Abstract
Globally, liver cancer is the most frequent fatal malignancy; in the United States, it ranks fifth. Patients are often diagnosed with liver cancer in advanced stages, contributing to its poor prognosis. Of all liver cancer cases, >90% are hepatocellular carcinomas (HCCs) for which chemotherapy and immunotherapy are the best options for therapy. For liver cancer patients, new treatment options are necessary. Use of natural compounds and/or nanotechnology may provide patients with better outcomes with lower systemic toxicity and fewer side effects. Improved treatments can lead to better prognoses. Finally, in this review, we present some of the problems and current treatment options contributing to the poor outcomes for patients with liver cancer.
Keywords: Liver cancer, natural compound, immunotherapy, chemotherapeutics
1. Introduction
As the most frequent cause of cancer deaths across the globe and fifth most common in the United States, liver cancer is the only one of the top five deadliest cancers to have an annual percentage increase in occurrence [1]. Developing counties have more incidence of liver diseases [2]. Risk factors include hepatitis B virus, hepatitis C virus, fatty liver disease, alcohol-related cirrhosis, smoking, obesity, diabetes, iron overload, and various dietary exposures [3]. The prognosis for liver cancer is poor. Only 5% to 15% of patients are eligible for surgical removal, which is suitable only for early-stage patients and due to diminished hepatic regenerative capacity, typically without cirrhosis; right hepatectomy carry a higher risk for post-operative complications compared to left hepatectomy. Treatment options for more advanced stages include the following: (a) Trans-arterial chemoembolization (TACE), which leads to a 23% improvement in the 2-year survival in comparison to conservative therapy for intermediate stage HCC patients. (b) Oral dosing with sorafenib, a kinase inhibitor and the most accepted option for late-stage cases. However, fewer than one-third of patients benefit from the treatment, and drug resistance is evident within six months of initiating the regimen [4]. With long-term use, chemotherapeutic drugs, such as sorafenib, have additional issues such as toxicity and/or drug inefficacy. As a result, neither current ablation therapies nor chemotherapy is appreciably effective in improving outcomes of this devastating disease. Further research to find better methods for treating liver cancer are necessary.
Prevention, development, progression, and treatment of cancers is associated with the diet of patients. A European study showed that a higher dietary intake of fruits and vegetables is associated with a lower risk of cancer development [5]. Diverse natural compounds in fruits, vegetables, and spices function in suppressing mechanisms that are involved in development of cancers, and they stimulate mechanisms that are associated with prevention of the disease. These compounds activate anti-tumor, anti-proliferative, anti-inflammatory, and anti-oxidant systems that may provide therapeutic options for new cancer treatment regimens [6–8]. Some compounds demonstrate selectivity in causing cytotoxicity to cancer cells, leaving non-cancerous cells unaffected [9]. For instance, compounds such as piperine, inhibit enzymes necessary for drug metabolism, which may indicate a future use of co-administration with current or potential chemotherapeutic drugs to increase plasma concentrations [10]. Other natural compounds may enhance the efficacy of current drug regimens without increasing host toxicity. As an example, for H22 cells, polysaccharides from Lentinus edodes and Tricholoma matsutake enhance the inhibitory effect of 5-fluorouracil (5-FU)[11].
The immune system is commonly involved in killing cancer cells. In this process, antigen-presenting cells present the peptides of tumor fragments to class I and II major histocompatibility complex (MHC) molecules. However, this mechanism may be ineffective due to the capacity of malignancies to evade these functions [12, 13]. Moreover, biomarkers of tumor growth and desmoplasia can be targeted to prevent tumor progression [14]. With chemotherapeutic agents, multidrug resistance is an issue. Cancer stem-like cells/cancer-initiating cells are responsible for resistance, providing a pathway for tumor recurrence and metastasis. Immunotherapy helps fight resistance to common chemotherapies by targeting stem cells [15]. Other useful approaches, through immune checkpoint inhibitors, such as PD-1 and PD-L1 targeting, and cancer vaccines hinder cancer progression and kill cancer cells. Sorafenib prevents immunosuppression, giving reason to consider combination therapy with this drug [16, 17].
Nanotechnology, which may be utilized to create or enhance treatments that lead to better results for neoplasms, can improve the activity of minimally effective drugs in targeting and killing cancer cells. This is accomplished by optimizing the size and surface properties of drugs and/or utilizing tissue-specific homing devices to target sites that lower the likelihood of systemic toxicity and side-effects [18]. Nanotechnology may alter current combination therapy approaches and improve permeability, retention, and pharmacokinetic profiles, and thereby reduce side effects [19, 20]. Nanoparticle techniques provide a promising future through treatment programs that combine separate agents to improve the effects of drugs [21, 22].
The poor prognosis for liver cancer leads scientists and physicians to search for novel treatment options to improve patient survival. Combining medications and altering drug administration/delivery methods give new horizons to improving the outcomes for malignancies. In this study, we describe some of the most common agents used for treatment of advanced HCCs, including chemotherapies, immunotherapies, and nanoparticles, and the rationale behind some current clinical trials.
2. Natural compounds and their outcomes in liver cancer
Piperine, an alkaloid extracted from black and long peppers, has anti-tumor, anti-mutagenic, anti-oxidant, and anti-proliferative activities [23]. It lowers lipid peroxidation and inhibits enzymes involved in drug metabolism, such as aryl hydrocarbon hydroxylase and UDP-glucuronyl transferase, which increase the bioavailability of drugs and phytochemicals [24]. It also improves intestinal absorption upon interaction with the lipid environment of the gut [25].
Drug resistance is an issue for a variety of drugs that could otherwise be more effective in the treatment of cancers. Concentrations of piperine that were toxic to liver cancer cells were nontoxic to normal hepatocytes, showing selectivity. Moreover, in cells, the alkaloid increased caspase-3 and caspase-9 activity and inhibited catalase, leading to stimulation of peroxi-dedriven, mitochondria-mediated apoptosis. Furthermore, piperine inhibited receptor tyrosine kinase and human HCC progression; in rats with diethylnitrosamine-induced HCC cells, piperine therapy caused increases of apoptotic cell death [26]. By suppressing cytochrome P450-mediated bioactivation of mycotoxin, piperine counteracted the toxicity of aflatoxin B1 and the formation of micronuclei in hepatoma cells of rats [27]. Thus, piperine may contribute to the activity of other natural compounds and thereby aid in the treatment of liver cancer.
Curcumin, a component of turmeric, has numerous biological effects in various diseases, including liver cancer [28]. For rats, curcumin enhanced the effect of piperine on diethylnitrosamine-induced HCC. Fewer morphological, biochemical, apoptotic, and proliferative changes occurred with the combination compared to curcumin alone or to a placebo, an indication of synergistic activity in the liver and serum of the rats [29].
Oleocanthal is a phenolic compound present in extra-virgin olive oil, the main source of oil in the Mediterranean diet. There is a negative correlation between the intake of extra-virgin olive oil and the incidence of cancer, metabolic, cardiovascular, Alzheimer’s, and osteoporosis diseases [30]. Oleocanthal kills cancer cells and stimulates apoptosis [31]. Additionally, Chronic inflammation may lead to the development of fibrosis, cirrhosis, and HCC, due to its correlation with persistent hepatic injury and regeneration [32]. Inflammation, associated with HCC, worsens liver cancer. Compared to normal cells, COX-2 is up-regulated in HCC cells, and higher concentrations are evident as cancer cells differentiate [33]. Oleocanthal has anti-inflammatory effects via inhibition of COX-1 and COX-2. Oleocanthal caused more growth inhibition of HCC cells than ibuprofen, indomethacin, and nimesulide, which are NSAIDs and COX inhibitors. With oleocanthal treatment, inhibition of colony formation and induction of apoptosis were associated with PARP cleavage, caspase-3/caspase-7 activation, and chromatin condensation. Expression of γH2AX, a marker of DNA damage, increased, resulting in elevated production of intracellular reactive oxygen species and mitochondrial depolarization. Oleocanthal was toxic to HepG2, Huh7, and Hep3b cells, but normal human hepatocytes were unharmed, showing selectivity [34]. Various concentrations of oleocanthal increased PARP cleavage and caspase cleavage with G0/G1 cell cycle arrest. For HepG2, Huh-7, and Hcclm3 HCC cells, oleocanthal inhibited cell progression via cell cycle arrest and apoptosis yet had no evident effect on normal human (LO2) cells. For HCC cells, oleocanthal reduced migration and invasion, and HCC metastasis was blocked in vivo. This herbal extract reduced the nuclear translocation of STAT3 and DNA binding activity, causing decreases of Bcl-2 family, survivin, cyclin D1, and MMP 2. Furthermore, the treatment decreased p-JAK1 and p-JAK-2m, which are positive regulators of STAT3, and increased the negative STAT3 regulator, SHP-1. mRNA and protein expression of the transcription factor, Twist, were decreased, leading to suppression of the epithelial-mesenchymal transition (EMT), a contributor to metastasis [35].
Allium extracts have tumor-inhibiting properties and are associated with decreased risk for various cancers [36]. These extracts are composed of various organosulfur compounds and flavanols that block various stages of carcinogenesis [37]. One constituent, diallyl sulfide, inhibited hepatocarcinogenesis and diethylnitrosamine-induced hepatocellular adenomas [38]. S-Allyl cysteine (SAC), another component of allium extracts, demonstrated anti-proliferative effects. Treatment of MHCC97L cells with SAC showed complete inhibition of colony formation by down-regulation of the proliferation biomarkers, Ki-67 and proliferation cell nuclear antigen. In the presence of SAC, an increased number of cells were necrotic or in early/late apoptotic stages; increased levels of caspase-3 and caspase-9, with lower levels of Bcl-xL and Bcl-2, were evident. Higher levels of S-phase cells were apparent, along with lower levels of G2/M phase cells. These observations were supported by suppression of cell cycle proteins (cdc2, cdc25c, and cyclin B1). For the treated cells, migration and invasion were reduced. This was accompanied by up-regulation of E-cadherin and down-regulation of vascular endothelial growth factor (VEGF) mRNA, two factors associated with metastasis. Similarly, in animals, there was inhibition of metastasis [39]. Methanol extracts of allium bulbs caused a G0/G1 block in HepG2 cells but not in other cancer cells. For the other cells, there was S and G2/M phase arrest. In addition, treatment of cancer cells with allium bulb extract increased the number of apoptotic cells. These cells showed an elevated caspase-9 activity and expression of the tumor suppressor, p53. Combination of an allium extract with doxorubicin, a common cancer chemotherapeutic drug, produced a synergistic effect and led to decreases in plasma concentrations necessary to induce cytotoxicity. This effect was seen for HepG2 cells but not for normal cells [37].
An herb used in traditional medicine, Cnidium officinale Makino, is native to China, where it is utilized to manage pain, inflammation, menstrual disturbances, and depressed blood pressure. Extracts of this herb reduce tumor angiogenesis and metastasis [40] and inhibit human oral and colorectal cancers [41, 42]. HepG2 cells exposed to Makino extracts showed reductions of cell viability and an increase in apoptotic bodies, but these effects were not evident for Chang liver cells. For HepG2 cells, the extract increased the number of cells in the G0/G1 phases, in turn, reduced the number of cells in the S-phase, indicating cell cycle arrest. This was accompanied by up-regulation of p53, caspase-3 in a dose-dependent manner, and lower expressions of Bcl-2, CDK4, and cyclin D [43].
The plant Viscum album var (VAV), commonly known as the Korean or European mistletoe, is used in Asia as an herbal medicine for chronic hepatic disorders. Extracts of the herb stimulate proliferation of normal liver cells, without cytotoxicity. However, for SK-Hep-1 cells, there were anti-proliferative effects at the same concentrations as for the normal liver cells. In addition, for cancer cells, these were more cells in the G0/G1 phases, with lower numbers in the S and G2/M phases. Moreover, with treatment, there was down-regulation of the S-phase regulator, Cdk2, and cyclin D1 levels and up-regulation of p21 gene expression. Thus, in addition to inhibition of proliferation, cell cycle arrest was evident [44].
For both HepG2 and Hep3B cells, two congeners isolated from Juglans mandshurica Maxim had moderate cytotoxicity (compound 4) or caused 50% cell death (compound 5) [45]. One of its alkaloids had anti-hepatoma properties, such as stimulation of apoptosis and autophagy of HepG2 cells, and induced autophagic death of Hep3B cells [46]. Isolated from extracts of Maxim, juglanthraquinone proved to be a promising drug for treatment of liver cancer, showing cytotoxic effects and reduced cell viability of HepG2 cells in comparison with normal L02 cells. An anti-proliferative effect was confirmed with S phase arrest and reduced G2/M populations, along with lowered expression of Ki67, cyclin A, and cdk2, and increased expression of Cip1/p21. Apoptosis was evident, as determined by the presence of DNA fragmentation, chromatin condensation, caspase-3 and −9 activation, higher levels of Bax, and lower levels of Bcl-2 [47]. Thus, these natural herbs contain compounds that are promising agents for treating liver cancer.
3. Chemotherapy for treating liver cancer
Oral administration of the multi-kinase inhibitor, sorafenib, is recommended worldwide as the first-line therapy for advanced stages of HCC supported by the results of several trials[48, 49]. This clinically approved drug suppresses tumor angiogenesis, cell division, and proliferation through inhibition of the MAP kinase cascade, and it induces apoptosis of cancer cells. Proteins inhibited by sorafenib include serine-threonine kinase Raf-1, platelet-derived growth factor receptor-β, c-KIT, FLT-3, VEGF receptor-2 and −3, and RET [50, 51]. In 2007, FDA approved sorafenib as an agent for treatment of HCC, although the average survival time of patients increased by only 3 to 5 months compared to the placebo group, an outcome far below ideal [52–54]. With extended administration of sorafenib, cancer cells become resistant to the drug, making it ineffective. Furthermore, sorafenib, administered to cancer patients causes adverse side effects. There are increased concentrations of serum lipase and amylase, hypertension, hemorrhage, neuropathy, leukopenia, lymphopenia, diarrhea, nausea, vomiting, and dyspnea. Moreover, 10% of patients treated with sorafenib may develop cutaneous squamous cell carcinomas [55, 56]. A new treatment, transarterial chemoembolization plus sorafenib, is superior to sorafenib alone [57].
Due to the marginal improvement in HCC prognosis by sorafenib, current clinical trials involve combinations of this multi-kinase inhibitor with other drugs to produce more favorable outcomes for patients, including treatment effectiveness and fewer side effects (Table 1). In several cell lines, including HepG2 cells, vorinostat and sorafenib synergistically induce apoptosis by up-regulating activity of Bax, Bid, Bak, Bim, and Bad and down-regulation of the anti-apoptotic proteins, Bcl-xl, Bcl-2, and MCL-1 [58]. The combination of sorafenib and doxorubicin, which is well tolerated by HCC patients, shows a synergistic effect in control of the disease. Studies with cultured cells indicate a reduced likelihood of development of resistance, partially by inhibition of Raf-1 by sorafenib. With the combination, progression-free and overall survival increased in compared to individual treatments of the two agents [59]. An ongoing phase II trial of Sorafenib + doxorubicin () is expected to be completed by April 2019.
Table 1:
Current ongoing clinical trials overview on liver cancer
| Patient | Intervention | Current Phase | Initiation date | Identifier |
|---|---|---|---|---|
| Advanced hepatocellular carcinoma | Sorafenib tosylate + vorinostat | Phase 1 | August 2010 | |
| Primary hepatic malignancy | Floxuridine + dexamethasone w/ bevacizumab | Phase 2 | May 2007 | |
| Advanced hepatocellular carcinoma | Bevacizumab + erlotinib | Phase 2 | April 2011 | |
| Advanced hepatocellular carcinoma | Leucovorin + 5-FU + oxaliplatin + sorafenib | January 2013 | ||
| Advanced hepatocellular carcinoma, non-resectable | 5-FU + cisplatin | Phase 2 | November 2016 | |
| Fibrolamellar carcinoma | -Everolimus -Letrozole + leuprolide -Everolimus + letrozole + leuprolide | Phase 2 | July 2012 | |
| Advanced/metastatic hepatocellular carcinoma | -MLN0128 -MLN0128 (RP2D) -Sorafenib | Phase 1/Phase 2 | July 2016 | |
| Hepatocellular carcinoma | -BMS-986183 -Nivolumab |
Phase 1/Phase 2 | August 2016 | |
| Advanced/non-resectable hepatocellular carcinoma | Tivozanib | Phase 1/Phase 2 | July 11, 2013 | |
| Advanced hepatocellular carcinoma | -Nivolumab + ipilimumab Sorafenib -Cabozantinib |
Phase 1/Phase 2 | September 26, 2012 | |
| Advanced hepatocellular carcinoma | Sorafenib + doxorubicin | Phase 2 | April 2013 | |
| Advanced hepatocellular carcinoma | -LY2157299 -Sorafenib -LY2157299 + sorafenib |
Phase 2 | August 2014 | |
| Hepatocellular carcinoma | -Ethiodized oil + doxorubicin | Phase 3 | August 2011 | |
| Advanced hepatocellular carcinoma | -Regorafenib | Phase 3 | May 14, 2013 | |
| Advanced hepatocellular carcinoma | Temsirolimus + sorafenib | Phase 2 | October 5, 2012 | |
| Hepatocellular carcinoma | LY2157299 + sorafenib LY2157299 + ramucirumab | Phase 2 | March 2011 | |
| Advanced hepatocellular carcinoma | Pexa-Vec + nivolumab | Phase 1/Phase 2 | July 27, 2017 | |
| Advanced hepatocellular carcinoma | Pex-Vec + sorafenib | Phase 3 | October 2015 | |
| Hepatocellular carcinoma | NBTXR3, intralesional or intra-arterial injection + SBRT | Phase 1/Phase 2 | November 2015 | NTC02721056 |
| Advanced hepatocellular carcinoma | MTL-CEBPA | Phase 1 | March 1, 2016 | NTC02716012 |
Evaluations of tivozanib, which downregulates VEGF receptor-1, −2, and −3, have been accomplished for renal, breast, and colorectal cancers, but there is limited research on liver cancers [60]. This drug inhibits angiogenesis suppressing VEGF receptor activity in selected forms of solid tumors in mice with HCC and shows low sensitivity in another set of HCC mice due to the variation of genetic oncogenic pathways and tumor suppressor genes which is consistent in human HCC patients. These limited positive effects are similar to those of sorafenib for patients with HCC [61]. Nonetheless, there is a current trial testing the effect of tivozanib for advanced HCC, although a previous phase 2 trial was terminated due to not meeting the set primary endpoint (, ).
5-FU, a drug used to inhibit the growth of various cancers, inhibits cell progression at the S-phase and up-regulates p53. Moreover, miR-22, which is down-regulated in HCCs, is elevated by 5-FU treatment, enhancing the growth inhibition of HepG2 and Huh7 cells compared to normal cells [62]. However, for many cancers, including liver cancers, chemoresistance to 5-FU is an issue. Acting through long non-coding RNAs, HCC cells activate protective autophagy against the drug. Liver cancer stem cells, which are associated with resistance to chemotherapy, have a higher survival rate compared to SK-Hep-1 cells, indicating their involvement in chemoresistance development and tumor growth [63]. Still, the efficacy of 5-FU can be enhanced by combining it with other chemotherapies. Hepatic arterial infusion of 5-FU with cisplatin increased survival (14 months) of HCC patients compared to patients who did not receive the combination (5.2 months) [64]. HCC patients who have developed sorafenib resistance are presently being recruiting for a clinical trial involving co-administration of cisplatin and 5-FU (NTC02967887).
4. Immunotherapy for Liver Cancer
Cancers can be treated by modifying the immune systems of patients so that they recognize specific antigens on cancer cells, by enhancing immune activity through blocking immune checkpoints responsible for immunosuppressive signaling, by cancer vaccines to prevent infection or inflammatory responses, and by non-specific cancer immunotherapies that provide a general boost to the immune system. In 2013, this field of research was selected as the Advance of the Year by the American Society of Clinical Oncology [65]. A benefit of this area of study is that immunotherapy may be combined with drugs currently used to treat liver cancer to give a combined/synergistic effect [17]. Clinical trials are underway for the monoclonal antibodies (mAbs), ramucirumab, which targets VEGF receptor-2, and bevacizumab, which inhibits VEGF receptor binding, in combination therapy with chemotherapeutic, immunotherapeutic, or other agents used in cancer treatment (, , ) [66].
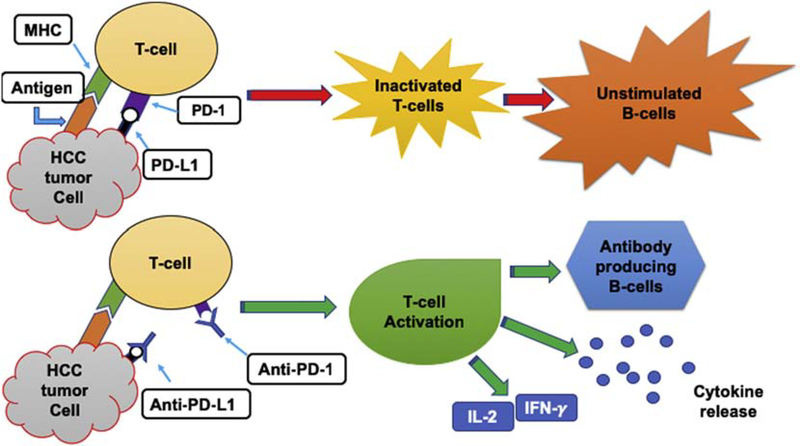
Immune checkpoint inhibitors target proteins that are responsible for decreasing the activity of the human immune system in attacking cancer cells that express these proteins. These checkpoints occur by the way of programmed cell death protein 1 (PD-1) and programmed cell death 1 ligand 1 (PD-L1) binding to cells [67]. PD-1 is a protein expressed on active CD8+ and CD4+ T-cells, B-cells, Treg cells, natural killer cells, myeloid cells, monocytes, and dendritic cells; PD-L1 is expressed on a variety of nonimmune cells as well as on B-cells, T-cells, dendritic cells, macrophages, antigen-presenting cells, myeloid-derived suppressor cells, and some tumor cells [68, 69]. The interaction of PD-1 and PD-L1 inhibits T-cell activity and suppresses release of IFN-γ, interleukin-2, and other cytokines, resulting in temporary immune-inhibiting signals and lessening the ability for a patient to develop antitumor responses that reduce the survival of cancer cells (Fig. 1) [69]. Accordingly, tumor aggression and recurrence are associated with PD-L1 expression, which leads to activated tumor-specific T-cell apoptosis. Thus, PD-L1 can be viewed as a biomarker. T-Cell evasion is enhanced when PD-L1 levels are high in patients. The prognosis for HCC patients is poor when there is high expression of PD-L1 (PD-L1 positive) compared to patients with low PD-L1 expression (PD-L1 negative). In addition, PD-L1 positive patients, in comparison with PD-L1 negative patients, are twice as likely to undergo relapse and have greater numbers of tumors with vascular invasion [69]. Immunosuppression enhances therapeutic outcomes, as demonstrated by the triple combination of anti-PD-1, anti-CXCR4, and sorafenib, which causes a reduction in metastasis and HCC growth [17].
Fig. 1: Schematic demonstrating HCC cells inducing T-cells;
inactivating T-cells, leaving B-cells unstimulated necessary for class switching. T-cells are activated to induce the immune response via IFN-gamma, IL-2, cytokine release, and stimulating B-cells to undergo class switching.
Targeting the PD-L1/PD-1 blockade may reduce hepatitis B and hepatitis C infections, which are the risk factors for HCC. Since these viral infections are partially responsible for the recurrence of HCC, this therapy may prevent relapses. However, autoimmune disease may occur due to an overactive immune system bypassing the PD-1/PD-L1 and PD-L2 systems; there is a possible association between autoimmune liver diseases, primary biliary cirrhosis, and chronic hepatitis type C [70]. Despite this possibility, these checkpoint inhibitors enhance the immune systems of patients and kill cancer cells, while showing few signs of autoimmune diseases. As a result, PD-1 checkpoint inhibitor, nivolumab, has shown promising results and is now FDA approved for numerous malignant neoplasms including HCC. An ongoing clinical trial is co-administering nivolumab with ipilimumab, a CTLA-4 negative regulator, in HCC [71]. Previous, positive clinical outcomes of ipilimumab gained FDA’s approval for melanoma, colorectal cancer, and renal cell carcinoma treatment () [71–73].
The use of mAbs to treat cancer is an example of how specific cancer antigens can be targeted to kill cells. To be efficacious, these synthetic antibodies must bind to the appropriate antigens. Due to variation among cancer types, this approach is suitable for only some cancers. Even with proper antibody-antigen interactions, the effects vary by cancer type. Designing mAbs against the immune checkpoint regulator, PD-1, reduces T-cell apoptosis, potentiates antitumor immunity, and inhibits cell proliferation [74, 75]. Anti-PD-1 or anti-PD-L1 drugs stimulate T-cell activity and boost the immune system to attack cancer cells. Nivolumab and ipilimumab, utilized as immune checkpoint inhibitors, are mAbs that target PD-1 and CTLA-4 antigens, respectively, blocking their interaction on neoplastic cells and allowing the immune system to attack tumor cells. These immune checkpoint inhibitors are currently in clinical trials to assess their efficacy in comparison with sorafenib ().
Multidrug resistance can be reduced by attacking cancer stem-like cells/cancer-initiating cells, which are resistant to cancer treatments and responsible for metastasis and tumor recurrence. This is relevant for many of the therapeutic options for HCC. Annexin A3-transfected dendritic cells induce T cell activation to kill the stem cells in populations of HCC cells and in animal models [15], allowing current treatments to be effective for extended times. Cancer vaccines are useful for cancers that are caused by viruses. Vaccines against these Hepatitis B and C viruses reduce the likelihood of contracting liver cancers. However, some viruses promote neoplastic destruction. Oncolytic immunotherapeutics vaccinia virus such as Pexa-Vec (pexastimogene devacirepvec, JX-594) involve viruses that replicate and lyse cancer cells, induces antitumor immune responses, and disrupts tumor vascularity [76]. With high-dose treatment, Pexa-Vec improved the survival of HCC patients (14.1 months) compared to those on low-dose treatment (6.7 months) [77]. This virus can be also be used in combination with common therapies. Patients are presently being recruited for a clinical trial to test Pexa-Vec with nivolumab for treatment of advanced HCC as a first-line treatment (NTC03071094); another trial will utilize Pexa-Vec with sorafenib for patients with advanced HCC (NTC02562755).
5. Nanotechnology based approaches for treating liver cancer
Nanotechnology has allowed scientists to improve the effectiveness of drugs in treating liver cancers. This is accomplished by utilizing various nanocarrier-based drug delivery systems that, in turn, allow for decreases in the amounts of drug necessary to elevate the therapeutic index, lower occurrences of systematic toxicity, extended release of the medication for days after a single administration, and enhanced selective targeting of liver cancer cells. An in vitro study utilized luminescent, organic dye-doped, core-shell nanoparticles that leach minimally and are photostable to bind covalently with anti-human liver cancer mAbs. Fluorescent silica nanoparticles efficiently and selectively seek out HepG2 liver cancer cells [78]. This approach is useful for common chemotherapeutic drugs, since toxicity and drug resistance due to efflux pumps are common problems. Several investigators have taken advantage of these techniques to demonstrate improved outcomes in treating liver cancers [79, 80].
Silica with a mesoporous and rattle-type structure was loaded with docetaxel to measure its effectiveness for killing HepG2 cells and for treating mice. This method required only 7% of the amount of docetaxel compared to free docetaxel to kill liver cancer cells. Also, the therapy showed low systematic toxicity for ICR mice with increased anti-tumor activity and a 15% improvement in tumor inhibition for mice subcutaneously transplanted with hepatocarcinomas [81]. Another study demonstrated that drug-eluting microspheres/beads minimized the amount of doxorubicin needed to treat hepatic tumors compared to intra-arterial administration. These microspheres resulted in plasma concentrations of doxorubicin ranging from 9–50 nmol/L, which were 70% to 85% lower compared to intra-arterial administration of doxorubicin. Tumor necrosis increased from one hour after treatment and reached optimum levels at seven days, indicating further elution of the drug from the microspheres; there were minimal side effects [82].
Combination therapies are used to chemo-sensitize cancer cells that have become resistant to drugs and to enhance the performance of drugs in treating tumors [83]. Nanoparticles have improved these outcomes by adding another element to the agents’ mechanism of action (Fig. 2). For HepG2 cells, delivery of doxorubicin and the chemosensitizer curcumin as lipid nanoparticles allowed for sustained release over 48 hours and led to possible synergy, as suggested by higher cytotoxicity, enhanced apoptosis, and decreased IC50, and, for L02 cells, to a reduction in cytotoxicity compared to free doxorubicin and doxorubicin-nanoparticles. For diethylnitrosamine-induced liver cancers, a synergistic inhibition of growth of tumors was evident with the doxorubicin/curcumin approach compared to free doxorubicin/curcumin [17].
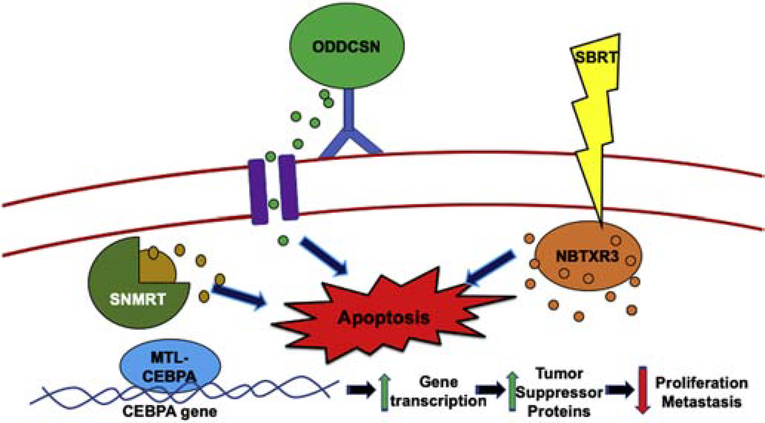
Fig. 2. Schematic of nanoparticle therapy on HCC cells.
Top-middle, ODDCSN (organic dye-doped core-shell nanoparticle) releasing contents while bound to antibody, inducing apoptosis. Bottom-left, MTL-CEBPA binds to the CEBPA gene, increasing transcription and leading to an increase in tumor suppressor proteins causing decreased cell proliferation and metastasis. Top-right, NBTXR3 activated by SBRT, leading to intracellular release of the drug and inducing cell death. Middle-left, silica nanoparticles with a mesoporous and rattle-type (SNMRT) structures provide center of a hollow mesoporous shell to be loaded with drug and released through pores upon stimuli. Here, the silica nanoparticle is releasing chemotherapeutics inside cell to induce apoptosis.
A clinical trial, underway for HCC patients with liver metastases, involves a single administration of NBTXR3, a nanoparticle formulation of hafnium oxide crystals, which is activated by stereotactic body radiation therapy (SBRT) at 24 hours post-injection. At present, patients are not experiencing dose-limiting toxicity, and the NBTRX3 injection is well-tolerated. Since the nanoparticles are safe and have anti-tumor activity, six other clinical studies are using the method [84]. Another trial is taking the advantage of lipid-based nanoparticles, called MTL-CEBPA, which target the CCAAT enhancer binding protein alpha (CEBPA) gene and restores transcription; the region is a tumor suppressor gene which involved in inhibition of cellular proliferation, metastasis, and hepatocyte function. The gene is downregulated in liver cancer cells. Upregulation of the CEBPA gene leads to increased mRNA and protein expression levels, causing a decline in cell proliferation; it prevents liver failure yet promotes normal liver function and increases albumin levels. These results were first demonstrated for HepG2 cells and cirrhotic/HCC rats with multifocal liver tumors [85]. Although many therapeutic methods to treat liver cancer are associated with decreased liver function, MLT-CEBPA improves liver function.
6. Conclusion
In some countries, there has been an improvement in the survival rates for patients with liver cancer [86]. Improvements in HCC patient outcomes are attributed to clinical trials optimizing individual treatment strategies and to the development of more complex therapeutic modalities. Constant development of new techniques and new drugs is providing hope of further advances. Given the various treatment options now available, including natural compounds, chemotherapeutics, immunotherapies, and new methods for delivery of drugs, there are now unprecedented opportunities to treat liver cancers, including use of combination therapy that can improve the effects of current agents. This report provides an overview of the rationales behind some current clinical trial interventions. Although new drugs could be useful, finding the ideal combinations will challenge scientists and physicians. Since the adjusted incidence rates and death rates have continued to increase, researchers must continue to work to reduce these rates and to remove liver cancer from the list of the most commonly diagnosed and fatal cancers.
Acknowledgments
This work was supported by the National Institute of Health [Award Numbers SC1CA193758; U54CA118638]; and from the Department of defense [Award Number W81XWH1810429].
Footnotes
Conflicts of interest
All authors have no conflict of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Siegel RL, Miller KD, Jemal A, Cancer statistics, 2019, CA Cancer J Clin, 69 (2019) 7–34. [DOI] [PubMed] [Google Scholar]
- [2].Starley BQ, Calcagno CJ, Harrison SA, Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection, Hepatology (Baltimore, Md.), 51 (2010) 1820–1832. [DOI] [PubMed] [Google Scholar]
- [3].Center MM, Jemal A, International trends in liver cancer incidence rates, Cancer Epidemiol Biomarkers Prev, 20 (2011) 2362–2368. [DOI] [PubMed] [Google Scholar]
- [4].El-Serag HB, Marrero JA, Rudolph L, Reddy KR, Diagnosis and treatment of hepatocellular carcinoma, Gastroenterology, 134 (2008) 1752–1763. [DOI] [PubMed] [Google Scholar]
- [5].Soerjomataram I, Oomen D, Lemmens V, Oenema A, Benetou V, Trichopoulou A, Coebergh JW, Barendregt J, de Vries E, Increased consumption of fruit and vegetables and future cancer incidence in selected European countries, Eur J Cancer, 46 (2010) 2563–2580. [DOI] [PubMed] [Google Scholar]
- [6].Banerjee S, Singh SK, Chowdhury I, Lillard JW Jr., Singh R, Combinatorial effect of curcumin with docetaxel modulates apoptotic and cell survival molecules in prostate cancer, Front Biosci (Elite Ed), 9 (2017) 235–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Singh SK, Banerjee S, Acosta EP, Lillard JW, Singh R, Resveratrol induces cell cycle arrest and apoptosis with docetaxel in prostate cancer cells via a p53/ p21WAF1/CIP1 and p27KIP1 pathway, Oncotarget, 8 (2017) 17216–17228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Li X, Sun R, Liu R, Natural products in licorice for the therapy of liver diseases: Progress and future opportunities, Pharmacol Res, 144 (2019) 210–226. [DOI] [PubMed] [Google Scholar]
- [9].Zhou Y, Li Y, Zhou T, Zheng J, Li S, Li HB, Dietary Natural Products for Prevention and Treatment of Liver Cancer, Nutrients, 8 (2016) 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Bhardwaj RK, Glaeser H, Becquemont L, Klotz U, Gupta SK, Fromm MF, Piperine, a major constituent of black pepper, inhibits human P-glycoprotein and CYP3A4, J Pharmacol Exp Ther, 302 (2002) 645–650. [DOI] [PubMed] [Google Scholar]
- [11].Ren M, Ye L, Hao X, Ren Z, Ren S, Xu K, Li J, Polysaccharides from Tricholoma matsutake and Lentinus edodes enhance 5-fluorouracil-mediated H22 cell growth inhibition, J Tradit Chin Med, 34 (2014) 309–316. [DOI] [PubMed] [Google Scholar]
- [12].Coffelt SB, de Visser KE, Revving Up Dendritic Cells while Braking PD-L1 to Jump-Start the Cancer-Immunity Cycle Motor, Immunity, 44 (2016) 722–724. [DOI] [PubMed] [Google Scholar]
- [13].Boon T, Coulie PG, Van den Eynde BJ, van der Bruggen P, Human T cell responses against melanoma, Annu Rev Immunol, 24 (2006) 175–208. [DOI] [PubMed] [Google Scholar]
- [14].Chen Y, Huang Y, Reiberger T, Duyverman AM, Huang P, Samuel R, Hiddingh L, Roberge S, Koppel C, Lauwers GY, Zhu AX, Jain RK, Duda DG, Differential effects of sorafenib on liver versus tumor fibrosis mediated by stromal-derived factor 1 alpha/C-X-C receptor type 4 axis and myeloid differentiation antigen-positive myeloid cell infiltration in mice, Hepatology (Baltimore, Md.), 59 (2014) 1435–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Pan QZ, Pan K, Wang QJ, Weng DS, Zhao JJ, Zheng HX, Zhang XF, Jiang SS, Lv L, Tang Y, Li YQ, He J, Liu Q, Chen CL, Zhang HX, Xia JC, Annexin A3 as a potential target for immunotherapy of liver cancer stem-like cells, Stem Cells, 33 (2015) 354–366. [DOI] [PubMed] [Google Scholar]
- [16].Kudo M, Immune Checkpoint Blockade in Hepatocellular Carcinoma: 2017 Update, Liver Cancer, 6 (2016) 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhao X, Chen Q, Liu W, Li Y, Tang H, Liu X, Yang X, Codelivery of doxorubicin and curcumin with lipid nanoparticles results in improved efficacy of chemotherapy in liver cancer, Int J Nanomedicine, 10 (2015) 257–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Reddy LH, Couvreur P, Nanotechnology for therapy and imaging of liver diseases, J Hepatol, 55 (2011) 1461–1466. [DOI] [PubMed] [Google Scholar]
- [19].Singh SK, Singh S, Lillard JW Jr., Singh R, Drug delivery approaches for breast cancer, Int J Nanomedicine, 12 (2017) 6205–6218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Davis ME, Chen ZG, Shin DM, Nanoparticle therapeutics: an emerging treatment modality for cancer, Nat Rev Drug Discov, 7 (2008) 771–782. [DOI] [PubMed] [Google Scholar]
- [21].Singh SK, Lillard JW Jr., Singh R, Reversal of drug resistance by planetary ball milled (PBM) nanoparticle loaded with resveratrol and docetaxel in prostate cancer, Cancer Lett, 427 (2018) 49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Livney YD, Assaraf YG, Rationally designed nanovehicles to overcome cancer chemoresistance, Adv Drug Deliv Rev, 65 (2013) 1716–1730. [DOI] [PubMed] [Google Scholar]
- [23].Tawani A, Amanullah A, Mishra A, Kumar A, Evidences for Piperine inhibiting cancer by targeting human G-quadruplex DNA sequences, Sci Rep, 6 (2016) 39239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Srinivasan K, Black pepper and its pungent principle-piperine: a review of diverse physiological effects, Crit Rev Food Sci Nutr, 47 (2007) 735–748. [DOI] [PubMed] [Google Scholar]
- [25].Johri RK, Thusu N, Khajuria A, Zutshi U, Piperine-mediated changes in the permeability of rat intestinal epithelial cells. The status of gamma-glutamyl transpeptidase activity, uptake of amino acids and lipid peroxidation, Biochem Pharmacol, 43 (1992) 1401–1407. [DOI] [PubMed] [Google Scholar]
- [26].Gunasekaran V, Elangovan K, Niranjali Devaraj S, Targeting hepatocellular carcinoma with piperine by radical-mediated mitochondrial pathway of apoptosis: An in vitro and in vivo study, Food Chem Toxicol, 105 (2017) 106–118. [DOI] [PubMed] [Google Scholar]
- [27].Singh J, Reen RK, Wiebel FJ, Piperine, a major ingredient of black and long peppers, protects against AFB1-induced cytotoxicity and micronuclei formation in H4IIEC3 rat hepatoma cells, Cancer Lett, 86 (1994) 195–200. [DOI] [PubMed] [Google Scholar]
- [28].Gupta SC, Patchva S, Aggarwal BB, Therapeutic roles of curcumin: lessons learned from clinical trials, AAPS J, 15 (2013) 195–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Patial V, Sharma MS,S, Pratap K, Singh D, Padwad YS, Synergistic effect of curcumin and piperine in suppression of DENA-induced hepatocellular carcinoma in rats, Environ Toxicol Pharmacol, 40 (2015) 445–452. [DOI] [PubMed] [Google Scholar]
- [30].Pang KL, Chin KY, The Biological Activities of Oleocanthal from a Molecular Perspective, Nutrients, 10 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Polini B, Digiacomo M, Carpi S, Bertini S, Gado F, Saccomanni G, Macchia M, Nieri P, Manera C, Fogli S, Oleocanthal and oleacein contribute to the in vitro therapeutic potential of extra virgin oil-derived extracts in non-melanoma skin cancer, Toxicol In Vitro, 52 (2018) 243–250. [DOI] [PubMed] [Google Scholar]
- [32].Bishayee A, The role of inflammation and liver cancer, Adv Exp Med Biol, 816 (2014) 401–435. [DOI] [PubMed] [Google Scholar]
- [33].Koga H, Sakisaka S, Ohishi M, Kawaguchi T, Taniguchi E, Sasatomi K, Harada M, Kusaba T, Tanaka M, Kimura R, Nakashima Y, Nakashima O, Kojiro M, Kurohiji T, Sata M, Expression of cyclooxygenase-2 in human hepatocellular carcinoma: relevance to tumor dedifferentiation, Hepatology (Baltimore, Md.), 29 (1999) 688–696. [DOI] [PubMed] [Google Scholar]
- [34].Cusimano A, Balasus D, Azzolina A, Augello G, Emma MR, Di Sano C, Gramignoli R, Strom SC, McCubrey JA, Montalto G, Cervello M, Oleocanthal exerts antitumor effects on human liver and colon cancer cells through ROS generation, Int J Oncol, 51 (2017) 533–544. [DOI] [PubMed] [Google Scholar]
- [35].Pei T, Meng Q, Han J, Sun H, Li L, Song R, Sun B, Pan S, Liang D, Liu L, (−)-Oleocanthal inhibits growth and metastasis by blocking activation of STAT3 in human hepatocellular carcinoma, Oncotarget, 7 (2016) 43475–43491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Petrovic V, Nepal A, Olaisen C, Bachke S, Hira J, Sogaard CK, Rost LM, Misund K, Andreassen T, Melo TM, Bartsova Z, Bruheim P, Otterlei M, Anti-Cancer Potential of Homemade Fresh Garlic Extract Is Related to Increased Endoplasmic Reticulum Stress, Nutrients, 10 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Khazaei S, Esa NM, Ramachandran V, Hamid RA, Pandurangan AK, Etemad A, Ismail P, In vitro Antiproliferative and Apoptosis Inducing Effect of Allium atroviolaceum Bulb Extract on Breast, Cervical, and Liver Cancer Cells, Front Pharmacol, 8 (2017) 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Sengupta A, Ghosh S, Bhattacharjee S, Allium vegetables in cancer prevention: an overview, Asian Pac J Cancer Prev, 5 (2004) 237–245. [PubMed] [Google Scholar]
- [39].Ng KT, Guo DY, Cheng Q, Geng W, Ling CC, Li CX, Liu XB, Ma YY, Lo CM, Poon RT, Fan ST, Man K, A garlic derivative, S-allylcysteine (SAC), suppresses proliferation and metastasis of hepatocellular carcinoma, PLoS One, 7 (2012) e31655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Jeong JB, Park JH, Lee HK, Ju SY, Hong SC, Lee JR, Chung GY, Lim JH, Jeong HJ, Protective effect of the extracts from Cnidium officinale against oxidative damage induced by hydrogen peroxide via antioxidant effect, Food Chem Toxicol, 47 (2009) 525–529. [DOI] [PubMed] [Google Scholar]
- [41].Lee KE, Shin JA, Hong IS, Cho NP, Cho SD, Effect of methanol extracts of Cnidium officinale Makino and Capsella bursa-pastoris on the apoptosis of HSC-2 human oral cancer cells, Exp Ther Med, 5 (2013) 789–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].de la Cruz J, Kim DH, Hwang SG, Anti cancer effects of Cnidium officinale Makino extract mediated through apoptosis and cell cycle arrest in the HT-29 human colorectal cancer cell line, Asian Pac J Cancer Prev, 15 (2014) 5117–5121. [PubMed] [Google Scholar]
- [43].Hong H, An JC, de La Cruz JF, Hwang SG, Cnidium officinale Makino extract induces apoptosis through activation of caspase-3 and p53 in human liver cancer HepG2 cells, Exp Ther Med, 14 (2017) 3191–3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].dela Cruz JF, Kim YS, Lumbera WM, Hwang SG, Viscum Album Var Hot Water Extract Mediates Anti-cancer Effects through G1 Phase Cell Cycle Arrest in SK-Hep1 Human Hepatocarcinoma cells, Asian Pac J Cancer Prev, 16 (2015) 6417–6421. [DOI] [PubMed] [Google Scholar]
- [45].Cheng ZY, Yao GD, Guo R, Huang XX, Song SJ, Phenylpropanoids from Juglans mandshurica exhibit cytotoxicities on liver cancer cell lines through apoptosis induction, Bioorg Med Chem Lett, 27 (2017) 597–601. [DOI] [PubMed] [Google Scholar]
- [46].Lou LL, Cheng ZY, Guo R, Yao GD, Song SJ, Alkaloids from Juglans Mandshurica maxim induce distinctive cell death in hepatocellular carcinoma cells, Nat Prod Res, (2017) 1–4. [DOI] [PubMed] [Google Scholar]
- [47].Yao Y, Zhang YW, Sun LG, Liu B, Bao YL, Lin H, Zhang Y, Zheng LH, Sun Y, Yu CL, Wu Y, Wang GN, Li YX, Juglanthraquinone C, a novel natural compound derived from Juglans mandshurica Maxim, induces S phase arrest and apoptosis in HepG2 cells, Apoptosis, 17 (2012) 832–841. [DOI] [PubMed] [Google Scholar]
- [48].Jelic S, Sotiropoulos GC, Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up, Ann Oncol, 21 Suppl 5 (2010) v59–64. [DOI] [PubMed] [Google Scholar]
- [49].Kane RC, Farrell AT, Madabushi R, Booth B, Chattopadhyay S, Sridhara R, Justice R, Pazdur R, Sorafenib for the treatment of unresectable hepatocellular carcinoma, Oncologist, 14 (2009) 95–100. [DOI] [PubMed] [Google Scholar]
- [50].Song J, Zhao W, Lu C, Shao X, LATS2 overexpression attenuates the therapeutic resistance of liver cancer HepG2 cells to sorafenib-mediated death via inhibiting the AMPK-Mfn2 signaling pathway, Cancer Cell Int, 19 (2019) 60. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [51].Cainap C, Qin S, Huang WT, Chung IJ, Pan H, Cheng Y, Kudo M, Kang YK, Chen PJ, Toh HC, Gorbunova V, Eskens FA, Qian J, McKee MD, Ricker JL, Carlson DM, El-Nowiem S, Linifanib versus Sorafenib in patients with advanced hepatocellular carcinoma: results of a randomized phase III trial, J Clin Oncol, 33 (2015) 172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Bruix J, Raoul JL, Sherman M, Mazzaferro V, Bolondi L, Craxi A, Galle PR, Santoro A, Beaugrand M, Sangiovanni A, Porta C, Gerken G, Marrero JA, Nadel A, Shan M, Moscovici M, Voliotis D, Llovet JM, Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial, J Hepatol, 57 (2012) 821–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J, Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D, Guan Z, Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial, Lancet Oncol, 10 (2009) 25–34. [DOI] [PubMed] [Google Scholar]
- [54].Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Haussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J, Group SIS, Sorafenib in advanced hepatocellular carcinoma, N Engl J Med, 359 (2008) 378–390. [DOI] [PubMed] [Google Scholar]
- [55].National Center for Biotechnology Information, Sorafenib, in: Information N.C.f.B. (Ed.), 2018.
- [56].Zhu YJ, Zheng B, Wang HY, Chen L, New knowledge of the mechanisms of sorafenib resistance in liver cancer, Acta Pharmacol Sin, 38 (2017) 614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Choi GH, Shim JH, Kim MJ, Ryu MH, Ryoo BY, Kang YK, Shin YM, Kim KM, Lim YS, Lee HC, Sorafenib alone versus sorafenib combined with transarterial chemoembolization for advanced-stage hepatocellular carcinoma: results of propensity score analyses, Radiology, 269 (2013) 603–611. [DOI] [PubMed] [Google Scholar]
- [58].Zhang G, Park MA, Mitchell C, Hamed H, Rahmani M, Martin AP, Curiel DT, Yacoub A, Graf M, Lee R, Roberts JD, Fisher PB, Grant S, Dent P, Vorinostat and sorafenib synergistically kill tumor cells via FLIP suppression and CD95 activation, Clin Cancer Res, 14 (2008) 5385–5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Richly H, Schultheis B, Adamietz IA, Kupsch P, Grubert M, Hilger RA, Ludwig M, Brendel E, Christensen O, Strumberg D, Combination of sorafenib and doxorubicin in patients with advanced hepatocellular carcinoma: results from a phase I extension trial, Eur J Cancer, 45 (2009) 579–587. [DOI] [PubMed] [Google Scholar]
- [60].Jamil MO, Hathaway A, Mehta A, Tivozanib: status of development, Curr Oncol Rep, 17 (2015) 24. [DOI] [PubMed] [Google Scholar]
- [61].Samuel Farlow DP, Zi Tong Sun Xiaojian, Lin Jie, Chiu Maria Isabel, Robinson Murray O., Heyer Joerg and Zhou Yinghui, Variation in response to triple VEGFR inhibitor tivozanib in mouse models of hepatocellular carcinoma, Molecular Cancer Therapeutics 8(2009) A12. [Google Scholar]
- [62].Luo LJ, Zhang LP, Duan CY, Wang B, He NN, Abulimiti P, Lin Y, The inhibition role of miR-22 in hepatocellular carcinoma cell migration and invasion via targeting CD147, Cancer Cell Int, 17 (2017) 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Xiaoqi Huo SH, Wu Guang, Latchoumanin Olivier, Zhou Gang, Hebbard Lionel, George Jacob, and Qiao Liang, Dysregulated long noncoding RNAs (lncRNAs) in hepatocellular carcinoma: implications for tumorigenesis, disease progression, and liver cancer stem cells, Molecular Cancer, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Nouso K, Miyahara K, Uchida D, Kuwaki K, Izumi N, Omata M, Ichida T, Kudo M, Ku Y, Kokudo N, Sakamoto M, Nakashima O, Takayama T, Matsui O, Matsuyama Y, Yamamoto K, Liver J Cancer Study Group of, Effect of hepatic arterial infusion chemotherapy of 5-fluorouracil and cisplatin for advanced hepatocellular carcinoma in the Nationwide Survey of Primary Liver Cancer in Japan, Br J Cancer, 109 (2013) 1904–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Couzin-Frankel J, Breakthrough of the year 2013. Cancer immunotherapy, Science, 342 (2013) 1432–1433. [DOI] [PubMed] [Google Scholar]
- [66].Morse MA, Sun W, Kim R, He AR, Abada PB, Mynderse M, Finn RS, The Role of Angiogenesis in Hepatocellular Carcinoma, Clin Cancer Res, 25 (2019) 912–920. [DOI] [PubMed] [Google Scholar]
- [67].Ho CM, Chen HL, Hu RH, Lee PH, Harnessing immunotherapy for liver recipients with hepatocellular carcinoma: a review from a transplant oncology perspective, Ther Adv Med Oncol, 11 (2019) 1758835919843463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Greten TF, Sangro B, Targets for immunotherapy of liver cancer, J Hepatol, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Gao Q, Wang XY, Qiu SJ, Yamato I, Sho M, Nakajima Y, Zhou J, Li BZ, Shi YH, Xiao YS, Xu Y, Fan J, Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma, Clin Cancer Res, 15 (2009) 971–979. [DOI] [PubMed] [Google Scholar]
- [70].Mataki N, Kikuchi K, Kawai T, Higashiyama M, Okada Y, Kurihara C, Hokari R, Kawaguchi A, Nagao S, Kondo T, Itoh K, Miyakawa H, Miura S, Expression of PD-1, PD-L1, and PD-L2 in the liver in autoimmune liver diseases, Am J Gastroenterol, 102 (2007) 302–312. [DOI] [PubMed] [Google Scholar]
- [71].Inarrairaegui M, Melero I, Sangro B, Immunotherapy of Hepatocellular Carcinoma: Facts and Hopes, Clin Cancer Res, 24 (2018) 1518–1524. [DOI] [PubMed] [Google Scholar]
- [72].Hodi FS, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Cowey CL, Lao CD, Schadendorf D, Wagstaff J, Dummer R, Ferrucci PF, Smylie M, Hill A, Hogg D, Marquez-Rodas I, Jiang J, Rizzo J, Larkin J, Wolchok JD, Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial, Lancet Oncol, 19 (2018) 1480–1492. [DOI] [PubMed] [Google Scholar]
- [73].Overman MJ, Lonardi S, Wong KYM, Lenz HJ, Gelsomino F, Aglietta M, Morse MA, Van Cutsem E, McDermott R, Hill A, Sawyer MB, Hendlisz A, Neyns B, Svrcek M, Moss RA, Ledeine JM, Cao ZA, Kamble S, Kopetz S, Andre T, Durable Clinical Benefit With Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer, J Clin Oncol, 36 (2018) 773–779. [DOI] [PubMed] [Google Scholar]
- [74].Keir ME, Butte MJ, Freeman GJ, Sharpe AH, PD-1 and its ligands in tolerance and immunity, Annu Rev Immunol, 26 (2008) 677–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Hirano F, Kaneko K, Tamura H, Dong H, Wang S, Ichikawa M, Rietz C, Flies DB, Lau JS, Zhu G, Tamada K, Chen L, Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity, Cancer Res, 65 (2005) 1089–1096. [PubMed] [Google Scholar]
- [76].Breitbach CJ, Bell JC, Hwang TH, Kirn DH, Burke J, The emerging therapeutic potential of the oncolytic immunotherapeutic Pexa-Vec (JX-594), Oncolytic Virother, 4 (2015) 25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Heo J, Reid T, Ruo L, Breitbach CJ, Rose S, Bloomston M, Cho M, Lim HY, Chung HC, Kim CW, Burke J, Lencioni R, Hickman T, Moon A, Lee YS, Kim MK, Daneshmand M, Dubois K, Longpre L, Ngo M, Rooney C, Bell JC, Rhee BG, Patt R, Hwang TH, Kirn DH, Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer, Nat Med, 19 (2013) 329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].He X, Duan J, Wang K, Tan W, Lin X, He C, A novel fluorescent label based on organic dye-doped silica nanoparticles for HepG liver cancer cell recognition, J Nanosci Nanotechnol, 4 (2004) 585–589. [DOI] [PubMed] [Google Scholar]
- [79].Ballout F, Habli Z, Rahal ON, Fatfat M, Gali-Muhtasib H, Thymoquinone-based nanotechnology for cancer therapy: promises and challenges, Drug Discov Today, 23 (2018) 1089–1098. [DOI] [PubMed] [Google Scholar]
- [80].Xia Q, Li L, Zhao L, Silica nanoparticle-based dual-responsive nanoprodrug system for liver cancer therapy, Exp Ther Med, 14 (2017) 2071–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Li L, Tang F, Liu H, Liu T, Hao N, Chen D, Teng X, He J, In vivo delivery of silica nanorattle encapsulated docetaxel for liver cancer therapy with low toxicity and high efficacy, ACS Nano, 4 (2010) 6874–6882. [DOI] [PubMed] [Google Scholar]
- [82].Hong K, Khwaja A, Liapi E, Torbenson MS, Georgiades CS, Geschwind JF, New intra-arterial drug delivery system for the treatment of liver cancer: preclinical assessment in a rabbit model of liver cancer, Clin Cancer Res, 12 (2006) 2563–2567. [DOI] [PubMed] [Google Scholar]
- [83].Saraswathy M, Gong S, Different strategies to overcome multidrug resistance in cancer, Biotechnol Adv, 31 (2013) 1397–1407. [DOI] [PubMed] [Google Scholar]
- [84].Enrique Chajon MP, De Baere Thierry, Nguyen France, Bronowicki Jean-Pierre, Vendrely Veronique, A phase I/II trial of NBTXR3 nanoparticles activated by SBRT in the treatment of liver cancers., (2018).
- [85].Reebye V, Saetrom P, Mintz PJ, Huang KW, Swiderski P, Peng L, Liu C, Liu X, Lindkaer-Jensen S, Zacharoulis D, Kostomitsopoulos N, Kasahara N, Nicholls JP, Jiao LR, Pai M, Spalding DR, Mizandari M, Chikovani T, Emara MM, Haoudi A, Tomalia DA, Rossi JJ, Habib NA, Novel RNA oligonucleotide improves liver function and inhibits liver carcinogenesis in vivo, Hepatology (Baltimore, Md.), 59 (2014) 216–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Kudo M, Surveillance, diagnosis, treatment, and outcome of liver cancer in Japan, Liver Cancer, 4 (2015) 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]