Abstract
Chronic demyelination and the concomitant loss of trophic support and increased energy demands in axons are thought to contribute to neurodegeneration in a number of neurological diseases such as multiple sclerosis (MS). Adult oligodendrocyte precursor cells (OPCs) play an important role in these demyelinating diseases by generating new myelinating oligodendrocytes that may help limit axonal degeneration. Thus, promoting the differentiation of OPCs and functional integration of newly generated oligodendrocytes is a crucial avenue for the next generation of therapies. Evidence to date suggests that the immune system may both positively and negatively impact OPC differentiation and endogenous remyelination in disease. Inflammatory cytokines not only suppress OPC differentiation but may also directly affect other functions of OPCs. Recent studies have demonstrated that OPCs and oligodendrocytes in both human multiple sclerosis lesions and mouse models of demyelination can express an immunogenic transcriptional signature and upregulate antigen presenting genes. In inflammatory demyelinating mouse models OPCs are capable of presenting antigen and activating CD8+ T cells. Here we review the evidence for this new role of oligodendroglia as antigen presenting cells and how these inflammatory OPCs (iOPCs) and inflammatory oligodendrocytes (iOLs) may influence myelin repair and other disease processes.
Keywords: Oligodendrocytes, multiple sclerosis, remyelination, myelin repair, antigen presentation, CD8 T cells
1. Adult oligodendrocyte precursor cell dynamics and plasticity
Oligodendrocytes serve an important role in the central nervous system (CNS), wrapping and insulating the axons of neurons with myelin which allows for fast saltatory conduction of action potentials and protects axons from inflammatory insults in a demyelinating environment. Mature myelin-forming oligodendrocytes arise from oligodendrocyte precursor cells (OPCs). During development OPCs proliferate, migrate and differentiate into mature oligodendrocytes that myelinate axons in the brain and spinal cord. OPCs remain widely distributed in the adult CNS and their density is maintained through constant homeostatic replacement, ensuring that there are progenitors capable of responding to oligodendrocyte loss and demyelination.
Recent application of advanced experimental techniques in mouse models has resulted in new insights into adult OPC plasticity and myelinating oligodendrocyte dynamics. In particular, in vivo two-photon fluorescence imaging of transgenic mice in which OPCs and oligodendrocytes can be visualized, revealed that in the mouse cerebral cortex oligodendrocytes continue to be generated throughout adulthood, with over half of mature oligodendrocytes generated after four months of age [1]. However, even in the absence of inflammation, the integration of mature oligodendrocytes in adult circuits is highly inefficient, with the majority of newly formed oligodendrocytes dying before they extend and compact myelin sheaths [1]. Two-photon imaging in the adult somatosensory cortex has also demonstrated that new myelin internodes are formed exclusively by newly generated oligodendrocytes, rather than through the extension of new processes from existing oligodendrocytes, and that once myelin sheaths are formed they are extremely stable [1]. In vivo genetic fate mapping using inducible expression of membrane-bound form of green fluorescent protein (GFP) to label adult generated myelinating oligodendrocytes also revealed that adult formed mature oligodendrocytes are remarkably stable in the adult CNS [2]. 14C (carbon) dating of oligodendrocyte lineage cells from post-mortem human tissue also demonstrated low turnover rates of oligodendrocytes in healthy control white matter [3]. In contrast, the rate of oligodendrocyte generation was much more heterogeneous in MS patients. Although there was no significant difference in oligodendrocyte turnover between normal appearing white matter (NAWM) of MS patients and healthy controls [3], MS patients with more aggressive MS (shorter disease course to death) had higher rates of oligodendrocyte turnover in NAWM compared to MS patients that experienced a longer disease course. However, this behavior was not universally seen, as some MS patients with rapid disease progression did not exhibit high rates of oligodendrogenesis in NAWM.
14C birth dating has also been used in MS patients to assess the age of oligodendrocytes within so-called “shadow plaques”, lesions with reduced myelin density that are thought to represent areas where remyelination is at an early stage, although such regions could also reflect myelin thinning by damaged oligodendrocytes. Unexpectedly, oligodendrocytes within these lesions had incorporated as much 14C as nearby NAWM, and overall less 14C than in healthy patients born during the same period, suggesting that there was limited production of new oligodendrocytes in these areas. These findings raise the further possibility that remyelination may occur through regeneration of myelin sheaths by surviving oligodendrocytes. Reduced oligodendrocyte turnover in shadow plaques may reflect influences of the inflammatory environment in preventing OPC proliferation and survival. However, interpretation based on post-mortem analysis of MS lesions is very challenging, due to uncertainties about the classification of lesions and NAWM, the timing of demyelination, the extent of oligodendrocyte death within lesions and assumptions about the behavior of OPCs, which in rodents, are able to directly differentiate into oligodendrocytes without cell division [4]. Nevertheless, the overall increase in oligodendrocyte generation in patients with more aggressive MS suggests an intrinsic capacity to increase oligodendrocyte generation in the human brain.
OPC density also appears to be under strong homeostatic control. Two-photon imaging of OPCs in the adult cortex of transgenic mice in which membrane anchored enhanced GFP is expressed under the control of the neuron-glial antigen 2 (NG2) promoter/enhancer revealed that loss of these cells through differentiation, transformation or death, resulted in rapid migration and proliferation of neighboring OPCs to restore their density [4]. This plasticity to maintain OPC density and tiling may be an adaptive mechanism that allows for an efficient oligodendrocyte response in the setting of injury and demyelination. In MS lesions, OPC density can be reduced [5-9] which may be a result of a variety of factors related to the inflammatory microenvironment including the presence of immune cells that could negatively impact OPC survival, differentiation and remyelination. Evidence suggesting a role for immune cells in influencing oligodendrocytes is further detailed below.
2. Adult oligodendrocyte heterogeneity
Transcriptional profiling with single-cell RNA sequencing (scRNA-seq) of oligodendrocyte lineage cells across developmental ages and different CNS regions indicates that regardless of embryonic origin and brain region, adult oligodendrocyte lineage cells exist on a common spectrum of distinct transcriptional sub-populations from progenitor to mature oligodendrocyte [10,11]. The transcriptional sub-population that an OPC or intermediate oligodendrocyte lineage cell belongs to may influence different functional capacities and their ability for integration and remyelination. One example of functional heterogeneity is the differential ion-channel expression and electrophysiological properties of adult OPCs across brain regions and with aging [12]. OPCs express voltage-gated ion channels and receive direct synaptic input from neurons [reviewed in [13]]. Neuronal signaling to OPCs has been shown to influence OPC properties, such as proliferation and differentiation [14-17] and mature oligodendrocyte myelin sheath dynamics [16-20]. Whether these differences in OPC ion-channel expression and electrophysiological properties correlate with distinct transcriptional sub-populations remains to be determined.
Changes in OPC ion-channel expression and electrophysiological properties across brain regions and with aging may influence an individual OPC’s capacity for survival, proliferation, differentiation and remyelination in a demyelinated inflammatory environment. Indeed, aged animals exhibit reduced remyelination capacity similar to human MS patients, and studies in mouse models have demonstrated a variety of factors influencing reduced remyelination capacity with aging, such as epigenetic changes in OPCs influencing their differentiation [21,22], changes in growth factor expression in lesion environment with age [23], reduced ability of aged infiltrating monocyte-derived macrophages to clear myelin debris [24,25], and stiffening of the extracellular matrix influencing OPC proliferation, differentiation and transcriptional signature [26]. Further characterizing the ability of individual OPCs to differentiate and remyelinate based on single cell molecular signature and electrophysiological properties in the setting of a demyelination inflammatory environment is a critically important area of further investigation.
3. Oligodendrocytes express antigen presenting molecules in inflammatory states in mouse and human
Interferon gamma (IFN-γ) is secreted by activated T cells and thought to play a deleterious role in immune-mediated demyelinating disorders including MS [27,28]. IFN-γ in vitro inhibits cell cycle exit and differentiation of OPCs [29]. Transgenic mice with IFN-γ expression driven by the myelin basic protein (MBP) promoter display developmental hypomyelination and reactive gliosis with macrophage and microglial infiltrates [30,31] and elevated major histocompatibility molecules (MHC) class I and class II mRNA transcript levels in white and gray matter [30]. GFAP promoter driven IFN-γ also results in developmental hypomyelination [32]. In an adult remyelinating setting, after cuprizone-mediated CNS demyelination, induction of IFN-γ expression (using doxycycline withdrawal in double transgenic mice GFAP/tTA;TRE/IFN-γ) during remyelination resulted in decreased mature oligodendrocyte numbers and impaired remyelination [33]. Together, these studies in transgenic mouse models of CNS IFN-γ expression suggest that OPCs can respond to IFN-γ and that it may impair OPC differentiation and survival.
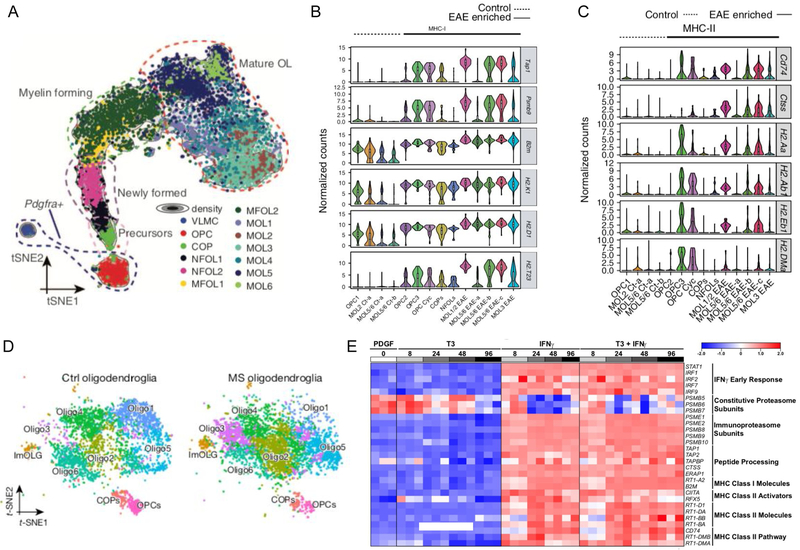
Several lines of experimental evidence indicate that a subset of oligodendroglial cells express genes involved in antigen processing and presentation in mouse [34,35] and human MS tissue [9]. This subset of oligodendroglia that have an inflammatory signature we will refer to as inflammatory OPCs (iOPCs) or inflammatory oligodendrocytes (iOLs). scRNA-seq is performed by isolating single cells and encapsulating each individual cell in a droplet containing primers with a unique identifying barcode. Reverse transcription occurs inside each droplet generating a barcoded cDNA library for each cell. Sequencing of cDNA libraries and mapping of data to a reference genome allows for establishing defined clusters of cells that express similar mRNA transcripts and distinguishing different cell types and maturation of a committed cell lineage, such as an oligodendrocyte precursor cell to mature oligodendrocyte ([10], Fig. 1a). scRNA-seq of oligodendrocyte lineage cells from the spinal cord of an experimental autoimmune encephalomyelitis (EAE) mouse model compared to complete Freund’s adjuvant (CFA) control revealed that some OPCs and mature oligodendrocytes upregulate genes involved in antigen processing and antigen presentation ([34], Fig. 1b,c). Moreover, scRNA-seq of white matter from human progressive MS patients also revealed enrichment of a cluster of oligodendrocytes that upregulate antigen presentation genes ([9], Fig. 1d). Single-nucleus RNA sequencing (snRNA-seq) from frozen post-mortem tissues of MS cortical, subcortical lesions and non-lesioned areas also revealed upregulation of MHC class I molecules, as well as cellular stress and iron overload transcripts in oligodendrocytes in periplaque white matter around subcortical lesion rims [36].
Fig. 1.
Oligodendrocytes upregulate antigen presentation transcripts in mouse EAE, human MS lesions, and after in vitro exposure to IFN-γ. (A) t-Distributed stochastic neighbor embedding projection showing the trajectory from OPCs to mature oligodendrocytes. (B) Violin plots depicting the expression of MHC class I and antigen processing genes in EAE and CFA control populations. (C) Violin plots depicting the expression of MHC class II genes in EAE and CFA control populations. (D) Oligodendrocyte clusters in control and MS tissue with enrichment of the immune oligodendroglial cluster “ImOLG” in MS tissue. (E) Oligodendrocytes exposed to IFN-γ and IFN-γ in the presence of differentiation conditions with T3 (triiodothyronine) in vitro upregulate genes involved in antigen presentation and antigen processing. (Panel A reprinted by permission from AAAS: Science, Oligodendrocyte heterogeneity in the mouse juvenile and adult central nervous system, Marques et al. 2016. Panels B and C reprinted by permission from Springer Nature: Nature Medicine, Disease specific oligodendrocyte lineage cells arise in multiple sclerosis, Falcao et al, 2018. Panel D reprinted by permission from Springer Nature: Nature, Altered human oligodendrocyte heterogeneity in multiple sclerosis, Jäkel et al. 2019).
Oligodendroglia have been shown to express a wide variety of immunomodulatory molecules such as cytokines (interleukin(IL)-1β, IL-17A), chemokines, MHC class I and class II, co-stimulatory molecules, complement and complement regulatory molecules [reviewed in [37]]. In human tissue, oligodendrocytes in MS lesions express beta-2 microglobulin (B2m), a component of MHC class I receptor complex [38]. B2m, proteasome subunit beta 9 (PSMB9) an immunoproteasome specific subunit, and MHC class II invariant chain molecule CD74 are also upregulated in oligodendrocytes in a mouse EAE model ([34], Fig. 1b,c). Moreover, oligodendrocytes exposed to IFN-γ in vitro upregulate genes involved in antigen presentation and peptide processing ([35], Fig. 1e).
These recent findings in inflammatory mouse models and human MS lesions suggest a role for oligodendrocyte lineage cells in antigen presentation and direct communication with T cells. Human MS scRNA-seq data suggest that the subset of oligodendroglia expressing antigen presentation and processing molecules most resemble intermediate oligodendrocytes [9], while mouse EAE models suggest that both OPCs [34,35] and mature oligodendrocytes upregulate antigen presentation molecules [34]. The expression dynamics of antigen presentation and processing molecules in the oligodendrocyte lineage in the setting of demyelination and remyelination warrants further investigation. The skewing of oligodendrocyte transcriptional profiles and increase in iOPCs/iOLs may contribute to functional differences in oligodendrocyte differentiation and their capacity for remyelination, and may increase susceptibility to damage by cytotoxic CD8+ T cells. The role of antigen presentation by oligodendrocytes and potential for direct communication with CD4+ and CD8+ T cells is an important avenue for further investigation.
4. Antigen presentation pathways
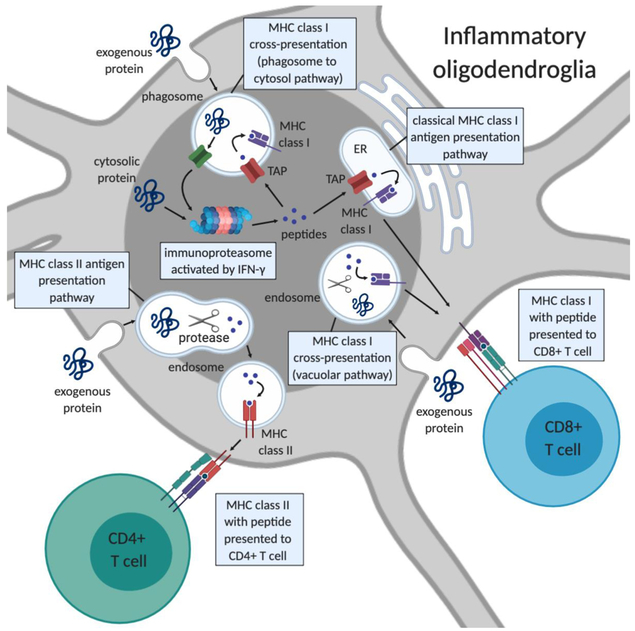
Recent research has revealed new complexities in MHC class I and class II antigen processing and presentation pathways [reviewed in [39,40]]. MHC class I and II molecules present peptides to CD8+ T cells and CD4+ T cells, respectively. Peptides presented by MHC class II molecules are exogenous to the antigen presenting cell, whereas antigens presented by MHC class I molecules are classically endogenous, which functions to identify and kill infected cells. Exogenous proteins however can be processed and presented on MHC class I molecules to CD8+ T cells in specialized antigen presenting cells (e.g. dendritic cells), a process called cross-presentation [reviewed in [41], Fig. 2].
Fig. 2.
Pathways of antigen presentation by inflammatory oligodendroglia to T cells. In MHC class II antigen presentation, exogenous proteins are processed by proteases in the endosome and assembled onto MHC class II molecules. MHC class II-peptide complexes are transported from the late endosome to the cell surface where they can engage T cell receptors on CD4+ T cells. In classical MHC class I antigen presentation, intracellular proteins are processed by the immunoproteasome and peptides are transported into the endoplasmic reticulum (ER) by TAP. Peptides are then loaded onto MHC class I molecules and the MHC class I-peptide complexes are transported to the plasma membrane where it can engage T cell receptors on CD8+ T cells. Cross-presentation pathways involve processing of exogenous proteins for presentation on MHC class I molecules to CD8+ T cells. In the phagosome-to-cytosol cross-presentation pathway exogenous proteins are taken up by phagosomes and transported to the cytosol where they undergo processing by the immunoproteasome and resulting peptides are transported back into the phagosome by TAP and loaded onto MHC class I molecules. In the vacuolar cross-presentation pathway exogenous proteins are taken up in vacuoles and transported to endosomes where they are processed by proteases and loaded onto MHC class I molecules. Not depicted in this figure are two other mechanisms for acquiring exogenous peptides for presentation on class I- transport of peptides through gap junctions from neighboring cells and transfer of plasma membrane MHC class I peptide loaded complexes by direct cell contact with a donor cell a mechanism termed cross-dressing that has been shown to occur in dendritic cells. Figure created with BioRender.com.
In the classic MHC class I antigen presentation pathway, intracellular antigens are degraded by the cytosolic immunoproteasome, which differs from standard proteasomes with three inducible beta subunits (PSMB8, 9, and 10) replacing the constitutively active beta subunits. In the setting of inflammatory conditions, the immunoproteasome is induced which has a higher catalytic capacity to generate a larger antigen pool [reviewed [42]]. Studies in autoimmune diseases suggest a role for inhibiting the immunoproteasome in treatment of various autoimmune diseases [43], including in mouse models of EAE [44]. Once peptides are processed by the immunoproteasome they are transported into the endoplasmic reticulum by the transporter associated with antigen presentation (TAP) where they can bind in the peptide binding groove of assembled MHC class I molecules. If peptide binding reaches a threshold for stable binding, the MHC class I peptide complex is transported to the plasma membrane where it can engage T cell receptors on CD8+ T cells. In MHC class I cross-presentation, exogenous internalized peptides are loaded onto MHC class I molecules via either the vacuolar pathway or phagosome-to-cytosol pathway [41].
In MHC class II antigen presentation, exogenous proteins are processed by proteases in the endosome and binding of peptides to MHC class II molecules occurs in the late endosome. High affinity stable MHC class II peptide complexes are transported from the late endosome to the plasma membrane where they can engage receptors on CD4+ T cells. Both MHC class II and MHC class I antigen presentation can occur through the acquisition and processing of exogenous proteins while intracellular antigen presentation occurs only through MHC class I. The ability of oligodendrocytes in vivo to process and present peptides by MHC class I and class II molecules and the mechanism by which they may present antigen to T cells remain to be fully determined.
5. Role of CD8+ T cells in inflammatory demyelinating diseases
MS is thought to occur in genetically predisposed hosts exposed to unknown environmental factors and triggers that result in activation of myelin-specific T cells in the periphery that migrate to the CNS. These myelin-specific T cells encounter CNS antigen presenting cells that further activate CD4+ T cells. This inflammatory cascade results in recruitment of naïve T cells from the periphery and monocyte derived macrophages that differentiate into antigen presenting cells. Despite the focus on CD4+ T cells in MS pathogenesis, cytotoxic CD8+ T cells are the predominant T-lymphocyte infiltrate in acute and chronic MS lesions [45-48]. CD8+ T cells with cytotoxic granules are often found in close proximity to oligodendrocytes and demyelinated axons, and axonal injury within lesions correlates with the density of CD8+ T cells and macrophages [49].
CD8+ T cell receptor recognition of MHC class I-peptide complexes and co-stimulatory molecules on antigen presenting cells results in maturation and activation of cytotoxic CD8+ T cells. Interaction of an antigen presenting cell and CD8+ T cell alone in some contexts, such as an inflammatory and infectious environment, may be sufficient to generate a CD8+ T cell response. However when the presence of inflammatory signals fail to generate an effective CD8+ T cell response, helper CD4+ T cells are required to prime antigen presenting cells to further activate CD8+ T cells [reviewed in [50]]. CD8+ T cells when bound to antigen presenting cells if sufficiently activated, secrete granzymes and perforin resulting in pore formation in the neighboring cell and entry of granzymes which stimulate programmed cell death. Cytotoxic mechanisms mediated by CD8+ T cells thus play an important role in the pathogenesis of CNS inflammatory diseases [reviewed in [51]].
The contribution of CD8+ T cells to MS pathogenesis is not well understood. Myelin-specific CD8+ T cells have been shown to worsen CD4+ T cell-mediated EAE [52] and induce EAE [53-55]. Transgenic mice with MBP-specific CD8+ T cells have been used to investigate which cell types present myelin antigens to CD8+ T cells in EAE [56]. At the peak of EAE, CNS cells were sorted and cells expressing MHC class I loaded with MBP peptide were isolated using an antibody that recognizes MBP peptide bound to MHC class I. The ability of different cells types to activate CD8+ T cells was determined ex vivo by co-culture with transgenic myelin-reactive CD8+ T cells followed by assessment of T cell activation [56].
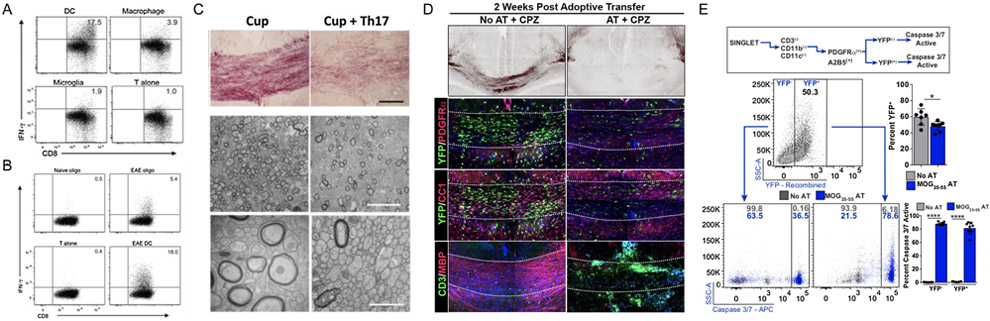
Of classical antigen presenting cells, both dendritic cells and macrophages expressed MBP peptide bound to MHC class I but only dendritic cells had the ability to activate CD8+ T cells ([56], Fig. 3a). Microglia did not express MBP peptide bound to MHC class I or activate CD8+ T cells. Dendritic cells presenting myelin peptide on MHC class I had the phenotype of peripherally derived inflammatory monocytes that infiltrate the CNS. The mechanism by which dendritic cells acquire myelin antigens is unclear. Analysis of MBP transcripts present in dendritic cells expressing myelin peptide on MHC class I indicated that they acquire MBP from the extracellular environment and present myelin antigen via cross-presentation. Dendritic cells could acquire endogenous myelin through phagocytosis, exosomes produced by oligodendrocytes [57], transport through neighboring cells via gap junctions, or transfer of loaded MHC class I molecules via direct cell contact, a process termed cross-dressing.
Fig. 3.
Oligodendrocytes present antigen and activate CD8+ T cells in an EAE model and adoptive transfer of myelin-reactive T cells results in reduced numbers of oligodendrocytes, reduced remyelination and increased numbers of caspase 3/7 positive oligodendrocytes. (A) Dendritic cells, not macrophages or microglia, present myelin peptide on MHC class I and activate CD8+ T cells in an EAE model. (B) Oligodendrocytes present myelin peptide on MHC class I and activate CD8+ T cells in an EAE model. (C) Adoptive transfer of Th17 CD4+ T cells after cuprizone-mediated demyelination impairs remyelination. (D) Reduced numbers of newly born yellow fluorescent protein (YFP)-positive OPCs and mature oligodendrocytes in an adoptive transfer cuprizone model. (E) Higher percentage of caspase 3/7-positive OPCs in an adoptive transfer cuprizone model compared to cuprizone alone. (Panel A and B reprinted by permission from Springer Nature: Nature Immunology, MHC class I-restricted myelin epitopes are cross-presented by Tip-DCs that promote determinant spreading to CD8 T cells, Ji et al. 2013).
6. Evidence that oligodendrocytes function as antigen presenting cells and activate T cells
Non-classical antigen presenting cells such as oligodendrocytes, astrocytes and endothelial cells, have been assessed for their ability to present myelin antigens and activate CD8+ T cells [56]. Of these CNS cell populations only oligodendrocytes expressed myelin peptide on MHC class I and activate CD8+ T cells ([56], Fig. 3b). It is also unclear if oligodendrocytes present endogenous or exogenous myelin peptides.
A combined mouse model of demyelination and T cell-mediated EAE has been used to further investigate the role of T cells in the context of oligodendrocyte remyelination [58]. After inducing CNS demyelination with the copper chelator cuprizone, transgenic myelin oligodendrocyte protein (MOG)-specific CD4+ T cells were polarized to Th17 phenotype and adoptively transferred into cuprizone-fed mice. Th17 polarized CD4+ T cells migrated efficiently to the corpus callosum and impaired remyelination after withdrawal of cuprizone diet ([58], Fig. 3c). Fate mapping experiments of newly born OPCs post-demyelination indicate that with T cell adoptive transfer there are reduced numbers of newly born surviving OPCs and mature oligodendrocytes, and higher number of OPCs express caspase 3/7 ([35], Fig. 3d,e), suggesting that OPC survival is impaired in the presence of T cell infiltrates. Inducing CNS IFN-γ expression in transgenic mice after cuprizone-mediated demyelination resulted in a reduced number of OPCs and mature oligodendrocytes, suggesting that exposure to IFN-γ is sufficient to reduce OPC survival [35].
The strategy of using T cells that recognize foreign peptides not normally expressed in the organism in combination with transgenic lines with cell-type specific expression of the foreign peptide has been used to investigate the ability of a specific cell type to present antigen and mediate autoimmunity. Several experiments using this approach have investigated the role of oligodendrocytes as antigen presenting cells. Transgenic mice with MBP promoter driven expression of ovalbumin protein (MBP-OVA) develop EAE and inflammatory infiltrates in the brain after immunization with ovalbumin, suggesting that oligodendrocytes are able to present antigen and activate T cells [59]. Crossing MBP-OVA transgenic mice with a T cell transgenic line with OVA-specific CD8+ T cells results in spontaneous EAE, CNS infiltrates and wide-spread demyelination [60]. In contrast, crossing MBP-OVA transgenic mice with a CD4+ T cell OVA-specific mice did not result in disease and CD4+ T cells remained naïve to CNS ovalbumin. Using adoptive transfer into MBP-OVA recipients, the authors found that CD4+ T cells were only activated with co-transfer of CD8+ T cells suggesting that CD8+ T cell engagement with antigen presenting oligodendrocytes and release sequestered ovalbumin from oligodendrocytes is required to activate CD4+ T cells. Experiments investigating different CNS cell types that express myelin peptide bound to MHC class I and activate CD8+ T cells as described in the previous section [56], revealed that in preclinical EAE CD45+ dendritic cells expressed myelin peptide bound to MHC class I prior to oligodendrocytes. These findings suggest that oligodendrocyte antigen presentation and subsequent lysis and release of myelin proteins was not necessary for presentation of myelin proteins by dendritic cells. One explanation for this unusual sequence of events may be that endogenous oligodendrocyte peptides are shed by oligodendrocytes by exosomes or breakdown of myelin and surface proteins independent of MHC class I CD8+ T-cell mediated cell lysis.
Similar experiments investigating the ability of oligodendrocytes to present antigen to CD8+ T cells have been performed with oligodendrocyte expression of influenza hemagglutinin (HA). Crossing a MOG-Cre transgenic to a Rosa26 line with floxed stop cassette flanked by HA (MOG-HA) results in HA expression in oligodendrocytes. MOG-HA mice crossed to HA-specific CD8+ T cell transgenic mice did not result in spontaneous EAE and CD8+ T cells remained naïve and did not experience T cell receptor down-regulation or deletion [61]. This result is in contrast to MBP-OVA mice crossed to OVA-specific CD8+ T cell mice that developed spontaneous EAE [60]. One potential explanation for this difference is that in MBP-OVA mice ovalbumin is released into the periphery allowing priming and activation of CD8+ T cells. In support of this conclusion, adoptive transfer of OVA-specific CD8+ T cells was only able to generate disease in pups 7-10 days-old and not greater than 12 days-old suggesting blood brain permeability and leakage of ovalbumin into the periphery was required for initiation of disease [60]. While crossing of MOG-HA mice to HA-specific T cell transgenic mice did not result in EAE, adoptive transfer of HA-specific CD8+ effector T cells into MOG-HA mice resulted in EAE with CNS T cell infiltrates and demyelination [61]. Adoptive transfer of naïve HA-specific CD8+ T cells did not result in disease. Moreover, adoptive transfer of GFP labeled HA-specific CD8+ effector T cells revealed GFP CD8+ T cells in close proximity to oligodendrocytes and granzyme B granules localized to the cell membrane in close opposition to oligodendrocytes.
Together, these in vivo genetic manipulations suggest that antigen presentation by oligodendrocytes is not sufficient in itself to activate and prime adoptively transferred CD4+ and CD8+ T cells, and that professional antigen presenting cells such as dendritic cells are required to license and prime T cells; however, once T cells are sufficiently activated, they are able to engage antigen presenting oligodendrocytes, which can further activate CD8+ T cells and potentially contribute to CD8+ T cell-mediated killing of oligodendrocytes. CD8+ T cell-mediated killing of oligodendrocytes could explain the reduction of OPCs in MS lesions. There may also be a role for cytotoxic CD4+ T cells in direct killing of oligodendrocytes in MS. Activated human myelin-reactive CD4+ T cells can upregulate natural killer (NK) receptors and kill oligodendrocytes in vitro [62]. Peripheral CD4+ T cells with NK receptors are found in greater proportions in MS patients compared to controls and display a cytotoxic profile.
The skewing of the oligodendroglia iOPC/iOL immune phenotype capable of presenting antigen may have an important role in oligodendrocyte survival and remyelination in CNS autoinflammatory disease. Further in vivo experiments using oligodendrocyte conditional lines with components of antigen presentation pathway will help to further elucidate the details of oligodendrocyte antigen presentation and their direct interaction with T cells to define how this communication influences OPC survival, differentiation and remyelination.
7. The role of microglia and monocyte-derived macrophages in remyelination
Microglia are resident macrophages in the brain that are derived from yolk sac myeloid precursors. These cells migrate into the brain during embryonic development and have diverse functions during development and in the adult CNS [reviewed in [63]]. Adult resting microglia express common surface markers such as CD11b, Iba1, Fc-gamma receptor 1 (CD64) and CD115 (Csf-1) that are also present on CNS infiltrating myeloid populations of dendritic cells and macrophages; however, resident microglia are distinguished by low expression of CD45 and high expression of Cx3cr1 [reviewed in [64]]. In the setting of inflammation, microglia become activated and change their morphology with shorter and thicker processes, proliferate and migrate to areas of injury and upregulate CD45, Iba1 and MHC class II presentation molecules and co-stimulatory molecules. In the setting of inflammation and blood brain barrier disruption [65,66], peripheral bone-marrow derived myeloid cells can infiltrate the CNS and differentiate into microglia-like cells. In the absence of inflammation, CNS nestin-positive precursors and not peripheral myeloid cells may be responsible for generating new microglia [67,68].
In the setting of CNS inflammation, peripheral bone-marrow derived monocytes cross the blood brain barrier and differentiate into macrophages and dendritic cells that express MHC class II, CD11c, and Ly6C. Activated macrophages in the presence of different cytokine environments can assume phenotypes ranging from a more reactive “M1” macrophage or suppressive “M2” macrophage, and this phenotype is likely a dynamic continuum rather than a static transformation. In the presence of lipopolysaccharide (LPS) or IFN-γ, macrophages develop a M1 phenotype and secrete tumor necrosis factor alpha (TNF-α), inducible nitric oxide synthase (iNOS), IL-1, IL-6 and IL-12. The presence of IL-4 and IL-10 can skew the macrophage phenotype to a more protective M2 phenotype characterized by production of Arginase-1 and suppression of T cell activity [reviewed in [69]].
Macrophages and microglia may have both detrimental and beneficial roles in demyelination and remyelination. Using a dual-reporter system consisting of Cx3cr1gfp/+ labeled microglia and Ccr2rfp/+ labeled monocyte derived macrophages in an EAE model, studies have demonstrated that microglia and macrophages have distinct roles and profiles in EAE [70]. Macrophages initiate demyelination and were found surrounding and invading internodes, while in the absence of macrophages (in Cx3cr1gfp/+; Ccr2rfp/rfp mice) demyelination was markedly reduced. Transcriptional profiling at EAE onset revealed microglia upregulated genes primarily involved in cell migration and chemoattraction and had repressed activation of genes involved in phagocytosis, reactive oxygen species and cytoskeletal reorganization compared to naïve microglia. Macrophages in contrast had upregulation of genes involved in phagocytosis and cell clearance. In focal demyelinating models, monocyte-derived macrophages have beneficial roles in promoting remyelination by clearing myelin debris, promoting oligodendrocyte differentiation and facilitating remyelination [25,71-73]. Microglia also have a role in myelin clearance suggested by experiments in Trem2 knockout mice [74]. Trem2 is a microglial surface receptor that binds polyanions such as LPS and dextrans. In the context of cuprizone-mediated demyelination, Trem2 knockout mice exhibited impaired myelin clearance, persistent demyelination and microglia that failed to upregulate transcripts involved in phagocytosis, activation and lipid metabolism [74]. Microglia and macrophages have also been shown to release factors that support oligodendrocyte proliferation and differentiation [reviewed in [75]]. Similar to oligodendroglia, with aging and in the context of demyelinating injury, subsets of microglia upregulate inflammatory transcripts and assume a more immunogenic profile [76].
Interpreting the distinct roles of microglia and macrophages in different models of demyelination and remyelination is challenging, as they have markedly different inflammatory contexts. EAE models may underestimate the reparative phenotype of microglia and macrophages, given the strong inflammatory environment, whereas lysolecithin and cuprizone models may underestimate the role of macrophages and microglia in antigen presentation, as they have more robust remyelination. Microglia and macrophages may also have different roles in early and late stages of disease, assuming a more immunogenic, detrimental role early in disease through antigen presentation and by initiating demyelination, and a more reparative role later in disease through clearance of myelin debris and promotion of oligodendrocyte remyelination.
8. Astrocytes
Similar to microglia and macrophages, astrocytes can display different levels of reactivity and can inhibit or promote remyelination, depending on the inflammatory context of growth factors and cytokines [reviewed in [77]]. Activated microglia stimulated by LPS exposure induce a reactive “A1” astrocyte phenotype, which was shown to be mediated through secretion of IL-1α, TNF-α and complement component 1q (C1q) [78]. A1 astrocytes lose the ability to promote neuronal survival and synaptogenesis, have reduced phagocytosis, and culture media from A1 reactive astrocytes induces neuronal and oligodendrocyte cell death. Blocking microglial-mediated activation of the A1 neurotoxic phenotype has been shown to be neuroprotective in a mouse model of Parkinson’s disease [79]. Similar to the macrophage M1/M2 continuum, there is likely a dynamic phenotype that astrocytes can assume, ranging from A2 protective to A1 neurotoxic phenotypes [reviewed in [80]].
Astrocytes also play a role in the recruitment of T cells [reviewed in [81,82]] and naïve dendritic cells [83] through production of cytokines and chemokines. Astrocytes may also present antigen and influence T cell responses, with some studies demonstrating that IFN-γ stimulated astrocytes are capable of activating naïve myelin-specific T cells [84-89]. Astrocytes can also influence microglia antigen presentation through secretion of cytokines, such as granulocyte macrophage colony stimulating factor (GM-CSF), macrophage colony stimulating factor (M-CSF) and transforming growth factor beta (TGF-β) [90-92]. Reactive astrocytes can also undergo morphological changes and form a glial scar that in EAE models has been shown to serve a protective role by limiting widespread T cell infiltration in the brain parenchyma and targeting T cell localization to the perivascular niche [93]. Reactive astrocytes can also modify the extracellular matrix environment, which may also limit oligodendrocyte precursor migration and repair within demyelinated lesions [reviewed in [94-96]].
9. Promoting remyelination in multiple sclerosis
MS is a CNS demyelinating disease likely caused by a complex combination of factors and environmental triggers that induce an inflammatory response in the CNS leading to an autoimmune attack on myelin. Myelin degeneration results in demyelinated plaques in the brain and spinal cord with areas of exposed axons. The inflammatory environment of MS is a challenging environment for myelin repair, which may require OPCs to migrate into demyelinating plaques (if the precursor pool is depleted), differentiate into mature oligodendrocytes and wrap denuded axons in the setting of a milieu of myelin debris, extracellular matrix changes, cytokines, neurotoxic reactive astrocytes, microglia, macrophages, T and B cells. Remyelination failure and resulting axonal degeneration is thought to underlie the progressive features of MS. Therapies targeted at overcoming these barriers to promote oligodendrogenesis and remyelination are thus a current area of focus in MS research. Understanding how oligodendrocytes and oligodendrocyte precursor cells interact with cells of the immune system and determine how these specific cellular interactions influence remyelination will be an important addition to our current knowledge about the influence of the inflammatory milieu on oligodendrocyte function and myelin repair.
10. Conclusions
In a neuroinflammatory environment, oligodendrocyte lineage cells in both mouse models and human MS lesions can develop an inflammatory signature characterized by upregulation of genes involved in antigen presentation. Adoptive transfer of Th17 polarized CD4+ T cells after cuprizone-mediated demyelination results in reduced numbers of oligodendrocytes and increased caspase-expressing dying OPCs, suggesting that both OPCs and mature oligodendrocytes may be targeted for killing by cytotoxic CD8+ T cells. Why these cells present antigens to T cells and potentially target themselves for CD8+ T cell-mediated killing is unclear, but may be a programmed response to chronic inflammation similar to that which occurs in viral infections of the CNS. Oligodendrocyte antigen presentation to T cells could have beneficial roles in the setting of CNS infection by assisting with T cell activation and recruitment; however, this pathway may be dysregulated in the setting of MS when healthy OPCs capable of forming remyelinating oligodendrocytes are necessary for repair. Further experiments are needed to define the role of antigen presentation by oligodendrocyte lineage cells in vivo and to assess whether inhibiting antigen presentation by these cells alters the timing and extent of myelin repair in MS.
Highlights.
Oligodendroglia can exhibit an immunogenic profile in demyelinating disease
Inflammation induces oligodendroglia to upregulate immune genes
Oligodendrocytes and their progenitors can present antigen to T cells
Antigen presentation by oligodendroglia can induce CD8+ T cell induced death
Depletion of OPCs by CD8+ T cells may contribute to impaired remyelination
Acknowledgments
Funding
EPH is supported by the National Multiple Sclerosis Society and American Brain Foundation Clinician Scientist Development Award. DEB is supported by the Dr. Miriam and Sheldon G Adelson Medical Research Foundation. PAC is supported in part by a Jacob Javitz Neuroscience Scholar Award R37NS041435 and the NMSS (collaborative center award).
Abbreviations
- B2m
beta-2 microglobulin
- CNS
central nervous system
- CFA
complete Freund’s adjuvant
- C1q
complement component 1q
- EAE
experimental autoimmune encephalomyelitis
- GM-CSF
granulocyte macrophage colony stimulating factor
- GFP
green fluorescent protein
- HA
hemagglutinin
- iNOS
inducible nitric oxide synthase
- iOPCs
inflammatory oligodendrocyte precursor cells
- iOLs
inflammatory oligodendrocytes
- IFN-γ
interferon gamma
- IL
interleukin
- LPS
lipopolysaccharide
- M-CSF
macrophage colony stimulating factor
- MHC
major histocompatibility molecules
- MS
multiple sclerosis
- MBP
myelin basic protein
- MOG
myelin oligodendrocyte protein
- NAWM
normal appearing white matter
- NG2
neuron-glial antigen 2
- NK
natural killer
- OPCs
oligodendrocyte precursor cells
- OVA
ovalbumin; proteasome subunit beta (PSMB)
- scRNA-seq
single-cell RNA sequencing
- snRNA-seq
single-nucleus RNA sequencing
- TGF-β
transforming growth factor beta
- TAP
transporter associated with antigen presentation
- TNF-α
tumor necrosis factor alpha
- YFP
yellow fluorescent protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Hughes EG, Orthmann-Murphy JL, Langseth AJ, Bergles DE, Myelin remodeling through experience-dependent oligodendrogenesis in the adult somatosensory cortex, Nat. Neurosci 21 (2018) 696–706. doi: 10.1038/s41593-018-0121-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Tripathi RB, Jackiewicz M, McKenzie IA, Kougioumtzidou E, Grist M, Richardson WD, Remarkable Stability of Myelinating Oligodendrocytes in Mice, Cell Rep. 21 (2017) 316–323. doi: 10.1016/j.celrep.2017.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Yeung MSY, Djelloul M, Steiner E, Bernard S, Salehpour M, Possnert G, Brundin L, Frisén J, Dynamics of oligodendrocyte generation in multiple sclerosis, Nature. 566 (2019) 538–542. doi: 10.1038/s41586-018-0842-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hughes EG, Kang SH, Fukaya M, Bergles DE, Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain, Nat. Neurosci 16 (2013) 668–676. doi: 10.1038/nn.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lucchinetti C, Bruck W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H, A quantitative analysis of oligodendrocytes in multiple sclerosis lesions. A study of 113 cases, Brain. 122(1999) 2279–2295. doi: 10.1093/brain/122.12.2279. [DOI] [PubMed] [Google Scholar]
- [6].Chang A, Nishiyama A, Peterson J, Prineas J, Trapp BD, NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions, J. Neurosci 20 (2000) 6404–6412. https://www.ncbi.nlm.nih.gov/pubmed/10964946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Boyd A, Zhang H, Williams A, Insufficient OPC migration into demyelinated lesions is a cause of poor remyelination in MS and mouse models, Acta Neuropathol. 125 (2013) 841–859. doi: 10.1007/s00401-013-1112-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kornek B, Storch MK, Weissert R, Wallstroem E, Stefferl A, Olsson T, Linington C, Schmidbauer M, Lassmann H, Multiple sclerosis and chronic autoimmune encephalomyelitis: a comparative quantitative study of axonal injury in active, inactive, and remyelinated lesions, Am. J. Pathol 157 (2000) 267–276. doi: 10.1016/S0002-9440(10)64537-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Jäkel S, Agirre E, Mendanha Falcão A, van Bruggen D, Lee KW, Knuesel I, Malhotra D, Ffrench-Constant C, Williams A, Castelo-Branco G, Altered human oligodendrocyte heterogeneity in multiple sclerosis, Nature. 566 (2019) 543–547. doi: 10.1038/s41586-019-0903-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Marques S, Zeisel A, Codeluppi S, van Bruggen D, Mendanha Falcão A, Xiao L, Li H, Häring M, Hochgerner H, Romanov RA, Gyllborg D, Muñoz Manchado A, La Manno G, Lönnerberg P, Floriddia EM, Rezayee F, Ernfors P, Arenas E, Hjerling-Leffler J, Harkany T, Richardson WD, Linnarsson S, Castelo-Branco G, Oligodendrocyte heterogeneity in the mouse juvenile and adult central nervous system, Science. 352 (2016) 1326–1329. doi: 10.1126/science.aaf6463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Marques S, van Bruggen D, Vanichkina DP, Floriddia EM, Munguba H, Väremo L, Giacomello S, Falcóo AM, Meijer M, Björklund ÅK, Hjerling-Leffler J, Taft RJ, Castelo-Branco G, Transcriptional Convergence of Oligodendrocyte Lineage Progenitors during Development, Dev. Cell 46 (2018) 504–517.e7. doi: 10.1016/j.devcel.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Spitzer SO, Sitnikov S, Kamen Y, Evans KA, Kronenberg-Versteeg D, Dietmann S, de Faria O Jr, Agathou S, Káradóttir RT, Oligodendrocyte Progenitor Cells Become Regionally Diverse and Heterogeneous with Age, Neuron. 101 (2019) 459–471.e5. doi: 10.1016/j.neuron.2018.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bergles DE, Richardson WD, Oligodendrocyte Development and Plasticity, Cold Spring Harb. Perspect. Biol 8 (2015) a020453. doi: 10.1101/cshperspect.a020453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Li Q, Brus-Ramer M, Martin JH, McDonald JW, Electrical stimulation of the medullary pyramid promotes proliferation and differentiation of oligodendrocyte progenitor cells in the corticospinal tract of the adult rat, Neurosci. Lett 479 (2010) 128–133. doi: 10.1016/j.neulet.2010.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Simon C, Götz M, Dimou L, Progenitors in the adult cerebral cortex: cell cycle properties and regulation by physiological stimuli and injury, Glia. 59 (2011) 869–881. doi: 10.1002/glia.21156. [DOI] [PubMed] [Google Scholar]
- [16].Gibson EM, Purger D, Mount CW, Goldstein AK, Lin GL, Wood LS, Inema I, Miller SE, Bieri G, Zuchero JB, Barres BA, Woo PJ, Vogel H, Monje M, Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain, Science. 344 (2014) 1252304. doi: 10.1126/science.1252304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mitew S, Gobius I, Fenlon LR, McDougall SJ, Hawkes D, Xing YL, Bujalka H, Gundlach AL, Richards LJ, Kilpatrick TJ, Merson TD, Emery B, Pharmacogenetic stimulation of neuronal activity increases myelination in an axon-specific manner, Nat. Commun 9 (2018) 306. doi: 10.1038/s41467-017-02719-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wake H, Lee PR, Fields RD, Control of local protein synthesis and initial events in myelination by action potentials, Science. 333 (2011) 1647–1651. doi: 10.1126/science.1206998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gautier HOB, Evans KA, Volbracht K, James R, Sitnikov S, Lundgaard I, James F, Lao-Peregrin C, Reynolds R, Franklin RJM, Káradóttir RT, Neuronal activity regulates remyelination via glutamate signalling to oligodendrocyte progenitors, Nat. Commun 6 (2015) 8518. doi: 10.1038/ncomms9518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Krasnow AM, Ford MC, Valdivia LE, Wilson SW, Attwell D, Regulation of developing myelin sheath elongation by oligodendrocyte calcium transients in vivo, Nat. Neurosci 21 (2018) 24–28. doi: 10.1038/s41593-017-0031-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Tang DG, Tokumoto YM, Raff MC, Long-term culture of purified postnatal oligodendrocyte precursor cells. Evidence for an intrinsic maturation program that plays out over months, J. Cell Biol 148 (2000) 971–984. doi: 10.1083/jcb.148.5.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Shen S, Sandoval J, Swiss VA, Li J, Dupree J, Franklin RJM, Casaccia-Bonnefil P, Age-dependent epigenetic control of differentiation inhibitors is critical for remyelination efficiency, Nat. Neurosci 11 (2008) 1024–1034. doi: 10.1038/nn.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hinks GL, Franklin RJ, Delayed changes in growth factor gene expression during slow remyelination in the CNS of aged rats, Mol. Cell. Neurosci 16 (2000) 542–556. doi: 10.1006/mcne.2000.0897. [DOI] [PubMed] [Google Scholar]
- [24].Neumann H, Kotter MR, Franklin RJM, Debris clearance by microglia: an essential link between degeneration and regeneration, Brain. 132 (2009) 288–295. doi: 10.1093/brain/awn109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ruckh JM, Zhao J-W, Shadrach JL, van Wijngaarden P, Rao TN, Wagers AJ, Franklin RJM, Rejuvenation of regeneration in the aging central nervous system, Cell Stem Cell. 10(2012) 96–103. doi: 10.1016/j.stem.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Segel M, Neumann B, Hill MFE, Weber IP, Viscomi C, Zhao C, Young A, Agley CC, Thompson AJ, Gonzalez GA, Sharma A, Holmqvist S, Rowitch DH, Franze K, Franklin RJM, Chalut KJ, Niche stiffness underlies the ageing of central nervous system progenitor cells, Nature. 573 (2019) 130–134. doi: 10.1038/s41586-019-1484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Panitch HS, Hirsch RL, Haley AS, Johnson KP, Exacerbations of multiple sclerosis in patients treated with gamma interferon, Lancet. 1 (1987) 893–895. doi: 10.1016/s0140-6736(87)92863-7. [DOI] [PubMed] [Google Scholar]
- [28].Vartanian T, Li Y, Zhao M, Stefansson K, Cell Death: Implications for the Pathogenesis of Multiple Sclerosis, Molecular Medicine. 1 (1995). https://www.ncbi.nlm.nih.gov/pubmed/8612196 . [PMC free article] [PubMed] [Google Scholar]
- [29].Chew L-J, King WC, Kennedy A, Gallo V, Interferon-gamma inhibits cell cycle exit in differentiating oligodendrocyte progenitor cells, Glia. 52 (2005) 127–143. doi: 10.1002/glia.20232. [DOI] [PubMed] [Google Scholar]
- [30].Corbin JG, Kelly D, Rath EM, Baerwald KD, Suzuki K, Popko B, Targeted CNS Expression of Interferon-γ in Transgenic Mice Leads to Hypomyelination, Reactive Gliosis, and Abnormal Cerebellar Development, Molecular and Cellular Neuroscience. 7 (1996) 354–370. doi: 10.1006/mcne.1996.0026. [DOI] [PubMed] [Google Scholar]
- [31].Horwitz MS, Evans CF, Mcgavern DB, Rodriguez M, Oldstone MBA, Primary demyelination in transgenic mice expressing interferon-gamma, Nat. Med 3 (1997) 1037–1041. doi: 10.1038/nm0997-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].LaFerla FM, Sugarman MC, Lane TE, Leissring MA, Regional hypomyelination and dysplasia in transgenic mice with astrocyte-directed expression of interferon-γ, J. Mol. Neurosci 15 (2000) 45–59. doi: 10.1385/JMN:15:1:45. [DOI] [PubMed] [Google Scholar]
- [33].Lin W, Kemper A, Dupree JL, Harding HP, Ron D, Popko B, Interferon-gamma inhibits central nervous system remyelination through a process modulated by endoplasmic reticulum stress, Brain. 129 (2006) 1306–1318. doi: 10.1093/brain/awl044. [DOI] [PubMed] [Google Scholar]
- [34].Falcão AM, van Bruggen D, Marques S, Meijer M, Jäkel S, Agirre E, Samudyata, Floriddia EM, Vanichkina DP, Ffrench-Constant C, Williams A, Guerreiro-Cacais AO, Castelo-Branco G, Disease-specific oligodendrocyte lineage cells arise in multiple sclerosis, Nat. Med 24 (2018) 1837–1844. doi: 10.1038/s41591-018-0236-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kirby L, Jin J, Cardona JG, Smith MD, Martin KA, Wang J, Strasburger H, Herbst L, Alexis M, Karnell J, Davidson T, Dutta R, Goverman J, Bergles D, Calabresi PA, Oligodendrocyte precursor cells present antigen and are cytotoxic targets in inflammatory demyelination, Nat. Commun 10 (2019) 3887. doi: 10.1038/s41467-019-11638-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Schirmer L, Velmeshev D, Holmqvist S, Kaufmann M, Werneburg S, Jung D, Vistnes S, Stockley JH, Young A, Steindel M, Tung B, Goyal N, Bhaduri A, Mayer S, Engler JB, Bayraktar OA, Franklin RJM, Haeussler M, Reynolds R, Schafer P, Friese MA, Shiow LR, Kriegstein AR, Rowitch DH, Neuronal vulnerability and multilineage diversity in multiple sclerosis, Nature. 573 (2019) 75–82. doi: 10.1038/s41586-019-1404-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zeis T, Enz L, Schaeren-Wiemers N, The immunomodulatory oligodendrocyte, Brain Res. 1641 (2016) 139–148. doi: 10.1016/j.brainres.2015.09.021. [DOI] [PubMed] [Google Scholar]
- [38].Höftberger R, Aboul-Enein F, Brueck W, Lucchinetti C, Rodriguez M, Schmidbauer M, Jellinger K, Lassmann H, Expression of major histocompatibility complex class I molecules on the different cell types in multiple sclerosis lesions, Brain Pathol. 14 (2004) 43–50. https://www.ncbi.nlm.nih.gov/pubmed/14997936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Neefjes J, Jongsma MLM, Paul P, Bakke O, Towards a systems understanding of MHC class I and MHC class II antigen presentation, Nat. Rev. Immunol 11 (2011) 823–836. doi: 10.1038/nri3084. [DOI] [PubMed] [Google Scholar]
- [40].van de Weijer ML, Luteijn RD, Wiertz EJHJ, Viral immune evasion: Lessons in MHC class I antigen presentation, Semin. Immunol 27 (2015) 125–137. doi: 10.1016/j.smim.2015.03.010. [DOI] [PubMed] [Google Scholar]
- [41].Kreer C, Rauen J, Zehner M, Burgdorf S, Cross-presentation: how to get there – or how to get the ER, Frontiers in Immunology. (2012). doi: 10.3389/fimmu.2011.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ferrington DA, Gregerson DS, Chapter 3 - Immunoproteasomes: Structure, Function, and Antigen Presentation, in: Grune T (Ed.), Progress in Molecular Biology and Translational Science, Academic Press, 2012: pp. 75–112. doi: 10.1016/B978-0-12-397863-9.00003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Basler M, Mundt S, Bitzer A, Schmidt C, Groettrup M, The immunoproteasome: a novel drug target for autoimmune diseases, Clin. Exp. Rheumatol 33 (2015) S74–9. https://www.ncbi.nlm.nih.gov/pubmed/26458097. [PubMed] [Google Scholar]
- [44].Basler M, Mundt S, Muchamuel T, Moll C, Jiang J, Groettrup M, Kirk CJ, Inhibition of the immunoproteasome ameliorates experimental autoimmune encephalomyelitis, EMBO Mol. Med 6 (2014) 226–238. doi: 10.1002/emmm.201303543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Booss J, Esiri MM, Tourtellotte WW, Mason DY, Immunohistological analysis of T lymphocyte subsets in the central nervous system in chronic progressive multiple sclerosis, Journal of the Neurological Sciences. 62 (1983) 219–232. doi: 10.1016/0022-510x(83)90201-0. [DOI] [PubMed] [Google Scholar]
- [46].Hauser SL, Bhan AK, Gilles F, Kemp M, Kerr C, Weiner HL, Immunohistochemical analysis of the cellular infiltrate in multiple sclerosis lesions, Ann. Neurol 19 (1986) 578–587. doi: 10.1002/ana.410190610. [DOI] [PubMed] [Google Scholar]
- [47].Gay FW, Drye TJ, Dick GWA, Esiri MM, Identification and characterization of the primary demyelinating lesion, Brain. 120 (1997) 1461–1483. [DOI] [PubMed] [Google Scholar]
- [48].Babbe H, Roers A, Waisman A, Lassmann H, Goebels N, Hohlfeld R, Friese M, Schröder R, Deckert M, Schmidt S, Ravid R, Rajewsky K, Clonal Expansions of Cd8 + T Cells Dominate the T Cell Infiltrate in Active Multiple Sclerosis Lesions as Shown by Micromanipulation and Single Cell Polymerase Chain Reaction, J. Exp. Med 192 (2000) 393–404. doi: 10.1084/jem.192.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Bitsch A, Acute axonal injury in multiple sclerosis: Correlation with demyelination and inflammation, Brain. 123 (2000) 1174–1183. doi: 10.1093/brain/123.6.1174. [DOI] [PubMed] [Google Scholar]
- [50].Bedoui S, Heath WR, Mueller SN, CD4(+) T-cell help amplifies innate signals for primary CD8(+) T-cell immunity, Immunol. Rev 272 (2016) 52–64. doi: 10.1111/imr.12426. [DOI] [PubMed] [Google Scholar]
- [51].Neumann H, Medana IM, Bauer J, Lassmann H, Cytotoxic T lymphocytes in autoimmune and degenerative CNS diseases, Trends Neurosci. 25 (2002) 313–319. doi: 10.1016/s0166-2236(02)02154-9. [DOI] [PubMed] [Google Scholar]
- [52].Mars LT, Bauer J, Gross DA, Bucciarelli F, Firat H, Hudrisier D, Lemonnier F, Kosmatopoulos K, Liblau RS, CD8 T cell responses to myelin oligodendrocyte glycoprotein-derived peptides in humanized HLA-A*0201-transgenic mice, J. Immunol 179 (2007) 5090–5098. doi: 10.4049/jimmunol.179.8.5090. [DOI] [PubMed] [Google Scholar]
- [53].Huseby ES, Liggitt D, Brabb T, Schnabel B, Öhlén C, Goverman J, A Pathogenic Role for Myelin-Specific Cd8 + T Cells in a Model for Multiple Sclerosis, J. Exp. Med 194 (2001) 669–676. doi: 10.1084/jem.194.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Sun D, Whitaker JN, Huang Z, Liu D, Coleclough C, Wekerle H, Raine CS, Myelin antigen-specific CD8+ T cells are encephalitogenic and produce severe disease in C57BL/6 mice, J. Immunol 166 (2001) 7579–7587. doi: 10.4049/jimmunol.166.12.7579. [DOI] [PubMed] [Google Scholar]
- [55].Friese MA, Jakobsen KB, Friis L, Etzensperger R, Craner MJ, McMahon RM, Jensen T, Huygelen V, Jones EY, J.I. Bell, L. Fugger, Opposing effects of HLA class I molecules in tuning autoreactive CD8+ T cells in multiple sclerosis, Nat. Med 14 (2008) 1227–1235. doi: 10.1038/nm.1881. [DOI] [PubMed] [Google Scholar]
- [56].Ji Q, Castelli L, Goverman JM, MHC class I–restricted myelin epitopes are cross-presented by Tip-DCs that promote determinant spreading to CD8+ T cells, Nat. Immunol 14 (2013) 254. doi: 10.1038/ni.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Fitzner D, Schnaars M, van Rossum D, Krishnamoorthy G, Dibaj P, Bakhti M, Regen T, Hanisch U-K, Simons M, Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis, J. Cell Sci 124 (2011) 447–458. doi: 10.1242/jcs.074088. [DOI] [PubMed] [Google Scholar]
- [58].Baxi EG, DeBruin J, Tosi DM, Grishkan IV, Smith MD, Kirby LA, Strasburger HJ, Fairchild AN, Calabresi PA, Gocke AR, Transfer of myelin-reactive th17 cells impairs endogenous remyelination in the central nervous system of cuprizone-fed mice, J. Neurosci 35 (2015) 8626–8639. doi: 10.1523/JNEUROSCI.3817-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Cao Y, Toben C, Na S-Y, Stark K, Nitschke L, Peterson A, Gold R, Schimpl A, Hünig T, Induction of experimental autoimmune encephalomyelitis in transgenic mice expressing ovalbumin in oligodendrocytes, Eur. J. Immunol 36 (2006) 207–215. doi: 10.1002/eji.200535211. [DOI] [PubMed] [Google Scholar]
- [60].Na S-Y, Cao Y, Toben C, Nitschke L, Stadelmann C, Gold R, Schimpl A, Hünig T, Naive CD8 T-cells initiate spontaneous autoimmunity to a sequestered model antigen of the central nervous system, Brain. 131 (2008) 2353–2365. doi: 10.1093/brain/awn148. [DOI] [PubMed] [Google Scholar]
- [61].Saxena A, Bauer J, Scheikl T, Zappulla J, Audebert M, Desbois S, Waisman A, Lassmann H, Liblau RS, Mars LT, Cutting edge: Multiple sclerosis-like lesions induced by effector CD8 T cells recognizing a sequestered antigen on oligodendrocytes, J. Immunol 181 (2008) 1617–1621. doi: 10.4049/jimmunol.181.3.1617. [DOI] [PubMed] [Google Scholar]
- [62].Zaguia F, Saikali P, Ludwin S, Newcombe J, Beauseigle D, McCrea E, Duquette P, Prat A, Antel JP, Arbour N, Cytotoxic NKG2C+ CD4 T cells target oligodendrocytes in multiple sclerosis, J. Immunol 190 (2013) 2510–2518. doi: 10.4049/jimmunol.1202725. [DOI] [PubMed] [Google Scholar]
- [63].Li Q, Barres BA, Microglia and macrophages in brain homeostasis and disease, Nat. Rev. Immunol 18 (2018) 225–242. doi: 10.1038/nri.2017.125. [DOI] [PubMed] [Google Scholar]
- [64].Greter M, Lelios I, Croxford AL, Microglia Versus Myeloid Cell Nomenclature during Brain Inflammation, Front. Immunol 6 (2015) 249. doi: 10.3389/fimmu.2015.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Kennedy DW, Abkowitz JL, Kinetics of central nervous system microglial and macrophage engraftment: analysis using a transgenic bone marrow transplantation model, Blood. 90 (1997) 986–993. https://www.ncbi.nlm.nih.gov/pubmed/9242527. [PubMed] [Google Scholar]
- [66].Varvel NH, Grathwohl SA, Baumann F, Liebig C, Bosch A, Brawek B, Thal DR, Charo IF, Heppner FL, Aguzzi A, Garaschuk O, Ransohoff RM, Jucker M, Microglial repopulation model reveals a robust homeostatic process for replacing CNS myeloid cells, Proc. Natl. Acad. Sci. U. S. A 109 (2012) 18150–18155. doi: 10.1073/pnas.1210150109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Elmore MRP, Najafi AR, Koike MA, Dagher NN, Spangenberg EE, Rice RA, Kitazawa M, Matusow B, Nguyen H, West BL, Green KN, Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain, Neuron. 82 (2014) 380–397. doi: 10.1016/j.neuron.2014.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Elmore MRP, Lee RJ, West BL, Green KN, Characterizing newly repopulated microglia in the adult mouse: impacts on animal behavior, cell morphology, and neuroinflammation, PLoS One. 10 (2015) e0122912. doi: 10.1371/journal.pone.0122912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Mosser DM, Edwards JP, Exploring the full spectrum of macrophage activation, Nat. Rev. Immunol 8 (2008) 958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Yamasaki R, Lu H, Butovsky O, Ohno N, Rietsch AM, Cialic R, Wu PM, Doykan CE, Lin J, Cotleur AC, Kidd G, Zorlu MM, Sun N, Hu W, Liu L, Lee J-C, Taylor SE, Uehlein L, Dixon D, Gu J, Floruta CM, Zhu M, Charo IF, Weiner HL, Ransohoff RM, Differential roles of microglia and monocytes in the inflamed central nervous system, J. Exp. Med 211 (2014) 1533–1549. doi: 10.1084/jem.20132477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Kotter MR, Setzu A, Sim FJ, Van Rooijen N, Franklin RJ, Macrophage depletion impairs oligodendrocyte remyelination following lysolecithin-induced demyelination, Glia. 35 (2001) 204–212. https://www.ncbi.nlm.nih.gov/pubmed/11494411. [DOI] [PubMed] [Google Scholar]
- [72].Miron VE, Boyd A, Zhao J-W, Yuen TJ, Ruckh JM, Shadrach JL, van Wijngaarden P, Wagers AJ, Williams A, Franklin RJM, Ffrench-Constant C, M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination, Nat. Neurosci 16 (2013) 1211–1218. doi: 10.1038/nn.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Natrajan MS, de la Fuente AG, Crawford AH, Linehan E, Nuñez V, Johnson KR, Wu T, Fitzgerald DC, Ricote M, Bielekova B, Franklin RJM, Retinoid X receptor activation reverses age-related deficiencies in myelin debris phagocytosis and remyelination, Brain. 138 (2015) 3581–3597. doi: 10.1093/brain/awv289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Poliani PL, Wang Y, Fontana E, Robinette ML, Yamanishi Y, Gilfillan S, Colonna M, TREM2 sustains microglial expansion during aging and response to demyelination, J. Clin. Invest 125 (2015) 2161–2170. doi: 10.1172/JCI77983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Miron VE, Microglia-driven regulation of oligodendrocyte lineage cells, myelination, and remyelination, J. Leukoc. Biol 101 (2017) 1103–1108. doi: 10.1189/jlb.3RI1116-494R. [DOI] [PubMed] [Google Scholar]
- [76].Hammond TR, Dufort C, Dissing-Olesen L, Giera S, Young A, Wysoker A, Walker AJ, Gergits F, Segel M, Nemesh J, Marsh SE, Saunders A, Macosko E, Ginhoux F, Chen J, Franklin RJM, Piao X, McCarroll SA, Stevens B, Single-Cell RNA Sequencing of Microglia throughout the Mouse Lifespan and in the Injured Brain Reveals Complex Cell-State Changes, Immunity. 50 (2019) 253–271.e6. doi: 10.1016/j.immuni.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Kiray H, Lindsay SL, Hosseinzadeh S, Barnett SC, The multifaceted role of astrocytes in regulating myelination, Exp. Neurol 283 (2016) 541–549. doi: 10.1016/j.expneurol.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Münch AE, Chung W-S, Peterson TC, Wilton DK, Frouin A, Napier BA, Panicker N, Kumar M, Buckwalter MS, Rowitch DH, Dawson VL, Dawson TM, Stevens B, Barres BA, Neurotoxic reactive astrocytes are induced by activated microglia, Nature. 541 (2017) 481–487. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Yun SP, Kam TI, Panicker N, Kim S, Oh Y, Park J-S, Kwon S-H, Park YJ, Karuppagounder SS, Park H, Kim S, Oh N, Kim NA, Lee S, Brahmachari S, Mao X, Lee JH, Kumar M, An D, Kang S-U, Lee Y, Lee KC, Na DH, Kim D, Lee SH, Roschke VV, Liddelow SA, Mari Z, Barres BA, Dawson VL, Lee S, Dawson TM, Ko HS, Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson’s disease, Nat. Med 24 (2018) 931–938. doi: 10.1038/s41591-018-0051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Liddelow SA, Barres BA, Reactive Astrocytes: Production, Function, and Therapeutic Potential, Immunity. 46 (2017) 957–967. doi: 10.1016/j.immuni.2017.06.006. [DOI] [PubMed] [Google Scholar]
- [81].Chastain EML, Duncan DS, Rodgers JM, Miller SD, The role of antigen presenting cells in multiple sclerosis, Biochim. Biophys. Acta 1812 (2011) 265–274. doi: 10.1016/j.bbadis.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Ding X, Yan Y, Li X, Li K, Ciric B, Yang J, Zhang Y, Wu S, Xu H, Chen W, Lovett-Racke AE, Zhang G-X, Rostami A, Silencing IFN-γ binding/signaling in astrocytes versus microglia leads to opposite effects on central nervous system autoimmunity, J. Immunol 194 (2015) 4251–4264. doi: 10.4049/jimmunol.1303321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Ambrosini E, Remoli ME, Giacomini E, Rosicarelli B, Serafini B, Lande R, Aloisi F, Coccia EM, Astrocytes produce dendritic cell-attracting chemokines in vitro and in multiple sclerosis lesions, J. Neuropathol. Exp. Neurol 64 (2005) 706–715. doi: 10.1097/01.jnen.0000173893.01929.fc. [DOI] [PubMed] [Google Scholar]
- [84].Tan L, Gordon KB, Mueller JP, Matis LA, Miller SD, Presentation of Proteolipid Protein Epitopes and B7-1-Dependent Activation of Encephalitogenic T Cells by IFN-g-Activated SJL/J Astrocytes1, J. Immunol 160 (1998) 4271–4279. [PubMed] [Google Scholar]
- [85].Soos JM, Ashley TA, Morrow J, Patarroyo JC, Szente BE, Zamvil SS, Differential expression of B7 co-stimulatory molecules by astrocytes correlates with T cell activation and cytokine production, Int. Immunol 11 (1999) 1169–1179. doi: 10.1093/intimm/11.7.1169. [DOI] [PubMed] [Google Scholar]
- [86].Øren A, Falk K, Rötzschke O, Bechmann I, Nitsch R, Gimsa U, Production of neuroprotective NGF in astrocyte-T helper cell cocultures is upregulated following antigen recognition, J. Neuroimmunol 149 (2004) 59–65. doi: 10.1016/j.jneuroim.2003.12.009. [DOI] [PubMed] [Google Scholar]
- [87].Carpentier PA, Begolka WS, Olson JK, Elhofy A, Karpus WJ, Miller SD, Differential activation of astrocytes by innate and adaptive immune stimuli, Glia. 49 (2005) 360–374. doi: 10.1002/glia.20117. [DOI] [PubMed] [Google Scholar]
- [88].Constantinescu CS, Tani M, Ransohoff RM, Wysocka M, Hilliard B, Fujioka T, Murphy S, Tighe PJ, Das Sarma J, Trinchieri G, Rostami A, Astrocytes as antigen-presenting cells: expression of IL-12/IL-23, J. Neurochem 95 (2005) 331–340. doi: 10.1111/j.1471-4159.2005.03368.x. [DOI] [PubMed] [Google Scholar]
- [89].Kort JJ, Kawamura K, Fugger L, Weissert R, Forsthuber TG, Efficient presentation of myelin oligodendrocyte glycoprotein peptides but not protein by astrocytes from HLA-DR2 and HLA-DR4 transgenic mice, J. Neuroimmunol 173 (2006) 23–34. doi: 10.1016/j.jneuroim.2005.11.014. [DOI] [PubMed] [Google Scholar]
- [90].Hailer NP, Heppner FL, Haas D, Nitsch R, Astrocytic factors deactivate antigen presenting cells that invade the central nervous system, Brain Pathol. 8 (1998) 459–474. https://www.ncbi.nlm.nih.gov/pubmed/9669697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Fischer HG, Bielinsky AK, Antigen presentation function of brain-derived dendriform cells depends on astrocyte help, Int. Immunol 11 (1999) 1265–1274. doi: 10.1093/intimm/11.8.1265. [DOI] [PubMed] [Google Scholar]
- [92].Aloisi F, Ria F, Adorini L, Regulation of T-cell responses by CNS antigen-presenting cells: different roles for microglia and astrocytes, Immunol. Today 21 (2000) 141–147. https://www.ncbi.nlm.nih.gov/pubmed/10689302. [DOI] [PubMed] [Google Scholar]
- [93].Voskuhl RR, Peterson RS, Song B, Ao Y, Morales LBJ, Tiwari-Woodruff S, Sofroniew MV, Reactive astrocytes form scar-like perivascular barriers to leukocytes during adaptive immune inflammation of the CNS, J. Neurosci 29 (2009) 11511–11522. doi: 10.1523/JNEUROSCI.1514-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Nair A, Frederick TJ, Miller SD, Astrocytes in multiple sclerosis: a product of their environment, Cell. Mol. Life Sci 65 (2008) 2702–2720. doi: 10.1007/s00018-008-8059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Clemente D, Ortega MC, Melero-Jerez C, de Castro F, The effect of glia–glia interactions on oligodendrocyte precursor cell biology during development and in demyelinating diseases, Frontiers in Cellular Neuroscience. (2013). doi: 10.3389/fncel.2013.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Domingues HS, Portugal CC, Socodato R, Relvas JB, Oligodendrocyte, Astrocyte, and Microglia Crosstalk in Myelin Development, Damage, and Repair, Front Cell Dev Biol. 4 (2016) 71. doi: 10.3389/fcell.2016.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]