Abstract
A concurrent increase in the prevalence of hepatocellular carcinoma (HCC) with that of type 2 diabetes (T2D) and obesity has been reported in the absence of hepatitis B virus surface antigen-negative/hepatitis C virus antibody-negative HCC (NBNC-HCC). However, the prognostic relevance of this association remains unclear. Promoter methylation (PM) of the dihydropyrimidinase-like 3 gene (DPYSL3) has been implicated in virus-related HCC. However, it remains unclear whether T2D influences PM in NBNC-HCC. We determined the influence of T2D on clinicopathological profile and PM of DPYSL3 and CDK2NA in patients with NBNC-HCC who were divided into two groups: non-diabetes (non-DM; n = 46) and diabetes (DM; n = 47). DM was associated with a higher Union for International Cancer Control grade, marginal vascular invasion and tumour cell proliferation irrespective of the duration of T2D as well as higher rates of PM of DPYSL3 than non-DM; however, PM of CDK2NA was similar between both groups. PM of DPYSL3 reduced its expression which inversely correlated with reduced patient survival. In conclusion, T2D is associated with poor prognosis of NBNC-HCC in which a high frequency of PM of DPYSL3 may play a pivotal role in its pathogenesis.
Subject terms: Cancer, Genetics, Diseases, Endocrinology, Gastroenterology, Oncology, Pathogenesis
Introduction
The rapid increase in the prevalence of type 2 diabetes mellitus (T2D) globally warrants an urgent need for improved disease prevention and management strategies1–3. Additionally, T2D is associated with an elevated risk of various cancers including hepatocellular carcinoma (HCC)4. Moreover, cancer is the leading cause of death in patients with T2D, with HCC being the most prevalent5.
HCC is the sixth most common neoplasm as well as the third leading cause of cancer-related death worldwide and fifth in Japan6,7. Viral hepatitis due to hepatitis B virus (HBV) and hepatitis C virus (HCV) infections was previously the leading cause of HCC. However, the number of patients with HCC in the absence of HBV surface antigen-negative/HCV antibody-negative HCC (NBNC-HCC) is rapidly increasing in Japan8,9, indicating that HCC developed independent of new HBV and HCV infections.
There is a close link between T2D and NBNC-HCC due to their association with non-alcoholic fatty liver disease (NAFLD) and obesity. More than 70% of individuals with T2D are estimated to have NAFLD10,11, of which 20% exhibit clinically relevant hepatic fibrosis, conferring an increased risk of progression to HCC12,13. While T2D and obesity elicit hepatic/peripheral insulin resistance, lipotoxicity, increased oxidative stress and chronic inflammation which result in the development of NBNC-HCC, the impact of T2D on the prognosis of NBNC-HCC has been partially evaluated14.
The molecular pathogenesis and progression of HCC involve multistep pathways encompassing the cooperation of genetic and epigenetic components at all stages of liver carcinogenesis15. In particular, promoter methylation (PM, an epigenetic DNA modification mechanism) is known to inactivate tumour suppressor genes, leading to the development of HCC16. Thus, the PM status of tumour suppressor genes can be related to the prognosis of HCC17,18. One such tumour suppressor gene is dihydropyrimidinase-like 3 (DPYSL3), a cell adhesion molecule expressed in the heart, brain and liver19. The expression of DPYSL3 is downregulated by PM, which may contribute to metastasis in prostate and pancreatic cancers as well as poor prognosis of gastric cancer20–22. In HCC, the reduced expression level of DPYSL3 through PM is inversely correlated with the expression of vascular endothelial growth factor (VEGF) and focal adhesion kinase (FAK), resulting in poor prognosis of HCC23.
Notably, PM is also implicated not only in the pathogenesis of T2D but also in cancer development complicated with T2D24–26. We previously demonstrated that enhanced PM of CDH1 in long-term T2D is associated with poor prognosis of pancreatic ductal cancer (PDC)26. Therefore, we hypothesised that T2D similarly enhances PM of DPYSL3 and deteriorates the prognosis of NBNC-HCC.
In this study, we evaluated the change in clinical outcome in NBNC-HCC confounded by T2D and the epigenetic modification of DPYSL3.
Results
Minimum impacts of T2D on clinicopathological changes in NBNC-HCC
The clinicopathological characteristics of the study patients are summarised in Table 1. The median age was 69 years (range, 48–82) in non-diabetic subjects (non-DM) and 67 years (range, 41–79) in diabetic subjects (DM). BMI was similar between non-DM (24.0; range, 19.5–32.7) and DM (23.7; range, 15.1–30.8). The median duration of diabetes in all patients was 9 years (range, 1–40). The pre- and post-surgery blood glucose and glycated haemoglobin (HbA1c) levels were significantly higher in DM than in non-DM (p < 0.01). The post-surgery elevated HbA1c levels (ΔHbA1c) were comparable between both groups. There were 22 patients (12 in DM and 10 in non-DM) with alcoholic liver disease (ALD) and 66 (32 in DM and 34 in non-DM) with a history of alcohol use. Transaminase, γ-GTP and tumour marker levels were comparable between both groups. Incidence of non-cancerous tumour of the liver was not significantly different between DM and non-DM. Recurrence after surgery was observed in 45 patients (24 in DM and 21 in non-DM), with 14 (8 in DM and 6 in non-DM) showing metastatic recurrence. There was no significant difference in terms of the site of recurrence between both groups. Pathological evaluation showed that the distribution of histological grade (wel and mod/por) was comparable between DM (41 and 6 cases, respectively) and non-DM (40 and 6 cases, respectively). There were no significant differences between the two groups in terms of capsule formation, capsule infiltration, septal formation and serosal invasion. Vascular invasion and tumour number had marginally, but not significantly, increased in DM compared with those in non-DM (p = 0.09 for vascular invasion and p = 0.07 for tumour number). The prevalence of high-grade tumour [Union for International Cancer control (UICC) stage II or III] was significantly higher in DM than in non-DM (p < 0.05).
Table 1.
Clinical and pathological profiles of examined subjects.
| non-DM | DM | p-value | |
|---|---|---|---|
| Number (male/female) | 46 (36/10) | 47 (42/5) | 0.17 |
| Age (years) | 67 (41−79) | 69 (48−82) | 0.30 |
| Body mass index | 24.0 (19.5−32.7) | 23.7 (15.1−30.8) | 0.39 |
| Duration of diabetes (years) | 9.0 (1.0−40.0) | ||
|
HbA1c (NGSP, %) (pre-operation) |
5.7 (5.1−6.2) | 7.0 (5.5−11.4) | <0.01 |
|
HbA1c (NGSP, %) (post-operation) |
5.7 (5.3−6.2) | 6.3 (4.9−7.5) | <0.01 |
|
ΔHbA1c (NGSP, %) (pre-operation minus post-operation) |
0.0 (−0.4–0.3) | 1.8 (−1.0–5.3) | 0.20 |
| Diabetes therapy: | |||
| Unknown | 6.4% (3/47) | ||
| Diet | 27.7% (13/47) | ||
| Oral hypoglycemic agent | 48.9% (23/47) | ||
| Insulin | 17.0% (8/47) | ||
| History of dyslipidemia | 8.7% (4/46) | 17.0% (8/47) | 0.36 |
| History of hypertension | 43.5% (20/46) | 57.5% (27/47) | 0.22 |
| Smoking habits (overall) | 47.8% (22/46) | 55.3% (26/47) | 0.53 |
|
Smoking habits (Brinkman index ≧400) |
30.4% (14/46) | 38.3% (18/47) | 0.51 |
| Alcohol habit | 71.7% (33/46) | 68.1% (32/47) | 0.20 |
| AST (IU/L) | 39.0 (18.0−136.0) | 32.0 (13.0−193.0) | 0.28 |
| ALT (IU/L) | 32.0 (15.0−131.0) | 35.0 (8.0−157.0) | 0.92 |
| γ-GTP (IU/L) | 89.5 (17−339) | 78.0 (19−592) | 0.38 |
| AFP (ng/mL) | 7.6 (1.2−24596) | 7.1 (1.3−8209) | 0.80 |
| PIVKA-II (μg/mL) | 90.0 (16.0−75000.0) | 332.0 (15.0−60973.0) | 0.16 |
| Alcoholic liver | 26.1% (12/46) | 21.3% (10/47) | 0.63 |
| Background liver: | 0.91 | ||
| Normal liver | 13.0% (6/46) | 17.0% (8/47) | |
| Chronic hepatitis | 60.9% (28/46) | 59.6% (28/47) | |
| Cirrhosis | 26.1% (12/46) | 21.3% (10/47) | |
| Histological grade: | 0.53 | ||
| wel or mod | 87.0% (40/46) | 87.2% (41/47) | |
| por | 13.0% (6/46) | 12.8% (6/47) | |
| Growth type: | 0.53 | ||
| Expansive growth | 87.0% (40/46) | 85.1% (40/47) | |
| Invasive growth | 8.7% (4/46) | 14.9% (7/47) | |
| Formation of capsule | 78.3% (36/46) | 80.9% (38/47) | 1.00 |
| Infiltration to capsule | 58.7% (27/46) | 61.7% (29/47) | 1.00 |
| Septum formation | 69.6% (32/46) | 76.6% (36/47) | 0.81 |
| Serosal infiltration | 4.3% (2/46) | 6.4% (3/47) | 1.00 |
| Total vascular invasion: | 30.4% (14/46) | 51.1% (24/47) | 0.09 |
| Portal vein invasion | 24.0% (11/46) | 38.3% (18/47) | 0.18 |
| Hepatic vein invasion | 19.6% (9/46) | 25.3% (12/47) | 0.62 |
| Hepatic artery invasion | 4.4% (2/46) | 6.4% (3/47) | 1.00 |
| Biliary duct invasion | 4.4% (2/46) | 8.5% (4/47) | 0.68 |
| Tumor number | 1.0 (1.0−2.0) | 1.0 (1.0−5.0) | 0.07 |
| Tumor size (mm) | 51.0 (10.0−180.0) | 45.0 (12.0–180.0) | 0.68 |
| UICC stage(8th): | 0.02 | ||
| I | 63% (29/46) | 34% (16/47) | |
| II | 33% (15/46) | 55% (26/47) | |
| III | 4% (2/46) | 11% (5/47) | |
| Recurrence | 46% (21/46) | 51% (24/47) | 0.68 |
| Recurrence by metastasis | 13% (6/46) | 17% (8/47) | 1.00 |
NGSP; National Glycohemoglobin Standardization Program, AST; Aspartate transaminase, ALT; Alanine transaminase, γ-GTP; γ-glutamyl transpeptidase, AFP; α-fetoprotein, PIVKA-II; protein induced by vitamin K absence or antagonist-II, wel; well-differentiated adenocarcinoma, mod; moderate-differentiated adenocarcinoma, por; poorly differentiated adenocarcinoma. Median(range).
Accelerated PM of DPYSL3 in T2D
Methylation-specific polymerase chain reaction (MSP) was performed using primers designed for DPYSL3 and CDK2NA to examine methylation (M) and unmethylation (U) in promoter regions in 10 cases followed by DNA sequencing. A positive M band indicates significant methylation of the CpG region of the promoter region (Supplemental Fig. S1a). DNA sequencing of the MSP product confirmed methylation (Supplemental Fig. S1b) of cytosine, which was not converted to thymine. All CpG motifs in the promoter region of DPYSL3 were methylated.
Based on previously confirmed accuracy and efficacy of MSP primers for CDK2NA in formalin-fixed, paraffin embedded (FFPE) specimens26, we found that the frequency of PM of CDK2NA in NBNC-HCC was not significantly different between DM (43%) and non-DM controls (33%) (p = 0.39) (Table 2). Similarly, the frequency of PM of CDK2NA in the non-cancerous tissue was not significantly different between DM (26%) and non-DM (11%) (p = 0.11). On the other hand, the frequency of PM of DPYSL3 significantly increased in DM (77%) compared with that in non-DM (22%) in the cancerous tissue (p < 0.01). This pattern was also observed in the non-cancerous tissue wherein the frequency of PM of DPYSL3 was significantly higher in DM (66%) than in non-DM (33%) (p < 0.01). Thus, DPYSL3 was significantly methylated in DM compared with that in non-DM irrespective of the occurrence of NBNC-HCC.
Table 2.
Promoter methylation analysis.
| Gene | non-DM (n = 46) | DM (n = 47) | ||
|---|---|---|---|---|
| Tumor | Non-tumor | Tumor | Non-tumor | |
| CDKN2A/P16 | 33% (15/46) | 11% (5/46) | 43% (20/47) | 26% (12/47) |
| DPYSL3 | 22% (10/46) | 33% (15/46) | 77% (36/47)* | 66% (31/47)* |
*p < 0.01 vs non-DM.
DPYSL3 and p16 expression evaluated by immunohistochemistry
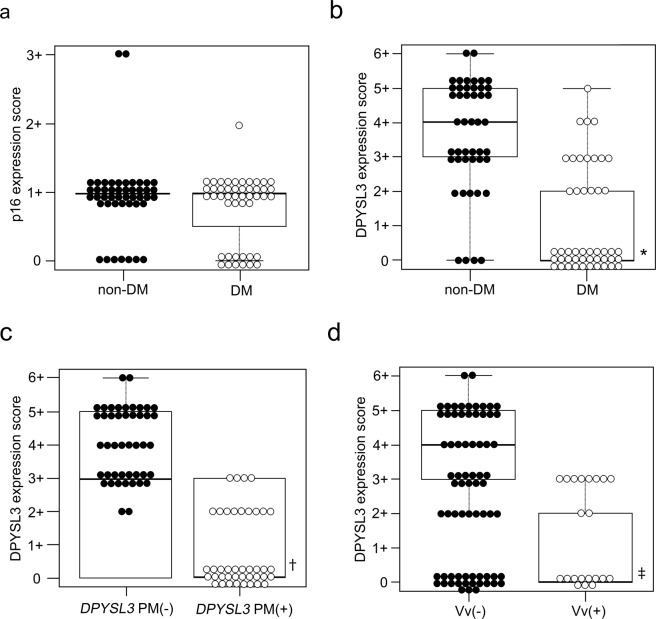
Immunohistochemical evaluation revealed that the diagnosis of T2D did not affect the frequency of p16, coded by CDK2NA, cells labelled positive in non-cancerous and cancerous tissues in NBNC-HCC (Figs. 1a,d and 2a). Supporting this data, the expression score of Ki67 was comparable between non-DM and DM in the cancerous tissue (Supplemental Fig. S2). On the other hand, the expression of DPYSL3 was preserved in both non-cancerous and cancerous tissues in non-DM controls, whereas its expression significantly reduced in DM (p < 0.01) (Figs. 1e–h and 2b). The protein expression score of DPYSL3 in cancerous tissue was irreversibly correlated with PM of DPYSL3 (p < 0.01) (Fig. 2c), and it reduced in patients with NBNC-HCC showing hepatic vein invasion (Vv+) compared with that in patients showing non-venous invasion (Vv−) (p < 0.01) (Fig. 2d).
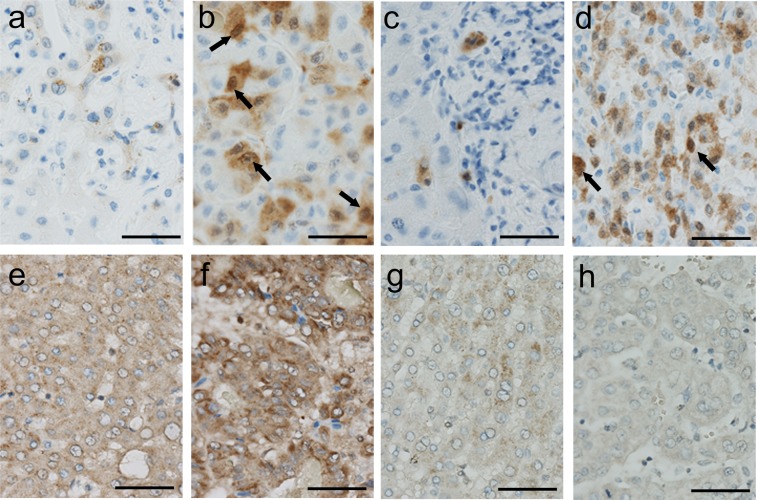
Figure 1.
Expression of p16 and DPYSL3 in NBNC-HCC subjects. p16 expression (arrows) was sparse in the non-cancerous tissue (a) but was retained in the cancerous tissue (b) in non-DM. T2D failed to influence the expression of p16 in non-cancerous (c) and cancerous (d) tissues in NBNC-HCC. Diffuse expression of DPYSL3 was apparent in the non-cancerous (e) tissue and cancerous tissue (f) in non-DM, but its expression was attenuated in the non-cancerous (g) and cancerous (h) tissues in DM (scale bar = 50 μm).
Figure 2.
P16 and DPYSL3 expression score. p16 expression score in the cancerous tissue was comparable between non-DM and DM (a); Lower expression of DPYSL3 was evident in NBNC-HCC complicated with T2D (p < 0.01) (b); The expression of DPYSL3 significantly reduced in DPYSL3-PM (+) compared with that in DPYSL3-PM (−) (p < 0.001) (c); the DPYSL3 expression score was attenuated in the hepatic vein invasion group (d). Vv; hepatic vein invasion. *p < 0.01 vs non-DM, †p < 0.01 vs DPYSL3-PM (−), ‡p < 0.01 vs Vv (−).
Survival of patients with NBNC-HCC complicated with T2DM or PM of DPYSL3
Univariate analysis for disease-specific survival (DSS) showed that tumour multiplicity (multiple), serosal invasion, portal vein invasion, hepatic vein invasion, T2D history and PM of DPYSL3 were the significant risk factors for reduced survival (Table 3). Smoking habit and history of metabolic disorders other than T2D such as hypertension and dyslipidaemia were not correlated with DSS (Table 3). Diabetes treatment was not correlated with DSS (Supplemental Table S1). Multivariate analysis further confirmed that hepatic vein invasion, tumour multiplicity (multiple), history of T2D and PM of DPYSL3 remained the significant risk factors for reduced survival (Table 4).
Table 3.
Univariate analysis of clinicopathological factors and disease-specific survival after resection of NBNC hepatocellular carcinoma (log-rank test).
| Variable | Median DSS (month) | p-value |
|---|---|---|
| Age: <68 vs ≧68 | 56.0 vs 46.5 | 0.481 |
| Male vs Female | 43.0 vs 54.0 | 0.599 |
| BMI: <23.9 vs ≧23.9 | 48.0 vs 52.0 | 0.453 |
| Tumor multiplicity: single vs multiple | 52.0 vs 40.5 | 0.0028 |
| Tumor size (mm): <20 vs ≧20 | 59.0 vs 47.0 | 0.129 |
| Tumor differentiation: wel, mod vs por | 44.0 vs 42.5 | 0.333 |
| Growth type: Expansive growth vs invasive growth | 49.5 vs 51.5 | 0.963 |
| Serosal infiltration: (−) vs (+) | 53.0 vs 38.0 | 0.007 |
| Formation of capsule: (−) vs (+) | 69.5 vs 45.5 | 0.131 |
| Infiltration to capsule: (−) vs (+) | 59.0 vs 45.5 | 0.392 |
| Septum formation: (−) vs (+) | 59.0 vs 45.5 | 0.110 |
| Bile duct invasion: (−) vs (+) | 52.0 vs 51.0 | 0.697 |
| Hepatic artery invasion:(−) vs (+) | 51.0 vs 56.0 | 0.193 |
| Portal vein invasion: (−) vs (+) | 54.0 vs 43.0 | 0.007 |
| Hepatic vein invasion: (−) vs (+) | 56.5 vs 38.0 | <0.001 |
| T1-2 vs T3 (UICC) | 51.5 vs 41.0 | 0.394 |
| Alcoholic liver damage: (−) vs (+) | 51.5 vs 82.25 | 0.841 |
| Alcohol habit: (−) vs (+) | 46.0 vs 54.0 | 0.243 |
| History of T2D: (−) vs (+) | 62.0 vs 41.0 | 0.002 |
| HbA1c (%): <6.1 vs ≧6.1 | 50.5 vs 45.5 | 0.700 |
| Blood glucose (mmol/L): <112.5 vs ≧112.5 | 60.0 vs 42.0 | 0.555 |
| History of hypertension: (−) vs (+) | 58.0 vs 46.0 | 0.832 |
| History of dyslipidemia: (−) vs (+) | 53.0 vs 44.0 | 0.644 |
| Smoking habits (overall): (−) vs (+) | 59.0 vs 45.0 | 0.726 |
| Smoking habits (Brinkman index≧400): (−) vs (+) | 54.0 vs 49.0 | 0.900 |
| DPYSL3 promoter methylation: (−) vs (+) | 62.0 vs 43.5 | 0.009 |
| CDK2NA promoter methylation: (−) vs (+) | 54.0 vs 42.0 | 0.552 |
DSS; Disease-specific survival, BMI; body mass index, T2D; type2 diabetes, wel; well-differentiated adenocarcinoma, mod; moderate-differentiated adenocarcinoma, por; poorly differentiated adenocarcinoma.
Table 4.
Multivariate analysis of clinicopathological factors and disease-specific survival after resection of NBNC hepatocellular carcinoma (Cox proportional hazards model).
| Variable | Hazard ratio | 95%CI | p-value |
|---|---|---|---|
| Hepatic vein invasion | 2.489 | 1.128–5.493 | 0.024 |
| Tumor multiplicity (multiple) | 2.520 | 1.091–6.318 | 0.011 |
| History of T2D | 2.017 | 1.061–5.194 | 0.035 |
| DPYSL3 promoter methylation | 2.656 | 1.210–6.483 | 0.024 |
95% CI; confidence interval, T2D; type2 diabetes.
Univariate analysis for overall survival (OS) showed that tumour multiplicity (multiple), serosal invasion, portal vein invasion, hepatic vein invasion, T3-4 cancer [UICC stage (8th)], history of T2D and PM of DPYSL3 were the significant risk factors for reduced survival (Supplemental Table S2). However, smoking habit as well as a history of dyslipidaemia and hypertension was not correlated with OS (Supplemental Table S2). Diabetes treatment was also not correlated with OS (Supplementary Table S3). Multivariate analysis further confirmed that hepatic vein invasion, serosal invasion and history of T2D remained the significant risk factors for reduced survival (Supplemental Table S4).
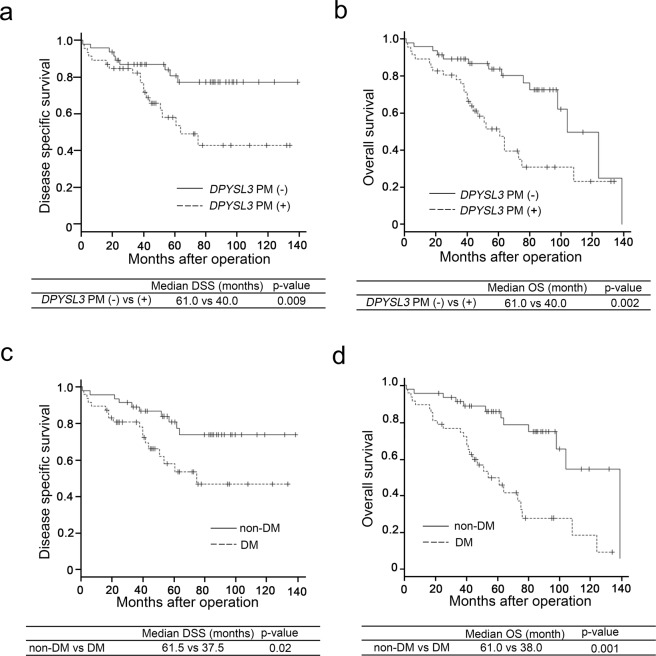
The Kaplan–Meier survival curve indicated a shortened DSS in DM compared with that in non-DM (Fig. 3a). OS also decreased in DM compared with that in non-DM (Fig. 3b). In addition, PM of DPYSL3 reduced DSS and OS (Fig. 3c,d).
Figure 3.
Survival curves based on disease-specific survival (DSS) and overall survival (OS). Results for survival curve of the DPYSL3-PM (+) group (fine break line) were significantly lower than those of the DPYSL3-PM (−) group (solid line) in terms of DSS (a) (p < 0.05) and OS (b) (p < 0.01). DM (fine break line) showed significantly worsened DSS (c) (p < 0.01) and OS (d) (p < 0.01) in NBNC-HCC than non-DM (solid line).
Discussion
To the best of our knowledge, for the first time, we demonstrated that T2D had a negative impact on DSS and OS of patients with NBNC-HCC. The UICC stage of NBNC-HCC was significantly higher in DM than in non-DM. While DM exhibited enhanced PM of DPYSL3 compared with non-DM, PM of CDK2NA was not influenced by T2D. The protein expression of DPYSL3 in tissues was inversely correlated with PM of DPYSL3, whose methylation status was also found to be an independent prognostic factor in multivariate analysis of DSS in patients with NBNC-HCC.
Metabolic disorders such as T2D and obesity are risk factors for HCC4,12–14. In this study, we identified that T2D was an independent prognostic factor for NBNC-HCC apart from known prognostic factors such as hepatic vein invasion, tumour multiplicity and epigenetic modifications involving PM of DPYSL318,27. While a previous report showed significantly poorer long-term prognosis in obese patients with recurrent HCC mainly arising from viral hepatitis than in non-obese patients28, obesity showed minimum effects on the prognosis of NBNC-HCC in the present study. In parallel with our result, Nishikawa et al. showed that obesity did not affect survival in patients with NBNC-HCC after curative therapy29. Therefore, obesity may not be a stronger risk factor for worsening of the prognosis of NBNC-HCC than diabetes. Diabetes can exacerbate chronic inflammation, formation of advanced glycation end-products and aberrant insulin signalling, which trigger tumour formation and progression30–32. These indicate the importance of intervening for the improvement of lifestyle and of maintaining good glycaemic control, leading to the prevention of not only the onset of NBNC-HCC but also an unfavourable outcome.
Smoking habits are known to be a prognostic factor for HCC, whereas our results showed that only diabetes was correlated with reduced OS and DSS. Evans et al. showed that a significant increase in the risk of HCC was demonstrated among female smokers but not among male smokers33. Because our cohort was male dominant, no significant correlation was found between smoking habit and DSS/OS.
Long-term T2D (>3 years) was also a significant factor in the poor prognosis of PDC. In short-term T2D, tumour resection significantly lowered HbA1c level26,34, suggesting that short-term T2D is a transient diabetic state exerted by PDC. However, regardless of the duration, T2D was a poor prognostic factor for NBNC-HCC. We showed that ΔHbA1c remained unchanged after resection in NBNC-HCC, indicating its poor influence on glucose homeostasis. Therefore, T2D manifested in NBNC-HCC is‘real’ diabetes which impacts the prognosis of NBNC-HCC similar to long-term T2D in PDC.
As described earlier, T2D deteriorated OS and DSS in PDC wherein long-term T2D was associated with a poor histological grade, vein invasion and a high frequency of PM of CDH126. In contrast, except the UICC stage obtained in our study, the impact of T2D on each clinical and pathological characteristic of NBNC-HCC was minimum although the patients with T2D exhibited shorter DSS and OS than those without T2D. UICC stages are graded by tumour size (≤5 or >5 cm), tumour number (solitary or multiple), vascular invasion and direct invasion of adjacent organs35. The marginal, but non-significant, increase in tumour number and vascular invasion found in the presence of T2D suggest that the combined effect of these variables increases the UICC stage, leading to worse prognosis even if each variable has a small effect.
Suppression of DPYSL3 expression in HCC specimens was inversely correlated with a high expression of VEGF and FAK, which are associated with epithelial–mesenchymal transition (EMT)23. A hallmark of EMT is a change in cell morphology into spindle-shaped mesenchymal cells and notable downregulation of DPYSL3 which caused morphological changes into spindle- and fibroblast-like shape in a lung cancer cell line36, conferring these cells high invasion ability. This was borne out in NBNC-HCC complicated with T2D where low DPYSL3 expression enhanced hepatic vein invasion. However, regardless of T2D, tumour differentiation was comparable in patients with HCC accompanied by low DPYSL3 expression, corroborating previous findings showing increased capsule infiltration and vascular invasion23. These results suggest that EMT can partially be implicated in the poor prognosis of NBNC-HCC complicated with T2D and that mechanisms other than EMT regulate invasion in HCC complicated with PM of DPYSL3.
Although the prevalence of PM of DPYSL3 did not increase in the non-cancerous tissue in a previous report23, we observed this characteristic in NBNC-HCC complicated with T2D. This may be ascribed to a strong potential of diabetes to elicit PM24–26, leading to an increase in tumour malignancy in NBNC-HCC cases with T2D.
On the other hand, CDK2NA is a common target of PM in HCC37–39. The frequency of CDK2NA PM-positive cases was lower in NBNC-HCC cases complicated with DM than in non-DM in our study. We attributed this to differential translational regulation of CDK2NA and DPYSL3 because the prevalence and pattern of PM may be organ and carcinoma specific. Further, diabetes may modify the methylation pattern in each organ and carcinoma; therefore, it may be necessary to perform a comprehensive PM analysis using next-generation sequencing to characterise the PM status elicited by T2D in NBNC-HCC.
The progression of hepatic fibrosis and non-alcoholic steatohepatitis (NASH)/NAFLD score was not affected by T2D in this study, with the caveat being that NASH in patients with HCC was difficult to evaluate because of the need for its histological diagnosis. In end-stage NASH, the pathological characteristics can be lost and be, in effect, ‘burned out’ in which case a diagnosis of cryptogenic cirrhosis is made instead of NASH40. Thus, it has been acknowledged that a substantial proportion of patients with cryptogenic cirrhosis may actually have previously unrecognised NASH because patients with cryptogenic cirrhosis have a high prevalence of obesity and/or T2D41–43.
Treatment for T2D can also influence the onset of HCC and its associated mortality. While insulin and sulfonyl urea are associated with an increased risk of HCC, metformin treatment lowers this risk44,45. We found that metformin and insulin were not independent prognostic factors for DSS and OS in patients with NBNC-HCC. This might be attributed to the relatively small sample size (27 cases including 8 on metformin therapy and 10 on insulin therapy) in this study. We propose a future evaluation of T2D treatment effect with a larger number of cases.
NASH- and ALD-related HCC have different clinical course and prognosis, with the most inferior OS being identified in NASH-related HCC46,47. ALD-related HCC exhibited marginally high frequency of extra-hepatic malignancy compared with NASH-related HCC48. Because our cohort included patients with ALD-related HCC and those with non-ALD-related HCC, their distribution may have influenced the worse prognosis of diabetes. However, the ratio of patients with ALD-related HCC was comparable between non-DM and DM (26% vs 21%). Further, while OS and DSS were evaluated for different types of HCC including ALD-related HCC, no significant difference was identified among these types in this study; this was possibly because of the small number of ALD-related HCC cases (22/93, 23.7%). As another possibility, the aetiology in some non-ALD-related HCC cases was not associated with NASH because the pathological confirmation for NASH was not achieved in all non-ALD-related HCC cases due to the ‘burned out’ phenomenon. In addition to the ‘burned out’ phenomenon, because a significant ratio of our patients had a history of alcohol use, it is difficult to completely distinguish the aetiology of NASH- and ALD-related HCC.
This study has certain limitations. First, this study was a retrospective study conducted using only FFPE specimens; therefore, a future study should involve appropriate specimens including fresh specimens to examine dynamic glucose metabolism. Second, both alcoholic and non-alcoholic livers formed the study sample for NBNC-HCC, and it may be necessary to independently examine these groups. However, we based our design on a previous study which showed comparable pathological grading and parameters of HCC between alcoholic and non-alcoholic fatty livers49. Third, we investigated PM of only CDK2NA and DPYSL3 in this study. Methylated genes other than CDK2NA and DPYSL3 may be implicated in an unfavourable prognosis of NBNC-HCC complicated with T2D, particularly because a recent genome-wide study showed PM of several tumour suppresser genes implicated in the tumorigenesis of NBNC-HCC50. Nevertheless, the results of our study support evidence in favour of interventions to alleviate metabolic disorders for better prognosis of NBNC-HCC complicated with T2D. It is hoped that future studies will explore the possibility of developing effective demethylating agents for these diseases.
Methods
Patients
Between January 2005 and December 2016, patients who were diagnosed with NBNC-HCC and underwent initial hepatic resection at Hirosaki University Hospital, Aomori Prefectural Central Hospital, Hakodate Municipal Hospital and Hachinohe City Hospital were included in this retrospective study based on clinical data extracted from medical records. All investigations and experiments were performed after receiving the permission of the Ethical Committee of Hirosaki University Graduate School of Medicine (approved number #2017-162), and they were performed according to the guidelines of the Ethics Committee on Human Research Samples at the Japanese Society of Pathology. Informed consent was obtained from all participants and/or their legal guardians.
Among patients with HCC, those who were seronegative for HBVAg (HBsAg), HBVAb (HBsAb and HBcAb), and HCVAb without autoimmune liver disease, Wilson’s disease or hemochromatosis was considered to indicate NBNC-HCC. Diabetic patients with a history of hyperglycaemia fulfilled the criteria of diabetes proposed by the Japan Diabetes Society51. Patients with ALD were diagnosed based on habitual daily alcohol consumption of >40 g for men and >20 g for women and negativity for markers of HBV and HCV in their medical records. Hypertension was defined as blood pressure of ≥140/90 mm Hg or history of treatment for hypertension. Dyslipidaemia was defined as a total serum cholesterol level of ≥220 mg/dL, triglyceride level of ≥150 mg/dL or a prescription for dyslipidaemia. ‘Smoking habits (overall)’ was defined as a condition in which an individual continued to smoke within pre-surgery 1 year. A total of 94 assessed patients were screened and 93 were enrolled and split into a diabetic group (DM, 42 male/5 female) and non-diabetic group (non-DM, 36 male/10 female).
Histopathological assessment
Histopathological assessment was independently performed using H&E-stained sections of each sample by three pathologists (HM, KK and CI). Pathological diagnosis of HCC was performed according to the 2010 WHO Classification of Tumours of the Digestive System, whereas staging was performed based on the 8th edition of UICC51. Histological differentiation was divided into three stages according to cell and structural variants52: well-differentiated carcinoma (wel), moderately differentiated adenocarcinoma (mod) and poorly differentiated adenocarcinoma (por). When ≥2 tissue types and regions showing various degrees of differentiation were mixed, they were grouped according to the predominant tissue type and degree of differentiation.
Genetic analysis
DNA extraction and bisulfite DNA modification
Tumour tissues without haemorrhage, necrosis or severe inflammation were selected. Non-tumour tissues adjacent to the tumour were selected. DNA extraction and bisulfite DNA modification were performed following the protocol reported previously26. In brief, DNA was extracted from FFPE sections (10-μm thickness) following the manufacturer’s instructions provided in the DNA extraction kit for FFPE (Qiagen K.K., Tokyo, Japan). Bisulfite DNA modification was conducted on the extracted samples with a commercially available kit (EpiTect Fast Bisulfite Conversion Kits, Qiagen K.K.). This process converts unmethylated cytosine residues to uracil while methylated cytosine residues remain unchanged53.
MSP
MSP was performed as previously described using primers for DPYSL354 and CDK2NA26. The PCR product was electrophoresed on a 3% agarose gel. A positive methylated band (M) indicated high rates of methylation of the CpG region. PM of DPYSL3 and CDK2NA was confirmed by Sanger sequencing26. MSP products were ligated using the TOPO cloning vector (Thermo Fisher Scientific K.K., Yokohama, Japan). After blue–white selection, a purified vector was digested with EcoRI. A 150-bp insert was sequenced using the ABI Prism 310 sequence analyser (Thermo Fisher Scientific K.K.) on the positive clone labelled using the VIC dye sequence kit (Thermo Fisher Scientific K.K.).
Immunohistochemical analysis
An automated immunohistochemistry instrument was applied for immunohistochemical analysis (Benchmark Ultra Automated Slide Preparation system, Ventana Medical Systems, Inc., Tucson, AZ, USA) as shown in our previous study26. Antibodies for p16 (Clone E6H4, pre-diluted, Ventana Medical Systems, Inc.) and DPYSL3 (Clone 1B8, LS-C133161, LifeSpan BioSciences, Seattle, WA, USA) were used. Staining intensity (score 0: no staining, 1: weaker staining than normal liver, 2: similar staining as normal liver and 3: stronger staining than normal liver) and staining range (score 0: no staining, 1: <30% staining, 2: 30%–69.9% staining and 3: >70% staining) were semiquantitatively scored. Further, the sum (minimum 0 to maximum 6) was graded as the degree of DPYSL3 expression. A total score of <4 indicated reduction in protein expression. p16 was evaluated as previously described by Saito et al.26.
Statistical analysis
All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan) and the graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria)55. This modified version of R commander was designed to add statistical functions frequently used in biostatistics. DSS was defined as the time between surgery and death from HCC. OS was calculated as the time between surgery and death from any cause. Continuous variables were analysed using non-parametric methods for non-normally distributed data (Mann –Whitney U-test) and are expressed as median (range). Categorical variables were analysed using the chi-squared test or Fisher’s exact test as appropriate and are expressed as number (percentage). Any variable with p-value of <0.05 on univariate analysis using the above test was considered a candidate for multivariate analysis using a Cox proportional hazards model. Survival curves were constructed using Kaplan–Meier analysis, and p-values were determined by the log-rank test for censored survival data. A p-value of <0.05 was considered statistically significant.
Supplementary information
Acknowledgements
This study was in part by KAKENHI (Grants-in-Aid for Scientific Research) from the Japanese Ministry of Education, Culture, Sports, Science and Technology to HM (#15K09374). Technical assistances from Ms. Saori Ogasawara, Misato Sakamoto and Hiroko Mori of the Department Pathology and Molecular Medicine of Hirosaki University Graduate of Medicine, are highly appreciated. This study was supported in part by KAKENHI (Grants-in-Aid for Scientific Research) from the Japanese Ministry of Education, Culture, Sports, Science and Technology to HM (#18K08462).
Author contributions
Umetsu S.; designed the study, conducted the study for all tables and figures, discussed and interpreted the results and wrote the manuscript. Mizukami H.; designed the study, conducted the study, discussed and interpreted the results and wrote the manuscript. Saito T.; conducted the study for Table 2 and supplemental Fig. 1, interpreted and discussed the results.Uchida C.; conducted the study for Tables 1 and 2, interpreted and discussed the results. Igawa A.; conducted the study for Tables 3 and 4, supplemental Tables S3 and S4. interpreted and discussed the results. Kudo K.; conducted the study for Table 2, interpreted and discussed the results. Itabashi C.; conducted the study for Table 1, interpreted and discussed the results. Osonoi S.; conducted the study for Table 1, interpreted and discussed the results. Guo Danyang; conducted the study for Table 1, interpreted and discussed the results. Sasaki T.; conducted the study for Figure 1, interpreted and discussed the results. Yagihashi S.; designed the study, conducted the study, interpreted and discussed the results and wrote the manuscript. Hakamada K.; designed the study, conducted the study, interpreted and discussed the results.
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-57883-1.
References
- 1.Worldwide trends in diabetes since 1980 a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387:1513–1530. doi: 10.1016/S0140-6736(16)00618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.International Diabetes Federation. IDF Diabetes Atlas, 8th edition, https://www.idf.org/e-library/epidemiology-research/diabetes-atlas.html (2019).
- 3.World Health Organization. Global report on diabetes, https://www.who.int/diabetes/global-report/en/ (2016).
- 4.Wang P, Kang D, Cao W, Wang Y, Liu Z. Diabetes mellitus and risk of hepatocellular carcinoma: a systematic review and meta-analysis. Diabetes/metabolism research and reviews. 2012;28:109–122. doi: 10.1002/dmrr.1291. [DOI] [PubMed] [Google Scholar]
- 5.Kasuga M, et al. Report of the Japan Diabetes Society/Japanese Cancer Association joint committee on diabetes and cancer. Cancer Science. 2013;104:965–976. doi: 10.1111/cas.12203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301–1314. doi: 10.1016/S0140-6736(18)30010-2. [DOI] [PubMed] [Google Scholar]
- 7.Statistics and Information Department, Ministry of Health, Labor and Welfare. Vital statistics 2014, https://www.mhlw.go.jp/toukei/saikin/hw/jinkou/kakutei14/index.html (2015).
- 8.Hiwatashi K, et al. Problems of Long Survival Following Surgery in Patients with NonBNonC-HCC: Comparison with HBV and HCV Related-HCC. Journal of Cancer. 2015;6:438–447. doi: 10.7150/jca.10398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hatanaka K, et al. Clinical characteristics of NonBNonC- HCC: Comparison with HBV and HCV related HCC. Intervirology. 2007;50:24–31. doi: 10.1159/000096309. [DOI] [PubMed] [Google Scholar]
- 10.Williams CD, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–131. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 11.Loomba R, et al. Association between diabetes, family history of diabetes, and risk of nonalcoholic steatohepatitis and fibrosis. Hepatology. 2012;56:943–951. doi: 10.1002/hep.25772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kwok R, et al. Screening diabetic patients for non-alcoholic fatty liver disease with controlled attenuation parameter and liver stiffness measurements: a prospective cohort study. Gut. 2016;65:1359–1368. doi: 10.1136/gutjnl-2015-309265. [DOI] [PubMed] [Google Scholar]
- 13.Koehler EM, et al. Presence of diabetes mellitus and steatosis is associated with liver stiffness in a general population: The Rotterdam study. Hepatology. 2016;63:138–147. doi: 10.1002/hep.27981. [DOI] [PubMed] [Google Scholar]
- 14.Hashimoto E, Tokushige K. Hepatocellular carcinoma in non-alcoholic steatohepatitis: Growing evidence of an epidemic? Hepatology research. 2012;42:1–14. doi: 10.1111/j.1872-034X.2011.00872.x. [DOI] [PubMed] [Google Scholar]
- 15.Nishida N, Goel A. Genetic and epigenetic signatures in human hepatocellular carcinoma: a systematic review. Current genomics. 2011;12:130–137. doi: 10.2174/138920211795564359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pogribny IP, Rusyn I. Role of epigenetic aberrations in the development and progression of human hepatocellular carcinoma. Cancer letters. 2014;342:223–230. doi: 10.1016/j.canlet.2012.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sceusi EL, Loose DS, Wray CJ. Clinical implications of DNA methylation in hepatocellular carcinoma. HPB: the official journal of the International Hepato Pancreato Biliary Association. 2011;13:369–376. doi: 10.1111/j.1477-2574.2011.00303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanda M, Sugimoto H, Kodera Y. Genetic and epigenetic aspects of initiation and progression of hepatocellular carcinoma. World journal of gastroenterology. 2015;21:10584–10597. doi: 10.3748/wjg.v21.i37.10584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blasco H, et al. A rare motor neuron deleterious missense mutation in the DPYSL3 (CRMP4) gene is associated with ALS. Human mutation. 2013;34:953–960. doi: 10.1002/humu.22329. [DOI] [PubMed] [Google Scholar]
- 20.Kawahara T, et al. Quantitative proteomic profiling identifies DPYSL3 as pancreatic ductal adenocarcinoma-associated molecule that regulates cell adhesion and migration by stabilization of focal adhesion complex. PloS one. 2013;8:e79654. doi: 10.1371/journal.pone.0079654.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao X, et al. Expression profiling identifies new function of collapsin response mediator protein 4 as a metastasis-suppressor in prostate cancer. Oncogene. 2010;29:4555–4566. doi: 10.1038/onc.2010.213. [DOI] [PubMed] [Google Scholar]
- 22.Kanda M, et al. Dihydropyrimidinase-like 3 facilitates malignant behavior of gastric cancer. Journal of experimental & clinical cancer research. 2014;33:66. doi: 10.1186/s13046-014-0066-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oya H, et al. Dihydropyrimidinase-like 3 is a putative hepatocellular carcinoma tumor suppressor. Journal of gastroenterology. 2015;50:590–600. doi: 10.1007/s00535-014-0993-4. [DOI] [PubMed] [Google Scholar]
- 24.Crujeiras AB, et al. Genome-wide DNA methylation pattern in visceral adipose tissue differentiates insulin-resistant from insulin-sensitive obese subjects. Translational research. 2016;178:13–24.e15. doi: 10.1016/j.trsl.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Yuan W, et al. An integrated epigenomic analysis for type 2 diabetes susceptibility loci in monozygotic twins. Nature communications. 2014;5:5719. doi: 10.1038/ncomms6719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saito T, et al. Worsened outcome in patients with pancreatic ductal carcinoma on long-term diabetes: association with E-cadherin1 (CDH1) promoter methylation. Scientific reports. 2017;7:18056. doi: 10.1038/s41598-017-18438-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sonohara F, et al. Serosal invasion strongly associated with recurrence after curative hepatic resection of hepatocellular carcinoma: a retrospective study of 214 consecutive cases. Medicine. 2015;94:e602. doi: 10.1097/md.0000000000000602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Utsunomiya T, et al. Impact of obesity on the surgical outcome following repeat hepatic resection in Japanese patients with recurrent hepatocellular carcinoma. World journal of gastroenterology. 2008;14:1553–1558. doi: 10.3748/wjg.14.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nishikawa H, et al. Effect of body mass index on survival after curative therapy for non-B non-C hepatocellular carcinoma. J Gastrointestin Liver Dis. 2013;22:173–181. [PubMed] [Google Scholar]
- 30.Sherry CL, O’Connor JC, Kramer JM, Freund GG. Augmented lipopolysaccharide-induced TNF-alpha production by peritoneal macrophages in type 2 diabetic mice is dependent on elevated glucose and requires p38 MAPK. Journal of immunology. 2007;178:663–670. doi: 10.4049/jimmunol.178.2.663. [DOI] [PubMed] [Google Scholar]
- 31.Basta G, et al. Advanced glycation end products activate endothelium through signal-transduction receptor RAGE: a mechanism for amplification of inflammatory responses. Circulation. 2002;105:816–822. doi: 10.1161/hc0702.104183. [DOI] [PubMed] [Google Scholar]
- 32.Masters SL, et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1beta in type 2 diabetes. Nature immunology. 2010;11:897–904. doi: 10.1038/ni.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Evans AA, et al. Eight-year follow-up of the 90,000-person Haimen City cohort: I. Hepatocellular carcinoma mortality, risk factors, and gender differences. Cancer Epidemiol Biomarkers Prev. 2002;11:369–376. [PubMed] [Google Scholar]
- 34.Pannala R, et al. Prevalence and clinical profile of pancreatic cancer-associated diabetes mellitus. Gastroenterology. 2008;134:981–987. doi: 10.1053/j.gastro.2008.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Annals of surgical oncology. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 36.Yang Y, et al. Inhibition of cell-adhesion protein DPYSL3 promotes metastasis of lung cancer. Respiratory research. 2018;19:41. doi: 10.1186/s12931-018-0740-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hinrichsen I, et al. Promoter methylation of MLH1, PMS2, MSH2 and p16 is a phenomenon of advanced-stage HCCs. PloS one. 2014;9:e84453. doi: 10.1371/journal.pone.0084453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang JC, et al. Promoter hypermethylation of p14 (ARF), RB, and INK4 gene family in hepatocellular carcinoma with hepatitis B virus infection. Tumour biology. 2014;35:2795–2802. doi: 10.1007/s13277-013-1372-0. [DOI] [PubMed] [Google Scholar]
- 39.Nishida N, et al. Aberrant methylation of multiple tumor suppressor genes in aging liver, chronic hepatitis, and hepatocellular carcinoma. Hepatology. 2008;47:908–918. doi: 10.1002/hep.22110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoshioka Y, et al. Nonalcoholic steatohepatitis: cirrhosis, hepatocellular carcinoma, and burnt-out NASH. Journal of gastroenterology. 2004;39:1215–1218. doi: 10.1007/s00535-004-1475-x. [DOI] [PubMed] [Google Scholar]
- 41.Caldwell SH, et al. Cryptogenic cirrhosis: clinical characterization and risk factors for underlying disease. Hepatology. 1999;29:664–669. doi: 10.1002/hep.510290347. [DOI] [PubMed] [Google Scholar]
- 42.Poonawala A, Nair SP, Thuluvath PJ. Prevalence of obesity and diabetes in patients with cryptogenic cirrhosis: a case-control study. Hepatology. 2000;32:689–692. doi: 10.1053/jhep.2000.17894. [DOI] [PubMed] [Google Scholar]
- 43.Yalamanchili K, Saadeh S, Klintmalm GB, Jennings LW, Davis GL. Nonalcoholic fatty liver disease after liver transplantation for cryptogenic cirrhosis or nonalcoholic fatty liver disease. Liver transplantation: official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2010;16:431–439. doi: 10.1002/lt.22004. [DOI] [PubMed] [Google Scholar]
- 44.Donadon V, et al. Antidiabetic therapy and increased risk of hepatocellular carcinoma in chronic liver disease. World journal of gastroenterology. 2009;15:2506–2511. doi: 10.3748/wjg.15.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fujita K, et al. Diabetes mellitus and metformin in hepatocellular carcinoma. World journal of gastroenterology. 2016;22:6100–6113. doi: 10.3748/wjg.v22.i27.6100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Younossi ZM, et al. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology. 2015;62:1723–1730. doi: 10.1002/hep.28123. [DOI] [PubMed] [Google Scholar]
- 47.Hester CA, Rich NE, Singal AG, Yopp AC. Comparative Analysis of Nonalcoholic Steatohepatitis- Versus Viral Hepatitis- and Alcohol-Related Liver Disease-Related Hepatocellular Carcinoma. J Natl Compr Canc Netw. 2019;17:322–329. doi: 10.6004/jnccn.2018.7105. [DOI] [PubMed] [Google Scholar]
- 48.Kodama K, Tokushige K, Hashimoto E, Taniai M, Shiratori K. Hepatic and extrahepatic malignancies in cirrhosis caused by nonalcoholic steatohepatitis and alcoholic liver disease. Alcohol Clin Exp Res. 2013;37(suppl 1):E247–52. doi: 10.1111/j.1530-0277.2012.01900.x. [DOI] [PubMed] [Google Scholar]
- 49.Kimura T, et al. Clinicopathological characteristics of non-B non-C hepatocellular carcinoma without past hepatitis B virus infection. Hepatology research. 2017;47:405–418. doi: 10.1111/hepr.12762. [DOI] [PubMed] [Google Scholar]
- 50.Dreval K, Tryndyak V, de Conti A, Beland FA, Pogribny IP. Gene Expression and DNA Methylation Alterations During Non-alcoholic Steatohepatitis-Associated Liver Carcinogenesis. Frontiers in genetics. 2019;10:486. doi: 10.3389/fgene.2019.00486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seino Y, et al. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. Journal of diabetes investigation. 2010;1:212–228. doi: 10.1111/j.2040-1124.2010.00074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bosman, F. T., Carneiro, F., Hruban, R. H. & Theise, N. D. WHO classification of tumours of the digestive system. (World Health Organization, 2010).
- 53.Clark SJ, Harrison J, Paul CL, Frommer M. High sensitivity mapping of methylated cytosines. Nucleic acids research. 1994;22:2990–2997. doi: 10.1093/nar/22.15.2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Herman JG, Graff JR, Myohanen S, Nelkin BD, Baylin SB. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:9821–9826. doi: 10.1073/pnas.93.18.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone marrow transplantation. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during the current study are available from the corresponding author on reasonable request.