease with reference to findings determined by intra-operative enteroscopy. Scand J Gastroenterol. 2005;40:1431–1437. doi: 10.1080/00365520510023729. [DOI] [PubMed] [Google Scholar]
- 24.Konno M, Takahashi M, Toita N, et al. Long-term therapeutic effectiveness of maintenance enteral nutrition for Crohn's disease. Pediatr Int. 2015;57:276–280. doi: 10.1111/ped.12494. [DOI] [PubMed] [Google Scholar]
- 25.Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359:1541–1549. doi: 10.1016/S0140-6736(02)08512-4. [DOI] [PubMed] [Google Scholar]
- 26.Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med. 2004;350:876–885. doi: 10.1056/NEJMoa030815. [DOI] [PubMed] [Google Scholar]
- 27.Sandborn WJ, Hanauer SB, Rutgeerts P, et al. Adalimumab for maintenance treatment of Crohn’s disease: results of the CLASSIC II trial. Gut. 2007;56:1232–1239. doi: 10.1136/gut.2006.106781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colombel JF, Sandborn WJ, Rutgeerts P, et al. Comparison of two adalimumab treatment schedule strategies for moderate-to-severe Crohn’s disease: results from the CHARM trial. Am J Gastroenterol. 2009;104:1170–1179. doi: 10.1038/ajg.2009.59. [DOI] [PubMed] [Google Scholar]
- 29.Singh S, Heien HC, Sangaralingham LR, et al. Comparative effectiveness and safety of anti-tumor necrosis factor agents in biologic-naive patients with Crohn's disease. Clin Gastroenterol Hepatol. 2016;14:1120–1129. doi: 10.1016/j.cgh.2016.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rungoe C, Langholz E, Andersson M, et al. Changes in medical treatment and surgery rates in inflammatory bowel disease: a nationwide cohort study 1979–2011. Gut. 2014;63:1607–1616. doi: 10.1136/gutjnl-2013-305607. [DOI] [PubMed] [Google Scholar]
- 31.Bouguen G, Peyrin-Biroulet L. Surgery for adult Crohn’s disease: what is the actual risk? Gut. 2011;60:1178–1181. doi: 10.1136/gut.2010.234617. [DOI] [PubMed] [Google Scholar]
- 32.Gisbert JP, Panés J. Loss of response and requirement of infliximab dose intensification in Crohn’s disease: a review. Am J Gastroenterol. 2009;104:760–767. doi: 10.1038/ajg.2008.88. [DOI] [PubMed] [Google Scholar]
- 33.Billioud V, Sandborn WJ, Peyrin-Biroulet L. Loss of response and need for adalimumab dose intensification in Crohn’s disease: a systematic review. Am J Gastroenterol. 2011;106:674–684. doi: 10.1038/ajg.2011.60. [DOI] [PubMed] [Google Scholar]
- 34.Qiu Y, Chen BL, Mao R, et al. Systematic review with meta-analysis: loss of response and requirement of anti-TNFα dose intensification in Crohn's disease. J Gastroenterol. 2017;52:535–554. doi: 10.1007/s00535-017-1324-3. [DOI] [PubMed] [Google Scholar]
- 35.Ding NS, Hart A, De Cruz P. Systematic review: predicting and optimising response to anti-TNF therapy in Crohn's disease—algorithm for practical management. Aliment Pharmacol Ther. 2016;43:30–51. doi: 10.1111/apt.13445. [DOI] [PubMed] [Google Scholar]
- 36.Ben-Horin S, Chowers Y. Review article: loss of response to anti-TNF treatments in Crohn's disease. Aliment Pharmacol Ther. 2011;33:987–995. doi: 10.1111/j.1365-2036.2011.04612.x. [DOI] [PubMed] [Google Scholar]
- 37.Park KT, Colletti RB, Rubin DT, et al. Health insurance paid costs and drivers of costs for patients with Crohn's disease in the United States. Am J Gastroenterol. 2016;111:15–23. doi: 10.1038/ajg.2015.207. [DOI] [PubMed] [Google Scholar]
- 38.Williams CJ, Peyrin-Biroulet L, Ford AC. Systematic review with meta-analysis: malignancies with anti-tumour necrosis factor-α therapy in inflammatory bowel disease. Aliment Pharmacol Ther. 2014;39:447–458. doi: 10.1111/apt.12624. [DOI] [PubMed] [Google Scholar]
- 39.Johnson FR, Ozdemir S, Mansfield C, et al. Crohn's disease patients' risk-benefit preferences: serious adverse event risks versus treatment efficacy. Gastroenterology. 2007;133:769–779. doi: 10.1053/j.gastro.2007.04.075. [DOI] [PubMed] [Google Scholar]
- 40.Tanaka T, Takahama K, Kimura T, et al. Effect of concurrent elemental diet on infliximab treatment for Crohn's disease. J Gastroenterol Hepatol. 2006;21:1143–1149. doi: 10.1111/j.1440-1746.2006.04317.x. [DOI] [PubMed] [Google Scholar]
- 41.Yamamoto T, Nakahigashi M, Umegae S, et al. Prospective clinical trial: enteral nutrition during maintenance infliximab in Crohn's disease. J Gastroenterol. 2010;45:24–29. doi: 10.1007/s00535-009-0136-5. [DOI] [PubMed] [Google Scholar]
- 42.Sazuka S, Katsuno T, Nakagawa T, et al. Concomitant use of enteral nutrition therapy is associated with sustained response to infliximab in patients with Crohn's disease. Eur J Clin Nutr. 2012;66:1219–1223. doi: 10.1038/ejcn.2012.120. [DOI] [PubMed] [Google Scholar]
- 43.Hirai F, Ishihara H, Yada S, et al. Effectiveness of concomitant enteral nutrition therapy and infliximab for maintenance treatment of Crohn's disease in adults. Dig Dis Sci. 2013;58:1329–1334. doi: 10.1007/s10620-012-2374-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kamata N, Oshitani N, Watanabe K, et al. Efficacy of concomitant elemental diet therapy in scheduled infliximab therapy in patients with Crohn's disease to prevent loss of response. Dig Dis Sci. 2015;60:1382–1388. doi: 10.1007/s10620-014-3493-8. [DOI] [PubMed] [Google Scholar]
- 45.Hirai F, Ishida T, Takeshima F, et al. Effect of a concomitant elemental diet with maintenance anti-tumor necrosis factor-α antibody therapy in patients with Crohn's disease: a multicenter prospective cohort study. J Gastroenterol Hepatol. 2019;34:132–139. doi: 10.1111/jgh.14361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Triantafillidis JK, Vagianos C, Papalois AE. The role of enteral nutrition in patients with inflammatory Bowel disease: current aspects. Biomed Res Int. 2015;2015:197167. doi: 10.1155/2015/197167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 48.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 49.Breslow NE, Day NE. Statistical methods in cancer research volume i: the analysis of case-control studies. IARC Sci Publ; 1980; 32:5–338. [PubMed]
- 50.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 51.Hisamatsu T, Kunisaki R, Nakamura S, et al. Effect of elemental diet combined with infliximab dose escalation in patients with Crohn's disease with loss of response to infliximab: CERISIER trial. Intest Res. 2018;16:494–498. doi: 10.5217/ir.2018.16.3.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moroi R, Endo K, Yamamoto K, et al. Long-term prognosis of Japanese patients with biologic-naïve Crohn's disease treated with anti-tumor necrosis factor-α antibodies. Intest Res. 2019;17:94–106. doi: 10.5217/ir.2018.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sugita N, Watanabe K, Kamata N, et al. Efficacy of a concomitant elemental diet to reduce the loss of response to adalimumab in patients with intractable Crohn's disease. J Gastroenterol Hepatol. 2018;33:631–637. doi: 10.1111/jgh.13969. [DOI] [PubMed] [Google Scholar]
- 54.Colombel JF, Narula N, Peyrin-Biroulet L. Management strategies to improve outcomes of patients with inflammatory Bowel diseases. Gastroenterology. 2017;152:351–361. doi: 10.1053/j.gastro.2016.09.046. [DOI] [PubMed] [Google Scholar]
- 55.Nguyen GC, Nugent Z, Shaw S, et al. Outcomes of patients with Crohn’s disease improved from 1988 to 2008 and were associated with increased specialist care. Gastroenterology. 2011;141:90–97. doi: 10.1053/j.gastro.2011.03.050. [DOI] [PubMed] [Google Scholar]
- 56.Ben-Horin S, Waterman M, Kopylov U, et al. Addition of an immunomodulator to infliximab therapy eliminates antidrug antibodies in serum and restores clinical response of patients with inflammatory Bowel disease. Clin Gastroenterol Hepatol. 2013;11:444–447. doi: 10.1016/j.cgh.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 57.Ungar B, Kopylov U, Engel T, et al. Addition of an immunomodulator can reverse antibody formation and loss of response in patients treated with adalimumab. Aliment Pharmacol Ther. 2017;45:276–282. doi: 10.1111/apt.13862. [DOI] [PubMed] [Google Scholar]
- 58.Nguyen DL, Palmer LB, Nguyen ET, et al. Specialized enteral nutrition therapy in Crohn's disease patients on maintenance infliximab therapy: a meta-analysis. Therap Adv Gastroenterol. 2015;8:168–175. doi: 10.1177/1756283X15578607. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.