Abstract
Introduction:
Dry powder inhalers (DPIs) are popular for pulmonary drug delivery. Various techniques have been employed to produce inhalation drug particles and improve the delivery efficiency of DPI formulations. Physical stability of these DPI formulations is critical to ensure the delivery of a reproducible dose to the airways over the shelf-life.
Areas covered:
This review focuses on the impact of solid-state stability on aerosolization performance of DPI drug particles manufactured by powder production approaches and particle engineering techniques. It also highlights the different analytical tools that can be used to characterize the physical instability originating from production and storage.
Expert opinion:
A majority of the DPI literature focuses on the effects of physico-chemical properties such as size, morphology and density on aerosolization. While little has been reported on the physical stability, particularly the stability of engineered drug particles for use in DPIs. Literature data have shown that different particle engineering methods and storage conditions may cause physical instability of dry powders for inhalation and can significantly change the aerosol performance. A systematic examination of physical instability mechanisms in DPI formulations is necessary during formulation development in order to select the optimum formulation with satisfactory stability. In addition, the use of appropriate characterization tools are critical to detect and understand physical instability during the development of DPI formulations.
Keywords: Dry powder inhaler, physical stability, aerosol performance, powder characterization, particle engineering
1. Introduction
1.1. Inhalation medicines
Pulmonary drug delivery for treatment of lung disease has advantages over systemically administered therapy for a number of reasons, including: to avoid first pass metabolism and the possibility for enzymatic inactivation, to enable delivery of higher drug concentrations to the lungs for improved efficacy, and to reduce systemic side effects [1, 2]. Thus, pulmonary drug delivery has been explored extensively in the recent years for the treatment of various respiratory tract diseases such as chronic obstructive pulmonary disorder (COPD), asthma, pneumonia and chronic pulmonary infections. Inhalation also offers a non-invasive route for delivery of therapeutic drugs to treat systemic diseases [3, 4]. Pulmonary drug delivery has become one of the most accepted systems for treatment of respiratory disorders [5].
Inhalation medicines are known as “combination products” as they consist of not only formulations but also a device. Advancements in the device and particle technology over the past decade have made the inhaler easier to use and the delivery of drugs to the lungs more efficient [6, 7]. The most commonly used devices for pulmonary drug delivery are nebulizers, metered dose inhalers (MDIs), soft mist inhalers (SMIs), and dry powder inhalers (DPIs) [8, 9, 10]. Nebulizers have been widely used in hospitals, which usually requires professional oversight [11], but are also commonly used at home because even children and elderly patients can execute the tidal inhalation maneuver required of a nebulizer [12]. While traditional jet nebulizers are bulky and inconvenient to use, with poor delivery efficiency of 10-15% of the drug typically delivered to the lower respiratory tract [13], the newer generation of vibrating mesh nebulizers can achieve lung doses of 40 to 50% of the loaded dose. However, many vibrating mesh nebulizers are battery-powered and more expensive [14]. MDIs have historically been popular due to their simple design and inexpensive cost, but they usually have low drug delivery efficiency [15]. Improper use of MDIs (e.g., the inability to accurately coordinate device actuation with inspiration) often leads to poor compliance, low delivery efficiency and poor disease control [16]. In contrast, portable SMIs are propellant-free devices that generate an aqueous aerosol mist that is independent of inspiratory effort and thus reduces patient administration errors [17]. The disadvantage of both SMIs and MDIs is that they are generally only compatible with low-dose potent drugs (e.g., 10 to 200 μg dose per puff). There is a growing interest in DPIs because they are usually simple and portable devices with improved chemical stability of the drug in the solid form [18, 19].
Dry powder inhalers (DPIs) do not need propellants. They are portable devices that enable relatively easy administration of the formulation by the patient [20, 21]. Due to the solid form and relative chemical stability of the powder, DPIs do not require cold chain storage [22]. In the last two decades, particle engineering techniques such as spray drying, lyophilization, and super critical fluid technology have been employed to produce DPI formulations [5]. Effects of physico-chemical properties of particulate systems such as particle size, density, porosity, surface morphology, inter-particle force, surface energy etc. on the aerosol performance of DPIs have been extensively studied [23, 24, 25, 26]. However, the solid-state characteristics and physical stability of the powder formulations in DPIs are frequently overlooked in the literature even though they are critical to the quality and performance of the inhalation products. In this review article the physical stability of DPI formulations and its importance to the quality of DPIs is discussed.
1.2. Challenges with DPI formulation design
It is well recognized that in DPI formulations, the drug particles in the aerodynamic particle size range of 1-5 μm have the potential to reach the lower respiratory tract [27]. However, the fine particles in this size range are extremely cohesive and adhesive, which usually causes poor aerosolization performance. In certain formulations, large carrier particles are blended with small drug particles to improve powder flow from the inhaler device [28]. To achieve improved aerosol performance and effective lung deposition, various particle-engineering methods are employed to develop DPI formulations.
Inhalable drug particles have traditionally been produced by jet milling of crystalline drug particles into smaller micron-sized particles. However, these jet-milled particles often have a high surface energy and strong electrostatic charge, which makes the drug particles extremely cohesive. For low-dose drugs, carriers such as lactose are added to improve the flowability and aerosolization properties as well as act as a filler; however, it is not feasible to add large amounts of excipient for high-dose products such as antibiotics because it increases the total powder load and the number of inhalations required for the patient to complete the dose [5, 6]. Thus, for high-dose products, particle-engineering techniques such as spray drying have been employed to produce respirable-sized particles free of carriers. The mechanical treatment in both the milling and spray drying operations could impact not only the physico-chemical properties of the drug [29], but also the solid-state properties and physical stability, which may exert substantial impact on aerosolization performance and product quality [30].
Many powder production methods such as spray drying and jet milling form amorphous particles or amorphous regions on the crystalline particles that are metastable and tend to re-crystallize at increased relative humidity (RH) because water acts as a plasticizer [29, 31]. Such changes in the solid-state property of powders during manufacturing and storage could significantly impact particle size, morphology, dissolution and aerosol performance of the DPI formulation [32, 33]. For example, a 50% reduction in the plasma concentrations of fluticasone in healthy subjects treated with the Seretide Diskus dry powder inhaler (DPI) was observed when the devices were stored at 75% RH at 40 °C for 3 months, compared to the subjects treated with a DPI stored at 25 °C/30% RH [34]. This change in pharmacokinetics (PK) correlated well with the in-vitro data where the fine particle dose (FPD) (i.e., the drug particle mass with aerodynamic diameter ≤5 μm) decreased by more than 50% after storage at the higher humidity [35]. Therefore, understanding the influence of storage conditions on solid-state properties and physical stability of drug particles is critical for DPI formulations.
2. Effect of powder production methods on stability of dry powder inhalers
Drug particles for DPIs can be produced using different particle processing techniques, as shown in Figure 1. The aerosol performance of dry powder inhalers can be significantly impacted by changes in morphology, size, surface energy and surface composition of the drug particles [23]. Physico-chemical properties of the powders can be altered using various techniques, or by modifying operation parameters and/or formulation compositions [30, 36] in order to improve powder flow and aerosolization. However, it is also important to understand the effects of the various production methods that provide mechanical and thermal treatments on the solid-state properties and physical stability of the powders for DPIs.
Figure 1:
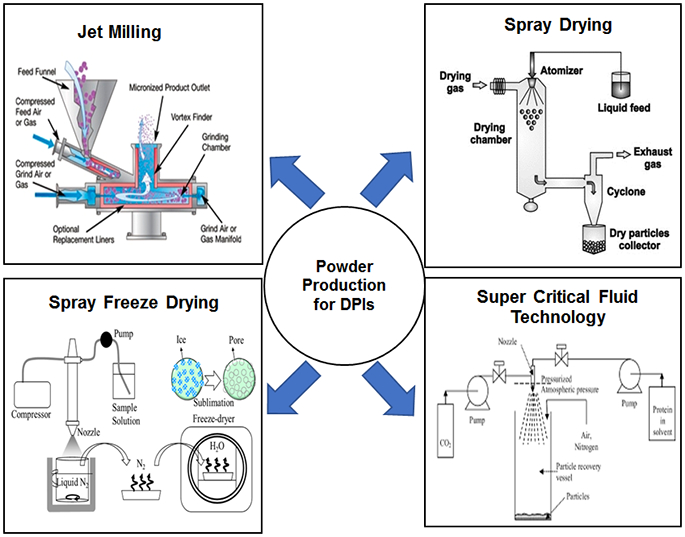
Schematic illustration of different powder production techniques used for the production of DPIs. (Reprinted with permission from [37], [38], [39], [40])
2.1. Milling
Micronization is a process that reduces the particle size down to micrometers by fracturing particles with mechanical energy or shear [41]. Jet milling the coarse particles into the inhalable size range is a common practice in the pharmaceutical industry. Jet mills have different types of action or design including fluid impact mills, opposed jet mills, spiral jet mills, oval chamber jet mills and fluidized bed opposed jet mills [30].
Jet milling uses pressurized gas to create high particle velocity and high-energy impaction between particles and/or between particles and the chamber walls to achieve micronization of particles. A jet mill essentially consists of a product inlet and a grinding chamber. The particles undergo multiple particle–particle and particle–wall impactions, which induce particle fracture and subsequent size reduction. Advantages of the jet milling process include convenience and low cost. Jet milling also micronizes the particles to a size suitable for inhalation [42].
Though jet milling is commonly used for DPIs, there are certain unfavorable characteristics of the inhalable particles produced by jet milling. The jet-milled powder is often extremely cohesive and prone to strong agglomeration due to the increase in surface energy and electrostatic charge [43]. The excess energy supplied during the milling process can lead to mechanical activation and development of local amorphous sites at the surface of the particle, which can result in re-crystallization during storage at high humidity environments [30, 44, 45, 46]. Young et al. studied the effect of freshly milled and milled-recrystallized lactose monohydrate on aerosol performance of 5% (w/w) nedocromil sodium trihydrate prepared by ball milling. The amorphous regions in freshly milled lactose monohydrate was intentionally re-crystallized upon storage at the relative humidity of 85% after milling for up to 60 minutes. It was shown that ball milling of a carrier such as α- Lactose monohydrate reduced the particle size while increasing the level of fine lactose and amorphous content [47]. For the freshly milled lactose, the amorphous content increased with milling time. However, the milled-recrystallized lactose remained completely crystalline during milling with 5% (w/w) nedocromil sodium trihydrate. The freshly milled lactose sample was physically unstable and the presence of increasing amorphous content resulted in a decrease in the fine particle fraction (FPF) compared to recrystallized lactose monohydrate (particles with aerodynamic diameter ≤ 5μm) [47].
This phenomenon has also been reported for jet-milled drugs such as revetropate hydrobromide, which agglomerated when subjected to a grinding pressure of 0.31MPa and 0.52MPa [48]. The results indicate that the intensity of micronization had an impact on the amount of amorphous content which is associated with a higher degree of agglomeration [48]. It has been reported that a low-level of amorphous materials present in the micronized samples can be identified using isothermal microcalorimetry and dynamic water vapor sorption (DVS). Using isothermal microcalorimetry it was observed that the micronized revatropate hydrobromide recrystallized at RH >30% [48]. Boshhiha et al. made a similar observation on salbutamol [49]. They found that amorphous regions can be generated in crystalline materials during the jet milling process, which had significant impacts on physical stability [49]. Thus, re-crystallization of the amorphous regions can lead to changes in the physico-chemical properties of the micronized particles and formation of strong agglomerates, which has the potential to significantly impact the aerosol performance of the formulation [50, 51, 52].
“Conditioning” or allowing the particles to “relax” following jet milling is one way to overcome these problems [30]. In the conditioning process, the storage environment is controlled with respect to relative humidity and temperature to enable intentional conversion of amorphous to crystalline content. The temperature of the surrounding environment is kept above the glass transition temperature (Tg) and the relative humidity is such that the solid powder adsorbs water reducing the Tg of the sample and thereby increasing the molecular mobility and the process of re-crystallization [53]. Exposure to accelerated temperature and relative humidity can improve the physical stability of the dry powder post-micronization by enhancing the structural relaxation rate and reducing the amorphous content and associated powder aggregation [54].
Another effective way to solve the problem is to coat the surface of jet-milled particles with anti-sticking materials [55]. Surface coating of jet-milled particles with appropriate excipients can be achieved using a high-shear dry coating approach such as mechanofusion with little or no reduction in particle size [56, 57]. Significant improvements in powder flowability and aerosol performance of the mechanofused formulations have been demonstrated for both carrier-based and carrier-free DPI formulations attributed to the reduction in inter-particulate forces [58, 59, 60, 61]. A high-shear dry coating technique can be employed to de-agglomerate the strong aggregates of jet-milled particles and coat the individual particles with a force control agent such as magnesium stearate [42, 62].
Co-milling of an active pharmaceutical ingredient (API) such as beclomethasone dipropionate (BDP) with additives such as magnesium stearate has also been reported to improve the aerosolization performance and aerosol stability [42]. Co-milling involves co-processing two or more components through a milling apparatus to achieve a homogenous powder blend, associated with particle size reduction. Lau et al. reported that the addition of magnesium stearate 5% (w/w) had a significant impact on the physical stability and aerosol performance of co-milled BDP with lactose by reducing the adhesive force between BDP and lactose [32], which improved de-agglomeration. The formulation of 1% w/w BDP co-jet-milled with 99% w/w lactose but without magnesium stearate agglomerated at the elevated humidity of 75% RH at 25°C due to re-crystallization of the amorphous regions of micronized lactose, which led to a decrease in aerosol performance (p <0.05) as compared to the 1% BDP/94% lactose formulation with 5% magnesium stearate [32]. A co-milling method may also prevent changes to the solid-state property of the API upon exposure to elevated humidity. For example, the crystallization inhibitors, a-lactose monohydrate (LAC), adipic acid (AA) and magnesium stearate (MgSt) prevented crystallization of salbutamol sulphate [63, 64, 65].
Wet milling was also studied by El-Gendy et al. to improve the aerosol performance of DPIs [66]. They used wet milling to produce budesonide NanoClusters (NCs), an excipient-free dry powder formulation. The NCs are irregularly shaped agglomerates of drug nanoparticles which possess superior aerosol performance due to reduced contact area and particulate interactions. As the particles are milled in aqueous media, the amorphous regions undergo crystallization immediately. Thus, the wet milling process minimizes crystallization of the particle surface during storage [67]. In comparison to the commercial budesonide DPI formulation, the NCs showed superior aerosol performance due to lower density and lower adhesive and/or cohesive forces [66]. Further investigation is warranted into the generation of crystalline particles without amorphous regions to improve the physical stability of respirable formulations.
2.2. Spray drying
Spray drying is one of the commonly used particle engineering methods to produce fine particles suitable for inhalation [68, 69, 70]. In this method a feed solution or suspension is sprayed into a hot drying medium (air/nitrogen gas) so as to generate particles [71]. The unique advantage of this technology is its potential to control and tune the particle characteristics such as size, density, morphology and surface composition via the adjustment of formulation or process parameters [70]. In addition, it is possible to incorporate multiple components into a single particle in this one-step continuous manufacturing process. This method is being increasingly used by the pharmaceutical industry to produce dry powders for inhalation within the aerodynamic size range of 1-5 μm, as exemplified by Aridol® (mannitol inhalation powder) and TOBI® Podhaler (tobramycin DPI).
2.2.1. Crystallization of spray dried amorphous particles
One challenge in the production of respirable dry powders using spray drying techniques is ensuring their long-term physical stability and minimizing their impact on aerosol performance. Many amorphous powders (particularly small molecules) obtained through the spray drying technique are physically unstable and tend to crystallize (especially when they are exposed to moisture and high temperatures) [72]. In a study by Shetty et al., it was revealed that amorphous spray-dried drug particles, such as ciprofloxacin hydrochloride, tend to re-crystallize on storage at 55% RH and above thereby altering their aerosol performance [73]. In their review article, Weers et al. described different approaches to maintaining the crystallinity of drug particles during the spray drying process [74]. One suggested method is to spray suspensions of micronized drug(s) from a liquid feed. However, this creates a need to minimize dissolution of drug particles in the liquid feed [74]. The review further discusses different formulations and engineering techniques useful to maintaining the physical and chemical stability of the crystalline drug in the formulation [74].
Challenges with physical instability due to spray-dried amorphous materials may be overcome by controlling formulation and process parameters. Adding suitable excipients to the spray-dried formulation could solve the crystallization problem and help to retain the physical stability and optimal aerosol performance in DPIs. Bosquillon et al. studied dependence of in-vitro aerosol performance on excipients (e.g., lactose, albumin, dipalmitoylphosphatidylcholine (DPPC), trehalose and mannitol). The aerosolization property of human serum albumin powder was improved when formulated as albumin/lactose/DPPC (30/10/60 w/w/w) but reduced in the presence of mannitol and trehalose. The physical characteristics of the particles were heavily influenced by the composition and type of excipient added: DPCC reduced the surface energy and thereby the powder cohesiveness whereas lactose and albumin reduced the density and inter-particle adhesion forces enabling improved aerosol performance [75]. Recently, the impact of excipients such as lactose, sucrose, trehalose, mannitol and leucine on the physical stability and aerosolization of spray-dried ciprofloxacin formulations was examined upon storage at 55% RH [76]. Leucine showed the most improvement in aerosol performance and enhanced physical stability for the spray-dried ciprofloxacin formulations due to its surface-enrichment properties, even at a low concentration (10% w/w) [76].
Similarly, Li et al. studied the moisture protection effect of varying leucine concentration (5-80% w/w) on the aerosol performance of spray-dried salbutamol sulfate. In their study, formulations containing 40% (w/w) leucine did not absorb much water due to increased crystalline leucine content; thus deterioration in the aerosol performance of the spray-dried amorphous salbutamol sulfate stored at 60% RH and 75% RH was significantly reduced compared to the formulations containing only 5-20% (w/w) leucine [77]. In addition, storage-induced crystallization associated with high-dose spray-dried amorphous powders (such as antibiotics) has been avoided through co-spray drying a combination of antibiotics. Shetty et al. observed that co-spray drying ciprofloxacin with the polypeptide antibiotic colistin enhances the physical and aerosol stability of amorphous spray dried ciprofloxacin powder formulation stored at 55% RH. Colistin formed a polymer-like matrix and was enriched on the composite particle surface during co-spray drying, reducing the molecular mobility of ciprofloxacin and completely preventing its crystallization at 55% RH in the mass ratio of 1:1. The composite formulation has also been shown to improve aerosol stability through intermolecular hydrogen bonding [78].
2.2.2. Hygroscopicity of spray-dried amorphous particles
Some spray-dried drugs can remain amorphous at elevated humidity conditions without crystallization, but usually these amorphous powders are hygroscopic and have a tendency to absorb moisture resulting in particle agglomeration and a subsequent decrease in aerosol performance. For instance, colistin remains in the amorphous form for up to three months upon storage at a relative humidity (RH) above 60%. However, upon exposure to high humidity (>70%), the amorphous powders tend to absorb excess moisture and form stronger agglomerates due to increased capillary forces [79, 80, 81, 82]. Zhou et al. was able to achieve enhanced aerosol performance at a high relative humidity (>70%) by co-spray drying hygroscopic colistin with other hydrophobic antibiotics like rifampicin and azithromycin [83, 84]. It was observed that with 50% (w/w) azithromycin in the formulation, the surface contained 96.5% azithromycin in the composite co-spray dried formulation as measured by the specialized surface characterization techniques such as X-ray photoelectron spectroscopy (XPS) and time of flight secondary ion mass spectrometry (ToF-SIMS). Thus, surface enrichment with hydrophobic drugs such as azithromycin prevented degradation in the aerosol performance of co-spray dried colistin at 75% RH.
After spray drying, disodium cromoglycate (DSCG) powders became amorphous and experienced significant mass increase of 50% due to water absorption at 90% RH [85]. After storage at 75% RH for a week, the fine particle fraction (FPF) of the spray-dried DSCG particles fell so drastically that the particles were no longer inhalable. To address this concern, the addition of sodium stearate protected DSCG from moisture resulting in better aerosolization performance. This was attributed to enrichment of sodium stearate on the particle surface resulting in a reduction in particle-particle and particle–moisture interactions [85]. Li et al. observed that adding L-leucine as an excipient not only improved aerosol performance but also prevented moisture-induced deterioration in the aerosol performance of DSCG powders by surface enrichment [86]. Similarly, Cui et al. improved the aerosol performance of the hygroscopic drug netilmicin by co-spray drying with different L-leucine ratios [87]. Yu et al. used different hydrophobic amino acids like valine, isoleucine and methionine as excipients in order to prevent the deterioration of aerosol performance in highly hygroscopic spray-dried powders that were stored at high humidity. These amino acids also acted by creating protective surfaces [81].
2.2.3. Spray-dried crystalline particles
In some cases, altering process parameters such as using high outlet temperatures with an insulated drying chamber and using ‘Spray blending’ methods have also been shown to improve physical stability [30]. The study by Abiad et al. clearly showed that varying the spray drying and storage conditions produced different structures in the material. According to the study, initial relaxation during spray drying affected the tendency of the dried powders to crystallize. Abiad et al. believed there is a need to monitor variations in spray drying which may impact nucleation and subsequent crystal growth [88].
Spray drying has also been shown to be a suitable method for preparing co-crystals; i.e., crystalline solid forms composed of a drug and a conformer [89]. Co-crystals alter the solid-state properties of the drug often leading to improved solubility, dissolution, stability and bioavailability of the drug molecules, which are usually less hygroscopic than the amorphous materials. Studies by Alhalaweh et al. on highly crystalline co-crystals of theophylline by spray drying demonstrated the potential of co-crystallization technology to control particle physico-chemical properties [90]. In comparison to milled theophylline, spray-dried co-crystals have considerably lower surface energy and different micromeritic properties [90]. Micronized co-crystals were also found to enhance the pulmonary absorption of poorly absorbed drugs like itraconazole. Karashima et al. prepared poorly soluble Itraconazole co-crystals with succinic acid (SA) or l-tartaric acid (TA) and successfully micronized them using a jet milling system [91]. The particle shapes of micronized Itraconzole co-crystal powders differed from those of the crystalline or spray-dried amorphous itraconazole, which showed better intrinsic dissolution rates (IDR) and pulmonary absorption profiles. DPI formulations of these co-crystals usually have superior physical stability than the amorphous DPI formulation for pulmonary administration [91].
2.2.4. Spray-dried biologics
More recently, spray drying has been explored as an effective means of producing inhalable formulations of peptides and proteins [6, 92], most notably with inhaled insulin [93]. One of the first evaluations of the potential for spray drying of an inhaled protein was recombinant human deoxyribonuclease (rhDNase) [94]. While no stabilizing excipients were required for rhDNase to retain its functional activity, sodium chloride was added to improve the dispersibility of the powder [94]. However, for a majority of proteins, denaturation occurred during spray drying due to the high drying temperature and exposure to the air–water interface that was generated [95]. During the process of spray drying, the protein solution is exposed to shear as it flows through tubing, broken up into droplets and subjected to hot air to strip the protein from its aqueous environment. However, the extent of protein degradation depends on the type of protein (and the presence or absence of stabilizing excipients), and the specific process conditions and configuration of the spray dryer [96]. A lower inlet air temperature can reduce the potential thermal stress but may not provide adequate thermal energy to fully dry the particles. Additionally, even at spray drying temperatures above 100°C, protein denaturation may not occur because the temperature of the droplet barely exceeds the wet bulb temperature of water (~40°C) [70, 71]. Also, incorporating a stabilizer (e.g., sugars, amino acids) into the protein formulation can reduce the likelihood for protein denaturation by immobilizing the protein in a glassy matrix with the stabilizer replacing the hydrogen bonds between water molecules and the protein (i.e., the water-replacement hypothesis) [97, 98]. However, phase separation or inhomogeneous distribution of different components within a particle may occur during the spray drying process due to differences in the solubility and diffusion rate, which could impact physical stability resulting in the denaturation of proteins [98, 99].
Solid-state stability is a critical quality concern for successful development of dry powder protein/peptide formulations. Spray-dried inhaled biomolecules must have a stable amorphous glass structure to prevent molecular mobility of the biomolecule and keep it stable in the dry solid-state [100]. Mannitol in the amorphous state has been shown to stabilize proteins; however, due to its low Tg (11°C), it tends to re-crystallize [98, 101]. This re-crystallization process may cause protein denaturation during the drying process [99]. The use of a high Tg excipient like trehalose (117°C) allows the formation of amorphous glasses after spray drying which can stabilize proteins during storage [102]. However, spray-dried lysozyme containing 50% trehalose was not stable after storage at elevated temperatures and humidity due to increased water uptake by trehalose compared to the sample containing a combination of mannitol and trehalose [102]. This study emphasizes the possibility that a mixed excipient system may stabilize a protein like lysozyme more effectively than single excipients.
Pulmonary vaccines are being tested as a non-invasive route to induce mucosal and systemic immunity [103]. This alternative delivery route may be more advantageous than parenteral vaccination because the antigen (e.g., macromolecules, peptides or proteins) can be administered directly to the site of infection resulting in a better immune response. Compared to traditional liquid formulations, dry powder pulmonary vaccine formulations may have better chemical and physical stability and eliminate the need for cold chain storage. Spray drying is a viable technique to produce dry powder vaccine formulations as it enables easy incorporation of excipients to preserve the integrity of antigenic macromolecules. Spray-dried influenza vaccine was found to be biochemically and physically stable for up to 3 years at 20°C with inulin as a stabilizer [104]. Pulmonary delivery of short interfering RNA (siRNA) is another possibility for treating various viral respiratory infectious diseases. The siRNA inhibit specific gene expression, degrading viral mRNA and preventing viral replication [105, 106]. Chow et al. produced a dry powder formulation of naked siRNA (2% (w/w)) without delivery vectors using a spray drying technique. L-leucine was added as an excipient and helped improve aerosol performance by surface-enrichment [107]. Liang et al. showed that dry powder formulation of siRNA co-spray dried with pH-responsive peptides such as LAH (histidine-rich peptides) and LADap (2,3-diaminopropionic acid (Dap)-rich peptides) and the excipient mannitol not only protected the siRNA from enzymatic degradation, but also enhanced local delivery for treatment of influenza [106]. The spray-dried powders containing siRNA/peptides/mannitol were found to be stable in the crystalline form for up to 5 months storage at 4°C due to low moisture absorption tendency.
Excipient selection for spray-dried biologics could have a dual function of enhancing aerosol performance and stabilizing the biologics [108]. Lechuga- Ballesteros et al. studied the impact of the excipient trileucine on the aerosol performance and solid-state property of spray-dried proteins and peptides such as human growth hormone and salmon calcitonin [108, 109]. Protein denaturation and aggregation in the sold state is reduced in the presence of trileucine as it lowers the surface tension of the co-spray dried formulation by competing with the protein at the air/liquid interface. Proteins are known to remain stable in an amorphous matrix. For this reason, the glass transition temperature should be higher than the storage temperature to ensure protein stability on storage [110]. Trileucine also has a high glass transition temperature (104°C) enabling better stability at room temperature. Enrichment of trileucine at particle surface during spray drying (due to its low aqueous solubility) also gives superior aerosol performance.
2.3. Spray freeze drying
Spray freeze drying (SFD) presents an alternative drying method for heat labile therapeutics. Typically porous particles with a larger surface area can be obtained by spray freeze drying [111], and are suitable for inhalation delivery. Lyophilization or freeze drying has been the gold standard method for protein powder formulations administered through the injectable routes [70, 112, 113]. However, spray freeze drying has advantages over lyophilization because the particle size, surface area and powder density can be controlled by altering process parameters such as atomization and freeze-drying conditions [114, 115]. These advantages are particularly important for pulmonary drug delivery because the powders can be engineered with desirable aerodynamic properties to ensure delivery to the deep lung [116].
Spray freeze drying consists of two steps: atomizing the feedstock into a freezing medium, followed by lyophilization to remove the ice via sublimation, thereby leaving behind a powder [30]. The freeze-drying itself involves three essential steps: (1) freezing, (2) primary drying, and (3) secondary drying [117]. Relatively low temperature drying conditions provided by lyophilization is a safe and effective approach for maintaining the stability of many biopharmaceutical products [118]. Spray freeze drying to produce light and porous particles has the advantage of preserving the integrity of biotherapeutics at a very high production yield. Attempts have been made to produce inhalable biological powder formulations using spray freeze drying including insulin, bovine serum albumin, trypsinogen [119], small interfering RNA (siRNA) [120] as well influenza vaccine [30].
As mentioned above, though spray freeze drying has many advantages for producing protein particles, the limitation is that some proteins experience extensive degradation during the atomizing, freezing and drying processing steps [121, 122]. The proteins are exposed to shear stress and air-water interface during atomization, which increases the likelihood of protein unfolding and aggregation [123]. Excipients such as sugars, polyols, surfactants and buffers are commonly added to stabilize proteins [124, 125]. Webb et al. demonstrated that adsorption of recombinant human interferon-gamma (rhIFN-γ) at the air-liquid interface during the atomization step resulted in loss of native protein structure [126]. The adsorption was significantly reduced by the addition of a surfactant-like polysorbate 20., which reduced the amount of protein molecules that could unfold at the surface and thus improved the stability [126].
It has also been shown that the stability of spray freeze dried powders can be improved by annealing the frozen particles prior to drying to decrease their specific surface area [97]. Audouy et al. have shown that whole inactivated virus influenza vaccine (WIV) can be spray freeze dried in the presence of inulin without loss of its bioactivity and the resultant powder was suitable for pulmonary administration [127]. Murugappan et al. further investigated the physical and immunogenic stability of spray freeze dried whole inactivated virus with inulin, dextran and a mixture of dextran and trehalose as protectants. They noted that the spray freeze dried whole inactivated virus was stable when stored at 30°C for 3 months with these different protectants. However, they found that the WIV incorporated in dextran alone was stable at temperatures even as high as 40°C for 3 months with no change in particle size and specific surface area [128]. In contrast, the higher storage temperature of 40°C reduced the particle size and specific surface area of SFD inulin and SFD dextran/trehalose. The initial glass transition temperature (Tg) for SFD dextran (220°C) was higher compared to SFD inulin and SFD dextran/trehalose (154°C) and thus the residual moisture associated with the spray freeze dried dextran could not sufficiently decrease the Tg at 40°C to cause a change in its physical stability [129].
However, the inhalation performance of large porous powders produced by SFD could be impaired as the porous particles with greater surface area tend to absorb moisture [130]. Sooner et al. showed in their study that SFD trehalose powder stored at 33% RH/25°C was more hygroscopic than spray-dried trehalose powders due to the highly porous nature and resulting high specific surface area [119]. Leung et al. spray freeze dried trehalose-mannitol-leucine in different ratios (F1 = 60:20:20 and F2 = 40:40:20 respectively) with phage as the active ingredient and observed that with a high concentration of hygroscopic trehalose (F1) the SFD particles lost their porous structure [131]. They also compared it to a spray-dried formulation which did not have a porous structure and observed that the degree of recrystallization of trehalose in the spray freeze dried particles was greater due to the increased moisture uptake [131]. Because the porous structure was lost, the SFD-F1 had a lower FPF as compared to SFD-F2.
Physico-chemical properties, such as the residual moisture and crystallinity of a spray freeze dried formulation, are important apart from their aerodynamic properties and biological activities for developing a suitable pharmaceutical product. Liang et al. used a two-fluid nozzle to produce an improved spray freeze dried powder of small interfering RNA (siRNA) [132]. The formulations were prepared with 2% (w/w) siRNA and with mannitol as a bulking excipient. Spray freeze dried siRNA powders were produced at various atomization gas flow rates which impacted the particle size. PXRD showed that mannitol was crystalline in all spray freeze dried formulations but exhibited different polymorphs (delta form for the spray freeze dried formulations) compared to the beta form for the raw mannitol [132]. The polymorphic transition in mannitol was attributed to the change in the atomization gas flow rate but could also be due to the freezing process during spray freeze drying [133]. As mannitol was in the crystalline state, it gave stability for this particular formulation; however, this might not be the case with other amorphous excipients and the suitability of SFD needs to be determined for other DPI carriers such as sucrose, lactose, etc. [132]. Ferrati et al. have developed a systematic fast screening approach to understand the influence of formulation parameters on the aerosol performance and stability of lysozyme powders [134]. Respirable protein powders with pre-determined aerodynamic properties can be prepared faster using such tools.
2.4. Supercritical fluid technology
Super critical fluid technology (SCF) is a relatively new technology which has been exploited for production of crystalline particles with desirable physico-chemical properties (e.g. size, shape etc.) for use in DPIs [135, 136, 137, 138, 139]. SCF uses a supercritical fluid such as ethanol, ethylene, and CO2 (instead of a drying gas) above their critical temperature and pressure [125]. By altering the pressure and temperature of the process, the density of the SCF and the solubility of the solute can be altered. SCF technology has been successfully used to produce inhaled APIs like ipratropium [140], budesonide [141], salbutamol, salmeterol [142] and terbutaline [143]. Though the conventional processes like crushing/milling and crystallization/ precipitation are still commonly used, supercritical fluid is a unique technology which uses a very different mechanism to form particles. The supercritical process generates micron- or even nano- sized particles with a relatively narrow size distribution and have the following advantages: it is an environment-friendly, single-step process, allowing control of crystal polymorphism with enhanced product purity. Most of these advantages are due to the use of carbon dioxide (CO2) as a substitute to organic solvents and to low critical temperature (Tc = 31.1°C) [70]. The sustainable, safe, and environment-friendly characteristics of this technology have resulted in SCF being one of the new generation of particle production technologies [144].
Supercritical methods can be broadly divided into two types: (1) the SCF is used as a solvent (Rapid expansion of supercritical solution) where the solute is dissolved in an SCF such as CO2 and made to precipitate mechanically by rapid expansion [145, 146] (however, most pharmaceuticals have poor solubility in supercritical CO2; for this reason a process known as solution enhanced dispersion by supercritical fluids (SEDS) is employed in which co-solvents such as methanol are added to enhance solubility) [147], or (2): the SCF is used as an antisolvent (Gas Anti-solvent or supercritical antisolvent process) and is responsible for precipitation [125, 148]. During precipitation the solute is forced to crystallize due to low solubility in an SCF such as CO2. The anti-solvent method employs organic solvents such as dimethylsulfoxide or methylene chloride which are not environmentally friendly and can also disrupt native protein structure [147]. Apart from these methods, Sellers et al. has developed a method which involves an emulsion formation between supercritical CO2 and an aqueous solution of a water-soluble drug and excipient called supercritical CO2-assisted nebulization and dehydration of aqueous solutions for producing dry powders for inhalation [147, 149].
Different polymorphic forms of the same drug with different surface properties can be obtained using the SCF technology. This method can improve flow and dispersion by altering particle surface energy [150]. The controlled crystallization from supercritical CO2 (as an antisolvent) can overcome the drawback of producing amorphous regions on the particle surface after traditional jet-milling [30]. Young et al. compared the properties of salbutamol sulfate produced by either micronized or solution-enhanced dispersion by supercritical fluids (SEDS). Particles produced by the two techniques demonstrated the different influence of humidity on aerosolization. There was a decrease in aerosol performance when humidity was raised (>63%) for the micronized salbutamol sulfate due to re-crystallization of amorphous content present on the surface, but no decrease in aerosol performance was observed for the powders produced by SCF technology [51]. This study highlights the significance of the choice of the powder production method on the resulting physical and aerosol stability of the DPI formulation.
Supercritical fluid (SCF) drying has achieved limited reported success with protein formulations due to the potential concern for protein aggregation. As such, there is limited published data on the stability of proteins dried through SCF. More systematic investigations are warranted because proteins dried by SCF processes are exposed to different stress conditions compared to freeze-drying. Sellers et al. produced amorphous and crystalline dry powders for inhalation of lysozyme and lactate dehydrogenase using excipients such as 10% (w/w) mannitol, 10% (w/w) sucrose and 10% (w/w) sucrose with 0.01% (w/w) polysorbate 20 [147]. They observed that amorphous sucrose prevented unfolding of lysozyme better than crystalline mannitol during CO2 associated nebulization and drying. In contrast, in the case of lactate dehydrogenase, both sucrose and polysorbate 20 helped to preserve the native structure [147].
Surfactants such as polysorbate 20 may prevent aggregation by saturating hydrophobic sites on the protein surface. Jovanović et al. attempted to produce stable, sugar-containing lysozyme formulations with sucrose and trehalose as stabilizers using SCF drying with CO2 and an ethanol mixture or CO2 alone [151]. Lysozyme-sucrose and lysozyme-trehalose formulations in a 1:10 (w/w) ratio crystallized during storage at 4°C after one and three months, respectively, due to the higher Tg of the latter. However, crystallization was not observed with a 1:1 or 1:4 (w/w) protein-sugar formulation possibly due to sufficient hydrogen bonding. In contrast, lysozyme was found to be structurally stable in the 1:4 and 1:10 (w/w) ratio but reversible protein aggregation was observed for the pure protein with no excipient and for the 1:1 (w/w) lysozyme-sugar formulation as the amount of sugar was insufficient to prevent protein-protein interaction. Thus, this study highlights that an optimum protein-sugar ratio is essential to prevent crystallization of the excipient and aggregation of the protein and thereby enhances stability of particles prepared by SCF drying. They also reported that eliminating ethanol during SCF drying helped to obtain a stable formulation with a high Tg; in this case, ethanol acts as a plasticizer and may lower the Tg causing crystallization of amorphous excipients [151]. In another study [40], insulin was dissolved in organic solvents such as DMSO or DMFA and dried by spraying in SCF. The SCF dried insulin powders showed increased ß-sheet content and concomitant loss of α- helicity, as detected by a shift of the amide I band in Raman and FTIR spectra. Higher operating temperatures and pressures led to more extensive β-sheet-mediated intermolecular interactions in the precipitates; the drying process itself most likely contributes to the conformational changes [40].
3. Novel particle engineering techniques
Pulmosphere™ is a novel particle engineering technique producing phospholipid-based small porous particles for inhalation of high-dose drugs [152]. The liquid feed consists of an oil-in-water emulsion which is stabilized by distearoylphosphotidylcholine (DSPC) and CaCl2 in a 2:1 molar ratio [74]. Miller et al. showed in their study (using the PulmoSphere process) that the porous and hydrophobic layer controls inter particle forces and enhances the fluidization and dispersion of powders. The phospholipid shell improves dispersion by minimizing the contact between the drug particles, reducing the particle density, and decreasing the surface energy [153]. Drug incorporation into the small porous Pulmosphere particle can be achieved by either a solution-based format in which drug is dissolved in the continuous phase of the emulsion or a suspension-based format in which drug is micronized by jet milling and then suspended within the emulsion prior to spray drying [74].
Tobramycin Inhalation Powder (TIP) produced by this technique is marketed as the TOBI Podhaler for treatment of lung infections in cystic fibrosis patients [154]. In this formulation, tobramycin exists in an amorphous form. In general, amorphous materials are unstable and could recrystallize upon storage posing challenges to both their physical and chemical stability. The physical instability can be avoided by adding suitable excipients to the formulation that can hydrogen bond with the active ingredient or if they have a sufficiently high Tg and low residual moisture, can prevent phase transition. It is critical to understand the relationship between moisture content and glass transition temperature (Tg) to predict the long-term physical stability of amorphous formulations. Miller et al. studied the physical stability of TIP upon exposure to a broad range of relative humidity (RH) and temperature conditions [153]. No re-crystallization of amorphous TIP was observed when they were exposed to a relative humidity ranging from 0% to 60% at 25°C. The physical stability was attributed to the enrichment of the DSPC excipient on the surface of TIP particles thereby impeding crystallization of tobramycin sulfate [154]. It is worth nothing that unpackaged bulk powder underwent viscoelastic collapse during storage at ≥60%RH at 25°C and ≥48%RH at 40°C. These changes in the solid-state properties of unpackaged drug under the extreme storage conditions resulted from a decrease in the glass transition temperature (Tg) below the storage temperature as a result of the plasticizing effect of water [153]. Miller et al. have further integrated the results obtained from different characterization techniques to predict the long-term stability of TIP under a broad range of temperature and water content (Figure 3). They have predicted that a storage temperature of 30°C would be well below the Tg even if the water content is as high as 10% w/w; therefore TIP can remain stable in an amorphous form for as long as 3 years when powders are well-packaged in the aluminum foil blister to maintain the water content in the package below 10% w/w (Figure 3).
Figure 3:
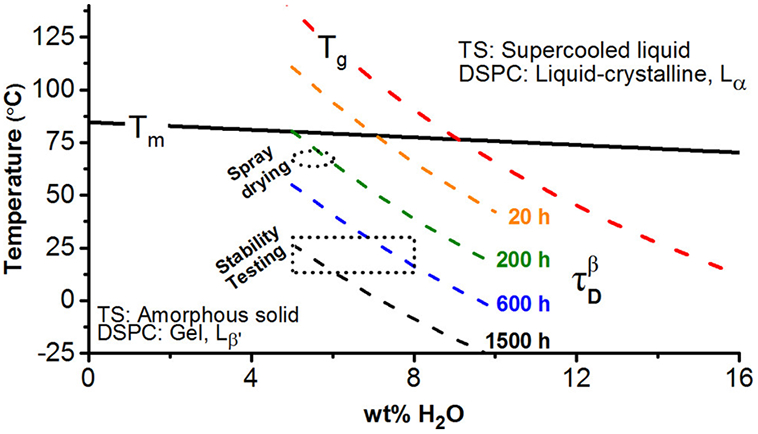
Solid state behavior of TIP as a function of water content and temperature. (TS = tobramycin sulfate, DSPC=distearoylphosphatidylcholine). The short, dashed curves represent iso-relaxation time contours (τDβ) of amorphous TS (Reprinted with permission from [153] Copyright (2017) American Chemical Society).
The manufacture of TIP using the solution-based PulmoSphere method produces amorphous drug product because the drug is fully dissolved and drying occurs rapidly, providing insufficient time for crystal growth and nucleation. Thus, amorphous powders obtained by solution-based spray drying create development risk and require additional characterization to maintain the drug in the amorphous form [74]. This can be avoided by employing a suspension-based spray drying process utilizing crystalline drug. Drug in the crystalline form results in better physical and chemical stability of the drug product. An example of a product produced using the suspension-based PulmoSphere technology is Ciprofloxacin DPI, which demonstrates consistent aerosol performance, particle size, density, and aerodynamic properties allowing efficient delivery to the lower respiratory tract [155]. In this technique the ciprofloxacin is in its neutral zwitterionic form which, due to its poor water solubility, maintains its crystalline form during spray drying [155].
Another novel particle engineering method employs a combination of microfluidics with spray drying to produce dry powders for inhalation [156, 157, 158, 159]. Saboti et al. developed budesonide fine particles (fine crystalline powders) using a microfluidic reactor coupled with ultrasonic spray freeze drying (Figure 4) [157]. No stabilizers were needed for this technique because of the crystalline nature of the fine particles as confirmed by PXRD patterns. Similarly, DVS results confirmed minimal moisture uptake by the budesonide (BDS) particles even at 90% RH with no indication of crystallization. This shows that microfluidics could be an alternative particle production technique for a DPI formulation [157].
Figure 4:
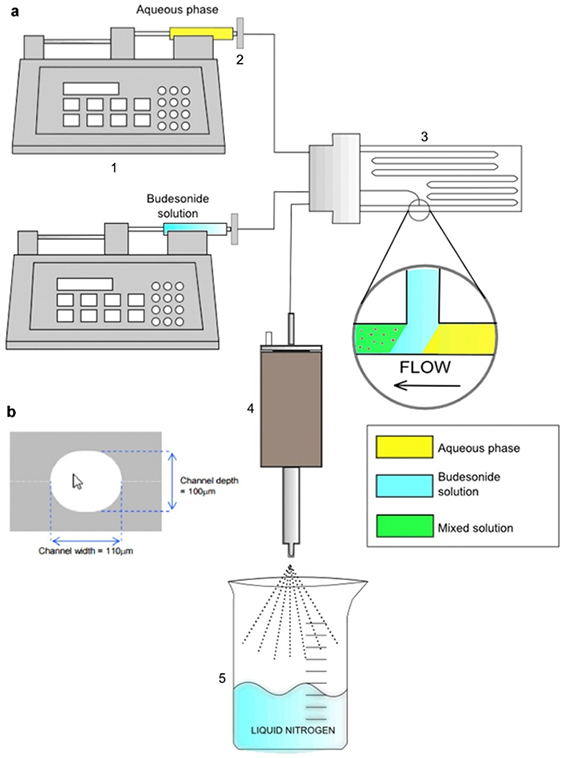
Schematic diagram of microfluidic reactor coupled with ultrasonic spray freeze drying [157]
A novel particle engineering method such as the particle replication in non-wetting template (PRINT) technology may be suitable for producing inhaled biologics [160, 161]. This technique claims complete control on particle morphology with no impact on protein structure or function. PRINT technology was shown to produce protein dry powders for inhalation using bovine serum albumin (BSA) and lysozyme 80% (w/w) and generated a high aerosol performance of 78.6% ± 0.3 and 84.6% ± 4.3 respectively while preserving the native structure and function of the model proteins BSA and lysozyme [160, 161].
4. Characterization techniques to detect the solid-state instability of Dry Powders
As discussed previously, dry powders for inhalation can exist in either amorphous or crystalline forms based on their manufacturing and storage conditions. The amorphous form of the drug may be relatively unstable unless it is present in the solid glassy state. However, as the relative humidity increases, materials may transform from the glassy state to the rubbery state, which can alter their molecular mobility resulting in crystallization [162]. The solid-state form of the API and excipients in the final product is critical for consistent product quality as solid-state transformation can lead to unpredictable and altered therapeutic effects. Thus, in the pharmaceutical industry it is essential to understand the solid-state behavior of powders during manufacturing and storage [163]. A wide-variety of analytical techniques have been used to determine the solid-state properties and physical stability of dry powders for inhalation.
Changes in solid-state characteristics of dry powders used for inhalation can be determined using methods such as powder X-ray diffraction (PXRD) and spectroscopic techniques such as Raman and Fourier-transform infrared spectroscopy (FTIR). These characterization techniques help us to understand phase changes when dry powders are stored at different relative humidity (RH) and temperature conditions [153]. In addition, quantitative information on glass transition temperature (Tg) and thermodynamic phase transition temperature can be determined using techniques such as differential scanning calorimetry (DSC), dynamic vapor sorption (DVS) and thermogravimetric analysis (TGA). Both temperature and humidity are key environmental variables during manufacturing, storage, packaging and product use. Understanding their impacts on phase transition and particle morphology is critical for DPIs. Also, surface diffusion in amorphous molecular materials is known to be much greater than bulk diffusion at temperatures below the Tg which can significantly impact the solid-state properties of powders [76, 154, 164]. Advanced surface characterization techniques such as Time-of-Flight Secondary Ion Mass Spectrometry (ToF-SIMS), X-ray photoelectron (XPS) spectroscopy and Energy dispersive X-ray spectrometer (EDX) are useful to determine the surface properties of dry powders for inhalation.
4.1. Solid-state characterization
The most commonly used solid-state characterization techniques for determining crystallinity in DPI formulations include PXRD, FTIR, FT-Raman spectroscopy, DSC, TGA and DVS [165]. Powder X-ray crystallography or PXRD measures the crystal structure and degree of crystallinity. It is a common tool to detect polymorphism and crystallinity in amorphous systems. X-ray diffraction is a classic technique based on the principle that when X-rays diffract from atoms that appear repeatedly in the same position within the unit cell (i.e., a crystalline structure), a sharp peak is observed [166]. In general, the limit of detection (LOD) for crystalline structures when using PXRD is about 2 % depending on the system. In contrast, amorphous structures have a short-range order which is observed as a broad baseline hump referred to as halo. Even if a sample is 10% - 20% amorphous, the halo may not be evident and cannot be separated completely with diffraction peaks. PXRD is useful for the detection of a small amount of crystalline, but it is difficult to obtain reliable results for samples with low amorphous content to access accurate amorphous content [167]. PXRD has been employed in investigating the crystallinity of α- lactose monohydrate and comparing it to spray-dried lactose which lacks crystalline long-range order [168]. PXRD is commonly used to determine and quantify the presence of crystallinity in spray-dried amorphous powder DPI formulations stored at higher humidity [73, 169, 170, 171].
FT-Raman spectroscopy has been used as a complementary technique to quantify crystallinity in powders [172, 173]. Mass et al. used FT-Raman spectroscopy in their study to identify and quantify different polymorphic forms present in spray-dried mannitol produced at different outlet temperatures [174]. Also, FTIR spectroscopy is commonly employed to study interactions between drug, carrier and excipient used in DPIs at the molecular level [175, 176, 177]. Both FT-Raman and FTIR can offer information on protein secondary structures and hydrogen bonding, which makes them useful tools to evaluate the stability during the preparation and storage of dry powder [178]. Characterization of the distribution and solid-state form of each component in a dry powder inhaler formulation can also be achieved using confocal Raman microscopy [179]. Duan et al. observed crystallization of moxifloxacin on the surface of spray-dried powders based on the Raman spectrum [180]. One limitation of FTIR is that it is a spectroscopic method which takes only a finite number of images and thus prevents bulk analysis. It is worth noting that the strengths of Raman and FTIR are different. For example, FTIR is better at observing changes in bond strength due to the molecular interaction of the specific molecular polar functional group with asymmetric vibration, while FT-Raman spectroscopy generally has an advantage of sensitivity for change in crystal packing and/or conformational arrangements as the symmetric vibration associated with the molecular skeleton deformations, librations, and translations in the low Raman shift range below 700 cm−1 [181, 182]. It is generally accepted that using the two methods in combination can better explain changes at the molecular level.
DSC is another solid-state characterization technique which measures the glass transition temperature (Tg) and helps in the identification of melting, crystallization, degree of crystallinity and thermal transition phenomena. Glass transition temperature is the temperature at which a transition from a ‘glassy state’ to a ‘rubbery state’ occurs in an amorphous material. The unique advantage of the DSC technique is its rapid analysis time and that it requires only a nominal sample. However, two major disadvantages of using DSC for measuring Tg are its relatively low sensitivity and the possibility that multiple thermal events may occur and overlap or interfere with determination of the glass transition [183]. To overcome these limitations of conventional DSC, the modulated DSC (mDSC) was introduced more than two decades ago and has been used to characterize amorphous solids [184]. Both DSC and PXRD can quantify moderate-to-high levels of amorphous materials whereas DVS is more sensitive and can detect very low amounts of amorphous content [49].
DVS measures the change in mass of substance with a change in relative humidity. Adi et al. have shown that pure spray-dried ciprofloxacin absorbed 7.9% (w/w) moisture as the relative humidity increased from 0% to 50%. However, beyond 50% RH, a decrease in mass was observed which was indicative of re-crystallization of ciprofloxacin [185]. The amount of moisture absorbed by amorphous materials is usually higher than their crystalline counterparts. Conversion from amorphous to crystalline form causes a dramatic decrease in water sorption capacity [186]. The amorphous content in the sample can be characterized by measuring the increase in weight from the start of the sorption run to the end of the first desorption run [187].
4.2. Characterization of the physical properties of the particle surface
The particle surface plays an important role in particle interactions, stability and dispersion [188, 189, 190]. The total surface area of inhalable particles is quite large considering the particles are relatively small. Naturally the large surface area makes the particles more susceptible to charging and moisture uptake. They are also more affected by the van der Waals forces than gravity due to their tiny mass. Surface properties such as morphology and surface energy of both the drug and carrier particle are known to have significant impacts on the inter-particulate contact area, dissolution, cohesion and adhesion forces, crystallization, and the aerosol performance of DPI formulations [191, 192]. Using surface analytical techniques such as scanning electron microscopy (SEM) and atomic force microscopy (AFM), Shetty et al. have shown that the crystallization of amorphous spray-dried ciprofloxacin can cause significant changes in particle morphology upon storage at high humidity [73]. AFM can determine the surface structure in the mesoscopic scale resolution (10−6-10−9m) [193] based on the deflection of the cantilever which helps measure morphological properties of the material [194]. AFM imaging helps us to understand the aerosol performance of particles in DPIs based on their morphology; rough surfaces reduce particle adhesive forces thereby improving aerosol performance [195]. AFM has also been used to characterize the force of adhesion between micronized drug particles and pulmonary surfactants [196]. The colloidal probe AFM was developed in the early 1990s with the advancement of the atomic force microscope (AFM) [197, 198]. The colloidal probe AFM can measure the cohesive and adhesive interactions as well as the mechanism of particle detachment between individual drug particles and between a drug particle and a substrate surface (such as carrier and device plastic material) [199].
Recently the tapping mode (TM) AFM was used to discriminate between different polymorphic forms and monitor crystallization on the powder surface [200, 201]. Young et al. employed AFM phase imaging to determine the surface stability of salbutamol sulfate powders produced by micronized and supercritical fluid. Phase imaging is an extension of the standard TM-AFM, where there is a phase lag as the tip makes contact with surfaces containing amorphous or crystalline regions [51]. Additionally, effects of capillary forces between particles due to absorption of moisture can be determined using AFM. Price et al. visualized subtle physical changes on the surface of amorphous spray-dried lactose using the high-resolution AFM imaging with an environmental control [168]. Similarly, AFM has been used as a preformulation tool to understand the effects of humidity on drug-drug interactions for cohesive drugs like salbutamol sulfate [202].
Surface morphology also has an impact on the surface area. It is known that corrugated particles with the same volume as smooth particles have a larger surface area [203]. Surface characterization techniques such as contact angle, isothermal microcalorimetry, gravimetric sorption and Inverse phase gas chromatography (IGC) can be used to assess the nature of powder surfaces. Contact angle is a traditional method used to measure the surface energy of powders but generally it is not the most accurate as powders have irregular geometries and powder properties may change in the presence of liquid [204]. On the other hand, newer techniques such as IGC have been shown to be useful in the surface characterization of DPI formulations as it is non-destructive, fast and requires only a small sample amount [23, 205].
Surface energy measurement for dry powder inhaler formulation can help differentiate amorphous and crystalline forms of the same material because they would have different surface energy, thereby exhibiting different interactions between drug/drug and drug/carrier particles [206, 207]. Also, amorphous substances tend to absorb more moisture than their crystalline counterparts, which is why the absorption of moisture could alter the surface energy of powders [208]. For this reason, the determination of surface energy is critical for understanding changes during dry powder manufacture or storage.
Inverse gas chromatography (IGC) is used in the study of DPI formulations as it helps understand powder quality for inhalation based on a wide range of physico-chemical properties of the solid, including surface energy and solubility parameters [209, 210, 211]. IGC is inverted to analyze the surface characteristics of a powder based on the interactions between the gaseous probe molecules and the stationary phase containing these powders [193]. The physicochemical properties of the powders can be determined by measuring the changes in retention time of the probe molecules [191]. IGC also helps to measure surface energy as a function of RH by exposing the carrier gas or pre-conditioning of the column to different RHs. Feeley et al. used IGC to detect a difference in surface energy between micronized and unmicronized material [46]. Jong et al. used IGC to differentiate between the surface energy for Colistin powders which were prepared using two different manufacturing techniques (i.e., spray drying and jet milling). These differences were then correlated to aerosol performance [212]. Das et al. reported that IGC can be used to investigate the correlation of aerosol performance with changes in surface energy for the carrier-based DPI formulation during storage at high RHs. Moisture absorption to the surface when stored at high RH increased specific surface energy. This increased interparticular interactions, leading to increased capillary interaction and/or solid bridging. Consequently, it resulted in an increase in emitted dose (ED) and a decrease in FPF (Das et al., 2009). A wide variety of literature has shown that IGC is a useful characterization technique for understanding the impact of milling, humidity and drug-carrier interactions on the surface energy of dry powder inhalation formulations [213].
4.3. Characterization of the chemical properties of the particle surface
The surface chemistry of a DPI formulation can determine the inter-particulate forces and their ability to be aerosolized. However, it is extremely difficult to obtain useful information about the surface chemistry of these fine particles because a high degree of surface-sensitivity and superior special resolution are necessary. Traditional spectroscopic techniques such as Raman and FT-IR typically have probing depths of several microns, which is not suitable for obtaining useful chemical information about the fine DPI particle surfaces as they measure both surface and core. Energy dispersive X-ray spectrometer (EDX) is a particle chemistry measurement technique with high spatial resolutions down to nanometers; however, the probing depth is often ≥ 1 μm. Recent advanced analytical techniques like time-of-flight secondary ion mass spectrometry (ToF-SIMS) and X-ray photoelectron (XPS) spectroscopy have achieved the chemical characterization of DPI particle surfaces with ultra-surface sensitivity and superior spatial resolution [214, 215].
EDX can determine the chemical composition of materials in dry powder for inhalation by elemental analysis. High energy electrons are bombarded on the sample surface, causing emission of an electron from the inner shell of an atom which is then replaced by a high energy electron from the next outer shell [216, 217]. The difference in energy due to the movement of a high energy electron from the outer to inner shell is characteristic to the atom and released as an X-ray [218, 219]. The surface composition of drug or excipient-coated materials by mechanofusion or spray drying methods for DPI formulations has been characterized using elemental mapping technique such as EDX [220, 221]. EDX detects X-rays which are characteristic to every element. However, EDX has a relatively low detection limit and is unable to detect elements with an atomic number less than 11 and the penetration depth of its X-rays is a few microns [78]. Advanced surface characterization techniques such as XPS or ToF-SIMS are used to provide both qualitative and quantitative characterization of the surface chemistry of fine DPI particles.
XPS is a quantitative spectroscopic technique that measures the surface composition of particles (2-10 nm probing depth) [222]. In this technique the powder samples are bombarded with X-rays causing electrons to be emitted from the particle surface. The kinetic energies of the photoelectrons are analyzed and their binding energies are determined [209]. This is a surface technique as the probing depth is typically less than 10 nm on the particle surface. ToF-SIMS is another technique used for qualitative characterization of particle surface chemistry. During the operation, energy-rich ions bombard the particle surface resulting in emission of secondary ions which are further analyzed by a mass spectrometer [222]. Using distinctive mass fragments, different molecules can be identified and mapped with a spatial resolution of 200 – 250 nm and an ultra-surface-sensitivity of < 1 nm. Recently, a quantitative correlation was established between surface lubricant coverage and aerosolization for carrier-free DPI formulations prepared by co-jet-milling (Mangal et al., 2019). These advanced surface characterization techniques are used more and more frequently to determine the surface composition of excipient or drug particles in dry powder formulations, and are useful for understanding the effects of the particle surface chemical properties on the dissolution and aerosol performance of DPIs [86, 107, 176, 223, 224, 225].
5. Conclusion
Production of inhalable drug particles traditionally involves milling or spray drying, but other diverse pharmaceutical operations have been evaluated including spray freeze drying, and supercritical fluid processes. Many of these processes expose the formulation to mechanical or thermal stresses, potentially leading to changes in the physical state and physico-chemical properties of the drug particles which may have an impact on their stability, particularly for use in DPIs. Certain precautions and methods such as surface coating, using novel excipient or particle engineering methods, can be taken to preserve the particle size, shape, density, polymorphism and surface morphology of the drug particles in order to maintain the stability of the DPI formulation. The use of advanced characterization techniques is critical to better understanding powder behavior in the early stages of DPI formulation development, which will help avoid stability issues later in development.
6. Expert opinion
Pulmonary drug delivery is an emerging research area and many new products are being developed for respiratory diseases. Understanding flow behavior, deposition profile and stability of the inhaled aerosols is fundamental to the successful use of this unique delivery system.
A significant challenge related to the development of formulations in DPIs is their stability. Manufacturing processes, pharmaceutical engineering techniques and storage conditions can significantly impact the physical and aerosol stability of inhalable particles; unfortunately, most of these stability issues are not yet fully understood.
Spray drying has been used for several decades as a method of producing/engineering fine particles for DPIs. Spray drying can engineer particles according to their physico-chemical properties; however, the physical and aerosol stability should be safeguarded especially for amorphous spray-dried particles. In addition, heterogeneous distribution of components in the spray-dried particles may lead to physical or chemical instability. Spray freeze drying is a more recent technology used to produce fine particles and is particularly suitable for thermolabile materials. Spray freeze drying combines two processes: spray drying and freeze drying. However, this technique is also time-consuming and generates fragile particles. Supercritical fluid technology is one of the latest technologies adopted for production of DPIs and has a superior stability profile due to the crystalline nature of the particles generated.
These technologies aim to provide superior inhalable particles; however, many of them are associated with challenges related to the stability of the inhalable particles (Figure 5). There is an urgent need for researchers to understand the mechanisms of instability in order to ensure product quality.
Figure 5:
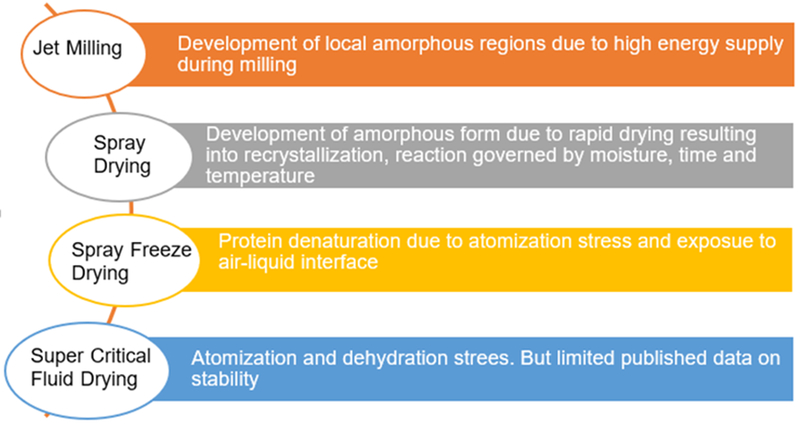
Summary of the underlying causes for potential physical instability in DPI formulations due to different powder production techniques (based on literature review).
It is worth noting that the commonly used solid-state techniques such as powder X-ray diffraction and differential scanning calorimetry may not be adequate for characterization of DPIs. This is largely due to the challenges in characterizing nonuniform, low-content modifications in the physical or chemical properties during the processing of very fine inhalation particles, e.g. partial amorphous contents generated on drug particle surface during jet milling. There is a need to exploit more sophisticated technologies with higher detection limits and superior spatial resolutions to understand the stability of very fine inhalable particles.
Figure 2:
Physical instability with spray-dried powders upon storage
Article highlights.
Dry powder inhalers (DPIs) have become popular pulmonary drug delivery systems
Various techniques have been applied to produce fine drug particles for inhalation.
There are many challenges to ensure physical and aerosolization stability of DPIs
Advanced characterization techniques are necessary to understand physical and aerosolization instability due to small particle size for inhaled drugs
Acknowledgments
Funding
This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number R01AI132681 and R01AI146160.
Footnotes
Declaration of interest
N Shetty is an employee of Genentech. QT Zhou is funded by National Institute of Health, Center for Pharmaceutical Processing Research, Genentech, Bill and Melinda Gates Foundation. D Cipolla is an employee of Insmed. he authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Nokhodchi A, Martin GP. Pulmonary Drug Delivery: Advances and Challenges. John Wiley & Sons; 2015. [Google Scholar]
- 2.Cipolla D, Chan HK. Current and Emerging Inhaled Therapies of Repositioned Drugs. Adv Drug Deliv Rev. 2018. August;133:1–4. doi: 10.1016/j.addr.2018.09.008. PubMed PMID: 30409263; eng. [DOI] [PubMed] [Google Scholar]
- 3.Anselmo AC, Gokarn Y, Mitragotri S. Non-invasive delivery strategies for biologics [Review Article]. Nature Reviews Drug Discovery. 2018. 11/30/online;18:19. doi: 10.1038/nrd.2018.18310.1038/nrd.2018.183https://www.nature.com/articles/nrd.2018.183#supplementary-informationhttps://www.nature.com/articles/nrd.2018.183#supplementary-information . [DOI] [PubMed] [Google Scholar]
- 4.Cipolla D. Will pulmonary drug delivery for systemic application ever fulfill its rich promise? Expert Opin Drug Deliv. 2016. October;13(10):1337–40. doi: 10.1080/17425247.2016.1218466. PubMed PMID: 27464271; eng. [DOI] [PubMed] [Google Scholar]
- 5.Zhou Q, Leung SSY, Tang P, et al. Inhaled formulations and pulmonary drug delivery systems for respiratory infections. Advanced Drug Delivery Reviews. 2015. 5//;85:83–99. doi: 10.1016/j.addr.2014.10.022. [DOI] [PubMed] [Google Scholar]
- 6.Zhou QT, Tang P, Leung SSY, et al. Emerging inhalation aerosol devices and strategies: where are we headed? Advanced drug delivery reviews. 2014;75:3–17. [DOI] [PubMed] [Google Scholar]
- 7.Weers JG, Bell J, Chan HK, et al. Pulmonary formulations: what remains to be done? J Aerosol Med Pulm Drug Deliv. 2010. December;23 Suppl 2:S5–23. doi: 10.1089/jamp.2010.0838. PubMed PMID: 21133800; eng. [DOI] [PubMed] [Google Scholar]
- 8.Ibrahim M, Verma R, Garcia-Contreras L. Inhalation drug delivery devices: technology update. Medical devices (Auckland, NZ). 2015;8:131–139. doi: 10.2147/MDER.S48888. PubMed PMID: 25709510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilbert I, Millette LA, Riebe M. Inhalation device options for the management of chronic obstructive pulmonary disease AU - DePietro, Michael. Postgraduate Medicine. 2018. 2018/January/02;130(1):83–97. doi: 10.1080/00325481.2018.1399042. [DOI] [PubMed] [Google Scholar]
- 10.Geller DE. Comparing clinical features of the nebulizer, metered-dose inhaler, and dry powder inhaler. Respiratory care. 2005;50(10):1313–1322. [PubMed] [Google Scholar]
- 11.Tiddens HA, Bos AC, Mouton JW, et al. Inhaled antibiotics: dry or wet? European Respiratory Journal. 2014;44(5):1308–1318. [DOI] [PubMed] [Google Scholar]
- 12.Cipolla D, Chan HK, Schuster J, et al. Personalizing aerosol medicine: development of delivery systems tailored to the individual. Ther Deliv. 2010. November;1(5):667–82. PubMed PMID: 22833956; eng. [DOI] [PubMed] [Google Scholar]
- 13.Reychler G, Keyeux A, Cremers C, et al. Comparison of lung deposition in two types of nebulization - Intrapulmonary percussive ventilation vs jet nebulization [Article]. Chest. 2004. February;125(2):502–508. doi: 10.1378/chest.125.2.502. PubMed PMID: WOS:000188978400025; English. [DOI] [PubMed] [Google Scholar]
- 14.Pham S, Ferguson GT, Kerwin E, et al. In Vitro Characterization of the eFlow Closed System Nebulizer with Glycopyrrolate Inhalation Solution. J Aerosol Med Pulm Drug Deliv. 2018. June;31(3):162–169. doi: 10.1089/jamp.2017.1384. PubMed PMID: 29125918; PubMed Central PMCID: PMCPMC5994673. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melani AS, Bonavia M, Cilenti V, et al. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med. 2011. June;105(6):930–8. doi: 10.1016/j.rmed.2011.01.005. PubMed PMID: 21367593; eng. [DOI] [PubMed] [Google Scholar]
- 16.Hamdan A-J, Ahmed A, Abdullah A-H, et al. Improper inhaler technique is associated with poor asthma control and frequent emergency department visits. Allergy, Asthma & Clinical Immunology. 2013;9(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hickey AJ, da Rocha SR. Pharmaceutical inhalation aerosol technology. CRC Press; 2019. [Google Scholar]
- 18.Kendre P Dry Powder Inhaler: A Review. Vol. 3 2016. [Google Scholar]
- 19.de Boer A, editor Factors affecting DPI development from bench to bedside JOURNAL OF AEROSOL MEDICINE AND PULMONARY DRUG DELIVERY; 2016: MARY ANN LIEBERT, INC 140 HUGUENOT STREET, 3RD FL, NEW ROCHELLE, NY 10801 USA. [Google Scholar]
- 20.Patil JS, Sarasija S. Pulmonary drug delivery strategies: A concise, systematic review. Lung India : official organ of Indian Chest Society. 2012. January;29(1):44–9. doi: 10.4103/0970-2113.92361. PubMed PMID: 22345913; PubMed Central PMCID: PMCPMC3276033. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crompton GK. Dry powder inhalers: advantages and limitations. Journal of aerosol medicine : the official journal of the International Society for Aerosols in Medicine. 1991. Fall;4(3):151–6. doi: 10.1089/jam.1991.4.151. PubMed PMID: 10147676; eng. [DOI] [PubMed] [Google Scholar]
- 22.Mehta P. Dry Powder Inhalers: A Focus on Advancements in Novel Drug Delivery Systems. Journal of Drug Delivery. 2016;2016:17. doi: 10.1155/2016/8290963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Telko MJ, Hickey AJ. Dry powder inhaler formulation. Respir Care. 2005. September;50(9):1209–27. PubMed PMID: 16122404; eng. [PubMed] [Google Scholar]
- 24.Zhang X, Zhao Z, Cui Y, et al. Effect of powder properties on the aerosolization performance of nanoporous mannitol particles as dry powder inhalation carriers. Powder Technology. 2018. 2018/August/23/. doi: 10.1016/j.powtec.2018.08.058. [DOI] [Google Scholar]
- 25.Peng T, Lin S, Niu B, et al. Influence of physical properties of carrier on the performance of dry powder inhalers. Acta Pharmaceutica Sinica B. 2016. 2016/July/01/;6(4):308–318. doi: 10.1016/j.apsb.2016.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.French DL, Edwards DA, Niven RW. The influence of formulation on emission, deaggregation and deposition of dry powders for inhalation. Journal of Aerosol Science. 1996;27(5):769–783. [Google Scholar]
- 27.Rees PJ, Clark TJ, Moren F. The importance of particle size in response to inhaled bronchodilators. European journal of respiratory diseases Supplement. 1982;119:73–8. PubMed PMID: 6954090; eng. [PubMed] [Google Scholar]
- 28.Sun Y Carrier free inhaled dry powder of budesonide tailored by supercritical fluid particle design. Powder Technology. 2016;304:248–260. [Google Scholar]
- 29.Ward GH, Schultz RK. Process-Induced Crystallinity Changes in Albuterol Sulfate and Its Effect on Powder Physical Stability. Pharmaceutical Research. 1995. 1995/May/01;12(5):773–779. doi: 10.1023/A:1016232230638.• Of interest: Demonstrated subtle difference in crystallinity induced by jet-milling.
- 30.Lin YW, Wong J, Qu L, et al. Powder production and particle engineering for dry powder inhaler formulations. Current pharmaceutical design. 2015;21(27):3902–16. PubMed PMID: 26290193. [DOI] [PubMed] [Google Scholar]
- 31.Zografi G States of water associated with solids. Drug Development and Industrial Pharmacy. 1988;14(14):1905–1926. [Google Scholar]
- 32.Lau M, Young PM, Traini D. Co-milled API-lactose systems for inhalation therapy: impact of magnesium stearate on physico-chemical stability and aerosolization performance. Drug Dev Ind Pharm. 2017. June;43(6):980–988. doi: 10.1080/03639045.2017.1287719. PubMed PMID: 28122460; eng. [DOI] [PubMed] [Google Scholar]
- 33.Depasquale R, Lee SL, Saluja B, et al. The influence of secondary processing on the structural relaxation dynamics of fluticasone propionate. AAPS PharmSciTech. 2015;16(3):589–600.• Of considerable interests: Demonstrated impact of storage considitions on amorphous disorder levels of micronized fluticasone propionate, cohesive-adhesive balance (CAB) between drug and carrier, and aerosol performance
- 34.Borgstrom L, Asking L, Lipniunas P. An in vivo and in vitro comparison of two powder inhalers following storage at hot/humid conditions. J Aerosol Med. 2005. Fall;18(3):304–10. doi: 10.1089/jam.2005.18.304. PubMed PMID: 16181005; eng. [DOI] [PubMed] [Google Scholar]
- 35.Ong MLH. The aerosol performance and physico-chemical properties of co-milled dry powder formulations for high dose delivery: University of Sydney; 2016. [Google Scholar]
- 36.Moore S, Harper K, Marshall S. Discovery and development of inhaled biopharmaceuticals. Drug Discovery. 2017;18:18. [Google Scholar]
- 37.Han X, Ghoroi C, To D, et al. Simultaneous micronization and surface modification for improvement of flow and dissolution of drug particles. International Journal of Pharmaceutics. 2011. 2011/August/30/;415(1):185–195. doi: 10.1016/j.ijpharm.2011.05.070. [DOI] [PubMed] [Google Scholar]
- 38.Sosnik A, Seremeta KP. Advantages and challenges of the spray-drying technology for the production of pure drug particles and drug-loaded polymeric carriers. Advances in Colloid and Interface Science. 2015. 2015/September/01/;223:40–54. doi: 10.1016/j.cis.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 39.Okuda T, Suzuki Y, Kobayashi Y, et al. Development of biodegradable polycation-based inhalable dry gene powders by spray freeze drying. Pharmaceutics. 2015;7(3):233–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jovanović N, Bouchard A, Hofland GW, et al. Stabilization of Proteins in Dry Powder Formulations Using Supercritical Fluid Technology [journal article]. Pharmaceutical Research. 2004. November 01;21(11):1955–1969. doi: 10.1023/B:PHAM.0000048185.09483.e7. [DOI] [PubMed] [Google Scholar]
- 41.Naik S, Chaudhuri B. Quantifying Dry Milling in Pharmaceutical Processing: A Review on Experimental and Modeling Approaches. J Pharm Sci. 2015. August;104(8):2401–13. doi: 10.1002/jps.24512. PubMed PMID: 26096636; eng. [DOI] [PubMed] [Google Scholar]
- 42.Lau M, Young PM, Traini D. A review of co-milling techniques for the production of high dose dry powder inhaler formulation. Drug Dev Ind Pharm. 2017. August;43(8):1229–1238. doi: 10.1080/03639045.2017.1313858. PubMed PMID: 28367654; eng. [DOI] [PubMed] [Google Scholar]
- 43.Malcolmson RJ, Embleton JK. Dry powder formulations for pulmonary delivery. Pharmaceutical Science & Technology Today. 1998;1(9):394–398. [Google Scholar]
- 44.Hoppentocht M, Hagedoorn P, Frijlink HW, et al. Technological and practical challenges of dry powder inhalers and formulations. Advanced drug delivery reviews. 2014. 2014/August/30/;75:18–31. doi: 10.1016/j.addr.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 45.Coelho MC, Harnby N. Moisture bonding in powders. Powder Technology. 1978. 1978/July/01/;20(2):201–205. doi: 10.1016/0032-5910(78)80049-7. [DOI] [Google Scholar]
- 46.Feeley JC, York P, Sumby BS, et al. Determination of surface properties and flow characteristics of salbutamol sulphate, before and after micronisation. International Journal of Pharmaceutics. 1998. 1998/October/15/;172(1):89–96. doi: 10.1016/S0378-5173(98)00179-3. [DOI] [Google Scholar]
- 47.Young PM, Chan HK, Chiou H, et al. The influence of mechanical processing of dry powder inhaler carriers on drug aerosolization performance. J Pharm Sci. 2007. May;96(5):1331–41. doi: 10.1002/jps.20933. PubMed PMID: 17455362; eng. [DOI] [PubMed] [Google Scholar]
- 48.Ticehurst MD, Basford PA, Dallman CI, et al. Characterisation of the influence of micronisation on the crystallinity and physical stability of revatropate hydrobromide. Int J Pharm. 2000. January 5;193(2):247–59. PubMed PMID: 10606789; eng. [DOI] [PubMed] [Google Scholar]
- 49.Boshhiha A, Urbanetz N. Influence of micronization on the physical properties of salbutamol sulfate as a model drug used in dry powder inhalation [Original Article]. Libyan International Medical University Journal. 2018. January 1, 2018;3(1):8–15. doi: 10.4103/liuj.Liuj_4_18. [DOI] [Google Scholar]
- 50.Padmadisastra Y, Kennedy RA, Stewart PJ. Influence of carrier moisture adsorption capacity on the degree of adhesion of interactive mixtures. International journal of pharmaceutics. 1994;104(1):R1–R4. [Google Scholar]
- 51.Young PM, Price R. The influence of humidity on the aerosolisation of micronised and SEDS produced salbutamol sulphate. European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences. 2004. July;22(4):235–40. doi: 10.1016/j.ejps.2004.03.006. PubMed PMID: 15196579; eng. [DOI] [PubMed] [Google Scholar]
- 52.Young PM, Price R, Tobyn MJ, et al. Effect of humidity on aerosolization of micronized drugs. Drug Dev Ind Pharm. 2003. October;29(9):959–66. doi: 10.1081/ddc-120025453. PubMed PMID: 14606660; eng. [DOI] [PubMed] [Google Scholar]
- 53.Brodka-Pfeiffer K, Hausler H, Grass P, et al. Conditioning following powder micronization: influence on particle growth of salbutamol sulfate. Drug Dev Ind Pharm. 2003. November;29(10):1077–84. doi: 10.1081/ddc-120025865. PubMed PMID: 14677768; eng. [DOI] [PubMed] [Google Scholar]
- 54.Depasquale R, Lee SL, Saluja B, et al. The influence of secondary processing on the structural relaxation dynamics of fluticasone propionate. AAPS PharmSciTech. 2014;16(3):589–600. doi: 10.1208/s12249-014-0222-8. PubMed PMID: 25398478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qu L, Morton DA, Zhou QT. Particle engineering via mechanical dry coating in the design of pharmaceutical solid dosage forms. Current pharmaceutical design. 2015;21(40):5802–5814. [DOI] [PubMed] [Google Scholar]
- 56.Zhou QT, Morton DA. Drug–lactose binding aspects in adhesive mixtures: controlling performance in dry powder inhaler formulations by altering lactose carrier surfaces. Advanced drug delivery reviews. 2012;64(3):275–284. [DOI] [PubMed] [Google Scholar]
- 57.Zhou QT, Qu L, Larson I, et al. Improving aerosolization of drug powders by reducing powder intrinsic cohesion via a mechanical dry coating approach. International journal of pharmaceutics. 2010;394(1-2):50–59. [DOI] [PubMed] [Google Scholar]
- 58.Begat P, Price R, Harris H, et al. The Influence of Force Control Agents on the Cohesive-Adhesive Balance in Dry Powder Inhaler Formulations. KONA Powder and Particle Journal. 2005;23:109–121. doi: 10.14356/kona.2005014. [DOI] [Google Scholar]
- 59.Begat P, Morton DA, Shur J, et al. The role of force control agents in high-dose dry powder inhaler formulations. J Pharm Sci. 2009. August;98(8):2770–83. doi: 10.1002/jps.21629. PubMed PMID: 19067395; eng. [DOI] [PubMed] [Google Scholar]
- 60.Zhou QT, Qu L, Gengenbach T, et al. Effect of surface coating with magnesium stearate via mechanical dry powder coating approach on the aerosol performance of micronized drug powders from dry powder inhalers. AAPS PharmSciTech. 2012;14(1):38–44. doi: 10.1208/s12249-012-9895-z. PubMed PMID: 23196863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhou QT, Armstrong B, Larson I, et al. Understanding the influence of powder flowability, fluidization and de-agglomeration characteristics on the aerosolization of pharmaceutical model powders. European Journal of Pharmaceutical Sciences. 2010. 2010/August/11/;40(5):412–421. doi: 10.1016/j.ejps.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 62.Kumon M, Suzuki M, Kusai A, et al. Novel approach to DPI carrier lactose with mechanofusion process with additives and evaluation by IGC. Chem Pharm Bull (Tokyo). 2006. November;54(11):1508–14. PubMed PMID: 17077547; eng. [DOI] [PubMed] [Google Scholar]
- 63.Balani PN, Ng WK, Tan RB, et al. Influence of excipients in comilling on mitigating milling-induced amorphization or structural disorder of crystalline pharmaceutical actives. J Pharm Sci. 2010. May;99(5):2462–74. doi: 10.1002/jps.21998. PubMed PMID: 19902526; eng. [DOI] [PubMed] [Google Scholar]
- 64.Balani PN, Wong SY, Ng WK, et al. Influence of polymer content on stabilizing milled amorphous salbutamol sulphate. Int J Pharm. 2010. May 31;391(1-2):125–36. doi: 10.1016/j.ijpharm.2010.02.029. PubMed PMID: 20211717; eng. [DOI] [PubMed] [Google Scholar]
- 65.Curtin V, Amharar Y, Gallagher KH, et al. Reducing mechanical activation-induced amorphisation of salbutamol sulphate by co-processing with selected carboxylic acids. Int J Pharm. 2013. November 18;456(2):508–16. doi: 10.1016/j.ijpharm.2013.08.025. PubMed PMID: 23994365; eng.• Of interests: Co-millling salbutamol sulphate with low glass transition temperature dicarboxylic acids resulted in minimized amorphisation of drug on mechanical activation.
- 66.El-Gendy N, Selvam P, Soni P, et al. Development of budesonide nanocluster dry powder aerosols: processing. J Pharm Sci. 2012. September;101(9):3425–33. doi: 10.1002/jps.23168. PubMed PMID: 22539360; eng. [DOI] [PubMed] [Google Scholar]
- 67.Chan HK, Chew NY. Novel alternative methods for the delivery of drugs for the treatment of asthma. Adv Drug Deliv Rev. 2003. July 18;55(7):793–805. PubMed PMID: 12842601; eng. [DOI] [PubMed] [Google Scholar]
- 68.Vehring R Pharmaceutical Particle Engineering via Spray Drying. Pharmaceutical Research. 2008. 2008/May/01;25(5):999–1022. doi: 10.1007/s11095-007-9475-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Saleem I, Petkar K, Somavarapu S. Chapter Nineteen - Rationale for Pulmonary Vaccine Delivery: Formulation and Device Considerations In: Skwarczynski M, Toth I, editors. Micro and Nanotechnology in Vaccine Development: William Andrew Publishing; 2017. p. 357–371. [Google Scholar]
- 70.Walters RH, Bhatnagar B, Tchessalov S, et al. Next generation drying technologies for pharmaceutical applications. Journal of pharmaceutical sciences. 2014;103(9):2673–2695. [DOI] [PubMed] [Google Scholar]
- 71.Bhatnagar B, Walters R, Tchessalov S, et al. Drying technologies for biopharmaceutical applications: Recent developments and future direction AU - Langford, Alex. Drying Technology. 2018. 2018/April/26;36(6):677–684. doi: 10.1080/07373937.2017.1355318. [DOI] [Google Scholar]
- 72.Chiou D, A. G. Langrish T Crystallization of Amorphous Components in Spray-Dried Powders. Vol. 25 2007. [Google Scholar]
- 73.Shetty N, Zeng L, Mangal S, et al. Effects of Moisture-Induced Crystallization on the Aerosol Performance of Spray Dried Amorphous Ciprofloxacin Powder Formulations. Pharm Res. 2018. January 2;35(1):7. doi: 10.1007/s11095-017-2281-5. PubMed PMID: 29294198; PubMed Central PMCID: PMCPMC5942560. eng.• Of interests: Demonstrated how crystallization of amorphous ciprofloxacin impacts aerosol performance.
- 74.Weers JG, Miller DP, Tarara TE. Spray-Dried PulmoSphere Formulations for Inhalation Comprising Crystalline Drug Particles. AAPS PharmSciTech. 2019. February 7;20(3):103. doi: 10.1208/s12249-018-1280-0. PubMed PMID: 30734187; eng. [DOI] [PubMed] [Google Scholar]
- 75.Bosquillon C, Lombry C, Préat V, et al. Influence of formulation excipients and physical characteristics of inhalation dry powders on their aerosolization performance. Journal of Controlled Release. 2001. 2001/February/23/;70(3):329–339. doi: 10.1016/S0168-3659(00)00362-X. [DOI] [PubMed] [Google Scholar]
- 76.Shetty N, Park H, Zemlyanov D, et al. Influence of excipients on physical and aerosolization stability of spray dried high-dose powder formulations for inhalation. International Journal of Pharmaceutics. 2018. 2018/June/10/;544(1):222–234. doi: 10.1016/j.ijpharm.2018.04.034.• Of consideration interests: Examined influence of excipients on physical and aerosolization stability of spray-dried DPIs and showed mechanisms of protective effects of leucine.
- 77.Li L, Leung SSY, Gengenbach T, et al. Investigation of L-leucine in reducing the moisture-induced deterioration of spray-dried salbutamol sulfate power for inhalation. International Journal of Pharmaceutics. 2017. 2017/September/15/;530(1):30–39. doi: 10.1016/j.ijpharm.2017.07.033. [DOI] [PubMed] [Google Scholar]
- 78.Shetty N, Ahn P, Park H, et al. Improved Physical Stability and Aerosolization of Inhalable Amorphous Ciprofloxacin Powder Formulations by Incorporating Synergistic Colistin. Mol Pharm. 2018. September 4;15(9):4004–4020. doi: 10.1021/acs.molpharmaceut.8b00445. PubMed PMID: 30028947; eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yu J, Romeo MC, Cavallaro AA, et al. Protective effect of sodium stearate on the moisture-induced deterioration of hygroscopic spray-dried powders. Int J Pharm. 2018. April 25;541(1-2):11–18. doi: 10.1016/j.ijpharm.2018.02.018. PubMed PMID: 29454904; eng. [DOI] [PubMed] [Google Scholar]
- 80.Zhou QT, Morton DA, Heidi HY, et al. Colistin powders with high aerosolisation efficiency for respiratory infection: preparation and in vitro evaluation. Journal of pharmaceutical sciences. 2013;102(10):3736–3747. [DOI] [PubMed] [Google Scholar]
- 81.Yu J, Chan H-K, Gengenbach T, et al. Protection of hydrophobic amino acids against moisture-induced deterioration in the aerosolization performance of highly hygroscopic spray-dried powders. European Journal of Pharmaceutics and Biopharmaceutics. 2017. 2017/October/01/;119:224–234. doi: 10.1016/j.ejpb.2017.06.023. [DOI] [PubMed] [Google Scholar]
- 82.Visser J. Van der Waals and other cohesive forces affecting powder fluidization. Powder Technology. 1989;58(1):1–10. [Google Scholar]
- 83.Zhou QT, Gengenbach T, Denman JA, et al. Synergistic antibiotic combination powders of colistin and rifampicin provide high aerosolization efficiency and moisture protection. The AAPS journal. 2014;16(1):37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhou Q, Loh ZH, Yu J, et al. How Much Surface Coating of Hydrophobic Azithromycin Is Sufficient to Prevent Moisture-Induced Decrease in Aerosolisation of Hygroscopic Amorphous Colistin Powder? The AAPS journal. 2016. 2016/September/01;18(5):1213–1224. doi: 10.1208/s12248-016-9934-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yu J, Romeo M-C, Cavallaro AA, et al. Protective effect of sodium stearate on the moisture-induced deterioration of hygroscopic spray-dried powders. International journal of pharmaceutics. 2018;541(1-2):11–18. [DOI] [PubMed] [Google Scholar]
- 86.Li L, Sun S, Parumasivam T, et al. l-Leucine as an excipient against moisture on in vitro aerosolization performances of highly hygroscopic spray-dried powders. European Journal of Pharmaceutics and Biopharmaceutics. 2016. 2016/May/01/;102:132–141. doi: 10.1016/j.ejpb.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 87.Cui Y, Zhang X, Wang W, et al. Moisture-Resistant Co-Spray-Dried Netilmicin with l-Leucine as Dry Powder Inhalation for the Treatment of Respiratory Infections. Pharmaceutics. 2018;10(4):252. doi: 10.3390/pharmaceutics10040252. PubMed PMID: 30513738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Abiad MG, Campanella OH, Carvajal MT. Effect of Spray Drying Conditions on the Physicochemical Properties and Enthalpy Relaxation of α-Lactose. International Journal of Food Properties. 2014. 2014/July/03;17(6):1303–1316. doi: 10.1080/10942912.2012.710287. [DOI] [Google Scholar]
- 89.Karimi-Jafari M, Padrela L, Walker GM, et al. Creating cocrystals: a review of pharmaceutical cocrystal preparation routes and applications. Crystal Growth & Design. 2018;18(10):6370–6387. [Google Scholar]
- 90.Alhalaweh A, Kaialy W, Buckton G, et al. Theophylline cocrystals prepared by spray drying: physicochemical properties and aerosolization performance. AAPS PharmSciTech. 2013. March;14(1):265–76. doi: 10.1208/s12249-012-9883-3. PubMed PMID: 23297166; PubMed Central PMCID: PMCPMC3581642. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Karashima M, Sano N, Yamamoto S, et al. Enhanced pulmonary absorption of poorly soluble itraconazole by micronized cocrystal dry powder formulations. Eur J Pharm Biopharm. 2017. June;115:65–72. doi: 10.1016/j.ejpb.2017.02.013. PubMed PMID: 28223260; eng. [DOI] [PubMed] [Google Scholar]
- 92.Lee G. Spray-drying of proteins Rational design of stable protein formulations: Springer; 2002. p. 135–158. [Google Scholar]
- 93.Cavaiola TS, Edelman S. Inhaled insulin: a breath of fresh air? A review of inhaled insulin. Clinical therapeutics. 2014;36(8):1275–1289. [DOI] [PubMed] [Google Scholar]
- 94.Chan H-K, Clark A, Gonda I, et al. Spray dried powders and powder blends of recombinant human deoxyribonuclease (rhDNase) for aerosol delivery. Pharmaceutical Research. 1997;14(4):431–437. [DOI] [PubMed] [Google Scholar]
- 95.Wang W. Advanced protein formulations. Protein Science. 2015;24(7):1031–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Grasmeijer N. Improving protein stabilization by spray drying. Ridderprint, Groningen: 2015. [Google Scholar]
- 97.Maa Y-F, Prestrelski SJ. Biopharmaceutical powders particle formation and formulation considerations. Current pharmaceutical biotechnology. 2000;1(3):283–302. [DOI] [PubMed] [Google Scholar]
- 98.Moussa EM, Wilson NE, Zhou QT, et al. Effects of Drying Process on an IgG1 Monoclonal Antibody Using Solid-State Hydrogen Deuterium Exchange with Mass Spectrometric Analysis (ssHDX-MS). Pharmaceutical research. 2018;35(1):12. [DOI] [PubMed] [Google Scholar]
- 99.Wilson NE, Topp EM, Zhou QT. Effects of drying method and excipient on structure and stability of protein solids using solid-state hydrogen/deuterium exchange mass spectrometry (ssHDX-MS). International Journal of Pharmaceutics. 2019. 2019/August/15/;567:118470. doi: 10.1016/j.ijpharm.2019.118470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chang LL, Pikal MJ. Mechanisms of protein stabilization in the solid state. Journal of pharmaceutical sciences. 2009;98(9):2886–2908. [DOI] [PubMed] [Google Scholar]
- 101.Costantino HR, Andya JD, Nguyen PA, et al. Effect of mannitol crystallization on the stability and aerosol performance of a spray-dried pharmaceutical protein, recombinant humanized anti-IgE monoclonal antibody. Journal of pharmaceutical sciences. 1998;87(11):1406–1411. [DOI] [PubMed] [Google Scholar]
- 102.Hulse WL, Forbes RT, Bonner MC, et al. Do co-spray dried excipients offer better lysozyme stabilisation than single excipients? european journal of pharmaceutical sciences. 2008;33(3):294–305. [DOI] [PubMed] [Google Scholar]
- 103.Sou T, Meeusen EN, de Veer M, et al. New developments in dry powder pulmonary vaccine delivery. Trends in biotechnology. 2011;29(4):191–198. [DOI] [PubMed] [Google Scholar]
- 104.Saluja V, Amorij J, Kapteyn J, et al. A comparison between spray drying and spray freeze drying to produce an influenza subunit vaccine powder for inhalation. Journal of Controlled Release. 2010;144(2):127–133. [DOI] [PubMed] [Google Scholar]
- 105.Chow MY, Qiu Y, Lo FF, et al. Inhaled powder formulation of naked siRNA using spray drying technology with L-leucine as dispersion enhancer. International journal of pharmaceutics. 2017;530(1-2):40–52. [DOI] [PubMed] [Google Scholar]
- 106.Liang W, Chow MY, Lau PN, et al. Inhalable dry powder formulations of siRNA and pH-responsive peptides with antiviral activity against H1N1 influenza virus. Molecular pharmaceutics. 2015;12(3):910–921. [DOI] [PubMed] [Google Scholar]
- 107.Chow MYT, Qiu Y, Lo FFK, et al. Inhaled powder formulation of naked siRNA using spray drying technology with l-leucine as dispersion enhancer. International Journal of Pharmaceutics. 2017. 2017/September/15/;530(1):40–52. doi: 10.1016/j.ijpharm.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 108.Lechuga-Ballesteros D, Charan C, Stults CLM, et al. Trileucine Improves Aerosol Performance and Stability of Spray-Dried Powders for Inhalation. Journal of Pharmaceutical Sciences. 2008. 2008/January/01/;97(1):287–302. doi: 10.1002/jps.21078. [DOI] [PubMed] [Google Scholar]
- 109.Emami F, Vatanara A, Park EJ, et al. Drying Technologies for the Stability and Bioavailability of Biopharmaceuticals. Pharmaceutics. 2018. August 17;10(3). doi: 10.3390/pharmaceutics10030131. PubMed PMID: 30126135; PubMed Central PMCID: PMCPMC6161129. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kanojia G, Have RT, Soema PC, et al. Developments in the formulation and delivery of spray dried vaccines. Human vaccines & immunotherapeutics. 2017. October 3;13(10):2364–2378. doi: 10.1080/21645515.2017.1356952. PubMed PMID: 28925794; PubMed Central PMCID: PMCPMC5647985. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Maa Y-F, Nguyen P-A, Sweeney T, et al. Protein inhalation powders: spray drying vs spray freeze drying. Pharmaceutical research. 1999;16(2):249–254. [DOI] [PubMed] [Google Scholar]
- 112.Wang W. Lyophilization and development of solid protein pharmaceuticals. International journal of pharmaceutics. 2000;203(1-2):1–60. [DOI] [PubMed] [Google Scholar]
- 113.Tang XC, Pikal MJ. Design of freeze-drying processes for pharmaceuticals: practical advice. Pharmaceutical research. 2004;21(2):191–200. [DOI] [PubMed] [Google Scholar]
- 114.Yu Z, Rogers TL, Hu J, et al. Preparation and characterization of microparticles containing peptide produced by a novel process: spray freezing into liquid. European journal of pharmaceutics and biopharmaceutics. 2002;54(2):221–228. [DOI] [PubMed] [Google Scholar]
- 115.Johnson KA. Preparation of peptide and protein powders for inhalation. Advanced drug delivery reviews. 1997;26(1):3–15. [DOI] [PubMed] [Google Scholar]
- 116.Ferrati S, Wu T, Fuentes O, et al. Influence of Formulation Factors on the Aerosol Performance and Stability of Lysozyme Powders: a Systematic Approach. AAPS PharmSciTech. 2018:1–12. [DOI] [PubMed] [Google Scholar]
- 117.Patel SM, Doen T, Pikal MJ. Determination of End Point of Primary Drying in Freeze-Drying Process Control. AAPS PharmSciTech. 2010. 01/0806/09/received 12/09/accepted;11(1):73–84. doi: 10.1208/s12249-009-9362-7. PubMed PMID: PMC2850457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rey L, May JC. Freeze-Drying/Lyophilization Of Pharmaceutical & Biological Products, Revised and Expanded. CRC Press; 2004. [Google Scholar]
- 119.Sonner C, Maa YF, Lee G. Spray-freeze-drying for protein powder preparation: Particle characterization and a case study with trypsinogen stability. Journal of Pharmaceutical Sciences. 2002. 2002/October/01/;91(10):2122–2139. doi: 10.1002/jps.10204. [DOI] [PubMed] [Google Scholar]
- 120.Liang W, Chan AYL, Chow MYT, et al. Spray freeze drying of small nucleic acids as inhaled powder for pulmonary delivery. Asian Journal of Pharmaceutical Sciences. 2018. 2018/March/01/;13(2):163–172. doi: 10.1016/j.ajps.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen L, Okuda T, Lu XY, et al. Amorphous powders for inhalation drug delivery. Adv Drug Deliv Rev. 2016. May 1;100:102–15. doi: 10.1016/j.addr.2016.01.002. PubMed PMID: 26780404; eng. [DOI] [PubMed] [Google Scholar]
- 122.Webb SD, Golledge SL, Cleland JL, et al. Surface adsorption of recombinant human interferon-gamma in lyophilized and spray-lyophilized formulations. J Pharm Sci. 2002. June;91(6):1474–87. doi: 10.1002/jps.10135. PubMed PMID: 12115847; eng. [DOI] [PubMed] [Google Scholar]
- 123.Costantino HR, Firouzabadian L, Hogeland K, et al. Protein spray-freeze drying. Effect of atomization conditions on particle size and stability. Pharmaceutical research. 2000;17(11):1374–1382. [DOI] [PubMed] [Google Scholar]
- 124.Carpenter JF, Pikal MJ, Chang BS, et al. Rational design of stable lyophilized protein formulations: some practical advice. Pharmaceutical research. 1997;14(8):969–975. [DOI] [PubMed] [Google Scholar]
- 125.Gradon L, Sosnowski TR. Formation of particles for dry powder inhalers. Advanced Powder Technology. 2014. 2014/January/01/;25(1):43–55. doi: 10.1016/j.apt.2013.09.012. [DOI] [Google Scholar]
- 126.Webb SD, Golledge SL, Cleland JL, et al. Surface adsorption of recombinant human interferon-γ in lyophilized and spray-lyophilized formulations. Journal of pharmaceutical sciences. 2002;91(6):1474–1487. [DOI] [PubMed] [Google Scholar]
- 127.Audouy SA, van der Schaaf G, Hinrichs WL, et al. Development of a dried influenza whole inactivated virus vaccine for pulmonary immunization. Vaccine. 2011;29(26):4345–4352. [DOI] [PubMed] [Google Scholar]
- 128.Murugappan S, Patil HP, Kanojia G, et al. Physical and immunogenic stability of spray freeze-dried influenza vaccine powder for pulmonary delivery: comparison of inulin, dextran, or a mixture of dextran and trehalose as protectants. European Journal of Pharmaceutics and Biopharmaceutics. 2013;85(3):716–725. [DOI] [PubMed] [Google Scholar]
- 129.Mauer L, Smith D, Labuza T. Effect of water content, temperature and storage on the glass transition, moisture sorption characteristics and stickiness of β-casein. International Journal of Food Properties. 2000;3(2):233–248. [Google Scholar]
- 130.Otake H, Okuda T, Okamoto H. Development of Spray-Freeze-Dried Powders for Inhalation with High Inhalation Performance and Antihygroscopic Property. Chem Pharm Bull (Tokyo). 2016;64(3):239–45. doi: 10.1248/cpb.c15-00824. PubMed PMID: 26725381; eng. [DOI] [PubMed] [Google Scholar]
- 131.Leung SSY, Parumasivam T, Gao FG, et al. Production of Inhalation Phage Powders Using Spray Freeze Drying and Spray Drying Techniques for Treatment of Respiratory Infections. Pharmaceutical research. 2016;33(6):1486–1496. doi: 10.1007/s11095-016-1892-6. PubMed PMID: 26928668; eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liang W, Chow MYT, Chow SF, et al. Using two-fluid nozzle for spray freeze drying to produce porous powder formulation of naked siRNA for inhalation. International Journal of Pharmaceutics. 2018. 2018/December/01/;552(1):67–75. doi: 10.1016/j.ijpharm.2018.09.045. [DOI] [PubMed] [Google Scholar]
- 133.Kaialy W, Nokhodchi A. Dry powder inhalers: Physicochemical and aerosolization properties of several size-fractions of a promising alterative carrier, freeze-dried mannitol. European Journal of Pharmaceutical Sciences. 2015. 2015/February/20/;68:56–67. doi: 10.1016/j.ejps.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 134.Ferrati S, Wu T, Fuentes O, et al. Influence of formulation factors on the aerosol performance and stability of lysozyme powders: A systematic approach. AAPS PharmSciTech. 2018;19(7):2755–2766. [DOI] [PubMed] [Google Scholar]
- 135.Shoyele SA, Cawthorne S. Particle engineering techniques for inhaled biopharmaceuticals. Advanced drug delivery reviews. 2006;58(9-10):1009–1029. [DOI] [PubMed] [Google Scholar]
- 136.Chow AH, Tong HH, Chattopadhyay P, et al. Particle engineering for pulmonary drug delivery. Pharmaceutical research. 2007;24(3):411–437. [DOI] [PubMed] [Google Scholar]
- 137.Emami F, Vatanara A, Park E, et al. Drying Technologies for the Stability and Bioavailability of Biopharmaceuticals. Pharmaceutics. 2018;10(3):131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Subramaniam B, Rajewski RA, Snavely K. Pharmaceutical processing with supercritical carbon dioxide. Journal of pharmaceutical sciences. 1997;86(8):885–890. [DOI] [PubMed] [Google Scholar]
- 139.Sievers R, Milewski P, Sellers S, et al. Supercritical CO2-assisted methods for the production and pulmonary administration of pharmaceutical aerosols. Journal of Aerosol Science. 1998. (29):S1271–S1272. [Google Scholar]
- 140.Kim YH, Shing KS. Supercritical fluid-micronized ipratropium bromide for pulmonary drug delivery. Powder Technology. 2008;182(1):25–32. [Google Scholar]
- 141.Steckel H, Pichert L, Müller BW. Influence of process parameters in the ASES process on particle properties of budesonide for pulmonary delivery. European journal of pharmaceutics and biopharmaceutics. 2004;57(3):507–512. [DOI] [PubMed] [Google Scholar]
- 142.Shekunov BY, Feeley JC, Chow AH, et al. Aerosolisation behaviour of micronised and supercritically-processed powders. Journal of Aerosol Science. 2003;34(5):553–568. [Google Scholar]
- 143.Rehman M, Shekunov BY, York P, et al. Optimisation of powders for pulmonary delivery using supercritical fluid technology. European journal of pharmaceutical sciences. 2004;22(1):1–17. [DOI] [PubMed] [Google Scholar]
- 144.Fages J, Lochard H, Letourneau J-J, et al. Particle generation for pharmaceutical applications using supercritical fluid technology. Powder Technology. 2004. 2004/March/30/;141(3):219–226. doi: 10.1016/j.powtec.2004.02.007. [DOI] [Google Scholar]
- 145.Debenedetti PG, Tom JW, Kwauk X, et al. Rapid expansion of supercritical solutions (RESS): fundamentals and applications. Fluid Phase Equilibria. 1993;82:311–321. [Google Scholar]
- 146.Matson DW, Fulton JL, Petersen RC, et al. Rapid expansion of supercritical fluid solutions: solute formation of powders, thin films, and fibers. Industrial & engineering chemistry research. 1987;26(11):2298–2306. [Google Scholar]
- 147.Sellers SP, Clark GS, Sievers RE, et al. Dry powders of stable protein formulations from aqueous solutions prepared using supercritical CO2-assisted aerosolization. Journal of Pharmaceutical Sciences. 2001. 2001/June/01/;90(6):785–797. doi: 10.1002/jps.1032. [DOI] [PubMed] [Google Scholar]
- 148.Yeo SD, Lim GB, Debendetti PG, et al. Formation of microparticulate protein powder using a supercritical fluid antisolvent. Biotechnology and Bioengineering. 1993;41(3):341–346. [DOI] [PubMed] [Google Scholar]
- 149.Sievers R Formation of aqueous small droplet aerosols assisted by supercritical carbon dioxide. Aerosol Science & Technology. 1999;30(1):3–15. [Google Scholar]
- 150.Rehman M, Shekunov BY, York P, et al. Optimisation of powders for pulmonary delivery using supercritical fluid technology. European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences. 2004. May;22(1):1–17. doi: 10.1016/j.ejps.2004.02.001. PubMed PMID: 15113578; eng. [DOI] [PubMed] [Google Scholar]
- 151.Jovanović N, Bouchard A, Sutter M, et al. Stable sugar-based protein formulations by supercritical fluid drying. International journal of pharmaceutics. 2008;346(1-2):102–108. [DOI] [PubMed] [Google Scholar]
- 152.Weers J, Tarara T. The PulmoSphere™ platform for pulmonary drug delivery. Therapeutic delivery. 2014;5(3):277–295. [DOI] [PubMed] [Google Scholar]
- 153.Miller DP, Tan T, Nakamura J, et al. Physical Characterization of Tobramycin Inhalation Powder: II. State Diagram of an Amorphous Engineered Particle Formulation. Mol Pharm. 2017. June 5;14(6):1950–1960. doi: 10.1021/acs.molpharmaceut.7b00036. PubMed PMID: 28418683; eng. [DOI] [PubMed] [Google Scholar]
- 154.Miller DP, Tan T, Tarara TE, et al. Physical Characterization of Tobramycin Inhalation Powder: I. Rational Design of a Stable Engineered-Particle Formulation for Delivery to the Lungs. Mol Pharm. 2015. August 3;12(8):2582–93. doi: 10.1021/acs.molpharmaceut.5b00147. PubMed PMID: 26052676; eng. [DOI] [PubMed] [Google Scholar]
- 155.McShane PJ, Weers JG, Tarara TE, et al. Ciprofloxacin Dry Powder for Inhalation (ciprofloxacin DPI): Technical design and features of an efficient drug–device combination. Pulmonary pharmacology & therapeutics. 2018. [DOI] [PubMed] [Google Scholar]
- 156.Xu L-M, Zhang Q-X, Zhou Y, et al. Engineering drug ultrafine particles of beclomethasone dipropionate for dry powder inhalation. International Journal of Pharmaceutics. 2012. 2012/October/15/;436(1):1–9. doi: 10.1016/j.ijpharm.2012.06.038. [DOI] [PubMed] [Google Scholar]
- 157.Saboti D, Maver U, Chan HK, et al. Novel Budesonide Particles for Dry Powder Inhalation Prepared Using a Microfluidic Reactor Coupled With Ultrasonic Spray Freeze Drying. J Pharm Sci. 2017. July;106(7):1881–1888. doi: 10.1016/j.xphs.2017.02.035. PubMed PMID: 28285981; eng. [DOI] [PubMed] [Google Scholar]
- 158.Thiele J, Windbergs M, Abate AR, et al. Early development drug formulation on a chip: fabrication of nanoparticles using a microfluidic spray dryer. Lab on a chip. 2011. July 21;11(14):2362–8. doi: 10.1039/c1lc20298g. PubMed PMID: 21617823; eng. [DOI] [PubMed] [Google Scholar]
- 159.Mansur EA, Ye M, Wang Y, et al. A State-of-the-Art Review of Mixing in Microfluidic Mixers. Chinese Journal of Chemical Engineering. 2008. 2008/January/01/;16(4):503–516. doi: 10.1016/S1004-9541(08)60114-7. [DOI] [Google Scholar]
- 160.Wilson EM, Luft JC, DeSimone JM. Formulation of High-Performance Dry Powder Aerosols for Pulmonary Protein Delivery. Pharm Res. 2018. August 23;35(10):195. doi: 10.1007/s11095-018-2452-z. PubMed PMID: 30141117; eng. [DOI] [PubMed] [Google Scholar]
- 161.Xu J, Wang J, Luft JC, et al. Rendering protein-based particles transiently insoluble for therapeutic applications. J Am Chem Soc. 2012. May 30;134(21):8774–7. doi: 10.1021/ja302363r. PubMed PMID: 22568387; PubMed Central PMCID: PMCPMC3365610. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Elamin AA, Sebhatu T, Ahlneck C. The use of amorphous model substances to study mechanically activated materials in the solid state. International Journal of Pharmaceutics. 1995. 1995/May/30/;119(1):25–36. doi: 10.1016/0378-5173(94)00364-B. [DOI] [Google Scholar]
- 163.Shah B, Kakumanu VK, Bansal AK. Analytical techniques for quantification of amorphous/crystalline phases in pharmaceutical solids. J Pharm Sci. 2006. August;95(8):1641–65. doi: 10.1002/jps.20644. PubMed PMID: 16802362; eng. [DOI] [PubMed] [Google Scholar]
- 164.Yu L Surface mobility of molecular glasses and its importance in physical stability. Advanced Drug Delivery Reviews. 2016. 2016/May/01/;100:3–9. doi: 10.1016/j.addr.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 165.Lehto V-P, Tenho M, Vähä-Heikkilä K, et al. The comparison of seven different methods to quantify the amorphous content of spray dried lactose. Powder Technology. 2006. 2006/September/15/;167(2):85–93. doi: 10.1016/j.powtec.2006.05.019. [DOI] [Google Scholar]
- 166.Saleki-Gerhardt A, Ahlneck C, Zografi G. Assessment of disorder in crystalline solids. International Journal of Pharmaceutics. 1994. 1994/January/25/;101(3):237–247. doi: 10.1016/0378-5173(94)90219-4. [DOI] [Google Scholar]
- 167.Newman A, Zografi G. Critical considerations for the qualitative and quantitative determination of process-induced disorder in crystalline solids. Journal of pharmaceutical sciences. 2014;103(9):2595–2604. [DOI] [PubMed] [Google Scholar]
- 168.Price R, Young PM. Visualization of the crystallization of lactose from the amorphous state. Journal of pharmaceutical sciences. 2004. 2004/January/01/;93(1):155–164. doi: 10.1002/jps.10513. [DOI] [PubMed] [Google Scholar]
- 169.Simon A, Amaro MI, Cabral LM, et al. Development of a novel dry powder inhalation formulation for the delivery of rivastigmine hydrogen tartrate. International Journal of Pharmaceutics. 2016. 2016/March/30/;501(1):124–138. doi: 10.1016/j.ijpharm.2016.01.066. [DOI] [PubMed] [Google Scholar]
- 170.Leung SSY, Parumasivam T, Nguyen A, et al. Effect of storage temperature on the stability of spray dried bacteriophage powders. European Journal of Pharmaceutics and Biopharmaceutics. 2018. 2018/June/01/;127:213–222. doi: 10.1016/j.ejpb.2018.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mangal S, Nie H, Xu R, et al. Physico-Chemical Properties, Aerosolization and Dissolution of Co-Spray Dried Azithromycin Particles with L-Leucine for Inhalation. Pharmaceutical Research. 2018. 2018/January/08;35(2):28. doi: 10.1007/s11095-017-2334-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Taylor LS, Zografi G. The Quantitative Analysis of Crystallinity Using FT-Raman Spectroscopy. Pharmaceutical Research. 1998. 1998/May/01;15(5):755–761. doi: 10.1023/A:1011979221685. [DOI] [PubMed] [Google Scholar]
- 173.Pajander JP, Matero S, Sloth J, et al. Raman mapping of mannitol/lysozyme particles produced via spray drying and single droplet drying. Pharmaceutical research. 2015;32(6):1993–2002. [DOI] [PubMed] [Google Scholar]
- 174.Maas SG, Schaldach G, Littringer EM, et al. The impact of spray drying outlet temperature on the particle morphology of mannitol. Powder Technology. 2011. 2011/November/10/;213(1):27–35. doi: 10.1016/j.powtec.2011.06.024. [DOI] [Google Scholar]
- 175.Molina C, Kaialy W, Chen Q, et al. Agglomerated novel spray-dried lactose-leucine tailored as a carrier to enhance the aerosolization performance of salbutamol sulfate from DPI formulations. Drug Delivery and Translational Research. 2018. 2018/December/01;8(6):1769–1780. doi: 10.1007/s13346-017-0462-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Eedara BB, Rangnekar B, Doyle C, et al. The influence of surface active l-leucine and 1,2-dipalmitoyl-sn-glycero-3-phosphatidylcholine (DPPC) in the improvement of aerosolization of pyrazinamide and moxifloxacin co-spray dried powders. International Journal of Pharmaceutics. 2018. 2018/May/05/;542(1):72–81. doi: 10.1016/j.ijpharm.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 177.Molina C, Kaialy W, Nokhodchi A. The crucial role of leucine concentration on spray dried mannitol-leucine as a single carrier to enhance the aerosolization performance of Albuterol sulfate. Journal of Drug Delivery Science and Technology. 2019. 2019/February/01/;49:97–106. doi: 10.1016/j.jddst.2018.11.007. [DOI] [Google Scholar]
- 178.Kwok PCL, Chan H-K. Pulmonary delivery of peptides and proteins Peptide and Protein Delivery: Elsevier; 2011. p. 23–46. [Google Scholar]
- 179.Toporski J, Dieing T, Hollricher O. Confocal Raman Microscopy. Vol. 66 Springer; 2018. [Google Scholar]
- 180.Duan J, Vogt FG, Li X, et al. Design, characterization, and aerosolization of organic solution advanced spray-dried moxifloxacin and ofloxacin dipalmitoylphosphatidylcholine (DPPC) microparticulate/nanoparticulate powders for pulmonary inhalation aerosol delivery. International journal of nanomedicine. 2013;8:3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ayala AP. Polymorphism in drugs investigated by low wavenumber Raman scattering. Vibrational Spectroscopy. 2007;45(2):112–116. [Google Scholar]
- 182.Láng P, Kiss V, Ambrus R, et al. Polymorph screening of an active material. Journal of pharmaceutical and biomedical analysis. 2013;84:177–183. [DOI] [PubMed] [Google Scholar]
- 183.Höhne G, Hemminger WF, Flammersheim H-J. Differential scanning calorimetry. Springer Science & Business Media; 2013. [Google Scholar]
- 184.Knopp MM, Löbmann K, Elder DP, et al. Recent advances and potential applications of modulated differential scanning calorimetry (mDSC) in drug development. European Journal of Pharmaceutical Sciences. 2016;87:164–173. [DOI] [PubMed] [Google Scholar]
- 185.Adi H, Young PM, Chan H-K, et al. Co-spray-dried mannitol–ciprofloxacin dry powder inhaler formulation for cystic fibrosis and chronic obstructive pulmonary disease. European Journal of Pharmaceutical Sciences. 2010. 2010/June/14/;40(3):239–247. doi: 10.1016/j.ejps.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 186.Burnett DJ, Thielmann F, Booth J. Determining the critical relative humidity for moisture-induced phase transitions. International Journal of Pharmaceutics. 2004. 2004/December/09/;287(1):123–133. doi: 10.1016/j.ijpharm.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 187.Buckton G, Darcy P. The use of gravimetric studies to assess the degree of crystallinity of predominantly crystalline powders. International Journal of Pharmaceutics. 1995. 1995/September/12/;123(2):265–271. doi: 10.1016/0378-5173(95)00083-U. [DOI] [Google Scholar]
- 188.Chew NY, Chan H-K. The role of particle properties in pharmaceutical powder inhalation formulations. Journal of aerosol medicine. 2002;15(3):325–330. [DOI] [PubMed] [Google Scholar]
- 189.Hickey AJ. Fundamentals of Dry Powder Inhaler Technology Particles and Nanoparticles in Pharmaceutical Products: Springer; 2018. p. 213–232. [Google Scholar]
- 190.Buckton G Characterisation of small changes in the physical properties of powders of significance for dry powder inhaler formulations. Advanced drug delivery reviews. 1997;26(1):17–27. [DOI] [PubMed] [Google Scholar]
- 191.Ho R, Heng JY. A review of inverse gas chromatography and its development as a tool to characterize anisotropic surface properties of pharmaceutical solids. KONA Powder and Particle Journal. 2013;30:164–180. [Google Scholar]
- 192.Adi H, Traini D, Chan H-K, et al. The Influence of Drug Morphology on Aerosolisation Efficiency of Dry Powder Inhaler Formulations. Journal of Pharmaceutical Sciences. 2008. 2008/July/01/;97(7):2780–2788. doi: 10.1002/jps.21195. [DOI] [PubMed] [Google Scholar]
- 193.Hickey AJ, Mansour HM, Telko MJ, et al. Physical characterization of component particles included in dry powder inhalers. I. Strategy review and static characteristics. J Pharm Sci. 2007. May;96(5):1282–301. doi: 10.1002/jps.20916. PubMed PMID: 17455324; eng. [DOI] [PubMed] [Google Scholar]
- 194.Weiss C, McLoughlin P, Cathcart H. Characterisation of dry powder inhaler formulations using atomic force microscopy. International Journal of Pharmaceutics. 2015. 2015/October/15/;494(1):393–407. doi: 10.1016/j.ijpharm.2015.08.051. [DOI] [PubMed] [Google Scholar]
- 195.Adi S, Adi H, Tang P, et al. Micro-particle corrugation, adhesion and inhalation aerosol efficiency. European Journal of Pharmaceutical Sciences. 2008. 2008/September/02/;35(1):12–18. doi: 10.1016/j.ejps.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 196.Davies MJ, Brindley A, Chen X, et al. A quantitative assessment of inhaled drug particle–pulmonary surfactant interaction by atomic force microscopy. Colloids and Surfaces B: Biointerfaces. 2009. 2009/October/01/;73(1):97–102. doi: 10.1016/j.colsurfb.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 197.Butt H-J. Measuring electrostatic, van der Waals, and hydration forces in electrolyte solutions with an atomic force microscope. Biophysical Journal. 1991;60(6):1438–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ducker WA, Senden TJ, Pashley RM. Direct measurement of colloidal forces using an atomic force microscope. Nature. 1991;353(6341):239. [Google Scholar]
- 199.Begat P, Morton DA, Staniforth JN, et al. The cohesive-adhesive balances in dry powder inhaler formulations I: direct quantification by atomic force microscopy. Pharmaceutical research. 2004;21(9):1591–1597. [DOI] [PubMed] [Google Scholar]
- 200.Danesh A, Chen X, Davies MC, et al. The discrimination of drug polymorphic forms from single crystals using atomic force microscopy. Pharm Res. 2000. July;17(7):887–90. PubMed PMID: 10990210; eng. [DOI] [PubMed] [Google Scholar]
- 201.Hooton JC, Jones MD, Harris H, et al. The influence of crystal habit on the prediction of dry powder inhalation formulation performance using the cohesive-adhesive force balance approach. Drug Dev Ind Pharm. 2008. September;34(9):974–83. doi: 10.1080/03639040802149087. PubMed PMID: 18622874; eng. [DOI] [PubMed] [Google Scholar]
- 202.Young PM, Price R, Tobyn MJ, et al. Investigation into the Effect of Humidity on Drug–Drug Interactions Using the Atomic Force Microscope. Journal of Pharmaceutical Sciences. 2003. 2003/04/January/;92(4):815–822. doi: 10.1002/jps.10250. [DOI] [PubMed] [Google Scholar]
- 203.Telko MJ, Hickey AJ. Dry powder inhaler formulation. Respiratory care. 2005;50(9):1209–1227. [PubMed] [Google Scholar]
- 204.Alhalaweh A, Vilinska A, Gavini E, et al. Surface thermodynamics of mucoadhesive dry powder formulation of zolmitriptan. Aaps Pharmscitech. 2011;12(4):1186–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Cline D, Dalby R. Predicting the quality of powders for inhalation from surface energy and area. Pharmaceutical Research. 2002;19(9):1274–1277. [DOI] [PubMed] [Google Scholar]
- 206.Newell HE, Buckton G, Butler DA, et al. The use of inverse phase gas chromatography to study the change of surface energy of amorphous lactose as a function of relative humidity and the processes of collapse and crystallisation. International journal of pharmaceutics. 2001;217(1-2):45–56. [DOI] [PubMed] [Google Scholar]
- 207.Das SC, Tucker IG, Stewart PJ . Surface energy determined by inverse gas chromatography as a tool to investigate particulate interactions in dry powder inhalers. Current pharmaceutical design. 2015;21(27):3932–3944. [DOI] [PubMed] [Google Scholar]
- 208.Newell HE, Buckton G, Butler DA, et al. The use of inverse phase gas chromatography to study the change of surface energy of amorphous lactose as a function of relative humidity and the processes of collapse and crystallisation. International Journal of Pharmaceutics. 2001. 2001/April/17/;217(1):45–56. doi: 10.1016/S0378-5173(01)00589-0. [DOI] [PubMed] [Google Scholar]
- 209.Wu X, Li X, Mansour HM. Surface analytical techniques in solid-state particle characterization for predicting performance in dry powder inhalers. KONA Powder and Particle Journal. 2010;28:3–19. [Google Scholar]
- 210.Zhou Q, Denman JA, Gengenbach T, et al. Characterization of the surface properties of a model pharmaceutical fine powder modified with a pharmaceutical lubricant to improve flow via a mechanical dry coating approach. Journal of Pharmaceutical Sciences. 2011. 2011/August/01/;100(8):3421–3430. doi: 10.1002/jps.22547.• Of interests: Developed a platform characterizating fine particle surface chemistry qualitatively and quantitatively with high surface sensitivity and superior spacial resolution.
- 211.Buckton G, Ambarkhane A, Pincott K. The Use of Inverse Phase Gas Chromatography to Study the Glass Transition Temperature of a Powder Surface. Pharmaceutical Research. 2004. 2004/September/01;21(9):1554–1557. doi: 10.1023/B:PHAM.0000041447.15874.f7. [DOI] [PubMed] [Google Scholar]
- 212.Jong T, Li J, Morton DAV, et al. Investigation of the Changes in Aerosolization Behavior Between the Jet-Milled and Spray-Dried Colistin Powders Through Surface Energy Characterization. Journal of Pharmaceutical Sciences. 2016. 2016/March/01/;105(3):1156–1163. doi: 10.1016/S0022-3549(15)00189-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Voelkel A, Strzemiecka B, Adamska K, et al. Inverse gas chromatography as a source of physiochemical data. Journal of chromatography A. 2009. March 6;1216(10):1551–66. doi: 10.1016/j.chroma.2008.10.096. PubMed PMID: 19010482; eng. [DOI] [PubMed] [Google Scholar]
- 214.Lin Y-W, Wong J, Qu L, et al. Powder production and particle engineering for dry powder inhaler formulations. Current pharmaceutical design. 2015;21(27):3902–3916. [DOI] [PubMed] [Google Scholar]
- 215.Qu L, Morton DA, Zhou QT. Particle Engineering Via Mechanical Dry Coating in the Design of Pharmaceutical Solid Dosage Forms. Curr Pharm Des. 2015;21(40):5802–14. doi: 10.2174/1381612821666151008151001. PubMed PMID: 26446461; eng. [DOI] [PubMed] [Google Scholar]
- 216.Kasemo B, Lausmaa J. Biomaterial and implant surfaces: a surface science approach. International Journal of Oral & Maxillofacial Implants. 1988;3(4). [PubMed] [Google Scholar]
- 217.Schulze M, Wagner N, Kaz T, et al. Combined electrochemical and surface analysis investigation of degradation processes in polymer electrolyte membrane fuel cells. Electrochimica Acta. 2007;52(6):2328–2336. [Google Scholar]
- 218.Muralidharan P, Acosta M, Hayes D, et al. Fundamentals of Solid-State Characterization of Dry Powder Inhalers. 2016. [Google Scholar]
- 219.Abd Mutalib M, Rahman MA, Othman MHD, et al. Chapter 9 - Scanning Electron Microscopy (SEM) and Energy-Dispersive X-Ray (EDX) Spectroscopy In: Hilal N, Ismail AF, Matsuura T, et al., editors. Membrane Characterization: Elsevier; 2017. p. 161–179. [Google Scholar]
- 220.Pfeffer R, Dave RN, Wei D, et al. Synthesis of engineered particulates with tailored properties using dry particle coating. Powder Technology. 2001. 2001/June/04/;117(1):40–67. doi: 10.1016/S0032-5910(01)00314-X. [DOI] [Google Scholar]
- 221.Stank K, Steckel H. Physico-chemical characterisation of surface modified particles for inhalation. International Journal of Pharmaceutics. 2013. 2013/May/01/;448(1):9–18. doi: 10.1016/j.ijpharm.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 222.Dahmash EZ, Mohammed AR. Characterisation and surface-profiling techniques for composite particles produced by dry powder coating in pharmaceutical drug delivery. Drug discovery today. 2016. 2016/April/01/;21(4):550–561. doi: 10.1016/j.drudis.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 223.Wang W, Zhou QT, Sun S-P, et al. Effects of Surface Composition on the Aerosolisation and Dissolution of Inhaled Antibiotic Combination Powders Consisting of Colistin and Rifampicin. The AAPS Journal. 2016. 2016//;18(2):372–384. doi: 10.1208/s12248-015-9848-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Mangal S, Xu R, Park H, et al. Understanding the Impacts of Surface Compositions on the In-Vitro Dissolution and Aerosolization of Co-Spray-Dried Composite Powder Formulations for Inhalation. Pharmaceutical Research. 2018. 2018/November/07;36(1):6. doi: 10.1007/s11095-018-2527-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Zhou Q, Qu L, Gengenbach T, et al. Investigation of the extent of surface coating via mechanofusion with varying additive levels and the influences on bulk powder flow properties. International Journal of Pharmaceutics. 2011. 2011/July/15/;413(1):36–43. doi: 10.1016/j.ijpharm.2011.04.014. [DOI] [PubMed] [Google Scholar]


