Abstract
The brain is worthy of study because it is in charge of behavior. A flurry of recent technical advances in measuring and quantifying naturalistic behaviors provide an important opportunity for advancing brain science. However, the problem of understanding unrestrained behavior in the context of neural recordings and manipulations remains unsolved, and developing approaches to addressing this challenge is critical. We discuss considerations in computational neuroethology — the science of quantifying naturalistic behaviors for understanding the brain — and propose strategies to evaluate progress. We point to open questions that require resolution and call upon the broader systems neuroscience community to further develop and leverage measures of naturalistic, unrestrained behavior, which will enable us to more effectively probe the richness and complexity of the brain.
In brief:
The goal of computational neuroethology is to understand the relationship between the brain and purposive behavior that evolved under natural selection. Technology is transforming how we measure and model naturalistic behavior, affording new insight into brain function.
Leveraging naturalistic behavior to explore brain function
Two distinct traditions have shaped how neuroscientists think about behavior in the lab (Gomez-Marin et al., 2014). Comparative psychology studies the ability of the brain to generate behaviors in response to rewards and punishments (Domjan, 1987). This perspective has led to a large body of work in which animals are trained in the laboratory to respond to specific sensory cues. By combining these behavioral methods with neural recordings and manipulations, modern neuroscience is now addressing fundamental questions about how task-related variables are encoded in the brain, and about how neurons and circuits generate task-related behaviors (Jazayeri and Afraz, 2017; Krakauer et al., 2017). Animals are typically trained to produce simple actions (e.g., to lick a port, or to reach for a target) that are easy to measure and readily correlated with neural activity patterns. In addition, animals are often (but not always) physically restrained, both to facilitate neural recordings and to avoid spurious movements that complicate inferences about the meaning and purpose of measured patterns of neural activity.
Ethology, on the other hand, has historically focused on natural behavior (Tinbergen, 1951; Tinbergen, 1963). The underlying hypothesis of ethology is that exposing the structure of behavior — how behavior in the natural environment is built from components and organized over time in response ecologically-relevant stimuli — will yield insights into how the brain creates behavior (Simmons and Young, 1999; Tinbergen, 1951). However, traditional ethology has focused on observing the behavior of animals without neural recordings or interventions. Exploring neural activity during the expression of “naturalistic” behaviors (which here is taken to mean behaviors that are representative of actions generated during complex real-world tasks, like exploring new environments, obtaining food, finding shelter, and identifying mates, and therefore largely self-motivated and expressed freely without physical restraint; see Glossary) has the potential to reveal how the brain does much of what the brain evolved to do. Furthermore, ethology has revealed that many naturalistic behaviors are built from components that are probabilistically expressed as sequences, a feature that in principle can be used to reveal dependencies in both neural activity and actions across multiple timescales, and to illuminate how longer-lasting brain states specify the moment-to-moment contents of behavior (Baerends, 1976; Manoli et al., 2006; Tinbergen, 1951).
We argue that understanding the relationship between brain and behavior will require bringing the traditions of psychology and ethology together, towards an integrated study of naturalistic behavior spanning a gamut of questions from brain mechanisms to evolution. Despite the compromises imposed by training and/or restraint, the comparative psychology framework for relating neural activity to behavior has yielded, and will continue to yield, key insights into the mechanisms that support perception, govern decision making and regulate action (Juavinett et al., 2018; Panzeri et al., 2017). Technical advances — ranging from the development of virtual reality-based tasks to the use of touchpads for operant conditioning — are integrating increasingly naturalistic behaviors into training-based experiments (Mar et al., 2013; Minderer et al., 2016). Furthermore, the development of deep-learning based frameworks for tracking e.g., paws during reaching (see below) has revealed the behavioral richness and variability that underlies even simple behavioral reports like pellet grabs or lever presses (Graving et al., 2019; Guo et al., 2015; Mathis et al., 2018; Nath et al., 2019; Pereira et al., 2019). In contrast, the technical and conceptual challenges of relating naturalistic, unrestrained and minimally shaped behavior to neural activity are formidable and only beginning to be addressed, leaving that area ripe for further development.
In the past decade, a field we now call “computational ethology” has begun to take shape. It involves the use of machine vision and machine learning to measure and analyze the patterns of action generated by animals in preparations designed to evoke ethologically-relevant behaviors (Anderson and Perona, 2014). Technical progress in statistical inference and deep learning, the democratization of high-performance computing (due to falling hardware costs and the ability to rent GPUs and CPUs in the cloud), and new and creative ideas about how to apply technology to measuring naturalistic behavior have dramatically accelerated progress in this research area.
Approaches from computational ethology may be particularly important in a future in which we have access to recordings from many thousands of neurons, with the richness of neural codes on full display. Indeed today, nearly all of the neurons in the brains of the worm C. elegans and the zebrafish D. Rerio can be recorded simultaneously, thereby allowing a large fraction of the brain’s neural dynamics to be observed (Cong et al., 2017; Kim et al., 2017; Nguyen et al., 2016; Symvoulidis et al., 2017; Venkatachalam et al., 2016). Given that — even in restrained animals — subtle movements can have pervasive effects on neural dynamics, obtaining unbiased and holistic measurements of an animal’s behavior will be important to understanding dense neural activity (Musall et al., 2019; Stringer et al., 2019). We further propose that making sense of high-dimensional neural data will ultimately be facilitated by access to behaviors whose complexity and dimensionality is of a similar order as the neural space that is concurrently being surveyed. This perspective makes urgent the need to develop methods that combine analysis of naturalistic, unrestrained behavior with measures of neural activity. Here we review progress towards a science of computational neuroethology — the problem of relating naturalistic behavior to neural recordings or manipulations in order to better understand brain function.
Challenges in computational neuroethology
Studying neural activity as animals behave freely has led to some of the most exciting discoveries in brain science over the past 50 years, including place cells (Hartley et al., 2014), grid cells (Rowland et al., 2016), replay (Foster, 2017), mechanisms of non-associative learning (Kandel et al., 2014), and the escape response (Medan and Preuss, 2014). However, to approach these problems without the benefit of restraint (i.e., head fixation), researchers have, by necessity, focused on those behaviors that are simplest to quantify. For example, an animal’s head direction can be quantified by a single angle; its location can be quantified by an (x,y) pair of spatial coordinates; and a gill-withdrawal or escape reflex can be quantified by a single binary variable. These behaviors are trivial to estimate by eye, easy to plot on a graph, and unambiguous in their timing.
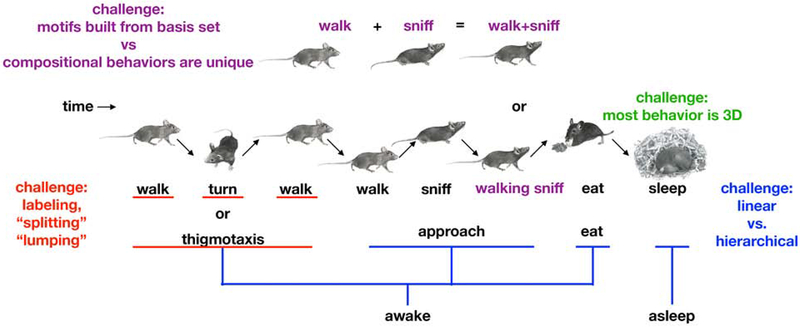
Unfortunately, most naturalistic behaviors — from exploration of a novel environment to mating rituals — are not well captured by simple parameters like centroid position or head direction (Fig. 1). First, naturalistic behaviors involve coordinated movements of limbs, facial features and other body parts, and often include rapid changes in the animal’s three-dimensional pose, i.e., its dynamics. Understanding dynamics requires simultaneous measurements of how the positions of many different body parts evolve over time. Second, although naturalistic behaviors are often built from stereotyped components (referred to variously as behavioral “motifs,” “modules”, “syllables,” “primitives,” and “movemes”), labeling behaviors on a moment-to-moment basis is made complicated by spatial and temporal variability in the execution of each behavior (Anderson and Perona, 2014; Berman et al., 2014; Flash and Hochner, 2005; Tinbergen, 1951; Wiltschko et al., 2015). This variability, taken with the observation that many spontaneous behaviors evolve continuously over time, makes it difficult to assign labels and explicit start and stop times to individual actions. Third, because naturalistic behaviors can be described at different levels of granularity, there are many simultaneously valid descriptions of an animal’s behavior at any given time point (Dawkins, 1976). For example, replaying a video of a mouse in slow-motion will reveal kinematics of limb movement as the animal turns its body, but the same movements when played on fast-forward will reveal whether the animal is in the midst of sleep, courtship or escape behaviors. Finally, identifying actions is complicated by the ability of animals to do more than one thing at once. Some of the authors of this review, for example, claim to be able to walk and chew gum at the same time. This parallelism undermines descriptions of action in which each moment in time is associated with only a single behavior.
Fig 1. Challenges in computational ethology.
A characteristic sequence of behaviors exhibited by a mouse in its home cage as it thigmotaxis around the walls, approaches some food, eats and then sleeps (center, mouse cartoons). Several key challenges face any segmentation of continuous behavior into components are illustrated. First, the behavior of most model organisms occurs in three dimensions (green). Second, behaviors need to be labeled, which raises the problem of “lumping” versus “splitting” (red); for example, mice thigmotax, a behavior in which mice exhibit locomotion and turning behaviors that are deterministically sequenced to generate an action where the animal circumnavigates its cage. Is thigmotaxis a singular behavior (because its elements are deterministically linked during its expression), or it is a sequences of walking, turning and walking behaviors? Third, should behavior be considered at a single timescale that serially progressed, or instead considered a hierarchical process organized at multiple timescales simultaneously (blue). Fourth, when the mouse is sniffing and running at the same time, is that a compositional behavior whose basis set includes “run” and “sniff,” or is “running+sniffing” a fundamentally new behavior (purple)?
It is no coincidence that naturalistic behavior shares many characteristics with neural activity: high dimensionality, time-evolving structure, variability and organization at multiple temporal and spatial scales (Panzeri et al., 2015). However, efforts to develop methods to understand the spontaneous behavior of untethered animals have lagged substantially behind those to measure neural activity. In part this is because of a longstanding focus on a limited set of assays that provide low-dimensional descriptions of complex patterns of action (e.g. the three-chamber social assay, the open field test, and the tail-suspension test), whose aim is to probe the psychological state of the animal, and which therefore have been heavily used in the pharmaceutical industry (Crawley, 2003, 2008). The availability of commercial tracking software has contributed to a perception that automatically measuring and characterizing naturalistic behavior is either a trivial problem, or one that has already been solved (Spink et al., 2001; Verbeek, 2005). To the contrary, a number of outstanding challenges need to be addressed if we seek to leverage the strengths of naturalistic behavior to better understand brain function. Below we describe the current conceptual and technical framework that supports efforts in computational neuroethology; we later discuss additional future progress that remains to be made.
Technology for quantifying behavior
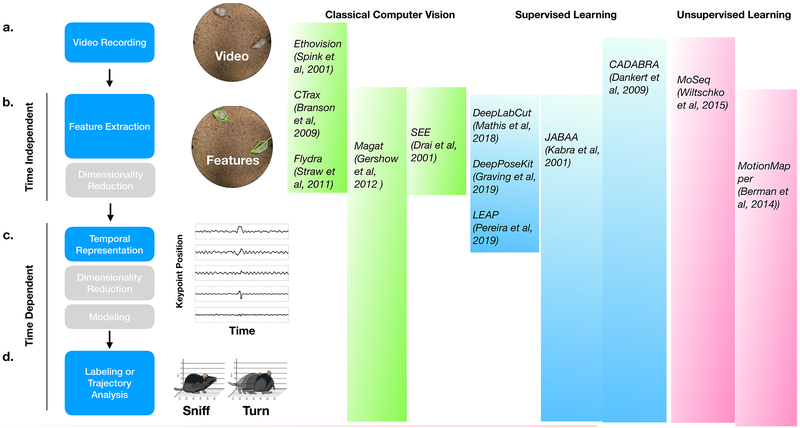
Animal behavior inherently evolves over time. Capturing its time-varying structure requires measuring features of an animal’s body and pose, tracking those features over time, and then identifying patterns that correspond to different movements, behaviors, or behavioral states (Fig. 2). Given the pervasive use of cameras as sensors, here we largely consider this process from the perspective of video data of worms, flies, fish and mice; however, all of the described steps have been applied to other animals (like bacteria and birds) and other types of datastreams (like accelerometry or ultrasonic vocalizations) (Berg, 1975; Coffey et al., 2019; Markowitz et al., 2013; Van Segbroeck et al., 2017; Venkatraman et al., 2010). It is also important to note that although video of behaving animals is most often obtained from a single viewpoint using standard video cameras (Drai and Golani, 2001; Spink et al., 2001; Verbeek, 2005), recent innovations (including depth cameras and image fusion approaches) allow researchers to track freely-behaving animals to be tracked via video in three dimensions (Günel et al., 2019; Hong et al., 2015; Nath et al., 2019; Straw et al., 2011; Wiltschko et al., 2015).
Fig. 2. Typical stages in a behavioral analysis pipeline.
Conceptual steps involved in any behavior analysis pipeline (left) with references to published example pipelines that are openly available for use (right). Shaded region indicates the steps that each method implements. a. Animal behavior is recorded. b. Features are extracted. Features may be interpretable, e.g. a mouse’s paw position; or abstract e.g. an algorithmically defined weighted set of pixels. Automatic feature extraction can roughly be divided into algorithms that use classical computer vision feature detection, supervised learning, or unsupervised learning. High-dimensional descriptions of behavior features can optionally be approximated with fewer-dimensions via dimensionality reduction. c. To leap from features to behavior requires first representing the temporal dynamics of the features. Feature dynamics can be represented in either the time- or frequency domain. Additional dimensionality reduction can optionally be performed at this stage. d. The resulting temporal dynamics are organized into behavior which can be either discrete or continuous. For discrete behavior representations, feature dynamics are clustered and labeled (e.g. ‘Sniffing’ or ‘Turning’) using either supervised or unsupervised machine learning. For continuous descriptions of behavior, trajectories through behavior space are analyzed and interpreted e.g. using dynamical systems models.
Feature extraction
A mouse’s pose (i.e., its posture), a bird’s beak, and the angle of a fly’s wing are all features that may be relevant to an analysis of behavior. When studying insect locomotion, for example, one may wish to measure the position of each insect leg in relation to the other legs (Wilson, 1966). Two decades ago, this meant recording video of the insect and manually identifying the location of each of its legs or joints at each point in time (Strauss and Heisenberg, 1990). Early automated techniques required painting the animal’s joint or leg, adding a small marker or dye, or using sophisticated imaging modalities like frustrated total internal reflection to highlight points of interest. Image processing algorithms could then automatically extract the location of these points of interests, obviating the need for manual identification (Bender et al., 2010; Kain et al., 2013; Mendes et al., 2013; Petrou and Webb, 2012).
Markerless approaches are an important alternative to these methods, as they enable automatic extraction of specific kinematic features without the use of surrogate markers. For animals whose anatomy is relatively simple, like worms or drosophila larvae, simple computer vision algorithms can automatically identify such features without any human supervision (Broekmans et al., 2016; Gershow et al., 2012; Liu et al., 2018b; Stephens et al., 2008). For animals whose anatomy is more complex, “supervised” machine learning approaches — in which humans identify which features to track and provide labeled data used to train a machine learning-based feature detection algorithm — can be used to facilitate feature identification and tracking from video. Platforms that use this strategy (including CADABRA and JAABA) have been widely used in a variety of contexts (Branson et al., 2009; Dankert et al., 2009; Kabra et al., 2013). Similarly, the LocoMouse platform uses supervised machine learning techniques to recognize the precise position of paws, joints, the snout, and the tail in mice walking on a track (Machado et al., 2015). Recently, there has been an explosion in artificial neural network-based algorithms that can track human-identified anatomical keypoints in video (Graving et al., 2019; Mathis et al., 2018; Pereira et al., 2019). By tracking several of these keypoints in parallel, aspects of an animal’s pose can be estimated using a limited amount of training data. These methods are gaining fast adoption for their versatility, ease of use, and accuracy. However, their use is limited to situations in which the relevant keypoints are readily identifiable by human researchers, and by themselves automated pose estimators are not sufficient to produce a classification of an animal’s behavior or reveal its time-varying structure.
Image properties can also be analyzed without human supervision to identify statistical regularities that recur in the pixel data themselves which can capture or reflect important features of an animal’s behavior. Such “unsupervised” algorithm-driven approaches can extract features that are recognizable to a human (like the degree to which the left wing is lifted, or a grimace in a mouse face) and can also yield features that a human would be hard-pressed to name (Berman et al., 2014; Musall et al., 2018; Stringer et al., 2019; Wiltschko et al., 2015). Because it is not always clear which behavioral features are most relevant or informative in a particular experiment, the ability to identify unexpected features is a potential benefit of taking this sort of approach.
Large numbers of behavioral features are often required to capture behavior within a given experiment. The high-dimensionality of behavioral data can be cumbersome, and thus dimensionality reduction is commonly used after feature extraction to generate a lower-dimensional dataset that approximates the original feature set. Many such approaches — like Principal Components Analysis (PCA) — are familiar from their use in neural data (Pang et al., 2016). Given a set of data about behavioral features, PCA identifies an ordered set of principal components (PCs), each of which constitutes a different “axis” representing progressively less variance present in the data. A reduced subset of these PCs can be used to approximately reconstruct the underlying features using fewer dimensions than present in the original data. In C. elegans, for example, the animal’s centerline captures most of the worm’s behavior (Croll, 1975) but reconstructing this sinusoidal centerline requires tens of (x,y) coordinates. When transformed into a new basis set defined by PCA, these centerlines can be represented nearly as well by just three numbers, aka the “Eigenworm” (Stephens et al., 2008), thus providing a more tractable representation for use in subsequent analysis. It is important to note that the largest sources of behavioral variance may not reveal those features that most meaningfully describe a particular behavior.
Temporal Dynamics
Imagine a video of a worm crawling on an agar plate. A single frame of video provides no information about the animal’s velocity, whether it is accelerating or decelerating, or even whether it is moving forward or backward. Multiple sequential frames, on the other hand, reveal the evolution of the worm’s position and pose, which can be used as building blocks to create a description of behavior. Behavioral representations can incorporate information about time by considering behavioral features in either the time domain or the frequency domain. Most commonly, analysis of behavior takes place exclusively in the time domain — researchers consider how a given behavioral feature (after extraction and dimensionality reduction) evolves over time, and then use that information to characterize behavioral dynamics or to identify behavioral motifs. For example, behavioral motifs in the worm have been identified by using a sliding time window to capture worm postures (Brown et al., 2013).
Alternatively, behavioral features can be first transformed into frequency space before considering how behavior evolves over time. Whereas a time domain analysis would represent a beating wing as the position of the wing over time, a frequency domain analysis would instead represent the same moving wing as its characteristic wing beat frequency. The MotionMapper platform (see below) takes this approach to format video data before downstream identification of behavioral motifs (Berman et al., 2016; Klibaite et al., 2017; Liu et al., 2018a; Pereira et al., 2019; Wang et al., 2016). Frequency domain analyses are well suited for representing cyclic motions (e.g., walking gaits, wing flapping, head swinging), and simplify the problem of identifying relationships between behaviors that are similar but out of phase (e.g., a walking bout starting with the right foot and a walking bout starting with the left foot). Importantly, both time- and frequency-domain approaches require the experimenter to select a relevant timescale at which behavior is thought to be organized. In the time domain, this timescale defines the duration or distribution of durations of each behavioral motif, and in the frequency domain this timescale sets the lowest frequency that can be represented. In both cases the choice of timescale plays an important role in determining whether an animal’s action is naturally represented as a single contiguous entity or as separate behavioral motifs.
Organizing data and assigning labels
Behavior can be described as being continuous (i.e., following a trajectory through a behavioral space), discrete, or a combination of the two (Fuchs, 1967). For example, the behavior of a worm exploring its environment can be elegantly described using dynamical systems approaches as a continuous trajectory through posture space (Stephens et al., 2010). Similarly, brief elements of action (frequently corresponding to semantically low levels of behavior) can follow simple trajectories that are best described as continuous processes (Katsov et al., 2017; Wiltschko et al., 2015). However, animals can also express one or more of a large number of possible discrete behaviors that are stereotyped, distinct from each other, and are organized at a variety of timescales. Behavior therefore often requires labeling to identify which behavioral motifs or states are being expressed at a particular time point: e.g., was the animal awake or asleep, mating or fighting? Labeling also provides access to information about when particular behaviors started and stopped, and reveals the sequences of actions taken during an experiment. Traditionally, labeling has been done by hand — ethologists inspected either raw video of behavior or extracted behavioral feature data, and then segmented those data using their own implicit criteria to label the types of actions being exhibited by a given subject. This sort of hand-labeling, when used to build transition probability matrices, leads to the generation of ethograms, the lingua franca of traditional ethologists (Baerends, 1976; Tinbergen, 1951). Relatively low-tech heuristic methods have automated some of these human intuitions — for example, when a mouse’s nose is high enough to break a laser beam placed in an open field, the mouse is labeled as “rearing” (Crawley, 2003).
Machine learning is now revolutionizing the process of labeling behavioral data and generating ethograms. As with feature extraction, automated labeling often takes advantage of supervised learning approaches, in which machine learning algorithms are trained to identify particular behaviors based upon a set of human-curated examples (Branson et al., 2009; Dankert et al., 2009; Kabra et al., 2013; Machado et al., 2015; Mueller et al., 2019; Ravbar et al., 2019). JAABA, for example, includes an interface that allows researchers to indicate which video snippets correspond to a particular behavior of interest, and which can then be used to train a classifier to identify when that behavior occurred (Kabra et al., 2013). Multiple classifiers can be built for different behaviors of interest, allowing researchers to flexibly extract complex information about when different behaviors are expressed (Robie et al., 2017).
Alternatively, unsupervised methods take advantage of statistical regularities in behavioral feature data to identify repeatedly-used motifs of action (Berman, 2018; Brown and De Bivort, 2018; Nater et al., 2010). Two exemplar methods (of many) highlight the different paths that can be taken in using the inherent structure of behavioral data to define a description of behavior. MotionMapper takes as its input video data, and then after pre-processing (which includes a PCA-based dimensionality reduction step, and the reformatting of these dimensionally-reduced data into a frequency domain representation), these data are further dimensionally reduced by projecting them into a two-dimensional space through a non-linear method called t-stochastic nearest neighbor (tSNE) embedding (Berman et al., 2016; Klibaite et al., 2017; Liu et al., 2018a; Pereira et al., 2019; Wang et al., 2016). All unsupervised behavioral methods have to address the problem of “lumping” versus “splitting” — the granularity at which a given behavioral datastream is broken up into parts. MotionMapper addresses this challenge by subjecting the behavioral tSNE embeddings to watershed-based clustering, which identifies reused motifs of action (which appear as peaks in the tSNE embedding) as well as behaviors that are less stereotyped (which appear as valleys). A comparison of clustering-based unsupervised behavioral classification methods can be found in (Todd et al., 2017).
Motion Sequencing (MoSeq), on the other hand, takes advantage of a classic method in time-series analysis: the hidden Markov model (HMM)(Eddy, 2004). MoSeq (reviewed in (Datta, 2019)) takes as its input 3D data obtained from depth cameras, and then uses statistical learning techniques to fit a hierarchical variant of the HMM, in which each behavioral component is modeled as a continuous auto-regressive process in pose space, while the components themselves (whose duration distributions are flexibly modeled based upon the data) are modeled using an HMM (Johnson et al., 2016; Markowitz et al., 2018; Pisanello et al., 2017; Wiltschko et al., 2015). The fitting procedures used by MoSeq allow it to flexibly learn the identity, number and ordering of 3D behavioral components (called “syllables”) for any given dataset. MoSeq — like all HMMs — is a generative model that after training can generate a synthetic 3D mouse (whose realism can be measured via statistical comparisons to held-out data). The fitting procedure underlying MoSeq explores different descriptions of behavior — “lumping” some movements together and “splitting” others — as it seeks an representation for behavior that best predicts held-out behavioral data.
It should be clear that all behavior pipelines — supervised or unsupervised — require the experimenter to make choices. These choices include which behavioral features to quantify, whether to analyze behavior in the time or frequency domain, which timescales to consider, whether behavior will be represented as continuous, discrete or both, and if discrete how to address the problem of granularizing behavior into elements. Optimal choices should reflect the nature of the ethological task the animal is solving, and the inherent structure of the data; as our ability to record neural activity improves, these choices should be made with an eye towards maximizing our ability to understand the relationship between brain and behavior (perhaps at the expense of understanding behavior per se).
Emerging methods in computational neuroethology are yielding insight into the relationship between brain and behavior.
Methods for monitoring naturalistic behavior in the laboratory are largely in their infancy, and yet have already made contributions to understanding the relationship between brain and behavior; below we review highlights. Note that for brevity here we largely focus on analysis of freely-behaving animals during neural recording or neural manipulation, although where relevant we point to examples of interesting analysis in more structured settings.
Forward screens to identify neurons and circuits for behavior
In Drosophila, modern genetics has yielded collections of driver lines that, either alone or in combination, afford specific access to nearly every neuron in the fly brain (Jenett et al., 2012). The near-simultaneous development of these driver libraries and methods for automated behavioral classification is enabling a new type of forward screen, one that seeks to identify neurons that are necessary or sufficient for particular behaviors or behavioral components. This strategy has been particularly successful at identifying and dissecting neural circuit that underlie fly behaviors (Albin et al., 2015; Hoopfer et al., 2015; von Philipsborn et al., 2011). For example, to identify neurons linked to aggression, researchers expressed neural actuators or inhibitors (such as the thermogenetic activator TrpA1 or the constitutive inhibitor Kir2.1) in specific neural populations, and used CADABRA and/or JAABA to quantify the behavioral influence of the targeted neuron (Asahina et al., 2014; Duistermars et al., 2018; Hoopfer et al., 2015). Because these automated methods dramatically reduce the time it takes to score videos, thousands of lines could be quickly analyzed, enabling both screens focused on likely neurons of interest (i.e., neurons that express neuromodulators) as well as screens that survey the entire brain. This work has revealed key roles for tachykinin-expressing neurons, octopamine receptor-expressing neurons, P1 neurons, and fruitless-positive aSP2 neurons in driving or modulating aggression, and has identified population of neurons that control discrete behavioral modules that collectively comprise threat-related behaviors (Asahina et al., 2014; Duistermars et al., 2018; Hoopfer et al., 2015). It has also revealed epistasis relationships between different identified neural populations, for example, that P1 neurons and octopamine receptor-expressing neurons likely functionally converge upon aSP2 neurons (Watanabe et al., 2017). Similar optogenetic-based screens that leverage machine vision at scale have been used to identify neural circuits related to fly feeding and courtship (Albin et al., 2015; von Philipsborn et al., 2011).
Recent work has built upon this success to characterize the effects of neural activation and silencing on fly behavior more broadly (Robie et al., 2017). In this work, 20 male and female flies were imaged in parallel; video data was then used to identify a set of 128 hand-engineered features describing the behavior of each fly, which in turn was submitted to a set of supervised classifiers (built using JAABA) to identify specific behaviors (e.g., walking, chasing, mating). The behavioral consequences of thermogenetically activating each of more than 2000 Gal4 lines (whose anatomy was previously characterized) was assessed using this method. The output of this process was a map linking sub-regions of the fly brain with particular behaviors; this map identified likely relationships (such as that between a series of visual areas and walking behaviors, and between fruitless-positive neurons and wing extension) as well as an online resource to mine the data for further hypothesis generation.
These screens demonstrate the power of scalable machine vision-based methods to reveal the neural substrates of behavior. Complementary experiments have also been carried out using unsupervised behavioral classification methods. For example, a variant of hierarchical clustering has been used to characterize the set of behavioral components and sequences that make up Drosophila larvae behavior before and after channelrhodopsin-based actuation of more than 1000 Gal4 lines (Vogelstein et al., 2014). This experiment identified a set of 29 atomic movements falling into four basic categories (e.g., avoid, escape, backup, turn), and generated a look-up table linking each line to its characteristic behavioral consequence. A related set of experiments has been performed in the adult fly through the use of MotionMapper (Berman et al., 2014; Cande et al., 2018). Flies whose descending neurons (which connect the CNS to effector motor centers in the ventral nerve cord) were optogenetically activated exhibited changes in each of the 5 behavioral categories identified by MotionMapper; furthermore, these experiments revealed dependencies between the optogenetically-induced behaviors and the behaviors that was expressed immediately prior (Cande et al., 2018).
Probing sensorimotor processing
Rich descriptions of innate animal behavior are proving critical for probing mechanisms of sensorimotor processing. For example, much of our understanding of neural mechanisms underlying sensory-driven navigation in drosophila larvae comes from machine vision-based behavioral quantification. Drosophila larvae exhibit a stereotyped head-swing behavior that probes the sensory environment before the animal commits to a new movement direction. Computer vision-based behavior quantification systems such as (Gershow et al., 2012) automatically detect these head swings, and have been used to demonstrate that larvae temporally compare light intensites or chemical concentrations during head swings to chart future movements (Gepner et al., 2015; Gershow et al., 2012; Hernandez-Nunez et al., 2015; Kane et al., 2013; Schulze et al., 2015). Combined behavioral measurements and cell-specific inactivations have also identified specific lateral neurons downstream of the Bolwig’s Organ that are crucial for mediating phototaxis (Kane et al., 2013). Similarly, work spanning many labs (reviewed in (Calabrese, 2015) ) has used automated measures of behavior to reveal neural loci where chemosensory signals are integrated with photosensory signals for driving multi-sensory behavioral decisions (Gepner et al., 2015; Hernandez-Nunez et al., 2015; Schulze et al., 2015).
One common thread to these experiments is the combined use of optogenetic stimulation of sensory neurons and simple neural models to link sensory inputs to the animal’s head-swings and turns. By automatically detecting a different set of behaviors — “hunching” and “bending” — Jovanic and colleagues conducted a complete neural dissection of the larvae’s aversive response to mechanical stimulation, which included functionally and anatomically mapping a set of specific reciprocal inhibitory circuits from sensory input to motor output (Jovanic et al., 2016; Ohyama et al., 2013); this work illustrates how the automated analysis of naturalistic behavior can be used in concert with connectomics, electrophysiology, optogenetics, genetics and modeling to probe a complete sensorimotor circuit. Similar work in C. elegans using a variety of methods (including clustering and MotionMapper) has quantified behavioral responses to the optogenetic activation of a nociceptive neuron, and to characterize the innate behaviors expressed by thousands of individual worms during optogenetic stimulation of their mechanosensory neurons (Liu et al., 2018a; Schwarz et al., 2015).
Automated behavior measures have also revealed new insights into the sensorimotor transformations driving social behaviors. For example, during courtship, adult Drosophila detect the sound and behavior of potential mates to dynamically coordinate its response. Male song production was long thought to be a fixed action pattern and any variability in the song was considered noise. Careful measures of social behaviors during song production instead showed that the details of the song, such as choice of pulse versus sine, could be quantitatively predicted from the kinematic details of inter-animal behavior (Coen et al., 2014). Further measures of song and inter-animal behavior during calcium imaging revealed new forms of song, detailed neural correlates of male song production, and the neural coding of male song in the female auditory system (Clemens et al., 2018; Clemens et al., 2015) as well as internal latent states that are correlated with neural processing (Calhoun et al., 2019). Generalized linear models were used throughout this body of work to mathematically relate inter-animal behavior, song features and neural coding (Clemens and Murthy, 2017).
Relating global brain dynamics to naturalistic behavior
Large-scale recording techniques now allow patterns of neural actively to be measured from hundreds to thousands of neurons at cellular resolution throughout the brain, providing an unprecedented view into neural computations and representational strategies (Williamson et al., 2019). While such experiments still sub-sample neural activity in rodents or non-human primates, progress is being made in methods characterize the global pattern of brain dynamics exhibited during naturalistic behavior in a variety of simpler model organisms. This work finds its origins in brain-scale recordings made at cellular resolution via calcium imaging in small transparent organisms like larval zebrafish or C. elegans during partial or complete immobilization. Investigations of whole-brain activity during fictive locomotion in zebrafish have been crucial for mapping and identifying functional roles of new brain areas, including those for motor adaptation learning (Ahrens et al., 2012), turning behavior (Dunn et al., 2016); and for discerning sensory vs motor areas (Chen et al., 2018). In C. elegans, measures of fictive locomotion inferred from interneuron activity has revealed stereotyped low-dimensional neural state-space trajectories that explain over 75% of the variance of brain-wide calcium activity during immobilization (Kato et al., 2015).
In the past few years, such whole-brain imaging approaches have been adapted to freely moving zebrafish and C. elegans (Cong et al., 2017; Kim et al., 2017; Nguyen et al., 2016; Symvoulidis et al., 2017; Venkatachalam et al., 2016). Recordings from neurons in the head of larval zebrafish during innate foraging behaviors have identified brain regions related to the animal’s swim bout angle and bout speed (Kim et al., 2017); similarly, recordings have been made during prey-capture to relate neural activity to the distance between the zebrafish and its paramecium prey, or to features of the animal’s eye convergence angle and head orientation (Cong et al., 2017). And in C. elegans, combining whole-brain recordings during unrestrained movements with whole body posture analysis has revealed new insights into where and how neural activity codes for locomotion (Scholz et al., 2018); in this work, a neural decoder of behavior was used to show that during unrestrained behavior only a small fraction of the brain’s neural dynamics (<5%) are explained by locomotory behavior, suggesting that the rest of the worm brain’s activity may be involved in other computations.
Probing the relationship between pose dynamics and motor circuits
Animals interact with the world by generating kinematics that support precise task-related movements (like grasping an object) and large-scale changes in pose (like rearing). Computational approaches have played a prominent role in quantifying the detailed kinematics of reaching or grasping behaviors in head-fixed primates and rodents, and to relate measured kinematics to neural dynamics (e.g.,(Churchland et al., 2012; Guo et al., 2015)). Improved measures of behavior now allow kinematic measurements during the self-generated locomotory behavior of unrestrained rodents. For example, LocoMouse has been used to recently demonstrate that the temporal and spatial aspects of adaptation to a split-belt treadmill are dissociated, and that a key locus within cerebellum is required to compensate for lateralized speed perturbations (Darmohray et al., 2019). While LocoMouse estimates gait parameters in a specialized apparatus (in which a camera images the mouse from two orientations), related future experiments exploring locomotion in the open field or other contexts will likely take advantage of deep learning-based point tracking methods like LEAP, DeepLabCut, and DeepPoseKit (Graving et al., 2019; Mathis et al., 2018; Nath et al., 2019; Pereira et al., 2019).
While point-tracking methods are well suited to monitor the position of easily-segmented features like paws or the base of the tail, clearly identifying keypoints can be difficult over much of the surface of many animals (like furry rodents). Alternative methods may therefore play an important complementary role in measuring the global pose dynamics expressed by animals as they generate naturalistic movements (Meyer et al., 2018; Venkatraman et al., 2010). For example, accelerometer data has also been used to parse spontaneous mouse behavior into motifs through the use of unsupervised affinity propagation-based techniques; this behavioral clustering has been combined with miniscope recordings to show that individual behavioral motifs are encoded by ensembles of direct and indirect striatal medium spiny neurons whose variance is systematically related to morphological similarities and differences in 3D behavior (Klaus et al., 2017).
Depth cameras have also been used to directly measure 3D information about an animal’s pose dynamics, and to use that information to explore brain circuits regulating action. By combining MoSeq with electrophysiological, multi-color photometry and miniscope methods, neural correlates for 3D behavioral syllables have recently been identified in dorsolateral striatum (DLS) (Markowitz et al., 2018). These experiments reveal a systematic fluctuation in neural activity associated with transitions between behavioral syllables over time, and an obligate role for DLS in generating appropriate behavioral sequences both during exploration and odor-guided naturalistic behaviors. Furthermore, MoSeq has been combined with optogenetic stimulation to reveal the differential consequences of activating the motor cortex, the dorsal and the ventral striatum (Pisanello et al., 2017; Wiltschko et al., 2015). These results are consistent with similar results recently obtained using marker-based approaches to explore the relationship between 3D posture and activity in posterior parietal cortex (Mimica et al., 2018). Future benchmarking will reveal the trade-offs (if any) between direct measurements of 3D pose with specialized hardware (like depth cameras) and indirect estimates using more accessible hardware (like standard CCDs used to generate 3D keypoint tracking through image fusion, or marker-based approaches).
Addressing the challenges that remain: a way forward for behavior-guided discovery in the brain
As is made plain by the examples above, although important progress is being made in relating brain activity to naturalistic behaviors, there are a host of conceptual and technical issues that remain. Behavior manifests itself as complex moment-to-moment trajectories; yet, it is driven by often long-lasting internal states like e.g., sleep, wakefulness, hunger, thirst, as well as external states such as the availability of resources. Thus a description of natural behavior must be hierarchically organized in time, and it remains unclear how to best identify behavioral hierarchies in a given dataset (Berman, 2018; Tao et al., 2019). Furthermore, identifying this sort of hierarchical structure requires large-scale data, and in particular, experimental set-ups and analysis pipelines that enable long-term assessment of behavior; this contrasts with most current naturalistic behavioral experiments, in which animal behavior is measured for minutes rather than hours (but see (Jhuang et al., 2010; Ohayon et al., 2013)).
Second, there is the problem of context — the richness of naturalistic behaviors is most fully observed in complex environments where sensory cues and affordances evoke the complete behavioral repertoire of the animal, and yet naturalistic behaviors in the lab are largely assessed in impoverished arenas. Future improvements in machine vision, virtual reality and robotics should allow animals explore increasingly realistic contexts while researchers monitor spontaneous naturalistic behavior (e.g., (Del Grosso and Sirota, 2019; Meyer et al., 2018)). One humble and yet not fully addressed challenge is segmentation — if a lab animal is in an arena with a lot of stuff (as will be the case if e.g., a mouse is imaged across its lifetime in its homecage during enrichment), it can be difficult to reliably tell the surface of the animal apart from objects around the animal. This is most difficult when considering social behaviors, which requires pose estimation of more than one animal at a time. Recent improvements in deep learning are helping to address this problem, as has the use of 3D cameras, but additional technical work will be required if we are to better understand the behavioral diversity of animals as they interact with realistic environments (which include conspecifics and predators) (Hong et al., 2015; Markowitz et al., 2018; Nath et al., 2019).
Finally, gaining access to multi-scale relationships between brain and behavior requires an understanding of how brain activity itself is organized and evolves over time. While much progress has been made in developing methods to infer structure in high-dimensional neural data, the field is still in flux (Cunningham and Yu, 2014; Williamson et al., 2019). As a consequence, most of the methods currently used to relate brain activity to complex naturalistic behavior — like linear regression — are drawn from the standard toolkit. An important road forward will be to build methods and model classes in which structure in the neural data and naturalistic behavioral data are jointly inferred (Glaser and Kording, 2016). Ideally, joint inference will allow trial-by-trial variability in neural data to be related to trial-by-trial behavioral performance (e.g, the kinematics that underlie the expression of any given instance of an identified behavioral motif, which in the context of naturalistic behavior can be considered a “trial”). It remains to be seen whether the tools used to solve the problem of organizing behavioral data will be the same as those used to jointly infer joint structure in neural and behavioral data.
Given that human-imposed design decisions suffuse any quantification of animal behavior, what types of behavioral representations are maximally informative for understanding brain function? Historically, a given behavioral analysis method has been judged to be successful if it can be used to discern a difference after an experimental manipulation. This standard arose from the tradition of behavioral neurogenetics, where observable behavioral differences are used to support forward screens whose goal is to identify individual genes that contribute to the generation of behavior (Benzer, 1971). Importantly, this standard ducks the key conceptual questions in computational neuroethology: how do we choose which distance metrics to use to tell us whether any two behaviors are similar or different, how do we balance “lumping” versus “splitting,” and in a given situation how do we decide whether to characterize behavior as continuous, discrete, or both?
We argue that future development of methods in computational neuroethology should adopt a set of simple design principles. These principles do not address all the key questions posed above, but instead are intended to prompt an ongoing conversation about how we measure behavior:
-
Timescales. Humans have traditionally applied labels to animal behavior that operate on timescales of seconds or longer — think running, grooming, rearing — because that is the timescale at which perception and language most conveniently intersect. The availability of automated behavioral analysis methods circumvents this limit. Thus behavioral measurements and segmentations should ideally include the timescale at which neural variability is expected to occur. Furthermore, since slow behaviors can reflect fast neural activity, and rapid behavioral events can reflect long-term neural dynamics, when possible behavioral descriptions should organize information hierarchically to facilitate multi-scale neurobehavioral discovery.
Successfully meeting this imperative for many naturalistic behaviors will require ongoing improvements both in behavioral measurements (e.g., faster and higher resolution cameras, better tracking, improved data compression) and in analytical methods for characterizing behavior at multiple timescales at once.
Interpretability. Descriptions of behavior should lend themselves to hypothesis generation. Representations or models whose latent variables can be directly related to neural activity are more useful than those whose underlying variables lack obvious meaning; from this perspective, a behavioral representation can be thought of as “making sense” in light of neural activity, and such representations are useful for generating hypotheses about neural mechanisms underlying action. In a similar vein, representations whose latent variables correspond to a human intuition about behavior are more useful than those that do not; an “Eigenworm” is interpretable by a human and therefore useful for articulating hypotheses about how worm behavior might be organized, whereas PCA over arbitrary collections of behavioral parameters may not yield to human intuition. While artificial neural networks are able to generate excellent predictions about behavior, it is often hard for a human to understand what the network actually learned that enabled it to make a prediction, or to relate what the network learned back to a latent variable that might be detected in the brain. This does not mean eschewing approaches like deep learning — indeed such methods are currently playing a central role in detecting behavioral features — but rather deploying them selectively to support hypothesis generation. One such example is the recent use of a variational autoencoder to reformat raw mouse videos before submission to an interpretable generative model (Johnson et al., 2016).
Prediction. When possible, prediction should be adopted as a standard for judging the quality of behavioral representations. Effective behavioral representations should be able to predict behavior (in those circumstances when behavior is expected to be predictable) or neural activity; conversely, an effective behavioral representation should enable actions to be predicted from neural activity. As neuroscientists, this goal of being able to predict brain from behavior and vice versa strikes to the heart of our motivation for studying behavior. Therefore, prediction quality is a natural arbiter for deciding amongst model parameters or competing behavioral representations. Prediction also affords the possibility of finding the right balance between parsimony and richness, as testing the predictive performance of different models of behavior potentially offers a solution to the problem of “lumping” versus “splitting.” Of course, one fundamental challenge that remains is deciding what sort of predictions are most relevant in a particular experimental context. In the long run, it is likely that multiple types of prediction (including of simultaneously measured neural data, of genomic or transcriptomic data, or of different treatment classes) will be required to fully assess and compare the performance of different behavioral analysis methods.
The future of computational neuroethology
We are now gaining access to powerful tools for recording behavior, including machine learning-based methods to extract key features like joint angles and body postures, and for organizing this information to understand how behavior is structured and evolves over time. These tools will only get better, and enable increasingly dense characterization of action. Thanks to ongoing technical progress in both brain recording and behavioral analysis, we will soon face the serious problem of relating high dimensional time-varying neural data to high dimensional time-varying behavioral data. A major goal for future research will be to identify those behavioral representations that will give us the most insight into how neural circuits create behavior. Meeting this goal will require a dedicated effort on the part of neuroscientists to build tools for characterizing naturalistic behavior with the same vigor and creativity that they have thus far largely reserved for measuring and characterizing neural activity.
In looking towards this future it is helpful to ask what it means to “understand” the relationship between naturalistic behavior and the brain. Psychologists would likely agree that this answer requires a full account of those brain circuits that regulate a given behavior, including testable predictions about how circuit manipulations will affect behavior. Ethologists would wish to understand how behavior helps a given species prosper in its ecological niche, including accounts of how behavior emerges through evolutionary pressures, and arises in each individual through the interplay of genetics and learning. These different levels of explanation are interdependent and equally valid (Barlow, 2012).
And yet, the conceptual difficulties in reaching this understanding may sometimes appear rather daunting (Gomez-Marin et al., 2014; Jazayeri and Afraz, 2017; Krakauer et al., 2017). How do we search the immense brain for the circuits that are relevant for a given naturalistic behavior? How should we interpret the neural signals that we record, some of which may be as complex as the behavior itself and many of which may be irrelevant? Which is the right representation of behavior, and at what temporal and semantic scale should we look? How do we know which behavioral features are meaningful and which are idiosyncratic? How do we relate the goal we presume the animal is pursuing with the observed behavior? And when we finally describe a circuit, how do we think about the computation that it is intrinsically carrying out to support behavior, independently of the circuit details?
We argue that progress in computational neuroethology will require the biologist to think, at times, like an engineer — to propose mechanisms that might allow an animal to reach an ethological goal. Identifying possible mechanisms requires answering three questions that echo Marr’s three levels of analysis of perception (Marr, 1982): First, what ethological goal is any given behavior to meant to address and how shall we measure whether a particular action helps an animal reach its goal? Second, given the available sensory inputs and physical constraints, what computational strategies could allow an animal to reach its goal? Third, how should algorithms that support goal-oriented behavior be implemented in the hardware of a nervous system? This engineering mindset is illustrated by the book ‘Vehicles’ by Valentino Braitenberg, which suggests that testable hypotheses about how the brain creates naturalistic task-driven behaviors can be generated by attempting to design simple automata that can accomplish that same task (Braitenberg, 1986).
Importantly, this process of mapping goals to algorithms produces normative models. These models are valuable in many ways: to generate hypotheses about which neural signals to look for in the brain with relation to behavior, to evaluate whether the observed neural signals are sufficient for a particular task, to assess which behavioral features are noise and which are diagnostic of the main design choices and trade-offs (for example sensitivity to input noise vs. circuit complexity), and to taxonomize behaviors in the context of a goal at different scales of temporal and semantic resolution. The use of methods in computational neuroethology — whether focused on simple trained behaviors or complex, unrestrained patterns of action, whether done in single animals or at scale — will teach us about the structure of behavior; extracting meaning from these experiments and understanding how behavior meaningfully relates to brain activity will require a notion of the animal’s goals in generating a behavior, and in the long run, normative models for how a brain might accomplish that goal. The engineering approach has been very fruitful in understanding perception and learning (Nakayama and Shimojo, 1992; Navalpakkam et al., 2010; Reichardt et al., 1983; Shadlen and Newsome, 1998; Sutton and Barto, 1998); when taken with the conceptual and technical advances in computational neuroethology, we predict this approach will play an equally powerful role in the study of naturalistic behavior.
Fig. 3. Pose estimation.
Recent advances in artificial neural networks allow identifiable points on an animal’s surface (e.g., joints) to be automatically detected from images with minimal human supervision, even when animals interact or are in rich and complex backgrounds (Graving et al., 2019; Mathis et al., 2018; Pereira et al., 2019). Examples of a. flies, b. giraffes and c. mice are shown from (Pereira et al., 2019 and unpublished).
Acknowledgements
We are unfortunately unable to comprehensively cite the rich literature on this topic due to space limitations — we thank the many talented colleagues working in this area for inspiration. This review was prompted by symposium sponsored by the Simons Collaboration on the Global Brain. SRD, AL, DJA and PP are supported by grants from the Simons Collaboration on the Global Brain. SRD is supported by NIH grants U24NS109520, RO1DC016222, U19NS108179, and U19NS112953. AL is supported by NSF CAREER Award 1845137.
GLOSSARY
- Behavioral representation
A quantitative distillation of any aspect of the time-varying behavior exhibited by an animal in an experiment. Such representations can vary in form from classical ethograms to low-dimensional plots capturing the trajectory of an animal in space
- Naturalistic
as with “ethologically-relevant,” (see below) there are many definitions for naturalistic, and indeed most experiments in behavioral neuroscience can justifiably be argued to be naturalistic at some level. Here, we take the word “naturalistic” to mean behaviors that are representative of actions generated during complex real-world tasks, like exploring new environments, obtaining food, finding shelter, and identifying mates; naturalistic behaviors as referred to herein are also largely self-motivated and expressed freely without physical restraint. This definition is meant to distinguish such behaviors from those that are imposed by researchers on animals through overtraining, or those that are more constrained due to e.g., head fixation (although, as mentioned above, there are contexts in which those types of behaviors are quite reasonably also referred to as “naturalistic”)
- Ethologically-relevant
as with “naturalistic,” (see above), “ethologically-relevant” is an adjective whose meaning is in the eye of the beholder; again, this term can be appropriately applied to many kinds of behavioral experiments, including those in which animals are subject to training and restraint. Here, we take “ethologically-relevant” to mean a set of behaviors that support tasks animals have to address as part of the existential challenge of living in a particular environmental niche
- Behavioral label
a behavioral label is a descriptor applied to an epoch of behavioral data. Behavioral labels can cover descriptions of behavior at many levels of granularity, and run the gamut from “a twitch of motor unit 72 in the soleus muscle” to “hibernating.”
- Behavioral motif
a stereotyped and re-used unit of movement. The terms “motif,” “moveme,” “module,” “primitive” and “syllable” have all been used interchangeably, and none of these terms is linked to a rigorous definition of the spatiotemporal scale at which a unit behavior is organized. Similarly, action and behavior here and elsewhere are used to refer to collections of units of behavior, but again, there is no rigorous line that separates these or related terms. Perona and Anderson have argued for a taxonomy in which moveme is the simplest movement associated with a behavior, an action is a sequence of movemes, and an activity is a species-characteristic set of movemes and actions (Anderson and Perona, 2014)
- Behavioral sequence
an epoch in which more than one behavioral motif is expressed; sequences cof motifs an be either deterministic (e.g., motif A always follows motif B), or probabilistic (e.g., motif A follows motif B fifty percent of the time)
- Artificial neural network
class of machine learning algorithms that operate by simulating a network of simplified neurons. These often are trained through supervised learning
- Behavioral feature
a relevant attribute of an animal that, when observed over time, helps define behavior. For example, the location of a paw
- Trajectory
the motion of a point in space over time. This can be either a physical object, like an animal’s paw through real space, or it can be abstract like the animal’s current behavior state as it travels through behavior state space
- Behavior analysis pipeline
a set of algorithms that, all together, take a raw recording of behavior (usually video) and returns high level representations of the animal’s behavior, such as trajectories, motifs, sequences or behavior labels
- Dimensionality
number of variables required to describe a dataset. For example the (x,y) position of a mouse paw has dimensionality of 2. A complete description of its pose requires many more variables and thus has higher dimensionality
- Dimensionality reduction
mathematically approximating a dataset using fewer than the original dimensions. For example, Stephens et al 2008 showed that a worm’s centerline originally requiring 100 points could be well-approximated by three numbers. Dimensionality reduction usually requires a change in the representation of the data
- Embedding
a type of dimensionality reduction that takes data, which is assumed to exists on a high manifold, and unwraps it into a lower dimensional space where it is more easily visualized. T-SNE and U-MAP are two examples of embeddings that are gaining adoption in the life sciences
- Temporal dynamics
here, how behavior features change over time. These can be mathematically represented in the time-domain, or in the frequency-domain
- Key-point
region of interest in an image, such as an animal’s joint or appendage
- Supervised learning
a computer algorithm that learns to performs a task, such as identifying an animal’s joint in an image, through human provided examples
- Behavior state-space
a mathematical space (possibly high dimensional) such that a point in this space corresponds to a specific instance of animal behavior
- Behavior map
visualization of a behavior state-space, usually refers to a two-dimensional visualization. Convenient for visualizing how an animal’s behavior is organized into clusters or how it might differ from another animal
- Principal Components Analysis (PCA)
A mathematical change of basis that is commonly used for dimensionality reduction in many behavioral analysis pipelines. PCA identifies a new basis set in which to represent data. A truncated version of this dataset serves as a useful lower-dimensional approximation of the original data
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
SRD is a is a founder of Syllable Life Sciences.
References
- Ahrens MB, Li JM, Orger MB, Robson DN, Schier AF, Engert F, and Portugues R (2012). Brain-wide neuronal dynamics during motor adaptation in zebrafish. Nature 485, 471–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albin SD, Kaun KR, Knapp J-M, Chung P, Heberlein U, and Simpson JH (2015). A Subset of Serotonergic Neurons Evokes Hunger in Adult Drosophila. Current biology : CB 25, 2435–2440. [DOI] [PubMed] [Google Scholar]
- Anderson DJ, and Perona P (2014). Toward a science of computational ethology. Neuron 84, 18–31. [DOI] [PubMed] [Google Scholar]
- Asahina K, Watanabe K, Duistermars BJ, Hoopfer E, González CR, Eyjólfsdóttir EA, Perona P, and Anderson DJ (2014). Tachykinin-Expressing Neurons Control Male-Specific Aggressive Arousal in Drosophila. Cell 156, 221–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baerends GP (1976). The functional organization of behaviour. Animal Behavior 24, 726–738. [Google Scholar]
- Barlow HB (2012). Possible Principles Underlying the Transformations of Sensory Messages In Sensory Communication (The MIT Press; ). [Google Scholar]
- Bender JA, Simpson EM, and Ritzmann RE (2010). Computer-assisted 3D kinematic analysis of all leg joints in walking insects. PloS One 5, e13617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzer S (1971). From the gene to behavior. JAMA 218, 1015–1022. [PubMed] [Google Scholar]
- Berg HC (1975). Chemotaxis in bacteria. Annual review of biophysics and bioengineering 4, 119–136. [DOI] [PubMed] [Google Scholar]
- Berman GJ (2018). Measuring behavior across scales. BMC biology 16, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman GJ, Bialek W, and Shaevitz JW (2016). Predictability and hierarchy in Drosophila behavior. Proceedings of the National Academy of Sciences 113, 11943–11948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman GJ, Choi DM, Bialek W, and Shaevitz JW (2014). Mapping the stereotyped behaviour of freely moving fruit flies. Journal of the Royal Society, Interface / the Royal Society 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braitenberg V (1986). Vehicles: Experiments in synthetic psychology (MIT press; ). [Google Scholar]
- Branson K, Robie AA, Bender J, Perona P, and Dickinson MH (2009). High-throughput ethomics in large groups of Drosophila. Nature methods 6, 451–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekmans OD, Rodgers JB, Ryu WS, and Stephens GJ (2016). Resolving coiled shapes reveals new reorientation behaviors in C. elegans. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AEX, and De Bivort B (2018). Ethology as a physical science. Nature Physics 14, 653–657. [Google Scholar]
- Brown AEX, Yemini EI, Grundy LJ, Jucikas T, and Schafer WR (2013). A dictionary of behavioral motifs reveals clusters of genes affecting Caenorhabditis elegans locomotion. Proceedings of the National Academy of Sciences 110, 791–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese RL (2015). In search of lost scent. eLife 4, e08715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun AJ, Pillow JW, and Murthy M (2019). Unsupervised identification of the internal states that shape natural behavior. bioRxiv, 691196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cande J, Namiki S, Qiu J, Korff W, Card GM, Shaevitz JW, Stern DL, and Berman GJ (2018). Optogenetic dissection of descending behavioral control in Drosophila. eLife 7, 970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Randi F, Leifer AM, and Bialek W (2018). Searching for collective behavior in a small brain. arXiv:181007623 [cond-mat, physics, qbio]. [DOI] [PubMed]
- Churchland MM, Cunningham JP, Kaufman MT, Foster JD, Nuyujukian P, Ryu SI, and Shenoy KV (2012). Neural population dynamics during reaching. Nature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens J, Coen P, Roemschied FA, Pereira TD, Mazumder D, Aldarondo DE, Pacheco DA, and Murthy M (2018). Discovery of a New Song Mode in Drosophila Reveals Hidden Structure in the Sensory and Neural Drivers of Behavior. Current Biology 28, 2400–2412.e2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens J, Girardin CC, Coen P, Guan X-J, Dickson BJ, and Murthy M (2015). Connecting Neural Codes with Behavior in the Auditory System of Drosophila. Neuron 87, 1332–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens J, and Murthy M (2017). The Use of Computational Modeling to Link Sensory Processing with Behavior in Drosophila In Decoding Neural Circuit Structure and Function: Cellular Dissection Using Genetic Model Organisms, Qelik A, and Wernet MF, eds. (Cham: Springer International Publishing; ), pp. 241–260. [Google Scholar]
- Coen P, Clemens J, Weinstein AJ, Pacheco DA, Deng Y, and Murthy M (2014). Dynamic sensory cues shape song structure in Drosophila Nature 507, 233–237. [DOI] [PubMed] [Google Scholar]
- Coffey KR, Marx RG, and Neumaier JF (2019). DeepSqueak: a deep learning-based system for detection and analysis of ultrasonic vocalizations. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 231, 909–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong L, Wang Z, Chai Y, Hang W, Shang C, Yang W, Bai L, Du J, Wang K, and Wen Q (2017). Rapid whole brain imaging of neural activity in freely behaving larval zebrafish (Danio rerio). eLife 6, e28158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN (2003). Behavioral phenotyping of rodents. Comparative medicine 53, 140–146. [PubMed] [Google Scholar]
- Crawley JN (2008). Behavioral phenotyping strategies for mutant mice. Neuron 57, 809–818. [DOI] [PubMed] [Google Scholar]
- Croll N (1975). Components and patterns in the behavior of the nematode Caenorhabditis elegans. Journal of Zoology 176, 159–176. [Google Scholar]
- Cunningham JP, and Yu BM (2014). Dimensionality reduction for large-scale neural recordings. Nature Publishing Group 17, 1500–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dankert H, Wang L, Hoopfer ED, Anderson DJ, and Perona P (2009). Automated monitoring and analysis of social behavior in Drosophila. Nature methods 6, 297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmohray DM, Jacobs JR, Marques HG, and Carey MR (2019). Spatial and Temporal Locomotor Learning in Mouse Cerebellum. Neuron 102, 217–231.e214. [DOI] [PubMed] [Google Scholar]
- Datta SR (2019). Q&A: Understanding the composition of behavior. BMC biology 17, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawkins R (1976). Hierarchical organisation: A candidate principle for ethology In Growing points in ethology (Oxford, England: Cambridge U Press; ). [Google Scholar]
- Del Grosso NA, and Sirota A (2019). Ratcave: A 3D graphics python package for cognitive psychology experiments. Behavior Research Methods 10, 433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domjan M (1987). Comparative psychology and the study of animal learning. Journal of Comparative Psychology 101, 237–241. [Google Scholar]
- Drai D, and Golani I (2001). SEE: a tool for the visualization and analysis of rodent exploratory behavior. Neuroscience and biobehavioral reviews 25, 409–426. [DOI] [PubMed] [Google Scholar]
- Duistermars BJ, Pfeiffer BD, Hoopfer ED, and Anderson DJ (2018). A Brain Module for Scalable Control of Complex, Multi-motor Threat Displays. Neuron 100, 1474–1490.e1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn TW, Mu Y, Narayan S, Randlett O, Naumann EA, Yang C-T, Schier AF, Freeman J, Engert F, and Ahrens MB (2016). Brain-wide mapping of neural activity controlling zebrafish exploratory locomotion. eLife 5, e12741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy SR (2004). What is a hidden Markov model? Nature Biotechnology 22, 1315–1316. [DOI] [PubMed] [Google Scholar]
- Flash T, and Hochner B (2005). Motor primitives in vertebrates and invertebrates. Current opinion in neurobiology 15, 660–666. [DOI] [PubMed] [Google Scholar]
- Foster DJ (2017). Replay Comes of Age. Annual review of neuroscience 40, 581–602. [DOI] [PubMed] [Google Scholar]
- Fuchs AF (1967). Saccadic and smooth pursuit eye movements in the monkey. The Journal of physiology 191, 609–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gepner R, Skanata MM, Bernat NM, Kaplow M, and Gershow M (2015). Computations underlying Drosophila photo-taxis, odor-taxis, and multi-sensory integration. eLife 4, e06229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershow M, Berck M, Mathew D, Luo L, Kane EA, Carlson JR, and Samuel ADT (2012). Controlling airborne cues to study small animal navigation. Nature methods 9, 290–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser JI, and Kording KP (2016). The Development and Analysis of Integrated Neuroscience Data. Frontiers in computational neuroscience 10, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Marin A, Paton JJ, Kampff AR, Costa RM, and Mainen ZM (2014). Big Behavioral Data: Psychology, Ethology and the Foundations of Neuroscience. 1–30. [DOI] [PubMed] [Google Scholar]
- Graving JM, Chae D, Naik H, Li L, and bioRxiv BK (2019). Fast and robust animal pose estimation. biorxivorg, http:--dx.doi.org. - 10.1101 - 620245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günel S, Rhodin H, Morales D, Campagnolo J, Ramdya P, and Fua P (2019). DeepFly3D: A deep learning-based approach for 3D limb and appendage tracking in tethered, adult Drosophila. bioRxiv, 640375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J-Z, Graves AR, Guo WW, Zheng J, Lee A, Rodríguez-González J, Li N, Macklin JJ, Phillips JW, Mensh BD, et al. (2015). Cortex commands the performance of skilled movement. eLife 4, e10774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley T, Lever C, Burgess N, and O’Keefe J (2014). Space in the brain: how the hippocampal formation supports spatial cognition. Philosophical Transactions of the Royal Society B: Biological Sciences 369, 20120510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Nunez L, Belina J, Klein M, Si G, Claus L, Carlson JR, and Samuel AD (2015). Reverse-correlation analysis of navigation dynamics in Drosophila larva using optogenetics. eLife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong W, Kennedy A, Burgos-Artizzu XP, Zelikowsky M, Navonne SG, Perona P, and Anderson DJ (2015). Automated measurement of mouse social behaviors using depth sensing, video tracking, and machine learning. Proceedings of the National Academy of Sciences 112, E5351–5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoopfer ED, Jung Y, Inagaki HK, Rubin GM, and Anderson DJ (2015). P1 interneurons promote a persistent internal state that enhances inter-male aggression in Drosophila. eLife 4, 2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazayeri M, and Afraz A (2017). Navigating the Neural Space in Search of the Neural Code. Neuron 93, 1003–1014. [DOI] [PubMed] [Google Scholar]
- Jenett A, Rubin GM, Ngo T-TB, Shepherd D, Murphy C, Dionne H, Pfeiffer BD, Cavallaro A, Hall D, Jeter J, et al. (2012). A GAL4-driver line resource for Drosophila neurobiology. CellReports 2, 991–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhuang H, Garrote E, Yu X, Khilnani V, Poggio T, Steele AD, and Serre T (2010). Automated home-cage behavioural phenotyping of mice. Nature Communications 1, 68. [DOI] [PubMed] [Google Scholar]
- Johnson M, Duvenaud DK, Wiltschko A, Adams RP, and Datta SR (2016). Composing graphical models with neural networks for structured representations and fast inference. Advances In Neural Information Processing … 29, 2946–2954. [Google Scholar]
- Jovanic T, Schneider-Mizell CM, Shao M, Masson J-B, Denisov G, Fetter RD, Mensh BD, Truman JW, Cardona A, and Zlatic M (2016). Competitive Disinhibition Mediates Behavioral Choice and Sequences in Drosophila. Cell 167, 858–870.e819. [DOI] [PubMed] [Google Scholar]
- Juavinett AL, Erlich JC, and Churchland AK (2018). Decision-making behaviors: weighing ethology, complexity, and sensorimotor compatibility. Current opinion in neurobiology 49, 42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabra M, Robie AA, Rivera-Alba M, Branson S, and Branson K (2013). JAABA: interactive machine learning for automatic annotation of animal behavior. Nature methods 10, 64–67. [DOI] [PubMed] [Google Scholar]
- Kain J, Stokes C, Gaudry Q, Song X, Foley J, Wilson R, and Bivort B.d. (2013). Legtracking and automated behavioural classification in Drosophila. Nature Communications 4, 1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER, Dudai Y, and Mayford MR (2014). The molecular and systems biology of memory. Cell 157, 163–186. [DOI] [PubMed] [Google Scholar]
- Kane EA, Gershow M, Afonso B, Larderet I, Klein M, Carter AR, Bivort B.L.d., Sprecher SG, and Samuel ADT (2013). Sensorimotor structure of Drosophila larva phototaxis. Proceedings of the National Academy of Sciences 110, E3868–E3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato S, Kaplan HS, Schrödel T, Skora S, Lindsay TH, Yemini E, Lockery S, and Zimmer M (2015). Global brain dynamics embed the motor command sequence of Caenorhabditis elegans. Cell 163, 656–669. [DOI] [PubMed] [Google Scholar]
- Katsov AY, Freifeld L, Horowitz M, Kuehn S, and Clandinin TR (2017). Dynamic structure of locomotor behavior in walking fruit flies. eLife 6, e26410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Kim J, Marques JC, Grama A, Hildebrand DGC, Gu W, Li JM, and Robson DN (2017). Pan-neuronal calcium imaging with cellular resolution in freely swimming zebrafish. Nature methods 14, 1107–1114. [DOI] [PubMed] [Google Scholar]
- Klaus A, Martins GJ, Paixao VB, Zhou P, Paninski L, and Costa RM (2017). The Spatiotemporal Organization of the Striatum Encodes Action Space. Neuron 95, 1171–1180.e1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klibaite U, Berman GJ, Cande J, Stern DL, and Shaevitz JW (2017). An unsupervised method for quantifying the behavior of paired animals. Phys Biol 14, 015006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer JW, Ghazanfar AA, Gomez-Marin A, MacIver MA, and Poeppel D (2017). Neuroscience Needs Behavior: Correcting a Reductionist Bias. Neuron 93, 480–490. [DOI] [PubMed] [Google Scholar]
- Liu M, Sharma AK, Shaevitz J, and Leifer AM (2018a). Temporal processing and context dependency in C. elegans response to mechanosensation. eLife 7, e36419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Sharma AK, Shaevitz JW, and Leifer AM (2018b). Temporal processing and context dependency in C. elegans mechanosensation. arXiv:180304085 [physics, q-bio]. [DOI] [PMC free article] [PubMed]
- Machado AS, Darmohray DM, Fayad J, Marques HG, and Carey MR (2015). A quantitative framework for whole-body coordination reveals specific deficits in freely walking ataxic mice. eLife 4, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoli DS, Meissner GW, and Baker BS (2006). Blueprints for behavior: genetic specification of neural circuitry for innate behaviors. Trends Neurosci 29, 444–451. [DOI] [PubMed] [Google Scholar]
- Mar AC, Horner AE, Nilsson SRO, Alsiö J, Kent BA, Kim CH, Holmes A, Saksida LM, and Bussey TJ (2013). The touchscreen operant platform for assessing executive function in rats and mice. Nature Protocols 8, 1985–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz JE, Gillis WF, Beron CC, Neufeld SQ, Robertson K, Bhagat ND, Peterson RE, Peterson E, Hyun M, Linderman SW, et al. (2018). The Striatum Organizes 3D Behavior via Moment-to-Moment Action Selection. Cell 174, 44–58.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz JE, Ivie E, Kligler L, and Gardner TJ (2013). Long-range Order in Canary Song. PLoS computational biology 9, e1003052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr D (1982). Vision: A computational investigation into the human representation and processing of visual information, henry holt and co. Inc, New York, NY: 2. [Google Scholar]
- Mathis A, Mamidanna P, Cury KM, Abe T, Murthy VN, Mathis MW, and Bethge M (2018). DeepLabCut: markerless pose estimation of user-defined body parts with deep learning. Nature Publishing Group 21, 1281–1289. [DOI] [PubMed] [Google Scholar]
- Medan V, and Preuss T (2014). The Mauthner-cell circuit of fish as a model system for startle plasticity. Journal of physiology, Paris 108, 129–140. [DOI] [PubMed] [Google Scholar]
- Mendes CS, Bartos I, Akay T, Márka S, and Mann RS (2013). Quantification of gait parameters in freely walking wild type and sensory deprived Drosophila melanogaster. eLife 2, e00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer AF, Poort J, O’Keefe J, Sahani M, and Linden JF (2018). A Head-Mounted Camera System Integrates Detailed Behavioral Monitoring with Multichannel Electrophysiology in Freely Moving Mice. Neuron 100, 46–60.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimica B, Dunn BA, Tombaz T, Bojja VPTNCS, and Whitlock JR (2018). Efficient cortical coding of 3D posture in freely behaving rats. Science (New York, NY) 362, 584–589. [DOI] [PubMed] [Google Scholar]
- Minderer M, Harvey CD, Donato F, and Moser EI (2016). Neuroscience: Virtual reality explored. Nature 533, 324–325. [DOI] [PubMed] [Google Scholar]
- Mueller JM, Ravbar P, Simpson JH, and Carlson JM (2019). Drosophila melanogaster grooming possesses syntax with distinct rules at different temporal scales. PLoS computational biology 15, e1007105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musall S, Kaufman MT, Gluf S, and Churchland A (2018). Movement-related activity dominates cortex during sensory-guided decision making. bioRxiv, 308288. [Google Scholar]
- Musall S, Urai A, Sussillo D, and Churchland A (2019). Harnessing behavioral diversity to understand circuits for cognition. arXivorg q-bio.NC. [DOI] [PMC free article] [PubMed]
- Nakayama K, and Shimojo S (1992). Experiencing and perceiving visual surfaces. Science (New York, NY) 257, 1357–1363. [DOI] [PubMed] [Google Scholar]
- Nater F, Grabner H, and Gool LV (2010). Exploiting simple hierarchies for unsupervised human behavior analysis. Paper presented at: 2010 IEEE Computer Society Conference on Computer Vision and Pattern Recognition. [Google Scholar]
- Nath T, Mathis A, Chen AC, Patel A, Bethge M, and Mathis MW (2019). Using DeepLabCut for 3D markerless pose estimation across species and behaviors. Nature Protocols 14, 2152–2176. [DOI] [PubMed] [Google Scholar]
- Navalpakkam V, Koch C, Rangel A, and Perona P (2010). Optimal reward harvesting in complex perceptual environments. Proceedings of the National Academy of Sciences 107, 5232–5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen JP, Shipley FB, Linder AN, Plummer GS, Liu M, Setru SU, Shaevitz JW, and Leifer AM (2016). Whole-brain calcium imaging with cellular resolution in freely behaving Caenorhabditis elegans. Proceedings of the National Academy of Sciences 113, E1074–E1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohayon S, Avni O, Taylor AL, Perona P, and Egnor SER (2013). Automated multi-day tracking of marked mice for the analysis of social behavior. Journal of Neuroscience Methods, 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama T, Jovanic T, Denisov G, Dang TC, Hoffmann D, Kerr RA, and Zlatic M (2013). High-Throughput Analysis of Stimulus-Evoked Behaviors in Drosophila Larva Reveals Multiple Modality-Specific Escape Strategies. PLOS ONE 8, e71706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang R, Lansdell BJ, and Fairhall AL (2016). Dimensionality reduction in neuroscience. Current biology : CB 26, R656–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panzeri S, Harvey CD, Piasini E, Latham PE, and Fellin T (2017). Cracking the Neural Code for Sensory Perception by Combining Statistics, Intervention, and Behavior. Neuron 93, 491–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panzeri S, Macke JH, Gross J, and Kayser C (2015). Neural population coding: combining insights from microscopic and mass signals. Trends in cognitive sciences 19, 162–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira TD, Aldarondo DE, Willmore L, Kislin M, Wang SSH, Murthy M, and Shaevitz JW (2019). Fast animal pose estimation using deep neural networks. Nature methods 16, 117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrou G, and Webb B (2012). Detailed tracking of body and leg movements of a freely walking female cricket during phonotaxis. Journal of Neuroscience Methods 203, 56–68. [DOI] [PubMed] [Google Scholar]
- Pisanello F, Mandelbaum G, Pisanello M, Oldenburg IA, Sileo L, Markowitz JE, Peterson RE, Della Patria A, Haynes TM, Emara MS, et al. (2017). Dynamic illumination of spatially restricted or large brain volumes via a single tapered optical fiber. Nature Publishing Group 111, 13972–11188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravbar P, Branson K, and Simpson JH (2019). An automatic behavior recognition system classifies animal behaviors using movements and their temporal context. Journal of Neuroscience Methods 326, 108352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichardt W, Poggio T, and Hausen K (1983). Figure-ground discrimination by relative movement in the visual system of the fly. Biological Cybernetics 46, 1–30. [Google Scholar]
- Robie AA, Hirokawa J, Edwards AW, Umayam LA, Lee A, Phillips ML, Card GM, Korff W, Rubin GM, Simpson JH, et al. (2017). Mapping the Neural Substrates of Behavior. Cell 170, 393–406.e328. [DOI] [PubMed] [Google Scholar]
- Rowland DC, Roudi Y, Moser M-B, and Moser EI (2016). Ten Years of Grid Cells. Annual review of neuroscience 39, 19–40. [DOI] [PubMed] [Google Scholar]
- Scholz M, Linder AN, Randi F, Sharma AK, Yu X, Shaevitz JW, and Leifer A (2018). Predicting natural behavior from whole-brain neural dynamics. bioRxiv, 445643. [Google Scholar]
- Schulze A, Gomez-Marin A, Rajendran VG, Lott G, Musy M, Ahammad P, Deogade A, Sharpe J, Riedl J, Jarriault D, et al. (2015). Dynamical feature extraction at the sensory periphery guides chemotaxis. eLife 4, e06694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz RF, Branicky R, Grundy LJ, Schafer WR, and Brown AEX (2015). Changes in Postural Syntax Characterize Sensory Modulation and Natural Variation of C. elegans Locomotion. PLOS Computational Biology 11, e1004322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadlen MN, and Newsome WT (1998). The variable discharge of cortical neurons: implications for connectivity, computation, and information coding. The Journal of neuroscience : the official journal of the Society for Neuroscience 18, 3870–3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons P, and Young D (1999). Nerve Cells and Animal Behavior.
- Spink AJ, Tegelenbosch RA, Buma MO, and Noldus LP (2001). The EthoVision video tracking system--a tool for behavioral phenotyping of transgenic mice. Physiology & behavior 73, 731–744. [DOI] [PubMed] [Google Scholar]
- Stephens GJ, Johnson-Kerner B, Bialek W, and Ryu WS (2008). Dimensionality and Dynamics in the Behavior of C. elegans. PLoS computational biology 4, e1000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens GJ, Johnson-Kerner B, Bialek W, and Ryu WS (2010). From Modes to Movement in the Behavior of Caenorhabditis elegans. PLoS ONE 5, e13914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss R, and Heisenberg M (1990). Coordination of legs during straight walking and turning in Drosophila melanogaster. Journal of Comparative Physiology A, Sensory, Neural, and Behavioral Physiology 167, 403–412. [DOI] [PubMed] [Google Scholar]
- Straw AD, Branson K, Neumann TR, and Dickinson MH (2011). Multi-camera real-time three-dimensional tracking of multiple flying animals. Journal of the Royal Society, Interface / the Royal Society 8, 395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer C, Pachitariu M, Steinmetz N, Reddy CB, Carandini M, and Harris KD (2019). Spontaneous behaviors drive multidimensional, brainwide activity. Science 364, eaav7893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton RS, and Barto AG (1998). Introduction to reinforcement learning, Vol 2 (MIT press Cambridge; ). [Google Scholar]
- Symvoulidis P, Lauri A, Stefanoiu A, Cappetta M, Schneider S, Jia H, Stelzl A, Koch M, Perez CC, Myklatun A, et al. (2017). NeuBtracker—imaging neurobehavioral dynamics in freely behaving fish. Nature methods 14, 1079–1082. [DOI] [PubMed] [Google Scholar]
- Tao L, Ozarkar S, Beck JM, and Bhandawat V (2019). Statistical structure of locomotion and its modulation by odors. eLife 8, 425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinbergen N (1951). The study of instinct (Oxford,: Clarendon Press; ). [Google Scholar]
- Tinbergen N (1963). On aims and methods of ethology. Zeitschrift fur Tierpsychologie 20, 410–433. [Google Scholar]
- Todd JG, Kain JS, and de Bivort BL (2017). Systematic exploration of unsupervised methods for mapping behavior. Phys Biol 14, 015002. [DOI] [PubMed] [Google Scholar]
- Van Segbroeck M, Knoll AT, Levitt P, and Narayanan S (2017). MUPET—Mouse Ultrasonic Profile ExTraction: A Signal Processing Tool for Rapid and Unsupervised Analysis of Ultrasonic Vocalizations. Neuron 94, 465–485.e465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam V, Ji N, Wang X, Clark C, Mitchell JK, Klein M, Tabone CJ, Florman J, Ji H, Greenwood J, et al. (2016). Pan-neuronal imaging in roaming Caenorhabditis elegans. Proceedings of the National Academy of Sciences of the United States of America 113, E1082–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatraman S, Jin X, Costa RM, and Carmena JM (2010). Investigating neural correlates of behavior in freely behaving rodents using inertial sensors. Journal of neurophysiology 104, 569–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeek J (2005). Rodent behavior annotation from video.
- Vogelstein JT, Vogelstein JT, Park Y, Park Y, Ohyama T, Kerr RA, Kerr RA, Truman JW, Truman JW, Priebe CE, et al. (2014). Discovery of brainwide neural-behavioral maps via multiscale unsupervised structure learning. Science (New York, NY) 344, 386–392. [DOI] [PubMed] [Google Scholar]
- von Philipsborn AC, Liu T, Yu JY, Masser C, Bidaye SS, and Dickson BJ (2011). Neuronal control of Drosophila courtship song. Neuron 69, 509–522. [DOI] [PubMed] [Google Scholar]
- Wang Q, Taliaferro JM, Klibaite U, Hilgers V, Shaevitz JW, and Rio DC (2016). The PSI-U1 snRNP interaction regulates male mating behavior in Drosophila. Proceedings of the National Academy of Sciences 113, 5269–5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Chiu H, Pfeiffer BD, Wong AM, Hoopfer ED, Rubin GM, and Anderson DJ (2017). A Circuit Node that Integrates Convergent Input from Neuromodulatory and Social Behavior-Promoting Neurons to Control Aggression in Drosophila. Neuron 95, 1112–1128.e1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson RC, Doiron B, Smith MA, and Yu BM (2019). Bridging large-scale neuronal recordings and large-scale network models using dimensionality reduction. Current opinion in neurobiology 55, 40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DM (1966). Insect walking. Annu Rev Entomol 11, 103–122. [DOI] [PubMed] [Google Scholar]
- Wiltschko AB, Johnson MJ, Iurilli G, Peterson RE, Katon JM, Pashkovski SL, Abraira VE, Adams RP, and Datta SR (2015). Mapping Sub-Second Structure in Mouse Behavior. Neuron 88, 1121–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]