Abstract
The pathogenesis of malaria is a multifactorial syndrome associated with a deleterious inflammatory response that is responsible for many of the clinical manifestations. While dendritic cells (DCs) play a critical role in initiating acquired immunity and host resistance to infection, they also play a pathogenic role in inflammatory diseases. In our recent studies, we found in different rodent malaria models that the monocyte-derived DCs (MO-DCs) become, transiently, a main DC population in spleens and inflamed non-lymphoid organs. These studies suggest that acute infection with P. berghei promotes the differentiation of splenic monocytes into inflammatory monocytes (iMOs) and thereafter into MO-DCs that play a pathogenic role by promoting inflammation and tissue damage. The recruitment of MO-DCs to the lungs and brain are dependent on expression of CCR4 and CCR5, respectively, and expression of respective chemokine ligands in each organ. Once they reach the target organ the MO-DCs produce the CXCR3 ligands (CXCL9 and CXCL10), recruit CD8+ T cells, and produce toxic metabolites that play an important role in the development of experimental cerebral malaria (ECM) and acute respiratory distress syndrome (ARDS).
Keywords: monocytes, inflammatory monocytes, monocyte derived dendritic cells, macrophages, dendritic cells, T lymphocytes, chemokines, Plasmodium, malaria, pathogenesis
Introduction
According to the World Health Organization (WHO 2017), in 2017, 216 million malaria cases were reported in 91 countries. Amongst those cases, 445,000 resulted in death, primarily in children under five years old. The clinical outcome of the disease is affected by factors such as parasite and host genetics, previous exposure to infection, age, nutritional, geographic and socioeconomic factors. The most common early signs and symptoms of malaria in non-immune individuals, such as fever (paroxysm), nausea, headache and lethargy, are associated with rupture of schizonts and consequent inflammatory response. Activation of immune cells and systemic inflammation in response to the infection also lead to tissue damage and severe clinical manifestations such as anemia, jaundice, metabolic acidosis, respiratory distress, cerebral malaria, multiple organ failure and death.
The clinical manifestations of malaria result from three main events: excessive inflammation, destruction of red blood cells (RBCs) and cytoadherence of infected RBCs (iRBCs). The pro-inflammatory cytokines produced by the host stimulate the expression of adhesion molecules in endothelial cells. This promotes cytoadherence of iRBCs to the small capillary veins, formation of blood clogs, edema, and leukocytes diapedesis to the inflamed areas. These inflammatory mediators also contribute to anemia by augmenting the phagocytosis of altered RBCs and inhibition of erythropoiesis [1,2].
In this review, we will discuss the importance of monocytes (MOs) and MO-derived dendritic cells (MO-DCs) as the main source of pro-inflammatory cytokines during acute infection with Plasmodium and their role on malaria pathogenesis.
Monocyte progenitors and differentiated effector cells
MOs are highly plastic cells that, when activated by microbial components and pro-inflammatory cytokines, can differentiate to more specialized cell types. MOs, macrophages and dendritic cells (DCs) are heterogeneous cell populations that have a critical role in sensing the presence of invading microorganisms and initiating protective immune responses. While presenting overlapping characteristics, MOs, macrophages and DCs can be distinguished based on specific surface markers, transcriptional signatures and certain specialized functions [3-6].
MOs originate from the myeloid lineage and are found in a resting stage in the bone marrow (BM), blood and peripheral lymphoid organs. Once activated by cytokines, such as IFNγ and TNFα, or pathogen associated microbial patterns (PAMPs), MOs differentiate into inflammatory MOs (iMOs) that migrate to non-lymphoid and lymphoid tissues, where they further differentiate into macrophages or MO-DCs. Additionally, iMOs may also act as effector cells by producing pro-inflammatory mediators and toxic metabolites that effectively kill microbes. MOs can be activated by IL-4 and differentiate into alternative macrophages (aMacs). This pathway is of particular relevance in worm infections, where aMacs play distinct roles in the immune response as well as on tissue homeostasis and repair [7]. In addition, both the iMOs and aMacs have distinct regulatory activities that protect the host from an excessive and a deleterious inflammatory reaction [8,9].
Each tissue has its own reservoir of embryonically developed cells that maintain the pool of tissue resident macrophages, without a major input of hematopoietic cells [4,6,10]. These resident phagocytes and aMacs have an important role on tissue homeostasis by clearing dead and senescent cells, as well as acting on tissue repair. Moreover, in the presence of invading microbes, these macrophages produce chemokines and pro-inflammatory cytokines that recruit myeloid and lymphoid cells, thus promoting local inflammation. Under these highly inflammatory conditions, the resident macrophages undergo cell death, which makes a niche accessible to the circulating MOs. The MOs rapidly replenishes the emptied space and differentiate into macrophages that, over time, assume similar phenotype and functional properties of the resident macrophages [4,6,10]. Furthermore, there is an intense infiltration of iMOs from the bone marrow (BM) and other lymphoid organs to the site of infection, enhancing the local inflammatory response.
DCs are efficient antigen presenting cells (APCs) for T cells that regulate the acquired immune response. Depending on their stage of differentiation and tissue location, DCs play a central role in initiating immune responses and leading the development of immunologic memory or, alternatively, mediating immune tolerance [11]. In healthy individuals, most DC progenitors found in peripheral tissues at homeostasis are not derived from MOs. MOs are, however, more abundant than DC progenitors both in the bone marrow and in the blood stream. Thus, the in vivo mobilization of the MO reservoir to generate competent antigen-presenting DCs is of central importance during acute microbial insults. The MO-DCs share many morphological and functional characteristics with conventional DCs (cDCs), such as high efficiency in antigen capture and processing, expression of co-stimulatory molecules, and the ability to present microbial peptides to CD4+ T cells or to cross-present to CD8+ T cells [12-20].
Understanding the origin, dynamics and emergence of MO-derived cells in lymphoid and non-lymphoid organs, as well as their roles, is essential for developing new strategies for immunological-based interventions to treat or prevent Plasmodium infection and disease.
DCs bridge the gap between innate and acquired immunity
DCs have the primary role of bridging innate and acquired immunity [3], playing an important role in host resistance to Plasmodium infection and in malaria pathogenesis [21,22]. After the infectious mosquito bite, CD11c+ as well as CD8α+CD11b− DCs located in the draining lymph nodes elicit a protective CD8+ T cell response in vivo against the P. yoelli liver stage [23-25]. In the blood stage, splenic DCs efficiently phagocytize iRBCs, contributing to the removal of iRBCs from the circulation [26]. Once internalized, the schizonts are destroyed and parasite components are released, which activate both CD8α−CD11b+and CD8α+CD11b− DCs. Those cells then migrate from the marginal zone to the T cell area and enhance expression of MHC II, co-stimulatory molecules, IL-12 and present antigens to CD4+ T and CD8+ T cells [27-29].
Various parasite [e.g., glycosylphosphatidylinositol anchors (GPI-anchors), DNA, RNA and hemozoin (Hz)] and host components (e.g., heme and uric acid) released from bursting iRBCs activate DCs during malaria [2]. Much attention has been given to the pro-inflammatory activity of Hz, the β-hematin crystals that are formed to detoxify the free heme generated from hemoglobin digestion by the parasites [30]. Hz is constantly released during malaria and is promptly internalized by phagocytic cells including DCs. Internalized Hz crystals rupture phagolysosome membranes, which releases its content in the cytosol, resulting in NLRP3 inflammasome assembly, caspase-1 activation and IL-1β release [31-33]. In addition, Hz acts as a carrier of nucleic acids from the parasite. This material activates multiple innate cognate receptors, such as TLR7 [34,35] and TLR9 [34,36-39], AIM2 [33,39], and other DNA/RNA sensing cytosolic receptors [35,40]. Among the cytosolic receptors that are activated by Plasmodium DNA is cGAS [41], which stimulates DCs to produce type I IFN. The TLR activation leads to IL-12 production by iMOs and DCs, which in turn activates NK cells, CD8+ T cells and promotes polarization of CD4+ T helper 1 (Th1) lymphocytes, all important sources of IFNγ [42,43].
IFNγ is a pleiotropic cytokine that promotes host resistance to infection in multiple ways, including: (i) differentiation of MOs to macrophages and MO-DCs, which have enhanced phagocytic activities; (ii) amplification of pro-inflammatory cytokines and toxic molecules release by macrophages, MO-DCs, and neutrophils that contribute to parasite killing; (iii) differentiation and activation of Th1 lymphocytes and cytotoxic T cells; and (iv) IgG switching and production of IgG isotypes that block parasite invasion, enhance the efficiency of phagocytosis and mediate cytotoxicity of opsonized iRBCs. The DCs have been shown to impact effector functions, maintain the pool of effector memory T cells, and promote long-term immunity to Plasmodium infection [29,38,44-49]. Importantly, in mice, virulent Plasmodium strains subvert the function of DCs, and thereby impair IL-12 production and the development of T cell-mediated immunity [50,51].
IFNγ is, however, the classic “double-edged sword”. While IFNγ-priming of innate immune cells enhances the capacity of fighting an ongoing infection, it makes innate immune cells hyper-responsive to microbial challenge. Thus, IFNγ also mediates the cytokine storm and a septic shock-like syndrome observed during malaria [37,52].
MO and DC subsets in malaria patients
Based on their function, human monocytes can be classified in at least 3 subsets that are defined by the level of CD14 and CD16 expression [53-55]. Classical MOs (cMOs) are CD14+CD16−, whereas intermediate MOs (intMOs) are CD14+CD16+, and patrolling MOs (pMOs) express low levels of CD14 and high levels of CD16 (CD14lowCD16+). In other studies, based on the pattern of CD14 and CD16 expression, intMOs and pMOs are defined as iMOs and non-classical monocytes (ncMOs), respectively [56,57]. In addition to the differential expression of CD14 and CD16, these MOs subsets vary on expression of molecules associated with cell adhesion, migration, innate immune response and phagocytosis [55,58]. In studies with human DCs, the term MO-DCs is used for DCs that are differentiated from monocytes in vitro, whereas term myeloid DCs (mDCs) is used for the equivalent DCs differentiated in vivo. Although some functional differences have been described between human MO-DCs and mDCs, they show high similarity in the pattern of cell surface markers expression [59,60]. Table 1 lists the nomenclature and cell surface markers of MOs and DCs used in various studies employing the peripheral blood mononuclear cells (PBMCs) from malaria patients. For better comparison with studies performed in rodent malaria models (Table 2), please notice that human mDCs is, most likely, the equivalent human DC subset to mouse MO-DCs.
Table 1 –
Cell surface markers and classification of monocytes and dendritic cells in malaria patients1
| Author, year, [ref] | Strain2 | Tissue | Population3 | Cell Surface Markers |
|---|---|---|---|---|
| Pinzon-Charry, 2013 [72] | Pf, Pv | Blood | mDCs | Lin− (CD3, CD14, CD19, CD20, CD56, CD34) HLA-DR+CD11c+ |
| Pinzon-Charry, 2013 [72] | Pf, Pv | Blood | mDCs subset | Lin− (CD3, CD14, CD19, CD20, CD56, CD34) HLA-DR+CD11c+CD141+ |
| Pinzon-Charry, 2013 [72] | Pf, Pv | Blood | mDCs subset | Lin− (CD3, CD14, CD19, CD20, CD56, CD34) HLA-DR+CD11c+CD1c+ |
| Pinzon-Charry, 2013 [72] | Pf, Pv | Blood | mDCs subset | Lin− (CD3, CD14, CD19, CD20, CD56, CD34) HLA-DR+CD11c+CD16+ |
| Pinzon-Charry, 2013 [72] | Pf, Pv | Blood | pDCs | Lin− (CD3, CD14, CD19, CD20, CD56, CD34) HLA-DR+CD11c+CD123+ |
| Franklin, 2009 [37] | Pf | Blood | MOs | CD14c+ |
| Franklin, 2009 [37] | Pf | Blood | DCs | CD11c+ |
| Urban, 2006 [68] | Pf | Blood | mDCs | Lin− (CD3, CD14, CD19) HLA-DR+CD11c+CD1c+ |
| Urban, 2006 [68] | Pf | Blood | mDCs | Lin− (CD3, CD14, CD19) HLA-DR+CD11c+BDCA3+ |
| Urban, 2006 [68] | Pf | Blood | pDCs | Lin− (CD3, CD14, CD19) HLA-DR+CD123+BDCA2+ |
| Urban, 2006 [68] | Pf | Blood | MOs | CD14+HLA-DR+ |
| Leoratti, 2012 [65] | Pv | Blood | MOs | CD14+CD16−CD66b− |
| Osier, 2014 [64] | Pf | Blood | MOs | CD14+ |
| Zhou, 2015 [56] | Pf | Blood | cMOs | CD14hiCD16− |
| Zhou, 2015 [56] | Pf | Blood | intMOs | CD14hiCD16+ |
| Zhou, 2015 [56] | Pf | Blood | ncMOs | CD14loCD16+ |
| Dobbs, 2017 [57] | Pf | Blood | cMOs | CD14++CD16− |
| Dobbs, 2017 [57] | Pf | Blood | intMOs | CD14++CD16+ |
| Dobbs, 2017 [57] | Pf | Blood | ncMOs | CD14+CD16++ |
| Jangpatarapongsa, 2008 [71] | Pv | Blood | pDCs | HLA-DR+CD123+Lin− (CD3, CD14, CD19, CD20, CD56, CD66b) |
| Jangpatarapongsa, 2008 [71] | Pv | Blood | mDCs | HLA-DR+CD11c+Lin− (CD3, CD14, CD19, CD20, CD56, CD66b) |
| Antonelli, 2014 [61] | Pv | Blood | cMOs | CD14+CD16− |
| Antonelli, 2014 [61] | Pv | Blood | iMOs | CD14+CD16+ |
| Antonelli, 2014 [61] | Pv | Blood | pMOs | CD14loCD16+ |
| Ataide, 2014 [52] | Pf, Pv | Blood | MOs | CD14+CD16− |
| Ataide, 2014 [52] | Pf, Pv | Blood | iMOs | CD14dimCD16+ |
| Ataide, 2014 [52] | Pf, Pv | Blood | mDCs | CD1c+CD19− |
| Gonçalves, 2010 [70] | Pf, Pv | Blood | mDCs | HLA-DR+CD11c+Lin− (CD3, CD14, CD16, CD20, CD56,) |
| Gonçalves, 2010 [70] | Pf, Pv | Blood | pDCs | HLA-DR+CD123+Lin− (CD3, CD14, CD16, CD20, CD56,)) |
| Kho, 2016 [76] | Pf, Pv | Blood | pDCs | CD303+(BDCA-2)+ |
| Kho, 2016 [76] | Pf, Pv | Blood | mDCs | CD1c+(BDCA-1)+ |
| Kho, 2016 [76] | Pf, Pv | Blood | mDCs | CD141+(BDCA-3)+ |
| Woodberry, 2017 [75] | Pv | Blood | pDCs | Lin−(CD3, CD14, CD19, CD20, CD56, CD34) HLA-DR+CD123+ |
| Woodberry, 2017 [75] | Pv | Blood | mDCs | Lin−(CD3, CD14, CD19, CD20, CD56, CD34) HLA-DR+CD11c+ |
| Woodberry, 2017 [75] | Pv | Blood | mDCs subset | Lin−(CD3, CD14, CD19, CD20, CD56, CD34) HLA-DR+CD11c+CD141+ |
| Woodberry, 2017 [75] | Pv | Blood | mDCs subset | Lin−(CD3, CD14, CD19, CD20, CD56, CD34) HLA-DR+CD11c+CD16+ |
| Woodberry, 2017 [75] | Pv | Blood | mDCs subset | Lin−(CD3, CD14, CD19, CD20, CD56, CD34) HLA-DR+CD11c+CD1c+ |
| Loughland, 2017 [73] | Pf | Blood | pDCs | Lin−(CD3, CD14, CD19, CD20, CD56,CD34) CD11c−HLA-DR+CD123+ |
| Loughland, 2019 [74] | Pf | Blood | mDCs | Lin−(CD3, CD14, CD19, CD20, CD34,CD56) CD11c+HLA-DR+CD123+ CD16+ |
We used the cell population classification provided by the authors in the original manuscript.
Pf, P. falciparum; Pv, P. vivax.
MOs, monocytes are equivalent to classical monocytes (cMOs); iMOs, inflammatory monocytes are equivalent to intermediate monocytes (intMOs); pMOs, patrolling monocytes are equivalent to non-classical monocytes (ncMOs); DCs, dendritic cells; pDCs, plasmocytoid dendritic cells; mDCs, myeloid dendritic cells are equivalent to monocyte-derived dendritic cells (MO-DCs) [76,77].
Table 2 –
Cell surface markers and classification of monocytes and dendritic cells in rodent malaria models1
| Author, year, [ref] | Strain2 | Tissue3 | Population4 | Cell Surface Markers |
|---|---|---|---|---|
| Schumak, 2015 [82] | PbA | Sp | MOs | Ly6ChiF4/80+ |
| Schumak, 2015 [82] | PbA | Sp | MOs | Ly6CintF4/80+ |
| Pai, 2014 [81] | PbA | Br | MOs | CD11b+Ly6G−Ly6Chi |
| Pai, 2014 [81] | PbA | Br | MOs | Ly6Clo |
| Franklin, 2007 [38] | Pc | Sp | MOs | CD11b+ |
| Haque, 2014 [105] | PbA | Bl | MOs | CD11b+Ly6Chi |
| Hirako, 2016 [20] | PbA | Sp | MOs | F480+CD11b+Ly6C−MHCII− |
| Ataide, 2014 [52] | Pc | Sp | MOs | CD11b+F4/80+ |
| Lai, 2018 [84] | Py17 | Sp, Lv, Lg | MOs | F4/80intCD11bhi |
| Galvão-Filho, 2018 [85] | PbN | Lg | MOs | CD11b+F4/80+Ly6C−CD11c−DC-SIGN−CD80intCD86int |
| Lagasse, 2015 [89] | PbA | Lg | iMOs | F4/80+CCR2+CD11b+Ly6Chi |
| Schumak, 2015 [82] | PbA | Br | iMOs | Ly6ChiLy6G− |
| Sorensen, 2018 [83] | PbA | Br | iMOs | CD45+CD11b+CD11c−Ly6Chi |
| Tamura, 2011 [107] | PbA | Sp | iMOs | CD11c−Ly6C+ |
| Hirako, 2016 [20] | PbA | Sp | iMOs | F480+CD11b+Ly6C+MHCII− |
| Sponaas, 2009 [80] | Pc | Sp | MO-DCs | CD11b+Ly6C+F4/80+CD68+CD11c+MHCII+CD40+CD86+ |
| Lai, 2018 [84] | Py17 | Sp, Lv, Lg | MO-MACs | F4/80intCD11bhiLy6ChiMHCII− |
| Lai, 2018 [84] | Py17 | Sp, Lv, Lg | MO-MACs | F4/80intCD11bhiLy6Chi/intMHCII+ |
| Lai, 2018 [84] | Py17 | Sp, Lv, Lg | MO-MACs | F4/80intCD11bhiLy6C−MHCII+ |
| Lai, 2018 [84] | Py17 | Sp, Lv, Lg | rMACs | F4/80hiCD11blo |
| Lai, 2018 [84] | Py17 | Sp, Lv, Lg | Act-rMACs | F4/80hiCD11b+CD11c+ |
| Chakravarty, 2007 [24] | Py | ALN, CLN, Sp | DCs | CD11c+ |
| Seixas, 2001 [27] | Pc | Sp | DCs | CD11c+MHCII+ |
| Leisewitz, 2004 [28] | Pc | Sp | DCs | CD11c+ |
| Ing, 2006 [29] | Pc | Sp | DCs | CD11c+ |
| Franklin, 2007, [38] | Pc | Sp | DCs | CD11c+CD40+CD86+ |
| da Silva, 2013 [44] | Pc | Sp | DCs | CD11c+MHCII+ |
| Perry, 2005 [51] | Py | Sp | DCs | CD11c+CD11b−MHCII+ |
| Perry, 2005 [51] | Py | Sp | DCs | CD11c+CD11b+MHCII+ |
| Ataide, 2014 [52] | Pc | Sp | DCs | CD11c+MHCII+ |
| Guermonprez, 2013 [69] | Pc | Sp | DCs | CD8α+CD103+ |
| Piva, 2012 [106] | PbA | Sp | DCs | CD11chighCD8+ |
| Piva, 2012 [106] | PbA | Sp | DCs | CD11chighCD8− |
| Tamura, 2011 [107] | PbA | Sp | DCs | CD11chighCD8+CD3e−CD19−DX5− |
| Gowda, 2012 [45] | Py17 | Sp | DCs | CD11c+ |
| Haque, 2014 [105] | PbA | Sp | DCs | CD8+CD11chiMHCIIhiCD86+TCRb−B220− |
| Haque, 2014 [105] | PbA | Sp | DCs | CD8−CD11chiMHCIIhiCD86+TCRb−B220− |
| deWalick, 2007 [88] | PbA | Sp | DCs | CD11c+ |
| Radtke, 2015 [25] | Py spr | LT | pDCs | B220+ |
| Piva, 2012 [106] | PbA | Sp | pDCs | CD11cintSiglec-H+ |
| deWalick, 2007 [88] | PbA | Sp | pDCs | B220+mPDCA-1+ |
| Sorensen, 2018 [83] | PbA | Br | MO-DCs | CD45+CD11b+CD11c+Ly6+F4/80+DC-SIGN+ |
| Hirako, 2016 [20] | PbA | Sp | MO-DCs | F480+CD11b+DC-SIGN+MHCII+CD11c+CD80+CD86+ |
| Hirako, 2016 [20] | PbA | Br | MO-DCs | CD45+CD8−CD3−Ly6C+CD11b+CD11c+DC-SIGN+ |
| Galvão-Filho, 2018 [85] | PbN | Lg | MO-DCs | CD11b+CD11c+F4/80+Ly6C+DC-SIGN+CD80+CD86+ |
| Galvão-Filho, 2018 [85] | PbN | Lg | Tip-DC | CD11b+CD11c+F4/80+DC-SIGN+TNF+iNOS+ |
We used the cell population classification provided by the authors in the original manuscript.
PbA, P. berghei ANKA; Pc, P. chabaudi; PbN, P. berghei NK65; Py17, P. yoelli 17XNL; Py, P. yoelli; spr, sporozoite.
Bl, blood; Br, brain; Lv, liver; Lg, lung; Sp, spleen; LT, lymphoid tissue.
MOs, monocytes; iMOs, inflammatory monocytes; MO-MACs, monocyte-derived macrophages; rMACs, resident macrophages; Act-rMACs, activated-resident macrophages; DCs, dendritic cells; pDCs, plasmocytoid dendritic cells; MO-DCs, monocyte derived-dendritic cells; Tip-DCs, TNF/iNOS producing dendritic cells; ALN, auricular lymph node; CLN, celiac lymph node.
Studies performed in our lab and elsewhere show that iMOs are the most efficient subset in phagocytizing both P. vivax and P. falciparum-infected RBC. Along with neutrophils, iMOs exposed to iRBC produce significantly higher levels of both intracellular ROS and mitochondrial ROS than the other MO subpopulations [56,61,62]. Additionally, antibody-dependent phagocytosis of iRBC, complement, and adhesion molecules are also known to contribute to protection against malaria caused by P. falciparum [57,63,64]. MOs are also an important source of pro-inflammatory cytokines during acute malaria episodes [2]. In children infected with P. falciparum there is an expansion mainly of iMOs (CD14+CD16+) from the pool of circulating MOs. This parallels the higher levels of circulating CXCL10, TNFα, IFNγ and IL-6 [57]. Either in P. vivax or P. falciparum malaria, the CD14+CD16+ monocytes produce high levels of the pro-inflammatory cytokines IL-1β, IL-6 and TNFα, upon stimulation with TLR agonists [37,52,61,65].
MOs also give rise to MO-DCs, which are important players in the immune response in Plasmodium-infected patients [21,66,67]. CD141 expressing MO-DCs (BDCA3+: Lineage− HLA-DR+CD11c+), but not CD1c+ expressing MO-DCs, are expanded in children infected with P. falciparum [68,69]. In contrast, adults infected with P. vivax- and P. falciparum show a decrease in the ratio of MO-DCs (BDCA2/CD303)+ to plasmocytoid DCs (pDCs) (HLA-DR+CD11c+) [70,71]. The reduction of both MO-DCs and pDCs in the circulation of P. falciparum and P. vivax-infected patients is associated with an impairment of DCs maturation and antigen presentation to T cells [72]. In another study, it was shown that expression of the co-stimulatory molecules CD86, CD80 and CD40 were not up-regulated and the surface expression of HLA-DR and CD123 (IL-3R-alpha) was reduced in pDCs during early infection, suggesting that they are not activated [73]. Indeed, it was shown that the CD16+ MO-DCs is the main subset activated during subpatent infection with P. falciparum. The CD16+ MO-DCs produce TNFα and IL-10 and are potentially involved on both inflammatory as well as regulatory immune processes [74]. In addition, it was shown that during primary P. vivax infection, the number of cells and HLA-DR expression are decreased in CD1c(+) MO-DCs, while increased in CD16+ MO-DCs [75]. Importantly, in asymptomatic malaria patients with sub-microscopic parasitemia, the function of the DCs is not affected [76]. In vitro, high concentrations of iRBCs or Hz impair monocyte differentiation. In contrast, smaller concentrations of iRBCs or under certain conditions Hz activate DCs leading to cytokine production, expression of adhesion and co-stimulatory molecules favoring antigen presentation [77-79].
MO-DCs in rodent malaria models
During acute Plasmodium infection in mice, the cellularity of spleen, blood, as well as non-lymphoid organs such as brain, liver, and lungs, changes drastically. An enhanced frequency of iMOs, activated macrophages, and MO-DCs is observed [20,80-85]. The aforementioned studies addressed the role of iMOs during malaria. Only few studies have suggested that aMacs have an anti-pathology effect and that they should be explored as potential therapeutic targets during malaria [86,87]. Hence , in this review we focus our discussion on the role of iMOs and MO-DCs during malaria.
In a study performed by Ruedl and colleagues [84], it was shown that at early stage of Plasmodium infection, there is a rapid disappearance of resident macrophages (F4/80hiCD11blo) in different organs, such as spleen, liver, and lungs and the distribution of MO subsets undergoes striking changes. In all these organs there is a transient emergence of activated MOs that express F4/80intCD11bhiMHCIIhiLy6Chi. During the healing process, these monocytic cells disappear and the splenic red pulp and kupffer cell compartments are restored, presumably by circulating MOs (F4/80hiCD11blo) that assume similar characteristic of the embryonic resident macrophages [84]. In the P. chabaudi-infeted mice, CD11b+Ly6C+ cells are important sources of inflammatory cytokines, reactive oxygen species, and nitric oxide that effectively phagocytize and destroy iRBCs, thus mediating resistance to infection [80]. In addition, both CD11c+ and Ly6C+ cells have been shown to play important roles in the development of experimental cerebral malaria (ECM) in P. berghei-infected mice [81-83,88]. Likewise, it has been shown that CCR2+CD11b+Ly6C+ cells mediate malaria-induced lung injury in a rodent malaria model [89]. However, the role of MO-DCs in rodent malaria models had not been explored.
Traditionally, iMOs are defined as F4/80int, CD11bhi, MHC II−, Ly6Chi and CCR2+, whereas MO-DCs are supposedly Ly6C− [18,19,90]. In the studies described below, we defined F4/80intCD11bhiMHC IIhiLy6C+ cells as MO-DCs, because they express high levels of CD11c, DC-Sign, MHC-IIhi and co-stimulatory molecules (CD86 and CD80), display large dendrites, are highly phagocytic, and efficiently present antigens to CD8+ T cells [20]. Similarly, in a second study by our group, F4/80intCD11bhiMHC IIhiLy6C+ cells also showed all the characteristic markers of DCs [85] and a subset of them simultaneously expressed TNFα and iNOS, suggesting that they are the TNF/iNOS producing DCs (Tip-DCs) subset. It is noteworthy that the MO-DCs defined in our studies showed a gradient of Ly6C expression, suggestive of a population that includes cells transitioning from iMOs to MO-DCs [20,85]. Hence, we believe that the role of MO-DCs in rodent malaria models has been underestimated. Most of the studies that addressed the role of DCs in rodent malaria have used a limited number of cell surface markers and have defined CD11c as a specific marker for cDCs. In addition, multiple studies have defined iMOs regarding the expression of CD11b, F4/80, and Ly6C, but did not evaluated CD11c [5]. This is of particular importance during the acute stage of infection, when the MO-DCs transiently emerge as a main population in the spleen and non-lymphoid organs [20,84,85]. Table 2 lists the nomenclature and cell surface markers used for iMOs and DCs employed in various studies using mouse malaria models.
MO-DCs in malaria acute respiratory distress syndrome (MA-ARDS)
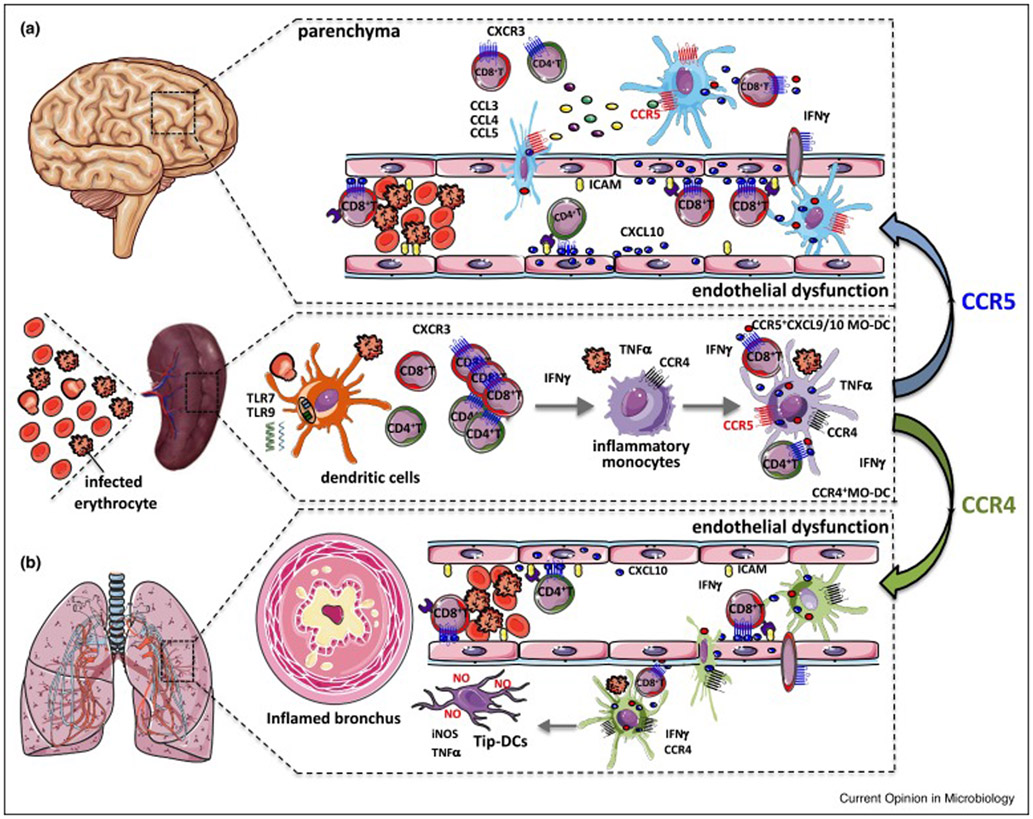
MA-ARDS is a severe and often fatal complication of P. falciparum and P. vivax infection that is related to the sequestration of iRBCs into the pulmonary microvasculature [91-95]. The lung inflammatory process and injury, both in humans and mice, are linked with the presence of Hz. This crystal induces the expression of various inflammatory mediators, such as chemokines (e.g., CXCL10 and CCL2), cytokines (e.g., IL-1β, IL-6, IL-10 and TNFα) and metabolic enzymes (e.g., iNOS and heme-oxygenase) [85,96]. In infections caused by P. berghei NK65 (PbN), the inflammatory infiltrate in the lungs is characterized by a Th1 response and a predominance of CD8+ T lymphocytes expressing Tbet and high levels of IFNγ [85,97,98].
Our recent study [85] demonstrated that development of murine MA-ARDS is dependent on a functional CCR4. This chemokine receptor is involved in lung pathologies, but is normally responsible for mediating the recruitment of Th2 and regulatory T cells. CCR4−/− mice infected with PbN are protected from lung damage and present less hemorrhagic lesions and edema. A deeper analysis of leukocyte subsets in the lung of MA-ARDS mice showed a high frequency of MOs. Most of these cells express MO-DC markers such as CD11c and DC-Sign, stimulatory molecules MHCII, CD80, and CD86, and did not express specific markers for blood MOs (CD115), macrophages (CD68) or cDCs (CD135). In addition, a significant proportion of these MO-DCs are Tip-DCs. Importantly, MOs from control mice constitutively express CCR4, and we found that the recruitment of MO-DCs to the lungs, and not of T lymphocytes, is profoundly impaired in CCR4−/− mice infected with PbN.
As observed in other models, the differentiation of MO-DCs in PbN infection is highly dependent on endogenous IFNγ [85,97-99]. It is also known that during experimental MA-ARDS, CD8+ T cells are the main source of IFNγ in the lungs. In fact, our studies suggest that the differentiation of MO-DCs into Tip-DCs that occurs in the lungs of PbN-infected mice is dependent of locally produced IFNγ by CD8+ T cells. Additionally, we found that Tip-DCs are the most important nitric oxide synthase 2 (NOS2) expressing cells in the MA-ARDS model and that NOS2−/− mice have attenuated pathology [85]. Thus, these results indicate that Tip-DCs are important effector cells mediating lung tissue damage and MA-ARDS (Figure 1).
Figure 1. MO-DC and Tip-DCs mediate cerebral malaria and ARDS in P. berghei-infected mice.
The systemic increase of circulating cytokines, elicited during malaria, induce the expression of adhesion molecules by endothelial cells, which mediate parasite sequestration. The sequestration of infected RBCs disrupts blood flow, promotes blood clots, injures endothelial cells and ruptures vascular walls, leading to the extravasation of vascular content and local tissue inflammation. These mechanisms contribute ECM and the acute respiratory distress in experimental malaria. (A) Infection with P. berghei ANKA leads to an IFNγ-dependent differentiation of inflammatory monocytes into splenic MO-DCs (CD45highCD8−CD11b+Ly6C+CD11c+MHCIIhigh CCR5+CXCL9+CXCL10+). After differentiation, these MO-DCs migrate to the CNS in response to CCR5 ligands and amplify recruitment of CD8+ T lymphocytes, initiated by endothelial cells producing CXCL10 and promote development of ECM. (B) Infection with P. berghei NK65 leads to differentiation of inflammatory monocytes into MO-DCs that migrate to the lung. In the lung these cells are further activated by IFNγ locally produced by CD8+ T cells and differentiate into Tip-DCs (CD45highCD8−CD11b+Ly6C+CD11c+MHCIIhighCCR4+TNF+iNOS+). The nitric oxide produce by the Tip-DCs is the main cause of lung damage. This figure is adapted from Figure 10 of Hirako et al. [17], which now includes the path of MO-DCs to the lungs and their differentiation to Tip-DCs during MA-ARDS.
MO-DCs in experimental cerebral malaria (ECM)
Cerebral malaria is a severe and often fatal neurological complication of P. falciparum infection [100-102]. It is characterized by cerebral dysfunctions that includes different degrees of impaired consciousness, delirium, abnormal neurological signs, as well as focal and generalized convulsions. The most studied model to study ECM is the infection of C57BL/6 mice with P. berghei ANKA (PbA). ECM develops within 6-12 days of infection and is characterized by ruffled fur, hunching, unbalancing, limb paralysis, convulsions, coma and death. The development of ECM is highly dependent on the inflammatory response, in particular of TNFα, IFNγ, Th1 and CD8+ T lymphocytes [103,104]. Intriguingly, it has been shown that Type I IFN promotes development of ECM by regulating the production of IFNγ by Th1 lymphocytes [105].
Different studies have demonstrated an intense infiltrate of blood iMOs in the CNS of PbA-infected mice [81-83]. In addition to iMOs, CD11c+DCs are also important for the development of ECM [88,106]. In contrast, it was shown that treatment with Flt3, a potent differentiation factor for DCs, promoted the expansion of CD8+ DCs resulting in lower levels of parasitemia and protection against ECM [107]. However, these studies have used a limited number of cell surface markers to define iMOs and DCs, and the precise sub-populations are not defined. Our recent study indicates that MO-DCs (CD45highCD8−CD11b+Ly6C+CD11c+MHCIIhighCCR5+) are pivotal cells in mediating PbA-induced neuroinflammation [20]. At five days post-infection, MO-DCs become a dominant DC population during the period of ECM development. Amongst CD11b+F480+ cells, the frequency of MO-DCs increases from 18% in uninfected controls to 74% in PbA-infected mice. These MO-DCs are highly phagocytic, when compared to splenic MOs and iMOs. They express high levels of CD80 and CD86 and cross-present soluble antigens, suggestive of a role in activating CD8+ T lymphocytes. Among members of chemokine family, the enhanced production of CXCL9, CXCL10, and expression of CCR5 by the differentiated MO-DCs stands out [20]. It is noteworthy that CXCL9 and CXCL10 play important roles on ECM [108,109]. Importantly, IFNγ or IFNγR deficiency leads to an impairment in the differentiation of iMOs into CXCL9/CXCL10-expressing MO-DCs. Furthermore, treatment with E6446 (a TLR7 and TLR9 antagonist) inhibits the expression of IFNγ by both CD4+ T and CD8+ T cells, CXCL9 and CXCL10 by MO-DCs, and prevents development of ECM [20,34].
Soon after their differentiation in the spleen, MO-DCs emerge in the CNS of PbA-infected mice. While endothelial cells express high levels of CXCL10, MO-DCs are the main hematopoietic source of CXCL9 and CXCL10 in the brain of PbA-infected mice undergoing ECM [83]. Importantly, the emergence of MO-DCs in the CNS is highly compromised in PbA-infected CCR5−/− mice, which are resistant to ECM. Different CCR5 ligands (i.e., CCL3, CCL4 and CCL5) are expressed in the brain of PbA-infected mice and CCL5 is highly efficient in mediating MO-DC migration in vitro [20].
In summary, our studies suggest that parasite nucleic acids activate cDCs to produce IL-12, which in turn stimulates the production of IFNγ by NK cells and T cells. The circulating IFNγ induces the expression of CXCL10 by endothelial cells in the CNS and promotes the differentiation of MOs into CXCL9/CXCL10-producing MO-DCs in the spleen of PbA-infected mice. After differentiation, MO-DCs migrate to the CNS in response to CCR5 ligands and amplify the recruitment of CXCR3+CD8+ T lymphocytes, initiated by endothelial cells producing CXCL10 and promote the development of ECM. Finally, CD8+ T cells act as effector cells causing tissue damage and lethal encephalitis (Figure 1).
MO-DC precursors in rodent malaria
Myelopoiesis-induced inflammation is critical to replenish myeloid cells in the periphery and control infectious pathogens. It has been shown that IL-27 promotes expansion of lineage negative (LIN−) LIN−Sca-1+c-Kit+ (LSK) cells, especially the long-term repopulating hematopoietic stem cells, and the common myeloid progenitor (CMP) cells that preferentially differentiate into mDCs, but not pDCs or cDCs. Importantly, the Plasmodium blood stage infection in mice production of IL-27 is enhanced through the induction of IFNγ. IL-27 then promotes the expansion of LSK cells in the bone marrow (BM), their differentiation and mobilization to the spleen [110]. Our unpublished results indicate that during rodent malaria, BM-MOs (CD11b+CCR2+F4/80+MHC-II−DC-Sign−Ly6cloCD11c−CCR5−CXCL9−CXCL10−) differentiate into BM-iMOs (CD11b+F4/80+MHC-II+CCR2+DC-Sign+Ly6cloCD11c−CCR5−CXCL9−CXCL10−) that express some, but not all markers from MO-DCs (CD11b+F4/80+MHC-II+CCR2+DC-Sign+Ly6c+CD11c+CCR5+CXCL9+CXCL10+). These findings suggest that the BM-iMOs may migrate to the spleen and then differentiate into MO-DCs [20,85]. In fact, it has also been observed a contraction in the population of LIN− cells as well as the CMP and the granulocyte monocyte progenitor (GMP) cells in the BM of P. chabaudi-infected mice. This contraction of BM progenitor cells during rodent malaria is dependent on IFNγ and CCR2 [111,112]. These results are consistent with the findings that the egress of MOs from the BM and their emergence in the site of infection is CCR2-dependent [80,113]. Altogether, these results support the hypothesis that myeloid precursors cells migrate out of the BM for extra-medullar myelopoiesis and lead to the generation of iMOs and MO-DCs in the spleen of Plasmodium-infected mice.
However, we found that the generation of splenic MO-DCs is only partially compromised, whereas the migration of MO-DCs to the CNS and the development of ECM are not affected in PbA-infected CCR2−/− mice [20]. Furthermore, BM-iMOs do not express CCR5 and could not be recruited in a CCR5-dependent manner directly from the BM to the CNS in the ECM model. Importantly, our results also demonstrate that there is an accumulation of iMOs in the spleen of IFNγ−/− mice infected with PbA, but these cells do not differentiate into MO-DCs. As a consequence, there is no emergence of MO-DCs in the brain and development of ECM in IFNγ−/− mice [20]. These results indicate that CCR2, and potentially the egress of precursor cells from the BM during PbA infection, is not strictly necessary for the generation of splenic iMOs and that the final differentiation of MO-DCs in the spleen is highly dependent on IFNγ.
By using adoptive cell transfer experiments, MOs (F4/80+CD11b+Ly6C−DC-SIGN−MHCIIlo) were enriched from spleens of uninfected CD45.2 C57BL/6 mice and transferred to either uninfected or PbA-infected CD45.1 congenic mice. Two days later, the frequencies of splenic CD45.2+ MOs, iMOs and MO-DCs were evaluated. In infected mice, like host cells (CD45.1+), most splenic CD45.2+ MOs differentiated into splenic MO-DCs (F4/80+CD11b+Ly6C+DC-SIGN+MHCN+). Thus, we favor the hypothesis that splenic MOs (F4/80+CD11b+Ly6C+DC-SIGN−MHCII−) [84] are the main precursors or, at least, sufficient to generate the pathogenic MO-DCs that then migrate and promote ECM.
Since MOs constitutively express CCR4, it is possible that they are being recruited directly from the BM to the lungs in the MA-ARDS model. However, the emergence of MO-DCs in the lungs and development of MA-ARDS is unaffected in the PbN-infected CCR2−/− mice. Thus, we speculate that MO-DCs differentiate in the spleen and then are recruited to the lungs. Once they emerge in the lungs, the final differentiation to Tip-DCs occurs after interaction with IFNγ-producing CD8+ T cells. Both in the ECM and MA-ARDS one can propose that MO-DCs differentiate from brain and lung resident macrophages (F4/80+CD11b+Ly6C−DC-SIGN−MHCII−) [84]. However, the requirement of CCR5 and CCR4 for MO-DCs emergence in these organs must be taken into consideration [20,85].
Conclusions
Our studies highlight an intense differentiation of MO-DCs during the MA-ARDS and ECM models. We emphasize the importance of endogenous IFNγ in mediating MO-DCs differentiation and their migration to the brain or lungs in a CCR5- or CCR4-dependent manner, respectively. Once they migrate to the target organs, MO-DCs amplify the local inflammation causing tissue damage and consequent clinical manifestations. We hypothesize that MO-DCs amplify local tissue inflammation by serving as professional APCs and producing the chemokines CXCL9 and CXCL10 which recruit CD8+ T cells that mediate pathology. Therefore, the molecular steps involved on differentiation and migration of MO-DCs to the inflamed tissues may be important targets for therapeutic intervention during malaria.
Acknowledgements
We are grateful to Dr. Milton Pereira for careful revision of this manuscript, comments and suggestions. We also thank an anonymous reviewer for the helpful comments and suggestions to improve this review. This work was supported by the National Institutes of Health (R01NS098747, R01AI079293; R21AI131632; R0AI116577-02); Rede Mineira de Biomoléculas from Fundação de Amparo a Pesquisa de Minas Gerais (Fapemig, RED-00012-14), Brazilian National Institute of Science and Technology for Vaccines granted by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)/Fundação de Amparo a Pesquisa do Estado de Minas Gerais (Fapemig)/ Coordenação de Aperfeiçoamento de Pessoal de Ensino Superior (CAPES) (465293/2014-0). ICH and BGF received CAPES and LMNP CNPq fellowships. RTG and LVA are research fellows from CNPq.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Papers of particular interest, published within the period of review, have been highlighted as
* of special interest
** of outstanding interest
- 1.Crompton PD, Moebius J, Portugal S, Waisberg M, Hart G, Garver LS, Miller LH, Barillas-Mury C, Pierce SK: Malaria immunity in man and mosquito: insights into unsolved mysteries of a deadly infectious disease. Annu Rev Immunol 2014, 32:157–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gazzinelli RT, Kalantari P, Fitzgerald KA, Golenbock DT: Innate sensing of malaria parasites. Nat Rev Immunol 2014, 14:744–757. [DOI] [PubMed] [Google Scholar]
- 3.Steinman RM: Decisions about dendritic cells: past, present, and future. Annu Rev Immunol 2012, 30:1–22. [DOI] [PubMed] [Google Scholar]
- 4.Epelman S, Lavine KJ, Randolph GJ: Origin and functions of tissue macrophages. Immunity 2014, 41:21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, et al. : Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 2014, 41:14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perdiguero EG, Geissmann F: The development and maintenance of resident macrophages. Nat Immunol 2016, 17:2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinez FO, Helming L, Gordon S: Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol 2009, 27:451–483. [DOI] [PubMed] [Google Scholar]
- 8.Grainger JR, Wohlfert EA, Fuss IJ, Bouladoux N, Askenase MH, Legrand F, Koo LY, Brenchley JM, Fraser ID, Belkaid Y: Inflammatory monocytes regulate pathologic responses to commensals during acute gastrointestinal infection. Nat Med 2013, 19:713–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gundra UM, Girgis NM, Gonzalez MA, San Tang M, Van Der Zande HJP, Lin JD, Ouimet M, Ma LJ, Poles J, Vozhilla N, et al. : Vitamin A mediates conversion of monocyte-derived macrophages into tissue-resident macrophages during alternative activation. Nat Immunol 2017, 18:642–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ginhoux F, Guilliams M: Tissue-Resident Macrophage Ontogeny and Homeostasis. Immunity 2016, 44:439–449. [DOI] [PubMed] [Google Scholar]
- 11.Idoyaga J, Fiorese C, Zbytnuik L, Lubkin A, Miller J, Malissen B, Mucida D, Merad M, Steinman RM: Specialized role of migratory dendritic cells in peripheral tolerance induction. J Clin Invest 2013, 123:844–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romani N, Gruner S, Brang D, Kampgen E, Lenz A, Trockenbacher B, Konwalinka G, Fritsch PO, Steinman RM, Schuler G: Proliferating dendritic cell progenitors in human blood. J Exp Med 1994, 180:83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sallusto F, Lanzavecchia A: Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med 1994, 179:1109–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leon B, Lopez-Bravo M, Ardavin C: Monocyte-derived dendritic cells formed at the infection site control the induction of protective T helper 1 responses against Leishmania. Immunity 2007, 26:519–531. [DOI] [PubMed] [Google Scholar]
- 15.Nakano H, Lin KL, Yanagita M, Charbonneau C, Cook DN, Kakiuchi T, Gunn MD: Blood-derived inflammatory dendritic cells in lymph nodes stimulate acute T helper type 1 immune responses. Nat Immunol 2009, 10:394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG: TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity 2003, 19:59–70. [DOI] [PubMed] [Google Scholar]
- 17.Siddiqui KR, Laffont S, Powrie F: E-cadherin marks a subset of inflammatory dendritic cells that promote T cell-mediated colitis. Immunity 2010, 32:557–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheong C, Matos I, Choi JH, Dandamudi DB, Shrestha E, Longhi MP, Jeffrey KL, Anthony RM, Kluger C, Nchinda G, et al. : Microbial stimulation fully differentiates monocytes to DC-SIGN/CD209(+) dendritic cells for immune T cell areas. Cell 2010, 143:416–429.** This study defines various surface markers and function of mouse MO-DCs
- 19.Goldszmid RS, Caspar P, Rivollier A, White S, Dzutsev A, Hieny S, Kelsall B, Trinchieri G, Sher A: NK cell-derived interferon-gamma orchestrates cellular dynamics and the differentiation of monocytes into dendritic cells at the site of infection. Immunity 2012, 36:1047–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirako IC, Ataide MA, Faustino L, Assis PA, Sorensen EW, Ueta H, Araujo NM, Menezes GB, Luster AD, Gazzinelli RT: Splenic differentiation and emergence of CCR5(+)CXCL9(+)CXCL10(+) monocyte-derived dendritic cells in the brain during cerebral malaria. Nat Commun 2016, 7:13277.** This study describes de emergence of splenic MO-DCs in the rodent malaria model and their role in the development of experimental cerebral malaria.
- 21.Amorim KN, Chagas DC, Sulczewski FB, Boscardin SB: Dendritic Cells and Their Multiple Roles during Malaria Infection. J Immunol Res 2016, 2016:2926436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wykes MN, Good MF: What really happens to dendritic cells during malaria? Nat Rev Microbiol 2008, 6:864–870. [DOI] [PubMed] [Google Scholar]
- 23.Bruna-Romero O, Rodriguez A: Dendritic cells can initiate protective immune responses against malaria. Infect Immun 2001, 69:5173–5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chakravarty S, Cockburn IA, Kuk S, Overstreet MG, Sacci JB, Zavala F: CD8+ T lymphocytes protective against malaria liver stages are primed in skin-draining lymph nodes. Nat Med 2007, 13:1035–1041. [DOI] [PubMed] [Google Scholar]
- 25.Radtke AJ, Kastenmuller W, Espinosa DA, Gerner MY, Tse SW, Sinnis P, Germain RN, Zavala FP, Cockburn IA: Lymph-node resident CD8alpha+ dendritic cells capture antigens from migratory malaria sporozoites and induce CD8+ T cell responses. PLoS Pathog 2015, 11:e1004637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borges da Silva H, Fonseca R, Cassado Ados A, Machado de Salles E, de Menezes MN, Langhorne J, Perez KR, Cuccovia IM, Ryffel B, Barreto VM, et al. : In vivo approaches reveal a key role for DCs in CD4+ T cell activation and parasite clearance during the acute phase of experimental blood-stage malaria. PLoS Pathog 2015, 11:e1004598.* This study describes the role of DCs in phagocytosis of infected red blood cells from Plasmodium-infected mice.
- 27.Seixas E, Cross C, Quin S, Langhorne J: Direct activation of dendritic cells by the malaria parasite, Plasmodium chabaudi chabaudi. Eur J Immunol 2001, 31:2970–2978. [DOI] [PubMed] [Google Scholar]
- 28.Leisewitz AL, Rockett KA, Gumede B, Jones M, Urban B, Kwiatkowski DP: Response of the splenic dendritic cell population to malaria infection. Infect Immun 2004, 72:4233–4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ing R, Segura M, Thawani N, Tam M, Stevenson MM: Interaction of mouse dendritic cells and malaria-infected erythrocytes: uptake, maturation, and antigen presentation. J Immunol 2006, 176:441–450. [DOI] [PubMed] [Google Scholar]
- 30.Rosenthal PJ, Meshnick SR: Hemoglobin catabolism and iron utilization by malaria parasites. Mol Biochem Parasitol 1996, 83:131–139. [DOI] [PubMed] [Google Scholar]
- 31.Shio MT, Eisenbarth SC, Savaria M, Vinet AF, Bellemare MJ, Harder KW, Sutterwala FS, Bohle DS, Descoteaux A, Flavell RA, et al. : Malarial hemozoin activates the NLRP3 inflammasome through Lyn and Syk kinases. PLoS Pathog 2009, 5:e1000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dostert C, Guarda G, Romero JF, Menu P, Gross O, Tardivel A, Suva ML, Stehle JC, Kopf M, Stamenkovic I, et al. : Malarial hemozoin is a Nalp3 inflammasome activating danger signal. PLoS One 2009, 4:e6510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalantari P, DeOliveira RB, Chan J, Corbett Y, Rathinam V, Stutz A, Latz E, Gazzinelli RT, Golenbock DT, Fitzgerald KA: Dual engagement of the NLRP3 and AIM2 inflammasomes by plasmodium-derived hemozoin and DNA during malaria. Cell Rep 2014, 6:196–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Franklin BS, Ishizaka ST, Lamphier M, Gusovsky F, Hansen H, Rose J, Zheng W, Ataide MA, de Oliveira RB, Golenbock DT, et al. : Therapeutical targeting of nucleic acid-sensing Toll-like receptors prevents experimental cerebral malaria. Proc Natl Acad Sci U S A 2011, 108:3689–3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu J, Tian L, Yu X, Pattaradilokrat S, Li J, Wang M, Yu W, Qi Y, Zeituni AE, Nair SC, et al. : Strain-specific innate immune signaling pathways determine malaria parasitemia dynamics and host mortality. Proc Natl Acad Sci U S A 2014, 111:E511–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parroche P, Lauw FN, Goutagny N, Latz E, Monks BG, Visintin A, Halmen KA, Lamphier M, Olivier M, Bartholomeu DC, et al. : Malaria hemozoin is immunologically inert but radically enhances innate responses by presenting malaria DNA to Toll-like receptor 9. Proc Natl Acad Sci U S A 2007, 104:1919–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Franklin BS, Parroche P, Ataide MA, Lauw F, Ropert C, de Oliveira RB, Pereira D, Tada MS, Nogueira P, da Silva LH, et al. : Malaria primes the innate immune response due to interferon-gamma induced enhancement of toll-like receptor expression and function. Proc Natl Acad Sci U S A 2009, 106:5789–5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Franklin BS, Rodrigues SO, Antonelli LR, Oliveira RV, Goncalves AM, Sales-Junior PA, Valente EP, Alvarez-Leite JI, Ropert C, Golenbock DT, et al. : MyD88-dependent activation of dendritic cells and CD4(+) T lymphocytes mediates symptoms, but is not required for the immunological control of parasites during rodent malaria. Microbes Infect 2007, 9:881–890. [DOI] [PubMed] [Google Scholar]
- 39.Hirako IC, Gallego-Marin C, Ataide MA, Andrade WA, Gravina H, Rocha BC, de Oliveira RB, Pereira DB, Vinetz J, Diamond B, et al. : DNA-Containing Immunocomplexes Promote Inflammasome Assembly and Release of Pyrogenic Cytokines by CD14+ CD16+ CD64high CD32low Inflammatory Monocytes from Malaria Patients. MBio 2015, 6:e01605–01615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharma S, DeOliveira RB, Kalantari P, Parroche P, Goutagny N, Jiang Z, Chan J, Bartholomeu DC, Lauw F, Hall JP, et al. : Innate immune recognition of an AT-rich stem-loop DNA motif in the Plasmodium falciparum genome. Immunity 2011, 35:194–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gallego-Marin C, Schrum JE, Andrade WA, Shaffer SA, Giraldo LF, Lasso AM, Kurt-Jones EA, Fitzgerald KA, Golenbock DT: Cyclic GMP-AMP Synthase Is the Cytosolic Sensor of Plasmodium falciparum Genomic DNA and Activates Type I IFN in Malaria. J Immunol 2018, 200:768–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stevenson MM, Tam MF, Wolf SF, Sher A: IL-12-induced protection against blood-stage Plasmodium chabaudi AS requires IFN-gamma and TNF-alpha and occurs via a nitric oxide-dependent mechanism. J Immunol 1995, 155:2545–2556. [PubMed] [Google Scholar]
- 43.Stevenson MM, Riley EM: Innate immunity to malaria. Nat Rev Immunol 2004, 4:169–180. [DOI] [PubMed] [Google Scholar]
- 44.da Silva HB, de Salles EM, Panatieri RH, Boscardin SB, Rodriguez-Malaga SM, Alvarez JM, D'Imperio Lima MR: IFN-gamma-induced priming maintains long-term strain-transcending immunity against blood-stage Plasmodium chabaudi malaria. J Immunol 2013, 191:5160–5169. [DOI] [PubMed] [Google Scholar]
- 45.Gowda NM, Wu X, Gowda DC: TLR9 and MyD88 are crucial for the development of protective immunity to malaria. J Immunol 2012, 188:5073–5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Griffith JW, O'Connor C, Bernard K, Town T, Goldstein DR, Bucala R: Toll-like receptor modulation of murine cerebral malaria is dependent on the genetic background of the host. J Infect Dis 2007, 196:1553–1564. [DOI] [PubMed] [Google Scholar]
- 47.Torgler R, Bongfen SE, Romero JC, Tardivel A, Thome M, Corradin G: Sporozoite-mediated hepatocyte wounding limits Plasmodium parasite development via MyD88-mediated NF-kappa B activation and inducible NO synthase expression. J Immunol 2008, 180:3990–3999. [DOI] [PubMed] [Google Scholar]
- 48.Cramer JP, Lepenies B, Kamena F, Holscher C, Freudenberg MA, Burchard GD, Wagner H, Kirschning CJ, Liu X, Seeberger PH, et al. : MyD88/IL-18-dependent pathways rather than TLRs control early parasitaemia in non-lethal Plasmodium yoelii infection. Microbes Infect 2008, 10:1259–1265. [DOI] [PubMed] [Google Scholar]
- 49.Su Z, Stevenson MM: IL-12 is required for antibody-mediated protective immunity against blood-stage Plasmodium chabaudi AS malaria infection in mice. J Immunol 2002, 168:1348–1355. [DOI] [PubMed] [Google Scholar]
- 50.Wykes MN, Liu XQ, Beattie L, Stanisic DI, Stacey KJ, Smyth MJ, Thomas R, Good MF: Plasmodium strain determines dendritic cell function essential for survival from malaria. PLoS Pathog 2007, 3:e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perry JA, Olver CS, Burnett RC, Avery AC: Cutting edge: the acquisition of TLR tolerance during malaria infection impacts T cell activation. J Immunol 2005, 174:5921–5925. [DOI] [PubMed] [Google Scholar]
- 52.Ataide MA, Andrade WA, Zamboni DS, Wang D, Souza Mdo C, Franklin BS, Elian S, Martins FS, Pereira D, Reed G, et al. : Malaria-induced NLRP12/NLRP3-dependent caspase-1 activation mediates inflammation and hypersensitivity to bacterial superinfection. PLoS Pathog 2014, 10:e1003885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Passlick B, Flieger D, Ziegler-Heitbrock HW: Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood 1989, 74:2527–2534. [PubMed] [Google Scholar]
- 54.Ziegler-Heitbrock HW: Heterogeneity of human blood monocytes: the CD14+ CD16+ subpopulation. Immunol Today 1996, 17:424–428. [DOI] [PubMed] [Google Scholar]
- 55.Cros J, Cagnard N, Woollard K, Patey N, Zhang SY, Senechal B, Puel A, Biswas SK, Moshous D, Picard C, et al. : Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity 2010, 33:375–386.** This sudy describes the markers and functions of different monocyte subsets in humans.
- 56.Zhou J, Feng G, Beeson J, Hogarth PM, Rogerson SJ, Yan Y, Jaworowski A: CD14(hi)CD16+ monocytes phagocytose antibody-opsonised Plasmodium falciparum infected erythrocytes more efficiently than other monocyte subsets, and require CD16 and complement to do so. BMC Med 2015, 13:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dobbs KR, Embury P, Vulule J, Odada PS, Rosa BA, Mitreva M, Kazura JW, Dent AE: Monocyte dysregulation and systemic inflammation during pediatric falciparum malaria. JCI Insight 2017, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ingersoll MA, Spanbroek R, Lottaz C, Gautier EL, Frankenberger M, Hoffmann R, Lang R, Haniffa M, Collin M, Tacke F, et al. : Comparison of gene expression profiles between human and mouse monocyte subsets. Blood 2010, 115:e10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Andersson LI, Cirkic E, Hellman P, Eriksson H: Myeloid blood dendritic cells and monocyte-derived dendritic cells differ in their endocytosing capability. Hum Immunol 2012, 73:1073–1081. [DOI] [PubMed] [Google Scholar]
- 60.Osugi Y, Vuckovic S, Hart DN: Myeloid blood CD11c(+) dendritic cells and monocyte-derived dendritic cells differ in their ability to stimulate T lymphocytes. Blood 2002, 100:2858–2866. [DOI] [PubMed] [Google Scholar]
- 61.Antonelli LRV, Leoratti FMS, Costa PAC, Rocha BC, Diniz SQ, M.S. T, Pereira DB, Teixeira-Carvalho A, Golenbock DT, Gonçalvez R, et al. : The CD14+CD16+ inflammatory monocyte subset displays increased mitochondrial activity and effector function during acute Plasmodium vivax malaria PLoS Pathog 2014, in press.** This study chracterize the cell surface markers and function of monocytes subsets during malaria.
- 62.Rocha BC, Marques PE, Leoratti FMS, Junqueira C, Pereira DB, Antonelli L, Menezes GB, Golenbock DT, Gazzinelli RT: Type I Interferon Transcriptional Signature in Neutrophils and Low-Density Granulocytes Are Associated with Tissue Damage in Malaria. Cell Rep 2015, 13:2829–2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bouharoun-Tayoun H, Oeuvray C, Lunel F, Druilhe P: Mechanisms underlying the monocyte-mediated antibody-dependent killing of Plasmodium falciparum asexual blood stages. J Exp Med 1995, 182:409–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Osier FH, Feng G, Boyle MJ, Langer C, Zhou J, Richards JS, McCallum FJ, Reiling L, Jaworowski A, Anders RF, et al. : Opsonic phagocytosis of Plasmodium falciparum merozoites: mechanism in human immunity and a correlate of protection against malaria. BMC Med 2014, 12:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Leoratti FM, Trevelin SC, Cunha FQ, Rocha BC, Costa PA, Gravina HD, Tada MS, Pereira DB, Golenbock DT, Antonelli LR, et al. : Neutrophil paralysis in Plasmodium vivax malaria. PLoS Negl Trop Dis 2012, 6:e1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Urban BC, Roberts DJ: Malaria, monocytes, macrophages and myeloid dendritic cells: sticking of infected erythrocytes switches off host cells. Curr Opin Immunol 2002, 14:458–465. [DOI] [PubMed] [Google Scholar]
- 67.Chua CL, Brown G, Hamilton JA, Rogerson S, Boeuf P: Monocytes and macrophages in malaria: protection or pathology? Trends Parasitol 2013, 29:26–34. [DOI] [PubMed] [Google Scholar]
- 68.Urban BC, Cordery D, Shafi MJ, Bull PC, Newbold CI, Williams TN, Marsh K: The frequency of BDCA3-positive dendritic cells is increased in the peripheral circulation of Kenyan children with severe malaria. Infect Immun 2006, 74:6700–6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guermonprez P, Helft J, Claser C, Deroubaix S, Karanje H, Gazumyan A, Darasse-Jeze G, Telerman SB, Breton G, Schreiber HA, et al. : Inflammatory Flt3l is essential to mobilize dendritic cells and for T cell responses during Plasmodium infection. Nat Med 2013, 19:730–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goncalves RM, Salmazi KC, Santos BA, Bastos MS, Rocha SC, Boscardin SB, Silber AM, Kallas EG, Ferreira MU, Scopel KK: CD4+ CD25+ Foxp3+ regulatory T cells, dendritic cells, and circulating cytokines in uncomplicated malaria: do different parasite species elicit similar host responses? Infect Immun 2010, 78:4763–4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jangpatarapongsa K, Chootong P, Sattabongkot J, Chotivanich K, Sirichaisinthop J, Tungpradabkul S, Hisaeda H, Troye-Blomberg M, Cui L, Udomsangpetch R: Plasmodium vivax parasites alter the balance of myeloid and plasmacytoid dendritic cells and the induction of regulatory T cells. Eur J Immunol 2008, 38:2697–2705.** This study describes a connection of dendritic cells and regulatory T cells during P. vivax malaria.
- 72.Pinzon-Charry A, Woodberry T, Kienzle V, McPhun V, Minigo G, Lampah DA, Kenangalem E, Engwerda C, Lopez JA, Anstey NM, et al. : Apoptosis and dysfunction of blood dendritic cells in patients with falciparum and vivax malaria. J Exp Med 2013, 210:1635–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Loughland JR, Minigo G, Sarovich DS, Field M, Tipping PE, Montes de Oca M, Piera KA, Amante FH, Barber BE, Grigg MJ, et al. : Plasmacytoid dendritic cells appear inactive during sub-microscopic Plasmodium falciparum blood-stage infection, yet retain their ability to respond to TLR stimulation. Sci Rep 2017, 7:2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Loughland JR, Woodberry T, Boyle MJ, Tipping PE, Piera KA, Amante FH, Kenangalem E, Price RN, Engwerda CR, Anstey NM, et al. : Plasmodium falciparum Activates CD16+ Dendritic Cells to Produce Tumor Necrosis Factor and Interleukin-10 in Subpatent Malaria. J Infect Dis 2019, 219:660–671.* This study suggest the potential pro-inflammatory and regulatory role of unique dendritic cell subset during P. falciparum malaria.
- 75.Woodberry T, Loughland JR, Minigo G, Burel JG, Amante FH, Piera KA, McNeil Y, Yeo TW, Good MF, Doolan DL, et al. : Early Immune Regulatory Changes in a Primary Controlled Human Plasmodium vivax Infection: CD1c(+) Myeloid Dendritic Cell Maturation Arrest, Induction of the Kynurenine Pathway, and Regulatory T Cell Activation. Infect Immun 2017, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kho S, Marfurt J, Handayuni I, Pava Z, Noviyanti R, Kusuma A, Piera KA, Burdam FH, Kenangalem E, Lampah DA, et al. : Characterization of blood dendritic and regulatory T cells in asymptomatic adults with sub-microscopic Plasmodium falciparum or Plasmodium vivax infection. Malar J 2016, 15:328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Urban BC, Ferguson DJ, Pain A, Willcox N, Plebanski M, Austyn JM, Roberts DJ: Plasmodium falciparum-infected erythrocytes modulate the maturation of dendritic cells. Nature 1999, 400:73–77. [DOI] [PubMed] [Google Scholar]
- 78.Skorokhod O, Schwarzer E, Grune T, Arese P: Role of 4-hydroxynonenal in the hemozoin-mediated inhibition of differentiation of human monocytes to dendritic cells induced by GM-CSF/IL-4. Biofactors 2005, 24:283–289. [DOI] [PubMed] [Google Scholar]
- 79.Skorokhod OA, Alessio M, Mordmuller B, Arese P, Schwarzer E: Hemozoin (malarial pigment) inhibits differentiation and maturation of human monocyte-derived dendritic cells: a peroxisome proliferator-activated receptor-gamma-mediated effect. J Immunol 2004, 173:4066–4074. [DOI] [PubMed] [Google Scholar]
- 80.Sponaas AM, Freitas do Rosario AP, Voisine C, Mastelic B, Thompson J, Koernig S, Jarra W, Renia L, Mauduit M, Potocnik AJ, et al. : Migrating monocytes recruited to the spleen play an important role in control of blood stage malaria. Blood 2009, 114:5522–5531.* This study demonstrates the role of CCR2/CCL2 in the recruitment of inflammatory monocytes, potentially MO-DCs, and their importance in host resistance to Plasmodium infection in mice.
- 81.Pai S, Qin J, Cavanagh L, Mitchell A, El-Assaad F, Jain R, Combes V, Hunt NH, Grau GE, Weninger W: Real-time imaging reveals the dynamics of leukocyte behaviour during experimental cerebral malaria pathogenesis. PLoS Pathog 2014, 10:e1004236.* Immaging by two-photon microscopy that visualizes the migration and accumualtion of inflammatory monocytes in the endothelial vaxcular cells from the central nervous system during experimental cerebral malaria.
- 82.Schumak B, Klocke K, Kuepper JM, Biswas A, Djie-Maletz A, Limmer A, van Rooijen N, Mack M, Hoerauf A, Dunay IR: Specific depletion of Ly6C(hi) inflammatory monocytes prevents immunopathology in experimental cerebral malaria. PLoS One 2015, 10:e0124080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sorensen EW, Lian J, Ozga AJ, Miyabe Y, Ji SW, Bromley SK, Mempel TR, Luster AD: CXCL10 stabilizes T cell-brain endothelial cell adhesion leading to the induction of cerebral malaria. JCI Insight 2018, 3.* Immaging by two photon microscopy that visualizes the expression of CXCL10 by endothelial cells as well as inflammatory monocytes that accumulate in vassels in the central nervous system during experimental cerebral malaria.
- 84.Lai SM, Sheng J, Gupta P, Renia L, Duan K, Zolezzi F, Karjalainen K, Newell EW, Ruedl C: Organ-Specific Fate, Recruitment, and Refilling Dynamics of Tissue-Resident Macrophages during Blood-Stage Malaria. Cell Rep 2018, 25:3099–3109 e3093.** This study describes the deletion of tissue resident macrophages and their replaciment by inflammatory monocytes in spleen, liver and lungs of Plasmodium-infected mice.
- 85.Galvao-Filho B, de Castro JT, Figueiredo MM, Rosmaninho CG, Antonelli L, Gazzinelli RT: The emergence of pathogenic TNF/iNOS producing dendritic cells (Tip-DCs) in a malaria model of acute respiratory distress syndrome (ARDS) is dependent on CCR4. Mucosal Immunol 2018.* This study describes the emergence of MO-DCs in the lungs, their differentiation on Tip-DCs and role in a malaria ARDS model.
- 86.Olagnier D, Lavergne RA, Meunier E, Lefevre L, Dardenne C, Aubouy A, Benoit-Vical F, Ryffel B, Coste A, Berry A, et al. : Nrf2, a PPARgamma alternative pathway to promote CD36 expression on inflammatory macrophages: implication for malaria. PLoS Pathog 2011, 7:e1002254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Alferink J, Specht S, Arends H, Schumak B, Schmidt K, Ruland C, Lundt R, Kemter A, Dlugos A, Kuepper JM, et al. : Cannabinoid Receptor 2 Modulates Susceptibility to Experimental Cerebral Malaria through a CCL17-dependent Mechanism. J Biol Chem 2016, 291:19517–19531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.deWalick S, Amante FH, McSweeney KA, Randall LM, Stanley AC, Haque A, Kuns RD, MacDonald KP, Hill GR, Engwerda CR: Cutting edge: conventional dendritic cells are the critical APC required for the induction of experimental cerebral malaria. J Immunol 2007, 178:6033–6037.* This study describes the role of DCs in the development of experimental cerebral malaria.
- 89.Lagasse HA, Anidi IU, Craig JM, Limjunyawong N, Poupore AK, Mitzner W, Scott AL: Recruited monocytes modulate malaria-induced lung injury through CD36-mediated clearance of sequestered infected erythrocytes. J Leukoc Biol 2016, 99:659–671.** This study describes the emergence of inflammatory monocytes in the lungs and their role in a malaria ARDS model.
- 90.Romano A, Carneiro MBH, Doria NA, Roma EH, Ribeiro-Gomes FL, Inbar E, Lee SH, Mendez J, Paun A, Sacks DL, et al. : Divergent roles for Ly6C+CCR2+CX3CR1+ inflammatory monocytes during primary or secondary infection of the skin with the intra-phagosomal pathogen Leishmania major. PLoS Pathog 2017, 13:e1006479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Anstey NM, Handojo T, Pain MC, Kenangalem E, Tjitra E, Price RN, Maguire GP: Lung injury in vivax malaria: pathophysiological evidence for pulmonary vascular sequestration and posttreatment alveolar-capillary inflammation. J Infect Dis 2007, 195:589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Maguire GP, Handojo T, Pain MC, Kenangalem E, Price RN, Tjitra E, Anstey NM: Lung injury in uncomplicated and severe falciparum malaria: a longitudinal study in papua, Indonesia. J Infect Dis 2005, 192:1966–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hanson JP, Lam SW, Mohanty S, Alam S, Pattnaik R, Mahanta KC, Hasan MU, Charunwatthana P, Mishra SK, Day NP, et al. : Fluid resuscitation of adults with severe falciparum malaria: effects on Acid-base status, renal function, and extravascular lung water. Crit Care Med 2013, 41:972–981. [DOI] [PubMed] [Google Scholar]
- 94.Atam V, Singh AS, Yathish BE, Das L: Acute pancreatitis and acute respiratory distress syndrome complicating Plasmodium vivax malaria. J Vector Borne Dis 2013, 50:151–154. [PubMed] [Google Scholar]
- 95.Lee HJ, Baek JH, Chae MH, Joo H, Lee JS, Chung MH, Park YK, Kim JT: A case of vivax malaria complicated by adult respiratory distress syndrome and successful management with extracorporeal membrane oxygenation. Korean J Parasitol 2013, 51:551–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Deroost K, Tyberghein A, Lays N, Noppen S, Schwarzer E, Vanstreels E, Komuta M, Prato M, Lin JW, Pamplona A, et al. : Hemozoin induces lung inflammation and correlates with malaria-associated acute respiratory distress syndrome. Am J Respir Cell Mol Biol 2013, 48:589–600. [DOI] [PubMed] [Google Scholar]
- 97.Van den Steen PE, Geurts N, Deroost K, Van Aelst I, Verhenne S, Heremans H, Van Damme J, Opdenakker G: Immunopathology and dexamethasone therapy in a new model for malaria-associated acute respiratory distress syndrome. Am J Respir Crit Care Med 2010, 181:957–968. [DOI] [PubMed] [Google Scholar]
- 98.Pham TT, Verheijen M, Vandermosten L, Deroost K, Knoops S, Van den Eynde K, Boon L, Janse CJ, Opdenakker G, Van den Steen PE: Pathogenic CD8(+) T Cells Cause Increased Levels of VEGF-A in Experimental Malaria-Associated Acute Respiratory Distress Syndrome, but Therapeutic VEGFR Inhibition Is Not Effective. Front Cell Infect Microbiol 2017, 7:416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zielinska KA, de Cauwer L, Knoops S, Van der Molen K, Sneyers A, Thommis J, De Souza JB, Opdenakker G, De Bosscher K, Van den Steen PE: Plasmodium berghei NK65 in Combination with IFN-gamma Induces Endothelial Glucocorticoid Resistance via Sustained Activation of p38 and JNK. Front Immunol 2017, 8:1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shikani HJ, Freeman BD, Lisanti MP, Weiss LM, Tanowitz HB, Desruisseaux MS: Cerebral malaria: we have come a long way. Am J Pathol 2012, 181:1484–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Idro R, Marsh K, John CC, Newton CR: Cerebral malaria: mechanisms of brain injury and strategies for improved neurocognitive outcome. Pediatr Res 2010, 68:267–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kwiatkowski D, Molyneux ME, Stephens S, Curtis N, Klein N, Pointaire P, Smit M, Allan R, Brewster DR, Grau GE, et al. : Anti-TNF therapy inhibits fever in cerebral malaria. Q J Med 1993, 86:91–98. [PubMed] [Google Scholar]
- 103.Grau GE, Fajardo LF, Piguet PF, Allet B, Lambert PH, Vassalli P: Tumor necrosis factor (cachectin) as an essential mediator in murine cerebral malaria. Science 1987, 237:1210–1212. [DOI] [PubMed] [Google Scholar]
- 104.Grau GE, Heremans H, Piguet PF, Pointaire P, Lambert PH, Billiau A, Vassalli P: Monoclonal antibody against interferon gamma can prevent experimental cerebral malaria and its associated overproduction of tumor necrosis factor. Proc Natl Acad Sci U S A 1989, 86:5572–5574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Haque A, Best SE, Montes de Oca M, James KR, Ammerdorffer A, Edwards CL, de Labastida Rivera F, Amante FH, Bunn PT, Sheel M, et al. : Type I IFN signaling in CD8-DCs impairs Th1-dependent malaria immunity. J Clin Invest 2014, 124:2483–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Piva L, Tetlak P, Claser C, Karjalainen K, Renia L, Ruedl C: Cutting edge: Clec9A+ dendritic cells mediate the development of experimental cerebral malaria. J Immunol 2012, 189:1128–1132. [DOI] [PubMed] [Google Scholar]
- 107.Tamura T, Kimura K, Yuda M, Yui K: Prevention of experimental cerebral malaria by Flt3 ligand during infection with Plasmodium berghei ANKA. Infect Immun 2011, 79:3947–3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Campanella GS, Tager AM, El Khoury JK, Thomas SY, Abrazinski TA, Manice LA, Colvin RA, Luster AD: Chemokine receptor CXCR3 and its ligands CXCL9 and CXCL10 are required for the development of murine cerebral malaria. Proc Natl Acad Sci U S A 2008, 105:4814–4819.** This study demonstrates the expression of CXCL9 and CXCL10 in the brain as well as CXCR3 on T cells and their role on development of experimental cerebral malaria.
- 109.Nie CQ, Bernard NJ, Norman MU, Amante FH, Lundie RJ, Crabb BS, Heath WR, Engwerda CR, Hickey MJ, Schofield L, et al. : IP-10-mediated T cell homing promotes cerebral inflammation over splenic immunity to malaria infection. PLoS Pathog 2009, 5:e1000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Furusawa J, Mizoguchi I, Chiba Y, Hisada M, Kobayashi F, Yoshida H, Nakae S, Tsuchida A, Matsumoto T, Ema H, et al. : Promotion of Expansion and Differentiation of Hematopoietic Stem Cells by Interleukin-27 into Myeloid Progenitors to Control Infection in Emergency Myelopoiesis. PLoS Pathog 2016, 12:e1005507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Belyaev NN, Biro J, Langhorne J, Potocnik AJ: Extramedullary myelopoiesis in malaria depends on mobilization of myeloid-restricted progenitors by IFN-gamma induced chemokines. PLoS Pathog 2013, 9:e1003406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Belyaev NN, Brown DE, Diaz AI, Rae A, Jarra W, Thompson J, Langhorne J, Potocnik AJ: Induction of an IL7-R(+)c-Kit(hi) myelolymphoid progenitor critically dependent on IFN-gamma signaling during acute malaria. Nat Immunol 2010, 11:477–485. [DOI] [PubMed] [Google Scholar]
- 113.Dunay IR, Damatta RA, Fux B, Presti R, Greco S, Colonna M, Sibley LD: Gr1(+) inflammatory monocytes are required for mucosal resistance to the pathogen Toxoplasma gondii. Immunity 2008, 29:306–317. [DOI] [PMC free article] [PubMed] [Google Scholar]