Abstract
Objectives:
We evaluated whether plasma miRNAs were specifically associated with sudden cardiac and/or arrhythmic death (SCD) in a cohort of coronary heart disease (CHD) patients, most of whom were without primary prevention ICDs.
Background:
Novel biomarkers for sudden death risk stratification are needed in patients with CHD to more precisely target preventive therapies such as implantable cardioverter defibrillators (ICDs). MiRNAs have been implicated in regulating inflammation and cardiac fibrosis in cells, and plasma miRNAs have been shown to predict cardiovascular death in patients with CHD.
Methods:
We performed a nested-case control study within a multi-center cohort of 5956 patients with CHD followed prospectively for SCD. Plasma levels of 18 candidate miRNAs previously associated with cardiac remodeling were measured in 129 SCD cases and 258 controls matched on age, sex, race, and LVEF.
Results:
MiR-150-5p, miR-29a-3p, and miR-30a-5p were associated with increased SCD risk (Odds ratios and 95% CI: 2.03 [1.12-3.67] p=0.02, 1.93 [1.07-3.50] p=0.02, and 0.55 [0.31-0.97] p=0.04, respectively for 3rd versus 1st tertile miRNA level). Unfavorable levels of all 3 miRNAs was associated with a 4.8-fold increased SCD risk (1.59-14.51) p=0.006. A bioinformatics-based approach predicted miR-150-5p, miR-29a-3p, and miR-30a-5p to be involved in apoptosis, fibrosis, and inflammation.
Conclusions:
These findings suggest that plasma miRNAs may regulate pathways important for remodeling and may be useful in identifying CHD patients at increased risk of SCD.
Keywords: sudden death, microRNAs, coronary heart disease, risk prediction
Condensed Abstract
Novel biomarkers for sudden death risk stratification are needed to more precisely target preventive therapies. We evaluated whether plasma miRNAs were specifically associated with sudden cardiac and/or arrhythmic death (SCD) in a nested-case control study within a multi-center cohort of 5956 CHD patients followed prospectively for SCD. MiR-150-5p, miR-29a-3p, and miR-30a-5p were associated with increased SCD risk. A bioinformatics-based approach predicted these miRNAs to be involved in apoptosis, fibrosis, and inflammation. These findings suggest that plasma miRNAs may regulate pathways important for remodeling and may be useful in identifying CHD patients at increased SCD risk.
Introduction
Sudden death accounts for nearly 50% of all coronary heart disease (CHD) related mortality.(1,2) Current prevention strategies focus on the use of implantable cardioverter-defibrillators (ICDs) in patients considered high risk for sudden death based on a reduced left ventricular ejection fraction (LVEF).(3) However, 70-80% of sudden deaths occur in individuals who do not have a reduced LVEF in a range that qualifies them for ICD implantation.(2) Therefore, there is an urgent need to identify patients at high risk for sudden death who do not meet these traditional clinical criteria.(3)
Although there has been much interest in identifying circulating/plasma biomarkers to enhance SCD risk prediction, given the complexities and unheralded nature of SCD, few circulating biomarkers have previously been identified, and none of these biomarkers have progressed to clinical use for SCD.(4,5) Thus, there remains a critical need to identify circulating biomarkers of SCD.
MicroRNAs (miRNAs) are small noncoding RNAs that regulate post-transcriptional gene expression and play a role in intercellular communication.(6) Given their stability in peripheral blood, as well as their potential role in cardiovascular physiology, circulating miRNAs have garnered enthusiasm as possible biomarkers with a functional role in disease pathogenesis.(6) MiRNAs have been implicated in regulating inflammation and cardiac fibrosis, both of which have been linked to sudden cardiac death.(7-9) Furthermore, plasma miRNAs have been found to predict cardiovascular death in patients with CHD.(10) We have previously demonstrated an association between circulating miRNAs and adverse ventricular remodeling,(7) a known risk factor for sudden death.(11) Thus, miRNAs may be promising candidate markers of SCD risk, though little investigation to date has shed light on their role in this deadly condition.
Our cohort of 5956 individuals with CHD is a carefully-phenotyped population with sudden cardiac and/or arrhythmic death (SCD) adjudicated by a clinical end point committee.(12) Recent studies have demonstrated the difficulties in ascertaining the cause of sudden death in community populations;(13) hence this cohort allows the unique opportunity to derive the association of novel miRNA biomarkers with SCD. Therefore, in this study, we evaluated the association between several of these miRNAs and risk of SCD in patients with CHD, the majority of whom did not have a sufficiently reduced LVEF to meet current guidelines for ICD implantation.
Methods
Study Population
We performed a nested case-control study within a multi-center cohort of 5956 patients with CHD who were followed prospectively for SCD.(12) Each participant with incident SCD during follow-up (case) was matched (on age, sex, race, LVEF, time of blood draw, and fasting status) to two randomly selected participants without incident SCD during follow up (control) using risk set sampling. Of the initial 129 cases and 258 controls, samples from 35 participants (11 cases, 24 controls) were excluded from analysis (33 had significant hemolysis, and 2 had undetectable miRNA levels). This study complies with the declaration of Helsinki, the locally appointed ethics committee has approved the research protocol, and all subjects gave written informed consent.
Data Collection and Endpoint Classification
A comprehensive baseline assessment was performed at the time of enrollment, which included a comprehensive medical history, electrocardiogram, and venous blood sampling for laboratory analysis. During the baseline evaluation, venous blood was collected via peripheral venipuncture, centrifuged, and stored at −80°C until analysis. The baseline LVEF was recorded as the most recent clinically indicated assessment of LVEF at the time of study entry.
Following enrollment, participants were followed for clinical endpoints by a central Clinical Coordinating Center at Brigham and Women’s Hospital through questionnaires administered by mail or telephone. Medical records pertaining to all deaths and cardiac arrests were requested to confirm study endpoints. Endpoints were confirmed using data from emergency service reports, emergency room and other medical records, autopsies, and witness reports. Due to the known limitations for sudden death determination, information from the death certificate was not used in determination of sudden or arrhythmic death. All deaths were adjudicated by a clinical endpoints committee at Brigham and Women’s Hospital.
The primary endpoint was sudden cardiac and/or arrhythmic death (SCD). Details of the endpoint classification have been published previously, but are summarized here.(12) Definite sudden cardiac death was defined as death or fatal cardiac arrest occurring within 1 hour of symptom onset without evidence for a non-cardiac cause by history or autopsy. Probable sudden cardiac death was defined as an unwitnessed death or death that occurred during sleep where the participant was observed to be symptom-free within the preceding 24 hours. Arrhythmic death was defined as an abrupt spontaneous loss of pulse without evidence of preceding circulatory impairment or neurological dysfunction.(14) Out-of-hospital cardiac arrests due to ventricular fibrillation requiring external electrical defibrillation for resuscitation were considered aborted arrhythmic deaths and included as an arrhythmic death.
Laboratory Methods: miRNA and cytokine measurements
We quantified plasma levels of 18 candidate miRNAs that were identified from 2 sources: 1) Previously identified miRNAs in our laboratory using next-generation sequencing from plasma in post MI patients;(7) and 2) published literature.(15-17) Plasma miRNA levels were measured using the FirePlex® miRNA assay (Abcam, Cambridge, MA).(18,19)
Plasma levels of Interleukin (IL)--6, IL-10, Tumor Necrosis Factor-alpha (TNF-α), and Monocyte Chemoattractant Protein-1 (MCP-1), chosen based on prior data,(20,21) were measured using the FirePlex® immunoassay(22) in a subset of controls with adequate sample availability (n=57).
MiRNA data normalization
A total of 5 plates were used for the FirePlex® miRNA assay. To help normalize the intensity data between plates, replicates of the same pooled human serum (PHS) sample were run on each plate (ranging from 2-4 samples). The geometric mean of all PHS samples was used to calculate the scaling factors for each miRNA within each sample across all plates. The geometric mean of the scaling factors for each plate for each miRNA was then computed. Finally, the distinct scaling factor for each plate was then computed as the median of scaling factors across all miRNAs for each individual plate (thus yielding 1 scaling factor per plate).
To account for sample input volume and other technical factors, three miRNAs, namely hsa-miR-103a-3p, hsa-let-7g-5p and hsa-miR-140-3p, were chosen as normalizers based on the expression and their low coefficient of variation (CV) with relative invariance in healthy subjects and those with cardiovascular disease in prior work.(7,19) Levels of these miRNAs were measured (distribution plots can be found in the supplement), and the geometric mean for each of these three miRNAs across all samples was used to compute three scaling factors for each individual sample. The geometric mean of the three scaling factors was then used to normalize each sample. Following normalization, expression values for each miRNA were then log transformed for analysis.
Statistical analysis
The baseline characteristics of the study population were presented as means (+/− standard deviation [SD]), medians (interquartile range), and proportions. The associations between miRNAs and risk of SCD were evaluated using conditional logistic regression (to account for the matched case-control study design) adjusted for prior MI, NYHA class, and history of diabetes. MiRNA levels were evaluated across tertiles based upon the distribution of values in the control group (distribution plots can be found in the supplement [supplemental figures 1-3]). A linear test for trend was performed across tertiles using the median value in each tertile. Significant miRNAs were also evaluated as dichotomous variables: above vs below the median (based on the median value in controls) and combined into a multi-miRNA score. Spearman correlation coefficients were used to evaluate relationships between miRNAs among the controls, and between significant miRNAs and inflammatory cytokines in a subset of controls. Analyses were performed with SAS version 9.4. Data normalization was performed using R version 3.4.2.
Bioinformatics based approach: Network Reconstruction and Enrichment Analysis
Targets of the 3 SCD-associated miRNAs were extracted from miRTarBase version 6.1(23) using Target Interaction Finder(24) as integrated into the Genboree Workbench.(25) The xGMML output was imported into Cytoscape,(26) revealing a network of 1402 edges connecting 3 miRNA nodes to 1352 target gene nodes. The network was filtered in Cytoscape based on evidence type (“Functional MTI”, excluding “Non-Functional”) or topology (targets of at least 2 of 3 candidate miRNAs), producing a subnetwork of 160 target gene nodes and 210 edges. Working with only the subset of experimentally validated interactions, MiRTarBase provides additional annotations distinguishing interactions as ‘strong’ vs ‘weak’ depending on the experimental method used in the original data collection.(27) Given this information, we chose to prioritize interactions with "strong" evidence, as well as "any" experimental evidence involving 2 of the 3 query miRNAs.
The set of 160 target genes defined by network reconstruction were used as input for enrichment analysis using Enrichr.(28) Tabular results were downloaded for Gene Ontology: Biological Process, OMIM, Jensen Disease and WikiPathways.(29) Ontology and pathway terms were selected from the significant results based on low redundancy and relevance.
Cell based experiments
We performed cell-based experiments to evaluate whether the 3 miRNAs of interest (miR-150-5p, miR-29a-3p, and miR-30a-5p) originate from subtypes of bone marrow derived monocytes and whether they are secreted in extracellular vesicles. Detailed methods are available in the supplement.
Results
Plasma miRNAs associated with SCD
A total of 118 SCD cases and 234 matched controls were included in the nested case-control study. Baseline characteristics are presented in Table 1. Mean age of the cases and controls was 66 years and the majority (80%) were men. Most (86%) had a prior MI and more than one-third had diabetes. The median LVEF was 45% (IQR = 13%; range =10-75%), and slightly more than one in four participants had symptomatic heart failure. Participants were followed for 3.6 (± 1.6) years.
Table 1:
Baseline Characteristics
| Characteristic | Overall Cohort (N=352) |
Controls (N=234) |
Cases (N=118) |
|---|---|---|---|
| Age* | 66 (11) years | 66 (11) years | 66 (12) years |
| Female | 20% | 19% | 20% |
| Prior MI | 86% | 86% | 86% |
| History of diabetes | 38% | 32% | 48% |
| NYHA class ≥2 | 26% | 27% | 25% |
| History of hypertension | 80% | 78% | 84% |
| History of atrial arrhythmia | 20% | 18% | 23% |
| Family history of SCD | 31% | 30% | 34% |
| Current smoker | 14% | 12% | 18% |
| Non-white | 8% | 7% | 8% |
| BMI** | 29.6 (26.2 – 34.0) | 29.6 (25.8 – 34.0) | 29.8 (26.9 – 34.0) |
| Ejection fraction** | 45 (40 – 53) | 45 (40 – 53) | 45 (40 – 52) |
| Aspirin | 83% | 85% | 78% |
| Betablocker | 78% | 78% | 79% |
| Statin | 90% | 91 % | 87% |
| Follow up years* | 3.6 (1.6) | 4.2 (1.3) | 2.4 (1.5) |
mean (standard deviation)
median (quartile 1 – quartile 3)
The results of the conditional logistic regression models (adjusted for prior MI, NYHA class, and diabetes) for each of the miRNAs tested can be found in Supplemental Table 1. Of the 18 miRNAs evaluated, miR-150-5p, miR-29a-3p, and miR-30a-5p were each significantly associated with an increased risk of SCD (p trend <0.05, Figure 1) with ORs of 2.03 (95% CI, 1.12-3.67), 1.93 (95% CI, 1.07-3.50) and 0.55 (95% CI, 0.31-0.97), respectively, for individuals with miRNA levels in the 3rd versus 1st tertile (Figure 1). When miRNA levels were modeled as above vs. below the median, all three miRNAs were each independently associated with risk of SCD (Figure 2). When combined as part of a multiple miRNA biomarker score, an unfavorable level of all three miRNAs (above the median for miR-150–5p and miR-29a-3p; below the median for miR-30a-5p) was associated with a 4.8-fold increased risk of SCD (95% CI 1.59 – 14.51; p=0.006).
Figure 1: miRNAs and Risk of Sudden Cardiac and/or Arrhythmic Death.
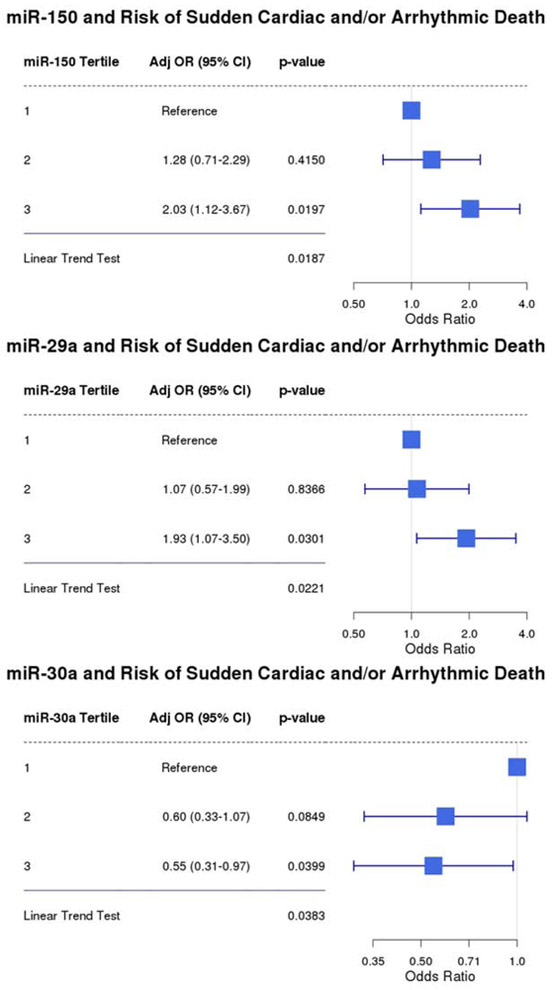
Conditional Logistic Regression Model adjusted for prior MI, NYHA class, and history of diabetes
Figure 2. (Central Illustration): miRNAs and Risk of Sudden Cardiac and/or Arrhythmic Death.
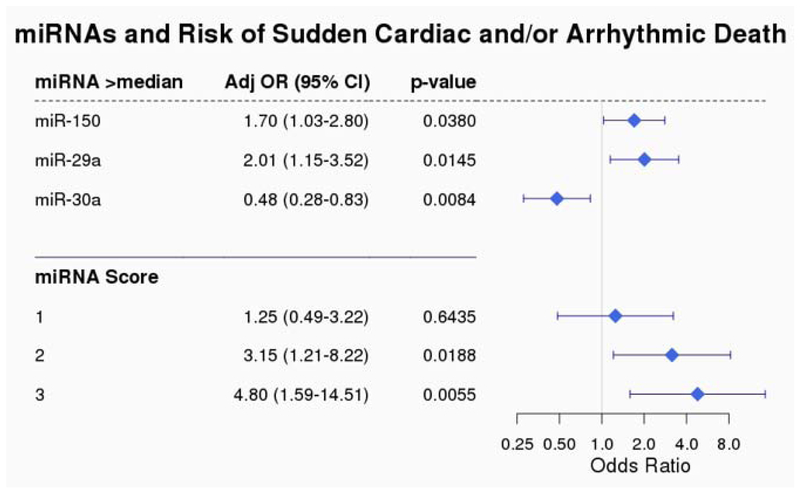
Conditional Logistic Regression Model adjusted for prior MI, NYHA class, history of diabetes miR-150-5p, miR-29a-5p, and miR-30a-5p. Levels above the median for each of the miRNAs is associated with risk of SCD. When combined into a multi-marker risk score, unfavorable levels of all three miRNAs is associated with a 4.8 fold increased risk of SCD.
Spearman correlation coefficients were calculated to evaluate the relationship between the three significant miRNAs in the 234 controls. Mir-150-5p and miR-29a-3p were moderately correlated (r=0.43, p<0.0001), and miR-29a-3p and miR-30a-5p were modestly correlated (r=0.30, p<0.0001), whereas miR-150-5p and miR-30a-5p were not (r=0.06, p=0.38).
Bioinformatics based approach: Network Reconstruction and Enrichment Analysis
Targets of the 3 SCD-associated miRNAs produced a subnetwork of 160 target gene nodes and 210 edges (Figure 3). The set of 160 target genes defined by network reconstruction were used as input for enrichment analysis. Ontology and pathway terms were selected from the significant results based on low redundancy and relevance. These selected terms were visualized as a bar graph (Figure 4). The key pathways identified are related to fibrosis, inflammation, and apoptosis/cell death; each of which have been related to ventricular arrhythmias and/or SCD. Given the potential associations between the miRNAs and pathways known to be associated with ventricular arrhythmias, an additional exploratory analysis was performed to evaluate the relationship between the three miRNAs (as above/below the median) and SCD in a limited subset of 21 cases where ventricular tachycardia (VT) or ventricular fibrillation (VF) was documented at the time of SCD. When compared with 40 matched controls, miR-150-5p was significantly associated with risk of VT/VF (OR 38.1 [95% CI 2.6-552.2; p=0.0076), whereas miR-29a-3p and miR-30a-5p were not. When modeled as continuous variables, miR-150-5p and miR-30a-5p were each significantly associated with risk of VT/VF (respective ORs of 5.18 [95% CI 1.27-21.09; p=0.02] and 0.05 [95% CI 0.004 – 0.62; p=0.02] per unit increase), whereas miR-29a-3p was not (OR 0.97 [95% CI 0.33-2.84; p=0.95] per unit increase).
Figure 3: Network interactions with experimentally determined targets of miR-150-5p, miR-29a-3p, and miR-30a-5p.
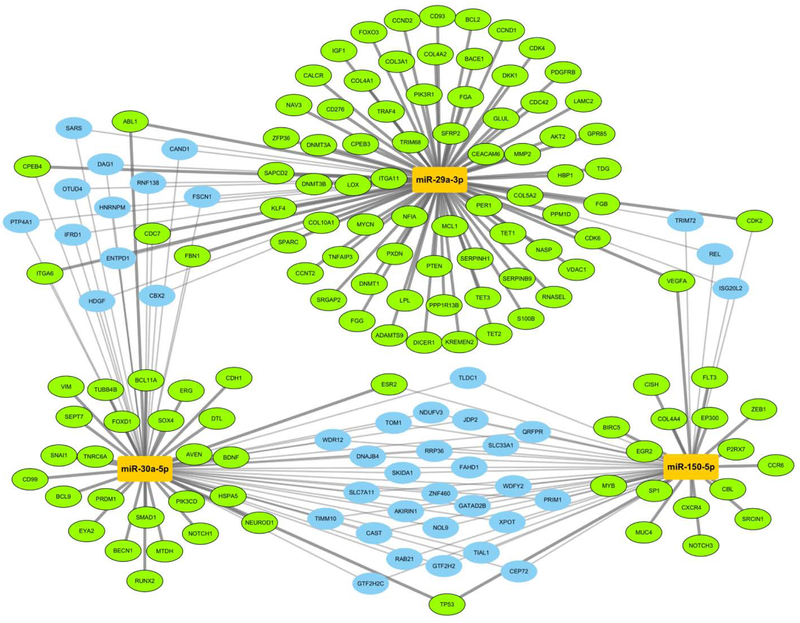
Gene targets (ovals) of the three miRNAs (orange rectangles) are connected by strong (thick) and weak (thin) evidence types according to miRTarBase. This subnetwork view focuses on 160 targets that either of have strong evidence for interacting with only one miRNA (green, radial layout), weak evidence to two or more miRNAs (blue, interstitial layout), or a mix of both (green, interstitial layout).
Figure 4: Enrichment analysis for ontology terms and pathways related to miR-150-5p, miR-29a-3p, and miR-30a-5p targets.
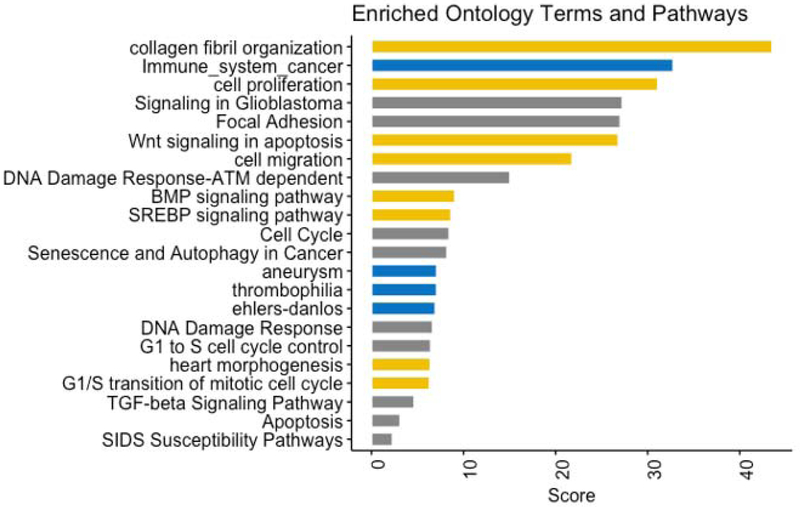
Combing results for Gene Ontology: Biological Process (gold), Jensen Disease Ontology and OMIM (blue), and WikiPathways (gray), the bar graph shows the enrichment score calculated as −log(p value) * Z score.
Plasma miRNA associations with cytokines
In a subset of controls (n=57, baseline characteristics in Supplemental Table 2), miR-150-5p was inversely correlated with IL-10 (r=−0.32, p=0.03), whereas miR-29a-3p and miR-30a-5p were each correlated with IL-6: r=0.32, p=0.01; and r=0.28, p=0.03 respectively. None of the miRNAs were correlated with TNF-α or MCP-1 (Supplemental Table 3).
Plasma miRNAs and bone marrow derived monocytes
Given the bioinformatics-imputed association of these miRNAs and inflammation, we measured levels of miR-150-5p, miR-29a-3p, and miR-30a-5p in murine bone marrow derived monocytes polarized to M1 (pro-inflammatory) or M2 (reparative) sub-types (supplemental figure 4). While miR-150-5p is expressed in these cells, surprisingly it does not appear to be secreted in extracellular vesicles, suggesting that the source of circulating miR-150 is from some other cellular source. In contrast, both miR-29a-3p and miR-30a-5p levels were elevated in the cellular lysates from bone marrow derived monocytes. Interestingly, miR-29a-3p appears to originate from M1 polarized (inflammatory) monocytes, whereas miR-30a-5p appears to originate from M2 polarized (reparative) monocytes, consistent with the idea that higher levels of miR-29a-3p may be associated with pathologic processes, whereas higher levels of miR-30a-5p may be associated with protective ones. Furthermore, miR-29a-3p was also elevated in the exosomal fraction of media, suggesting that miR-29a-3p may be secreted in extracellular vesicles by M1 macrophages.
Discussion
In this contemporary and well phenotyped nested case-control study of individuals with CHD, we evaluated the association between plasma miRNAs and SCD over 3.6 years of mean follow-up. From a list of 18 candidate miRNAs curated from our previous discovery efforts to identify RNA biomarkers for post-myocardial infarction remodeling,(7) or previously linked with cardiovascular disease,(15-17) we identified 3 plasma miRNAs that were independently associated with SCD; miR-150-5p, miR-29a-3p, and miR-30a-5p. These miRNAs were modestly correlated, and when combined into a multiple miRNA biomarker score (based on values being above vs. below the median), an unfavorable level of all three miRNAs was associated with a 4.8-fold increased risk of SCD.
To our knowledge, our study is the first to demonstrate an association between specific circulating miRNAs and SCD risk. The potential to more efficiently identify CHD patients at high risk of SCD is a key unmet need that has been underscored by guidelines and working groups.(3) Given that the majority of participants (94%) in the study did not fulfill current guidelines for ICDs based on LVEF and NYHA class, these miRNAs may be helpful in advancing SCD risk prediction in this broad population where SCD risk stratification measures are lacking and needed.(3) Since miRNAs may also regulate specific pathophysiologic pathways associated with ventricular arrhythmias,(30) circulating miRNAs appear to be promising candidate markers for more precise risk stratification, and may also shed light towards possible pharmacologic targets for novel preventive therapies.
We have previously identified miR-150-5p, miR-29a-3p, and miR-30a-5p to be associated with adverse left ventricular remodeling after myocardial infarction,(7) and the association between these miRNAs and SCD observed in our current study serves as potential validation for these earlier findings. Furthermore, miR-150-5p, miR-29a-3p, and miR-30a-5p have each been linked to pathophysiologic pathways (apoptosis, fibrosis, and inflammation) thought to predispose to both adverse remodeling and ventricular arrhythmias.(30,31) These miRNAs were also found to be correlated with inflammatory cytokines in our exploratory analysis, with miR-150-5p being inversely correlated with the anti-inflammatory cytokine IL-10, whereas miR-29a-3p and miR-30a-5p were each directly correlated with the pro-inflammatory cytokine IL-6. These data provide support for possible involvement of these diverse mechanistic pathways in the pathophysiology of SCD(8,31). These miRNAs appear to be associated with different pathways (through previous experimental and our current bioinformatic analyses), and only modestly correlated with each other, likely reflecting the broad pathways related to SCD (such as fibrosis, inflammation or electrical remodeling). Furthermore, the modest correlation likely accounts for the additive value of each miRNA in the combined biomarker score.
Of these miRNAs, miR-150 has recently been reported to predict cardiovascular death in CHD patients enrolled in the large AtheroGene study(10), and the association between miR-150 and SCD observed in our study may provide a mechanism underlying this association. Furthermore, miR-150 has been shown to be actively secreted in extracellular vesicles from monocytes in response to various stressors,(32) and has previously been implicated in reactive oxygen species (ROS)-mediated cardiac myocyte apoptosis(33) and inflammation (higher levels of IL-6, MCP-1, TNF-α; and lower levels of the anti-inflammatory cytokine IL-10).(20) Given the association with adverse outcomes across multiple populations using different platforms for miRNA measurement, miR-150 appears to be a promising marker of SCD risk.
Additionally, we identified that miR-29a-3p was predicted to target genes related to fibrosis, another pathophysiologic process associated with ventricular arrhythmias and SCD.(9,30) Higher levels of miR-29a have previously been associated with increased LV hypertrophy and fibrosis in patients with hypertrophic cardiomyopathy(34) and adverse LV remodeling in patients post MI.(35) Finally, like other members of the miR-30 family,(36) miR-30a-5p appears to be part of an adaptive/protective response to pathologic stressors including inflammation, and may serve to limit propagation of the inflammatory cascade.(21) Both miR-29a-3p and miR-30a-5p appear to originate (at least in part) from bone marrow derived monocytes, with our data suggesting that miR-29a-3p is in extracellular vesicles. Interestingly, the specific type of bone marrow derived monocytes that miR-29a-3p (M1 polarized) and miR-30a-5p (M2 polarized) appear to originate from fit with the concept that miR-29a-3p may be associated with a pathologic process, whereas miR-30a-5p may be associated with a protective/adaptive one.
Study Limitations
Our study has several limitations. Although rigorous and widely accepted methods of adjudication were used, the possibility of death misclassification cannot be excluded. Nonetheless, compared to other studies of SCD,(13) our study cohort has the significant advantage of adjudication by a clinical end point committee. Second, our power to both detect associations and to adequately adjust for multiple testing was limited by the small number of SCD cases. The low incidence of SCD in patients with relatively preserved ejection fraction, and the lack of available cohorts with detailed adjudication of SCD in this population prevented us from performing a second validation study. Thus, for all these reasons, we urge cautious interpretation of our results. While the potentially mechanistic associations observed through both bioinformatics and experimental based approaches are reassuring, the results must be interpreted accordingly. Additionally, because this nested case-control study matched participants by established risk predictors of SCD (ie age and LVEF), we could not evaluate the additional discriminative ability of the miRNAs (either individually or as part of a score) when added to established predictors of SCD. Prior to clinical translation, these results would need to be verified in a prospective fashion and further validated in an additional patient cohort. Fourth, from a laboratory analysis perspective, we used 3 prespecified miRNAs to normalize miRNA measures. Although the choice of these normalizers was justified based on their relative invariant expression across multiple data sets in a number of different measurement assays, use of a different normalization method could possibly result in differently normalized data and ultimately lead to different results. The cytokine analysis should also be viewed as exploratory and hypothesis generating, although the results lend support to the concept that these miRNAs may associate with inflammatory pathways. Finally, our observational study does not address whether the miRNAs play an active and/or causative role in the pathways related to SCD or whether they are a sequela or epiphenomenon.
Conclusion
In patients with CHD, plasma levels of miR-150-5p, miR-29a-3p, and miR-30a-5p were each independently associated with the risk of SCD in patients not traditionally identified as high risk for SCD and thus not candidates for ICD therapy. These miRNAs, individually or as part of a multi-marker risk score, may have the potential to enhance SCD risk prediction, and warrant further study.
Supplementary Material
Acknowledgements
This publication was supported by the National Institutes of Health Common Fund’s exRNA Communication Program.
Funding:
This work was supported by a research grant from the National Heart, Lung, and Blood Institute (R01HL091069, R01HL122547); the National Institutes of Health Common Fund’s exRNA Communication Program (UH3-TR000901); St Jude Medical Inc and St Jude Medical Foundation; MGS was supported by the John S. Ladue Memorial Fellowship from Harvard Medical School.
Abbreviations
- SCD
sudden cardiac and/or arrhythmic death
- CHD
coronary heart disease
- miRNA
microRNA
- ICD
implantable cardioverter defibrillator
- LVEF
left ventricular ejection fraction
- VT
ventricular tachycardia
- VF
ventricular fibrillation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Relationship with industry:
RVS is a consultant for MyoKardia, Inc., Amgen, Inc., Best Doctors, Inc., and KOLGroups, Inc., none of which participated in this study. SD is a founding member of Dyrnamix which had no role in this study.
Social Media Summary
We identified 3 miRNAs, miR-150-5p, miR-29a-3p, and miR-30a-5p, to be associated with an increased risk of sudden death. Further studies are warranted to evaluate whether these miRNAs can identify individuals who might benefit from specific preventive therapies such as ICDs.
There are no conflicts for any of the authors related to design, execution or analysis of the experiments presented in this manuscript. CMA reports being a consultant for MyoKardia and Sanofi and receiving grants from St Jude Medical, National Institutes of Health, Abbott, and Roche Diagnostics outside the submitted work.
Competency in medical knowledge:
Our study demonstrates a relationship between circulating microRNAs and risk of SCD in patients with CHD. We identified 3 microRNAs, miR-150-5p, miR-29a-3p, and miR-30a-5p (previously associated with structural remodeling of the heart) to be associated with an increased risk of SCD. These miRNAs seem to be related to plausible mechanisms of SCD including inflammation, fibrosis, and apoptosis.
Translational Outlook
Further studies are warranted to evaluate whether these microRNAs can identify individuals who might benefit from specific preventive therapies such as implantable cardioverter defibrillators.
References:
- 1.Wellens HJ, Schwartz PJ, Lindemans FW et al. Risk stratification for sudden cardiac death: current status and challenges for the future. Eur Heart J 2014;35:1642–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Myerburg RJ, Goldberger JJ. Sudden Cardiac Arrest Risk Assessment: Population Science and the Individual Risk Mandate. JAMA Cardiol 2017;2:689–694. [DOI] [PubMed] [Google Scholar]
- 3.Al-Khatib SM, Stevenson WG, Ackerman MJ et al. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2017. [DOI] [PubMed] [Google Scholar]
- 4.Havmoller R, Chugh SS. Plasma biomarkers for prediction of sudden cardiac death: another piece of the risk stratification puzzle? Circ Arrhythm Electrophysiol 2012;5:237–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wieneke H, Svendsen JH, Lande J et al. Polymorphisms in the GNAS Gene as Predictors of Ventricular Tachyarrhythmias and Sudden Cardiac Death: Results From the DISCOVERY Trial and Oregon Sudden Unexpected Death Study. J Am Heart Assoc 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santovito D, Weber C. Zooming in on microRNAs for refining cardiovascular risk prediction in secondary prevention. Eur Heart J 2017;38:524–528. [DOI] [PubMed] [Google Scholar]
- 7.Danielson KM, Shah R, Yeri A et al. Plasma circulating extracellular RNAs in left ventricular remodeling post-myocardial infarction. EBioMedicine 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hussein AA, Gottdiener JS, Bartz TM et al. Inflammation and sudden cardiac death in a community-based population of older adults: the Cardiovascular Health Study. Heart Rhythm 2013;10:1425–32. [DOI] [PubMed] [Google Scholar]
- 9.Halliday BP, Gulati A, Ali A et al. Association Between Midwall Late Gadolinium Enhancement and Sudden Cardiac Death in Patients With Dilated Cardiomyopathy and Mild and Moderate Left Ventricular Systolic Dysfunction. Circulation 2017;135:2106–2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karakas M, Schulte C, Appelbaum S et al. Circulating microRNAs strongly predict cardiovascular death in patients with coronary artery disease-results from the large AtheroGene study. Eur Heart J 2017;38:516–523. [DOI] [PubMed] [Google Scholar]
- 11.Phan D, Aro AL, Reinier K et al. Left Ventricular Geometry and Risk of Sudden Cardiac Arrest in Patients With Severely Reduced Ejection Fraction. J Am Heart Assoc 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chatterjee NA, Moorthy MV, Pester J et al. Sudden death in coronary heart disease patients without severe systolic dysfunction. JAMA Cardiol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Myerburg RJ. Cardiac and Noncardiac Causes of Apparent Sudden Arrhythmic Deaths: Shadows in a Spectrum. Circulation 2018;137:2701–2704. [DOI] [PubMed] [Google Scholar]
- 14.Hinkle LE, Thaler HT. Clinical classification of cardiac deaths. Circulation 1982;65:457–64. [DOI] [PubMed] [Google Scholar]
- 15.Bronze-da-Rocha E MicroRNAs expression profiles in cardiovascular diseases. Biomed Res Int 2014;2014:985408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuwabara Y, Ono K, Horie T et al. Increased microRNA-1 and microRNA-133a levels in serum of patients with cardiovascular disease indicate myocardial damage. Circ Cardiovasc Genet 2011;4:446–54. [DOI] [PubMed] [Google Scholar]
- 17.Marques FZ, Vizi D, Khammy O, Mariani JA, Kaye DM. The transcardiac gradient of cardio-microRNAs in the failing heart. Eur J Heart Fail 2016;18:1000–8. [DOI] [PubMed] [Google Scholar]
- 18.Chapin SC, Appleyard DC, Pregibon DC, Doyle PS. Rapid microRNA profiling on encoded gel microparticles. Angew Chem Int Ed Engl 2011;50:2289–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shah R, Ziegler O, Yeri A et al. MicroRNAs Associated With Reverse Left Ventricular Remodeling in Humans Identify Pathways of Heart Failure Progression. Circ Heart Fail 2018;11:e004278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo NG T; Wang D; Fu Y; . MicroRNA-150 regulates lipid metabolism and inflammatory response. J Metabolic Syndrome 2013;2. [Google Scholar]
- 21.Jiang X, Xu C, Lei F et al. MiR-30a targets IL-1alpha and regulates islet functions as an inflammation buffer and response factor. Sci Rep 2017;7:5270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lefrancais E, Mallavia B, Zhuo H, Calfee CS, Looney MR. Maladaptive role of neutrophil extracellular traps in pathogen-induced lung injury. JCI Insight 2018;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsu SD, Lin FM, Wu WY et al. miRTarBase: a database curates experimentally validated microRNA-target interactions. Nucleic Acids Res 2011;39:D163–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riutta A, Hanspers K, Pico AR. Target interaction finder. GitHub Repository, 2017. [Google Scholar]
- 25.Subramanian SL, Kitchen RR, Alexander R et al. Integration of extracellular RNA profiling data using metadata, biomedical ontologies and Linked Data technologies. J Extracell Vesicles 2015;4:27497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shannon P, Markiel A, Ozier O et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 2003;13:2498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chou CH, Shrestha S, Yang CD et al. miRTarBase update 2018: a resource for experimentally validated microRNA-target interactions. Nucleic Acids Res 2018;46:D296–D302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuleshov MV, Jones MR, Rouillard AD et al. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res 2016;44:W90–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kutmon M, Riutta A, Nunes N et al. WikiPathways: capturing the full diversity of pathway knowledge. Nucleic Acids Res 2016;44:D488–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang B, Lu Y, Wang Z. Control of cardiac excitability by microRNAs. Cardiovasc Res 2008;79:571–80. [DOI] [PubMed] [Google Scholar]
- 31.Wang M, Li S, Zhou X et al. Increased inflammation promotes ventricular arrhythmia through aggravating left stellate ganglion remodeling in a canine ischemia model. International Journal of Cardiology 2017;248:286–293. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y, Liu D, Chen X et al. Secreted monocytic miR-150 enhances targeted endothelial cell migration. Mol Cell 2010;39:133–44. [DOI] [PubMed] [Google Scholar]
- 33.Li X, Kong M, Jiang D, Qian J, Duan Q, Dong A. MicroRNA-150 aggravates H2O2-induced cardiac myocyte injury by down-regulating c-myb gene. Acta Biochim Biophys Sin (Shanghai) 2013;45:734–41. [DOI] [PubMed] [Google Scholar]
- 34.Roncarati R, Viviani Anselmi C, Losi MA et al. Circulating miR-29a, among other up-regulated microRNAs, is the only biomarker for both hypertrophy and fibrosis in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol 2014;63:920–7. [DOI] [PubMed] [Google Scholar]
- 35.Zile MR, Mehurg SM, Arroyo JE, Stroud RE, DeSantis SM, Spinale FG. Relationship between the temporal profile of plasma microRNA and left ventricular remodeling in patients after myocardial infarction. Circ Cardiovasc Genet 2011;4:614–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Melman YF, Shah R, Danielson K et al. Circulating MicroRNA-30d Is Associated With Response to Cardiac Resynchronization Therapy in Heart Failure and Regulates Cardiomyocyte Apoptosis: A Translational Pilot Study. Circulation 2015;131:2202–2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


