Abstract
Objective:
To estimate the prevalence of severe maternal morbidity (SMM) among very preterm births and determine its association with very preterm infant mortality and morbidity.
Study design:
This study used New York City Vital Statistics birth and death records linked with maternal and newborn discharge abstract data for live births between 2010 and 2014. We included 6901 infants without congenital anomalies born between 24+0 and 31+6 weeks of gestation. SMM was identified as life-threatening conditions or life-saving procedures. Outcomes were first-year infant mortality, severe neonatal morbidity (bronchopulmonary dysplasia, severe necrotizing enterocolitis, Stage 3–5 retinopathy of prematurity and intraventricular hemorrhage Grades 3–4) and a combined outcome of death or morbidity.
Results:
12% of very preterm live-born infants had a mother with SMM. Maternal and pregnancy characteristics associated with occurrence of SMM were multiparity, being non-Hispanic black and preexisting health conditions, but gestational age and the percentage small for gestational age did not differ. Infants whose mothers experienced SMM had higher first-year mortality, 11.2% versus 7.7% without SMM, yielding a relative risk of 1.39 (95% confidence interval: 1.14–1.70) after adjustment for maternal characteristics, preexisting co-morbidities, pregnancy complications and hospital factors. Severe neonatal morbidity was not associated with SMM.
Conclusions:
Severe maternal morbidity is an independent risk factor for mortality in the first year of life among very preterm infants after consideration of other maternal and pregnancy risk factors.
Severe maternal morbidity, defined as a life-threatening diagnosis or a lifesaving procedure during the delivery hospitalization (1, 2) occurs more frequently in very preterm than term deliveries. In high income countries, 1–2%of women experience SMM during their delivery hospitalization, with an estimated 20% of cases occurring before 31+6 weeks of gestation (3). There has been a significant focus on the life threatening nature of SMM for moms, but no inquiry on how it impacts vulnerable very preterm babies. Very preterm babies account for over one-half of infant deaths and face high risks of morbidity and developmental impairment (4–7).
Research on mothers and very preterm infants is often conducted in silos and therefore it is not known what percent of very preterm infants have a mother who experiences a severe morbid event. This information may be particularly relevant for very preterm infants because the context and events around birth, including the quality of obstetrical management, are determinant for their prognosis (8–12). Sub-optimal care during the delivery hospitalization has been shown to raise a mother’s risks of SMM (13, 14) and this may extend to higher risks for her infant. SMM could also be a marker for the severity of some pregnancy complications which have a negative impact on the infant.
Our goal was to estimate the prevalence of SMM among births before 31+6 weeks of gestation, to identify risk factors for co-occurrence of SMM in the setting of very preterm birth and to investigate its association with risks of infant mortality and severe neonatal morbidity.
Methods
We used New York City Vital Statistics birth and death records linked with New York State discharge abstract data, The Statewide Planning and Research Cooperative System (SPARCS), for all live births in New York City hospitals in the years 2010 to 2014. The New York State Department of Health and Human Services linked the data using a probabilistic linking methodology and more than 98% of all infant records were linked to vital statistics data and a further 98% of maternal discharge abstracts were linked with infant records. The linked dataset included birth data, death data, and the infant’s and mother’s hospital discharge records for all infants born from 2010 to 2014 who were discharged by December 31, 2014, and all infant deaths from 2010 to 2015. Institutional Review Board approvals were obtained from the New York City Department of Health and Mental Hygiene, the New York State Department of Health, and the Icahn School of Medicine at Mount Sinai.
The study population included births without congenital anomalies between 24+0 and 31+6 weeks of gestation (6901 infants who were born to 6138 mothers). We focused on very preterm births, defined as births under 31+6 weeks (15) as these infants face highest risks of mortality and morbidity, and care quality in the perinatal period has been associated with their risks of mortality and morbidity (8–12). We excluded births less than 24+0 weeks of gestational age and those with congenital anomalies because of their high risks of mortality and the possibility that death may have reflected a decision to abstain from active treatment. Cases with congenital anomalies were excluded based on diagnosis codes from the infant’s SPARCS record, following previous studies (16, 17).
Our primary outcome variable was first year mortality, including deaths in-hospital and after discharge. We also investigated severe neonatal morbidity, defined by the presence on the infant birth hospital record of the ICD-9 codes of any of the following diagnoses: bronchopulmonary dysplasia, necrotizing enterocolitis (Unspecified, Stage 2–3, Laparotomy), retinopathy of prematurity (Stage 3–5), intraventricular hemorrhage (Grade 3–4), as in previous studies (18, 19); detailed codes are provided in Table 1 (available at www.jpeds.com). We created a composite variable of either first-year death or any neonatal morbidity during the delivery hospitalization. We assumed that children discharged home without these diagnoses were at very low risk of developing them after discharge.
Table 1.
(on-line) Severe neonatal morbidity ICD-9 diagnosis and procedure codes
| Outcome | Subgroup | ICD-9 code |
|---|---|---|
| BPD | 770.7 | |
| NEC | ||
| NEC_unspec | 777.5 | |
| NEC_stage2 | 777.52 | |
| NEC_stage3 | 777.53 | |
| Laparotomy | 456, 4561, 4562, 4563, 457, 4571, 4572, 4573, 4574, 4575, 4576, 4579, 458, 459, 4590, 4591, 4592,4593, 4594, 4595, 460, 4601, 4602, 4603, 4604, 4605, 461, 4610, 4611, 4613, 4614, 462, 4620, 4621 4622, 4623, 4624, 463, 4631, 4632, 4639, 464, 4640, 4641, 4642, 4643, 468, 4680, 4681, 4682, 4685, 4686, 4687, 541, 541,5411, 5412, 5419 | |
| ROP | ||
| ROP_stage3 | 362.25 | |
| ROP_stage4 | 362.26 | |
| ROP_stage5 | 362.27 | |
| ROP_surg | 142, 1421, 1422, 1423, 1424, 1425, 1426, 1427, 1429, 1434, 144, 1441, 1449, 145, 1451, 1452, 1453, 1454, 1455, 1459 | |
| IVH | ||
| IVH_grade3 | 772.13 | |
| IVH_grade4 | 772.14 |
We used a published algorithm to identify severe maternal morbidity, using diagnoses for life-threatening conditions and procedure codes for life-saving procedures defined by investigators from the Centers for Disease Control and Prevention (20, 21). The algorithm excludes severe maternal diagnoses for hospitalizations with a short length of stay in order to reduce misclassification associated with coding errors. A short length of stay is defined as less than the 90th percentile corresponding to ≤2 days for vaginal, ≤4 days for primary cesarean, and ≤3 days for repeat cesarean deliveries) However, length of stay reclassification is not applied to in-hospital maternal deaths, transfers or delivery hospitalizations with severe complications identified by procedure codes (20). We calculated in-hospital stay based on postpartum stay, as done by other researchers developing SMM indicators (22). Because pregnant women with high risk pregnancies can have long antenatal hospitalizations unrelated to severe maternal morbidity, postpartum stay is more relevant for our population. As this deviates from the published algorithm, we also computed SMM using total hospital stay and found an almost identical proportion of SMM cases.
Co-variables included socioeconomic and clinical characteristics known to influence both maternal and infant outcomes that were available on the birth certificate, including self-identified race and ethnicity, maternal age, country of birth, multiple pregnancy, history of previous cesarean delivery, body mass index and prenatal care. Maternal co-morbidities were ascertained from the mother’s SPARCS record as well as the birth certificate in order to maximize sensitivity of our measures (23). We included medical risk factors that could lead to maternal morbidity, but were likely present on admission to the hospital (e.g., diabetes, hypertension, disorders of placentation) (24, 25). We also took into consideration newborn characteristics in our models, including gestational age, sex and small for gestational age based on US birthweight charts (26). We also described mode of delivery, but did not include it in our models as we did not have information on its timing with respect to SMM. Finally, we included hospital characteristics, including public/private ownership and nursery level.
Missing data were infrequent in this dataset; variables with the most missing data were the number of prenatal visits (3.9%), maternal prepregnancy body mass index (1.6%) and maternal educational level (0.8%).
Statistical Analyses
We first estimated the prevalence of SMM in our very preterm cohort, defined as the percent of newborns who had a mother with SMM. To assess risk factors for co-occurrence of SMM, we described the distribution of co-variables among newborns with and without mothers with SMM and modeled adjusted risk ratios, considering demographic and social variables and pregnancy complications. To evaluate the impact of SMM on infant outcomes, we compared our outcomes based on SMM co-occurrence using Chi squared tests. We then estimated risk ratios, adjusting for maternal, infant and hospital factors and taking into consideration clustering within hospitals and within multiple pairs (27, 28). Adjusted relative risks were derived using a modified Poisson regression model with robust standard errors (27, 28). To describe risks of mortality over time, we constructed Kaplan-Meier survival curves and survival estimates over the first six months of life were derived from the life table. Log rank tests were used to compare survival. We also looked at the proportion of deaths occurring before and after discharge from hospital. We used SAS 9.4 for all analyses.
Results
In our cohort of births between 24+0 and 31+6 weeks of gestation, 843 of 6901 (12.2%) of infants had a mother with a severe morbid event. Co-occurrence of SMM occurred more often among infants with older, multiparous and non-Hispanic black mothers, as shown in Table 2. Other sociodemographic characteristics and prenatal care visits were not significantly different. Pregestational diabetes, hypertensive disorders and preeclampsia and higher order multiple pregnancies were associated with SMM, and chorioamnionitis and gestational diabetes were similar in the 2 groups, and preterm premature rupture of membranes and precipitous labor were more common in preterm births without SMM. There were more cesarean deliveries among cases with SMM. The gestational age and percentage of small for gestational age infants did not differ by occurrence of SMM. There was also no difference in the distribution of SMM and non-SMM cases in public vs. private hospitals, but SMM cases occurred more often in hospitals with higher level nurseries.
TABLE 2.
Characteristics of live born very preterm newborns overall and by whether their mother had severe maternal morbidity (SMM)
| All newborns | Mother without SMM | Mother with SMM | P value | |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Total | 6901 (100) | 6057 (87.8) | 843 (12.2) | |
| Mother’s age | <0.001 | |||
| <20 | 369 (5.4) | 339 (5.6) | 30 (3.6) | |
| 20–34 | 4620 (67.0) | 41056 (67.8) | 515 (61.1) | |
| 35–39 | 1400 (20.3) | 1187 (19.6) | 213 (25.3) | |
| 40 or older | 512 (7.4) | 427 (7.0) | 85 (9.1) | |
| Parity | <0.001 | |||
| Nulliparous | 3060 (44.3) | 2757 (45.5) | 303 (35.9) | |
| Multiparous | 3819 (55.3) | 3288 (54.3) | 531 (63.0) | |
| Mother’s race | 0.008 | |||
| Non-Hispanic black | 2639 (38.2) | 2274 (37.5) | 365 (43.3) | |
| Hispanic | 2089 (30.3) | 1842 (30.4) | 247 (29.3) | |
| Non-Hispanic white | 1385 (20.1) | 1241 (20.5) | 144 (17.1) | |
| Asian | 749 (10.9) | 669 (11.0) | 80 (9.5) | |
| Mother’s education | 0.9à | |||
| Less than high school | 1557 (22.6) | 1365 (22.5) | 192 (22.8) | |
| High school | 1608 (23.3) | 1416 (23.4) | 192 (22.8) | |
| Greater than high school | 3682 (53.4) | 3241 (53.5) | 443 (52.6) | |
| Mother’s insurance | 0.07 | |||
| Governmental | 4209(61.0) | 3665(60.5) | 544(64.5) | |
| Commercial | 2493(36.1) | 2214(36.5) | 279(33.1) | |
| Uninsured | 199(2.9) | 179(3.0) | 20(2.4) | |
| Mother’s prenatal visits | 0.9 | |||
| 0–5 | 1923 (27.9) | 1682 (27.8) | 241 (28.6) | |
| 6–8 | 2006 (29.1) | 1760 (29.1) | 246 (29.3) | |
| 9 plus | 2717 (39.4) | 2394 (39.5) | 323 (38.3) | |
| missing | 255 (3.7) | 222 (3.7) | 33 (3.9) | |
| Smoked during pregnancy | 270 (3.9) | 238 (3.9) | 32 (3.8) | 0.90 |
| Pre-pregnancy body mass index | 0.55 | |||
| Underweight (<18.5) | 315(4.6) | 278(4.6) | 37(4.5) | |
| Normal weight (18.5–24.9) | 3078(45.3) | 2695(45.1) | 383(46.8) | |
| Overweight (25.0–29.9) | 1751(25.8) | 1536(25.7) | 215(26.3) | |
| Obese (≥30) | 1655(24.3) | 1472(24.6) | 183(22.4) | |
| Plurality | ||||
| Singleton | 5124 (74.3) | 4506 (74.4) | 618 (73.3) | 0.02 |
| Twins | 1580 (22.9) | 1393 (23.0) | 187 (22.2) | |
| Triplets or quadruplets | 197 (2.9) | 159 (2.6) | 38 (4.5) | |
| Vaginal delivery | 2118 (30.7) | 2040 (33.7) | 78 (9.2) | <0.001 |
| Cesarean section | 4783 (69.3) | 4018 (66.3) | 765 (90.8) | <0.001 |
| Trial of labor | 672 (9.7) | 574 (9.5) | 98 (11.6) | 0.001 |
| Maternal complications | ||||
| Pregestational diabetes | 185 (2.7) | 140 (2.3) | 45 (5.3) | <0.001 |
| Gestational diabetes | 676 (9.8) | 591 (9.8) | 85 (10.1) | 0.76 |
| Pregestational hypertension | 592 (8.6) | 472 (7.8) | 120 (14.2) | <0.001 |
| Gestational hypertension | 1749 (25.3) | 1449 (23.9) | 300 (35.6) | <0.001 |
| Preeclampsia | 1243 (18.0) | 1031 (17.0) | 212 (25.2) | <0.001 |
| Chorioamnionitis | 1197 (17.4) | 1059 (17.5) | 138 (16.4) | 0.42 |
| Premature rupture of the membranes | 2537 (36.8) | 2331 (38.5) | 206 (24.4) | <0.001 |
| Precipitous labor | 316 (4.6) | 300 (5.0) | 16 (1.9) | <0.001 |
| Gestational age (wks) | ||||
| 24–25 | 1014 (14.7) | 899 (14.8) | 115 (13.6) | 0.32 |
| 26–27 | 1283 (18.6) | 1113 (18.4) | 170 (20.2) | |
| 28–29 | 1707 (24.7) | 1487 (24.6) | 220 (26.1) | |
| 30–31 | 2897 (42.0) | 2559 (42.2) | 338 (40.1) | |
| Infant small for gestational age1 | 911 (13.2) | 785 (13.0) | 126 (15.0) | 0.11 |
| Sex | ||||
| Female | 3301 (47.8) | 2871 (47.4) | 430 (51.0) | 0.05 |
| Male | 3600 (52.2) | 3187 (52.6) | 413 (50.0) | |
| Hospital characteristics | ||||
| Public | 1364 (19.8) | 1181 (19.5) | 183 (21.7) | 0.13 |
| Private | 5537 (80.2) | 4877 (80.5) | 660 (78.3) | |
| Nursery level | 0.002 | |||
| 2 | 235 (3.4) | 218 (3.6) | 17 (2.0) | |
| 3 | 3585 (52.0) | 3177 (52.4) | 408 (48.4) | |
| 4 | 3081 (44.7) | 2663 (44.0) | 418 (50.0) |
Note:
Birthweight <10th percentile of US birthweight charts (26).
In adjusted models (Table 3), older maternal age, multiparity, non-Hispanic black race/ethnicity and pregestational diabetes and hypertension were related to a higher likelihood of co-occurrence of SMM. Prepregnancy obesity was negatively related to the probability of co-occurrence of SMM.
Table 3:
Characteristics associated with having a mother with SMM for very preterm newborns
| Unadjusted Risk Ratios with 95% CI | Adjusted Risk Ratios, with 95% CI1 | |
|---|---|---|
| Mother’s age | ||
| <20 | 0.72 (0.50–1.03) | 0.79 (0.54–1.14) |
| 20–34 | Ref | Ref |
| 35–39 | 1.34 (1.17–1.57) | 1.29 (1.11–1.51) |
| 40 or older | 1.46 (1.17–1.83) | 1.41 (1.12–1.78) |
| Parity | ||
| Nulliparous | Ref | Ref |
| Multiparous | 1.40 (1.23–1.60) | 1.30 (1.13–1.50) |
| Mother’s race | ||
| Non-Hispanic black | 1.33 (1.11–1.59) | 1.27 (1.04–1.54) |
| Hispanic | 1.14 (0.94–1.38) | 1.13 (0.92–1.39) |
| Non-Hispanic white | Ref | Ref |
| Asian | 1.03 (0.79–1.33) | 1.00 (0.77–1.31) |
| Mother’s education | ||
| Less than high school | 1.03 (0.88–1.20) | 0.97 (0.81–1.17) |
| High school | 0.99 (0.85–1.16) | 0.94 (0.79–1.11) |
| Greater than high school | Ref | Ref |
| Mother’s insurance | ||
| Governmental | 1.15 (1.01–1.32) | 1.16 (0.98–1.37) |
| Commercial | Ref | Ref |
| Uninsured | 0.90 (0.58–1.38) | 0.92 (0.58–1.45) |
| Mother’s prenatal visits | ||
| 0–5 | 1.05 (0.90–1.23) | 1.02 (0.87–1.21) |
| 6–8 | 1.03 (0.88–1.21) | 1.01 (0.87–1.18) |
| 9 plus | Ref | Ref |
| Smoked during pregnancy | 0.97 (0.70–1.35) | 1.01 (0.72–1.40) |
| Body mass index | ||
| Underweight (<18.5) | 0.92 (0.67–1.26) | 1.02 (0.74–1.40) |
| Normal weight (18.5–24.9) | Ref | Ref |
| Overweight (25.0–29.9) | 0.96 (0.82–1.12) | 0.86 (0.73–1.01) |
| Obese (≥30) | 0.86 (0.73–1.02) | 0.72 (0.60–0.85) |
| Multiple pregnancy | 1.06 (0.91–1.21) | 1.05 (0.90–1.23) |
| Maternal complications | ||
| Pregestational diabetes | 2.05 (1.57–2.66) | 1.76 (1.34–2.31) |
| Gestational diabetes | 1.03 (0.83–1.27) | 1.00 (0.80–1.25) |
| Pregestational hypertension | 1.77 (1.49–2.10) | 1.29 (1.05–1.58) |
| Gestational hypertension | 1.63 (1.42–1.85) | 1.20 (0.95–1.52) |
| Preeclampsia | 1.53 (1.32–1.76) | 1.14 (0.90–1.46) |
| Chorioamnionitis | 0.93 (0.79–1.11) | 1.08 (0.90–1.29) |
| Premature rupture of the membranes | 0.56 (0.48–0.65) | 0.60 (0.51–0.71) |
| Precipitous labor | 0.40 (0.25–0.65) | 0.45 (0.28–0.73) |
| Newborn characteristics | ||
| Gestational age (wks) | ||
| 24–25 | 0.97 (0.80–1.19) | 1.15 (0.93–1.42) |
| 26–27 | 1.14 (0.96–1.35) | 1.20 (1.00–1.43) |
| 28–29 | 1.10 (0.94–1.29) | 1.14 (0.97–1.34) |
| 30–31 | Ref | Ref |
| Small for gestational age2 | 1.15 (0.97–1.38) | 0.89 (0.74–1.07) |
| Sex | ||
| Female | Ref | Ref |
| Male | 0.88 (0.77–1.00) | 0.93 (0.82–1.05) |
| Hospital characteristics | ||
| Public | 1.13 (0.97–1.31) | 1.08 (0.91–1.29) |
| Private | Ref | |
| Nursery level | ||
| 2 | Ref | |
| 3 | 1.57 (0.99–2.51) | 1.53 (0.94–2.49) |
| 4 | 1.88 (1.18–2.99) | 1.88 (1.16–3.06) |
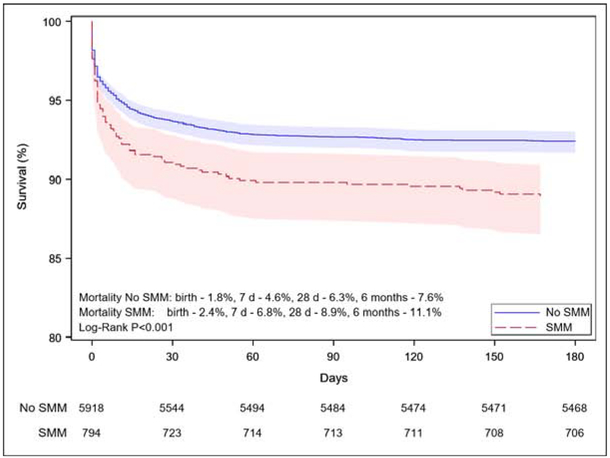
First-year mortality was higher for infants with a mother who experienced SMM: 11.2% versus 7.7% when SMM did not occur (P < .001). A majority of deaths in both groups took place in the first week, but relative risks remained elevated after delivery: mortality in the first day of life was 2.5% versus 1.9% (p=0.004) for children born to mothers with and without SMM and 6.8% versus 4.6% (p=0.004) over the next six days respectively, as reported in the Figure, which shows mortality risks over the first six months. Almost all first year deaths occurred within this time frame. The proportion of deaths that occurred during the neonatal hospitalization versus after discharge was similar: 92.9% of babies in the SMM group and 92.2 % in the group without SMM.
Figure.
Infant survival over the first six months of life by occurrence of severe maternal morbidity
Adjusting for sociodemographic, clinical and hospital factors reduced the relative risk of first year mortality associated with SMM from 1.47 (95% CI 1.15–1.84) to 1.39 (95% CI 1.14–1.70). Severe neonatal morbidity was not associated with the occurrence of SMM in unadjusted or adjusted models and SMM was not significant in the composite mortality and morbidity model (Table 4).
Table 4.
Impact of occurrence of severe maternal morbidity on very preterm outcomes
| Neonatal outcomes | Mother without SMM n (%) | Mother with SMM n (%) | p-value | Unadjusted RR | 95%CI | Adjusted RR1 | 95%CI |
|---|---|---|---|---|---|---|---|
| 1 year mortality | 462 (7.8) | 91 (11.4) | <0.001 | 1.47 | 1.17–1.83 | 1.39 | 1.14–1.70 |
| Severe neonatal morbidity | 1294 (21.9) | 178 (22.4) | 0.75 | 1.02 | 0.87–1.20 | 0.97 | 0.85–1.11 |
| Composite death or morbidity | 1615 (27.3) | 249 (31.3) | 0.02 | 1.15 | 1.04–1.31 | 1.10 | 0.99–1.22 |
NOTE:
Adjusted for variables in Table 2 and controlling for clustering patients within the hospital of delivery and multiples.
Discussion
Severe maternal morbidity occurred in 12% of very preterm live births and was associated with a 39% increased risk of infant death after adjustment for sociodemographic and clinical factors. These higher mortality risks were constant across the first 6 months of life. SMM was unrelated to gestational age or growth restriction, the main prognostic factors for very preterm mortality. By identifying SMM as a risk factor for these infants, our study raises the possibility that quality initiatives targeting SMM could achieve reductions in their mortality.
Our results are consistent with a Canadian study that found higher mortality among infants admitted to a neonatal unit when mothers were also admitted to an intensive care unit (29), but few studies have investigated the association between maternal morbidity and very preterm outcomes. The relatively high occurrence of SMM in our study is concordant with research showing that many cases of SMM are associated with very preterm delivery (30). In addition, mortality rates are in line with those reported in other cohorts of non-anomalous live births between 24+0 and 31+6 weeks of gestation (31, 32).
Our results corroborate studies showing that older maternal age, being non-Hispanic black and hypertensive disorders are risk factors for SMM (14, 24). In contrast, maternal education, number of prenatal visits, gestational age and growth restriction were not associated with SMM among very preterm births. Obesity was negatively associated with SMM risk, after adjustment for pregnancy complications such as hypertension and diabetes, which may reflect the high risk of spontaneous extremely preterm delivery among obese women (33–35). Cesarean delivery was more common in SMM cases, as seen in other studies (36, 37).
SMM did not affect risks of severe neonatal morbidity. If mortality risks are raised among the most vulnerable infants, survivors may be less likely to experience morbidity, giving the erroneous impression that morbidity risk is not impacted by SMM. Alternatively, SMM could truly have a lesser impact on morbidity risk; some studies have found that composite morbidity measures containing similar pathologies were less sensitive to perinatal management than mortality (9, 38). Additionally, there may be an impact on other morbidities, such as sepsis, which affect risks of death (39, 40), but cannot be measured reliably with hospital discharge data (41).
There are several explanations for the association between SMM and infant mortality. Quality of care is a determinant of adverse maternal as well as very preterm outcomes (9, 13), including in our prior research in New York City (14, 17). Higher risks for infants could be observed if poorly performing hospitals for mothers also performed poorly for babies, as suggested in a previous study which found a correlation between hospital performance for SMM and term newborn morbidity (25). Future research on SMM and infant outcomes should include an expanded population of moderate preterm and term infants to evaluate whether these adverse effects are observed after the very preterm period.
Another explanation could be a negative impact of some maternal complications, such as preeclampsia or placental abruption, which increase risks for an SMM event and are also at the origin of the preterm birth. However, although these complications are risk factors for poor outcome in general population studies (42, 43), research on very preterm infants has not shown that they increase mortality risks (44), although abruption may be related to neonatal morbidity (45). It is possible, nonetheless, that SMM may be a marker of the severity of some complications which are poorly captured by available data on co-morbidities.
Our study calls attention to the relevance of a research framework based on the mother-child dyad. This paradigm could have broader application. For instance, SMM may have longer-term effects on very preterm children’s health and development. It is negatively associated with breastfeeding (46), which requires initiation and frequent milk expression soon after delivery, and leads to longer maternal hospitalization, often in a different facility (29), which could affect the process of bonding with the newborn. More broadly, mothers who experience severe maternal morbidity may be at increased risk for postpartum depression or suffer from other health sequelae which could impact the mother-baby relationship (47, 48). There is some evidence that maternal depression and anxiety are related to adverse developmental outcomes for children born very preterm (49). Furthermore, child outcomes may be associated with maternal risks of SMM or with maternal recovery and resilience after SMM, as suggested in the previously cited Canadian study, where the mother’s risks of death in the ICU was higher when her infant was admitted to a neonatal intensive care unit (29).
This study’s strengths are its population-based design, use of variables from hospital discharge and vital statistics, linkage of maternal and newborn data, the large sample enabling investigation of two uncommon outcomes and consideration of clustering within hospitals and multiple pairs. Because we had data on timing of death, we were able to compare changes in mortality risks over time; however, information on timing was not available for neonatal or maternal morbidities. Other limitations include the exclusion of stillbirths. Given that SMM may affect risks of intrapartum death, adding stillbirths could lead to a strengthening of the association between SMM and mortality. Further, coding errors for diagnoses and procedures are of concern when using hospital discharge and birth certificate data (50), although using both sources improves the sensitivity of data on maternal co-morbidites (23). Finally, it was not possible to differentiate between maternal and fetal indications for delivery.
Severe maternal morbidity occurred in over one in ten very preterm deliveries and was a risk factor for infant death, but not severe neonatal morbidity, after adjusting for other known prognostic factors. Further investigation is needed to understand the causes of this excess mortality risk in order to develop strategies to improve perinatal management. These results should encourage researchers to include data on maternal outcomes related to childbirth in research network databases and cohort studies of very preterm infants.
Acknowledgments
Supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health (R01HD078565) and the National Institute on Minority Health and Health Disparities (R01MD007651). The authors declare no conflicts of interest.
List of abbreviations
- SPARCS
Statewide Planning and Research Cooperative System
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.D’Alton ME, Bonanno CA, Berkowitz RL, Brown HL, Copel JA, Cunningham FG, et al. Putting the “M” back in maternal-fetal medicine. Am J Obstet Gynecol. 2013;208(6):442–8. [DOI] [PubMed] [Google Scholar]
- 2.Callaghan WM, Mackay AP, Berg CJ. Identification of severe maternal morbidity during delivery hospitalizations, United States, 1991–2003. Am J Obstet Gynecol. 2008;199(2):133 e1–8. [DOI] [PubMed] [Google Scholar]
- 3.Kilpatrick SJ, Abreo A, Gould J, Greene N, Main EK. Confirmed severe maternal morbidity is associated with high rate of preterm delivery. Am J Obstet Gynecol. 2016;215(2):233 e1–7. [DOI] [PubMed] [Google Scholar]
- 4.Linsell L, Malouf R, Morris J, Kurinczuk JJ, Marlow N. Prognostic Factors for Poor Cognitive Development in Children Born Very Preterm or With Very Low Birth Weight: A Systematic Review. JAMA pediatrics. 2015;169(12):1162–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371(9608):261–9. [DOI] [PubMed] [Google Scholar]
- 6.Foix-L’Helias L, Marret S, Ancel PY, Marchand L, Arnaud C, Fresson J, et al. Impact of the use of antenatal corticosteroids on mortality, cerebral lesions and 5-year neurodevelopmental outcomes of very preterm infants: the EPIPAGE cohort study. Bjog. 2008;115(2):275–82. [DOI] [PubMed] [Google Scholar]
- 7.Serenius F, Kallen K, Blennow M, Ewald U, Fellman V, Holmstrom G, et al. Neurodevelopmental outcome in extremely preterm infants at 2.5 years after active perinatal care in Sweden. JAMA. 2013;309(17):1810–20. [DOI] [PubMed] [Google Scholar]
- 8.Kallen K, Serenius F, Westgren M, Marsal K, Group E. Impact of obstetric factors on outcome of extremely preterm births in Sweden: prospective population-based observational study (EXPRESS). Acta obstetricia et gynecologica Scandinavica. 2015;94(11):1203–14. [DOI] [PubMed] [Google Scholar]
- 9.Zeitlin J, Manktelow BN, Piedvache A, Cuttini M, Boyle E, van Heijst A, et al. Use of evidence based practices to improve survival without severe morbidity for very preterm infants: results from the EPICE population based cohort. BMJ. 2016;354:i2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rogowski JA, Horbar JD, Staiger DO, Kenny M, Carpenter J, Geppert J. Indirect vs direct hospital quality indicators for very low-birth-weight infants. Jama. 2004;291(2):202–9. [DOI] [PubMed] [Google Scholar]
- 11.Lasswell SM, Barfield WD, Rochat RW, Blackmon L. Perinatal regionalization for very low-birth-weight and very preterm infants: a meta-analysis. JAMA. 2010;304(9):992–1000. [DOI] [PubMed] [Google Scholar]
- 12.Horbar JD, Edwards EM, Greenberg LT, Morrow KA, Soll RF, Buus-Frank ME, et al. Variation in Performance of Neonatal Intensive Care Units in the United States. JAMA pediatrics. 2017;171(3):e164396. [DOI] [PubMed] [Google Scholar]
- 13.Ozimek JA, Eddins RM, Greene N, Karagyozyan D, Pak S, Wong M, et al. Opportunities for improvement in care among women with severe maternal morbidity. Am J Obstet Gynecol. 2016;215(4):509 e1–6. [DOI] [PubMed] [Google Scholar]
- 14.Howell EA, Egorova NN, Balbierz A, Zeitlin J, Hebert PL. Site of delivery contribution to black-white severe maternal morbidity disparity. Am J Obstet Gynecol. 2016;215(2):143–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howson C, Kinney M, Lawn J. Born Too Soon: the global action report on preterm birth March of Dimes, PMNCH, Save the Children, WHO, https://apps.who.int/iris/bitstream/handle/10665/44864/9789241503433_eng.pdf;jsessionid=11AC05786228B899E33BEDCAAA8AAFE2?sequence=1 (accessed 6/22/19), 2012. [Google Scholar]
- 16.Phibbs CS, Baker LC, Caughey AB, Danielsen B, Schmitt SK, Phibbs RH. Level and volume of neonatal intensive care and mortality in very-low-birth-weight infants. N Engl J Med. 2007;356(21):2165–75. [DOI] [PubMed] [Google Scholar]
- 17.Howell EA, Zeitlin J. Quality of Care and Disparities in Obstetrics. Obstet Gynecol Clin North Am. 2017;44(1):13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jensen EA, Lorch SA. Effects of a Birth Hospital’s Neonatal Intensive Care Unit Level and Annual Volume of Very Low-Birth-Weight Infant Deliveries on Morbidity and Mortality. JAMA Pediatr. 2015;169(8):e151906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howell EA, Janevic T, Hebert PL, Egorova NN, Balbierz A, Zeitlin J. Differences in Morbidity and Mortality Rates in Black, White, and Hispanic Very Preterm Infants Among New York City Hospitals. JAMA Pediatr. 2018;172(3):269–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Callaghan WM, Creanga AA, Kuklina EV. Severe maternal morbidity among delivery and postpartum hospitalizations in the United States. Obstet Gynecol. 2012;120(5):1029–36. [DOI] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention. Severe Maternal Morbidity in the United States. 2013; https://www.cdc.gov/reproductivehealth/maternalinfanthealth/severematernalmorbidity.htm Accessed 1 July 2017.
- 22.Main EK, Abreo A, McNulty J, Gilbert W, McNally C, Poeltler D, et al. Measuring severe maternal morbidity: validation of potential measures. Am J Obstet Gynecol. 2016;214(5):643 e1–e10. [DOI] [PubMed] [Google Scholar]
- 23.Lydon-Rochelle MT, Holt VL, Cardenas V, Nelson JC, Easterling TR, Gardella C, et al. The reporting of pre-existing maternal medical conditions and complications of pregnancy on birth certificates and in hospital discharge data. Am J Obstet Gynecol. 2005;193(1):125–34. [DOI] [PubMed] [Google Scholar]
- 24.Gray KE, Wallace ER, Nelson KR, Reed SD, Schiff MA. Population-based study of risk factors for severe maternal morbidity. Paediatr Perinat Epidemiol. 2012;26(6):506–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howell EA, Zeitlin J, Hebert PL, Balbierz A, Egorova N. Association between hospital-level obstetric quality indicators and maternal and neonatal morbidity. JAMA. 2014;312(15):1531–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olsen IE, Groveman SA, Lawson ML, Clark RH, Zemel BS. New intrauterine growth curves based on United States data. Pediatrics. 2010;125(2):e214–24. [DOI] [PubMed] [Google Scholar]
- 27.Zou GY, Donner A. Extension of the modified Poisson regression model to prospective studies with correlated binary data. Stat Methods Med Res. 2013;22(6):661–70. [DOI] [PubMed] [Google Scholar]
- 28.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162(3):199–200. [DOI] [PubMed] [Google Scholar]
- 29.Ray JG, Urquia ML, Berger H, Vermeulen MJ. Maternal and neonatal separation and mortality associated with concurrent admissions to intensive care units. CMAJ. 2012;184(18):E956–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kilpatrick SJ, Abreo A, Gould J, Greene N, Main EK. Confirmed severe maternal morbidity is associated with high rate of preterm delivery. Am J Obstet Gynecol. 2016. [DOI] [PubMed] [Google Scholar]
- 31.Edstedt Bonamy AK, Zeitlin J, Piedvache A, Maier RF, van Heijst A, Varendi H, et al. Wide variation in severe neonatal morbidity among very preterm infants in European regions. Arch Dis Child Fetal Neonatal Ed. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manuck TA, Rice MM, Bailit JL, Grobman WA, Reddy UM, Wapner RJ, et al. Preterm neonatal morbidity and mortality by gestational age: a contemporary cohort. Am J Obstet Gynecol. 2016;215(1):103 e1–e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cnattingius S, Villamor E, Johansson S, Edstedt Bonamy AK, Persson M, Wikström AK, et al. Maternal obesity and risk of preterm delivery. JAMA. 2103;309(22):2362–70. [DOI] [PubMed] [Google Scholar]
- 34.Lynch AM, Hart JE, Agwu OC, Fisher BM, West NA, Gibbs RS. Association of extremes of prepregnancy BMI with the clinical presentations of preterm birth. Am J Obstet Gynecol. 2014;210(5):428 e1–9. [DOI] [PubMed] [Google Scholar]
- 35.Shaw GM, Wise PH, Mayo J, Carmichael SL, Ley C, Lyell DJ, et al. Maternal prepregnancy body mass index and risk of spontaneous preterm birth. Paediatr Perinat Epidemiol. 2014;28(4):302–11. [DOI] [PubMed] [Google Scholar]
- 36.Korb D, Goffinet F, Seco A, Chevret S, Deneux-Tharaux C, Group ES. Risk of severe maternal morbidity associated with cesarean delivery and the role of maternal age: a population-based propensity score analysis. CMAJ. 2019;191(13):E352–E60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leonard SA, Main EK, Carmichael SL. The contribution of maternal characteristics and cesarean delivery to an increasing trend of severe maternal morbidity. BMC Pregnancy Childbirth. 2019;19(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilson E, Maier RF, Norman M, Misselwitz B, Howell EA, Zeitlin J, et al. Admission Hypothermia in Very Preterm Infants and Neonatal Mortality and Morbidity. J Pediatr. 2016;175:61–7 e4. [DOI] [PubMed] [Google Scholar]
- 39.Corchia C, Ferrante P, Da Fre M, Di Lallo D, Gagliardi L, Carnielli V, et al. Cause-specific mortality of very preterm infants and antenatal events. J Pediatr. 2013;162(6):1125–32, 32 e1–4. [DOI] [PubMed] [Google Scholar]
- 40.Schindler T, Koller-Smith L, Lui K, Bajuk B, Bolisetty S, New South W, et al. Causes of death in very preterm infants cared for in neonatal intensive care units: a population-based retrospective cohort study. BMC pediatrics. 2017;17(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ford JB, Roberts CL, Algert CS, Bowen JR, Bajuk B, Henderson-Smart DJ, et al. Using hospital discharge data for determining neonatal morbidity and mortality: a validation study. BMC health services research. 2007;7:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Downes KL, Shenassa ED, Grantz KL. Neonatal Outcomes Associated With Placental Abruption. American journal of epidemiology. 2017;186(12):1319–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ananth CV, Friedman AM. Ischemic placental disease and risks of perinatal mortality and morbidity and neurodevelopmental outcomes. Semin Perinatol. 2014;38(3):151–8. [DOI] [PubMed] [Google Scholar]
- 44.Delorme P, Goffinet F, Ancel PY, Foix-L’Helias L, Langer B, Lebeaux C, et al. Cause of Preterm Birth as a Prognostic Factor for Mortality. Obstet Gynecol. 2016;127(1):40–8. [DOI] [PubMed] [Google Scholar]
- 45.Chevallier M, Debillon T, Pierrat V, Delorme P, Kayem G, Durox M, et al. Leading causes of preterm delivery as risk factors for intraventricular hemorrhage in very preterm infants: results of the EPIPAGE 2 cohort study. Am J Obstet Gynecol. 2017;216(5):518 e1–e12. [DOI] [PubMed] [Google Scholar]
- 46.Thompson JF, Heal LJ, Roberts CL, Ellwood DA. Women’s breastfeeding experiences following a significant primary postpartum haemorrhage: A multicentre cohort study. Int Breastfeed J. 2010;5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Furuta M, Sandall J, Cooper D, Bick D. The relationship between severe maternal morbidity and psychological health symptoms at 6–8 weeks postpartum: a prospective cohort study in one English maternity unit. BMC Pregnancy Childbirth. 2014;14:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Furuta M, Sandall J, Bick D. A systematic review of the relationship between severe maternal morbidity and post-traumatic stress disorder. BMC Pregnancy Childbirth. 2012;12:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huhtala M, Korja R, Lehtonen L, Haataja L, Lapinleimu H, Rautava P, et al. Parental psychological well-being and behavioral outcome of very low birth weight infants at 3 years. Pediatrics. 2012;129(4):e937–44. [DOI] [PubMed] [Google Scholar]
- 50.Lain SJ, Hadfield RM, Raynes-Greenow CH, Ford JB, Mealing NM, Algert CS, et al. Quality of data in perinatal population health databases: a systematic review. Med Care. 2012;50(4):e7–20. [DOI] [PubMed] [Google Scholar]