Abstract
Background:
History of a hypertensive disorder of pregnancy (HDP) among women may be useful to refine atherosclerotic cardiovascular disease risk assessments. However, future risk of diverse cardiovascular conditions in asymptomatic middle-aged women with prior HDP remains unknown.
Objectives:
To examine the long-term incidence of diverse cardiovascular conditions among middle-aged women with and without prior HDP.
Methods:
We included women in the prospective, observational UK Biobank aged 40-69 years who reported ≥1 live birth. Non-invasive arterial stiffness measurement was performed in a subset of women. Cox models were fitted to associate HDP with incident cardiovascular diseases. Causal mediation analyses estimated the contribution of conventional risk factors to observed associations.
Results:
Of 220,024 women included, 2,808 (1.3%) had prior HDP. Mean (±SD) age at baseline was 57.4±7.8 years, and women were followed for median 7 (interquartile range 6.3-7.7) years. Women with HDP had elevated arterial stiffness indices and greater prevalence of chronic hypertension compared to women without HDP. Overall, 7.0 vs. 5.3 age-adjusted incident cardiovascular conditions occurred per 1,000 women-years for women with vs. without prior HDP, respectively (P=0.001). In analysis of time-to-first incident cardiovascular diagnosis, prior HDP was associated with a hazard ratio (HR) of 1.3 (95%CI 1.04-1.6, P=0.02). HDP was associated with greater incidence of CAD (HR 1.8, 95%CI 1.3-2.6, P<0.001), heart failure (HR 1.7, 95%CI 1.04-2.6, P=0.03), aortic stenosis (HR 2.9, 95%CI 1.5-5.4, P<0.001), and mitral regurgitation (HR 5.0, 95%CI 1.5-17.1, P=0.01). In causal mediation analyses, chronic hypertension explained 64% of HDP’s association with CAD and 49% of HDP’s association with heart failure.
Conclusions:
Hypertensive disorders of pregnancy are associated with accelerated cardiovascular aging and more diverse cardiovascular conditions than previously appreciated, including valvular heart disease. Cardiovascular risk after HDP is largely but incompletely mediated by development of chronic hypertension.
Keywords: Cardiovascular epidemiology, women’s health, pregnancy, cardio-obstetrics, hypertension in pregnancy, preeclampsia
Condensed Abstract
Among 220,024 women in the UK Biobank, history of a hypertensive disorder of pregnancy (HDP) was associated with elevated arterial stiffness indices and greater prevalence of chronic hypertension. In prospective follow-up, prior HDP was associated with shorter time to first incident cardiovascular diagnosis and increased hazards of coronary artery disease, heart failure, aortic stenosis, and mitral regurgitation. In causal mediation analysis, chronic hypertension largely but incompletely explained HDP’s association with incident coronary artery disease and heart failure. Taken together, these findings suggest HDP are associated with accelerated cardiovascular aging and greater long-term incidence of diverse cardiovascular conditions than previously appreciated, including valvular heart disease.
Introduction
Complications arising in pregnancy, including hypertensive disorders of pregnancy (HDP, i.e., gestational hypertension, preeclampsia, eclampsia, and HELLP [hemolysis, elevated liver enzymes, low platelet count] syndrome) (1-3), preterm delivery (4), and gestational diabetes (5), appear to portend elevated future risk of cardiovascular disease among affected women. Incidence of gestational hypertension and preeclampsia is rising in the United States, and >10% of women will experience at least one hypertensive pregnancy (6,7). Prior studies suggest that HDP portends a two-fold risk of future atherosclerotic cardiovascular disease (ASCVD), such as myocardial infarction and stroke (1,8). This understanding is reflected in the 2018 ACC/AHA cholesterol guidelines, which endorse using a history of preeclampsia to strengthen statin prescription recommendations among asymptomatic middle-aged women with intermediate 10-year ASCVD risk by conventional risk calculation (9).
However, unanswered questions about the links between HDP and cardiovascular risk remain. First, the degree to which HDP-associated cardiovascular disease risk persists into midlife and beyond is unclear, due in part to incomplete phenotyping and relatively short durations of follow-up in most studies performed to-date (1,10-14), with very few studies following women into their 60s and 70s (15,16), and with attenuated effects observed in studies with longer-term follow-up (15,17). Absolute risk for cardiovascular disease still remains low for a number of years after pregnancy due to young age. However, the long-term risks of diverse cardiovascular diseases among women in midlife who previously experienced HDP, the group now considered in prevention guidelines, remain largely unknown. Second, the risk of non-atherosclerotic cardiovascular diseases in women with HDP is currently unclear. While prior studies reported increased risks of incident heart failure (1,18), atrial fibrillation (19), and venous thromboembolism (3), associations have been inconsistent after adjustments for comorbidities and over the longer term (15,17).
Here, we examined the incidence of diverse cardiovascular conditions among parous women with and without prior hypertensive disorders of pregnancy in the UK Biobank.
Methods
Study cohort
The UK Biobank is a prospective, observational, population-based cohort study that recruited >500,000 adult residents of the United Kingdom between 2006-2010 (20,21). The baseline study visit included health and sociodemographic questionnaires and physical assessment (e.g., blood pressure, anthropometrics), and phlebotomy, as well as additional non-invasive phenotyping in subsets of individuals. Methods for follow-up were previously described (20); briefly, incident disease diagnoses were gleaned from linkage of study records to national hospital inpatient and outpatient records, death registrations, and primary care diagnoses.
We included women who were 40-69 years old and reported ≥1 prior live birth at the baseline study visit. Given prior associations between some types of congenital heart disease and HDP (22) as well as multiple outcomes of interest, we excluded women with congenital heart disease (Online Appendix 1 for relevant diagnosis codes).
Arterial stiffness index
Arterial stiffness index (ASI) is a non-invasive marker of vascular aging and an independent indicator of cardiovascular risk (23). Approximately 170,000 UK Biobank participants had a finger photoplethysmography-derived ASI measured with the PulseTrace PCA2 (CareFusion, San Diego, California) at baseline (24). ASI is calculated by dividing standing height by the time between the initial systolic peak and reflected wave, and expressed as meters per second. Extreme ASI outliers were excluded as before to remove spurious values (25).
Exposure, comorbidities, and outcomes
Women’s reproductive history, including parity and menopausal status, was obtained at the baseline study visit. A diagnosis of HDP (i.e., gestational hypertension, preeclampsia, eclampsia, or HELLP syndrome) was ascertained by qualifying ICD code or self-report at enrollment (Online Appendix 1). Prevalent comorbidities, including prevalent cardiovascular conditions, were captured from ICD codes and/or self-report at the first study visit. Because the cardiovascular conditions under study are not independent, the primary outcome was a composite of incident CAD, heart failure, aortic stenosis, mitral regurgitation, atrial fibrillation or flutter, ischemic stroke, peripheral artery disease, and venous thromboembolism. Individual diagnoses included in the composite primary outcome constituted secondary outcomes. Only new cardiovascular disease diagnoses from a qualifying ICD code (Online Appendix 2) were counted as incident diagnoses (e.g., recurrent myocardial infarction in a participant with prevalent CAD at enrollment did not count as an incident event).
Statistical analysis
Baseline continuous variables were compared between women with and without HDP using the Student’s t-test and categorical variables using either the Pearson’s chi-squared test or Fisher’s exact test as appropriate. Odds ratios for prevalent comorbidities and cardiovascular conditions among women with HDP were calculated using multivariable logistic regression models, adjusted for age at enrollment and race. Multivariable linear regression models were used to compare ASI between groups. Age was incorporated as a quadratic term in ASI models to account for non-linear effects.
To calculate overall age-adjusted incidence rates, the age-specific incidence of cardiovascular disease for each age group was calculated (in 5-year age bins) and then weighted for the proportion of the UK population belonging to each age group. Cox proportional hazard models estimated the association between HDP and first incident cardiovascular disease diagnosis, as well as between HDP and each of the 8 individual cardiovascular outcomes studied. Primary models were sparsely adjusted for age at enrollment and race. Secondary Cox models further adjusted for prevalent chronic hypertension to assess effects independent of hypertension, and additional models were stratified by age at enrollment. Women were excluded from each model for which they had a corresponding prevalent disease diagnosis (e.g., women with prevalent CAD were excluded from the models for incident CAD, etc.) (Online Appendix 3). For each participant, follow-up began at enrollment and was measured separately for each cardiovascular diagnosis. Time-to-censoring for each outcome was determined by the date a diagnosis newly appeared in the medical record or last encounter with the healthcare system. The proportional hazards assumption was tested with Schoenfeld residuals.
We performed causal mediation analysis to evaluate the proportional contribution of prevalent hypertension, hyperlipidemia, and diabetes mellitus to the association between HDP and cardiovascular disease risk using the “mediation” package in R (26). Briefly, causal mediation analysis attempts to estimate the proportional direct and indirect effects of an exposure-outcome relationship through mediators of the total exposure-outcome relationship (27). Each mediator model was adjusted for age, race, body mass index (BMI), ever-smoking, and the prevalent hypertension, hyperlipidemia, and diabetes mellitus statuses not under consideration. Each incident cardiovascular outcome model was adjusted for age, race, BMI, ever-smoking, and prevalent hypertension, hyperlipidemia, and diabetes mellitus. Each mediation analysis model was run using 100 simulations with a quasi-Bayesian approach to estimate variance. Mediation effects were bounded at 0 and 1.
Two-sided p-values less than α=0.05 were deemed statistically significant. All analyses were performed using R 3.5 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Description of study cohort
After exclusions (Online Figure 1), the study cohort consisted of 220,024 women, who were followed for a median 7 (interquartile range 6.3-7.7, overall range 0-10) years. Distribution of follow-up time is shown in Online Figure 2. Mean (±SD) age at study enrollment was 57.4±7.8 years, corresponding to mean duration since first birth of 31.4±10.2 years, and 94.3% of the cohort was white (Table 1). Of the total cohort, 2,808 women (1.3%) had prior HDP. Women with prior HDP were younger at study enrollment (mean (±SD) age 52.3±8.7 vs. 57.4±7.8 years, P<0.001) and older at first birth (28.7±6.2 vs. 25.9±5.1 years, P<0.001) (Table 1). Prevalence of ever-smoking was lower in women with prior HDP than in those without (33.0% vs. 40.7%, P<0.001). Although women with prior HDP were more likely to be taking antihypertensive medications at enrollment (22.4% vs. 18.2%, P<0.001), their blood pressures were higher at enrollment compared to women without prior HDP (systolic blood pressure 141.6 vs. 137.7 mmHg, P<0.001, and diastolic blood pressure 84.7 vs. 80.7 mmHg, P<0.001). Of women with prior HDP and chronic hypertension, 32.8% were taking antihypertensive medication at enrollment.
Table 1.
Baseline characteristics of the study cohort.
| Overall cohort (n = 220,024) |
Women with prior hypertensive disorder of pregnancy (n = 2,808) |
Women without prior hypertensive disorder of pregnancy (n = 217,216) |
P- value |
|
|---|---|---|---|---|
| Age, years | 57.4 ± 7.8 | 52.3 ± 8.7 | 57.4 ± 7.8 | <0.001 |
| Caucasian, % | 207,517 (94.3%) | 2,623 (93.4%) | 204,894 (94.3%) | 0.06 |
| Number of live births at baseline | 2.2 ± 0.9 | 2.16 ± 0.9 | 2.24 ± 0.9 | <0.001 |
| Age at first live birth, years | 25.9 ± 5.1 | 28.7 ± 6.2 | 25.9 ± 5.1 | <0.001 |
| Time from first birth to baseline study visit, years |
31.4 ± 10.2 | 23.7 ± 12.4 | 31.5 ± 10.1 | <0.001 |
| Mean age at menopause, years | 49.8 ± 5.1 | 49.4 ± 5.3 | 49.8 ± 5.1 | 0.02 |
| Menopause < age 40, % | 5,110 (4.0%) | 45 (4.3%) | 5,065 (4.0%) | 0.61 |
| Body mass index, kg/m2 | 27.2 ± 5.1 | 28.1 ± 5.6 | 27.1 ± 5.1 | <0.001 |
| Systolic blood pressure, mmHg | 137.7 ± 20.4 | 141.6 ± 20.4 | 137.7 ± 20.3 | <0.001 |
| Diastolic blood pressure, mmHg | 80.7 ± 10.5 | 84.7 ± 10.7 | 80.7 ± 10.5 | <0.001 |
| Hypertension, % | 56,987 (25.9%) | 1,889 (67.3%) | 55,098 (25.4%) | <0.001 |
| Hyperlipidemia, % | 24,828 (11.3%) | 305 (10.9%) | 24,523 (11.3%) | 0.49 |
| Diabetes mellitus, % | 9,005 (4.1%) | 177 (6.3%) | 8,828 (4.1%) | <0.001 |
| Ever smoking, % | 89,327 (40.6%) | 928 (33.0%) | 88,399 (40.7%) | <0.001 |
| Chronic kidney disease, % | 446 (0.2%) | 19 (0.7%) | 427 (0.2%) | <0.001 |
| Medication use at baseline | ||||
| • Aspirin | 21,863 (9.9%) | 246 (8.8%) | 21,617 (10.0%) | 0.04 |
| • Antihypertensive medication | 40,084 (18.2%) | 628 (22.4%) | 39,456 (18.2%) | <0.001 |
| • Cholesterol-lowering medication | 29,042 (13.2%) | 336 (12.0%) | 28,706 (13.2%) | 0.06 |
Values are displayed as mean ± SD or n (%).
Prevalent comorbidities and cardiovascular diagnoses
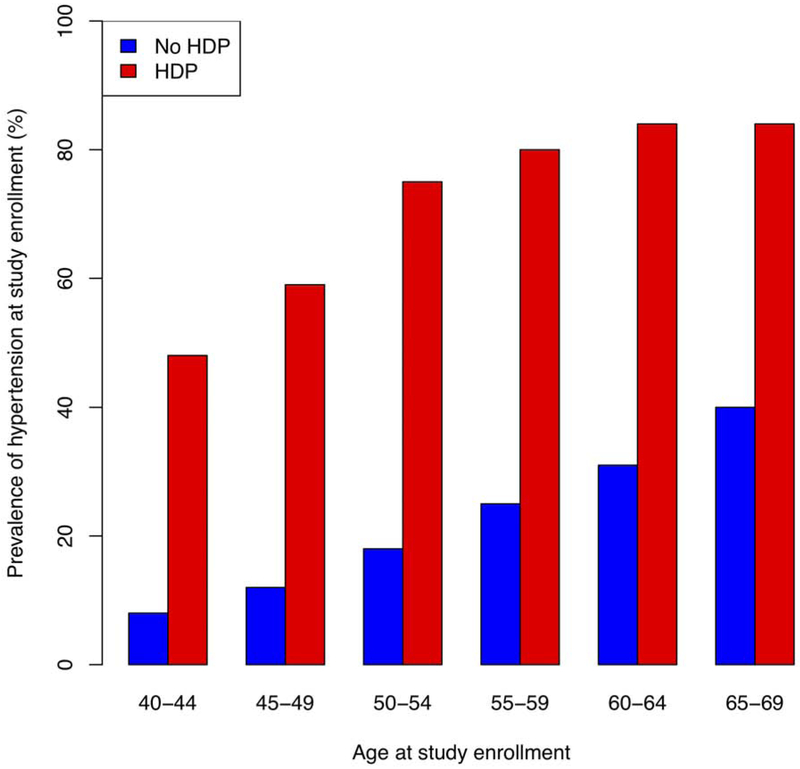
Women with prior HDP had a higher unadjusted prevalence of chronic hypertension than women without HDP (67.3% vs. 25.4%, P<0.001, Figure 1, Central Illustration). After adjustment for age and use of antihypertensive medication, women with prior HDP had higher systolic blood pressure by 9.0 mmHg (95% CI 8.3-9.8 mmHg, P<0.001) and higher diastolic blood pressure by 4.6 mmHg (95% CI 4.2-5.0 mmHg, P<0.001, Central Illustration). After adjustment for age at enrollment and race, women with prior HDP were more likely to have prevalent hypertension (odds ratio [OR] 11.6, 95% CI 10.6-12.7, P<0.001), as well as prevalent diagnoses of hyperlipidemia, diabetes mellitus, and chronic kidney disease (Online Table 1). Women with prior HDP had higher likelihood of prevalent CAD (OR 1.6, 95% CI 1.1-2.2, P=0.008), heart failure (OR 2.4, 95% CI 1.3-4.2, P=0.002), and venous thromboembolism (OR 1.5, 95% CI 1.2-1.9, P<0.001) (Online Table 1).
Figure 1. Prevalence of chronic hypertension stratified by age at study enrollment among women with and without prior hypertensive disorders of pregnancy.
Women with prior HDP had increased prevalence of chronic hypertension across age groups. HDP = hypertensive disorder or pregnancy.
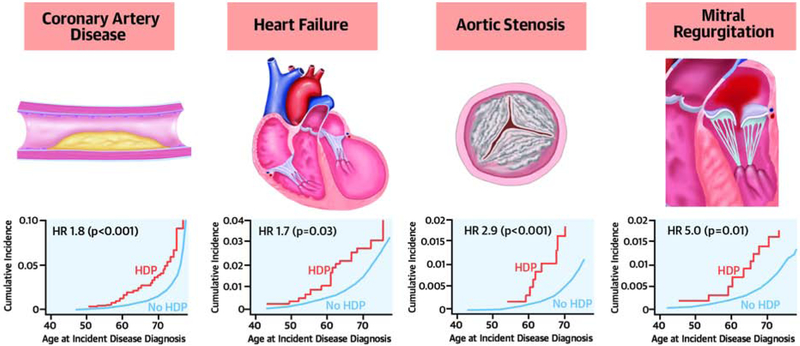
Central Illustration. Hypertensive disorders of pregnancy are associated with long-term risk of chronic hypertension and diverse cardiovascular diseases.
Women with a history of hypertensive disorders of pregnancy had greater prevalence of hypertension and elevated arterial stiffness indices compared to women without prior hypertensive pregnancy. Hypertensive pregnancy was associated with long-term risk of incident coronary artery disease, heart failure, aortic stenosis, and mitral regurgitation. HDP = hypertensive disorder of pregnancy. ASI = arterial stiffness index. HR = hazard ratio.
The cumulative incidence plots on the right reflect incident cardiovascular disease diagnoses plotted against the age at the time of diagnosis on the x-axis. The hazard ratios displayed reflect results of the primary survival (Cox proportional hazards) analysis, which were adjusted for age at study enrollment and race.
Arterial stiffness index
After exclusion of extreme outliers (n = 30), finger photoplethysmography-derived ASI data were available for 81,557 women in our cohort, including 1,191 with prior HDP (42.4% of women with HDP and 37.0% without HDP). Characteristics of the subgroup undergoing ASI measurement were similar to those of the rest of the cohort, including age at enrollment (57.5 vs. 57.3 years), time since first birth (31.4 vs. 31.6 years), and prevalence of chronic hypertension (25.6% vs. 26.1%).
Prior HDP was associated with higher ASI by 0.32 m/s (95% CI 0.13-0.51 m/s, P=0.001) after adjustment for age at enrollment and menopausal status. This association persisted after additionally adjusting for diabetes, ever-smoking, and BMI (+0.28 m/s, 95% CI 0.09-0.47, P=0.004). After further adjustment for prevalent hypertension, the association between HDP and ASI was no longer statistically significant (+0.18 m/s, 95% CI −0.01-0.8, P=0.07). In models stratified by age and menopausal status (28), prior HDP was associated with higher ASI among pre-menopausal women by 0.42 m/s (95% CI 0.21-0.63 m/s, P<0.001) and no significant association among post-menopausal women (+0.20 m/s, 95% CI −0.09-0.49, P=0.18) (Central Illustration). The estimated effect difference between pre- and post-menopausal women did not reach statistical significance in formal interaction testing (P(interaction)=0.26).
Incident cardiovascular disease diagnoses
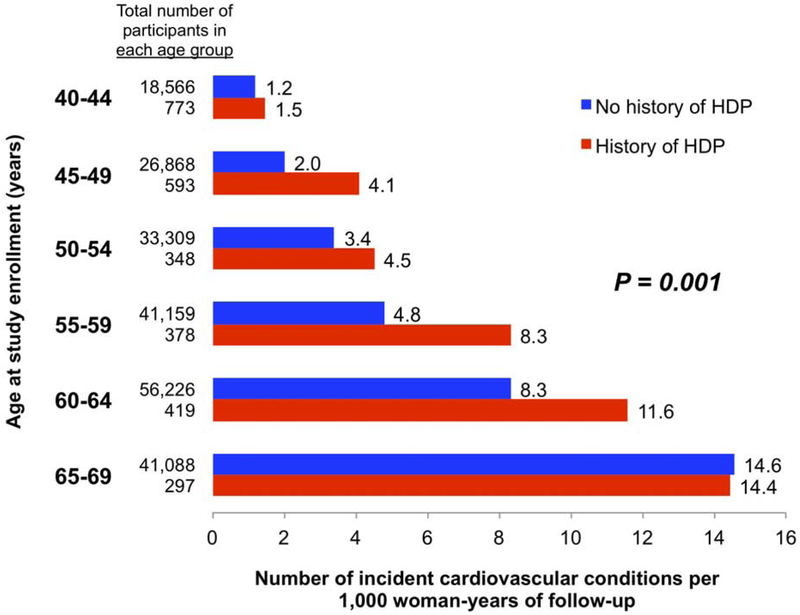
Overall, 7.0 vs. 5.3 total age-adjusted incident cardiovascular disease diagnoses occurred per 1,000 woman-years of follow-up in women with and without prior HDP, respectively (P=0.001, Figure 2), with persistent differences observed among women into their early 60s. In analysis of time-to-first incident cardiovascular diagnosis, prior HDP was associated with a hazard ratio (HR) of 1.3 (95% CI 1.04-1.6, P=0.02). Counts of unadjusted prevalent and incident disease diagnoses are summarized in Online Table 2. Notably, among women with prior HDP, 85% of incident cardiovascular diagnoses occurred in women also with prevalent chronic hypertension.
Figure 2. Age-specific unadjusted rates of incident cardiovascular diagnoses per 1,000 woman-years of follow-up in women with and without prior hypertensive disorders of pregnancy.
Women with prior HDP had increased incidence of cardiovascular diagnoses into their 60s. Incident cardiovascular disease diagnoses included coronary artery disease, heart failure, aortic stenosis, mitral regurgitation, atrial fibrillation or flutter, ischemic stroke, peripheral artery disease, and venous thromboembolism. HDP = hypertensive disorder of pregnancy.
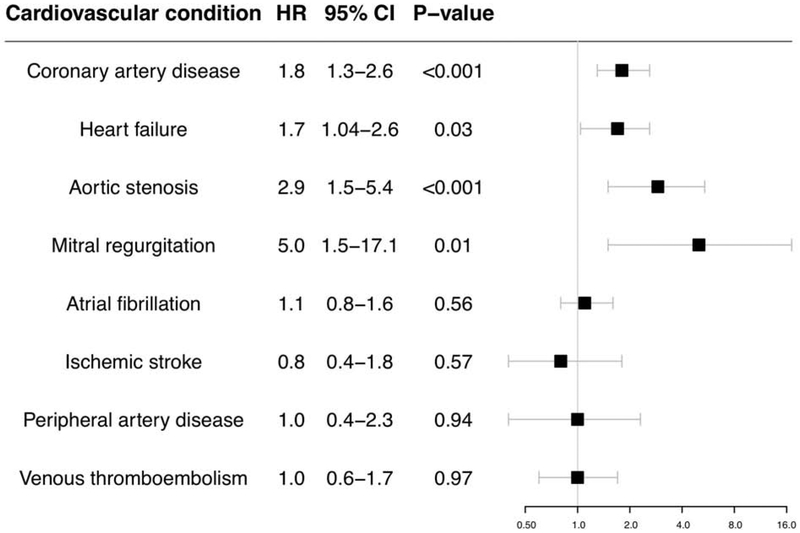
The proportional hazards assumption was met for all incident disease models except for mitral regurgitation; for mitral regurgitation, an interaction term between HDP and follow-up time was added to satisfy the proportional hazards assumption. Figure 3 summarizes the hazards of individual incident cardiovascular diagnoses associated with prior HDP. After adjustment for age at enrollment and race, significant associations were observed between prior HDP and incident CAD (HR 1.8, 95% CI 1.3-2.6, P<0.001), heart failure (HR 1.7, 95% CI 1.04-2.6, P=0.03), aortic stenosis (HR 2.9, 95% CI 1.5-5.4, P<0.001), and mitral regurgitation (HR 5.0, 95% CI 1.5-17.1, P=0.01) (Central Illustration), but not with incident atrial fibrillation or flutter, ischemic stroke, peripheral artery disease, or venous thromboembolism. In models stratified by age at study enrollment, risk of incident cardiovascular diseases persisted among women with prior HDP into their 60s, except for heart failure (Online Table 3). Associations between HDP and each incident cardiovascular diagnosis attenuated after incorporating prevalent hypertension into the models (Online Table 4).
Figure 3. Hazard ratios for incident cardiovascular diagnoses in women with prior hypertensive disorders of pregnancy.
Women with prior hypertensive pregnancy had increased hazards of coronary artery disease, heart failure, aortic stenosis, and mitral regurgitation. Cox proportional hazards models begin follow-up at study enrollment and are adjusted for age at enrollment and race. X-axis is presented in log scale. HR = hazard ratio. CI = confidence interval.
In a secondary model for incident heart failure incorporating aortic stenosis and mitral regurgitation as covariates, the estimated effect of HDP was unchanged from the primary model (HR 1.8, 95% CI 1.1-2.9, P=0.02). In exploratory analyses, HDP appeared to contribute additively with CAD for incident heart failure risk (Online Table 5).
In sensitivity analyses excluding 12,121 women in the cohort with any prevalent cardiovascular disease diagnosis, we still observed significant associations between HDP and incident CAD (HR 1.7, 95% CI 1.1-2.5, P=0.01), aortic stenosis (HR 2.2, 95% CI 1.0-5.0, P=0.04), and mitral regurgitation (HR 2.2, 95% CI 1.1-4.4, P=0.03). In sensitivity analyses excluding 2,628 women with prevalent CAD, we observed similar results to the primary analysis with significant hazards of incident heart failure (HR 1.7, 95% CI 1.01-2.7, P=0.04), aortic stenosis (HR 2.9, 95% CI 1.5-3.6, P=0.002), and mitral regurgitation (HR 2.0, 95% CI 1.04-3.9, P=0.04). Finally, in sensitivity analyses excluding 3,943 women with any prevalent ASCVD (CAD, ischemic stroke, or peripheral artery disease), results were again unchanged (for CAD, HR 1.8, 95% CI 1.3-2.6, P=0.001; for heart failure, HR 1.7, 95% CI 1.1-2. P=0.03; for aortic stenosis, HR 2.6, 95% CI 1.3-5.3, P=0.007; for mitral regurgitation, HR 2.1, 95% 1.1-4.0, P=0.03).
In sensitivity analyses excluding women with prevalent or incident heart failure to evaluate possible reverse causation bias, associations between HDP and CAD, aortic stenosis, and mitral regurgitation remained unchanged (Online Table 6). Results were additionally unchanged after excluding women with <1 year of follow-up (Online Table 7) and those with prevalent cancer (Online Table 8).
Mediation analysis
We performed causal mediation analysis to investigate the proportional contribution of chronic hypertension, hyperlipidemia, and diabetes mellitus to the risk of incident cardiovascular diagnoses associated with HDP, adjusting for other conventional ASCVD risk factors (age, race, BMI at study enrollment, and ever-smoking, in addition to hypertension, hyperlipidemia, and diabetes mellitus). Mediation analyses (Table 2) suggested that chronic hypertension accounted for 64% of the association between HDP and incident CAD (95% CI 36-100%, P<0.001) and 49% of HDP’s association with incident heart failure (95% CI 27-100%, P=0.02). There were no significant mediation effects observed for prevalent hyperlipidemia or diabetes mellitus.
Table 2.
Causal mediation analysis to assess the mediation effect of prevalent chronic hypertension, hyperlipidemia, and diabetes mellitus for observed associations between hypertensive disorders of pregnancy and incident cardiovascular disease risk.
| Hypertension | Hyperlipidemia | Diabetes mellitus | ||||
|---|---|---|---|---|---|---|
| Proportion of association with HDP mediated by hypertension (95% CI) |
P-value | Proportion of association with HDP mediated by hyperlipidemia (95% CI) |
P-value | Proportion of association with HDP mediated by diabetes mellitus (95% CI) |
P-value | |
| Coronary artery disease | 0.64 (0.36-1) | <0.001 | 0 (0-0.44) | 0.60 | 0 (0-0.05) | 0.50 |
| Heart failure | 0.49 (0.27-1) | 0.02 | 0 (0-0.10) | 0.26 | 0 (0-0.01) | 0.32 |
| Aortic stenosis | 0.43 (0-1) | 0.20 | 0 (0-0.18) | 0.44 | 0 (0-0.01) | 0.50 |
| Mitral regurgitation | 0.47 (0-1) | 0.26 | 0 (0-0.06) | 0.64 | 0 (0-0.01) | 0.88 |
These analyses test the extent to which conventional cardiovascular risk factors mediate the associations of hypertensive disorders of pregnancy with coronary artery disease, heart failure, aortic stenosis, and mitral regurgitation. For example, a mediation effect of 0 would indicate that a risk factor does not mediate the association with HDP, and a mediation effect of 1 would indicate that a risk factor mediates all of the association with HDP. The range of possible mediation effect is 0-1.
The p-value in this analysis reflects whether the proportion of the association with HDP mediated by each risk factor (hypertension, hyperlipidemia, and diabetes mellitus) is 0% (the null hypothesis) vs. not 0%.
Each mediator model incorporated age, race, body mass index at study enrollment, ever-smoking, and the prevalent hypertension, hyperlipidemia, and diabetes mellitus statuses not under consideration. Each outcome model incorporated age, race, body mass index at study enrollment, ever-smoking, and prevalent hypertension, hyperlipidemia, and diabetes mellitus.
CI = confidence interval; HDP = hypertensive disorder of pregnancy.
Discussion
In a large, prospective, population-based cohort of middle-aged women, including 2,808 women with HDP, we found that prior HDP was associated with a range of cardiovascular conditions later in life. Specifically, HDP was associated with greater risk of incident CAD, heart failure, aortic stenosis, and mitral regurgitation. We also observed that HDP history was associated with persistently increased arterial stiffness. Furthermore, increased prevalence of hypertension among women with HDP largely, but incompletely, mediated the relationship between HDP and long-term cardiovascular risk.
Several findings are notable with respect to the development of cardiovascular disease in women with a history of hypertensive pregnancy complications. First, the findings of increased arterial stiffness decades after pregnancy and increased risk of both vascular and non-vascular (e.g., valvular) pathology suggest that HDP are associated with a syndrome of accelerated cardiovascular aging. This hypothesis aligns with a prior study which found greater prevalence of subclinical atherosclerosis among women aged 45-55 with prior preeclampsia compared to a reference population (29). Women with HDP have greater endothelial dysfunction and arterial stiffness during pregnancy and the postpartum period (30), but prior work suggested that differences in affected women attenuate with time after pregnancy (23,31). Persistent differences in arterial stiffness beyond 10 years postpartum have not previously been demonstrated (32). Here, we observe a persistent association between HDP and elevated ASI >2 decades after first birth.
Second, our findings suggest that HDP may be associated with different cardiovascular conditions in the short and long term following pregnancy. Most prior studies of cardiovascular risk after HDP began follow-up at the time of pregnancy and/or have had short durations of follow-up (1,10), and few studies have followed women into middle age and beyond (15,16,33). Our study is unique in beginning prospective follow-up in women at midlife, which is the scenario specifically considered in the latest ACC/AHA cholesterol and prevention guidelines when using HDP history to support statin initiation. Prior work consistently showed a larger relative risk of cardiovascular disease closer to the time of pregnancy in women with HDP compared to unaffected women but still with low absolute risk in these young women. Whether the increased risk of cardiovascular disease associated with HDP persists long-term, particularly as general cardiovascular disease risk increases in midlife, has been unclear (10). Our findings suggest that women with prior HDP face increased early risk of CAD and heart failure and retain increased risk for incident CAD and heart failure into midlife. Conversely, we observed an increased risk of prevalent, but not incident, venous thromboembolism in women with prior HDP, suggesting that the previously observed heightened risk (3) may not persist long-term. Meanwhile, we observed increased risk of premature valvular disease in the form of aortic stenosis and mitral regurgitation, associations that have not been previously described. Of note, sFlt-1, a circulating antiangiogenic protein implicated in the pathogenesis of preeclampsia (34), was recently found to have a strong association with calcific aortic stenosis in the general population (35). Additionally, a recent Mendelian randomization analysis found that genetically associated elevation in systolic blood pressure was associated with incident aortic stenosis, aortic regurgitation, and mitral regurgitation, further supporting the plausibility of our observed associations between HDP and valvular heart disease (36). Although hazards for aortic stenosis and mitral regurgitation among women with prior HDP were significant, absolute risks of both conditions remained low given lower incidence rates of valvular heart disease.
Importantly, cardiovascular disease risk after HDP is largely, but incompletely, mediated by the development of chronic hypertension. Elevated arterial stiffness may be a strong precursor to the development of hypertension (25), and accelerated arterial stiffening may underlie the greater prevalence of chronic hypertension that we observed among women with prior HDP; this elevated prevalence of hypertension is consistent with prior studies (15,33). Our findings additionally reinforce those of two recent studies (16,18) suggesting that chronic hypertension accounts for one-half to two-third of the excess cardiovascular disease risk women with prior HDP. This suggests that blood pressure may represent an important therapeutic target for cardiovascular risk reduction in this population. Although 67% of women with prior HDP in our cohort had a diagnosis of hypertension at enrollment, only 33% of women with both HDP and chronic hypertension (and 22% of all women with prior HDP) were taking an antihypertensive medication, as similarly observed in another recent analysis from the UK Biobank (37). Current hypertension guidelines do not incorporate HDP into ASCVD risk assessment or discuss tailored blood pressure management in affected women (38,39). Strategies to optimize blood pressure management, including identification of suitable targets, and other interventions to slow vascular aging in women with HDP warrant dedicated study.
Notably, we did not observe an increased risk of prevalent or incident stroke in women with prior HDP, in contrast with some prior studies (1,15,17), although this association has not been consistent (5). We cannot rule out the possibility of low power hindering our ability to detect HDP-associated stroke risk.
Limitations
While our study has several strengths, our findings should be interpreted in the context of some limitations. First, the exposure (any prior history of HDP) was ascertained largely by participant self-report. Prior work on maternal recall of HDP indicates limited sensitivity but high specificity for true HDP (40,41). Additionally, a “healthy volunteer” selection bias has been noted in the UK Biobank (42), which may explain a lower prevalence of HDP than expected in our cohort. “Sicker” women with a prior history of HDP may be less likely to enroll than healthier women with prior HDP as indicated by observed age differences; this would serve to minimize the effects we observed and bias our results to the null. HDP were combined as a lump exposure, as data were not available to allow stratification of gestational hypertension, mild preeclampsia, severe preeclampsia, preeclampsia superimposed on chronic hypertension, eclampsia, and HELLP syndrome. Further, data on other associated pregnancy complications (e.g., preterm delivery, growth restriction) were not available, nor were data on recurrent HDP; it is possible that risks are higher among women with preeclampsia plus growth restriction and/or preterm delivery, as previous analyses have suggested for CAD (43). Further research is needed to show whether this effect modification extends to the diverse (non-ASCVD) conditions studied in the present analysis. Because women with HDP had higher baseline prevalence of CAD, heart failure, and venous thromboembolism, and because women with prevalent cardiovascular disease were excluded from incident disease models, our results may underestimate the magnitude of association between HDP and these conditions. However, the focus of our analysis was incident cardiovascular disease diagnoses among asymptomatic women in midlife, and we still found significant hazards of incident CAD and heart failure. Although ours is one of the first large, long-term cohort studies of HDP from outside of Scandinavia (15-17), as with these prior cohorts, the UK Biobank is predominantly (>90%) white, and further study is warranted to determine whether our findings generalize to other racial and ethnic groups.
In summary, hypertensive disorders of pregnancy are associated with accelerated cardiovascular aging and greater incidence of diverse cardiovascular conditions than previously appreciated, including valvular heart disease, later in life. Further research is needed to further elucidate the mechanisms linking hypertensive pregnancy complications with long-term cardiovascular risk and to determine optimal strategies for screening, prevention, and management.
Supplementary Material
CLINICAL PERSPECTIVES.
Competency in Medical Knowledge: Women with hypertension during pregnancy are at increased risk of later cardiovascular diseases, including coronary artery disease, heart failure, aortic stenosis, and mitral regurgitation.
Translational Outlook: Strategies to mitigate long-term cardiovascular risk in women with prior hypertensive pregnancies, including specific blood pressure management strategies and other interventions to slow vascular aging, warrant dedicated study.
Acknowledgments
Funding: M.C.H. is supported by the National Institutes of Health (T32HL094301-07). P.N. and G.M.P. are supported by a grant from the National Heart, Lung, and Blood Institute (R01HL142711). P.N. is also supported by a Hassenfeld Scholar Award from the Massachusetts General Hospital, a grant from Fondation Leducq (TNE-18CVD04), and National Heart, Lung, and Blood Institute grants (R01HL148565, R01HL148050).
Abbreviations and Acronyms
- ASCVD
Atherosclerotic cardiovascular disease
- ASI
Arterial stiffness index
- BMI
Body mass index
- CAD
Coronary artery disease
- CKD
Chronic kidney disease
- HDP
Hypertensive disorder(s) of pregnancy
- HELLP
Hemolysis, elevated liver enzymes, low platelet count
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: D.L.B. discloses the following relationships - Advisory Board: Cardax, Elsevier Practice Update Cardiology, Medscape Cardiology, PhaseBio, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care, TobeSoft; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Vice-Chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Medtelligence/ReachMD (CME steering committees), Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Abbott, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, Eisai, Ethicon, Forest Laboratories, Idorsia, Ironwood, Ischemix, Lilly, Medtronic, PhaseBio, Pfizer, Regeneron, Roche, Sanofi Aventis, Synaptic, The Medicines Company; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); Site Co-Investigator: Biotronik, Boston Scientific, St. Jude Medical (now Abbott), Svelte; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Fractyl, Merck, Novo Nordisk, PLx Pharma, Takeda. P.N. reports grant support from Amgen, Apple, and Boston Scientific, and is a scientific advisor to Apple and Blackstone Life Sciences. All other authors have no disclosures.
References
- 1.Wu P, Haththotuwa R, Kwok CS, et al. Preeclampsia and Future Cardiovascular Health: A Systematic Review and Meta-Analysis. Circ Cardiovasc Qual Outcomes. 2017;10(2). [DOI] [PubMed] [Google Scholar]
- 2.Tooher J, Thornton C, Makris A, Ogle R, Korda A, Hennessy A. All Hypertensive Disorders of Pregnancy Increase the Risk of Future Cardiovascular Disease. Hypertension. 2017;70(4):798–803. [DOI] [PubMed] [Google Scholar]
- 3.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335(7627):974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanz LJ, Stuart JJ, Williams PL, et al. Preterm Delivery and Maternal Cardiovascular Disease in Young and Middle-Aged Adult Women. Circulation. 2017;135(6):578–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grandi SM, Filion KB, Yoon S, et al. Cardiovascular Disease-Related Morbidity and Mortality in Women With a History of Pregnancy Complications. Circulation. 2019;139(8):1069–1079. [DOI] [PubMed] [Google Scholar]
- 6.Wallis AB, Saftlas AF, Hsia J, Atrash HK. Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension, United States, 1987-2004. Am J Hypertens. 2008;21(5):521–526. [DOI] [PubMed] [Google Scholar]
- 7.Ananth CV, Keyes KM, Wapner RJ. Pre-eclampsia rates in the United States, 1980-2010: age-period-cohort analysis. BMJ. 2013;347:f6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stuart JJ, Tanz LJ, Cook NR, et al. Hypertensive Disorders of Pregnancy and 10-Year Cardiovascular Risk Prediction. J Am Coll Cardiol. 2018;72(11):1252–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(24):3168–3209. [DOI] [PubMed] [Google Scholar]
- 10.Seely EW, Tsigas E, Rich-Edwards JW. Preeclampsia and future cardiovascular disease in women: How good are the data and how can we manage our patients? Semin Perinatol. 2015;39(4):276–283. [DOI] [PubMed] [Google Scholar]
- 11.Lin YS, Tang CH, Yang CY, et al. Effect of pre-eclampsia-eclampsia on major cardiovascular events among peripartum women in Taiwan. Am J Cardiol. 2011;107(2):325–330. [DOI] [PubMed] [Google Scholar]
- 12.Hovsepian DA, Sriram N, Kamel H, Fink ME, Navi BB. Acute cerebrovascular disease occurring after hospital discharge for labor and delivery. Stroke. 2014;45(7):1947–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melchiorre K, Sutherland GR, Liberati M, Thilaganathan B. Preeclampsia is associated with persistent postpartum cardiovascular impairment. Hypertension. 2011;58(4):709–715. [DOI] [PubMed] [Google Scholar]
- 14.Savitz DA, Danilack VA, Elston B, Lipkind HS. Pregnancy-induced hypertension and diabetes and the risk of cardiovascular disease, stroke, and diabetes hospitalization in the year following delivery. Am J Epidemiol. 2014;180(1):41–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Männistö T, Mendola P, Vääräsmäki M, et al. Elevated blood pressure in pregnancy and subsequent chronic disease risk. Circulation. 2013;127(6):681–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haug EB, Horn J, Markovitz AR, et al. Association of Conventional Cardiovascular Risk Factors With Cardiovascular Disease After Hypertensive Disorders of Pregnancy: Analysis of the Nord-Trøndelag Health Study. JAMA Cardiol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lykke JA, Langhoff-Roos J, Sibai BM, Funai EF, Triche EW, Paidas MJ. Hypertensive pregnancy disorders and subsequent cardiovascular morbidity and type 2 diabetes mellitus in the mother. Hypertension. 2009;53(6):944–951. [DOI] [PubMed] [Google Scholar]
- 18.Behrens I, Basit S, Lykke JA, et al. Association Between Hypertensive Disorders of Pregnancy and Later Risk of Cardiomyopathy. JAMA. 2016;315(10):1026–1033. [DOI] [PubMed] [Google Scholar]
- 19.Scantlebury DC, Kattah AG, Weissgerber TL, et al. Impact of a History of Hypertension in Pregnancy on Later Diagnosis of Atrial Fibrillation. J Am Heart Assoc. 2018;7(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sudlow C, Gallacher J, Allen N, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3):e1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bycroft C, Freeman C, Petkova D, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562(7726):203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drenthen W, Boersma E, Balci A, et al. Predictors of pregnancy complications in women with congenital heart disease. Eur Heart J. 2010;31(17):2124–2132. [DOI] [PubMed] [Google Scholar]
- 23.Hausvater A, Giannone T, Sandoval YH, et al. The association between preeclampsia and arterial stiffness. J Hypertens. 2012;30(1):17–33. [DOI] [PubMed] [Google Scholar]
- 24.Said MA, Eppinga RN, Lipsic E, Verweij N, van der Harst P. Relationship of Arterial Stiffness Index and Pulse Pressure With Cardiovascular Disease and Mortality. J Am Heart Assoc. 2018;7(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zekavat SM, Aragam K, Emdin C, et al. Genetic Association of Finger Photoplethysmography-Derived Arterial Stiffness Index With Blood Pressure and Coronary Artery Disease. Arterioscler Thromb Vasc Biol. 2019:ATVBAHA119312626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.'mediation' package [computer program]. 2019. https://cran.r-project.org/web/packages/mediation/vignettes/mediation.pdf
- 27.Lee H, Herbert RD, McAuley JH. Mediation Analysis. JAMA. 2019;321(7):697–698. [DOI] [PubMed] [Google Scholar]
- 28.Khan ZA, Janssen I, Mazzarelli JK, et al. Serial Studies in Subclinical Atherosclerosis During Menopausal Transition (from the Study of Women's Health Across the Nation). Am J Cardiol. 2018;122(7):1161–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zoet GA, Benschop L, Boersma E, et al. Prevalence of Subclinical Coronary Artery Disease Assessed by Coronary Computed Tomography Angiography in 45- to 55-Year-Old Women With a History of Preeclampsia. Circulation. 2018;137(8):877–879. [DOI] [PubMed] [Google Scholar]
- 30.Weissgerber TL, Milic NM, Milin-Lazovic JS, Garovic VD. Impaired Flow-Mediated Dilation Before, During, and After Preeclampsia: A Systematic Review and Meta-Analysis. Hypertension. 2016;67(2):415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grand'Maison S, Pilote L, Okano M, Landry T, Dayan N. Markers of Vascular Dysfunction After Hypertensive Disorders of Pregnancy: A Systematic Review and Meta-Analysis. Hypertension. 2016;68(6):1447–1458. [DOI] [PubMed] [Google Scholar]
- 32.Rönnback M, Lampinen K, Groop PH, Kaaja R. Pulse wave reflection in currently and previously preeclamptic women. Hypertens Pregnancy. 2005;24(2):171–180. [DOI] [PubMed] [Google Scholar]
- 33.Stuart JJ, Tanz LJ, Missmer SA, et al. Hypertensive Disorders of Pregnancy and Maternal Cardiovascular Disease Risk Factor Development: An Observational Cohort Study. Ann Intern Med. 2018;169(4):224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350(7):672–683. [DOI] [PubMed] [Google Scholar]
- 35.Small AM, Kiss DH, Anwaruddin S, et al. Soluble FMS-Like Tyrosine Kinase-1 Is a Circulating Biomarker Associated With Calcific Aortic Stenosis. J Am Coll Cardiol. 2019;73(11):1364–1365. [DOI] [PubMed] [Google Scholar]
- 36.Nazarzadeh M, Pinho-Gomes AC, Smith Byrne K, et al. Systolic Blood Pressure and Risk of Valvular Heart Disease: A Mendelian Randomization Study. JAMA Cardiol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Warren HR, Evangelou E, Cabrera CP, et al. Genome-wide association analysis identifies novel blood pressure loci and offers biological insights into cardiovascular risk. Nat Genet. 2017;49(3):403–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2018;138(17):e484–e594. [DOI] [PubMed] [Google Scholar]
- 39.Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021–3104. [DOI] [PubMed] [Google Scholar]
- 40.Stuart JJ, Bairey Merz CN, Berga SL, et al. Maternal recall of hypertensive disorders in pregnancy: a systematic review. J Womens Health (Larchmt). 2013;22(1):37–47. [DOI] [PubMed] [Google Scholar]
- 41.Carter EB, Stuart JJ, Farland LV, et al. Pregnancy Complications as Markers for Subsequent Maternal Cardiovascular Disease: Validation of a Maternal Recall Questionnaire. J Womens Health (Larchmt). 2015;24(9):702–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fry A, Littlejohns TJ, Sudlow C, et al. Comparison of Sociodemographic and Health-Related Characteristics of UK Biobank Participants With Those of the General Population. Am J Epidemiol. 2017;186(9):1026–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Riise HK, Sulo G, Tell GS, et al. Incident Coronary Heart Disease After Preeclampsia: Role of Reduced Fetal Growth, Preterm Delivery, and Parity. J Am Heart Assoc. 2017;6(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.